Process for producing a complex of a lanthanide with a macrocyclic ligand
A technology of macrocyclic ligands and lanthanides, which can be used in pharmaceutical formulations, drug delivery, preparations for in vivo tests, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
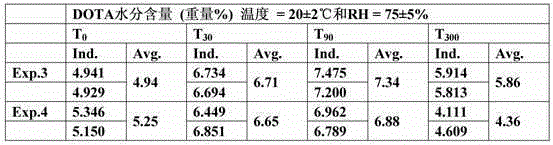
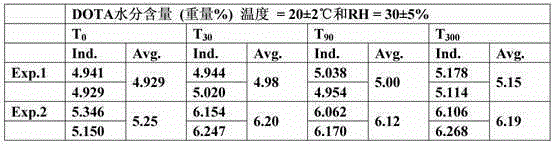
[0073] Measurement
[0074] Moisture content in DOTA
[0075] The measurement of the moisture content in DOTA was performed as described in Karl Fischer's semi-micro method (Fischer, Karl - Pharm. Eur. Section 2.5.12).
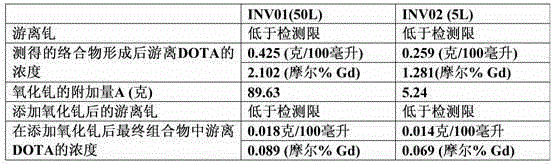
[0076] Analysis of free form of DOTA in solution (free DOTA)
[0077] Solution A: Dissolve 50 g of sodium acetate in 10 ml of glacial acetic acid and adjust the volume to 1000.0 ml with carbon dioxide-free water. The obtained solution was adjusted to pH (5±0.05) with 0.1 M sodium hydroxide solution or glacial acetic acid.
[0078] Solution B: Dissolve 50.8 mg of xylenol orange in carbon dioxide-free water and adjust the volume to 100.0 ml with the same solvent. Use freshly prepared solutions.
[0079] Solution C: 3 mL of Solution B was added to 30 mL of Solution A, and the volume of this solution was increased to 200.0 mL with carbon dioxide-free water.
[0080] A 0.005M solution of gadolinium sulfate was prepared as follows: 3.735 grams of gadolinium sul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com