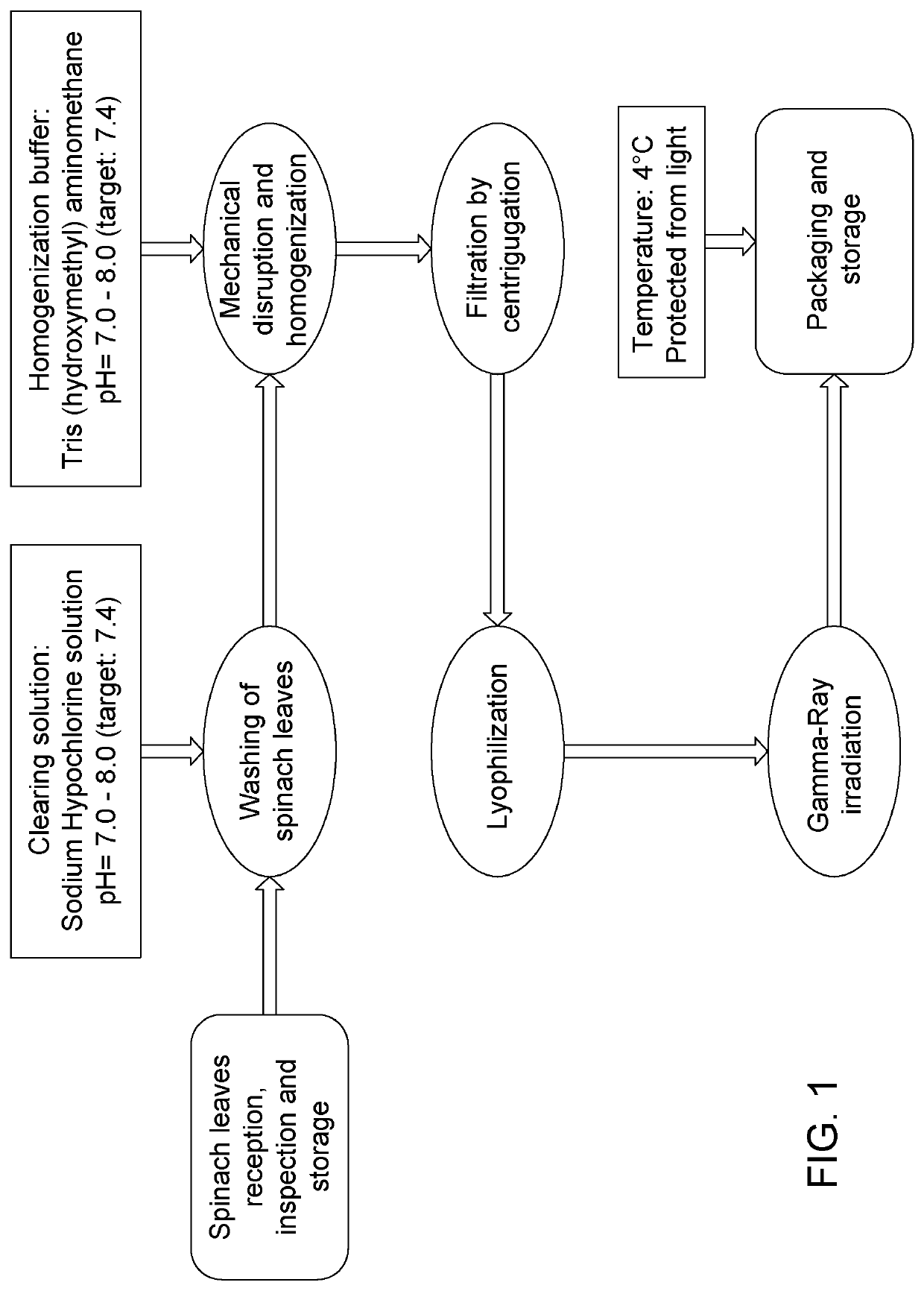
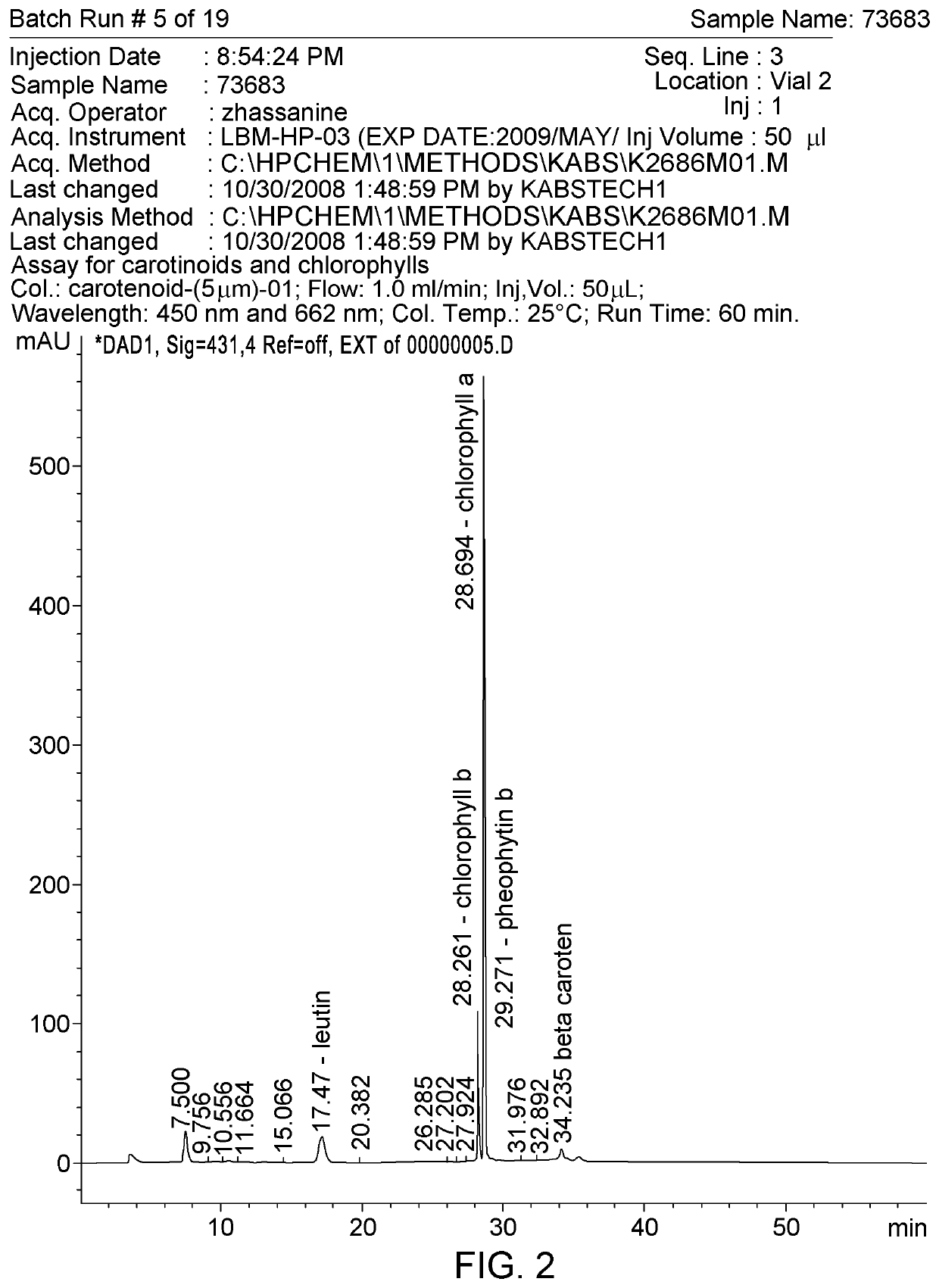
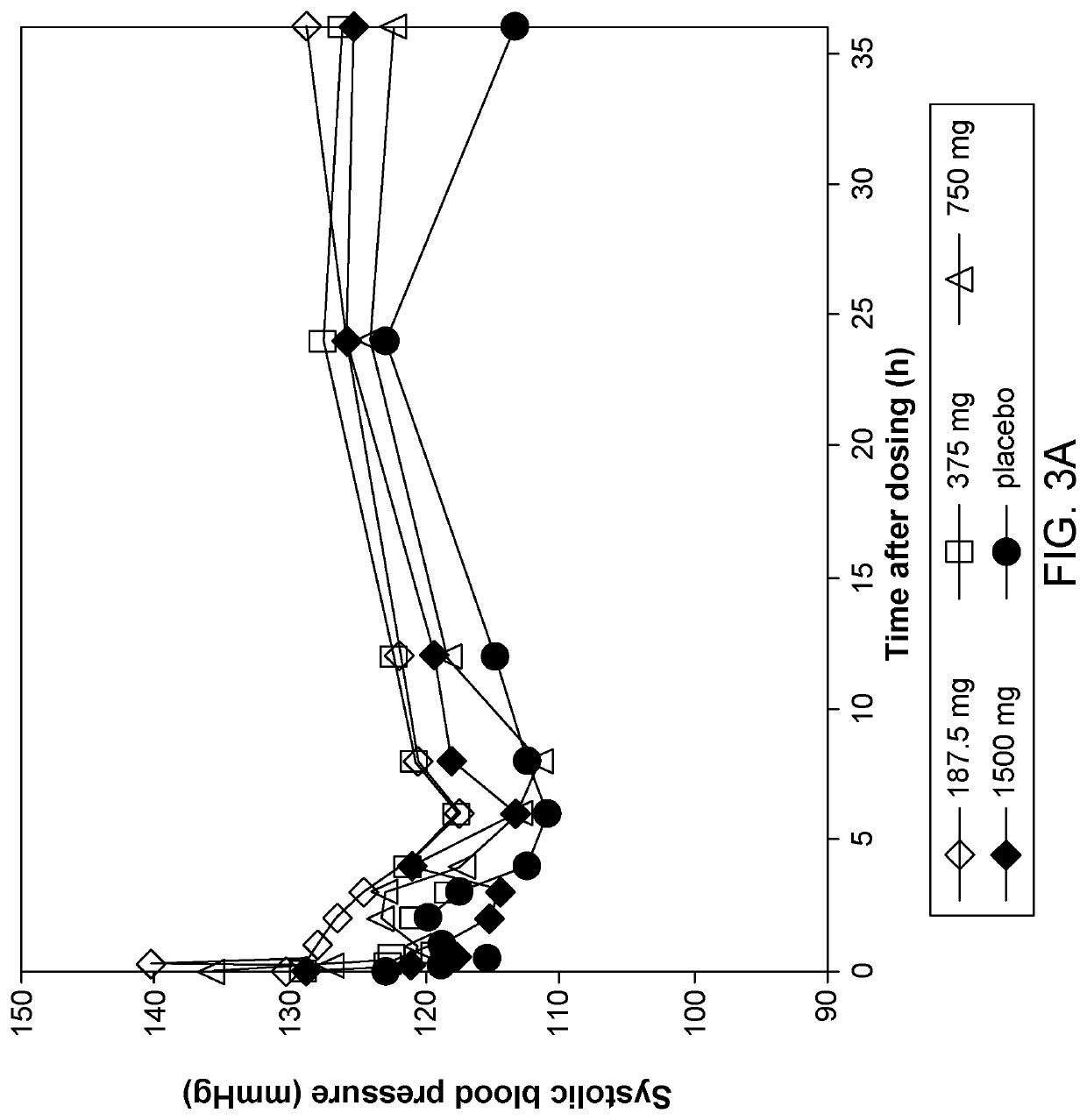
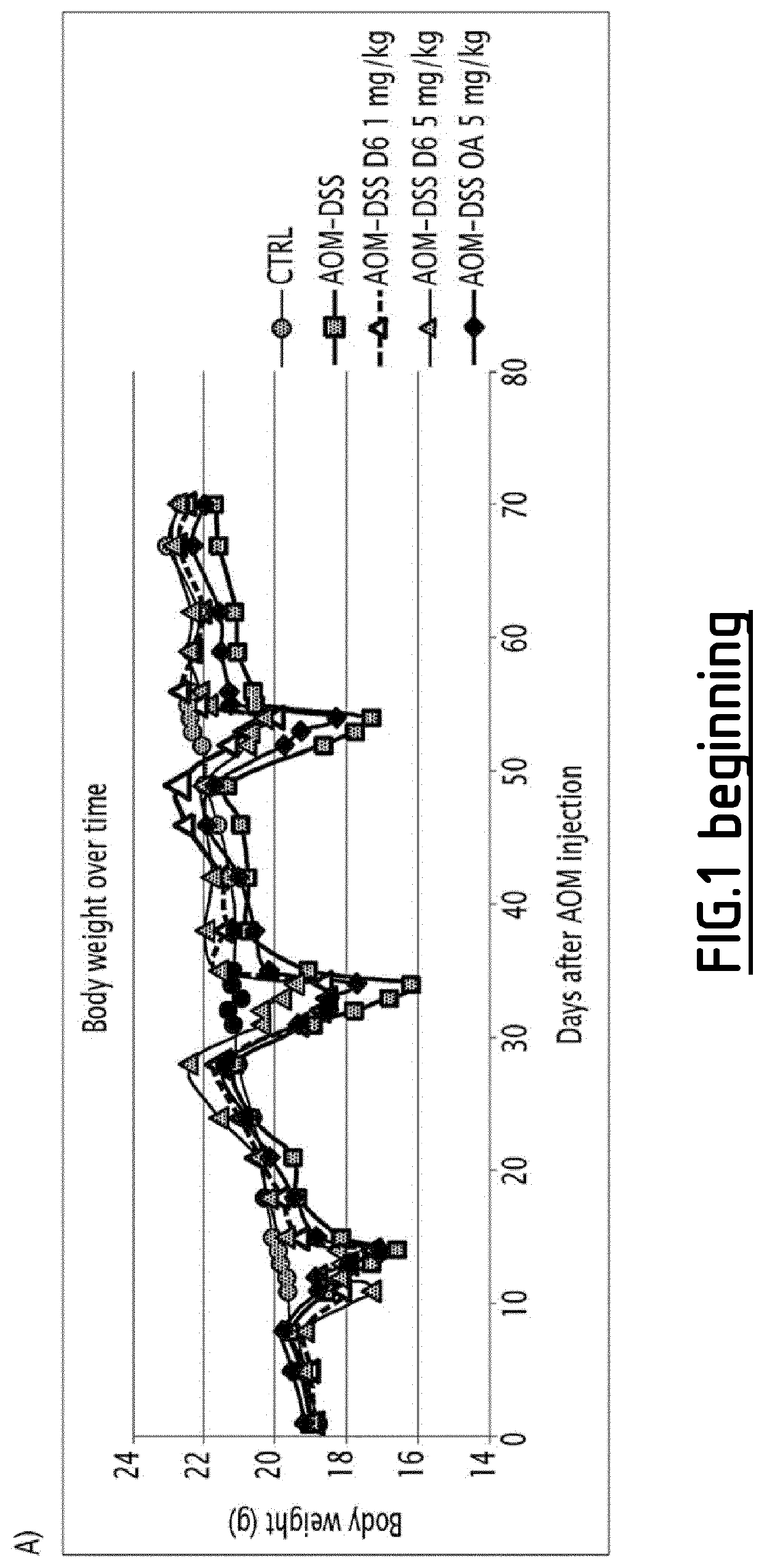
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Colon rectum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
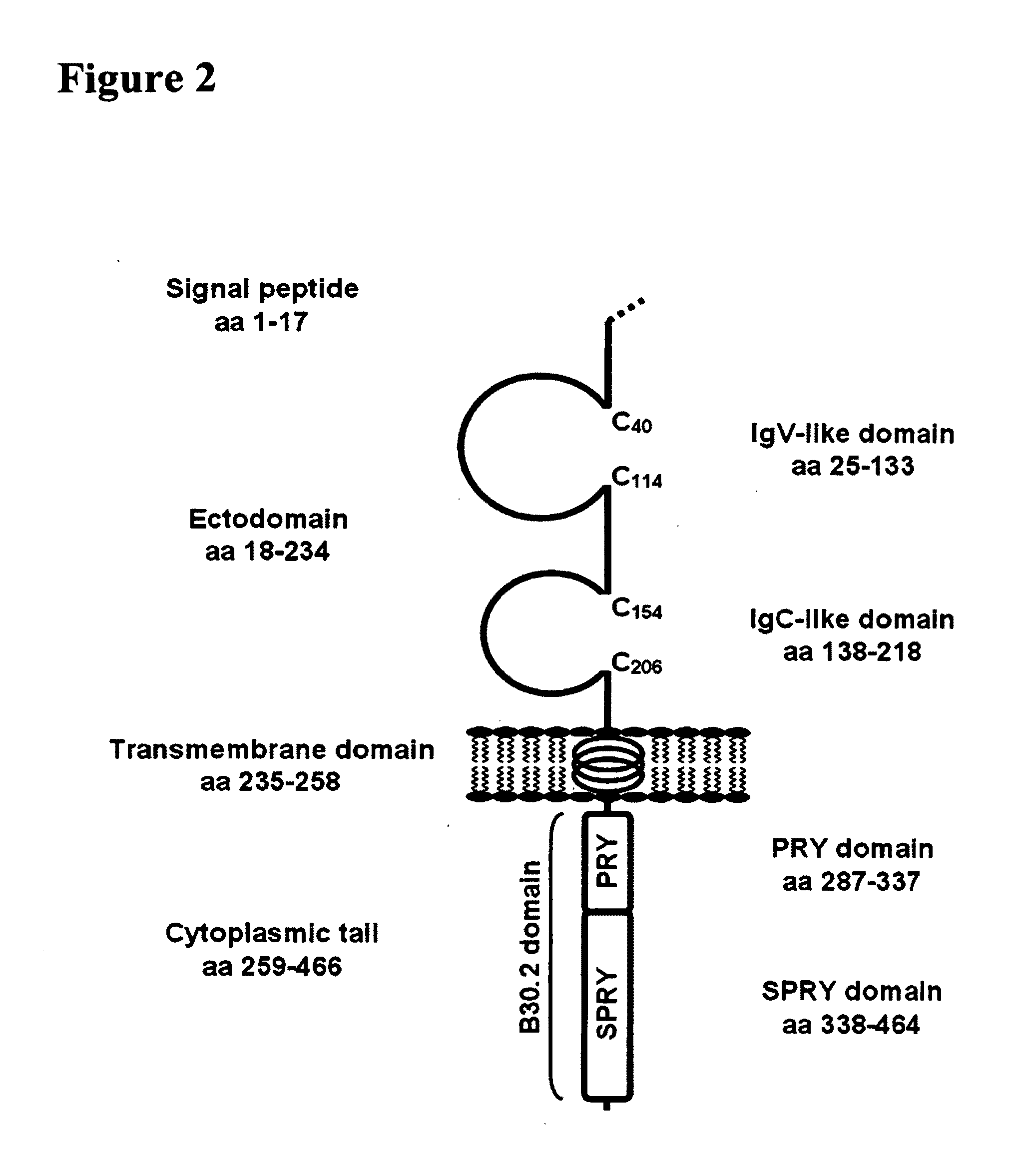
Molecular assay to predict recurrence of Duke's B colon cancer
A method of providing a prognosis of colorectal cancer is conducted by analyzing the expression of a group of genes. Gene expression profiles in a variety of medium such as microarrays are included as are kits that contain them.
Owner:VERIDEX LCC
Methods and compositions for diagnosis and treatment of cancer
ActiveUS20110318264A1Level avoidInhibition productionOrganic active ingredientsFungiDiseaseWilms' tumor
The present invention relates to the identification of nucleic acid and amino acid sequences that are characteristic of colorectal, in particular colonic, and gastric tumor tissues and colorectal, in particular colonic, and gastric tissues, and which represent targets for therapy or diagnosis of such tumor diseases in a subject.
Owner:BIONTECH SE +1
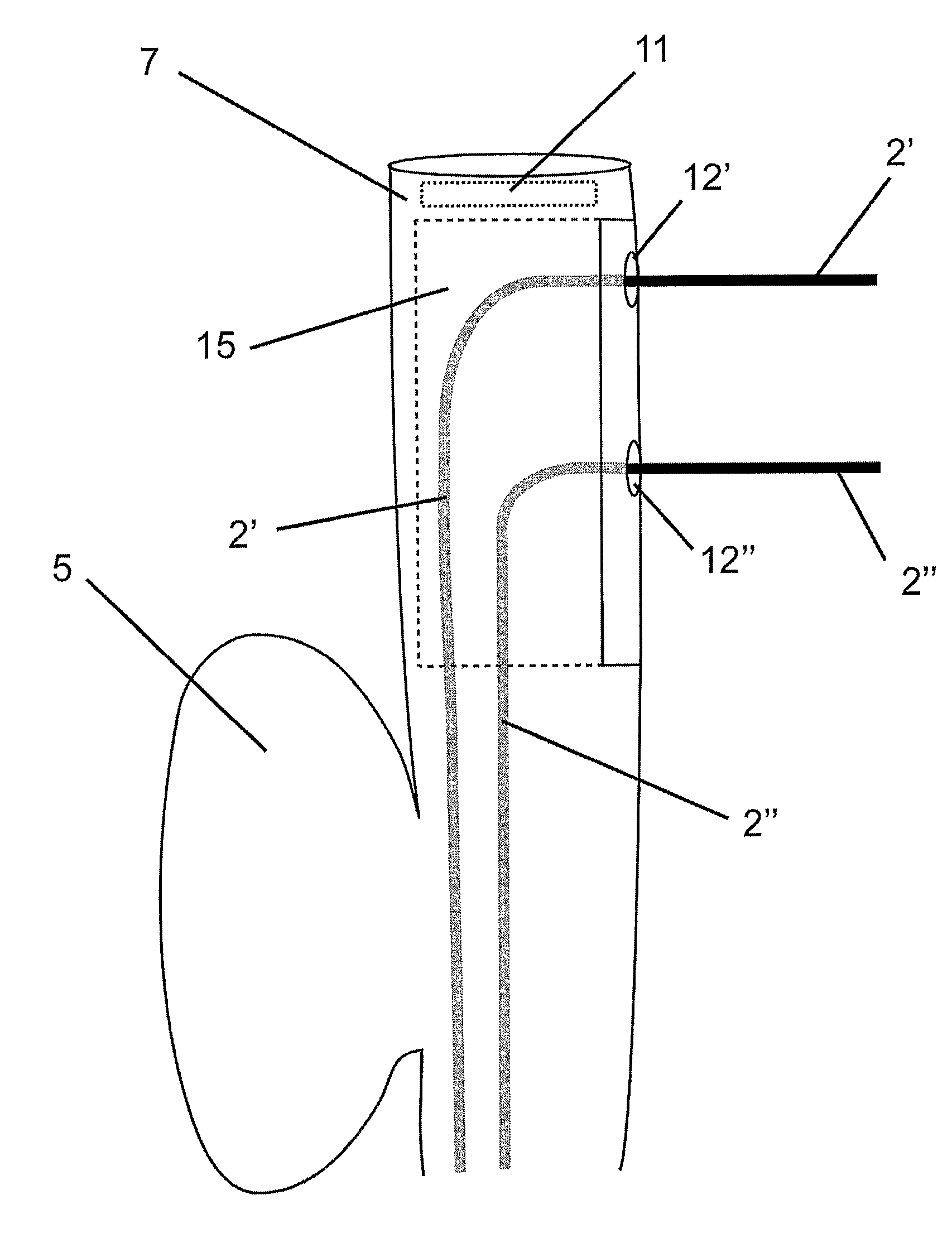
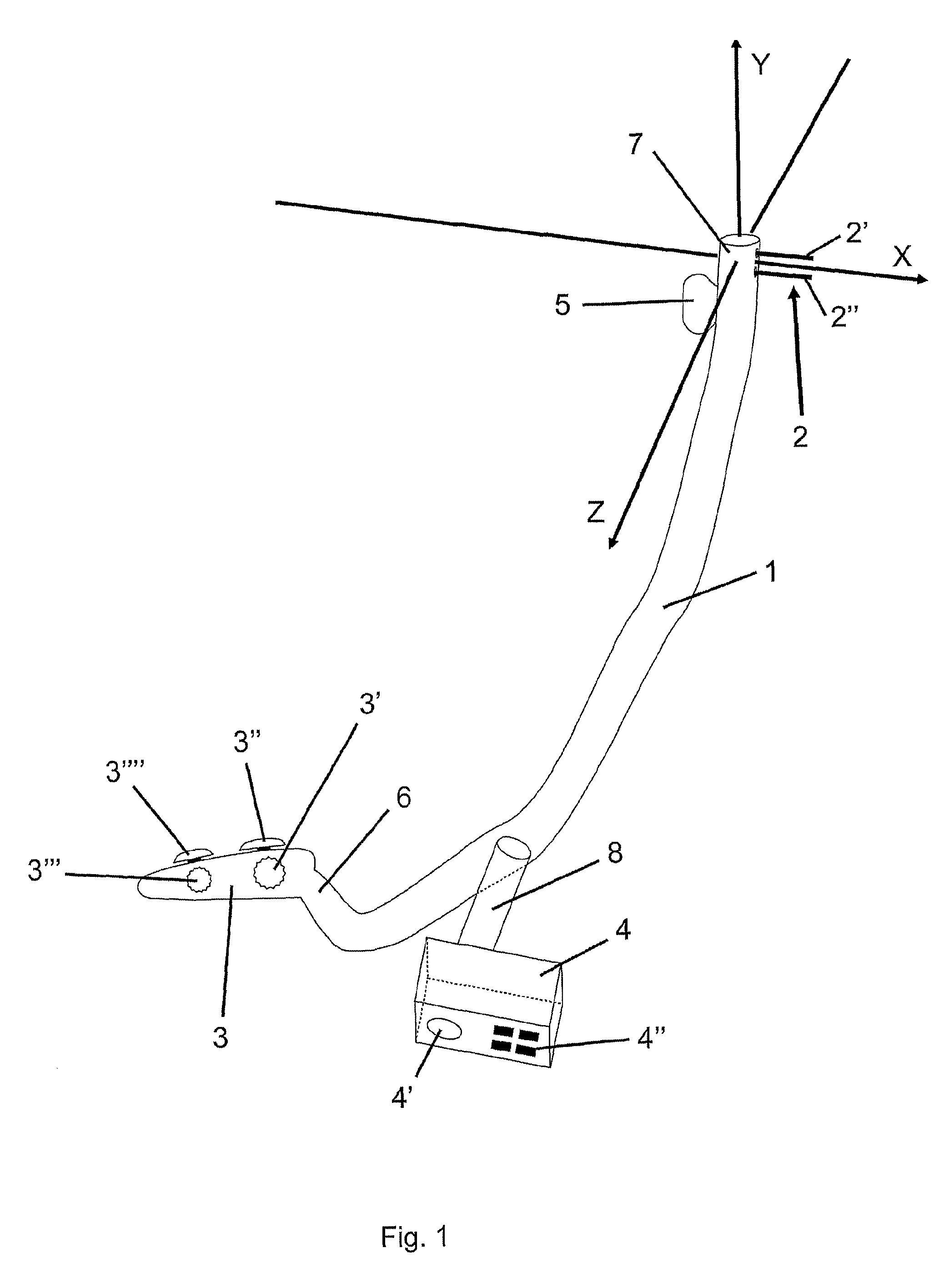
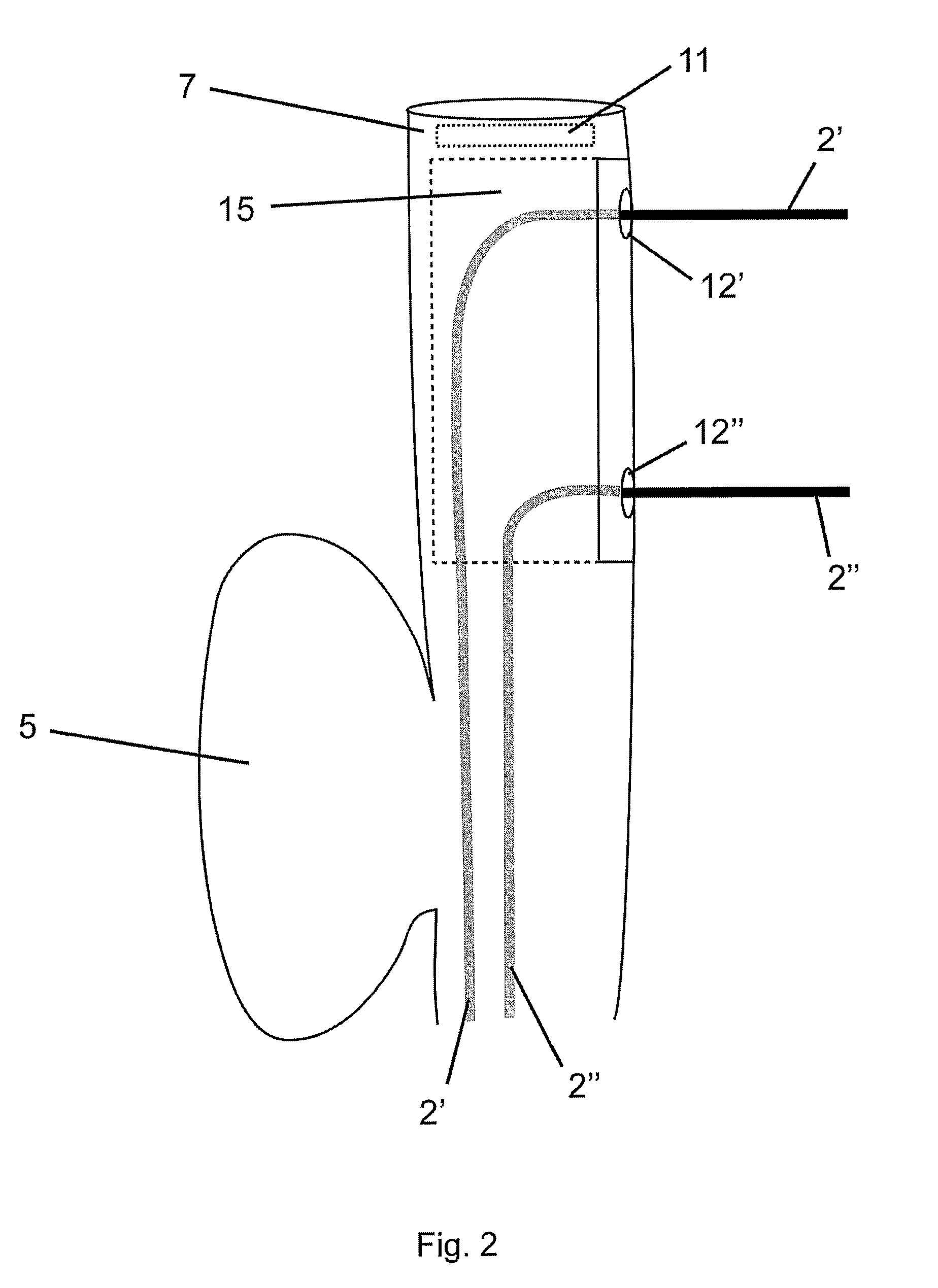
Flexible endoscope for endo luminal access radial frequency tumor ablation
A radio frequency (RF) treatment flexible endoscope, for the in depth destruction of tumors by endo luminal access, like in a lung, esophagus, colon, rectum, etc . . . The electrodes (2′,2″) working in bipolar mode, deploy laterally and in parallel from the endoscope main body (1), reaching a tumor deeply located in the organ parenchyma. The use of different disposable distal end portions (15) of the endoscope allows the deployment of the electrodes (2′,2″) at different distances one from each other, in order to fit the destruction are to the tumor's size and shape. The deployment of the parallel electrodes (2′,2″) from the endoscope's main body (1) is controlled by motor drives (4) or a mechanical handled system (3), in order to reach the tumor with near a millimeter precision. In order to fix the endoscope's distal end to the tissue, so to allow a perfect parallel deployment of the electrodes, an inflating cuff (5) is placed at the opposite side of the distal end, as well as a suction system, with its holes (21) placed near the electrodes' deployment location. An external RF generator and a imaging system are associated to the use of the device.
Owner:TROD MEDICAL NV
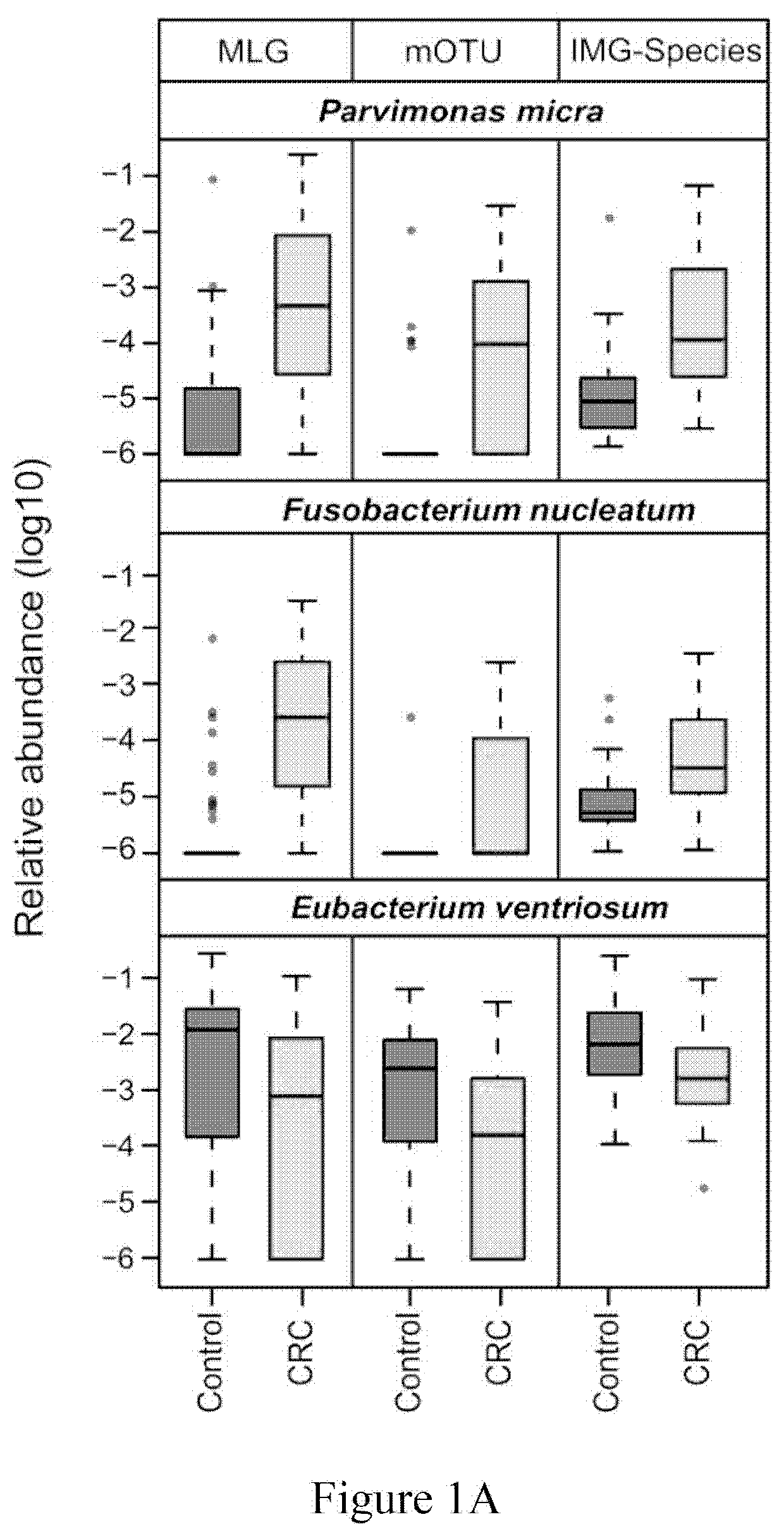
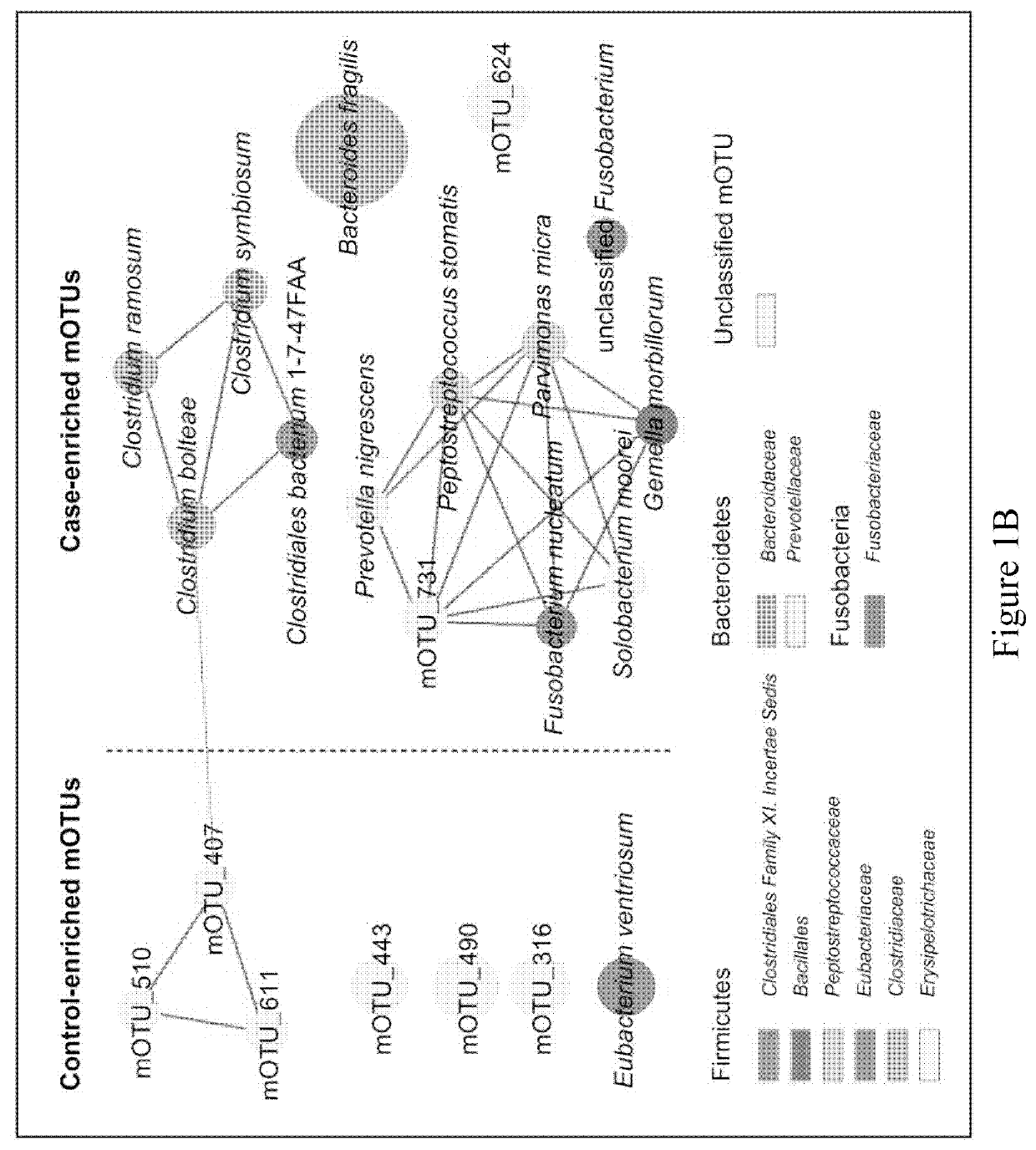
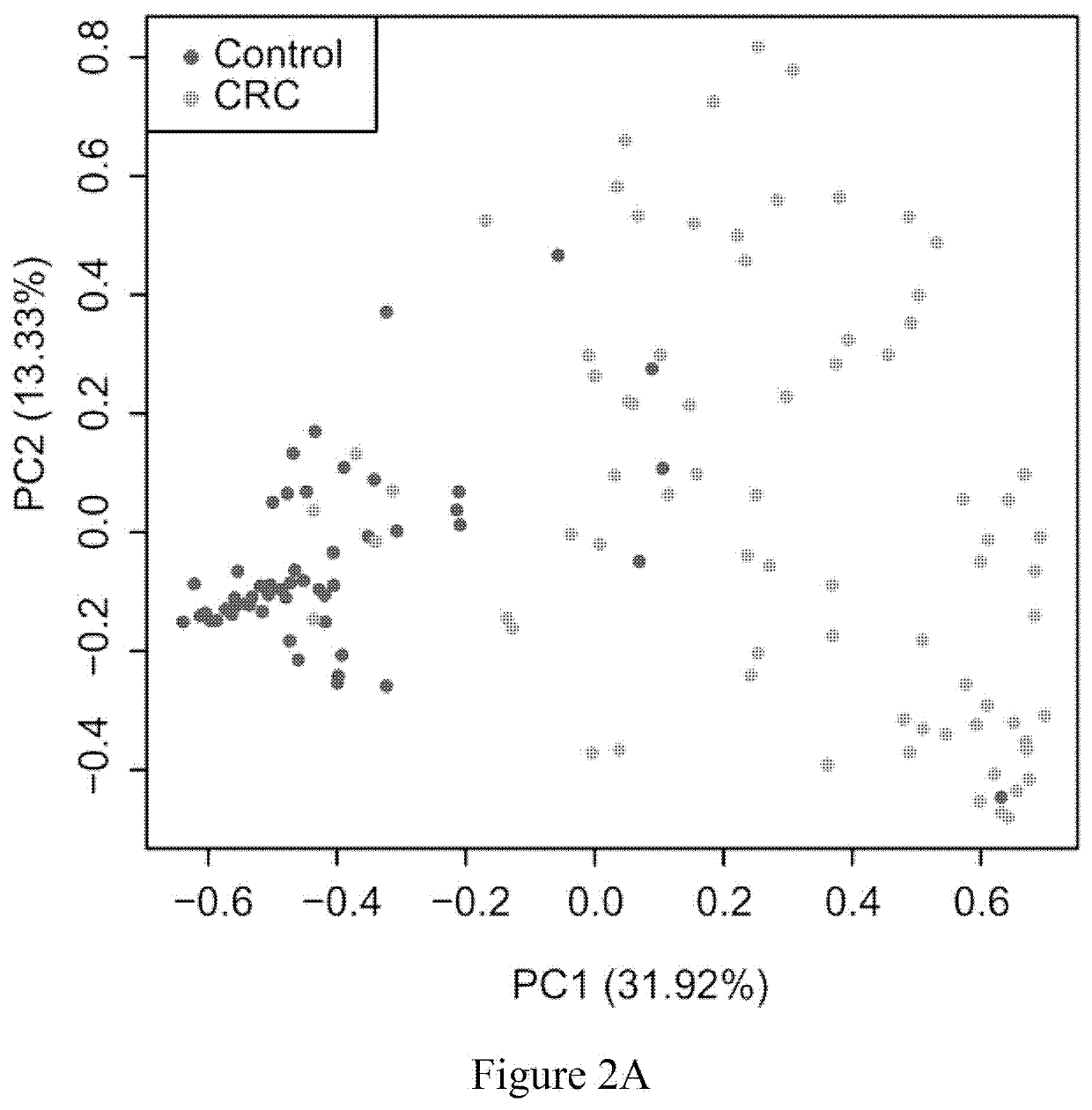
Fecal bacterial markers for colorectal cancer
Provided is a non-invasive method for diagnosing colorectal cancer in a subject by detecting enrichment or reduction of certain bacterial species. A kit and device useful for such methods are also provided. In addition, a method for reducing the risk of colon cancer by regulating the pertinent bacterial species in human colon is also provided.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Noninvasive detection of colorectal cancer and other gastrointestinal pathology
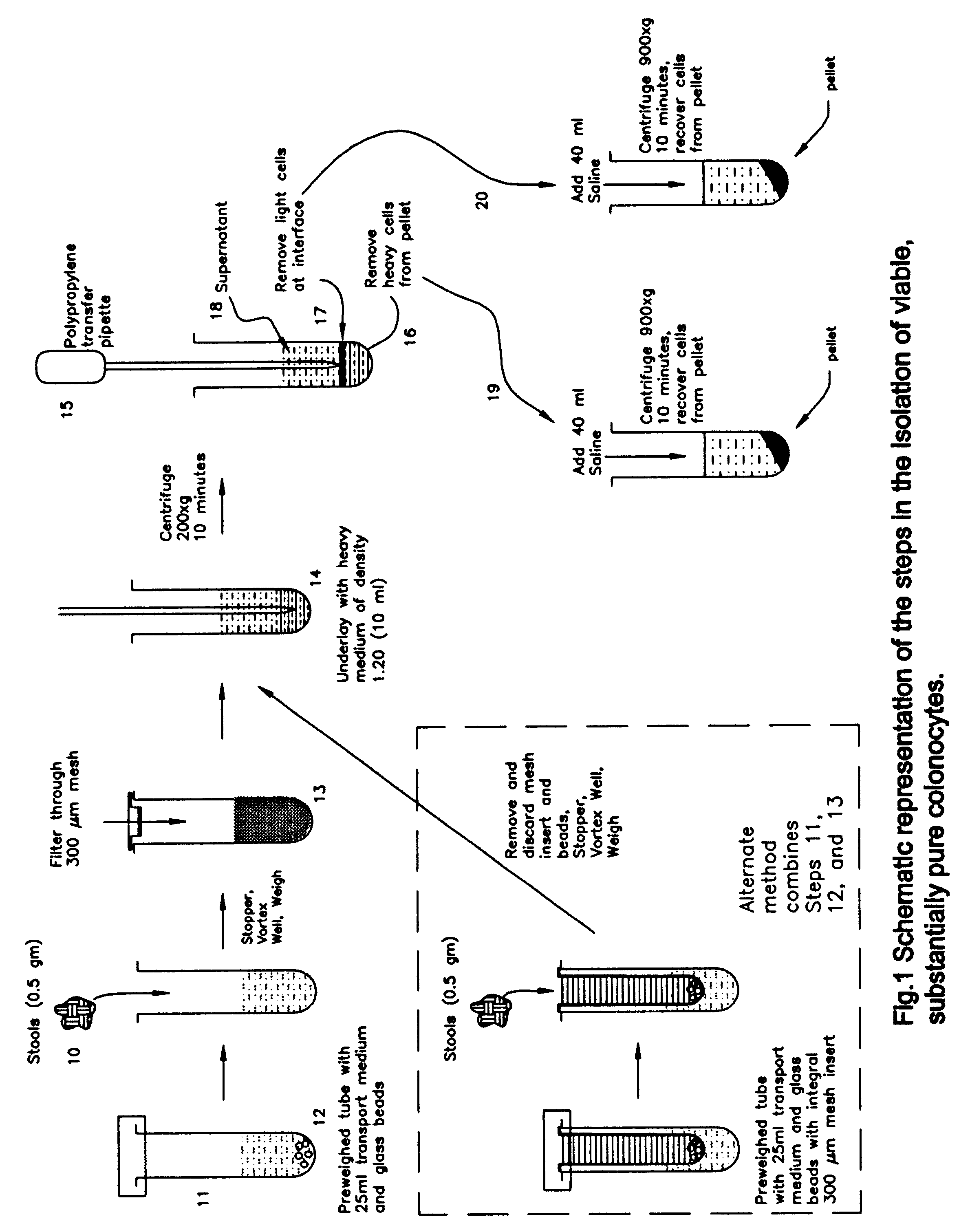
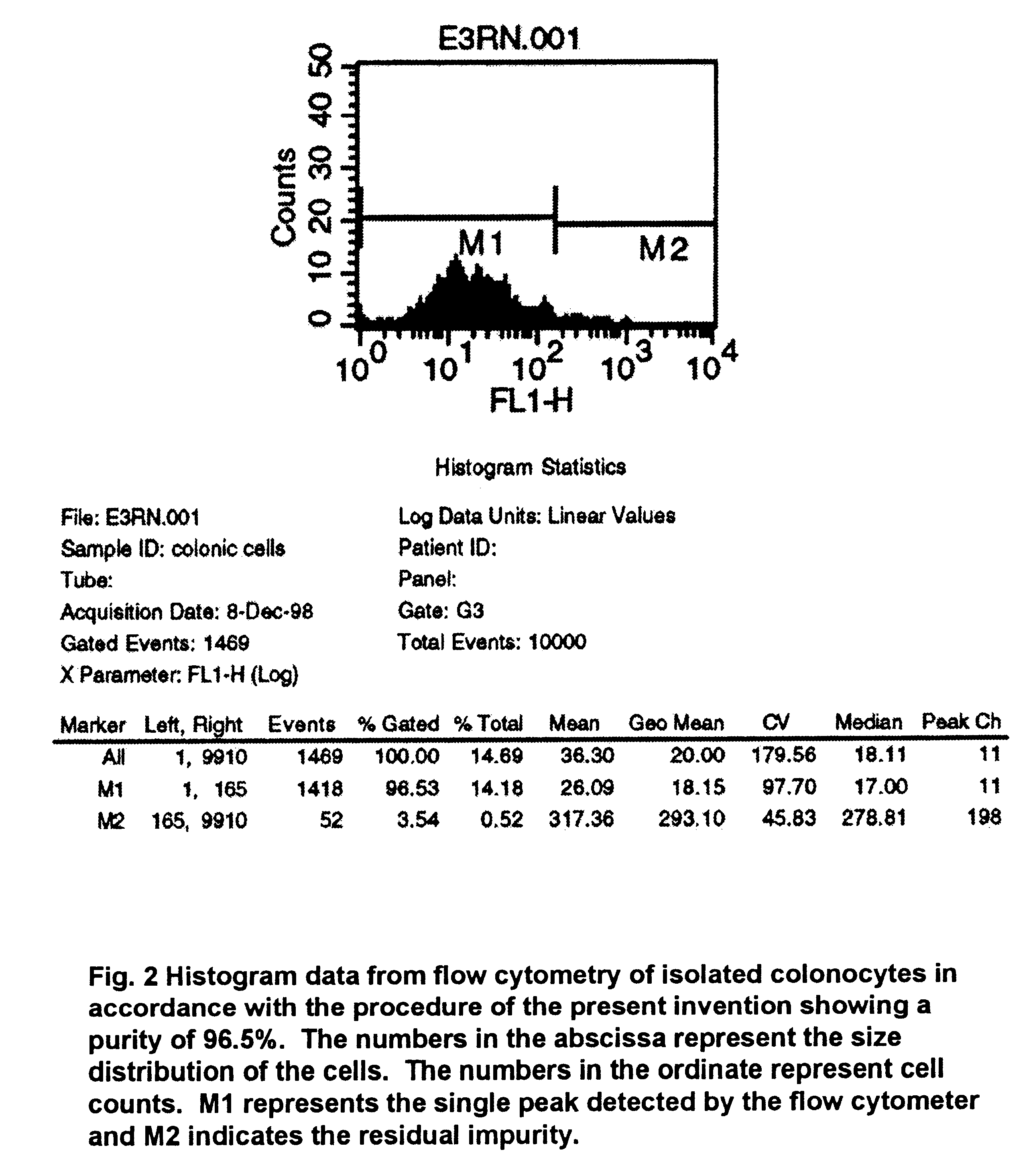
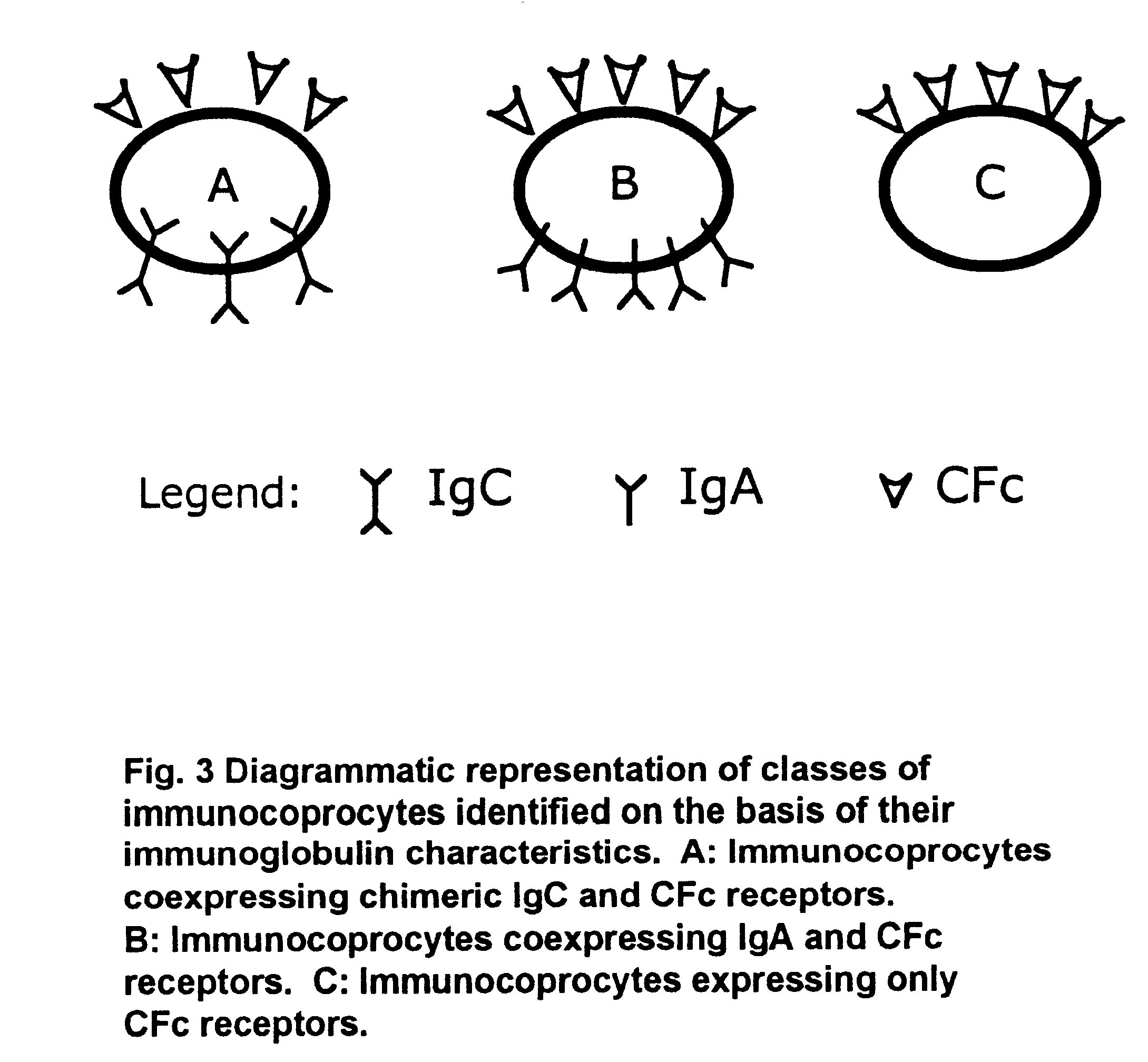
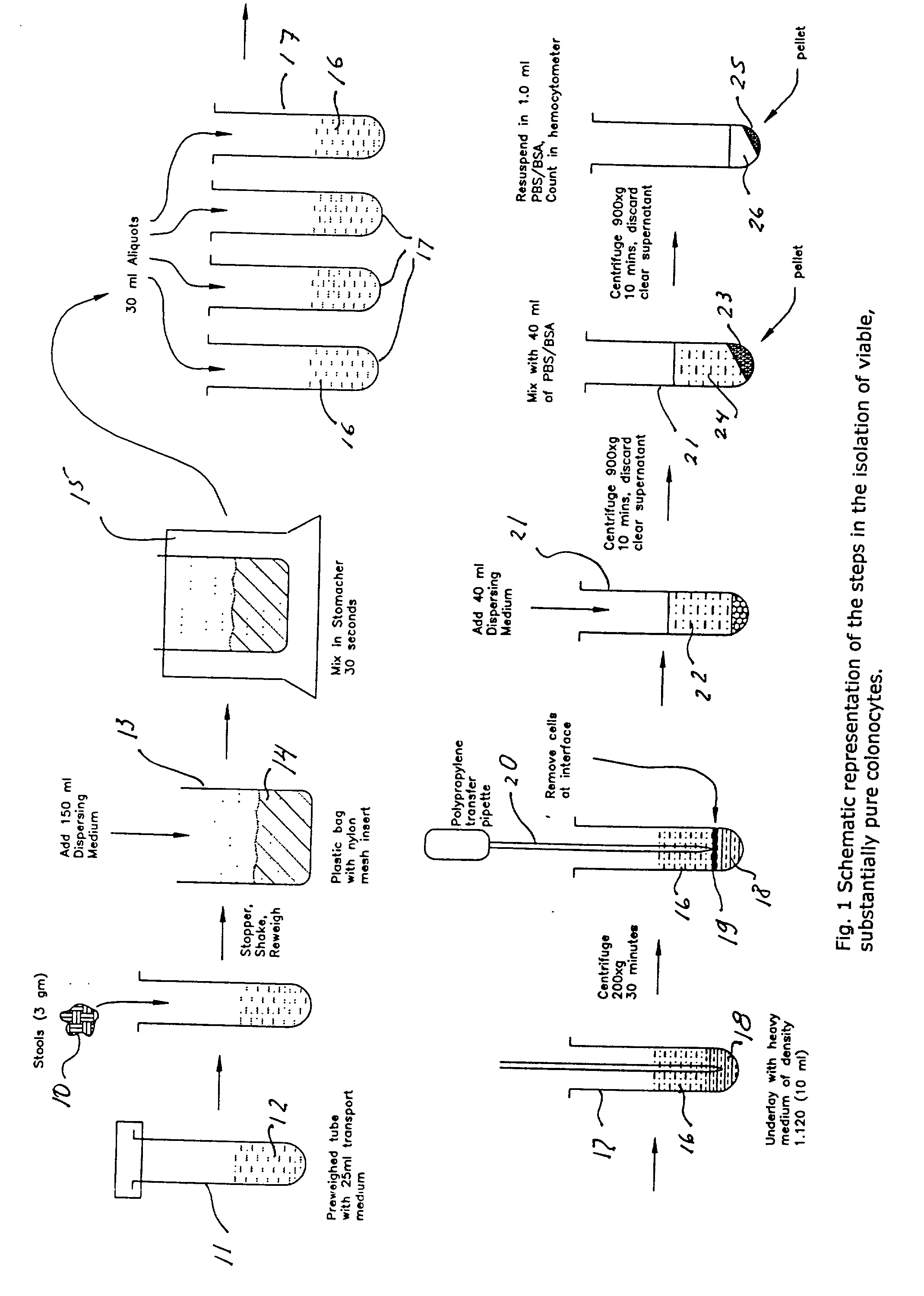
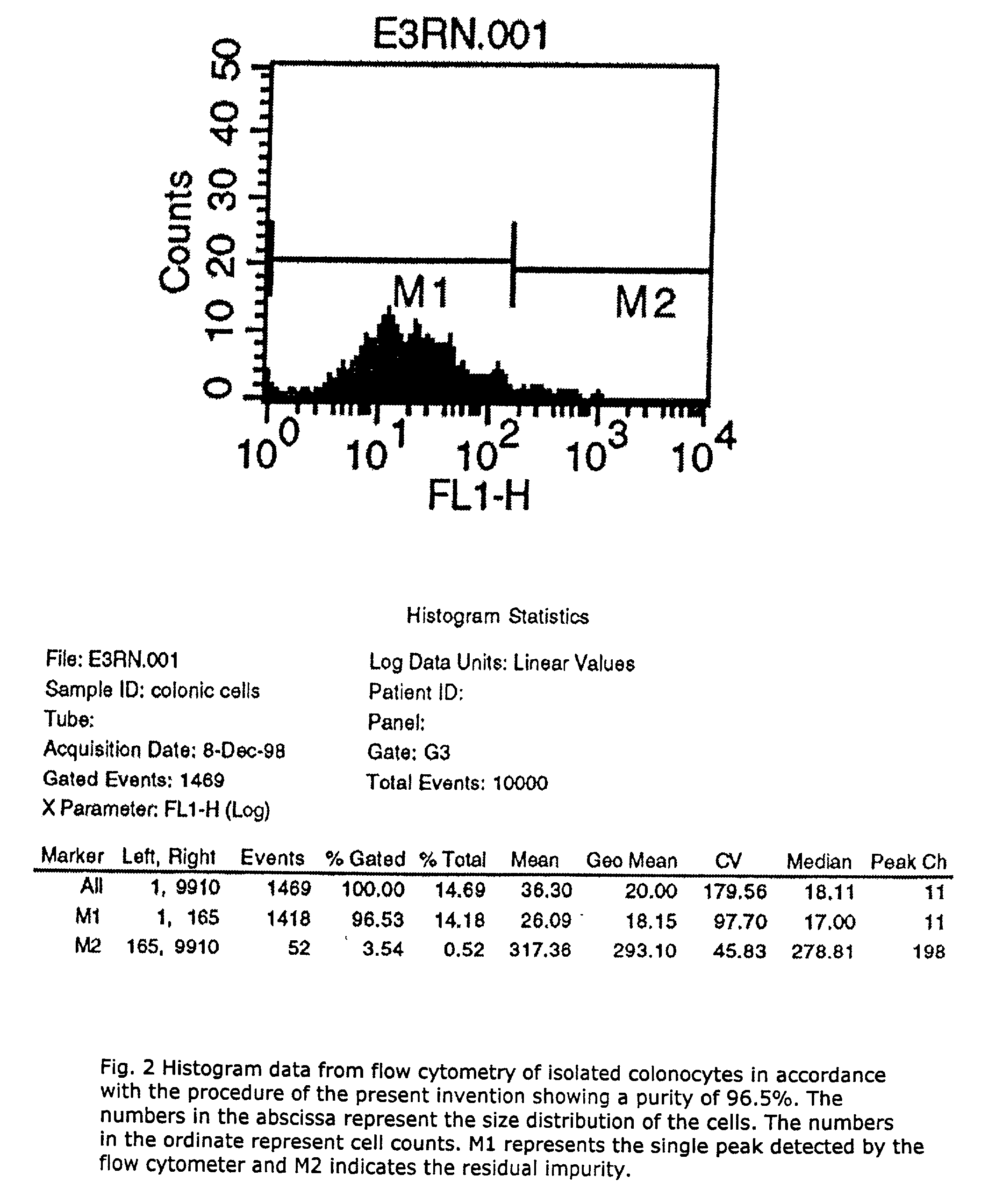
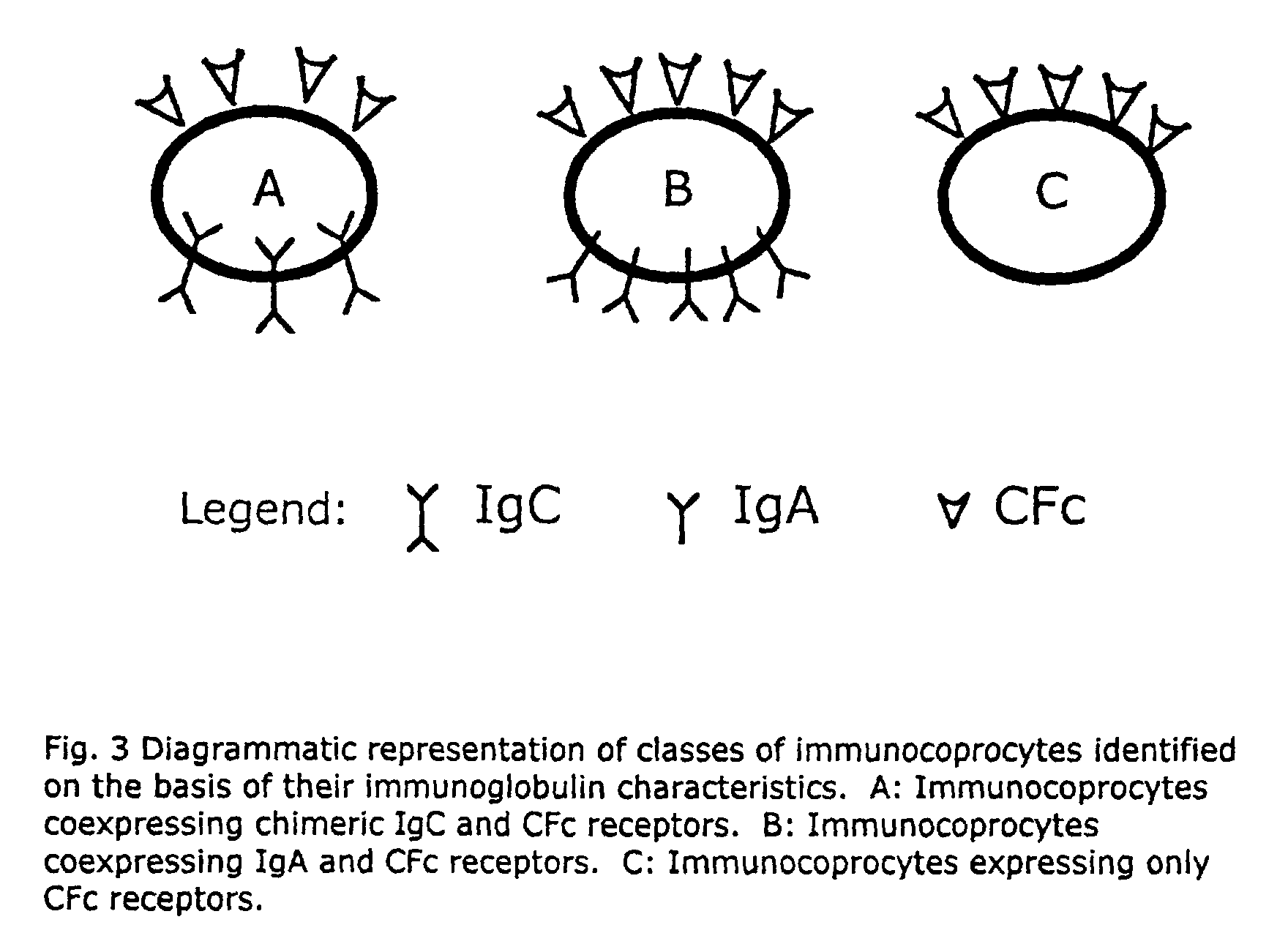
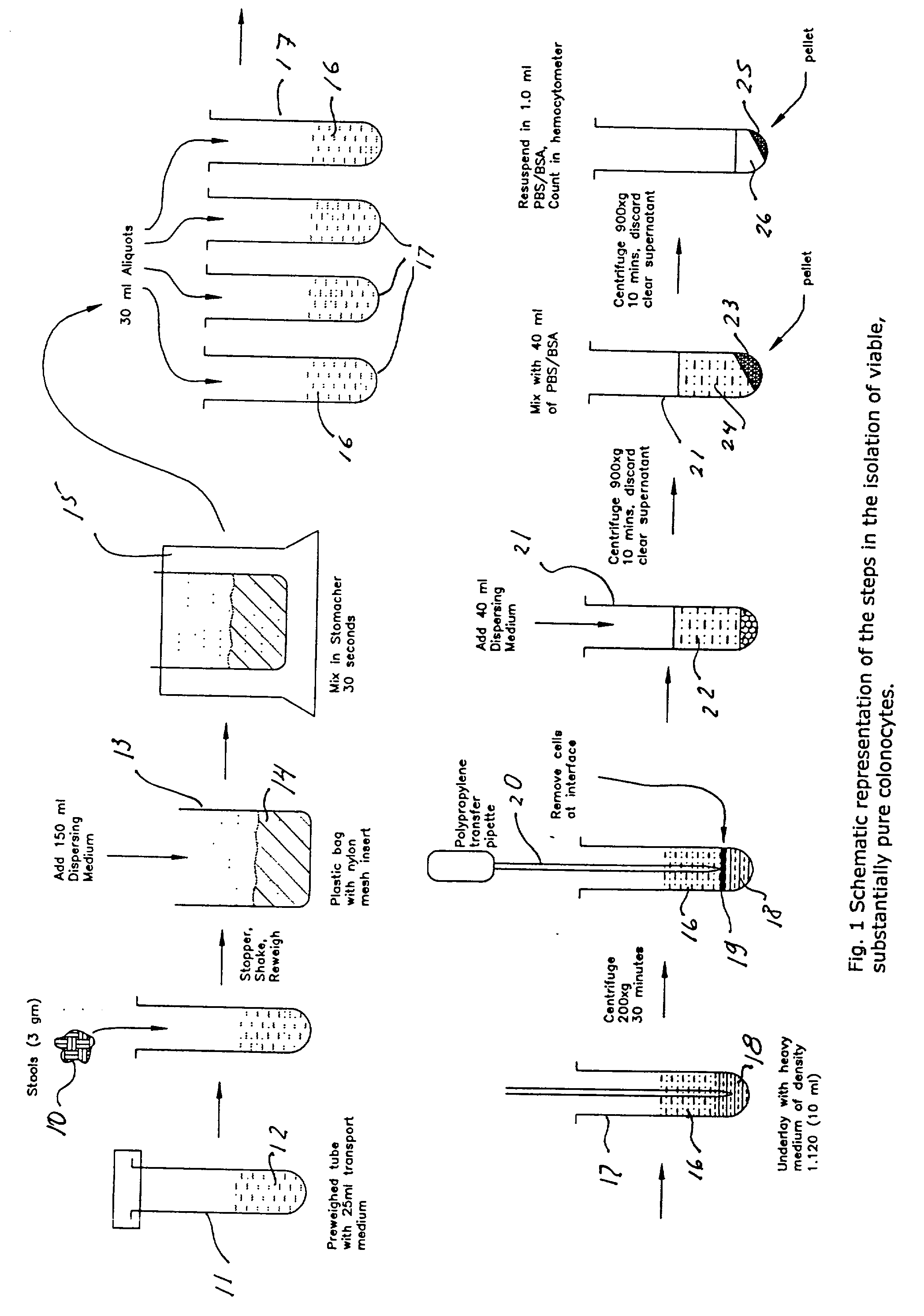
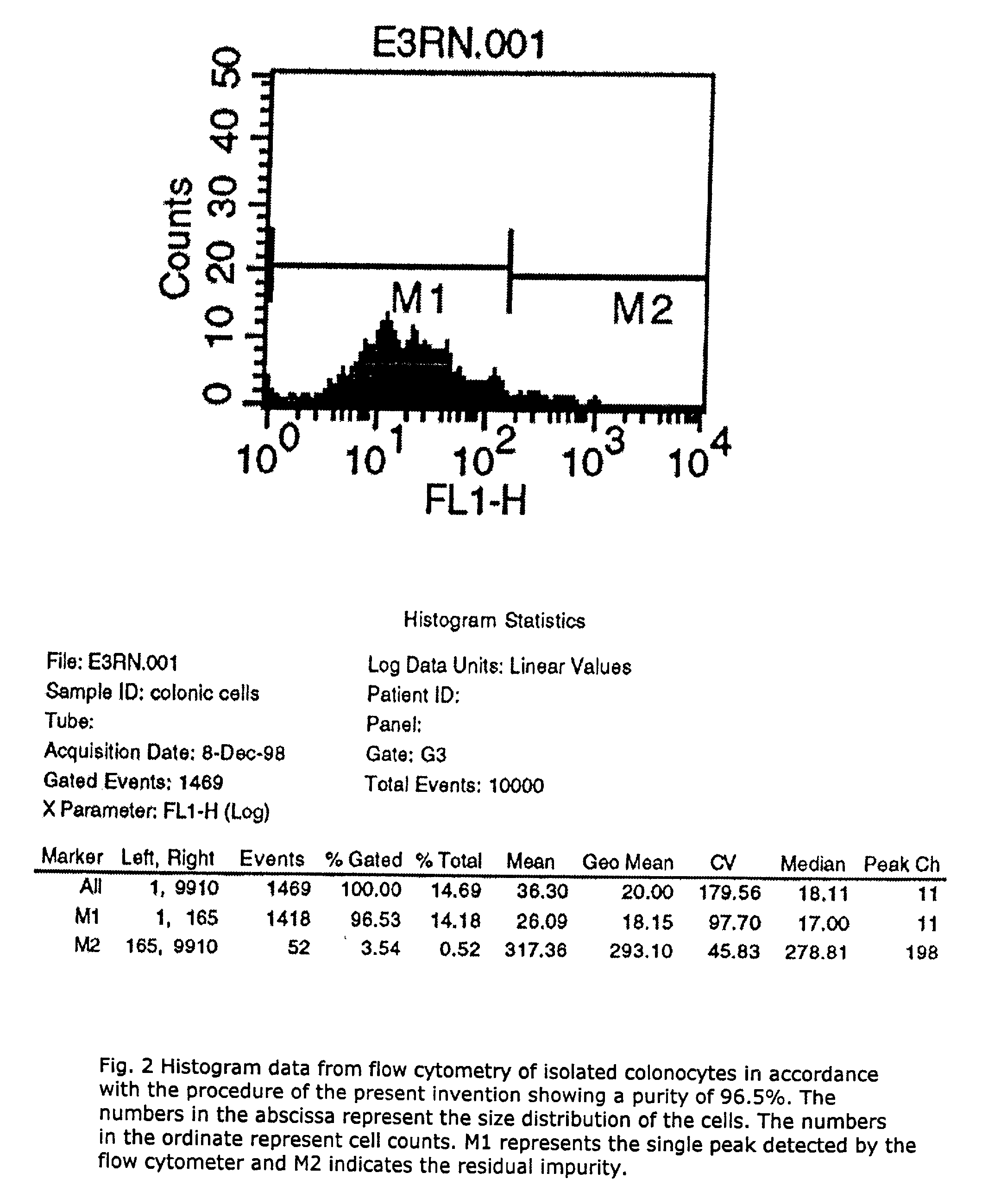
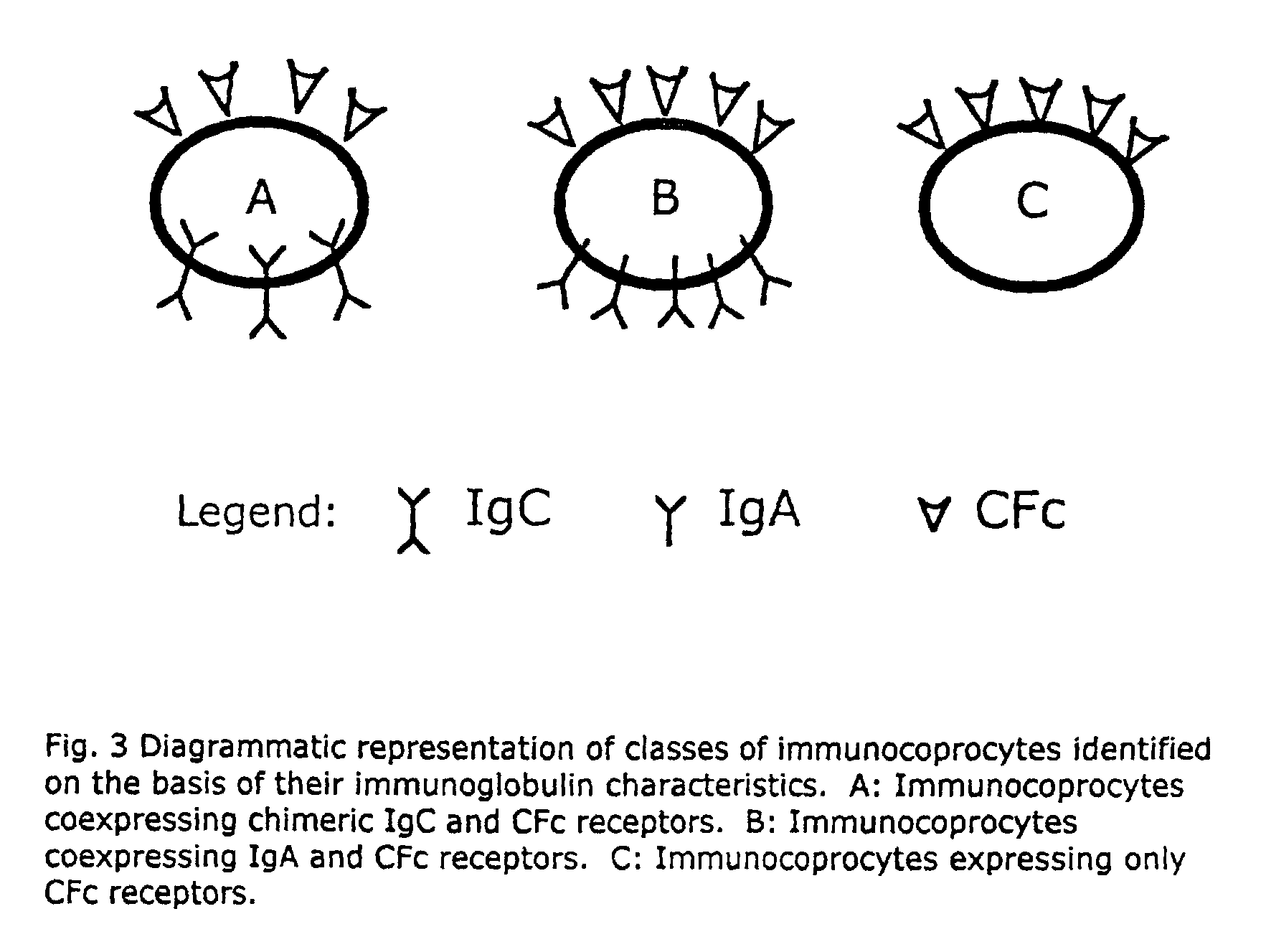
A method for isolating viable, biologically substantially pure exfoliated fecal colonocytes at normal ambient temperature is described. Immunocoprocytes and inflammatory cells indicative of certain gastrointestinal conditions and a noninvasive method for detecting colorectal cancer are set forth. Composition of transport and suspension media for isolation of colonocytes are detailed.
Owner:NAIR PADMANABHAN P
Noninvasive detection of colorectal cancer and other gastrointestinal pathology
InactiveUS20010024801A1Gastrointestinal cellsMicrobiological testing/measurementInflammatory cellColorectal cancer
A method for isolating viable, biologically substantially pure exfoliated fecal colonocytes at normal ambient temperature is described. Immunocoprocytes and inflammatory cells indicative of certain gastrointestinal conditions and a noninvasive method for detecting colorectal cancer are set forth. Composition of transport and suspension media for isolation of colonocytes are detailed.
Owner:NAIR PADMANABHAN P
Methods for detecting colon carcinoma
InactiveUS20090170132A1Reduce the possibilityMicrobiological testing/measurementBiological testingColon carcinomaExtent of resection
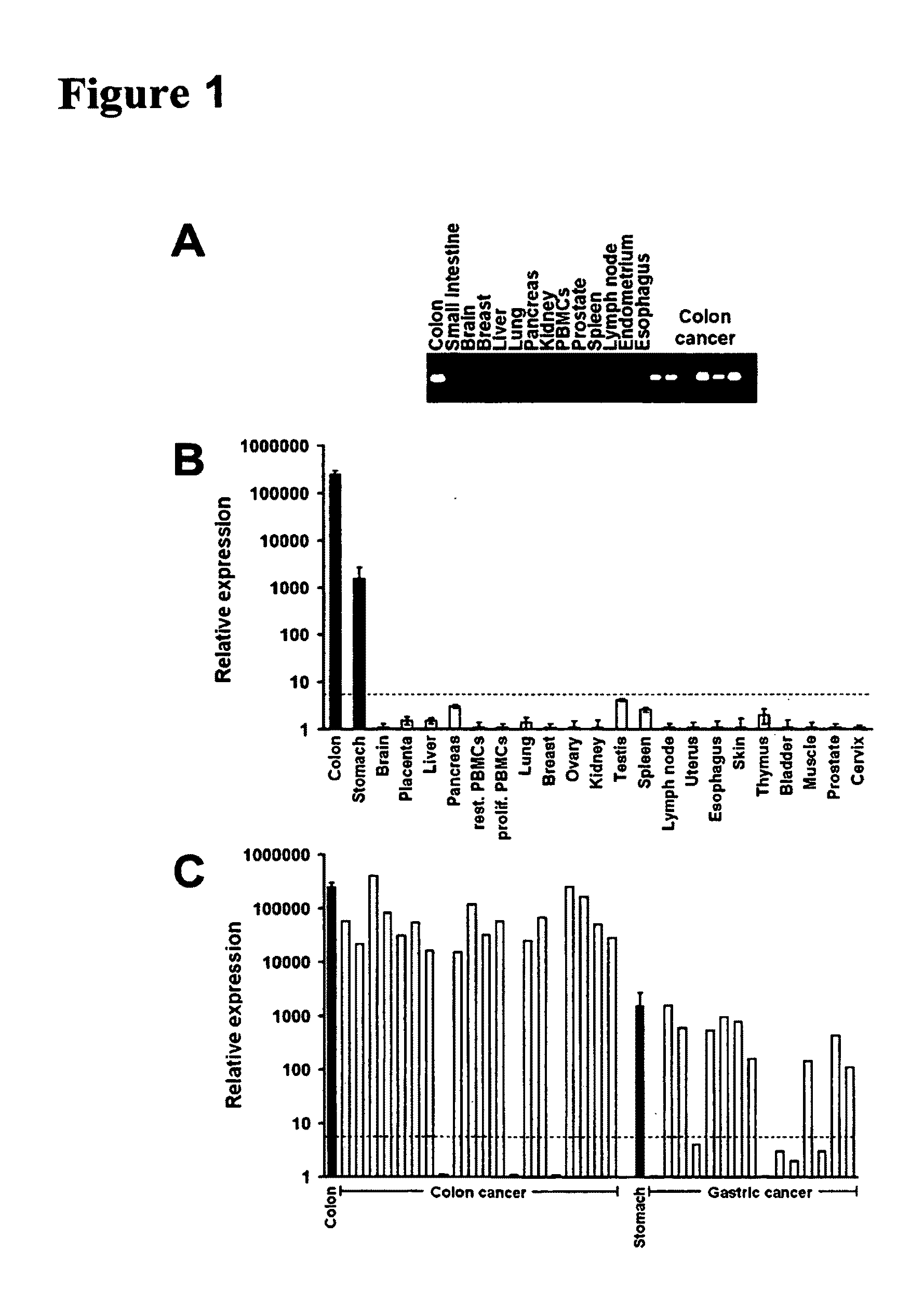
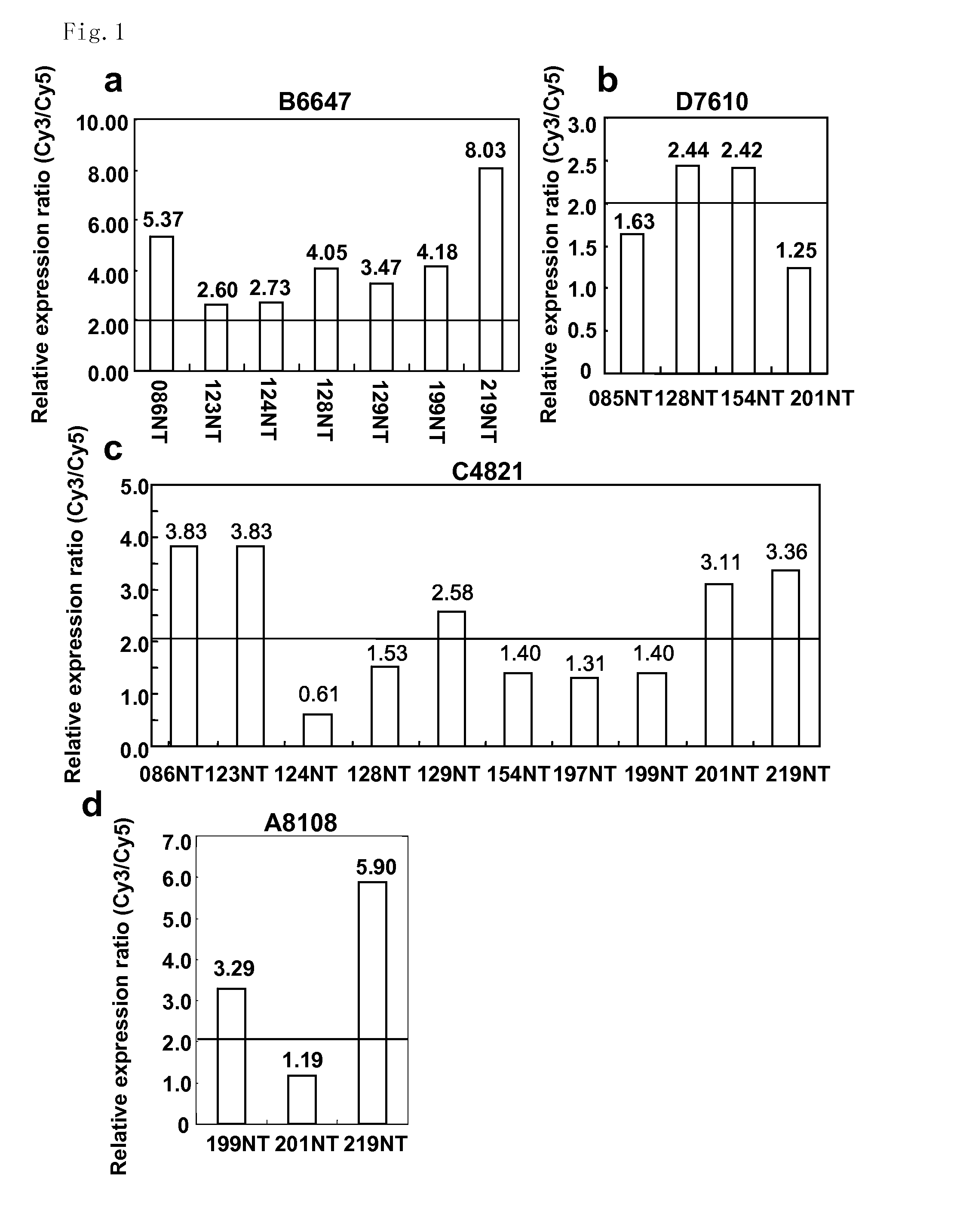
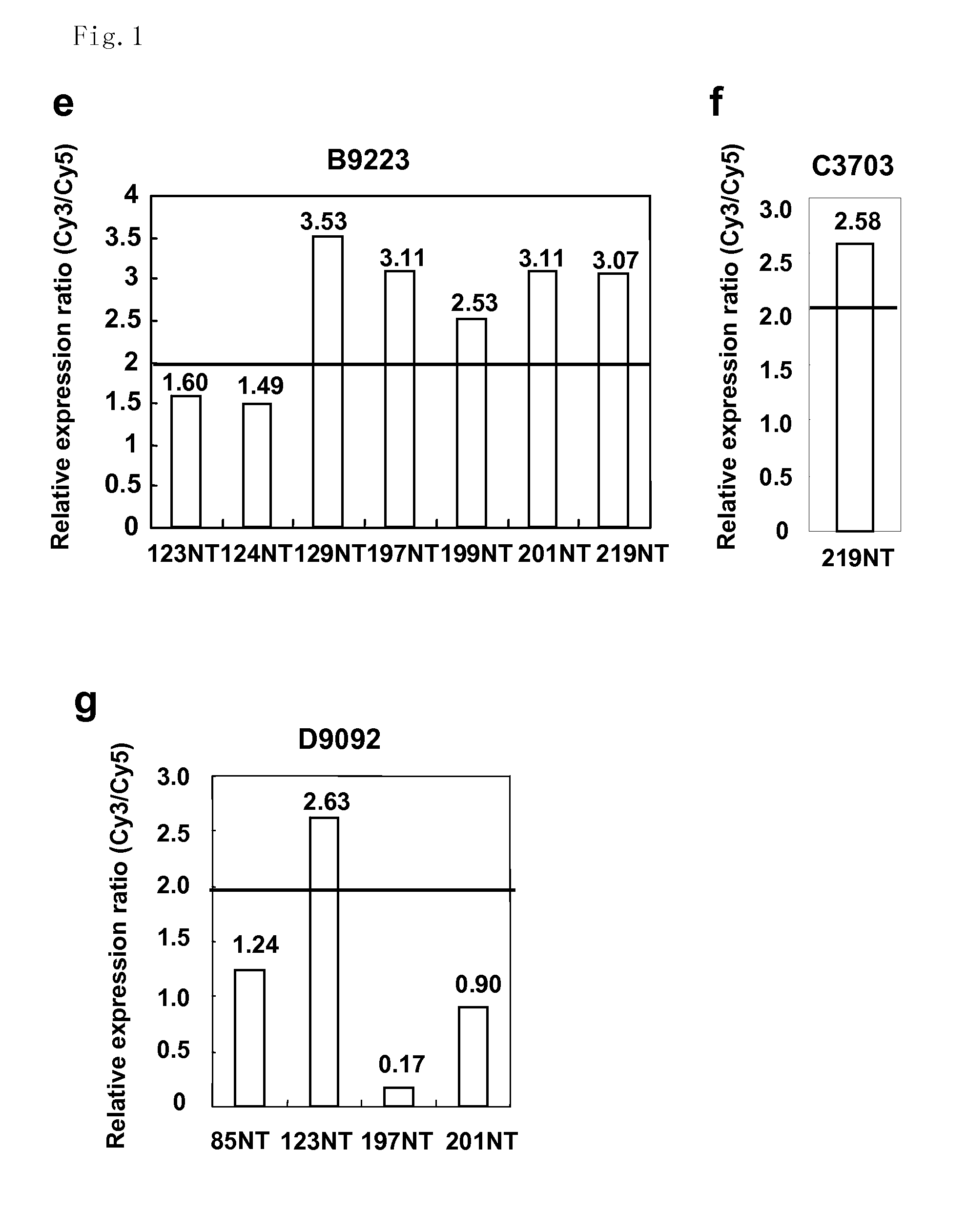
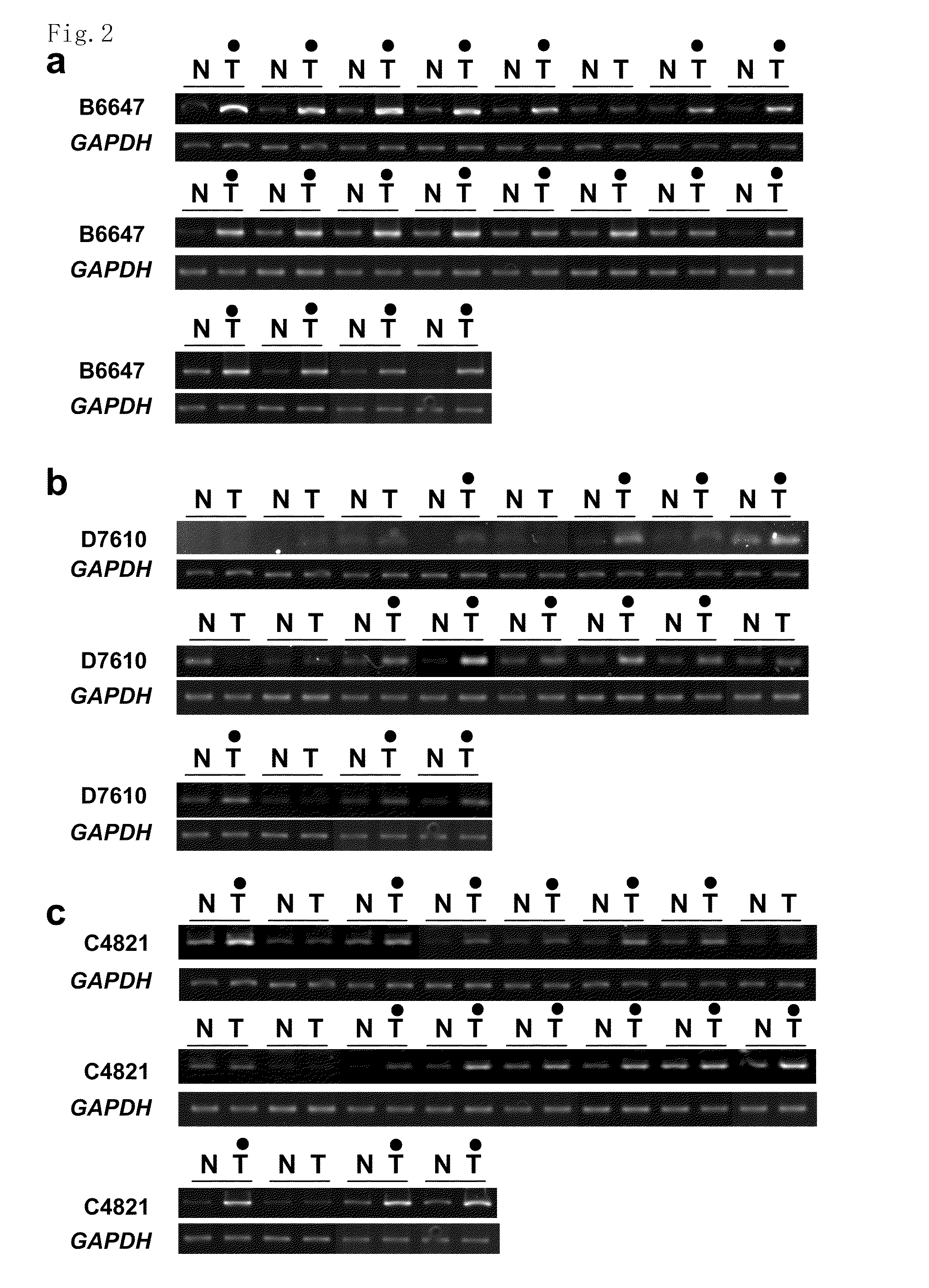
The present invention provides methods for diagnosing carcinoma, providing a prognosis for a carcinoma or assessing the likelihood that a tissue may become cancerous by identifying the presence or absence of or determining the amount of one or more carcinoma associated markers. Further, the present invention provides methods for determining whether a tissue should be surgically resected and for determining the territorial extent of resection. The carcinoma markers may be those provided in FIG. 1. The present invention also provides a diagnostic kit for diagnosing carcinoma, providing a prognosis for a carcinoma or assessing the likelihood that a tissue may become cancerous by identifying the presence of or determining the amount of one or more carcinoma associated markers. The methods and kits are especially useful regarding colon, rectal or colorectal carcinoma.
Owner:PEVSNER PAUL H
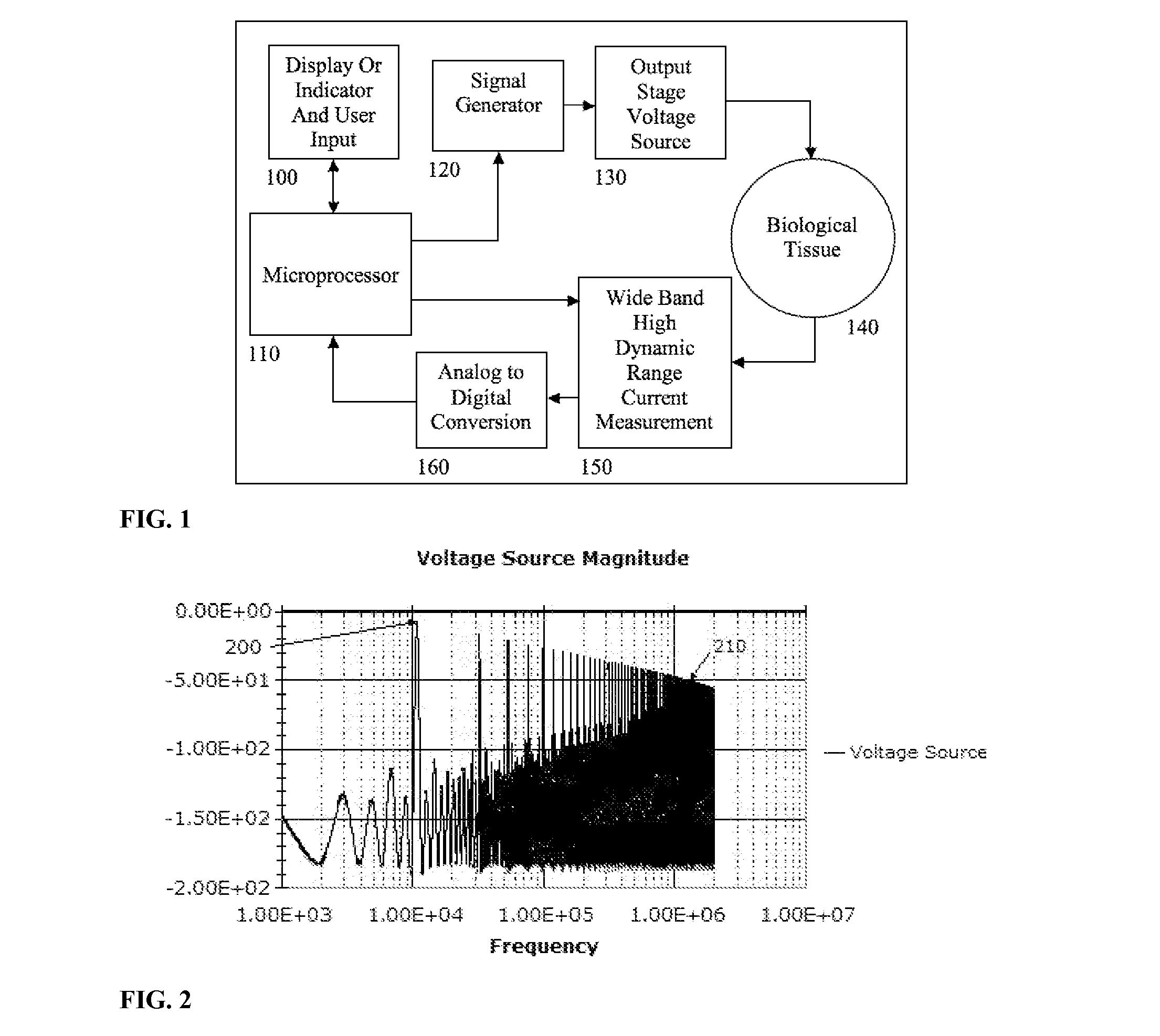
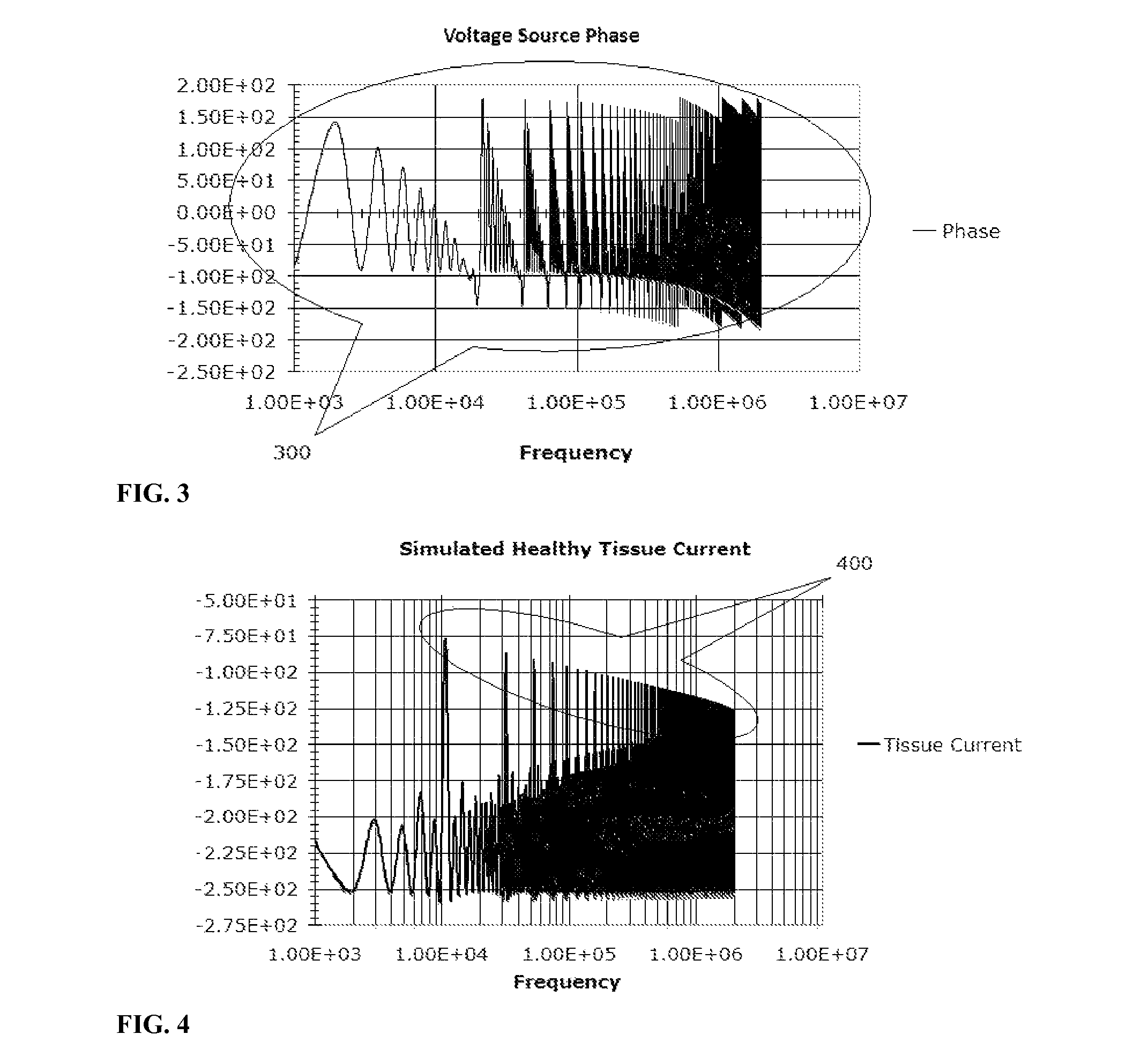
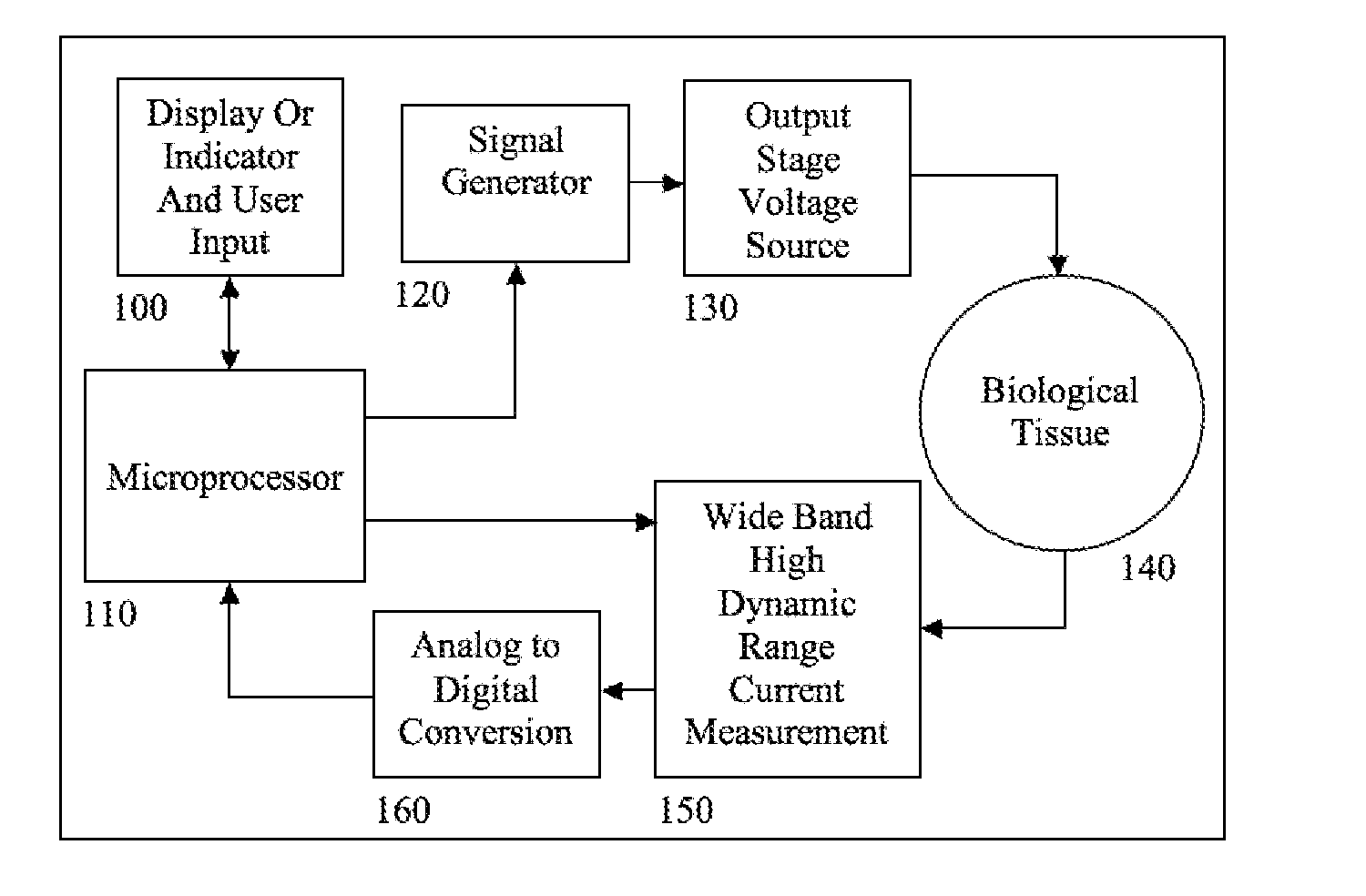
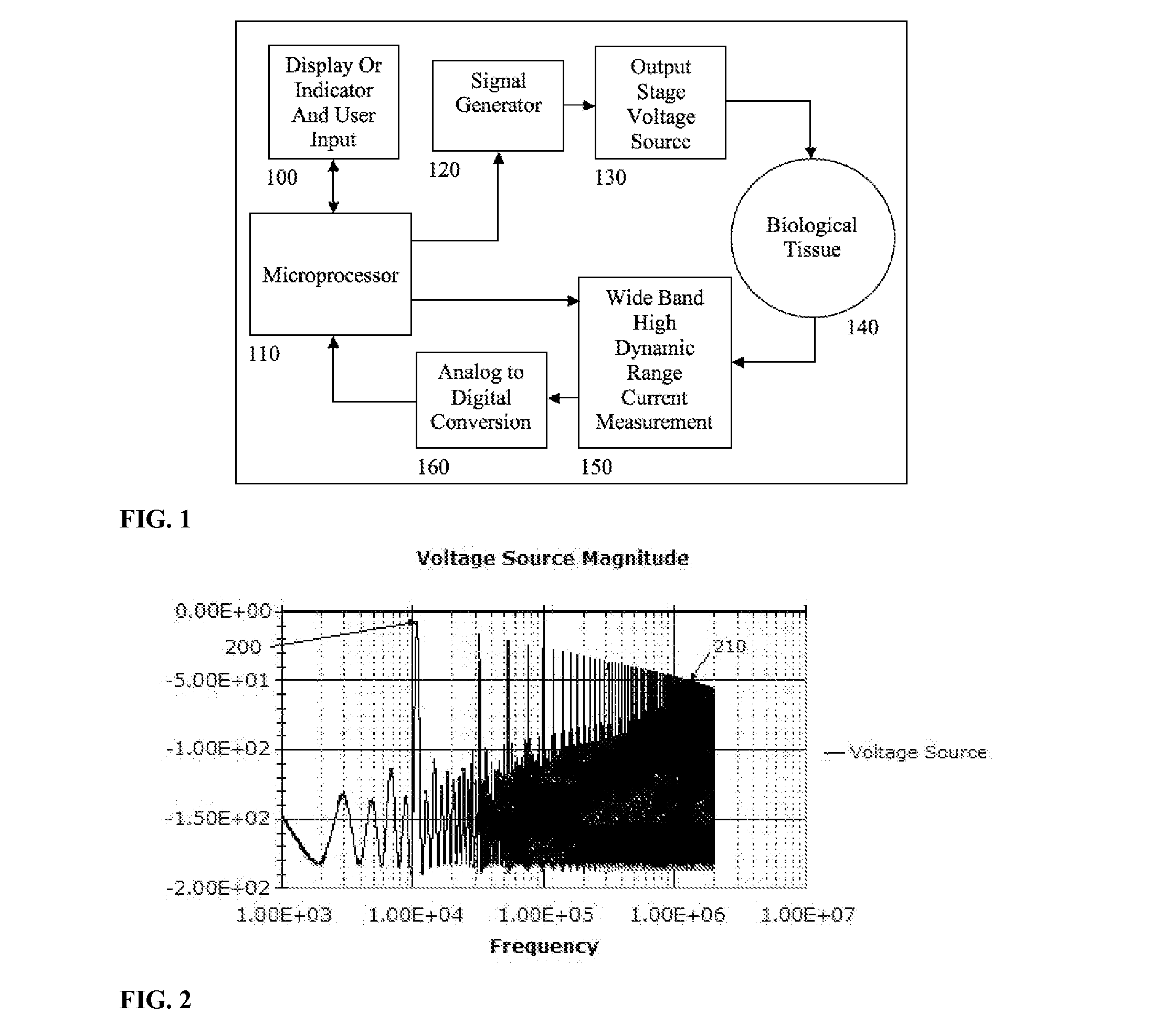
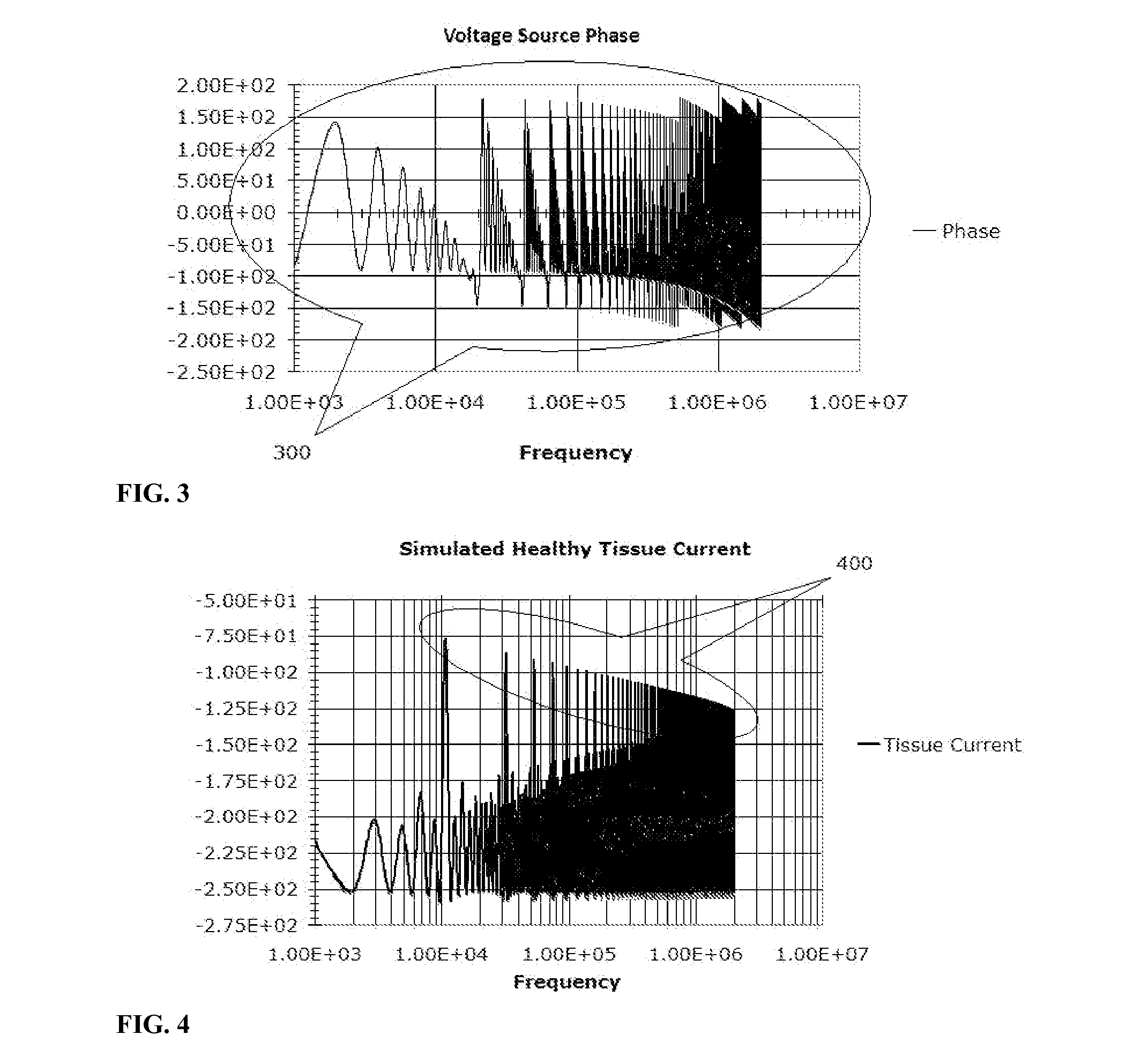
Electrical impedance techniques in tissue-mass detection and characterization
ActiveUS9445742B2Low costOrgan movement/changes detectionDiagnostic recording/measuringFrequency spectrumCancer cell
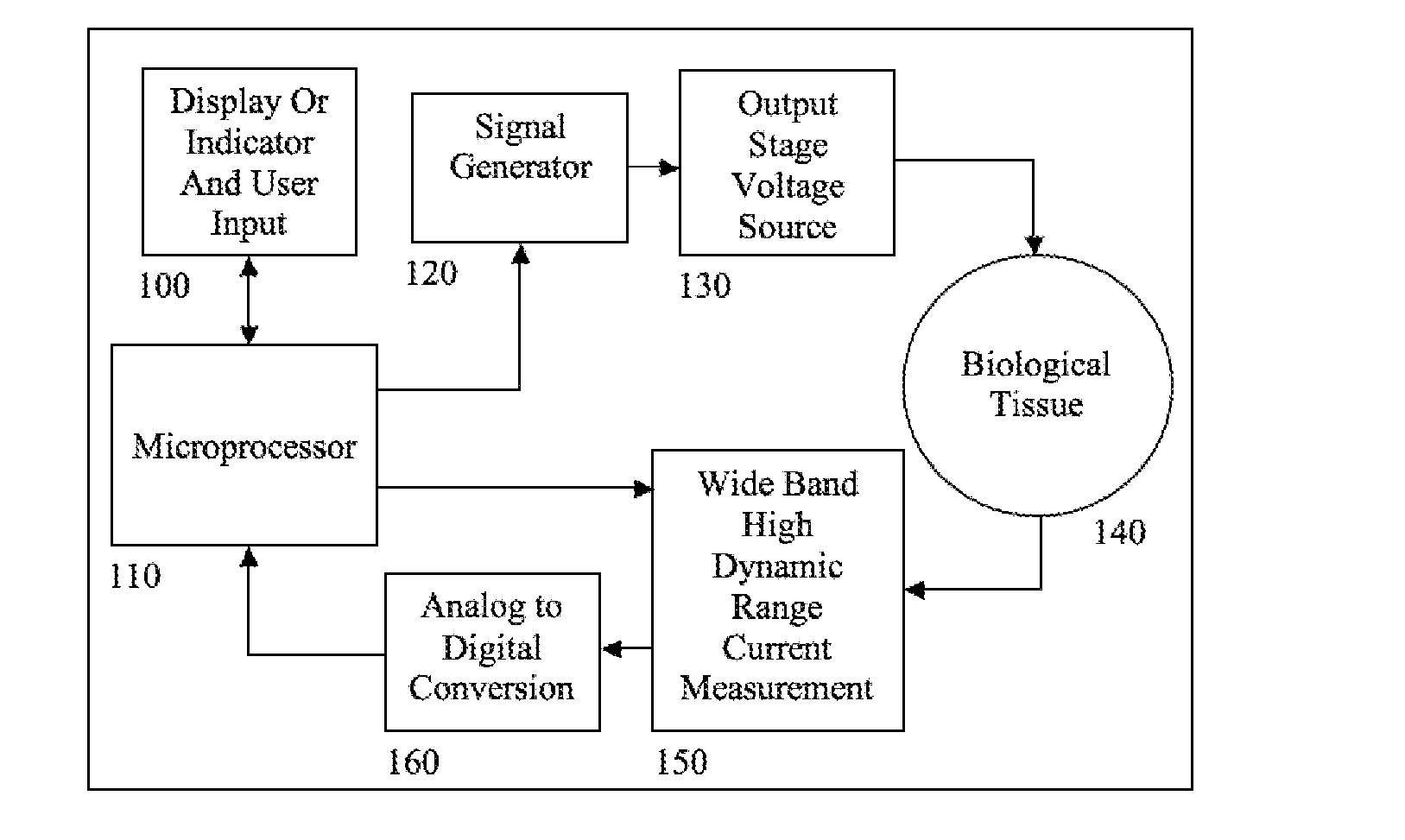
A device is described for measuring electrical characteristics of biological tissues with plurality of electrodes and a processor controlling the stimulation and measurement in order to detect the presence of abnormal tissue masses in organs. Examples of suitable organs are the breast, skin, oral cavity, lung, liver, colon, rectum, cervix, and prostate and determine probability of tumors containing malignant cancer cells being present in tissue. The approach can also be applied to biopsied tissue samples. The device has the capability of providing the location of the abnormality. The method for measuring electrical characteristics includes placing electrodes and applying a voltage waveform in conjunction with a current detector. A mathematical analysis method is then applied to the collected data, which computes spectrum of frequencies and correlates magnitudes and phases with given algebraic conditions to determine mass presence and type.
Owner:SLIZYNSKI ROMAN A +1
Genes and polypeptides relating to hepatocellular or colorectal carcinoma
Owner:ONCOTHERAPY SCI INC
Fecal bacterial markers for colorectal cancer
ActiveUS20200002769A1Reduce development riskIncreased riskMicrobiological testing/measurementDisease diagnosisColon rectal cancerFaecal bacteria
Provided is a non-invasive method for diagnosing colorectal cancer in a subject by detecting enrichment or reduction of certain bacterial species. A kit and device useful for such methods are also provided. In addition, a method for reducing the risk of colon cancer by regulating the pertinent bacterial species in human colon is also provided.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Noninvasive detection of colorectal cancer and other gastrointestinal pathology
InactiveUS20010026925A1Gastrointestinal cellsArtificial cell constructsInflammatory cellColorectal cancer
A method for isolating viable, biologically substantially pure exfoliated fecal colonocytes at normal ambient temperature is described. Immunocoprocytes and inflammatory cells indicative of certain gastrointestinal conditions and a noninvasive method for detecting colorectal cancer are set forth. Composition of transport and suspension media for isolation of colonocytes are detailed.
Owner:NAIR PADMANABHAN P
Monoclonal antibody CAb-1 heavy and light chain variable region gene of human carcinoma of large intestine, and its uses
ActiveCN1546664AOrganic active ingredientsSugar derivativesAntiendomysial antibodiesLarge Intestine Cancer
The invention relates to monoclonal antibody CAb--1 heavy and light chain variable region gene of human carcinoma of large intestine, the encoded polypeptides by the said genes, and the use of the genes and the polypeptides in preparing the medicament for diagnosis or treatment of colon tumor or rectum tumor, wherein a set of designed expanded primers are introduced. Based on the gene, gene engineering method can be employed to construct and express small molecular genetic engineering antibody of various forms, such as Fab antibody, chimeric antibody, and antibody fusion protein, which can be used in preparing medicament for diagnosis or treatment of large intestine (colon, rectum) cancers.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Electrical impedance techniques in tissue-mass detection and characterization
ActiveUS20150272470A1Low costResistance/reactance/impedenceOrgan movement/changes detectionCancer cellRadiology
A device is described for measuring electrical characteristics of biological tissues with plurality of electrodes and a processor controlling the stimulation and measurement in order to detect the presence of abnormal tissue masses in organs. Examples of suitable organs are the breast, skin, oral cavity, lung, liver, colon, rectum, cervix, and prostate and determine probability of tumors containing malignant cancer cells being present in tissue. The approach can also be applied to biopsied tissue samples. The device has the capability of providing the location of the abnormality. The method for measuring electrical characteristics includes placing electrodes and applying a voltage waveform in conjunction with a current detector. A mathematical analysis method is then applied to the collected data, which computes spectrum of frequencies and correlates magnitudes and phases with given algebraic conditions to determine mass presence and type.
Owner:SLIZYNSKI ROMAN A +1
Therapeutic and prophylactic treatment for colorectal cancer
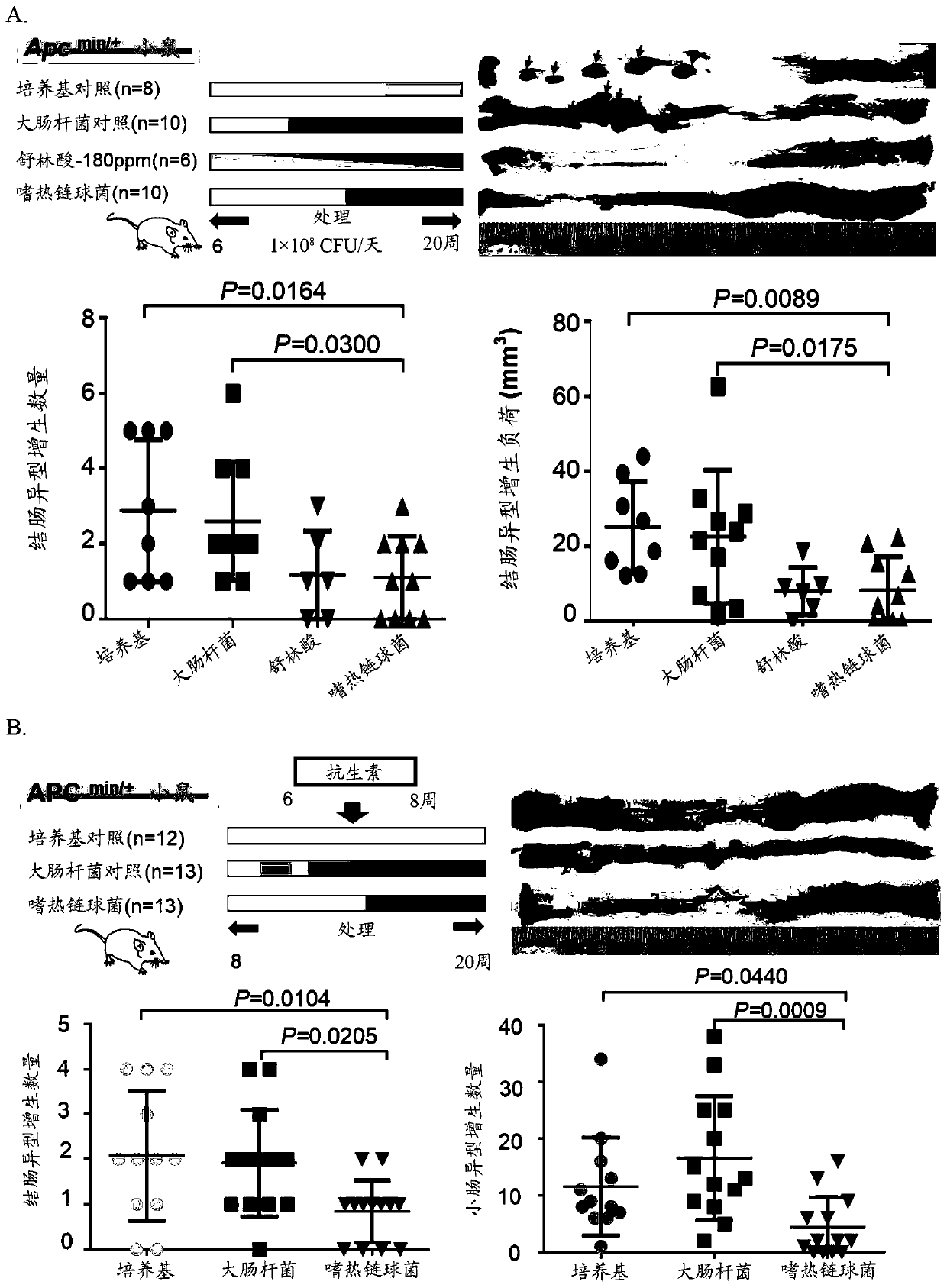
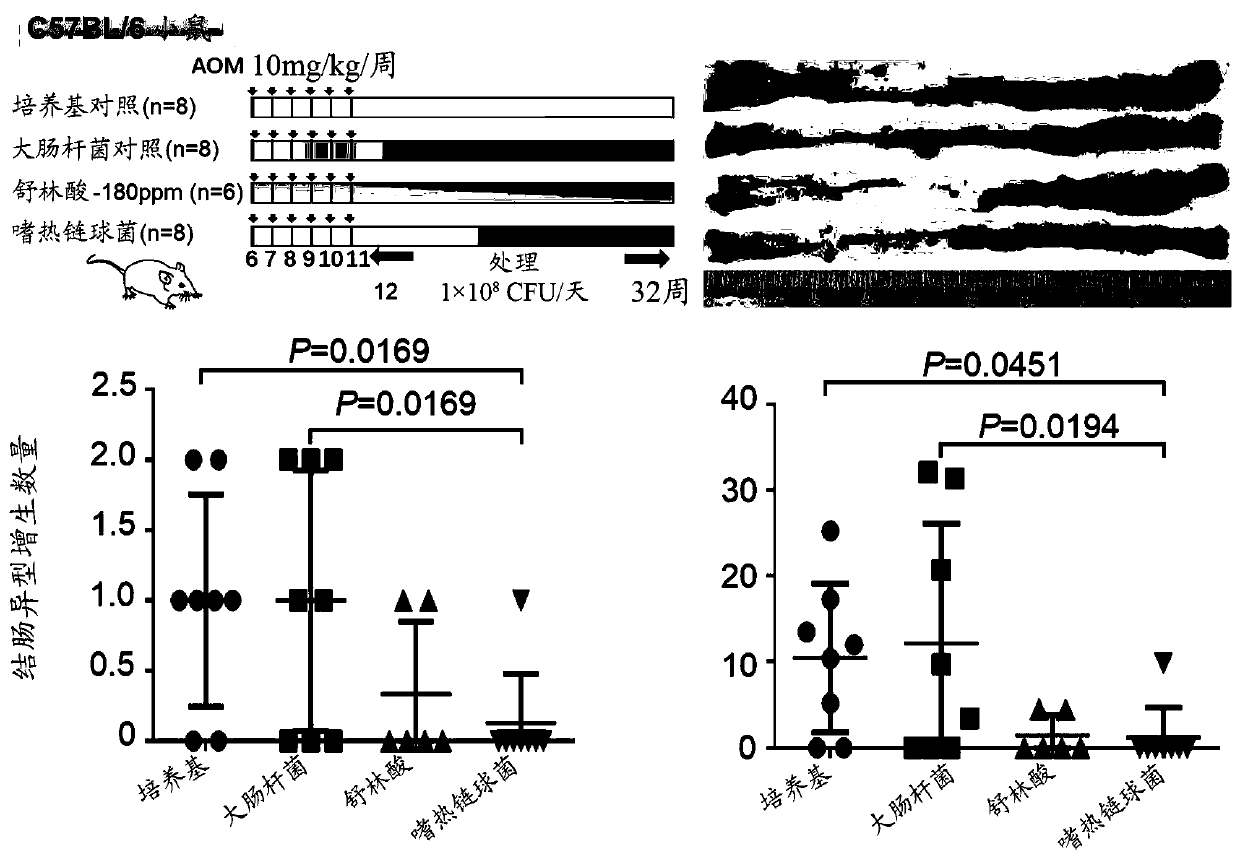
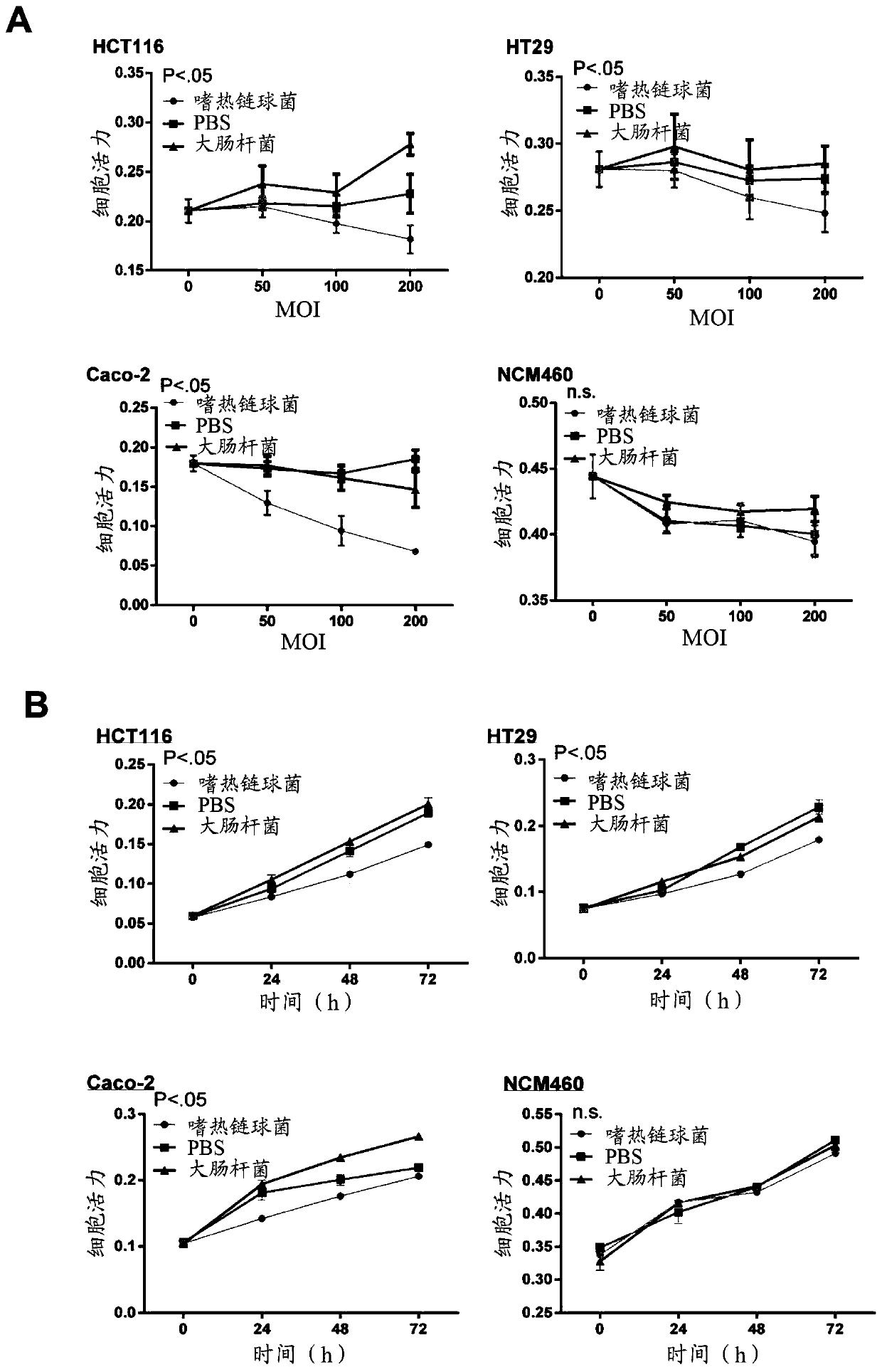
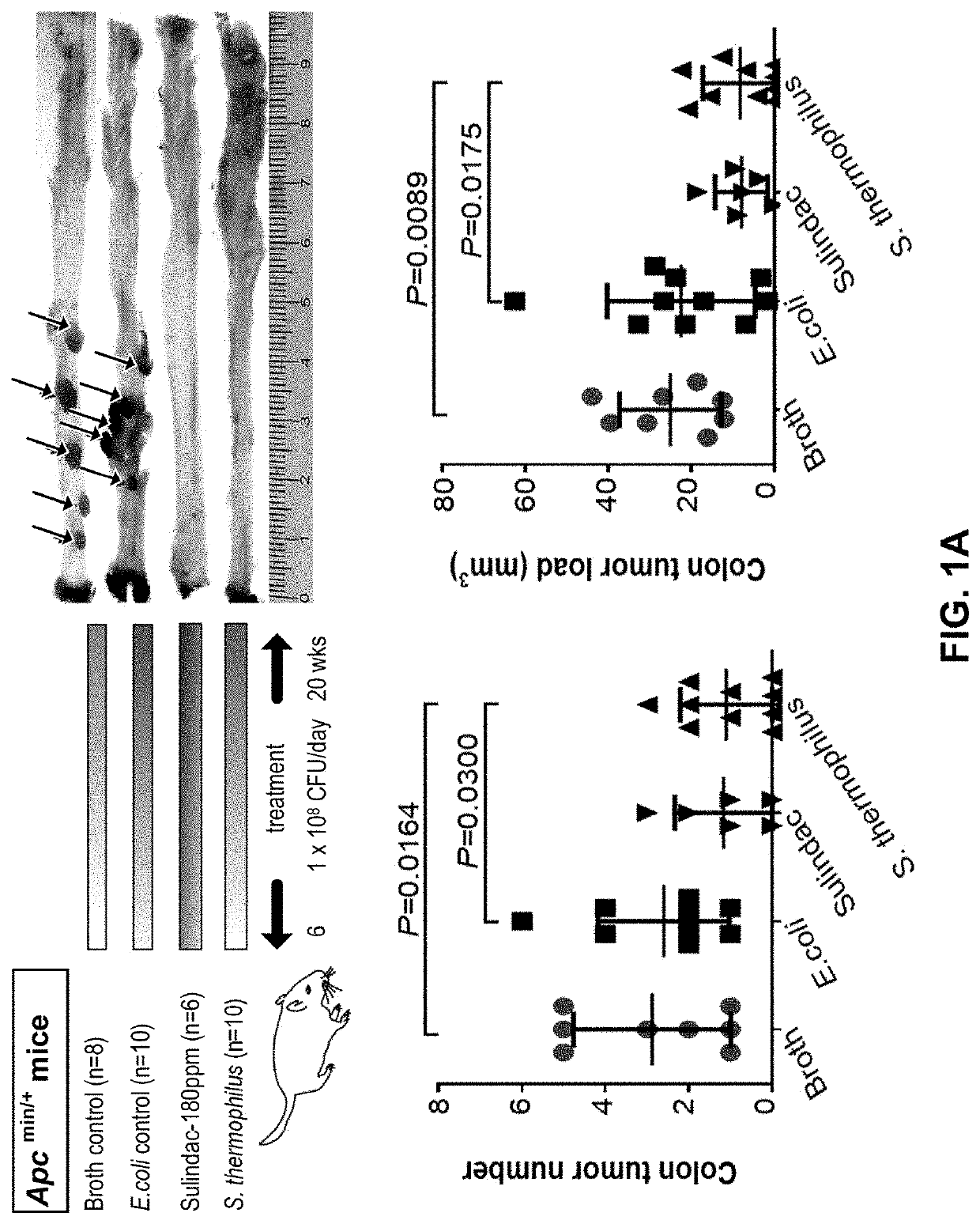
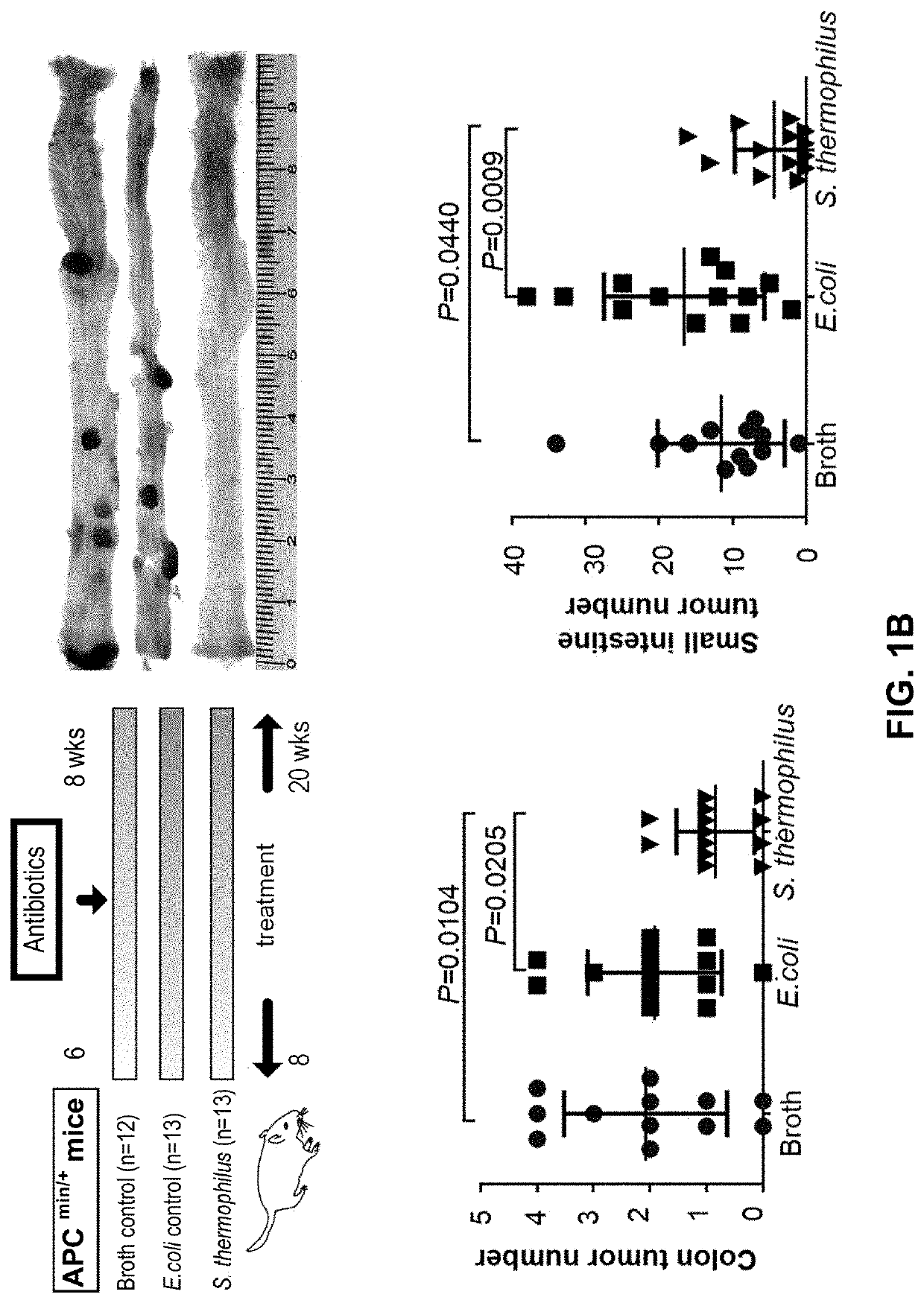
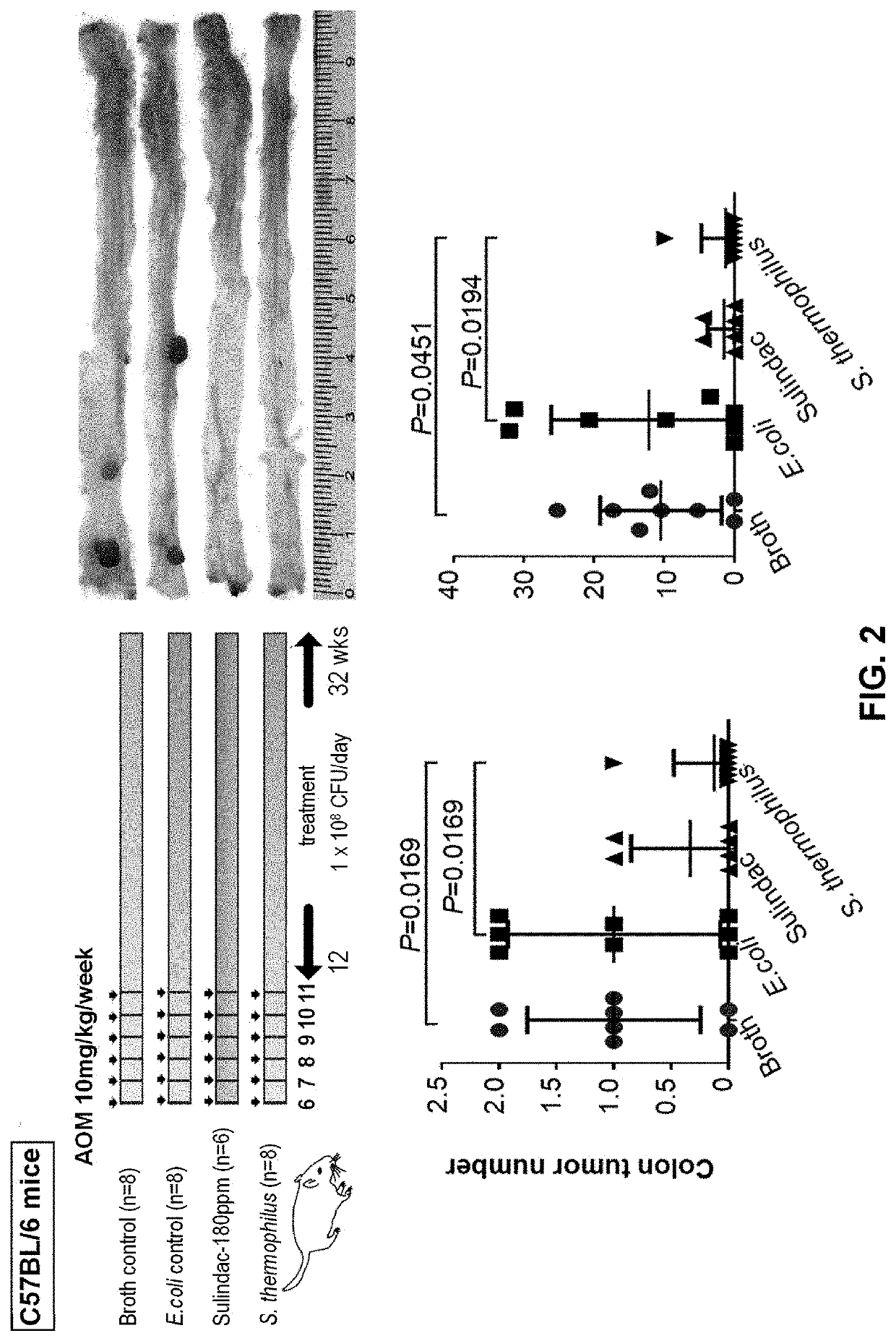
The present application provides novel methods of treating and preventing colorectal cancer in a subject by administering a composition comprising beta-galactosidase, a bacterial culture (e.g., a Streptococcus thermophilus culture) capable of producing beta-galactosidase, or a culture component comprising beta-galactosidase. The invention further provides a set product for the method. In addition,the present application provides a composition for treating or preventing colon cancer.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Combination test for colorectal cancer
Owner:벨지언볼리션에스알엘
Light and heavy chain variable region genes of monoclonal antibody CAb-2 against human colorectal carcinoma and their application
ActiveCN1546527AImmunoglobulins against animals/humansAntibody ingredientsLarge intestineGenetic engineering
The invention relates to monoclonal antibody CAb--2 heavy and light chain variable region gene of human carcinoma of large intestine, the encoded polypeptides by the said genes, and the use of the genes and the polypeptides in preparing the medicament for diagnosis or treatment of colon tumor or rectum tumor. By introducing a set of designed expanded primers, a strain of monoclonal antibody Cab-2 heavy and light chain variable region gene of human carcinoma of large intestine is cloned from the hybrid tumor cells. The gene can be applied in preparing the medicament for diagnosis or treatment of colon tumor or rectum tumor.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Chinese medicinal rectal administration capsule for treating colonitis
ActiveCN102793803AFast cureRealize heat-clearing and detoxificationDigestive systemAgainst vector-borne diseasesToxic materialRectal use
The invention discloses a Chinese medical rectal administration capsule for treating colonitis. Chinese medicinal powder is filled into a large-sized capsule shell. The Chinese medicinal powder is prepared from the following Chinese medical raw materials in parts by weight: 290-310 parts of underleaf pearl, 290-310 parts of geranium wilfordii, 290-310 parts of euphorbia humifusa willd, 190-210 parts of Chinese asystasiella herb, 290-310 parts of folium callicarpae pedunculatae, 140-160 parts of herba ajugae, 290-310 parts of herba agrimoniae, 390-410 parts of acalypha australis and 55-65 parts of frankincense. After the Chinese medicinal rectal administration capsule is put into anus, the capsule shell is dissolved, so that medicaments directly act on colon rectum, the effects of clearing away heat and toxic materials, scattering stasis, stopping bleeding, diminishing inflammation, sterilizing, softening hard lumps, dispelling nodes, stopping diarrhea, red white dysentery, hematuria, hematochezia, astringing dampness and relieving itching can be achieved, and chronic colonitis is cured quickly.
Owner:刘湘秋
Pro-drugs of nsaias with very high skin and membranes penetration rates and their new medicinal uses
InactiveUS20160272653A1Highly preventive effectToxic burdenOrganic active ingredientsNervous disorderSolubilityDISCOID LUPUS ERYTHEMATOSIS
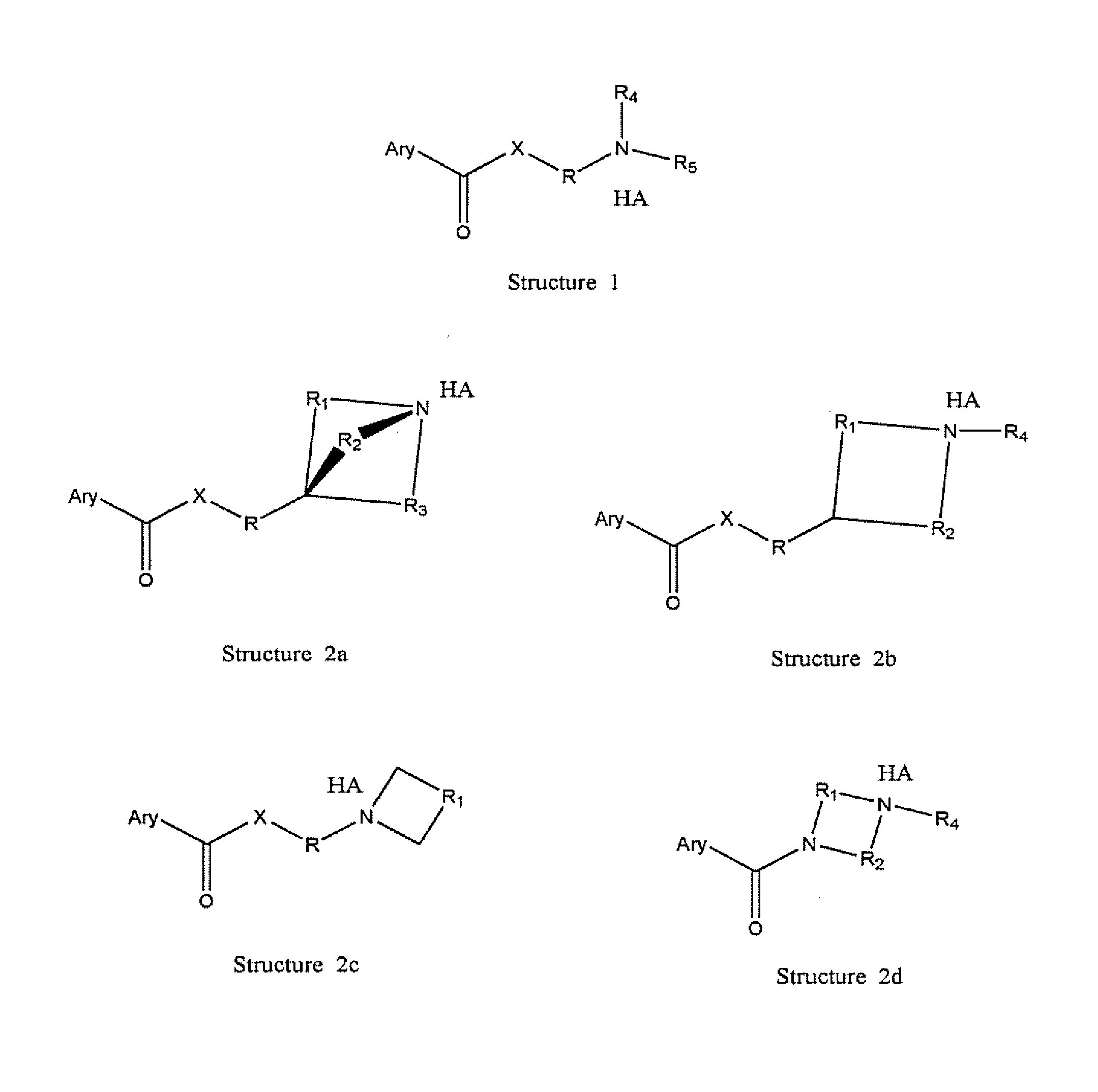
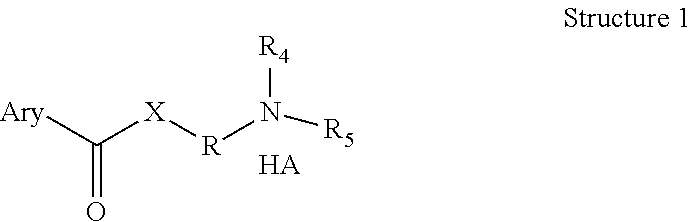
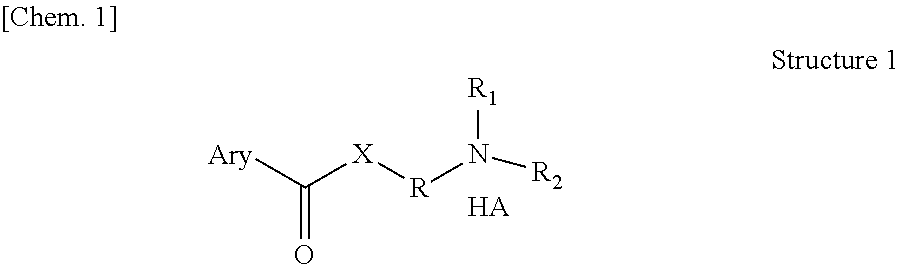
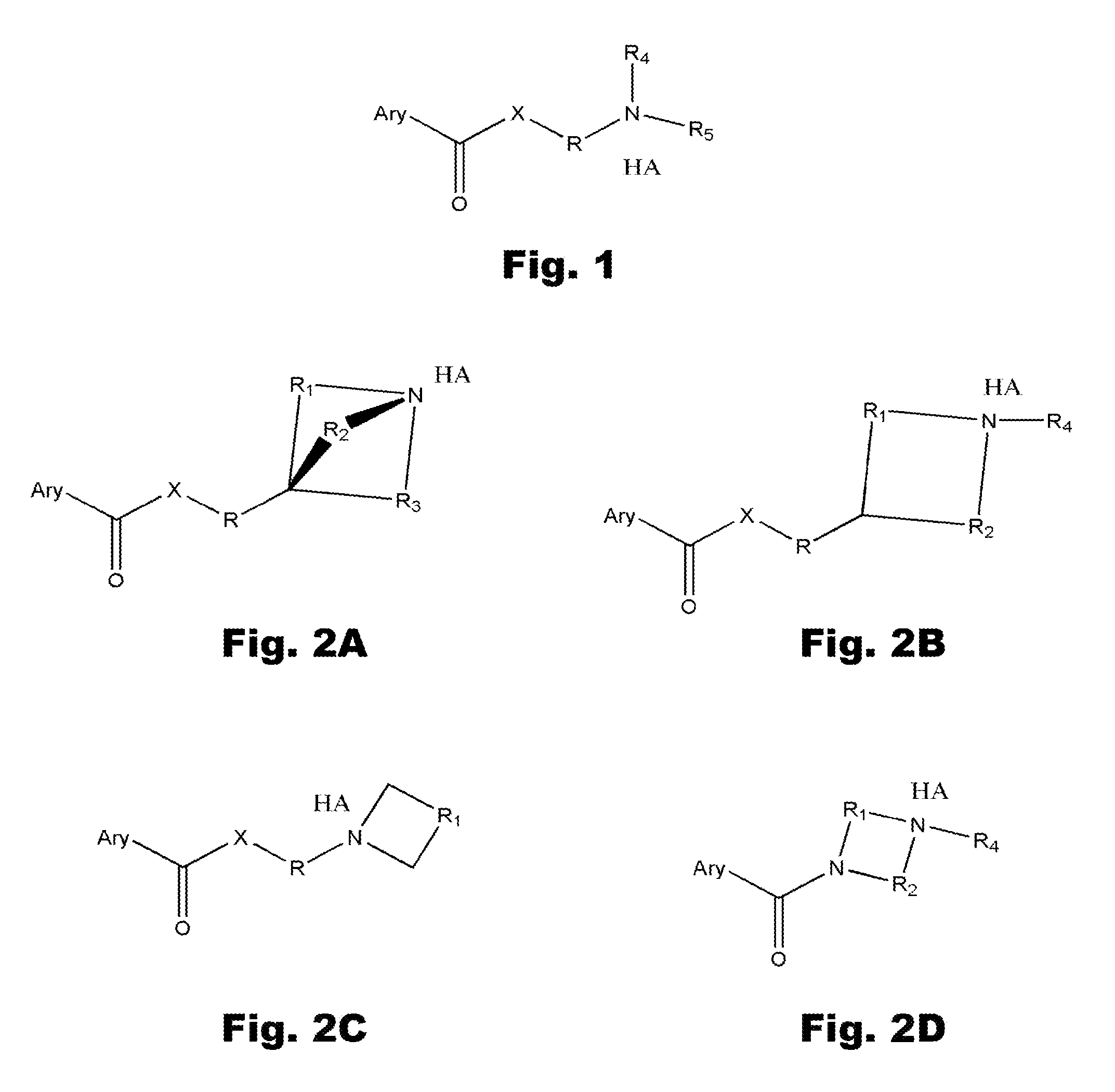
The novel positively charged pro-drugs of NSAIAs in the general formulas (1, 2a, 2b, 2c, or 2d) “Structure 1, 2a, 2b, 2c, or 2d” were designed and synthesized. The compounds of the general formulas (1, 2a, 2b, 2c, or 2d) “Structure 1, 2a, 2b, 2c, or 2d” indicated above can be prepared from metal salts, organic base salts, or immobilized base salts of NSAIAs with suitable halide compounds. The positively charged amino groups in the pro-drugs in this invention largely increase the solubility of the drugs in water and will bond to the negative charge on the phosphate head group of membrane. Thus, the local concentration of the outside of the membrane or skin will be very high and will facilitate the passage of these pro-drugs from a region of high concentration to a region of low concentration. This bonding will disturb the membrane a little bit and may make some room for the lipophilic portion of the pro-drug. When the molecules of membrane move, the membrane may “crack” a little bit due to the bonding of the pro-drug. This will let the pro-drug insert into the membrane. At pH 7.4, only about 99% of the amino group is protonated. When the amino group is not protonated, the bonding between the amino group of the pro-drug and the phosphate head group of the membrane will disassociate, and the pro-drug will enter the membrane completely. When the amino group of the pro-drug flips to the other side of the membrane and thus becomes protonated, then the pro-drug is pulled into the cytosol, a semi-liquid concentrated aqueous solution or suspension. These pro-drugs can be used for treating and preventing diabetes (type I or / and type II), abnormal blood glucose and lipid levels, stroke, heart attack, and other heart and vascular diseases Alzheimer's diseases, Parkinson's diseases and other neurodegenerative diseases, psoriasis, discoid lupus erythematosus, systemic lupus erythematosus (SLE), autoimmune hepatitis, multiple sclerosis (MS), and other autoimmune diseases, amyotrophic lateral sclerosis (ALS), oculopharyngeal muscular dystrophy (OPMD), and other muscle disorders, inflamed hemorrhoids, cryptitis, other inflammatory conditions of the anorectum, and pruritus ani, prostatitis, prostatocystitis, varicose veins, autoimmune liver inflammation, autoimmune kidney inflammation, vein inflammation and other inflammations, skin cancers, breast cancer, colon-rectum cancer, oral cancer, and other cancers, scars, abnormal vascular skin lesions, birthmarks, moles (nevi), skin tags, aging spots (liver spots), and other skin disorders. These pro-drugs can be administered transdermally without the help of skin penetration enhancers.
Owner:TECHFIELDS BIOCHEM CO LTD
Therapeutic and prophylactic treatment for colorectal cancer
ActiveUS20200261514A1Suppress proliferationSuppress viabilityPeptide/protein ingredientsDigestive systemProphylactic treatmentOncology
The present invention provides novel methods for treating and preventing colorectal cancer in a subject by administering a composition comprising β-galactosidase, a bacterial culture capable of producing β-galactosidase, such as an S. thermophilus culture, or a fraction of the culture comprising β-galactosidase. A kit useful for such methods is also provided. In addition, the present invention provides a composition for treating or preventing colon cancer.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Light and heavy chain variable region genes of monoclonal antibody CAb-2 against human colorectal carcinoma and their application
ActiveCN1303105CImmunoglobulins against animals/humansAntibody ingredientsAntiendomysial antibodiesLarge Intestine Cancer
The invention relates to monoclonal antibody CAb--2 heavy and light chain variable region gene of human carcinoma of large intestine, the encoded polypeptides by the said genes, and the use of the genes and the polypeptides in preparing the medicament for diagnosis or treatment of colon tumor or rectum tumor. By introducing a set of designed expanded primers, a strain of monoclonal antibody Cab-2 heavy and light chain variable region gene of human carcinoma of large intestine is cloned from the hybrid tumor cells. The gene can be applied in preparing the medicament for diagnosis or treatment of colon tumor or rectum tumor.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method of diagnosing colon and gastric cancers
Objective methods for detecting and diagnosing Colorectal and gastric carcinomas are described herein. In one embodiment, the diagnostic method involves the determining a expression level of colon or gastric cancer-associated gene that discriminate between colon or gastric cancer and normal cell. The present invention further provides methods of screening for therapeutic agents useful in the treatment of colonic cancer and method of vaccinating a subject against colon or gastric cancer.
Owner:ONCOTHERAPY SCI INC +1
Method for detecting and treating colon cancer by measuring heavy metal concentrations
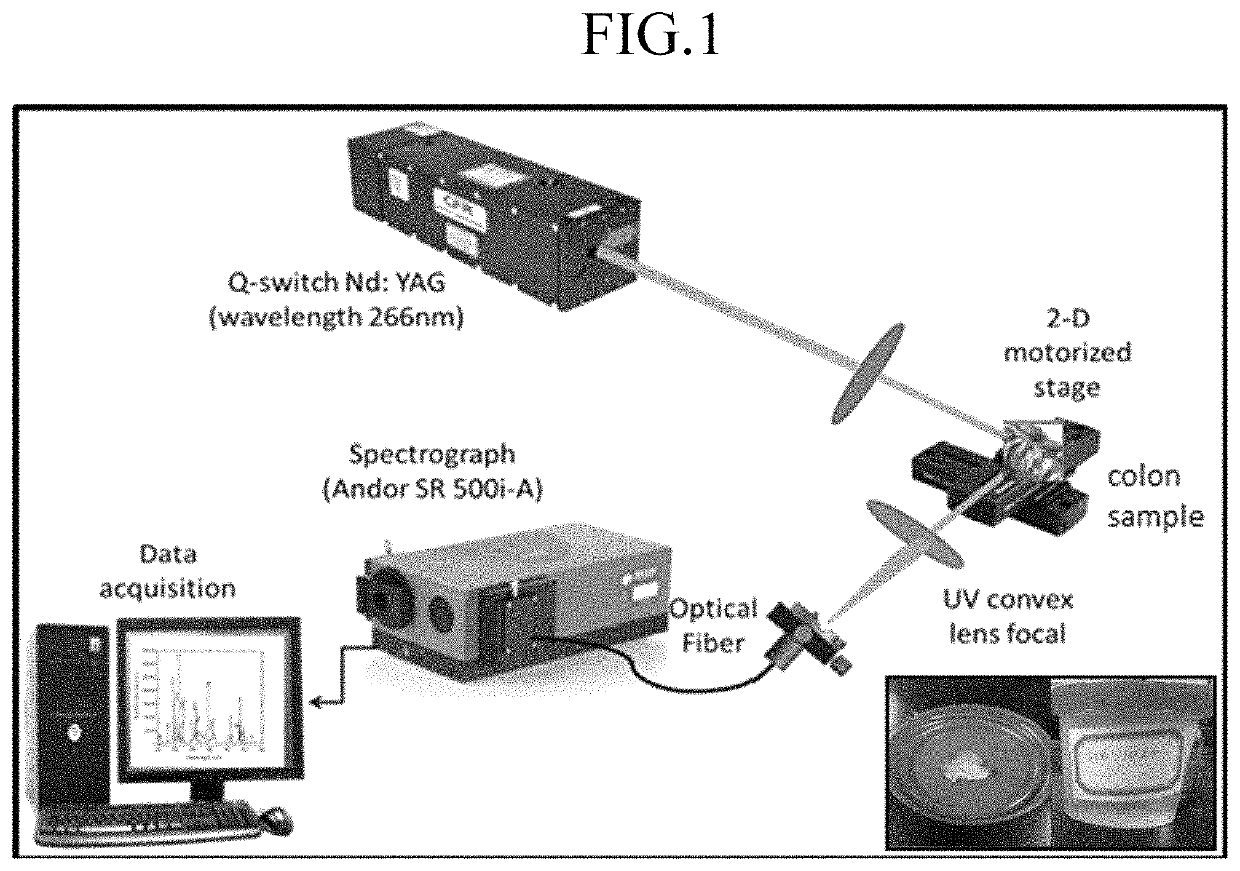
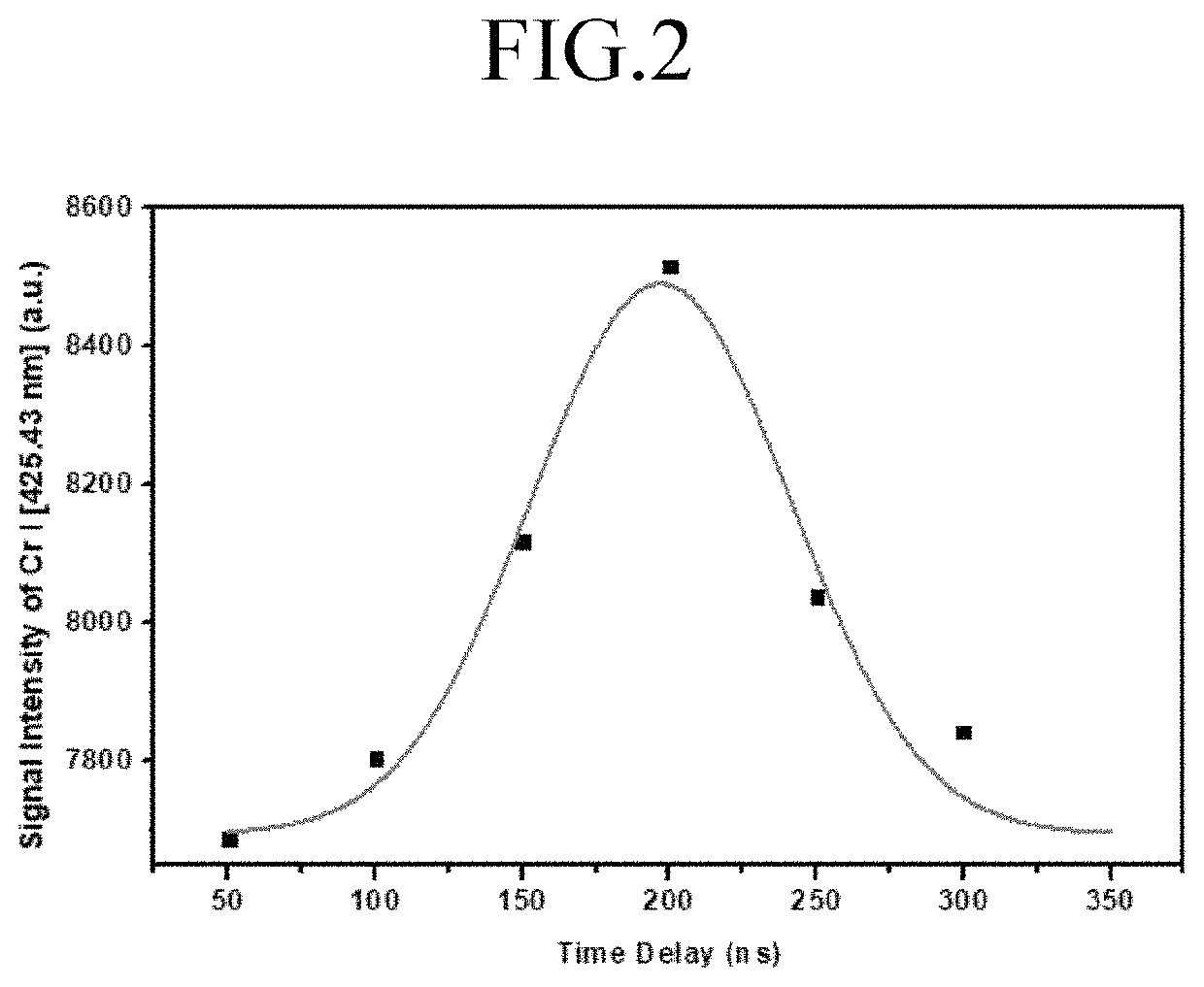
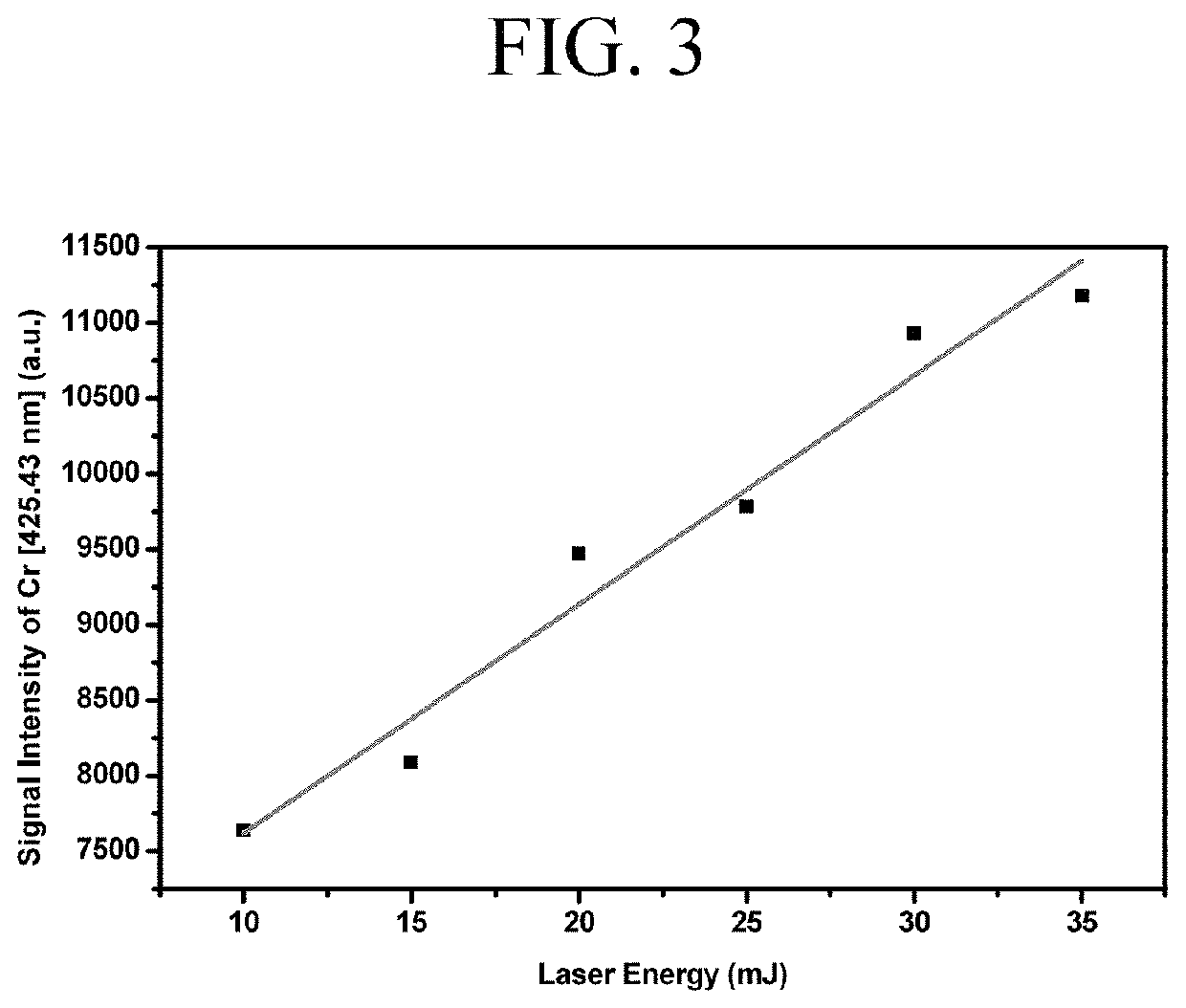
ActiveUS11415582B2Reduce noiseColor/spectral properties measurementsBiological testingLaser-induced breakdown spectroscopyOncology
Method for detecting colon or colorectal cancer by measuring heavy metal concentrations in colon or colorectal tissue using laser-induced breakdown spectroscopy (LIBS).
Owner:KING FAHD UNIVERSITY OF PETROLEUM AND MINERALS +1
Pro-drugs of NSAIAS with very high skin and membranes penetration rates and their new medicinal uses
ActiveUS9371284B2Highly preventive effectToxic burdenOrganic active ingredientsNervous disorderSolubilityDISCOID LUPUS ERYTHEMATOSIS
The novel positively charged pro-drugs of NSAIAs in the general formulas (1, 2a, 2b, 2c, or 2d) “Structure 1, 2a, 2b, 2c, or 2d” were designed and synthesized. The compounds of the general formulas (1, 2a, 2b, 2c, or 2d) “Structure 1, 2a, 2b, 2c, or 2d” indicated above can be prepared from metal salts, organic base salts, or immobilized base salts of NSAIAs with suitable halide compounds. The positively charged amino groups in the pro-drugs in this invention largely increase the solubility of the drugs in water and will bond to the negative charge on the phosphate head group of membrane. Thus, the local concentration of the outside of the membrane or skin will be very high and will facilitate the passage of these pro-drugs from a region of high concentration to a region of low concentration. This bonding will disturb the membrane a little bit and may make some room for the lipophilic portion of the pro-drug. When the molecules of membrane move, the membrane may “crack” a little bit due to the bonding of the pro-drug. This will let the pro-drug insert into the membrane. At pH 7.4, only about 99% of the amino group is protonated. When the amino group is not protonated, the bonding between the amino group of the pro-drug and the phosphate head group of the membrane will disassociate, and the pro-drug will enter the membrane completely. When the amino group of the pro-drug flips to the other side of the membrane and thus becomes protonated, then the pro-drug is pulled into the cytosol, a semi-liquid concentrated aqueous solution or suspension. These pro-drugs can be used for treating and preventing diabetes (type I or / and type II), abnormal blood glucose and lipid levels, stroke, heart attack, and other heart and vascular diseases Alzheimer's diseases, Parkinson's diseases and other neurodegenerative diseases, psoriasis, discoid lupus erythematosus, systemic lupus erythematosus (SLE), autoimmune hepatitis, multiple sclerosis (MS), and other autoimmune diseases, amyotrophic lateral sclerosis (ALS), oculopharyngeal muscular dystrophy (OPMD), and other muscle disorders, inflamed hemorrhoids, cryptitis, other inflammatory conditions of the anorectum, and pruritus ani, prostatitis, prostatocystitis, varicose veins, autoimmune liver inflammation, autoimmune kidney inflammation, vein inflammation and other inflammations, skin cancers, breast cancer, colon-rectum cancer, oral cancer, and other cancers, scars, abnormal vascular skin lesions, birthmarks, moles (nevi), skin tags, aging spots (liver spots), and other skin disorders. These pro-drugs can be administered transdermally without the help of skin penetration enhancers.
Owner:TECHFIELDS BIOCHEM CO LTD
Anal incontinence treatment apparatus
InactiveCN1679453BSimple and efficient energy transferWork reliablyAnti-incontinence devicesWireless transmissionDisease treatment
An anal incontinence disease treatment apparatus comprises a restriction device (4) implanted in a patient and engaging the colon (66), rectum or anus from a restricted faecal passageway therein. The restriction device is operable to change the restriction of the faecal passageway, i.e. to open or close the faecal passageway. An energy transmission device (10) is provided for wireless transmission of energy from outside the patient s body to inside the patient s body for use in connection with the operation of the restriction device including changing the restriction of the faecal passageway.
Owner:ABDOMICA
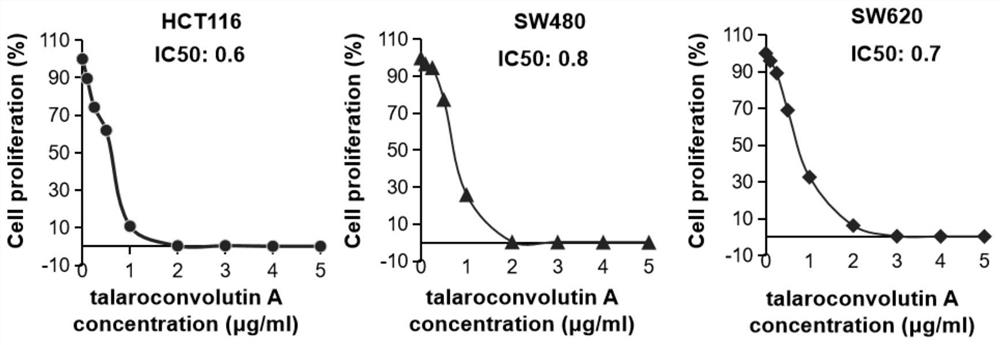
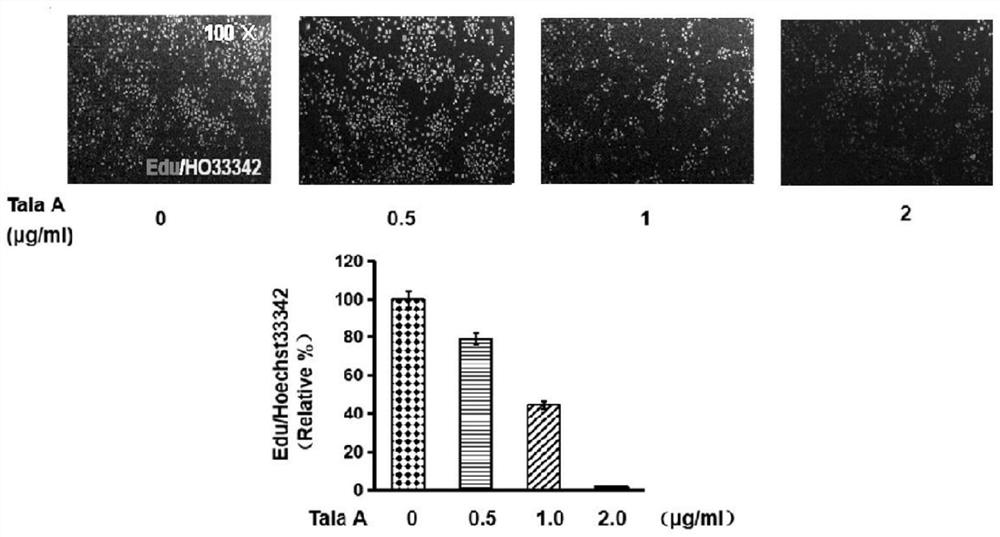
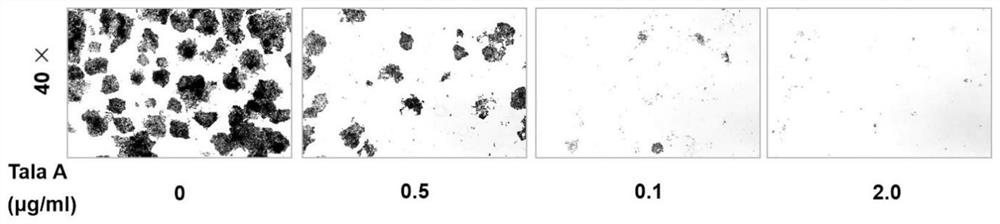
Application of talaroconvolutin A in anticancer drugs
ActiveCN111643497BAvoid side effectsDelay drug resistanceOrganic active ingredientsAntineoplastic agentsCancer cellSide effect
The invention belongs to the application field of microbial metabolites, and focuses on the application of Talaroconvolutin A in the preparation of drugs for treating colon, rectal cancer or lung cancer. Talaroconvolutin A kills cancer cells at a concentration of 0.5-50 μg / mL in vitro, and the lowest concentration that effectively kills cancer cells in 48 hours is 2 μg / mL; the effective concentration for inhibiting colon cancer cell colony formation is 2 μg / mL. IC 50 It is less than 1 μg / mL, and the effective concentration at the animal level is 5-20 mg / kg. The beneficial effects of the present invention are: IC 50 Low, minimal IC of Talaroconvolutin A at the cellular level 50 Less than 1μg / mL, the effective concentration in animals can be as low as 5mg / kg, which is obviously better than cisplatin chemotherapy drugs, and has less toxic and side effects to animals.
Owner:JINING MEDICAL UNIV
Chinese medicinal rectal administration capsule for treating colonitis
The invention discloses a Chinese medical rectal administration capsule for treating colonitis. Chinese medicinal powder is filled into a large-sized capsule shell. The Chinese medicinal powder is prepared from the following Chinese medical raw materials in parts by weight: 290-310 parts of underleaf pearl, 290-310 parts of geranium wilfordii, 290-310 parts of euphorbia humifusa willd, 190-210 parts of Chinese asystasiella herb, 290-310 parts of folium callicarpae pedunculatae, 140-160 parts of herba ajugae, 290-310 parts of herba agrimoniae, 390-410 parts of acalypha australis and 55-65 parts of frankincense. After the Chinese medicinal rectal administration capsule is put into anus, the capsule shell is dissolved, so that medicaments directly act on colon rectum, the effects of clearing away heat and toxic materials, scattering stasis, stopping bleeding, diminishing inflammation, sterilizing, softening hard lumps, dispelling nodes, stopping diarrhea, red white dysentery, hematuria, hematochezia, astringing dampness and relieving itching can be achieved, and chronic colonitis is cured quickly.
Owner:刘湘秋
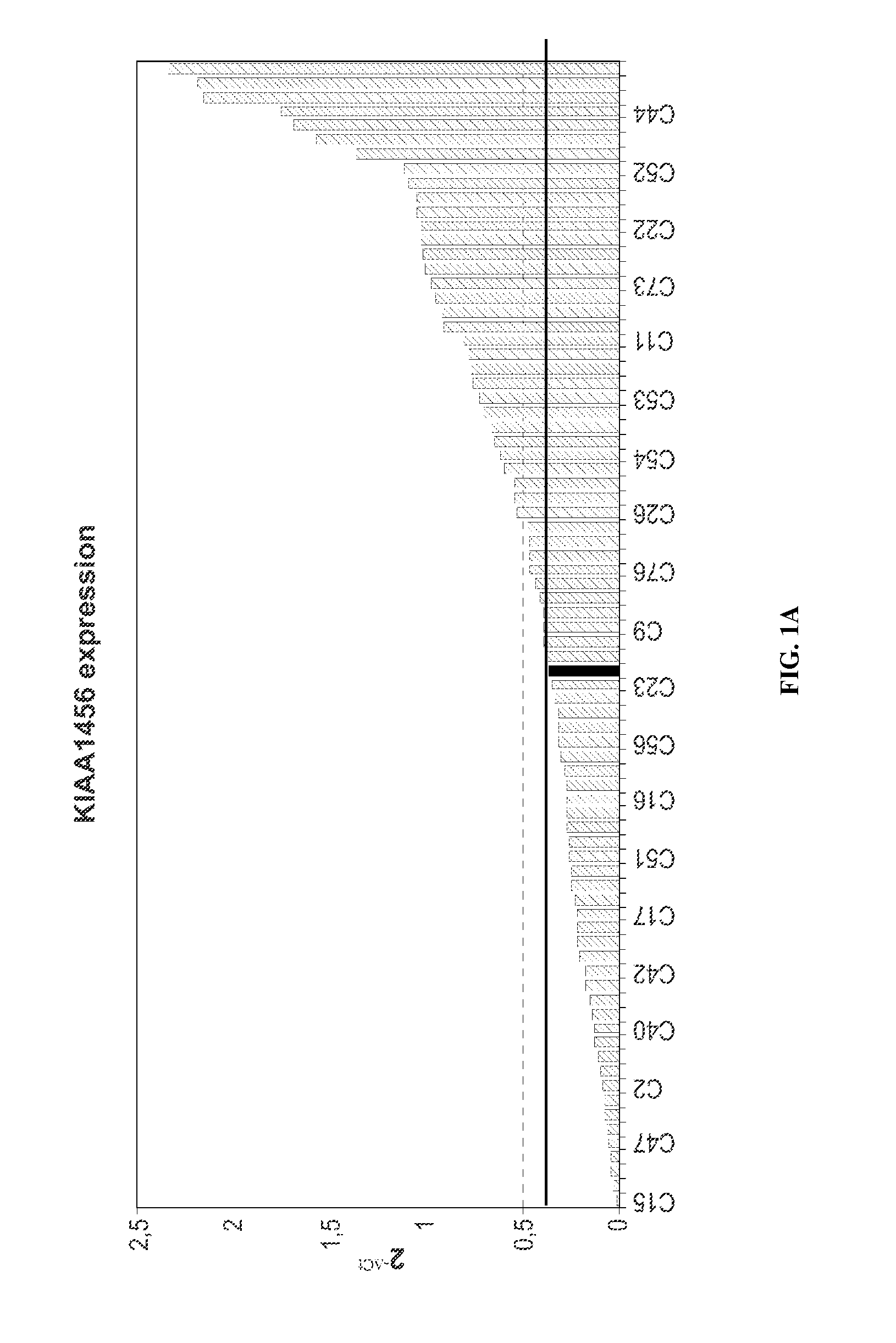
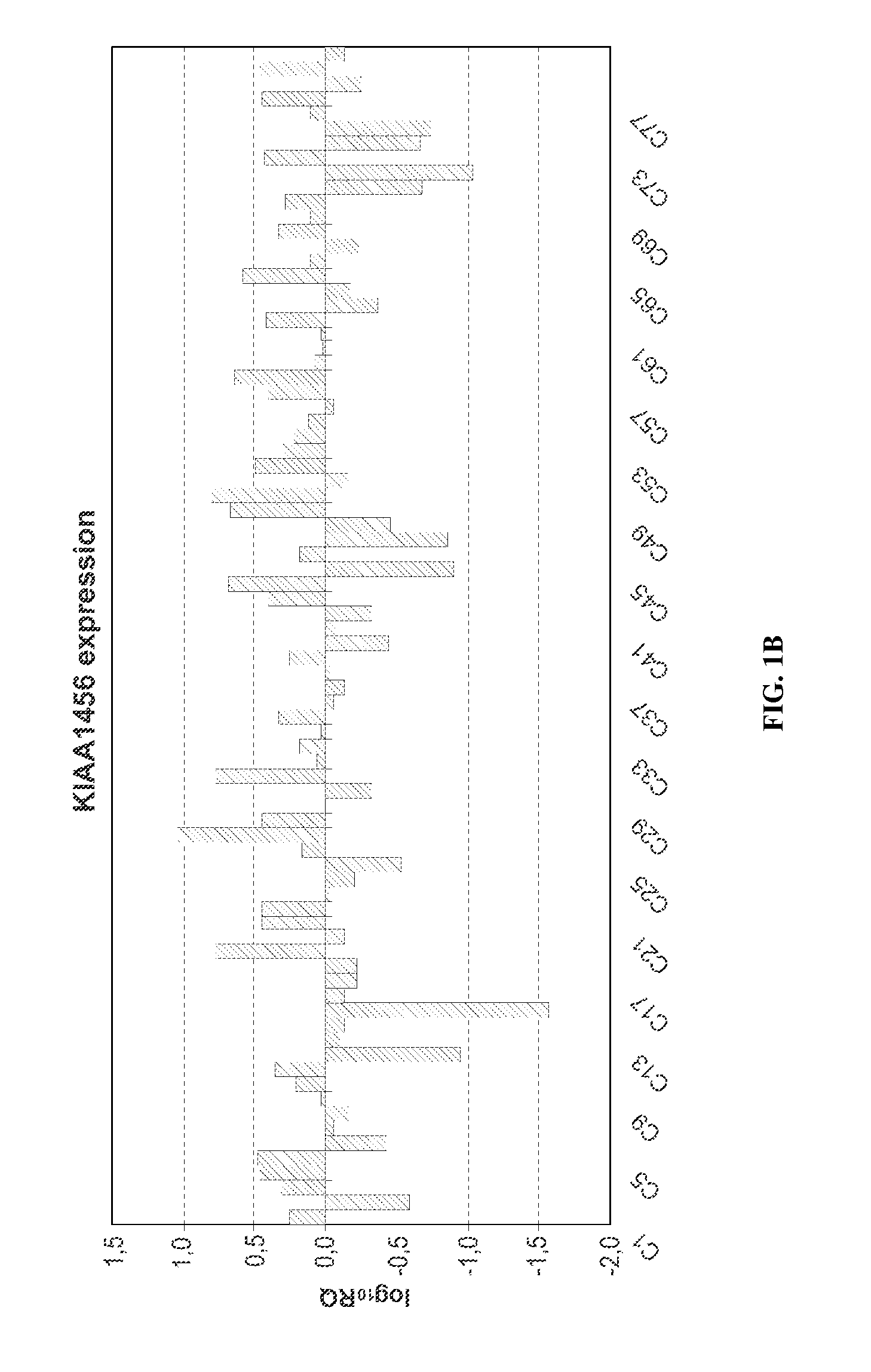
Kiaa1456 expression predicts survival in patients with colon cancer
InactiveUS20140329701A1Nucleotide librariesMicrobiological testing/measurementOncologyGene expression level
The invention relates to methods for deciding on the therapy in a subject suffering from colorectal cancer as well as for predicting the clinical outcome of a patient which suffers from colorectal cancer based on the expression level of KIAA1456 comprising determining the expression level of the KIAA1456 gene. The invention relates as well to kits and uses thereof comprising reagents adequate for the determination of the expression level of the KIAA1456 gene.
Owner:TRASLATIONAL CANCER DRUGS PHARMA SL
Nuclear matrix protein alterations associated with colon cancer and their use as markers for colorectal cancer
InactiveUS20090325214A1Gauging efficacyGood effectMicrobiological testing/measurementDisease diagnosisSerum samplesNuclear matrix
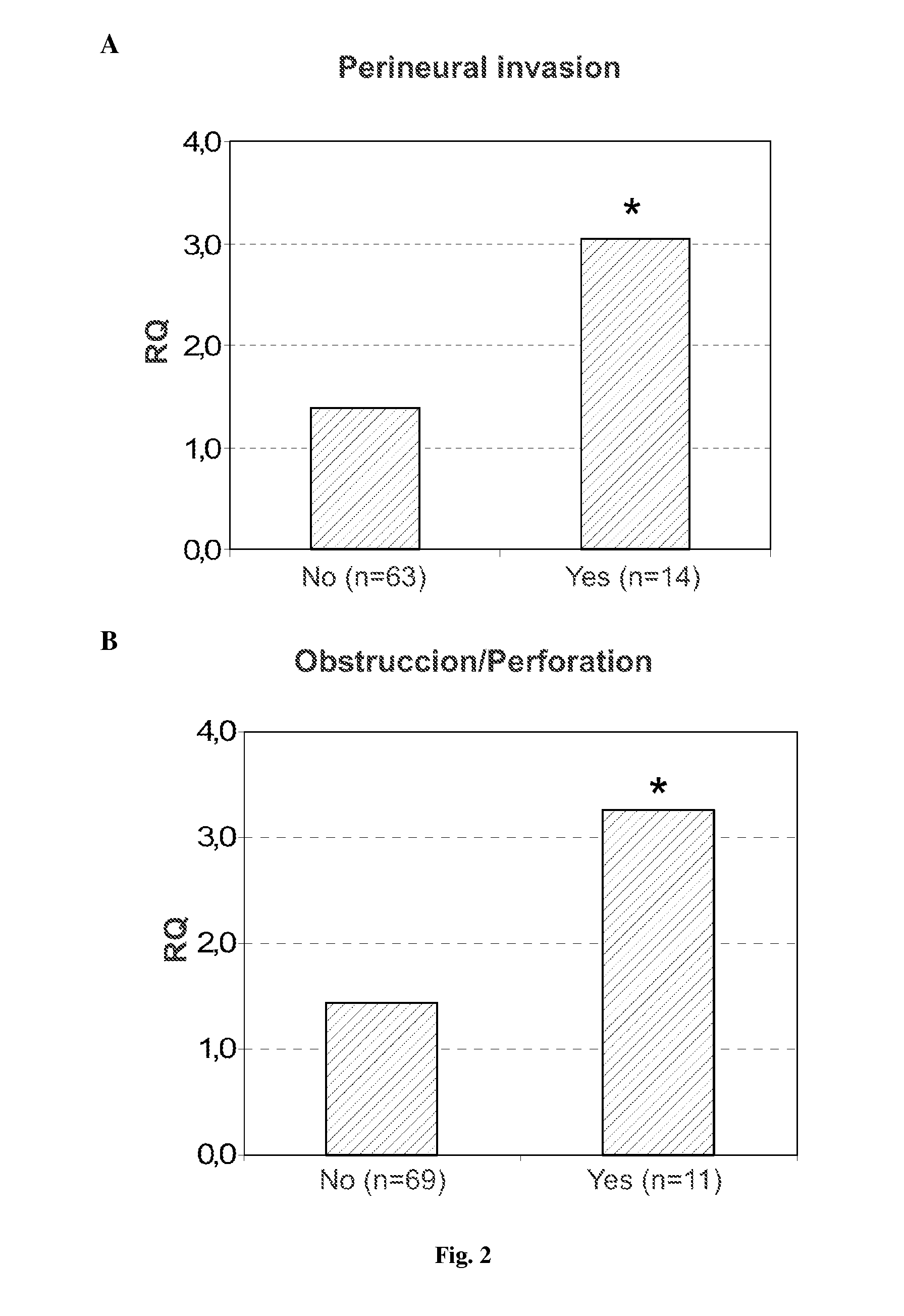
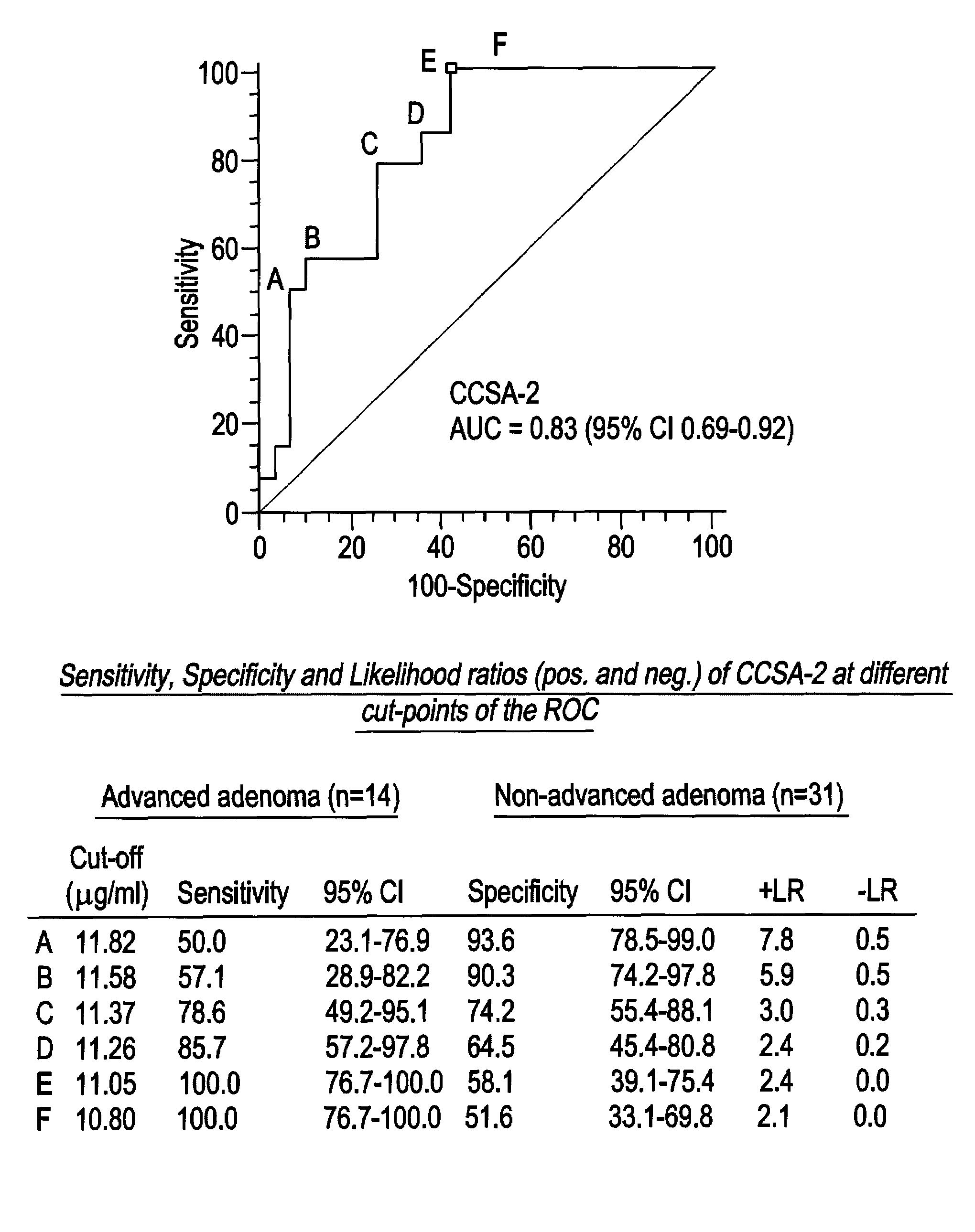
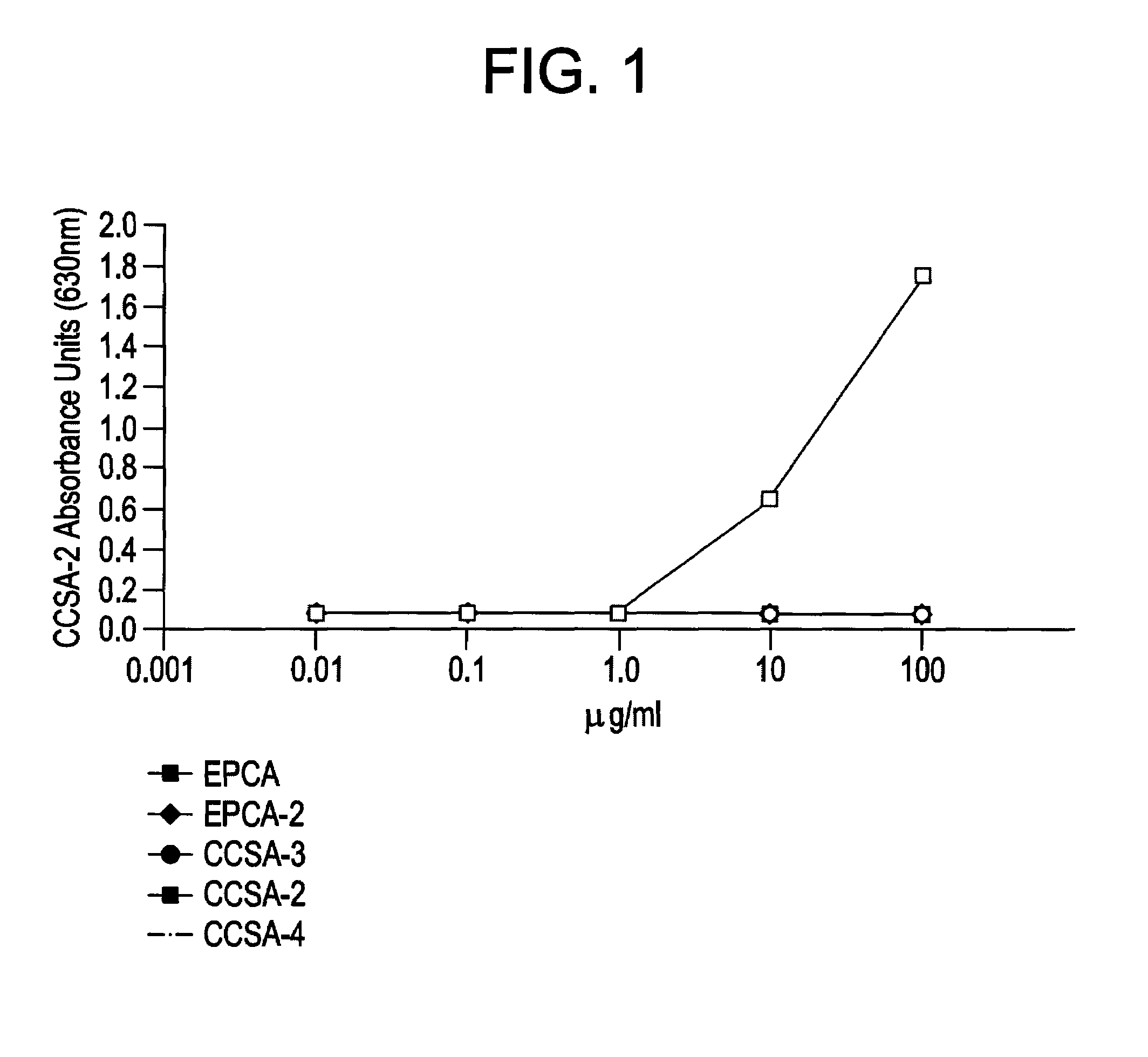
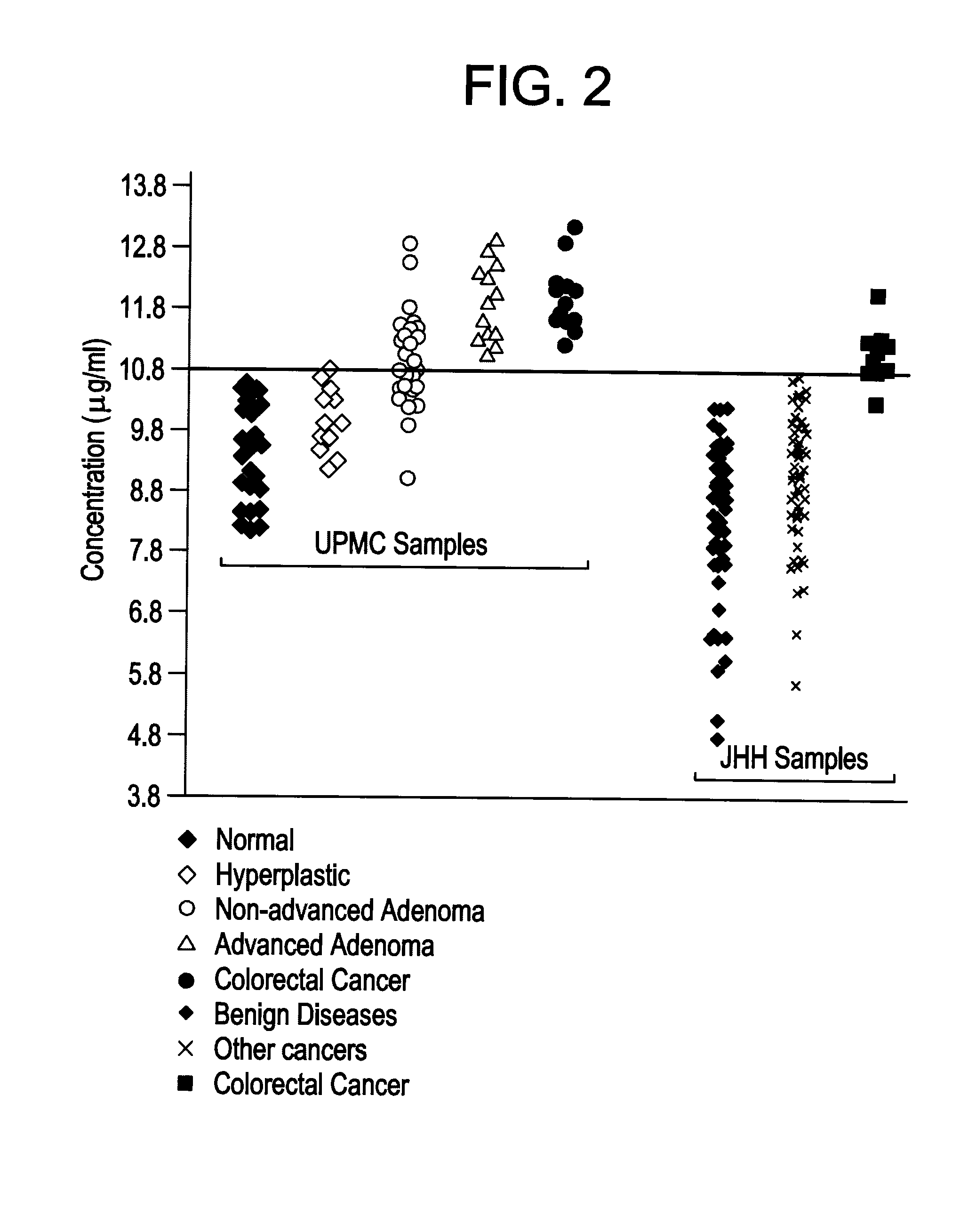
CCSA-2 levels in serum samples from individuals suffering from colorectal cancer can be used gauging the progression of a colorectal cancer condition. In particular, the progression of a colorectal cancer condition is gauged by comparing the level of serum CCSA-2 from individuals having colorectal cancer to predetermined CCSA-2 levels that correlate to the state of advancement of a cancer condition. Additionally, serum CCSA-2 levels can be used by a clinician to gauge the efficacy of a colorectal cancer treatment.
Owner:UNIVERSITY OF PITTSBURGH
Thylakoid extract composition and formulation for the treatment of inflammatory bowel disease
There is provided the use of a functional thylakoid extract, particularly in admixture with a physiologically acceptable carrier, in pharmaceutical application, in the treatment of inflammatory bowel disease, and method for the treatment thereof. The inflammatory bowel disease may comprise several diseases associated with inflammation of the small intestine, large intestine (colon), rectum or anus (anal sphincter), and may be particularly selected from ulcerative colitis and Crohn's disease.
Owner:GRP SANTE DEVONIAN INC
Hgf-met agonist for use in the treatment of cancer and colorectal fibrosis
PendingUS20210087281A1Relieve symptomsIncreased riskDigestive systemImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesFibrosis
The present invention relates to treatment of cancer using agonist anti-MET antibodies or fragments thereof. In particular, the invention relates to treatment of colorectal cancer using agonist anti-MET antibodies or fragments, typically colorectal cancer associated with chronic inflammation and / or gene mutations in the colon and in the gastrointestinal tract in general. The invention further relates to treating intestinal fibrosis using agonist anti-MET antibodies.
Owner:AGOMAB THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com