Preparation method of cycleanine
A technology of cyclamenine and tetraaza heterocycle is applied in the field of preparation of cyclamenine, can solve the problems of reduced reaction yield, complicated post-processing, high production and application cost, and achieves the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
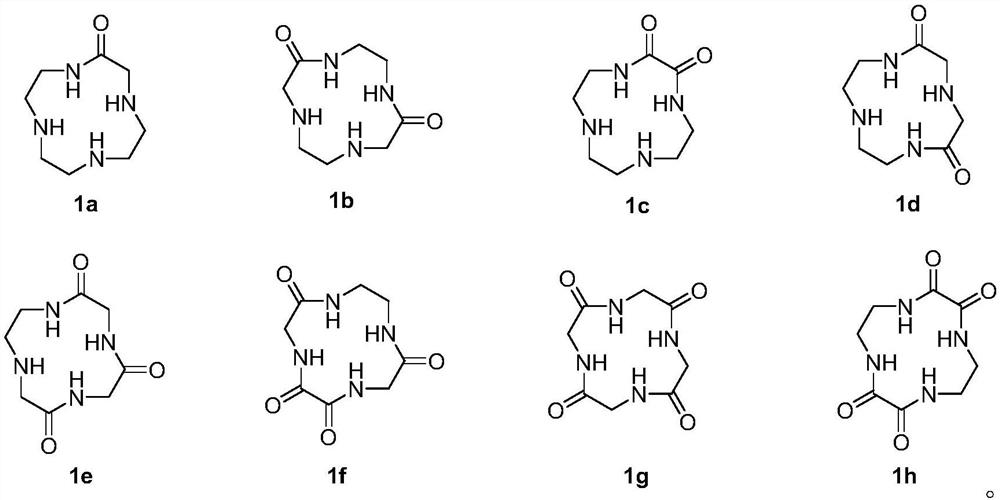
[0038] The preparation method of compound 1g
[0039] This embodiment provides a preparation method of compound 1g, the reaction equation is:
[0040]
[0041] Including the following reaction steps:
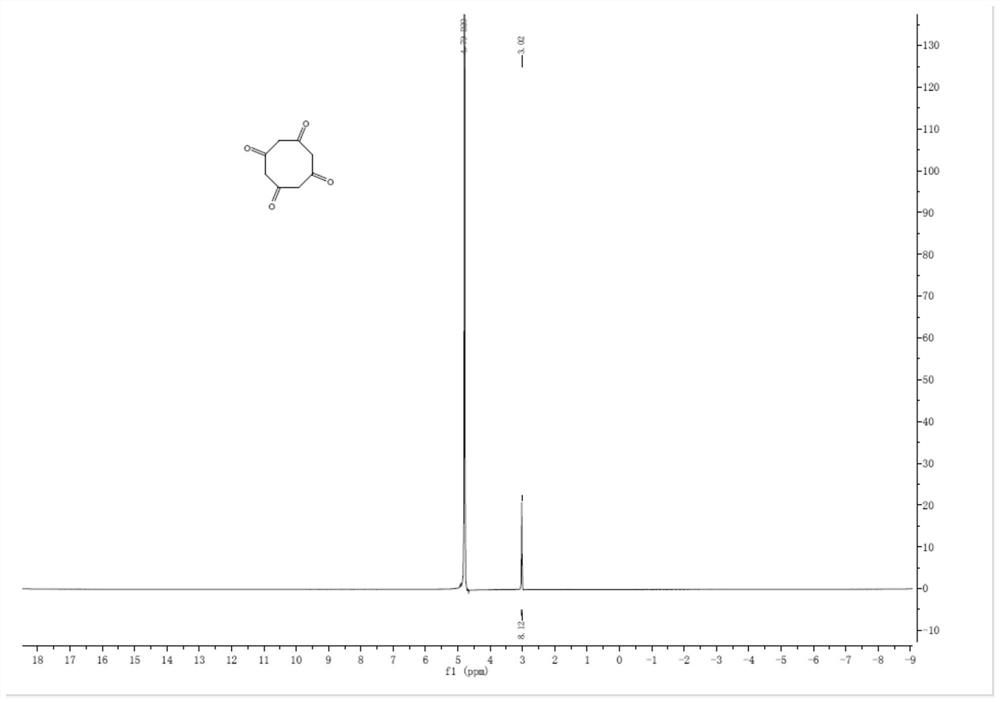
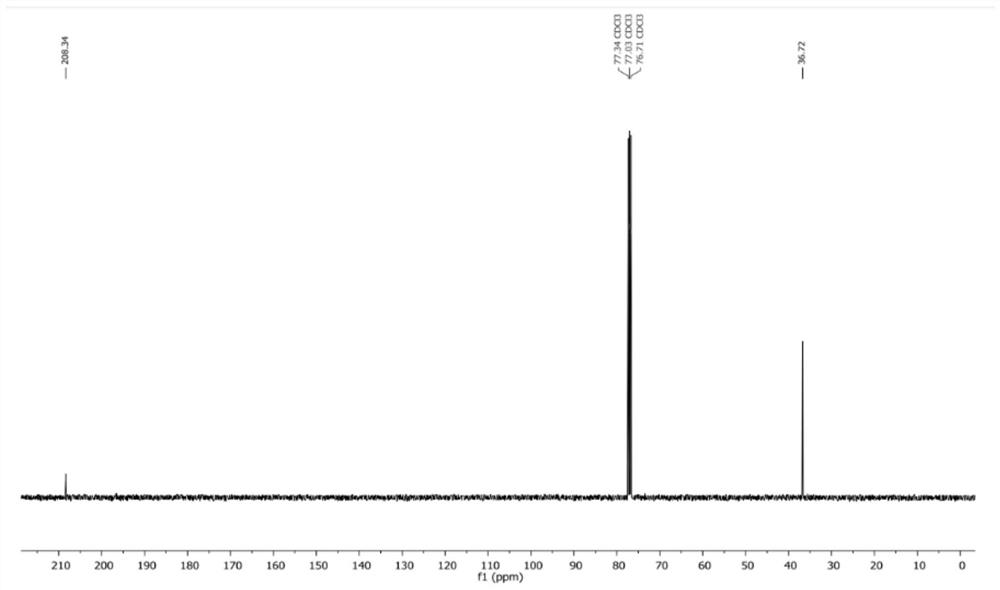
[0042] Step 1, step 1, 50g compound 5 (480mmol, 1eq) was dissolved in 200mL tetrahydrofuran to obtain a tetrahydrofuran solution of compound 5, and the tetrahydrofuran solution of compound 5 was added dropwise to 2.88L of 1mol / L borane in tetrahydrofuran solution, Stir the reaction at room temperature for 1 h, add dropwise 1.44L 5mol / L sodium hydroxide aqueous solution and 652g 30% hydrogen peroxide (5.76mol, 12eq) successively, stir the reaction for 6h, let stand, separate, take the organic phase, wash with saturated sodium carbonate aqueous solution ( 500mL×2), the organic phase was taken, concentrated under reduced pressure, and subjected to column chromatography to obtain 52.5g of compound 4 with a yield of 62.1%;
[0043] Step 2, dissolve 50g of compound 4 (283.8mmol, ...
Embodiment 8
[0050] Screening of Reaction Conditions in the Preparation Method of Compound 1
[0051] This example screens the specific reaction conditions used in step 3 of Example 1.
[0052] When adopting Lewis acid, reaction condition is as follows (hereinafter referred to as reaction condition A for short):
[0053] 20g of compound 3 (118.9mmol, 1eq), 49.5g of hydroxylamine hydrochloride (713.4mmol, 6eq) and 70.2g of sodium acetate (856.1mmol, 7.2eq) were dissolved in 150mL of ethanol, stirred for 1h, 100mL of DMF was added, and about 70mL of ethanol was added, then 100mL of DMF was added, and all remaining ethanol was distilled off, 120.4g of triethylamine (1.19mol, 10eq), Lewis acid, 1.45g of DMAP (11.9mmol, 0.1eq) were added successively, and the reaction was stirred for 3h, and 100mL of diethylamine was added Dilute with methyl chloride, wash with water (200mL×3), wash with saturated aqueous sodium chloride solution (200mL×1), combine the organic phases, dry over anhydrous sodium...
Embodiment 3
[0062] The preparation method of trigonine
[0063] The present embodiment provides a kind of preparation method of trigonine, and the reaction equation is as follows:
[0064]
[0065] Including the following reaction steps:
[0066] 20g of compound 1g (87.6mmol, 1eq, prepared by the method provided in Example 1) was dissolved in 120mL of tetrahydrofuran, and 182mL of 2.5mol / L lithium aluminum hydride in tetrahydrofuran was added dropwise under an ice-water bath. After the addition was completed, 68 Reflux reaction at ℃ for 8 hours. After the reaction, quench the reaction by adding 200mL 2mol / L aqueous sodium hydroxide solution in an ice-water bath, filter off the solid, extract with 200mL dichloromethane, combine the organic phases, and wash once with 200mL saturated brine. Dry over sodium sulfate, concentrate under reduced pressure, and perform flash column chromatography to obtain 11.0 g of compound 2 with a yield of 72.8%.
[0067] Compound 2 is characterized by:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com