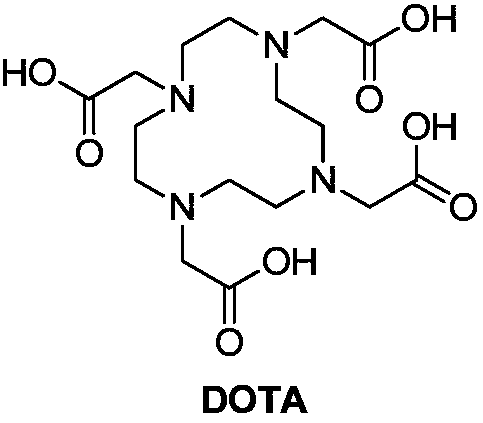
Preparation method of 1,4,7,10-tetraazacyclododecane-1,4,7-10-tetraacetic acid
一种酸性、烷基的技术,应用在有机化学等方向,能够解决耗能耗时长、不易操作、温度要求较高等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] At 0-10°C, add cyclen (17.27g, 100mmol), lithium hydroxide monohydrate (36.92g, 880mmol), and water (80mL) into a three-necked flask (1000mL). A solution of bromoacetic acid (61.14 g, 440 mmol) in water (30 mL) was added at 5-15 °C. Heat to 20-30° C. for 24 hours, and TLC detects that there is no remaining cyclen of the raw material. Add 36% hydrochloric acid (44.6g, 440mmol) to the system, add ethanol (600mL), precipitate a solid, filter, recrystallize and purify the obtained solid with ethanol / water (volume ratio: 3:1), and dry at 60°C to obtain DOTA.
[0065] Yield: 85.5%, HPLC: 99.7%, residue on ignition: 0.05%, moisture: 7.80%.
Embodiment 2
[0067] At 0-10°C, add cyclen (17.27g, 100mmol), lithium hydroxide monohydrate (36.92g, 880mmol), and water (80mL) into a three-necked flask (1000mL). A solution of bromoacetic acid (61.14 g, 440 mmol) in water (30 mL) was added at 5-15 °C. Incubate at 5-15°C for 24 hours, and TLC detects that there is no remaining cyclen of raw materials. Add 36% hydrochloric acid (44.6g, 440mmol) to the system, add ethanol (600mL), precipitate a solid, filter, recrystallize and purify the obtained solid with ethanol / water (volume ratio: 3:1), and dry at 60°C to obtain DOTA.
[0068] Yield: 78.0%, HPLC: 99.9%, residue on ignition: 0.05%, moisture: 6.25%.
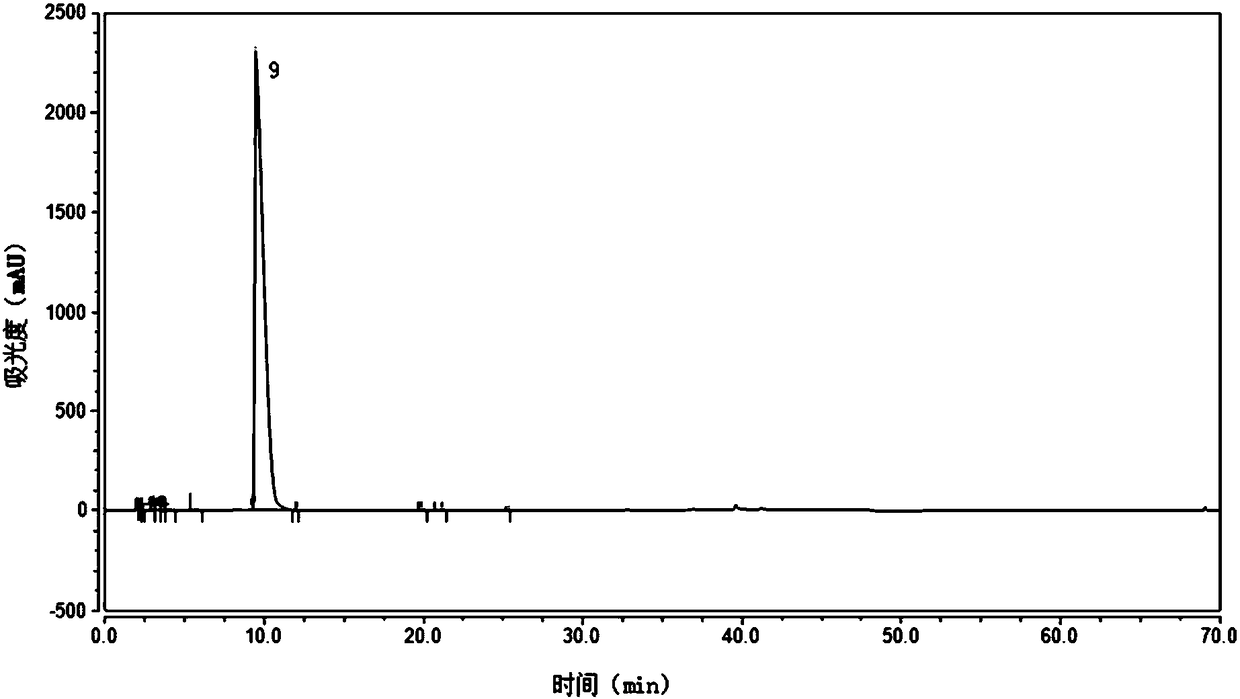
[0069] Wherein, the HPLC purity data of product sees the table below (see figure 1 ), its retention time is 9.447min.
[0070] Table 1
[0071]
[0072]
Embodiment 3
[0074] At 0-10°C, add cyclen (17.27g, 100mmol), lithium hydroxide monohydrate (36.92g, 880mmol), and water (80mL) into a three-necked flask (1000mL). A solution of bromoacetic acid (61.14 g, 440 mmol) in water (30 mL) was added at 5-15 °C. Heat to 35-45° C. for 24 hours, and TLC detects that there is no remaining cyclen of the raw material. Add 36% hydrochloric acid (44.6g, 440mmol) to the system, add ethanol (600mL), precipitate a solid, filter, recrystallize and purify the obtained solid with ethanol / water (volume ratio: 3:1), and dry at 60°C to obtain DOTA.
[0075] Yield: 82.3%, HPLC: 99.6%, residue on ignition: 0.06%, moisture: 5.60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com