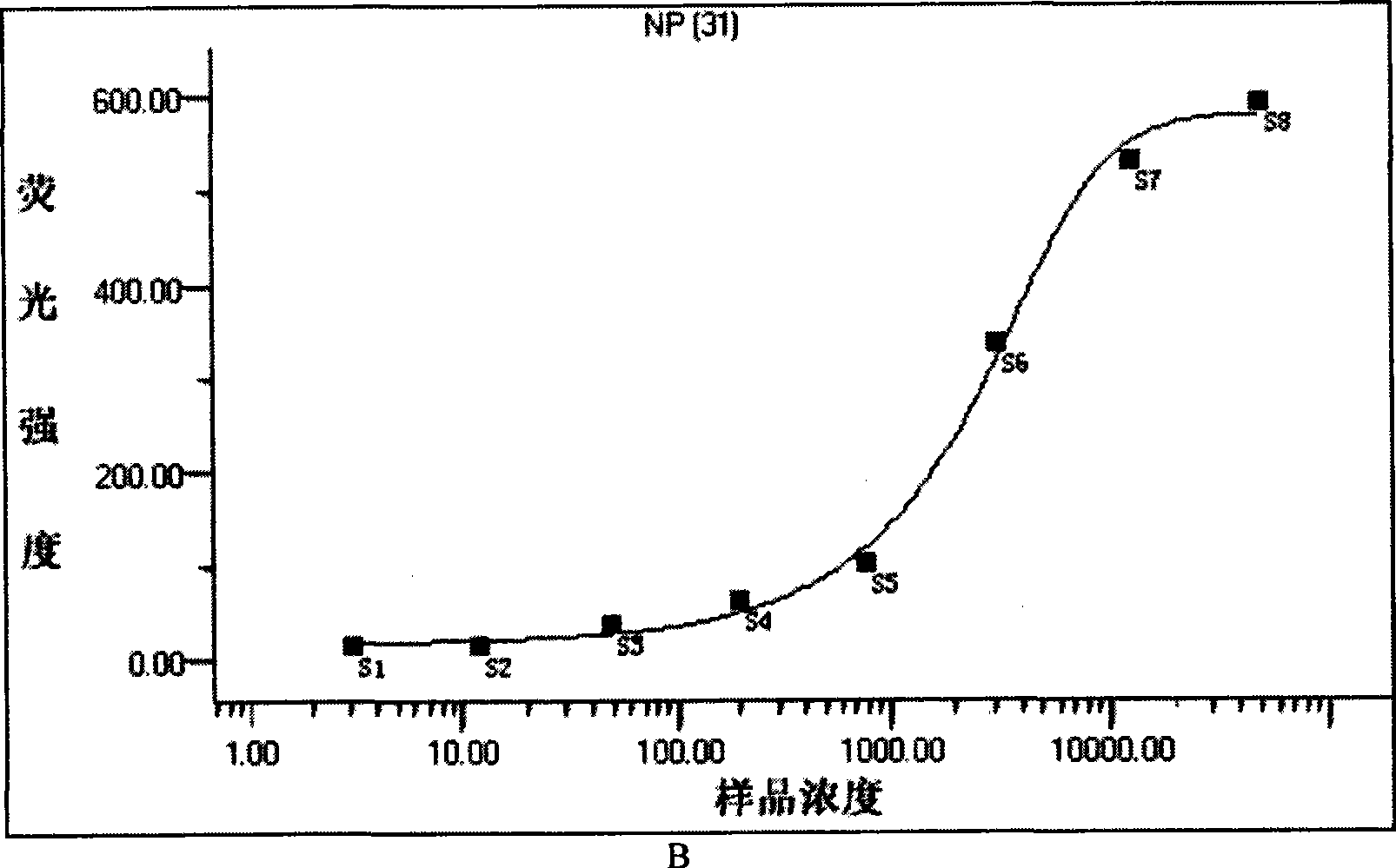
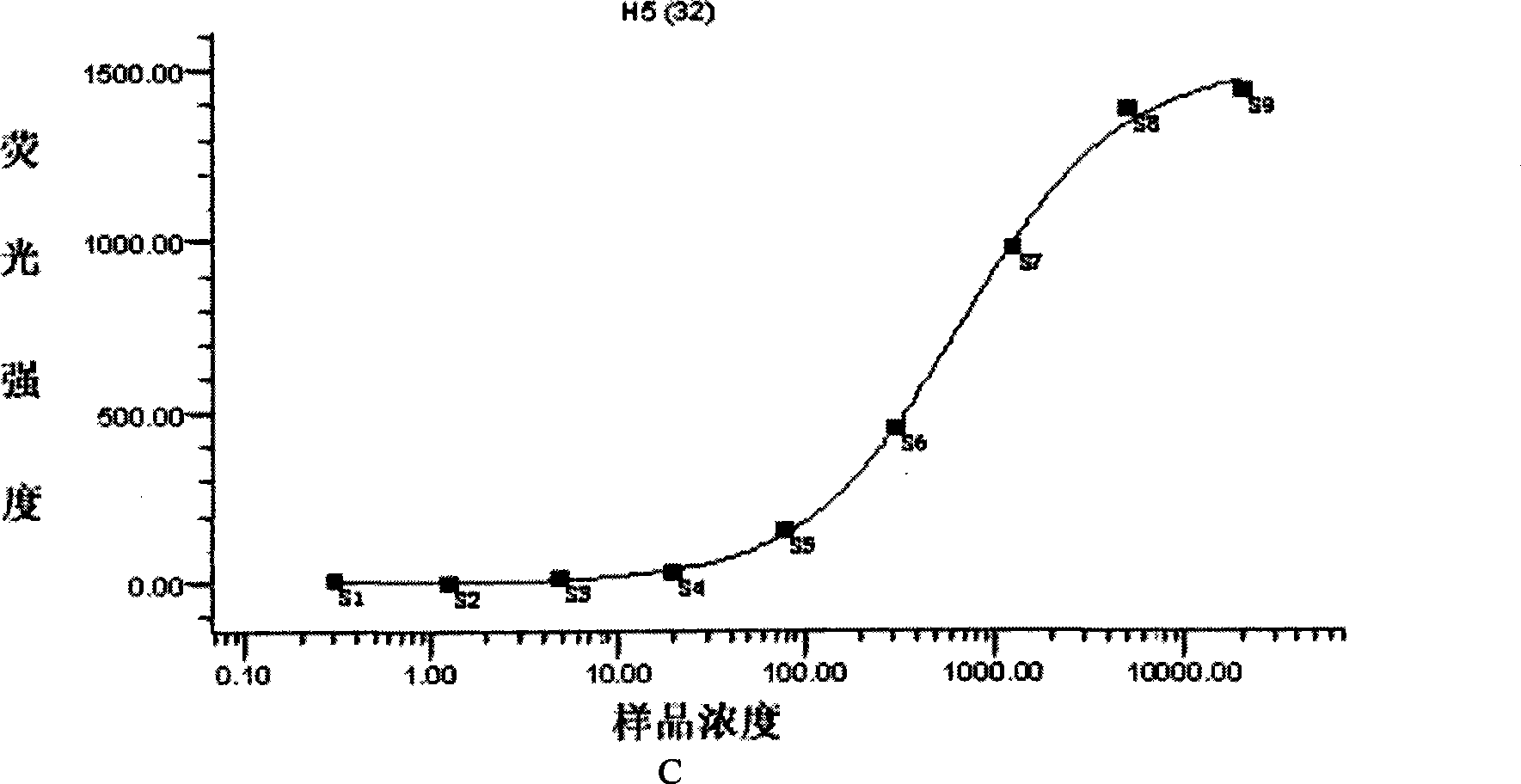
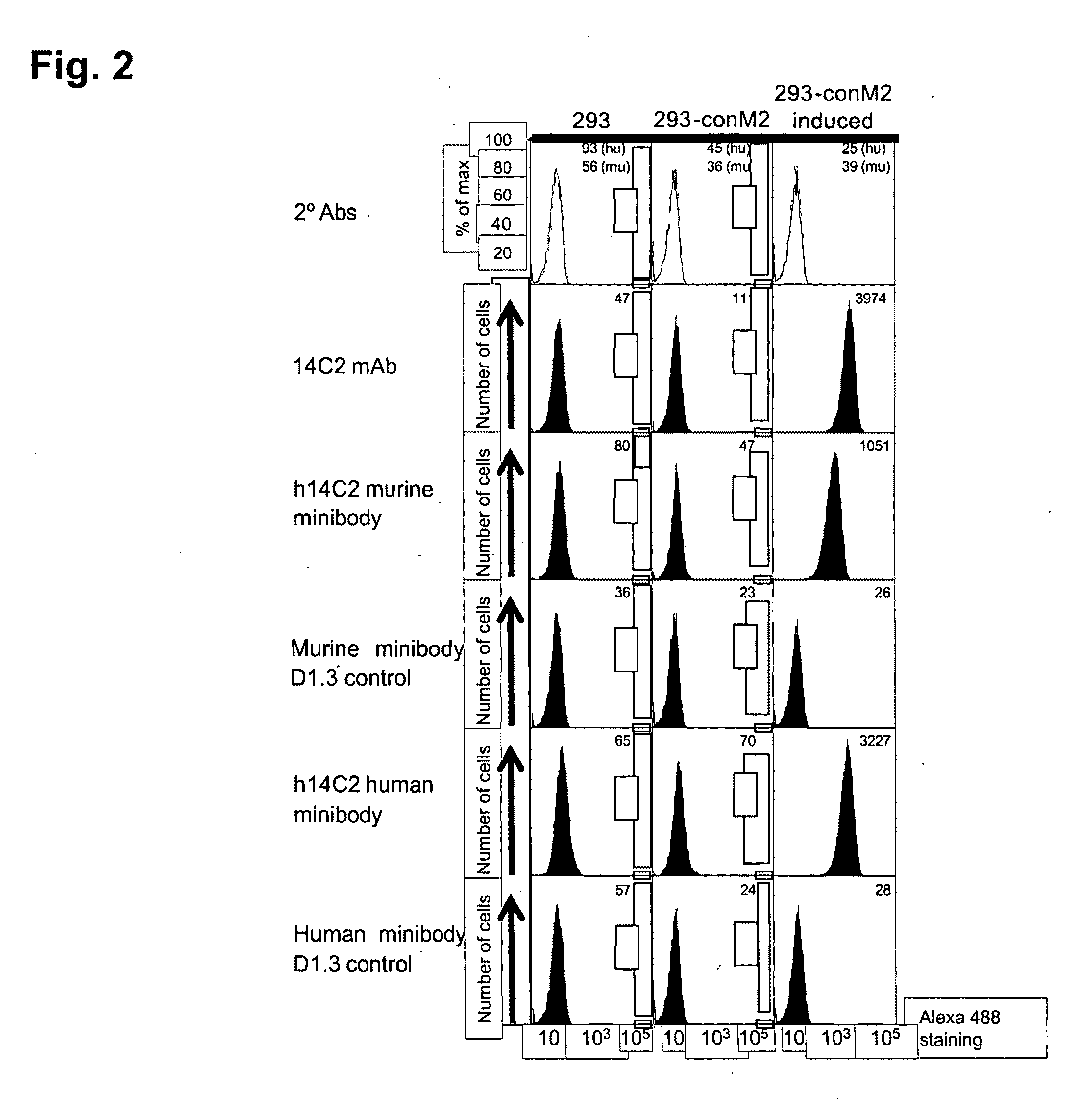
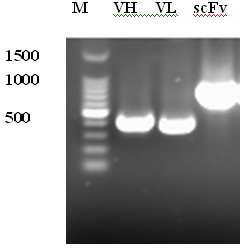
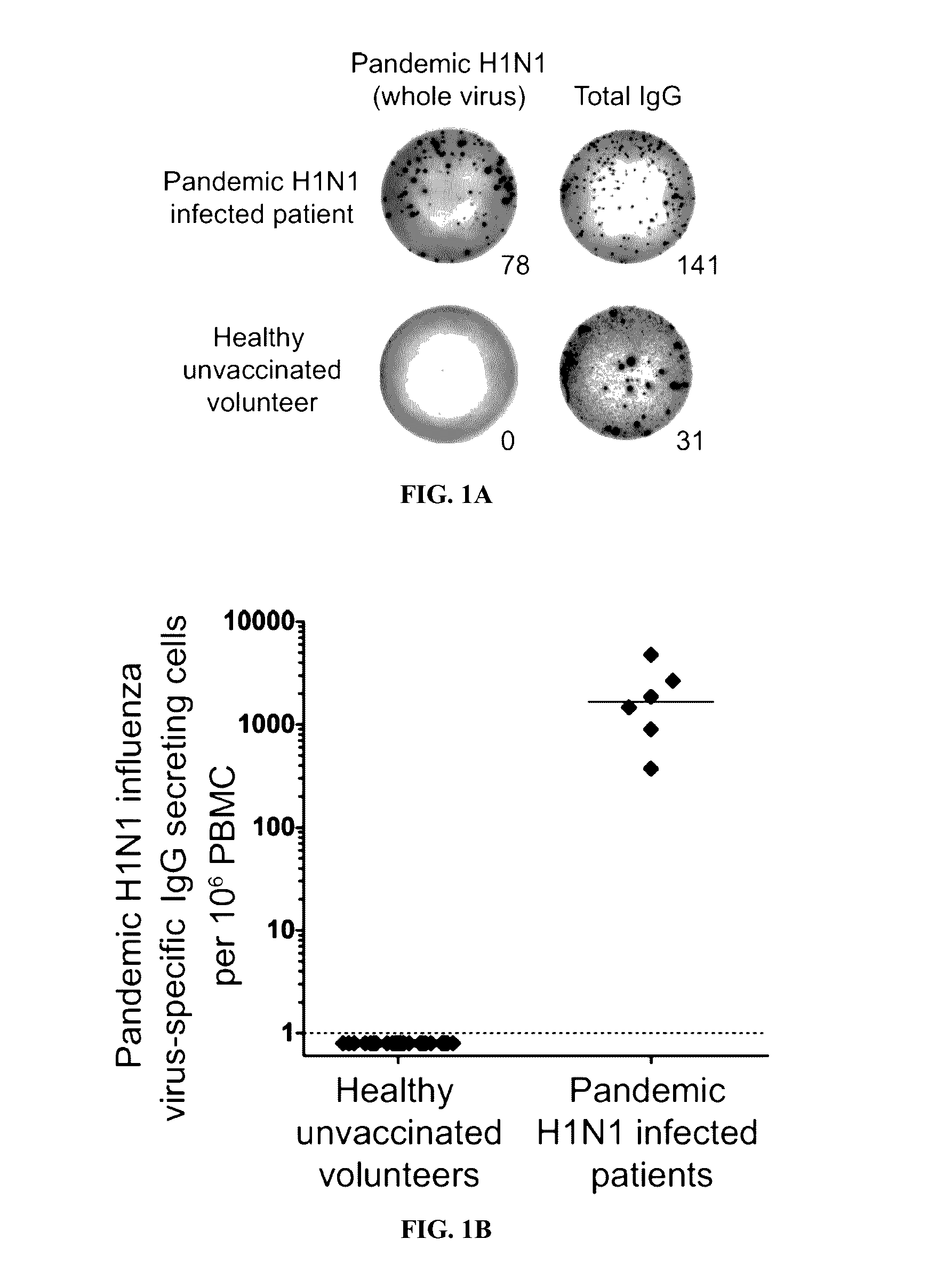
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Influenza antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Influenza antibodies, compositions, and related methods
InactiveUS20080124272A1Inhibitory activityImmunoglobulins against virusesAntiviralsProtein engineeringProtein antigen
The present invention relates to the intersection of the fields of immunology and protein engineering, and particularly to antigens and vaccines useful in prevention of infection by influenza virus. Provided are recombinant protein antigens, compositions, and methods for the production and use of such antigens and vaccine compositions.
Owner:FRAUNHOFER USA
Important heating pathogen fast screening system
The invention discloses a method for detecting a system for quickly screening a fever pathogen, and mainly relates to detection of a tuberculosis antibody, a flu antibody, a bird flu antibody, a plague antibody and an SARS antibody in blood serum. The chip mainly comprises coding microspheres, a coating antigen, a detection antibody, a biotinylated antibody and a streptavidin-phycoerythrin. The method comprises the following steps that: the coating antigen and the coding microshperes are coupled; the detection antibody is respectively coupled with the corresponding microspheres in separate specificity; the red laser excites the classified fluorescence on the spherical substrate; the type is determined according to different colors of the spherical substrates, wherein the biotinylated antibody is combined with the detection antibody; and the streptavidin-phycoerythrin is combined with the biotin of the detection antibody captured by the microshperes; the green laser excites the phycoerythrin, the number of report fluorescence molecules combined on the spherical substrate is measured, and the number is used for indirectly determining the content of the detection antibody combined onthe spherical substrate, so that whether the pathogen infection exists is determined, and the aim of quickly screening the fever pathogen is achieved.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Compositions and methods for the therapy and diagnosis of influenza
InactiveUS8057796B2SsRNA viruses negative-senseViral antigen ingredientsInfluenza antibodyInfluenza a
Owner:THERACLONE SCI
Anti-influenza M2e antibody
InactiveUS20100215662A1Inhibition of replicationSugar derivativesAntibody mimetics/scaffoldsIon Channel ProteinMonoclonal antibody
Humanized recombinant and monoclonal antibodies specific for the ectodomain of the influenza virus M2 ion channel protein are disclosed. The antibodies of the invention have anti-viral activity and may be useful as anti-viral therapeutics and / or prophylactic / vaccine agents for inhibiting influenza virus replication and for treating individuals infected with influenza.
Owner:TRIAD NAT SECURITY LLC
Compositions and methods for the therapy and diagnosis of influenza
InactiveUS20110033476A1SsRNA viruses negative-senseMicrobiological testing/measurementInfluenza antibodyInfluenza a
Owner:THERACLONE SCI
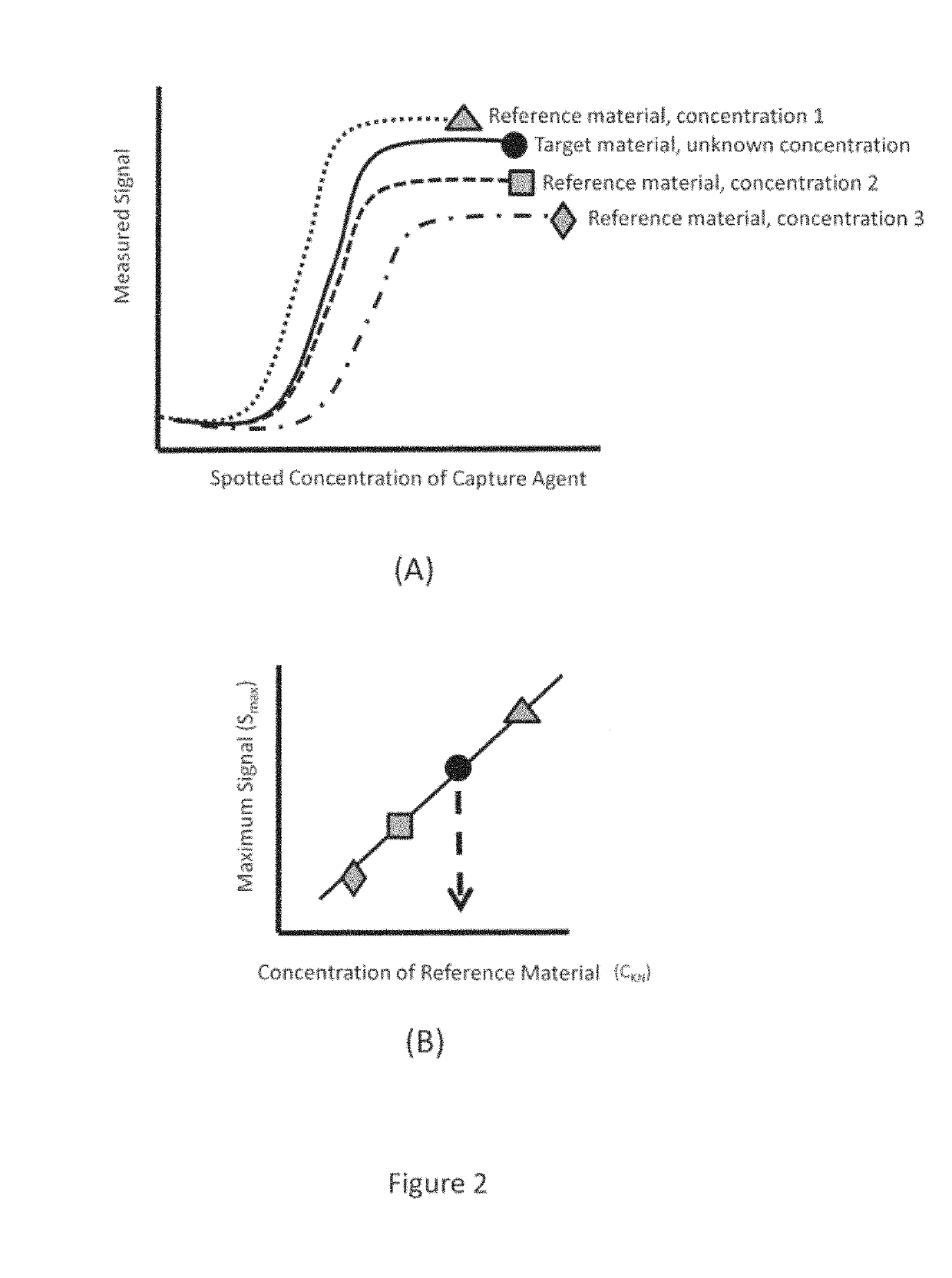
Low Density Microarrays for Vaccine Related Protein Quanitification, Potency Determination and Efficacy Evaluation
Methods for the quantification of influenza HA proteins and anti-influenza antibodies for the fields of vaccine-related protein quantification, potency determination, and efficacy evaluation are provided. According to the technology, quantification is achieved by providing capture agents attached to an array in a series of decreasing concentrations. Serial dilutions of a reference material also may be introduced. The reference material within each solution binds to the capture agents on the array and is labeled with a label agent capable of producing a detectable signal used to construct a calibration curve. A target material of unknown concentration is introduced to a separate identical array, and the target material binds to the capture agents and also is labeled by a label agent to produce a detectable signal. The calibration curve based on the reference material is then utilized to determine the concentration of the target material without the need to perform replicate experiments.
Owner:INDEVR
ScFv antibody for resisting H5N1 type highly-pathogenic avian influenza and application thereof
The invention relates to an scFv antibody for resisting H5N1 type highly-pathogenic avian influenza and an application thereof. A light chain amino acid sequence is shown as SEQIDNo: 1; a heavy chain amino acid sequence is shown as SEQIDNo: 2; a light chain nucleotide sequence is shown as SEQIDNo: 3; and a heavy chain nucleotide sequence is shown as SEQIDNo: 4. The scFv antibody for resisting the H5N1 type highly-pathogenic avian influenza is applied to the preparation of a medicine for resisting the H5N1 type avian influenza virus. Proven by protection tests of embryonated eggs, the 4F5 antibody has a 100% protective rate on prevention of the avian source and human source H5N1 type avian influenza virus infection, and the 4F5 antibody has a 100% protective rate on treatment of the avian source H5N1 type avian influenza virus infection. The highest protective rate on treatment of the human source H5N1 type highly-pathogenic avian influenza virus infection is 62.5%.
Owner:NANJING MEDICAL UNIV +1
Antibodies directed against influenza
ActiveUS9469685B2Preventing H1NReduce the risk of infectionImmunoglobulins against virusesAntiviralsAntibody fragmentsInfluenza antibody
Owner:EMORY UNIVERSITY +1
Broad-spectrum monoclonal Anti-flu b antibody and uses thereof
ActiveUS20190345230A1Preventing or treatingAvoid infectionSsRNA viruses negative-senseImmunoglobulins against virusesMonoclonalB Antibody
Provided are a broad-spectrum monoclonal anti-Flu B antibody, cell strains generating the antibody, and a composition comprising the antibody; also provided are uses of the antibody for diagnosing, preventing and / or treating an infection of the Flu B and / or diseases caused by the infection.
Owner:XIAMEN UNIV
Compositions and Methods for the Therapy and Diagnosis of Influenza
Owner:THERACLONE SCI
Compositions and methods for the therapy and diagnosis of influenza
InactiveUS20100178295A1SsRNA viruses negative-senseViral antigen ingredientsInfluenza antibodyInfluenza a
Owner:THERACLONE SCI
Protein suspension chip for synchronously detecting various antibodies in serum sample and preparation method and using method thereof
InactiveCN101936989AEasy to detectReduce the amount of coatingFluorescence/phosphorescenceAgainst vector-borne diseasesSerum igeSerum samples
The invention discloses a protein suspension chip for synchronously detecting various antibodies in serum sample and a preparation method and a using method thereof. The method has the advantages of good detection capacity, high sensitivity, strong specificity and wide dynamic range, and an open detection modular platform of the protein suspension chip for detecting microbial antibodies represented by West Nile antibody, Francisella tularensis antibody, dengue fever antibody and influenza antibody is established.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Low density microarrays for vaccine related protein quantification, potency determination and efficacy evaluation
Methods for the quantification of influenza HA proteins and anti-influenza antibodies for the fields of vaccine-related protein quantification, potency determination, and efficacy evaluation are provided. According to the technology, quantification is achieved by providing capture agents attached to an array in a series of decreasing concentrations. Serial dilutions of a reference material also may be introduced. The reference material within each solution binds to the capture agents on the array and is labeled with a label agent capable of producing a detectable signal used to construct a calibration curve. A target material of unknown concentration is introduced to a separate identical array, and the target material binds to the capture agents and also is labeled by a label agent to produce a detectable signal. The calibration curve based on the reference material is then utilized to determine the concentration of the target material without the need to perform replicate experiments.
Owner:INDEVR
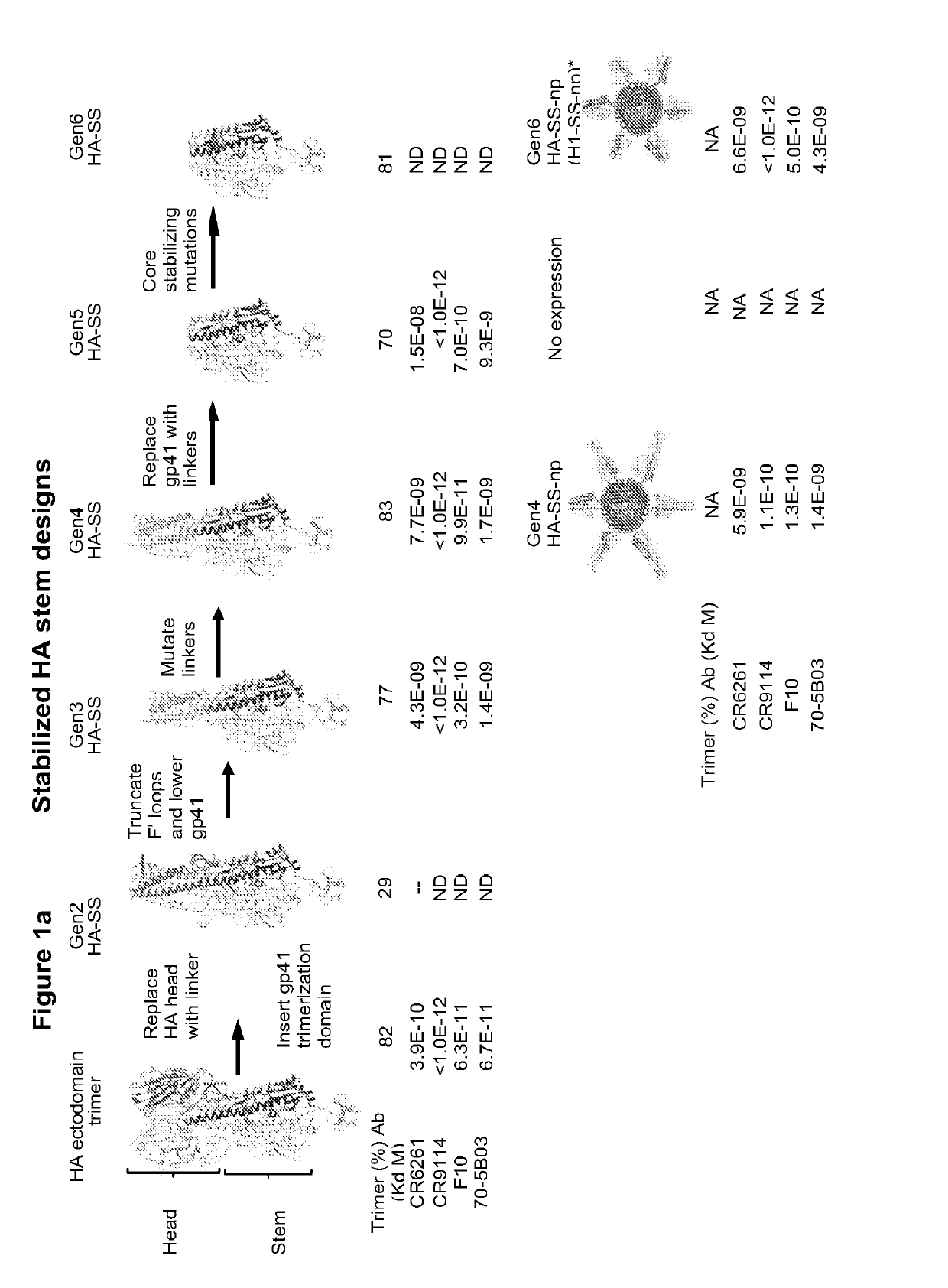
Stabilized influenza hemagglutinin stem region trimers and uses thereof
ActiveUS10363301B2Easy to manufactureProduct can be usedSsRNA viruses negative-senseViral antigen ingredientsHemagglutininPHA granule
Vaccines that elicit broadly protective anti-influenza antibodies. Some vaccines comprise nanoparticles that display HA trimers from influenza virus on their surface. The nanoparticles are fusion proteins comprising a monomeric subunit (e.g., ferritin) joined to the stem region of an influenza HA protein. The fusion proteins self-assemble to form the HA-displaying nanoparticles. The vaccines comprise only the stem region of an influenza HA protein joined to a trimerization domain. Also provided are fusion proteins, and nucleic acid molecules encoding such proteins, and assays using nanoparticles of the invention to detect anti-influenza antibodies.
Owner:UNITED STATES OF AMERICA
Compositions and Methods for the Therapy and Diagnosis of Influenza
InactiveUS20120315277A1Improve protectionImprove welfareAntiviralsAntibody ingredientsInfluenza antibodyInfluenza a
Owner:THERACLONE SCI
Compositions And Methods For The Therapy And Diagnosis Of Influenza
InactiveUS20120121603A1Microbiological testing/measurementImmunoglobulins against virusesInfluenza antibodyAntibody
Owner:THERACLONE SCI
Identification of influenza and common cold, and detecting method of influenza antibody
InactiveCN101017167AQuick distinctionAccurate distinctionMaterial analysisCommon coldInfluenza antibody
This invention belongs to disease dialogue field outside virus and its antigen rapid test method, which relates to flu virus film protein M1 and M2 with N end height conversion MSLLTEVET and its abnormal meter level to process dialogue case through decoration to process flu antigen level test; using MSLLTEVET and its abnormal meter antigen through decoration to process dialogue agent case for flu virus test.
Owner:河南省生物工程技术研究中心
Animal influenza antibody blocking test strip
InactiveCN110058018ARealize real-time monitoringRealize immune evaluationSsRNA viruses negative-senseVirus peptidesMaternal antibodyStaphylococcus aureus
The invention discloses an animal influenza antibody blocking test strip, which comprises a supporting plate, an antigen pad, a conjugate pad, a detection membrane and a water absorption pad, whereinthe antigen pad is used for adsorbing an influenza virus detection antigen; the conjugate pad is used for adsorbing a colloidal gold labeled anti-influenza virus monoclonal antibody mAb1; the detection film contains detection line T"|'' and quality control line C"|'' prints; the detection line T is an anti-influenza virus monoclonal antibody mAb2 or anti-influenza virus polyclonal antibody pAb1 print; and the quality control line C is an anti-mouse IgG rabbit antibody pAb2 or staphylococcus aureus SPA print. According to the test strip, the rapid detection of the neutralizing antibody of influenza virus is realized, the real-time monitoring of an animal influenza maternal antibody and the immune antibody level and the immune evaluation of an immune effect of a vaccine can be realized, thetest strip is easy to operate and can be operated by everyone, the requirements of people at different levels can be better met, and the test strip is easy to popularize and apply in a wide range, andhas a wide market prospect and relatively great economic and social benefits.
Owner:HENAN ACAD OF AGRI SCI
Stabilized group 2 influenza hemagglutinin stem region trimers and uses thereof
Vaccines that elicit broadly protective anti-influenza antibodies. The vaccines comprise nanoparticles that display HA trimers from Group 2 influenza virus on their surface. The nanoparticles are fusion proteins comprising a monomeric subunit (e.g., ferritin) joined to stabilized stem regions of Group 2 influenza virus HA proteins. The fusion proteins self-assemble to form the HA-displaying nanoparticles. Also provided are fusion proteins, and nucleic acid molecules encoding such proteins, and assays using nanoparticles of the invention to detect anti-influenza antibodies.
Owner:UNITED STATES OF AMERICA
Compositions and methods for the therapy and diagnosis of influenza
InactiveUS8858948B2Viral antigen ingredientsMicrobiological testing/measurementInfluenza antibodyInfluenza a
Owner:THERACLONE SCI
Stabilized influenza hemagglutinin stem region trimers and uses thereof
ActiveUS20190314490A1Easy to manufactureProduct can be usedSsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininAntiendomysial antibodies
Owner:UNITED STATES OF AMERICA
Avian H9 subtype avian influenza virus antibody colloidal gold test paper and test card
The invention provides avian H9 subtype avian influenza virus antibody colloidal gold test paper and test card. The avian H9 subtype avian influenza virus antibody colloidal gold test paper and test card can detect the immune level of various birds and are wide in application range, high in specificity, high in sensitivity, easy to operate and short in consumed time. The test paper comprises a sample pad, a gold-labeled pad and a nitrocellulose membrane which are sequentially connected, wherein the gold-labeled pad is wrapped with a colloidal gold label of a monoclonal antibody, namely anti-mouse IgY Fc CH3-CH4; and a detection line close to one end of the gold-labeled pad and a quality control line away from one end of the gold-labeled pad are arranged on the nitrocellulose membrane, thedetection line is wrapped with an AIV-HA19 recombinant protein, the quality control line is wrapped with a second antibody, and the DNA sequence for encoding the AIV-HA19 recombinant protein is shownas SEQ ID NO.1.
Owner:HUAZHONG AGRI UNIV
New method and product for detecting influenza antibody in serum sample
InactiveCN101533022AImproved antigen coatingEasy to detectFluorescence/phosphorescenceSerum igeViral antibody
The invention relates to a new quantitative detection method and a product for detecting influenza antibody in a serum sample. The method of the invention has good detection capability, high sensitivity, strong specificity and wide dynamic range. The invention establishes an open detection modularised platform of pathogenic bacteria antibody protein suspension chip detection with the influenza antibody as a representative.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Polypeptide sequence and application thereof
ActiveCN113278078AEnhanced inhibitory effectHave binding activityAntibacterial agentsSsRNA viruses negative-senseHemagglutininAntigen epitope
The invention discloses a polypeptide sequence and application thereof. The polypeptide sequence comprises a choline binding sequence at an N terminal and a specific epitope sequence at a C terminal, wherein the N terminal is a part with choline binding activity in a ChBp sequence; and the terminal C is an antigen epitope of influenza virus surface protein hemagglutinin HA. The amino acid sequence at the N terminal of the polypeptide sequence has streptococcus pneumoniae binding activity, the C terminal has influenza antigen characteristics, and the polypeptide sequence can induce influenza antibody binding in the presence of influenza antibodies, so that the growth rate of streptococcus pneumoniae, adhesion to the cell surfaces and autolysis are reduced; and the co-presence of the polypeptides and the influenza antibodies in mice can reduce the colonization of the Streptococcus pneumoniae.
Owner:SOUTHWEST MEDICAL UNIVERISTY
Based on self-assembled ferritin nanoantigen particles and influenza vaccine and preparation method
ActiveCN111825768BOperational securitySimple and fast operationViral antigen ingredientsAntiviralsEscherichia coliAntiendomysial antibodies
The invention discloses an influenza vaccine and a preparation method based on self-assembled ferritin nanometer antigen particles. In the present invention, the extracellular domain of the influenza virus hemagglutinin protein is fused and expressed with the N-terminal of the self-assembled ferritin nanoparticle subunit, and the influenza virus hemagglutinin protein is displayed on the surface of the self-assembled ferritin cage structure. The invention carries out mutation optimization of the influenza virus hemagglutinin protein, improves its soluble expression amount and expression efficiency, improves the immune efficacy and breadth of the vaccine, and improves the immunogenicity of the influenza virus hemagglutinin protein. The present invention utilizes E. coli prokaryotic expression system, silkworm and AcMNPV-insect cell eukaryotic expression system to express and prepare recombinant protein vaccine respectively, or generate antigen and induce antibody production by gene presentation of recombinant baculovirus in vertebrate in vivo tissue. The influenza vaccine of the invention can induce broadly neutralizing anti-influenza antibodies, can not only improve the immune efficacy but also expand the immune range, and has the potential of a universal vaccine with cross-immunity efficacy.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Stabilized group 2 influenza hemagglutinin stem region trimers and uses thereof
PendingUS20220339278A1Improve stabilitySsRNA viruses negative-senseViral antigen ingredientsHemagglutininAntiendomysial antibodies
Vaccines that elicit broadly protective anti-influenza antibodies. The vaccines comprise nanoparticles that display HA trimers from Group 2 influenza virus on their surface. The nanoparticles are fusion proteins comprising a monomeric subunit (e.g., ferritin) joined to stabilized stem regions of Group 2 influenza virus HA proteins. The fusion proteins self-assemble to form the HA-displaying nanoparticles. Also provided are fusion proteins, and nucleic acid molecules encoding such proteins, and assays using nanoparticles of the invention to detect anti-influenza antibodies.
Owner:UNITED STATES OF AMERICA
Influenza virus ha protein stem specific monoclonal antibody and its preparation method and application
ActiveCN111825763BProof of uniquenessBroad spectrum reactivitySsRNA viruses negative-senseVirus peptidesNucleotideAvian virus
The invention belongs to the field of biotechnology, and relates to a monoclonal antibody for recognizing the HA stem of influenza virus, an antigenic epitope, a coding gene and applications thereof. The present invention provides an influenza virus HA stalk-specific monoclonal antibody, including a heavy chain variable region and a light chain variable region, and the nucleotide and amino acid sequences of the heavy chain variable region are respectively shown in SEQ ID NO.1‑2 shown; the nucleotide and amino acid sequences of the light chain variable region are shown in SEQ ID NO.6-7 respectively. The monoclonal antibody specifically recognizes the epitope of the stem of the HA protein with the 431st W, 433rd Y, and 437th L as core motifs. The monoclonal antibody is obtained by immunizing mice with H7N9 subtype avian influenza virus HA protein as an antigen. It has high affinity and strong sensitivity, and has good cross-reactivity with group 1 and group 2 influenza viruses. It has high application value in scientific detection.
Owner:YANGZHOU UNIV
Polypeptides for generating anti-influenza antibodies and uses thereof
ActiveUS10344058B2SsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininImmunogenicity
Owner:INDIAN INSTITUTE OF SCIENCE
A multiple respiratory antigen detection card and kit
ActiveCN112362869BAdequate responseHigh sensitivityBiological testingImmunoassaysAntigen testingRespiratory syncytial virus antigen
The invention relates to a multi-respiratory tract antigen detection card, which includes influenza A virus antigen test strips, influenza B virus antigen test strips, respiratory adenovirus antigen test strips, respiratory syncytial virus antigen test strips and Mycoplasma pneumoniae antigen test strips, Influenza A virus antigen test strips, Influenza B virus antigen test strips, Respiratory adenovirus antigen test strips, Respiratory syncytial virus antigen test strips and Mycoplasma pneumoniae antigen test strips all include Colloidal gold conjugate pads, colloidal gold conjugate pads include streptavidin conjugate pads and double-nanoparticle double-labeled antibody conjugate pads; the test strips in this test card can fully react biological raw materials and improve the sensitivity of antigen detection , effectively reducing missed detection, and adding blocking agents to the sample pad to improve specificity, and can simultaneously detect influenza A virus, influenza B virus, respiratory adenovirus, respiratory syncytial virus, and Mycoplasma pneumoniae antigens.
Owner:山东康华生物医疗科技股份有限公司
Polypeptides for generating Anti-influenza antibodies and uses thereof
ActiveUS20170204142A1SsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininImmunogenicity
The present disclosure relates to a polypeptide comprising hemagglutinin stem domain fragments that can elicit broadly cross-reactive anti-influenza antibodies and confer protection against influenza virus. The disclosure also provides a method of preparing the polypeptide with biochemical and biophysical properties that enhance its immunogenic properties. Also provided are recombinant DNA constructs, vectors, and host cells comprising the nucleic acid encoding the polypeptide, as well as uses of the polypeptide, particularly in the prevention, and detection of influenza.
Owner:INDIAN INSTITUTE OF SCIENCE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com