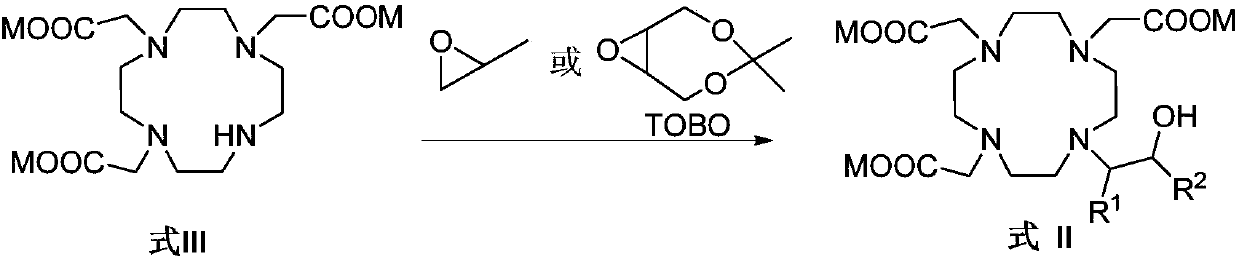
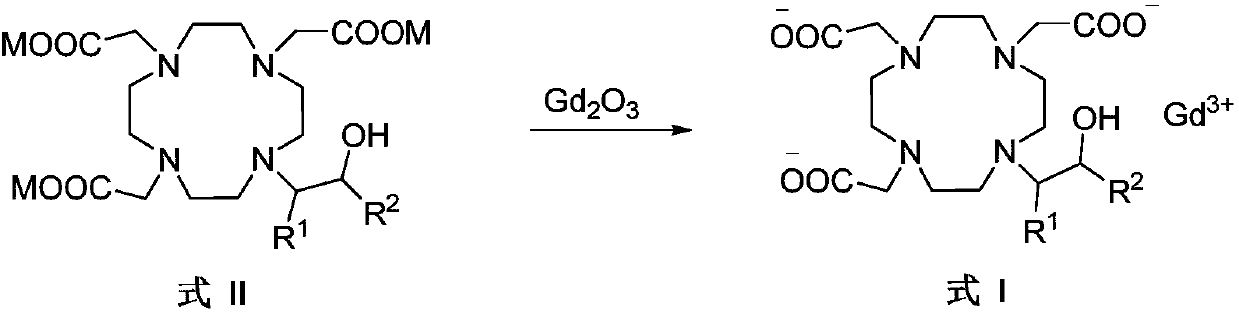Preparation method of macrocyclic chelating agent and intermediate thereof
A compound, the technology of cyclamenine, is applied in the field of preparation of gadoterol, gadobutrol and their intermediates, and can solve the problems of complicated purification, long reaction route, many by-products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] The preparation of embodiment 1t-Bu-DO3A
[0140]
[0141] Add 1.0Kg of cyclen to 7.5L of N,N-dimethylacetamide, add 1.57Kg of sodium acetate, and add N,N of tert-butyl bromoacetate at -5°C to 5°C -Dimethylacetamide solution (3.74Kg of tert-butyl bromoacetate dissolved in 2.5L of N,N-dimethylacetamide), after addition, react at room temperature for 24h, pour the reaction solution into 20L of purified water, drop Add 2.0 mol / L sodium hydroxide aqueous solution to adjust the pH to 8.5-9.5 to precipitate a solid, which is shaken and filtered to obtain a solid.
[0142] Dissolve the above solid in 16.0L of dichloromethane, wash the organic phase with 8.0L of purified water, dry the organic phase with anhydrous sodium sulfate, filter, and concentrate the filtrate to saturation under reduced pressure at 20°C to 30°C (very small amount of solid Precipitate), stop concentrating, add 30L methyl tert-butyl ether, separate out a large amount of white solids, shake filter, dry ...
Embodiment 2
[0143] The preparation of embodiment 2DO3A
[0144]
[0145] Dissolve 2.7Kg of t-Bu-DO3A in 13.5L of anhydrous methanol, add aqueous sodium hydroxide solution (dissolve 1.45Kg of NaOH in about 2.7L of water), heat up to 65°C to 75°C after the addition, and stir to react 3h, concentrated under reduced pressure at 50℃~60℃ to nearly dryness to obtain DO3A.
Embodiment 3
[0146] The preparation of embodiment 3HP-DO3A
[0147]
[0148] The DO3A that embodiment 2 obtains is dissolved with the purified water of 13.5L, adjusts pH to 11.0-12.5 with the concentrated hydrochloric acid of 12.0mol / L, under the condition of controlled temperature 0 ℃~15 ℃, drip propylene oxide aqueous solution (526.6g epoxy Dissolve propane in about 5.3L of purified water), after addition, react at 20°C to 30°C for about 20h, adjust the pH of the reaction solution to 10.5-11.5 with 4mol / L aqueous sodium hydroxide solution, and filter the nanofilter at 65 Concentrate under reduced pressure and close to dryness at ℃~75℃, add 1L purified water to dissolve the solid, heat to 55℃~65℃, slowly add 20L isopropanol while keeping warm, cool down to 0℃~10℃, stir and crystallize, filter, The solid was dried to obtain 1.9 Kg of white solid HP-DO3A, the yield was 89.0%, and the purity by HPLC was 99.86% (area normalization method).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com