Preparation method of cyclen and intermediate thereof
A compound, a technology of hydrazine hydrate, which is applied in the field of preparation of cyclamenine and its intermediates, and can solve the problems of single types of synthetic routes and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101]
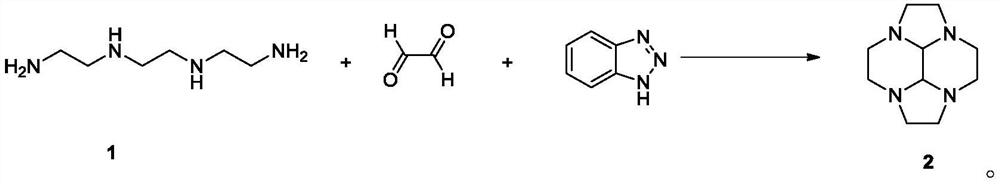
[0102] Dissolve 100 g of triethylenetetramine and 163 g of benzotriazole in 400 mL of ethanol, add dropwise 200 g of 40% glyoxal aqueous solution at 0°C, stir at 0°C for 3 hours, and pass the reaction control. Add 52 g of sodium borohydride at 0°C and stir for 3 hours. After the reaction control is qualified, remove the insoluble salt by filtration, concentrate the filtrate to remove ethanol, add 600mL of toluene, heat up and carry out normal pressure reflux to divide water, after completely removing the water, cool down to 20°C, and filter to remove the insoluble solid benzotriazole ( For recovery, the recovery rate is 92%), the filter cake is washed once with 100mL toluene, the filtrate is combined, and the filtrate is concentrated under reduced pressure until no obvious fractions are distilled out to obtain 100 grams of light yellow oily matter of the compound of formula 2, with a yield of 75% , with a content of about 70%. 1 H NMR (CDCl 3 ,400MHz):3.087(s,2H)...
Embodiment 2
[0104]
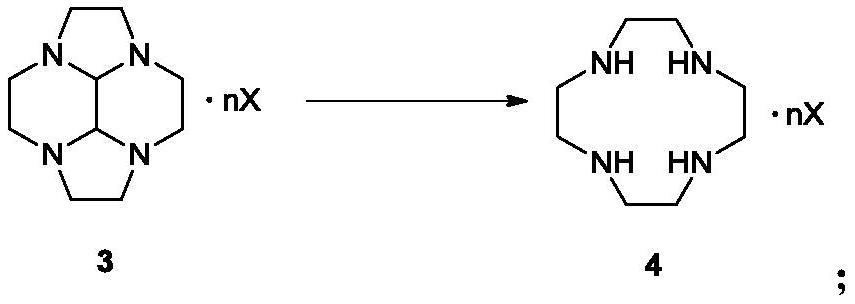
[0105] 56 grams of the compound of formula 2 (according to the purity of 100%) were dissolved in 1000 mL of ethanol, cooled to 0° C., and 68.6 grams of 98% sulfuric acid was added dropwise, and stirred at 0° C. for 5 hours. After filtering, the filter cake was washed with ethanol, and the filter cake was dried to obtain 107 g of the compound of formula 3-1, with a yield of 95%. The purity of compound 3-1 is not less than 96%, total nitrogen: 14.45%.
Embodiment 3
[0107]
[0108] 56 grams of the compound of formula 2 (according to the purity of 100%) were dissolved in 1000 mL of ethanol, cooled to 0° C., and 173 grams of 98% sulfuric acid was added dropwise, and stirred at 0° C. for 5 hours. After filtering, the filter cake was washed with ethanol, and the filter cake was dried to obtain 108 g of the compound of formula 3-1, with a yield of 95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com