Detection kit and detection method for medication of hypertension patients
A detection kit and detection method technology, applied in the field of genetic detection, can solve the problems of patients who cannot provide medication guidance, low specificity, and no obvious advantages, so as to achieve accurate drug guidance, improve accuracy and specificity Reasonable effect of gender and locus coverage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The amplification primer probe mainly contains 15 loci of 14 genes. The synthesis of the primers is to design the amplification primers according to the regions of the corresponding sites of the corresponding genes, and the sequences of the primers and the probes are as described in the summary of the present invention. The probe is a TaqMan probe, the fluorescent reporter group of the wild type is FAM, and the fluorescent reporter group of the mutant type is VIC.
Embodiment 2
[0076] The kit includes: detection reaction solution, wild-type quality control, homozygous mutant quality control, heterozygous mutant quality control and blank quality control. Detection reaction solution includes: 10×Buffer, 10×Solution, dNTP, MgSO 4 , primer mix, probe mix, KCl, Taq enzyme, sterilized purified water. Wild-type quality control: wild-type plasmid, homozygous mutant quality control: mutant plasmid, heterozygous mutant quality control: wild-type and mutant plasmid mixture, blank quality control: sterile purified water.
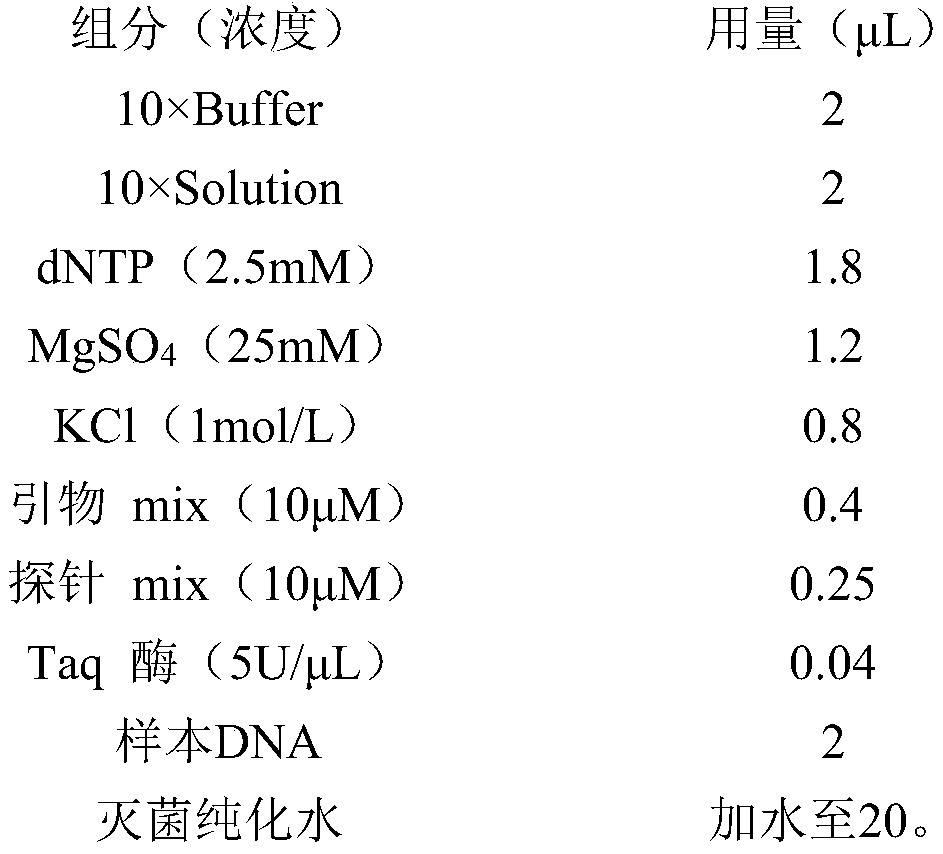
[0077] Specifically in a PCR amplification embodiment, the system used is as follows:
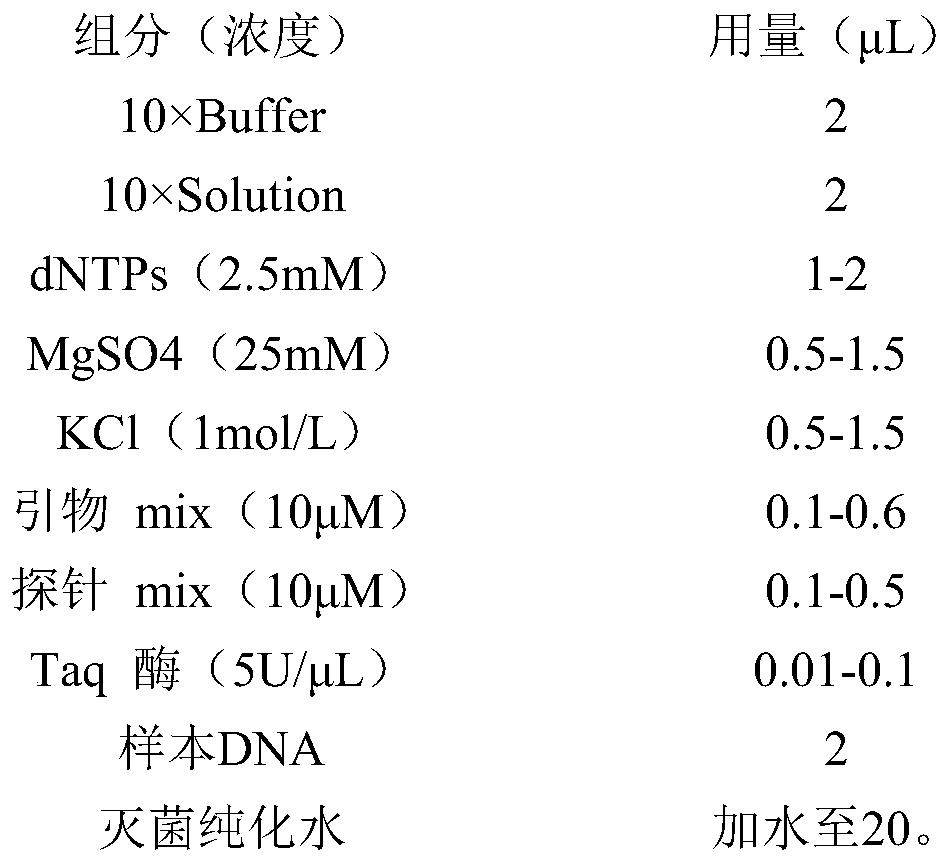
[0078] Component (concentration) Dosage (μL) 10×Buffer 2 10×Solution 2 dNTPs (2.5mM) 1-2 MgSO4(25mM) 0.5-1.5 KCl(1mol / L) 0.5-1.5 Primer mix(10μM) 0.1-0.6 Probe mix(10μM) 0.1-0.5 Taq enzyme (5U / μL) 0.01-0.1 sample DNA 2 Sterilized purified water Add water to 20
[0079] The primer...
Embodiment 3
[0087] The genotype detection of human samples using the above-mentioned kit is as follows:
[0088] 1) Take human peripheral anticoagulated blood, use a commercial DNA extraction kit to process the sample, and extract DNA. The extracted DNA should meet OD260 / OD280=1.8-2.0, concentration>0.5ng / μL;
[0089] 2) Dispense the detection reaction solution into 18 μL / well of the PCR reaction well, and add the DNA of the sample to be tested, the wild-type quality control, the homozygous mutant quality control, the heterozygous mutant quality control and the blank quality control 2 μL each;
[0090] 3) Run according to the following PCR amplification conditions;
[0091]
[0092] 4) Interpretation of results
[0093] Internal standard gene GAPDH: ROX channel Ct ≤ 33, the quality of nucleic acid is qualified, and the results can be analyzed.
[0094] Wild type: FAM channel Ct≤35, corresponding VIC channel Ct>35, the detection result is wild type;
[0095] Homozygous mutant: FAM ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com