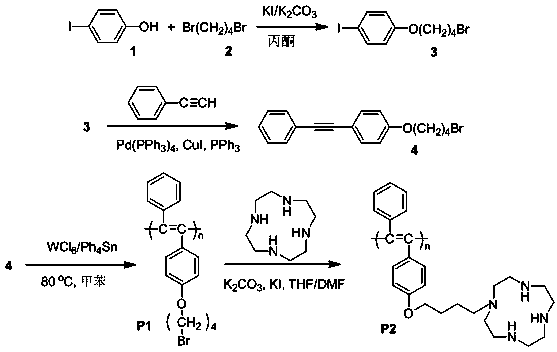
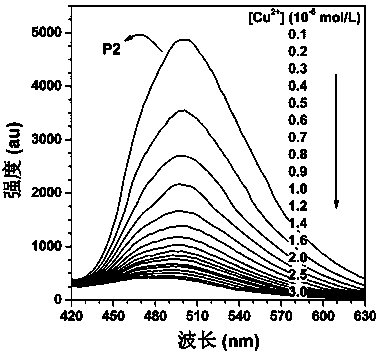
Macrocyclic polyamine-based polyacetylene fluorescent sensor and preparation method thereof
A technology of fluorescence sensor and polyacetylene, which is applied in the direction of fluorescence/phosphorescence, material excitation analysis, etc., can solve the problems of difficult double-substituted polyacetylene, less research on polyacetylene, and influence on the application of polyacetylene, so as to achieve the effect of broadening the application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] In order to better understand the content of the present invention, the content of the present invention will be further described below in conjunction with specific embodiments, but the protection content of the present invention is not limited to the following embodiments.
[0030] The raw materials used in the examples of the present invention can be purchased from the market.
[0031] (1) Synthesis of polyacetylene monomer containing alkyl bromide
[0032] Weigh compound 2.500g compound 3, CuI (0.040g), Pd (PPh 3 ) 4 (0.050g), PPh 3 (0.055g) was placed in a dry Schlenk tube, plugged with saline, and pumped through nitrogen for 5-6 times. Under nitrogen, Et3N (4.2mL), THF (30mL) and phenylacetylene (1.4mL) were added. , and stirred at room temperature for one day under the protection of nitrogen. After the reaction was completed, the excess salt was removed by normal pressure filtration, and the mixture was spin-dried. The crude product was separated by silica ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com