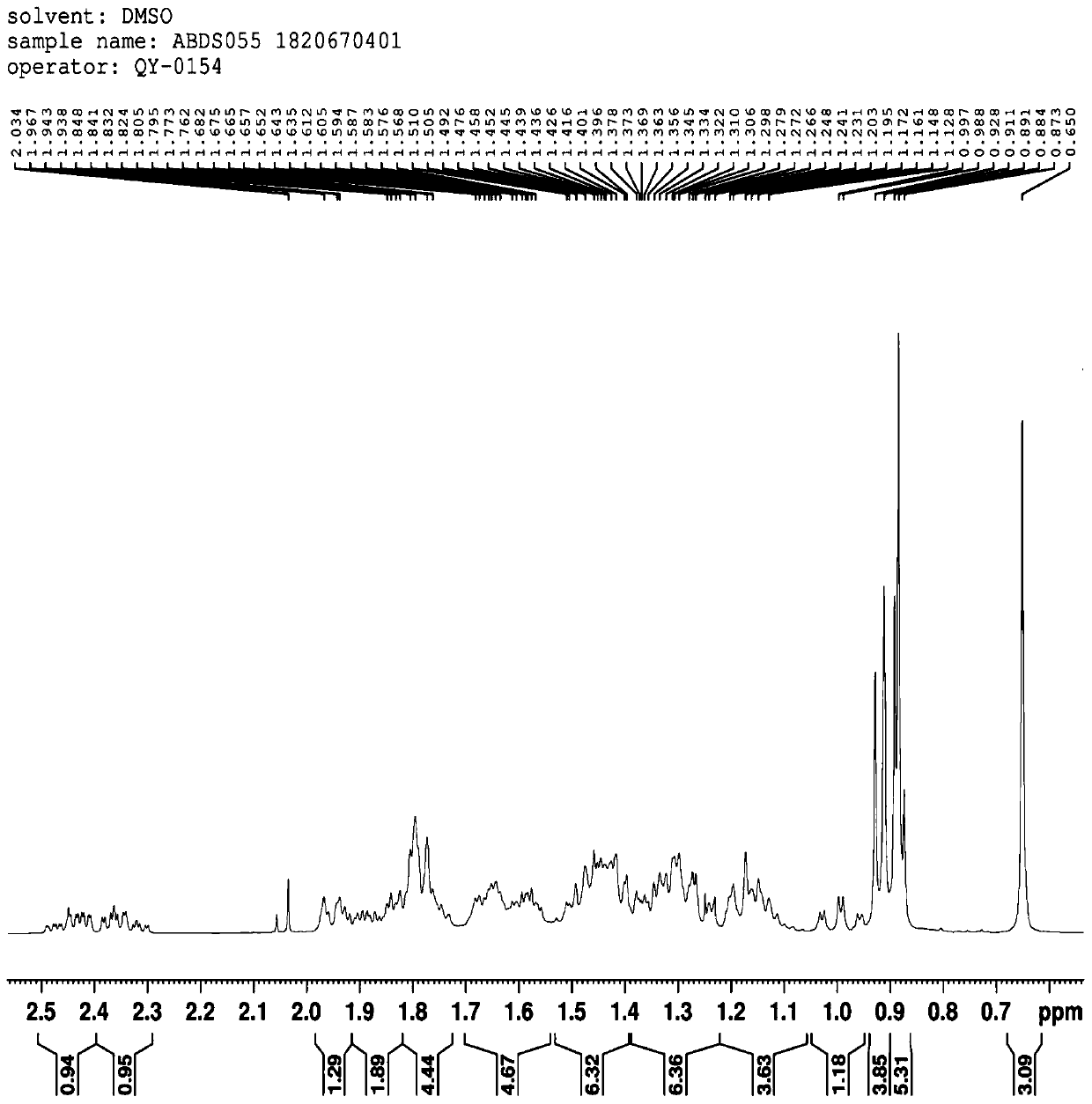
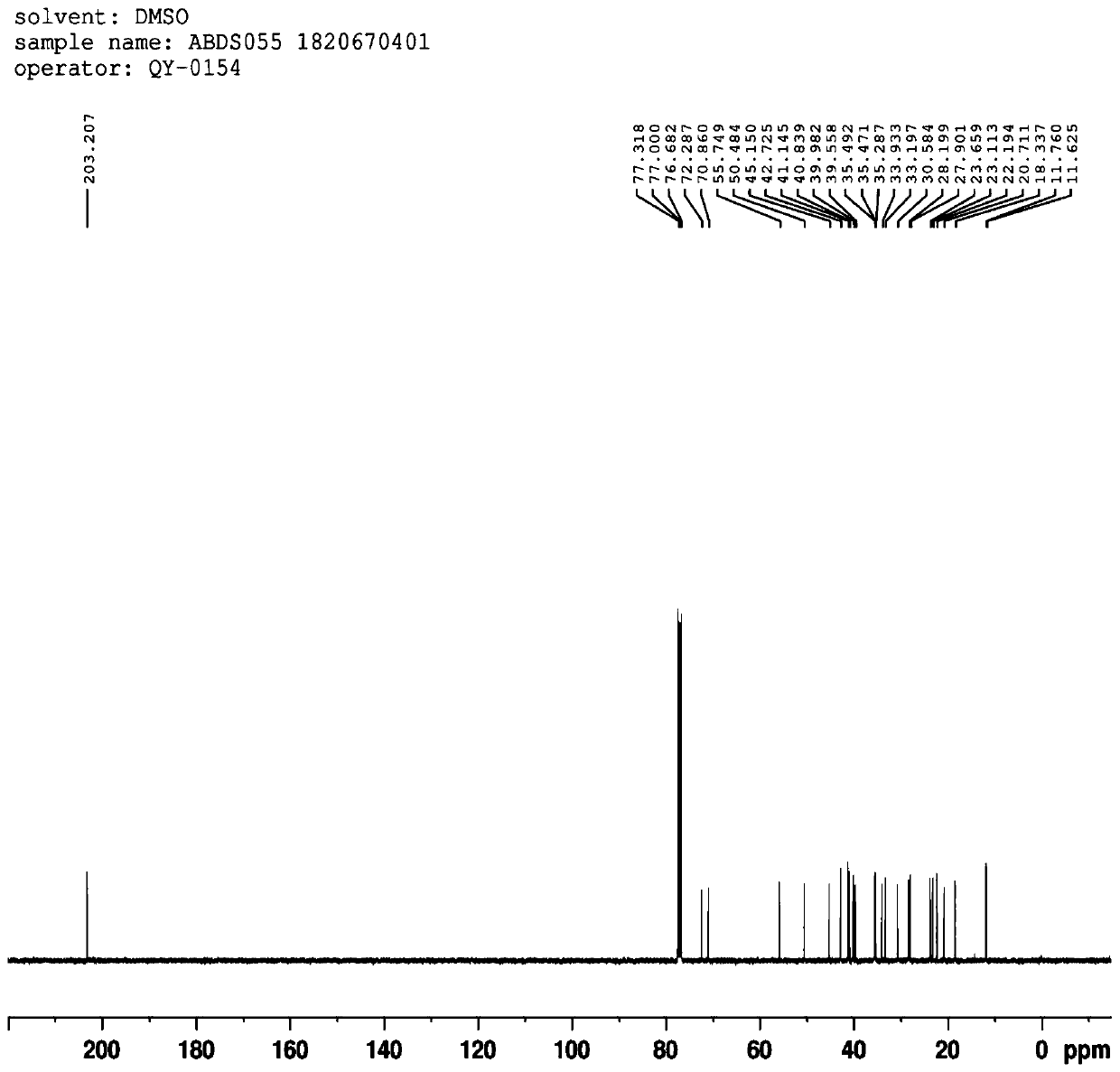
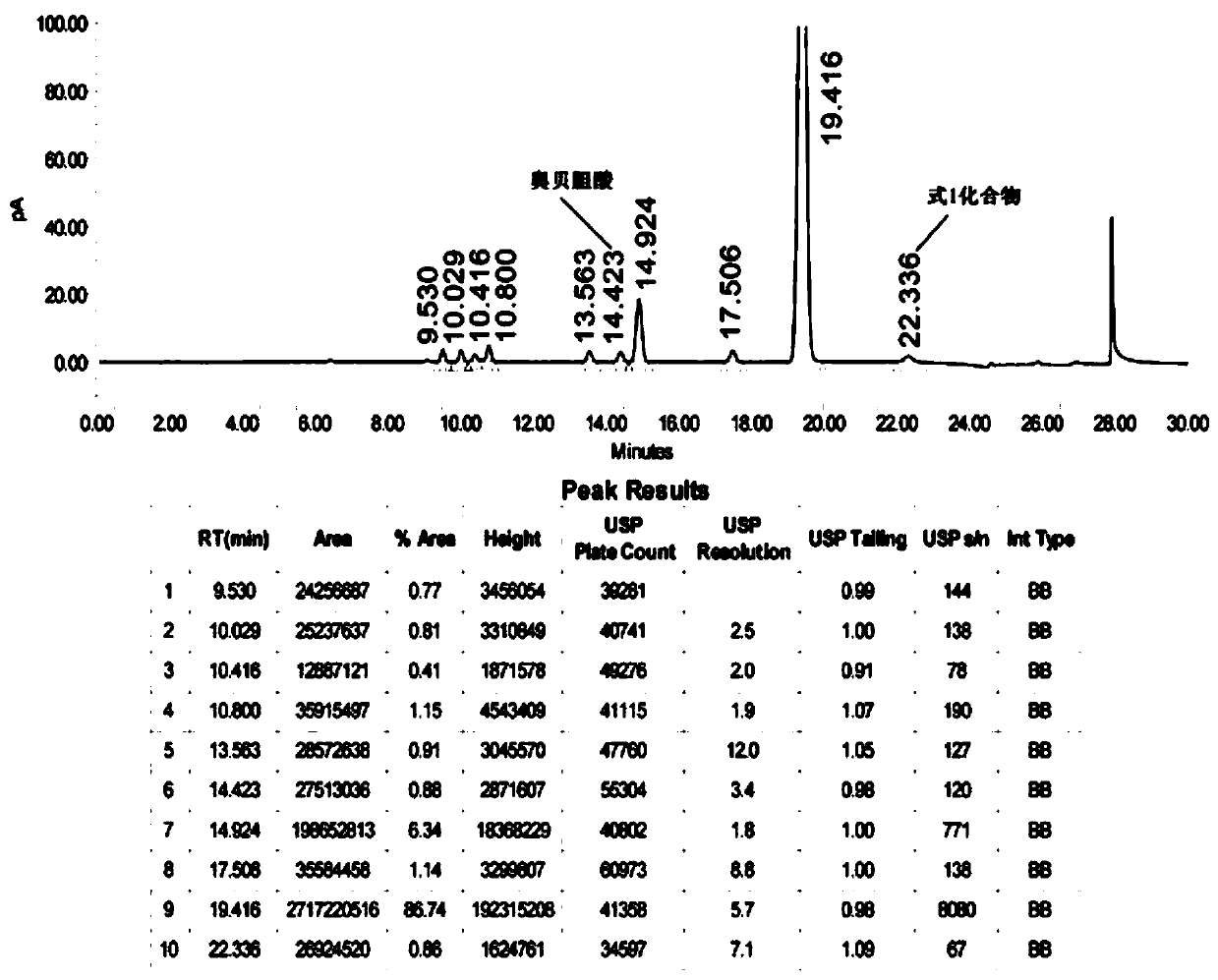
Preparation method and application of 3 alpha, 7 alpha-dihydroxy-6 alpha-ethyl-5 beta-cholane-24-aldehyde
A dihydroxy and ethyl technology, applied in the field of medicinal chemistry, can solve the problems of low content found, unfavorable for purification, etc., and achieve the effects of high yield and content, and simple and convenient preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of formula II compound
[0039] Add 100.0g of the compound of formula III, 29.3g of dimethylhydroxylamine hydrochloride into the reaction flask, add 2000ml of dichloromethane, then add 60.7g of triethylamine, 42.2g of 1-hydroxybenzotriazole and 59.8g of 1- (3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, after adding the reaction at room temperature, after HPLC confirms that the remaining raw materials are less than 2.0%, then water, sodium bicarbonate solution, saturated sodium chloride solution After washing and concentration under reduced pressure, 108.2 g of a light yellow solid compound of formula II was obtained.
Embodiment 2
[0040] Embodiment 2: the preparation of formula II compound
[0041] Add 100.0g of the compound of formula III, 29.3g of dimethylhydroxylamine hydrochloride into the reaction flask, add 2000ml of methyl tetrahydrofuran, then add 77.6g of diisopropylethylamine, 42.5g of 1-hydroxy-7-azabenzene Triazole and 59.8g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride were added and reacted at room temperature. After HPLC confirmed that the remaining raw materials were less than 2.0%, water, carbonic acid Wash with sodium hydrogen solution and saturated sodium chloride solution, and concentrate under reduced pressure to obtain 107.6 g of the compound of formula II as a white solid compound.
Embodiment 3
[0042] Embodiment 3: the preparation of formula II compound
[0043]Add 100.0g of the compound of formula III, 29.3g of dimethylhydroxylamine hydrochloride into the reaction flask, add 2000ml of ethyl acetate, then add 86.87g of diisopropylethylamine, 40.8g of 1-hydroxybenzotriazole and 37.8 g diisopropylcarbodiimide, after adding the reaction at room temperature, after HPLC confirms that the remaining raw material is less than 2.0%, wash with water, sodium bicarbonate solution, saturated sodium chloride solution successively, and concentrate under reduced pressure to obtain the compound of formula II as a white solid Compound 104.4g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com