Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
68 results about "Intra peritoneal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of intraperitoneal. : existing within or administered by entry into the peritoneum intraperitoneal chemotherapy.
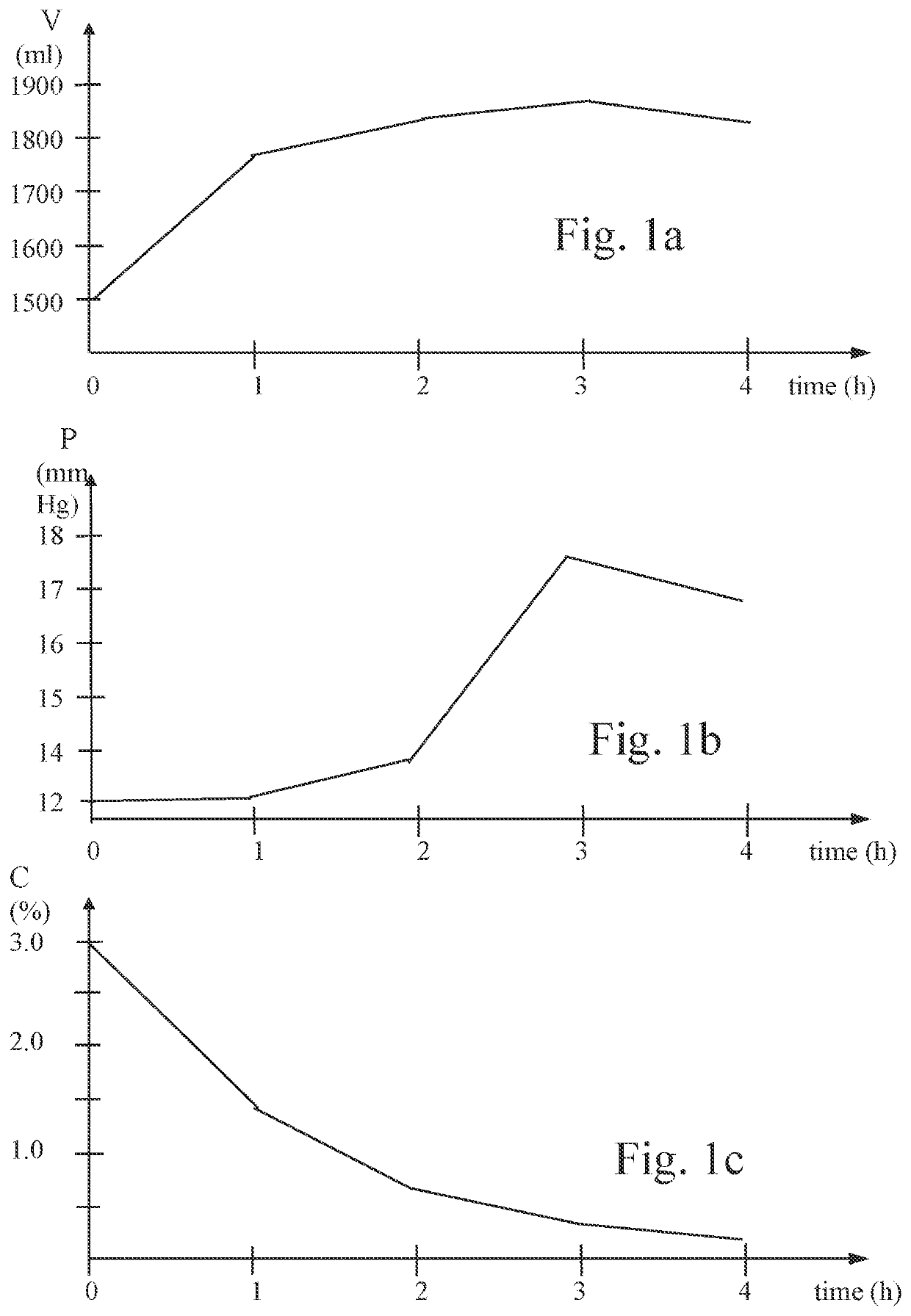
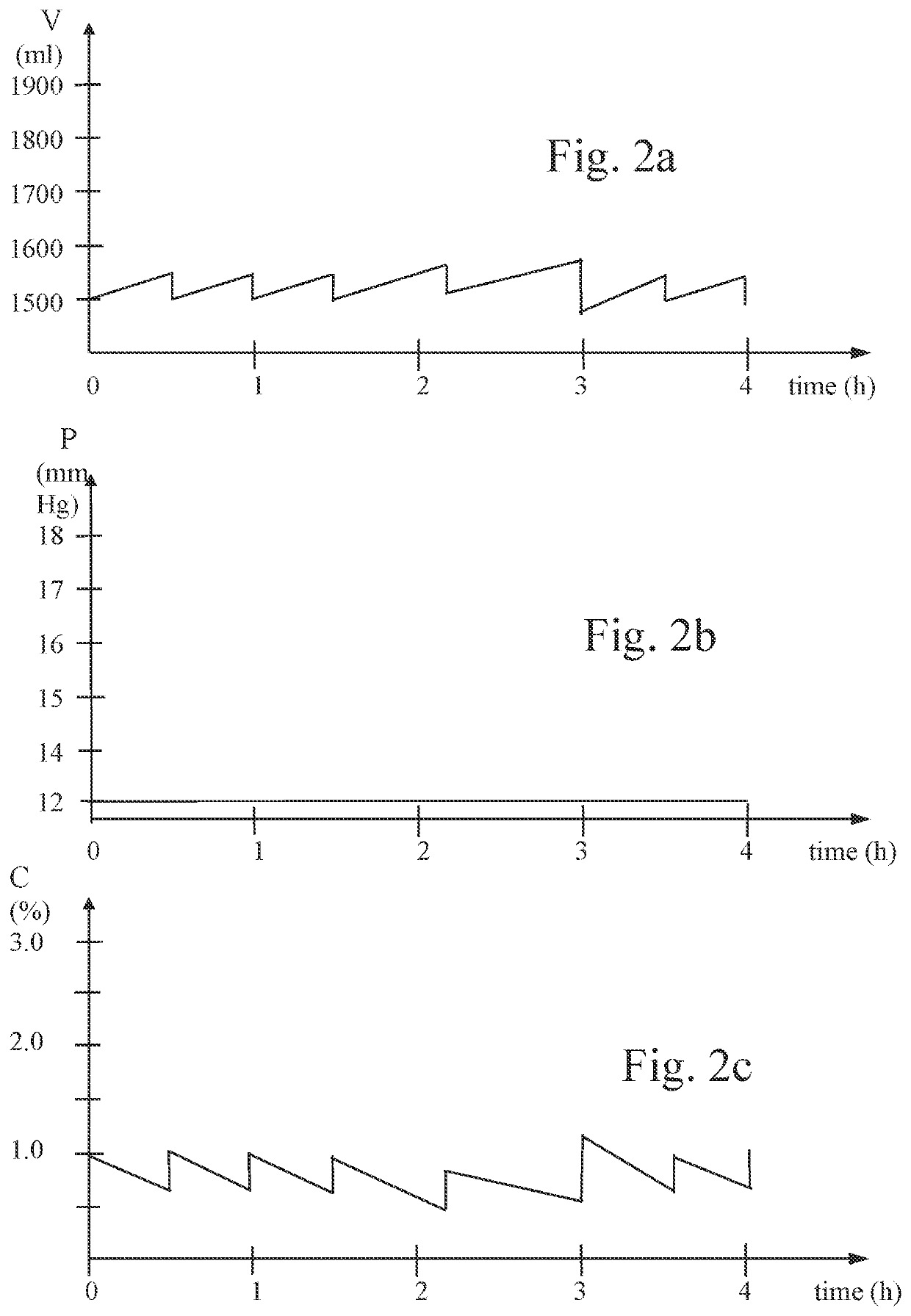
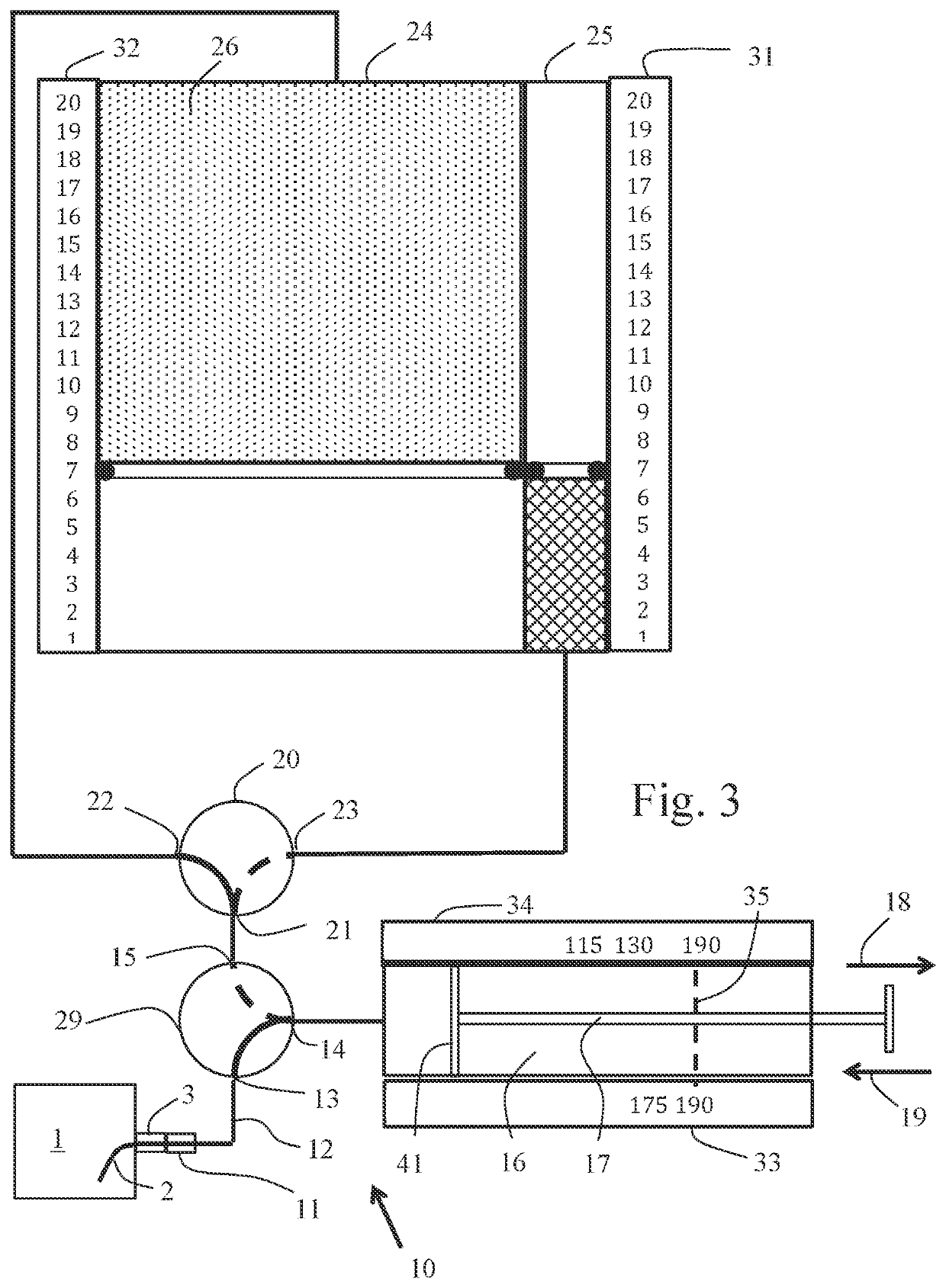
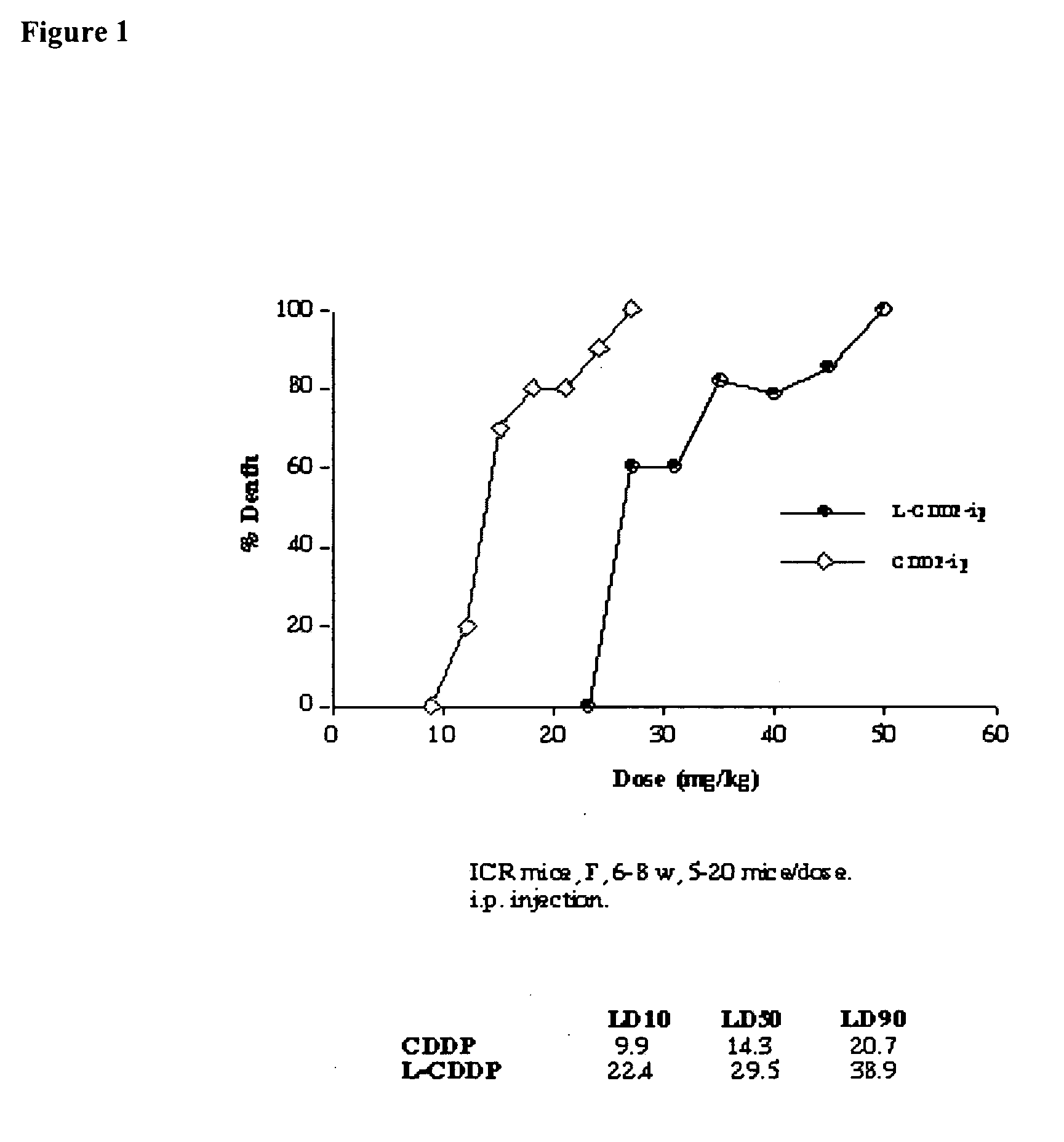
Method for inducing hypothermia
InactiveUS6962601B2Simple methodImprove the protective effectOther chemical processesLighting and heating apparatusParticulatesSlurry
Systems for phase-change particulate slurry cooling equipment and methods to induce hypothermia in a patient through internal and external cooling are provided. Subcutaneous, intravascular, intraperitoneal, gastrointestinal, and lung methods of cooling are carried out using saline ice slurries or other phase-change slurries compatible with human tissue. Perfluorocarbon slurries or other slurry types compatible with human tissue are used for pulmonary cooling. And traditional external cooling methods are improved by utilizing phase-change slurry materials in cooling caps and torso blankets.
Owner:UNIVERSITY OF CHICAGO +1
Therapy with clostridium perfringens enterotoxin to treat ovarian and uterine cancer
ActiveUS20060084594A1Low toxicityGood curative effectBacterial antigen ingredientsIn-vivo radioactive preparationsIntraperitoneal routeCancer cell
The invention discloses high levels of receptors for Clostridium perfringens enterotoxin (CPE) have been found in ovarian cancer and uterine cancer tissue samples. In addition, successful in vivo treatment of a mouse model of ovarian cancer with intraperitoneal injection of CPE is disclosed. High levels of Ep-CAM protein is also disclosed in ovarian cancer tissue samples. Thus, the invention provides a method of treating ovarian cancer and uterine cancer by administering CPE. The invention also provides a method of treating cancer in a mammal involving intraperitoneal administration of CPE, where at least some cancerous cells are located in or adjacent to the peritoneal cavity of the mammal. The invention also provides a method of treating ovarian cancer involving administering an anti-Ep-CAM antibody. The invention also provides a method of treating cancers expressing claudin-3 or claudin-4 by administering an antibody against claudin-3 and / or an antibody against claudin-4. The invention also provides a method of protecting a mammal from CPE toxicity involving administering a protective agent that binds to claudin-3 and / or claudin-4 and inhibits CPE binding to claudin-3 and / or claudin-4.
Owner:YALE UNIV
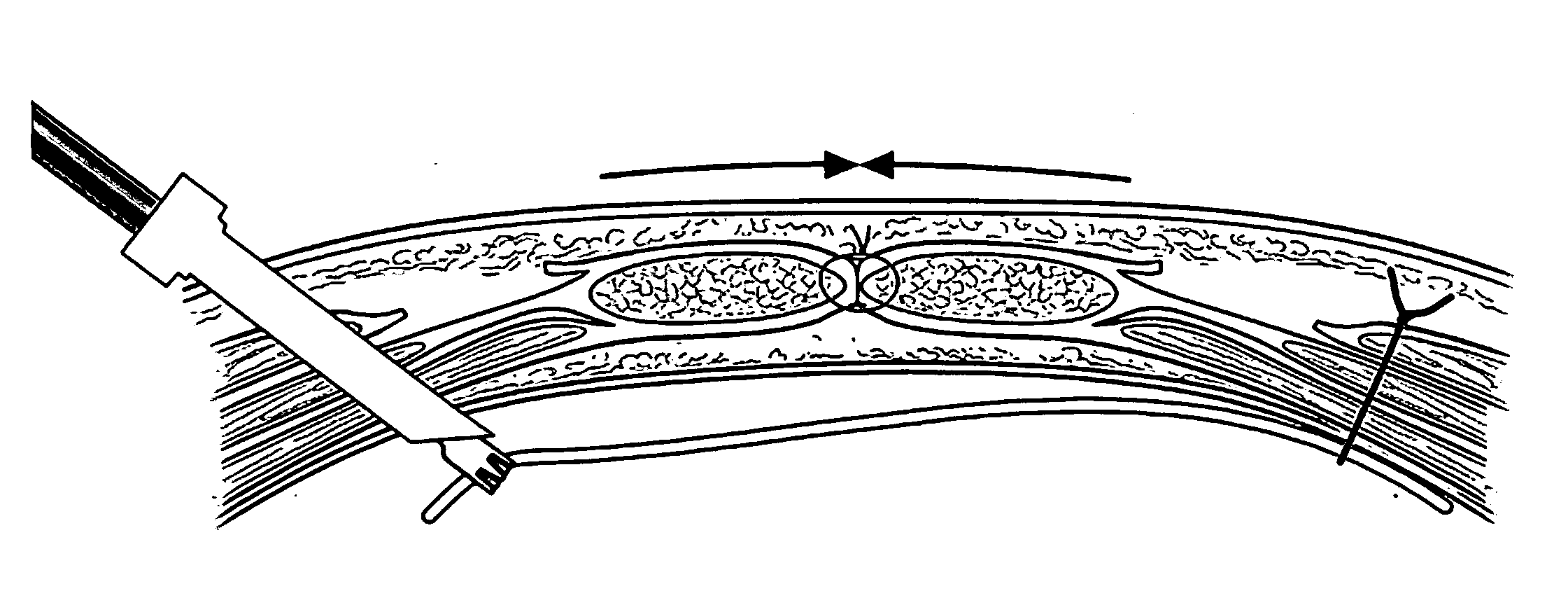
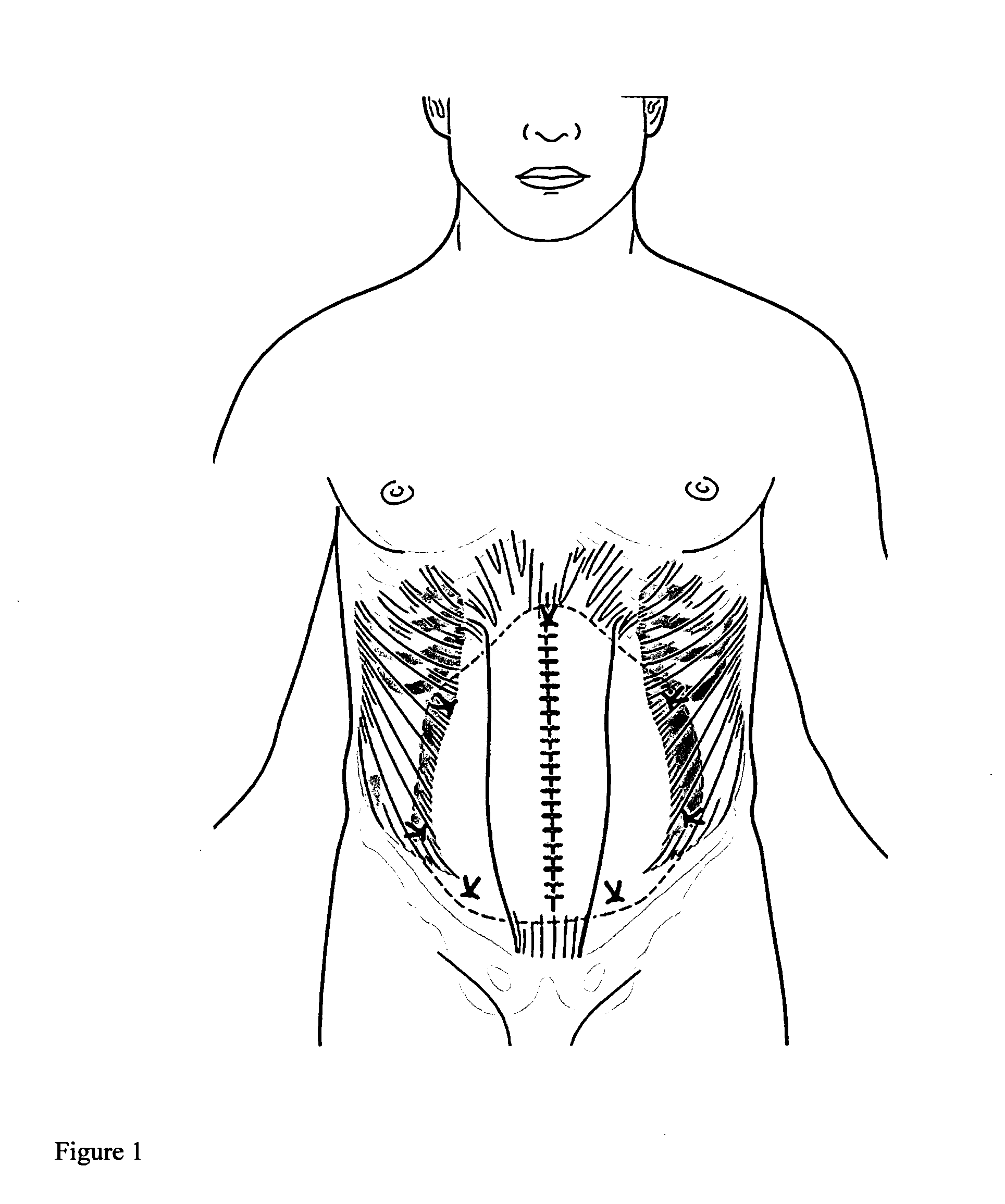
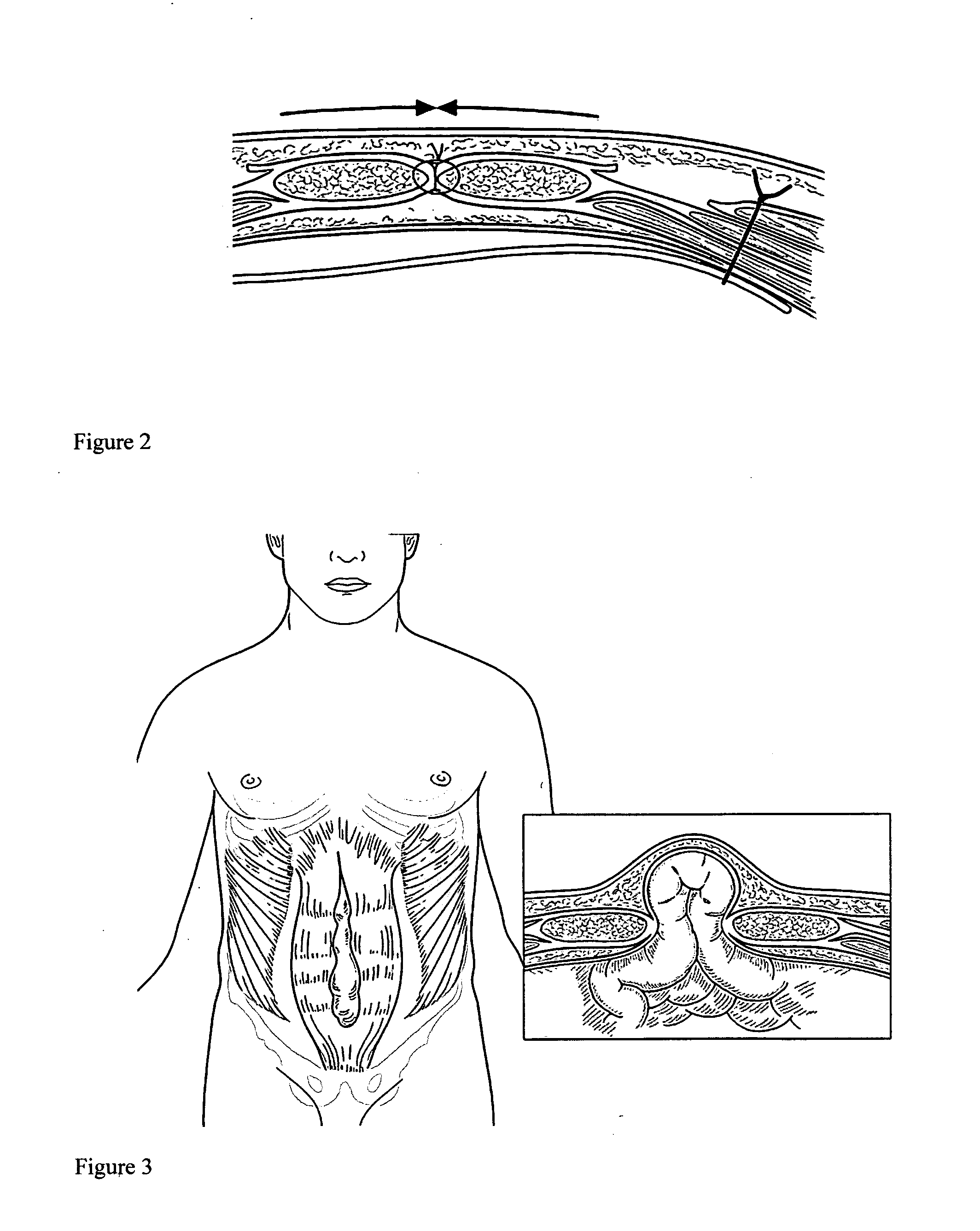
Apparatus and method for use of a biosurgical prosthetic rectus sheath
InactiveUS20090234461A1Promote healingEasy and efficient to manufactureSuture equipmentsStapling toolsAbdominal wall closureSurgical approach
A prosthetic mesh and method of use in surgical methods for closure of abdominal incisions and prosthetics for repair of ventral hernias. The mesh provides for a new prosthetic that can be used for closure of laparotomy incisions, in patients at high risk for hernia formation, and in the repair of existing ventral hernias. The mesh utilizes existing multi laminar technology for intra peritoneal mesh, and fashions it in the shape of an intact Rectus Sheath. The mesh matrix can be made of any of the various absorbable or permanent polymer filaments that are woven or knitted into a scaffold for added strength to the abdominal wall closure during the healing phase, and for delivery of bio active substances that contribute to a biochemical milieu that contributes to favorable healing. A permanent non absorbable prosthesis will be desirable in certain circumstances and for particular patients, a slowly dissolving mesh would be ideal in most cases. The absorption of the prosthesis must be slow, approximately one to two years, thus giving enough time to preserve support for the healing abdominal wall repair, as this is the period of time over which most incisional hernias present.
Owner:REHNKE ROBERT D
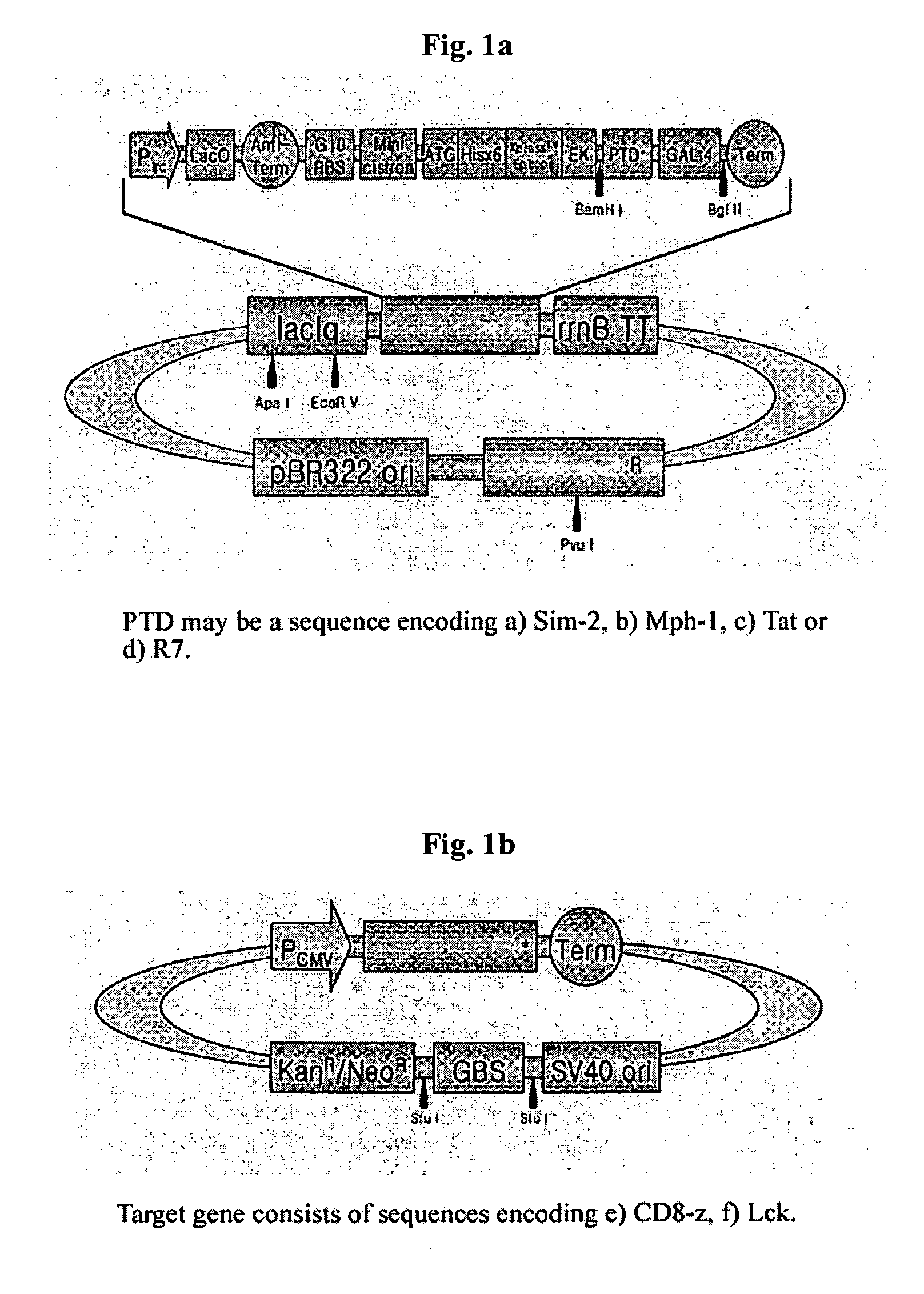
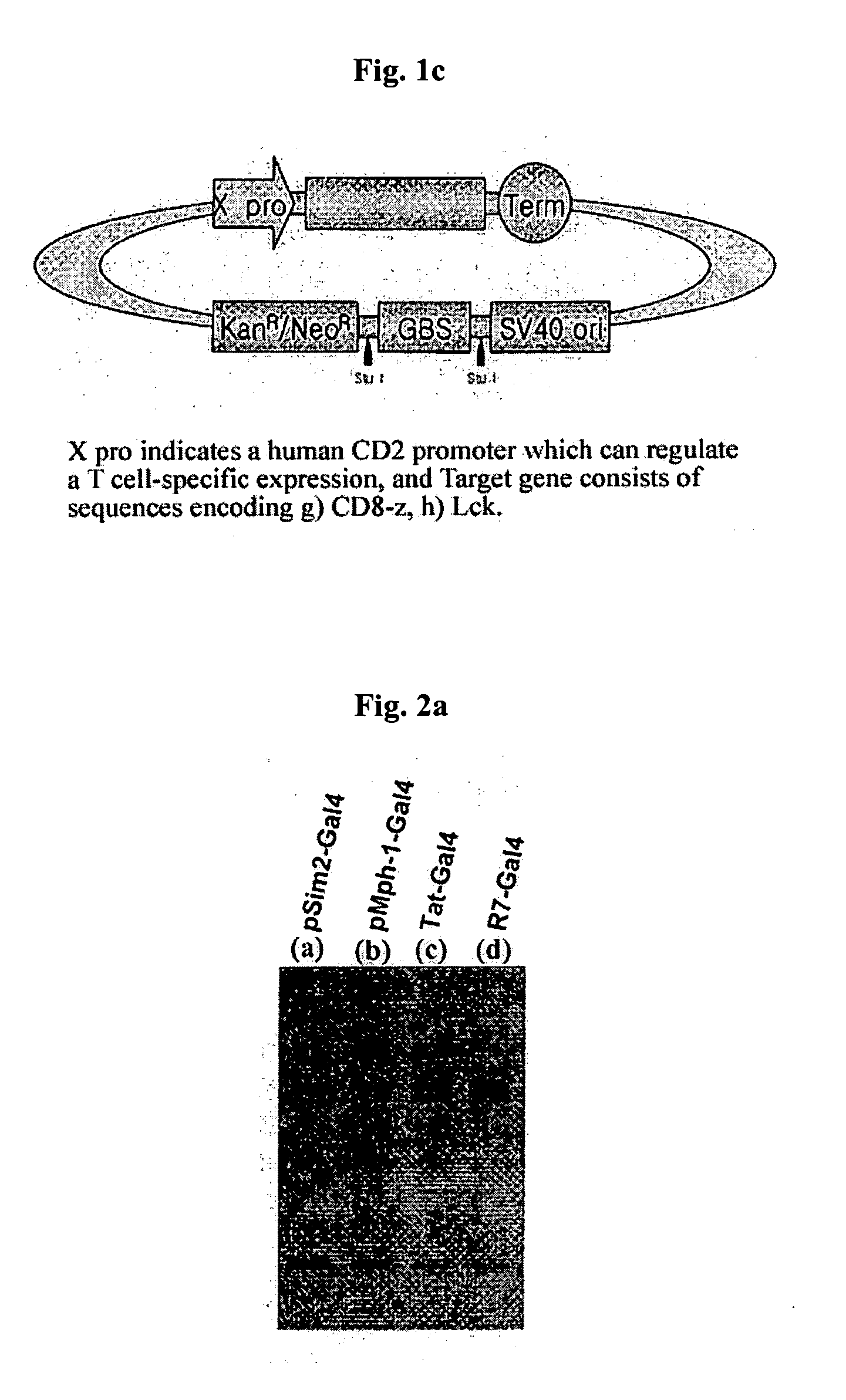
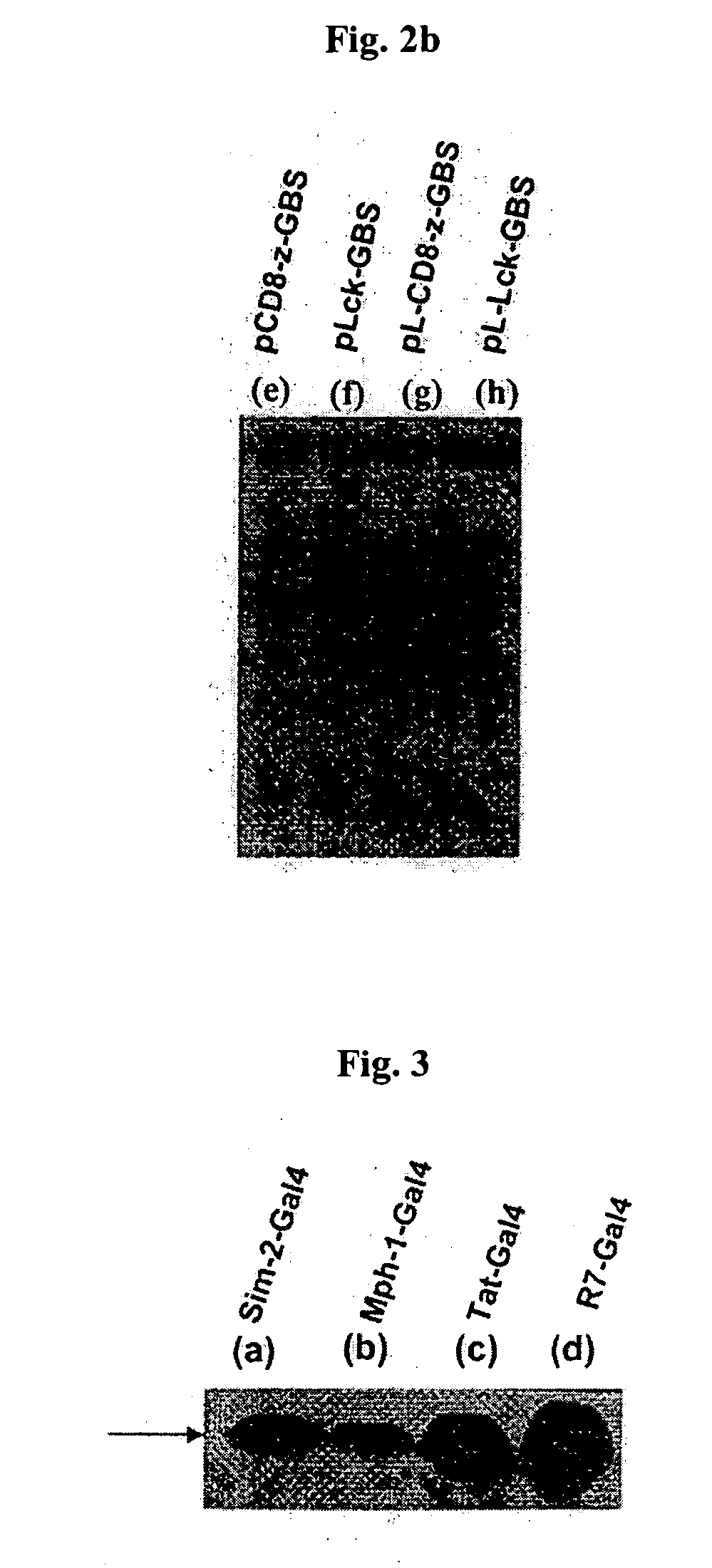
DNA/RNA transduction technology and its clinical and basic applications
InactiveUS20060099677A1Polypeptide with localisation/targeting motifFusion with DNA-binding domainIn vivoCytoplasm
This invention relates to a method for in vivo and in vitro transferring efficiently DNA / RNA coding materials regulating bio-function to cytoplasm or nucleus in eukaryotic or prokaryotic cells using PTD (Protein Transduction Domain) and DNA / RNA binding factor. Particularly, this invention provides a method for in vivo transferring the materials to cells through various routes comprising intramuscular, intraperitoneal, intravein, oral, nasal, subcutaneous, intradermal, mucosal, inhalation. Accordingly, the method of this invention can be used for technology to transfer DNA / RNA to various cell types and express them in the cells transiently or permanently in medicinal applications such as DNA / RNA vaccine and gene therapy, as well as basic applications.
Owner:FORHUMANTECH CO LTD
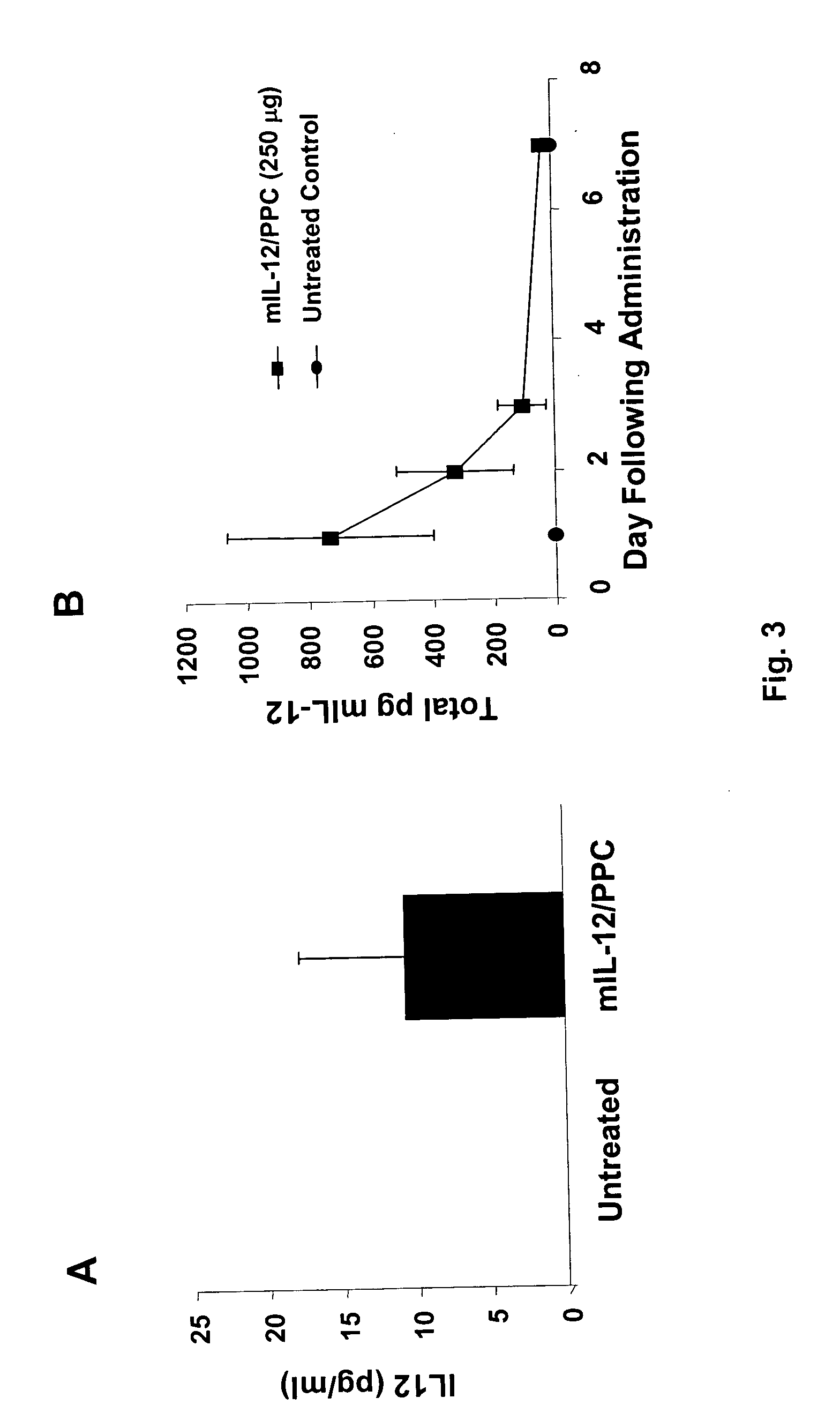
Combination of immuno gene therapy & chemotherapy for treatment of cancer & hyperproliferative diseases
ActiveUS20060127482A1Tumor shrinkageInhibiting growth and metastasisHeavy metal active ingredientsBiocideGene deliveryWhole body
Pharmaceutical compositions comprising a nucleic acid, a gene delivery polymer, and at least one adjunctive chemotherapeutic drug for the treatment of mammalian cancer or hyperproliferative disorders and methods of using thereof for the treatment of mammalian cancer or hyperproliferative disorders by intratumoral, intraperitoneal or systemic injection.
Owner:CLSN LAB
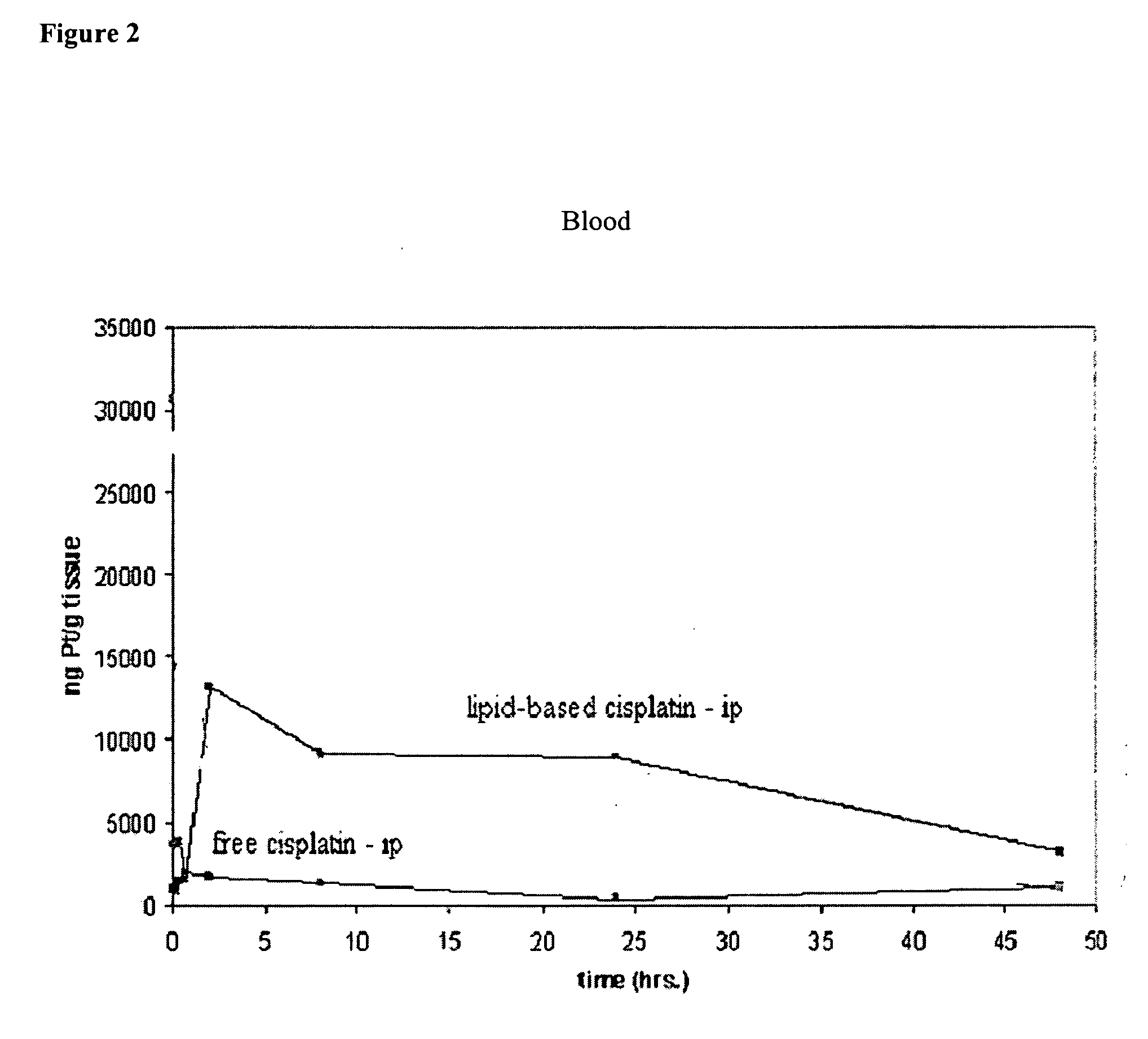
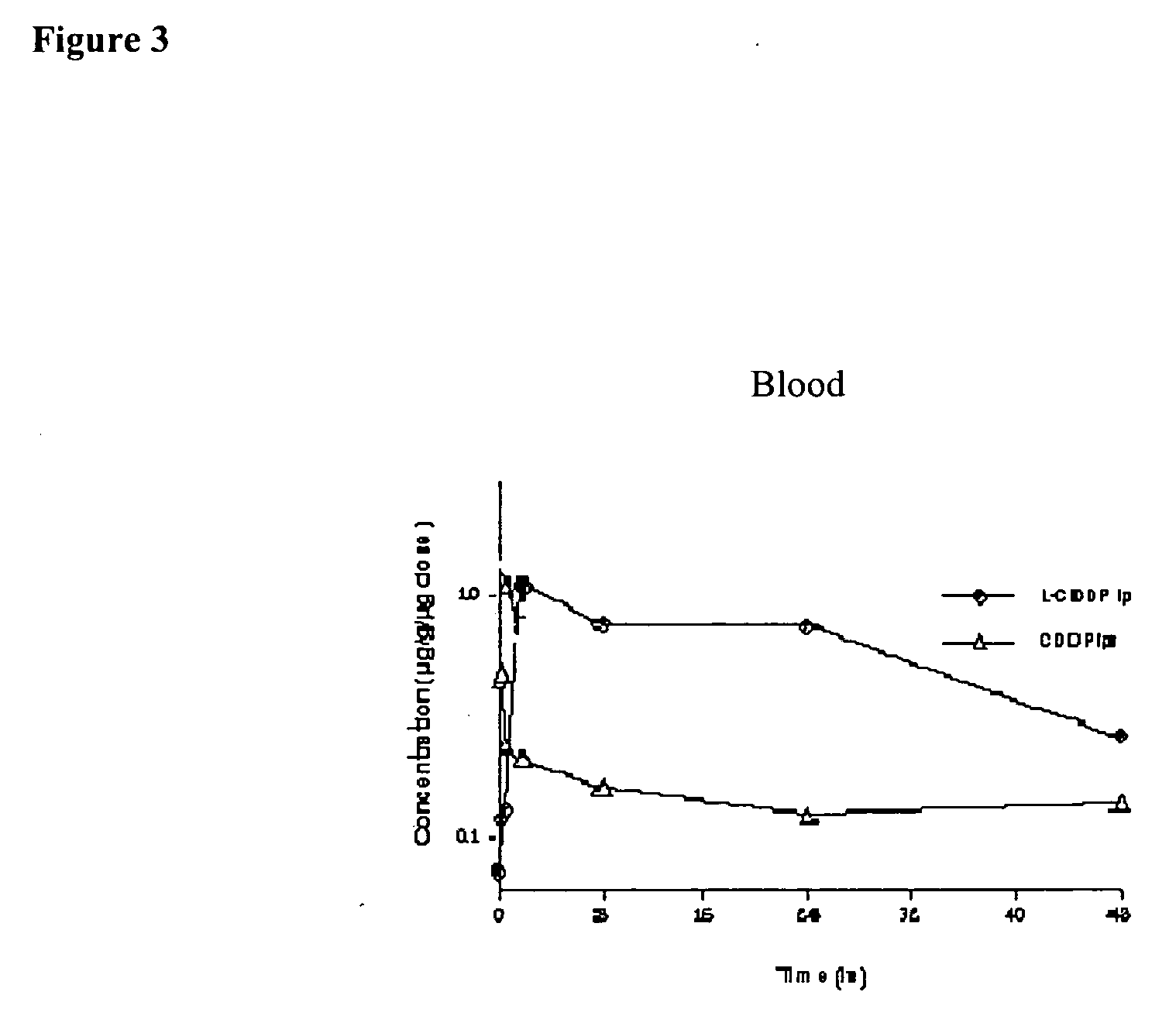
Methods of treating cancer with lipid-based platinum compound formulations administered intraperitoneally
InactiveUS20060246124A1Reduce nephrotoxicityPotent and efficient cancer treatmentBiocideHeavy metal active ingredientsLipid formationAbdominal cavity
Owner:PILKIEWICZ FR G +3
Use hydrolyzed medium containing microorganisms medicinally
InactiveUS20080102061A1OptimizationPromote high vitalityBiocideMilk preparationIntraperitoneal routeDisease
Owner:TECH COMMLIZATION
Pharmaceutical composition
A pharmaceutical composition for intraperitoneal delivery of an anti-neoplastic agent is provided for treating cancers associated with aberrant mucin expression, preferably ovarian cancer and pancreatic, prostate, metastatic breast, bladder and lung cancers. The composition comprises nanomicelles loaded with the anti-neoplastic agent, and antibodies such as anti-MUC16, anti-MUC1 or anti-MUC4 are conjugated to these nanomicelles. The antibody-bound nanomicelles are optionally embedded in a biodegradable pH- and thermo-responsive hydrogel capable of sol-gel transition at body temperature. The pharmaceutical composition is implantable in the peritoneum, where it transforms into a semi-solid gel at the body's core temperature. In response to pH, the hydrogel swells and releases the antibody-bound nanomicelles. The nanomicelles specifically target mucin antigens on cancer cells. The anti-mucin antibodies can be internalized by the tumour cells, enabling the drug-loaded nanomicelles to gain entry and deliver the chemotherapeutic drugs inside the tumour cell.
Owner:UNIVERSITY OF THE WITWATERSRAND
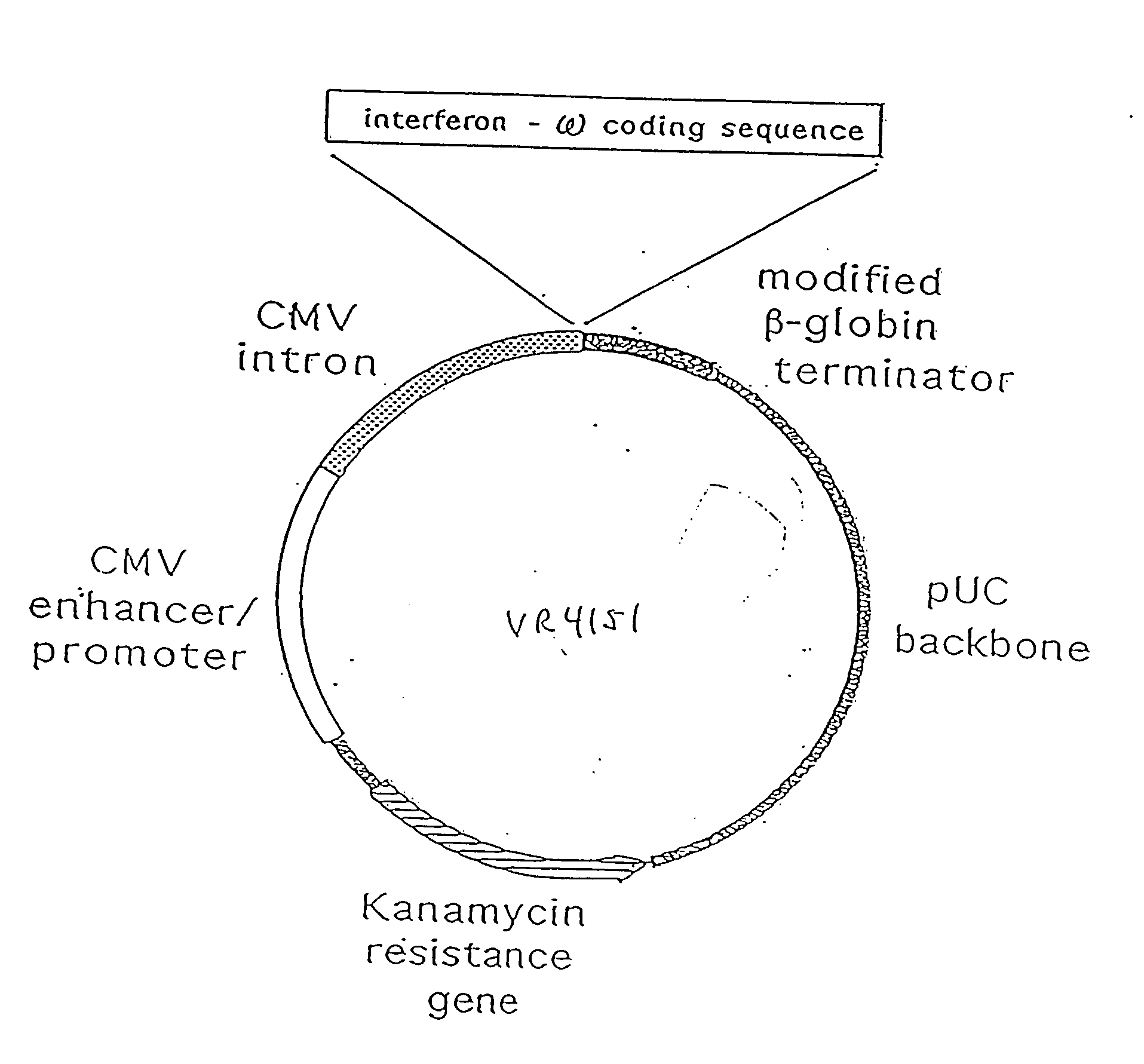
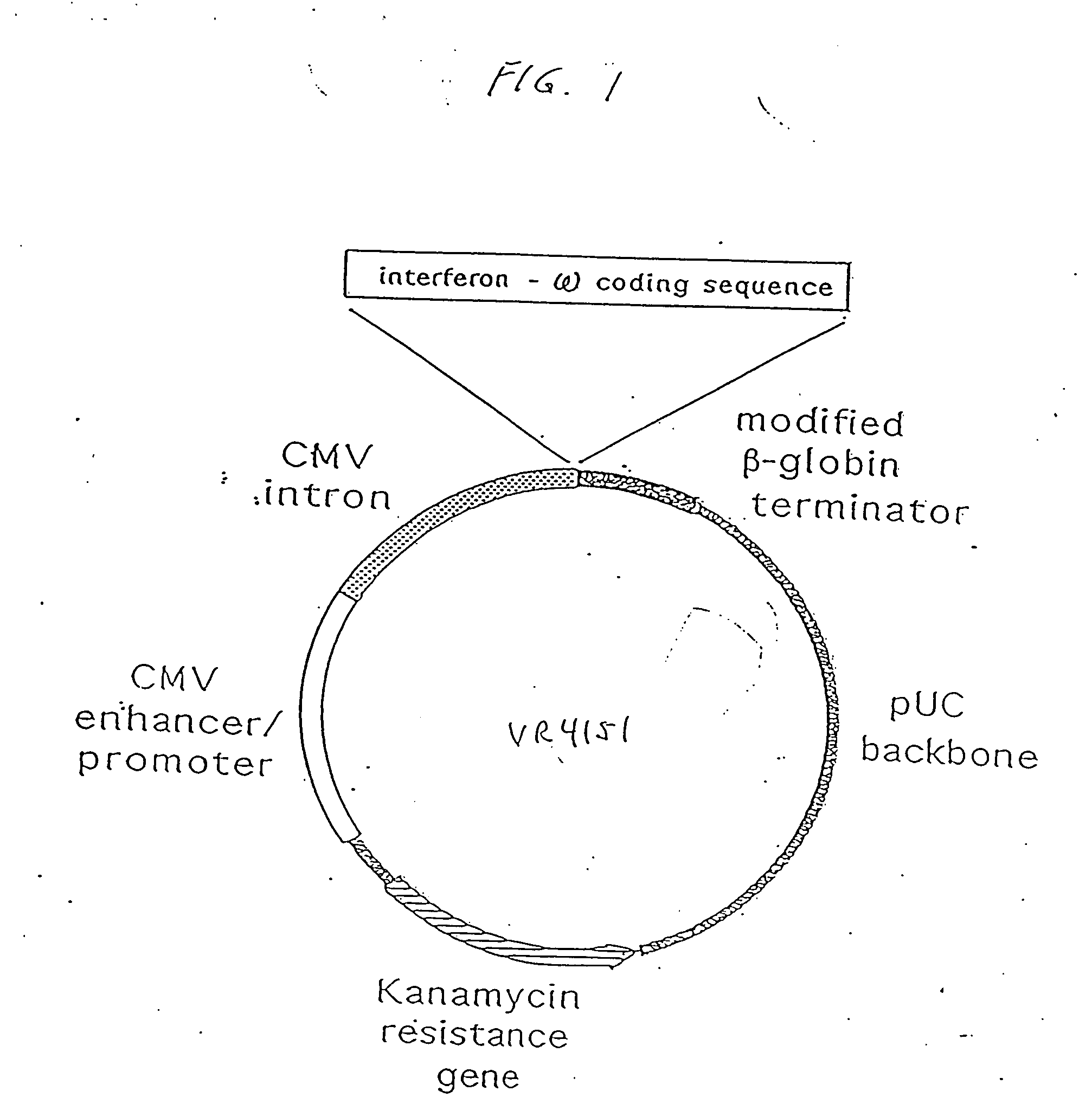
Treatment of cancer using cytokine-expressing polynucleotides and compositions therefor
InactiveUS20070225243A1Minimizing adverse side effectGood effectOrganic active ingredientsPeptide/protein ingredientsAbnormal tissue growthSodium phosphates
The present invention provides a pharmaceutical composition, comprising a non-infectious, non-integrating polynucleotide construct comprising a polynucleotide encoding an interferon ω and one or more cationic compounds. The present invention also provides methods of treating cancer in a mammal, comprising administering into a tissue of the mammal a non-infectious, non-integrating polynucleotide construct comprising a polynucleotide encoding a cytokine. In addition, the present invention also relates to the methodology for selective transfection of malignant cells with polynucleotides expressing therapeutic or prophylactic molecules in intra-cavity tumor bearing mammals. More specifically, the present invention provides a methodology for the suppression of an intra-cavity dissemination of malignant cells, such as intraperitoneal dissemination. Furthermore, the invention relates to compositions and methods to deliver polynucleotides encoding polypeptides to vertebrate cells in vivo, where the composition comprises an aqueous solution of sodium phosphate.
Owner:VICAL INC
Formulations comprising a nucleic acid in a high concentration
PendingCN111132663AImprove toleranceOrganic active ingredientsCosmetic preparationsHypoviscosityAcute myocardial ischaemia
Low-viscosity, high concentration nucleic acid compositions that can be administered by multiple parenteral routes may allow for less frequent dosing than nucleic acid products currently on the market. In particular, low-viscosity defibrotide formulations for subcutaneous, intramuscular, and intraperitoneal administration are more convenient to the patient and / or are administered outside of the hospital setting. Formulations of the invention may be used for the treatment of numerous conditions including for example, treatment of peripheral arteriopathies, treatment of acute renal insufficiency, treatment of acute myocardial ischemia, and treatment and prevention of sinusoidal obstruction syndrome or VOD.
Owner:JAZZ PHARMA IRELAND LTD
Neurotensin as a marker and therapeutic target for sepsis
InactiveUS20080213270A1Improve survivalReduce abilityCompound screeningApoptosis detectionNeurotensin receptor 1Mast cell
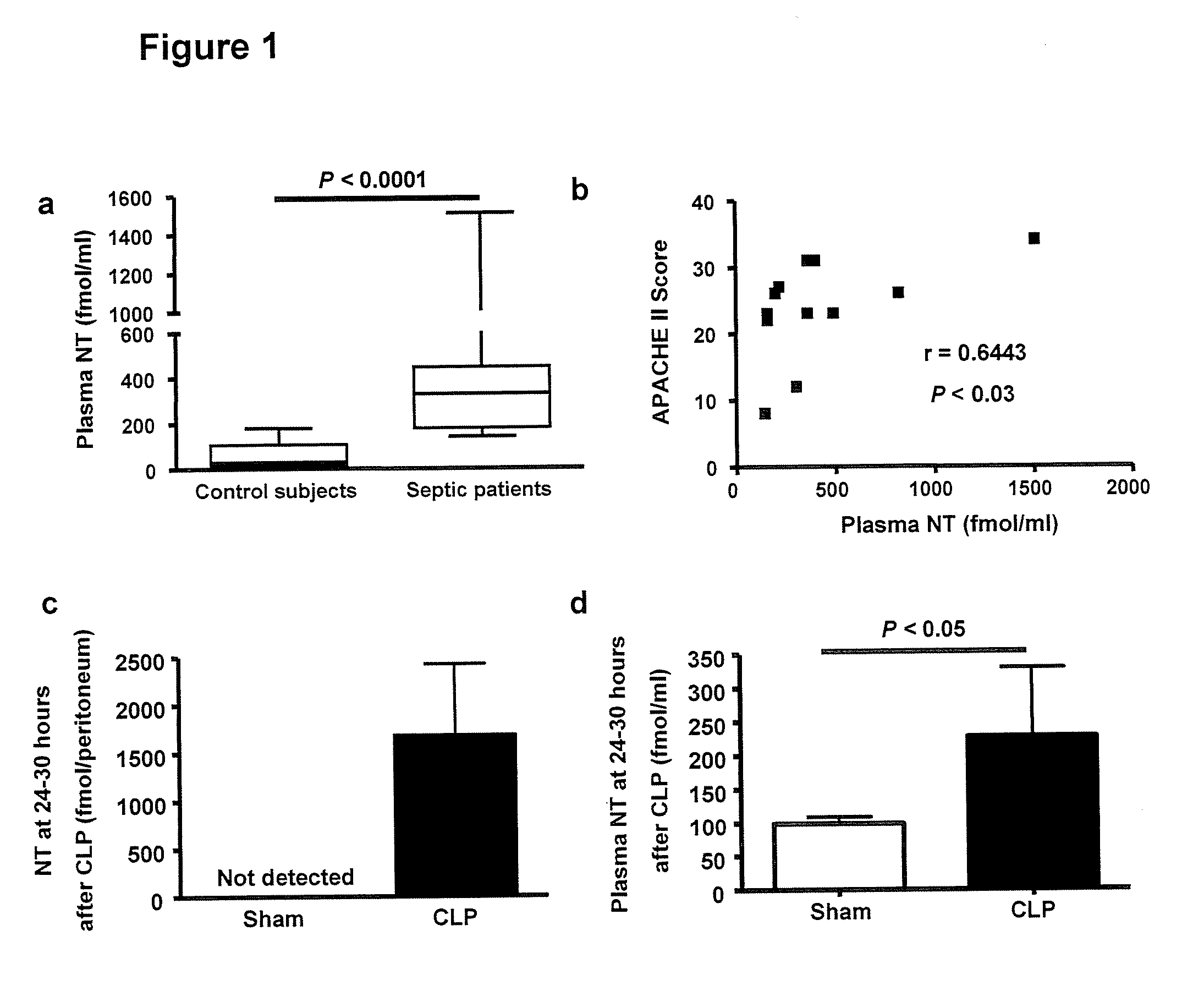
Sepsis is a complex, incompletely understood and often fatal disorder, typically accompanied by hypotension, that is considered to represent a dysregulated host response to an infection. Neurotensin (NT) is 13-amino-acid peptide that, among its multiple effects, induces hypotension. It is shown herein that plasma concentrations of NT are increased in humans with sepsis and in mice after caecal ligation and puncture (CLP), a model of sepsis. Mast cells can degrade NT through neurotensin receptor 1- and neurolysin-dependent mechanisms, diminishing the hypotensive effects of NT, reducing intraperitoneal NT concentrations, and improving survival. These findings show that mast cells can regulate NT concentrations, and identify NT as a biomarker and therapeutic target in sepsis.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
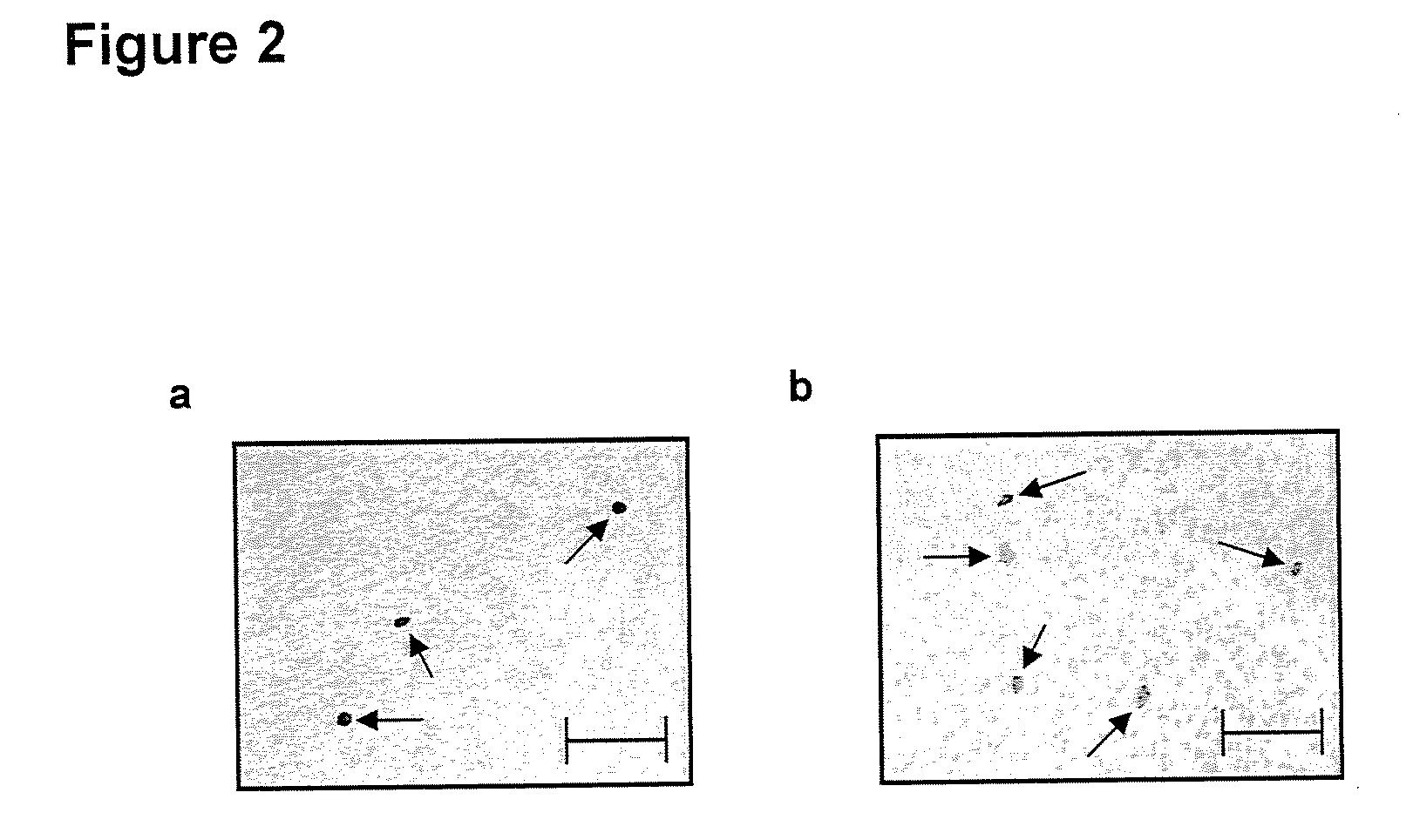
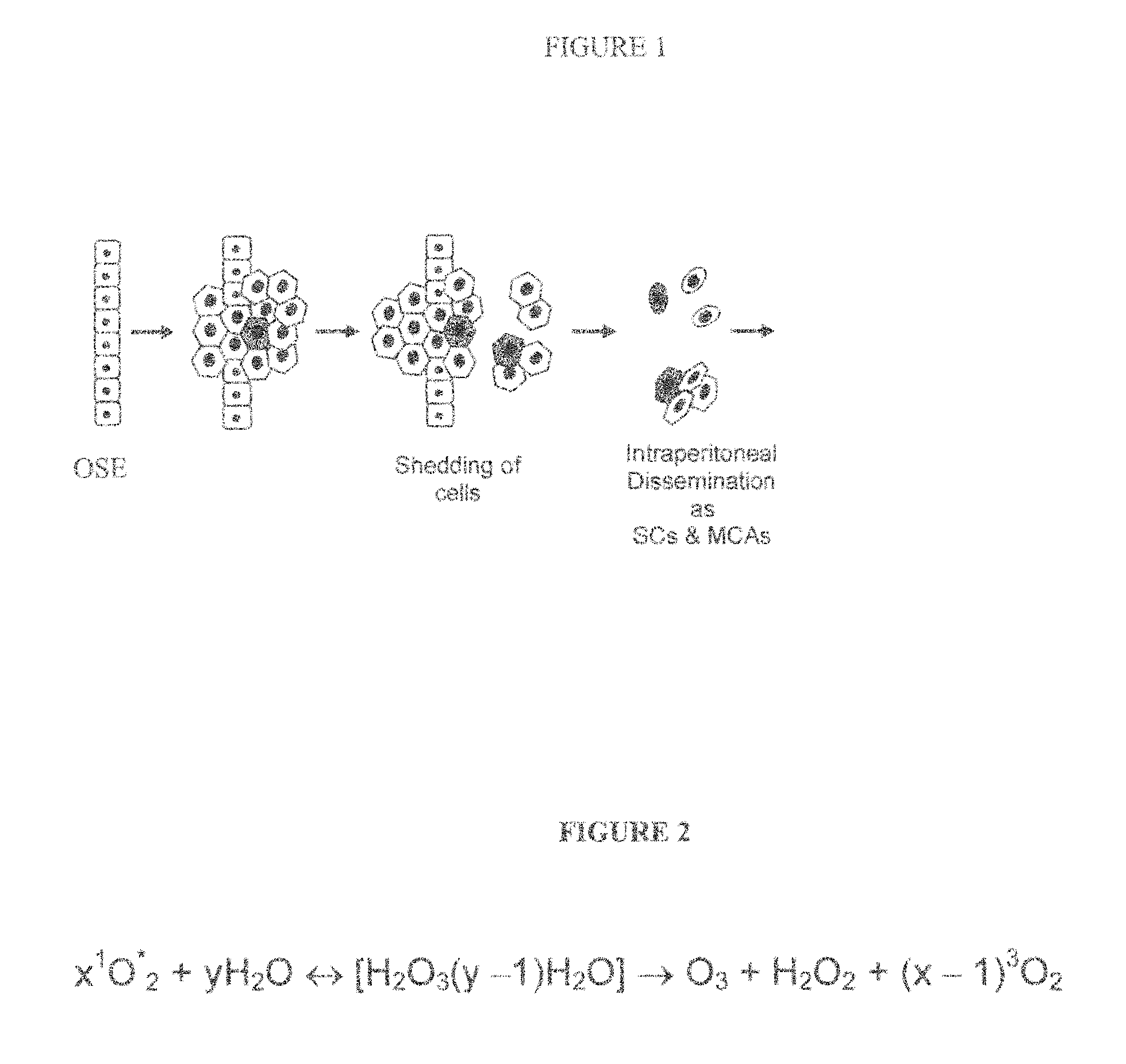
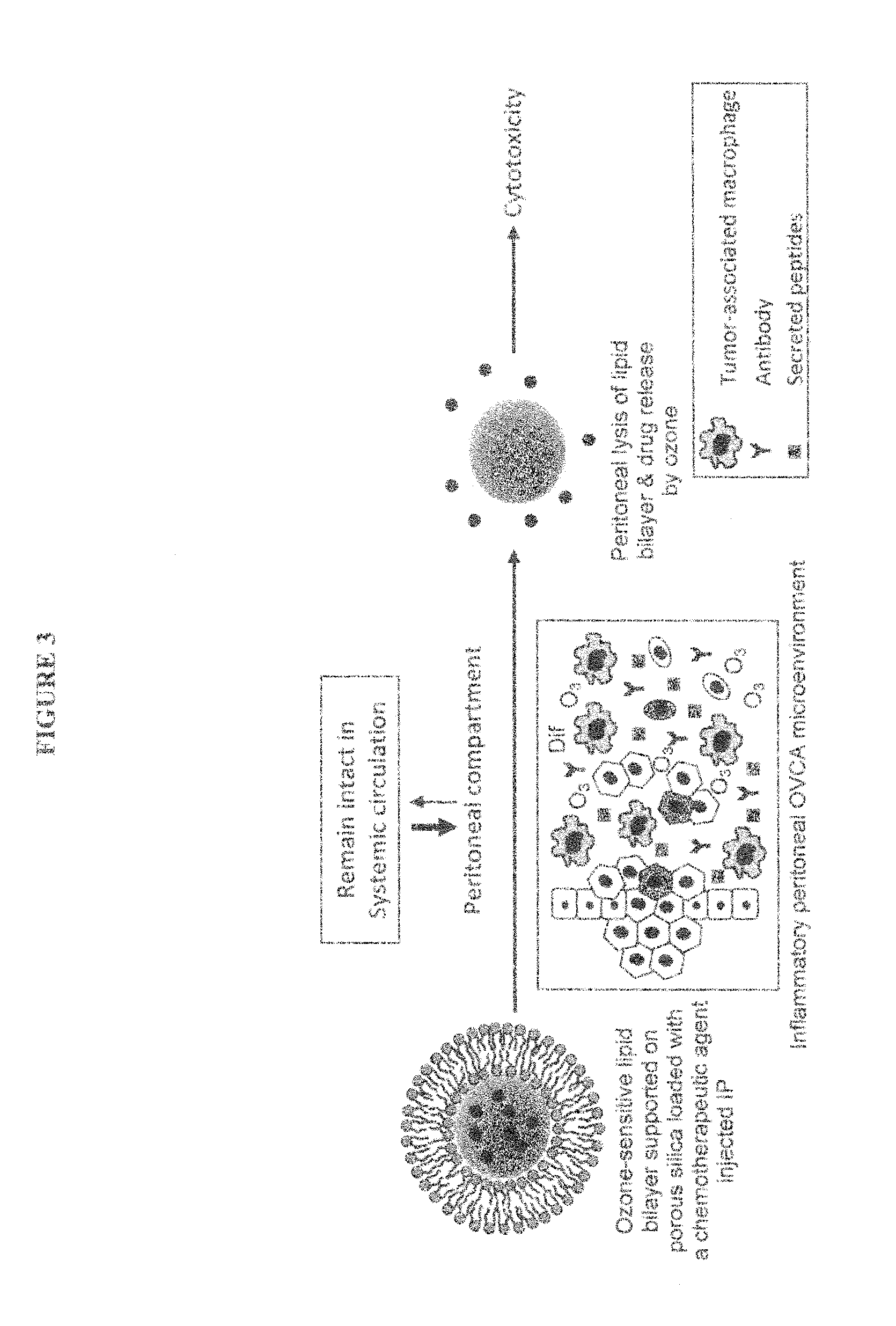
Intraperitoneally-administered nanocarriers that release their therapeutic load based on the inflammatory environment of cancers
In one embodiment, the invention provides a new design of nanocarrier compositions that release their therapeutic load specifically at intraperitoneal cancers' site. These nanocarriers are administered intraperitoneally and comprise a plurality of porous nanoparticulates that (a) are loaded with one or more pharmaceutically-active agents alone or in combination with imaging agents thus providing a theranostic value and (b) that are encapsulated by and that support a lipid bilayer which is disrupted upon contact with a reactive oxygen species generated within the environment of the cancer. In other embodiments, the invention provides methods of treatment and pharmaceutical compositions comprising nanocarriers as described herein.
Owner:STC UNM
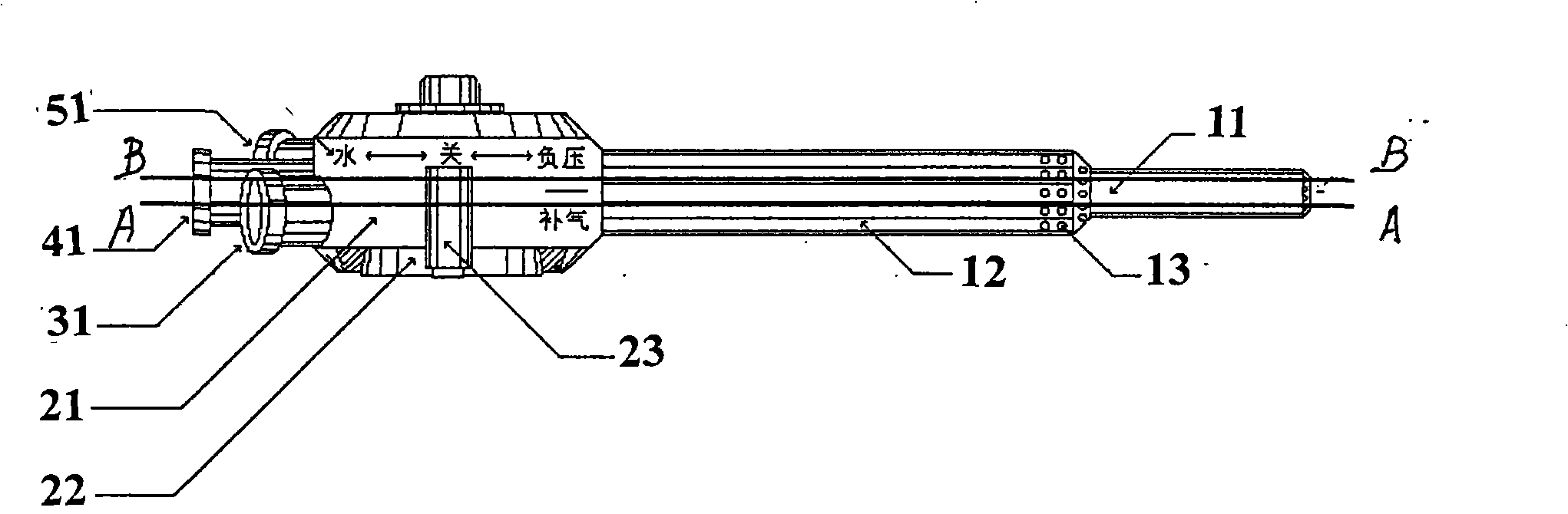
Intra-abdominal pressure balancing apparatus of peritoneal irrigation
InactiveCN101401718ARelieve painMaintain operating spaceLaproscopesEndoscopesSurgical operationAutomatic control
The invention discloses an intra-peritoneal pressure balancing apparatus for use in irrigation of peritoneal cavity. The apparatus comprises a peritoneal trocar sleeve, an irrigating fluid irrigation tube, a negative-pressure suction tube and a control device, wherein the peritoneal trocar sleeve is provided with an inner sleeve and an outer sleeve, and is connected and integrated with a constant-pressure irrigation and suction adjusting valve which is connected with the irrigating fluid irrigation tube, the negative-pressure suction tube and a carbon dioxide intake tube. A rear end of the carbon dioxide intake tube is provided with an automatic air pressure balancing device for balancing the intake pressure of carbon dioxide. An irrigation tube is connected with an external irrigating fluid source. A negative-pressure tube is connected with an external negative-pressure source. The carbon dioxide intake tube is connected with an external carbon dioxide source. A micro computer control board and a leading-out power wire are provided for automatic control. The intra-peritoneal pressure balancing apparatus has the advantages of maintain the balance of the intra-peritoneal pressure during a clinic surgical operation, ensures an operation space in a peritoneal cavity, reduces risks in the operation, improves the quality of the operation and is an intra-peritoneal pressure balancing apparatus for use in operations with a peritoneoscope during various surgeries to balance the intra-peritoneal pressure.
Owner:杨君
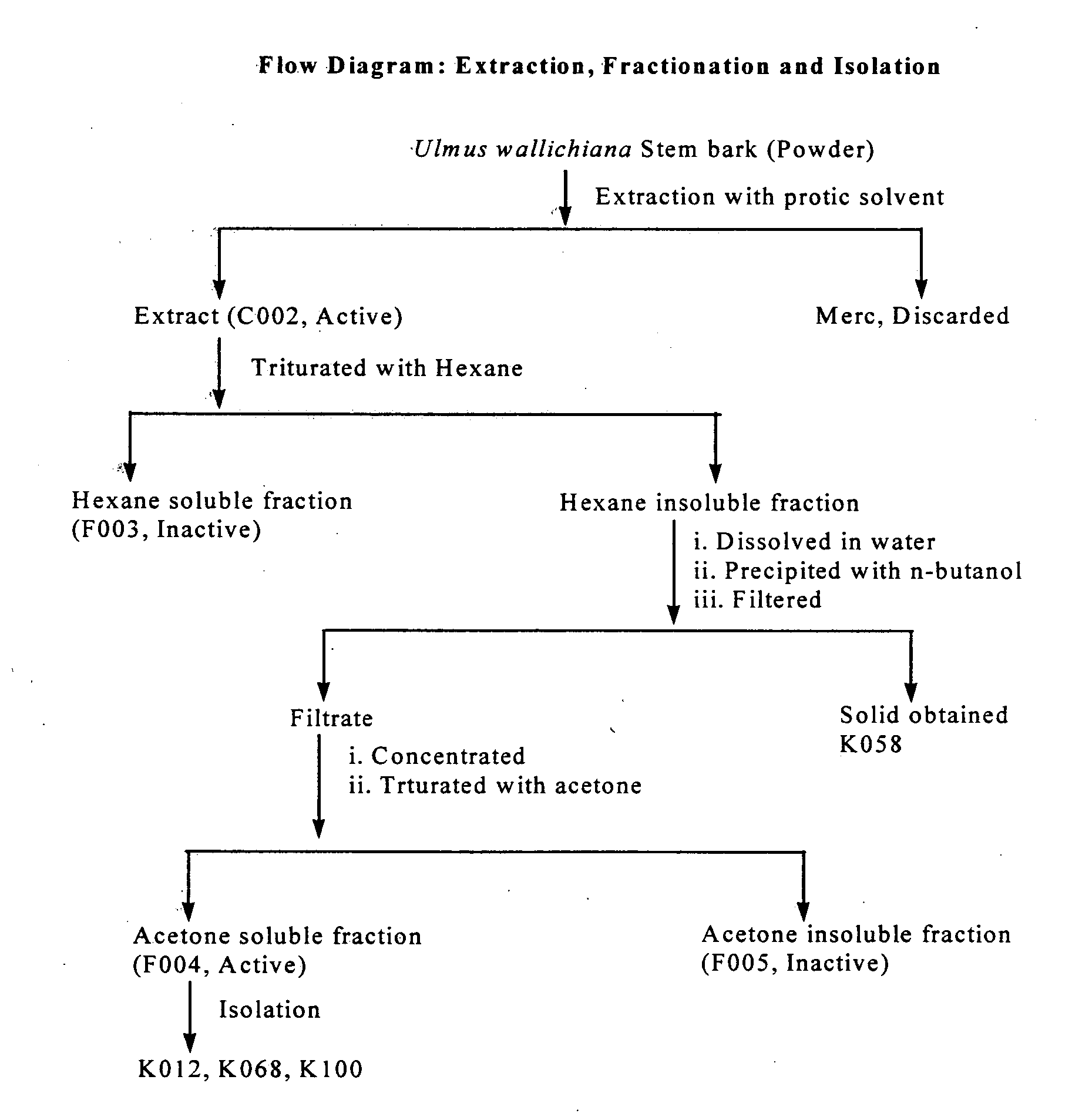
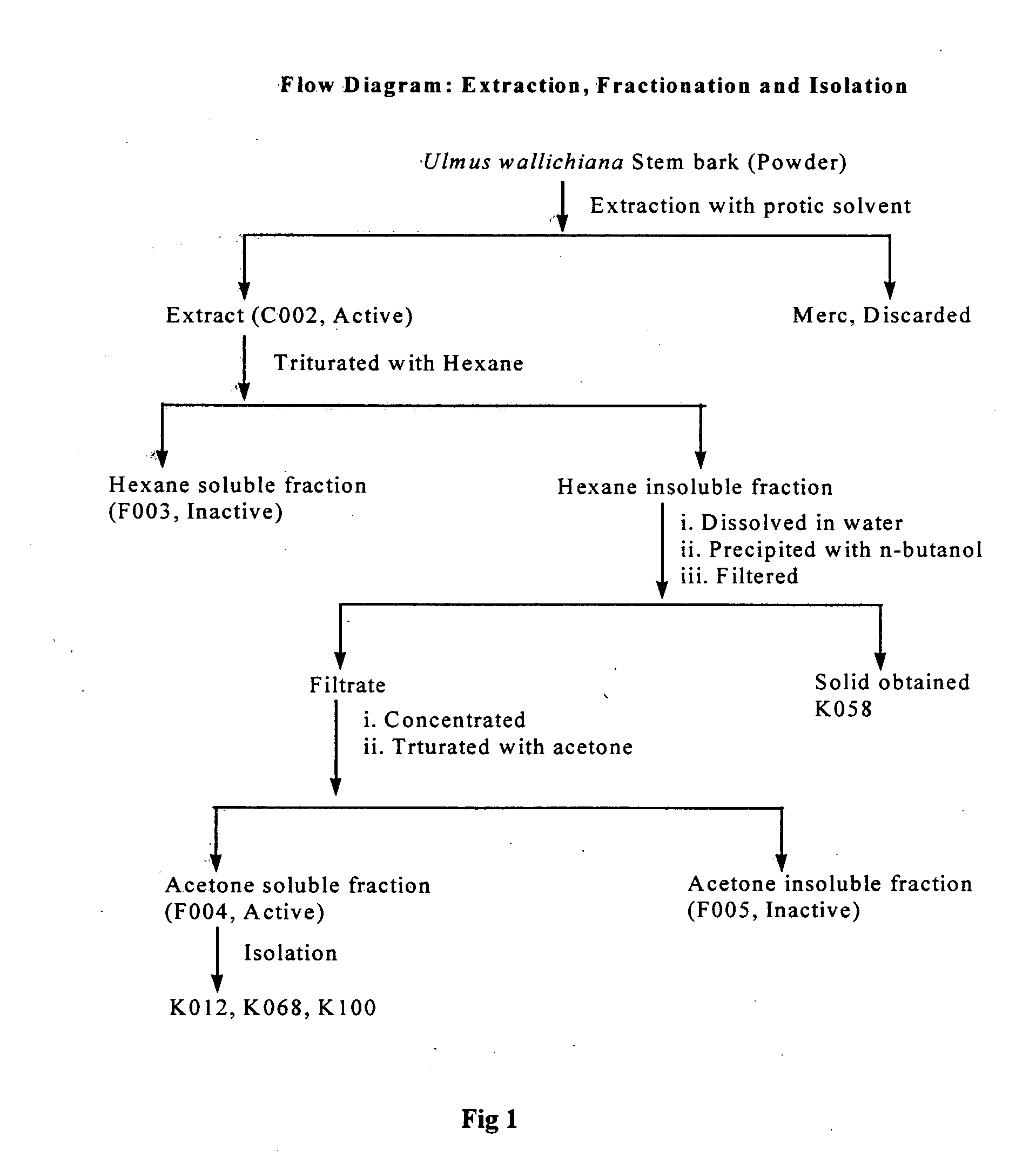
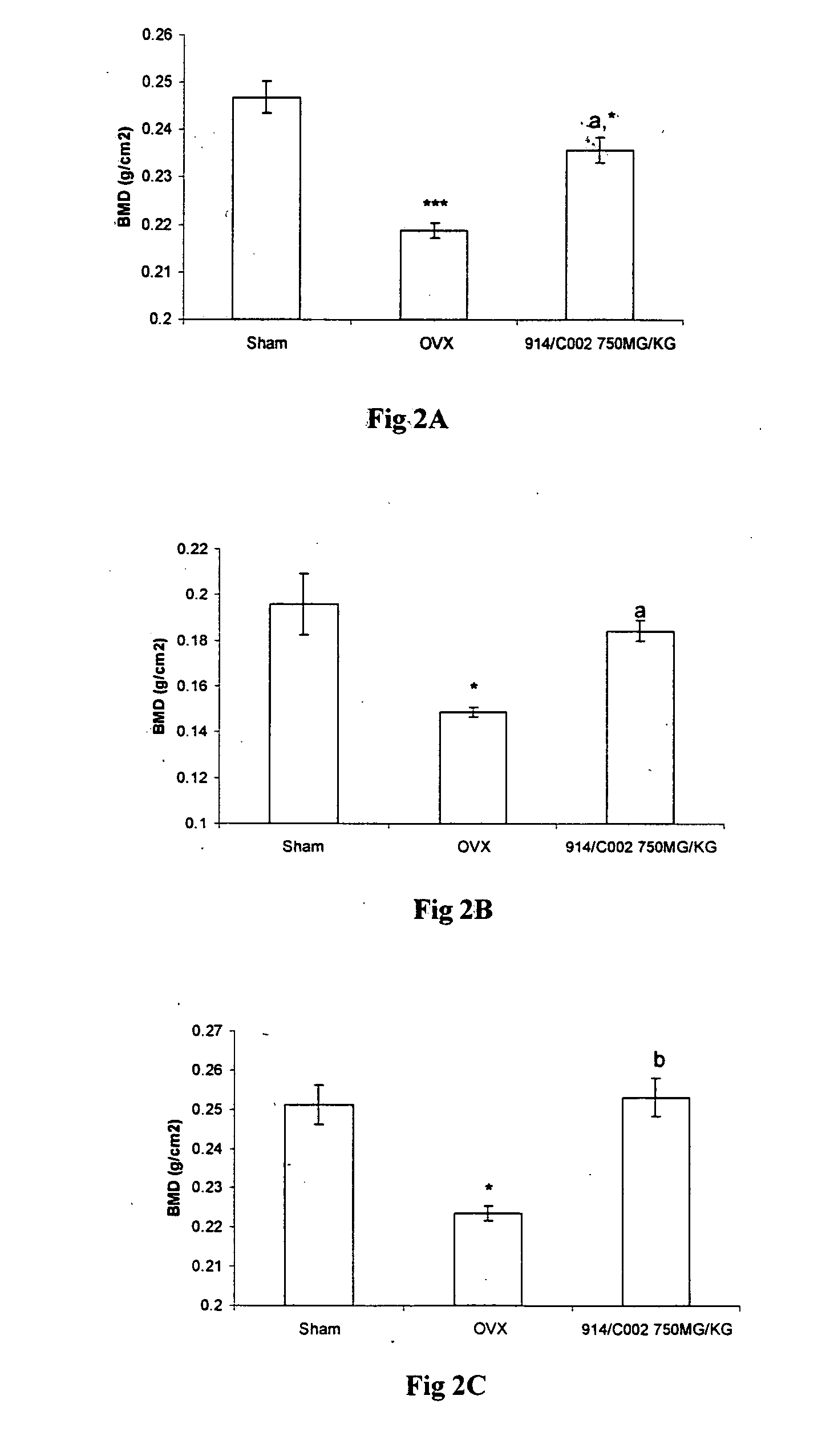
Novel flavonol compounds, a bioactive extract/fraction from ulmus wallichiana and its compounds for prevention for treatment of osteo-health related disorders
InactiveUS20110112042A1Reduces Ovx-induced bone lossBiocideSaccharide with heterocyclic radicalsDiseaseDouble bond
The present invention provides a flavonol compound and a bioactive extract / fraction from Ulmus wallichiana useful for the management or prevention or treatment of bone disorders. Said extract / fraction comprising marker compounds of general formula 2, K058: R1═R2═0H K012: R1═R2═OH, 2,3 double bond K068: R1═R2═H KIOOi R1═OH, R2═H mula 2 Wherein the marker compounds K012, K058, K068, K100 ranges 6.7-12%, 1.7-4.5%, 0.6-1.2%, 1.7-4.5% respectively in alcoholic extract and a process of extraction thereof. According to another aspect of the invention provides a pharmaceutical composition comprising the said compound. The present invention further provides a method of treating bone disorders by administering the pharmaceutical composition by oral, intravenous, subcutaneous, intra-peritoneal or intramuscular route.
Owner:COUNCIL OF SCI & IND RES
Composite absorbable/biodegradable rings for controlled drug delivery
A fiber-reinforced composite ring for the controlled release of at least one bioactive agent includes a biocompatible matrix reinforced with absorbable / biodegradable fibers capable of providing the mechanical properties needed for inserting and maintaining the ring in a body cavity for a desired period of time. Such ring system as can be used for the intravaginal, intraperitoneal, and subcutaneous delivery of at least one bioactive agent, including those used as contraceptives, antimicrobial agents, and / or antiviral agents, as well as those for the treatment of cancer.
Owner:POLY MED
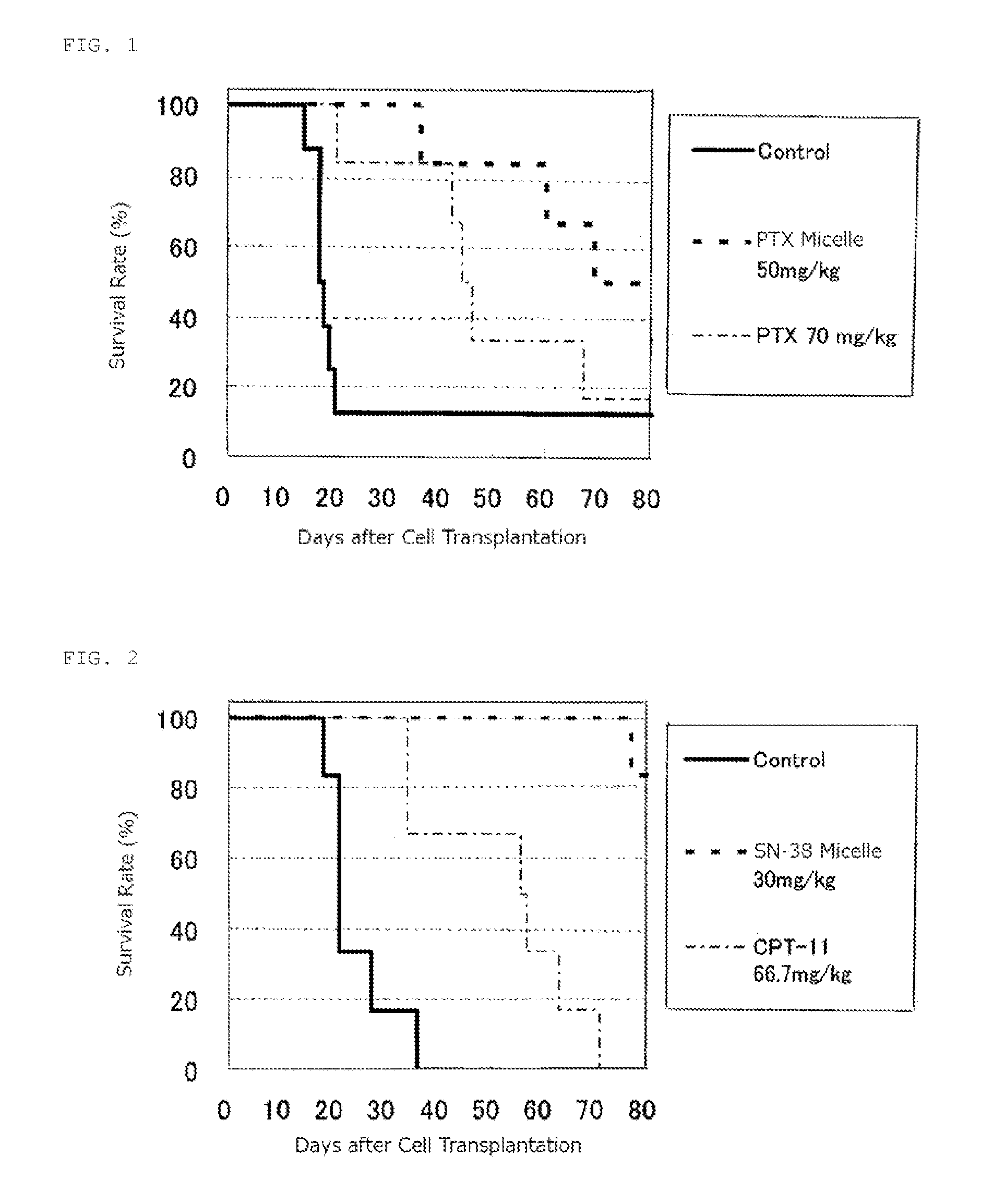
Block Copolymer For Intraperitoneal Administration Containing Anti-Cancer Agent, Micelle Preparation Thereof, And Cancer Therapeutic Agent Comprising The Micelle Preparation As Active Ingredient
InactiveUS20120231053A1Extending abdominal retention timeLess adverse side-effectsBiocidePowder deliveryAnticarcinogenSide effect
To provide a therapeutic method using a water soluble, high molecular weight block polymer to enable that an intraperitoneally administered anti-cancer agent may maintain for a long-term retention in the abdominal cavity to enoughly exert the effect of the anti-cancer agent and reduce adverse side-effects thereof.A therapeutic agent as a micelle preparation, comprising a copolymer having a hydrophilic polymeric moiety and a polycarboxylic acid derivative moiety; and an anti-cancer agent bonding to or encapsulated in the copolymer, wherein the micelle preparation may exhibit sustained drug release capability, and enables an extension of a retention time period of the anti-cancer agent in an abdominal cavity, is provided. A superior life-prolonging effect was found in an intraperitoneal administration mouse model compared with a case in which only an encapsulated drug is administered, and thus the present invention was completed accordingly.
Owner:NIPPON KAYAKU CO LTD
Combination of atr kinase inhibitors with 2,3-dihydroimidazo[1,2-c]quinazoline compounds
PendingUS20210369724A1Ease of preparation and detectabilityGood metabolic stabilityAntineoplastic agentsHeterocyclic compound active ingredientsPharmaceutical medicinePharmaceutical Substances
The present invention relates to combinations of: component A: one or more 2,3-dihydroimidazo[1,2-c]quinazoline compounds of general formula (A1) or (A2) as defined herein, or a stereoisomer, a hydrate, a solvate, or a pharmaceutically acceptable salt thereof; and component B: one or more ATR kinase inhibitor(s) as defined herein, or a stereoisomer, a tautomer, an N-oxide, a hydrate, a solvate, or a pharmaceutically acceptable salt thereof; and, optionally, component C: one or more further pharmaceutical agent(s); and, optionally, in which either or both of components A and B in any of the above-mentioned combinations are in the form of a pharmaceutical composition which is ready for use to be administered simultaneously, concurrently, separately or sequentially. The components may be administered independently of one another by the oral, intravenous, topical, local installations, intraperitoneal or nasal route.
Owner:BAYER AG
Peritoneal Dialysis Method
InactiveUS20130197428A1Easy to manageRecovery functionOrganic active ingredientsPeritoneal dialysisPhosphateMortality rate
A method of dialyzing renal failure patients via the peritoneum (P.O.) using a series of innovations in the dialysate composition aiming at preventing the intra vascular or intra peritoneal formation and deposition of calcium / phosphate compounds which having no excretory pathway are deposited in soft tissue and are the cause of cardiovascular related morbidity and mortality. Additionally this method allows for the use of the physiologic buffer (bicarbonate) which will improve the acid base balance and prolong the functional life of the peritoneum as the dialysis membrane.
Owner:CRUZ COSME
Composition and methods based on neutralizing antibodies delivered intranasally for enhanced therapeutic efficacy
ActiveUS20170002062A1Effective treatment and prophylaxis of viral infectionSuperior, more efficacious and effectivePharmaceutical delivery mechanismImmunoglobulins against virusesNeutralising antibodyInhalation
The present invention provides methods for treatment or prophylaxis of viruses, particularly influenza virus, by administration of agents, particularly neutralizing antibodies or active fragments thereof, directly to the respiratory tract, including by intranasal or inhalation administration. The invention provides compositions suitable for intranasal or inhalation treatment and administration. The invention includes methods for treatment or prophylaxis combining intranasal or inhalation administration with intraperitoneal or intravenous administration of antibodies.
Owner:CONTRAFECT CORP
Methods and compositions for adaptive immune modulation
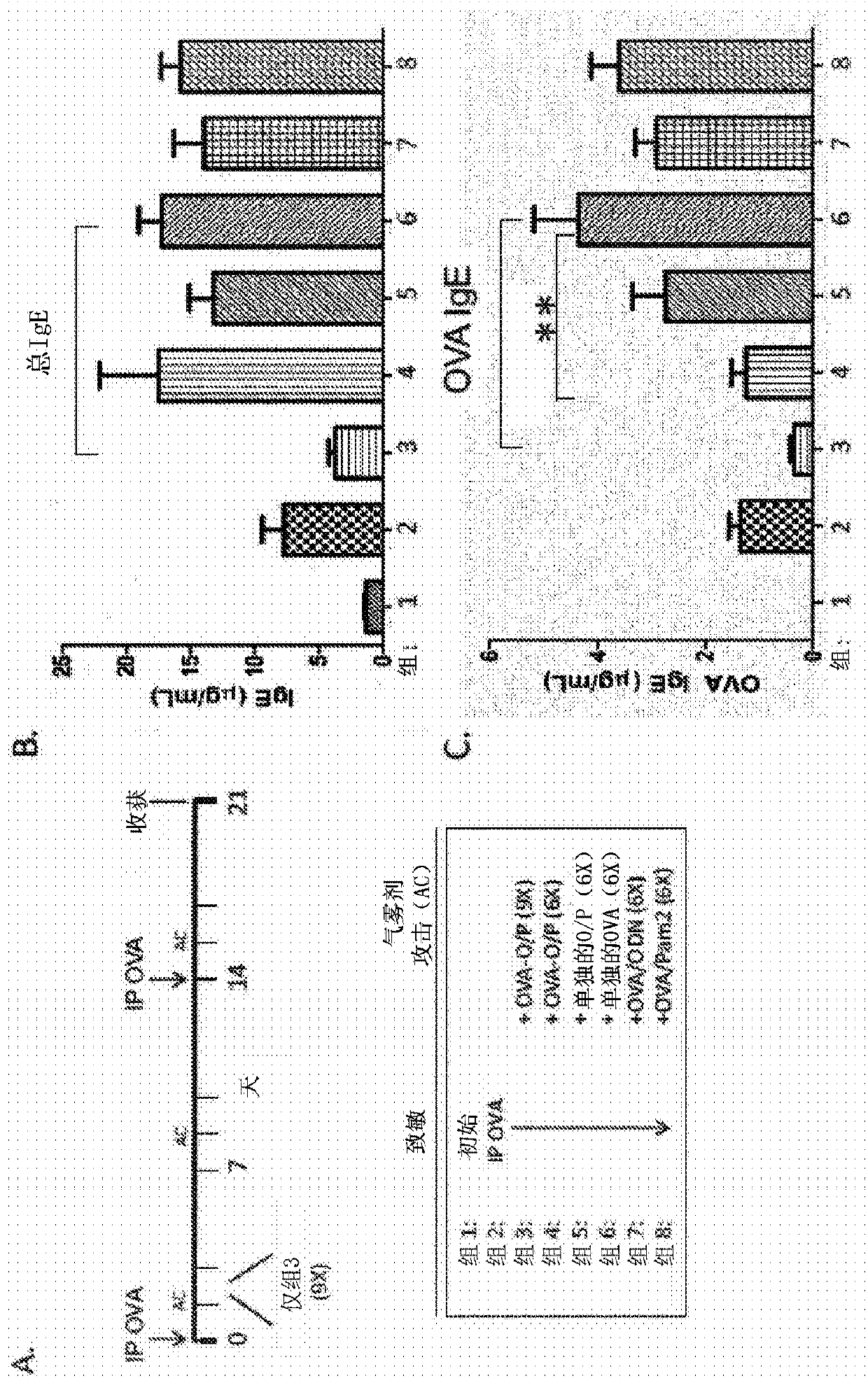
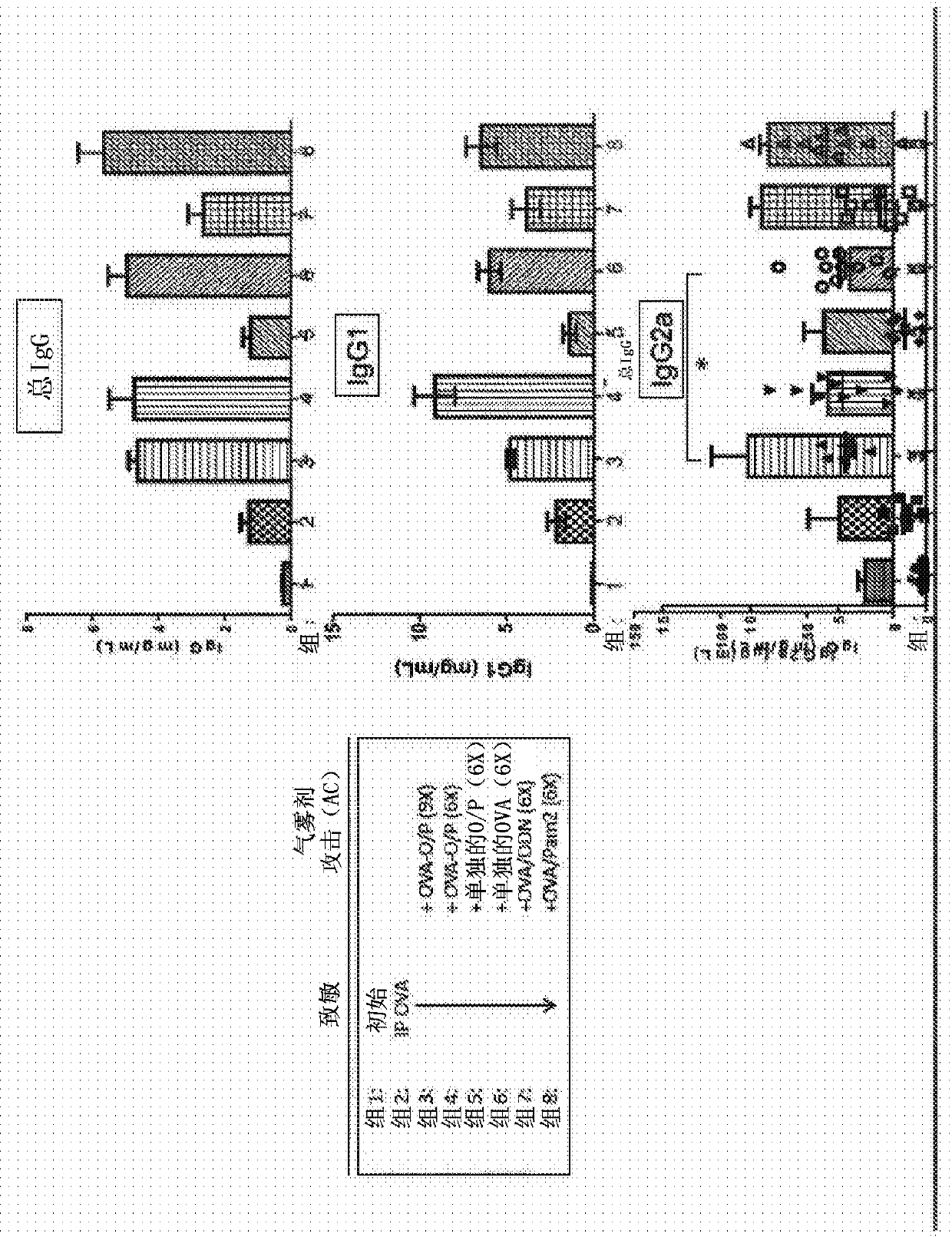
Embodiments are directed to methods and compositions for modulating an immune response. In certain aspects the immune response is a type I hypersensitivity response. In particular aspects the subjecthas allergic asthma or allergic rhinitis. Using a conventional experimental asthma mouse model (BALB / c), the inventors demonstrate that aerosol administration of TLR agonists, in particular a combination of TLR2 / 6 and TLR9 agonist (e.g., TLR9 oligonucleotide agonist / PAM2CSK4) along with an antigen (e.g., ovalbumin (OVA)) suppresses the immune response as exemplified by the production of antigen-specific IgE and decreases the number of airway eosinophils in bronchoalveolar lavage fluid (BAL) in response to intra-peritoneal (IP) immunization with an antigen mixed with alum.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +2
Method for detecting cancer cells and monitoring cancer therapy
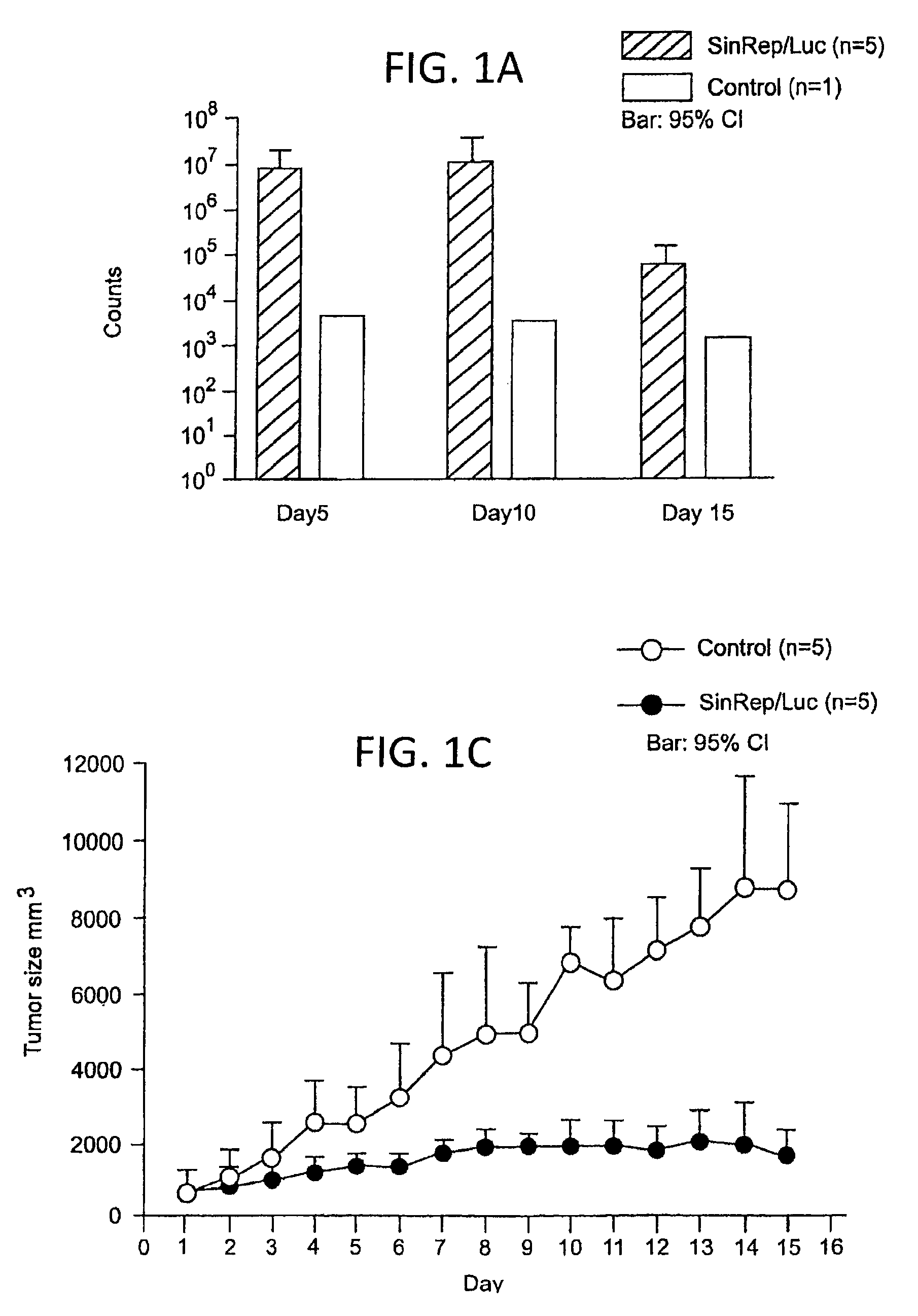
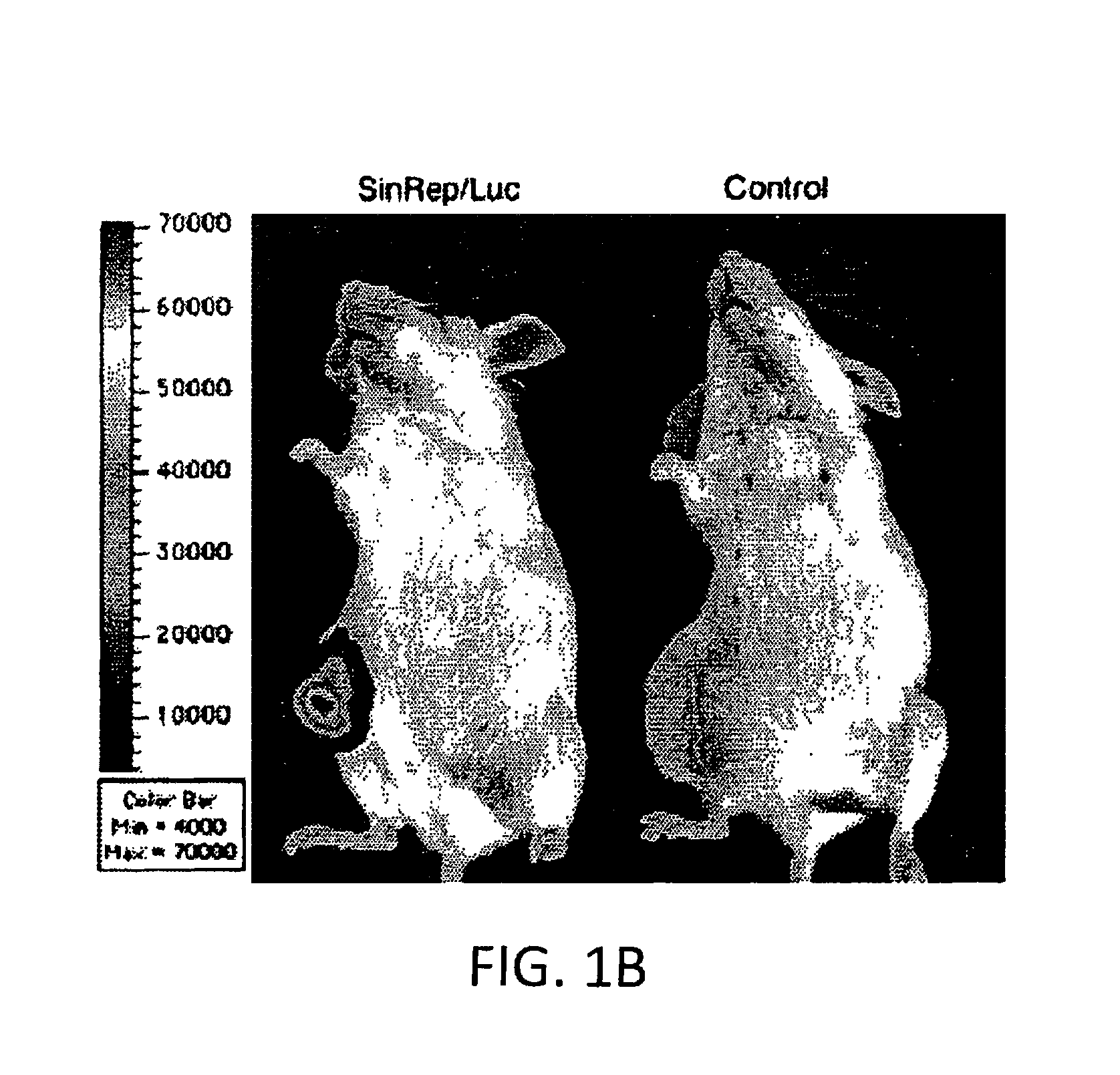
Disclosed herein are methods for identifying cancer cells and monitoring anti-cancer therapy in the body of a mammal by systemically delivering Sindbis viral vectors. The vector can specifically target and identify tumor cells in mice growing subcutaneously, intraperitoneally, intrapancreatically, or in the lungs. These findings demonstrate the remarkable specificity of the Sindbis vector system that is relatively safe and can specifically target tumor cells throughout the body via the bloodstream.
Owner:NEW YORK UNIV
Suturable woven implants from electrospun yarns for sustained drug release in body cavities
The invention discloses a saturable drug delivery implant made of a woven fabric material with variable packing density, dimension and weight. The woven fabric material includes electrospun continuous yarn with individual micro- or nano-fiber yarn made from a single polymer. The yarn is loaded with at least one therapeutic agent to provide controlled and sustained release when the device is sutured to a wall of the peritoneal cavity of a subject. The woven fabric is flexible, biodegradable and configured to be sutured to the wall of a body cavity to provide a sustained and controlled release of therapeutic agent in a subject. In some aspects, a peritoneal implant is sutured to the peritoneal wall cavity for continuous and sustained intraperitoneal release of a therapeutic agent.
Owner:AMRITA VISHWA VIDYAPEETHAM
Therapy with clostridium perfringens enterotoxin to treat ovarian and uterine cancer
InactiveUS20090291047A1Low toxicityGood curative effectIn-vivo radioactive preparationsPeptide/protein ingredientsIntraperitoneal routeCancer cell
The invention discloses high levels of receptors for Clostridium perfringens enterotoxin (CPE) have been found in ovarian cancer and uterine cancer tissue samples. In addition, successful in vivo treatment of a mouse model of ovarian cancer with intraperitoneal injection of CPE is disclosed. High levels of Ep-CAM protein is also disclosed in ovarian cancer tissue samples. Thus, the invention provides a method of treating ovarian cancer and uterine cancer by administering CPE. The invention also provides a method of treating cancer in a mammal involving intraperitoneal administration of CPE, where at least some cancerous cells are located in or adjacent to the peritoneal cavity of the mammal. The invention also provides a method of treating ovarian cancer involving administering an anti-Ep-CAM antibody. The invention also provides a method of treating cancers expressing claudin-3 or claudin-4 by administering an antibody against claudin-3 and / or an antibody against claudin-4. The invention also provides a method of protecting a mammal from CPE toxicity involving administering a protective agent that binds to claudin-3 and / or claudin-4 and inhibits CPE binding to claudin-3 and / or claudin-4.
Owner:YALE UNIV
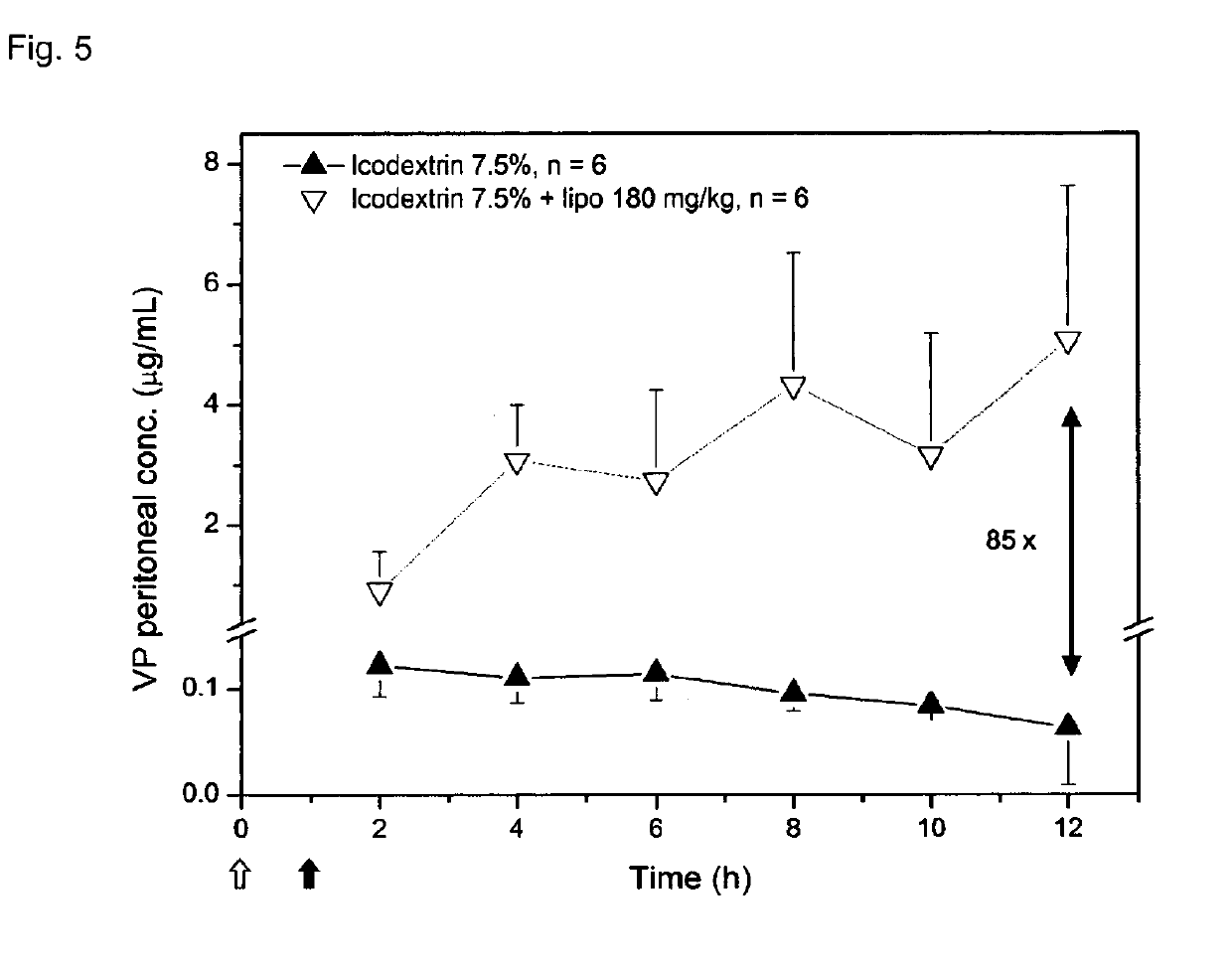
Liposome composition for use in peritoneal dialysis
InactiveUS20190105270A1Metabolism disorderAntinoxious agentsPeritoneal dialysis catheterPeritoneal space
The present invention is directed to a liposome composition for use in the peritoneal dialysis of patients suffering from endogenous or exogenous toxicopathies, wherein the pH within the liposomes differs from the pH in the intraperitoneal cavity and wherein the pH within the liposome results in a liposome-encapsulated charged toxin. The invention also relates to a pharmaceutical composition comprising said liposomes. A further aspect of the present invention relates to a method of treating patients suffering from endogenous or exogenous toxicopathies, preferably selected from drug, metabolite, pesticide, insecticide, toxin, and chemical warfare toxicopathies, more preferably hyperammonemia, comprising the step of administering liposomes of the invention in a therapeutically effective amount into the peritoneal space of a patient in need thereof. Next to human, the present invention is particularly suitable to veterinary aspects.
Owner:ETH ZZURICH
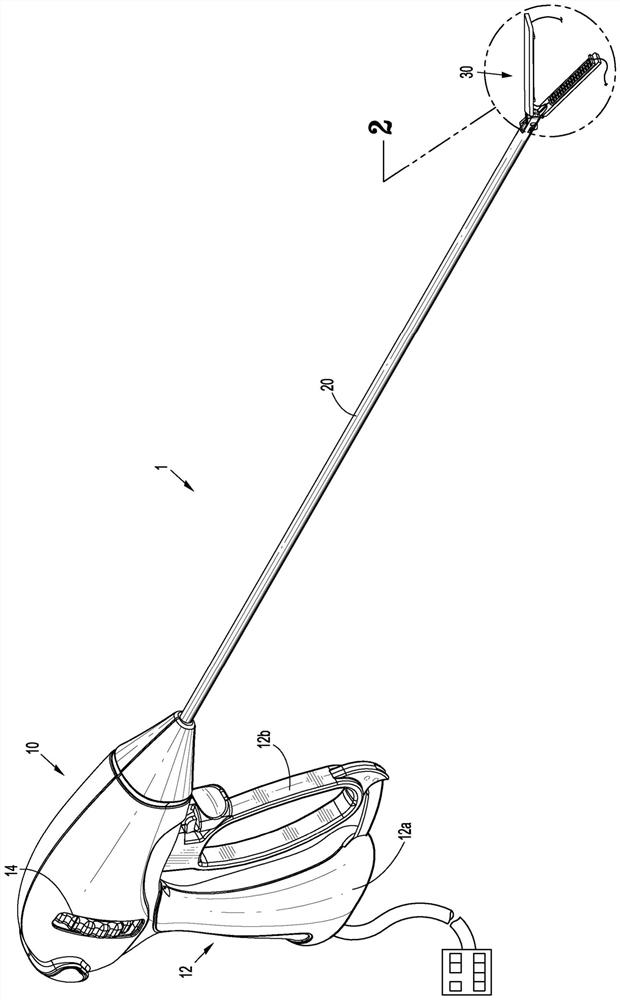
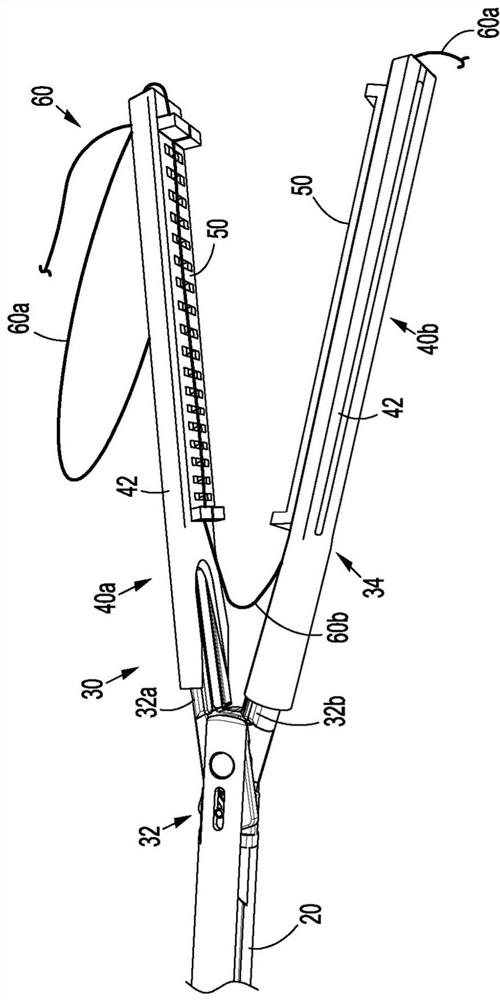
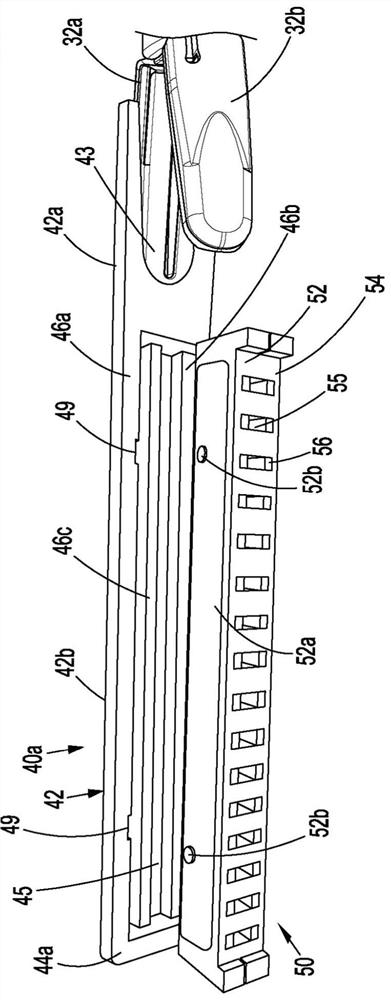
Surgical device and end effector
A surgical device or a portion of a surgical device that places a purse suture in tissue within a body (e.g., an intraperitoneal space) is disclosed. The surgical device includes, or an end effector for a surgical device includes a jaw assembly, a staple cartridge, and a suture.
Owner:TYCO HEALTHCARE GRP LP
Prevention and treatment of organ fibrosis
The disclosure provides a method of treating fibrosis of an organ comprising administering a c-P4H inhibitor to a patient suffering from fibrosis in an organ, to counteract pathological fibrosis. c-P4H inhibitors can be administered via various routes, including in an aerosolized state using a nebulizer, intravenous, intraperitoneal or subcutaneous injection.
Owner:CORNELL UNIVERSITY
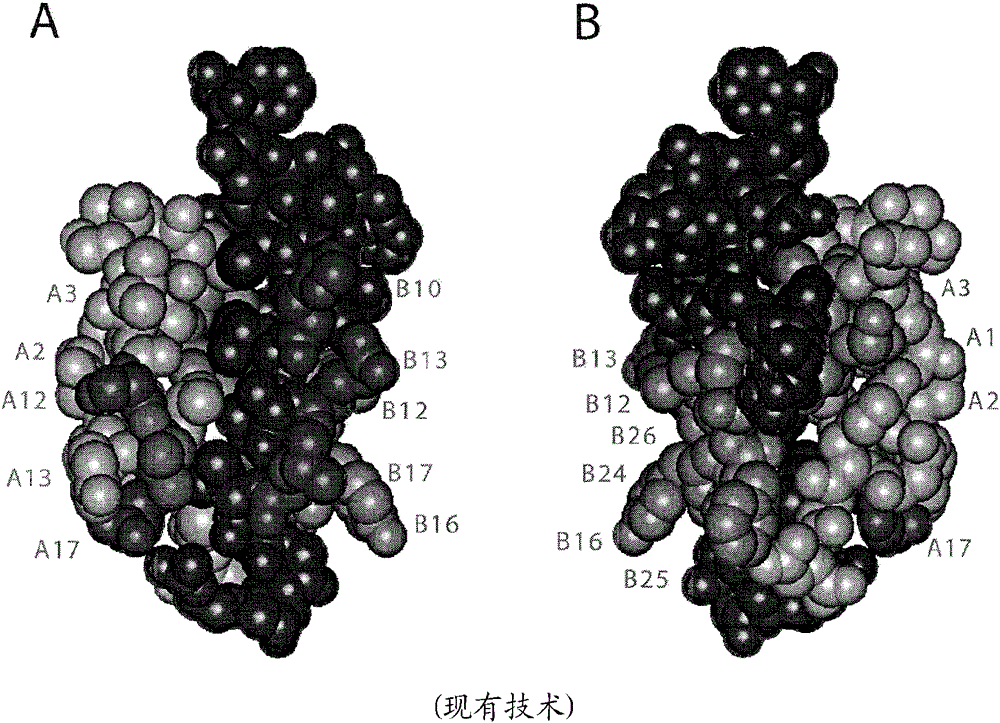
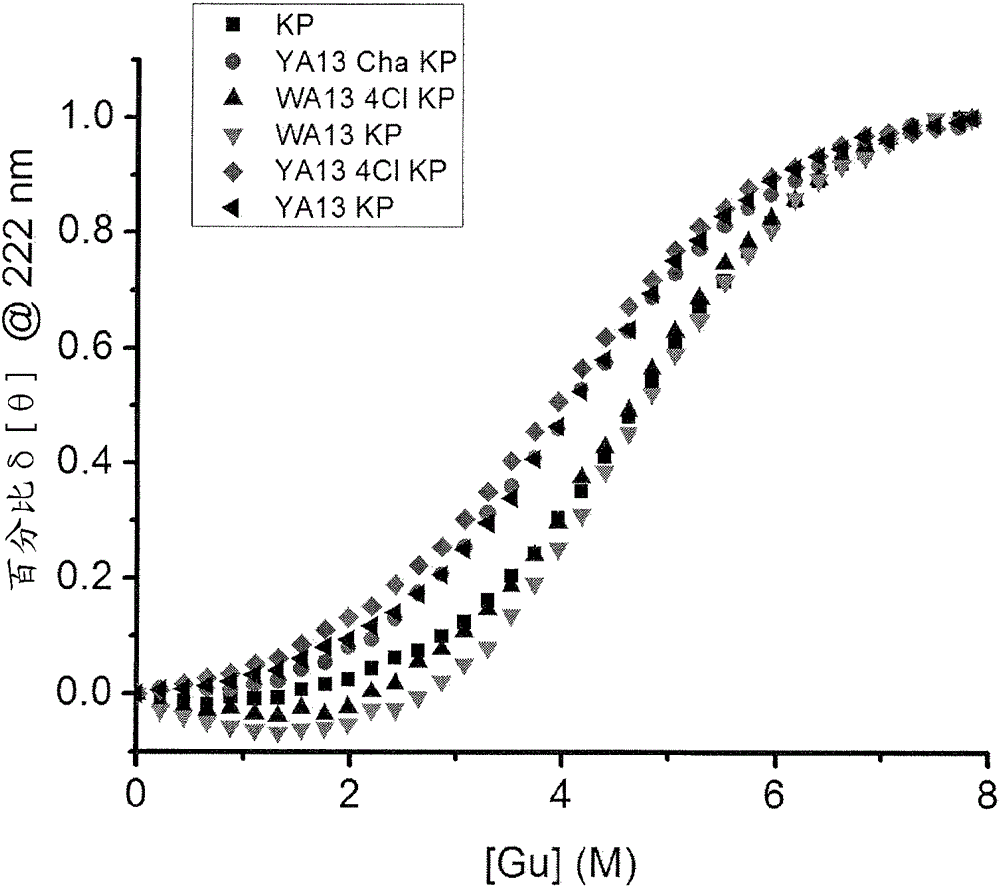
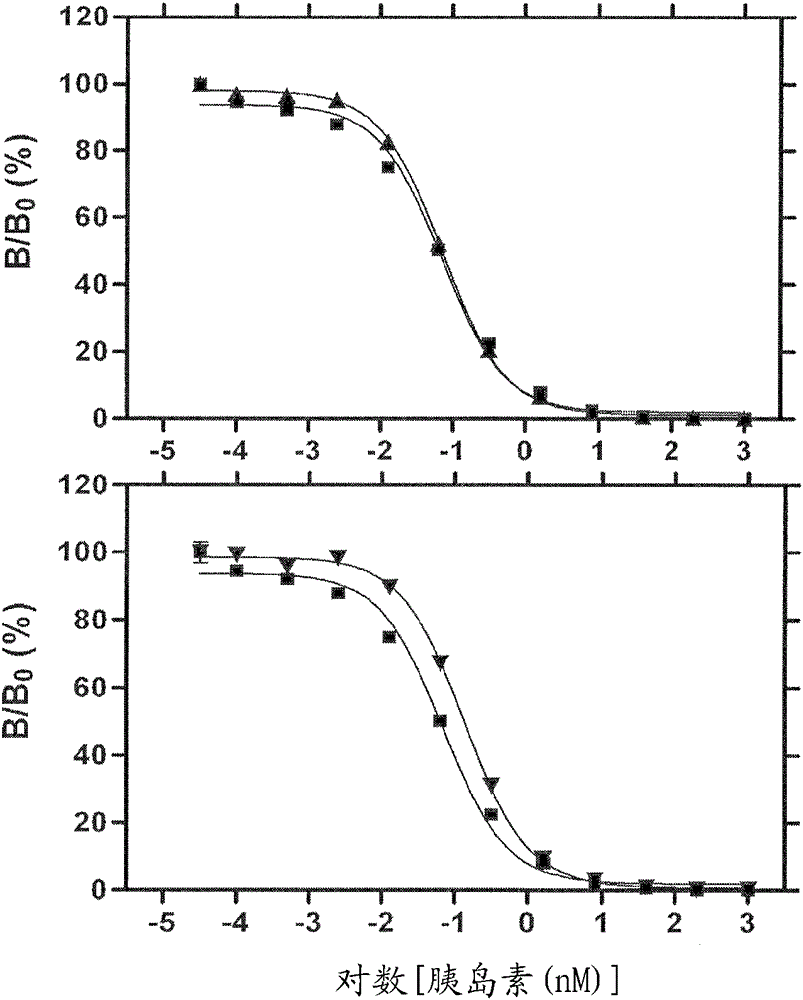
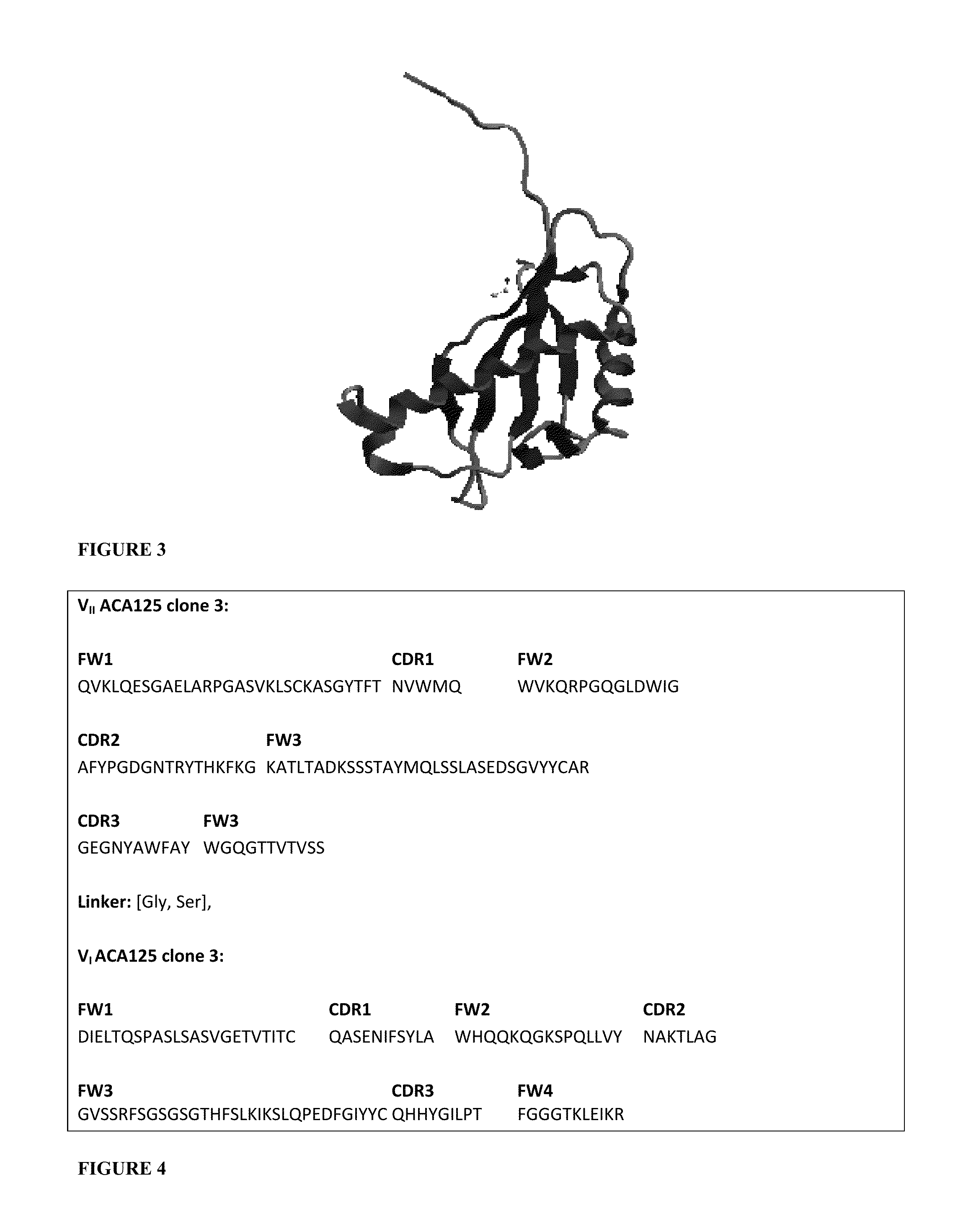
Site 2 insulin analogues
An insulin analogue contains one or more modifications at a distinct protein surface comprising one or more of the residues at position B13, B17, A12, A13, and / or A17. Formulations of the above analogues at successive strengths U-100 to U-1000 in soluble solutions a at least pH value in the range 6.8-8.0 either in the presence of zinc ions at a molar ratio of 2.2-10 zinc ions per six insulin analogue monomers or in the presence of fewer than 1 zinc ions per six insulin analogue monomers. Use of the above formulation in an insulin pump functionally integrated with a continuous glucose monitor and computer-based control algorithm as a closed-loop system. A method of treating a patient with diabetes mellitus comprises administering a physiologically effective amount of the insulin analogue to a patient by means of intravenous, intraperitoneal, or subcutaneous injection.
Owner:CASE WESTERN RESERVE UNIV
Pharmaceutical composition
InactiveUS9220773B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenAntiendomysial antibodies
A pharmaceutical composition for intraperitoneal delivery of an anti-neoplastic agent is provided for treating cancers associated with aberrant mucin expression, preferably ovarian cancer and pancreatic, prostate, metastatic breast, bladder and lung cancers. The composition comprises nanomicelles loaded with the anti-neoplastic agent, and antibodies such as anti-MUC16, anti-MUC1 or anti-MUC4 are conjugated to these nanomicelles. The antibody-bound nanomicelles are optionally embedded in a biodegradable pH- and thermo-responsive hydrogel capable of sol-gel transition at body temperature. The pharmaceutical composition is implantable in the peritoneum, where it transforms into a semi-solid gel at the body's core temperature. In response to pH, the hydrogel swells and releases the antibody-bound nanomicelles. The nanomicelles specifically target mucin antigens on cancer cells. The anti-mucin antibodies can be internalized by the tumor cells, enabling the drug-loaded nanomicelles to gain entry and deliver the chemotherapeutic drugs inside the tumor cell.
Owner:UNIVERSITY OF THE WITWATERSRAND
Line leaf inula flower lactone a and methods for preparing and using the same for treating myocarditis
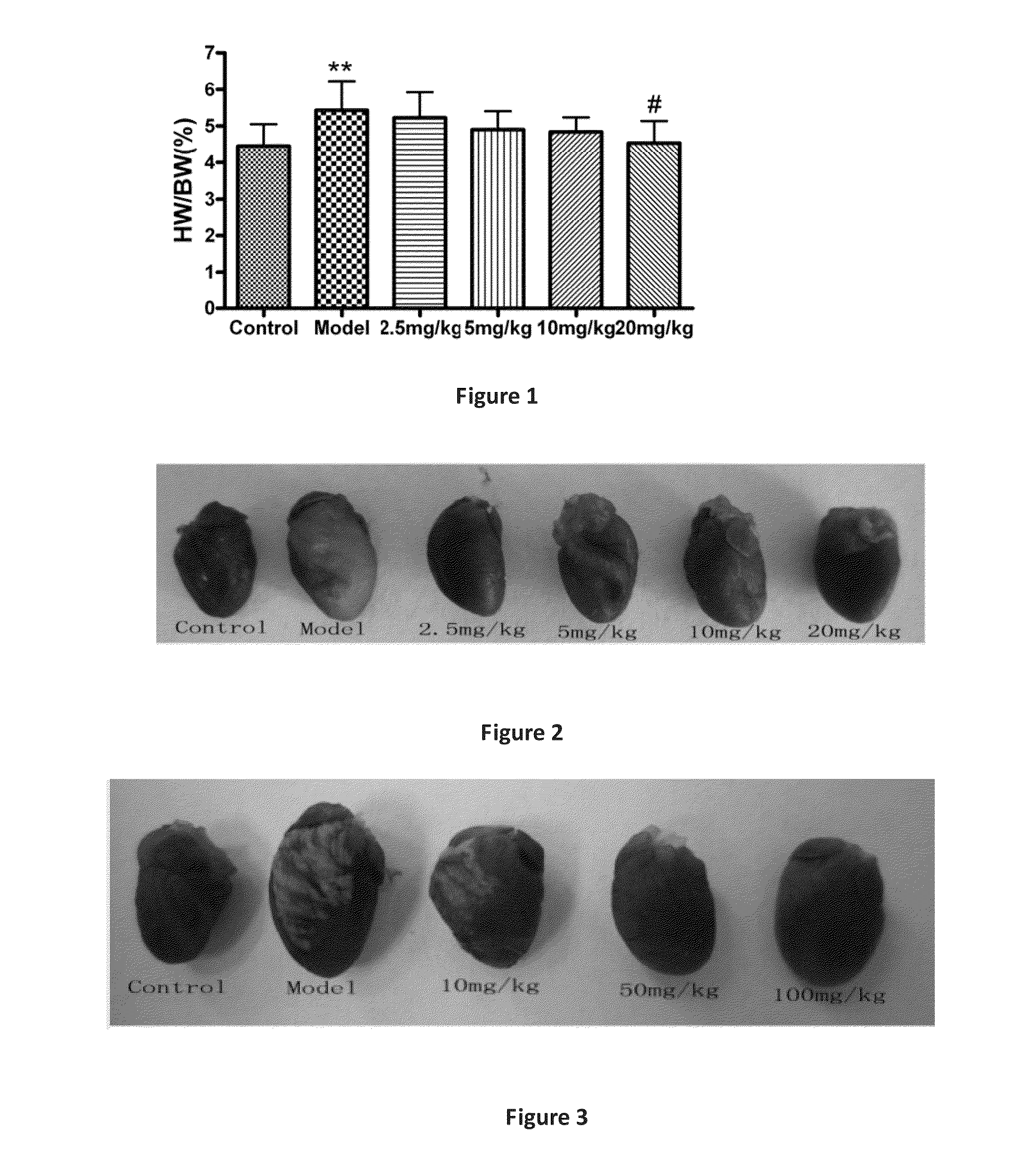
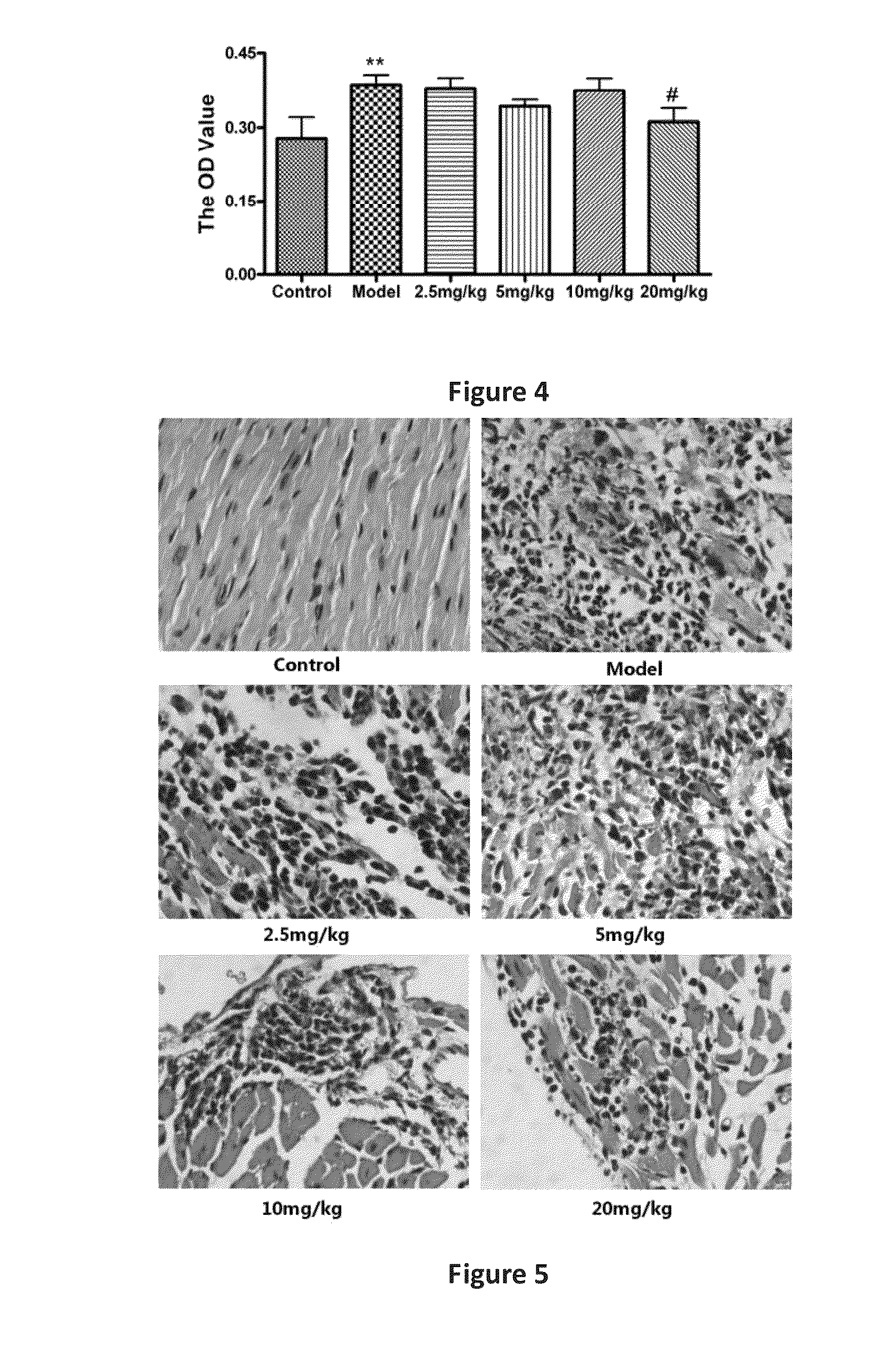
ActiveUS20150105457A1SignificantAvoid it happening againBiocideOrganic chemistryIntraperitoneal routeDisease
Preparation and application of line leaf inula flower lactone A for treating myocarditis having a structure ofThe compound shows positive therapeutic activity against Coxsackie virus and significant dose dependent correlation. The line leaf inula flower lactone A prevents disease in mouse models of experimental autoimmune myocarditis and onset of the process by intraperitoneal injection of a dose of 20 mg / kg / d. When increased to 100 mg / kg / d, the line leaf inula flower lactone A shows positive therapeutic effect on EAM. Line leaf inula flower lactone A is used as the sole active ingredient and combined with a conventional pharmaceutical carrier in a drug for treating myocarditis, and the pharmaceutical composition may be in the form of tablets, dispersible tablets, mouth collapse tablets, retard tablets, capsule, soft capsule, dropping pill, granules, injection, powder injection, or aerosol.
Owner:SHANXI ZHENDONG LEADING BIOTECH CO LTD
Apparatus for performing peritoneal ultrafiltration
ActiveUS10946130B2Constant volumeGlucose concentrationMedical devicesCatheterPeritoneal fluidConcentrations glucose
Owner:TRIOMED AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com












![Combination of atr kinase inhibitors with 2,3-dihydroimidazo[1,2-c]quinazoline compounds Combination of atr kinase inhibitors with 2,3-dihydroimidazo[1,2-c]quinazoline compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/14cc67dc-9d8d-4703-bbdf-cb10229b0075/US20210369724A1-D00001.png)
![Combination of atr kinase inhibitors with 2,3-dihydroimidazo[1,2-c]quinazoline compounds Combination of atr kinase inhibitors with 2,3-dihydroimidazo[1,2-c]quinazoline compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/14cc67dc-9d8d-4703-bbdf-cb10229b0075/US20210369724A1-D00002.png)
![Combination of atr kinase inhibitors with 2,3-dihydroimidazo[1,2-c]quinazoline compounds Combination of atr kinase inhibitors with 2,3-dihydroimidazo[1,2-c]quinazoline compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/14cc67dc-9d8d-4703-bbdf-cb10229b0075/US20210369724A1-D00003.png)