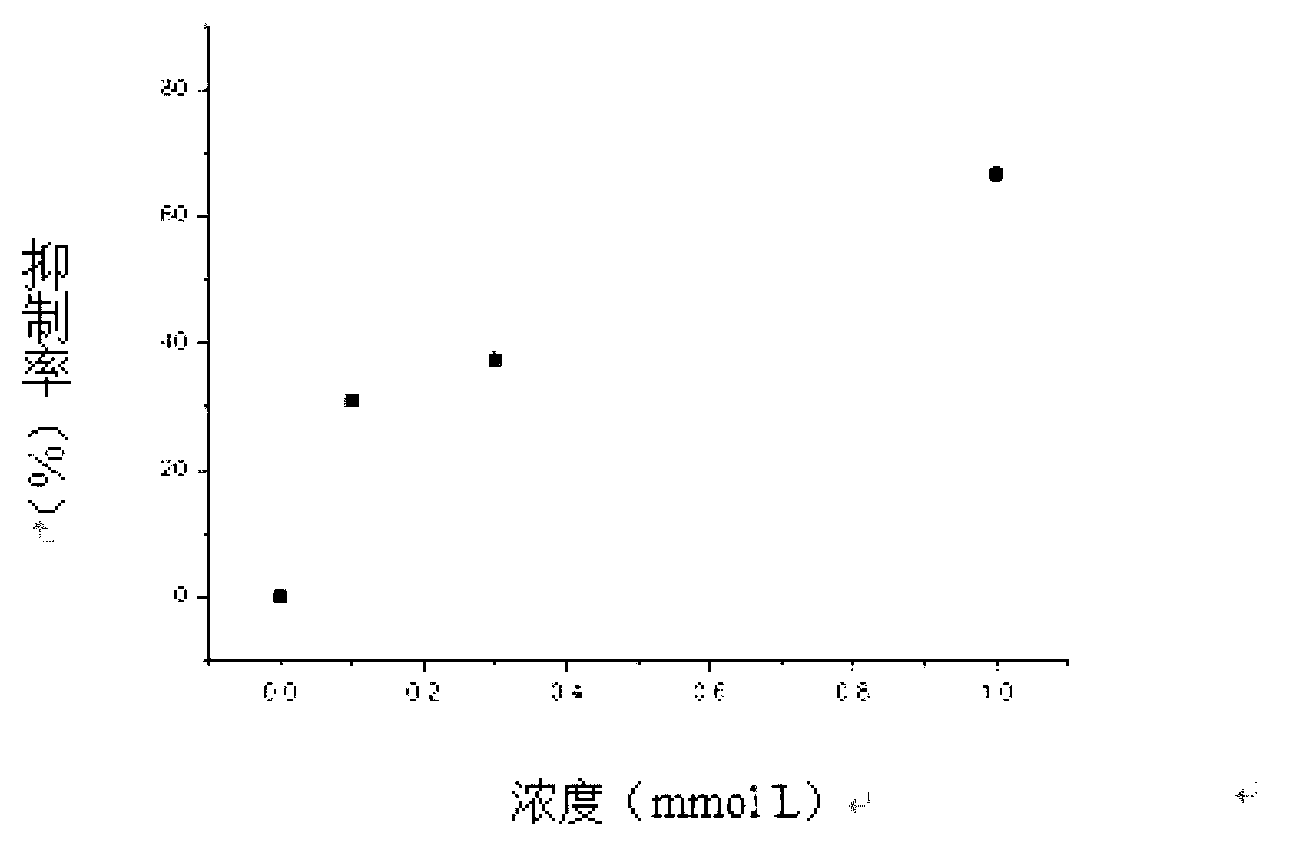
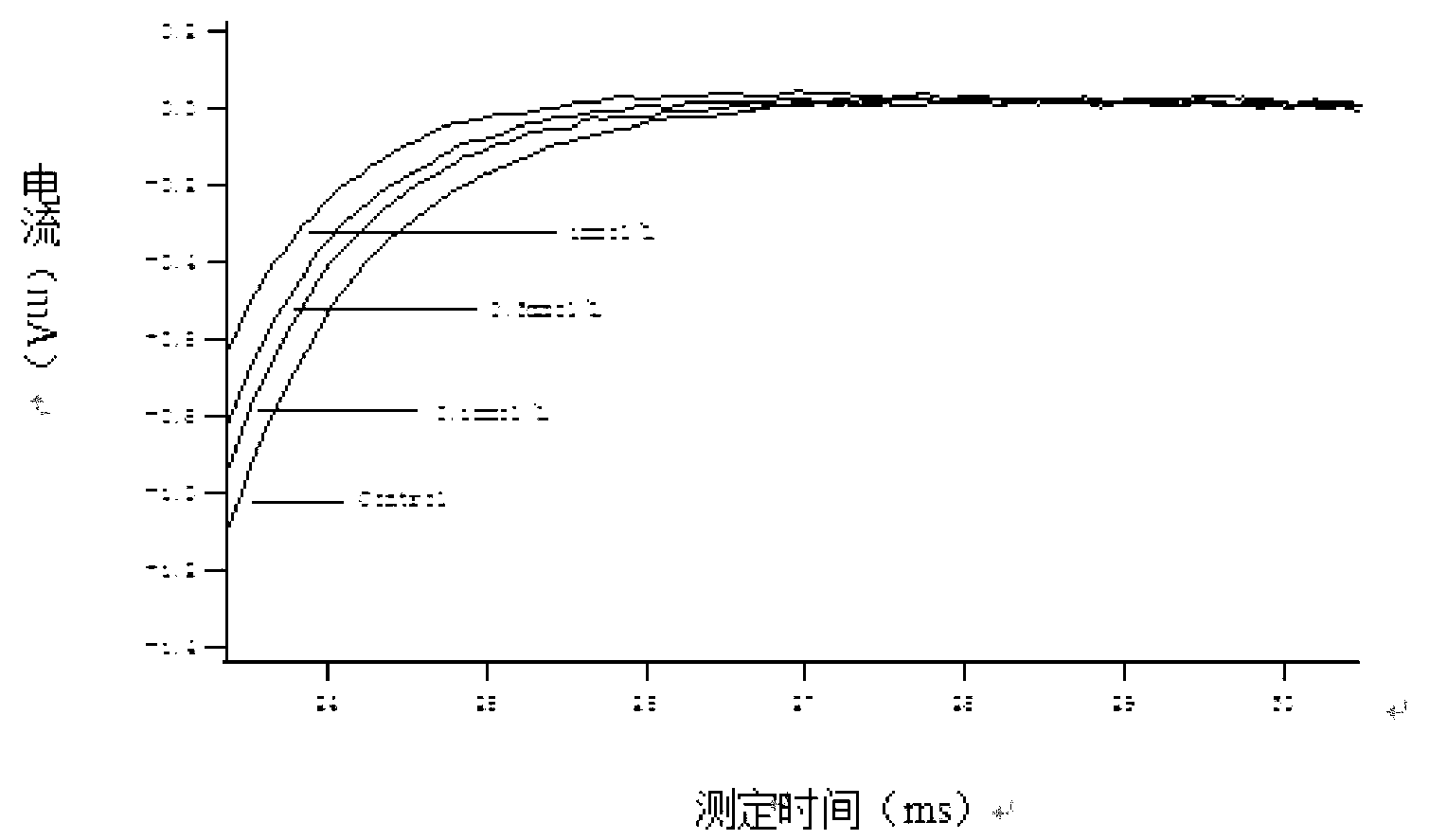
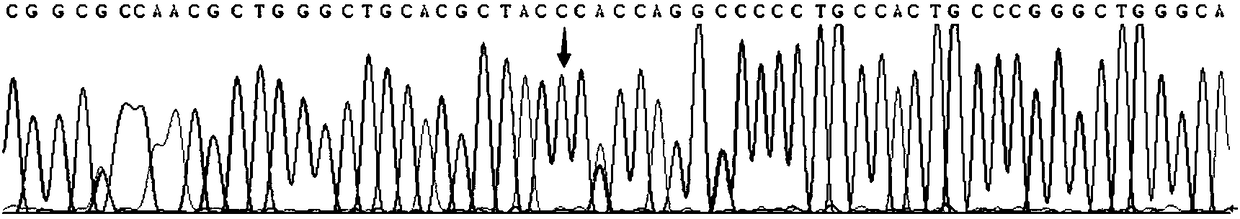
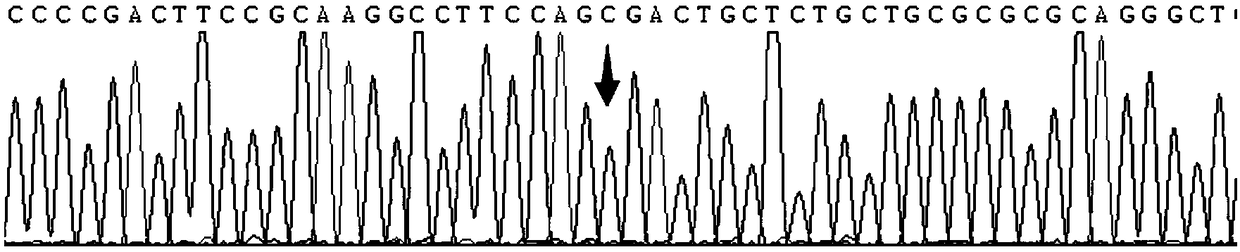
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
60 results about "Drug metabolizing enzymes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The “drug metabolizing enzymes” (DMEs) are a diverse group of proteins that are respon- sible for metabolizing a vast array of xenobiotic compounds including drugs, environmental pollutants, and endogenous compounds such as steroids and prostaglandins (1).
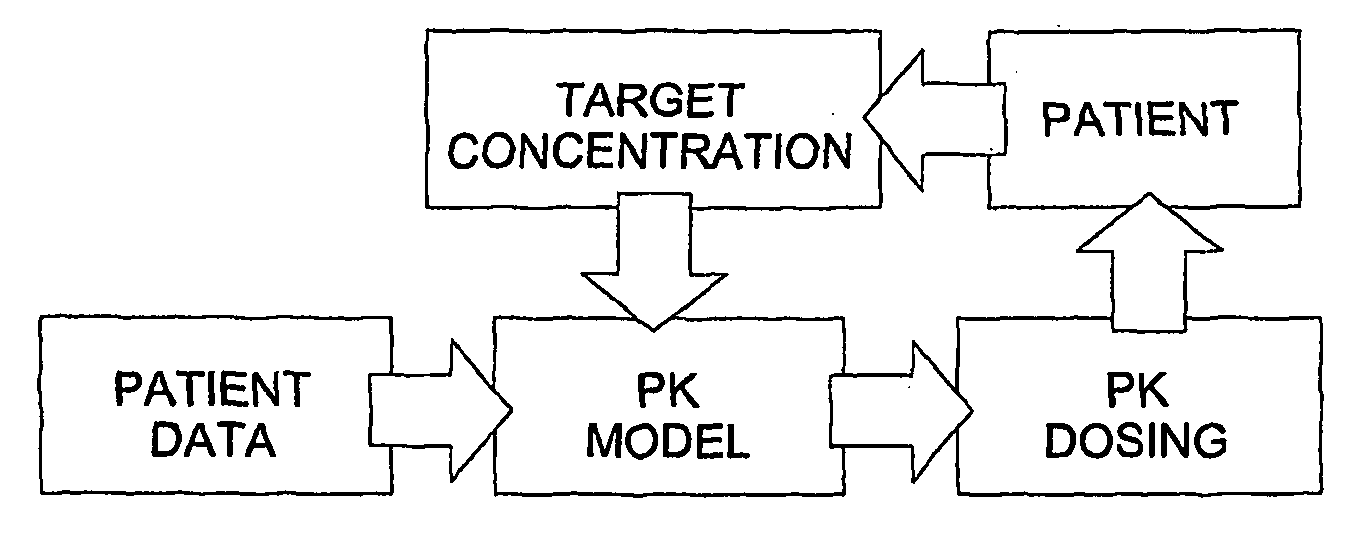
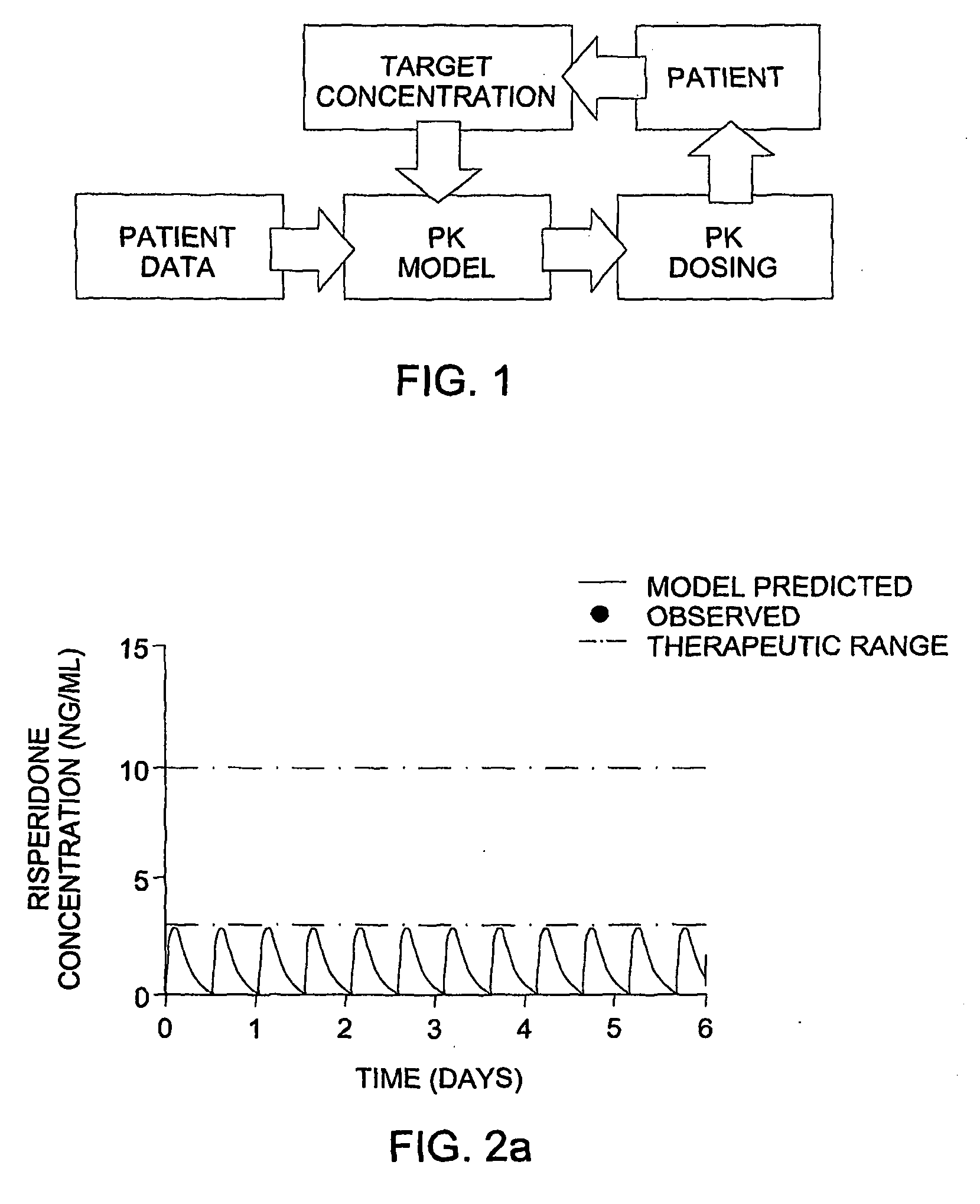
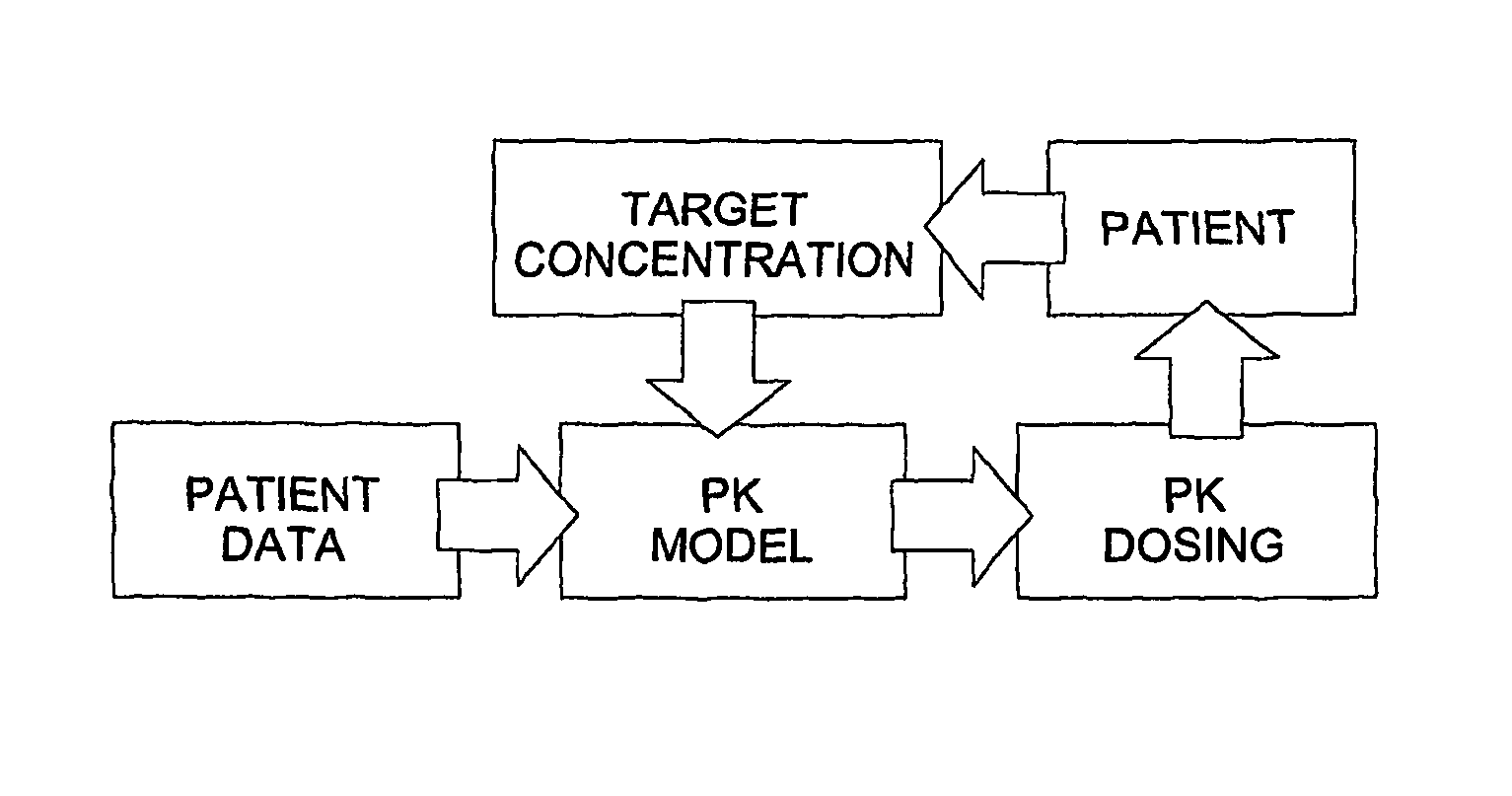
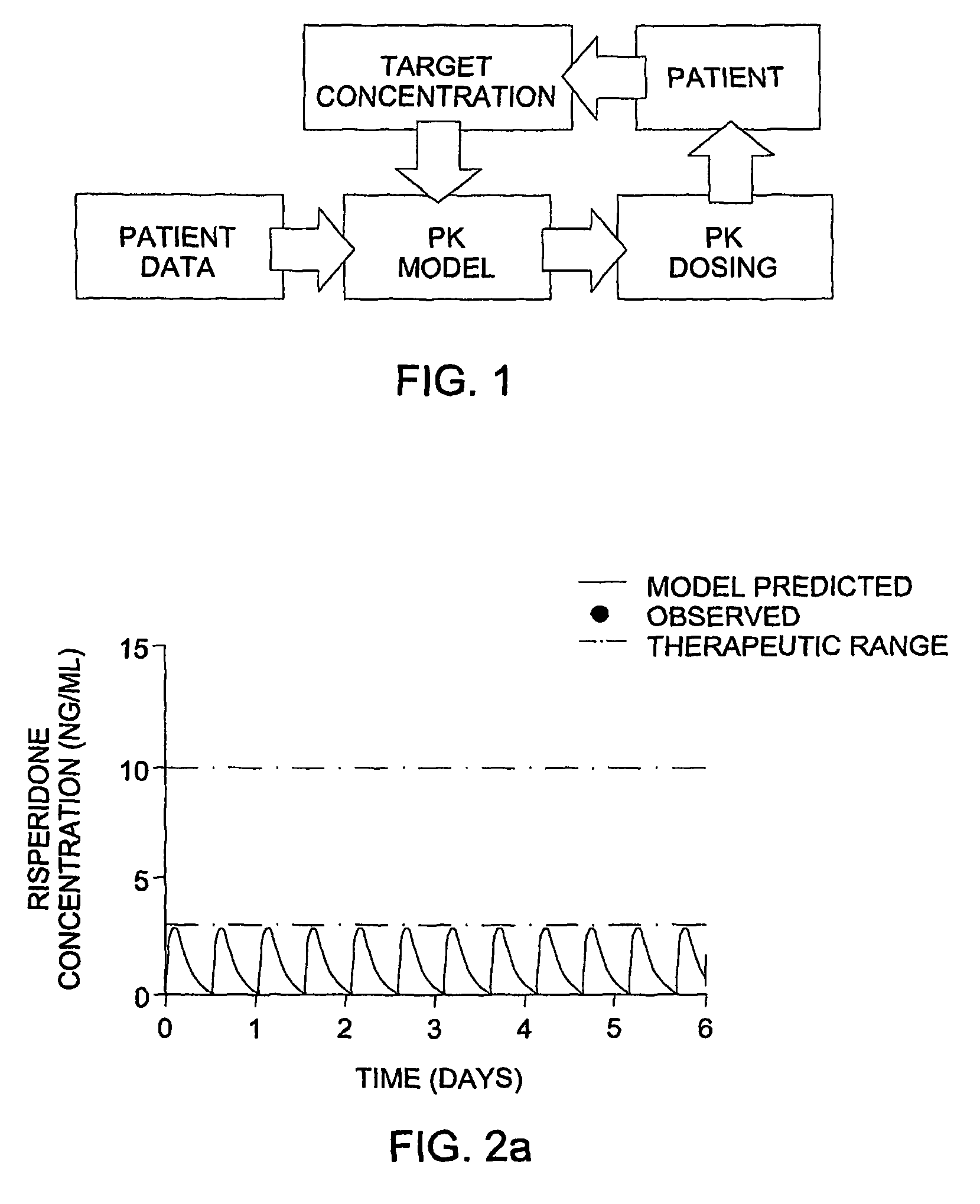
Optimization and Individualization of Medication Selection and Dosing
ActiveUS20090171697A1Easy to understandEasy to recommendationDrug and medicationsBiostatisticsPersonalizationDosing regimen
The invention provides population models, methods, and algorithms for targeting a dosing regimen or compound selection to an individual patient. The methods and algorithms of the invention utilize population models that incorporate genotype information for genes encoding drug metabolizing enzymes for one or more compounds of interest. The methods allow integration of genotype information for one or more genes encoding a drug metabolizing enzyme, particularly a cytochrome P450 gene with patient data. The methods allow integration of genotype information and the effect of one or more compounds on one or more drug metabolizing enzymes. The methods allow iterative feedback of drug metabolizing data obtained from a patient into the process of generating a dosage regimen recommendation for a compound of interest for an individual patient.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
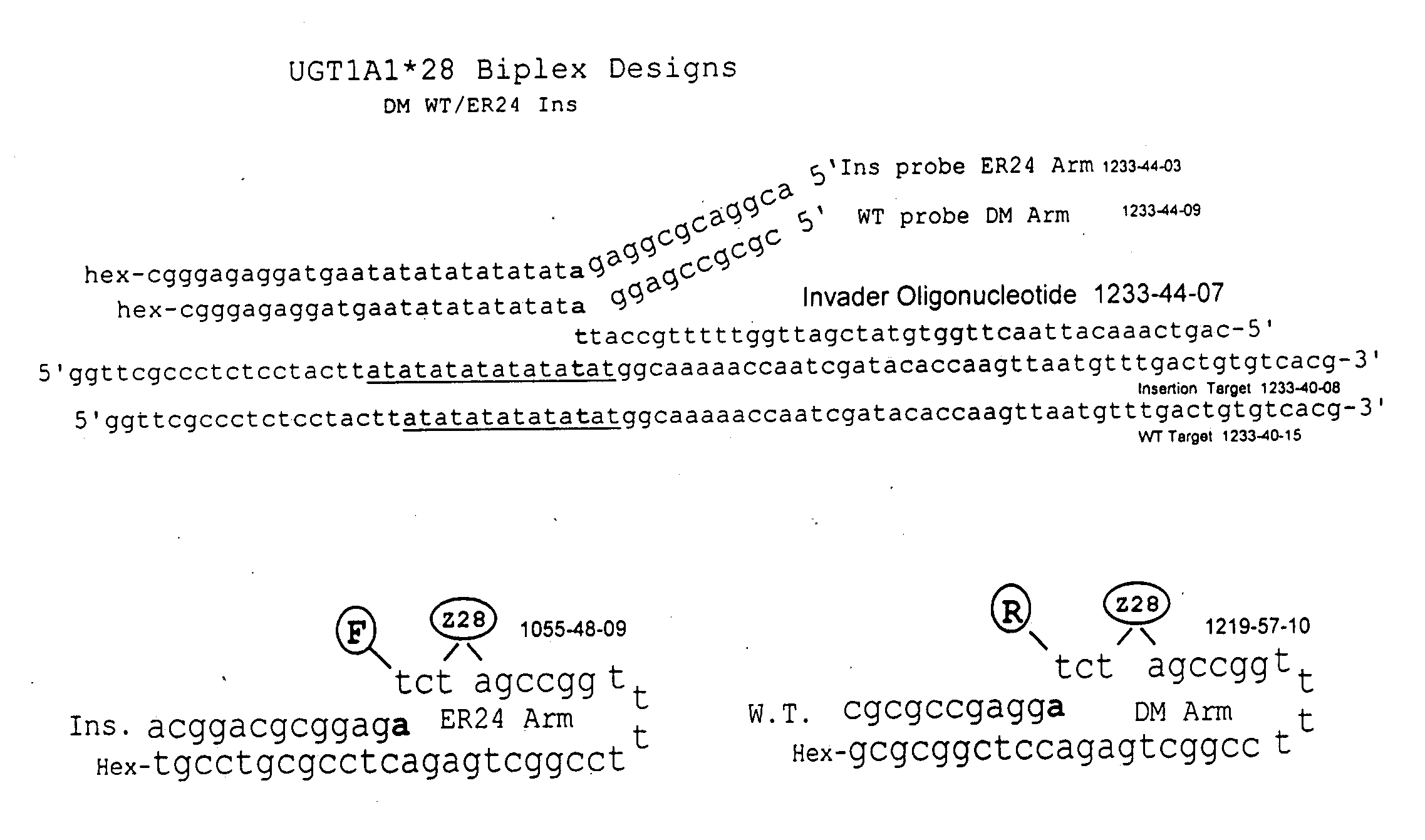
Pharmacogenetic DME detection assay methods and kits
InactiveUS20060160074A1Increase nucleic acid synthesis reaction rateHeating evenlySugar derivativesMicrobiological testing/measurementDrug metabolismPharmacogenetics
The present invention relates to methods for detecting polymorphisms in enzymes related to drug metabolizm (Drug Metabolizing Enzymes or DMEs) such as uridine diphosphate glucuronosyl transferase (UGT) gene promoter, cytochrome p450, with a non-amplified oligonucleotide detection assays. The present invention also relates to pharmacogenetic DME detection assay kits.
Owner:THIRD WAVE TECH
Optimization and individualization of medication selection and dosing
ActiveUS8589175B2Easy to understandEasy to recommendationDrug and medicationsBiostatisticsPersonalizationDosing regimen
The invention provides population models, methods, and algorithms for targeting a dosing regimen or compound selection to an individual patient. The methods and algorithms of the invention utilize population models that incorporate genotype information for genes encoding drug metabolizing enzymes for one or more compounds of interest. The methods allow integration of genotype information for one or more genes encoding a drug metabolizing enzyme, particularly a cytochrome P450 gene with patient data. The methods allow integration of genotype information and the effect of one or more compounds on one or more drug metabolizing enzymes. The methods allow iterative feedback of drug metabolizing data obtained from a patient into the process of generating a dosage regimen recommendation for a compound of interest for an individual patient.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Drug metabolizing enzymes
InactiveUS20040110259A1Efficiently translatedImprove expression efficiencyBacteriaSugar derivativesENCODEAgonist
The invention provides human drug metabolizing enzymes (DME) and polynucleotides which identify and encode DME. The invention also provides expression vectors, host cells, antibodies, agonists, and antagonists. The invention also provides methods for diagnosing, treating, or preventing disorders associated with aberrant expression of DME.
Owner:INCYTE
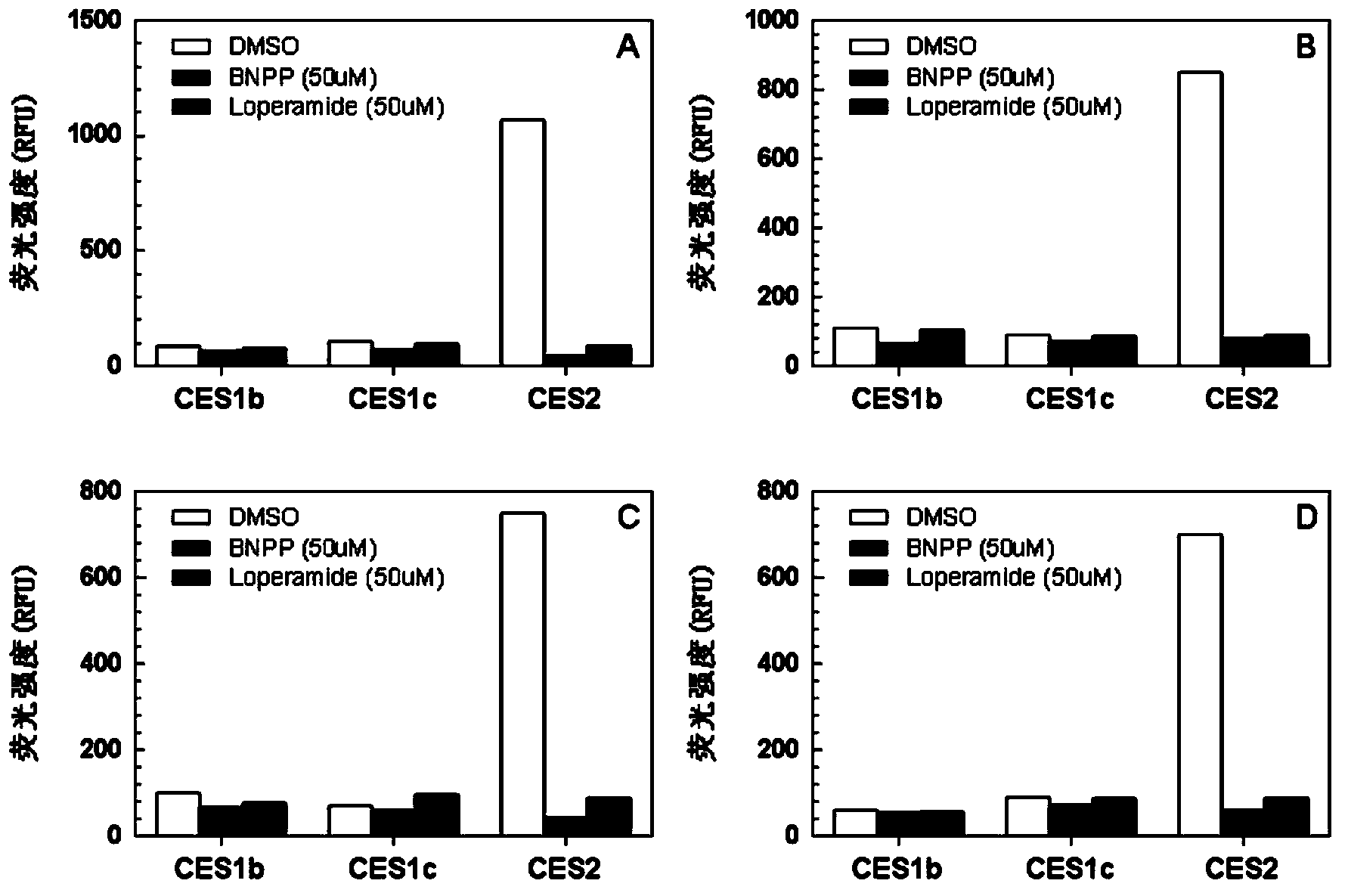
Specific fluorescence probe substrates of human carboxylesterase 2 and application thereof
InactiveCN104120164AEasy to detectThe synthesis process is simpleOrganic chemistryMicrobiological testing/measurementMetaboliteHydrolysis
The invention provides a specific fluorescence probe substrates of human carboxylesterase 2 (CES2) and application thereof. The specific probe substrate is a benzoateb compound of a C4 hydroxyl naphthalimide, and is applicable to determine the enzyme activity of CES2 in a biological system. The CES2 enzyme activity determination flow comprises: selecting a hydrolysis benzoyl-removal reaction of the benzoate compound of the C4 hydroxyl naphthalimide as a probe reaction, and quantitatively determining the generation amount of a hydrolysis metabolite of the compound in a unit time, so as to determine the enzyme activity of CES2 in all biological samples, cells, bodies and integral organs. The probe is applicable to quantitative assessment of CES2 enzyme activity in biological samples of different species and different individual sources, and quantitative determination on CES2 enzyme activity in different sources of animal tissue cell culture fluids and cell preparation substances, so that the probe is expected to help to realize assessment on medicine disposal capability of important drug metablic enzyme CES2. Additionally, the probe also is applicable as an inhibitor for rapidly screening CES2 in vitro by means of the probe reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Methods and compositions for analysis of UGT1A1 alleles
InactiveUS20080032305A1Facilitate drug therapyConvenient treatmentMicrobiological testing/measurementDrug metabolismNucleic acid detection
The present invention relates to methods for detecting polymorphisms in enzymes related to drug metabolizm (Drug Metabolizing Enzymes or DMEs) such as uridine diphosphate glucuronosyl transferase (UGT) gene promoter, with nucleic acid detection assays. The present invention also relates to detection assay kits.
Owner:THIRD WAVE TECH
Complete-set primer for SNP (Single Nucleotide Polymorphism) sites of genes of drug-metabolizing enzyme and application thereof
ActiveCN108192966AComprehensive detectionSimple and fast operationMicrobiological testing/measurementDNA/RNA fragmentationHuman DNA sequencingTime-of-flight mass spectrometry
The invention discloses a complete-set primer for SNP (Single Nucleotide Polymorphism) sites of drug-metabolizing enzyme genes and application thereof. The complete-set primer comprises amplificationprimers and extension primers of 9 SNP sites of 7 drug-metabolizing enzyme associated genes of human genome DNA, wherein a pair of multiple PCR amplification primers and a single-base extension primerare respectively designed for each site, and an MALDI-TOF MS (Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry) method is adopted to detect typing of the SNP sites of a sample to be detected. The complete-set primer disclosed by the invention has the beneficial effects that a detection method for the drug-metabolizing enzyme genes based on gene molecule typing is established, and the typing of multiple SNPs can be finished at the same time in the same reaction, so that the accurate and efficient effects are achieved and the cost is low; the covered genes and sitesare related to the metabolizing enzyme genes related to 8 major types of common drugs for children, and the coverage is most complete in gene detection products of safe drugs used for children so far.
Owner:北京天平永达生物科技发展有限公司
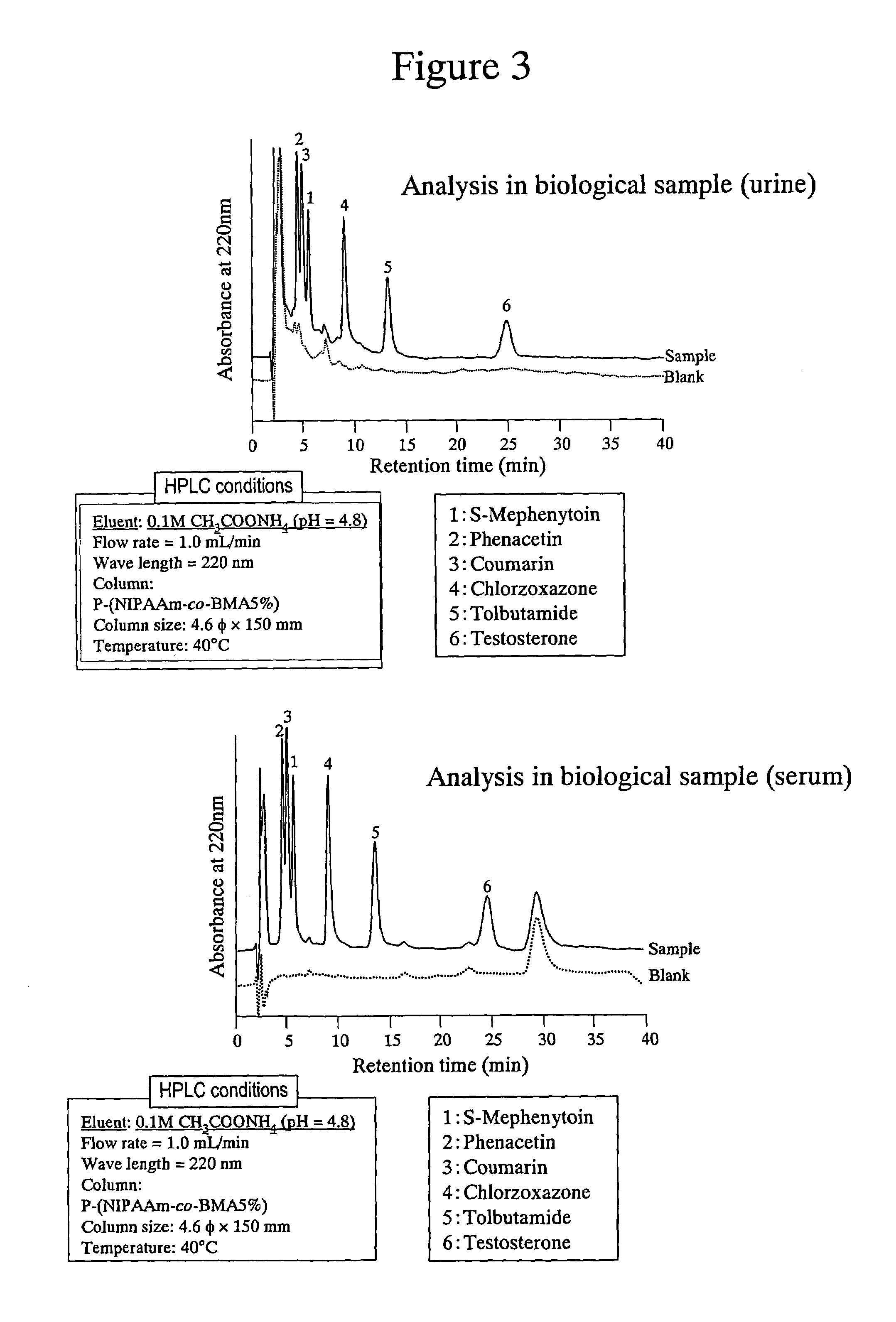
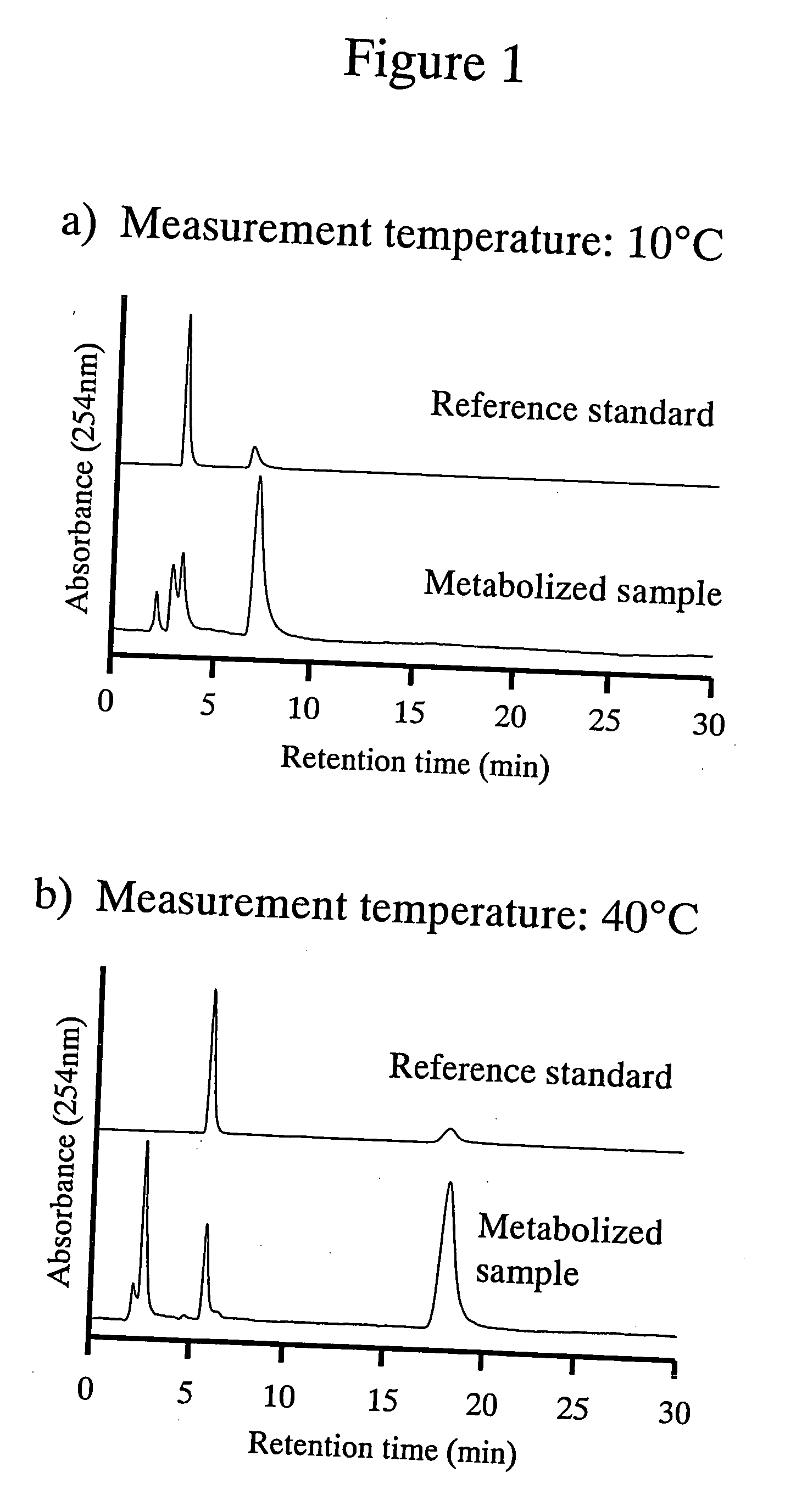
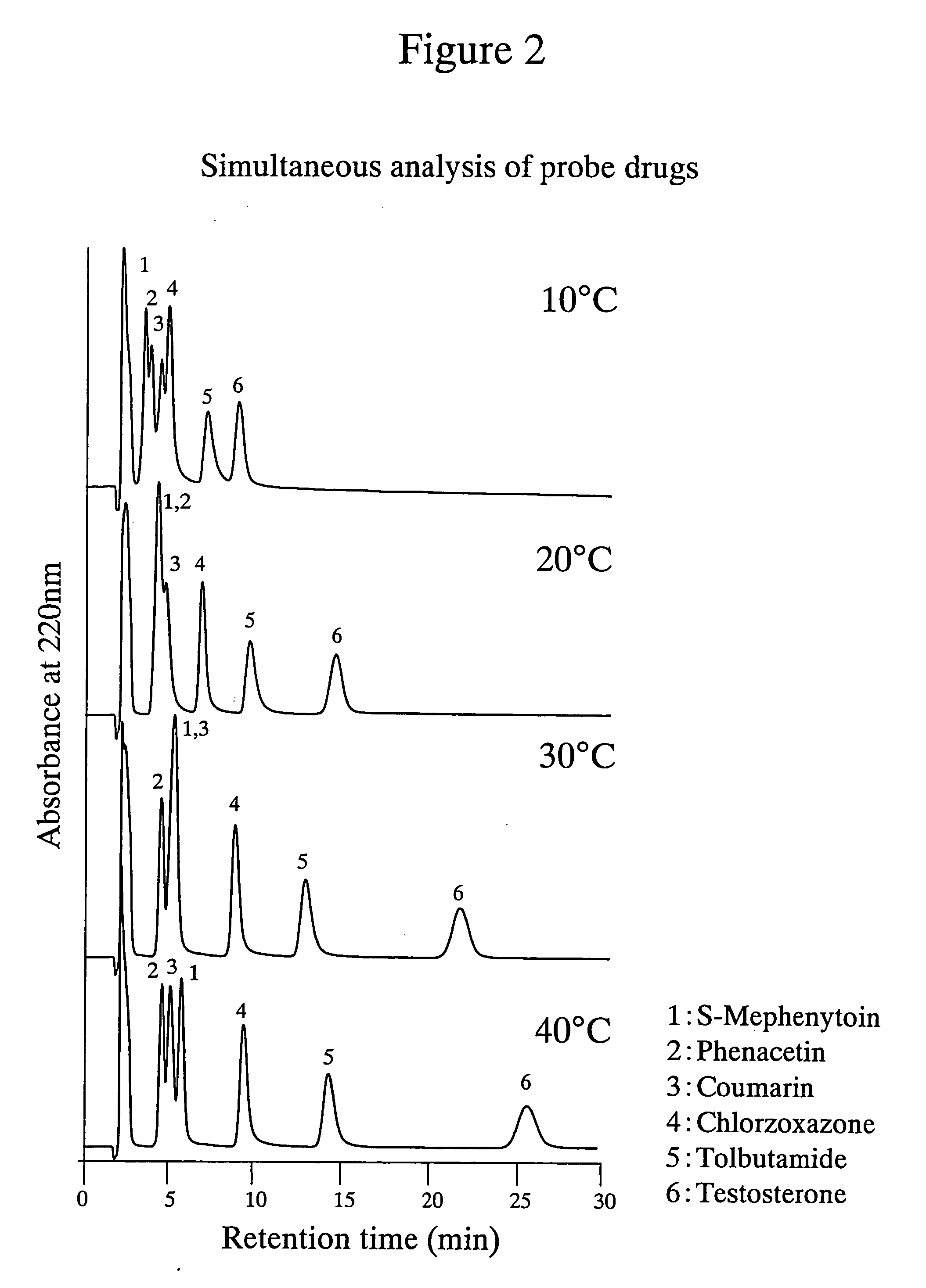
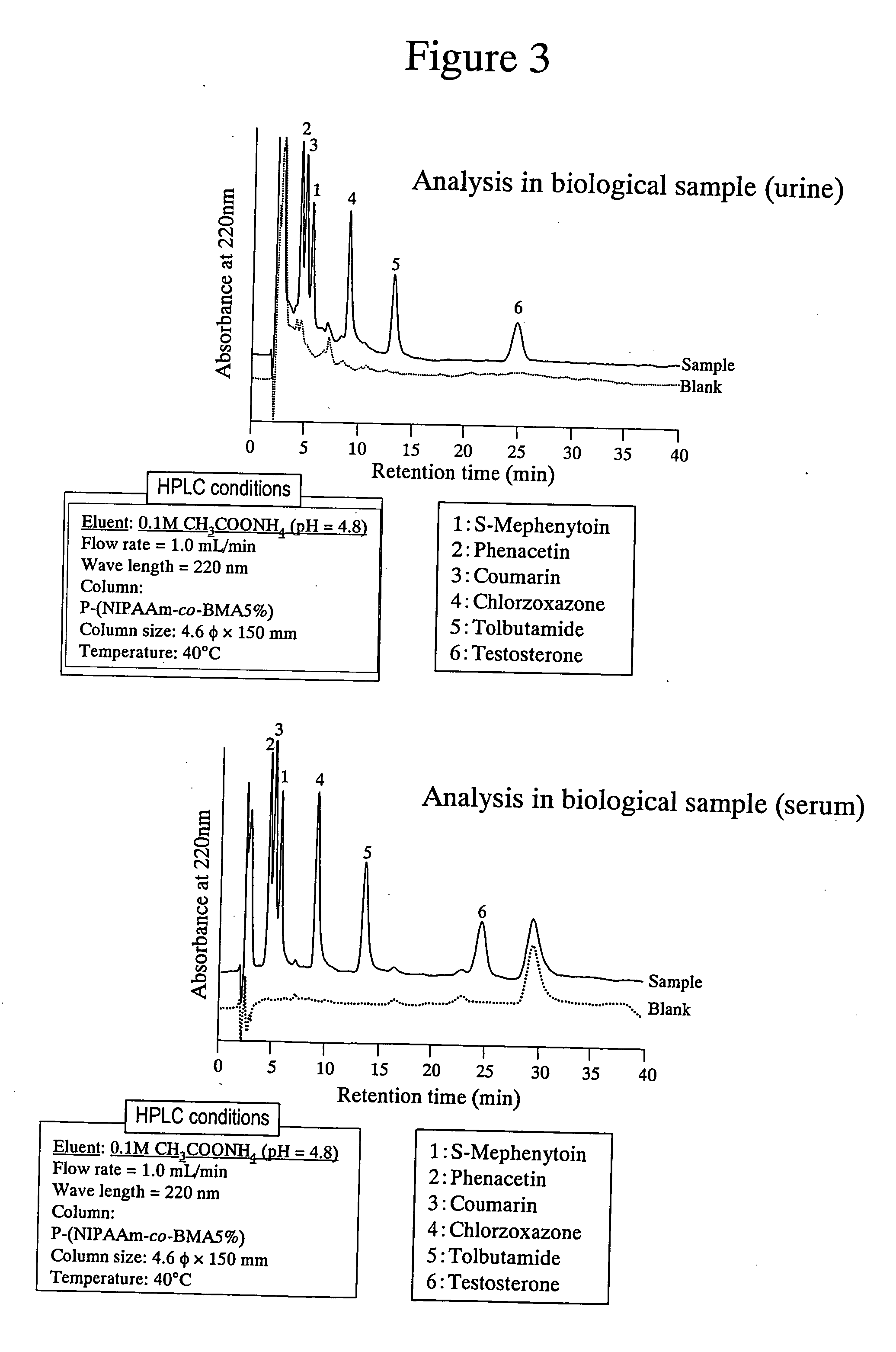
High performance liquid chromatography with an aqueous mobile phase for analysis of drug and its metabolite
InactiveUS7632656B2Easy to useMicrobiological testing/measurementBiological testingDrug metabolismTemperature control
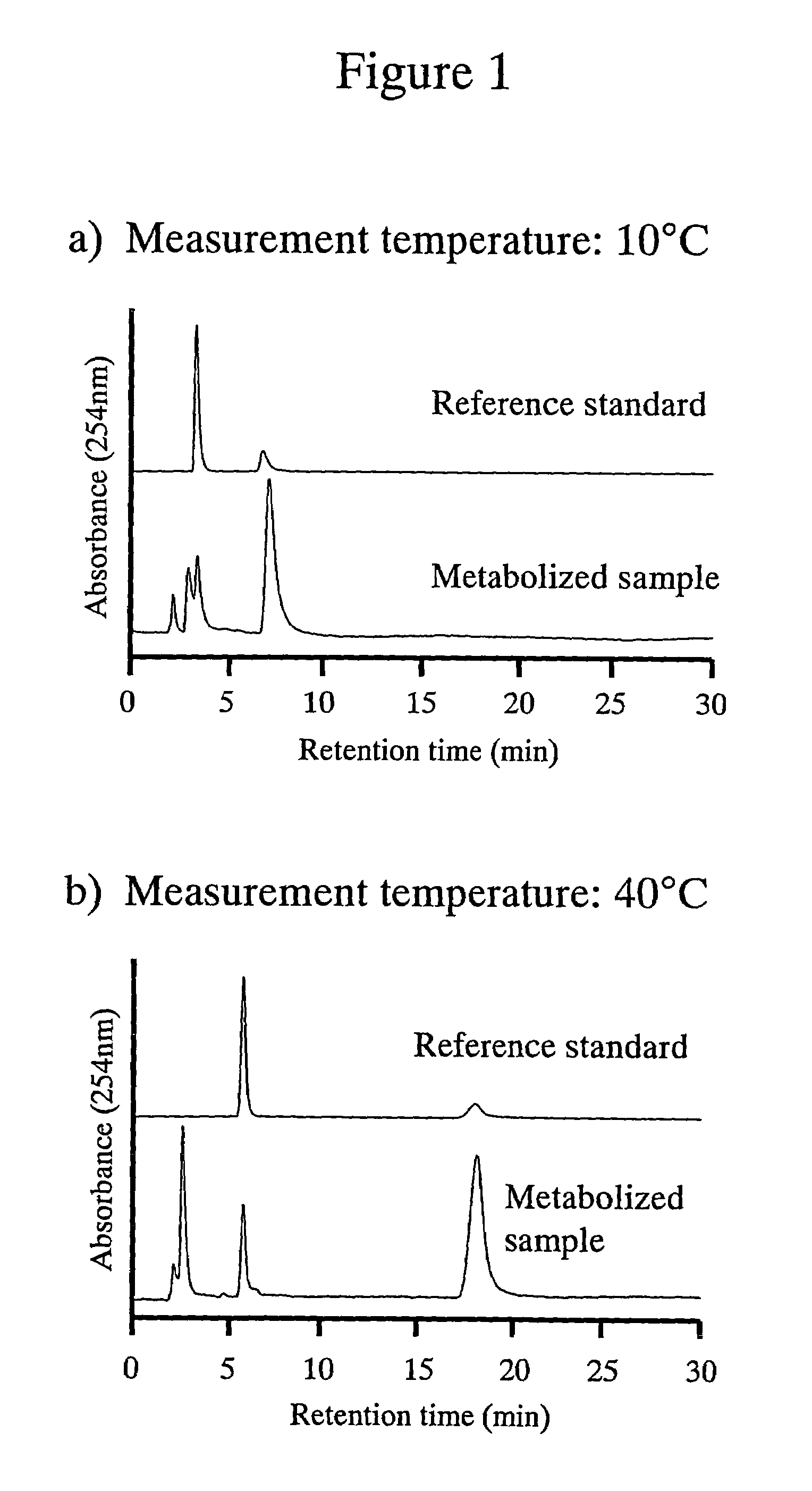
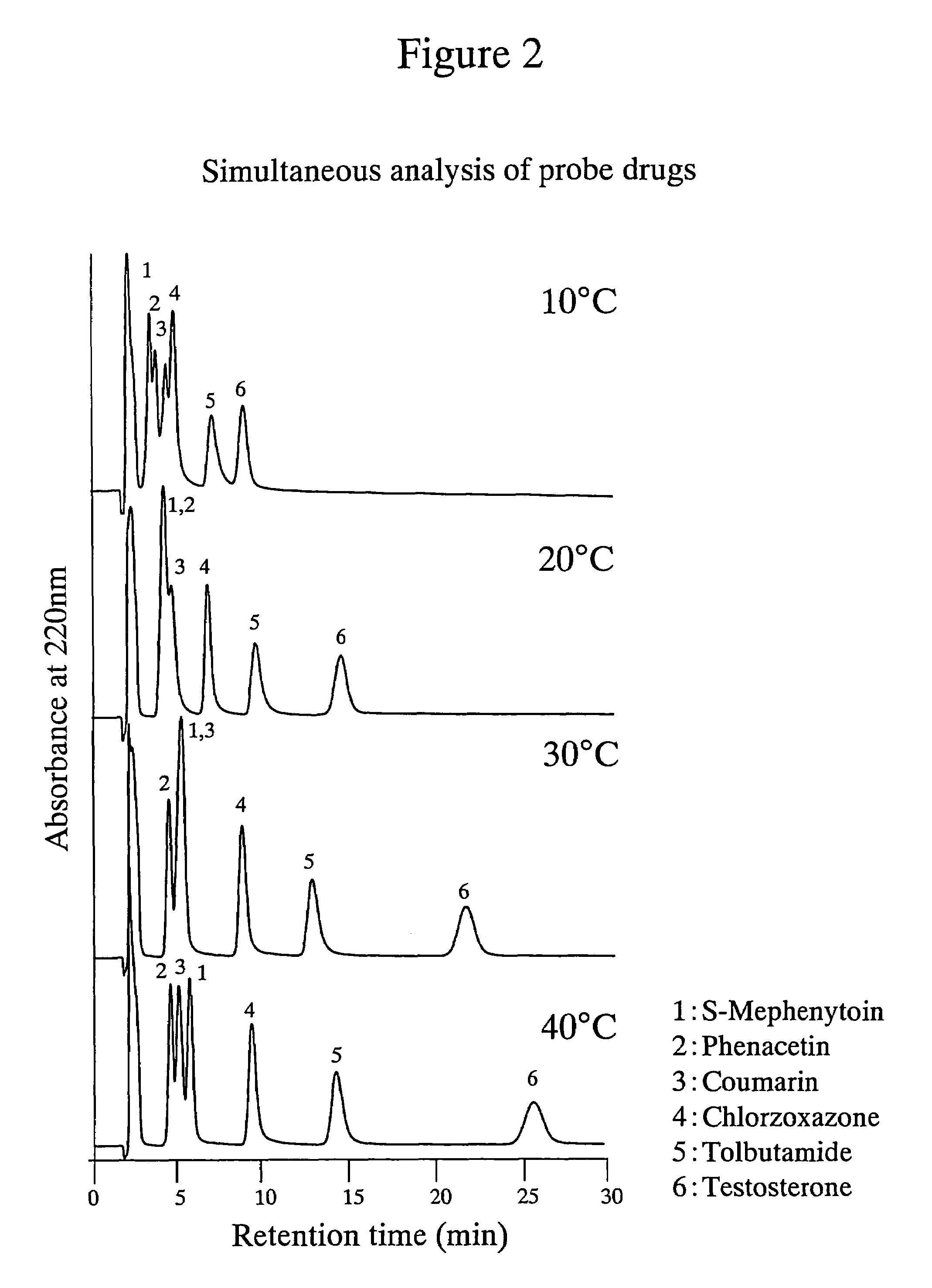
The amount of drug consumption caused by a specific drug-metabolizing enzyme and / or the amount of the resulting metabolite(s) is measured by chromatography with an aqueous mobile phase under temperature control using a packing material whose surface is coated with a polymer having a hydration force varying in the temperature range of 0° C. to 80° C. This system enables proper evaluation of drug-metabolizing capacity through simple means without adversely affecting the environment.
Owner:CELLSEED +1
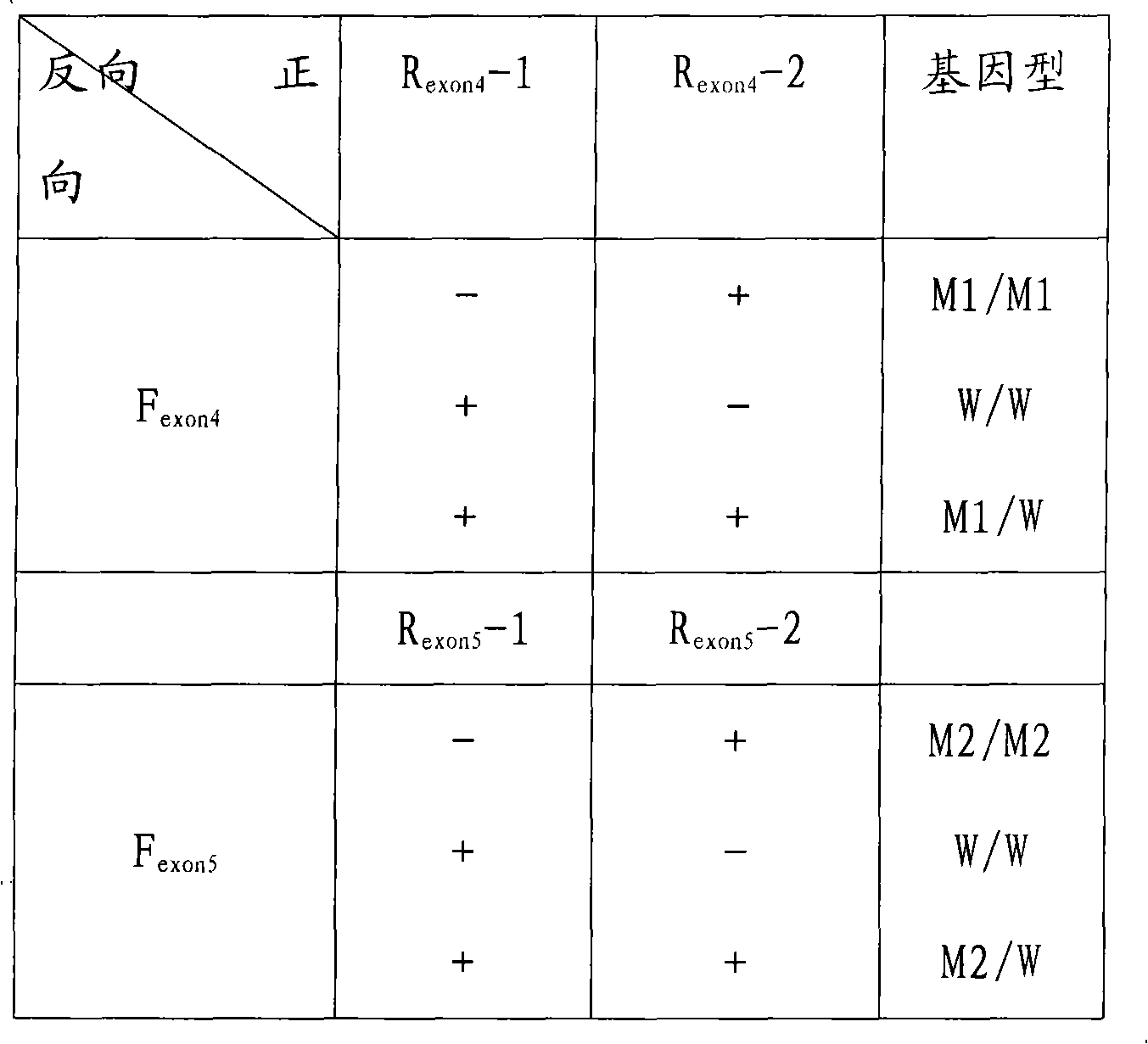
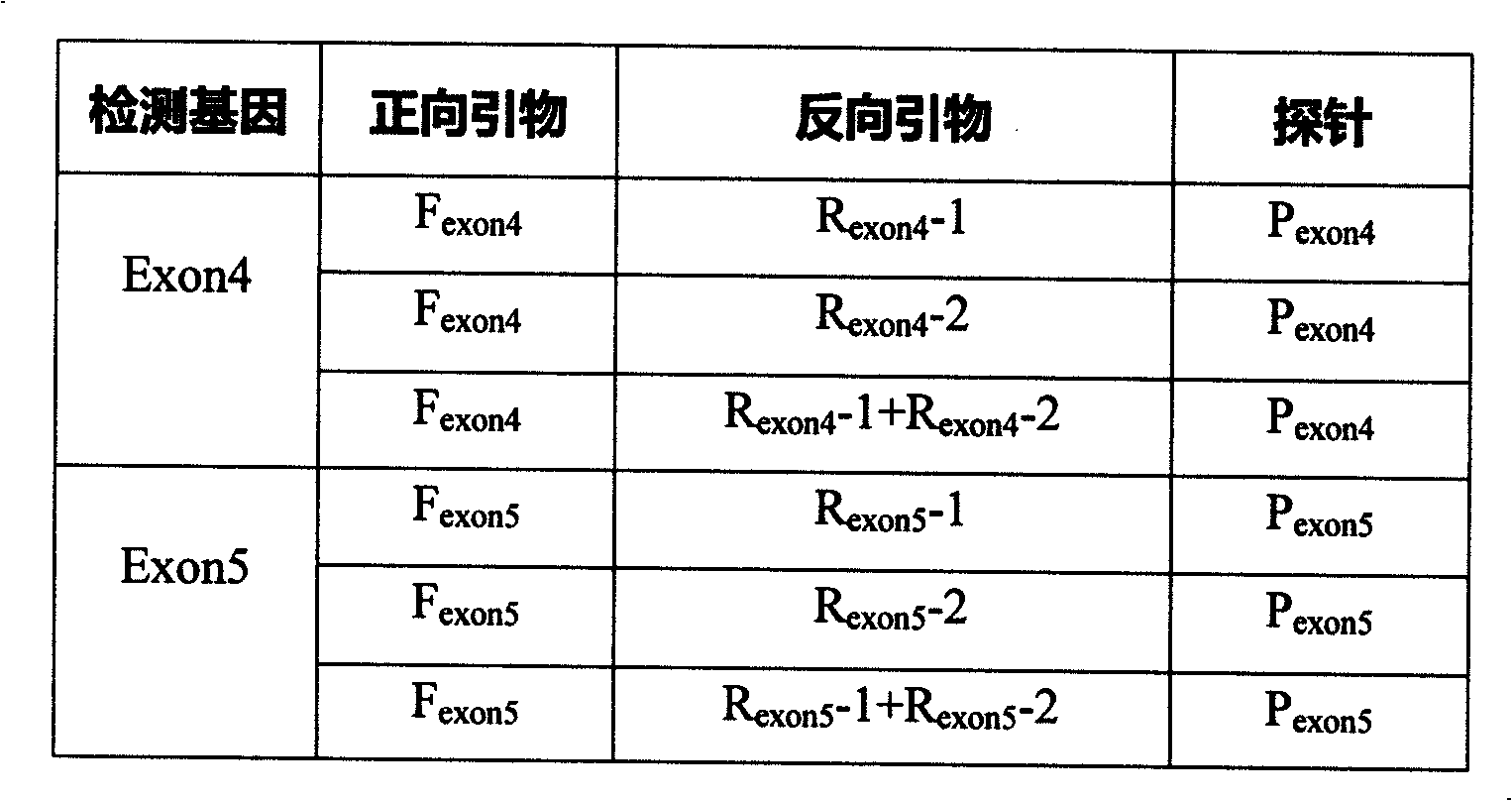
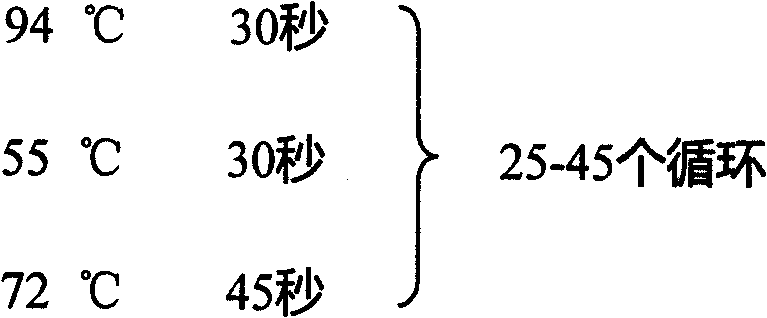
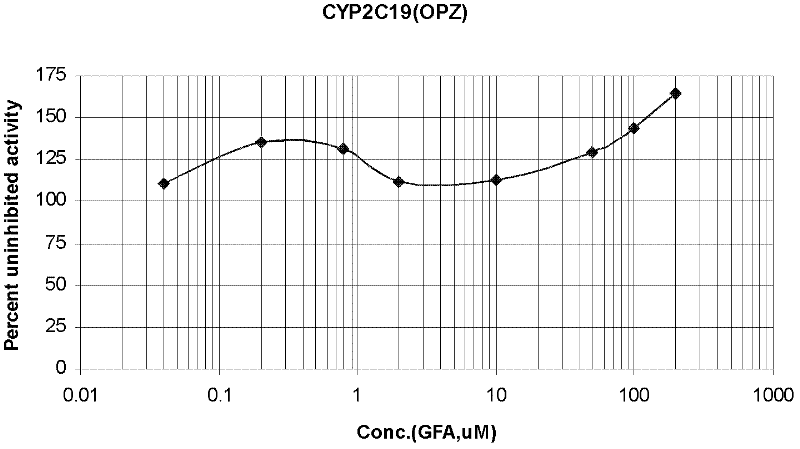
Fluorescence quantitative detecting method for CYP2C19 genotyping
The invention relates to a fluorescent quantitative detecting method for CYP2C19 genotyping fluorescent and a diagnostic kit. At present, CYP2C19 genotyping techniques at home and abroad are mainly RFLP technique and ALA technique which determine genotypes according to the size of a DNA fragment, with the disadvantages of time-consuming operation and low flux. The invention provides a novel point mutating or SNP detecting method which utilizes the ASA combination primer sequence designed for the polymorphic loci of the CYP2C19 gene exons 4 and 5, the referential ASA primer combination or the degenerated primer sequence for quality control, specific TaqMan-MGB probe sequences for amplified products, an ASA amplifying reaction method, the amplifying reaction result of real-time fluorescent quantitative detection, the fast analysis of mutation locus type and genotyping. The quantitative detecting method has the advantages of time saving compared with a conventional ASA method, no need for electrophoresis detection, fastness, accuracy, and the like, and can be used for detecting other drug-metabolizing enzymes, or more extensive genetic variation or mutation.
Owner:樊世斌 +1
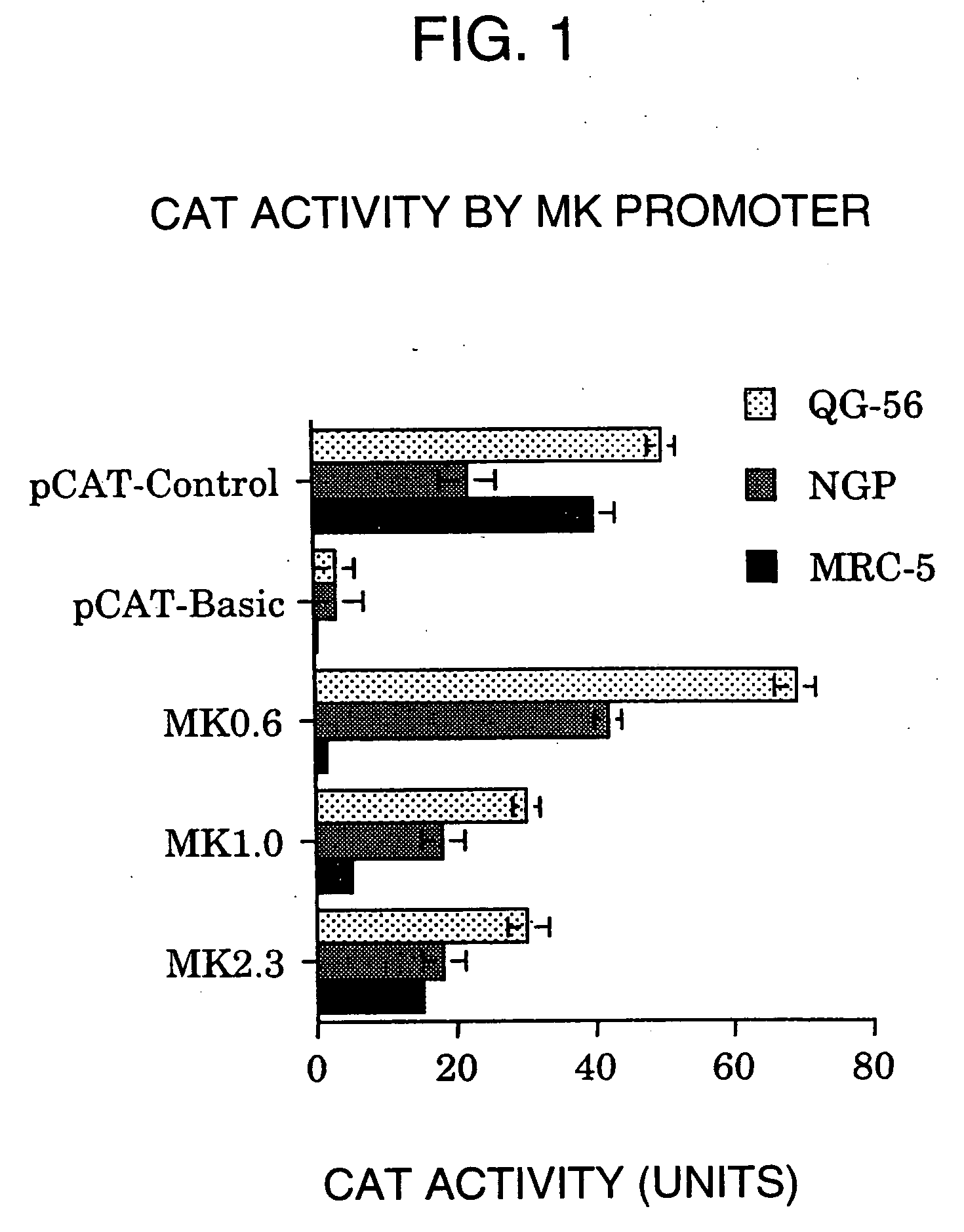
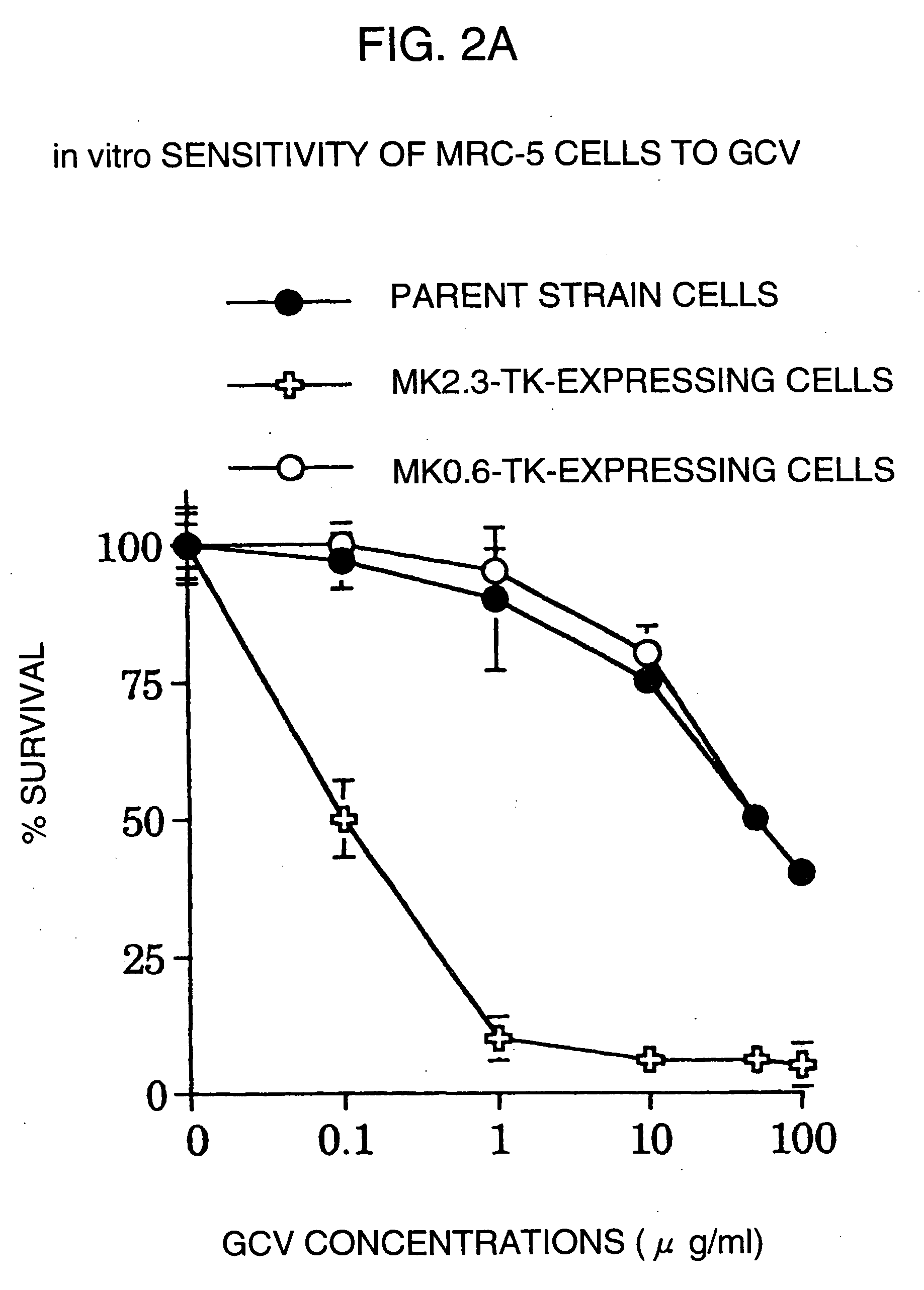
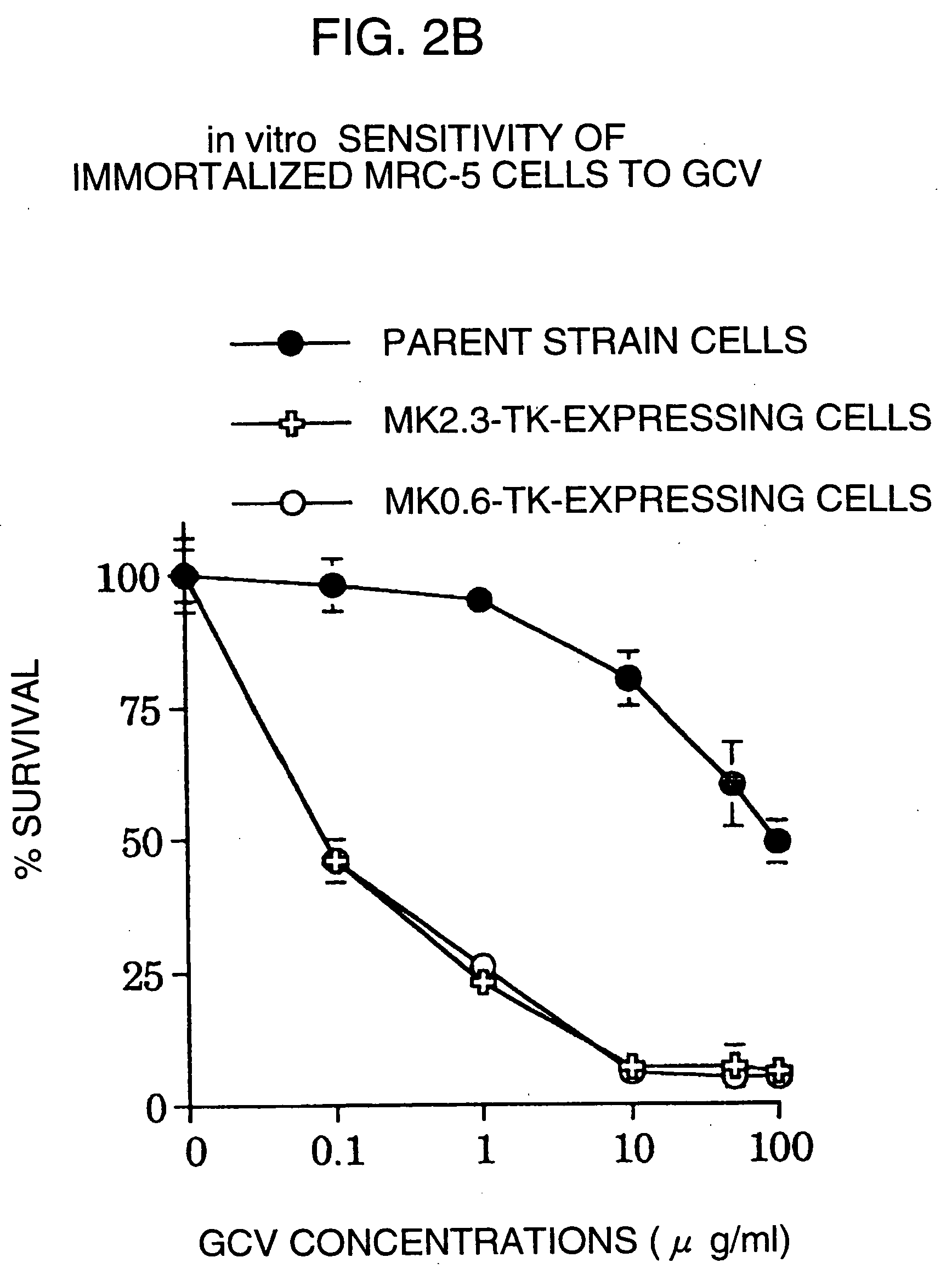
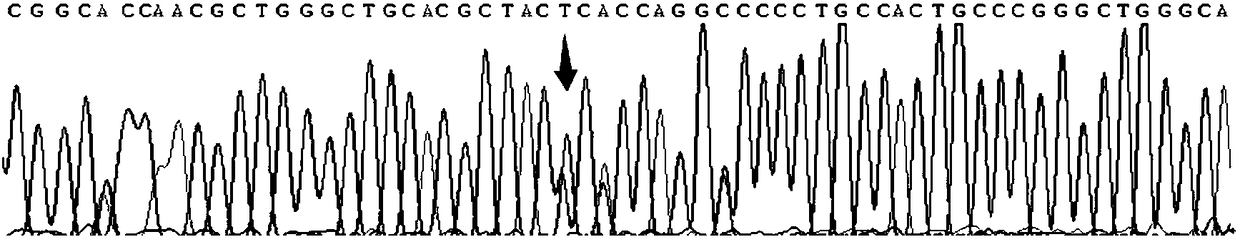
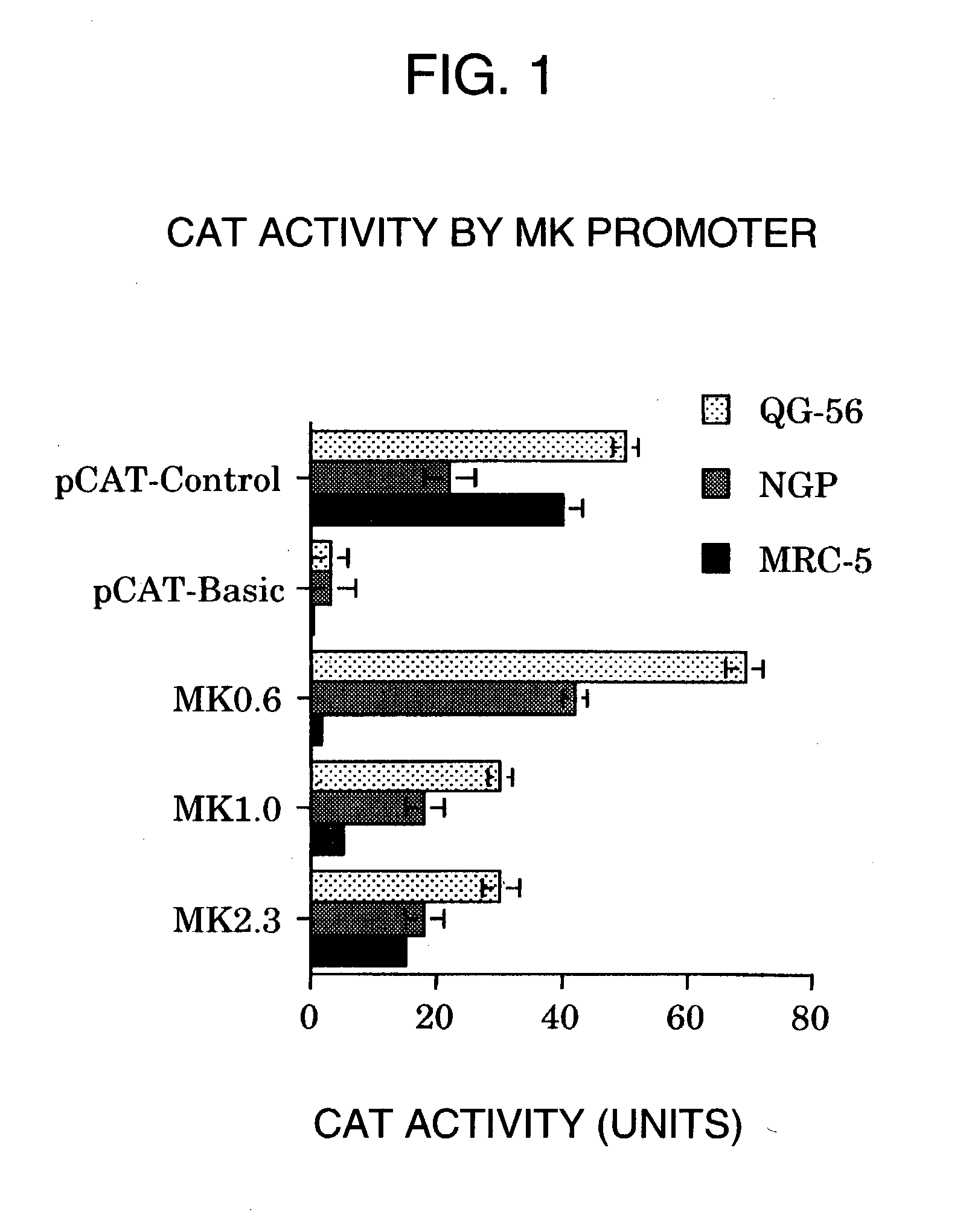
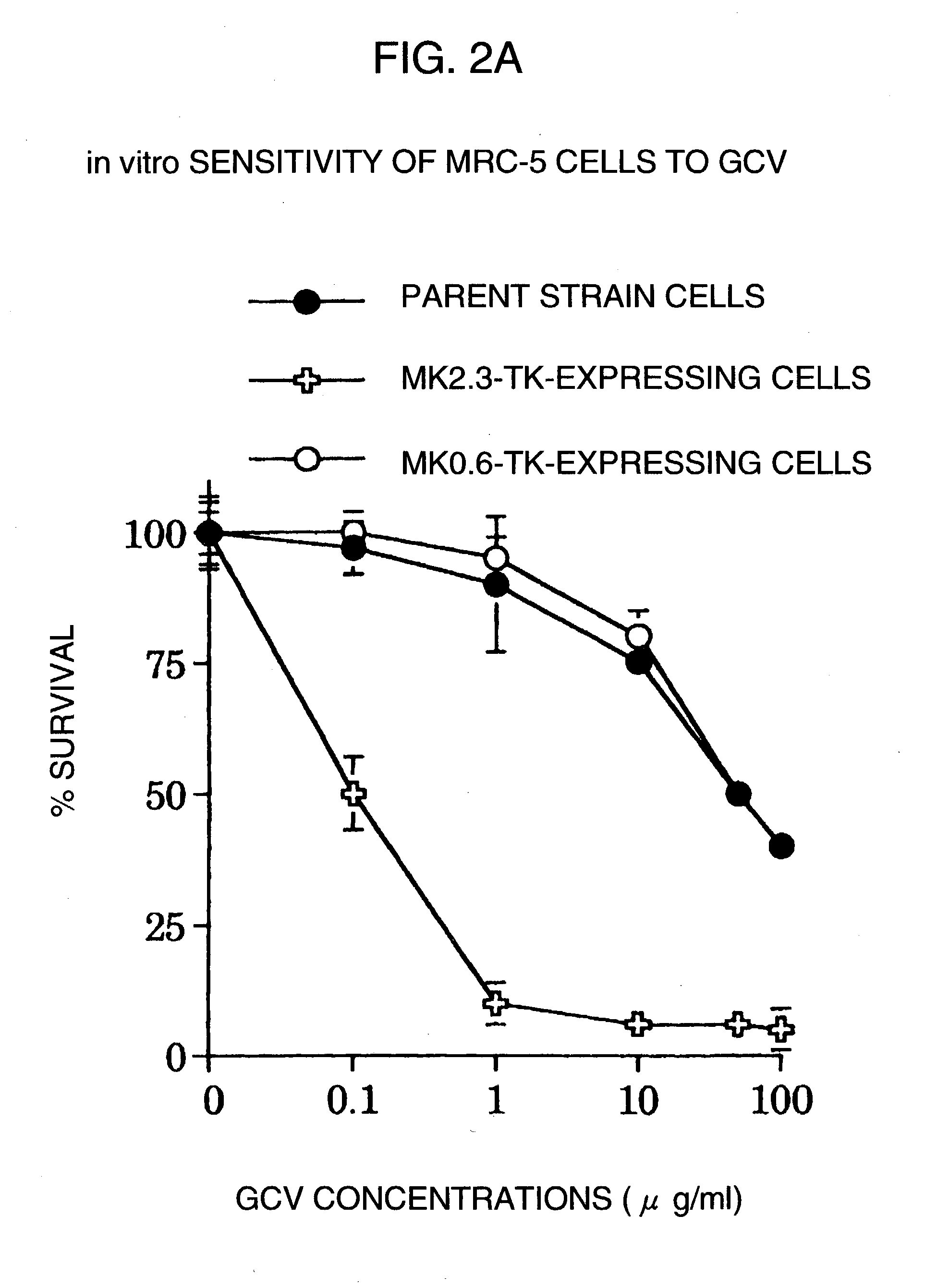
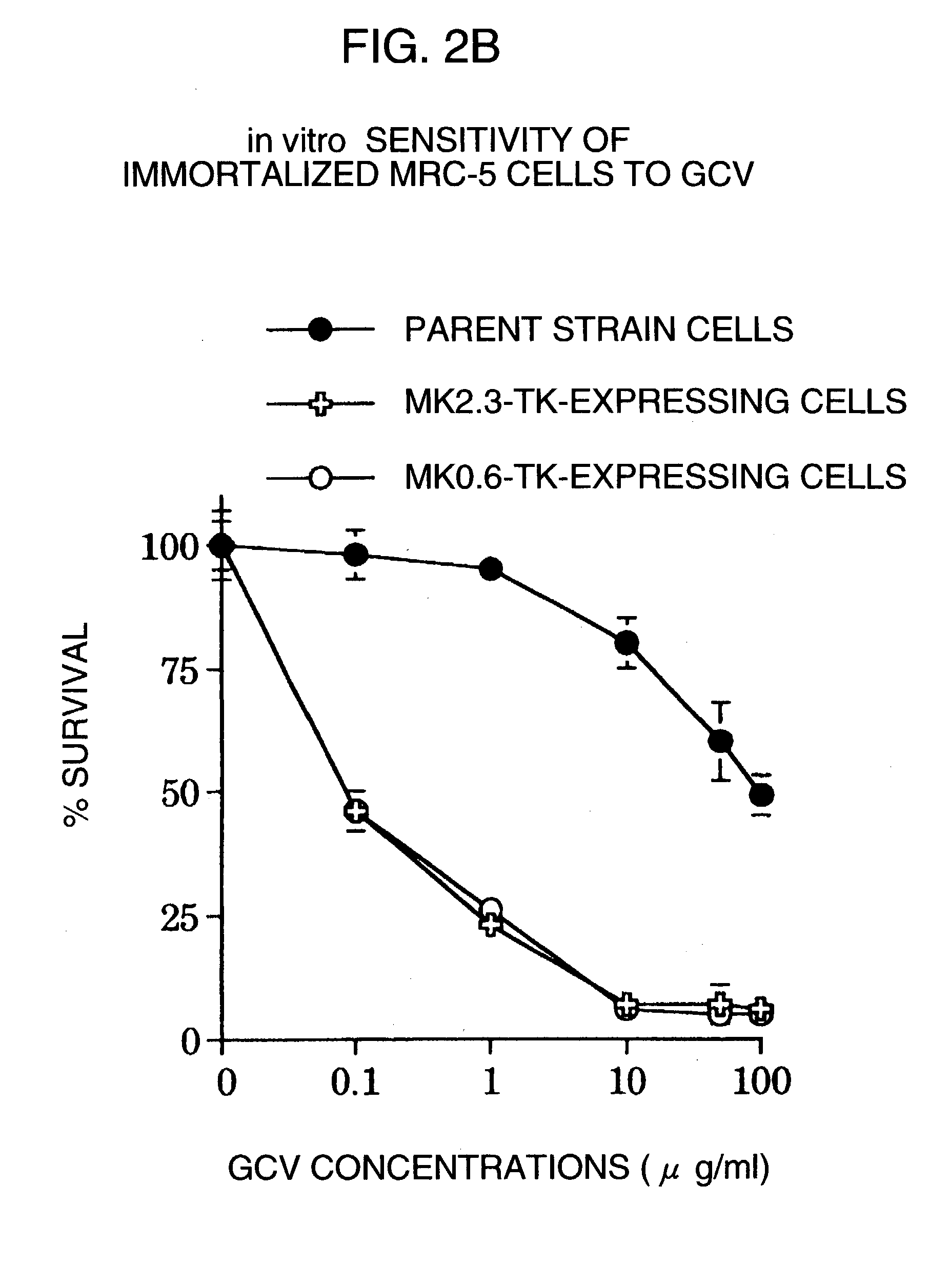
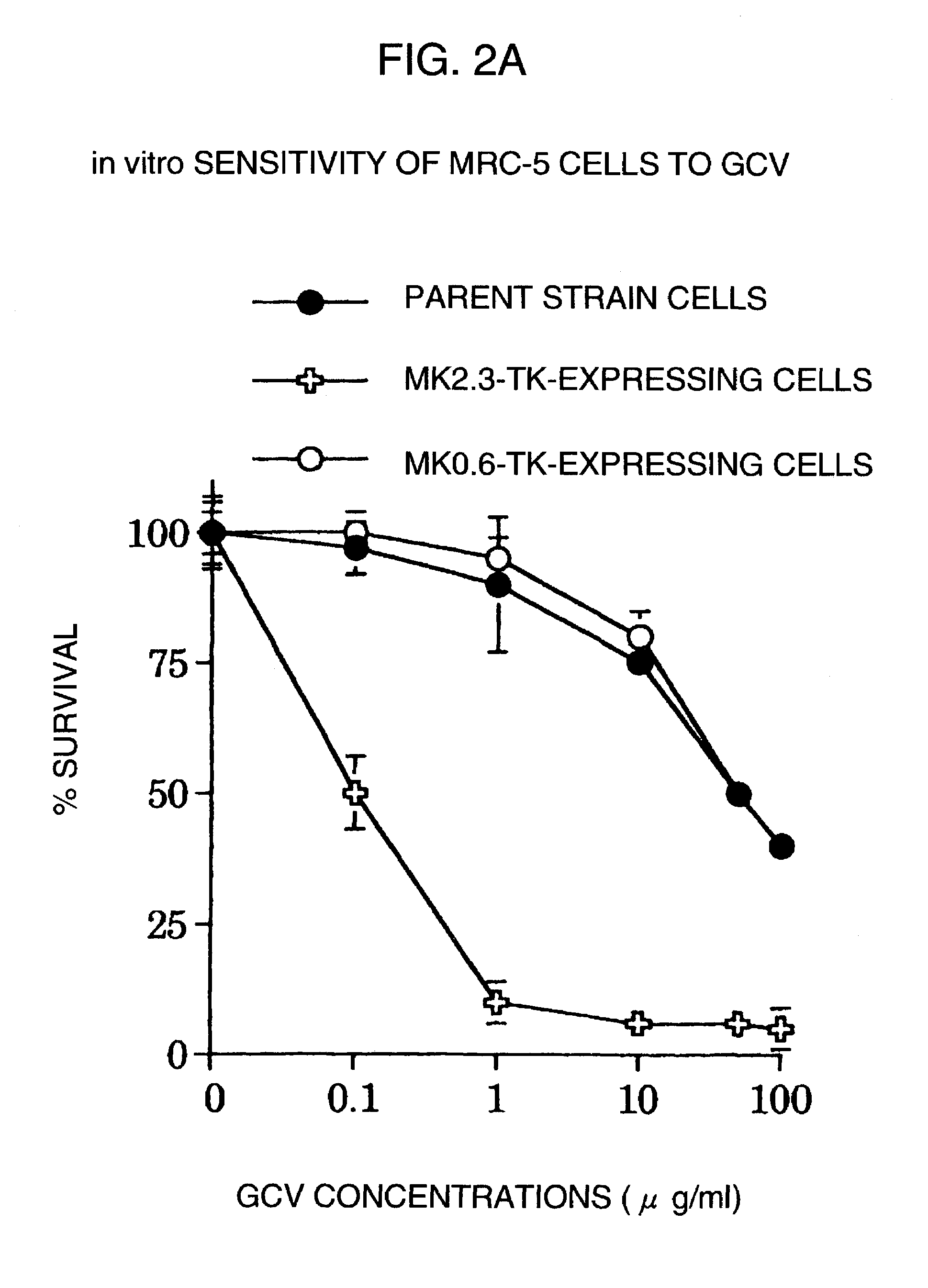
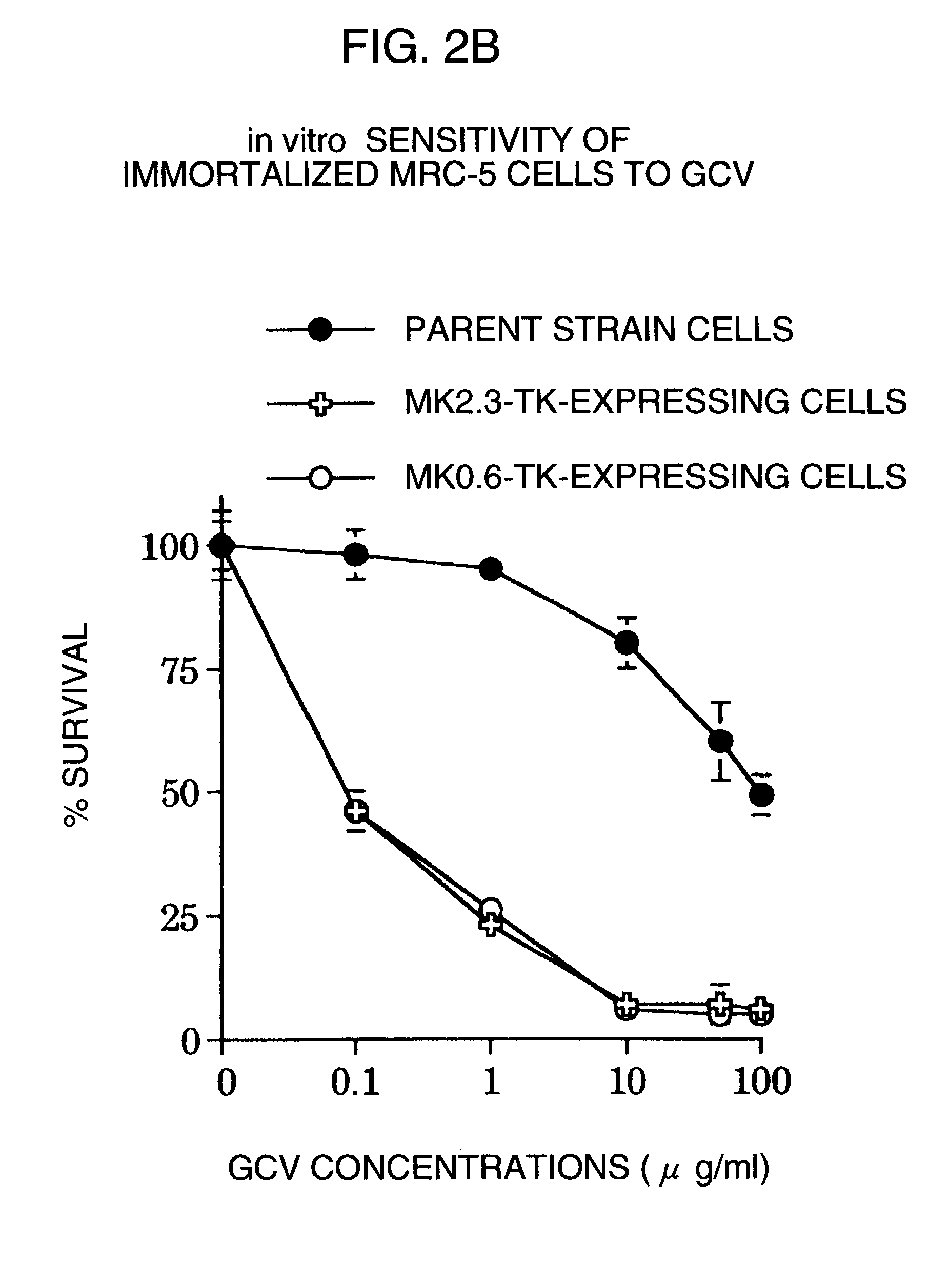
Tumor-specific promoter
InactiveUS20060099188A1High tumor-specificity and promoter activityHigh promoter activityBiocideGenetic material ingredientsPromoter activityC erbb 2
A DNA comprising a 609 bp base sequence from −559 to +50 when the first base sequence of exon 1 of the midkine gene, a human retinoic acid-responsive growth / differentiation factor was set as +1, or a DNA comprising a 251 bp base sequence from −213 to +38 when the transcription initiation point of the c-erbB-2 gene belonging to the EGF receptor family and having a tyrosine kinase activity was set as +1 has a tumor-specific transcription activity, and the promoter activity thereof is high, and therefore is very important as a tumor-specific promoter for use in the suicide gene therapy that combines the use of a gene for a drug metabolizing enzyme and a prodrug for cancer therapy, the gene therapy of cancer using an expression vector that contains a gene encoding a cytokine, and the gene therapy of cancer using an oncolytic virus.
Owner:PRIMMUNE CORP +1
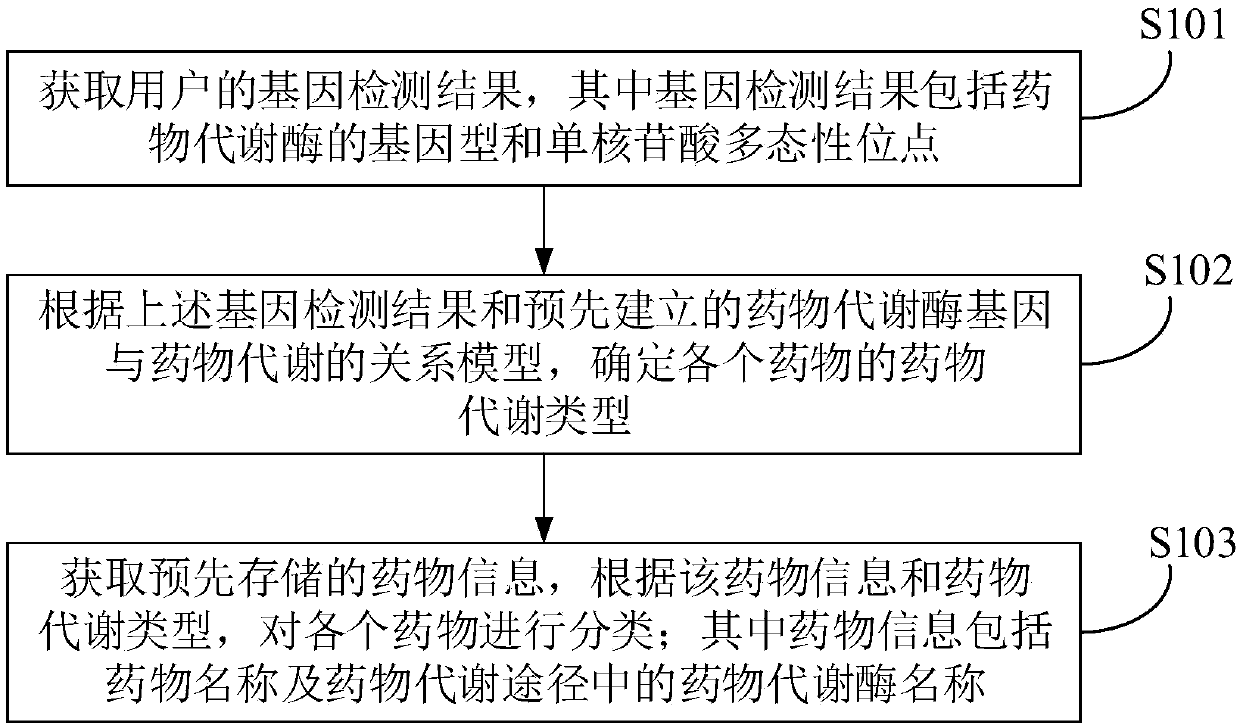
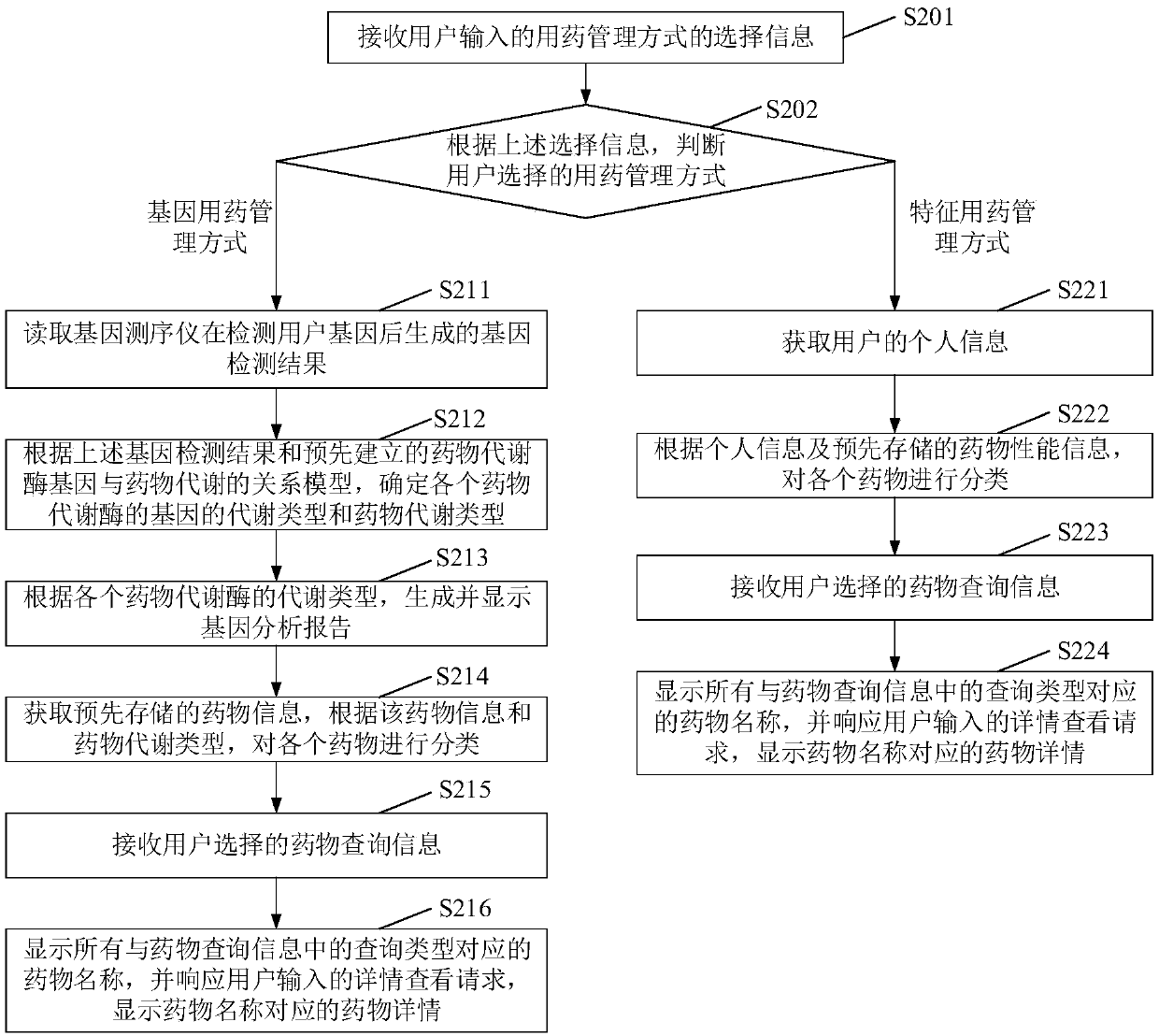
Drug use management method and device and electronic equipment
InactiveCN108682458ARealize scientific managementDrug referencesSystems biologyPersonalizationGenomics
The invention provides a drug use management method and device and electronic equipment. According to the method, first a gene detection result of a user is obtained, wherein the gene detection resultincludes a genotype of a drug metabolizing enzyme and a single nucleotide polymorphism site; then, according to the gene detection result and a pre-established relationship model between drug metabolizing enzyme genes and drug metabolism, the drug metabolism type of each drug is determined; and finally, pre-stored drug information is obtained, and each drug is classified according to the drug information and drug metabolism type, wherein the drug information includes drug names. In the technical scheme provided by the embodiment of the invention, the difference of the drug metabolizing enzymes in personal genetic genes is detected through a gene detection means, thereby obtaining the drug metabolism capabilities of the drug metabolizing enzymes, and then the drugs are classified accordingto a pharmacogenomics theory, thereby assisting the user to correctly use drugs and realizing scientific management of personalized drug use.
Owner:北京岙特杰诺生物科技有限公司
Detection kit and detection method for medication of hypertension patients
InactiveCN110129430AEffective treatmentAccurate guidanceMicrobiological testing/measurementFluorescencePcr ctpp
The invention belongs to the technical field of gene detection, in particular to a detection kit and a detection method for medication of hypertension patients. The medication gene locus combination for hypertension patients totally comprises 15 gene loci, comprising common drug metabolizing enzyme gene loci, drug targeting gene loci and gene loci of common drugs for H-type hypertension and otherhypertension complications. The gene loci are based on a fluorescence quantitative PCR technology and uses a specific Taqman primer probe set to greatly improve the accuracy and specificity of the product; the locus coverage is reasonable, the guiding effect on drugs is more accurate, a comprehensive reference is provided for the combined medication and individualized medication of hypertension patients, guidance can be provided for people suffering from hypertension and complications thereof, patients can receive the most effective treatment as soon as possible, and the whole detection process only needs 4 hours from extraction to result determination.
Owner:广州海思医疗科技有限公司
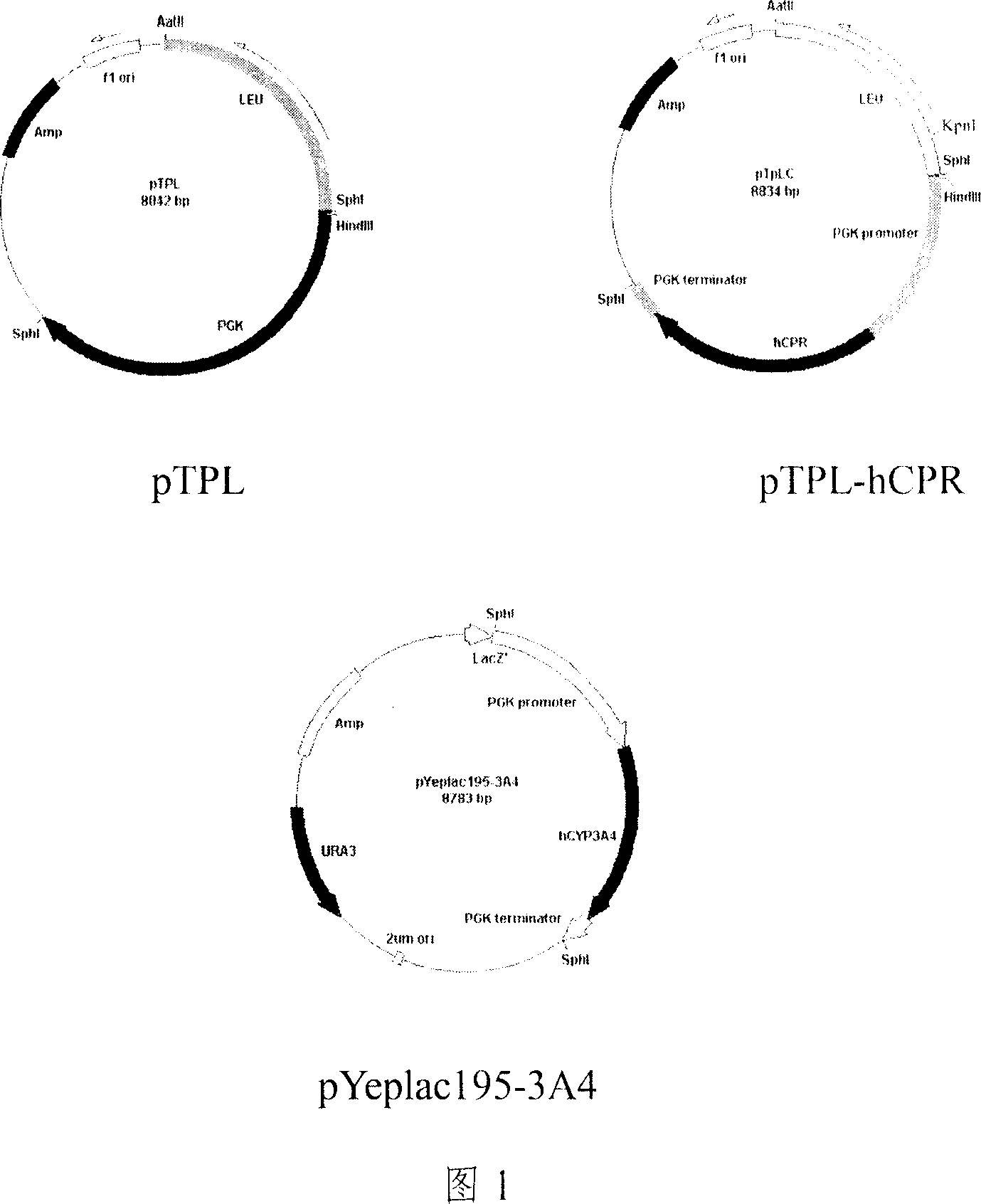
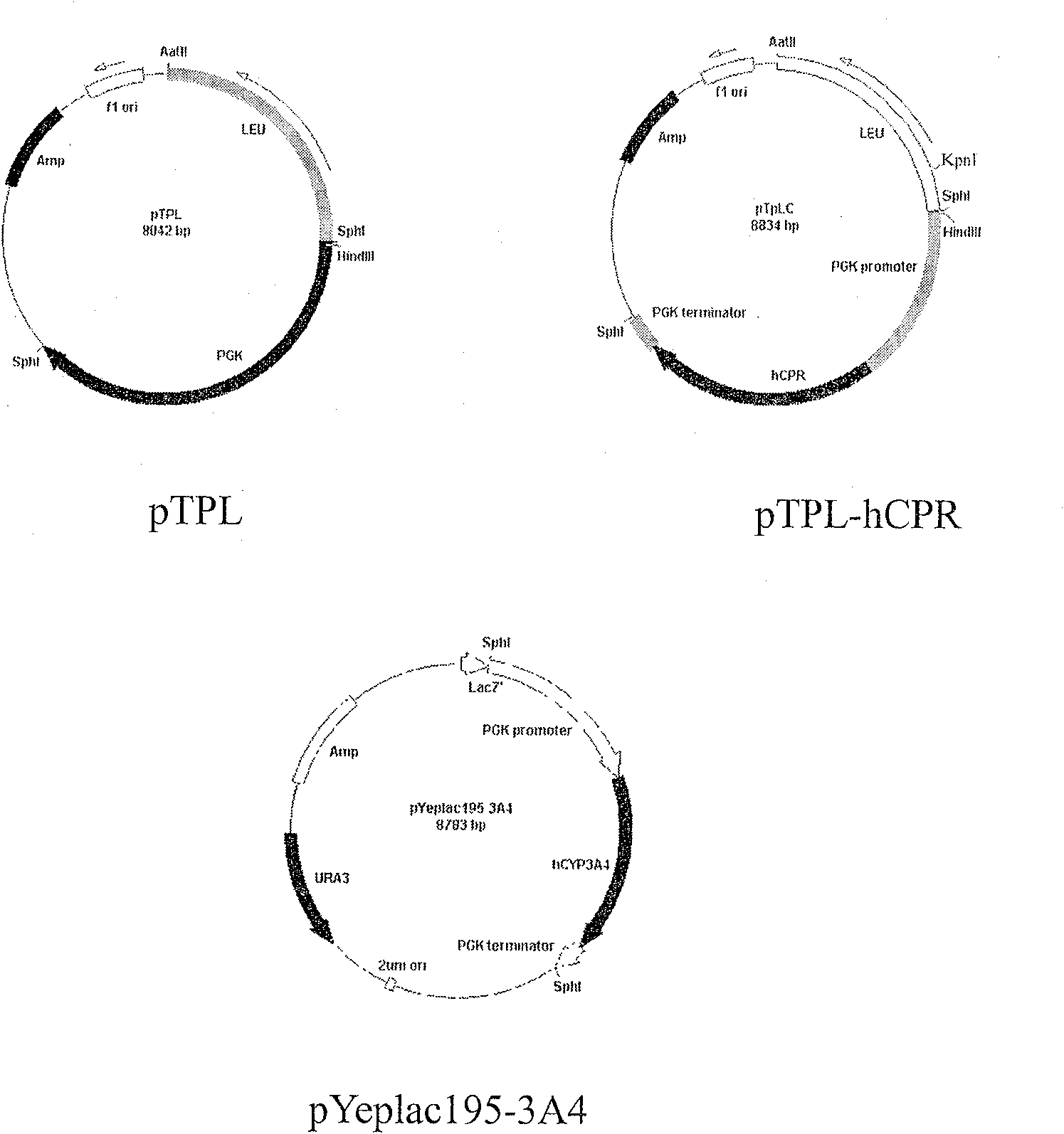
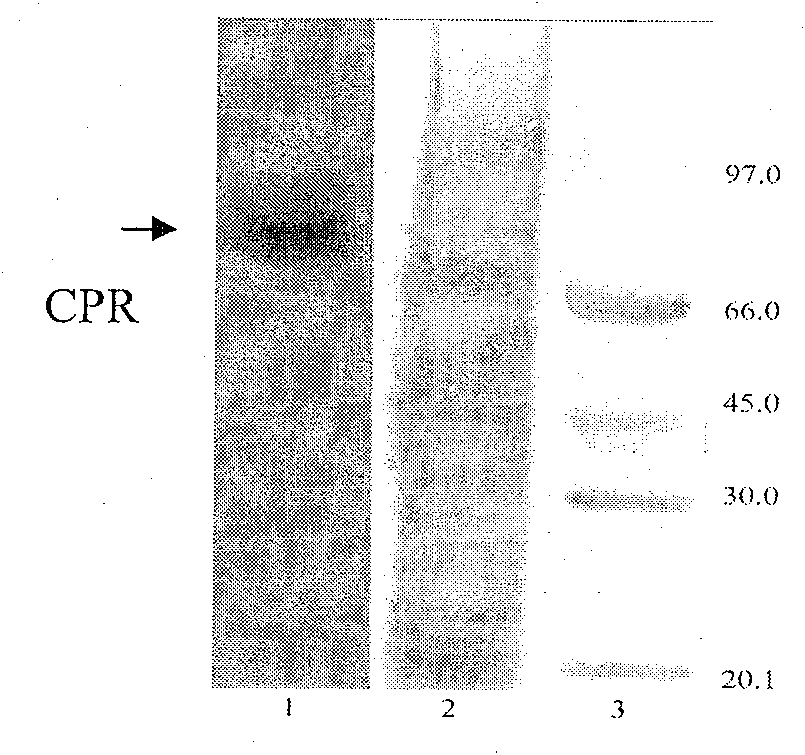
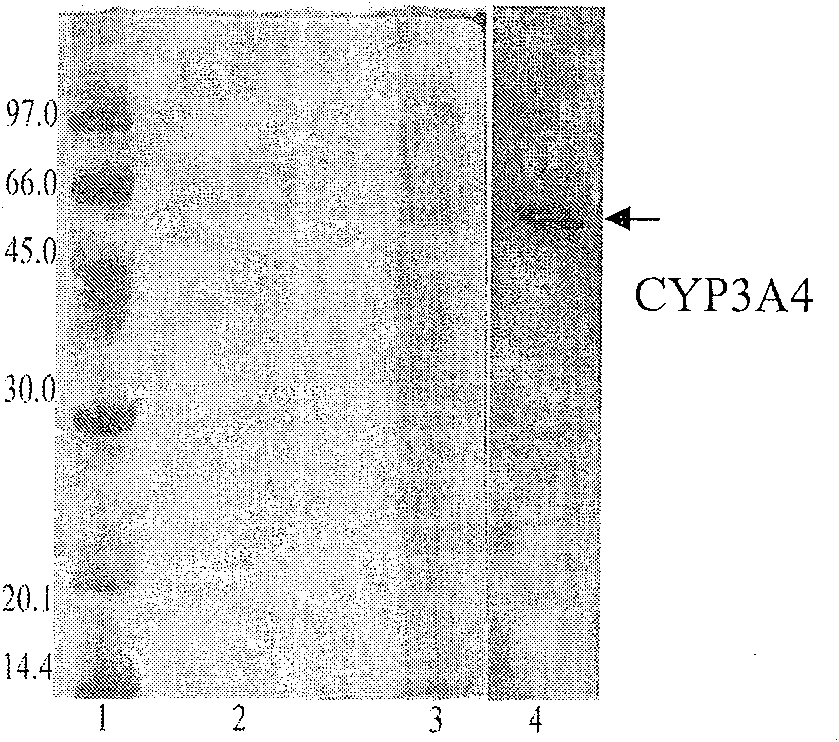
Yeast system capable of coexpressing CYP and CPR
This invention relates to a drug-metabolizing enzyme heterogenesis expression system used for out-of-body drug metabolism property evaluation, exactly to say a yeast expression system of coexpressing CYP and CPR, the yeast expression vector which respectively has CPR DNA fragment and CYP DNA fragment is transduced into yeast cell, this two expression vector express in yeast cell by means of occlusal pattern and liberation respectively , custom-crafted yeast cell is fermented and induced, getting yeast cell product which is purpose expression system, it can be used for CYP enzyme system out-of-body drug metabolism property evaluation. The constructed new Saccharomyces cerevisia co-expression system can be extensively used for oxidation reduction enzyme system such as cytopigment P450 enzyme system, the latter one is extensively used for screening, application and development of drug metabolism involved in ADME / T and pharmacokinetics.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Chip Production, Hybridization and Data Interpretation for Antibody and Protein Microarrays
InactiveUS20080058215A1Enhanced detection signalBioreactor/fermenter combinationsPeptide librariesCytochrome P450ADAMTS Proteins
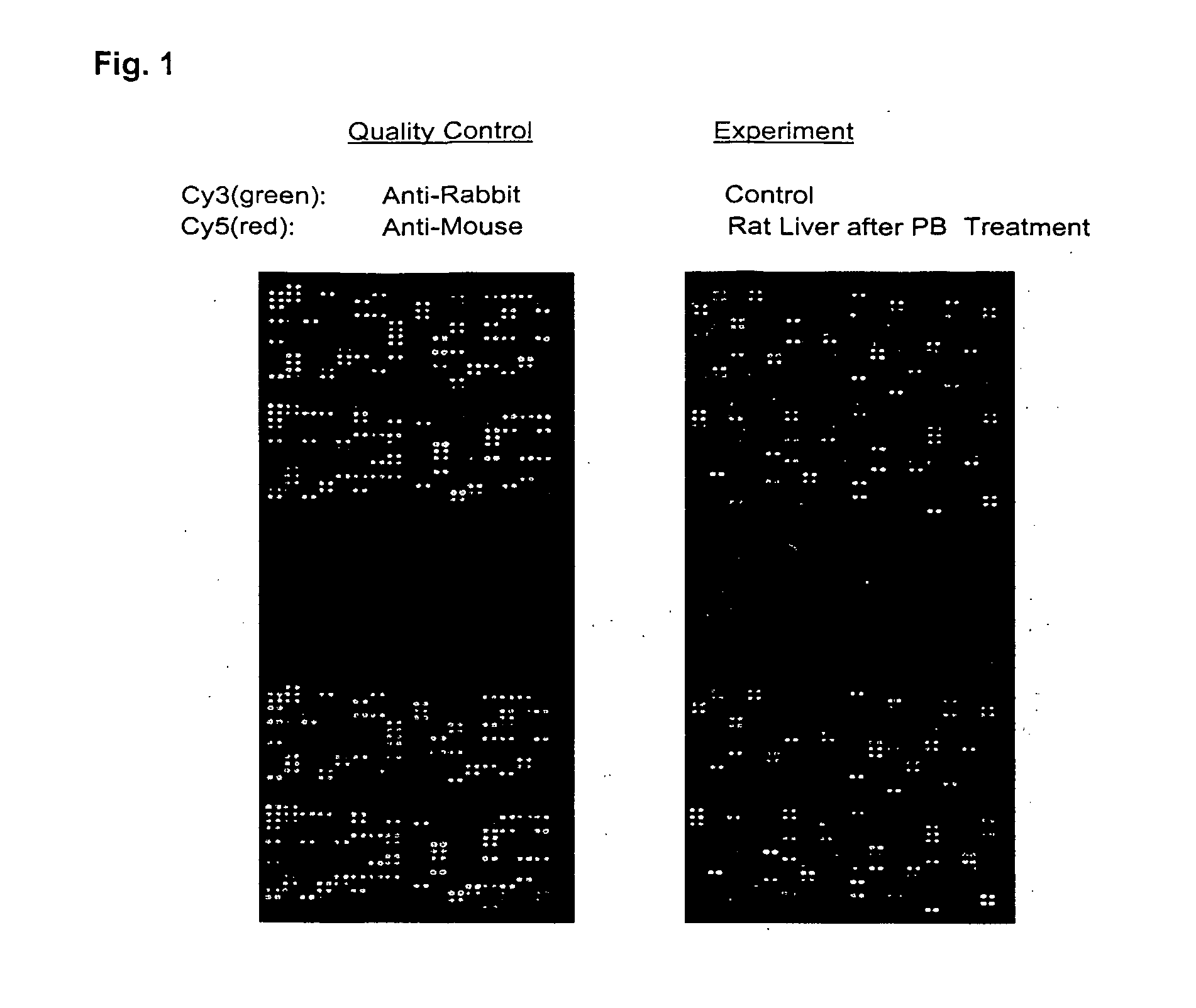
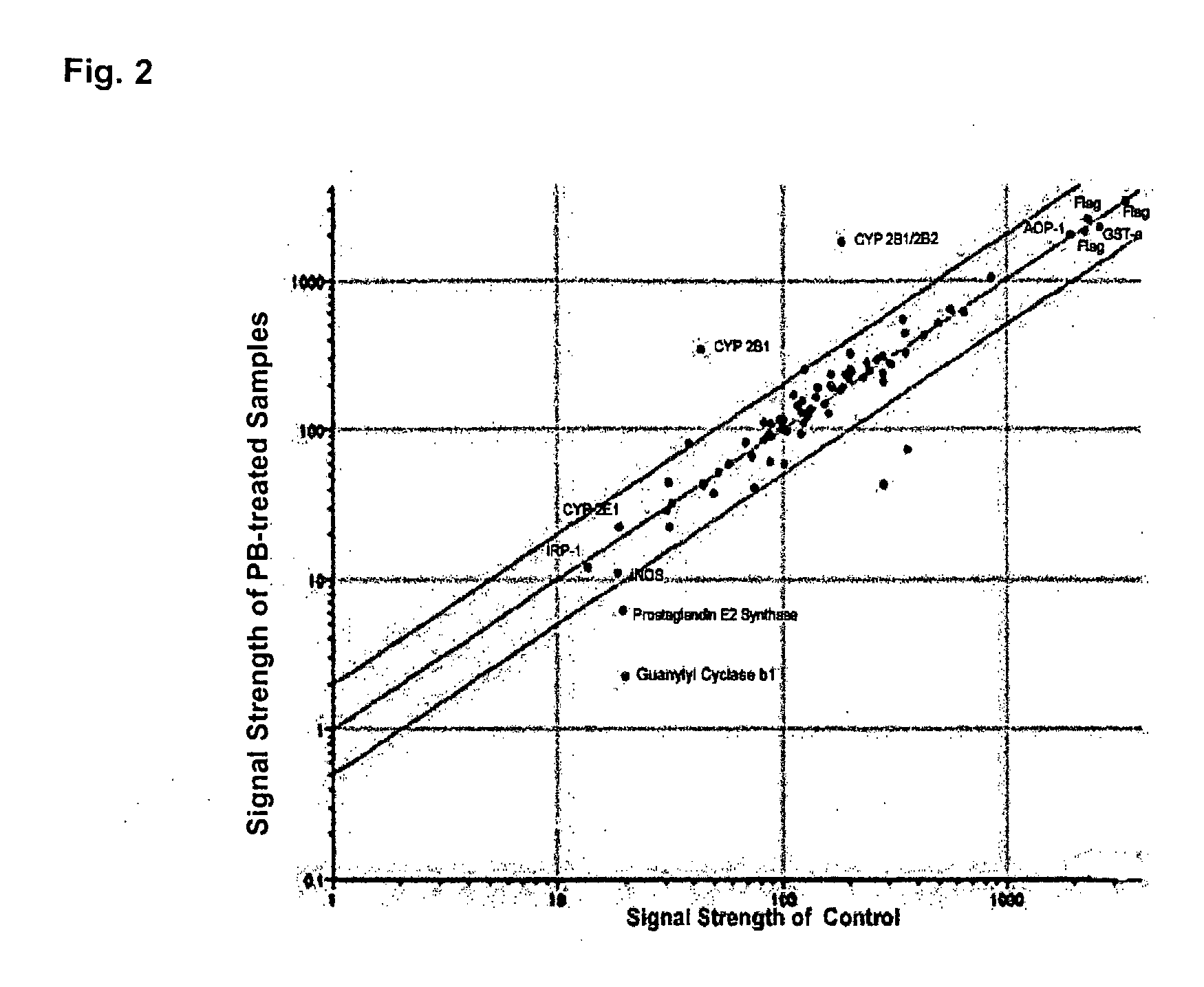
An antibody microarray screen including a substrate, monoclonal and polyclonal antibodies that are purified immunoglobins, wherein the antibodies are spotted on predetermined positions on the substrate, and fluids unprocessed for immunoglobulin isolation (e.g., anti-sera, ascites fluids, or hybridoma culture media), wherein the unprocessed fluids are spotted on the predetermined positions on the substrate. Production of drug-metabolizing enzyme antibody microarrays containing closely related cytochromes P450 is disclosed. Methods of manufacturing an antibody microarray, an internal control molecule for use in an antibody microarray, a method of determining optimal spotting concentrations of IgG and a method to increase a detectable signal with microarray analysis are disclosed.
Owner:DETROIT R&D
Protein arrays and uses thereof
The inventors herein describe methods for the production of a functional human, animal, plant or microbe protein arrays and methods to assay for interactions between the proteins on the array with molecules of interest, for example, using such arrays to determine the in vitro metabolite profile of any drug. Such protein arrays can be used, for example, to assay, in a parallel fashion, the protein products of DNA sequences encoding drug metabolizing enzymes (DMES) to obtain a toxicology profile. Also described herein is a novel DME expression and purification strategy using detergents and not requiring an ultra-centrifugation step.
Owner:SENSE PROTEOMIC LTD
Method for guiding helicobacter pylori to eradicate drug use based on PGM high throughput sequencing technology
InactiveCN107236787AGood for guiding detectionGood for guiding eradicationMicrobiological testing/measurementMicroorganism based processesMetabolic enzymesLibrary preparation
The invention provides a method for guiding helicobacter pylori to eradicate drug use based on a PGM high throughput sequencing technology, and mainly aims to realize detection of helicobacter pylori on antibiotic drug resistance and host drug metabolic enzyme types. The method mainly comprises the steps of designing a specific primer for covering VacA, 23SrRNA, gyrA, CYP2C19*2 and CYP2C19*3 gene to-be-detected regions to form a Primer Pool; extracting host and helicobacter pylori genome to perform amplification; performing PGM library preparation, purification, quantifying and sequencing; and working out drug resistance proportion and drug metabolic enzyme types. By adoption of multiple PCR technologies, synchronous amplification of multiple sites can be realized; and by combination with PGM sequencing, guiding to clinical detection and eradiation of the helicobacter pylori can be facilitated.
Owner:杭州致远医学检验所有限公司
New application of Korean mondshood root total alkaloids and guanfu base VIIII
The invention provides a preparation method of Korean mondshood root total alkaloids and applications in preparing a slow sodium-ion passage blocking agent and preparing drugs for resisting arrhythmia. Both the Korean mondshood root total alkaloids and the guanfu base I are provided to have an effect for inhibiting slow sodium current of ventricular muscles of cavies. The invention particularly relates to an application of guanfu base VIIII in preparing a drug-metabolizing enzyme inhibitor. The invention also provides an application of guanfu base VIIII in preparing a slow sodium-ion passage blocking agent.
Owner:CHINA PHARM UNIV +1
Individualized medication gene testing reagent kit for beta-receptor blocker
InactiveCN109136360AGood curative effectReduce adverse reactionsMicrobiological testing/measurementBeta blockerCurative effect
The invention belongs to the technical field of biology, and particularly relates to an individualized medication gene testing reagent kit for a beta-receptor blocker. The individualized gene testingreagent kit for the beta-receptor blocker mainly comprises: (1) two pairs of CYP2D6*10 and ADRBB1(1165 G) [C] locus amplification and sequencing primers, (2) PCR amplification reagents, (3) a PCR product purification reagent, and (4) a DNA sequencing reagent. The reagent kit is used for detecting the loci of the beta-receptor blocker drug metabolizing enzyme and the receptor CYP2D6*10 and ADRBB1 (1165 G) [C] of the hypertension patient, uses the key technology for Sanger sequencing (the accuracy rate is more than 99.9%). The genotypes of the two loci are proved to be closely associated with the curative effect of medication of the beta-receptor blocker, so that the gene testing reagent kit can be used for guiding the hypertension patient in using the beta-receptor blocker drug reasonably and safely according to the testing result of the gene testing reagent kit.
Owner:中科基因生物科技(江苏)有限公司
Tumor-specific promoters
InactiveUS20030157065A1High tumor-specificityHigh promoter activityBiocideGenetic material ingredientsPromoter activityC erbb 2
A DNA comprising a 609 bp base sequence from -559 to +50 when the first base sequence of exon 1 of the midkine gene, a human retinoic acid-responsive growth / differentiation factor was set as +1, or a DNA comprising a 251 bp base sequence from -213 to +38 when the transcription initiation point of the c-erbB-2 gene belonging to the EGF receptor family and having a tyrosine kinase activity was set as +1 has a tumor-specific transcription activity, and the promoter activity thereof is high, and therefore is very important as a tumor-specific promoter for use in the suicide gene therapy that combines the use of a gene for a drug metabolizing enzyme and a prodrug for cancer therapy, the gene therapy of cancer using an expression vector that contains a gene encoding a cytokine, and the gene therapy of cancer using an oncolytic virus that exhibits cytotoxic effects only on tumor cells, etc.
Owner:CHIBA PREFECTURE +1
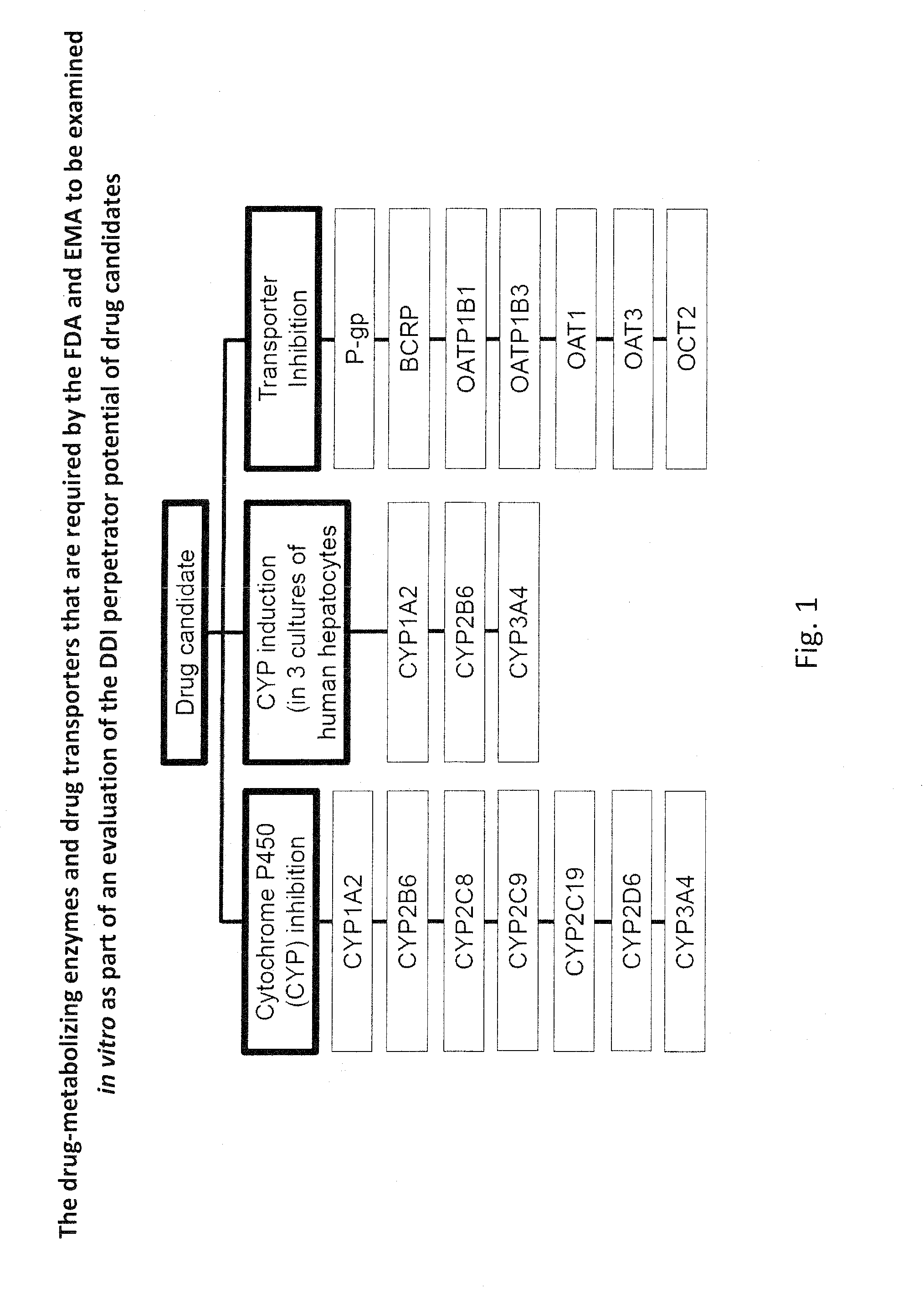
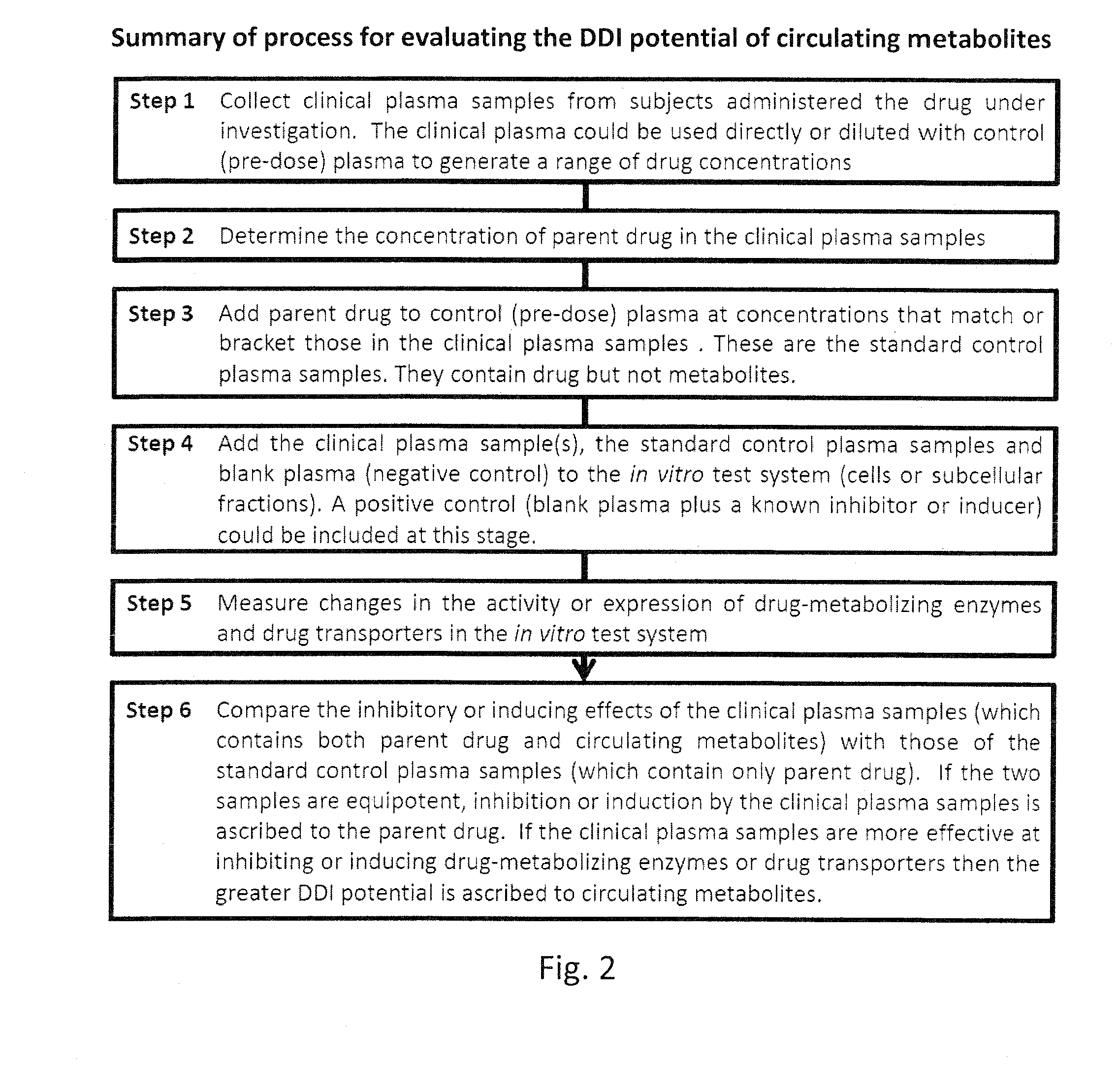
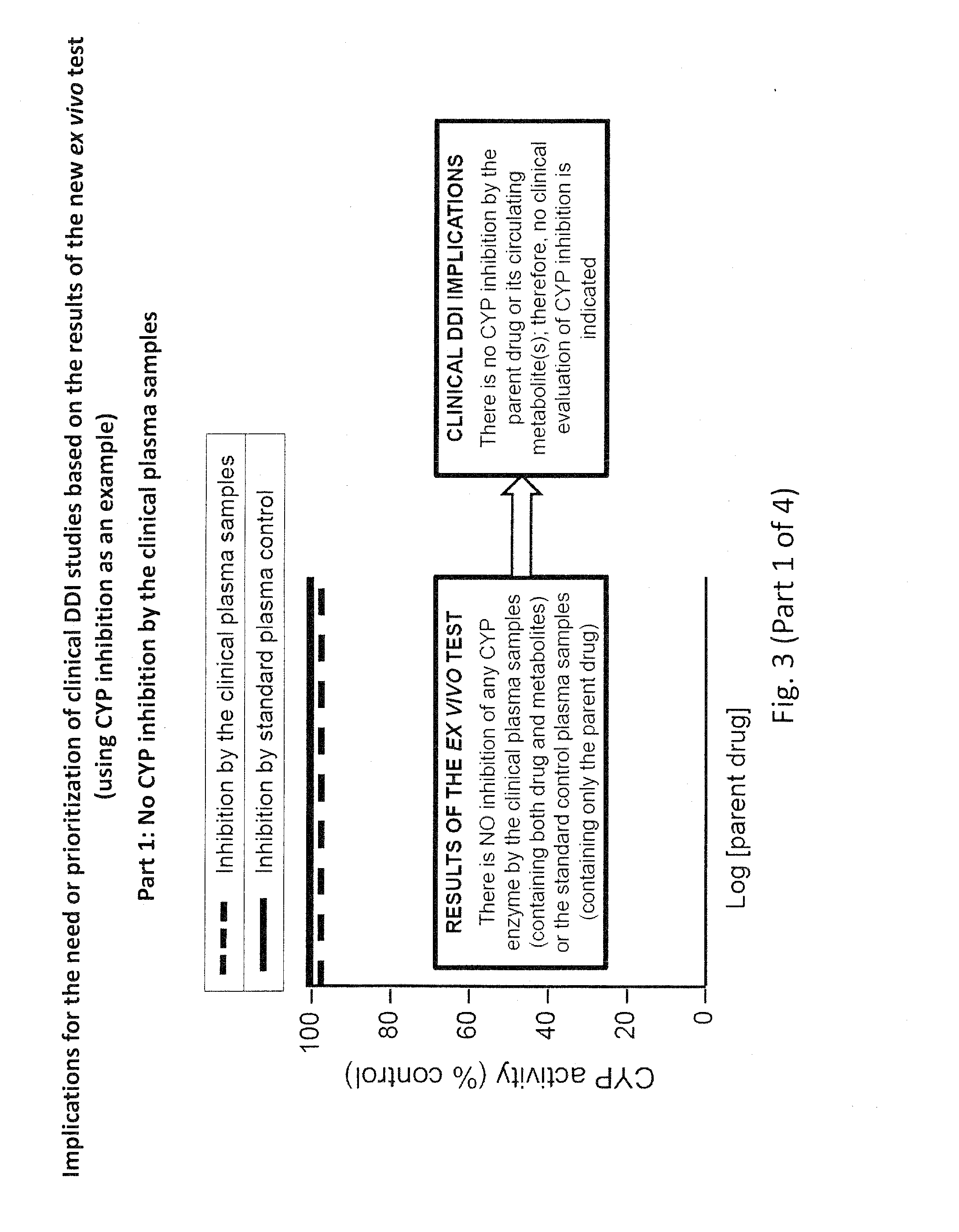
Ex vivo methods to identify circulating drug metabolites with drug interaction potential
InactiveUS20130330737A1Microbiological testing/measurementBiological testingMetaboliteDrug interaction
Ex vivo methods of detecting and analyzing circulating drug metabolites with drug interaction potential are provided. The methods include the use of clinical plasma samples from subjects who have been administered an investigational drug in vivo. The clinical plasma samples will contain both the parent drug and associated metabolites. A control plasma sample spiked directly with the drug of interest can be used as a standard reference. The plasma samples can be applied to an in vitro test system to evaluate the changes in activity or expression of drug-metabolizing enzymes and / or drug transporters in the test systems to determine circulating drug metabolites with drug interaction potential. By comparing the clinical plasma sample to the drug-spiked control, the inhibitory and / or inducing effects on drug-metabolizing enzymes and / or drug transporters can be correctly attributed to the parent drug or its associated metabolites.
Owner:XPD CONSULTING
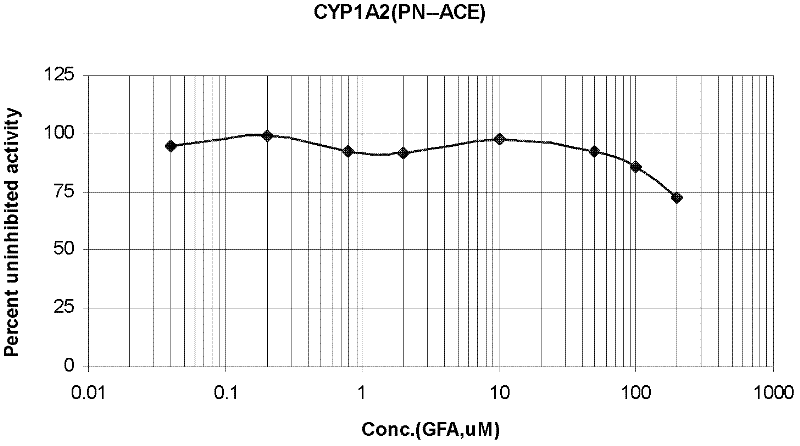
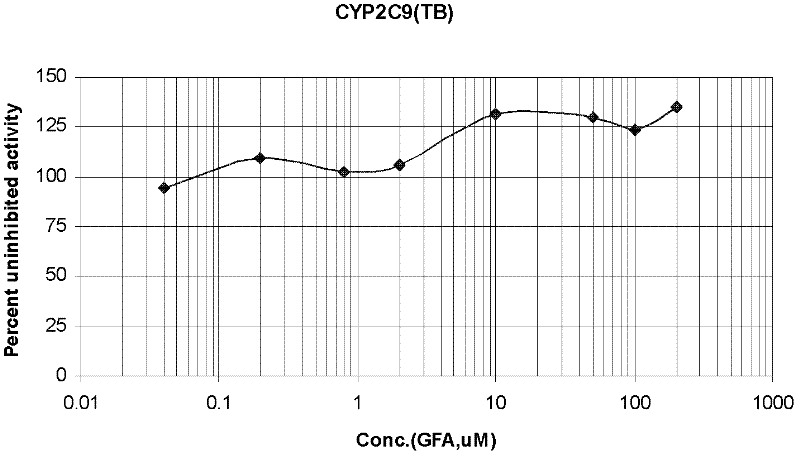
Evaluation system for drug-metabolizing capacity and use thereof
InactiveUS20060228770A1Easy to useMicrobiological testing/measurementBiological testingDrug metabolismTemperature control
The amount of drug consumption caused by a specific drug-metabolizing enzyme and / or the amount of the resulting metabolite(s) is measured by chromatography with an aqueous mobile phase under temperature control using a packing material whose surface is coated with a polymer having a hydration force varying in the temperature range of 0° C. to 80° C. This system enables proper evaluation of drug-metabolizing capacity through simple means without adversely affecting the environment.
Owner:CELLSEED +1
Isolated human UDP-glycosyltransferase, nucleic acid molecules encoding human UDP-glycosyltransferase, and uses thereof
The present invention provides amino acid sequences of peptides that are encoded by genes within the human genome, the drug-metabolizing enzyme peptides of the present invention. The present invention specifically provides isolated peptide and nucleic acid molecules, methods of identifying orthologs and paralogs of the drug-metabolizing enzyme peptides, and methods of identifying modulators of the drug-metabolizing enzyme peptides.
Owner:CELERA CORPORATION
Yeast system capable of coexpressing CYP and CPR
InactiveCN100591754CEasy to buildLow costFungiMicroorganism based processesBiotechnologyHeterologous
This invention relates to a drug-metabolizing enzyme heterogenesis expression system used for out-of-body drug metabolism property evaluation, exactly to say a yeast expression system of coexpressingCYP and CPR, the yeast expression vector which respectively has CPR DNA fragment and CYP DNA fragment is transduced into yeast cell, this two expression vector express in yeast cell by means of occlusal pattern and liberation respectively , custom-crafted yeast cell is fermented and induced, getting yeast cell product which is purpose expression system, it can be used for CYP enzyme system out-of-body drug metabolism property evaluation. The constructed new Saccharomyces cerevisia co-expression system can be extensively used for oxidation reduction enzyme system such as cytopigment P450 enzymesystem, the latter one is extensively used for screening, application and development of drug metabolism involved in ADME / T and pharmacokinetics.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Tumor specific promoters of the midkine gene that allow for selective expression in P53-inactivated cells
InactiveUS7030099B2High tumor-specificity and promoter activityHigh promoter activityBiocideSugar derivativesPromoter activityC erbb 2
A DNA comprising a 609 bp base sequence from −559 to +50 when the first base sequence of exon 1 of the midkine gene, a human retinoic acid-responsive growth / differentiation factor was set as +1, or a DNA comprising a 251 bp base sequence from −213 to +38 when the transcription initiation point of the c-erbB-2 gene belonging to the EGF receptor family and having a tyrosine kinase activity was set as +1 has a tumor-specific transcription activity, and the promoter activity thereof is high, and therefore is very important as a tumor-specific promoter for use in the suicide gene therapy that combines the use of a gene for a drug metabolizing enzyme and a prodrug for cancer therapy, the gene therapy of cancer using an expression vector that contains a gene encoding a cytokine, and the gene therapy of cancer using an oncolytic virus.
Owner:CHIBA PREFECTURE +1
Macaca fascicularis P450 2C9 medical metabolic enzyme and co-expression recombinant carrier with macaca fascicularis P450 oxidoreductase
The invention discloses cynomolgus monkey P450 2C9 drug metabolizing enzyme and a co-expression recombinant vector of the cynomolgus monkey P450 2C9 drug metabolizing enzyme and cynomolgus monkey P450 oxidoreductase. The cynomolgus monkey P450 2C9 drug metabolizing enzyme can catalyze 4'-hydroxylation of diclofenac, the 4'-hydroxylation of tolbutamide and the 7'-hydroxylation of warfarin and a gene sequence of the cynomolgus monkey P450 2C9 drug metabolizing enzyme or a complementary sequence of the cynomolgus monkey P450 2C9 drug metabolizing enzyme is a sequence in SEQ ID NO:1 with the mutation rate of 0-1%. The co-expression recombinant vector of the cynomolgus monkey P450 2C9 drug metabolizing enzyme and the cynomolgus monkey P450 oxidoreductase comprises an open reading frame of the sequence of the cynomolgus monkey P450 2C9 drug metabolizing enzyme and the sequence of the cynomolgus monkey P450 oxidoreductase. The sequence or the complementary sequence of the cynomolgus monkey P450 oxidoreductase is shown in SEQ ID NO:2. Protein which is expressed by a heterogeneous source of the invention only represents the cynomolgus monkey P450 2C9 and the system is closer to the real situation of metabolism in vivo of cynomolgus monkeys.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Novel application of guanfu base A and guanfu base G
ActiveCN102641268AInhibition of activityInhibition of oxygen demethylation reactionOrganic active ingredientsDrug compositionsAlkaloidDrug effect
The invention provides the application of norditerpenoid alkaloids which are guanfu base A and guanfu base G in Korean mondshood roots in the aspect of suppressing the activity of drug metabolizing enzymes. By the guanfu base A and the guanfu base G, the activity of CYP2D6 can be directly suppressed, the metabolism of drugs (substrates) is retarded, and the drug effect is reinforced; and moreover, the guanfu base A and the guanfu base G have no influence to the activity of other metabolizing enzymes.
Owner:CHINA PHARM UNIV +1
Macaca fascicularis P450 2B6 medical metabolic enzyme and co-expression recombinant carrier with macaca fascicularis P450 oxidoreductase
The invention discloses cynomolgus monkey P450 2B6 drug metabolizing enzyme and a co-expression recombinant vector of the cynomolgus monkey P450 2B6 drug metabolizing enzyme and cynomolgus monkey P450 oxidoreductase. The cynomolgus monkey P450 2B6 drug metabolizing enzyme can catalyze hydroxylation of bupropion hydrochloride and N end demethylation of 3-methyl benzene phenytoin, a gene sequence of the cynomolgus monkey P450 2B6 drug metabolizing enzyme or a complementary sequence of the cynomolgus monkey P450 2B6 drug metabolizing enzyme is a sequence in SEQ ID NO:1 with the mutation rate of 0-1%. The co-expression recombinant vector of the cynomolgus monkey P450 2B6 drug metabolizing enzyme and the cynomolgus monkey P450 oxidoreductase comprises an open reading frame of the sequence of the cynomolgus monkey P450 2B6 drug metabolizing enzyme and the open reading frame of the sequence of the cynomolgus monkey P450 oxidoreductase. The sequence or the complementary sequence of the cynomolgus monkey P450 oxidoreductase is shown in SEQ ID NO:2. Protein which is expressed by a heterogeneous source of the invention only represents the cynomolgus monkey P450 2B6 hypotype and the system is closet to the real situation of metabolism in vivo of cynomolgus monkeys.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Macaca fascicularis P450 2C18 medical metabolic enzyme and co-expression recombinant carrier with macaca fascicularis P450 oxidoreductase
The invention discloses cynomolgus monkey P450 2C18 drug metabolizing enzyme and a co-expression recombinant vector of the cynomolgus monkey P450 2C18 drug metabolizing enzyme and cynomolgus monkey P450 oxidoreductase. The cynomolgus monkey P450 2C18 drug metabolizing enzyme can catalyze hydroxylation of piroxicam and a gene sequence of the cynomolgus monkey P450 2C18 drug metabolizing enzyme or a complementary sequence of the cynomolgus monkey P450 2C18 drug metabolizing enzyme is a sequence in SEQ ID NO:1 with the mutation rate of 0-1%. The co-expression recombinant vector of the cynomolgus monkey P450 2C18 drug metabolizing enzyme and the cynomolgus monkey P450 oxidoreductase comprises an open reading frame of the sequence of the cynomolgus monkey P450 2C18 drug metabolizing enzyme and the open reading frame of the sequence of the cynomolgus monkey P450 oxidoreductase. The sequence or the complementary sequence of the cynomolgus monkey P450 oxidoreductase is shown in SEQ ID NO:2. Protein which is expressed by a heterogeneous source of the invention only represents the cynomolgus monkey P450 2C18 hypotype and the system is closer to the real situation of metabolism in vivo of cynomolgus monkeys.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
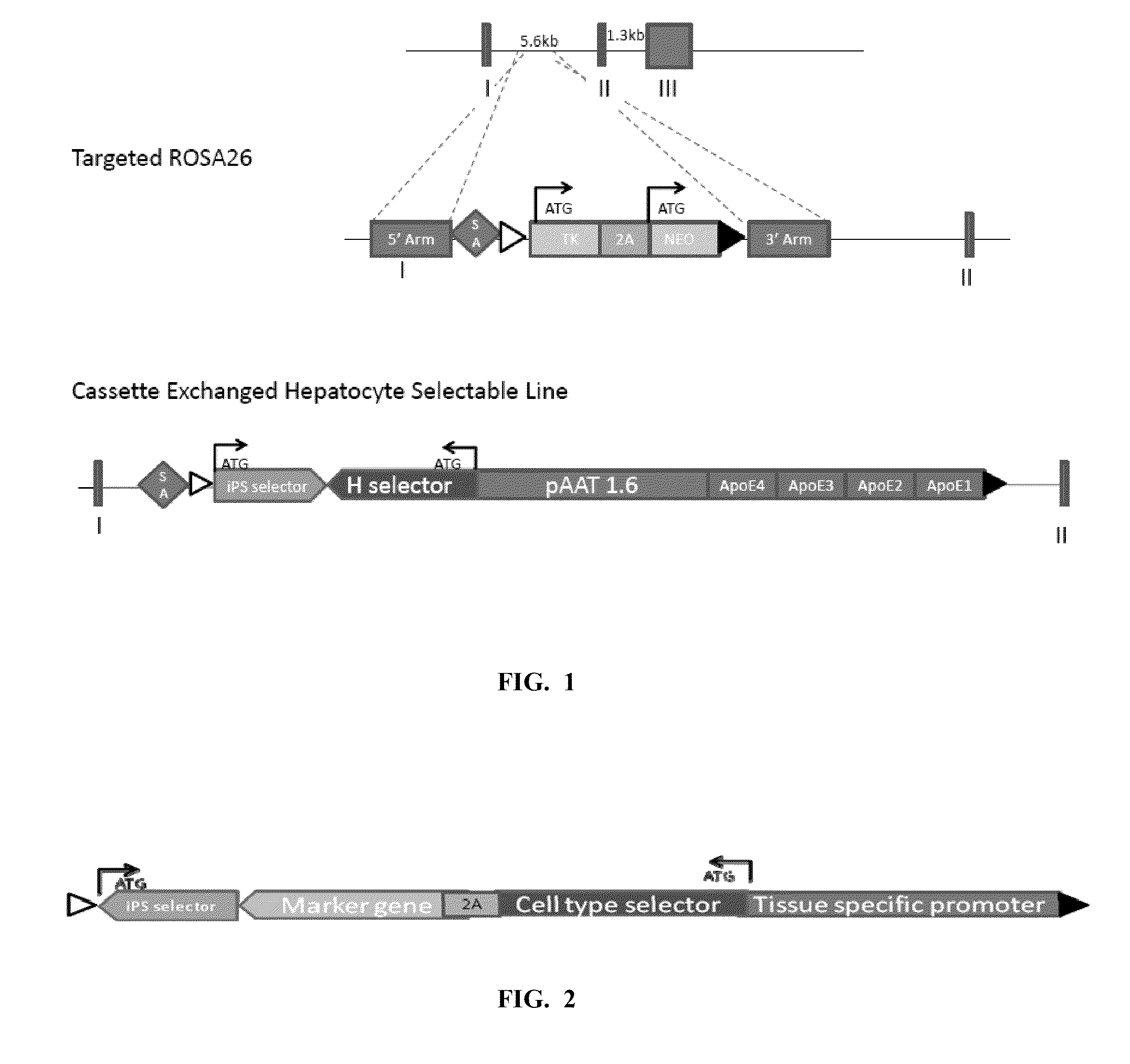
Compendium of ready-built stem cell models for interrogation of biological response
InactiveUS20110312001A1Microbiological testing/measurementFermentationSignaling Pathway GeneDrug target
The invention generally features methods for providing engineered pluripotent stem cells that can be used to study biological response and pathways, including differentiation and drug effects. For example, these cells are provided comprising two or more exogenous expression cassettes including a selectable or screenable marker under the control of different condition-responsive regulatory elements, such as differentiation-responsive promoters or regulatory element of a receptor, drug target, drug metabolizing enzyme or signaling pathway gene. Also provided are sets of stem cell lines each comprising a different exogenous expression cassette including a selectable or screenable marker under the control of a different condition-responsive regulatory element.
Owner:FUJIFILM CELLULAR DYNAMICS INC
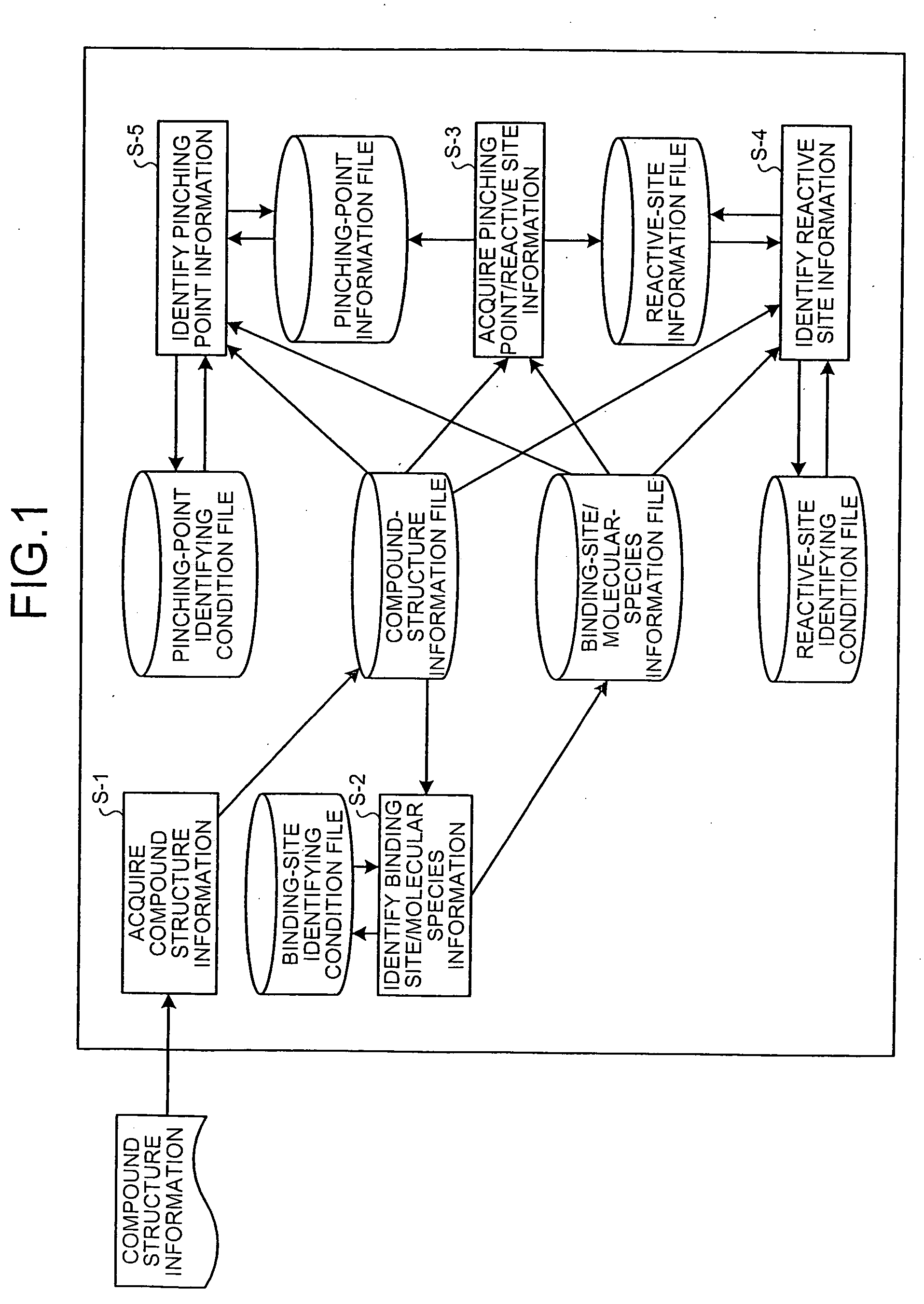
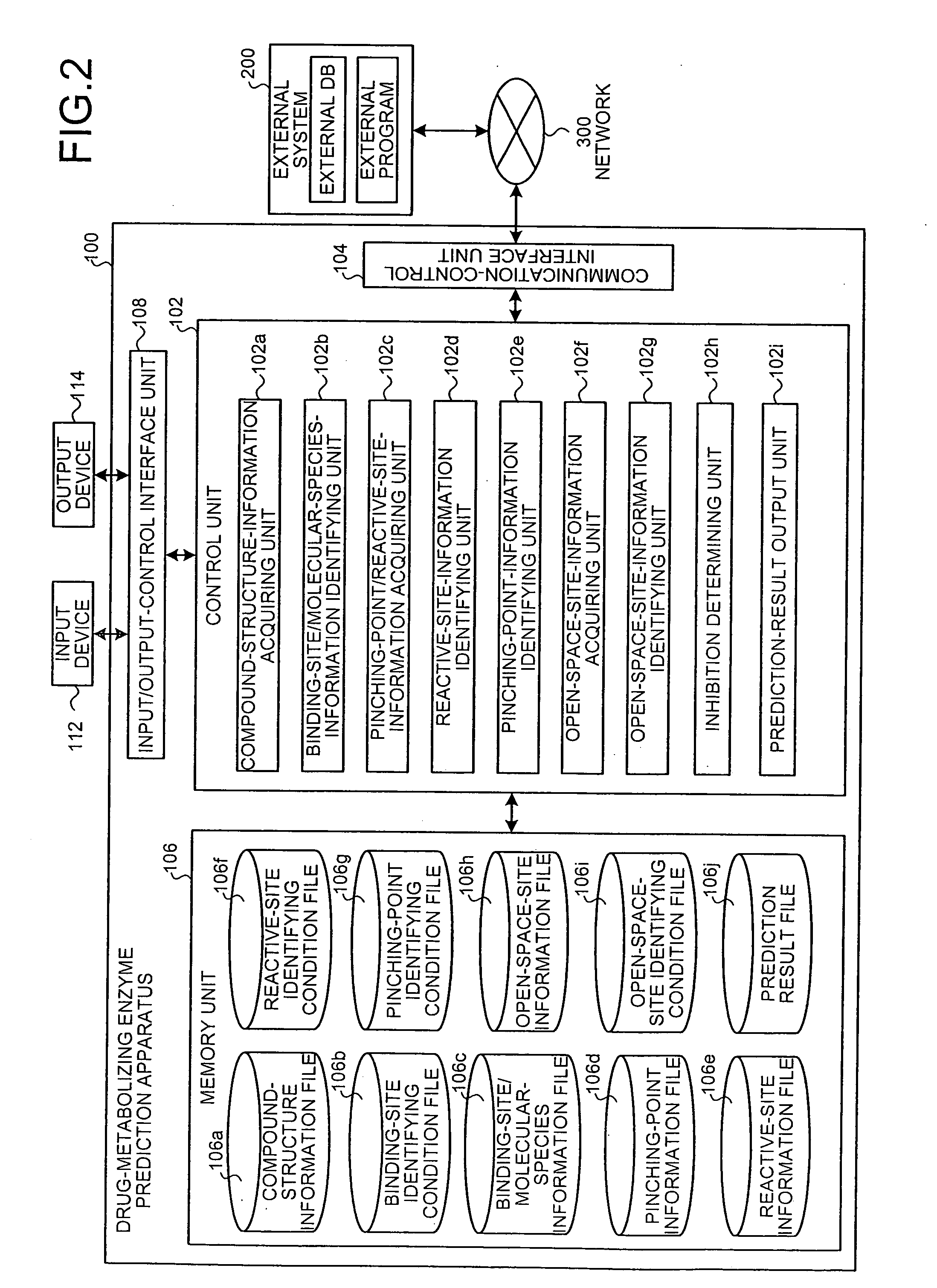
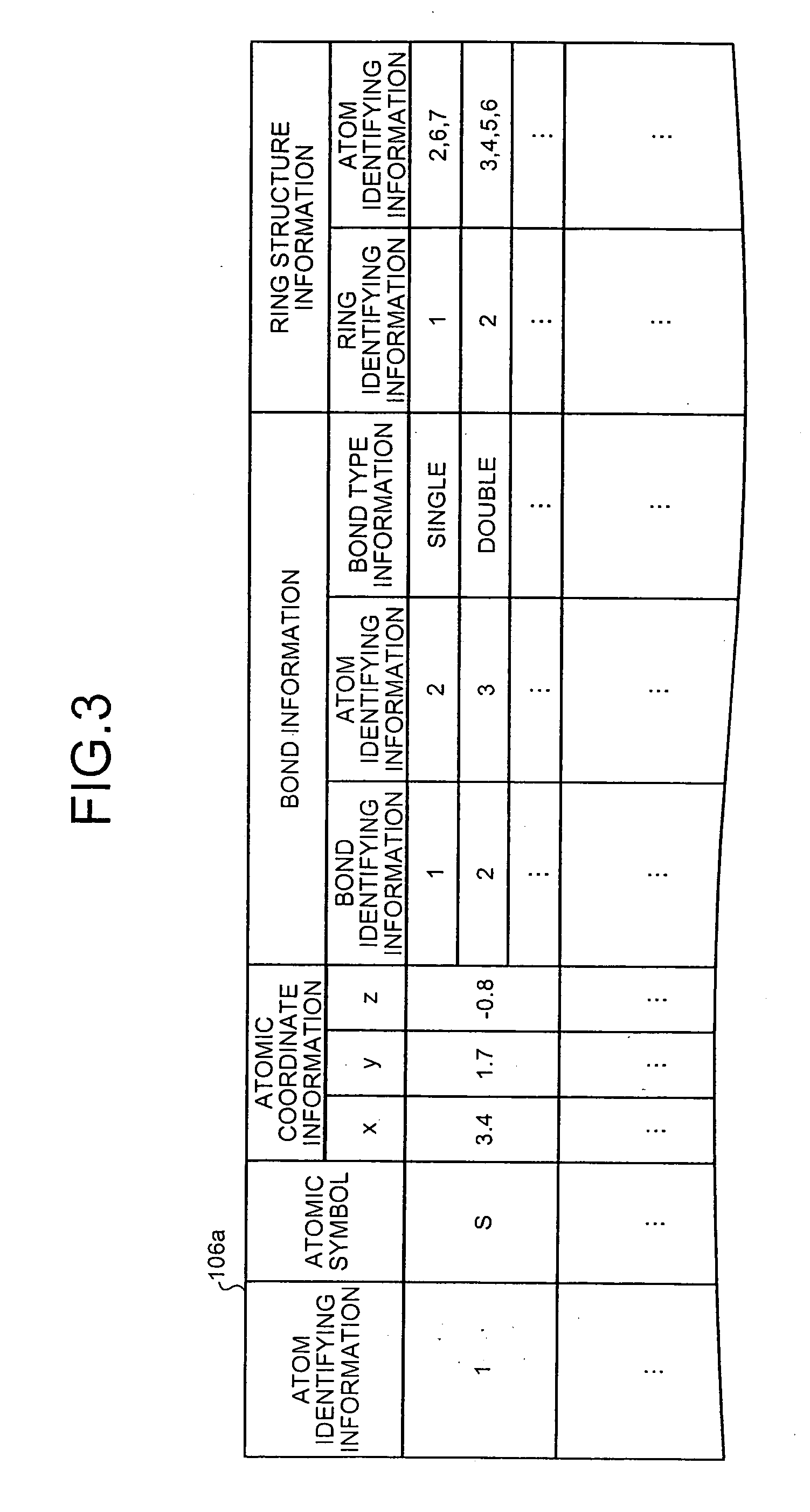
Drug-metabolizing enzyme prediction apparatus
A drug-metabolizing enzyme prediction apparatus acquires compound structure information, identifies binding site information and molecular species information based on a binding-site identifying condition, specifies a pinching point that is an atom binding at least between a reactive site and a binding site, acquires pinching point information on the specified pinching point and reactive site information on the reactive site bound to the pinching point, and identifies the acquired reactive site information based on a reactive-site identifying condition and the acquired pinching point information based on a pinching-point identifying condition.
Owner:FUJITSU LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com