Spirulina polysaccharide immune adjuvant and influenza vaccine containing the same
A technology of spirulina polysaccharide and immune adjuvant, which is applied in the direction of medical preparations containing active ingredients, antiviral agents, antibody medical components, etc., can solve the problem of limited supply of vaccine production, unavoidable side effects, slow vaccine onset time, etc. problems, to achieve the effect of weakened activity, obvious injection local swelling, and small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
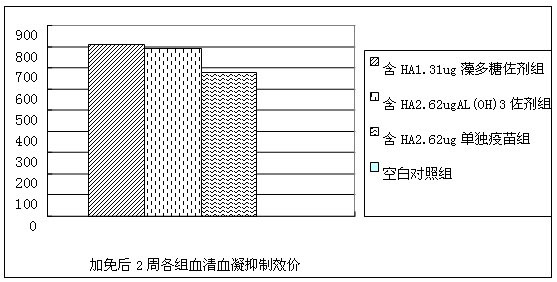
Image
Examples
Embodiment 1
[0045] Preparation of nanoemulsion vehicle:
[0046] 1) Prepare materials according to the following mass ratio: 3g water for injection, 3g soybean oil for injection, 2g polyethylene glycol 400, 1.2g Tween80, 0.8g Span80;
[0047]2) Add 1.2g Tween80, 0.8g Span80, and 2g polyethylene glycol 400 into the mixing bottle, keep stirring for 1 to 10 minutes at a speed of 200 rpm at room temperature, mix well and add 3g injection Soybean oil, mixed well to obtain a mixture;
[0048] 3) Drop 3 g of water for injection into the mixture of the above 2), and at the same time, stir the mixture at a rotation speed of 200 rpm at room temperature until the liquid is transparent to obtain a nanoemulsion;
[0049] 4) The nanoemulsion prepared in 3) was tested as follows: 1. The particle size range was 28.985-56.493nm, which was qualified by the Malvern particle size detector; 3. No change in the nanoemulsion system after being placed at 4°C for 50 days, qualified.
Embodiment 2
[0051] 1) Weigh 10 mg of commercially available spirulina polysaccharide and dissolve it in 1000 μl of absolute ethanol to make a spirulina polysaccharide solution for later use;
[0052] 2) Mix the spirulina polysaccharide solution prepared in the above 1) with 1075 μl of the commercially available H3N2 influenza virus split vaccine (with a hemagglutinin content of 43.67 μg / ml), and then add 925 μl of normal saline to the mixture to obtain a total volume of 3000 μl of influenza vaccine containing spirulina polysaccharide, spare;
[0053] 3) Weigh 3g of soybean oil for injection, 2g of polyethylene glycol 400, 1.2g of Tween80, and 0.8g of Span80 in a stirring bottle, keep the rotation speed at 200 rpm, and stir at room temperature for 1 to 10 minutes until fully mixed to obtain mixture;
[0054] 4) Slowly drop 3000 μl of the influenza vaccine containing spirulina polysaccharide in 2) into the mixed solution of 3) above, while maintaining the rotation speed at 200 rpm, stir un...
Embodiment 3
[0057] 1) Weigh 7.6 mg of commercially available spirulina polysaccharide and dissolve it in 400 μl of absolute ethanol to obtain a spirulina polysaccharide solution for later use;
[0058] 2) Mix the spirulina polysaccharide solution in 1) with 3.4ml of commercially available H3N2 influenza virus split vaccine (hemagglutinin content 43.67μg / ml) to obtain 3.8ml influenza vaccine containing spirulina polysaccharide;
[0059] 3) Take 1.1g of polyoxyethylene castor oil, 1.6g of Span80, 6g of 1,2-propanediol, and 3g of isopropyl myristate in a stirring bottle, keep the rotation speed at 200 rpm, and stir at room temperature for 10 minutes until fully Mix well to obtain a mixed solution;
[0060] 4) Slowly drop 3.8ml of the influenza vaccine containing spirulina polysaccharide prepared in 2) into the mixed solution above 3), while maintaining the rotation speed at 200 rpm, stir until the solution is light yellow and transparent, and obtain spirulina polysaccharide, The nanoemulsio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com