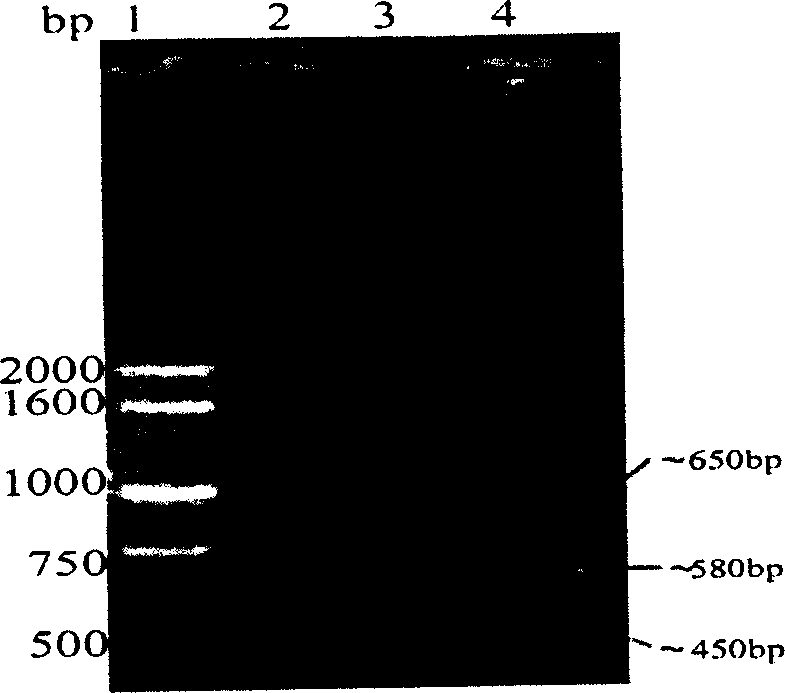Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Clone human" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
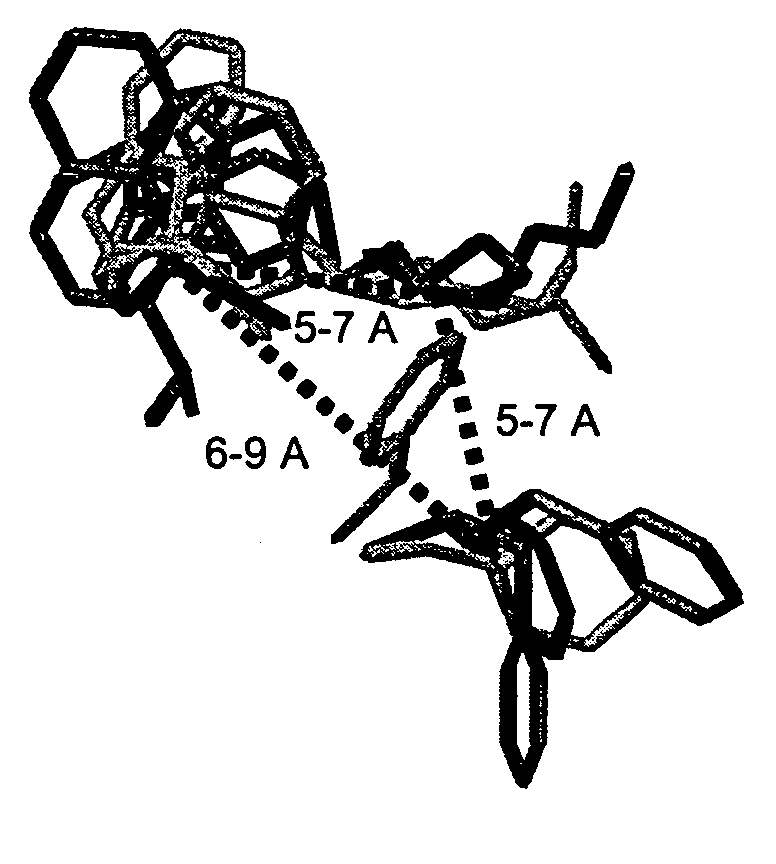
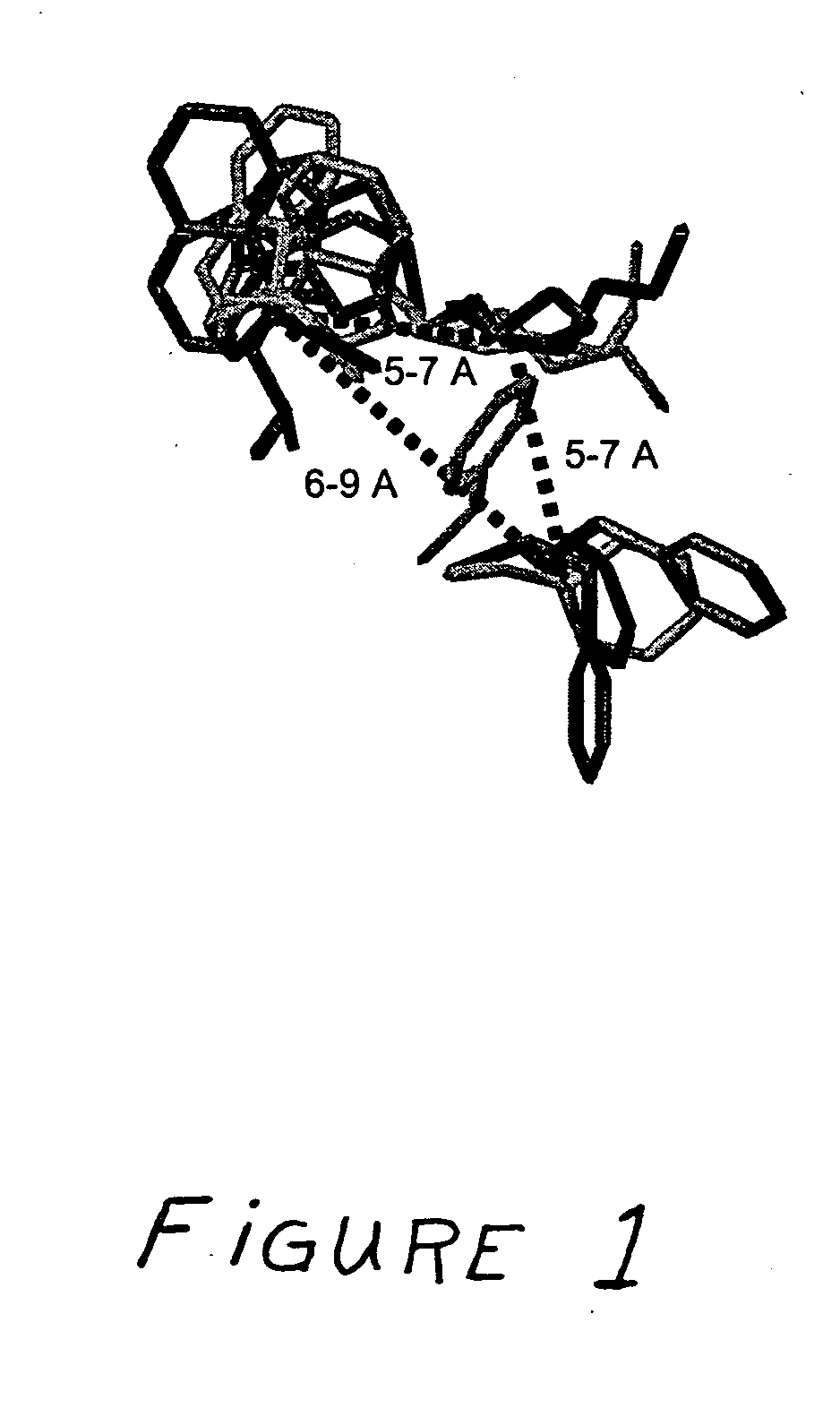
SSTR1-selective analogs
InactiveUS7019109B2High affinityEasy to markPeptide sourcesRadioactive preparation carriersNervous systemScreening method
Analogs of SRIF which are selective for SSTR1 in contrast to the other cloned SRIF receptors. These analogs are useful in determining the tissue and cellular expression of the receptor SSTR1 and its biological role in the endocrine, exocrine and nervous system, as well as in regulating tumor growth. SRIF analog peptides, such as des-AA1,2,5[D-Trp8, NαMeIAmp9, Tyr11]-SRIF and counterparts incorporating Cbm at the N-terminus and / or NαSer13, inhibit the binding of a universal SRIF radioligand to the cloned human receptor SSTR1, but they do not bind with significant affinity to human SSTR2, SSTR3, SSTR4 or SSTR5. By incorporating an iodinated tyrosine in position-2 or in position-11 in these SSTR1-selective SRIF analogs, a labeled compound useful in drug-screening methods is provided. The N-terminus accommodates bulky moieties without loss of selectivity, and a carbamoyl moiety or a conjugating agent that will accept a radioactive nuclide or will link to a cytotoxin may be present at the N-terminus.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Receptor(SSTR4)-selective somatostatin analogs
Analogs of SRIF which are selective for SSTR4 in contrast to the other cloned SRIF receptors are useful in determining tissue and cellular expression of the receptor SSTR4 and its biological role in regulating tumor growth. SRIF analog peptides, such as des-AA1,2,4,5,12,13[Ala7]-SRIF; des-AA1,2,4,5,12,13[Aph7]-SRIF, des-AA1,2,4,5,12,13[Aph7]Cbm-SRIF; des-AA1,2,4,5,12,13[Tyr2,Ala7]-Cbm-SRIF, and des-AA1,2,4,5,12,13[Tyr7,CβMe-L-2Nal8]-SRIF, and counterparts incorporating D-Cys3 and / or D-Trp8 and / or Ala11, bind with high affinity to the cloned human receptor SSTR4 and activate the receptor, but they do not bind with significant affinity to human SSTR1, SSTR2, SSTR3 or SSTR5. By incorporating an iodinated tyrosine in position-2 in these SSTR4-selective SRIF analogs, a labeled compound useful in drug-screening methods is provided. Alternatively, for use in therapy, cytotoxins or highly radioactive elements can be N-terminally coupled or complexed thereto.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Gene promoter of ginseng PgPDR3 responded by methyl jasmonate and application thereof
InactiveCN103103194AVector-based foreign material introductionAngiosperms/flowering plantsNicotiana tabacumSalicylic acid
The invention discloses a gene promoter of ginseng PgPDR3 responded by methyl jasmonate and an application thereof. The sequences of the gene promoter of the ginseng PgPDR3 are shown in SEQ ID NO.1, or are the DNA sequences of which the homology with SEQ ID NO.1 is over 99%. According to the invention, a Genomewalker technology is adopted to clone, later the sequences of the gene promoter of the PDR transport protein is obtained, and then the expression vector Pcambia1301-ProPDR3::GUS of the promoter is built; if the promoter in the transgenic tobacco drives the GUS to express, and can be controlled by ginsenoside, salicylic acid, gibberellins and abscisic acid, the promoter participates in the transport and accumulation of the ginsenoside, which provides a basis for cloning the gene of key control recording factor in the ginsenoside transport signal pathway and controlling the transport and accumulation of the terpenoid, such as ginsenoside; and fine plants containing rich ginsenoside can be obtained by using a method for controlling or improving the ginseng and other plants via the promoter or the derivative thereof.
Owner:CENT SOUTH UNIV
Receptor(SSTR2)-selective somatostatin antagonists
ActiveUS20080260638A1Easy to markEasy to usePeptide/protein ingredientsMetabolism disorderSide effectHigh affinity binding
SRIF peptide antagonists, which are selective for SSTR2 in contrast to the other cloned SRIF receptors and which bind with high affinity to the cloned human receptor SSTR2 but do not activate the receptor, have many useful functions. Because they do not bind with significant affinity to SSTR1, SSTR3, SSTR4 or SSTR5, their administration avoids potential undesirable side effects. Because they block the receptor function, they can be used therapeutically to block certain physiological effects which SSTR2 mediates. By incorporating radioiodine or the like in these SSTR2-selective SRIF antagonists, a labeled compound useful in drug-screening methods is provided. Alternatively, for use in therapy, highly radioactive moieties can be N-terminally coupled, complexed or chelated thereto.
Owner:SALK INST FOR BIOLOGICAL STUDIES +2
Receptor(SSTR4)-selective somatostatin analogs
Owner:SALK INST FOR BIOLOGICAL STUDIES
SSTR1-selective analogs
InactiveUS20060155107A1Improve bindingHigh selectivityPeptide/protein ingredientsSomatostatinsNervous systemScreening method
Analogs of SRIF which are selective for SSTR1 in contrast to the other cloned SRIF receptors. These analogs are useful in determining the tissue and cellular expression of the receptor SSTR1 and its biological role in the endocrine, exocrine and nervous system, as well as in regulating tumor growth. SRIF analog peptides, such as des-AA1,2,5[D-Trp8, IAmp9, Tyr11]-SRIF and counterparts incorporating Cbm at the N-terminus, as well as radioiodinated versions thereof, inhibit the binding of a universal SRIF radioligand to the cloned human receptor SSTR1, but they do not bind with significant affinity to human SSTR2, SSTR3, SSTR4 or SSTR5. By incorporating an iodinated tyrosine in position-2 or in position-11 in these SSTR1-selective SRIF analogs, a labeled compound useful in drug-screening methods is provided. The N-terminus accommodates bulky moieties without loss of selectivity, and a carbamoyl moiety or a conjugating agent that will accept a radioactive nuclide or will link to a cytotoxin may be present at the N-terminus.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Receptor(SSTR2)-selective somatostatin antagonists
ActiveUS7960342B2Easy to markEasy to useIn-vivo radioactive preparationsPeptide/protein ingredientsSide effectMedicine
SRIF peptide antagonists, which are selective for SSTR2 in contrast to the other cloned SRIF receptors and which bind with high affinity to the cloned human receptor SSTR2 but do not activate the receptor, have many useful functions. Because they do not bind with significant affinity to SSTR1, SSTR3, SSTR4 or SSTR5, their administration avoids potential undesirable side effects. Because they block the receptor function, they can be used therapeutically to block certain physiological effects which SSTR2 mediates. By incorporating radioiodine or the like in these SSTR2-selective SRIF antagonists, a labeled compound useful in drug-screening methods is provided. Alternatively, for use in therapy, highly radioactive moieties can be N-terminally coupled, complexed or chelated thereto.
Owner:SALK INST FOR BIOLOGICAL STUDIES +2
Recombinant Adeno-Associated Virus Expressing human Antisense Gene Cyp2j2 and Its Preparation Methods
ActiveUS20080131403A1Inhibit migrationPromote apoptosisBiocideGenetic material ingredientsHuman tumorLymphatic Spread
A recombinant adeno-associated virus expressing antisense human CYP2J2 gene and preparation method thereof are provided, the recombinant adeno-associated virus is prepared through cloning human CYP2J2 cDNA (1509 bp, which encode the protein containing 503 amino acids) from human leucocyte DNA by PCR, cotransfecting three plasmids by the calcium phosphate precipitation technique to pack and prepare the recombinant adeno-associated virus containing the antisense target gene, and purifying. The recombinant adeno-associated virus obtained from the above method can be transfected into different kinds of human tumor cell lines, inhibiting the proliferation and migration of tumor cells, promoting the apoptosis of tumor cells and suppressing the growth and metastases of tumor. Therefore, it can prove that the selective inhibitor of CYP2J2 and the recombinant adeno-associated virus expressing antisense human CYP2J2 gene are the potential medicine for treating tumor.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Method for expressing and purifying human recombinant interleukin-3
ActiveCN102146413AAvoid degradationLow toxicityPeptide preparation methodsFermentationPichia pastorisMass spectrometric
The invention discloses a Pichia pastoris transformant capable of expressing human recombinant interleukin-3 (rhIL-3) with high efficiency and a purification method thereof. In the method, the eukaryotic host is pichia pastorisX-33. The purification method comprises the following steps: cloning a human IL-3 gene; establishing a eukaryotic expression vector, and transforming the eukaryotic expression vector into the eukaryotic yeast host; obtaining a yeast transformant for high-level secretory expression by screening, wherein the IL-3 expressed by the yeasts are available in a glycosylated mode and a non-glycosylated module; and performing amplified culture by using a shake flask, and subjecting the supernate of the culture solution to dialysis, nickel affinity purification and further purification by diethylaminoethanol (DEAE) anion column. The purified product is subjected to mass spectrometric identification and analysis, and the result of the mass spectometric identification and analysis indicates that the expressed IL-3 is modified by different glycosyls and that the IL-3 has an his*6 tag and a C-MYC tag and is easy for purification and detection of expression product. In the invention, different from the conventional method using a prokaryotic host to express the rhIL-3, the method for expressing a large amount of rhIL-3 by using a Pichia pastoris expression system is adopted for the first time, quick purification is realized by using a His-tag protein, the purified rhIL-3 is high-activity rhIL-3 protein which is glycosylated to different extents and of which the molecular weight is 19kDa and 22kDa. The method ensures that the high-activity rhIL-3 recombinant protein is obtained quickly.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
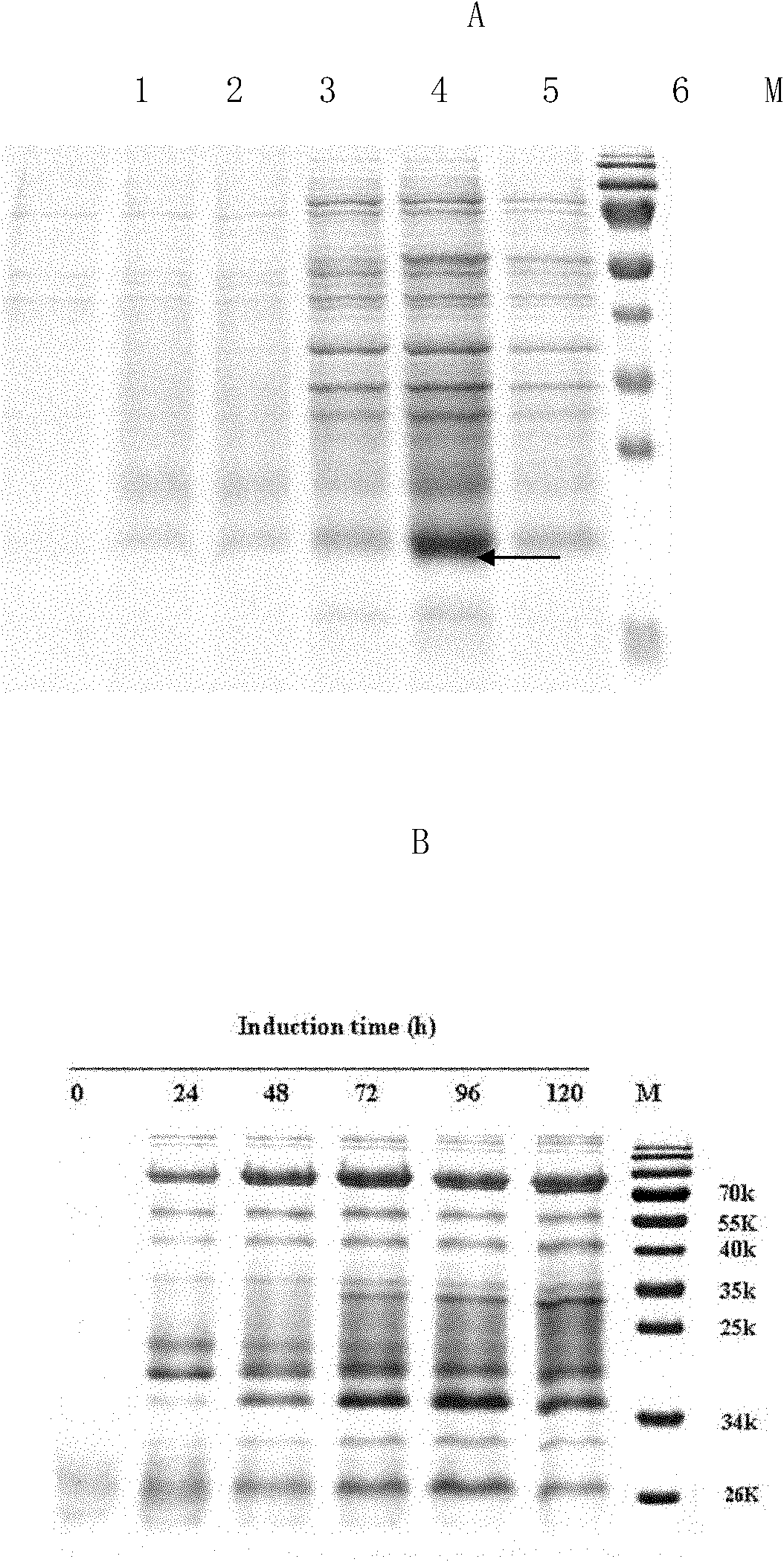
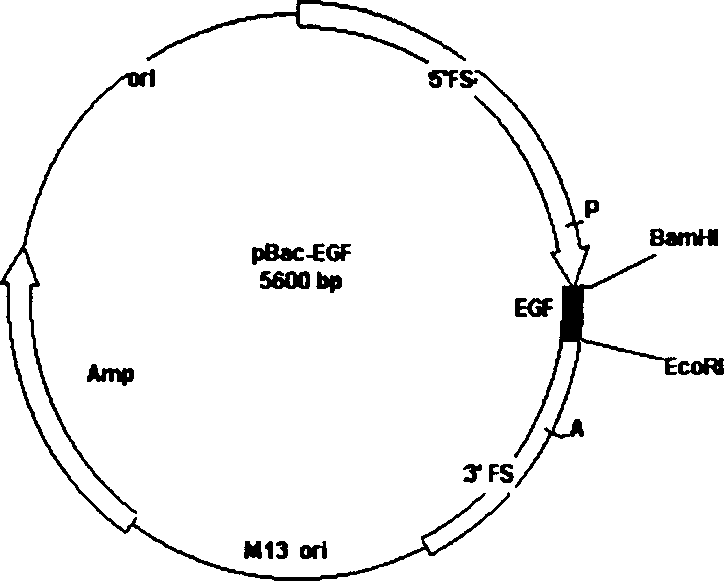
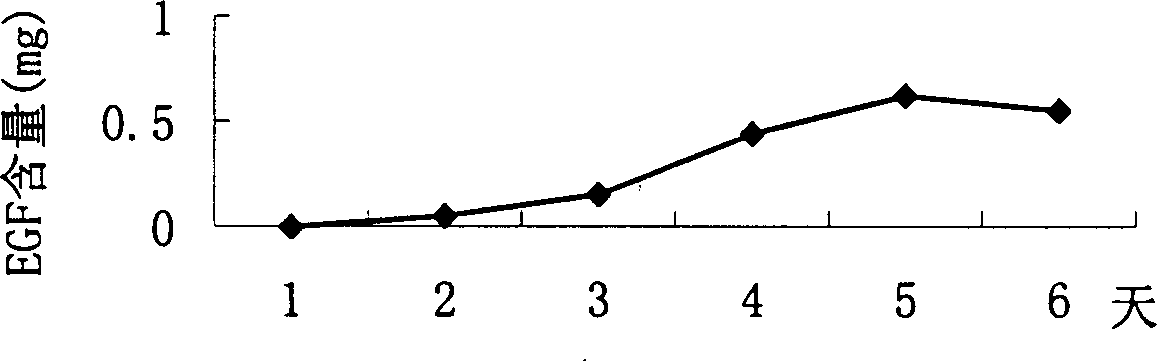
Method for producing medicine using silkworm expressed numan epidermal growth factor
InactiveCN1405310ADetermine therapeutic functionPeptide/protein ingredientsMicroencapsulation basedDiseaseCuticle
The invention belongs to the technique field of producing multi-peptide drugs by gene engineering. It clones human EGF gene into the silkworm bacilliform virus (Bombyx mori Nuclear polyhedrosis Virus) transferring carrier pBacPAK8 and makes them carry through homologous reformation in the silkworm cells to obtain the reformed virus BmBacEGF with human-coat growing gene. Use the reformed virus to to inocualte the silkworm grub and chrysalis by acupuncture in order to make the human-coat growing gene efficient expressed. Then make the grub and chrysalis into oral drugs which has remarkable action on the digestible ulcer by examination on animal.
Owner:正源堂(天津)生物科技有限公司
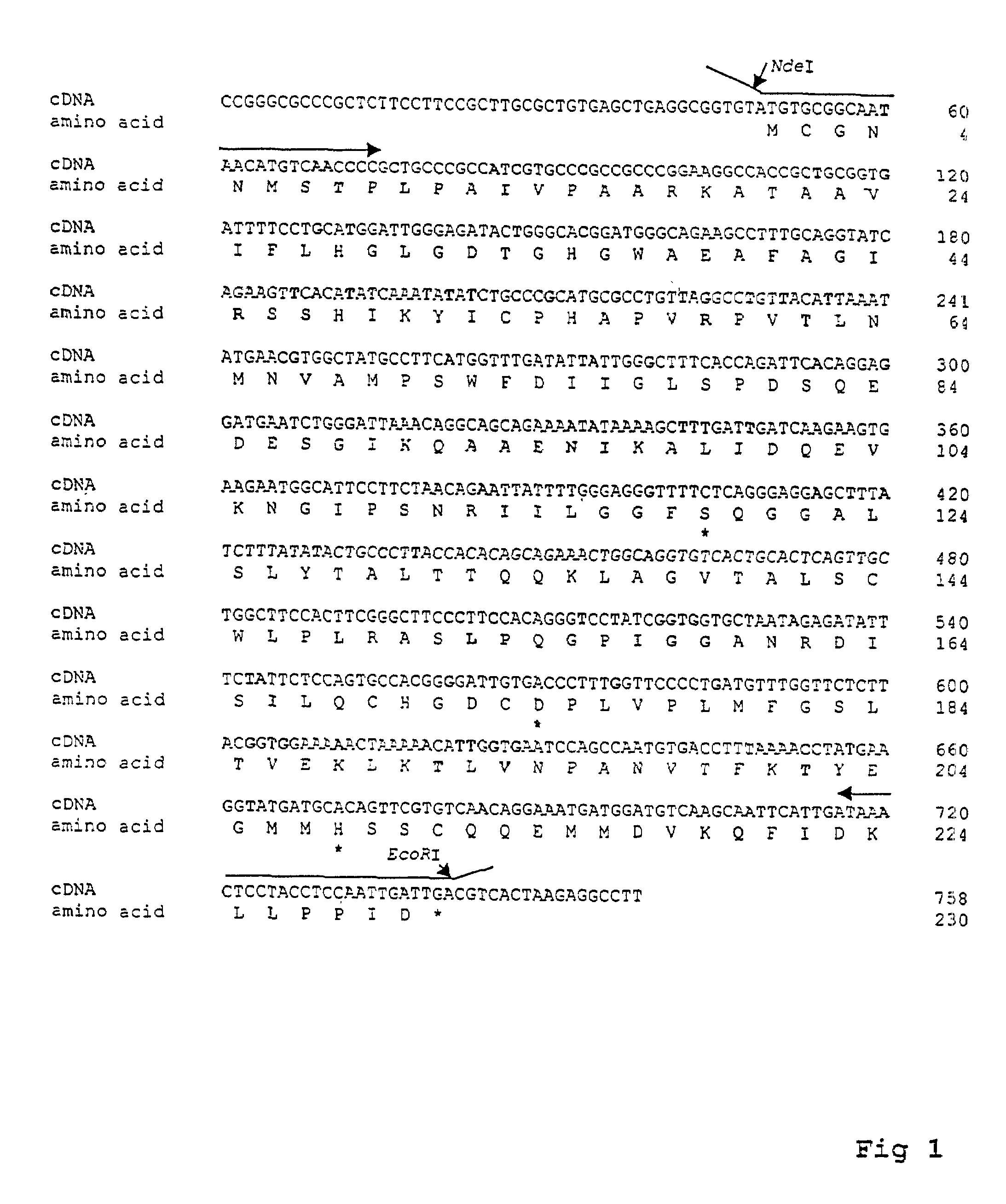
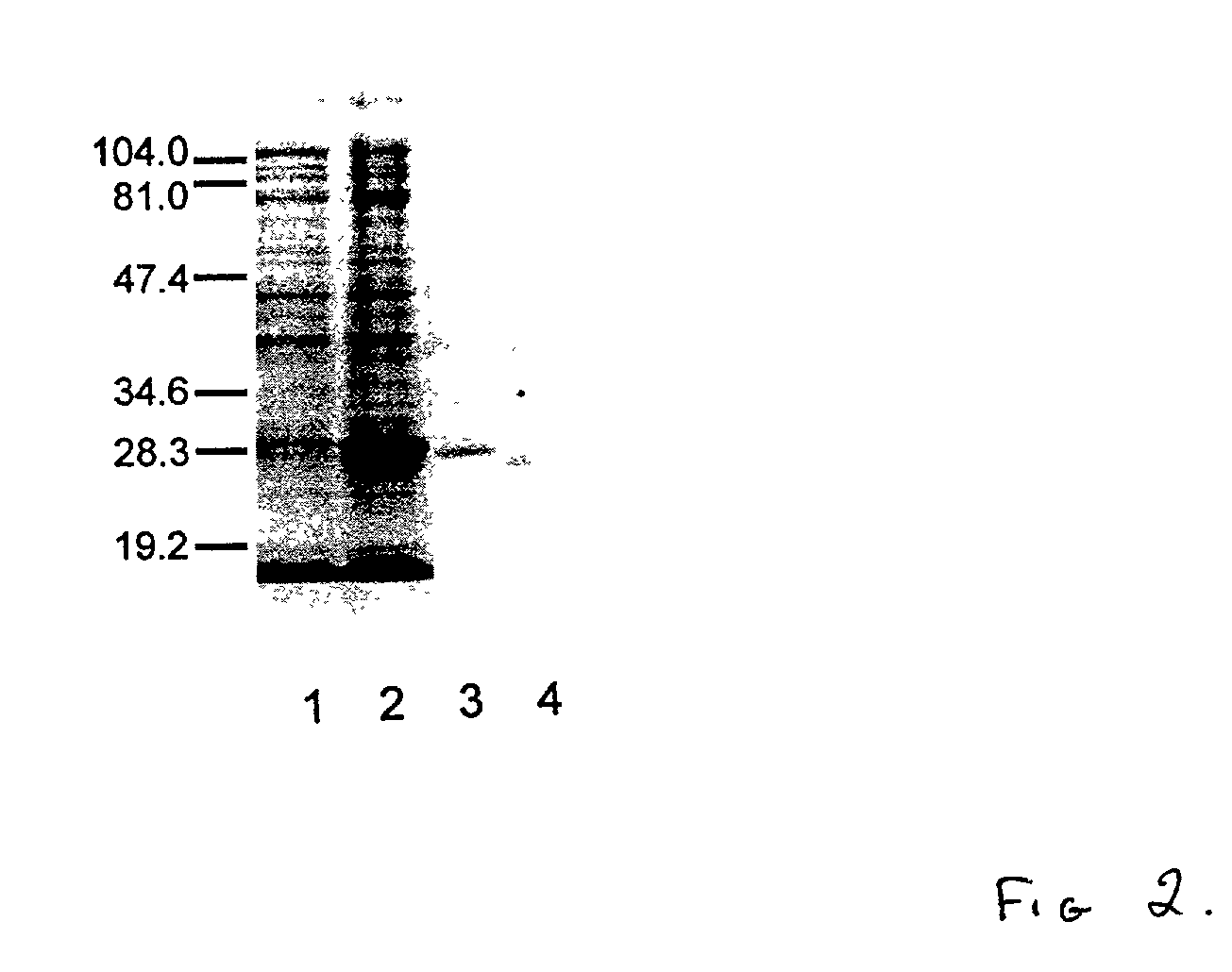
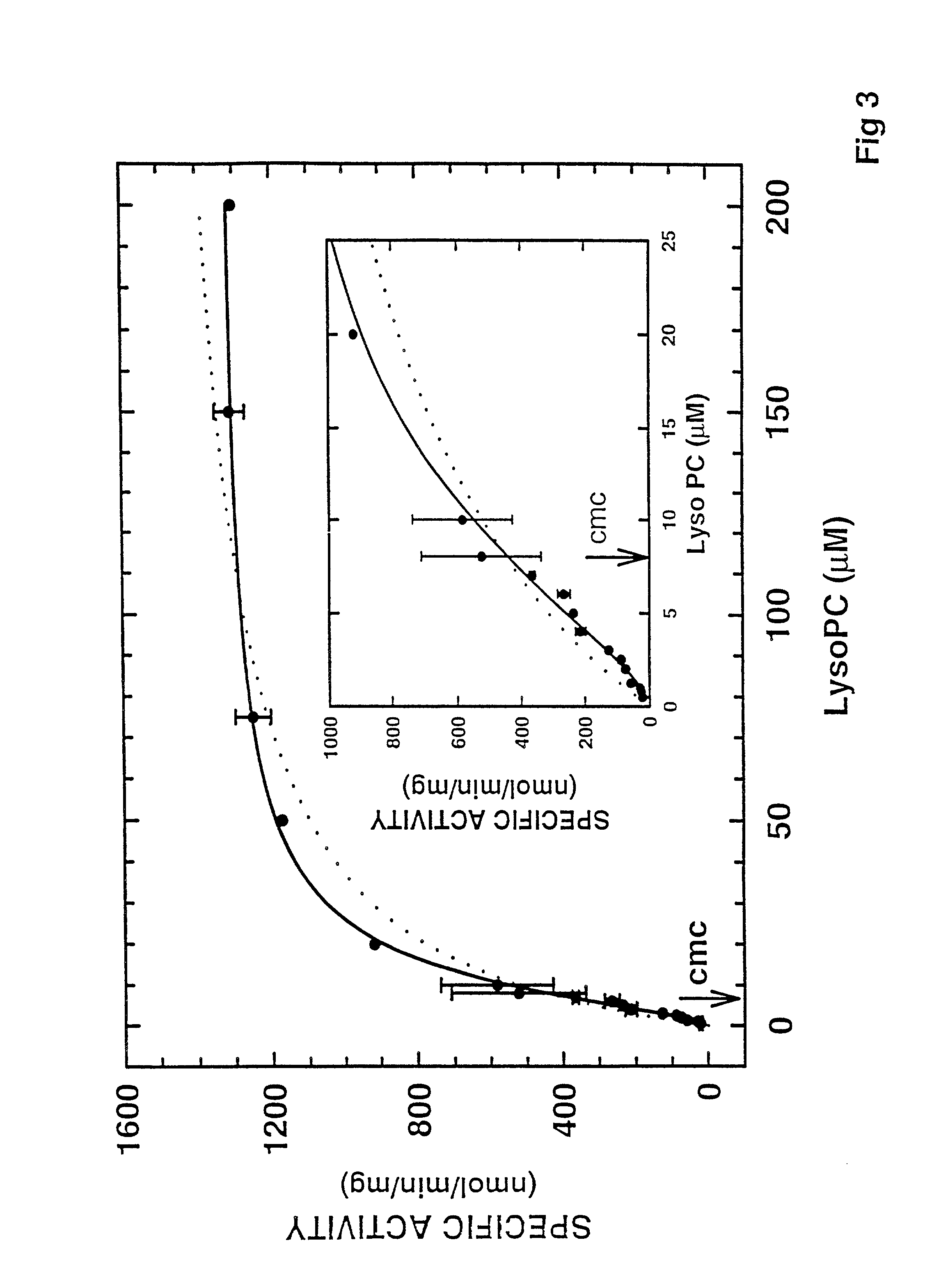
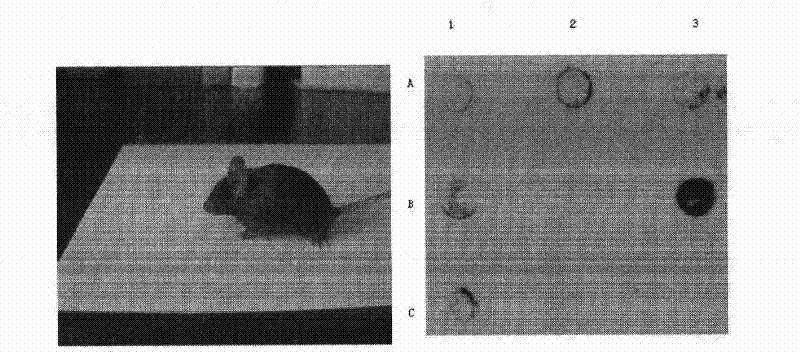
Cloned human lysophospholipase
InactiveUS7294496B1Inhibition is irreversibleImprove the level ofSugar derivativesBacteriaDiseaseBinding site
A cloned of a human brain lysophospholipid-specific lysophospholipase enzyme molecule, its potential use for treatment of a host of diseases and method of inactivation are disclosed. Also disclosed are its distribution in tissue sand detailed kinetic analysis. hLysoPLA has a single substrate binding site and a surface recognition site. In contrast to many nonspecific lipolytic enzymes that exhibit lysophospholipase activity, hLysoPLA hydrolyzes only lysophospholipids and has no other significant enzymatic activity.
Owner:RGT UNIV OF CALIFORNIA
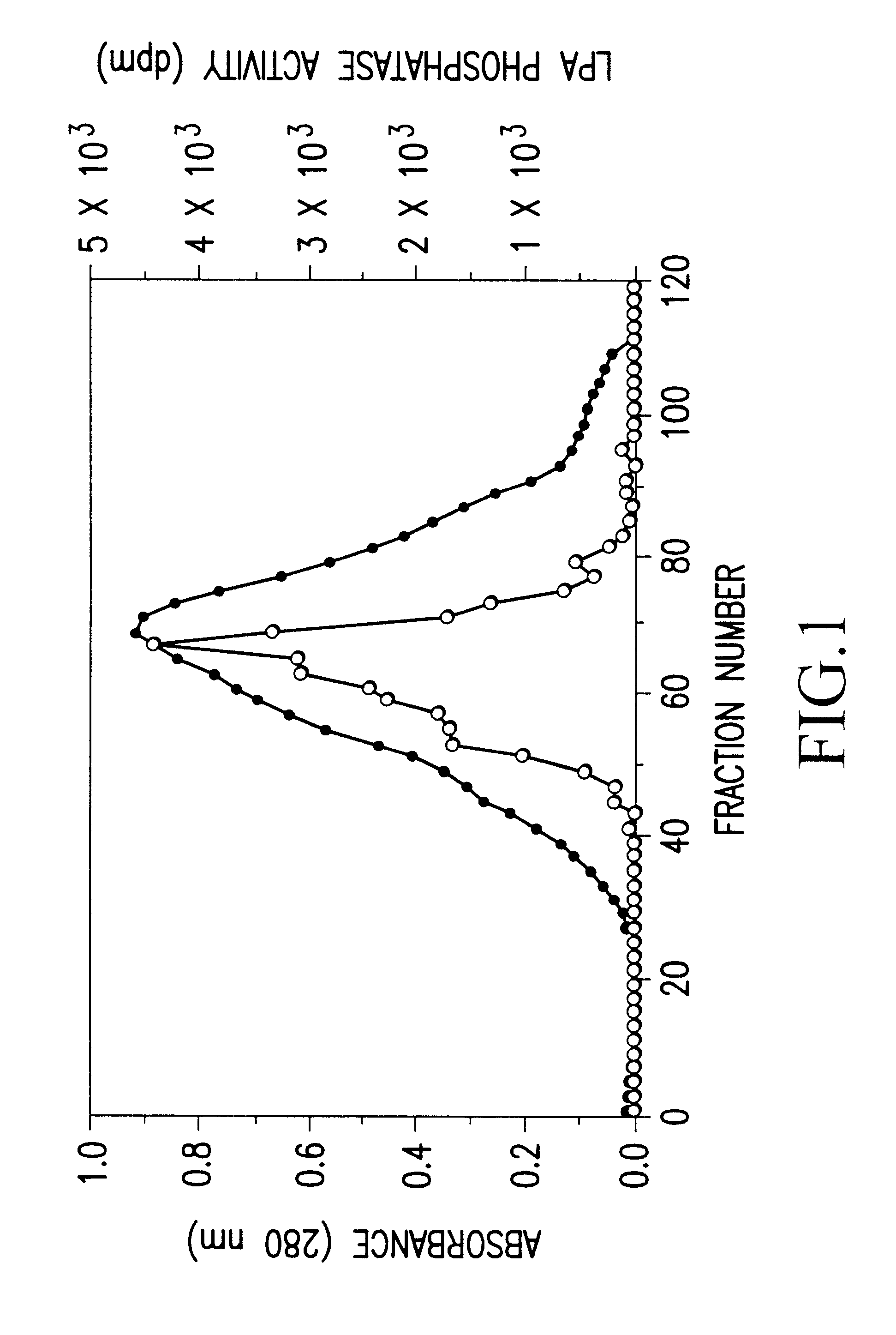
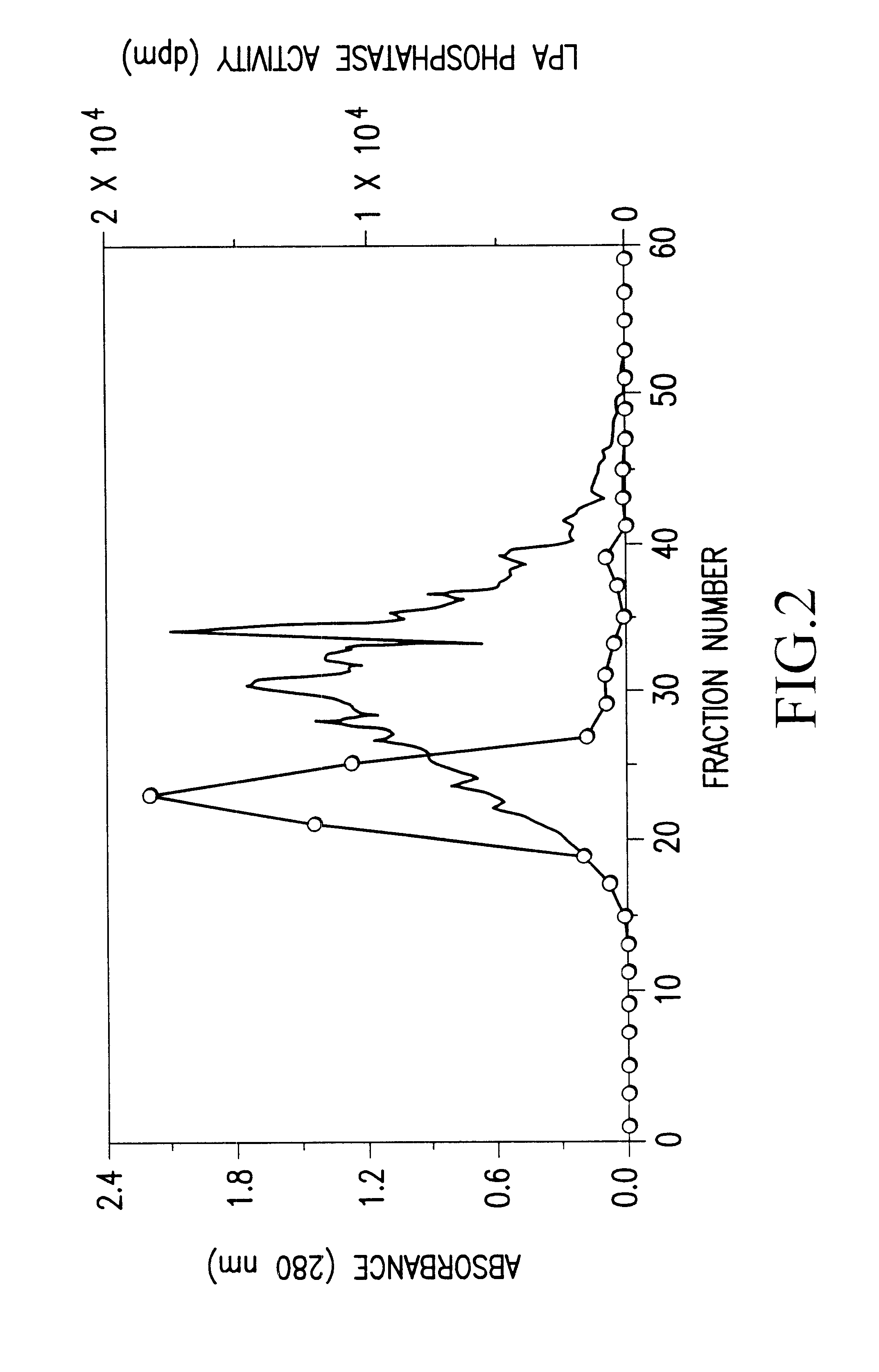
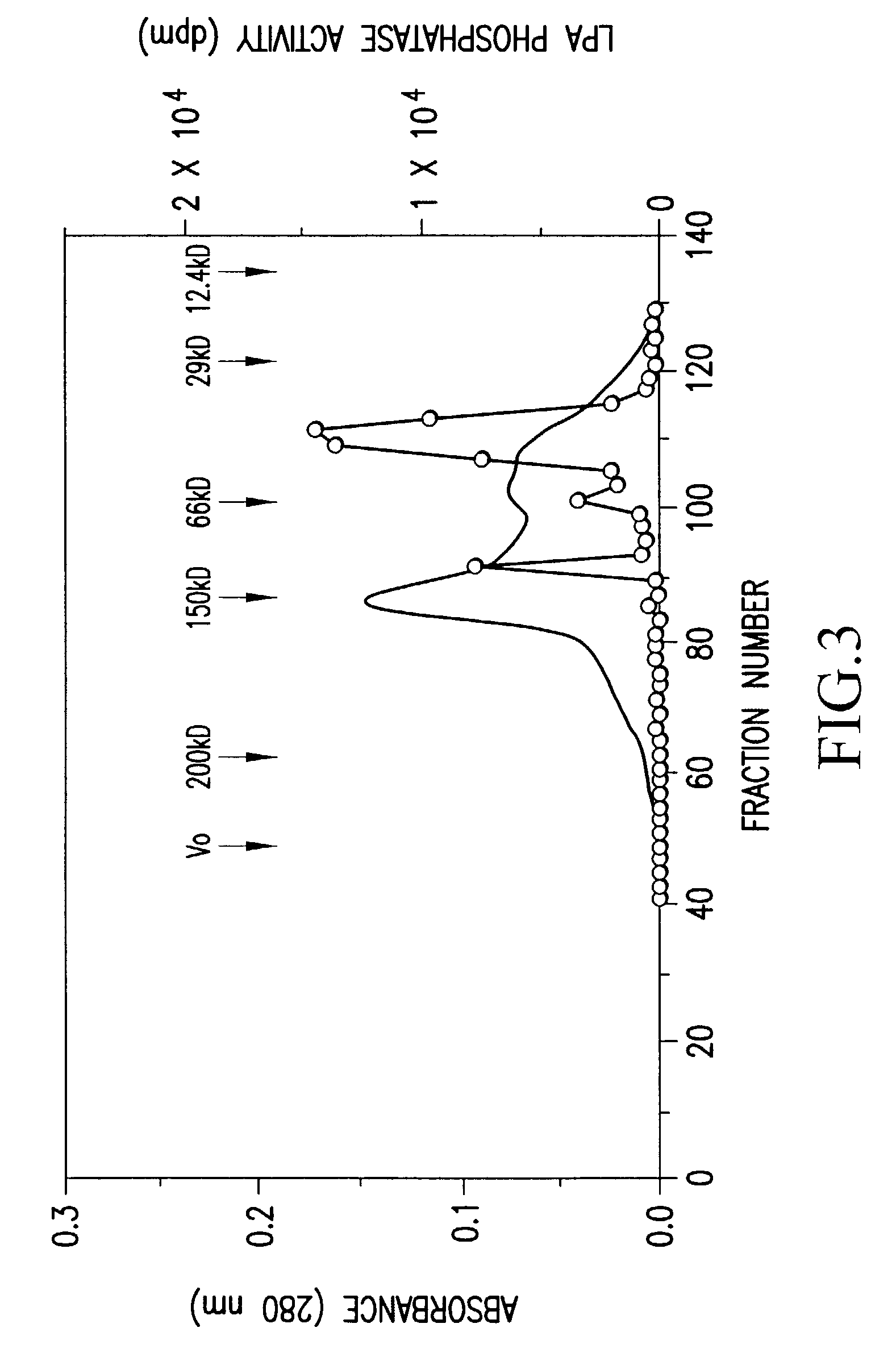
Recombinant lysophosphatidic acid phosphatase
InactiveUS6472193B1Simply and inexpensively determiningEasy to prepareBacteriaSugar derivativesNucleotidePhosphate
An object of the present invention is to provide a recombinant LPA phosphates capable of specifically hydrolyzing LPA, which is useful for elucidation of the metabolic pathway of LPA, and also for diagnosis and treatment of various diseases with which LPA is associated. The present invention also provides for a method capable of simply and inexpensively determining LPA associated with various diseases. The present invention also provides for a kit for determination suitable for the method. The present invention has succeeded in purifying the LPA phosphatase using bovine brain as a raw material, and further in cloning human LPA phosphatase gene. The present invention specifically relates to a DNA encoding a peptide comprising the amino acid sequence of SEQ ID NO:1; a DNA comprising the nucleotide sequence of SEQ ID NO:2; a protein encoded by the DNA; and expression vector carrying the DNA; a transformant harboring the expression vector; a method for producing a recombinant lysophosphatidic acid phosphatase by the transformant; a method for determination of LPA by the protein; a determination reagent for LPA by the protein; a kit for diagnosis, comprising the reagent for determination, and the like.
Owner:AZWELL
Antibodies directed against influenza
ActiveUS9469685B2Preventing H1NReduce the risk of infectionImmunoglobulins against virusesAntiviralsAntibody fragmentsInfluenza antibody
Owner:EMORY UNIVERSITY +1
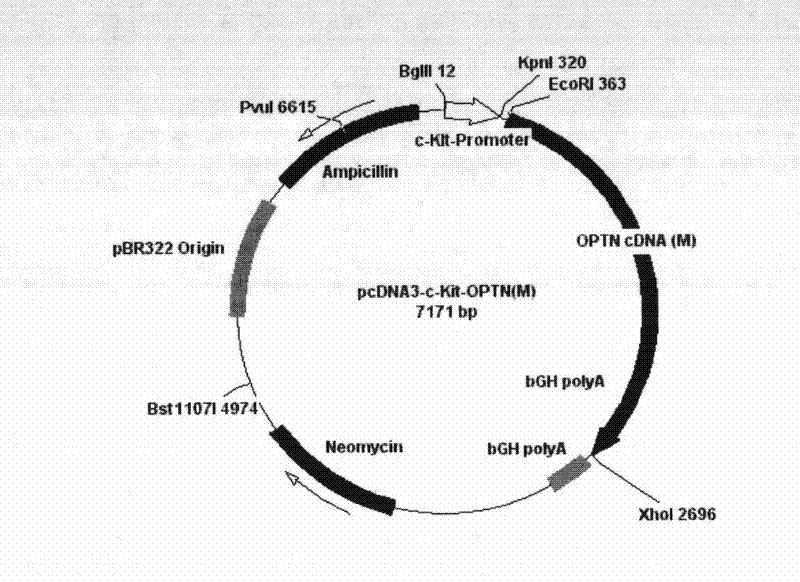
Disease gene animal model of primary open angle glaucoma and construction method thereof
InactiveCN102229959AShort reproductive cyclePromote growthVector-based foreign material introductionAnimal husbandryOpen angle glaucomaClone human
The invention discloses a disease gene animal model of primary open angle glaucoma and a construction method thereof. The method comprises the following steps: (1) cloning human an OPTN (optineurin, E50K) mutant gene to an expression vector with a retinal specific promoter c-kit to construct a recombinant mammal expression vector; and (2) introducing the recombinant mammal expression vector into the bodies of mice and screening and identifying to obtain transgenic mice. In consideration of the difference between the mouse OPTN (E50K) and the human gene, the human OPTN (E50K) is introduced into the retinal specific promoter so that the human OPTN (E50K) gene is specifically expressed in the retina of the mouse. The model of primary open angle glaucoma due to the mutation of OPTN (E50K) is constructed, and the laboratory animals are provided for the systemic researches on pathogenic mechanism, development trend and disease prognosis and treatment of primary open angle glaucoma (POAG) due to the mutation of OPTN (E50K) in human beings.
Owner:HARBIN MEDICAL UNIVERSITY
Method for preparing transgenic bombyx mori and its application in pharmacy
InactiveCN1670215AClear efficacyHigh biosecurityAnthropod material medical ingredientsMicrobiological testing/measurementBiologyGene carrier
This invention discloses a method for preparing trans-gene cultivated silkworm with human granulocyte, namely macrophage colony stimulating factor, characterized by the following steps: a, through gene engineering operation, cloning human granulocyte under the control of cultivated silkworm's karyotype polyhedrosis virogene promoter into a carrier with reporting gene based on piggyBAC factor; b, constructing and recombining trans-gene carrier; c, at the presence of subsidiary particles, attaining silkworm with reporting gene by spermium conducting method, and testing out human granulocyte, breeding to silkworms.
Owner:SUZHOU UNIV +1
Human olfactory receptors and genes encoding same
The invention relates to cloned human olfactory receptors and the use thereof for identifying compounds that modulate olfaction.
Owner:SENOMYX INC
Recombinant lysophosphatidic acid phosphatase
An object of the present invention is to provide a recombinant LPA phosphatase capable of specifically hydrolyzing LPA, which is useful for elucidation of the metabolism pathway of LPA, and also for diagnosis and treatment of various diseases with which LPA is associated; a method capable of simply and inexpensively determining LPA associated with various diseases; and a kit for determination suitable for the method. The present invention has succeeded in purifying the LPA phosphatase using bovine brain as raw material, and further in cloning human LPA phosphatase gene. The present invention is accomplished by a DNA encoding a peptide comprising the amino acid sequence of SEQ ID NO:1; a DNA comprising the nucleotide sequence of SEQ ID NO:2; a protein encoded by the DNA; an expression vector carrying the DNA; a transformant harboring the expression vector; a method for producing a recombinant lysophosphatidic acid phosphatase by the transformant; a method for determination of LPA by the protein; a determination reagent for LPA by the protein; a kit for diagnosis, comprising the reagent for determination, and the like.
Owner:AZWELL
Optimized porcine circovivus type 2 recombinant adenovirus construction method
InactiveCN106399262AIncrease target gene expressionImprove stabilityVectorsVirus peptidesShuttle vectorAutoimmune responses
The invention relates to an optimized porcine circovivus type 2 recombinant adenovirus construction method. The construction of recombinant adenovirus is completed by cloning Human cytomegalovirus first intron (Intron A) and woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) into an adenovirus shuttle vector. The construction method provided by the invention has the advantages that a Cap expression quantity is improved, so that adenovirus usage dose and adenovirus protein autoimmune response can be relieved and the preparation efficiency of the porcine circovivus type 2 recombinant adenovirus can be improved.
Owner:NORTHWEST A & F UNIV
Human recombinant amelogenin and preparation method thereof
InactiveCN101565463AFor long-term storageArtificial synthesis is convenientFermentationAnimals/human peptidesEscherichia coliX chromosome
The invention relates to protein, in particular to human recombinant amelogenin and a preparation method thereof. The human recombinant amelogenin is prepared by the following method: a, cloning human amelogenin mature peptide gene hAm coded by the X chromosome; b, constructing recombinant prokaryotic expression plasmid PGEX4T1-hAm containing the human amelogenin mature peptide gene; c, transfecting the recombinant prokaryotic expression plasmid PGEX4T1-hAm obtained from the step b into an expression strain E.coli.Rosetta; d, inducing the cloning expression strain obtained from the step c to express fusion protein GST-hAm; and e, purifying the fusion protein by GST affinity chromatography. The invention firstly obtains the encoding sequence of human amelogenin mature peptide from human deciduous dental germ through cloning, and successfully expresses the fusion protein in bacillus through the prokaryotic expression plasmid; and the artificially synthesized recombinant amelogenin can be obtained through the purification by the GST affinity chromatography.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
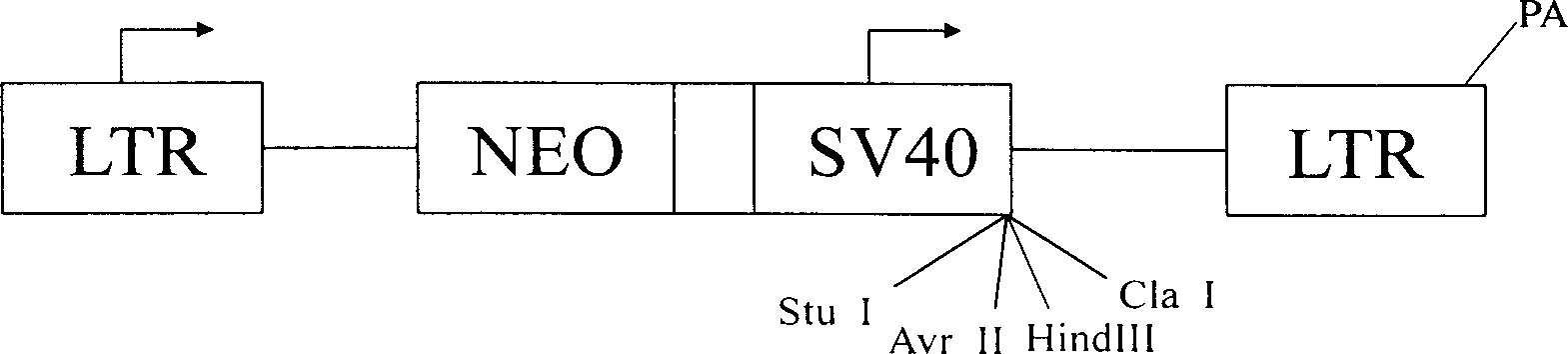
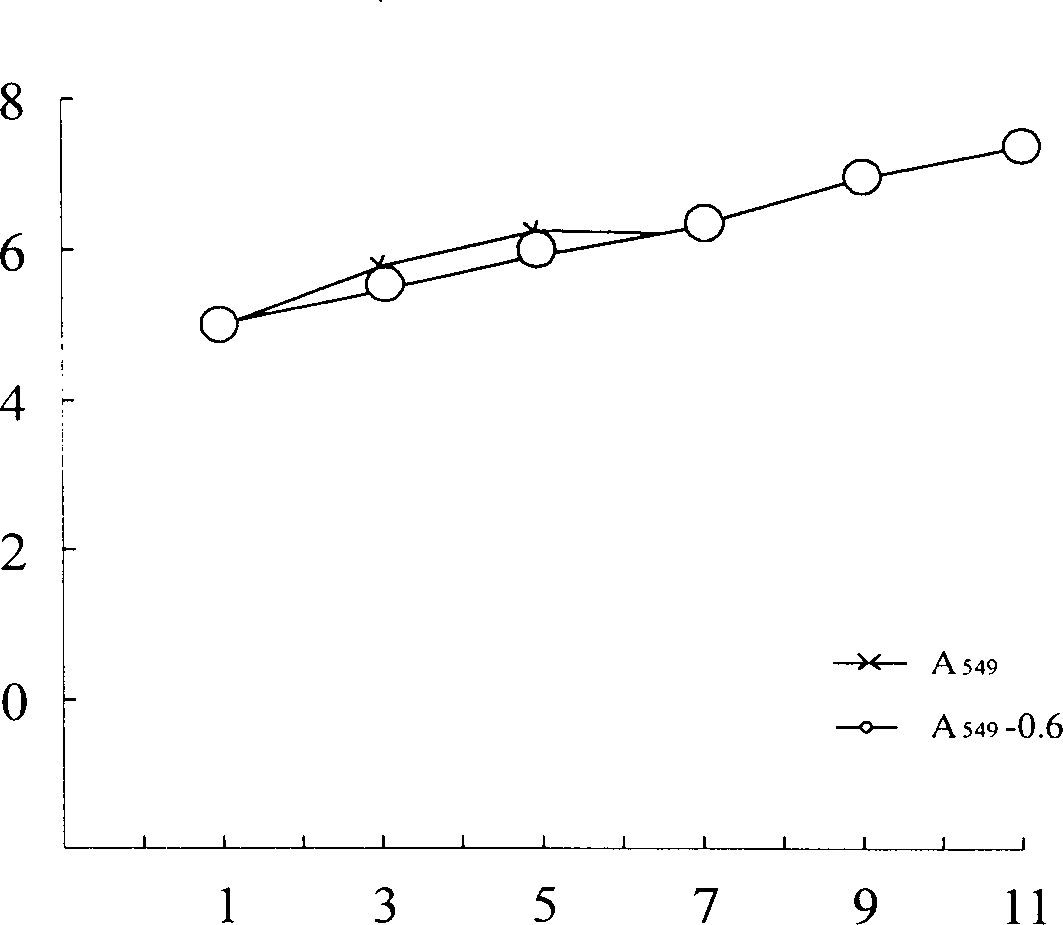
Lung cancer transgene vaccine and its preparation method
InactiveCN1429624AEnhance active immune rejectionPrevent relapseAntibody medical ingredientsAntineoplastic agentsPulmonary adenocarcinomaFull length cdna
A transgenic vaccine for lung cancer is prepared through targeting the cloned human IFN-gamma whole length CDMA to the cell strain A548 of human pulmonary adenocarcinoma by retroviral rector PLXSN, resistance screen to obatain cellstrain hu gamma-IFN-A549, and conventional culture to obtain the vaccine. It can prevent recurrence and transfer of tumor.
Owner:HANGZHOU FIRST PEOPLES HOSPITAL
Method for acquiring human alpha fetoproteins
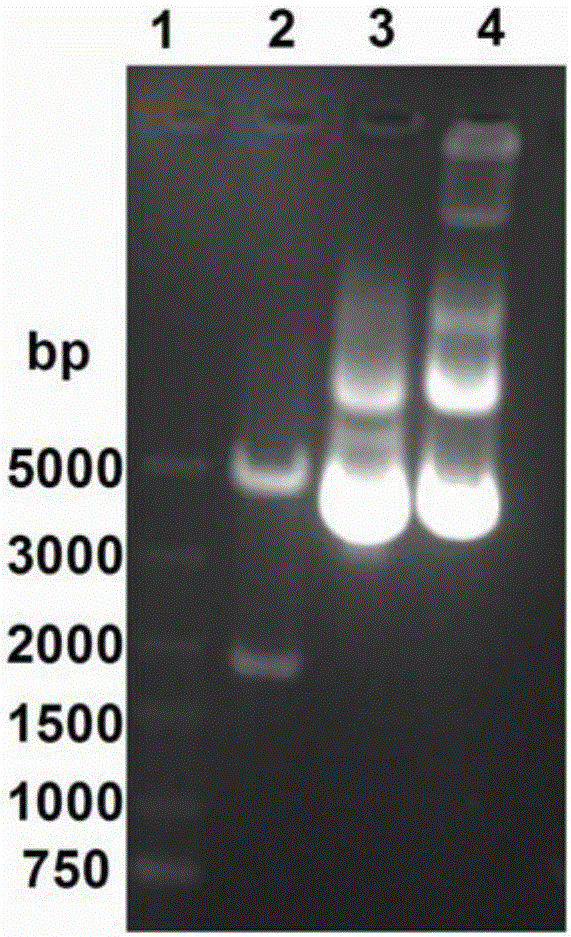
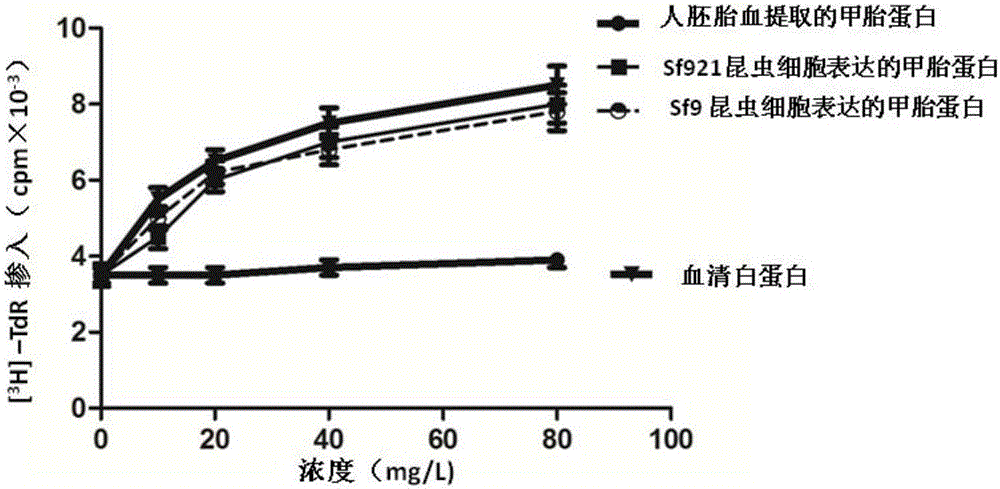
InactiveCN105732795AHigh activityHigh purityAnimals/human peptidesVector-based foreign material introductionEmbryoAlpha-fetoprotein
The invention discloses a method for obtaining human alpha-fetoprotein, which comprises cloning the human alpha-fetoprotein gene into insect cell expression vectors pFastBac 1, pFastBac Dual or pFastBac1‑Gus, and transforming DH10Bac to obtain human alpha-fetoprotein-containing Bacmid gene, Bacmid transfected insect cells 2 to 4 days after the secretion of human alpha-fetoprotein into the culture medium, human alpha-fetoprotein secreted by nickel column and gel chromatographic column after purification can be active and extracted from human embryonic blood of alpha-fetoprotein. The invention utilizes the eukaryotic expression of Sf9 and Sf21 insect cells to obtain human alpha-fetoprotein with better activity.
Owner:HAINAN MEDICAL COLLEGE
Human platelet-derived growth factor A homologous dimers and production method thereof
InactiveCN102154305AImprove biological activityMaintain biological activityPeptide preparation methodsFermentationBiotechnologyHuman platelet
The invention discloses a human platelet-derived growth factor (PDGF) A homologous dimers and a high-efficiency production method thereof. The method mainly comprises the following steps: cloning a human platelet-derived growth factor A spliceosome 2 gene; constructing an eukaryotic expression vector and transferring the eukaryotic expression vector into an eukaryotic yeast host; screening a yeast transformant for high level secretion expression, wherein the PDGF-As expressed by the yeast are in a glycosylated form and a non-glycosylated form; and performing amplified culture in a shake flask, dialyzing supernate of culture solution, purifying the supernate efficiency by using a chromatography (CM) cation exchange column and G-75 molecular sieve, and obtaining the novel modified protein of the PDGF-AA dimers, of which one PDGF-A monomer is glycosylated and of which the other PDGF-A monomer is not glycosylated, by using a CM cation exchange process and optimizing the pH value of dislysate, wherein the separated and purified new glycosylation modified protein of the PDGF-AA dimers has high uniformity.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Method for building immortalized preadipocytes of chicken
The invention relates to a method for building the immortalized preadipocytes of chicken, which can be used for solving the problems that the stable and reliable result is difficult to obtain by the research as the preadipocytes of primary chicken can not go down to the future generation infinitely and is heterogeneous, cells with different sources have different genetic backgrounds, and the like. The method comprises the steps of firstly, cloning human chicken telomerase reverse transcriptase (chTERT) genes; secondly, cloning chicken telomerase (chTR) RNA genes; thirdly, constructing a reverse transcribing virus expression vector; fourthly, packaging and preparing reverse transcribing virus; and fifthly, obtaining an immortalized chicken preadipocyte system ICPA-1 and an immortalized chicken preadipocyte system ICPA-2. The method is applied to the field of building the immortalized preadipocytes of the chicken.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Method for producing blood vessel diseases gene medicine-blood vessel endothelium growth gene-2 naked DNA by microorganism cloning vehicle
InactiveCN1225557CGood treatment effectEliminate potentially dangerousGenetic material ingredientsMicrobiological testing/measurementVascular endotheliumPromoter
A process for constructing the vector pEGVPV, which can copy a lot of DNAs in procaryotic cell and is able to express in procaryon and mammal cell, includes such steps as cloning murinal VEGF-2 (mVEGF-2) gene from mouse embryo, cloning human VEGF promoter, linking them and inserting the gene into mammal's expression carrier pEGSH. After said carrier is used to transform E. coli DH5 alpha, the output of DNA separated from the culture medium of cells can reach 1.5 mg / L. A process for preparing the vascular endothelial growth factor 2 as DNA medicine from microbe for treating vascular diseases is also disclosed.
Owner:甘肃亚盛盐化工业集团有限责任公司
Recombinant lysophosphatidic acid phosphatase
InactiveUS20030104600A1Simply and inexpensively determiningEasy to prepareSugar derivativesBacteriaDiseaseNucleotide
An object of the present invention is to provide a recombinant LPA phosphatase capable of specifically hydrolyzing LPA, which is useful for elucidation of the metabolism pathway of LPA, and also for diagnosis and treatment of various diseases with which LPA is associated; a method capable of simply and inexpensively determining LPA associated with various diseases; and a kit for determination suitable for the method. The present invention has succeeded in purifying the LPA phosphatase using bovine brain as a raw material, and further in cloning human LPA phosphatase gene. The present invention is accomplished by a DNA encoding a peptide comprising the amino acid sequence of SEQ ID NO: 1; a DNA comprising the nucleotide sequence of SEQ ID NO: 2; a protein encoded by the DNA; an expression vector carrying the DNA; a transformant harboring the expression vector; a method for producing a recombinant lysophosphatidic acid phosphatase by the transformant; a method for determination of LPA by the protein; a determination reagent for LPA by the protein; a kit for diagnosis, comprising the reagent for determination, and the like.
Owner:AZWELL
High-efficiency non-integrating human iPSC induction platform
ActiveCN104673741BImprove induction efficiencyEasy to separateArtificial cell constructsVertebrate cellsHydrolase inhibitorThe Internet
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Method for preparing transgenic bombyx mori and its application in pharmacy
InactiveCN1320120CClear efficacyHigh biosecurityAnthropod material medical ingredientsMicrobiological testing/measurementBiologyGene carrier
This invention discloses a method for preparing trans-gene cultivated silkworm with human granulocyte, namely macrophage colony stimulating factor, characterized by the following steps: a, through gene engineering operation, cloning human granulocyte under the control of cultivated silkworm's karyotype polyhedrosis virogene promoter into a carrier with reporting gene based on piggyBAC factor; b, constructing and recombining trans-gene carrier; c, at the presence of subsidiary particles, attaining silkworm with reporting gene by spermium conducting method, and testing out human granulocyte, breeding to silkworms.
Owner:SUZHOU UNIV +1
Method for producing medicine using silkworm expressed numan epidermal growth factor
InactiveCN1170927CDetermine therapeutic functionPeptide/protein ingredientsMicroencapsulation basedDiseaseCuticle
The invention belongs to the technique field of producing multi-peptide drugs by gene engineering. It clones human EGF gene into the silkworm bacilliform virus (Bombyx mori Nuclear polyhedrosis Virus) transferring carrier pBacPAK8 and makes them carry through homologous reformation in the silkworm cells to obtain the reformed virus BmBacEGF with human-coat growing gene. Use the reformed virus to to inocualte the silkworm grub and chrysalis by acupuncture in order to make the human-coat growing gene efficient expressed. Then make the grub and chrysalis into oral drugs which has remarkable action on the digestible ulcer by examination on animal.
Owner:正源堂(天津)生物科技有限公司
TPO gene modifying human marrow stroma stem cell and its preparation method and use
The invention discloses human marrow-interstitial stem cell modified by TPO gene, its preparing process and use. The human marrow-interstitial stem cell is obtained by recombination gland relevant virus meso-guide TPO gene decoration. The preparation method comprises the following steps consequently cloning human tyre liver TPO gene, constructing carrier plasmid Paav-tpo-ires-hrGFP, preparing Raav-TPO viral vectors, culturing human marrow-interstitial stem cell, transfecting Raav-TPO on human marrow-interstitial stem cell. The human marrow-interstitial stem cell modified by TPO gene or human marrow-interstitial stem cell exudate modified by TPO gene can be used for preparing medicine of urging blood platelet-generating. The MSCs modified by TPO gene generates internal source TPO through cell secreting for treating blood platelet reduction so the side-effect generated from injection human recombination TPO is not generated.
Owner:CENT SOUTH UNIV
Method for the generation of monoclonal antibodies derived from human b cells
InactiveUS20130029424A1Other foreign material introduction processesImmunoglobulinsMonoclonal antibodyClone human
The present invention relates, in general, to human B cells, and, in particular to a method of immortalizing and cloning human B cells and to monoclonal antibodies derived therefrom. The invention further relates to methods of using the monoclonal antibodies for therapeutic and diagnostic purposes.
Owner:DUKE UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com