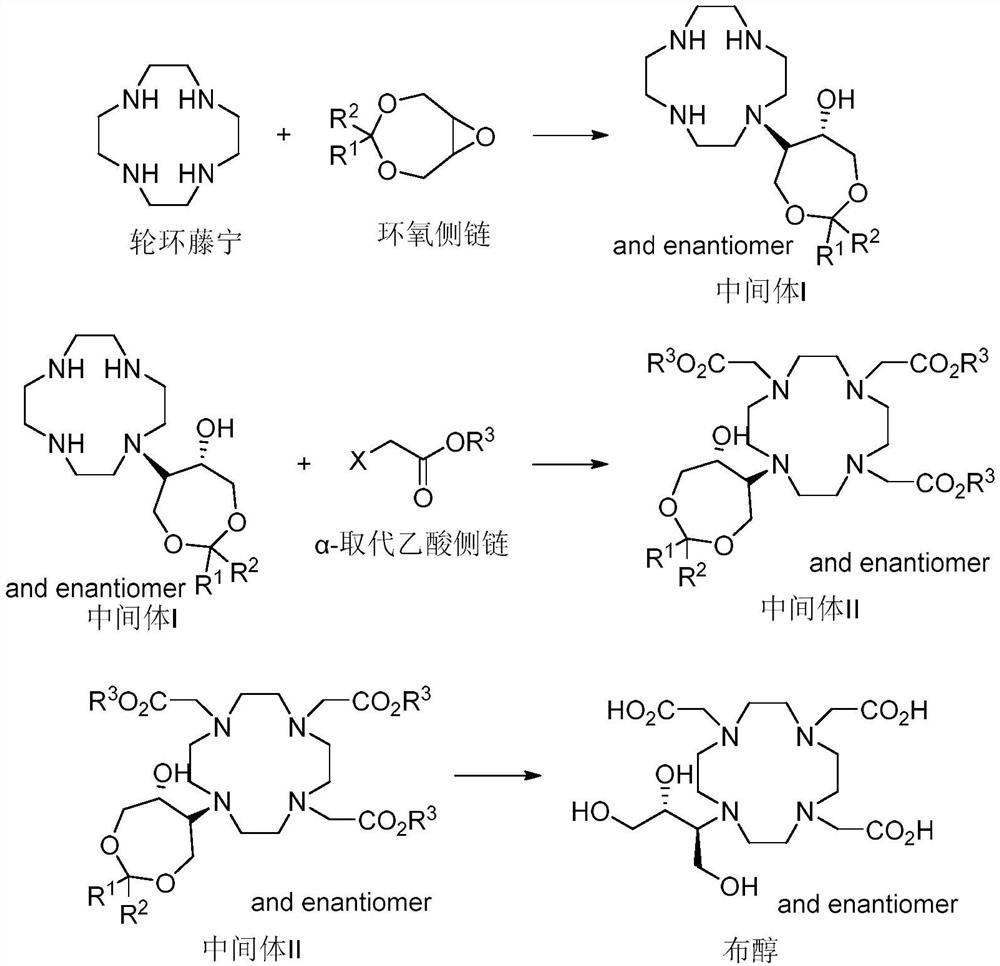
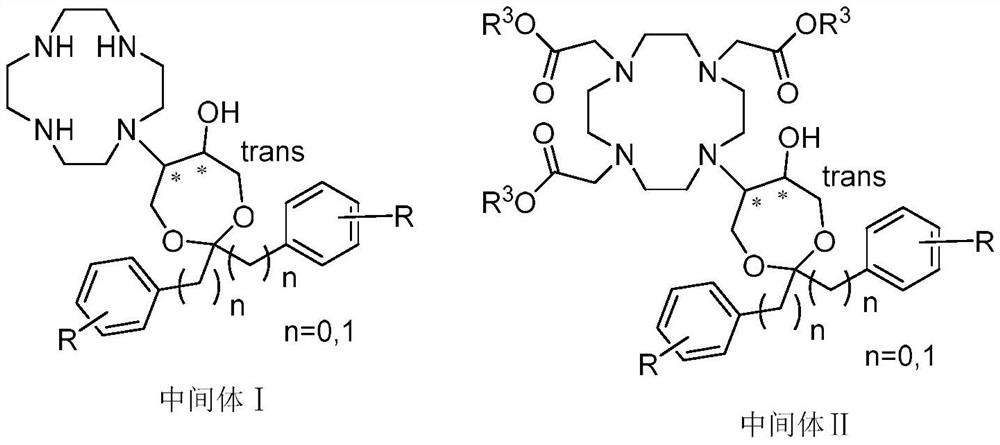
Preparation method of butenol
A technology of butanol and intermediates, which is applied in the field of preparation of high-purity butanol, can solve problems such as unfavorable quality control, difficult purification, cumbersome process, etc., and achieve improved atom economy, easy recrystallization and purification, and simple quality research Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] Preparation of epoxy side chains
[0057] Preparation of 4,4-dibenzyl-3,5,8-trioxabicyclo[5,1,0]octane:
[0058]
[0059] 2,2-Dimethoxypropane-1,3-diyl-diphenyl
[0060] 1,3-diphenylacetone (25.7g), trimethyl orthoformate (26g), iron p-toluenesulfonate (1.4g) and methanol (150g) were added to the reaction flask, all the materials were dissolved, the reaction was carried out at room temperature, and the system There was solid precipitation, TLC tracked the reaction progress, after the reaction, filtered and dried to obtain 2,2-dimethoxypropane-1,3-diyl-diphenyl (white solid, 26.9 g, yield: 85.9%) ,HPLC:99.13%). 1 H NMR (400MHz, Chloroform-d) δ 7.56-7.41 (m, 6H), 7.26-7.11 (m, 4H), 3.35 (s, 6H), 2.86 (s, 4H). MS: m / e 257.1[ (M+H) + ].
[0061] 2,2-Dibenzyl-4,7-dihydro-1,3-dioxoheptane
[0062] Into the reaction flask were added 2,2-dimethoxypropane-1,3-diyl-diphenyl (22.7g), iron p-toluenesulfonate (0.5g), maleic-1,4-diol (10g) and dichloromethane (100g), all th...
Embodiment 1
[0082] The reaction equation is as follows:
[0083]
[0084] Preparation of Compound Ⅰ-A
[0085] Under nitrogen protection, cyclamenine (10 g) and 4,4-dibenzyl-3,5,8-trioxabicyclo[5,1,0]octane (10 g) were mixed in ethanol (50 mL) , stirred, heated to reflux, followed the progress of the reaction by HPLC, stopped heating, added to purified water, filtered, and dried to obtain 13.6 g of crude product, which was added to ethyl acetate and ethanol for recrystallization to obtain compound Ⅰ-A: 10 g, yield Yield: 63.3%, HPLC: 99.1%. 1 H NMR (400MHz, Chloroform-d) δ 7.31–7.21(m, 6H), 7.18–7.14 (m, 2H), 7.10–7.06 (m, 2H), 4.25–3.60 (m, 6H), 2.87 (s) ,4H),2.80–2.42(m,16H).MS: m / e469.3[(M+H) + ]. Preparation of compound Ⅱ-A
[0086] Intermediate I-A (8 g) and acetonitrile (40 mL) were added to the three-necked flask, stirred, potassium carbonate (11.8 g) and tert-butyl chloroacetate (8.5 g) were added, and the system was heated to 55° C. to react for 3 hours. Cool the temperat...
Embodiment 2
[0090] The reaction equation is as follows:
[0091]
[0092] Preparation of compound I-B
[0093] Into the reaction flask was added cyclamenine (50g), 4,4-diphenyl-3,5,8-trioxabicyclo[5,1,0]octane (50g) and acetonitrile (500mL), Stir, heat to reflux, follow the reaction process by HPLC, stop heating, add the reaction solution to purified water, filter with suction, wash, and dry to obtain 80 g of crude product. The crude product was recrystallized from methanol / ethyl acetate system to obtain compound I-B: 70.6 g, yield: 86%, HPLC: 98.9%. 1 H NMR (400MHz, DMSO-d6) δ 7.50 (d, J=7.7Hz, 4H), 7.31 (t, J=7.5Hz, 4H), 7.23 (d, J=7.2Hz, 2H), 3.71–3.22 (m,6H),2.71–2.34(m,16H).MS: m / e 441.3[(M+H) + ].
[0094] The following compounds were prepared with reference to the same method
[0095]
[0096]
[0097] Preparation of compound Ⅱ-B
[0098] Intermediate I-B (50g) and acetonitrile (800mL) were added to the three-necked flask, stirred, potassium carbonate (63g) and tert-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com