Polypeptide, immunogenicity conjugate and influenza vaccine
A technology of immunogenicity and influenza vaccine, which is applied in the field of immunogenic conjugates, influenza vaccine and polypeptide, can solve the problems of poor immunogenicity and small molecular weight, and achieve the effect of improving survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Design and synthesis of polypeptide M2A5:
[0076] 1. Design of polypeptide M2A5
[0077] Due to the high conservation of influenza virus M2e protein, it has become the research target of general influenza vaccine. The present invention designs a polypeptide M2A5 by comparing the sequences of about 13,000 influenza strains from NCBI, and its amino acid sequence is shown in SEQ ID NO.1, and it is named as polypeptide M2A5.
[0078] 2. Synthesis of polypeptide M2A5
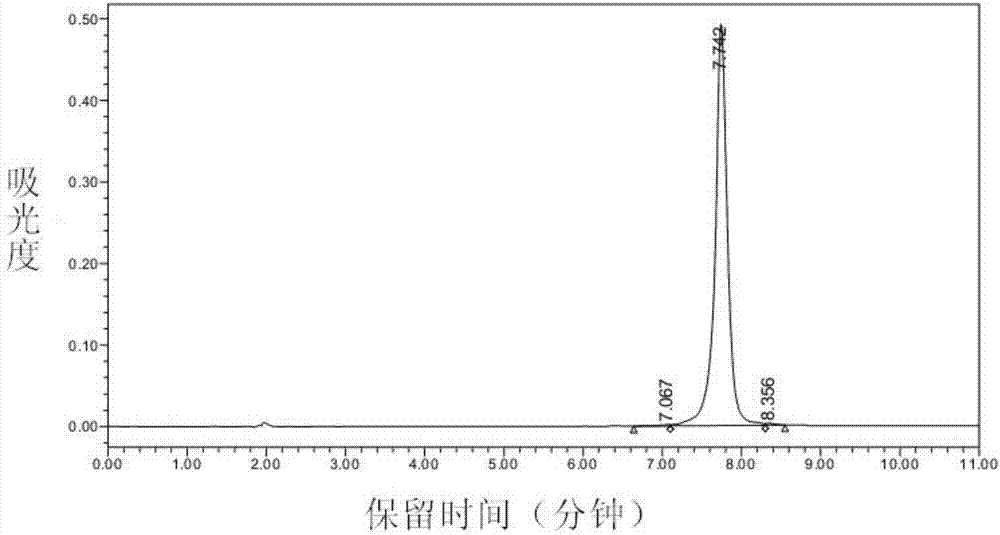
[0079] The present invention adopts polypeptide solid-phase synthesis method to synthesize polypeptide M2A5. Based on Fmoc chemical synthesis, the carboxyl group of the C-terminal amino acid of the target polypeptide to be synthesized is first connected with an insoluble polymer resin in the form of a covalent bond, and then the amino acid As the starting point of peptide synthesis, the amino group of the amino acid reacts with the activated carboxyl group of other amino acids to form a peptide bond. This p...
Embodiment 2
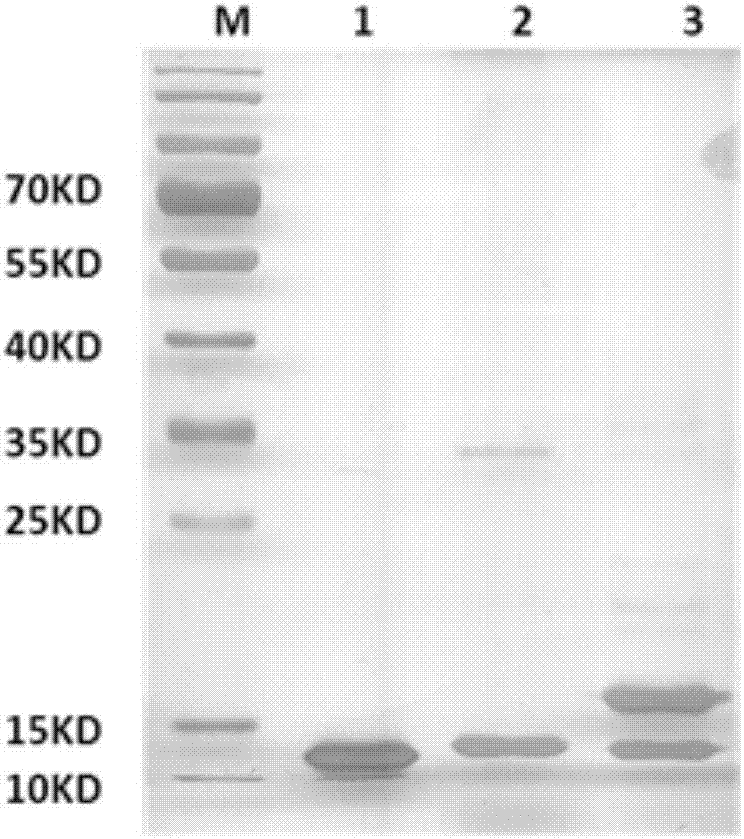
[0095] Preparation of immunogen HM4-M2A5 and its immune effect
[0096] 1. Preparation of immunogen HM4-M2A5
[0097] 1. Preparation of carrier protein solution:
[0098] (1) Preparation of seed solution: the HM4 gene (its base sequence is shown in SEQ ID NO.3, and the amino acid sequence of its encoded HM4 protein is shown in SEQ ID NO.2) is recombined into a plasmid and introduced into Escherichia coli to obtain For the strain capable of producing human HM4, the glycerol strain was first expanded to obtain the seed liquid, that is, 100ul of the glycerol strain was inoculated per 100ml LB, and cultivated overnight at 37°C and 200rpm. 5L tank fermentation: the fermentation medium is TB medium, the inoculum size is 1%, cultivated at 37°C and 200rpm until the OD600 is about 1.0, cool down to 28°C, add IPIG with a final concentration of 0.5mM to induce fermentation, and the fermentation period is about 15h. Harvest the cells: centrifuge the fermentation broth at 4000rpm for 1 h...
Embodiment 3
[0119] Preparation of HM4-M2A5 conjugate vaccine and its immune effect
[0120] 1. Preparation of HM4-M2A5 conjugate vaccine
[0121] Dilute the immunogen HM4-M2A5 prepared in Example 2 with PBS buffer (20 mM phosphate, 150 mM sodium chloride, pH7.4) to a concentration of 250 μg / mL, and then slowly add aluminum hydroxide adjuvant , ensure that the final concentration of aluminum hydroxide adjuvant is 2.5 mg / mL, and shake the sample slowly during the dropping process. After adding aluminum hydroxide, seal the sample well, place it at 2-8°C and slowly shake it for 15-18 hours after adsorption, 15-18 hours (16 hours is the best, according to the adsorption time, 16 hours has the best immune effect). Afterwards, it was diluted to a final antigen concentration of 100 μg / mL to obtain the HM4-M2A5 conjugate vaccine.
[0122] Second, the detection of antibody titer
[0123] The HM4-M2A5 conjugate vaccine prepared in the above steps was injected by intraperitoneal injection. The im...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com