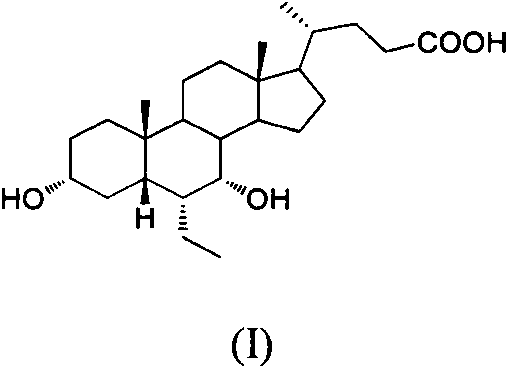
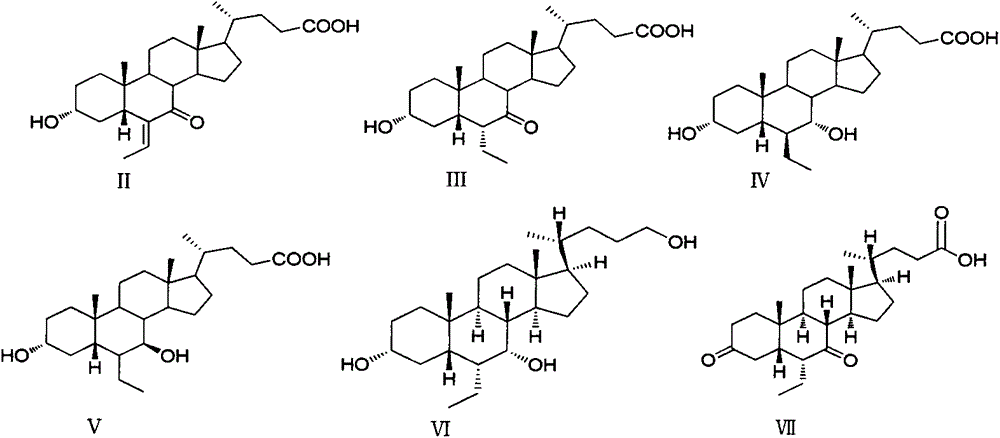
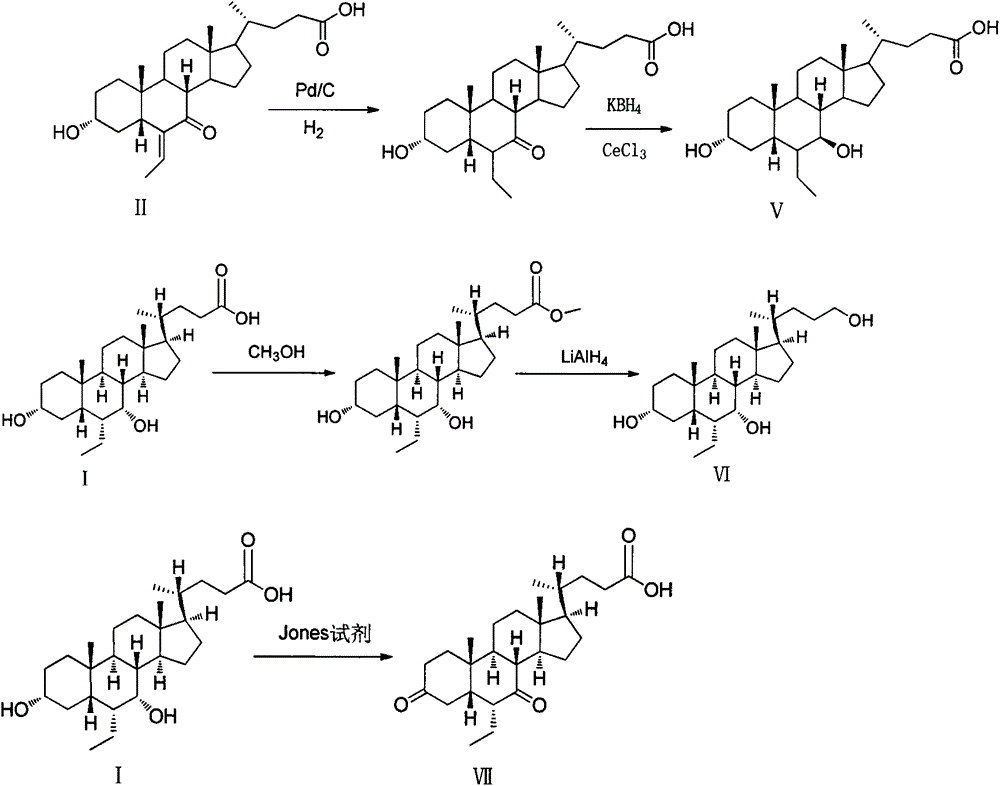
Preparation method of impurities of obeticholic acid
A technology of obeticholic acid and impurities, which is applied in the field of preparation of obeticholic acid impurities, and can solve the problems of few reports on the synthesis of obeticholic acid impurities, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation of 3α, 7β-dihydroxy-6-ethyl-5β-cholan-24-acid (V)
[0038]Put 2.0g of 3α-hydroxyl-6-ethylene-7-keto-5β-cholan-24-acid in a 100ml single-necked bottle, add 50ml of methanol-30% sodium hydroxide aqueous solution (2:1) mixed solution, and stir Dissolve, add 0.2g 5% βd / C, pass H 2 , stir the reaction at 25°C, absorb hydrogen under normal pressure until the reaction is complete, filter off palladium carbon, adjust the filtrate to acidity with phosphoric acid, and when concentrated to a white solid, pour it into 20ml of ice water, let stand to precipitate the solid, suction filter, and dry , 1.25 g of white solid was obtained.
[0039] Under the protection of nitrogen, the above white solid 0.95g, CeCl 3 0.85g, dissolve with 10ml methanol tetrahydrofuran mixed solution (v / v=1 / 1), cool down to -25°C after complete dissolution, then quickly add potassium borohydride, monitor the reaction by TLC until the raw materials disappear, add 10% hydrochloric aci...
Embodiment 23
[0043] Example 23α, the preparation of 7α-dihydroxy-6α-ethyl-5β-cholan-24-ol (VI)
[0044] Put 2.0g of obeticholic acid in a 25ml reaction bottle, add 10ml of methanol and stir to dissolve, add 0.1ml of concentrated hydrochloric acid, stir at room temperature (25-30°C) for 24h, monitor by TLC until the reaction of the raw materials is complete, adjust with 2M sodium hydroxide solution pH is about 7, concentrated under reduced pressure, the residue is dissolved in dichloromethane, separated, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate is concentrated to dryness under reduced pressure to obtain 1.8 g of methyl obeticholic acid;
[0045] -10°C, add 20ml dry tetrahydrofuran, 0.35g lithium aluminum hydride, nitrogen protection, keep at -10°C into the reaction flask, slowly add a solution of methyl obeticholic acid (1.8g) in tetrahydrofuran (10ml) dropwise, After dropping, rise to room temperature and react for 10h. Slowly add 1ml of...
Embodiment 33
[0049] Example 33, Preparation of 7-diketone-6α-ethyl-5β-cholan-24-acid (VII)
[0050] Put 3.75g of obeticholic acid in a 250ml single-necked bottle, add 75ml of acetone at room temperature, stir to dissolve, under nitrogen protection, under ice-water bath, slowly add 7ml of Jones reagent dropwise, after dropping, slowly rise to room temperature, and continue stirring for 2h. Slowly add 30ml of isopropanol to quench, filter, concentrate the filtrate, add 40ml of ethyl acetate to the residue, wash with saturated sodium bicarbonate, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and the concentrate is separated by column chromatography to obtain Off-white solid 1.52g.
[0051] Its structural identification data are as follows:
[0052] 1 H-NMR (300Mz, CDCl 3 )δ: 0.69(S, 3H), 0.80(t, J=7.3Hz, 3H), 0.94(d, J=6.3Hz, 3H), 1.48-1.77(m, 6H), 2.3-2.5(m, 2H ), 2.73 (m, 1H).
[0053] ESI-MS(m / z): 439.3[M+Na] + , 415.3[M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com