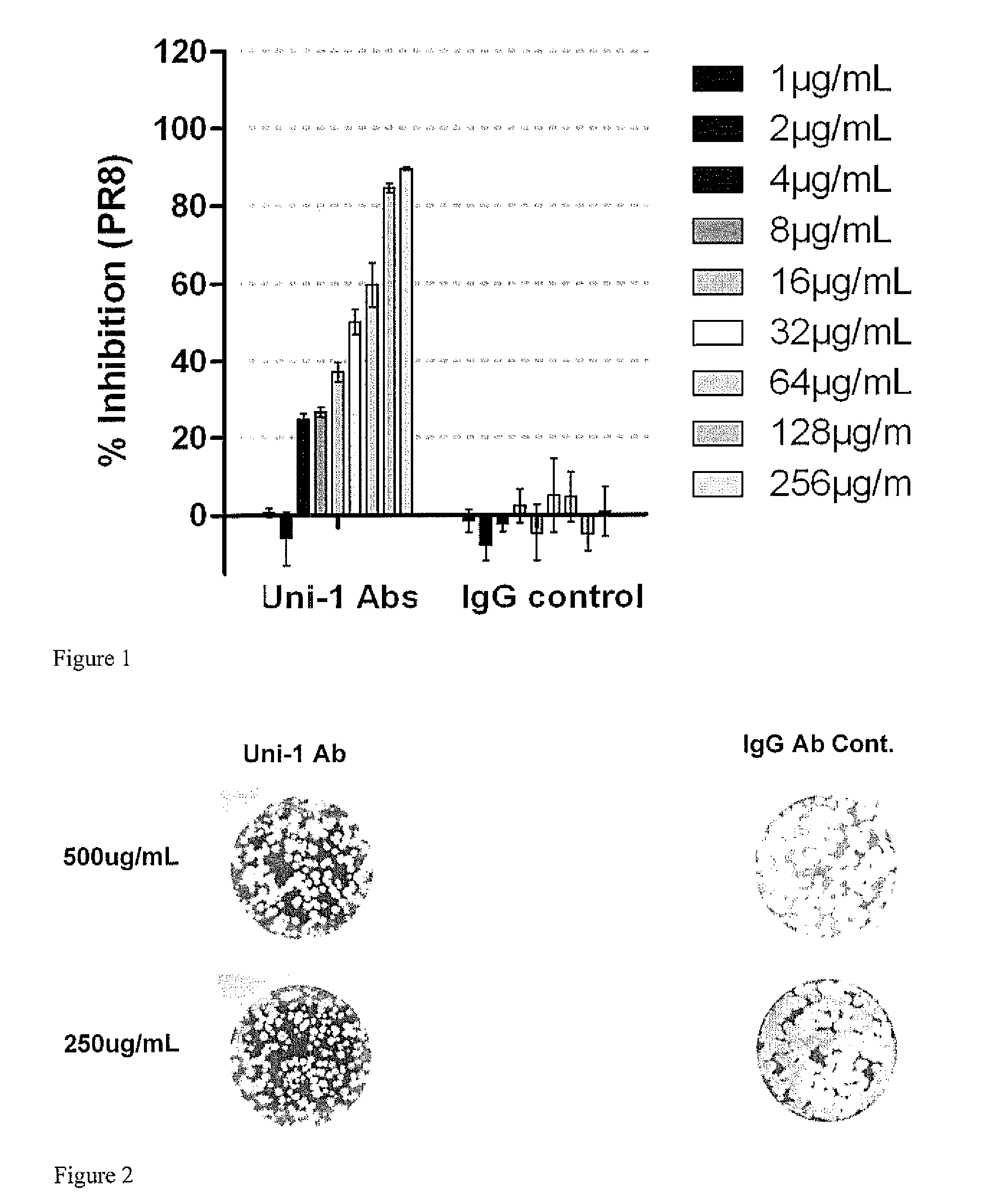
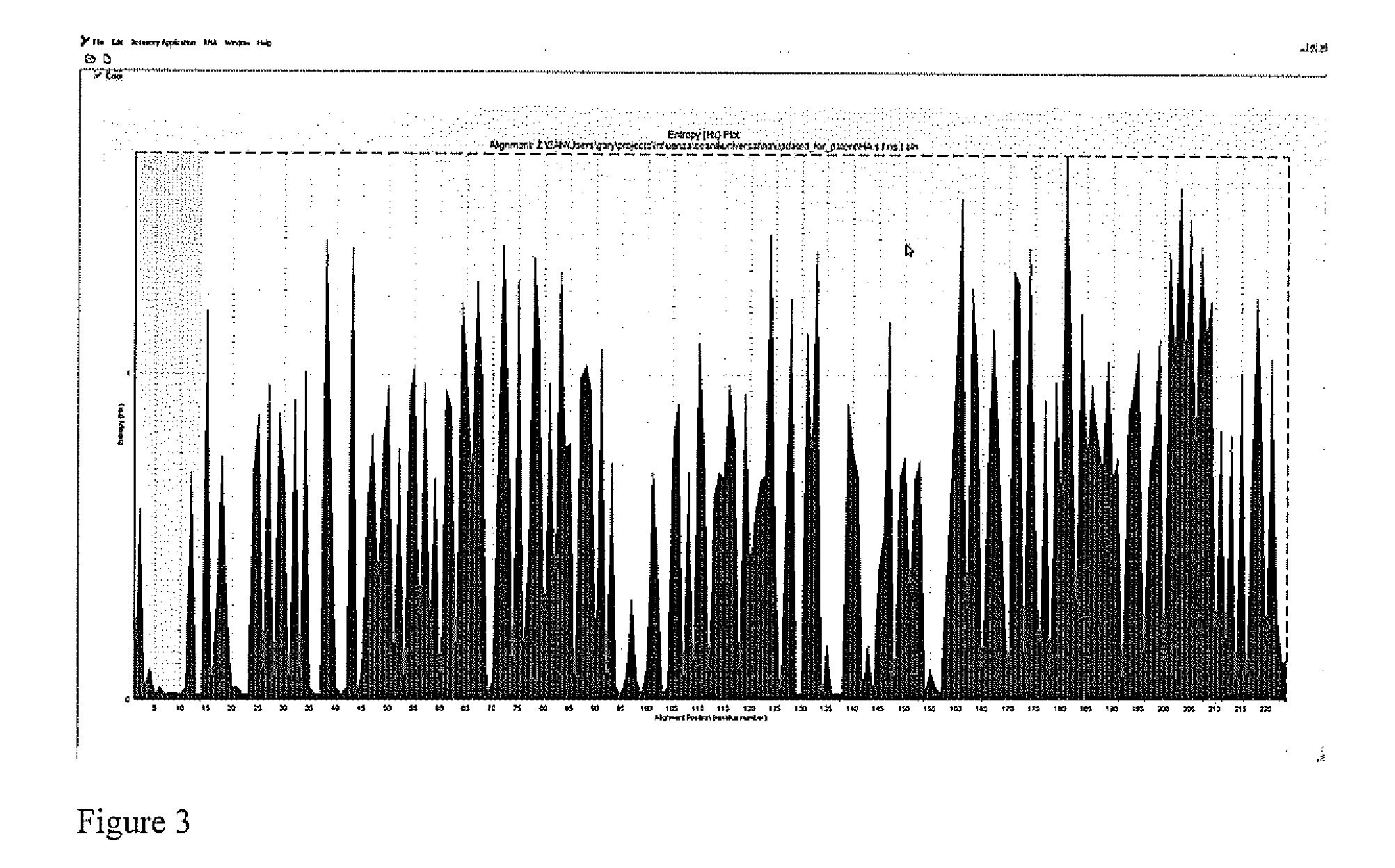
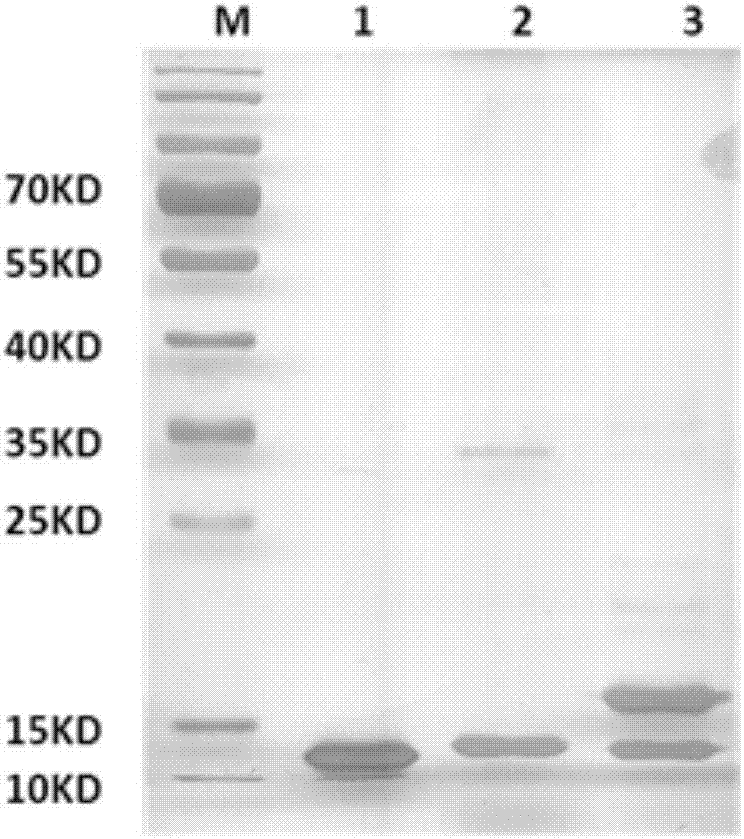
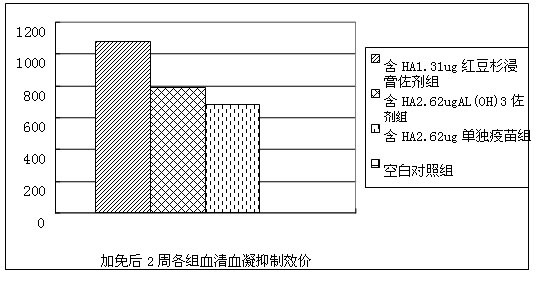
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67 results about "Fluad vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fluad (influenza virus vaccine, surface antigen, inactivated, adjuvanted with MF59C.1) is a trivalent vaccine produced from three influenza virus strains (two subtype A and one type B), approved for the prevention of seasonal influenza in people 65 years of age and older.
Rescue of influenza virus
The invention relates to the field of influenza vaccine production. Influenza vaccines have been produced in embryonated hens' eggs for over 50 years, but recently there have been considerable efforts to develop cell culture systems for vaccine production. The invention provides a nucleic acid comprising an influenza gene segment and a bacteriophage polymerase promotor or a complementary strand of said nucleic acid, and a cell comprising such a nucleic acid capable of producing desired influenza virus. Furthermore, the invention provides a composition comprising a cell or material derived from a cell according to the invention and a virus or material derived from a viral particle according to the invention.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC +1
Influenza virus-like particles (VLPS) comprising hemagglutinin
ActiveUS20110293650A1Easy to captureEnhance immune responseVirusesAntiviralsHemagglutininVirus-like particle
A method for synthesizing influenza virus-like particles (VLPs) within a plant or a portion of a plant is provided. The method involves expression of influenza HA of type A / California / 04 / 09 in plants and the purification by size exclusion chromatography. The invention is also directed towards a VLP comprising influenza HA protein of type A / California / 04 / 09 and plants lipids. The invention is also directed to a nucleic acid encoding influenza HA of type A / California / 04 / 09 as well as vectors. The VLPs may be used to formulate influenza vaccines, or may be used to enrich existing vaccines.
Owner:MEDICAGO INC
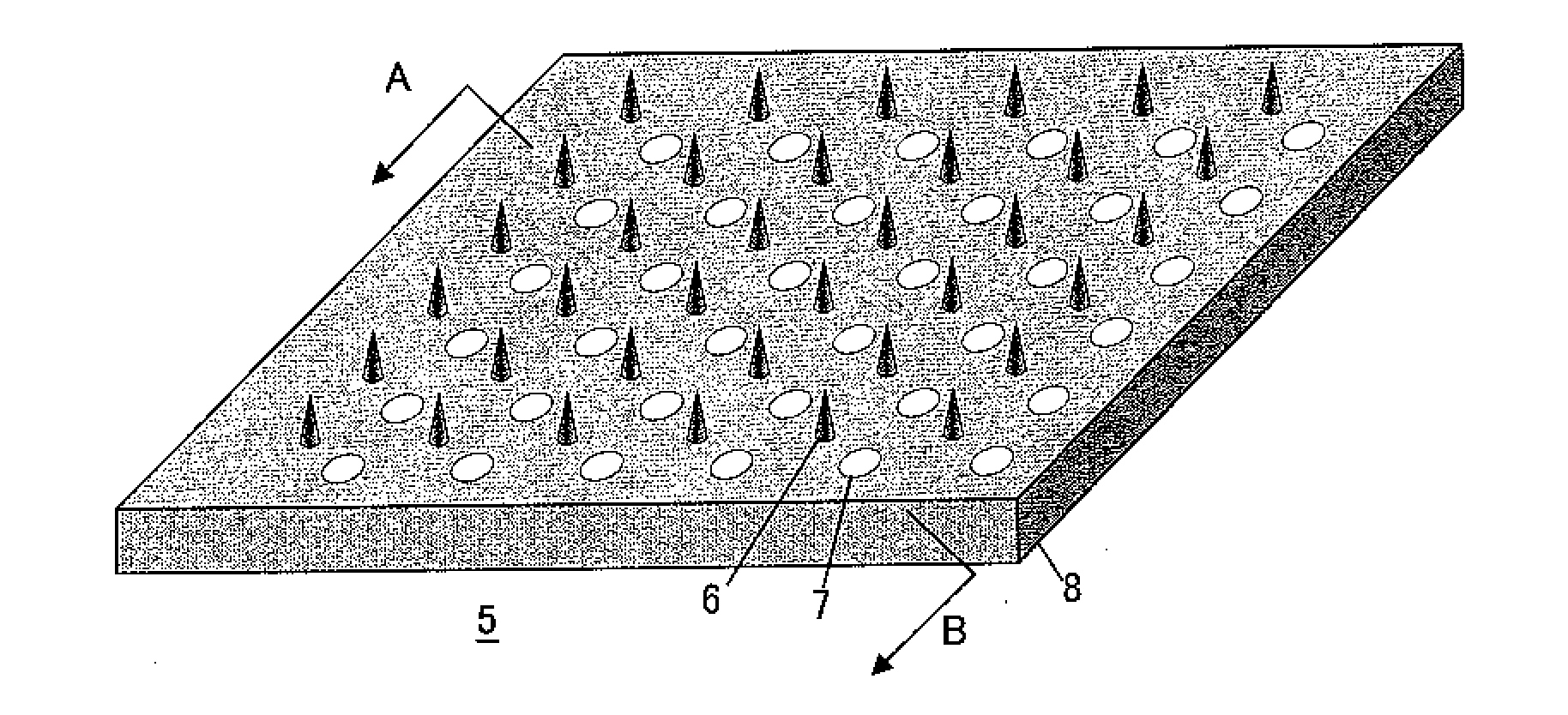
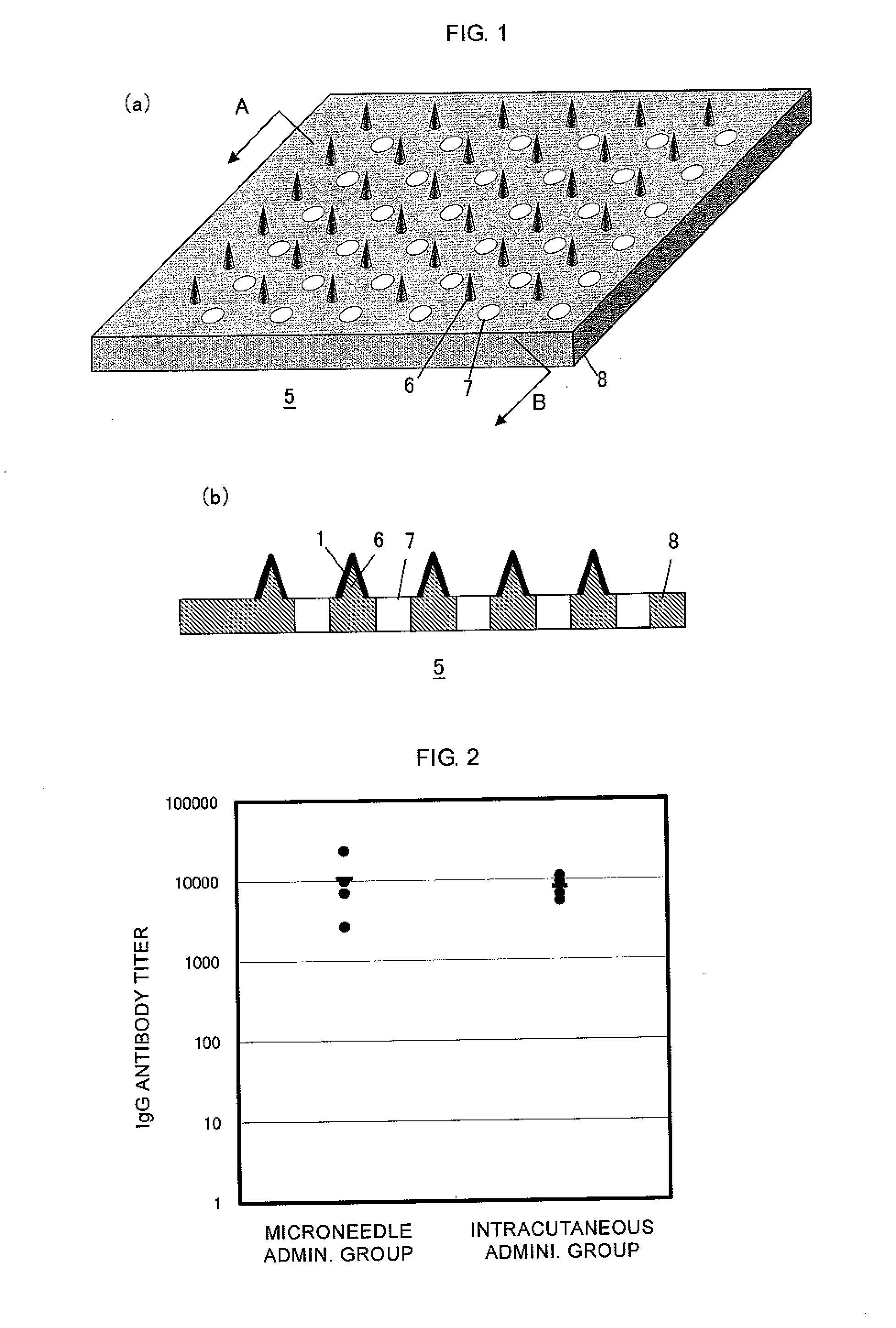
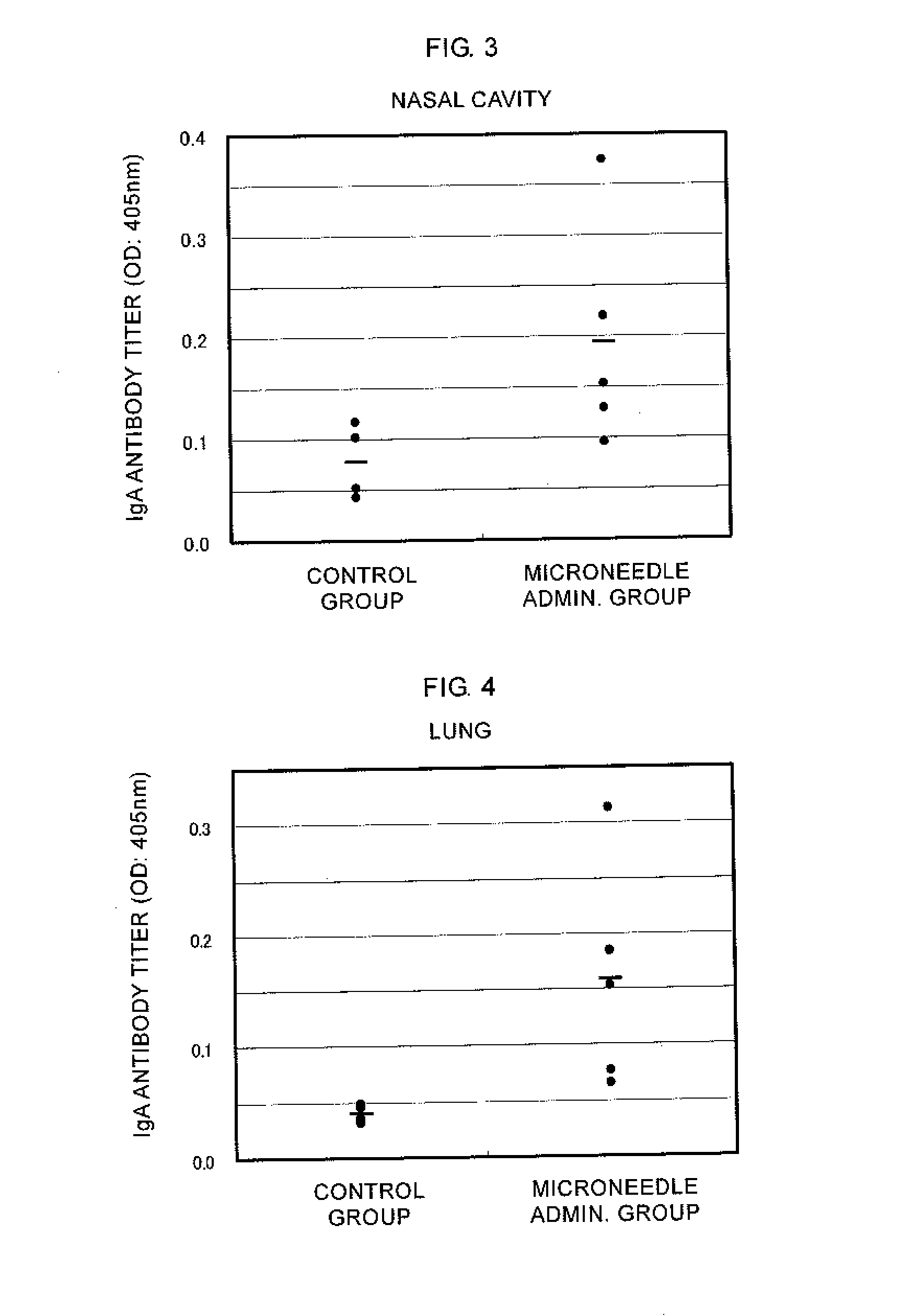
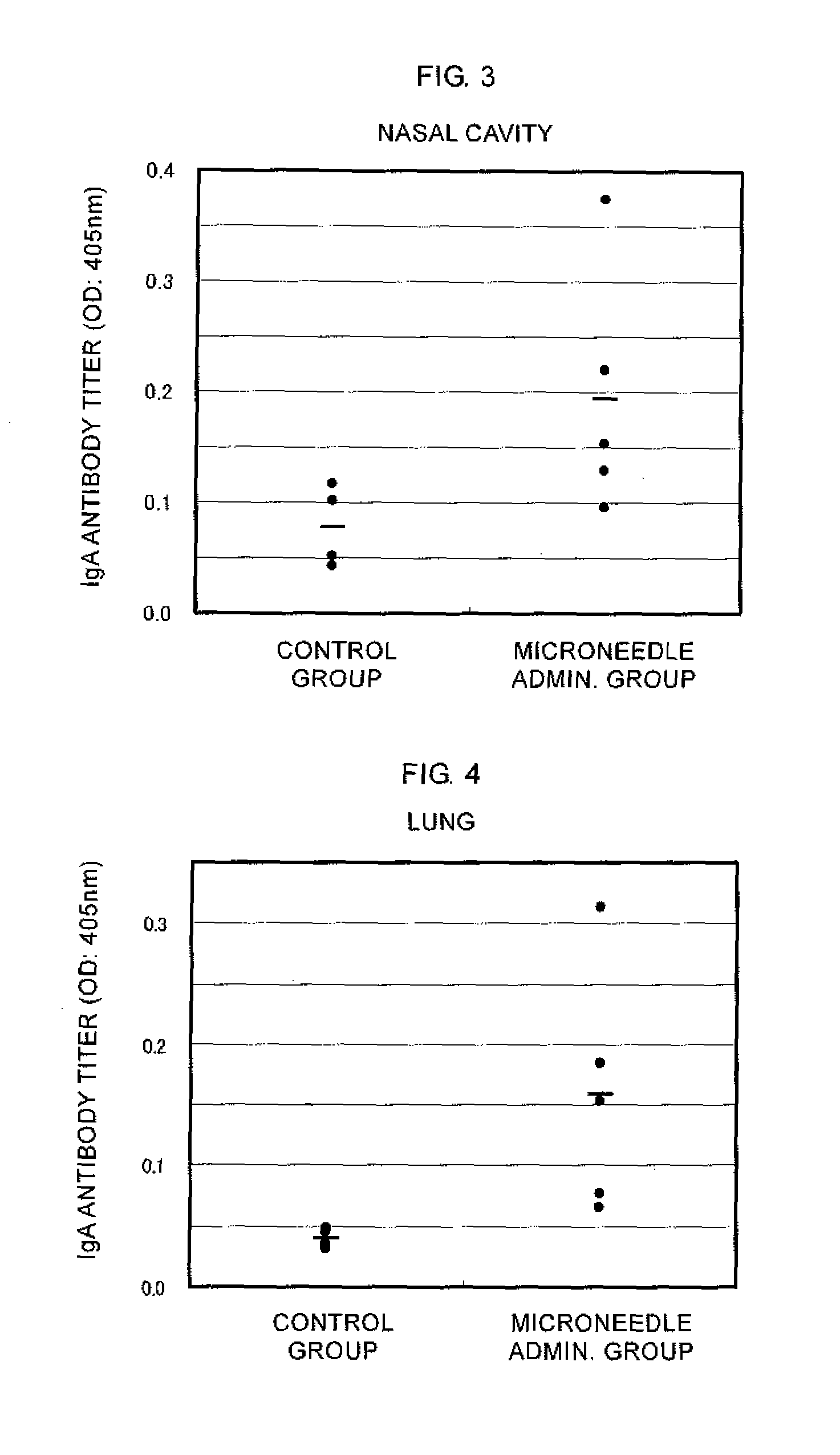
Microneedle device, and method for enhancing the efficacy of influenza vaccine by using microneedle device
ActiveUS20110112509A1Low effective doseSimple and efficient operationSsRNA viruses negative-senseViral antigen ingredientsInfluenza vaccineBULK ACTIVE INGREDIENT
The present invention provides a method for enhancing the immunogenicity using a microneedle device capable of enhancing the immunogenicity of an influenza vaccine. According to the method for enhancing the immunogenicity using the present microneedle device, a microneedle device having microneedles made of polylactic acid, coated with an influenza vaccine composed of an antigen having type A strain (H1N1), type A strain (H3N2), and type B strain as active ingredients is brought into direct contact with the skin so as to transcutaneously administer the aforementioned influenza vaccine. After the transcutaneous administration, lauryl alcohol is applied to the site of the skin where the microneedle device has been brought into direct contact.
Owner:HISAMITSU PHARM CO INC
Soluble Recombinant Influenza Antigens
ActiveUS20110104753A1Improve the level ofReduce complexitySsRNA viruses negative-senseAntibody mimetics/scaffoldsSequence signalHemagglutinin
The present invention provides a recombinant soluble trimeric hemagglutinin (rHA) protein comprising a hemagglutinin ectodomain and an oligomerization domain. The rHA is produced as a soluble homotrimer, and may further comprises a signal peptide and / or an endoplasmic reticulum (ER) retention signal. The invention is also directed to nucleic acids encoding the rHA of the invention, as well as vectors and chimeric constructs comprising the nucleic acid. Methods of producing the rHA are also provided. The rHA described herein may be used to formulate influenza vaccines, or may be used to enrich existing vaccines.
Owner:MEDICAGO INC
Influenza virus splitting vaccine and preparation method thereof
InactiveCN102068692AGood immune effectLess antigenAntiviralsAntibody medical ingredientsHemagglutininMedicine
The invention provides an influenza virus splitting vaccine and a preparation method thereof. Each dose of influenza virus splitting vaccine contains three influenza hemagglutinins, namely H1N1, H3N2 and type B, a CpG ODN adjuvant and an aluminium adjuvant. The invention also provides a preparation method for the influenza vaccine, which comprises the following steps of: multiplying influenza viruses in chick embryo; performing ultrafitration and concentration; centrifuging and purifying; splitting; performing secondary purification; inactivating; and diluting and packaging. The influenza virus splitting vaccine can reduce the dosage of the influenza virus antigen and production cost of the influenza vaccine, and is suitable for quickly improving influenza vaccine supplying capacity under the threat of global influenza pandemic.
Owner:BEIJING MINHAI BIOTECH
Novel use
InactiveUS20110123568A1Enhance immune responseImprove responseSsRNA viruses negative-senseViral antigen ingredientsDiseaseAntigen
The present invention relates to influenza vaccine formulations and vaccination regimes for immunizing against influenza disease. In particular the invention relates to vaccine formulations comprising an oil-in-water emulsion adjuvant and optionally 3D-MPL, their use in medicine, in particular their use in augmenting immune responses to influenza antigens, and to methods of preparation, wherein the oil in water emulsion comprises a sterol, a metabolizable oil and an emulsifying agent. The present invention also provides for new prime-boost vaccination regimes for immunizing humans against influenza disease, and in particular for ensuring and ameliorating the immune response to the booster administration, in which a first influenza virus vaccine is administered in the presence of an adjuvant.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Method for proliferating avian influenza viruses in bioreactor with cell carrier
InactiveCN102127524AUniform product qualityStable virulenceMicroorganism based processesViruses/bacteriophagesAutomatic controlVaccine Production
The invention discloses a method for replicating and proliferating avian influenza viruses in the passage cells which are absorbed and grow in the microcarrier of a bioreactor. The invention is based on the method which uses cells as carrier to proliferate viruses in the bioreactor. The obtained avian influenza viruses and the avian influenza virus vaccine product have uniform quality and stable toxicity. The automatic control of vaccine production can be realized, the problem of large-scale production and application can be solved, the production matrix of the influenza vaccine can be completely changed, the technology that chick embryo is adopted to culture viruses can be changed, the problems caused by chick embryo such as adventitious agent pollution and viral pollution and the interferences caused by heterologous proteins such as chick embryo can be reduced, the purity, safety and immune effect of vaccine can be increased and the method can play an important role in the prevention of avian influenza.
Owner:深圳市南海潮生物技术有限公司
Neuraminidase-deficient live influenza vaccines
ActiveUS8597661B2SsRNA viruses negative-senseViral antigen ingredientsNeuraminidaseInfluenza vaccine
Attenuated, neuraminidase deficient influenza virus, and compositions and methods to prepare that virus, are provided.
Owner:WISCONSIN ALUMNI RES FOUND
Influenza vaccine
ActiveUS20120070455A1Enhance immune responseSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutinin proteinInfluenza vaccine
The present invention discloses isolated peptides encoding an antigen or fragments thereof from the N-terminus of hemagglutinin protein of influenza, methods for isolating such antigens and specific uses thereof. The peptide can be used as a vaccine to generate an antibody response that neutralizes influenza infectivtty against a variety of influenza strains.
Owner:HE RUNTAO +2
Polypeptide, immunogenicity conjugate and influenza vaccine
InactiveCN107488217AReduce morbidityAvoid deathSsRNA viruses negative-senseAntibody mimetics/scaffoldsVirus influenzaImmunogenicity
The invention discloses a polypeptide, an immunogenicity conjugate and an influenza vaccine, and relates to the bio-technical field. The polypeptide is any one of a), b) or c): a) a polypeptide with the amino acid sequence as shown in SEQ ID NO.1; b) fused protein obtained by connecting a label to the end N and / or the end C of the polypeptide with the amino acid sequence as shown in SEQ ID NO.1; c) a polypeptide which is obtained by substitution and / or deletion and / or adding of one or several amino acid residues for the amino acid sequence as shown in SEQ ID NO.1 and has the same function. The polypeptide can be used for preventing or treating influenza virus, and can be used as an active component for preparing a medicine for preventing or treating the influenza virus. The immunogenicity conjugate disclosed by the invention can improve the immunogenicity and the immune effect on an antigen at a conformational dependency site.
Owner:华兰生物疫苗股份有限公司
Quadrivalent influenza virus subunit vaccine and preparation method thereof
InactiveCN107537032AHigh purityQuality improvementAntiviralsViruses/bacteriophagesHemagglutininAdjuvant
The invention discloses a quadrivalent influenza virus subunit vaccine and a preparation method thereof, wherein virus protein after lysis is further purified by using a lysis agent and a new purification method to prepare the tetravalent influenza virus subunit vaccine, the content of four influenza hemagglutinins such as influenza A virus H1N1, influenza A virus H3N2 and two kinds of influenza Bviruses in each dose of the vaccine is more than 80%, and the quadrivalent influenza virus subunit vaccine does not contain adjuvant and does not contain thimerosal and other preservatives. The invention further provides a preparation method of the quadrivalent influenza virus subunit vaccine, wherein the preparation method comprises: virus inoculation, virus proliferation culture, allantoic fluid harvesting, clarification, ultra-filtration concentration, inactivation, lysis and ultracentrifugation purification, gel filtration chromatography purification (ultra-filtration), blending, filtration sterilization, sub-packaging, packaging and other steps. According to the present invention, the quadrivalent influenza virus subunit vaccine can improve the safety of the influenza vaccine, can eliminate the adverse reaction caused by the adjuvant, and can eliminate the toxic-side effects caused by thimerosal.
Owner:ZHONGYI ANKE BIOTECH CO LTD
Influenza vaccine constructs
InactiveCN104582714ASsRNA viruses negative-senseViral antigen ingredientsInfluenza vaccineTGE VACCINE
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Recombinant avian influenza vaccine and uses thereof
ActiveUS8394384B2Highly immunogenicImprove protectionSsRNA viruses negative-sensePeptide/protein ingredientsEpitopeHemagglutinin
Owner:MERIAL INC
Universal influenza vaccine and preparation method thereof
ActiveCN105148264AImproving immunogenicityInhibit productionAntiviralsAntibody medical ingredientsHemagglutinin proteinViral Vaccine
The invention relates to universal influenza vaccine and a preparation method thereof. After an influenza virus HA is subjected to gene transfection to eukaryocyte, influenza haemagglutinin (HA) protein subjected to eukaryotic expression modification is inserted into a host plasma membrane, then vesicles largely displaying influenza virus envelope glycoprotein is produced with a method that a surface active agent induces a cell to bud, and the vesicles are changed into controllable and nanometer mimicry virus vesicles with uniform dimensions after purification and re-adding of the surface active agent for treatment. At the same time, by the aid of establishment of stably expressing an HA cell strain and usage of a bioreactor, the method is also applicable to large scale influenza vaccine production. The method is simple and feasible, hemagglutinin of most influenza virus (including avian influenza and swine influenza) can be displayed, and the method has excellent universality, has a great potential and can be used for designing emerging highly lethal influenza virus vaccines.
Owner:XIAMEN UNIV
An avian influenza vaccine composition, a preparing method thereof and applications of the composition
InactiveCN104288759AImprove presentationStrong penetrating powerSsRNA viruses negative-senseViral antigen ingredientsMicrosphereLiposome
The invention provides an avian influenza vaccine composition. The composition comprises a liposome, and an immunizing dose of M2 and / or 3M2e protein. The sequence of the M2 protein is SEQ ID NO.2. The sequence of the 3M2e protein is SEQ ID NO.4. According to the avian influenza vaccine, the M2 protein and / or the 3M2e protein which are the main components in an antigen in an influenza vaccine are included by utilization of vesicular microspheres formed by biological membrane bilayers of the liposome. Under functions of the liposome, the vaccine can efficiently carry the antigen included to an antigen presenting cell in a targeted manner, stimulates a body to generate humoral immunity and cellular immunity, has a broad spectrum and durability, resists a plurality of types or subtypes of avian influenza viruses, has a function of slowly releasing the antigen under the actions of the liposome, and is capable of being degraded by the body and free of side and toxic effects. The vaccine is beneficial to large-scale production.
Owner:PU LIKE BIO ENG
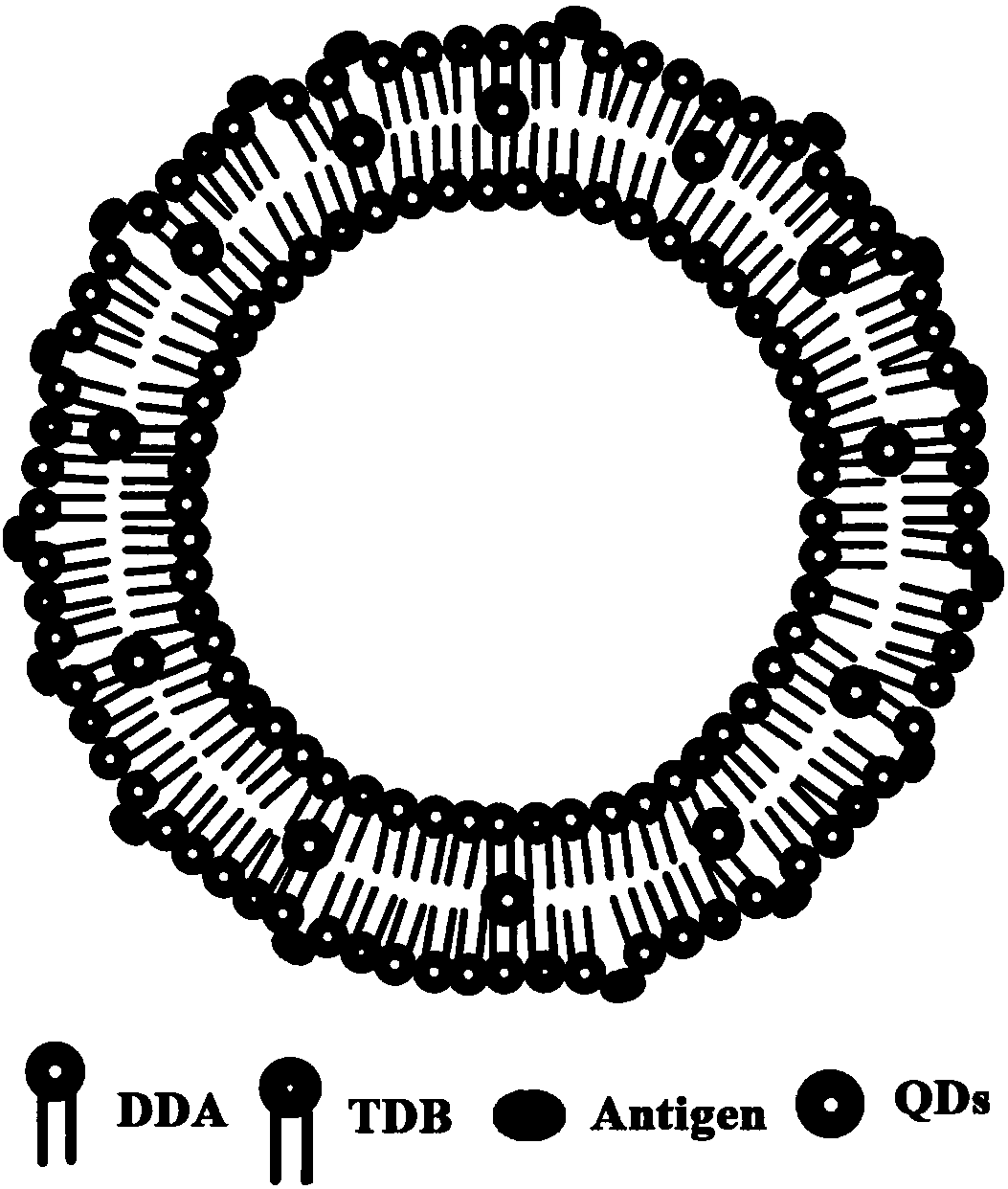
Cationic liposome influenza vaccine capable of entrapping quantum dots and preparation method thereof
ActiveCN107583059AHigh encapsulation efficiencyHigh drug loadingAntiviralsAntibody medical ingredientsFreeze thawingZeta potential
The invention relates to a cationic liposome influenza vaccine capable of entrapping quantum dots and a preparation method thereof. The cationic liposome influenza vaccine adopts a cationic complex asa vaccine adjuvant and a carrier, and is prepared by entrapping the quantum dots and influenza vaccine stock into the cationic liposome complex by adopting a film scattering method, a freeze-thaw method or a freeze drying method; and the particle size of the obtained cationic liposome influenza vaccine is between 100nm and 3 micrometers, a zeta potential is 30 to 90 mV, the entrapping rate is 45percent to 95 percent, and the drug carrying capacity is 2 to 10 percent. The cationic liposome influenza vaccine entrapping the quantum dots prepared by the invention can be used as a biological probe to capture and image cells and is good in stability; and by virtue of the immunization of mouse nasal mucosa, the novel vaccine can significantly improve the mouse humoral immunity and mucosal immunity response levels.
Owner:NINGXIA MEDICAL UNIV
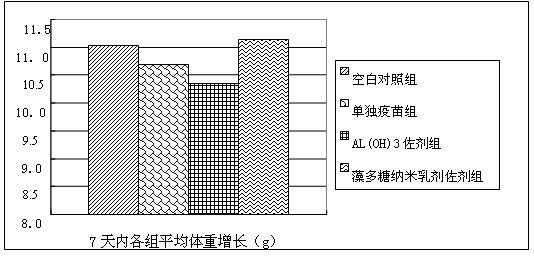
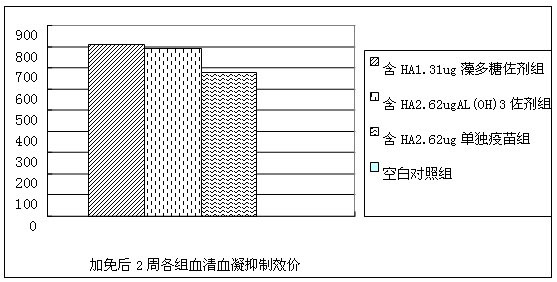
Spirulina polysaccharide immune adjuvant and influenza vaccine containing the same
ActiveCN102028945AEasy to prepareReduced activityAntiviralsAntibody medical ingredientsSide effectVaccine antigen
The invention provides a spirulina polysaccharide immune adjuvant and an influenza vaccine containing the same, wherein the spirulina polysaccharide immune adjuvant is prepared from the components in mass ratio as follows: 0.001-100% of spirulina polysaccharide and 0-99.999% of carrier, which is capable of preferably improving immunogenicity of the vaccine and greatly reducing dosage of antigen vaccine; the dosage of the antigen vaccine taking the spirulina polysaccharide as adjuvant is only a half of the dosage of the antigen vaccine taking the aluminum hydroxide as adjuvant or less, which can achieve ideal immune protection effect as well with high safety and small side effect; the influenza vaccine taking the spirulina polysaccharide as adjuvant is convenient for preparation and the quality thereof is easy to control.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
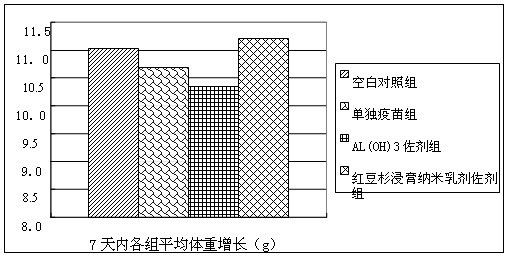
Taxus mairei immunological adjuvant and influenza vaccine containing same
ActiveCN102028946AEasy to prepareReduced activityAntiviralsAntibody medical ingredientsSide effectVaccine antigen
The invention provides a taxus mairei immunological adjuvant and an influenza vaccine containing the same. The taxus mairei immunological adjuvant is characterized by consisting of the following components in percentage by mass: 0.001 to 100 percent of taxus madia extract, and 0 to 99.999 percent of vector. The immunological adjuvant can better improve the immunogenicity of the vaccine, and greatly reduces the consumption of vaccine antigens; the consumption of the vaccine antigens used by using the taxus mairei extract as the adjuvant is half of the consumption of vaccine antigens used by only using aluminum hydroxide as the adjuvant or less, and ideal immunological protective effect can be achieved likewise; the immunological adjuvant has high safety and low side effect; and a method for preparing the influenza vaccine by using the taxus mairei extract as the adjuvant is simple and convenient, and the quality of the vaccine is easy to control.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
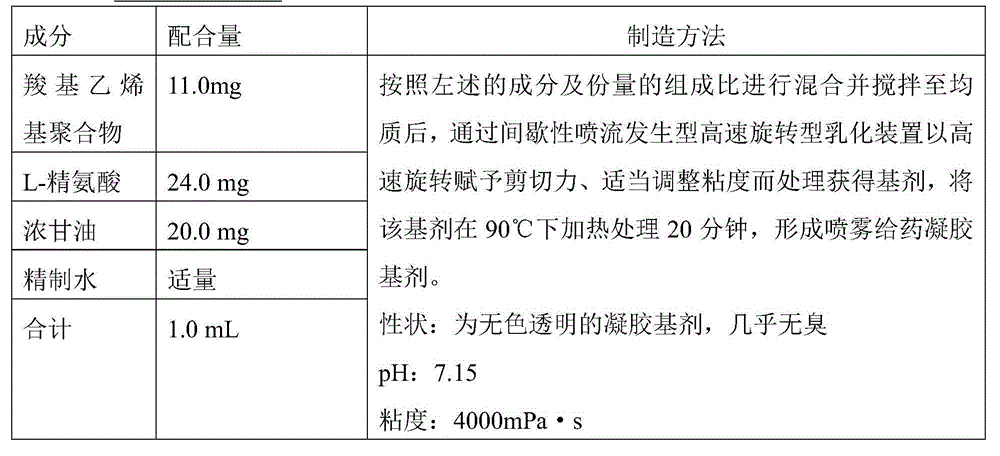
Nasal influenza vaccine composition
InactiveCN104884086ATo deal with the epidemicEffective immune responseSsRNA viruses negative-senseViral antigen ingredientsAntigenAdjuvant
The present invention relates to a nasal mucosal spray delivery type influenza vaccine composition which is characterized in that the composition comprises a gel base including an influenza virus inactivated whole particle antigen and a carboxyvinyl polymer but does not comprise an adjuvant.
Owner:JAPAN AS REPRESENTED BY DIRECTOR GENERAL OF NAT INST OF INFECT IOUS DISEASES +1
Method for detecting allergen ovomucoid or ovotransferrin in influenza vaccine quantificationally
InactiveCN102650640AImprove and control qualityReduce and prevent allergic reactionsBiological testingEmbryoInfluenza vaccine
The invention discloses a method for detecting allergen ovomucoid or ovotransferrin in influenza vaccine quantificationally. Based on the preparation of anti-ovomucoid or anti-ovotransferrin monoclonal antibody, after paired antibodies are screened, a double antibody sandwich ELIsA (enzyme linked immunosorbent assay) adsorption test is established to quantificationally detect allergen ovomucoid or ovotransferrin in influenza vaccine. The method aims to reduce the incidence rate of allergic reaction of inoculated people caused by ovomucoid or ovotransferrin remaining in influenza vaccine prepared in a chick embryo allantoic cavity in a purified manner, has the advantages of strong specificity, high sensitivity, good repeatability and simplicity and convenience in implementation, and is very suitable for quickly and quantificationally detecting ovomucoid and ovotransferrin in food or influenza vaccine in disease control.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Influenza vaccine and therapy
ActiveUS20150086560A1Extended half-lifeReduce severitySsRNA viruses negative-senseViral antigen ingredientsProphylactic treatmentInfluenza vaccine
The present invention is directed generally to M1 polypeptides that can be utilized as vaccines and / or antigens for generation of anti-M1 polypeptide antibodies for prophylactic treatment of individuals who are susceptible to infection by influenza virus. The anti-M1 polypeptide antibodies of the invention are useful for treatment of individuals infected with influenza virus, or useful for prophylactic treatment of individuals who are susceptible to infection by influenza virus, or for immune-suppressed individuals who cannot generate an effective antibody response.
Owner:ENGEN BIO
Epitope peptide capable of inducing broad-spectrum protective antibody by H1N1 influenza virus hemagglutinin and application
The invention provides an epitope peptide capable of inducing a broad-spectrum protective antibody by H1N1 influenza virus hemagglutinin. The epitope peptide is from an influenza A virus (IAV) H1N1 hemagglutinin (HA) cleavage site. Besides the H14 subtype, the epitope peptide almost 100% conservatively exists in 17 IAV-H subtype HA proteins of partial H8. The epitope peptide can be used for preparing anti-influenza broad-spectrum therapeutic antibody medicines and detection antigens, or used for preparing univalent / multivalent preventive influenza vaccines for human and / or poultry.
Owner:SHANGHAI INST OF PLANNED PARENTHOOD RES
Process for producing influenza vaccine
ActiveUS20120219587A1SsRNA viruses negative-senseViral antigen ingredientsOctylphenoxy PolyethoxyethanolInfluenza vaccine
A process for producing a split influenza virus preparation or subunit influenza preparation comprising the steps of: (i) providing a whole virus preparation; (ii) splitting the whole virus preparation in the presence of a first detergent; (iii) adding t-octylphenoxypolyethoxyethanol (TRITON X-100™) to the resulting split virus preparation; and (iv) filtering the split virus preparation.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Microneedle device, and method for enhancing the efficacy of influenza vaccine by using microneedle device
ActiveUS9028463B2Low effective doseSimple and efficient operationSsRNA viruses negative-senseViral antigen ingredientsBULK ACTIVE INGREDIENTInfluenza vaccine
The present invention provides a method for enhancing the immunogenicity using a microneedle device capable of enhancing the immunogenicity of an influenza vaccine. According to the method for enhancing the immunogenicity using the present microneedle device, a microneedle device having microneedles made of polylactic acid, coated with an influenza vaccine composed of an antigen having type A strain (H1N1), type A strain (H3N2), and type B strain as active ingredients is brought into direct contact with the skin so as to transcutaneously administer the aforementioned influenza vaccine. After the transcutaneous administration, lauryl alcohol is applied to the site of the skin where the microneedle device has been brought into direct contact.
Owner:HISAMITSU PHARM CO INC
Preparation method of recombinant avian influenza subunit vaccine
InactiveCN104667268ASimple stepsGood immune effectMicroorganism based processesAntiviralsImmune effectsCulture cell
The invention relates to a preparation method of a recombinant avian influenza subunit vaccine. The preparation method of the recombinant avian influenza subunit vaccine comprises the following steps: culturing a virus strain A / Anhui / 1 / 2013(H7N9), and extracting RNA in the virus strain; obtaining cDNA by utilizing reverse transcription PCR; amplifying a gene HA by taking the obtained cDNA as a template; constructing a recombinant expressing plasmid containing the gene HA, and expressing in Sf9 cells; and culturing cells containing the recombinant expressing plasmid, collecting, and purifying a recombinant avian influenza vaccine. The preparation method of the recombinant avian influenza subunit vaccine has the advantages of simple steps, good immune effect, low production cost and high yield, and is easy to operate and applicable to large-scale production.
Owner:WUHAN SIQIYUAN BIOTECH
Polypeptides for treating and/or limiting influenza infection
InactiveUS20150038408A1Infection controlBiocidePeptide/protein ingredientsHemagglutininInfluenza vaccine
The present invention provides polypeptides that recognize and are strong binders to Influenza A hemagglutinin and can be used, for example, to treat and / or limit development of an influenza infection. The present invention further provides isolated nucleic acids encoding the polypeptides of the invention, recombinant expression vectors comprising the nucleic acids encoding the polypeptides of the invention operatively linked to a suitable control sequence, and recombinant host cells comprising the recombinant expression vectors of the invention. The present invention also provides antibodies that selectively bind to the polypeptides of the invention, and pharmaceutical compositions comprising one or more polypeptides according to the invention and a pharmaceutically acceptable carrier. Additionally, the present invention provides methods for treating and / or limiting an influenza infection, methods for diagnosing an influenza infection, or monitoring progression of an influenza infection, methods for identifying candidate influenza vaccines, and methods for identifying candidate compounds for treating, limiting, and / or diagnosing influenza infection.
Owner:UNIV OF WASHINGTON CENT FOR COMMERICIALIZATION
Method for detecting neutralizing antibodies aiming at avian influenza virus in serum of animals immune to avian influenza vaccine
InactiveCN101587121AImprove objectivityImprove standardizationMaterial analysisSerum igeAvian influenza virus
The invention discloses a method for detecting neutralizing antibodies aiming at avian influenza virus in serum of animals immune to avian influenza vaccine. The method provided by the invention comprises the steps: mixing inactivated to-be-detected serum with avian influenza virus; co-culturing obtained virus-serum mixture and a host cell of the avian influenza virus; taking an antibody resisting avian influenza virus NP protein as a first antibody to perform enzyme-linked immune response; and determining the titer of a neutralizing antibody of avian influenza virus serum in the to-be-detected serum. The method can be used to detect the effectiveness of the avian influenza vaccine. The method provided by the invention has the advantages of strong objectivity, convenience for standardization, short required time, capability of being performed in BSL-2 laboratories and performing high-throughput detection, objective results, stability and reliability.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Adjuvated Influenza Vaccine and Use Thereof
InactiveUS20120269852A1Reduce the amount requiredSsRNA viruses negative-senseViral antigen ingredientsViral VaccineInfluenza vaccine
Owner:ABBOTT BIOLOGICALS
Nano influenza vaccine, construction method and application
ActiveCN107344969AOperational securitySimple and fast operationSsRNA viruses negative-senseAntibody mimetics/scaffoldsEscherichia coliChick embryos
The invention discloses a nano influenza vaccine, a construction method and application. The nano influenza vaccine is a recombinant protein 3M2e-rHF and has a sequence shown in SEQ ID No. 2. According to the recombinant protein disclosed by the invention, 3M2e is displayed on the surface of a cage structure of ferritin, so that the immunogenicity of M2e is remarkably improved, and thus, a novel influenza vaccine, i.e., nanoparticles 3M2e-rHF is constructed. According to the nano influenza vaccine, the construction method and the application, a recombinant protein vaccine is expressed by using a procaryotic expression system, i.e., Escherichia coli, and no live virus is involved in a vaccine preparation process, so that compared with the traditional methods for preparing influenza vaccines from chick embryos, the method has the advantages of being safer, simpler and more convenient in operation and being suitable for carrying out rapid large-scale production; by using the high sequence conservation of the M2e in different subtype influenza viruses, proven by experiments, the 3M2e-rHF protects mice from resisting the infection of homotype and heterotype influenza viruses, thereby being advantageously developed into general influenza vaccines with cross protection potency.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Influenza vaccines
ActiveCN102170902ASsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusInfluenza vaccine
Owner:GAMMA VACCINES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com