A kind of preparation method of ursodeoxycholic acid and its preparation enzyme 2
A technology of ursodeoxycholic acid and cholan acid, applied in the field of β-steroid dehydrogenase, which can solve the problems of organic solvent residue, long reaction time, and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The specific implementation process of the preparation method of ursodeoxycholic acid provided by the invention is as follows:
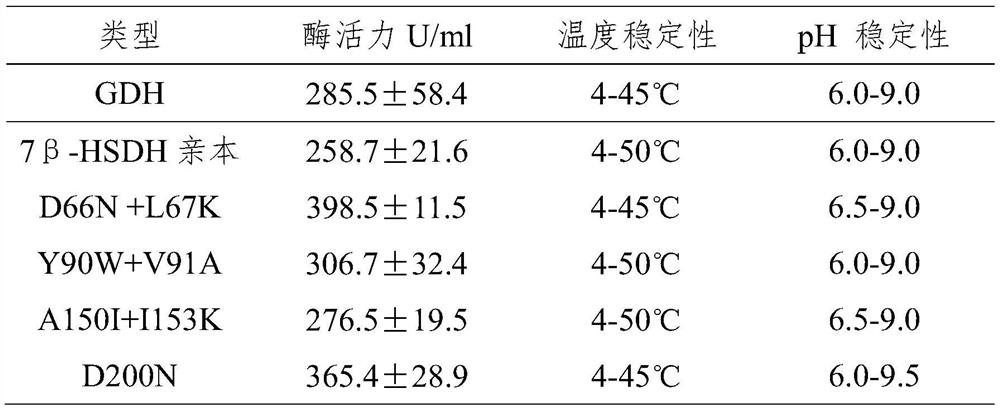
[0026] Suspend 3α-hydroxy-7oxo-5β-cholanic acid in 50-100mM potassium phosphate buffer (pH8.0), adjust the pH to 8.0 with 10M NaOH, and then add the final concentration of 30-50mg / mL Glucose and use 10M NaOH to adjust the pH to 7.8-8.0, add 7β-steroid dehydrogenase and glucose dehydrogenase, and finally add NADP with a final concentration of 0.01-0.25 mg / mL, and the final concentration of the substrate is 50-100 mg / mL. The reaction is carried out at a temperature of 25-35° C., 200-400 rpm and a pH of 7.5-8.5, and the reaction time is 8-15 hours. Take the reaction solution at regular intervals and dilute it 50-100 times with the mobile phase, and inject samples for liquid phase analysis after microporous filtration. For liquid phase detection, use Phenomenx Gemini 5μmNX-C18 110A 250×4.6mm as the analytical column, and the mobile phase is aceto...
Embodiment 1
[0029] Preparation of co-expression recombinant plasmid pET22b-BHSDH5-GDH containing parental gene
[0030]The 7β-steroid dehydrogenase gene BHSDH5 derived from Enterococcus silesiacus and the glucose dehydrogenase gene GDH derived from Bacillus megaterium were respectively used with the primer pair 5'CGCCATATGATGTATACAGATTTAAAAGA3' and 5'CCGGAATTCTTAGCCTCTTCCCGTTTGGA3' and the primer 5'CCGGAATTCAAGGAGATATACATATGATGTATACAGATTTAAAAGA3' and 5'CCGCTCGAGTTAGCTCTTCCCGTTTGGA3' were amplified by PCR to obtain the PCR product and then inserted into the Nde I and EcoR I sites, EcoR I site and Xho I site of the expression vector pET22b(+) point, the co-expression recombinant plasmid pET22b-BHSDH5-GDH was obtained. Through DNA sequencing, it is determined that the nucleotide sequence of the cloned parent 7β-steroid dehydrogenase is shown in SEQ ID NO: 1, and its amino acid sequence is shown in SEQ ID NO: 2; it is determined that the cloned parent glucose dehydrogenase The nucleotide seq...
Embodiment 2
[0032] Preparation of co-expression recombinant plasmids containing 7β-steroid dehydrogenase mutants
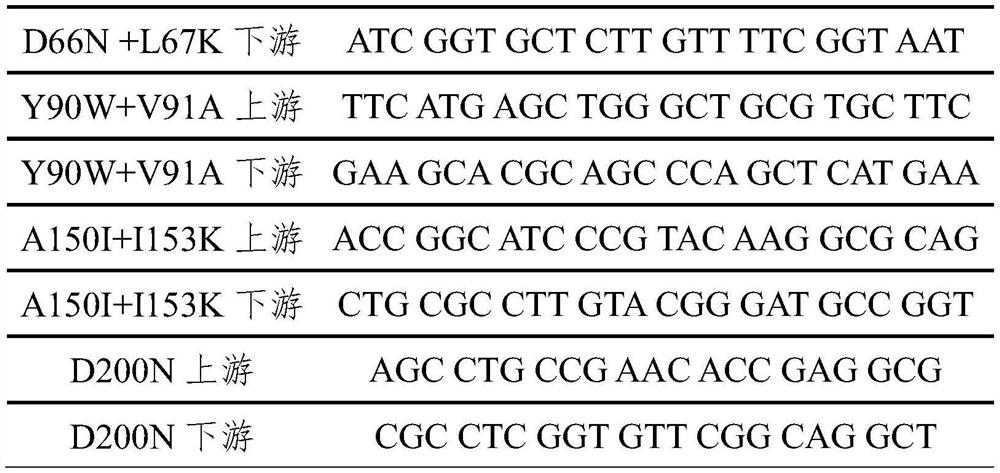
[0033] Perform site-directed mutation on the 7β-steroid dehydrogenase parent by reverse PCR technology, design reverse primers at the mutation position, use upstream and downstream mutation primers to amplify the target fragment, and introduce corresponding mutations on the primers to recombine plasmid pET22b-BHSDH5 -GDH was used as a template for inverse PCR, and the PCR product was transformed into Escherichia coli Rosetta (de3) after being digested with Dpn I enzyme, and colonies were picked and sent for sequencing after being screened by Amp. The mutation sites and primer design are shown in Table 1.
[0034] The PCR system is: TaKaRa EX Taq HS 0.25ul; 10×Ex Taq Buffer 5ul; template plasmid 1ul; dNTP (2.5mM each) 4ul; upstream primer 1ul; downstream primer 1ul; sterile water up to 50ul.
[0035] The PCR program is: first 98°C for 2min; then 98°C for 10s, 50-65°C for 30s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com