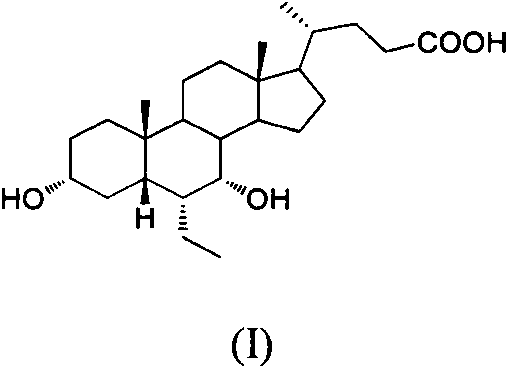
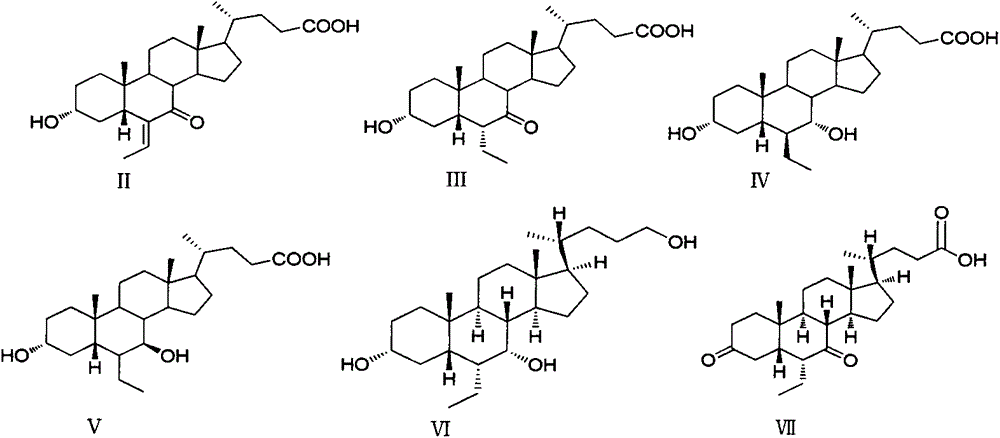
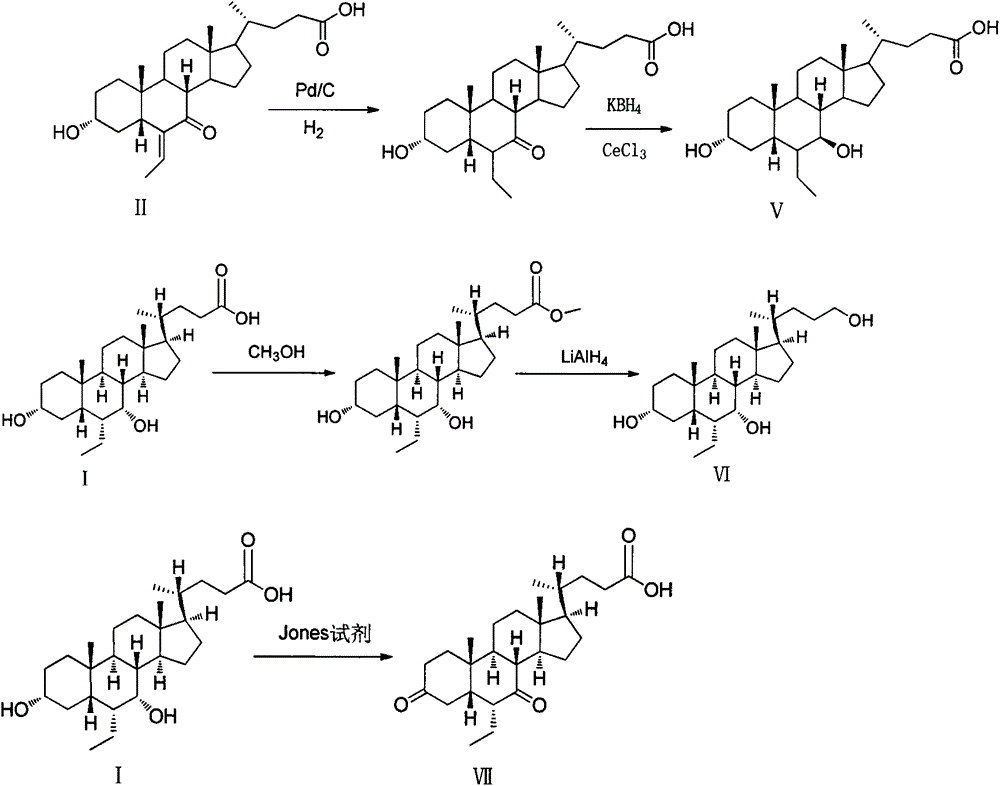
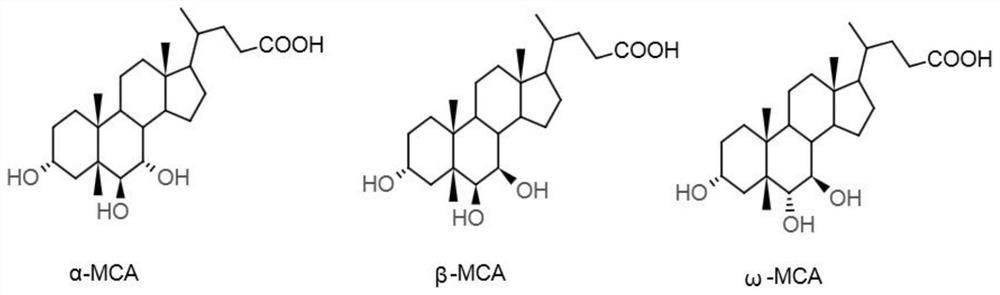
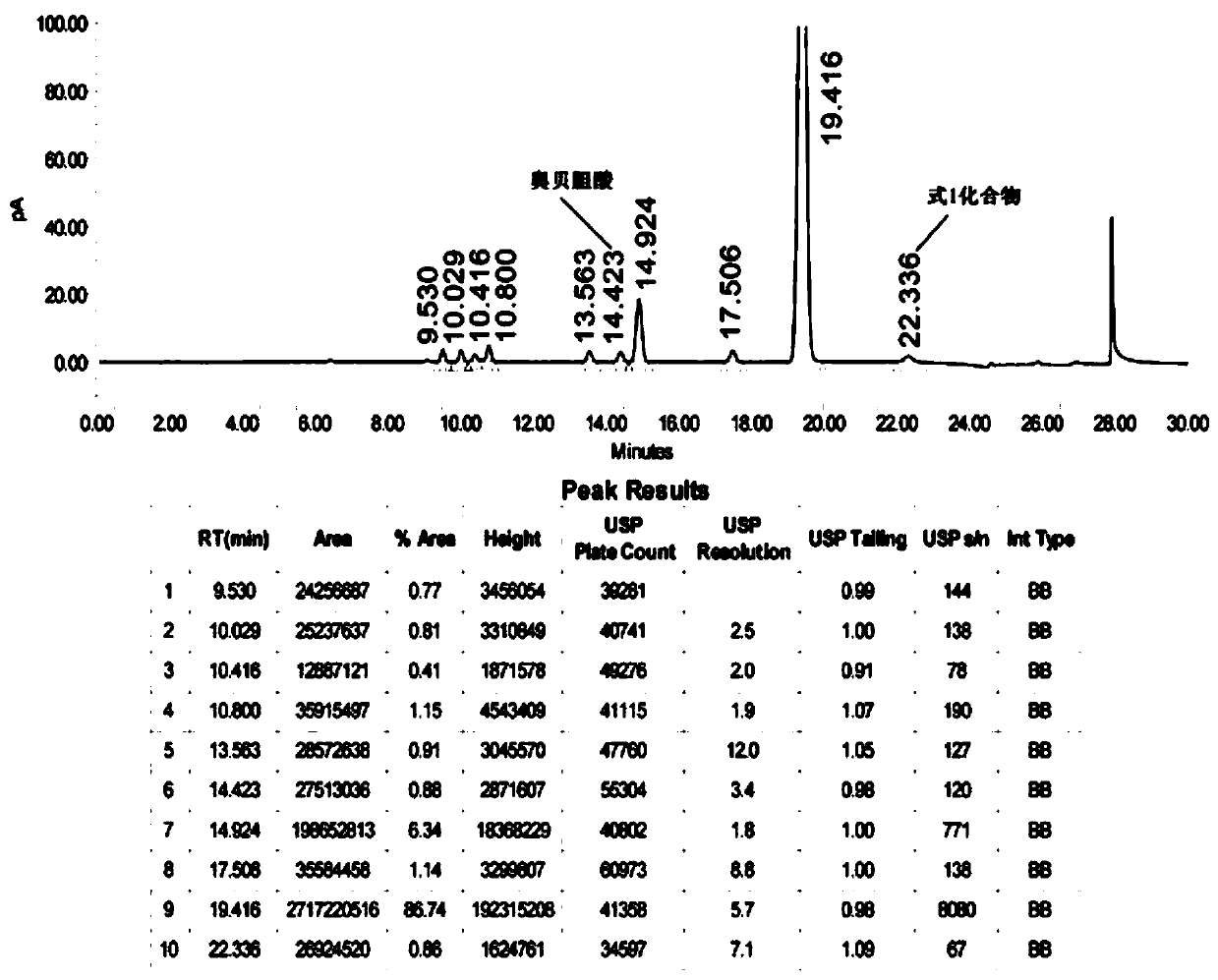
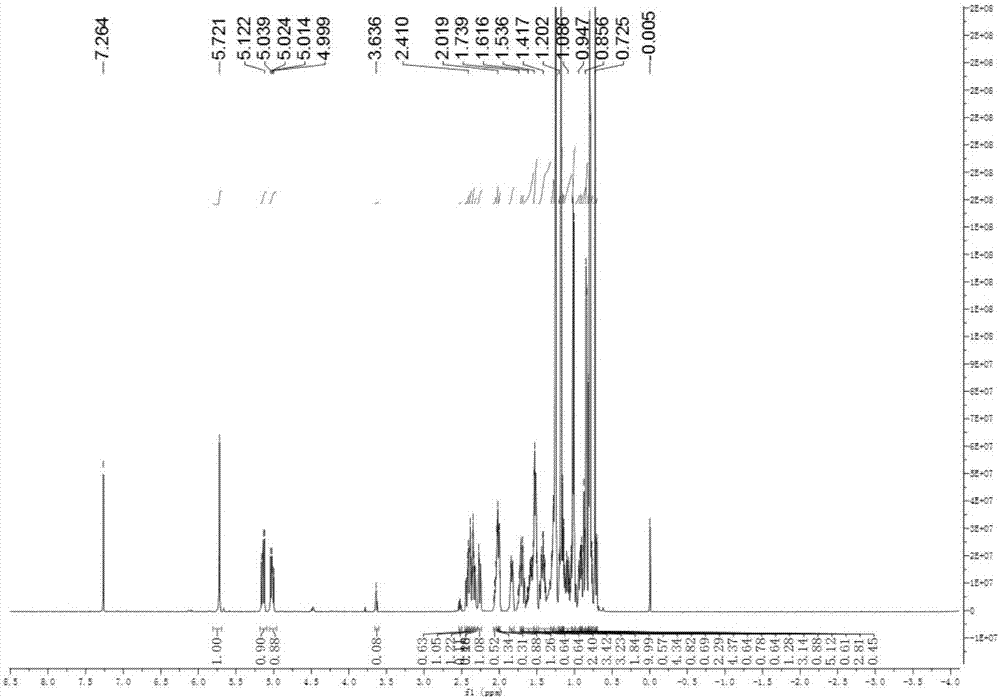
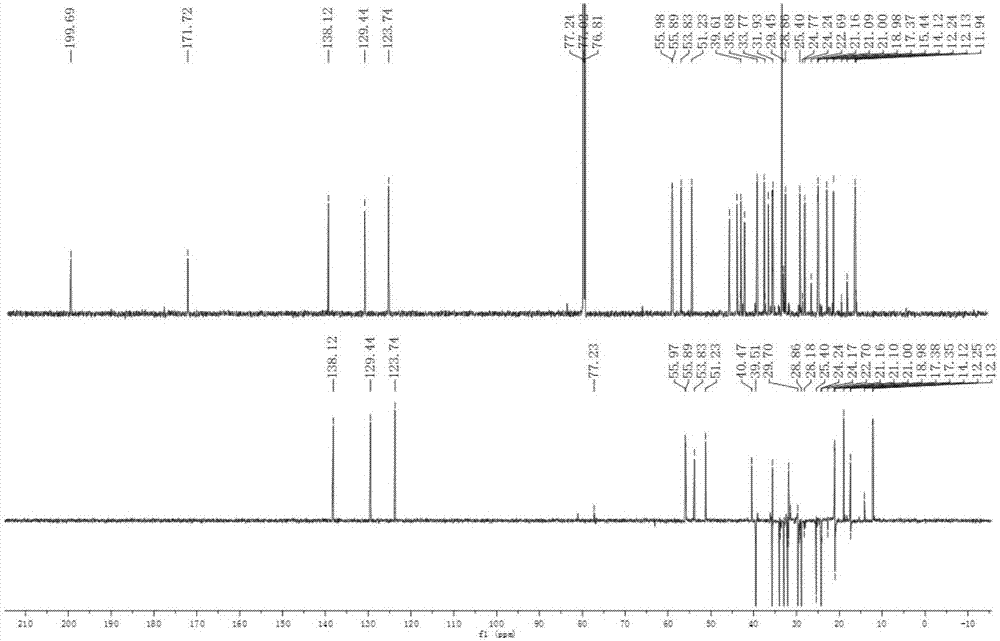
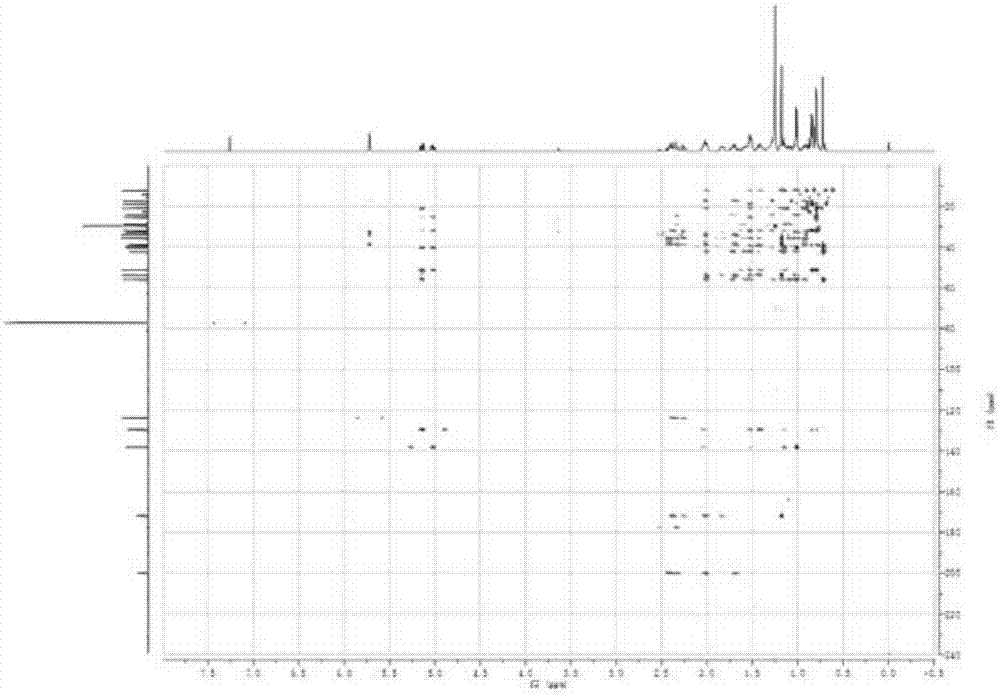
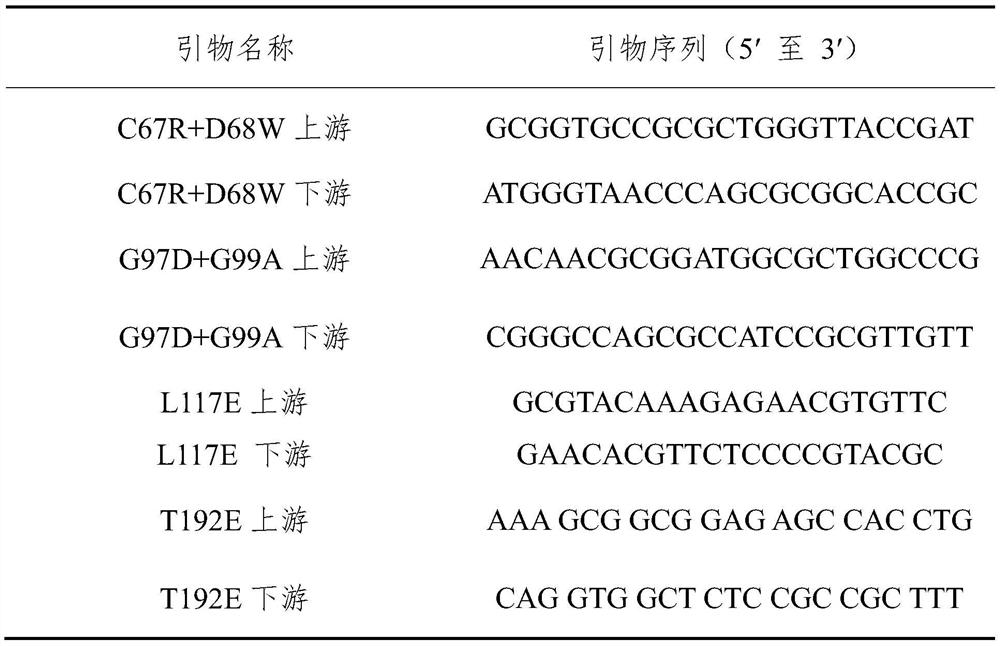
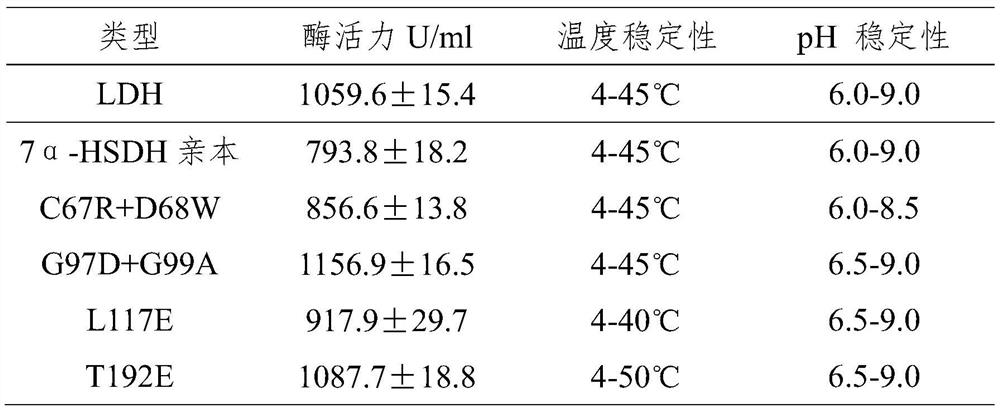
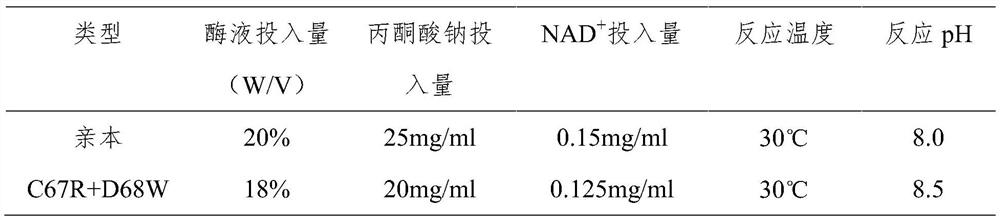
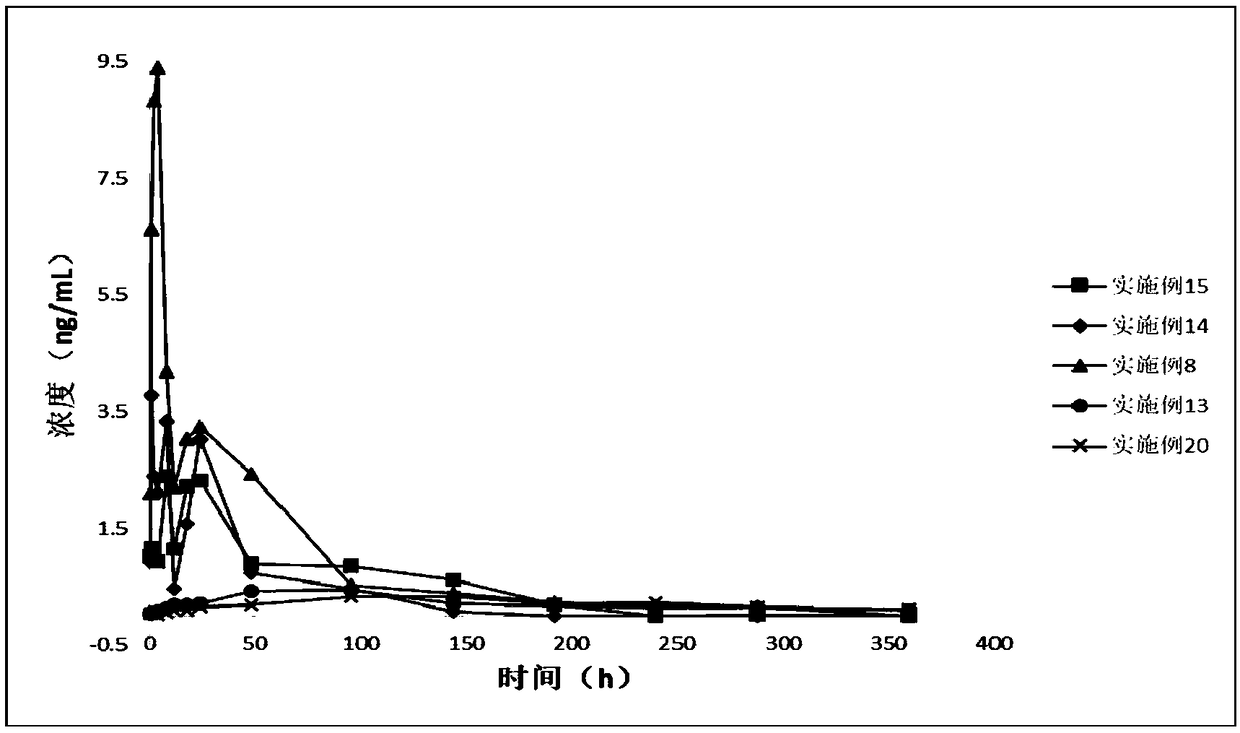
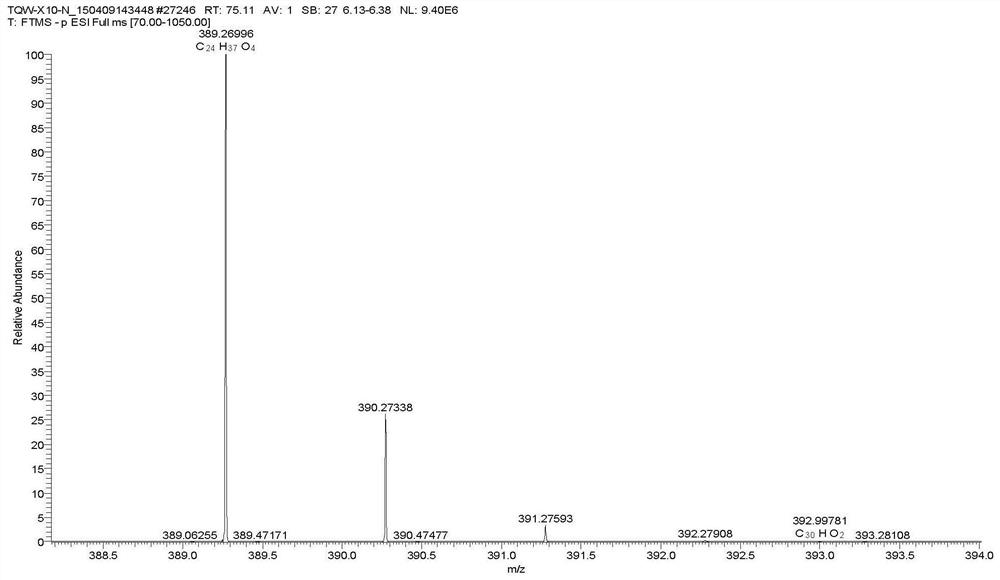
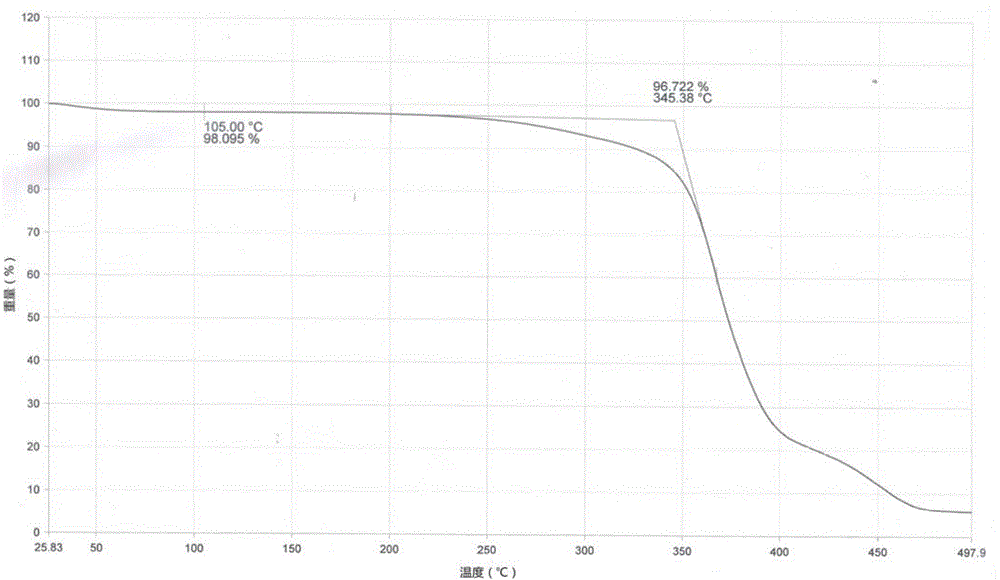
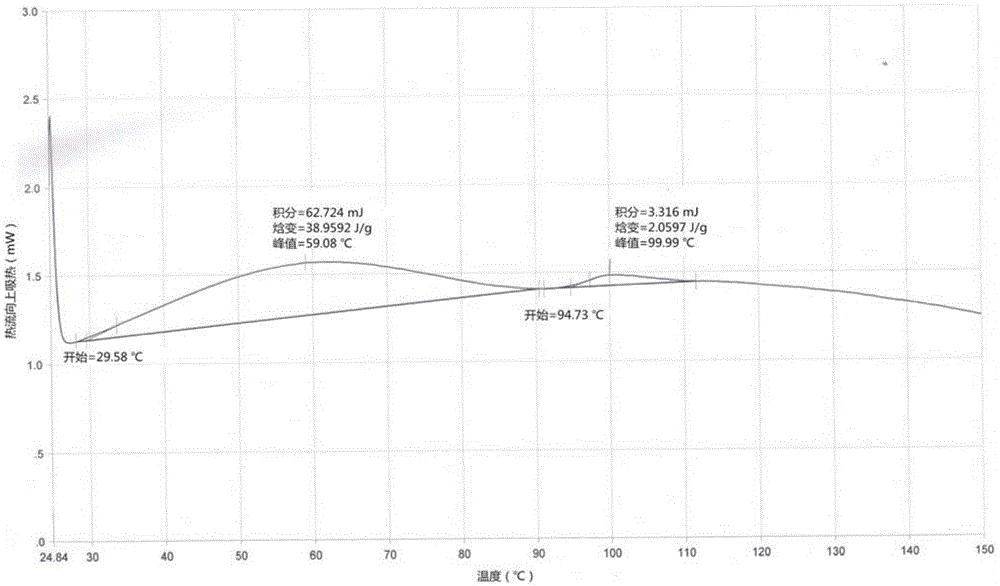
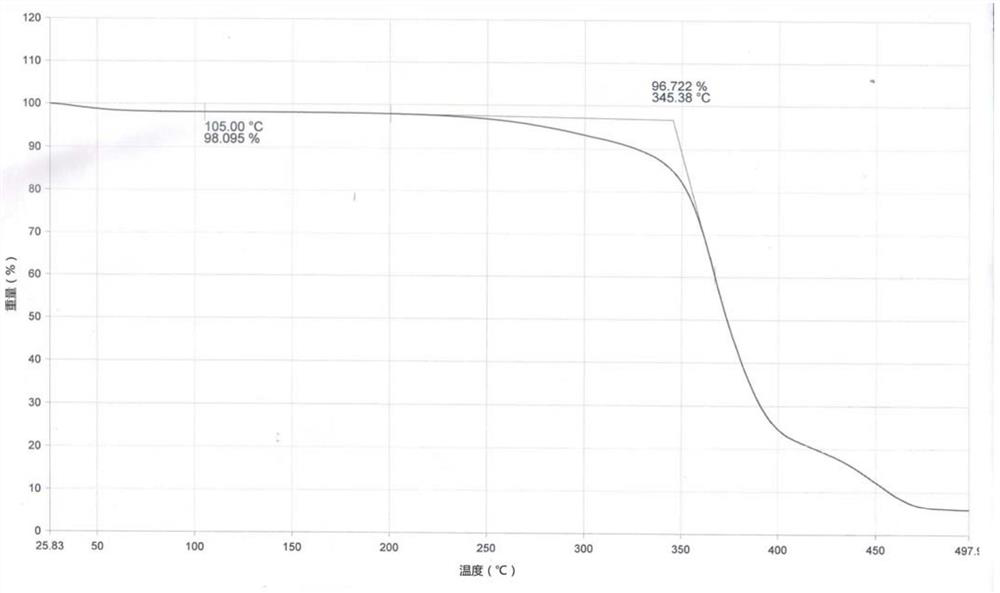
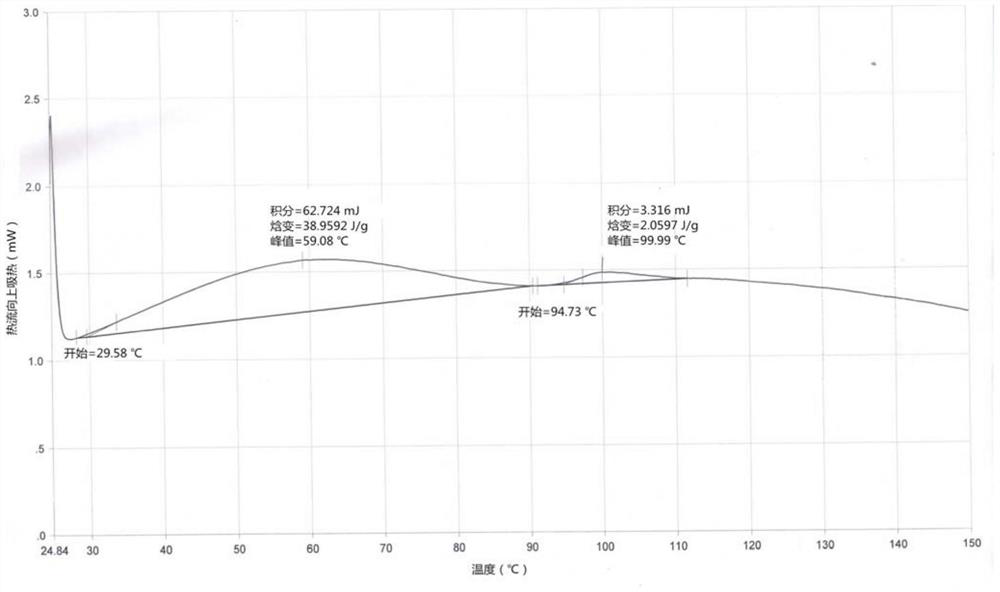
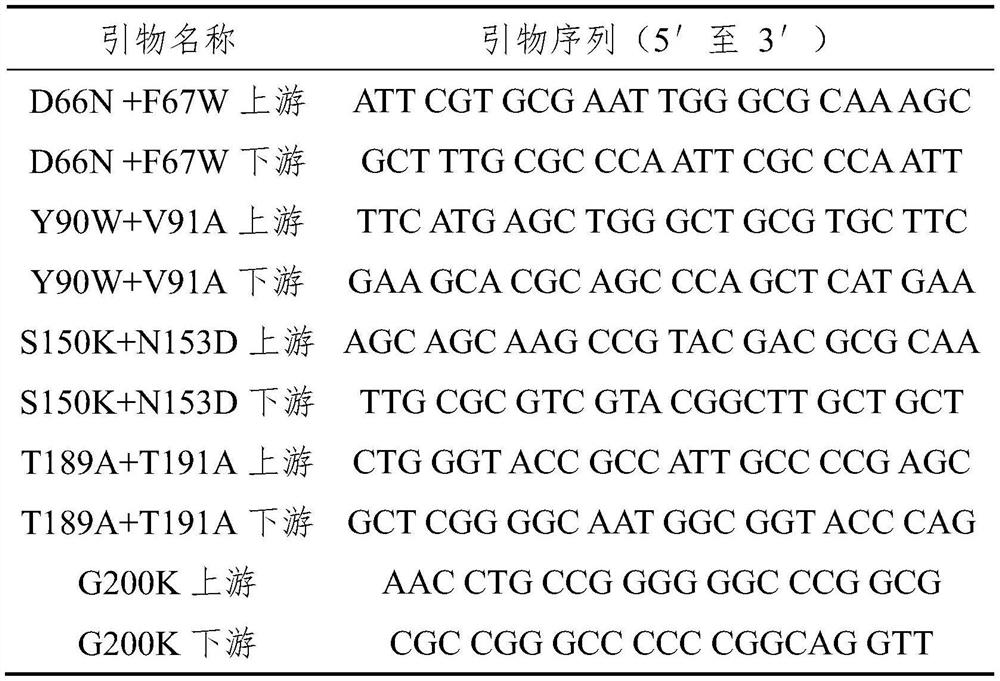
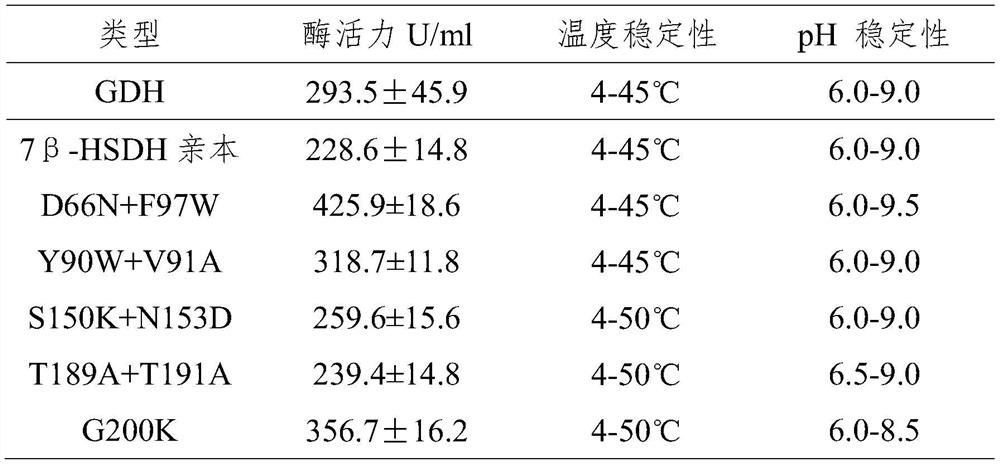
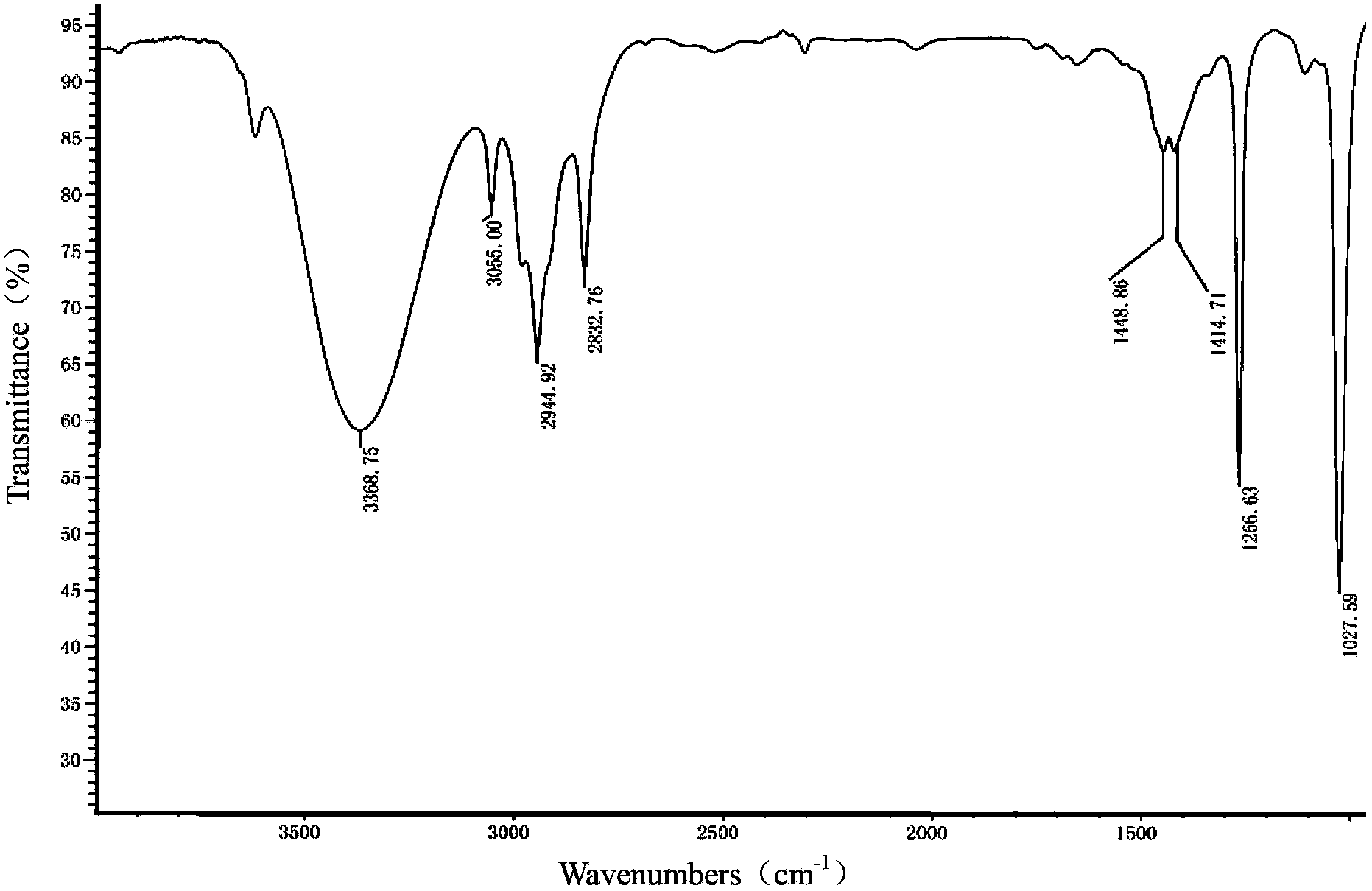
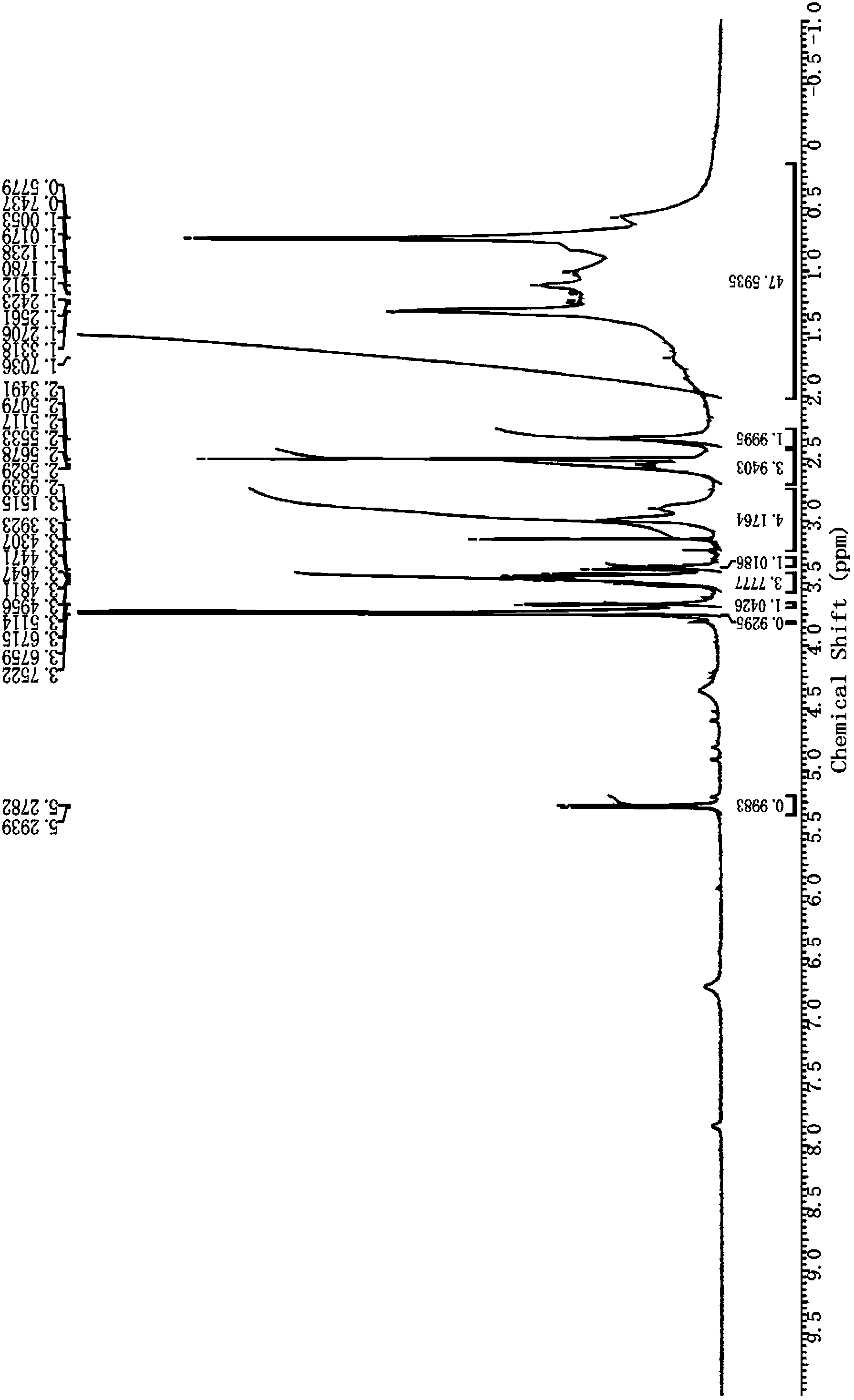
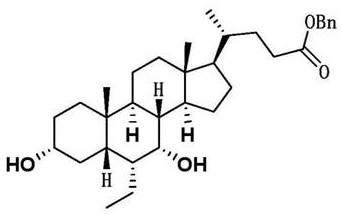
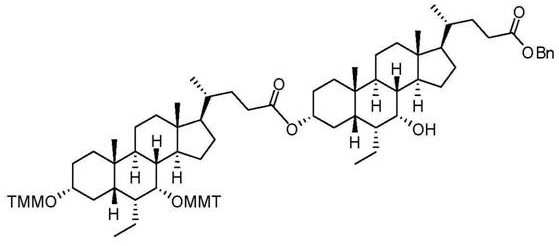
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Cholane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
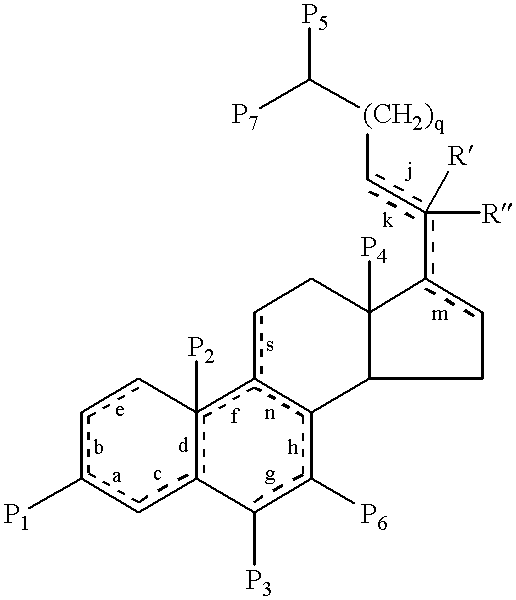
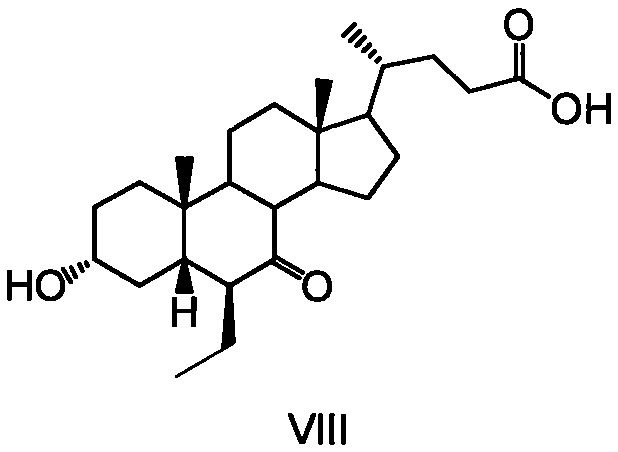
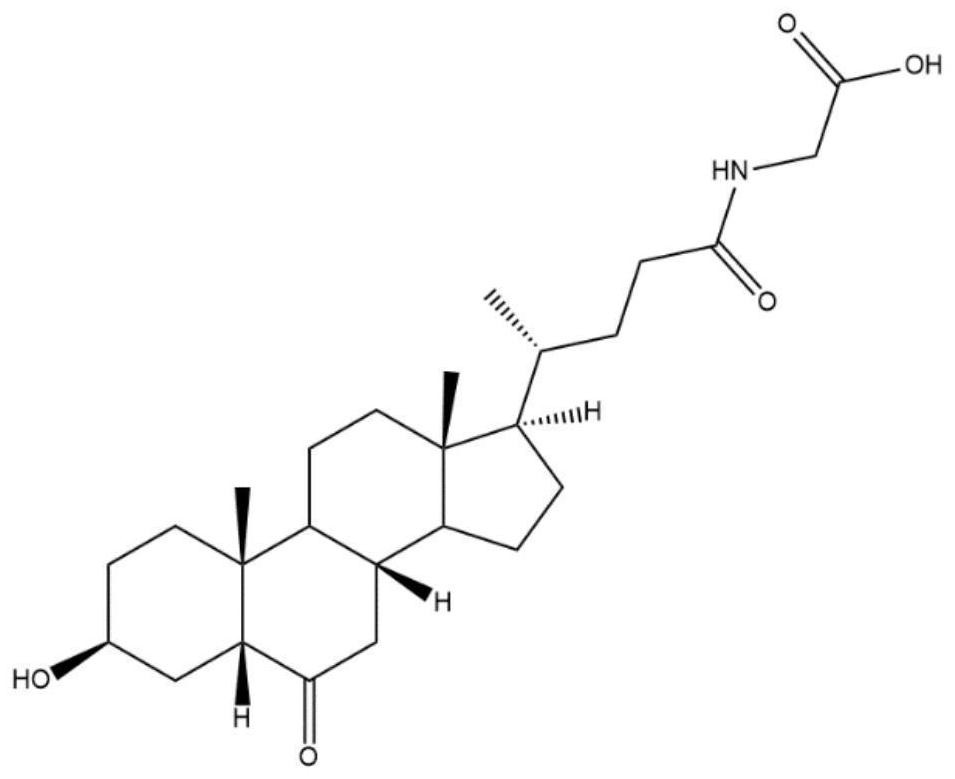
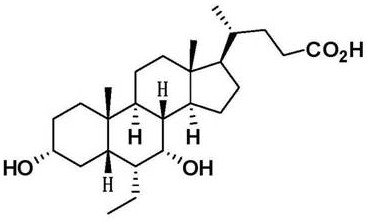
Cholane is a triterpene which can exist as either of two stereoisomers, 5α-cholane and 5β-cholane. Its name is derived from the Greek work for bile (χολή, chole) in reference to its original discovery from the bile of the American bullfrog (Rana catesbeiana). The compound itself has no known uses; however, various functionalized analogues are produced by plants and animals, typically in the form of sterols, steroids and bile acids (e.g. cholic acid).
Antigen antibody complex dissociation liquid kit and application thereof
InactiveCN101832999AFacilitate dissociationIncrease the absolute concentration valueBiological testingSerum igeBlood plasma
The invention discloses an antigen antibody complex dissociation liquid kit, which is prepared by blending reagents of 0.3 volume percent of Triton-X100, 1.5 volume percent of lauryl sodium sulfate and 1.5 volume percent of 3-[(3-cholane amidopropyl)-dimethylammonium]propanesulfonic acid in equal amount. The invention also discloses application of the kit in separating a pathogenic antigen antibody complex. The kit of the invention effectively realizes rapid dissociation of the pathogenic antigen antibody complex in a serum or blood plasma sample to be detected, increases the absolute concentration value of an antigen and an antibody, and greatly improves the detection sensitivity of the sample to be detected.
Owner:山东莱博生物科技有限公司
Ursodeoxycholic acid compound for preventing or treating FXR (farnesol X receptor)-mediated disease
ActiveCN106008639AGood pharmacokinetic parametersImprove securityOrganic active ingredientsMetabolism disorderFatty liverEnantiomer
Owner:SHENZHEN TARGETRX INC
19-nor-cholane steroids as neurochemical initators of change in human hypothalamic function
The invention relates to a method of altering hypothalamic function in an individual. The method comprises nasally administering a human vomeropherin, e.g. a 19-nor cholane steroid, or a pharmaceutical composition containing a vomeropherin, such that the vomeropherin binds to a specific neuroepithelial receptor. The steroid or steroids is / are preferably administered in the form of a pharmaceutical composition containing one or more pharmaceutically acceptable carriers. Other embodiments of the invention include pharmaceutical compositions containing the steroids.
Owner:PHERIN PHARMA INC
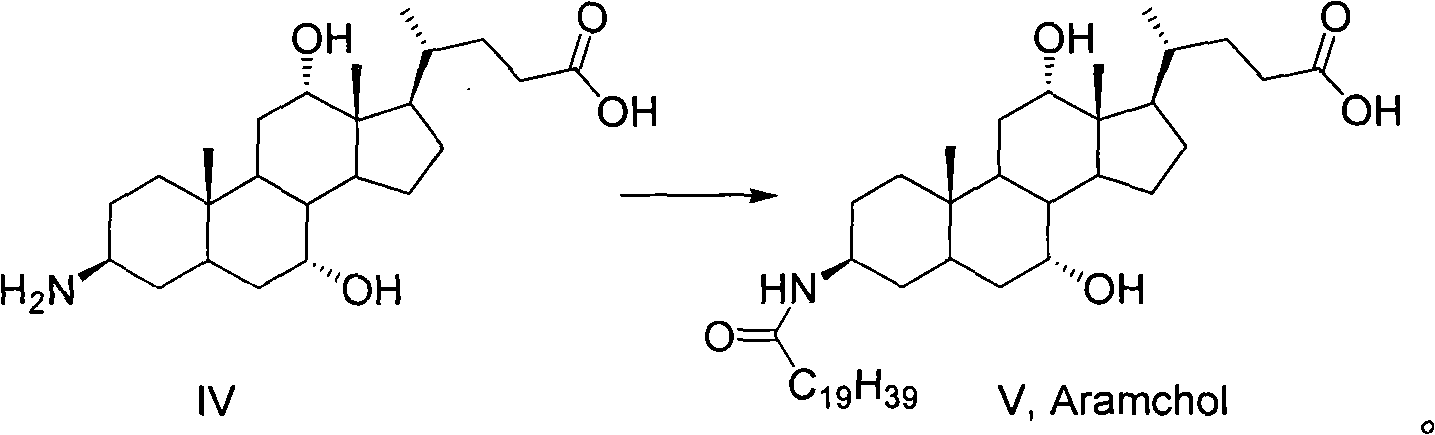
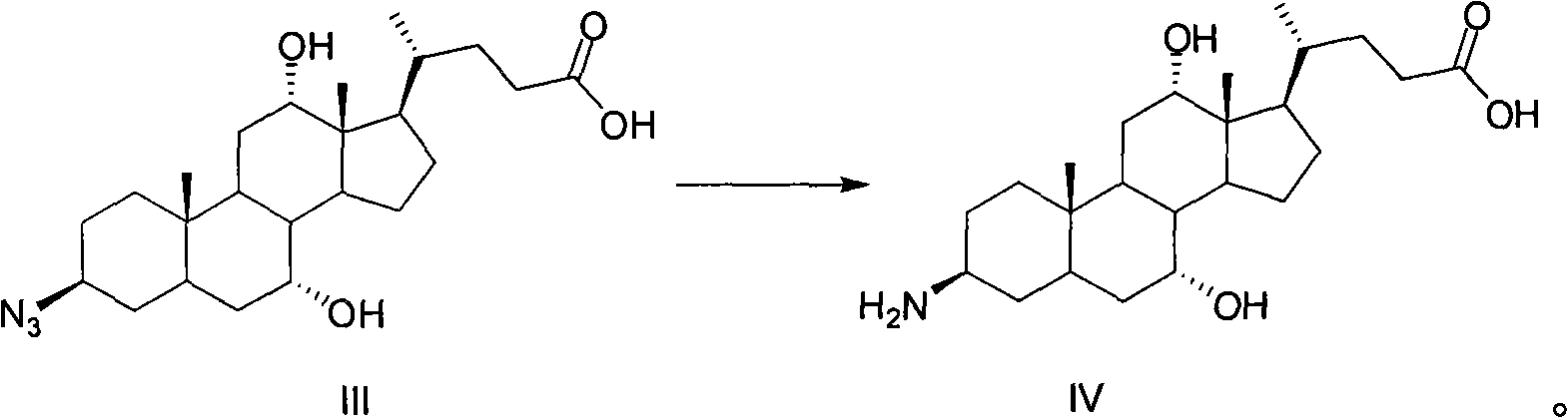
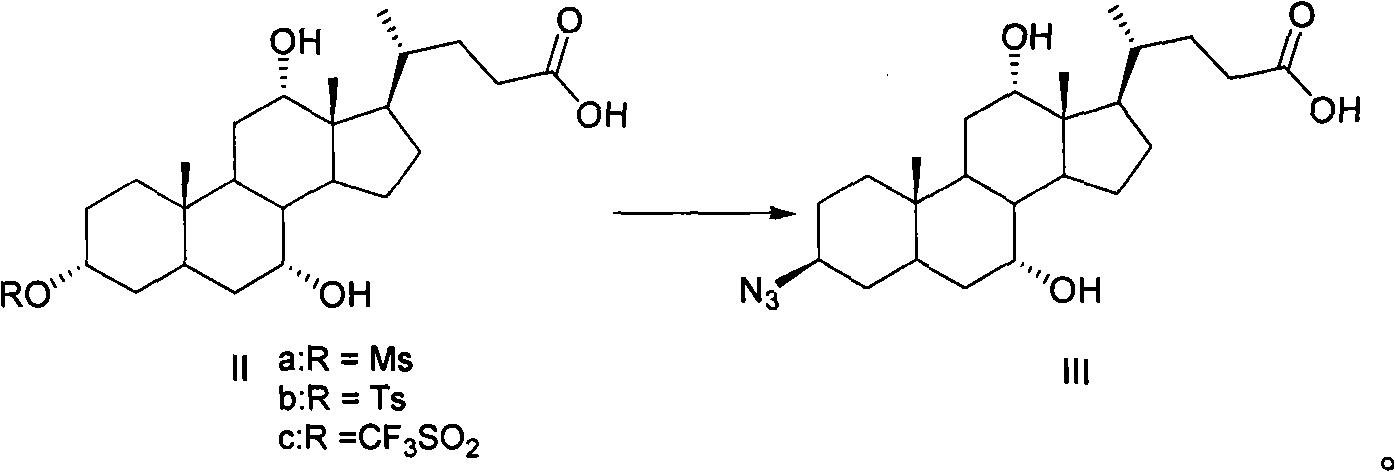
A method for preparing obeticholic acid and related compound
ActiveCN106589039AImprove refining efficiencyReduce the introductionSteroidsBulk chemical productionProtecting groupCholane
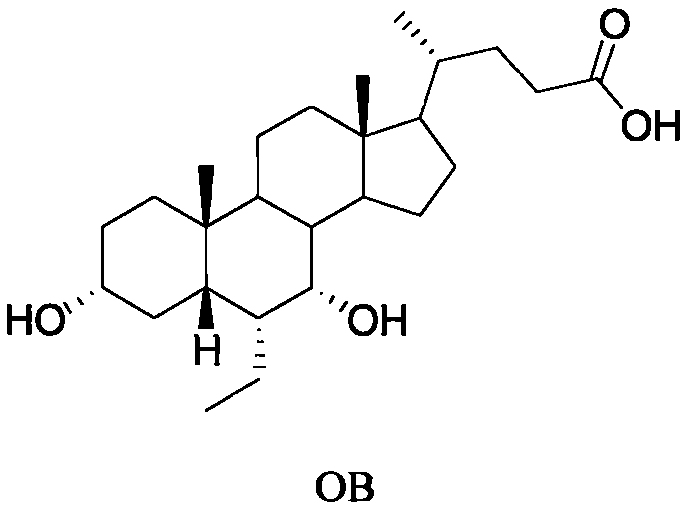
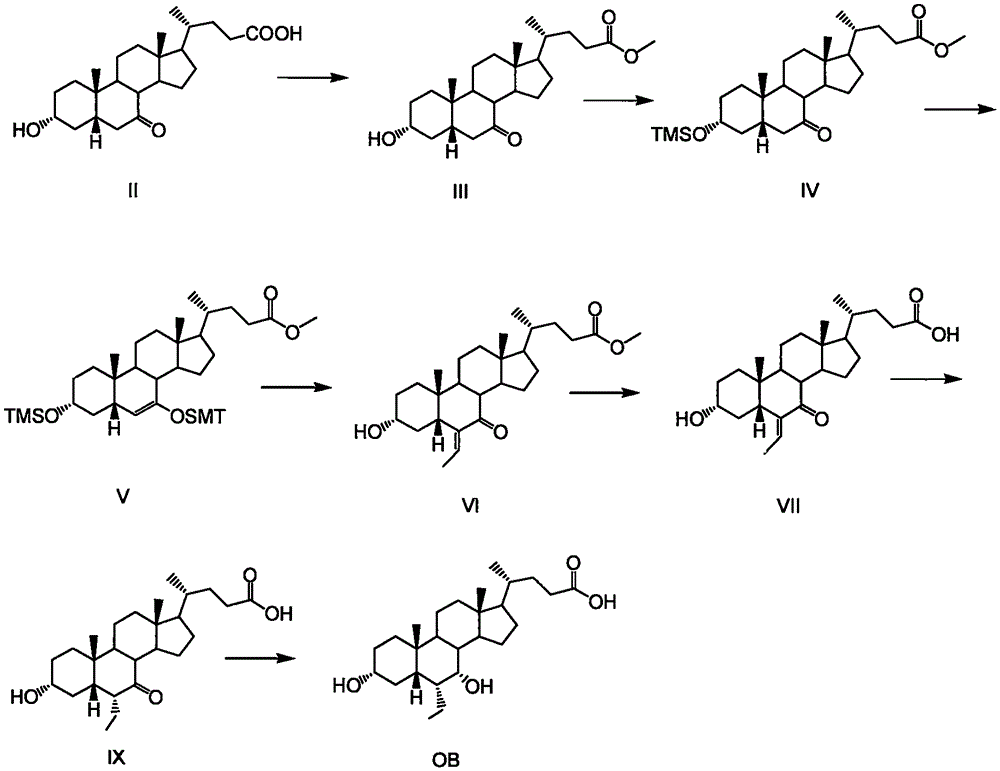
This invention provides a new method for preparing obeticholic acid which belongs to the technical field of medicine. Its raw material is E / 2 -3alpha-hydroxy-b-ethylidene-keto-5beta-cholane-24-acid methyl ester(OB-4)which can be gained easily. First, OB-3 can be produced through hydroxy-protection with tetra hydropyrane protecting group. Then, OB-2 can be produced through hydrogenation-reduction in alkaline aqueous solution. Then, OB-1 can be produced through reducing again. At last, the target product--obeticholic acid can be got through catalyzing and removing tetrahydropyrane. The method is simple in production process, the content of isomer impurity is low, and the method is a new synthetic method of obeticholic acid suitable for industrial production.
Owner:SUZHOU LANXITE BIOTECH
Method for preparing obeticholic acid type 1
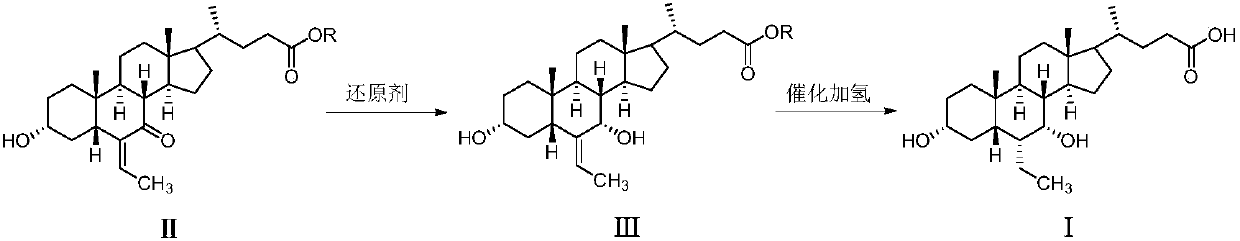
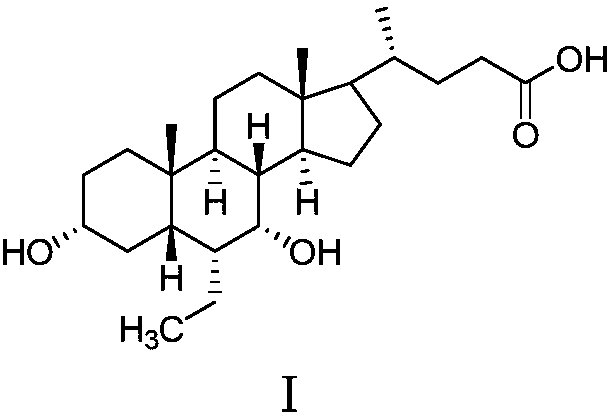
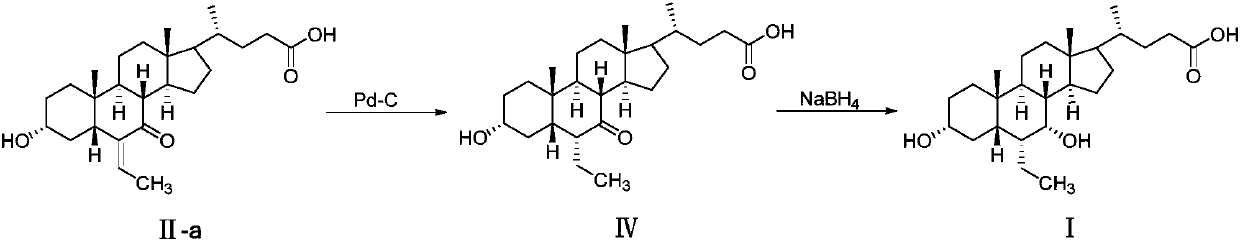
The invention discloses a method for preparing obeticholic acid type 1. The method comprises the steps that firstly, (E)-3 alpha,7 alpha-dyhydroxyl-6-ethylidene-5 beta-cholane-24-acid or (E)-3 alpha,7 alpha-dyhydroxyl-6-ethylidene-5 beta-cholane-24-acid ester (a compound II), alkali, a solvent and 5% palladium on carbon are loaded into a reactor, the mixture reacts under the pressure of 1-3 atmospheres, a hydrogenation reaction is carried out at the preserved temperature until it is indicated that hydrogen does not conduct absorption any more; reaction liquid is cooled to 40-50 DEG C and filtered, filter liquid is added with water and heated to 40-50 DEG C, and a solution is obtained; secondly, the obtained solution is dipped with a thin acid solution to regulate the pH value to be 1-6, the solution is cooled, stirred for crystallization and filtered, and obeticholic acid type 1 is obtained through vacuum drying. By means of the preparing method, crystallized obeticholic acid type C does not need to be obtained, and obeticholic acid type 1 is obtained in one step, so that production steps are reduced, operation is simplified, aftertreatment is easy, and cost is reduced.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Cyclic cholane carboxylate derivative, preparation method and uses thereof
InactiveCN109021059AReliable source of commercializationMild reaction conditionsSteroidsChromatographic separationSide chain
The present invention provides a cyclic cholane carboxylate derivative and a preparation method thereof, and uses of the cyclic cholane carboxylate derivative in industrial preparation of 25-hydroxycholesterol (1), wherein R1 in the cyclic cholane carboxylate derivative represented by a formula I is straight chain or branched chain C1-C12 alkyl, R2 is methyl or ethyl, and the formula (I) is defined in the specification. According to the present invention, the starting raw material stigmasterol of the 25-hydroxycholesterol (1) synthesis process route has characteristics of low price, easy obtaining and reliable commercial source; and particularly during the constructing of the 17-position side chain of 25-hydroxycholesterol (1), the reaction conditions are mild, the reaction steps are simple, the special and dangerous reagents are not required, the harsh reaction conditions are not required, the redundant reaction steps are not required, the chromatographic separation and purification is not required, the industrial production is easily achieved, and the significant progress is achieved compared to the prior art.
Owner:SHANGHAI INST OF PHARMA IND
Preparation method of impurities of obeticholic acid
The invention relates to a synthesis method of three impurities of obeticholic acid. The method has important significance in synthesizing high-quality obeticholic acid. The method mainly studies 3 alpha,7 beta-dyhydroxyl-6-ethyl-5 beta-cholane-24-acid (V), 3 alpha,7 beta-dyhydroxyl-6 alpha-ethyl-5 beta-cholane-24-alcohol (VI) and 3,7-dicarbonyl-6 alpha-ethyl-5 beta-cholane-24-acid (VII). The synthesis route is shown in the description.
Owner:CHINA PHARM UNIV
Preparation method of alpha-murine cholic acid
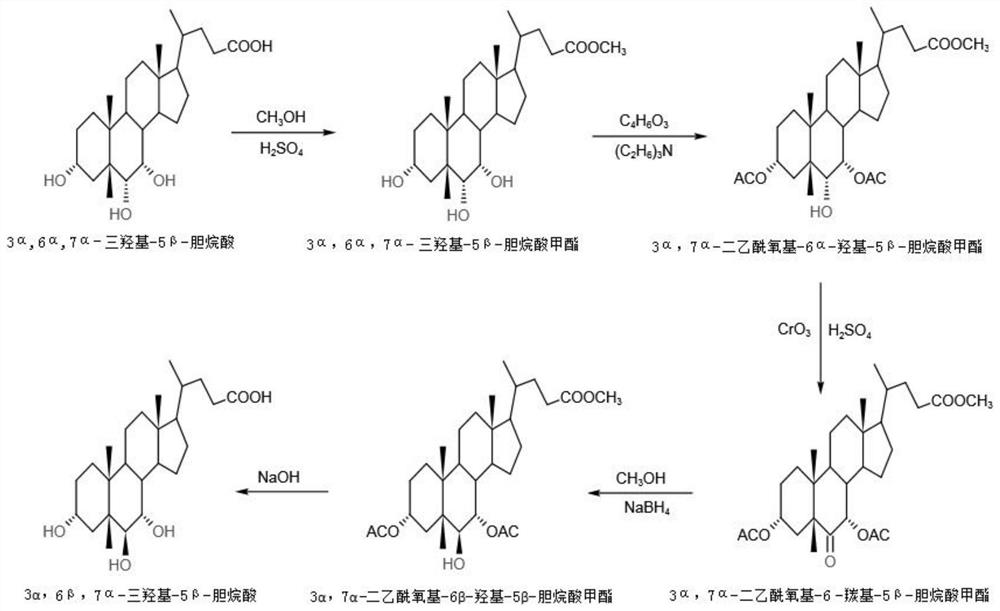
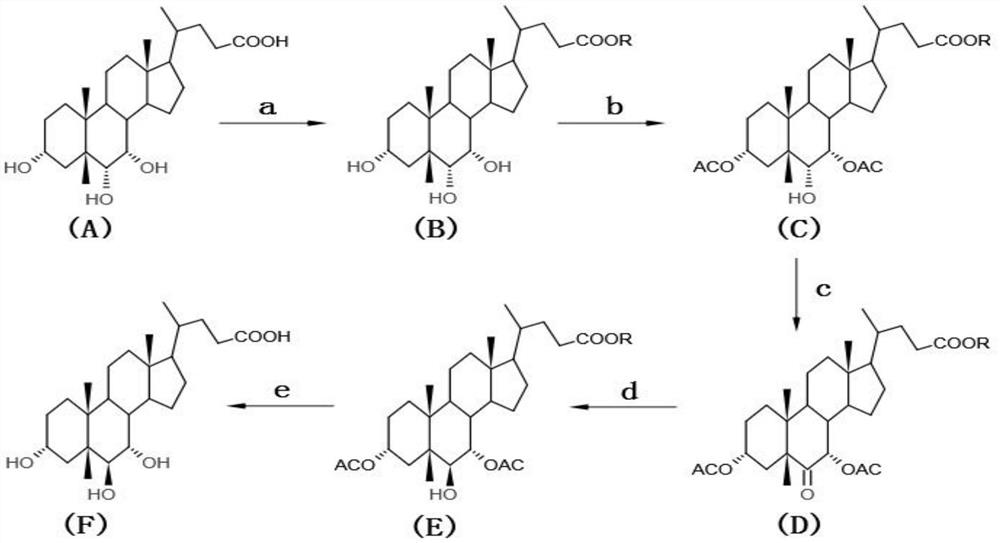
The invention discloses a preparation method of alpha-murine cholic acid. The method takes 3alpha, 6alpha, 7alpha-trihydroxy-5beta-cholanic acid (hyocholic acid) as an initial raw material, and comprises the following steps: carboxyl esterification, acetylation of 3-site and 7-site hydroxyl, oxidation of 6-site hydroxyl, reduction of 6-site carbonyl, hydrolysis and recrystallization to obtain high-purity 3alpha, 6beta, 7alpha-trihydroxy-5beta-cholanic acid, namely alpha-murine cholic acid. The alpha-murine cholic acid synthesized and prepared by the route is wide in raw material source; and the method has the advantages of high yield, high purity and few side reactions, and is suitable for large-scale preparation.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
19-nor-cholane steroids as neurochemical initiators of change in human hypothalamic function
InactiveUS6437156B1Organic active ingredientsNanostructure manufactureNeuroepithelial receptorCholane
The invention relates to a method of altering hypothalamic function in an individual. The method comprises nasally administering a human vomeropherin, e.g. a 19-nor cholane steroid, or a pharmaceutical composition containing a vomeropherin, such that the vomeropherin binds to a specific neuroepithelial receptor. The steroid or steroids is / are preferably administered in the form of a pharmaceutical composition containing one or more pharmaceutically acceptable carriers. Other embodiments of the invention include pharmaceutical compositions containing the steroids.
Owner:PHERIN PHARMA INC
Preparation method of 3-beta-peanut amide-7alpha, 12alpha, 5beta-cholane-24-carboxylic acid
ActiveCN102115486AHigh purityQuality improvementSteroidsBulk chemical productionCarboxylic acidTwo step
The invention relates to a preparation method of 3-beta-peanut amide-7alpha, 12alpha, 5beta-cholane-24-carboxylic acid. The method adopts a two-step reaction route for preparation, which avoids the utilization of protecting groups, reduces reaction steps, decreases unnecessary raw material consumption, increases production efficiency, reduces environment pollution, and has the advantages of high yield and low cost.
Owner:TOPHARMAN SHANGHAI CO LTD +1
Preparation method of OCA (obeticholic acid)
The invention discloses a preparation method of OCA (obeticholic acid). (E)-3 alpha-hydroxy-6-ethylidene-7-one-5 beta-cholane-24-acid or (E)-3 alpha-hydroxy-6-ethylidene-7-one-5 beta-cholane-24-acid ester, a solvent and a reducing agent are placed in a reactor to react to produce (E)-3 alpha, 7 alpha-dihydroxy-6-ethylidene-5 beta-cholane-24-acid or (E)-3 alpha, 7 alpha-dihydroxy-6-ethylidene-5 beta-cholane-24-acid ester. (E)-3 alpha, 7 alpha-dihydroxy-6-ethylidene-5 beta-cholane-24-acid or (E)-3 alpha, 7 alpha-dihydroxy-6-ethylidene-5 beta-cholane-24-acid ester, alkali, a solvent and a catalyst are placed in the reactor for hydrogenation to produce OCA. OCA with the purity of 99.5% or above can be obtained with the preparation method, the process is simple and good in reproducibility, thesolvent is safe, non-toxic, conventional and easy to obtain, post-treatment is simple, and the production cycle is short.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
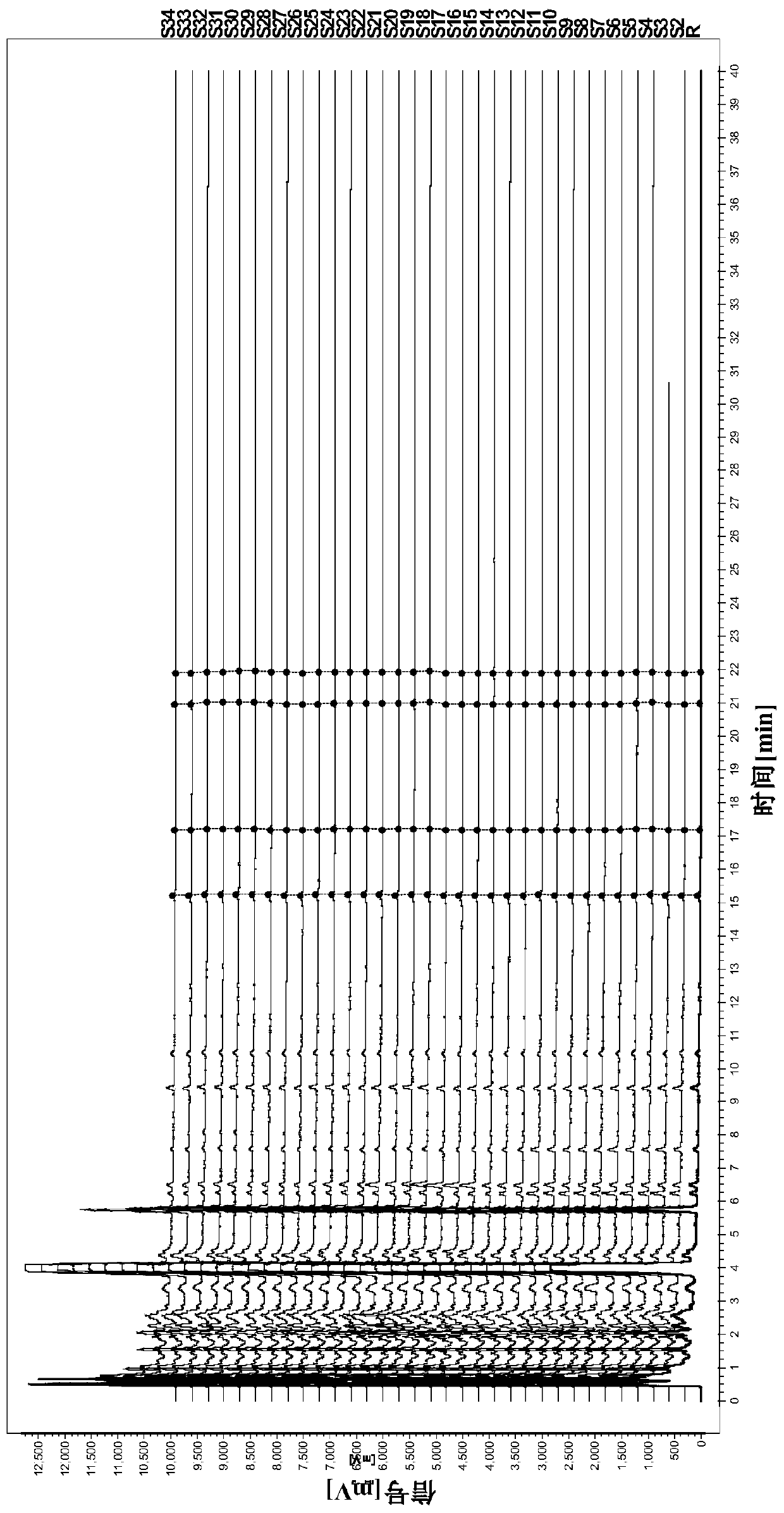
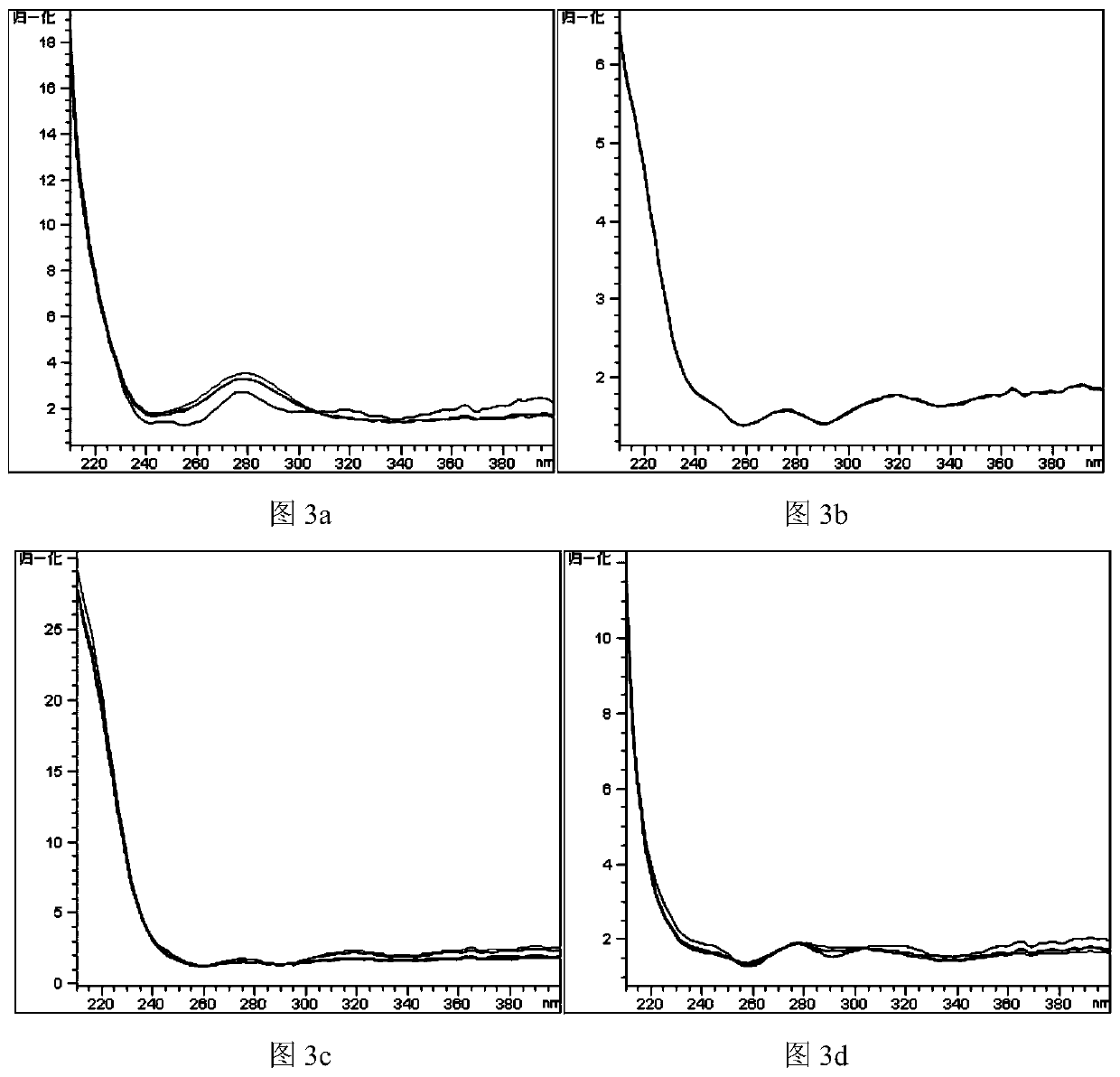
Limit detection method of bear gall powder extract in phlegm-heat clearing injection and fingerprint spectrum thereof
ActiveCN111272940ASimple processing methodEasy to operateComponent separationAgainst vector-borne diseasesCholic acidChenodeoxycholic acid
The invention relates to a limit detection method for a bear gall powder extract in a phlegm-heat clearing injection. The bear gall powder extract comprises ursodeoxycholic acid, chenodeoxycholic acid, 3 [alpha]-hydroxyl-7-oxo-5[beta]-cholanic acid and 7 [alpha]-hydroxyl-3-oxo-5[beta]-cholanic acid. The limit detection method is an HPLC-DAD method and comprises the following steps: S1, preparing areference substance solution; S2, preparing a test solution; and S3, performing limit detection. The invention also relates to a fingerprint spectrum obtained by adopting the limit detection method,the fingerprint spectrum has four characteristic peaks in total, and each peak takes an ursodeoxycholic acid peak as a reference peak. According to the limit detection method disclosed by the invention, limit detection is carried out on the bear gall powder extract by calculating the peak area of a test sample and the peak area of a reference substance through adoption of an external standard method; by calculating the similarity between the fingerprint spectrum of the test sample and the reference fingerprint spectrum, the product quality is accurately, efficiently and comprehensively monitored, and meanwhile, the authenticity of the product is identified.
Owner:SHANGHAI KAIBAO PHARMA +1
Preparation method and application of 3 alpha, 7 alpha-dihydroxy-6 alpha-ethyl-5 beta-cholane-24-aldehyde
ActiveCN111518152AImprove quality controlEasy to prepareComponent separationSteroidsCholic acidEthyl group
The invention relates to a preparation method of 3 alpha, 7 alpha-dihydroxy-6 alpha-ethyl-5 beta-cholane-24-aldehyde. The method is simple, convenient and high in yield and content. The3 alpha, 7 alpha-dihydroxy-6 alpha-ethyl-5 beta-cholane-24-aldehyde can be conveniently used as a reference substance of related substances for quality control of obeticholic acid or derivatives of obeticholic acid,or used for impurity identification of obeticholic acid or diffracted substances of obeticholic acid, and is beneficial to detection control in the production process of obeticholic acid or derivatives of obeticholic acid and quality control of drugs containing obeticholic acid or derivatives of obeticholic acid.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Method for extracting (20R)-22E-24-ethyl cholane-4,22-diene-3-ketone from stems and leaves of Coix lacryma-jobi
The invention discloses a method for extracting (20R)-22E-24-ethyl cholane-4,22-diene-3-ketone from stems and leaves of Coix lacryma-jobi. The method comprises the following steps: dried stems and leaves of Coix lacryma-jobi are crushed, ethanol is added and heated, reflux extraction is carried out, and an extracting solution is condensed in order to obtain an extract A; water is added into the extract A and the extract A is dispersed, petroleum ether is used for extraction, and petroleum ether is partially condensed in order to obtain an extract B; silica gel column chromatography is carried out for the extract B firstly, petroleum ether-ethyl acetate gradient elution is carried out, separated and purified components are collected, chromatography is carried out, pure dichloromethane is used for elution, separated and purified components are separated and purified by preparation type high performance liquid chromatography, a tR=31 min component is collected, condensation and crystallization are carried out, and the product is obtained. (20R)-22E-24-ethyl cholane-4,22-diene-3-ketone is extracted from waste stems and leaves of Coix lachrymal-jobi, environmental pollution is reduced, utilization rate of stems and leaves of Coix lacryma-jobi is improved, an industry chain of Coix lacryma-jobi is prolonged, income of Coix lacryma-jobi industry is increased, and the method has good economic benefits and ecological benefits.
Owner:右江民族医学院
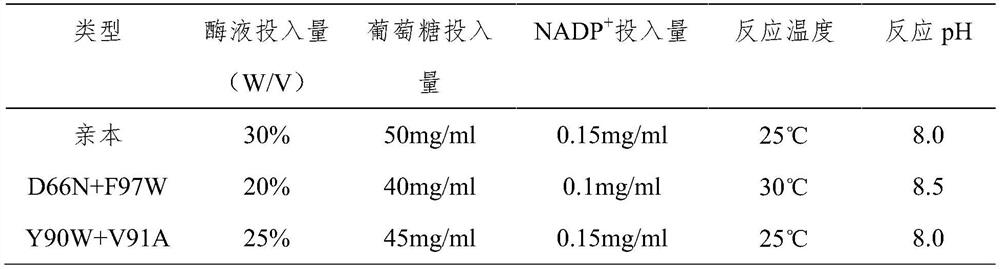
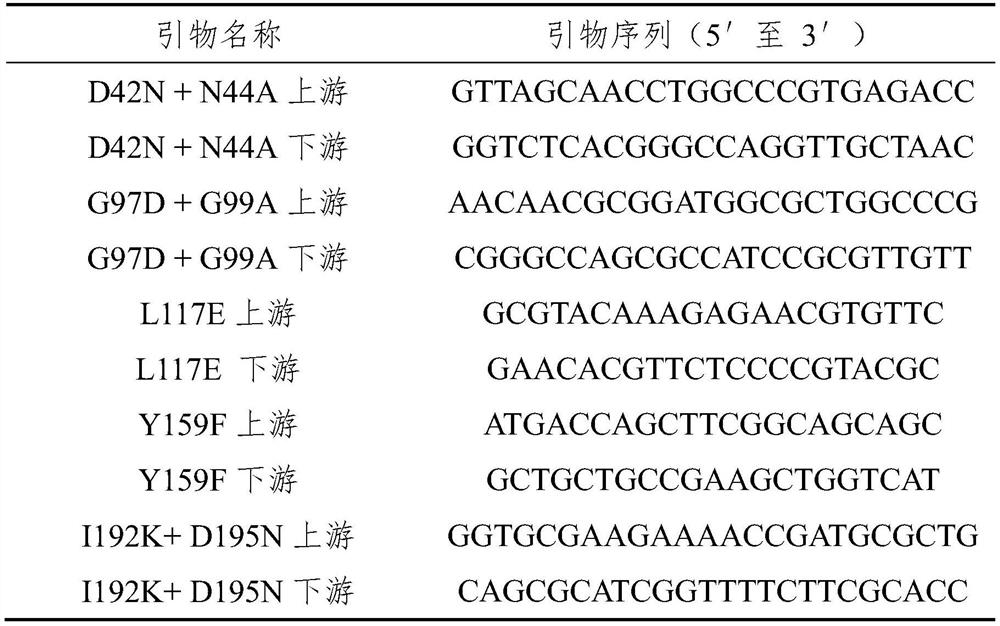
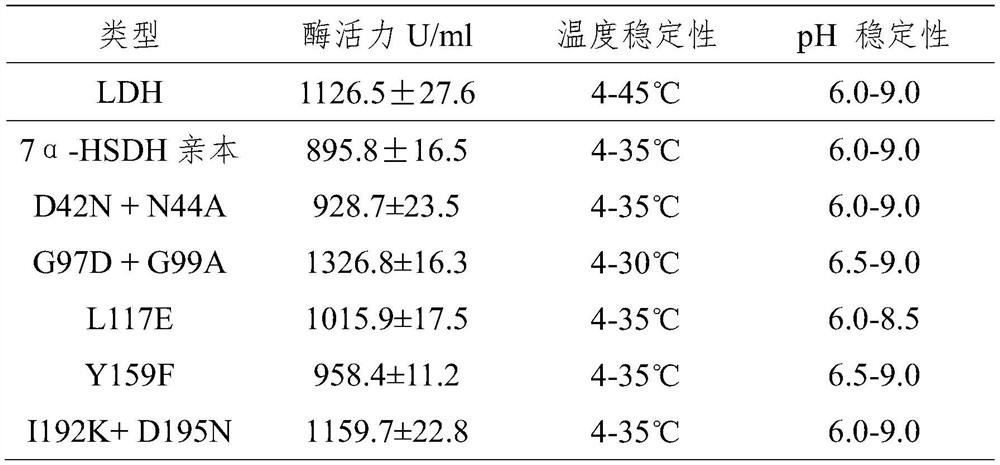
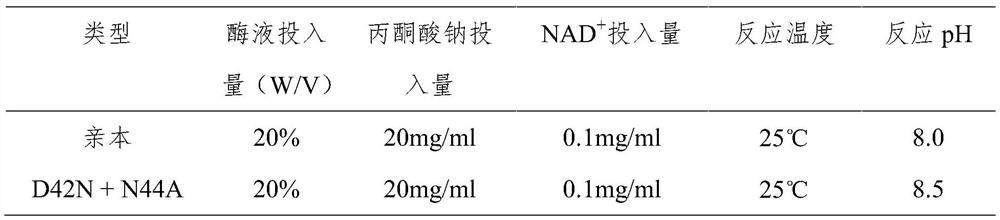
A kind of preparation method of 3α-hydroxyl-7 oxo-5β-cholanic acid and its preparation enzyme 1
ActiveCN107995928BEasy to operateThe reaction conditions are mild and easy to controlOxidoreductasesFermentationHalomonas salinaCholic acid
The invention relates to a method for preparing 3α-hydroxy-7-oxo-5β-cholanic acid by using biological enzyme catalysis technology and 7α-steroid dehydrogenase for preparation thereof. The method uses chenodeoxycholic acid as a substrate, and in the presence of NAD, lactate dehydrogenase, sodium pyruvate and buffer solution, uses 7α-steroid dehydrogenase to catalyze chenodeoxycholic acid to prepare 3α-hydroxy-7 Oxo-5β-cholanic acid with 7α-steroid dehydrogenase from Halomonas Halomonas jeotgali sp. The method has the advantages of simple operation, mild and easy-to-control reaction conditions, short reaction time, a substrate conversion rate as high as over 99.7%, and the obtained product content is over 97.5%.
Owner:ZHONGSHAN BONTAC BIO TECH CO LTD
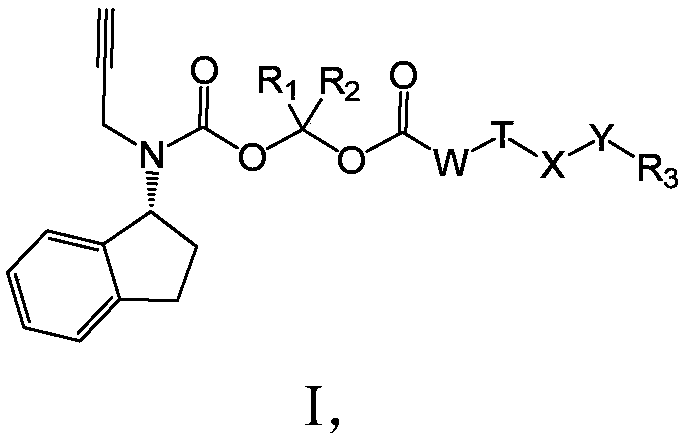
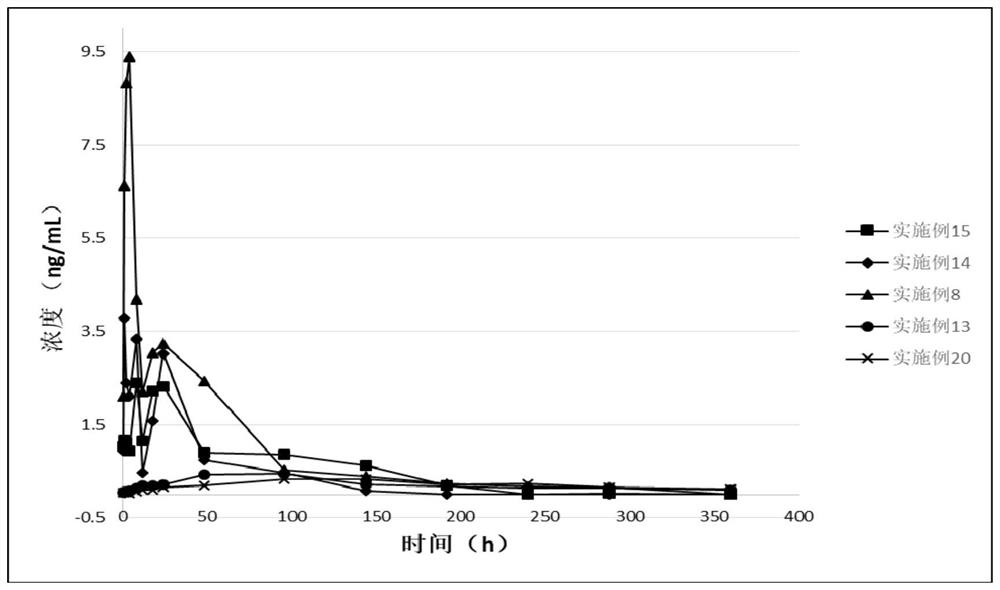
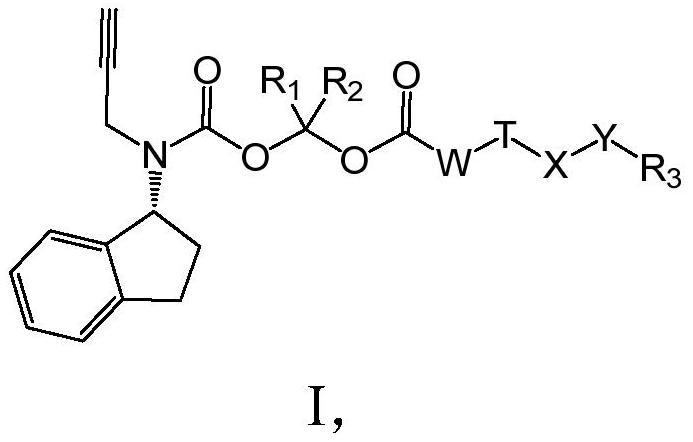
Long-acting Rasagiline prodrug as well as preparation method and application thereof
ActiveCN109422668AHigh melting pointReduce solubilityNervous disorderCarbamic acid derivatives preparationSolubilityHydrogen
The invention relates to a long-acting Rasagiline prodrug as well as a preparation method and application thereof. The structure of the long-acting Rasagiline prodrug is as shown in formula I: (shownin the description), wherein T is selected from (shown in the description) or none; R1 and R2 are respectively independently selected from hydrogen, deuterium and methyl; W is selected from (CH2)N ornone, and n is selected from an integer of 1 to 15; X is selected from (CH2)m or none, and m is selected from an integer of 1 to 10; Y is selected from -C(=O)NH-, -NHC(=O) or none; and R3 is selectedfrom substituted or unsubstituted alkyl of C1 to C30, substituted or unsubstituted C2 to C3 alkenyl, substituted or unsubstituted C2 to C30 alkynyl, substituted or unsubstituted C3 to C30 naphthenic base, cholanic aliphatic chain, -R<3a>-C(=O)O-R<3b>, -R<3a>-OC(=O)-R<3b>, -R<3a>-C(=O)NH-R<3b>, -R<3a>-NHC(=O)-R<3b>, or -R<3a>-S(=O)1-2O-R<3b> and -R<3a>-OS(=O)1-2-R<3b>. The Rasagiline prodrug of theinvention is high in melting point, low in solubility, capable of forming a drug library by virtue of injection administration in a body, capable of increasing the release time of the drug in the body, and capable of playing the lasting-acting treatment.
Owner:GUANGZHOU HENOVCOM BIOSCI CO LTD
Method for determining content of related components of four bear gall powder in cooling-based phlegm eliminating injection
ActiveCN112198234AHigh precisionSimple and fast operationComponent separationAgainst vector-borne diseasesCholic acidChenodeoxycholic acid
The invention relates to the field of chemistry, in particular to a method for determining the content of related components of four bear gall powder in cooling-based phlegm eliminating injection. Themethod comprises the following steps: S1, preparing a reference solution; S2, preparing a test solution; S3, performing detecting by using a high performance liquid chromatograph-mass spectrometer; S4, carrying out qualitative detection; and S5, performing quantitative detection: according to the method, under the condition set by a high performance liquid chromatograph-mass spectrometer, respectively carrying out determination, repeating the process for 6 times, determining the peak area of the corresponding concentration, and carrying out linear regression to obtain a regression equation soas to obtain a correlation coefficient and a linear range and determine the contents of the four bear gall powder related components in the cooling-based phlegm eliminating injection. The method canbe used for qualitatively analyzing ursodeoxycholic acid, 3alpha- hydroxyl-7oxo-5beta-cholanic acid, chenodeoxycholic acid and 7alpha- hydroxyl-3-oxo-5beta-cholanic acid of the cooling-based phlegm eliminating injection and the bear gall powder extract, and is simple and convenient to operate, stable in result, good in reproducibility and high in precision.
Owner:SHANGHAI KAIBAO PHARMA
Type-1 obeticholic acid preparation method
ActiveCN105566429AHigh purityHigh recovery rateOrganic chemistry methodsSteroidsDistillationDissolution
The invention discloses a type-1 obeticholic acid preparation method. The type-1 obeticholic acid preparation method comprises the following steps that firstly, the mixture of 3 alpha-hydroxyl-6 alpha-ethyl-7-ketone-5 beta-cholane-24-acid ester, alkali and a solvent is heated to reach the temperature of 75-105 DEG C, a reducing agent is added for reaction, TLC tracking is performed till raw materials react completely, the reaction solution is cooled to reach the temperature of 30-50 DEG C, an organic solvent and water are added, a pH value of the solution is regulated to be 3-5 by using hydrochloric acid, solution separation is performed, and organic phase reduced-pressure drying by distillation is performed to obtain a colorless light yellow oily residue; secondly, water and alkali are added into the obtained residue, heating is performed for dissolution, and a solution is obtained; finally, a dilute hydrochloric acid is slowly and dropwise added into the obtained solution to regulate the pH value to be 3-4, and cooling, stirring for crystallization, filtering and vacuum drying are performed to obtain a type-1 obeticholic acid.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Preparation method of chenodeoxycholic acid derivatives
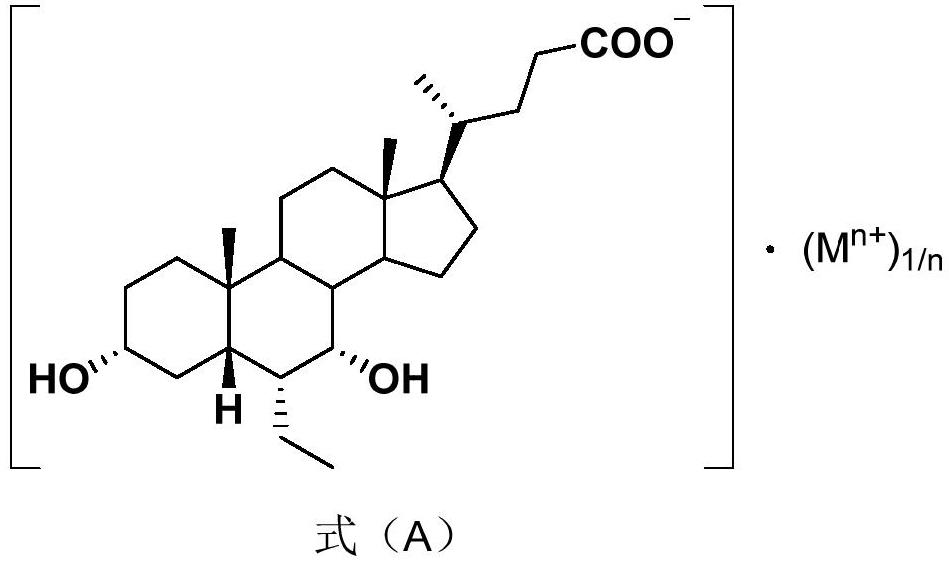
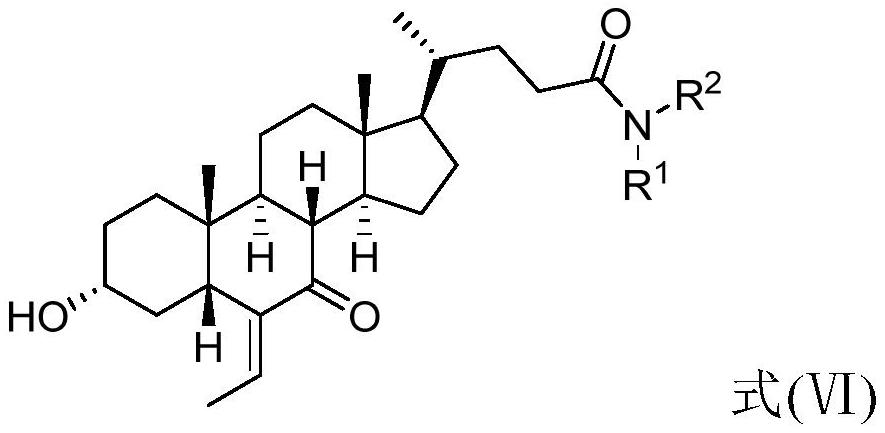
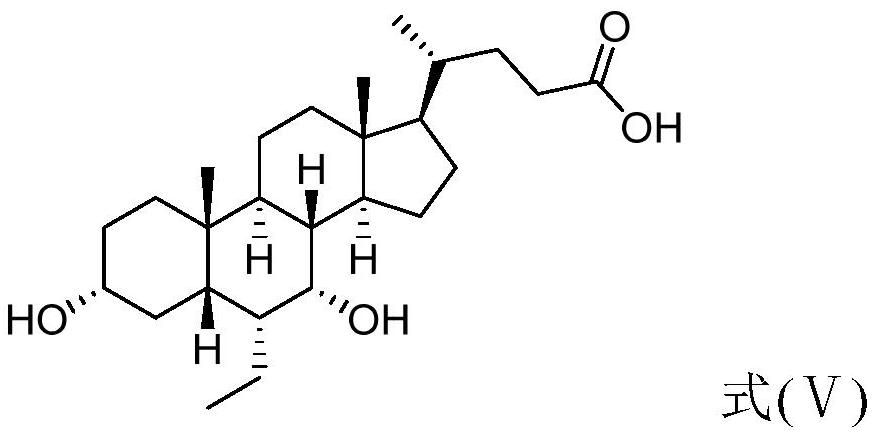
The invention relates to a preparation method of chenodeoxycholic acid derivatives. Specifically, the invention discloses a method for preparing high-purity obeticholic acid sodium salt, potassium salt, magnesium salt and calcium salt by performing one-pot reduction on a compound 3[alpha]-hydroxyl-ethylene-7-keto-5[beta]-cholanate-24-alkanolamide shown as a formula (VI) to obtain a compound namely3[alpha],7[alpha--dihydroxyl-6[alpha]-ethyl-5[beta]-cholanic acid shown as a formula (V) and performing salt-forming and crystallization.
Owner:SUZHOU ZELGEN BIOPHARML
A kind of preparation method of obeticholic acid type 1
ActiveCN105566429BHigh purityHigh recovery rateOrganic chemistry methodsSteroidsCholic acidDistillation
The invention discloses a type-1 obeticholic acid preparation method. The type-1 obeticholic acid preparation method comprises the following steps that firstly, the mixture of 3 alpha-hydroxyl-6 alpha-ethyl-7-ketone-5 beta-cholane-24-acid ester, alkali and a solvent is heated to reach the temperature of 75-105 DEG C, a reducing agent is added for reaction, TLC tracking is performed till raw materials react completely, the reaction solution is cooled to reach the temperature of 30-50 DEG C, an organic solvent and water are added, a pH value of the solution is regulated to be 3-5 by using hydrochloric acid, solution separation is performed, and organic phase reduced-pressure drying by distillation is performed to obtain a colorless light yellow oily residue; secondly, water and alkali are added into the obtained residue, heating is performed for dissolution, and a solution is obtained; finally, a dilute hydrochloric acid is slowly and dropwise added into the obtained solution to regulate the pH value to be 3-4, and cooling, stirring for crystallization, filtering and vacuum drying are performed to obtain a type-1 obeticholic acid.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
A kind of preparation method of ursodeoxycholic acid and its preparation enzyme 1
ActiveCN107995929BImprove conversion rateEasy to operateOxidoreductasesFermentationCholic acidAcyl CoA dehydrogenase
The invention relates to a method for preparing ursodeoxycholic acid by using biological enzyme catalysis technology and 7β-steroid dehydrogenase for preparation thereof. The method uses 3α-hydroxy-7 oxo-5β-cholanic acid as a substrate, and in the presence of NADP, glucose, glucose dehydrogenase and buffer solution, 3α-hydroxy-7 Preparation of ursodeoxycholic acid from oxo-5β-cholanic acid with 7β-steroid dehydrogenase from Ruminococcus twistersensis Ruminococcus torques ATCC 27756. The method has the advantages of simple operation, mild and easy-to-control reaction conditions, short reaction time, a substrate conversion rate as high as 99.7%, and the obtained product content is above 98.5%.
Owner:BONTAC INST OF GREEN BIOCATALYSIS
A kind of preparation method of 3α-hydroxyl-7 oxo-5β-cholanic acid and its preparation enzyme 2
ActiveCN107980060BImprove conversion rateEasy to operateOxidoreductasesFermentationCholic acidChenodeoxycholic acid
A method for preparing 3α-hydroxy-7oxo-5β-cholanic acid catalyzed by biological enzymes and 7α-steroid dehydrogenase for its preparation. The method uses chenodeoxycholic acid as a substrate, and in the presence of NAD, lactate dehydrogenase, sodium pyruvate and buffer solution, uses 7α-steroid dehydrogenase to catalyze chenodeoxycholic acid to prepare 3α-hydroxy-7 Oxo-5β-cholanic acid with 7α-steroid dehydrogenase from Cyanobacteria Cyanothece sp.ATCC 29155.
Owner:BONTAC INST OF GREEN BIOCATALYSIS
Cholanic acid compounds for preventing or treating fxr-mediated diseases
ActiveCN106008639BGood pharmacokinetic parametersImprove securityOrganic active ingredientsMetabolism disorderFatty liverPharmaceutical drug
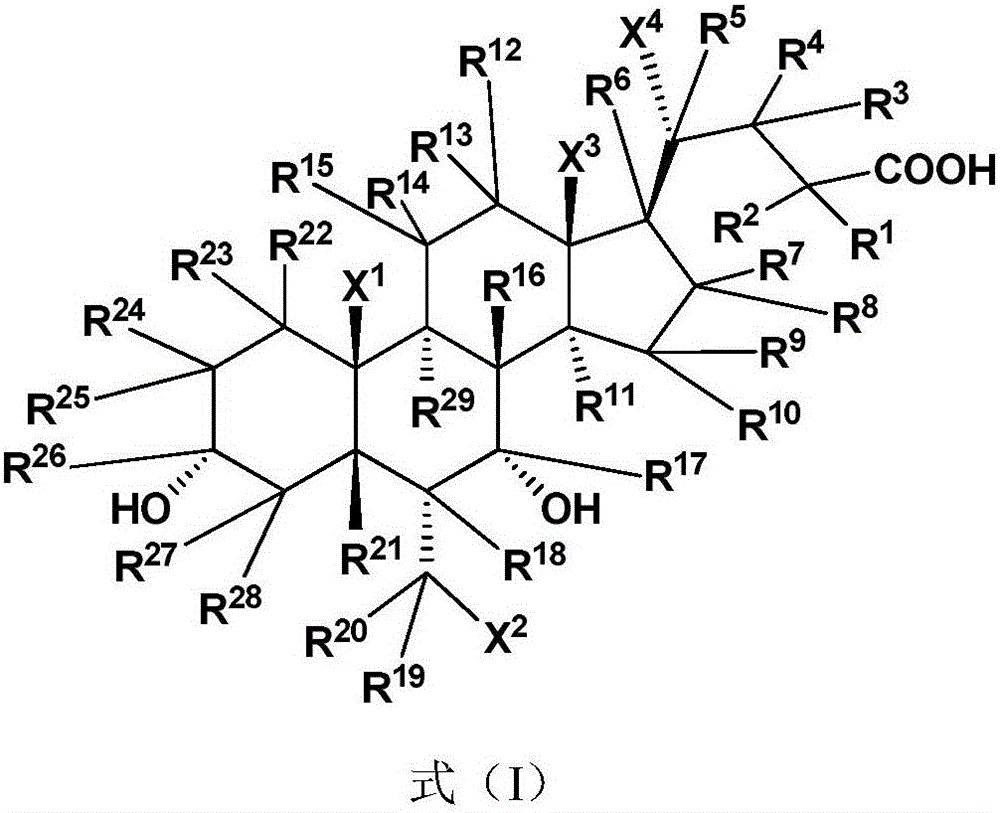
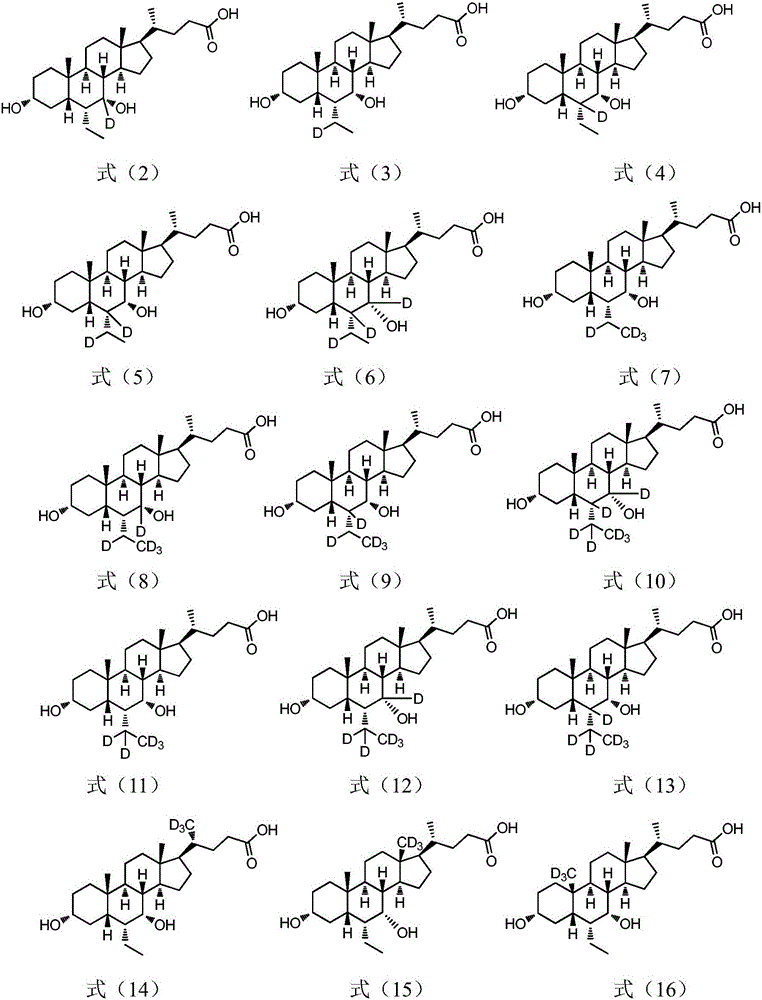
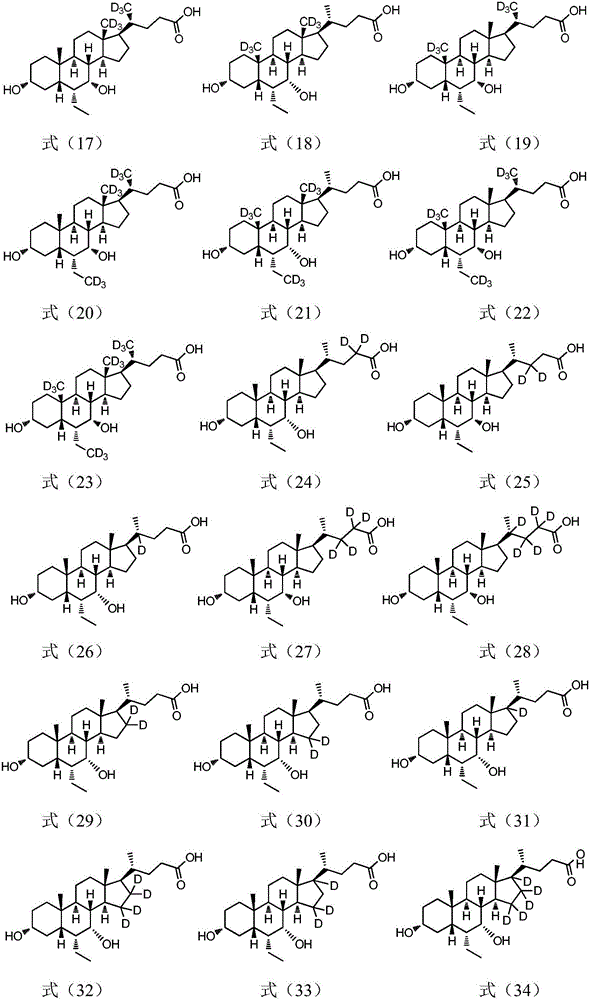
The present invention relates to a cholanic acid compound for preventing or treating FXR-mediated diseases. Specifically, the invention discloses cholanic acid compounds represented by formula (I) and compounds containing the compound, or its crystal form, pharmaceutically acceptable salts, prodrugs, tautomers and stereoisomers, and Pharmaceutical compositions of enantiomers, hydrates or solvates. The compound of the present invention can be used as a farnesoid X receptor agonist, and can be applied to the preparation of drugs for treating FXR-related diseases (such as fatty liver, etc.).
Owner:SHENZHEN TARGETRX INC
A long-acting rasagiline prodrug and its preparation method and application
ActiveCN109422668BHigh melting pointReduce solubilityNervous disorderCarbamic acid derivatives preparationDrug reservoirPharmaceutical drug
Owner:GUANGZHOU HENOVCOM BIOSCI CO LTD
Isomer of cholanic acid as well as detection method and application thereof
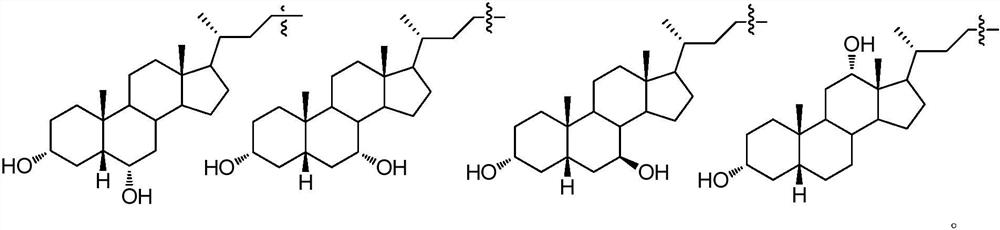
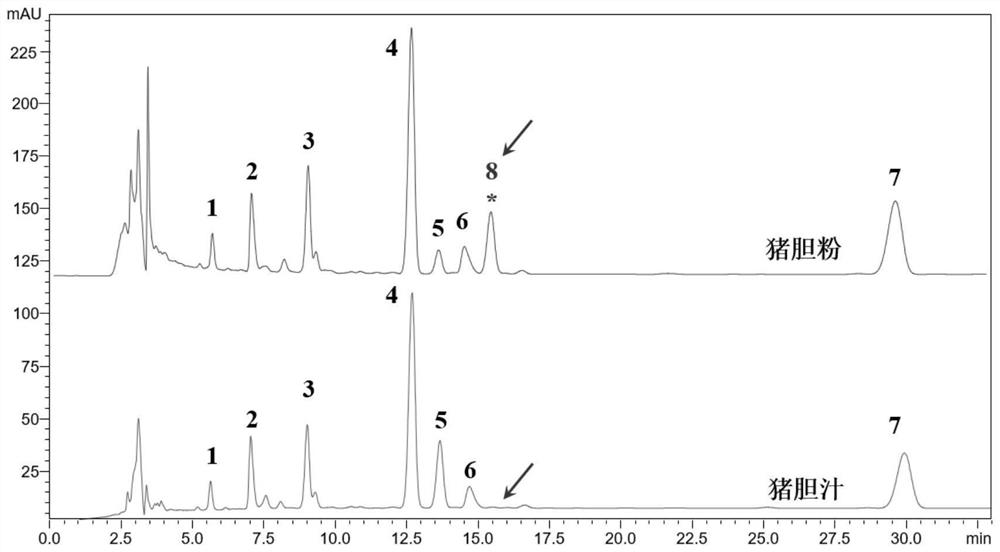
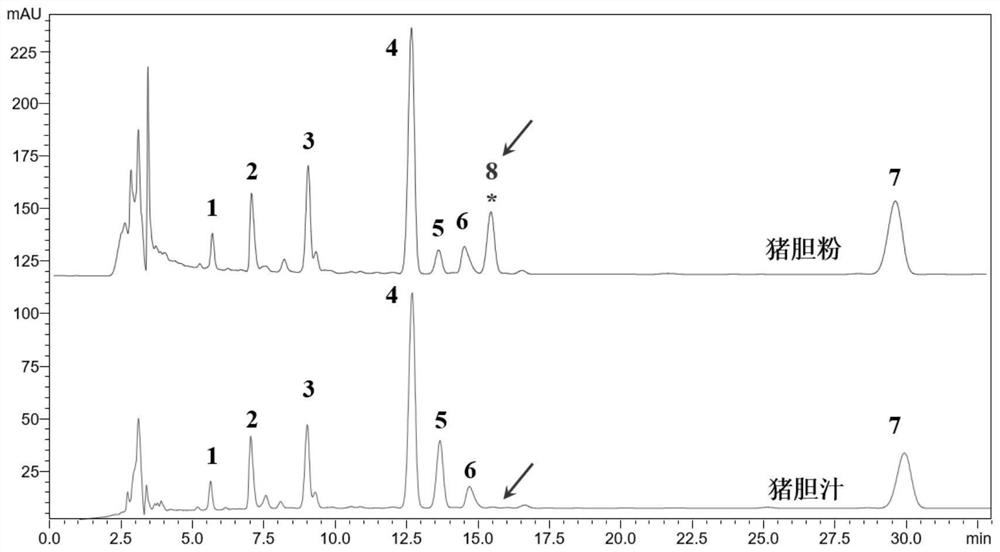
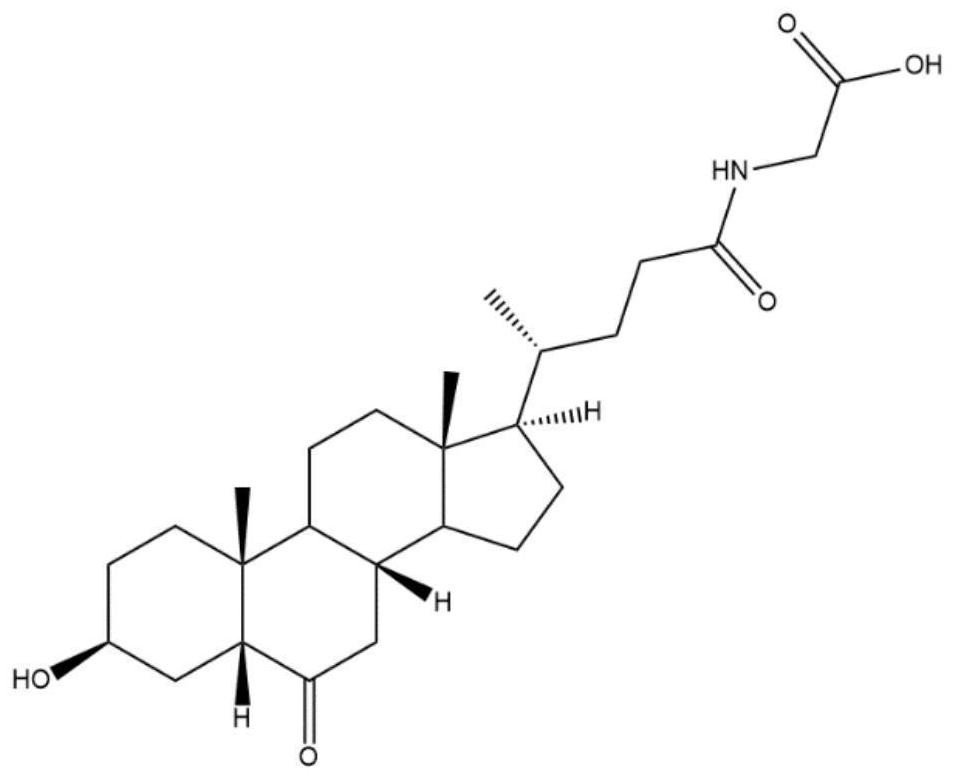
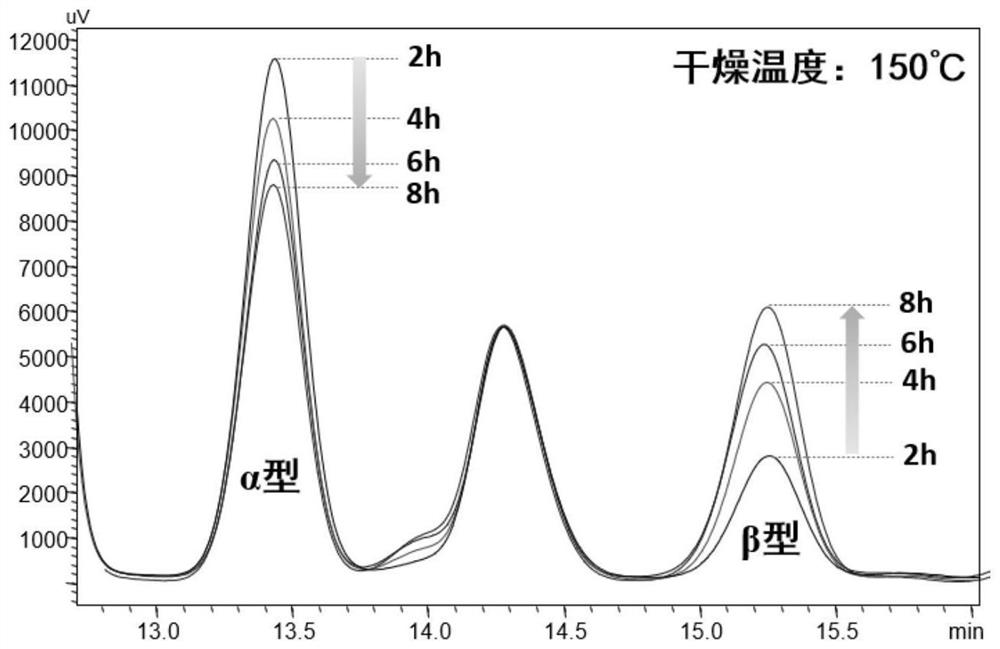
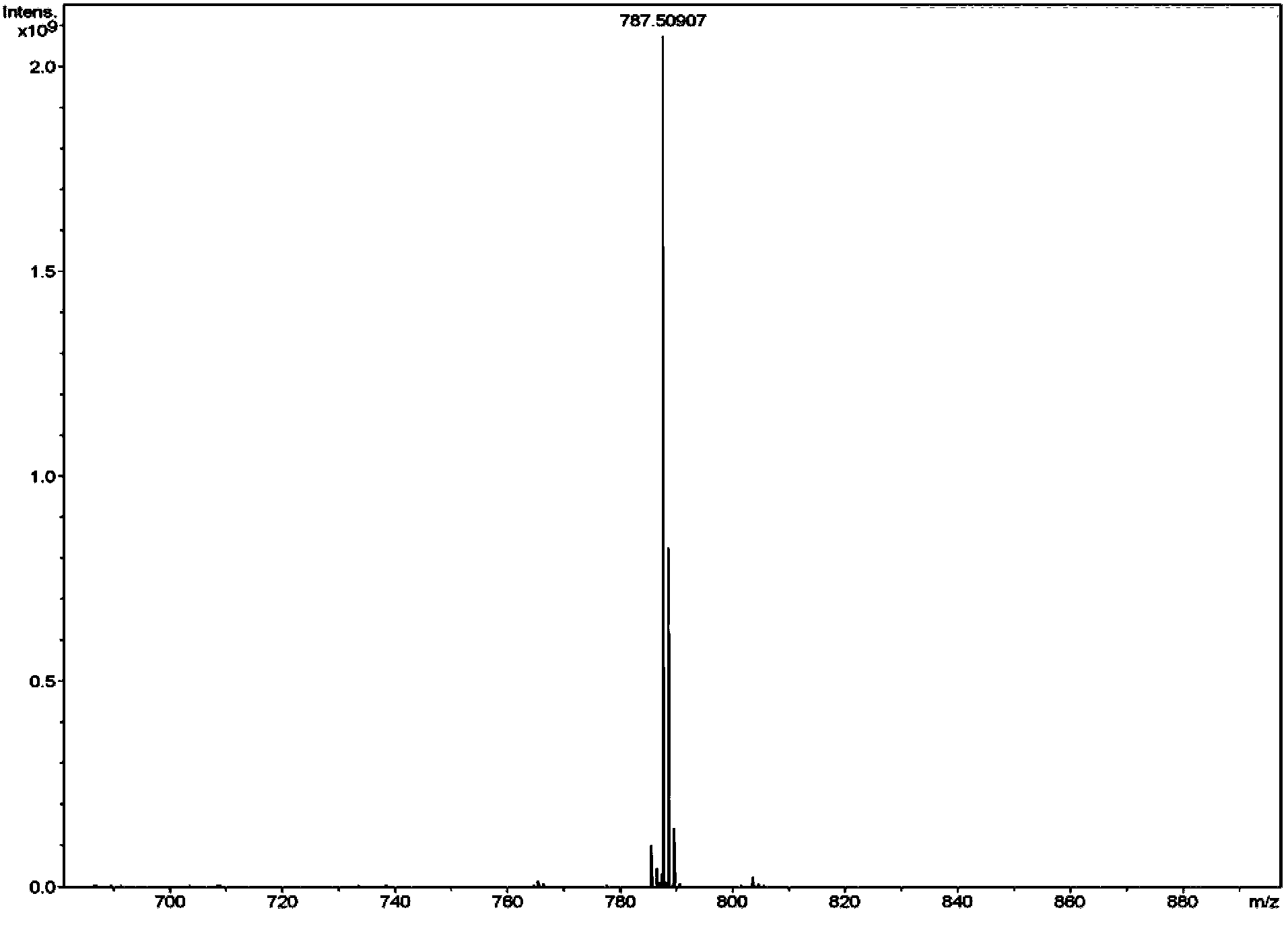
The invention discloses an isomer of cholanic acid as well as a detection method and application thereof, a novel isomer 3 beta type cholanic acid of cholanic acid is found in a pig gall substance serving as a Naoliqing preparation raw material, and the byproduct widely exists in pig gall powder and pig bile paste medicinal materials and is transferred into a Naoliqing preparation along with the production feeding process, and the substance is used as an index for detecting the quality of the pig gall raw material, so that the quality risk caused by component change can be avoided.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL
A kind of preparation method of ursodeoxycholic acid and its preparation enzyme 2
ActiveCN107980064BImprove conversion rateEasy to operateOxidoreductasesFermentationCholic acidEnzyme catalysis
The invention relates to a method for preparing ursodeoxycholic acid by using biological enzyme catalysis technology and 7β-steroid dehydrogenase for preparation thereof. The method uses 3α-hydroxy-7 oxo-5β-cholanic acid as a substrate, and in the presence of NADP, glucose, glucose dehydrogenase and buffer solution, 3α-hydroxy-7 Preparation of ursodeoxycholic acid from oxo-5β-cholanic acid with 7β-steroid dehydrogenase from Enterococcus Enterococcus silesiacus . The method has the advantages of simple operation, mild and easy-to-control reaction conditions, short reaction time, a substrate conversion rate as high as 99.6%, and a product content of more than 98.5%.
Owner:BONTAC INST OF GREEN BIOCATALYSIS
A kind of isomer of cholanic acid and its detection method and application
The invention discloses a cholanic acid isomer and its detection method and application. A new cholanic acid isomer 3β is found in pig bile substances used as the raw material of Naoliqing preparation Type cholanoic acid, the by-product widely exists in pig bile powder and pig bile ointment medicinal materials, and will be transferred into Naoliqing preparation following the production and feeding process. Using this substance as an indicator to detect the quality of pig bile raw materials can avoid due to composition changes. And the quality risk introduced.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL
Hexose ester, synthetic method thereof and use thereof
The invention relates to a hexose ester, and concretely relates to the hexose ester, a synthetic method thereof and a use thereof. The molecular general formula of the hexose ester is C36+m+nH60+2m+2nN2O10. The synthetic method comprises the following steps: dissolving an alpha, omega-diamine and a Boc acid anhydride in an organic solvent, and reacting to obtain a compound 1; dissolving the compound 1, cholesteryl chloroformate and an alkali in the organic solvent, and reacting to obtain a compound 2; dissolving acetyl chloride and the compound 2 in the organic solvent, and reacting to obtain a compound 3; dissolving the compound 3 and alpha, omega-dicarboxylic anhydride the an organic solvent, adding the alkali, and reacting to obtain a compound 4; dissolving 2,3,4,6-tetra-O-benzyl-hexose, the compound 4 and a condensing agent in the organic solvent, adding the alkali, and reacting to obtain a compound 5; and dissolving the compound 5 and Pd / C in the organic solvent, and reacting to obtain a compound 6(D-hexose-4[(4-{[(cholane-4-oxo)carbonyl]amino}A)amino]-4-carbonyl B ester).
Owner:XIAMEN UNIV
A kind of preparation method of obeticholic acid dimer
The present invention relates to a preparation method of obeticholic acid dimer. The carboxyl group and hydroxyl group of obeticholic acid are protected to obtain benzyl 3α,7α-dihydroxy-6α-ethyl-5β-cholanoate ( Ⅵ) and 3α, 7α-bis-(4-methoxytriphenylmethyl)-6α-ethyl-5β-cholanic acid (IV), and then esterify compounds Ⅳ and Ⅵ under alkaline conditions , and finally remove the protective groups of the hydroxyl group and the carboxyl group in turn to obtain the obeticholic acid dimer (I). The obeticholic acid dimer prepared in the present invention has high purity and can be well used for the research on impurities of obeticholic acid without further purification.
Owner:NCPC NEW DRUG RES & DEV
A kind of method for preparing obeticholic acid type 1
ActiveCN105646633BSimple preparation processGood reproducibilitySteroidsCholic acidPalladium on carbon
The invention discloses a method for preparing obeticholic acid type 1. The method comprises the steps that firstly, (E)-3 alpha,7 alpha-dyhydroxyl-6-ethylidene-5 beta-cholane-24-acid or (E)-3 alpha,7 alpha-dyhydroxyl-6-ethylidene-5 beta-cholane-24-acid ester (a compound II), alkali, a solvent and 5% palladium on carbon are loaded into a reactor, the mixture reacts under the pressure of 1-3 atmospheres, a hydrogenation reaction is carried out at the preserved temperature until it is indicated that hydrogen does not conduct absorption any more; reaction liquid is cooled to 40-50 DEG C and filtered, filter liquid is added with water and heated to 40-50 DEG C, and a solution is obtained; secondly, the obtained solution is dipped with a thin acid solution to regulate the pH value to be 1-6, the solution is cooled, stirred for crystallization and filtered, and obeticholic acid type 1 is obtained through vacuum drying. By means of the preparing method, crystallized obeticholic acid type C does not need to be obtained, and obeticholic acid type 1 is obtained in one step, so that production steps are reduced, operation is simplified, aftertreatment is easy, and cost is reduced.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com