Preparation method of high-purity cycleanine
A high-purity technology with ring vines, applied in the field of medicine, can solve the problems of high price, inconvenient use, and unsuitability for industrial production of lithium tetrahydrogen, and achieve the effects of less impurities, avoiding the use of sulfuric acid, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
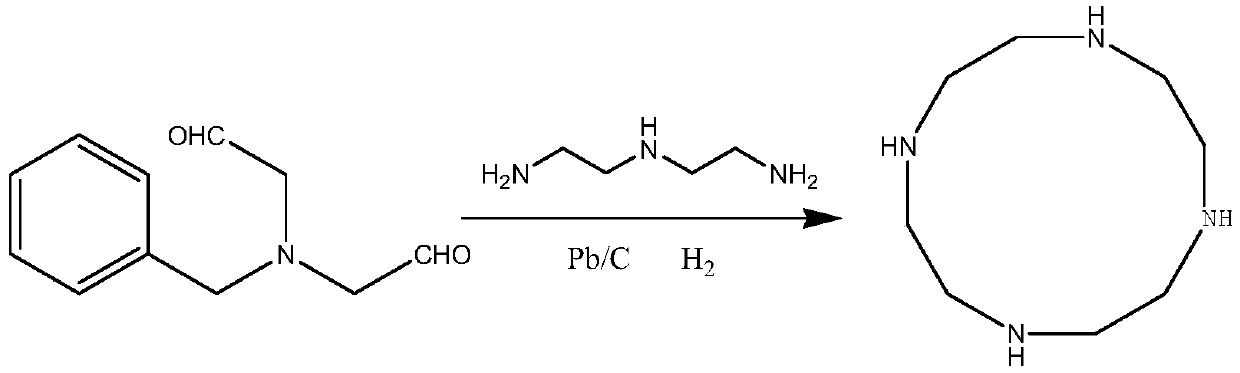
[0029] Take 191g of N-benzyldiacetaldehyde amine, 103g of diethylenetriamine, 1200ml of tetrahydrofuran, 20g of calcium chloride, 10g of 10% palladium carbon into a 2000ml reaction bottle, pass in hydrogen gas and react at 10°C for 5h, after the reaction is detected by GC, After suction filtration, the filtrate recovered tetrahydrofuran under normal pressure, then added 1500ml of toluene, recrystallized, and dried under reduced pressure at 50°C to obtain 160.3g of the product, with a yield of 93.0% and a purity of 99.5%.
Embodiment 2
[0031] Take 382g of N-benzyldiacetaldehyde amine, 225g of diethylenetriamine, 2000ml of 2-methyltetrahydrofuran, 50g of potassium carbonate, 30g of 5% palladium carbon into a 5000ml reaction flask, inject hydrogen into the reaction bottle at 15°C for 4h, and detect by GC After the reaction was completed, it was filtered with suction, and 2-methyltetrahydrofuran was recovered from the filtrate under normal pressure, then 2500ml of toluene was added, recrystallized, and dried under reduced pressure at 50°C to obtain 327.1g of the product, with a yield of 94.9% and a purity of 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com