Preparation method of chenodeoxycholic acid derivatives
A technology for compound and hydrochloride, applied in the field of preparation and purification of chenodeoxycholate, can solve the problems of difficult removal of impurities in final products, difficult separation of intermediates, and needs for intermediates and products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0109] Compared with the prior art, the preparation method of the present invention has a series of advantages. Its main advantages include:
[0110] (1) The intermediates obtained by the amide condensation of the present invention are all in solid form, easy to purify, pack and store;
[0111] (2) Compared with the disclosed preparation technology, the route of the present invention is shorter;
[0112] (3) Compared with the disclosed preparation technology, the present invention adopts a "one-pot" process, which has stronger production continuity and is more suitable for large-scale production.
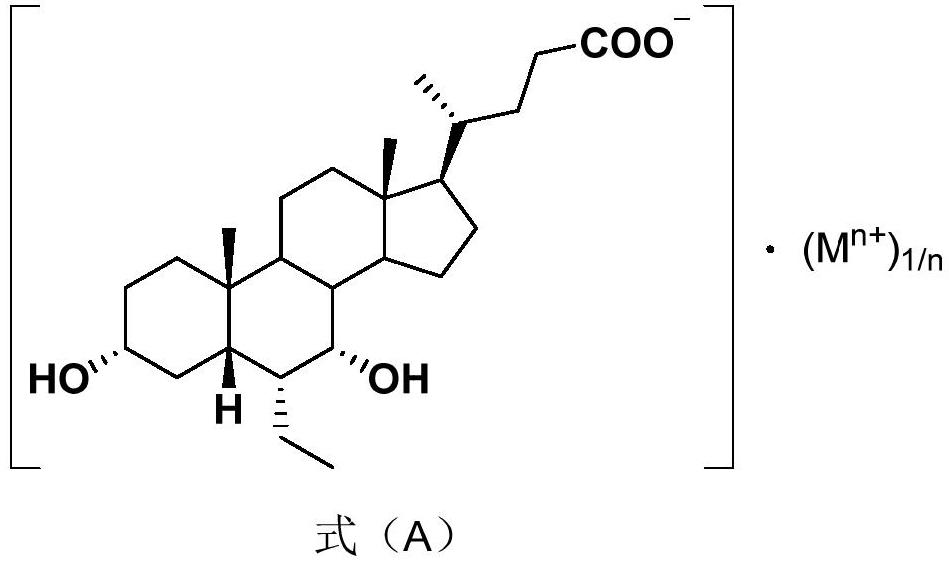
[0113] (4) Compared with the disclosed preparation technology, the corresponding bile salt obtained in the present invention has higher purity and better product quality, especially the magnesium salt, calcium salt, sodium salt and potassium salt of obeticholic acid prepared by the method of the present application Less impurities, suitable for pharmaceutical production.
Embodiment 1
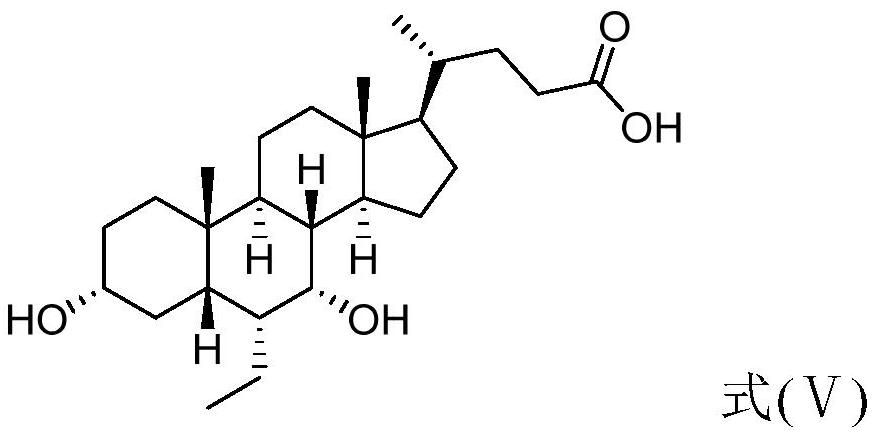
[0115] Example 1 Preparation of 3α, 7α-dihydroxy-6α-ethyl-5β-chole-24-acid (compound 1, compound of formula (Ⅴ))
[0116]
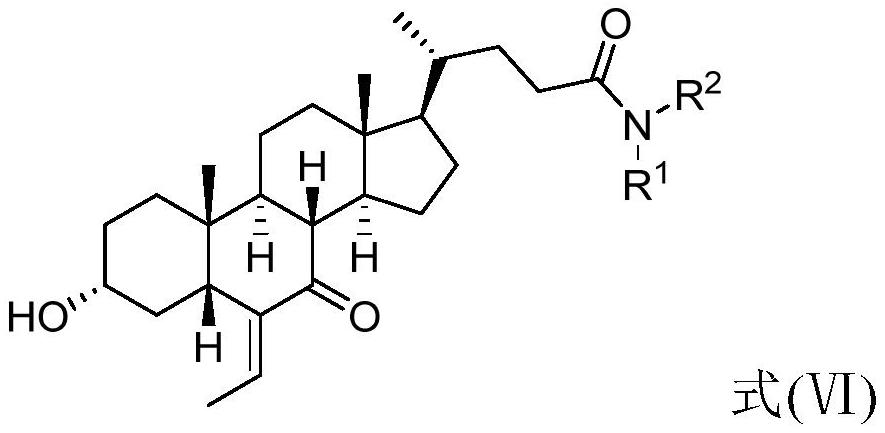
[0117] 1. Preparation of 3α-hydroxy-6-ethylene-7-ketone-5β-chol-24-amide (compound 2)
[0118] Add 3α-hydroxyl-6-ethylene-7-keto-5β-chole-24-acid (formula (VII), 60.0g, 0.144mol), PyBOP (90.0g, 0.173mol) and N successively in the flask, N-Dimethylformamide (400ml); stirred under ice bath, the temperature was lowered to 0°C, DIPEA (74.4g, 0.576mol) was added, and the temperature was controlled at 0°C to continue stirring for 30min. Under nitrogen protection, ammonium chloride (12.3 g, 0.230 mol) and N,N-dimethylformamide (100 ml) were added. Warm to room temperature and stir overnight. With stirring, the reaction mixture was slowly poured into 4L of 5% aqueous sodium bicarbonate solution, solids were precipitated, stirred for 2 h, filtered, washed with pure water (500 ml), and dried in vacuo to obtain a crude product. Add ethyl acetate (360ml) to the...
Embodiment 2
[0121] Example 2 Preparation of 3α, 7α-dihydroxy-6α-ethyl-5β-chole-24-acid (compound 1)
[0122]
[0123] 1. Preparation of 3α-hydroxy-6-ethylene-7-ketone-5β-chol-24-(N-methyl-N-methoxy)amide (compound 3)
[0124] Add 3α-hydroxyl-6-ethylene-7-keto-5β-chole-24-acid (formula (VII), 60.0g, 0.144mol), PyBOP (90.0g, 0.173mol) and N successively in the flask, N-Dimethylformamide (400ml); stirred under ice bath, the temperature was lowered to 0°C, DIPEA (74.4g, 0.576mol) was added, and the temperature was controlled at 0°C to continue stirring for 30min. Under nitrogen protection, N,O-dimethylhydroxylamine hydrochloride (128.4g, 0.288mol) and N,N-dimethylformamide (100ml) were added. Warm to room temperature and stir overnight. With stirring, the reaction mixture was slowly poured into water (4L), extracted with ethyl acetate (1L×3), and the organic phase was then mixed with saturated sodium bicarbonate solution (2L×3), water (2L) and saline (2L ), dried over anhydrous sodium s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com