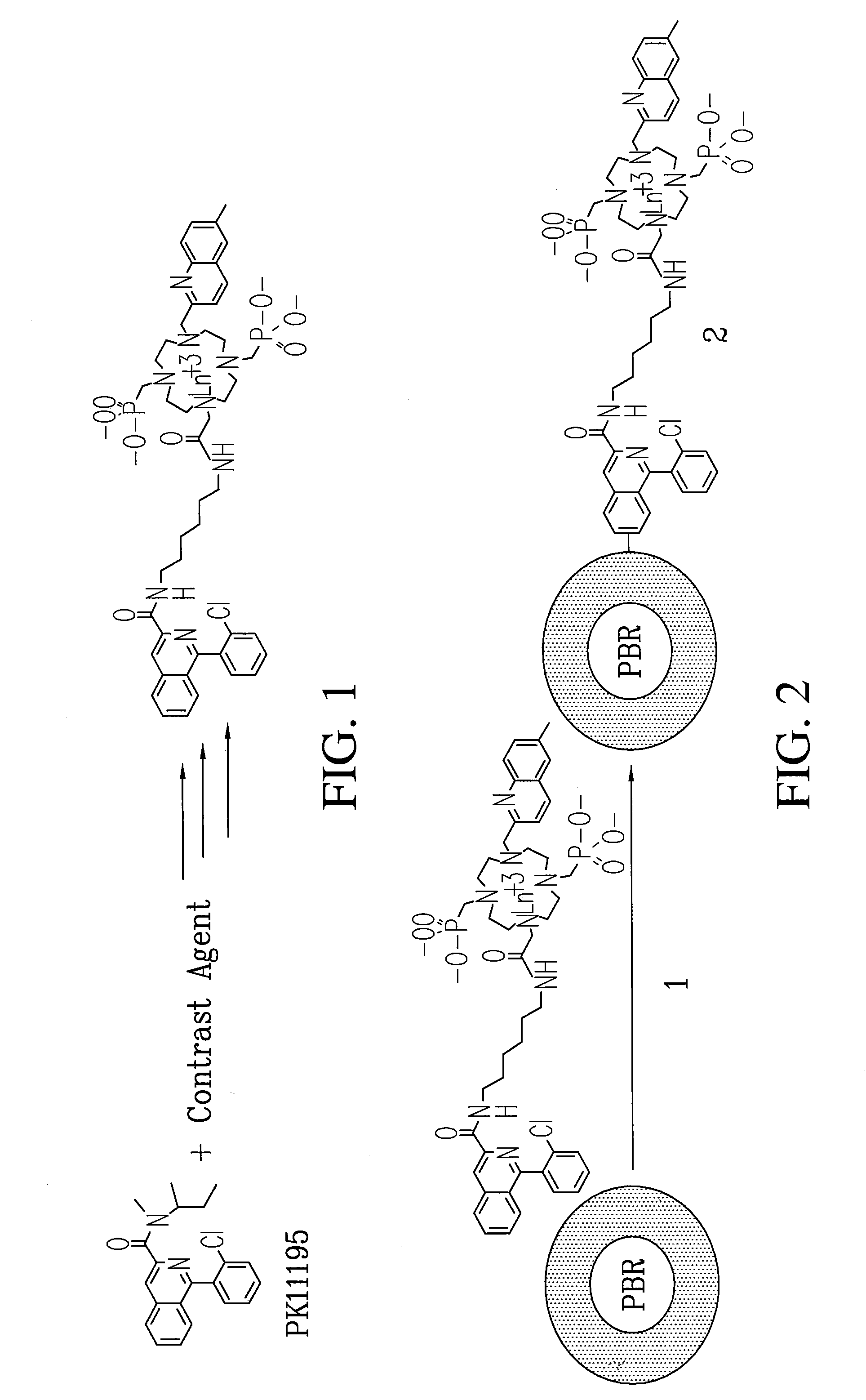
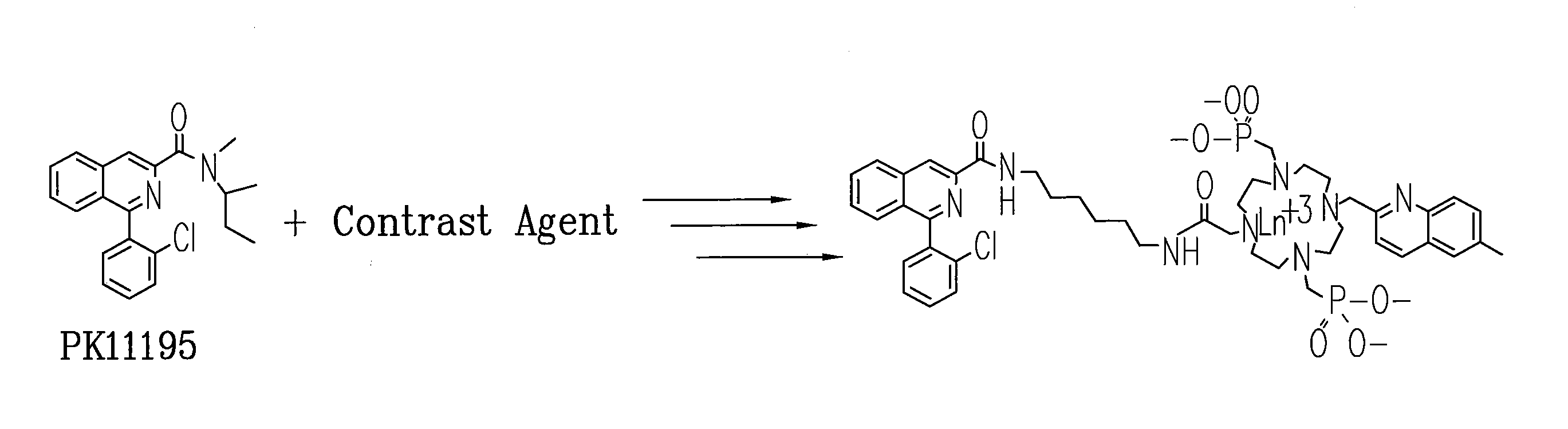
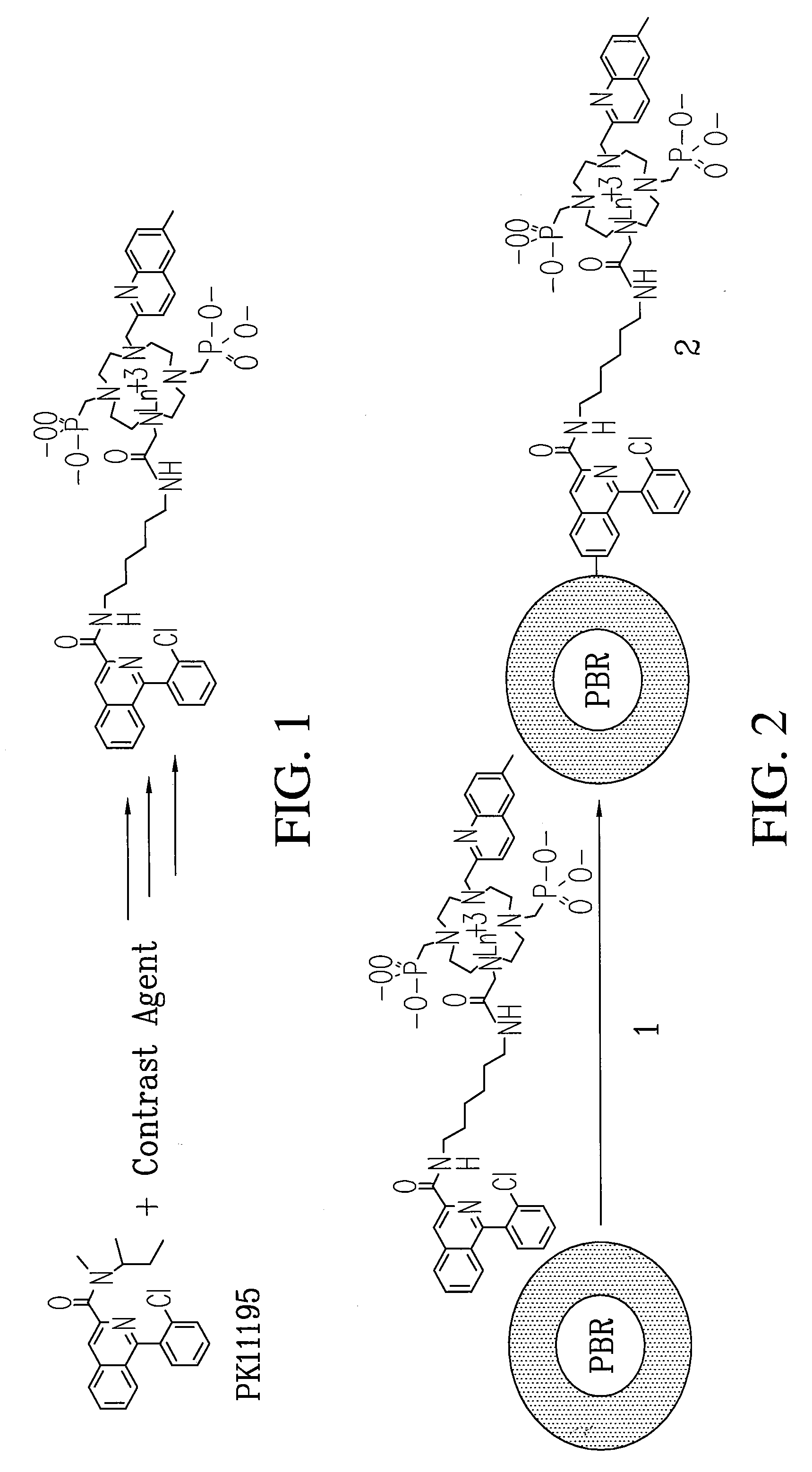
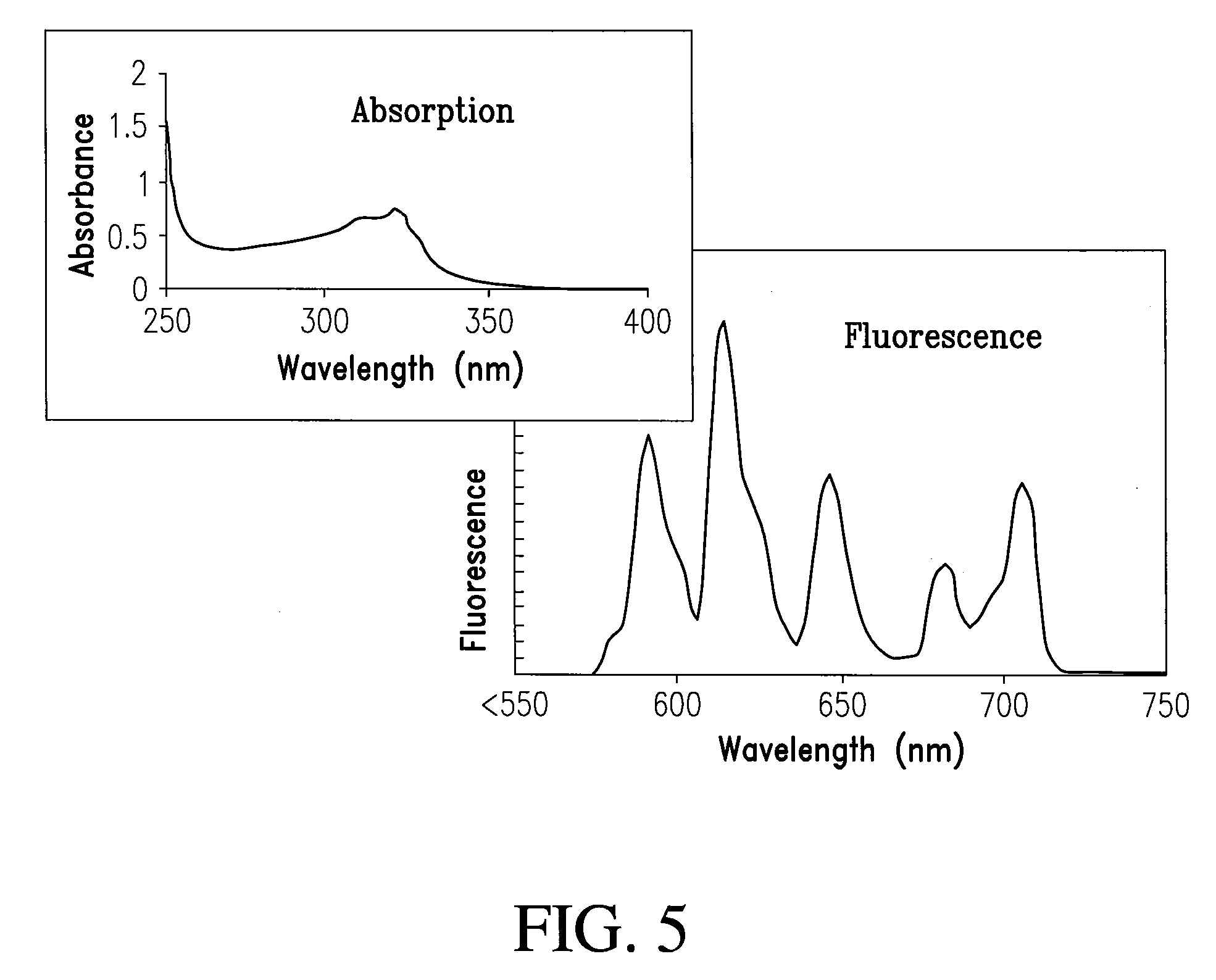
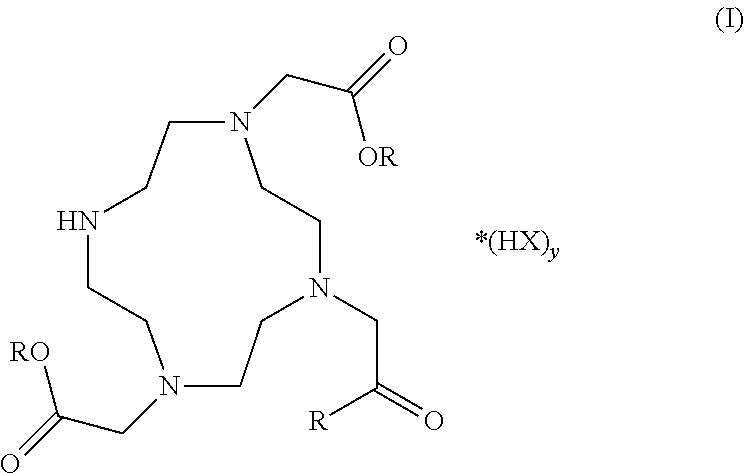
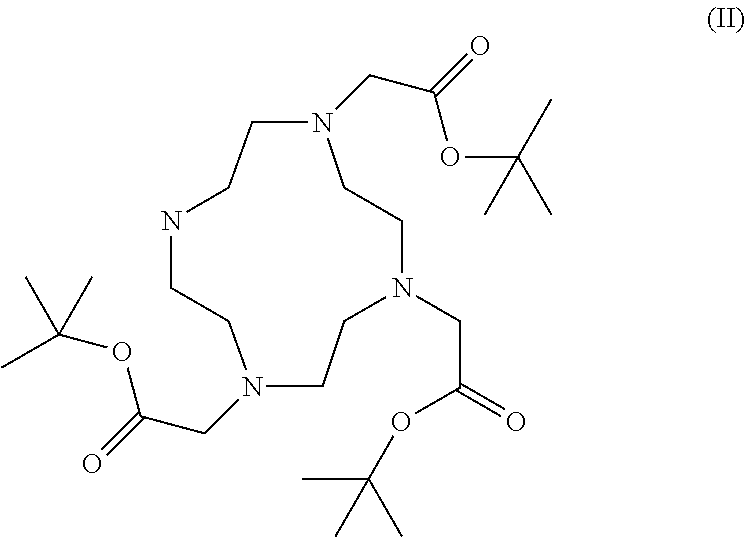
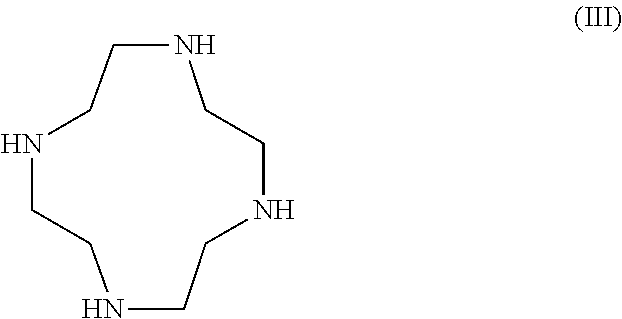
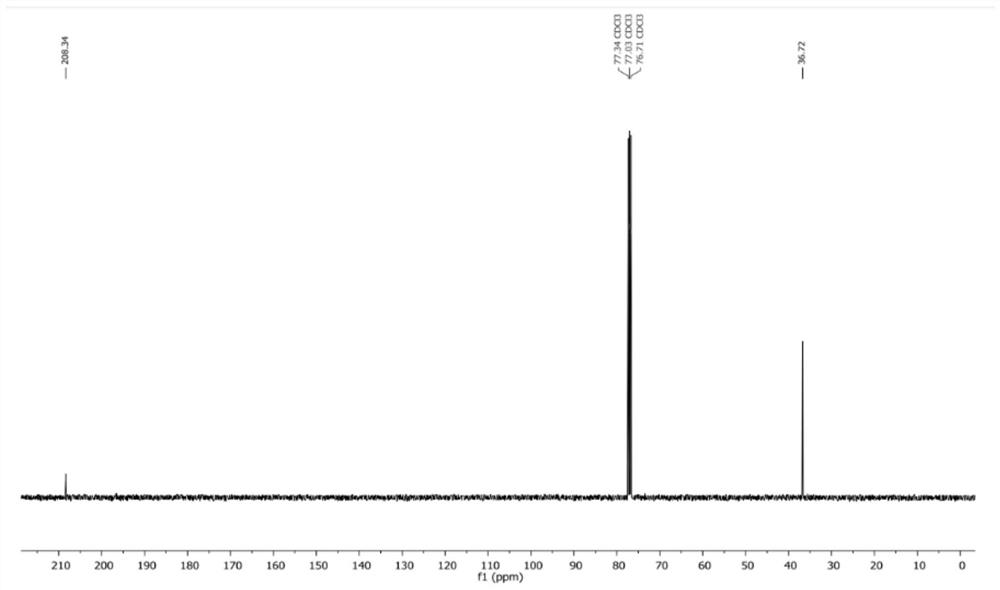
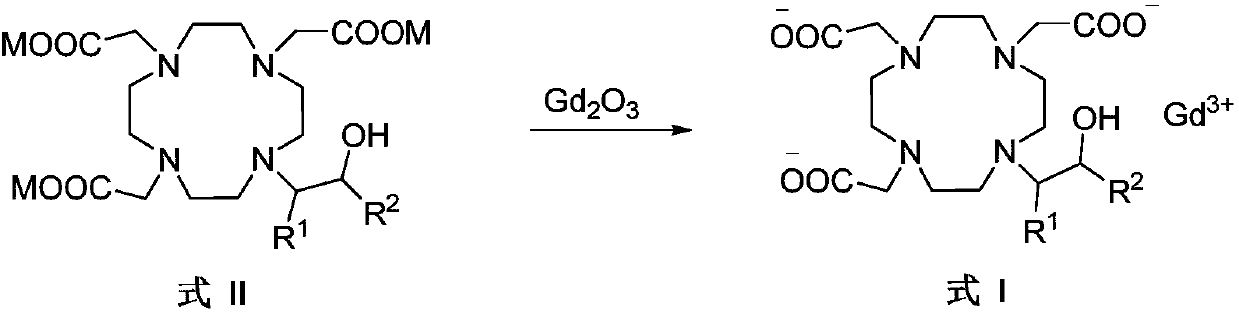Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Cyclen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
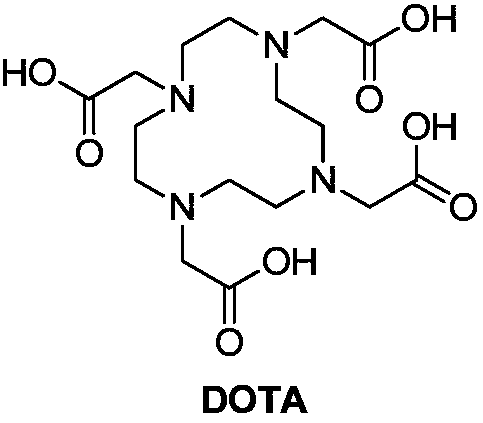
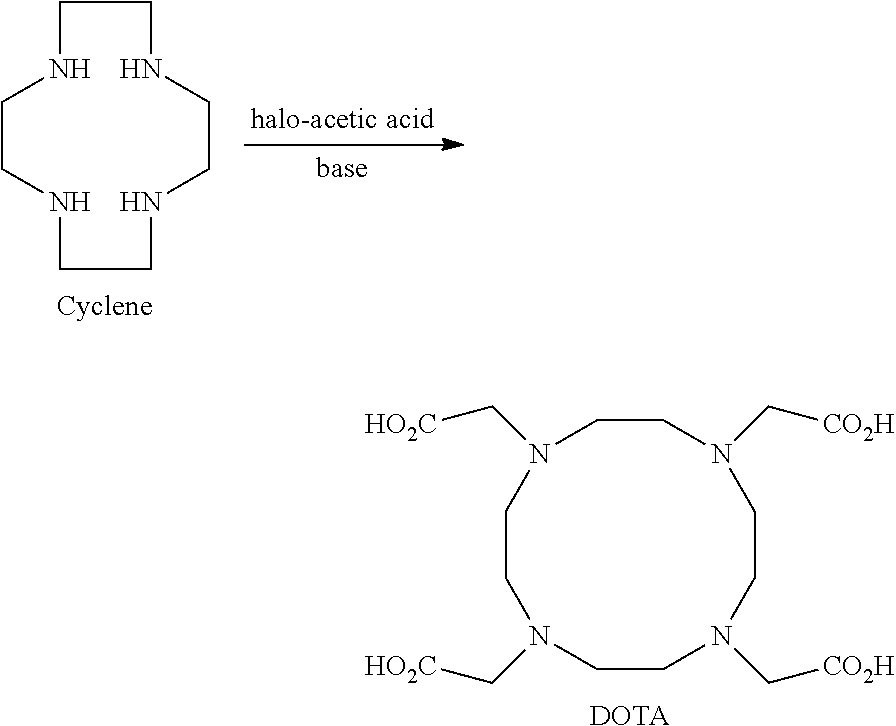
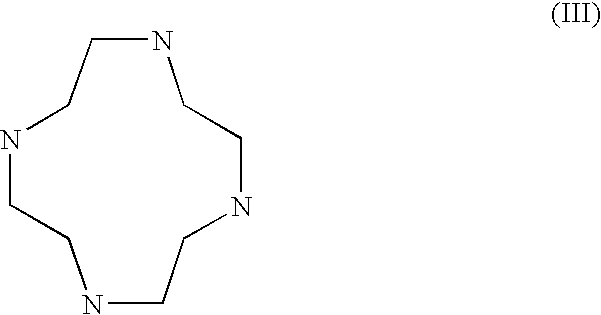
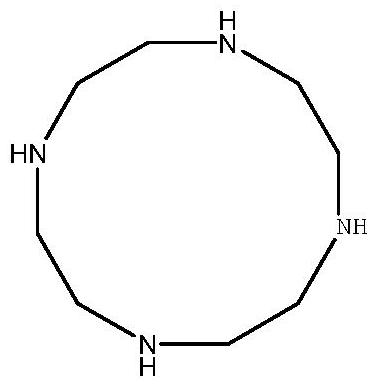
Cyclen (1,4,7,10-tetraazacyclododecane) is a macrocycle and the aza analog of the crown ether 12-crown-4. Derivatives of cyclen are larger cyclic polyamines but the repeating unit (ethyleneimine, –CH₂CH₂NH–) is always the same. Like crown ethers, cyclen compounds are capable of selectively binding cations. They are used as a ligand in chemistry for instance with chemicals used in MRI contrast agents.
Multi-use multimodal imaging chelates
InactiveUS7338651B2Improve discriminationEasy to detectUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsCancer cellFluorescence
Cyclen-based chelates can be used as contrast agents for multi-modal imaging of tissue cells. The cyclen-based chelates are preferably polyazamacrocyclic molecules formed from 1,4,7,10 tetraazacyclododecane (“cyclen”) having varying chelating ions, phosphoester chains, and light harvesting moieties. By changing the chelating ion, phosphoester chain length and / or the light harvesting moiety different imaging techniques, such as MRI, CT, fluorescence and absorption, x-ray and NIR, may be employed to image the tissue cells. Additionally, the cyclen-based chelates may be conjugated to provide for site-specific delivery of the cyclen-based chelate to the desired tissue cells. The cyclen-based chelates may also be delivered to the tissue cells by attaching the cyclen-based chelates to a polymeric delivery vehicle. Although these cyclen-based chelates have a wide variety of application, the preferred use is for imaging of cancer cells, such as brain cancer, for improving resection of a cancerous tissue.
Owner:TEXAS TECH UNIV SYST +1
Multi-use multimodal imaging chelates
InactiveUS20080241873A1Improve discriminationEasy to detectOrganic active ingredientsIn-vivo radioactive preparationsCancer cellFluorescence
Cyclen-based chelates can be used as contrast agents for multi-modal imaging of tissue cells. The cyclen-based chelates are preferably polyazamacrocyclic molecules formed from 1,4,7,10 tetraazacyclododecane (“cyclen”) having varying chelating ions, phosphoester chains, and light harvesting moieties. By changing the chelating ion, phosphoester chain length and / or the light harvesting moiety different imaging techniques, such as MRI, CT, fluorescence and absorption, x-ray and NIR, may be employed to image the tissue cells. Additionally, the cyclen-based chelates may be conjugated to provide for site-specific delivery of the cyclen-based chelate to the desired tissue cells. The cyclen-based chelates may also be delivered to the tissue cells by attaching the cyclen-based to a polymeric delivery vehicle. Although these cyclen-based chelates have a wide variety of application, the preferred use is for imaging of cancer cells, such as brain cancer, for improving resection of a cancerous tissue.
Owner:BORNHOP DARRYL J +2
Multi-use multimodal imaging chelates
InactiveUS20080241074A1Improve discriminationEasy to detectUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsCancer cellDelivery vehicle
Cyclen-based chelates can be used as contrast agents for multi-modal imaging of tissue cells. The cyclen-based chelates are preferably polyazamacrocyclic molecules formed from 1,4,7,10 tetraazacyclododecane (“cyclen”) having varying chelating ions, phosphoester chains, and light harvesting moieties. By changing the chelating ion, phosphoester chain length and / or the light harvesting moiety different imaging techniques, such as MRI, CT, fluorescence and absorption, x-ray and NIR, may be employed to image the tissue cells. Additionally, the cyclen-based chelates may be conjugated to provide for site-specific delivery of the cyclen-based chelate to the desired tissue cells. The cyclen-based chelates may also be delivered to the tissue cells by attaching the cyclen-based to a polymeric delivery vehicle. Although these cyclen-based chelates have a wide variety of application, the preferred use is for imaging of cancer cells, such as brain cancer, for improving resection of a cancerous tissue.
Owner:TEXAS TECH UNIV SYST +1
Preparation method of macrocyclic chelating agent and intermediate thereof
ActiveCN110835326AHigh yieldThe reaction route of the production process is shortOrganic chemistryBulk chemical productionBiochemical engineeringIon exchange
The invention relates to a preparation method of a macrocyclic chelating agent and an intermediate thereof, particularly to a preparation method of gadoteridol, gadobutrol and intermediates of gadoteridol and gadobutrol thereof. According to the method of the invention, high-purity t-Bu-DO3A can be simply and conveniently obtained from cyclen in one step, and gadoteridol or gadobutrol is obtainedthrough subsequent related reaction and purification; and the high purity of the final product is achieved by controlling the each link of the process so as to achieve the medicinal standard, the purification step of ion exchange chromatography is not needed, the operation is simple and convenient, the preparation is easy, the yield is high, and the method is particularly suitable for the requirements of industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Bifunctional chelators for sequestering lanthanides
ActiveUS20080107606A1Minimizes streakingSynthetic is simpleOrganic chemistryGeneral/multifunctional contrast agentsLanthanideTetra
The present invention relates to a method for preparing a bifunctional chelator for lanthanide. The method comprises the steps of providing a starting material which has an amino and carboxyl group; protecting the amino with an amino protecting group and the carboxyl with a carboxyl protecting group to produce a protected compound; reacting the protected compound with cyclen to generate a monoalkylated cyclen; reacting the monoalkylated cyclone with an activated compound to generated tetra-alkylated cyclone; removing the amino protecting group with a first protecting group removal reagent; and removing the carboxyl protecting groups with a second protecting group removal reagent to yield a bifunctional chelator having three more carboxyl groups and one or more amino groups.
Owner:SAN DIEGO STATE UNIVERSITY +1
Preparation method of 1,4,7,10-tetraazacyclododecane-1,4,7-10-tetraacetic acid
ActiveCN108264491AReaching the isoelectric pointAvoid strong acid resistance requirementsOrganic chemistryAlkyl transferAcetic acid
The invention discloses a preparation method of 1,4,7,10-tetraazacyclododecane-1,4,7-10-tetraacetic acid (DOTA). The preparation method includes the steps of in water phase, subjecting 1,4,7,10-tetraazacyclododecane (cyclen) and XCH2COOR to alkylation reaction under the action of an acid-binding agent; adjusting pH to isolate crude DOTA; recrystallizing. The preparation method is suitable for large-scale industrial production of DOTA; the whole process has no need for purification by ion exchange resin and low-temperature freezing; the product yield s high; the product purity is 99.0% and above; single impurity content is < / =0.05%; residue on ignition is <0.10%; the preparation method meets the quality standard for bulk pharmaceutical chemicals.
Owner:SHANGHAI VIWIT PHARMA CO LTD +1
Process for producing 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid and complexes thereof
ActiveUS20160024030A1High yieldQuality improvementOrganic chemistryIn-vivo testing preparationsAcetic acidDodecane
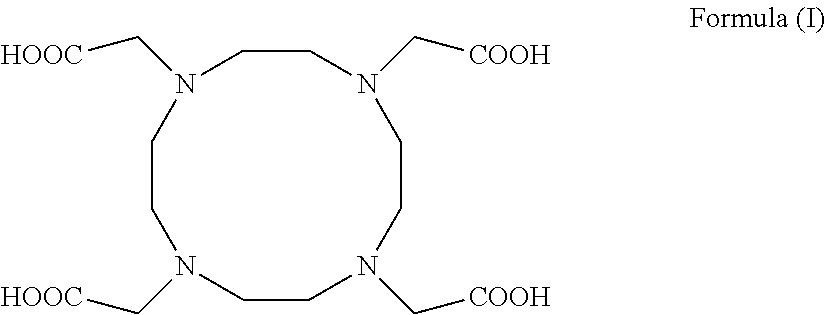
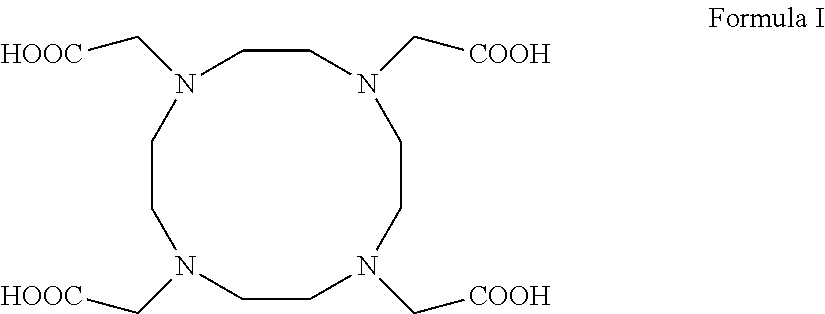
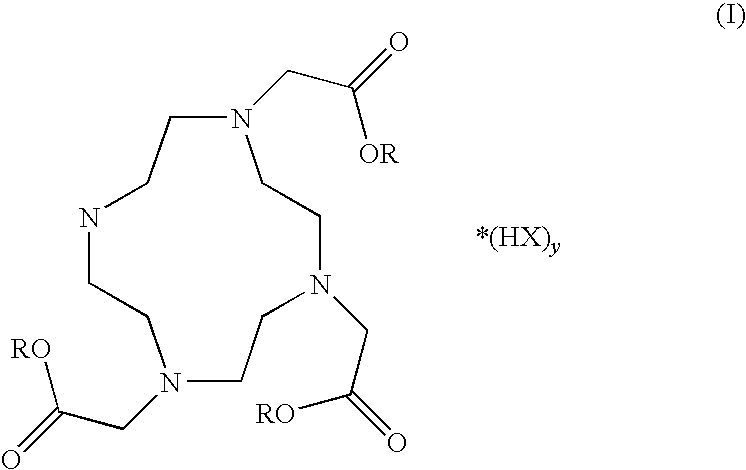
A process for producing 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) including salts and hydrates thereof of general formula (I) from the respective cyclen.The process involves the use of cationic- and anionic exchange resins and solvent treatments to remove the organic and inorganic contaminants. Any cations present in the raw DOTA or other contaminants resulting from the reaction of cyclen are largely reduced in early stages of the process allowing to obtain good yields of DOTA in a purified grade and in an easier and reliable way. The process is useful for the production of DOTA, of macrocyclic compounds including metal ions complexes thereof and of compositions including the macrocyclic compounds that can be used as contrast agents for magnetic resonance imaging.
Owner:T2PHARMA GMBH
Process for producing a complex of a lanthanide with a macrocyclic ligand
InactiveCN105073144ABook and dependent claims are obviousEmulsion deliveryIn-vivo testing preparationsCyclamGadolinium
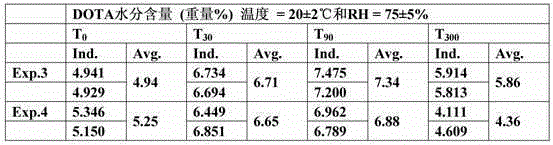
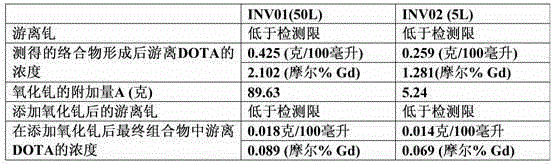
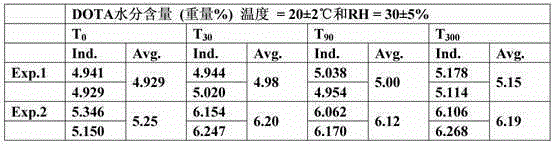
The present invention relates to a process for preparing a macrocyclic ligand and for producing a pharmaceutical liquid formulation comprising a complex of said ligand with a lanthanide or similar compounds. The macrocyclic ligand of the present invention is a derivative of tetraaza macrocycles such as 1,4,7,10-tetraazacyclododecane (cyclen), 1,4,7,10-tetrazacyclotridecan (homocyclen) and 1,4,8,11-tetraazacyclotetradecane (cyclam), such as DOTA, TRITA, TETA, DOTMA, TCE-DOTA, DOTA-pNB, D03A, HP-D03A, D03A-butrol, D03MA, ODOTRA, D03A-L2, DOTP, DOTMP, DO2a, THP, THED, DOTAM, DOTTA. The preferred lanthanide and similar compounds are Gadolinium (Gd), Yttrium (Y) and Terbium (Tb). The process of the present invention aims to obtain an accurate balance between the ligand and the lanthanide, and to avoid the presence of free lanthanide in said formulation, by calculating the necessary amount of ligand for a formulation batch, measuring the moisture content of a sample of the material in said batch, calculating the total amount of moisture present of the batch and calculating the total amount of material which is required to prepare the batch size. In this way, the production of a pharmaceutical liquid formulation comprising a complex of a macrocyclic ligand with lanthanide is more accurate, faster and easier. The present invention is thus useful for the production of pharmaceutical liquid formulations comprising a complex of a macrocyclic ligand with a lanthanide, which can be used as contrast agents for magnetic resonance imaging.
Owner:T2PHARMA GMBH
Chiral cyclen compounds and their uses
PendingCN109963839AOrganic active ingredientsIn-vivo radioactive preparationsMRI contrast agentSquare antiprism
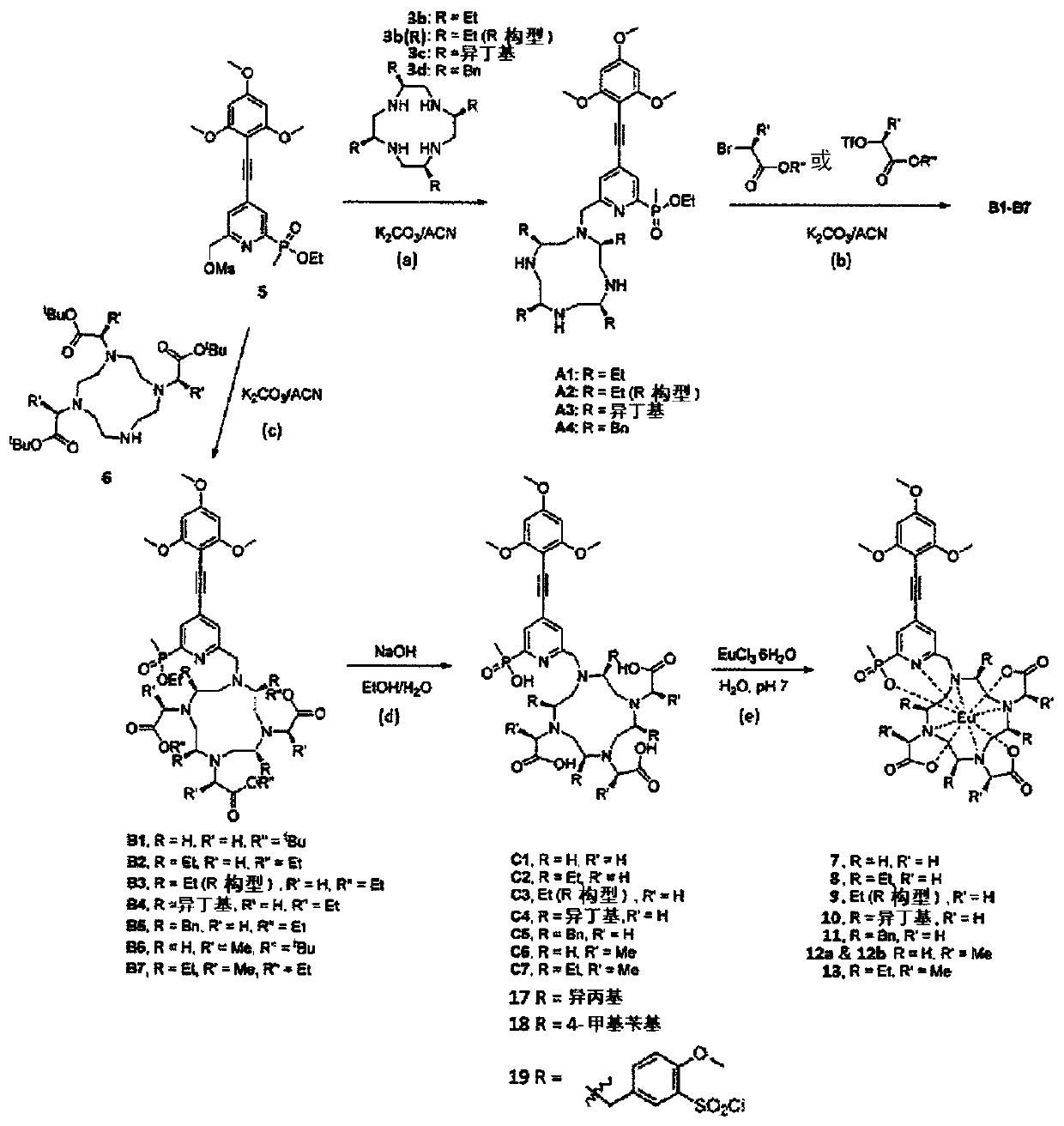
The present invention relates to the preparation of a series of chiral DOTA, D03A, D02A, DO1A, cyclen and their metal complexes, which display properties superior to those of previous DOTA- based compounds, and hence are potentially valuable as a platform for diagnostic applications. The chiral DOTAs reveal a high abundance of twisted square antiprism (TSA) geometry favoring them to be used as potential MRI contrast agents, whereas their rapid labelling properties at mild conditions make them excellent candidates for use as radiometal chelators.
Owner:THE HONG KONG POLYTECHNIC UNIV
Novel chelate resin containing tetraazacyclo and preparation method of chelate resin
InactiveCN104307495AStable structureThe fluctuation of adsorption amount is smallOther chemical processesWater contaminantsPolymer scienceMicrosphere
The invention discloses a novel chelate resin containing tetraazacyclo and a preparation method of chelate resin, and belongs to the technical field of resin synthesis. The method comprises the following steps: modifying chloromethylate crosslinked polystyrene microsphere (PsCl) by using 1,4,7,10-tetraazacyclodocecane (Cyclen) so as to prepare a novel aza-crown ether resin Ps-Cy, wherein the highest full exchange capacity can achieve 5.3mmol / g. The adsorption of the Ps-Cy to Cu(II) indicates that the maximum adsorption quantity achieves 158.9mg / g. The crosslinked polystyrene microsphere is used as a substrate and the cyclic polyamine compound Cyclen is used as functional group, the substrate is connected with the functional group by using a covalent bond through methylene, the resin structure is stable, the adsorption capacity to metal ion is strong, the adsorption quantity fluctuation is small, and the resin can be repeatedly used.
Owner:CHANGZHOU UNIV
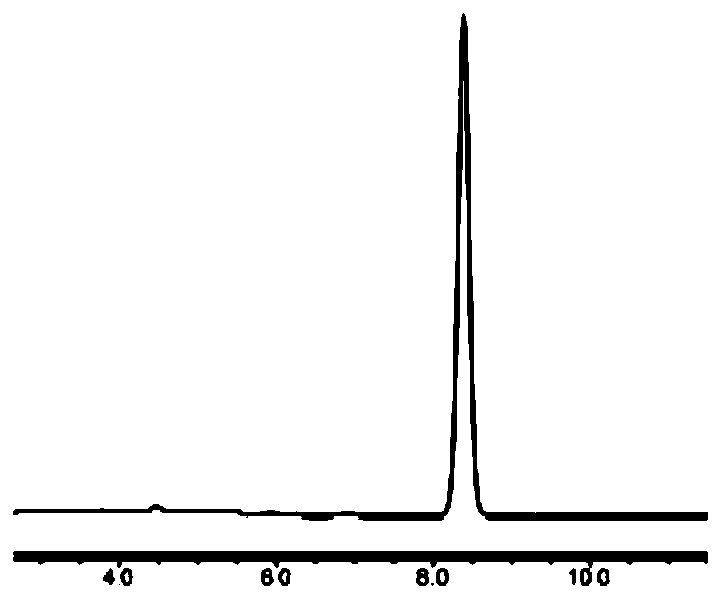
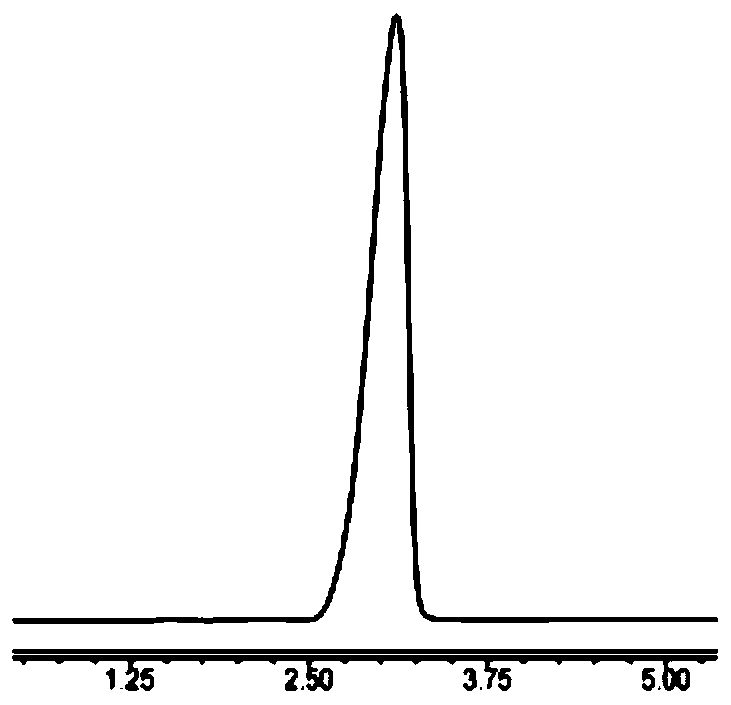
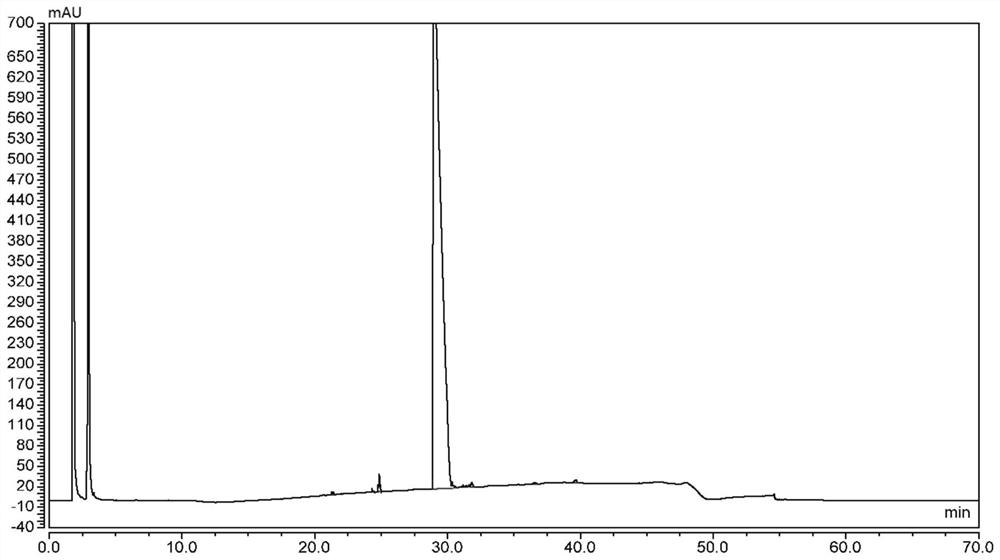
Method for detecting contents of 1, 4, 7, 10-tetra-azacyclo dodecane (Cyclen) and derivatives thereof by using HPLC-ELSD
The invention discloses a method for detecting contents of 1, 4, 7, 10-tetra-azacyclo dodecane (Cyclen) and derivatives thereof by using HPLC-ELSD. The invention comprises the following steps: 1) arranging HPLC-ELSD conditions for detection: an HPLC condition: the ratio of flowing phase acetonitrile to water being 30-50: 50-70, and performing isocratic elution; an ELSD condition: the temperature of a drift tube being 75-105 DEG C, the pressure of N2 being 0.4-0.6 M pa, the flow rate of N2 being 1.6-2.2 L / min, the gain being 2-4, and carrying out a non-shunt mode; 2) drawing a standard curve; and 3) measuring the content of samples of the Cyclen and the derivatives thereof. The method for detecting contents of the Cyclen and the derivatives thereof by using HPLC-ELSD is good in specificity, repeatability, degree of precision and recovery rate.
Owner:荆门医药工业技术研究院 +2
Macrocyclic polyamine-based polyacetylene fluorescent sensor and preparation method thereof
InactiveCN103396504APromote aggregationEfficient removalFluorescence/phosphorescenceFluorescenceTungsten hexachloride
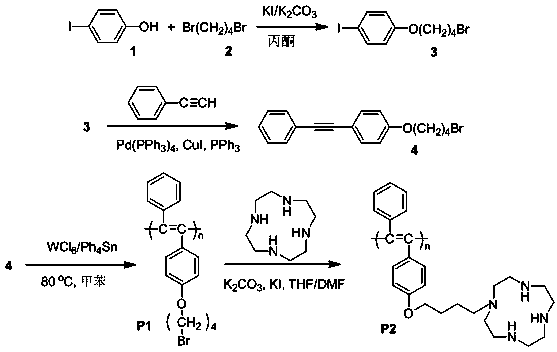
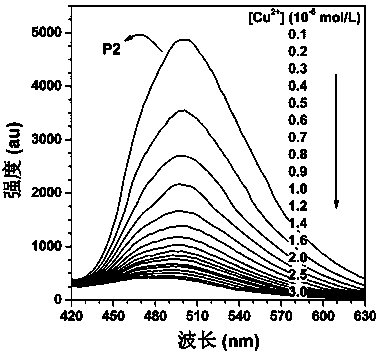
The invention discloses a macrocyclic polyamine-based polyacetylene fluorescent sensor and a preparation method thereof. The preparation method comprises three steps of preparation of polyacetylene monomer, preparation of polyacetylene macromolecule and post functionalization of the macromolecule. A Sonogashira reaction is carried out on phenylacetylene and 1-(4-bromine butoxy)-4-iodobenzene so as to obtain 1-(4-bromine butoxy)-4-(phenyl acetenyl) benzene, an addition reaction is carried out on the 1-(4-bromine butoxy)-4-(phenyl acetenyl) benzene under the catalytic actions of tungsten hexachloride and tetraphenyltin so as to obtain a polyacetylene macromolecule P1 free of post functionalization, and a substitution reaction is carried out on the polyacetylene macromolecule P1 and the macrocyclic polyamine cyclen so as to obtain a post-functionalized polyacetylene macromolecule P2, and the polyacetylene macromolecule P2 is the polyacetylene fluorescent sensor. A fluorescence titration result shows that the disubstituted polyacetylene macromolecule P2 can recognize Cu<2+> with high selectivity and high sensitivity, and also can detect S<2-> indirectly. The invention provides a simple method for synthesizing the disubstituted polyacetylene fluorescent sensor.
Owner:WUHAN UNIV
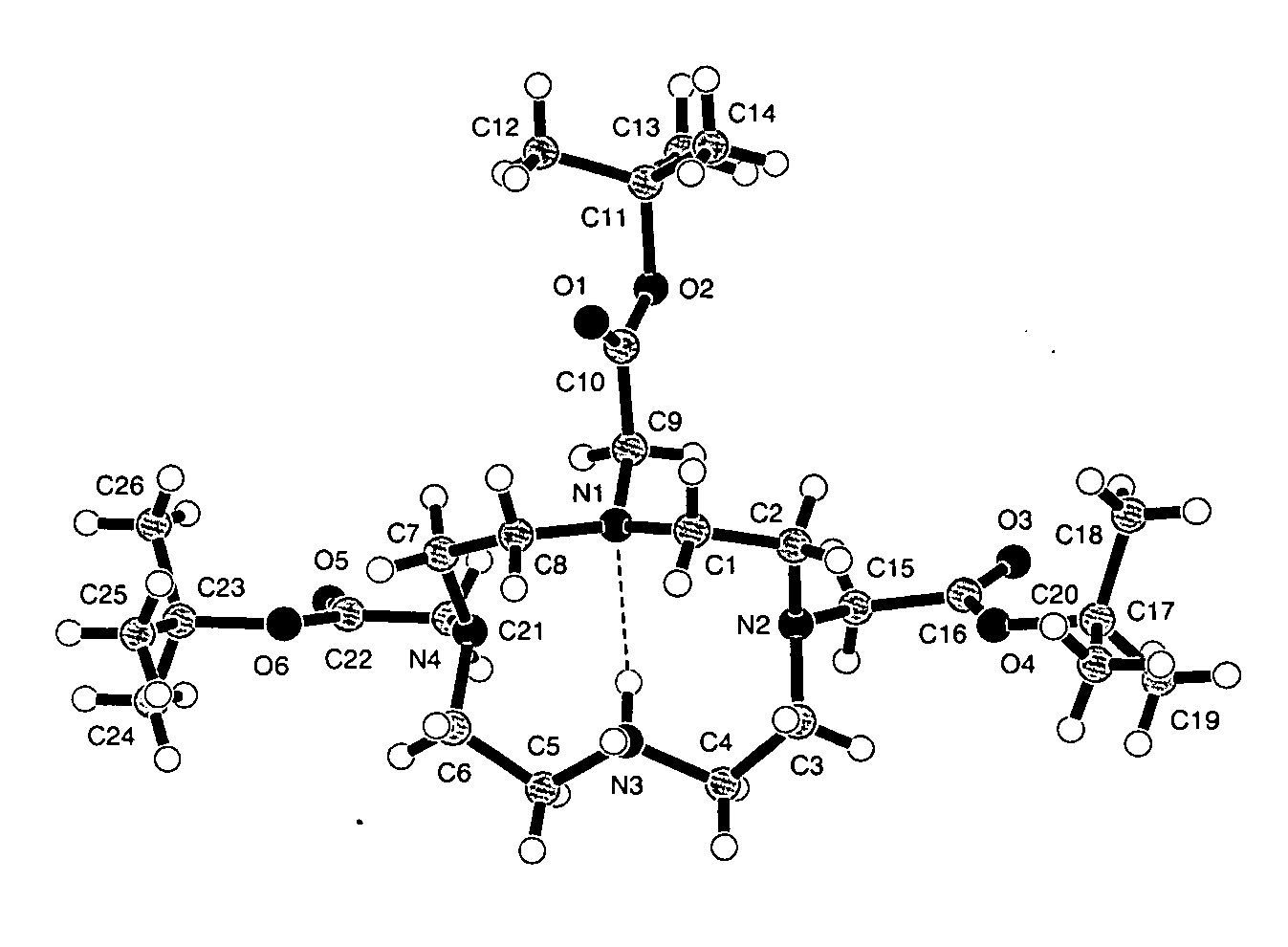
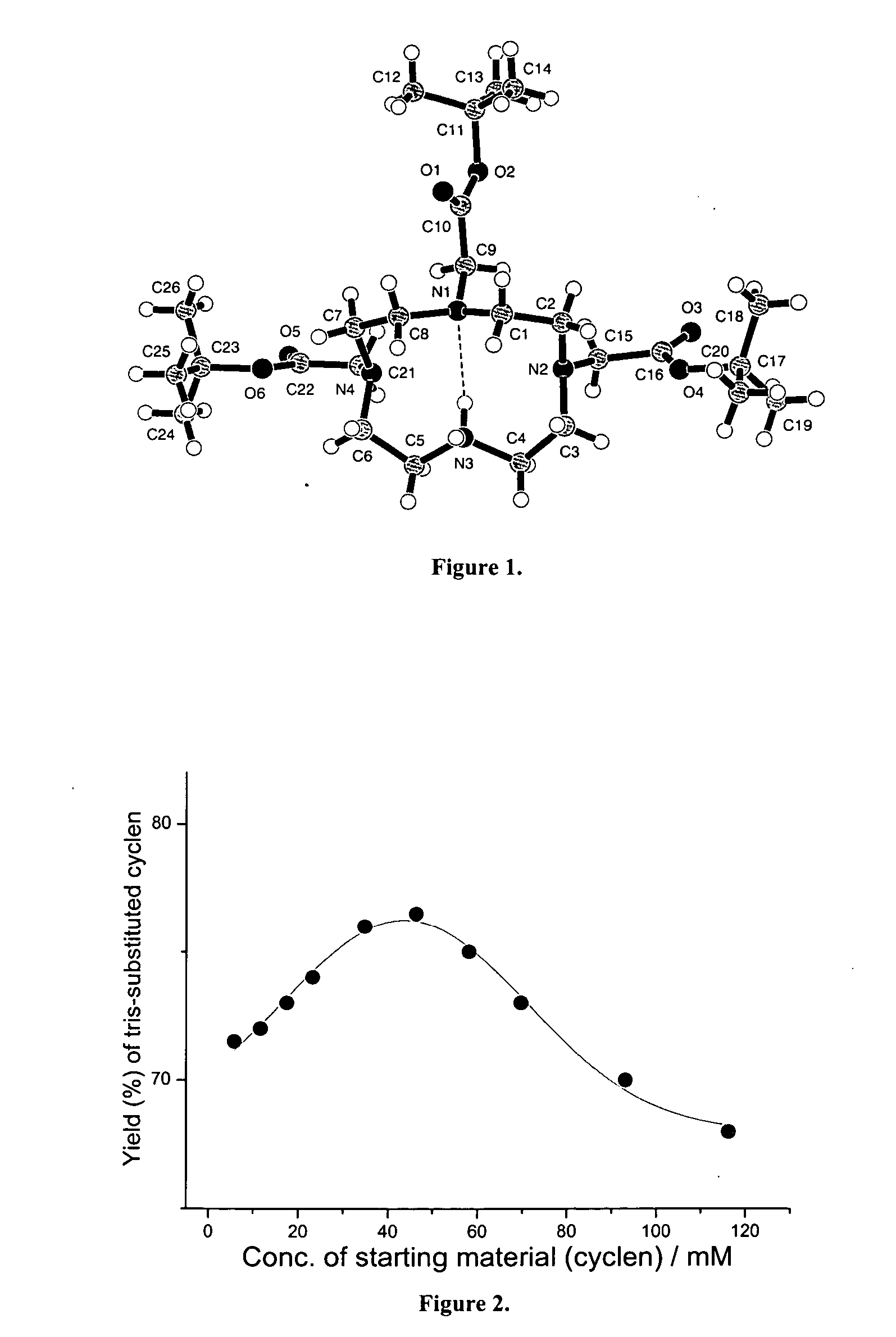
Synthesis of tris N-alkylated 1,4,7,10-tetraazacyclododecanes
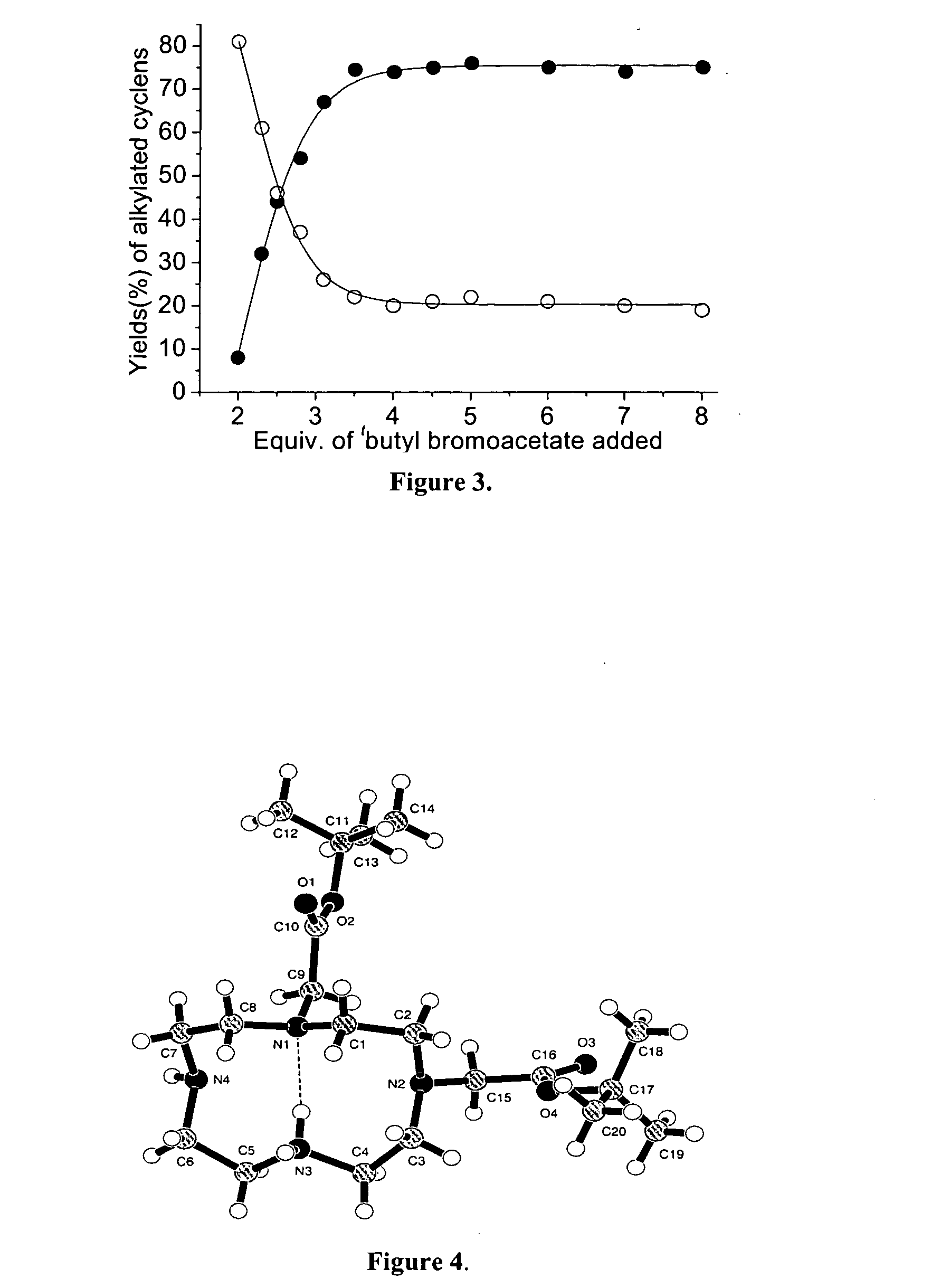
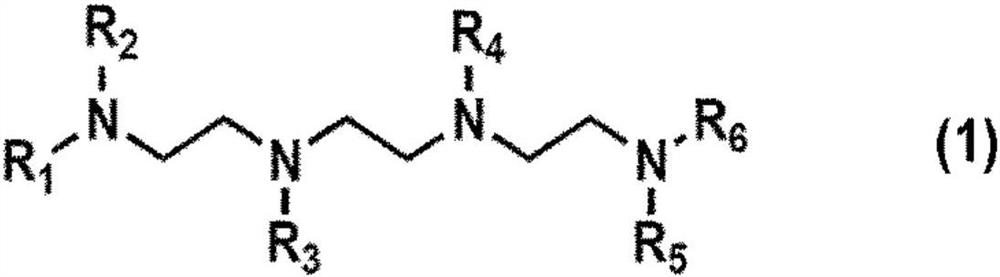
InactiveUS20050033106A1Good choiceHigh yieldOrganic chemistryMicrowave therapyDodecaneTert-Butyloxycarbonyl protecting group
A directly synthetic method for preparing tris-alkylated 1,4,7,10-tetraazacyclododecanes by the reactions of 1,4,7,10-tetraazacyclododecane (cyclen) and appropriate electrophiles is accomplished in high yield. The method provides operational convenience, starting material availability, cost economy, atom efficiency and reaction insensitivity to temperature, moisture, and concentrations of starting materials. With this method, the yield of tris-(tert-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane can be 77%, the highest reported. The yield of other tris-N alkylated products can be in the range of 65-84%.
Owner:THE UNIVERSITY OF HONG KONG
Compound for restoring contaminated soil or contaminated water
ActiveCN112262002AGood removal effectNo toxicityWater contaminantsNon-surface-active detergent compositionsCyclamEnvironmental engineering
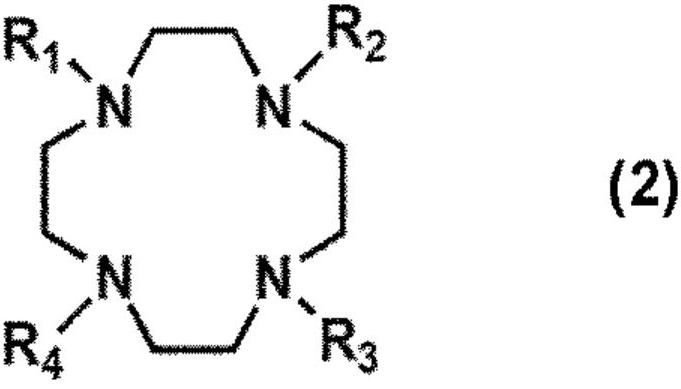
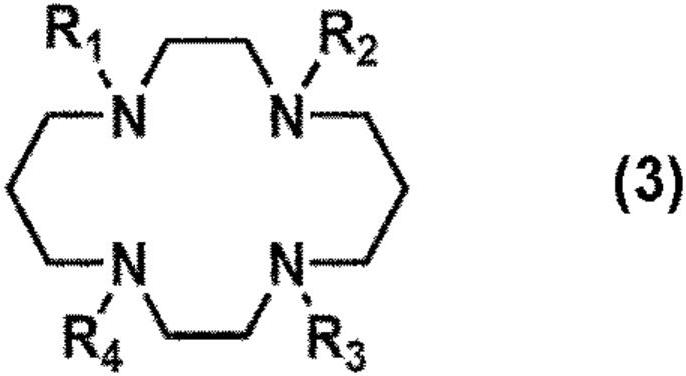
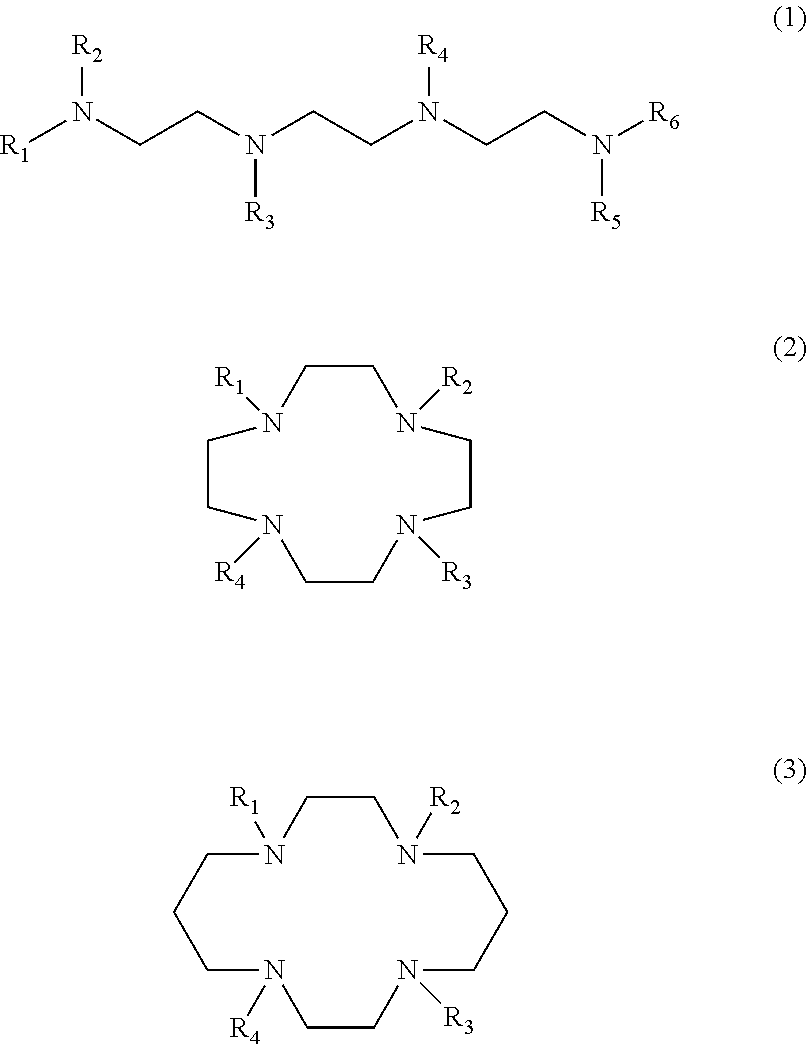
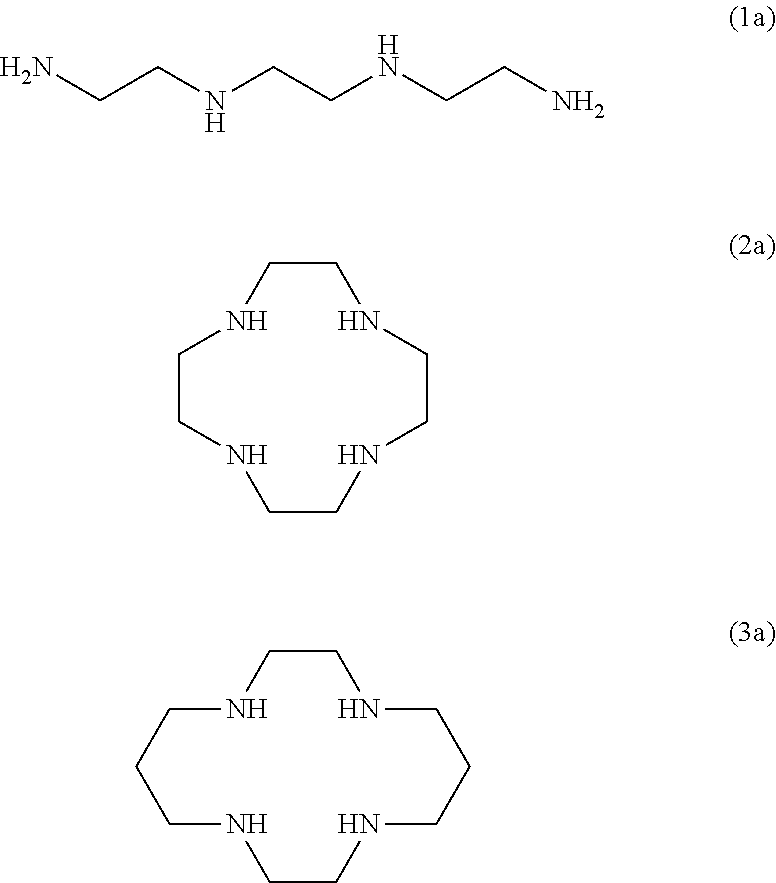
The present invention provides a compound for restoring contaminated soil or contaminated water for the removal of heavy metals and formaldehyde wherein the compound includes at least one selected from a trientine or a trientine derivative represented by following formula 1, a cyclen or a cyclen derivative represented by following formula 2, and a cyclam or cyclam derivative represented by following formula 3 as an active ingredient.
Owner:FNG RES
Synthesis of cyclen derivatives
Owner:GE HEALTHCARE AS
Preparation method of gadobutrol and intermediate thereof
PendingCN114573521AReduce generationReduce contentOrganic chemistryBulk chemical productionCompound aCycleanine
The invention discloses a preparation method of gadobutrol and an intermediate thereof. The invention provides a preparation method of a compound B or a salt thereof, which comprises the following step: in the presence of acetate and N, N-dimethylformamide, carrying out substitution reaction on cycleanine and a compound A to generate the compound B or the salt thereof. According to the method, side reactions such as primary esterification and secondary esterification can be well controlled and reduced, so that the content of impurities such as a monoester product, a diester product and a tetraester product in a reaction solution is low, and the impurities generated by the side reactions can be removed from a target product by using safe and nontoxic ethanol and water; the problems of drug safety and / or environmental pollution and the like possibly caused by using toxic solvents such as dichloromethane are effectively avoided, and the method is safe, environmentally friendly and more suitable for being applied to large-scale production.
Owner:SHANGHAI VIWIT PHARMA CO LTD
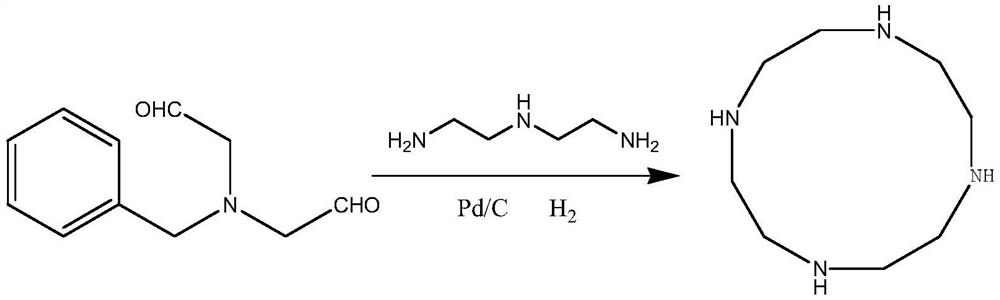
Preparation method of cyclen
InactiveCN110698419AImprove stabilityGood reaction selectivityOrganic chemistryBiochemical engineeringAcid hydrolysis
The invention provides a preparation method of cyclen. The method comprises the following step: taking triethylenetetramine (TETA) as a raw material to carry out refining, then carrying out a reactionon the refined triethylenetetramine with methylglyoxal to obtain an intermediate 2, carrying out cyclization on the intermediate 2 with glyoxal, carrying out reducing with sodium borohydride to obtain a cyclized intermediate 3, finally carrying out ring opening through acid hydrolysis, and then adjusting the pH value by using an alkali solution to free out the cyclen.
Owner:CHEN STONE GUANGZHOU CO LTD
Detergent compositions for removing heavy metals and formaldehyde
ActiveUS20200002649A1Strong heavy metal removal abilityEffectively used as detergentOrganic detergent compounding agentsDetergent mixture composition preparationCyclamPhysical chemistry
The present invention provides a detergent composition for removing heavy metals and formaldehyde, comprising at least one selected from the group consisting of trientine or trientine derivative of Formula (1), cyclen or cyclen derivative of Formula (2), cyclam or cyclam derivative of Formula (3), and a salt thereof.
Owner:FNG RES +2
Preparation method of cycleanine
InactiveCN110669020ALow one-time investment costReduce usageOrganic chemistryPtru catalystDimethyl acetal
The invention provides a preparation method of cycleanine, and belongs to the technical field of production and preparation of fine chemicals. The method comprises the following steps: 1) adding a solvent, a ring extender and an acid-binding catalyst into an intermediate bisimidazoline, and performing a reaction in an inert gas atmosphere for a period of time; 2) performing vacuumizing after the reaction is finished in order to remove light components, then adding water and an alkaline catalyst, raising the temperature, performing a reaction for a period of time, and performing vacuumizing toremove the light components; and 3) adding toluene, filtering out a product liquid while hot, and finally performing recrystallization with water-toluene to obtain the cycleanine final product. The intermediate bisimidazoline is prepared by taking toluene as a solvent and triethylenetetramine as a substrate, dropwise adding N,N'-dimethylformamide dimethyl acetal, and performing vacuumizing after the raw materials are dropwise added to extract light components so as to obtain a turbid liquid which is the intermediate bisimidazoline. A single kettle or a multi-kettle combined form can be adoptedin the preparation process of cycleanine, so that the one-time investment cost is reduced; and the solvent has the characteristics of low dosage, recycling realization and low loss.
Owner:SOUTHWEST RES & DESIGN INST OF CHEM IND
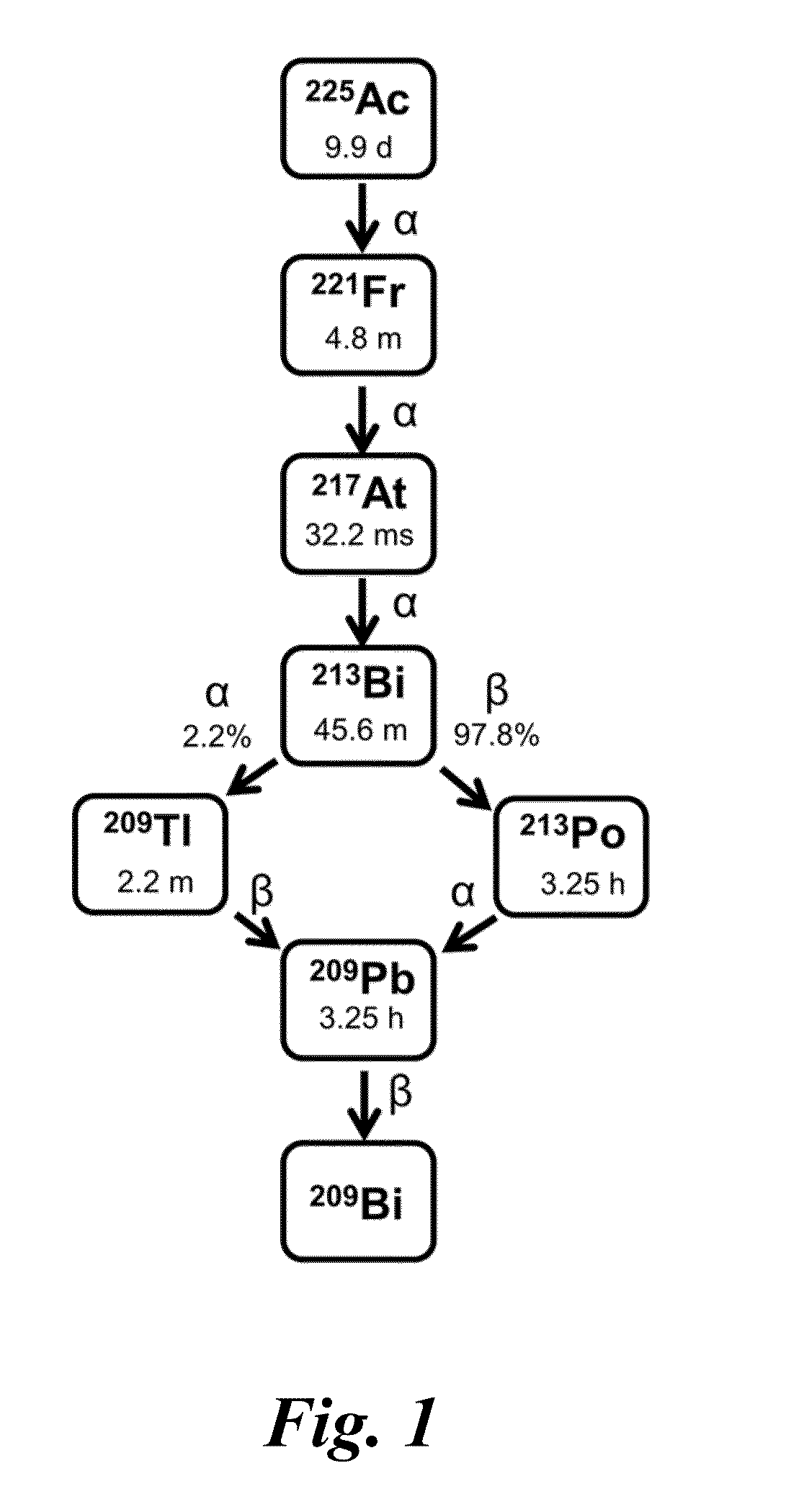
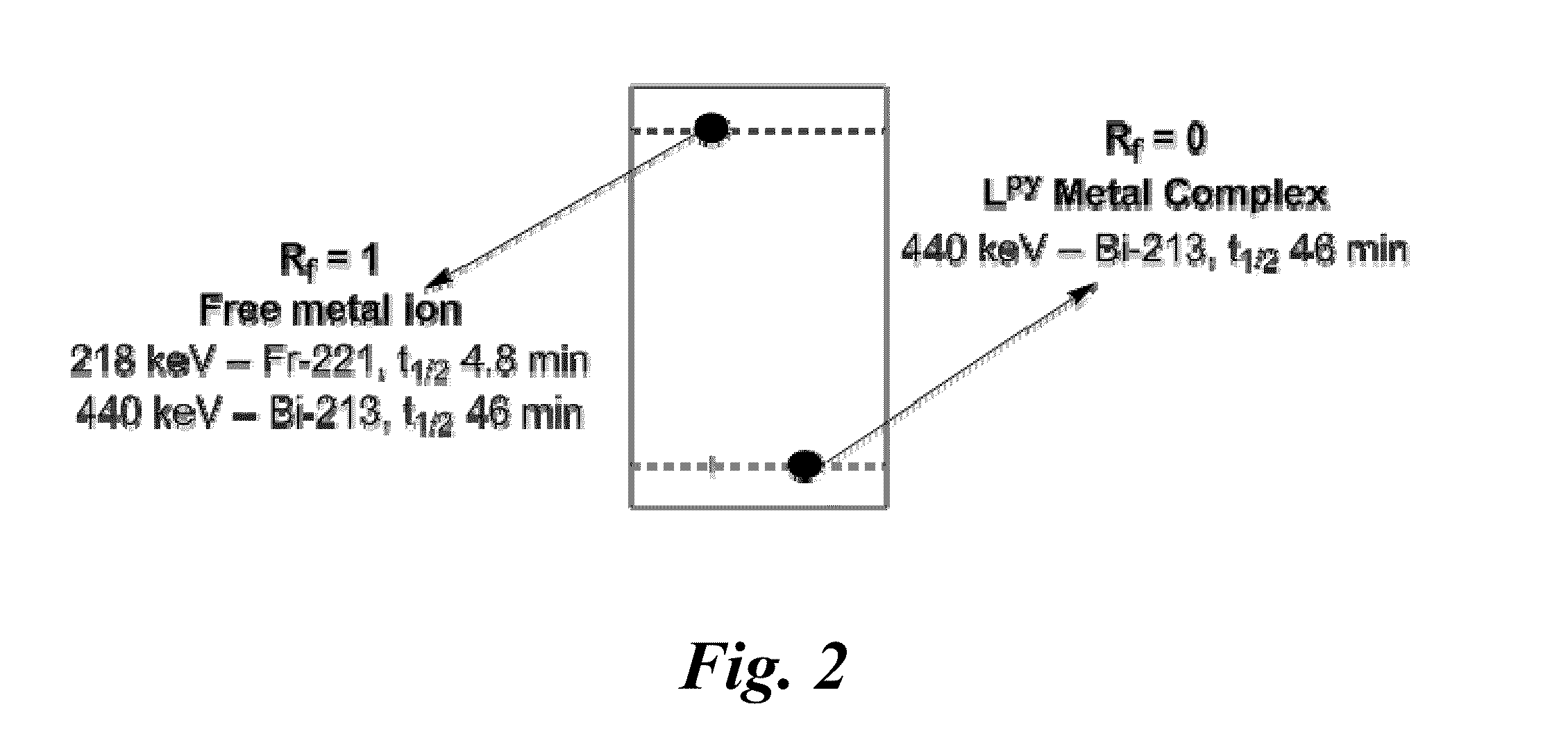
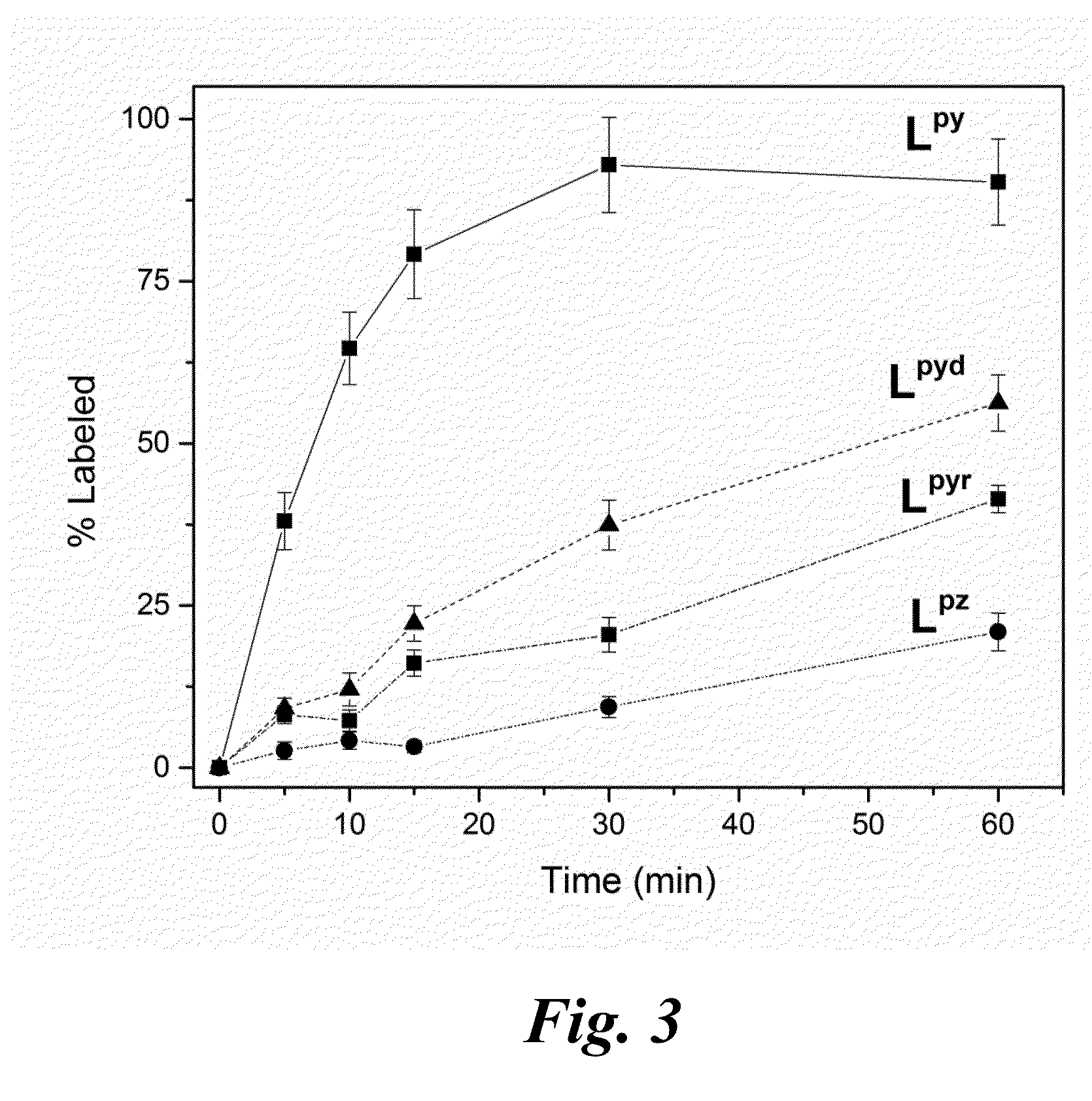
Nitrogen-rich macrocyclic ligands, chelation complexes thereof, and process for selective chelation of radioactive bismuth ions with the ligands
Selective chelation of bismuth radionuclide ions from a mixture including actinium radionuclide ions involves exposing a ligand to an aqueous solution that includes bismuth radionuclide ions and actinium radionuclide ions under conditions whereby the bismuth radionuclide ions selectively chelate to the ligand for form cationic complexes of the bismuth radionuclide ions. and separating the cationic complexes of the bismuth radionuclide ions from the actinium radionuclide ions. The ligands have a structure based on a 12-membered cyclen ring and may include pendant functional groups that can be derivatized with biological targeting vectors for targeted alpha therapy.
Owner:TRIAD NAT SECURITY LLC
Synthesis of cyclen derivatives
Owner:GE HEALTHCARE AS
Preparation method of high-purity cycleanine
ActiveCN110922365APromote generationPromote reductionOrganic chemistryCycleanineBiochemical engineering
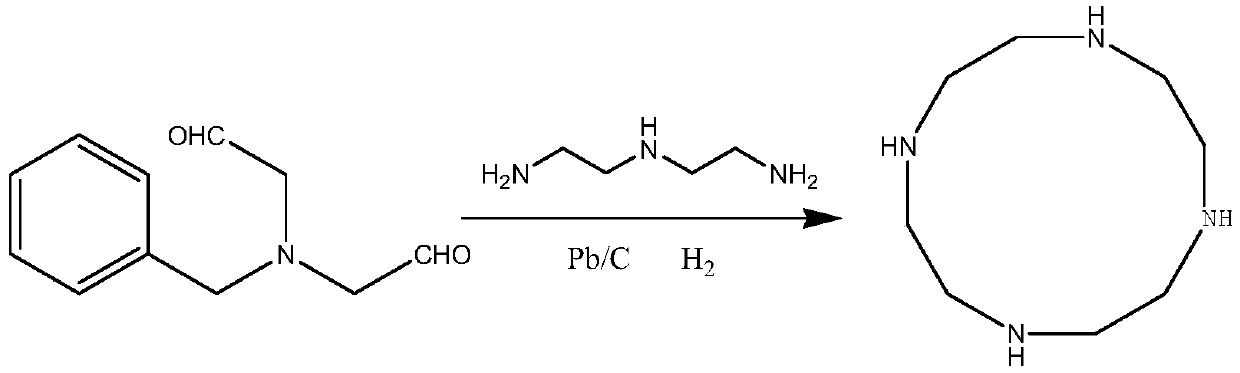
The invention discloses a preparation method of high-purity cycleanine. Diethylenetriamine and N-benzyl diacetaldehyde amine are used as main raw materials, a drying agent is added, generated water isabsorbed, the generation of imine is promoted, meanwhile, hydrogen is introduced to rapidly reduce the imine, the decomposition of imine is avoided, and the reaction is more thorough. The method is simple in step, low in cost, high in yield, low in equipment requirement, environmentally friendly and suitable for industrial production.
Owner:SHANDONG BOYUAN PHARM CO LTD
Detergent compositions for removing heavy metals and formaldehyde
ActiveUS11274268B2Strong heavy metal removal abilityEffectively used as detergentOrganic detergent compounding agentsDetergent mixture composition preparationCyclamPhysical chemistry
Owner:FNG RES +2
A kind of preparation method of high-purity trigonine
Owner:SHANDONG BOYUAN PHARM CO LTD
Preparation method of cycleanine
The invention provides a preparation method of cycleanine, and belongs to the field of organic synthesis. The invention provides a preparation method of cycleanine, which comprises the following reaction steps: a 1, 4, 7, 10-tetraazacyclododecane derivative is contacted with a carbonyl reducing agent to obtain cycleanine, the 1, 4, 7, 10-tetraazacyclododecane derivative is a compound with at least one carbon atom on the ring of 1, 4, 7, 10-tetraazacyclododecane being oxo, and the carbonyl reducing agent is a compound with at least one carbon atom on the ring of 1, 4, 7, 10-tetraazacyclododecane being oxo. The carbonyl reducing agent is lithium aluminum hydride, sodium borohydride or potassium borohydride. According to the technical scheme, the invention provides a brand-new cycleanine synthesis route which does not involve a ring-closing reaction and has high yield.
Owner:SHANGHAI LINKCHEM TECH CO LTD
A kind of preparation method of high-yield gadobutrol
ActiveCN108047151BReduce security risksAvoid concentrationOrganic chemistryCycleanineBromoacetic acid
The invention provides a preparation method of high field gadobutrol. Cycleanine monoformaldehyde is used as a raw material, alkylation reaction is carried out with tert-butyl bromoacetate, then the alkylation product is hydrolyzed, and tricarboxylate intermediate is obtained; the tricarboxylate intermediate and 4,4-dimethyl-3,5,8-three oxygen bicyclo [5.1.0] octane react, then complex reaction ofthe reaction product and gadolinium oxide is carried out, and gadobutrol with higher purity and good yield is obtained.
Owner:CHEN STONE GUANGZHOU CO LTD
Synthesis of tris n-alkylated 1,4,7,10-tetraazacyclododecanes
ActiveCN100418957COrganic chemistryDiagnostic recording/measuringDodecaneTert-Butyloxycarbonyl protecting group
A directly synthetic method for preparing tris-alkylated 1,4,7,10-tetraazacyclododecanes by the reactions of 1,4,7,10-tetraazacyclododecane (cyclen) and appropriate electrophiles is accomplished in high yield. The method provides operational convenience, starting material availability, cost economy, atom efficiency and reaction insensitivity to temperature, moisture, and concentrations of starting materials.
Owner:THE UNIVERSITY OF HONG KONG
Preparation method of butenol
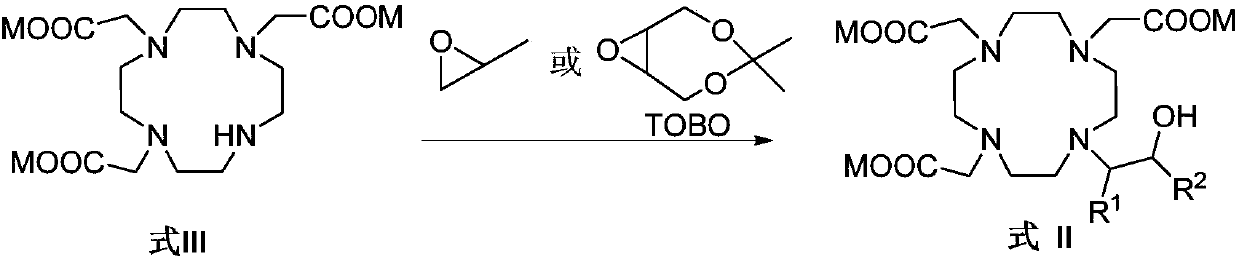
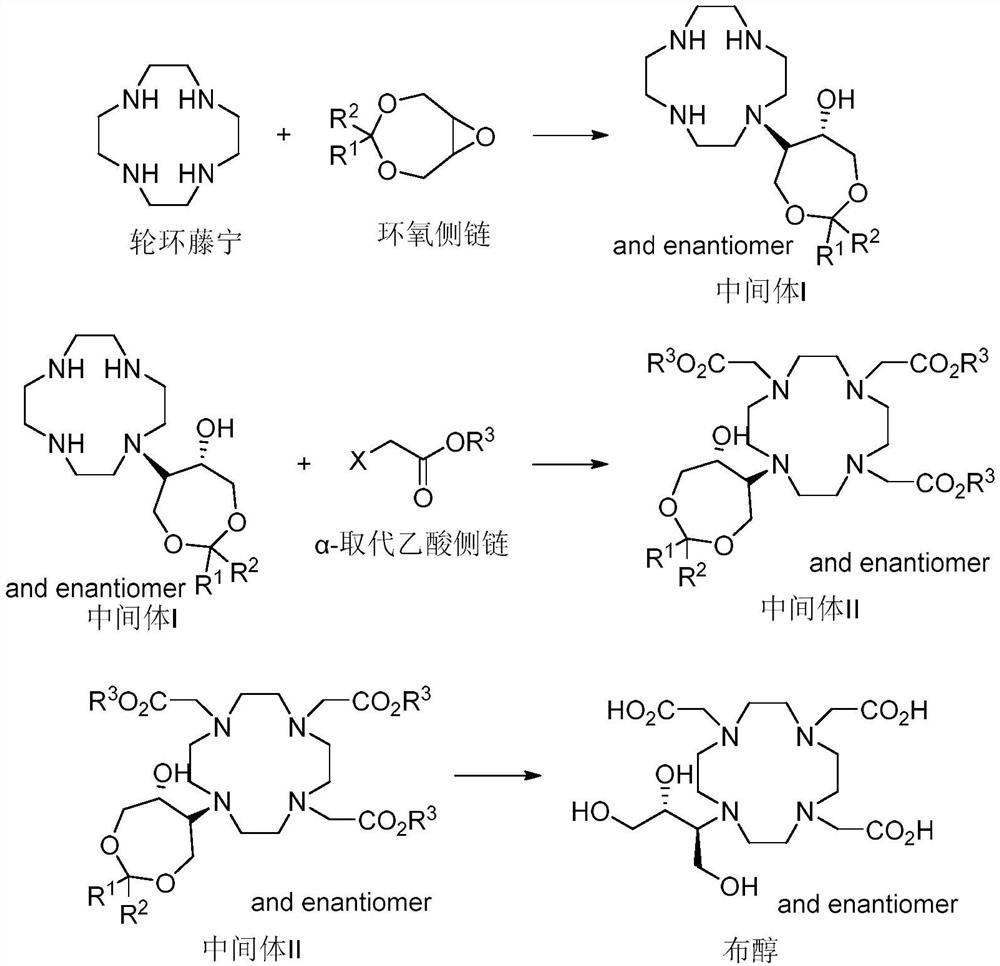
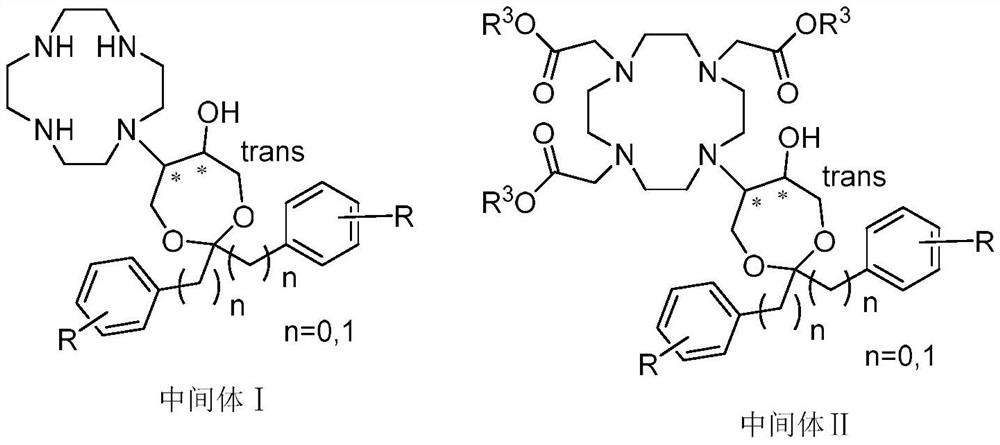
The invention belongs to the technical field of medicine synthesis, and particularly relates to a preparation method of high-purity bufol. The preparation method comprises the following steps: (1) reacting cycleanine with an epoxy side chain to generate an intermediate I, (2) carrying out nitrogen alkylation on the intermediate I and an alpha-substituted acetic acid side chain to generate an intermediate II, and (3) hydrolyzing an ester group and acetal (ketone) of the intermediate II to obtain the bufol.
Owner:SHANGHAI JIANHE PHARM & TECH CO LTD +1
Preparation method of cyclen and intermediate thereof
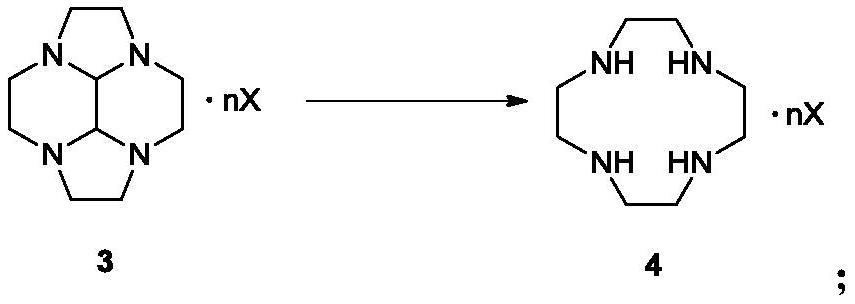
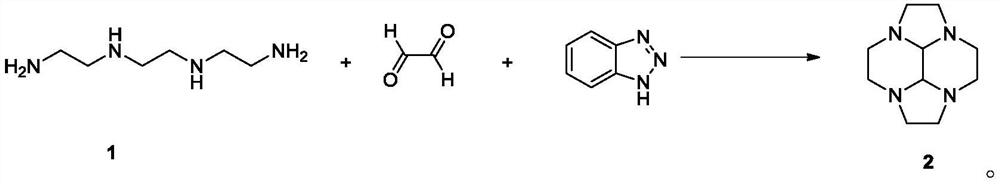
The invention discloses a preparation method of cyclen and an intermediate thereof, specifically a preparation method of a compound as shown in a formula 4. The method comprises the following step: in water, carrying out a reduction reaction as shown in the specification on a compound as shown in a formula 3 and hydrazine hydrate, wherein X is phosphoric acid or sulfuric acid, when X is phosphoric acid, n is 4 / 3, and when X is sulfuric acid, n is 2. The preparation method has the advantages of low cost, simple operation, easy purification of intermediates and products, high yield and purity, and suitableness for industrial production.
Owner:LIANHE CHEM TECH +2
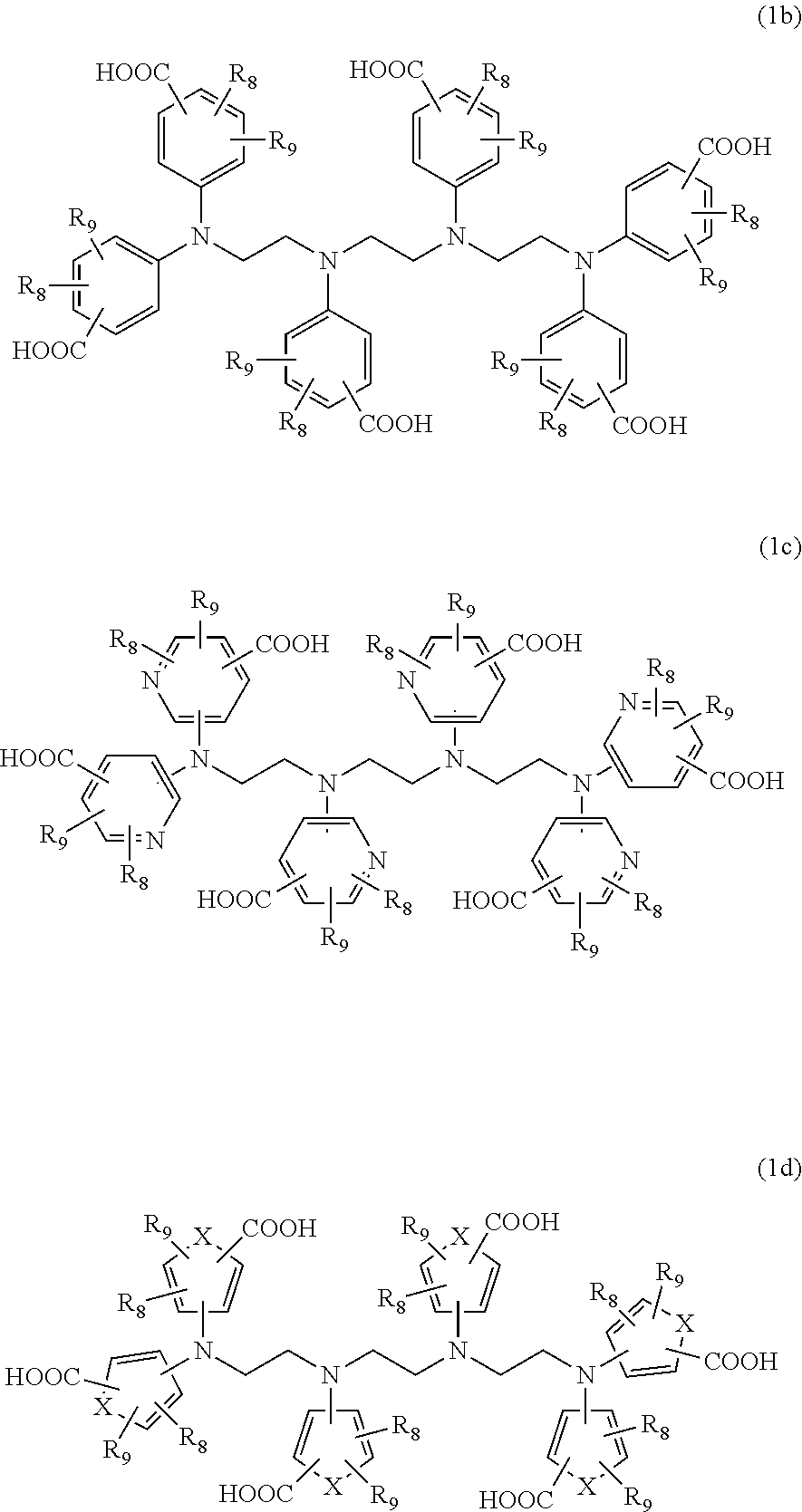
Cyclen based compounds, coordination compounds, peptides, pharmaceutical preparation, and use thereof
PendingUS20220218849A1Maintain positionRestricts conformational flexibility and rotational motionGroup 3/13 organic compounds without C-metal linkagesPeptidesMeth-Acyl group
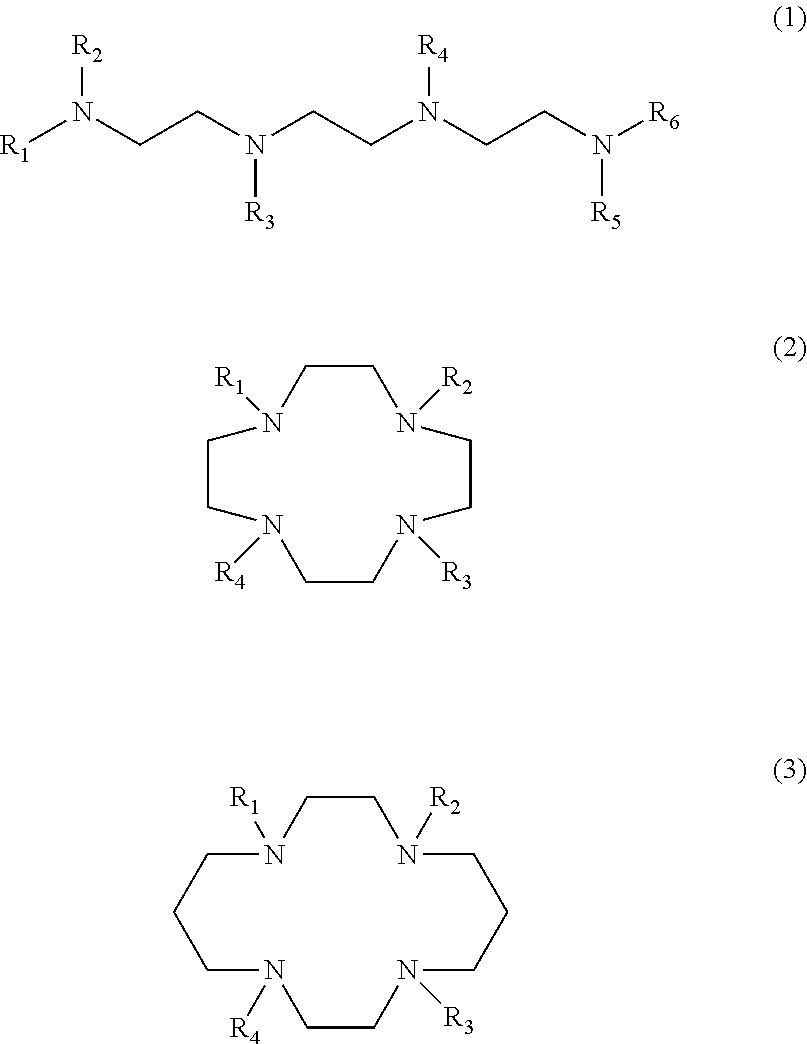
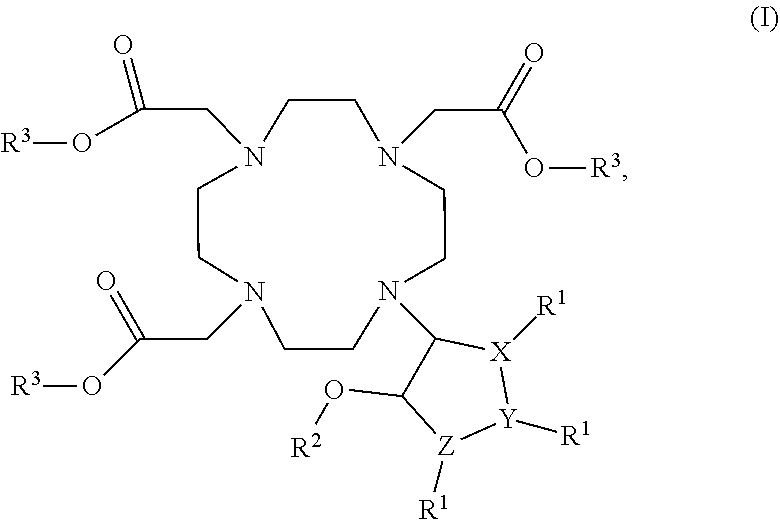
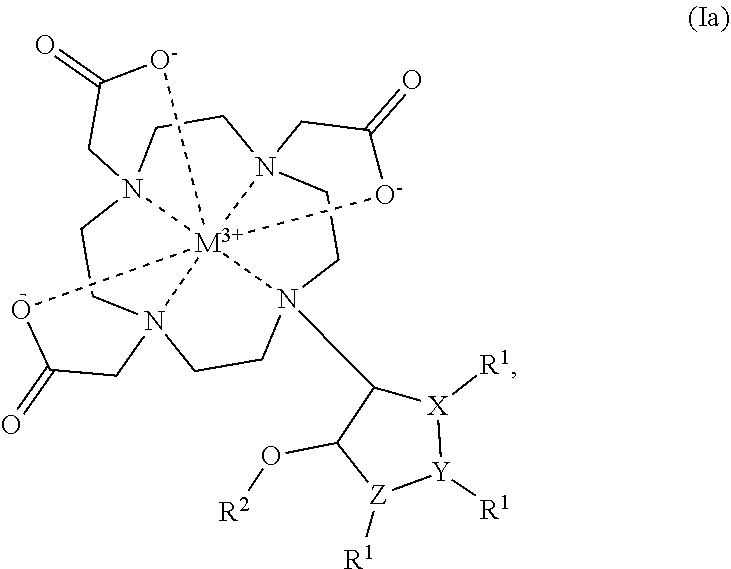
Cyclen based compounds of general formula (I) are disclosed. X is nitrogen and Y, Z are —CH—, or X, Z are —CH— and Y is nitrogen, or X, Y are —CH— and Z is nitrogen. R1 is independently selected from H; COOH; benzyloxycarbonyl; fluorenylmethyloxycarbonyl; tert-butoxycarbonyl; methylcarbonyl; trifluoromethylcarbonyl; benzyl; triphenylmethyl; tosyl; mesyl; benzyloxymethyl; phenylsulfonyl; ethoxycarbonyl; 2,2,2-trichloroethyloxycarbonyl; methoxycarbonyl; methoxymethyloxycarbonyl; R2 is selected from H; methylcarbonyl; tert-butyldimethylsilyl; (C1-C4)alkyl; R3 is independently selected from H; (C1-C6)alkyl.
Owner:INST OF ORGANIC CHEM & BIOCHEMISTRY OF THE ACAD OF SCI OF THE CZECH REPUBLIC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com