Method for determining content of related components of four bear gall powder in cooling-based phlegm eliminating injection
A measurement method and injection technology, applied in the field of chemistry, can solve the problems of low precision and poor reproducibility, and achieve the effects of high precision, stable results and remarkable curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
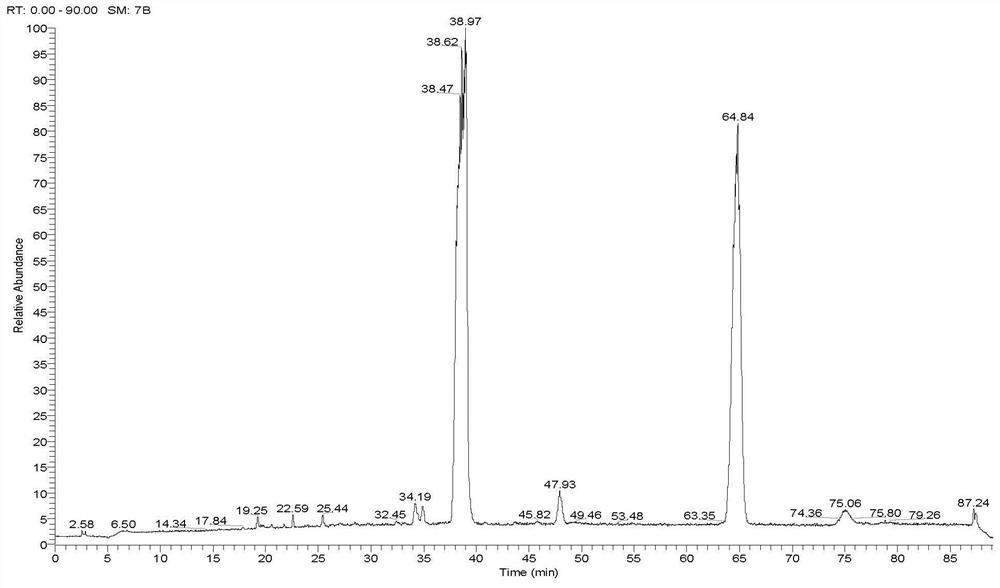
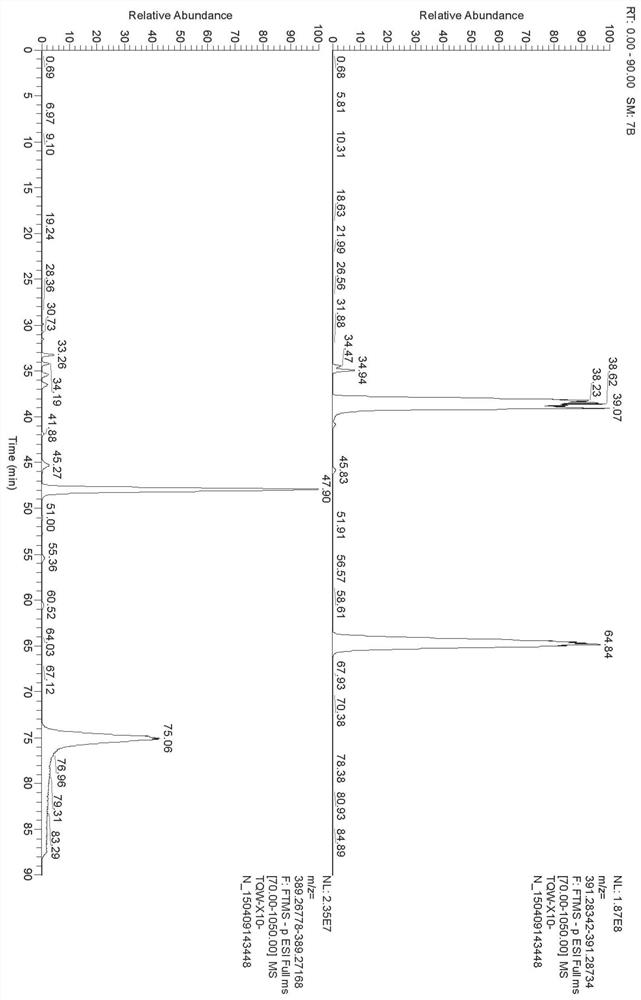
[0071]1. Select instrument: Waters Acquity high performance liquid chromatograph, Thermo Q-Exactive mass spectrometer;
[0072]2. Set chromatographic conditions: Chromatographic column: Pheomenex Luna C18 (5μm, 0.46 × 25cm); mobile phase: acetonitrile as mobile phase A, water as mobile phase B, gradient elution according to Table 2; column temperature: 30℃ ; Flow rate: 1.0ml / min; Injection volume: 1μl;
[0073]3. Set gradient elution conditions: 0~2min, volume percentage 10% mobile phase B, 2~3min, volume percentage 10-20% mobile phase B, 3~25min, volume percentage 20% – 40% mobile phase B, 25~55min, volume percentage concentration 40-42% mobile phase B;
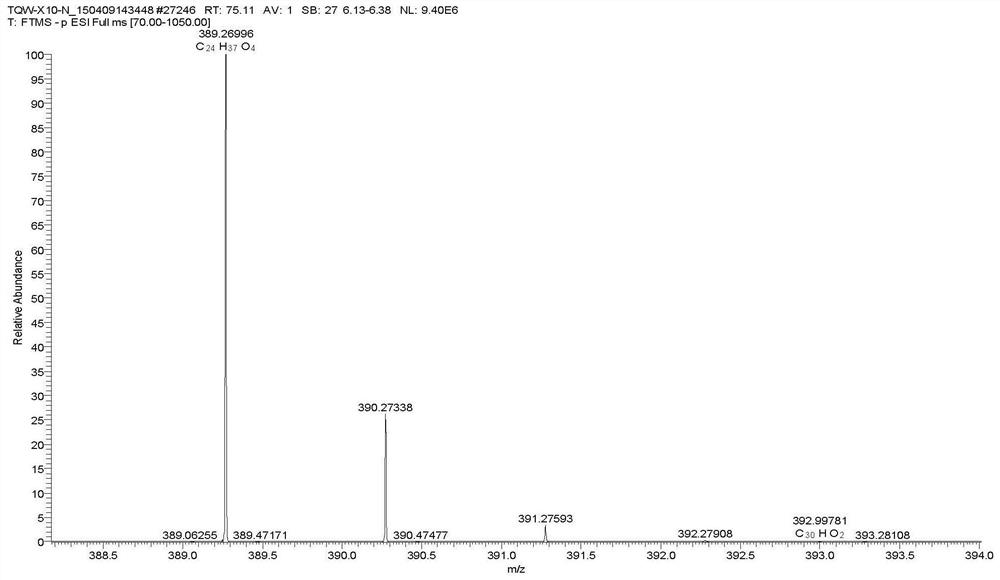
[0074]4. Set mass spectrometry conditions: acquisition mode: negative mode; scanning range m / z: 70~1050. Ion source parameters: ion source temperature 325℃; capillary voltage 3500V; sheath gas is 40arb, auxiliary gas is 10arb; resolution is 70000;
[0075]5. According to the above method, search with precise molecular weight and compare the r...
Embodiment 2
[0091]Determination of 4 components in bear bile powder extract
[0092]1. Select instruments and reagents: Agilent 1260 high performance liquid chromatograph, ursodeoxycholic acid reference substance and chenodeoxycholic acid reference substance from China Pharmaceutical and Biological Products Inspection Institute, 3α-hydroxy-7-oxo-5β- Cholanic acid (TRC, 1-ALB-5-1), 7α-hydroxy-3-oxo-5 β-cholanoic acid (UHN, 4185-00-6), acetonitrile and methanol are chromatographically pure, bear bile powder Extracts and other reagents are of analytical grade;
[0093]2. Setting conditions: Chromatographic column: Pheomenex luna omega C18 (5μm, 0.46 × 25cm), mobile phase: acetonitrile as mobile phase A and 0.1% phosphoric acid as mobile phase B, column temperature: 30℃, flow rate: 1.0ml / min, injection volume: 10μl, with 203nm as the UV absorption detection wavelength;
[0094]3. Set gradient elution conditions: 0~10min, 90-45% volume percentage mobile phase B, 10~35min, volume percentage 45% mobile phase ...
Embodiment 3
[0104]Determination of 4 kinds of bear bile powder related components in Tanreqing injection, chromatographic conditions are the same as in Example 2
[0105]1. Take a blank sample (lack of bear bile powder) and a sample, prepare a blank sample solution and a test solution in parallel according to the preliminarily developed method, and analyze the samples. The result is that the test product chromatogram contains a reference substance with ursodeoxycholic acid. , 3α-hydroxy-7-oxo-5β-cholanic acid reference substance, chenodeoxycholic acid reference substance and 7α-hydroxy-3-oxo-5β-cholanic acid reference substance have the same retention time chromatographic peaks, and Without interference from other components, the blank sample (ursodeoxycholic acid powder) is in ursodeoxycholic acid reference substance, 3α-hydroxy-7-oxo-5β-cholanoic acid reference substance, chenodeoxycholic acid reference substance and 7α-hydroxy There is no chromatographic peak at the retention time of the refere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com