Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49results about How to "Minimize the risk of damage" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
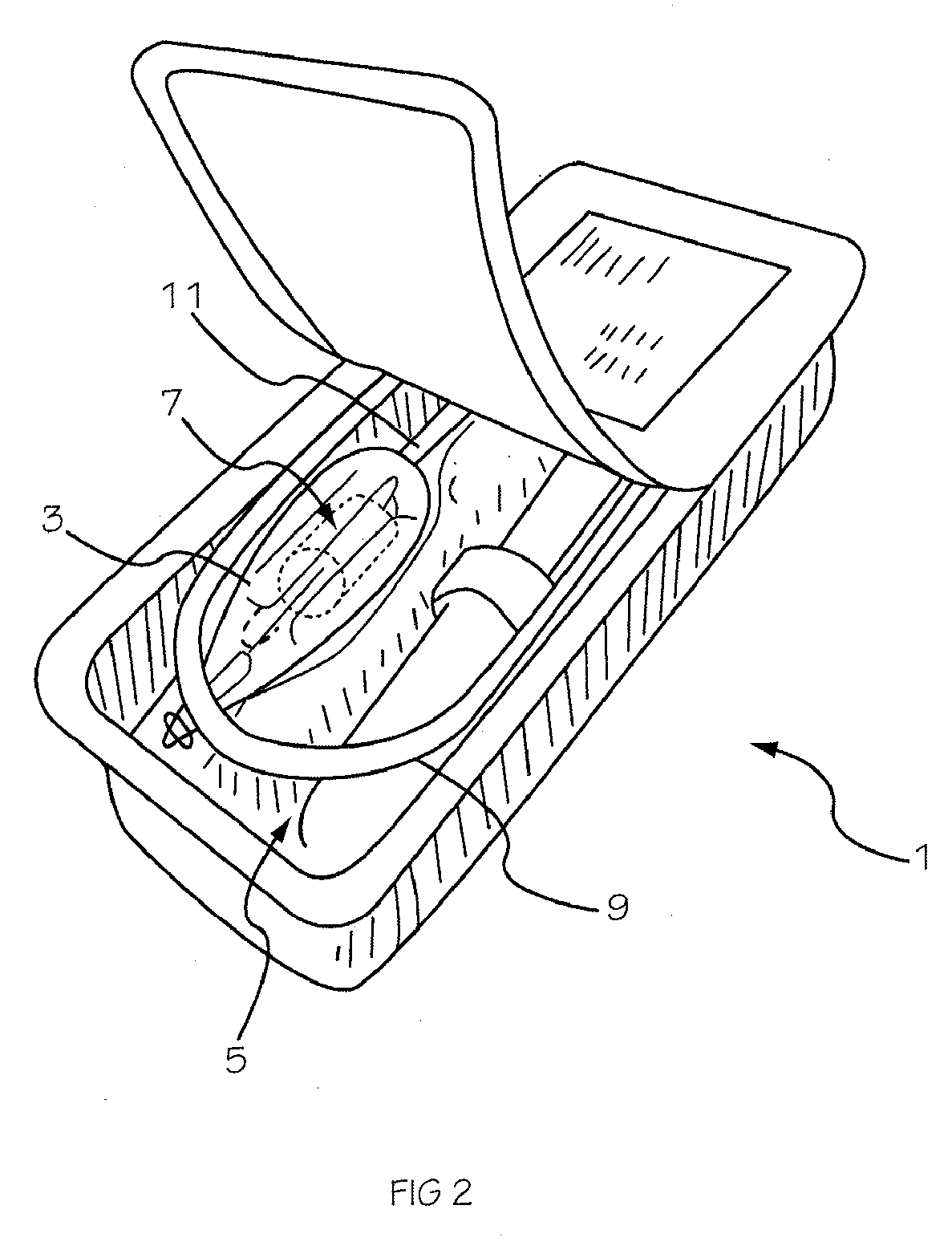
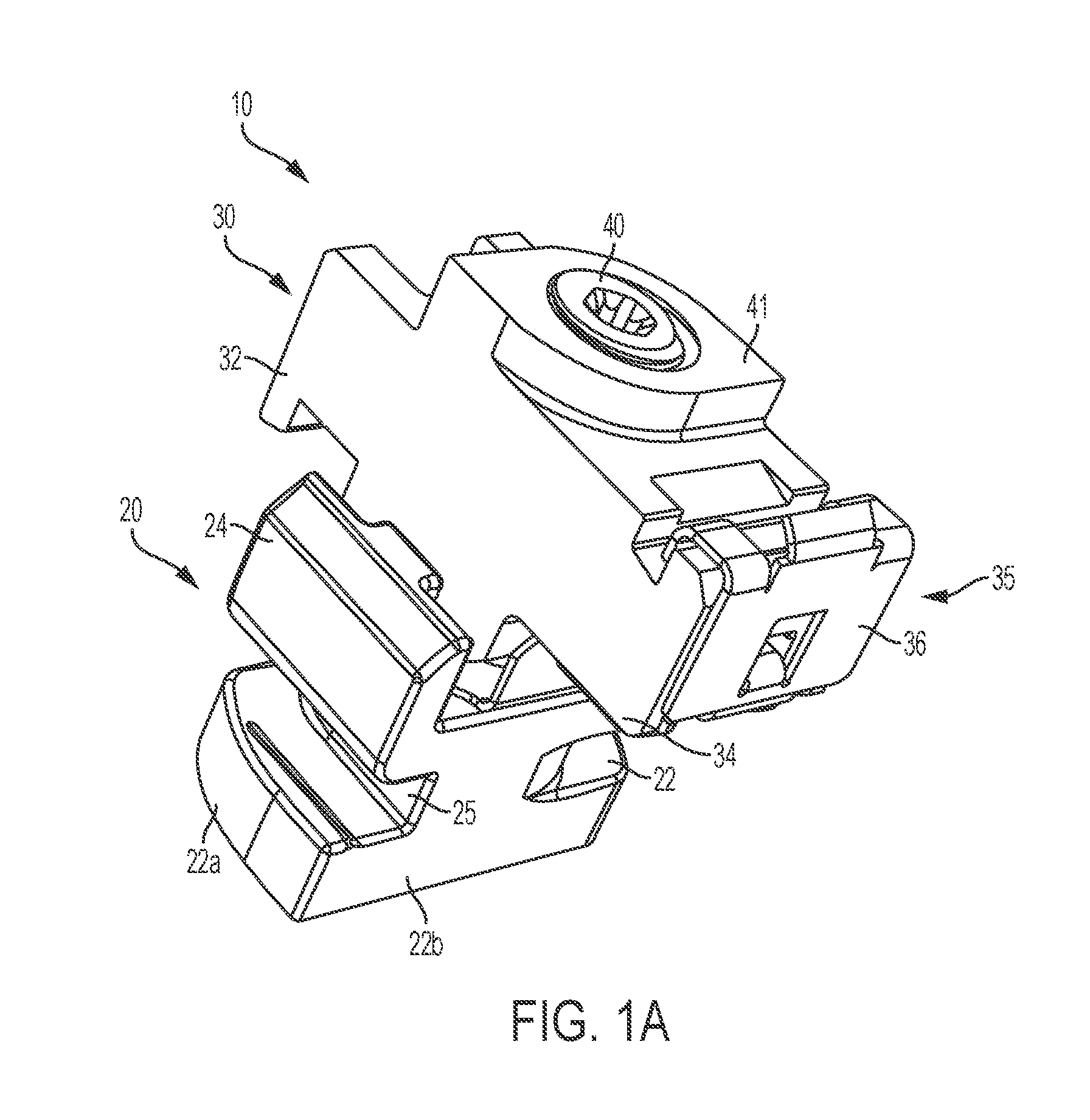
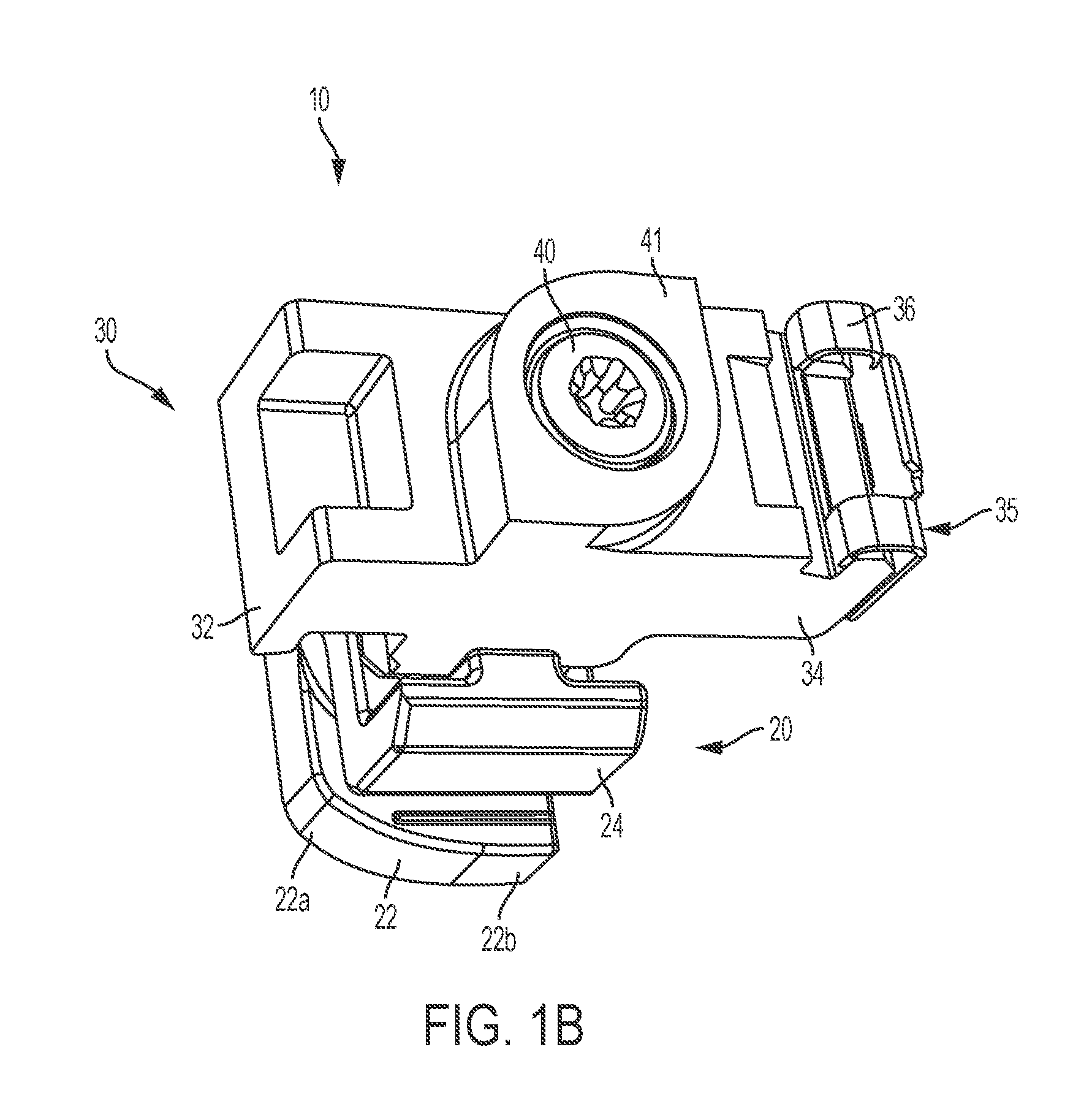
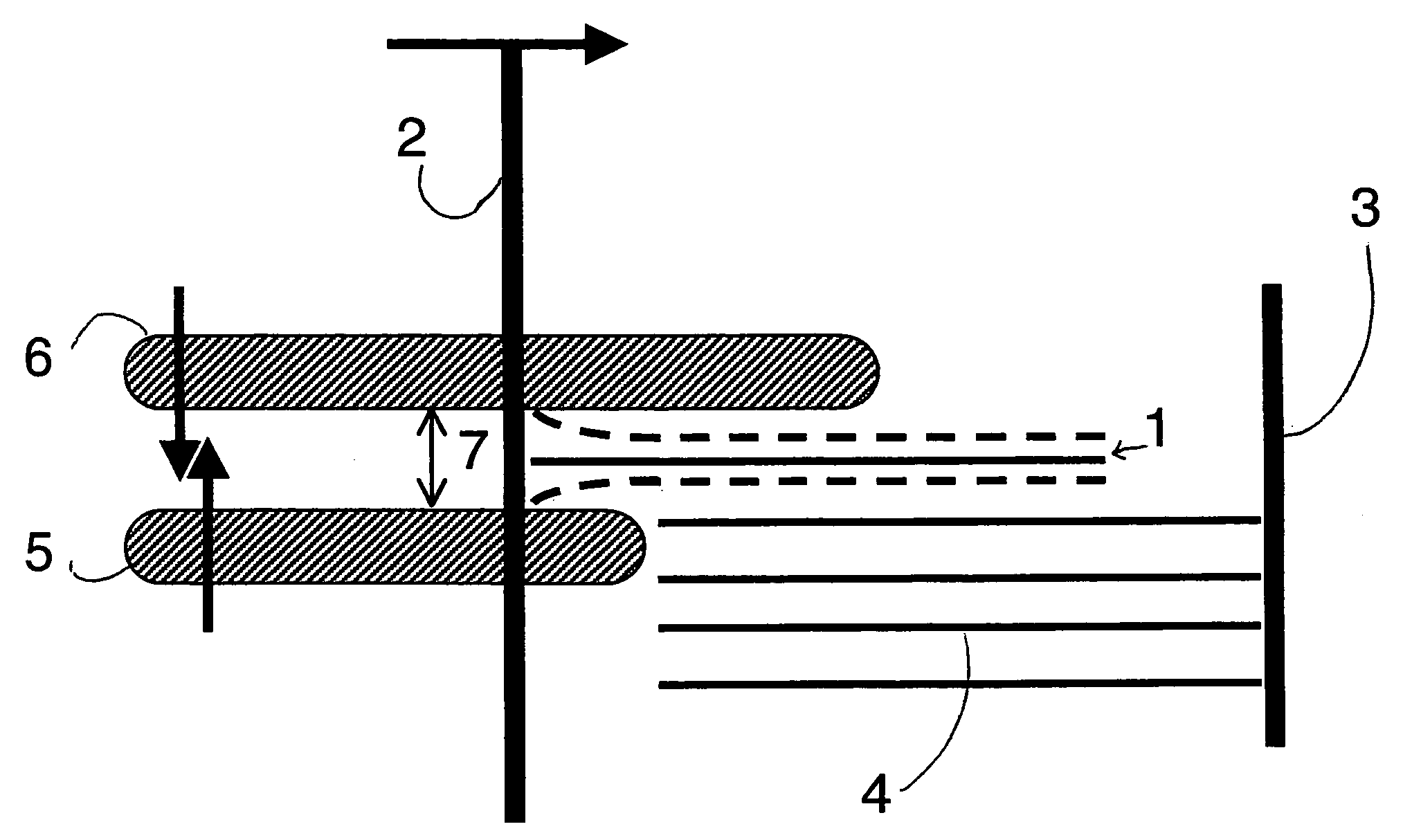
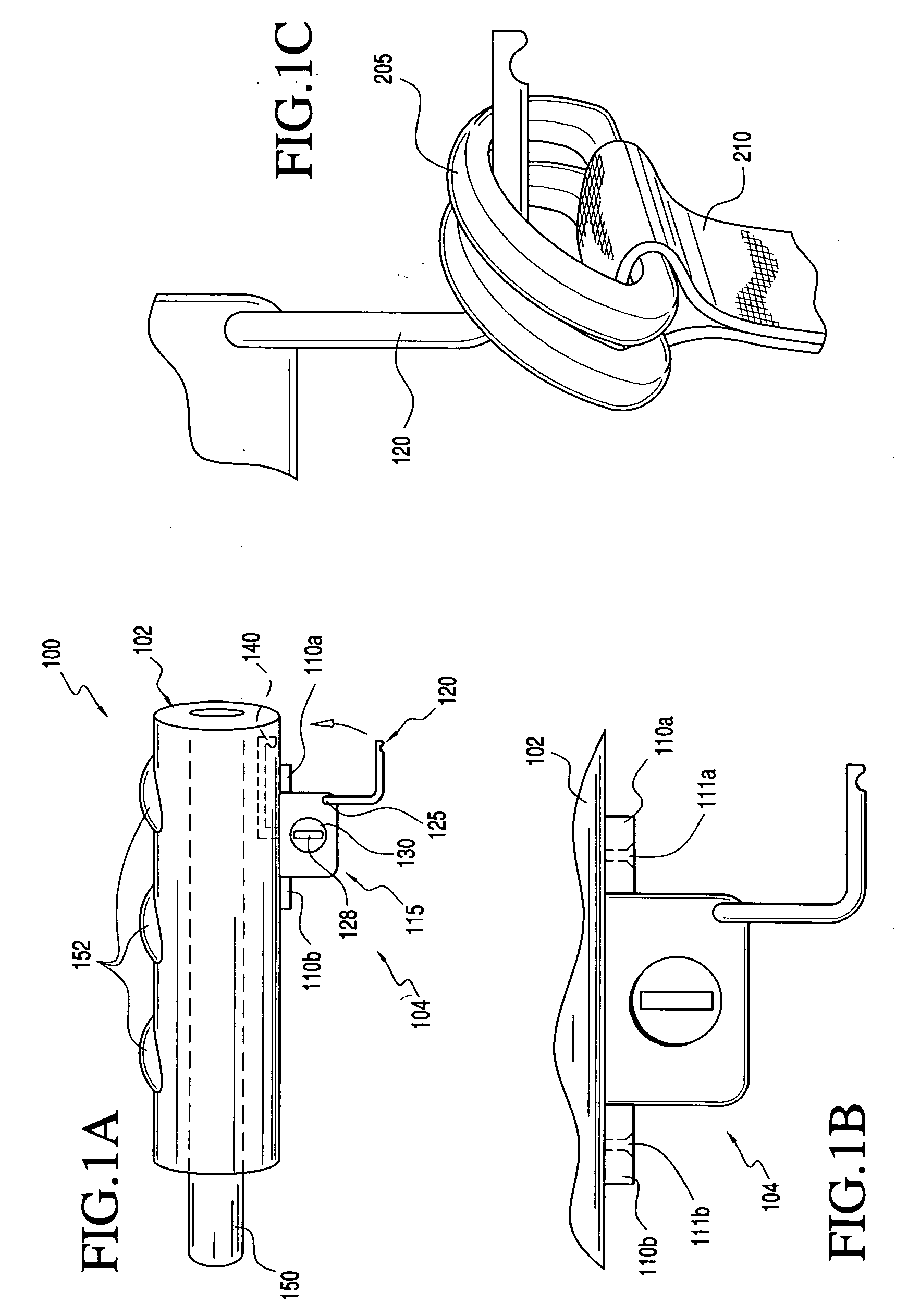
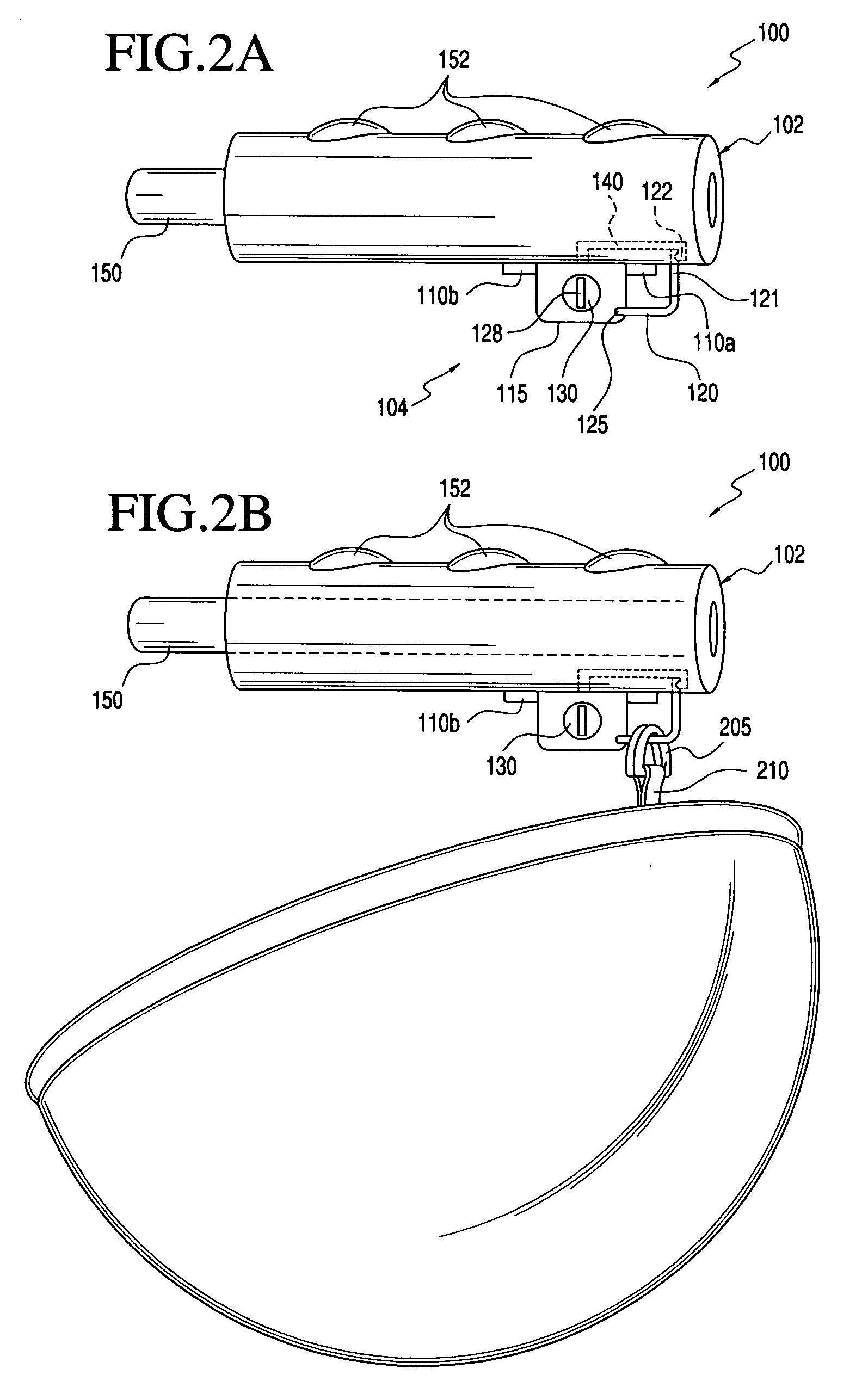
Two-Part Package For Medical Implant
ActiveUS20070061008A1Low costMinimize preparation timeDispensing apparatusHeart valvesBiomedical engineeringMedical treatment
The invention provides a two-part package and method of use for a pre-attached medical implant and delivery tool system. The package includes a wet compartment and a dry compartment and allows a pre-attached implant and delivery tool system to be at least partially stored immersed in a fluid in the wet compartment and at least partially stored in the dry compartment. In one embodiment the implant comprises a replacement heart valve, and the heart valve is stored inside the wet compartment while the heart valve delivery tool remains dry in the dry compartment.
Owner:BOSTON SCI SCIMED INC
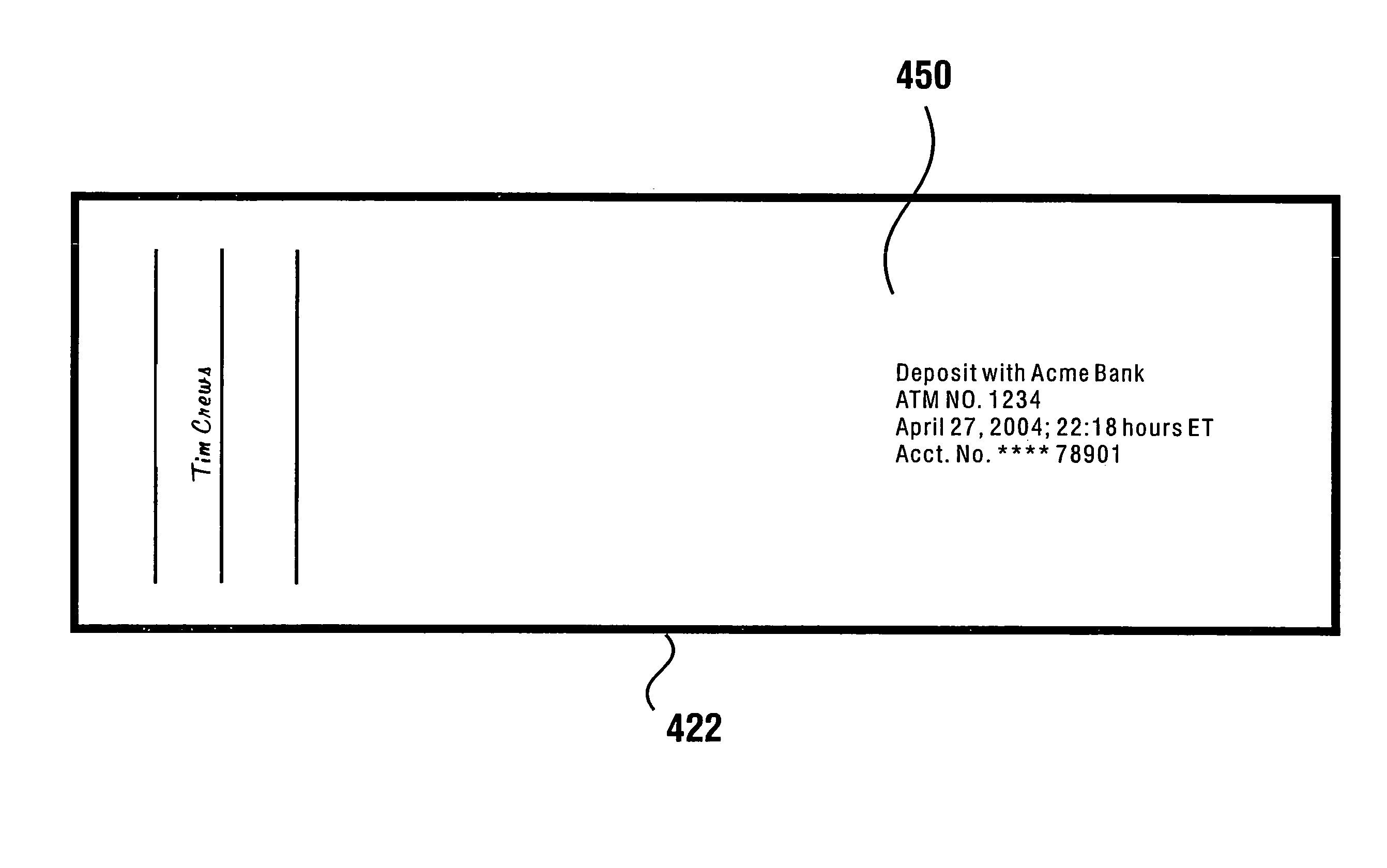
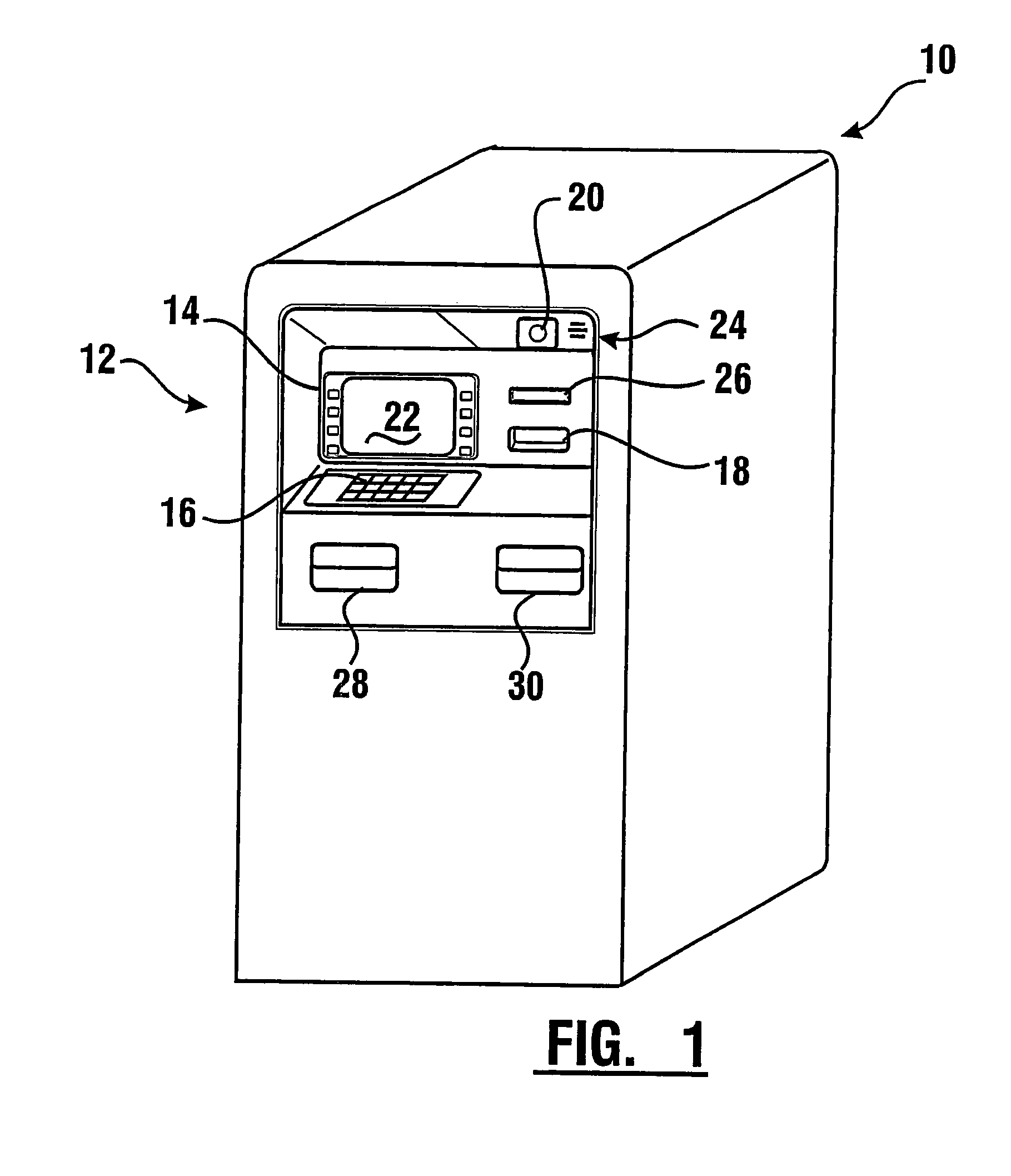
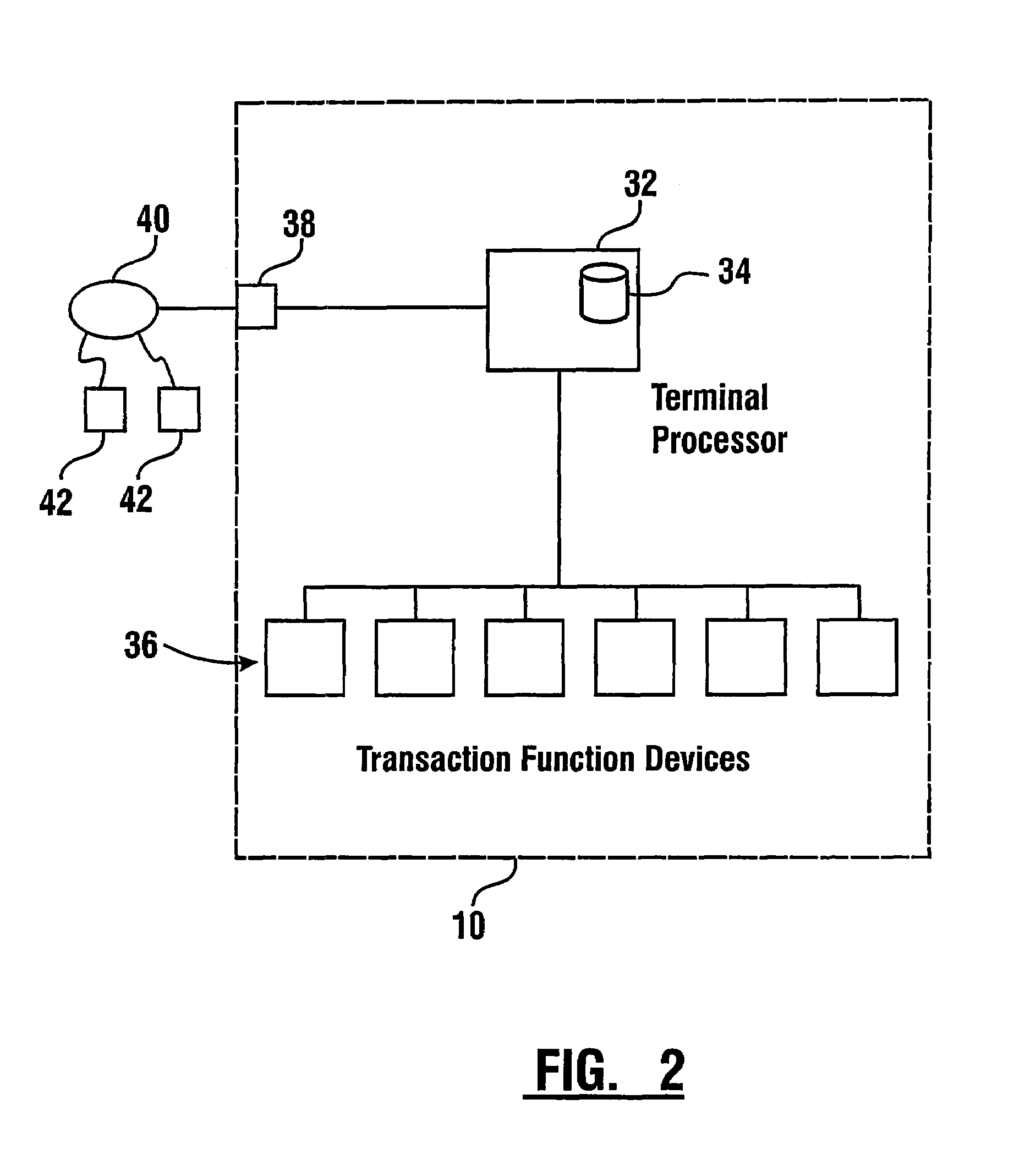
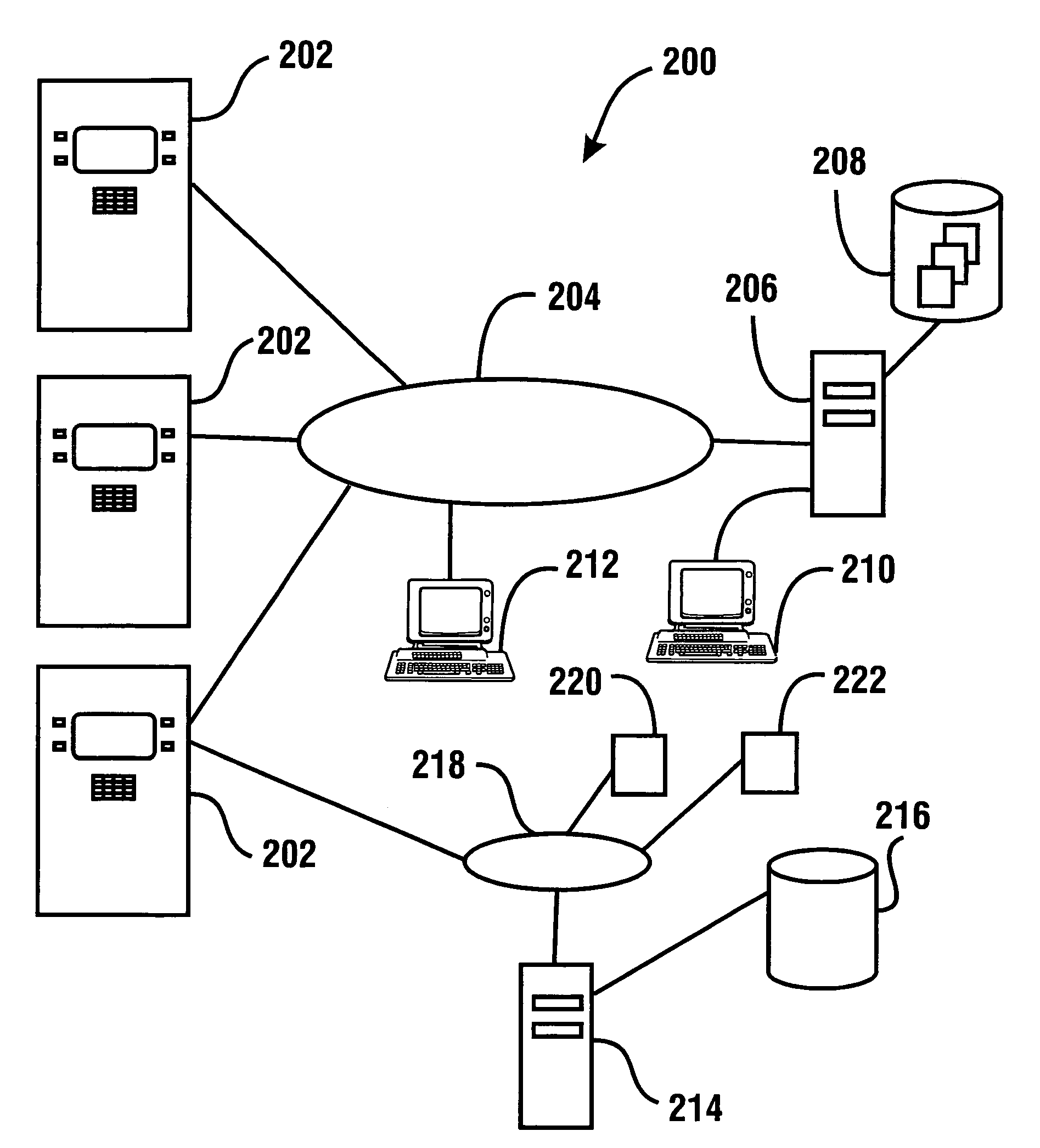
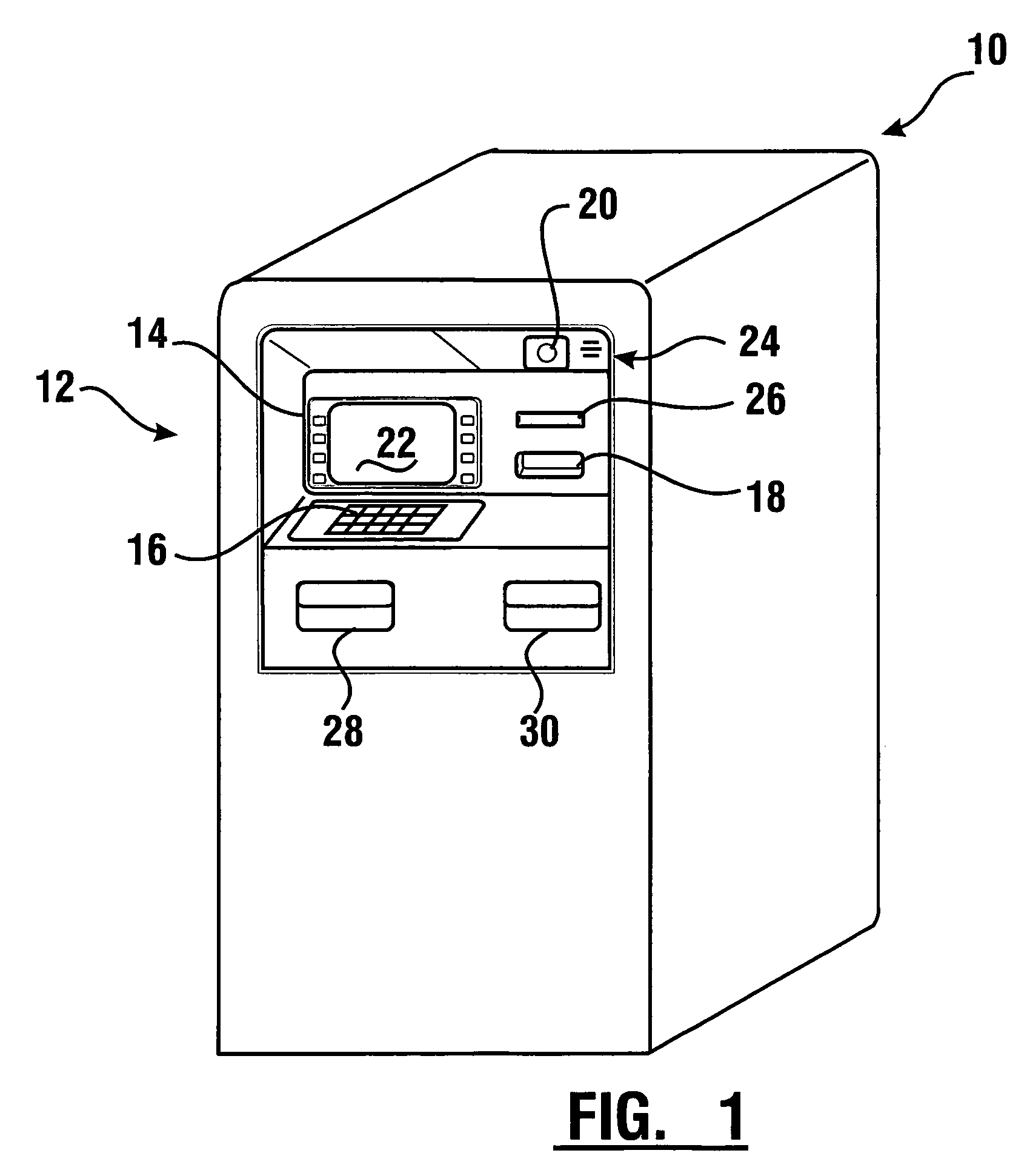
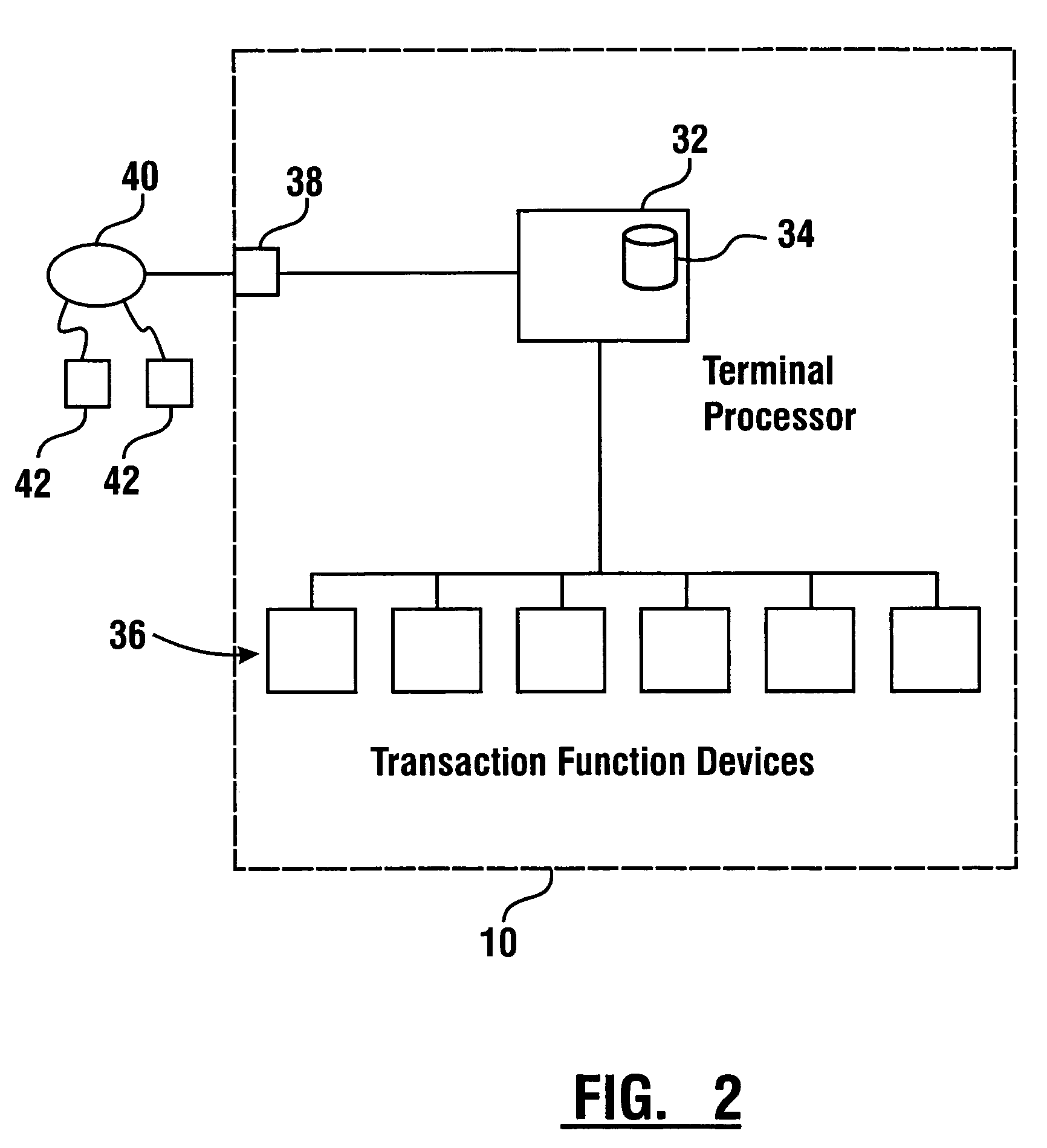
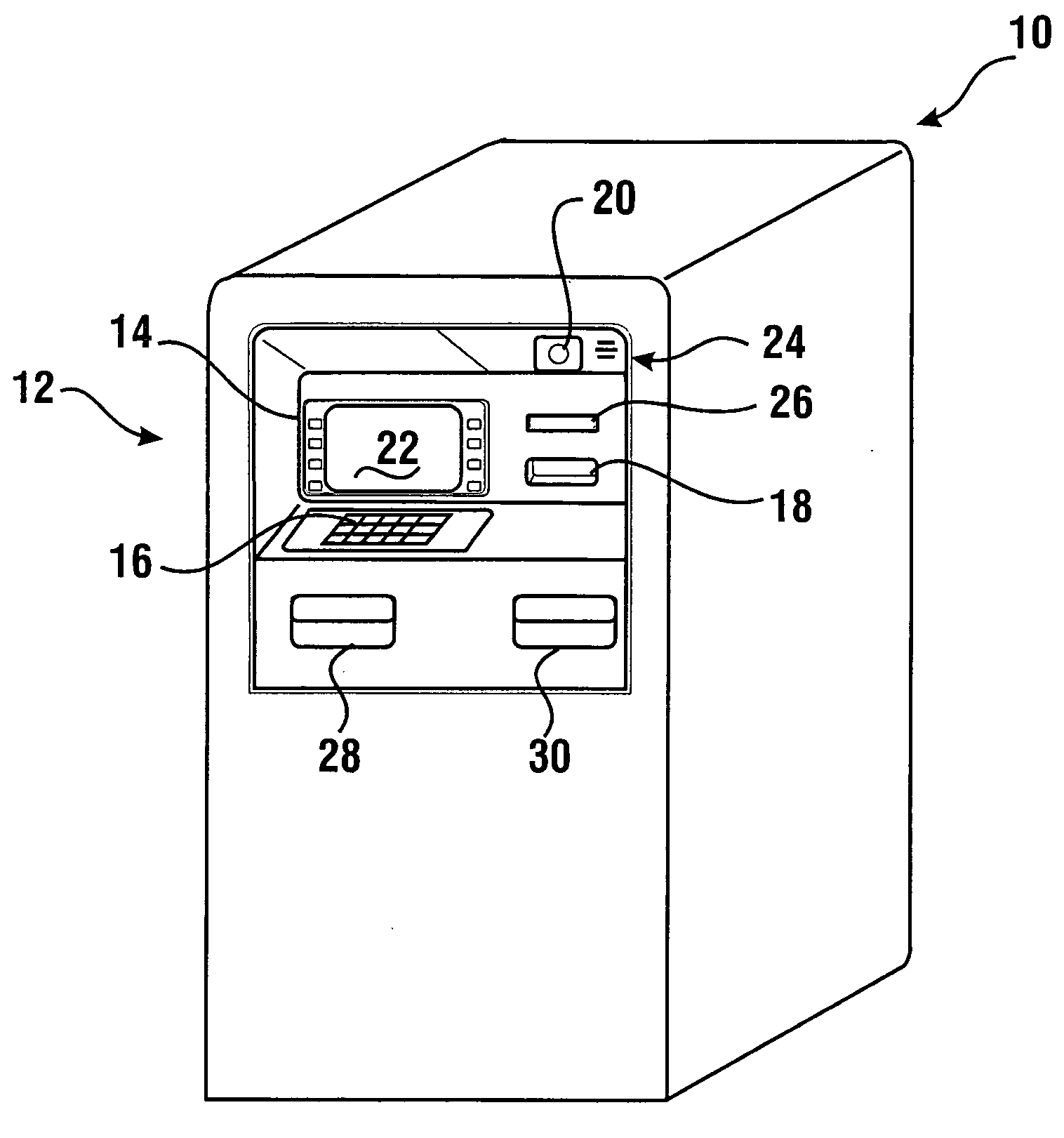
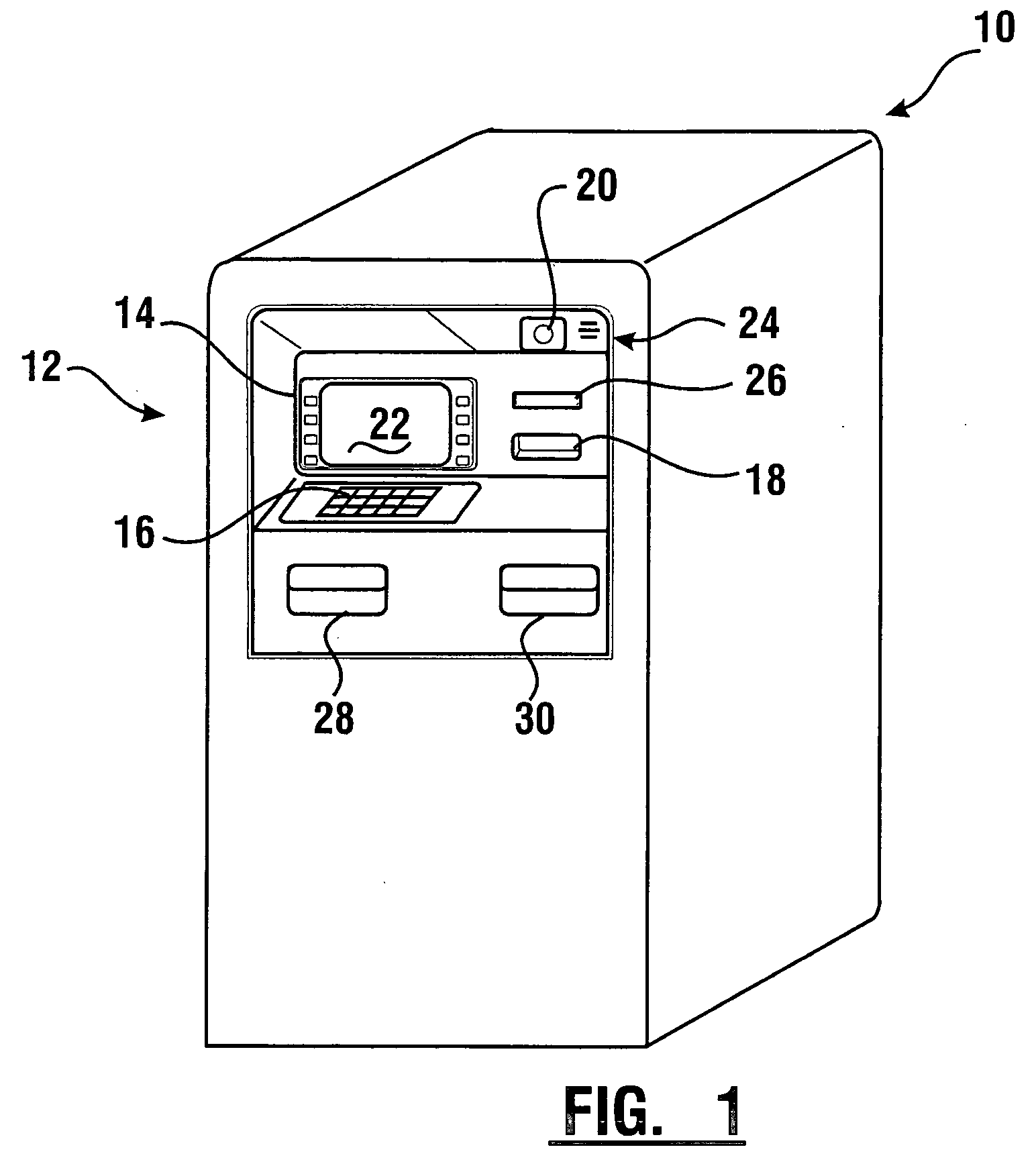
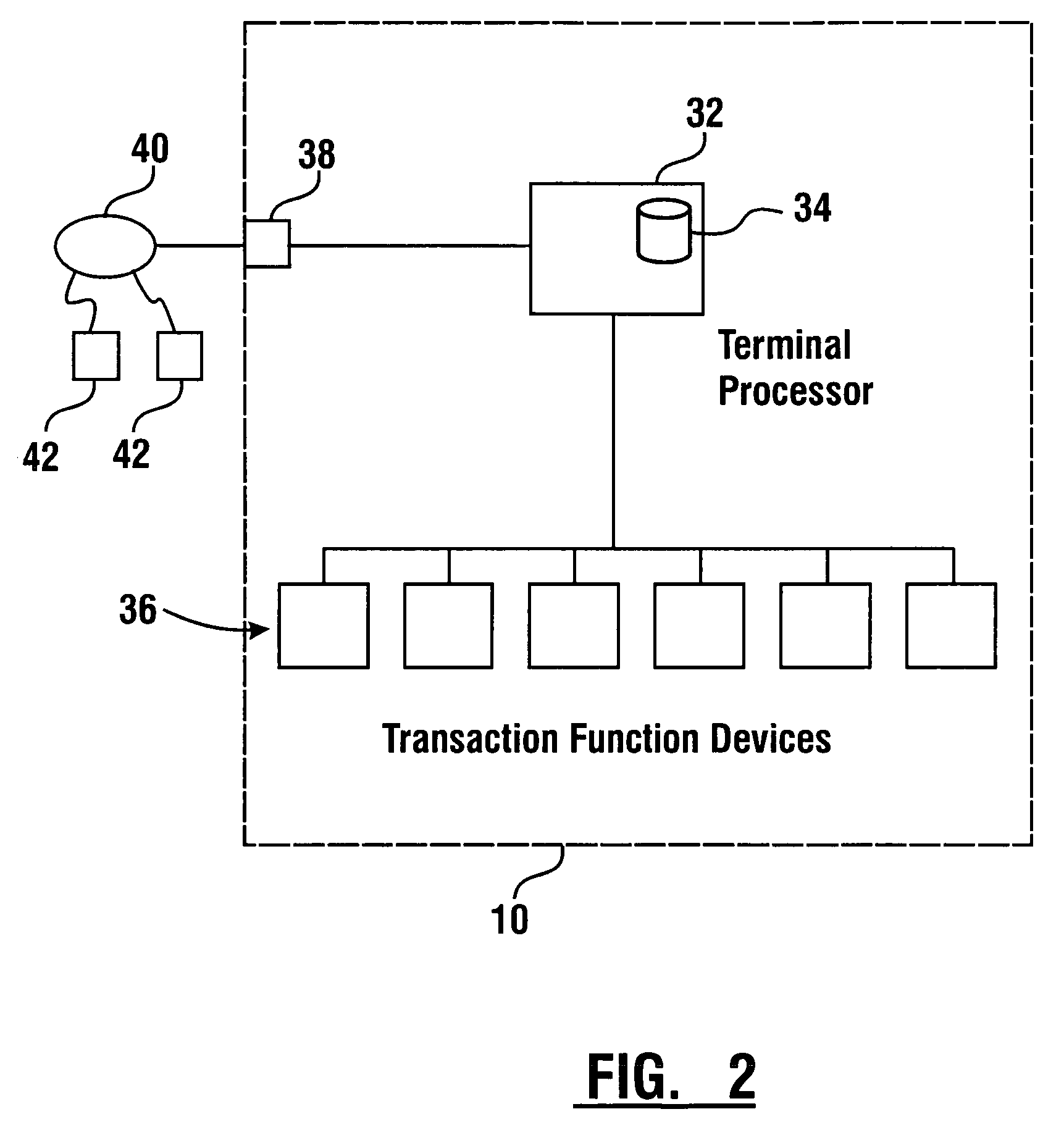
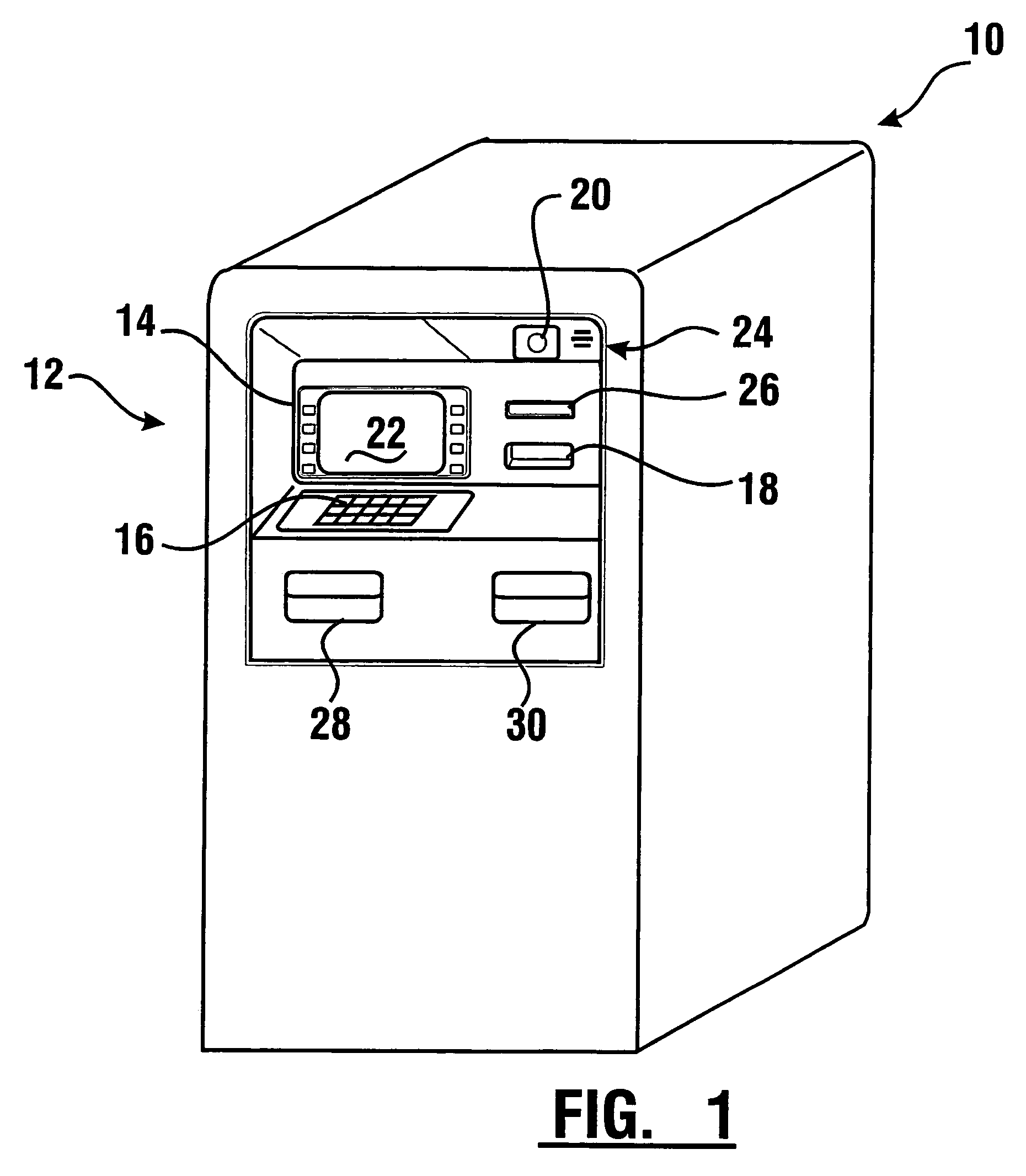
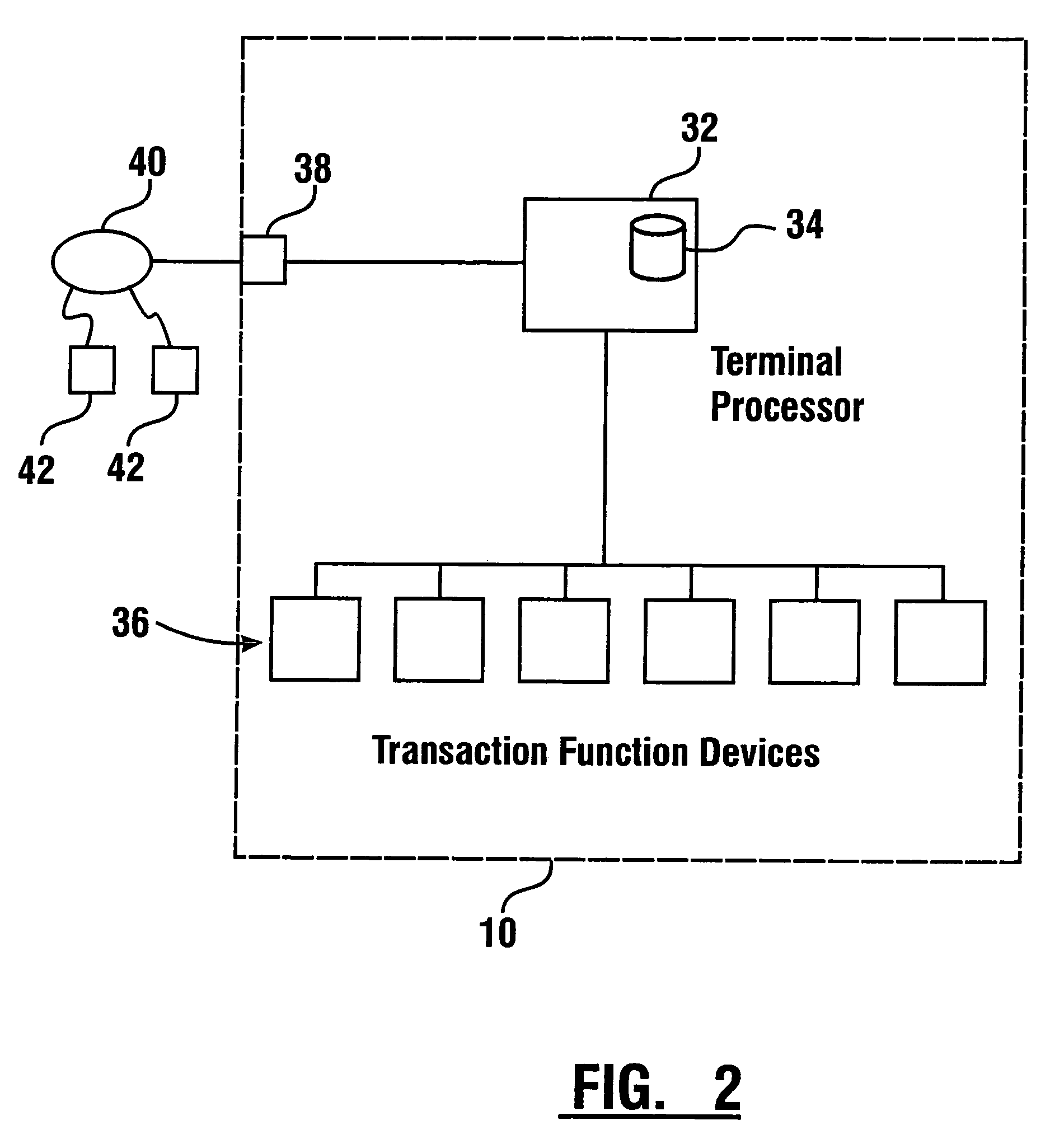
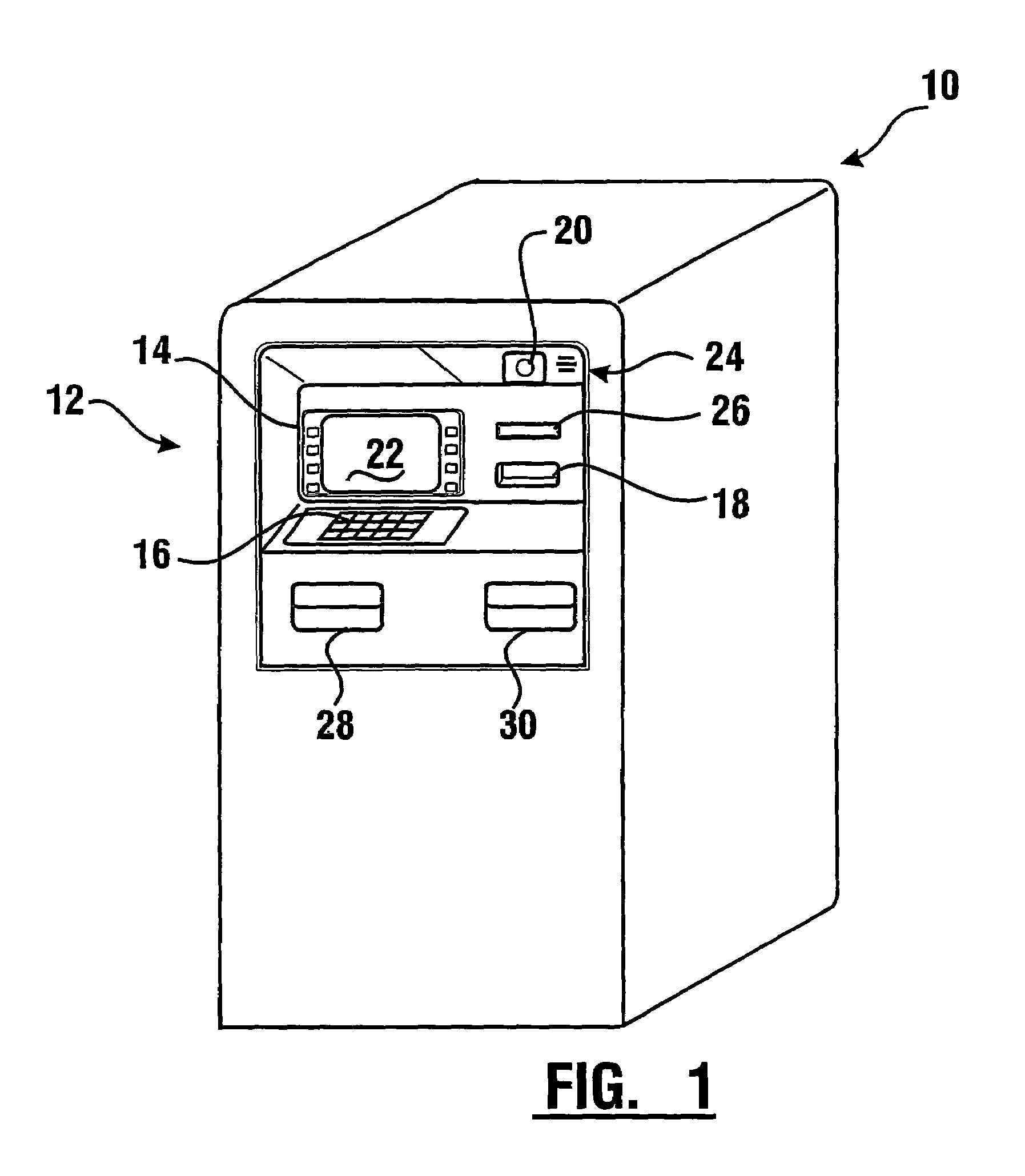
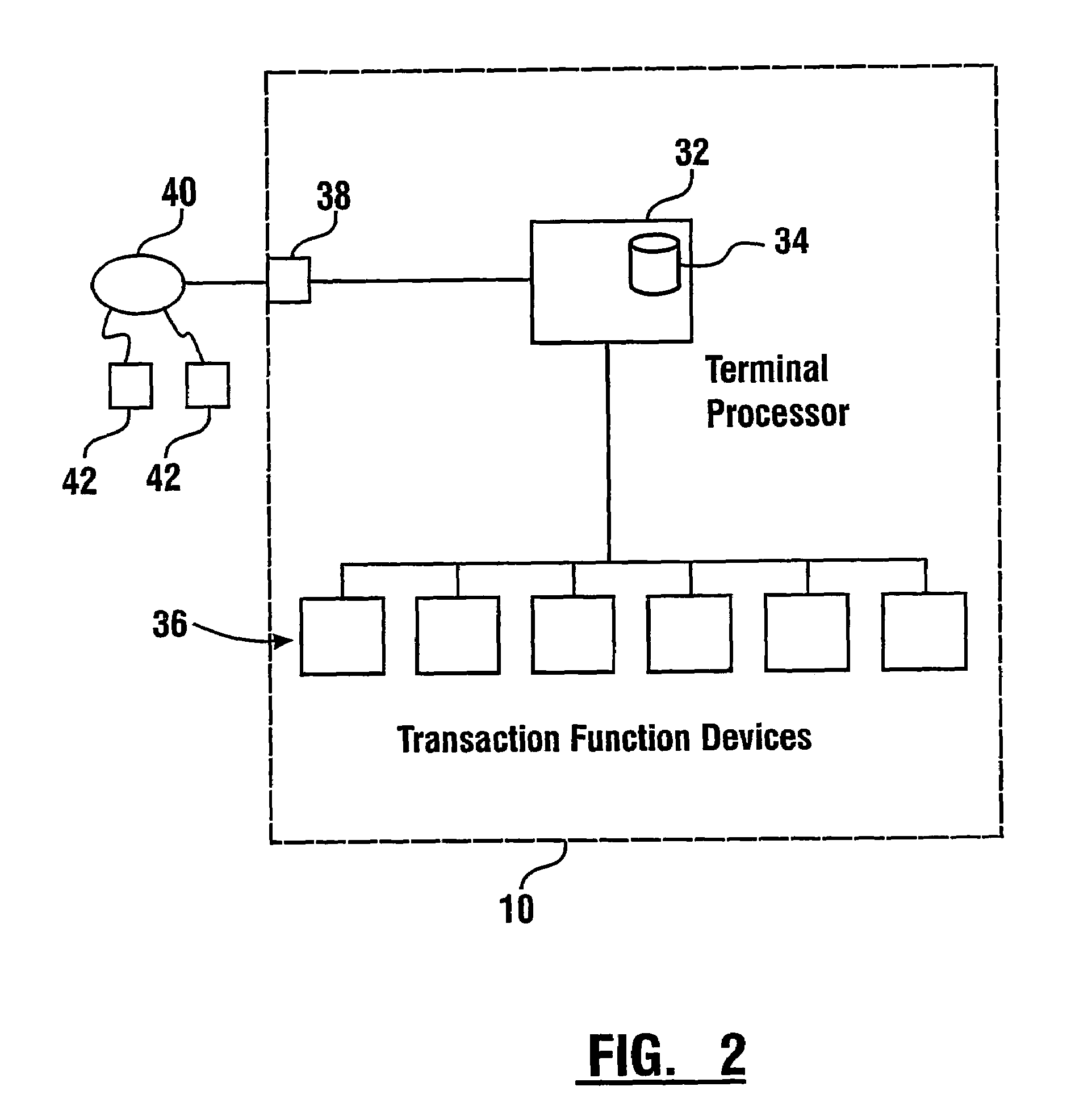
Check accepting and cash dispensing automated banking machine system and method
InactiveUS7314163B1Minimize the risk of damageReduce the possibilityComplete banking machinesFinanceTransaction dataCheque
An automated banking machine system includes ATMs that can accept checks. Cash may be dispensed from an ATM to the user in exchange for a check. Cancellation indicia can be marked on the check by the ATM. The ATM can acquire image data and magnetic data from a received check to determine the genuineness of the check and the authority of the user to cash the check. A check imaging device of the ATM can generate a digital image of a received check. The check image data can be stored in correlated relation with the related transaction data. The ATM can also modify the check image data to produce a modified check image that excludes sensitive check information, such as the user's account number in the micr line. The ATM can then print this modified check image on the user's transaction receipt.
Owner:DIEBOLD NIXDORF
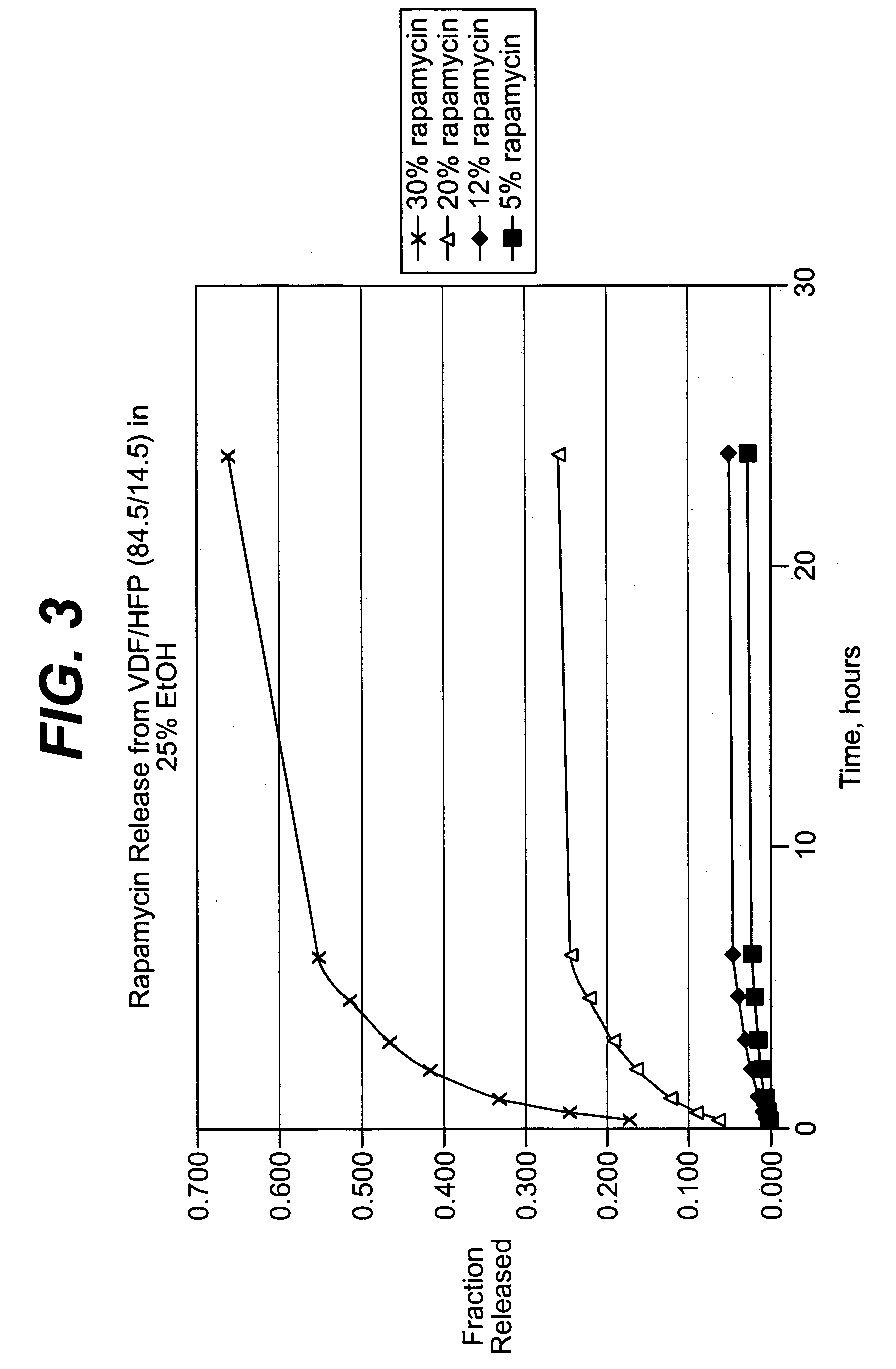
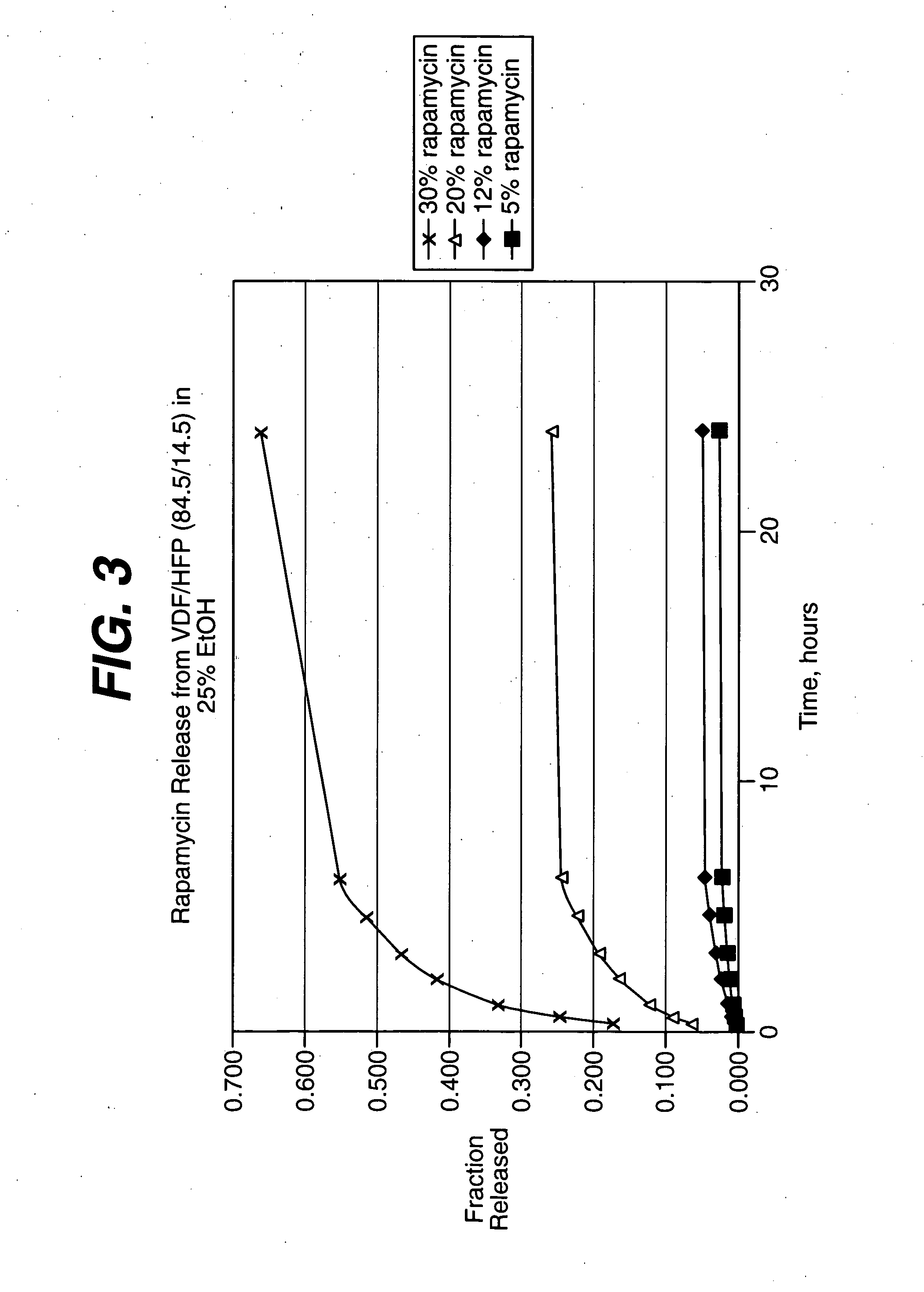
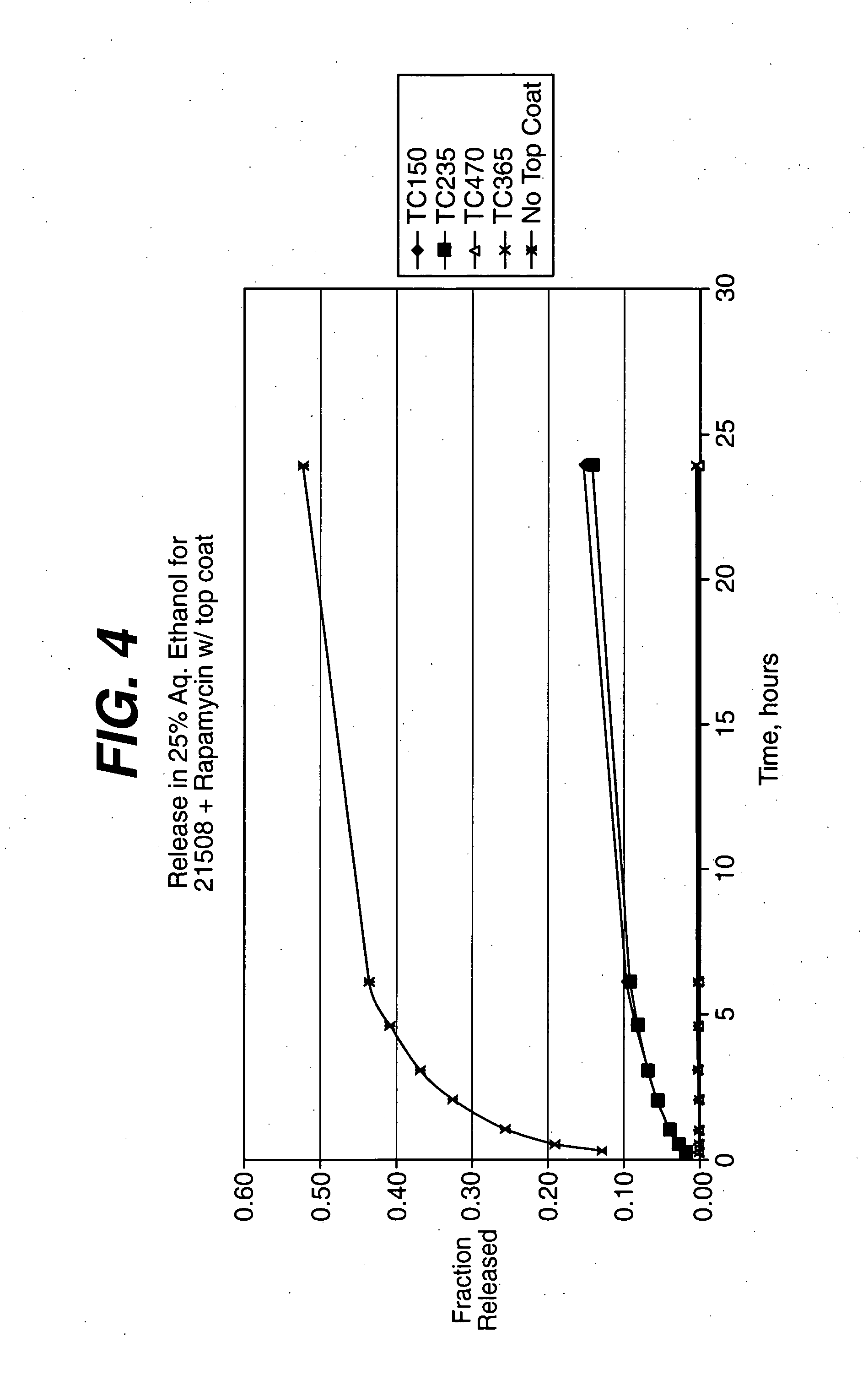
Solution formulations of sirolimus and its analogs for CAD treatment
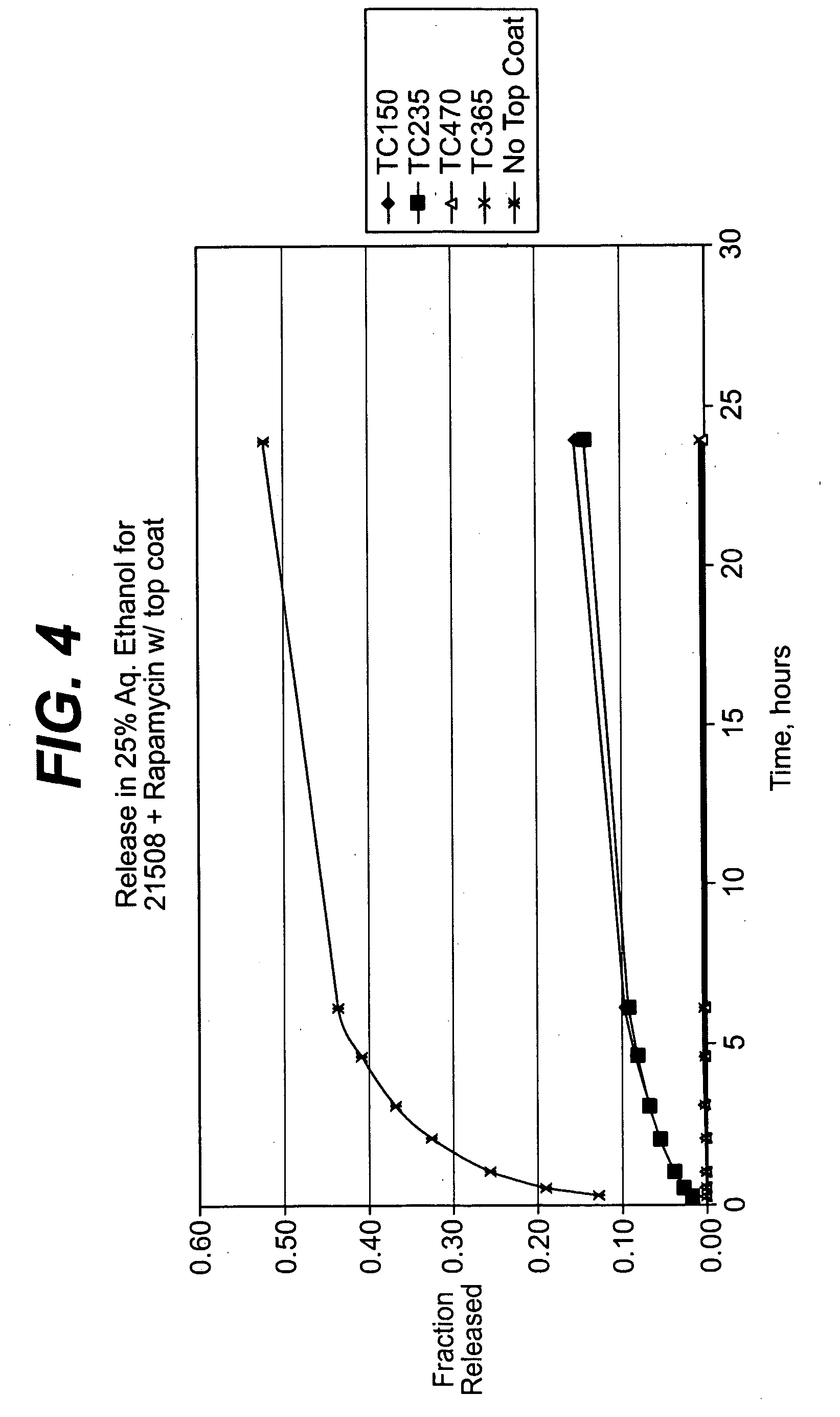
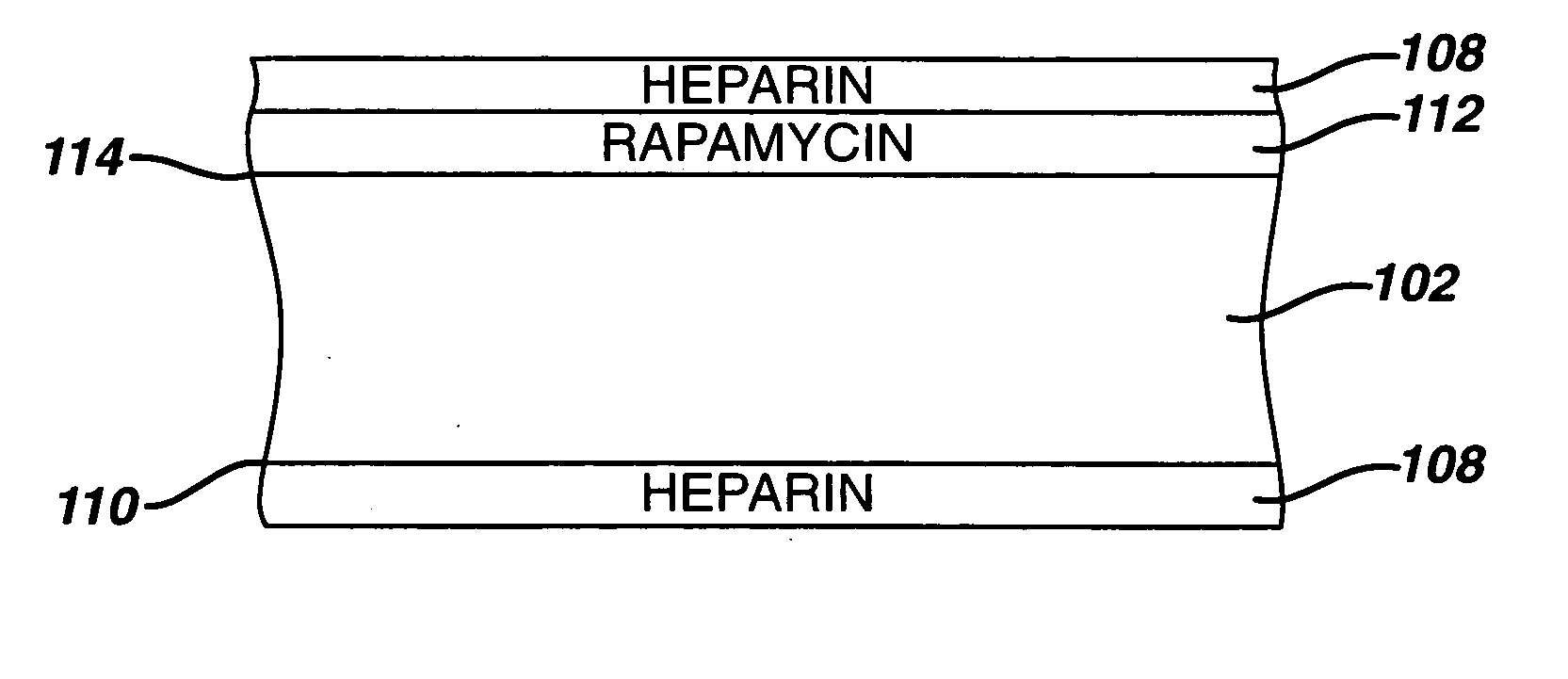
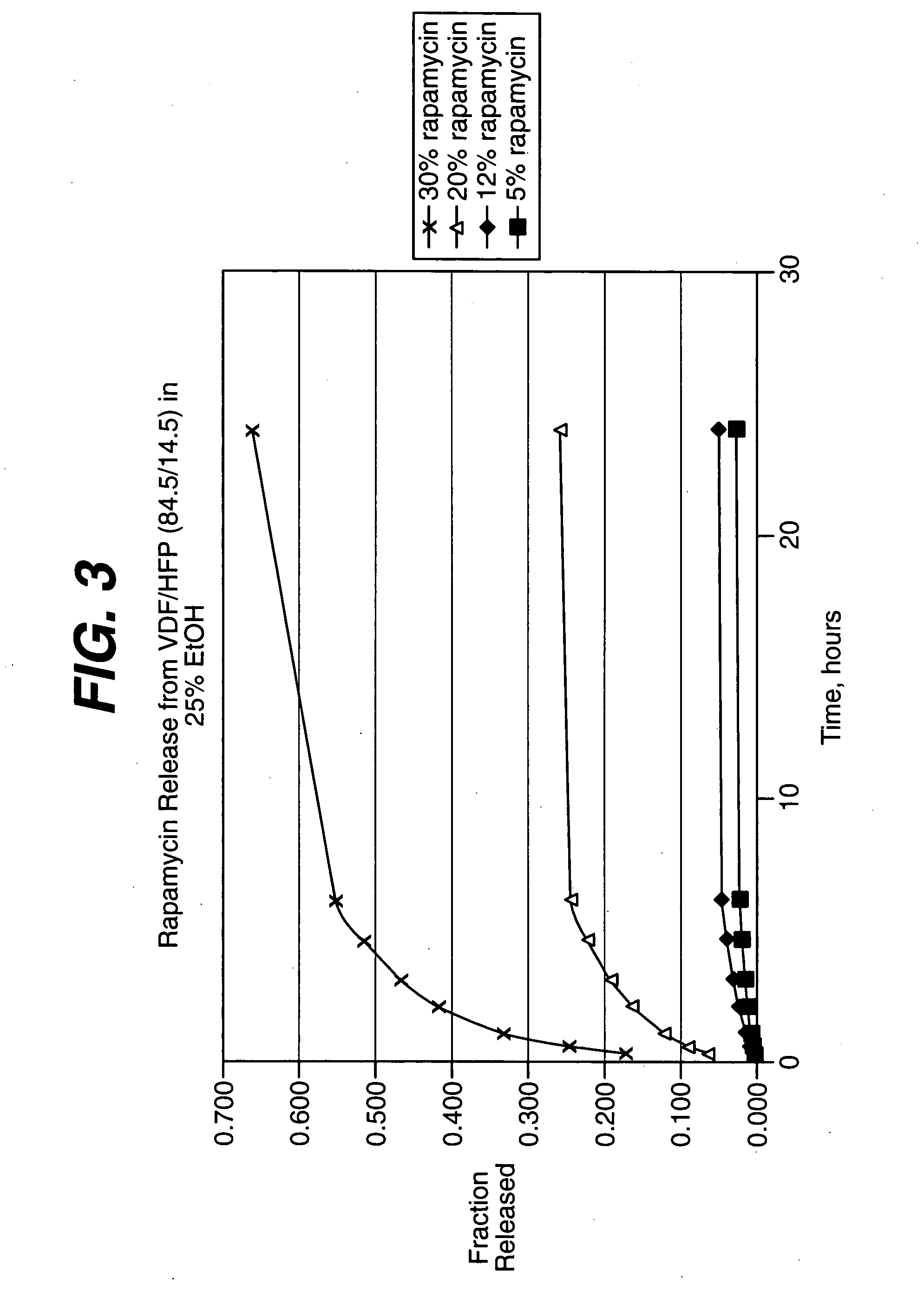
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Cash dispensing and check accepting ATM and method
InactiveUS7577614B1Minimize the risk of damageReduce the possibilityFinanceAutomatic teller machinesTransaction dataCheque
An automated banking machine system and method includes ATMs which accept checks and dispense cash to users. The ATMs are operated to acquire image and magnetic data from deposited checks to determine the genuineness of checks and the authority of a user to receive cash for such checks. Cash is then dispensed to the user from the ATM in exchange for the deposited check. The ATMs dispense cash responsive to communications with a transaction host. The transaction host provides transaction identifying data to the ATM. The ATM sends the transaction identifying data and check images to an image and transaction data server for processing.
Owner:DIEBOLD NIXDORF
SERRATOME vertebral cortical endplate cutter
ActiveUS20050131416A1Easy to controlEfficient and safe and waySurgeryJoint implantsIntervertebral spaceBone Cortex
The invention relates to spinal fusion implants or grafts and to apparatus for the installation thereof. More specifically, the invention is a serrated cutting or abrading tool designed to be pushed into an intervertebral space and thereby remove and partially penetrate the cortical bone layer that defines the endplates of the respective mutually adjacent vertebral bodies.
Owner:ALPHATEC SPINE INC
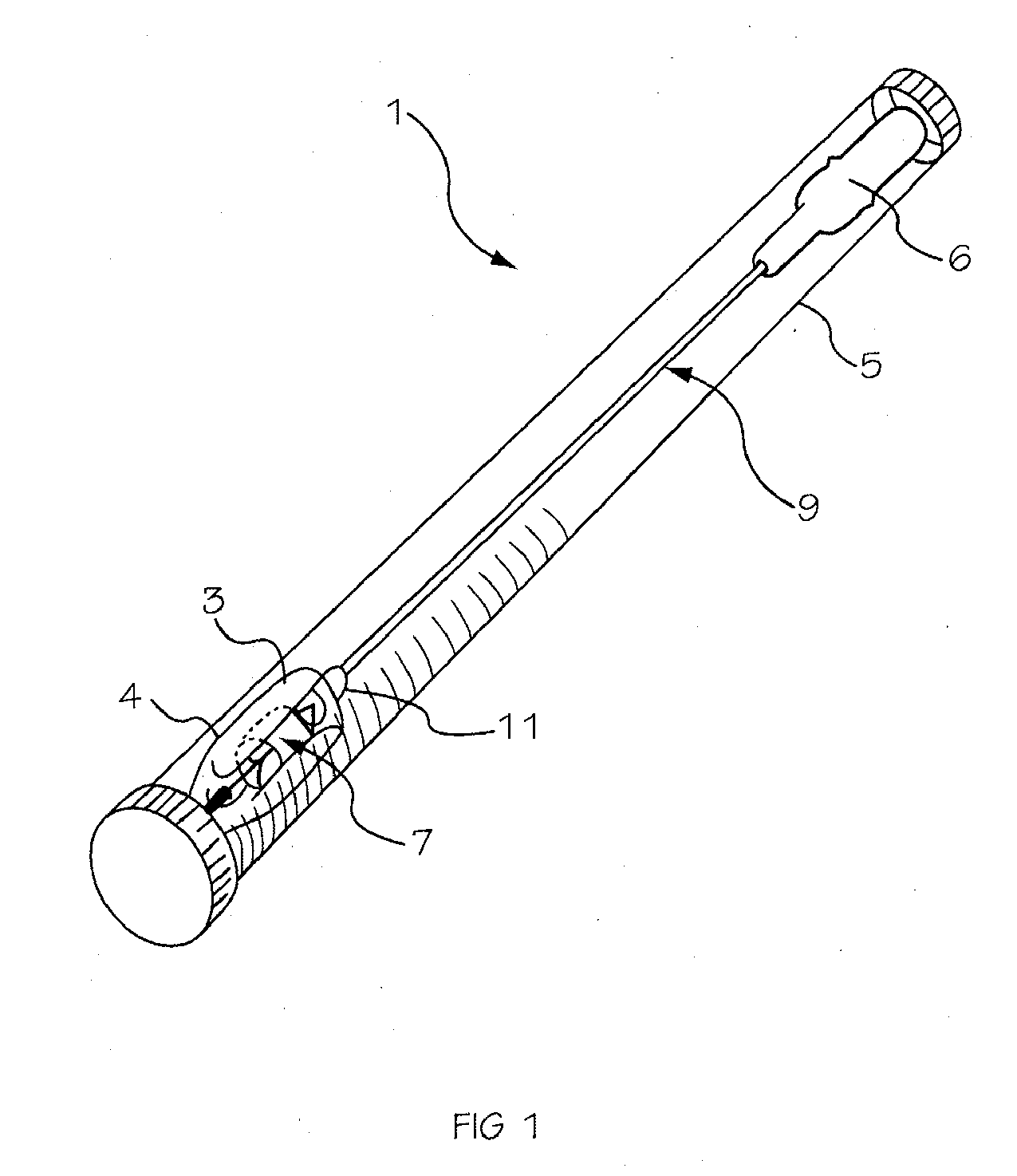
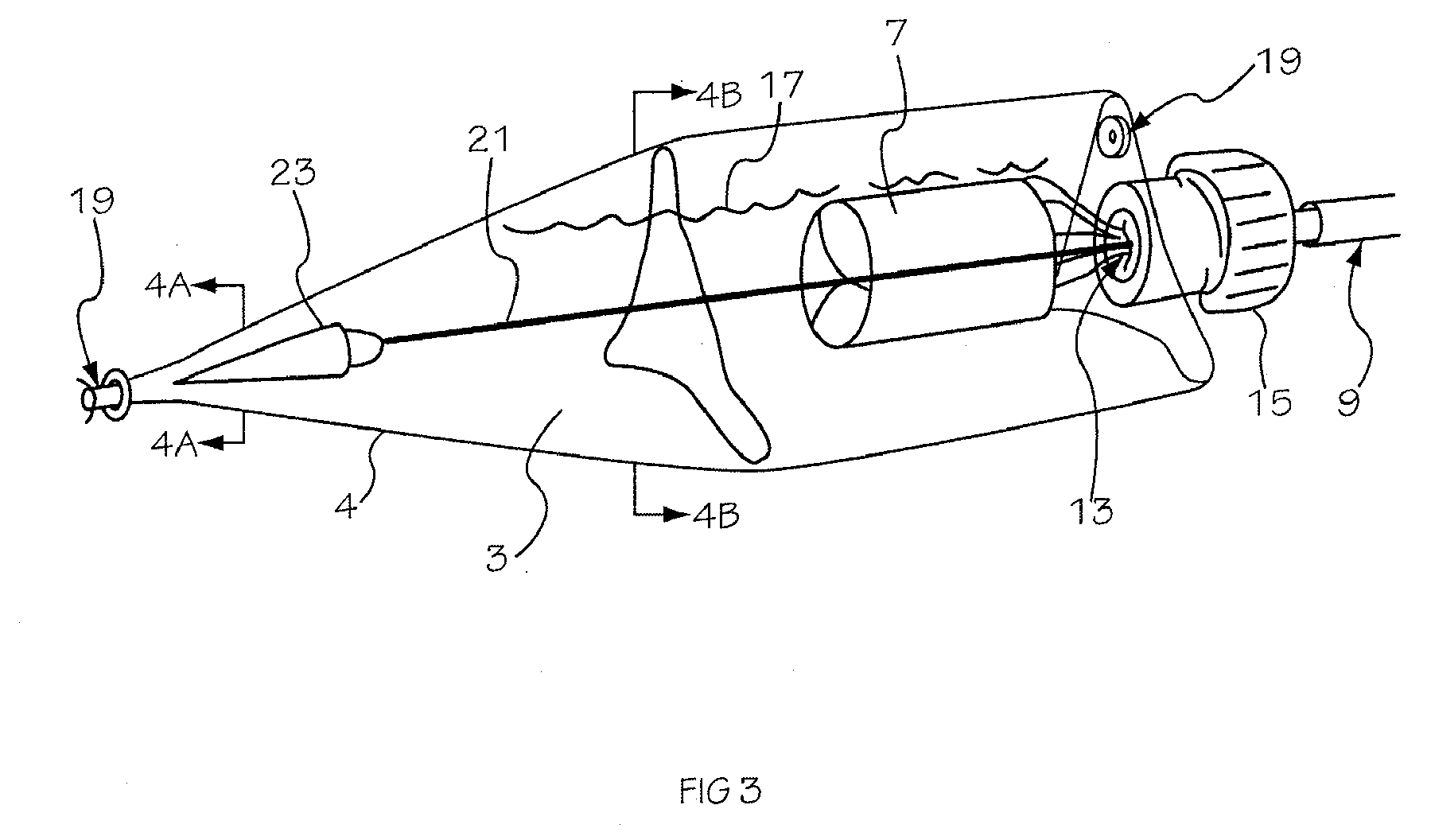
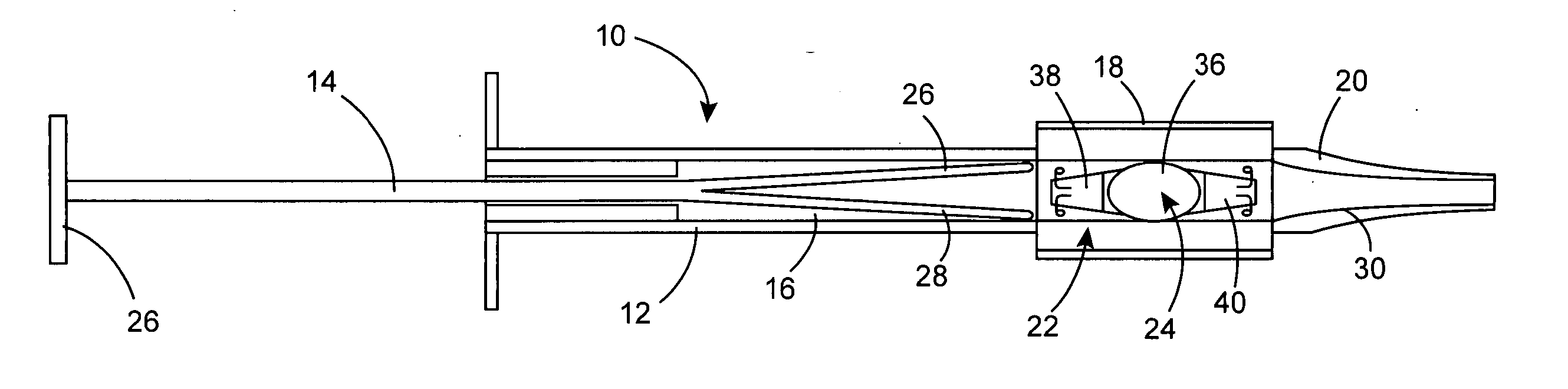
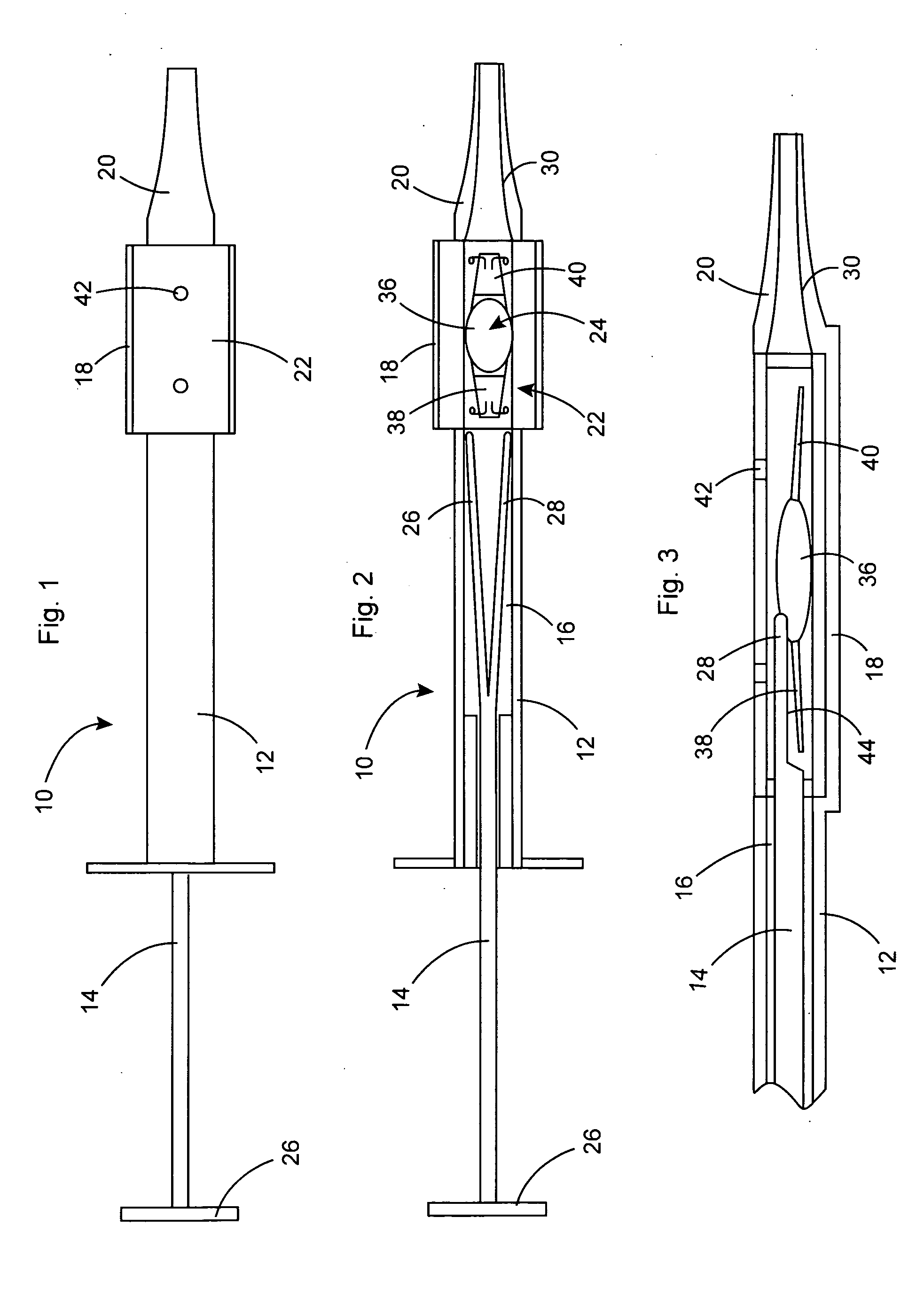
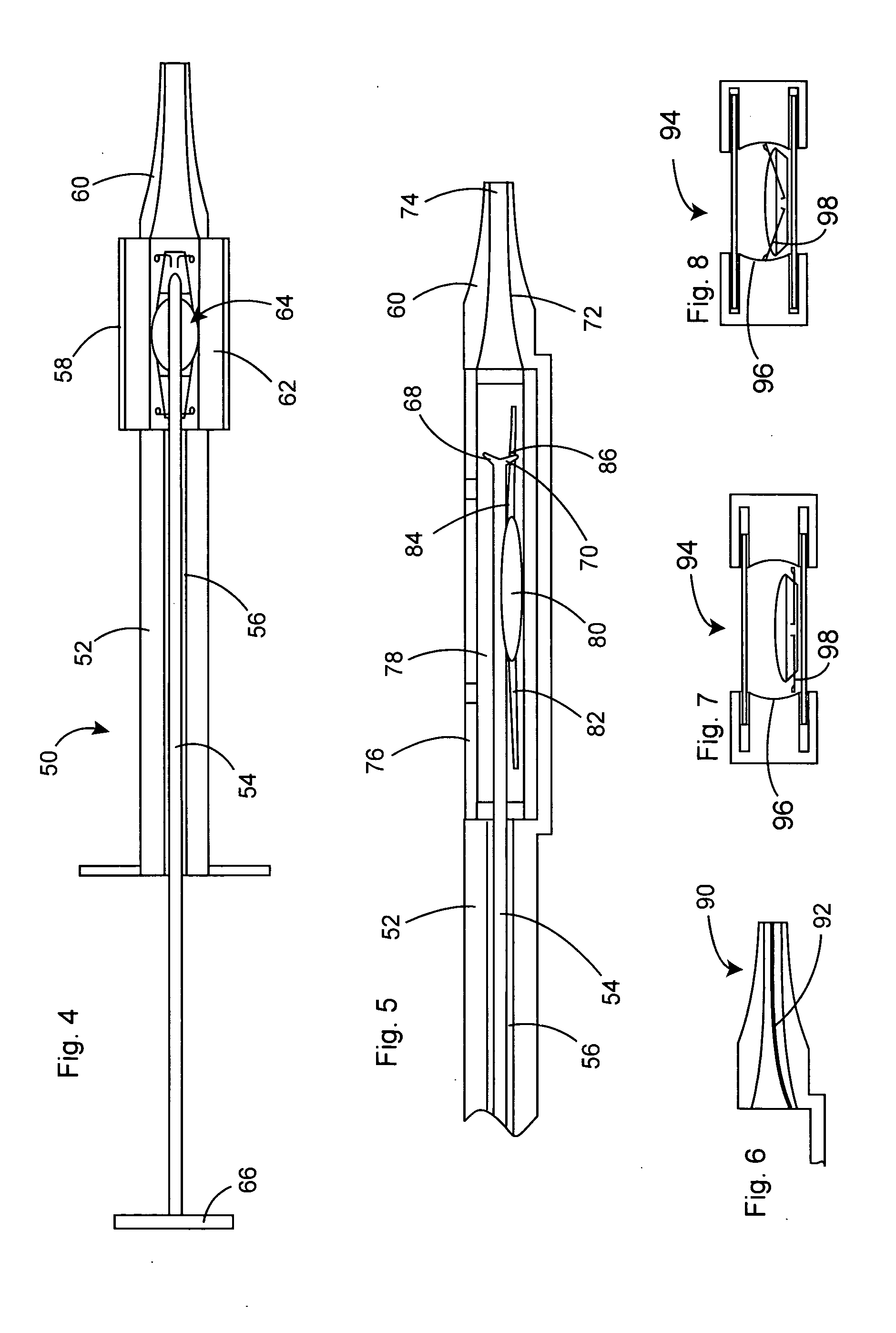
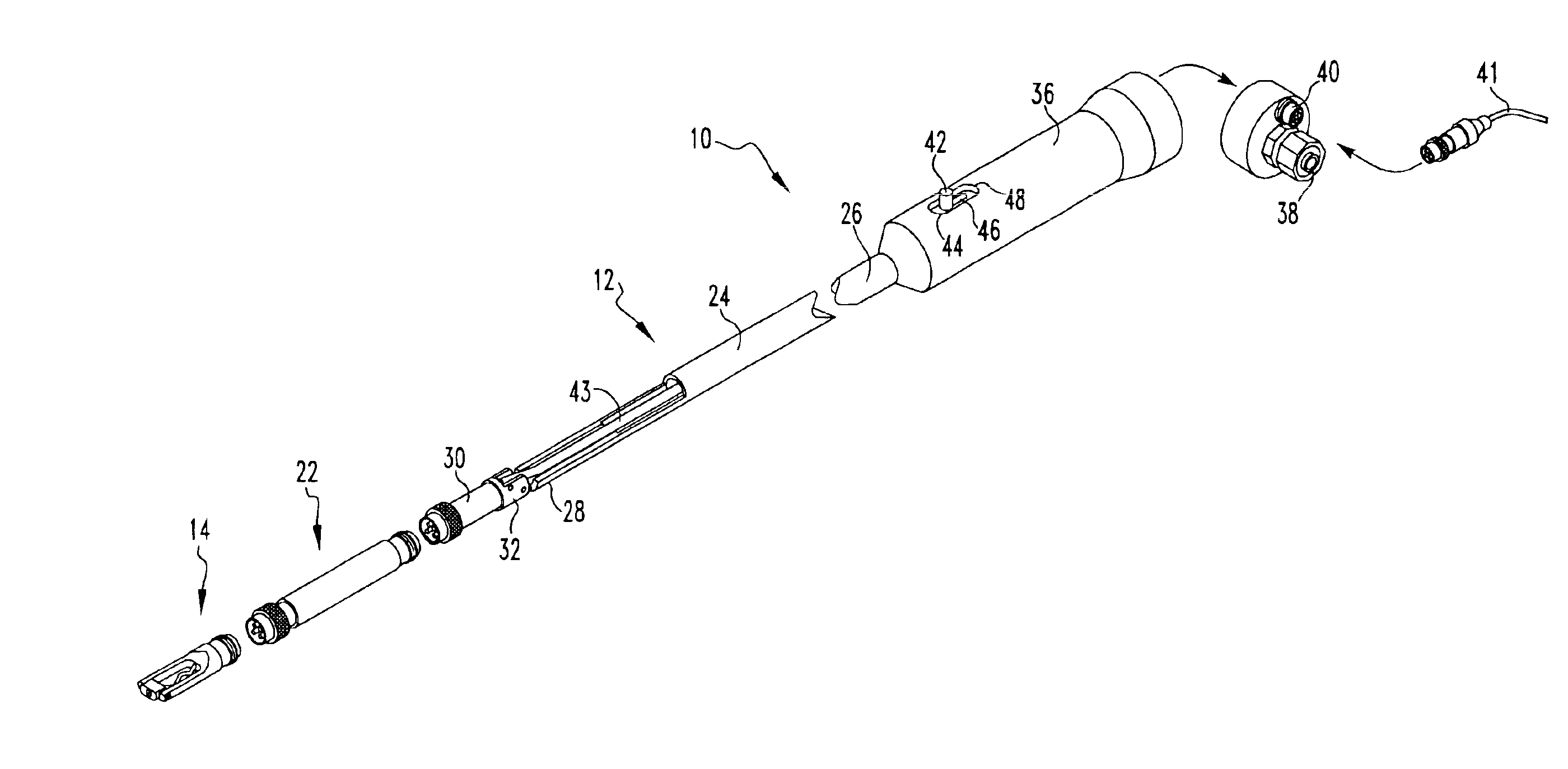
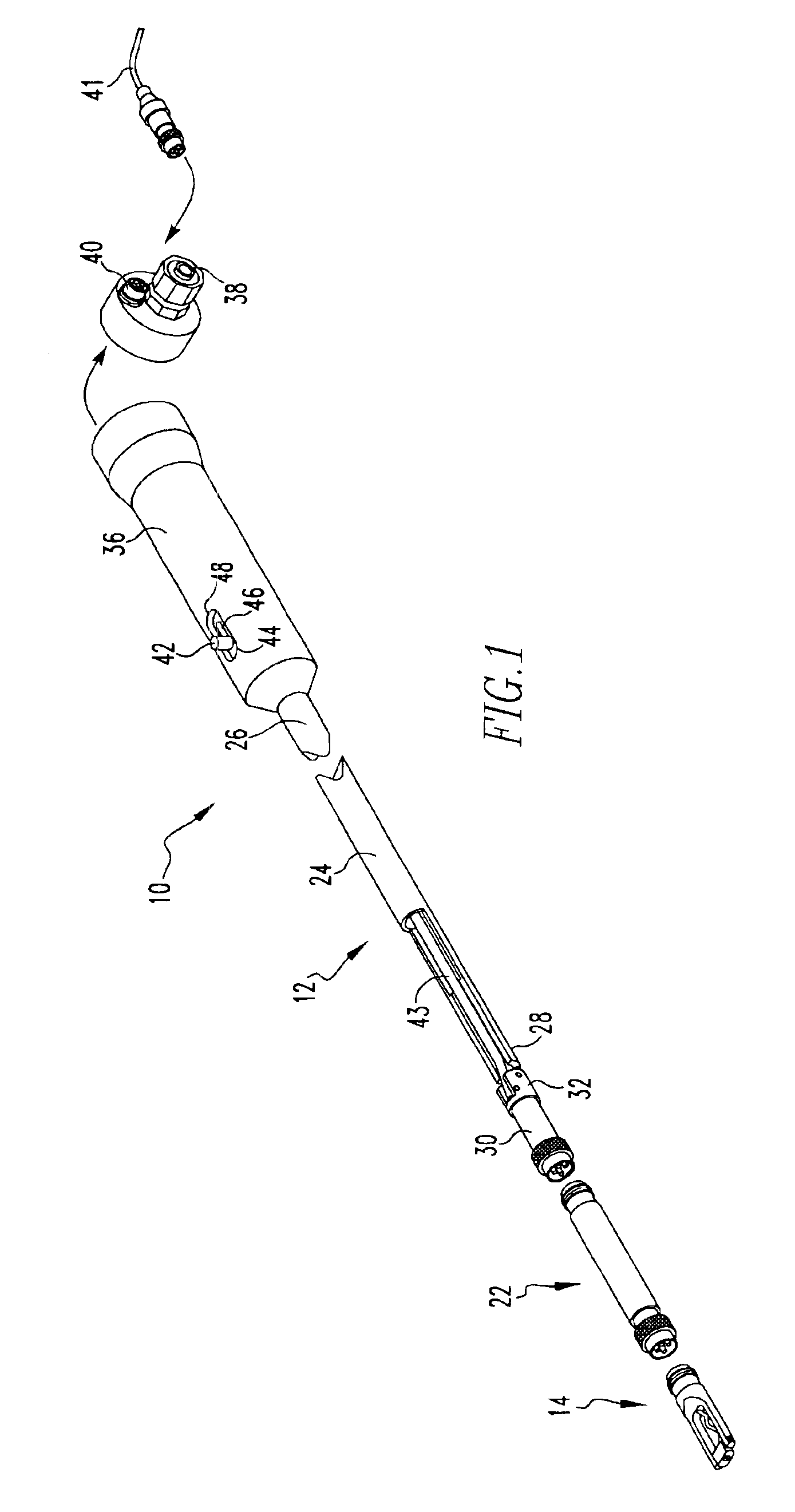
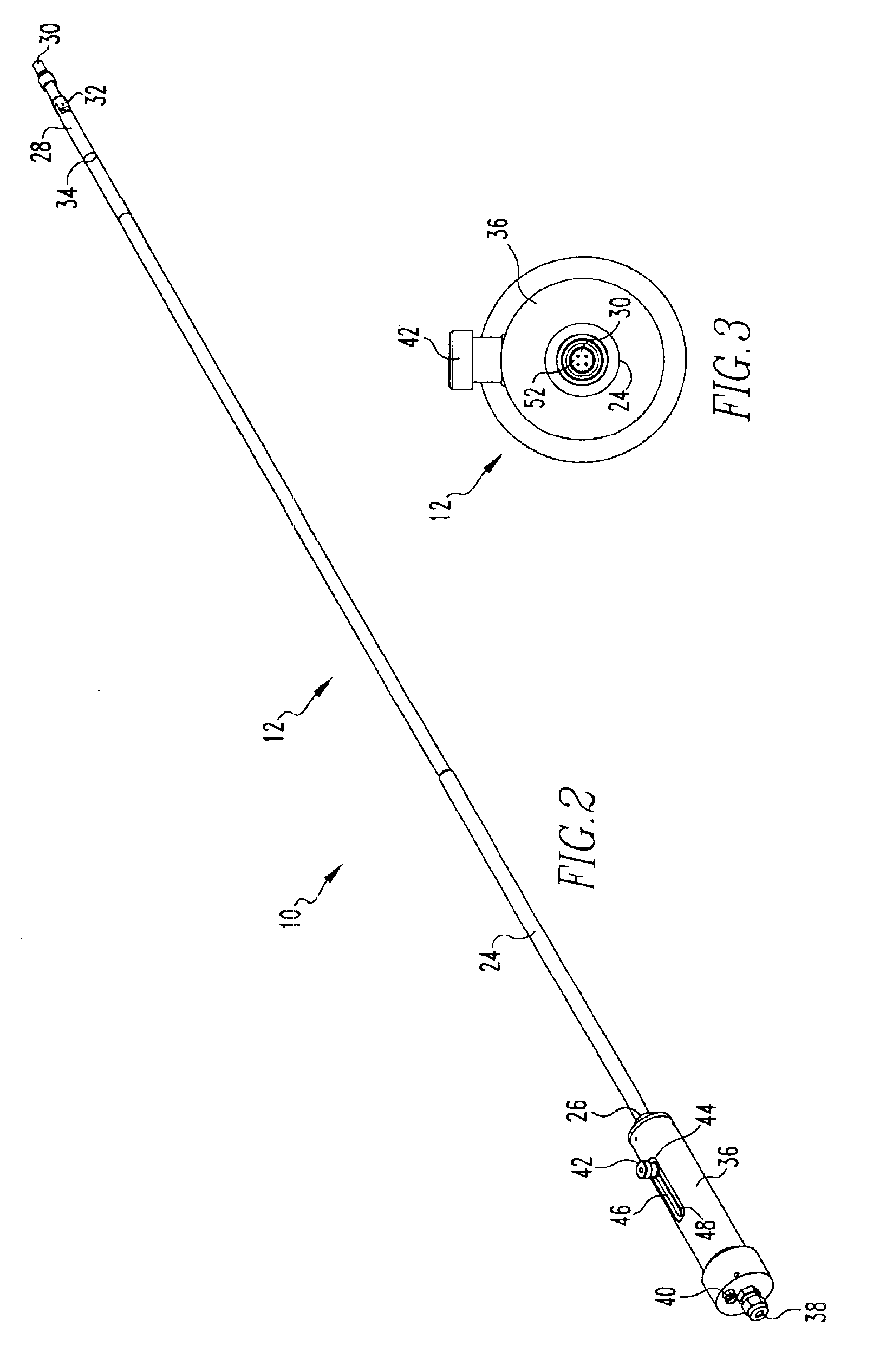
Intraocular lens inserter
InactiveUS20050283162A1Minimize the risk of damagePrecise compressionEye treatmentIntraocular lensCamera lensPhakic iol
An intraocular lens is laterally compressed prior to insertion through an eye incision using an inserter adapted to minimize damage to the intraocular lens. The inserter includes a handpiece with a longitudinal bore, a cartridge holder at the distal end of the handpiece bore, and a discharge nozzle with a tapered bore on the distal side of the cartridge holder. A prepackaged cartridge holding an intraocular lens is mounted in the cartridge holder. The intraocular lens has an optic with leading and trailing haptics axially aligned with the handpiece and nozzle bores. A plunger compresses the lens by moving the lens through the nozzle tapered bore. One plunger described includes flexible distal arms that compress inwardly to maintain the arm tips against the sides of the optic, while another plunger includes an angular tip that engages a hole in the leading haptic to pull the lens through the tapered bore.
Owner:STRATAS BYRON A
Check accepting and cash dispensing automated banking machine system and method
ActiveUS20060144923A1Minimize the risk of damageReduce the possibilityComplete banking machinesFinanceChequeMachining system
An automated banking machine system and method includes ATMs which accept checks and dispense cash to users. The ATMs are operated to acquire image and magnetic data from deposited checks to determine the genuineness of checks and the authority of a user to receive cash for such checks. Cash may be dispensed to the user from the ATM in exchange for the deposited check. The ATMs dispense cash responsive to communications with a transaction host.
Owner:DIEBOLD NIXDORF
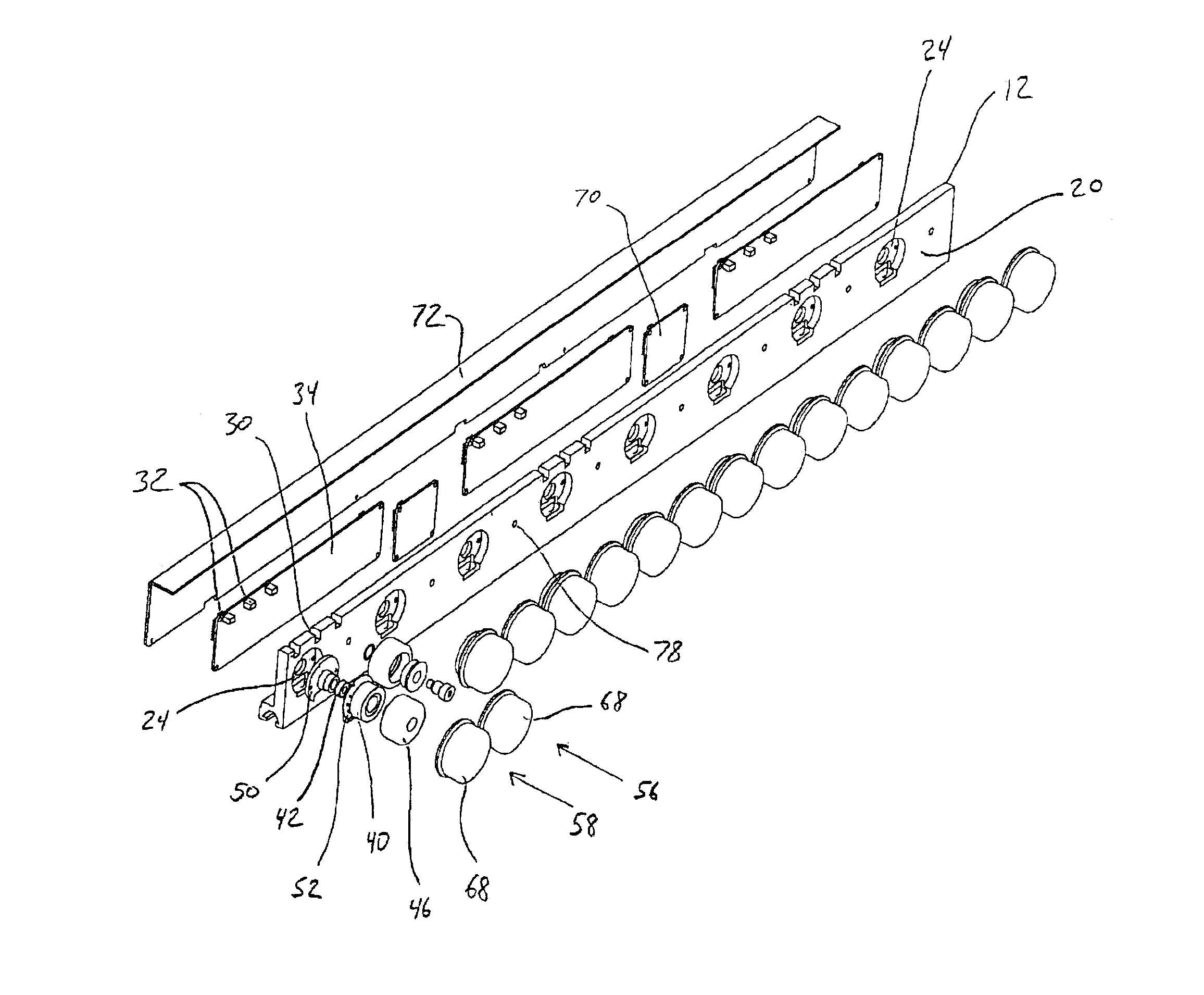
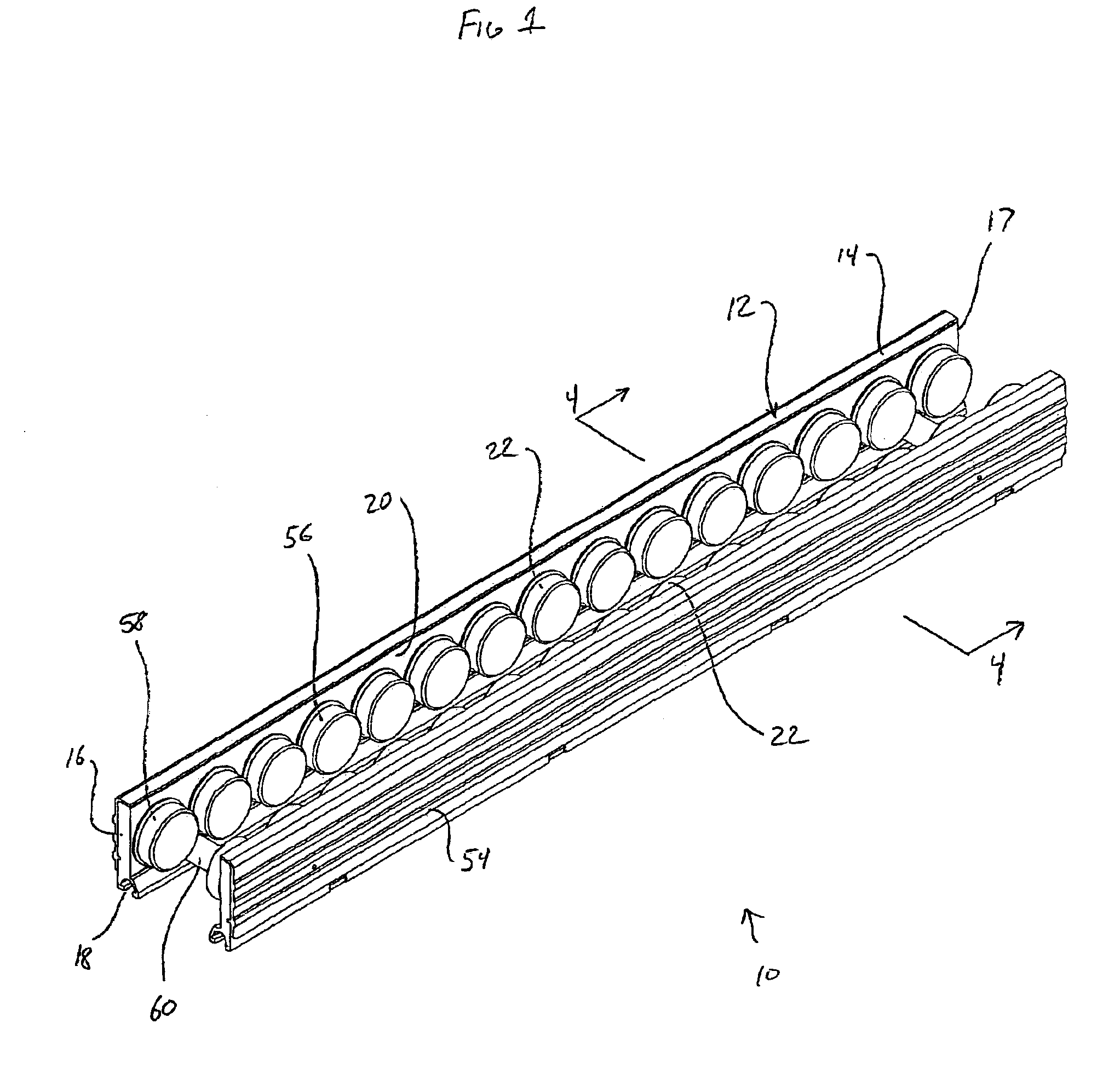
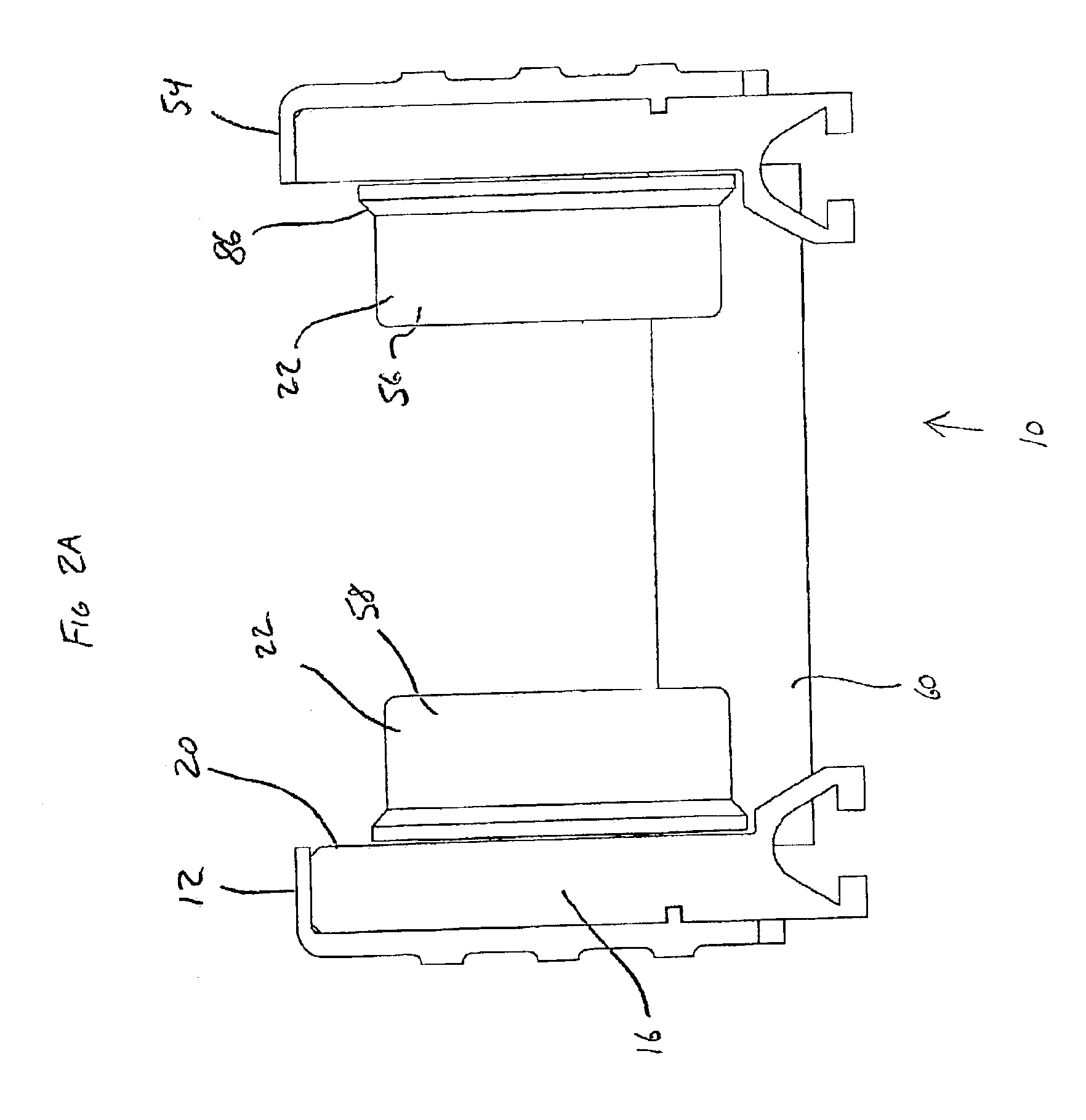
Conveyor assembly
InactiveUS6959804B2Simple and inexpensive componentMinimize the risk of damageConveyorsRollersEngineeringElectric motor
The conveyor apparatus disclosed herein is generally formed of a first rail and a second rail, each rail having a plurality of rollers. A portion of the rollers are desirably drive rollers, receiving power from an integral motor assembly. The motor generally comprises a stator that is coupled to the rail and an outer roller assembly rotatably coupled to the rail. The roller assembly includes an outer circumferential magnetized portion positioned in surrounding engagement to the motor stator and functions both as the motor rotor and the conveying surface of the roller. Non-slip roller covering material may be attached to the exterior of the roller assembly. A circuit board having logic controls is preferably in communication with at least one sensor and the motor rotor to regulate rotation of the motor rotor during use of the conveyor.
Owner:ACTIVAR TECHN PRODS GROUP
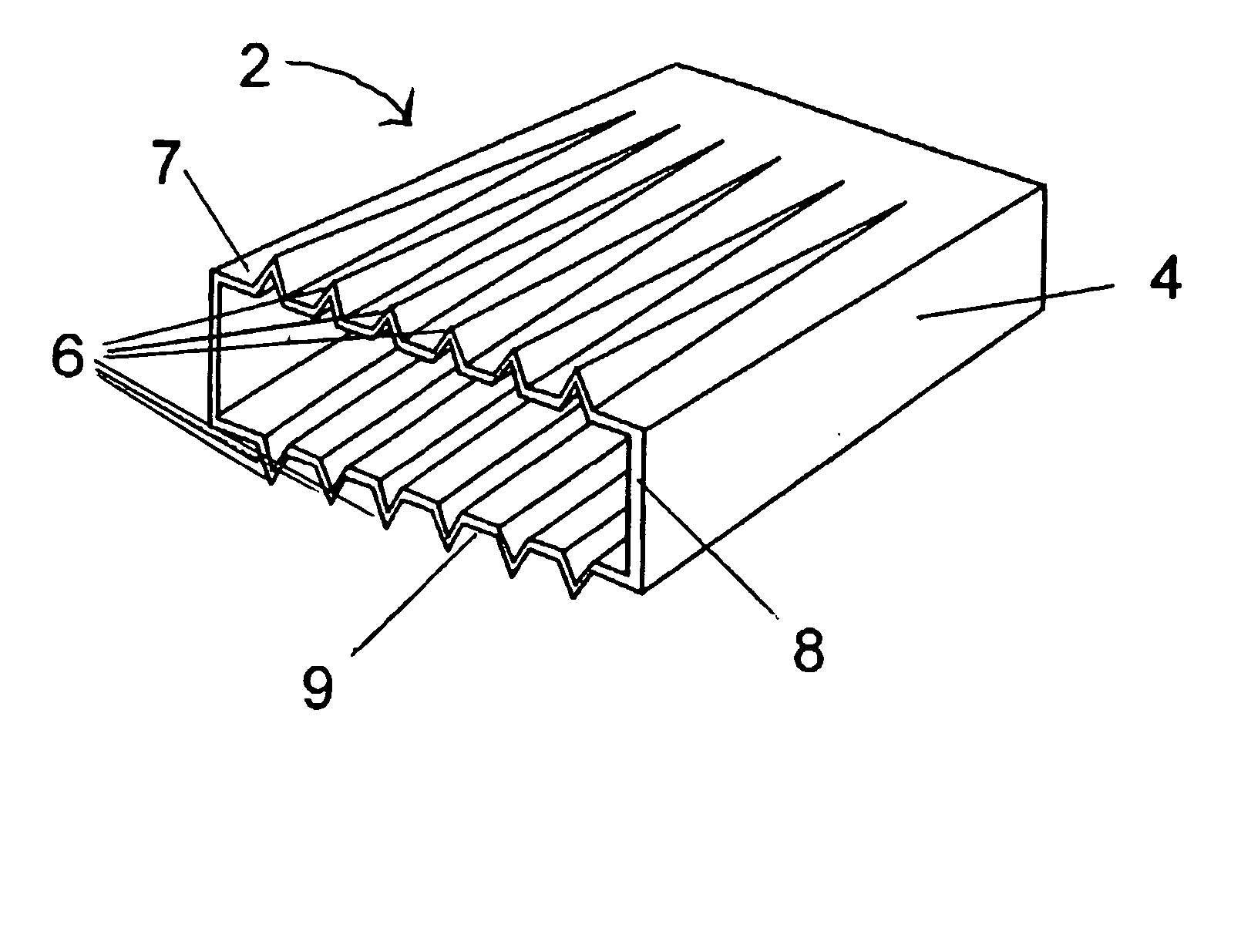
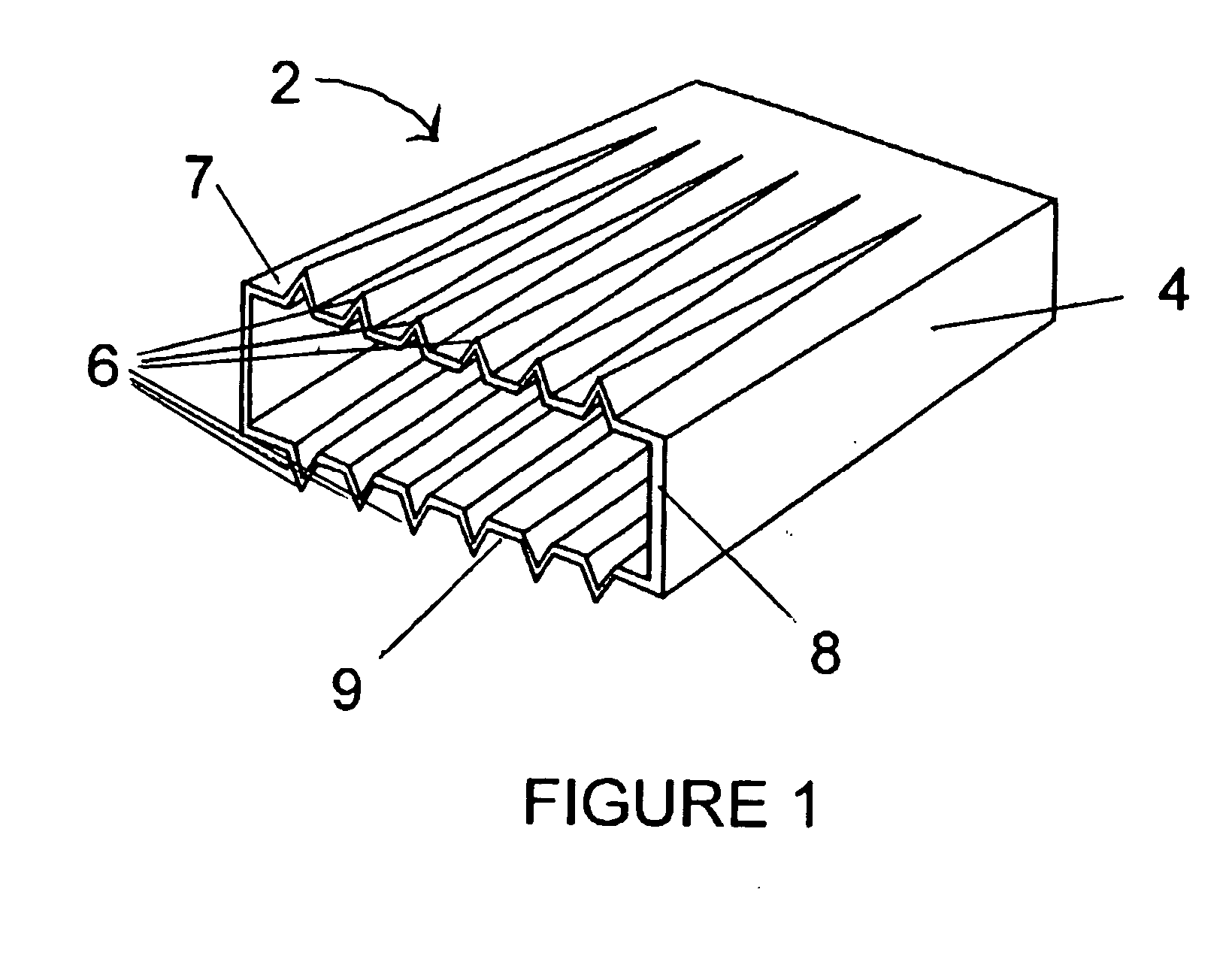
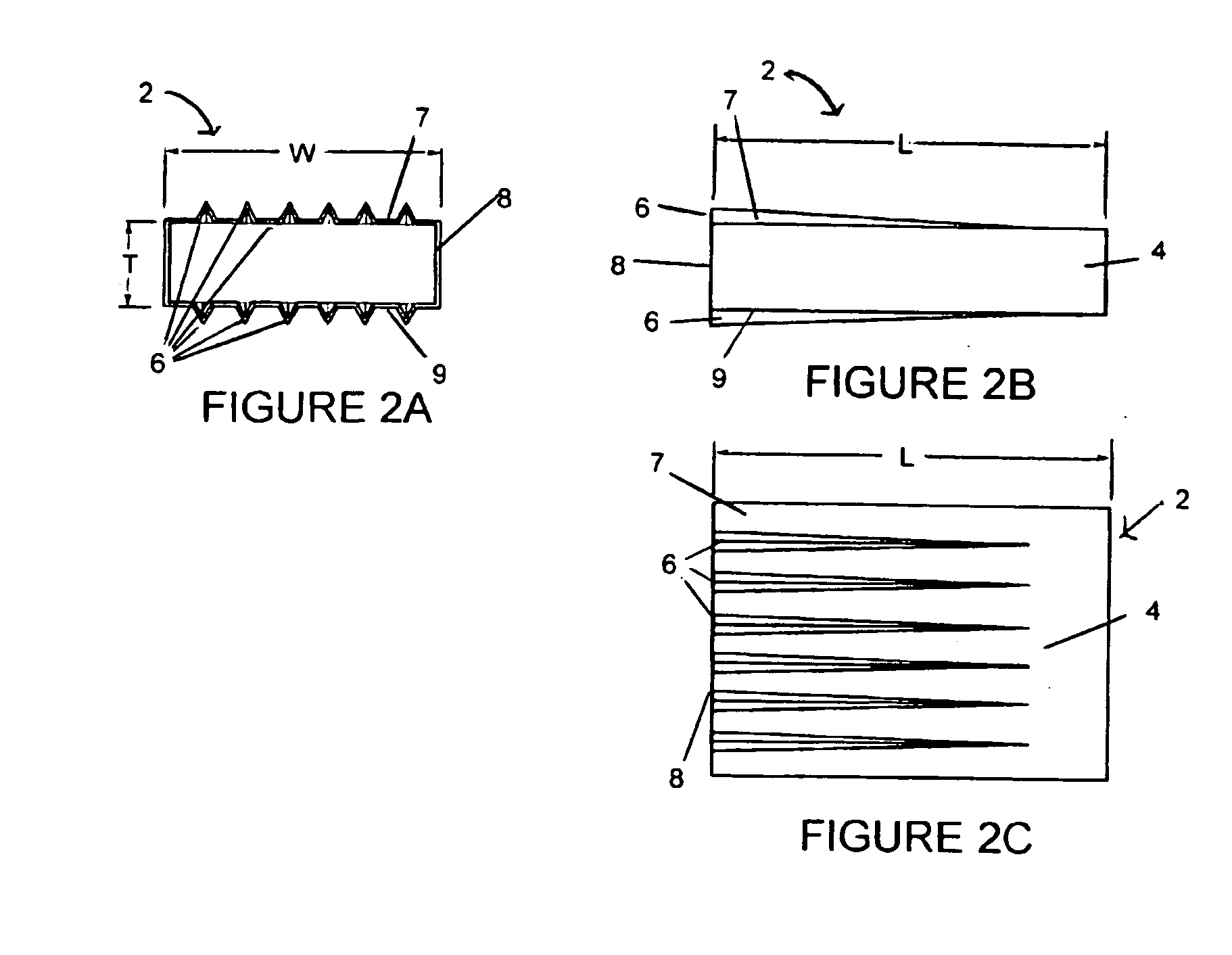
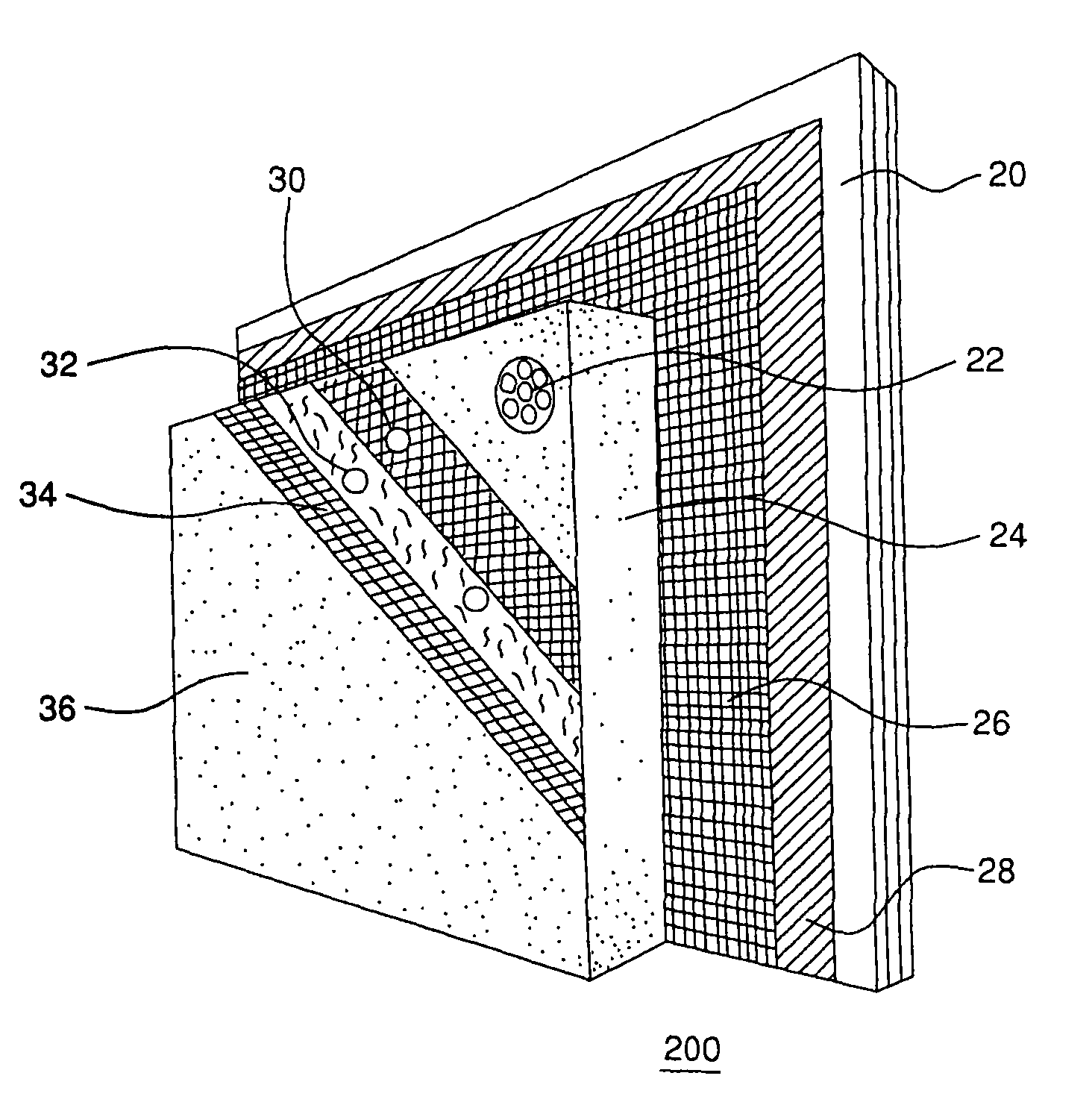
Exterior finishing system and building wall containing a corrosion-resistant enhanced thickness fabric and method of constructing same
A corrosion-resistant lath is provided for use in exterior finishing systems, such as stucco systems and exterior insulation and finish systems (“EIFS”). The lath includes in a first embodiment an open, woven fabric comprising weft and warp yarns containing non-metallic fibers, such as glass fibers. A portion of the weft yarns are undulated, resulting in an increased thickness for the fabric. The fabric is coated with a polymeric resin for substantially binding the weft yarns in the undulated condition. This invention also includes methods for making an exterior finish system and building wall including an exterior finish system using such a lath.
Owner:BASF LEC CONSTR CHEM
Local administration of a combination of rapamycin and cilostazol for the treatment of vascular disease
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Check accepting and cash dispensing automated banking machine system and method
InactiveUS7284695B1Minimize the risk of damageReduce the possibilityComplete banking machinesFinanceDelayed periodsCheque
An automated banking machine system and method includes ATMs which accept checks and dispense cash to users. The ATMs are operated to acquire image and magnetic data from deposited checks to determine the authority of a user to receive cash for such checks. The authority of the user is determined based on the user being associated in a data store with the maker of the check. The check cannot exceed certain programmed thresholds or be presented earlier than the expiration of a delay period from when a prior check from the same maker is presented. In exchange for acceptable checks cash is dispensed to the user from the ATM in exchange for the deposited check.
Owner:DIEBOLD NIXDORF
Eddy current inspection probe for inspecting multiple portions of a turbine blade having different geometric surfaces
InactiveUS6867586B2Quantity minimizationPrecise positioningMagnetic property measurementsMaterial magnetic variablesCombustionTurbine blade
An eddy current inspection probe provides multiple interchangeable configurations for enabling an inspector to reach most or all portions of most or all turbine blades within a combustion turbine by inserting the probe through an inspection port, without disassembling the turbine. The probe shaft contains the electronic signal wire for the inspection tip and a port for a video probe, thereby facilitating use of the video probe and protecting these components. The inspection tip connector at the end of the main shaft is pivotally secured to the shaft, and may be set at any desired angle by using a semirigid or rigid member passing through the shaft, and connected to lever within the handle. Any one of a plurality of probe tips and shaft extensions may be selected to configure the inspection probe to reach a desired location within the turbine.
Owner:SIEMENS ENERGY INC
Local vascular delivery of PI3 kinase inhibitors alone or in combination with sirolimus to prevent restinosis following vascular injury
InactiveUS20070116736A1Reduce frictionLittle strengthStentsCoatingsPI3-Kinase InhibitorsDisease cause
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Check accepting and cash dispensing automated banking machine system and method
InactiveUS7216801B1Minimize the risk of damageReduce the possibilityComplete banking machinesFinanceChequeMachining system
An automated banking machine system and method includes ATMs which accept checks and dispense cash to users. The ATMs are operated to acquire image and magnetic data from deposited checks to determine the genuineness of checks and the authority of a user to receive cash for such checks. Cash may be dispensed to the user from the ATM in exchange for the deposited checks.
Owner:DIEBOLD NIXDORF
Articulating pin for bicycle chains and relative chain
ActiveCN101561027AMinimize the risk of damageEffective connectionDriving chainsMetal chainsClassical mechanicsEngineering
The invention relates to an articulating pin for bicycle chains and relative chain. Concretely, the articulating pin for bicycle chains, of the type capable to be inserted in aligned holes of an outerlink and of the corresponding inner link of the chain, to hinge the links to one another, has a central portion and a distal portion. The distal portion is elastically deformable for the insertion th rough the holes. The invention also discloses a lock button for bicycle chains, which is characterized in that the lock button can deform plasticly to prevent the wall of the pin from deforming or moving along the related lognitudinal axes. The invention also discloses a lock assembly for chains including the said articulating pin and the corresponding lock button, a bicycle drive chain including the said assembly and tools for assembling the bicycle chain.
Owner:CAMPAGNOLO SRL
Method for generating a circular periodic structure on a basic support material
ActiveUS20060098566A1Improve throughputMinimize the risk of damageMechanical record carriersRecord information storageMagnetic storageLight beam
The present invention is directed to a method for the generation of periodic curved structures in a basic support material such as the basic layer for the magnetic bit cells of a magnetic storage device. The method includes the steps of generating a number of diffraction masks such that each mask comprises at least one transmission diffraction gratings having at least one of a different periodic concentric circular pattern, spiral-like periodic pattern and periodic radial spoke pattern; positioning at least one of the diffraction masks simultaneously or successively in a certain distance of the basic support material to be patterned, the distance being mask dependent; exposing the basic support material by directing light beams through each of the diffraction masks; and interfering the different light beams diffracted by the gratings on each mask in order to generate coincident light intensity patterns on the surface of the basic support material.
Owner:EULITHA
Test connector with metallic stiffener
InactiveUS20050233614A1Minimize the risk of damageMinimize damageEngagement/disengagement of coupling partsMeasurement instrument housingElectrical and Electronics engineering
Owner:HON HAI PRECISION IND CO LTD
Holding device
InactiveUS7425311B2Simplifies insertionEasy transferBioreactor/fermenter combinationsShaking/oscillating/vibrating mixersGraphiteEngineering
A holding device for at least one object carrier, the object carrier being suitable to receive one or more organic and / or inorganic samples and comprising materials such as glass, plastic, silicon, pyrolytic graphite, and / or metal, this holding device being configured to be gripped by grippers of a robot. The holding device comprising two essentially parallel lengthwise walls and two essentially parallel transverse walls which extend substantially at right angles from the lengthwise walls. Holding devices according to preferred embodiments are constructed in a frame shape, wherein the lengthwise and transverse walls define a frame surrounding at least one opening which completely transverses the device. Holding devices according to further embodiments are constructed a plate shape, in that the region between the lengthwise walls and transverse walls is implemented as a carrying surface. All embodiments comprising gripping surfaces on the external surface profile of the lengthwise and transverse walls.
Owner:TECAN TRADING AG
Method of manufacturing a semiconductor structure and a corresponding semiconductor structure
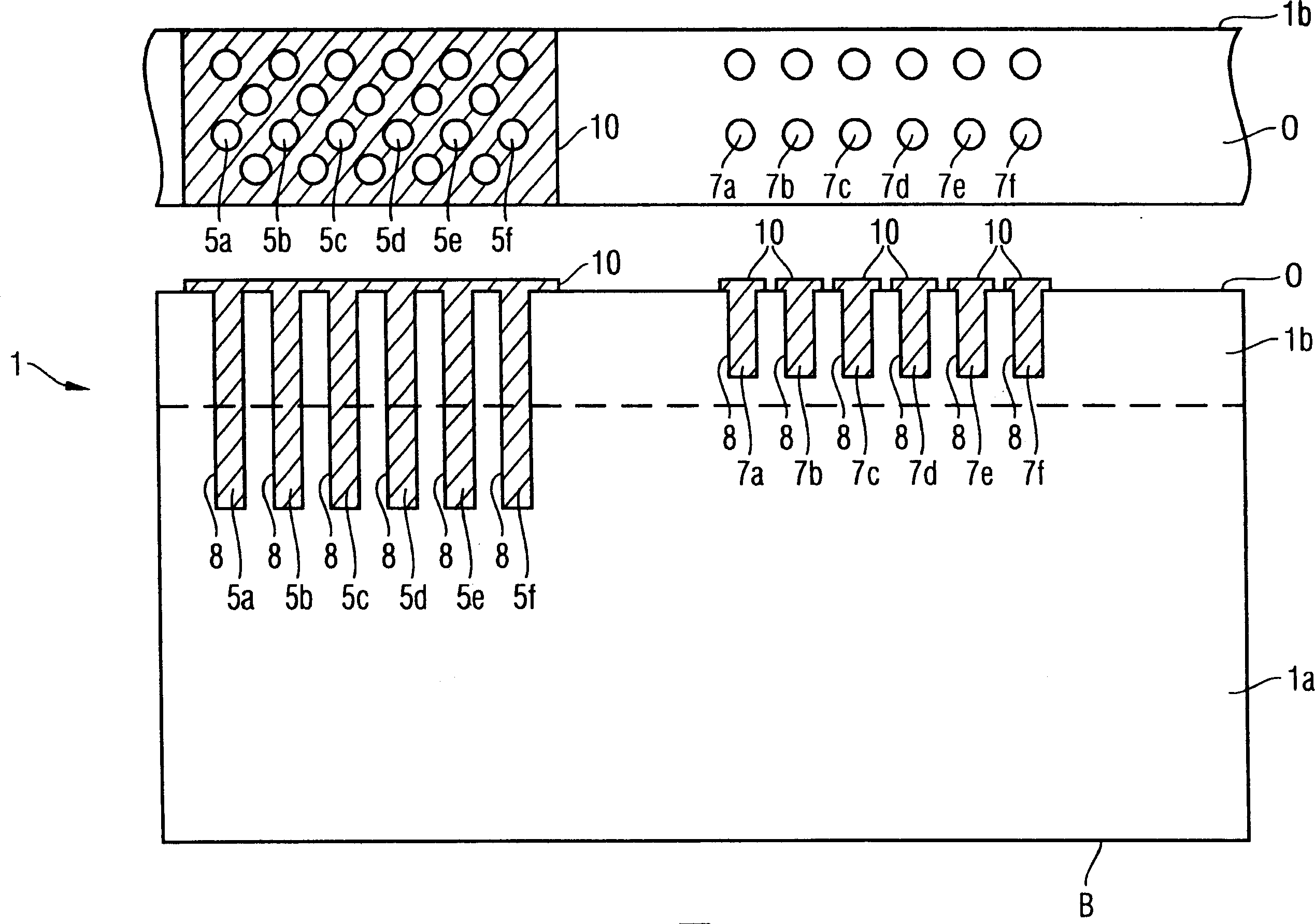
InactiveCN1909208AWill not modifySo as not to damageSemiconductor/solid-state device detailsSolid-state devicesSemiconductor structureEngineering
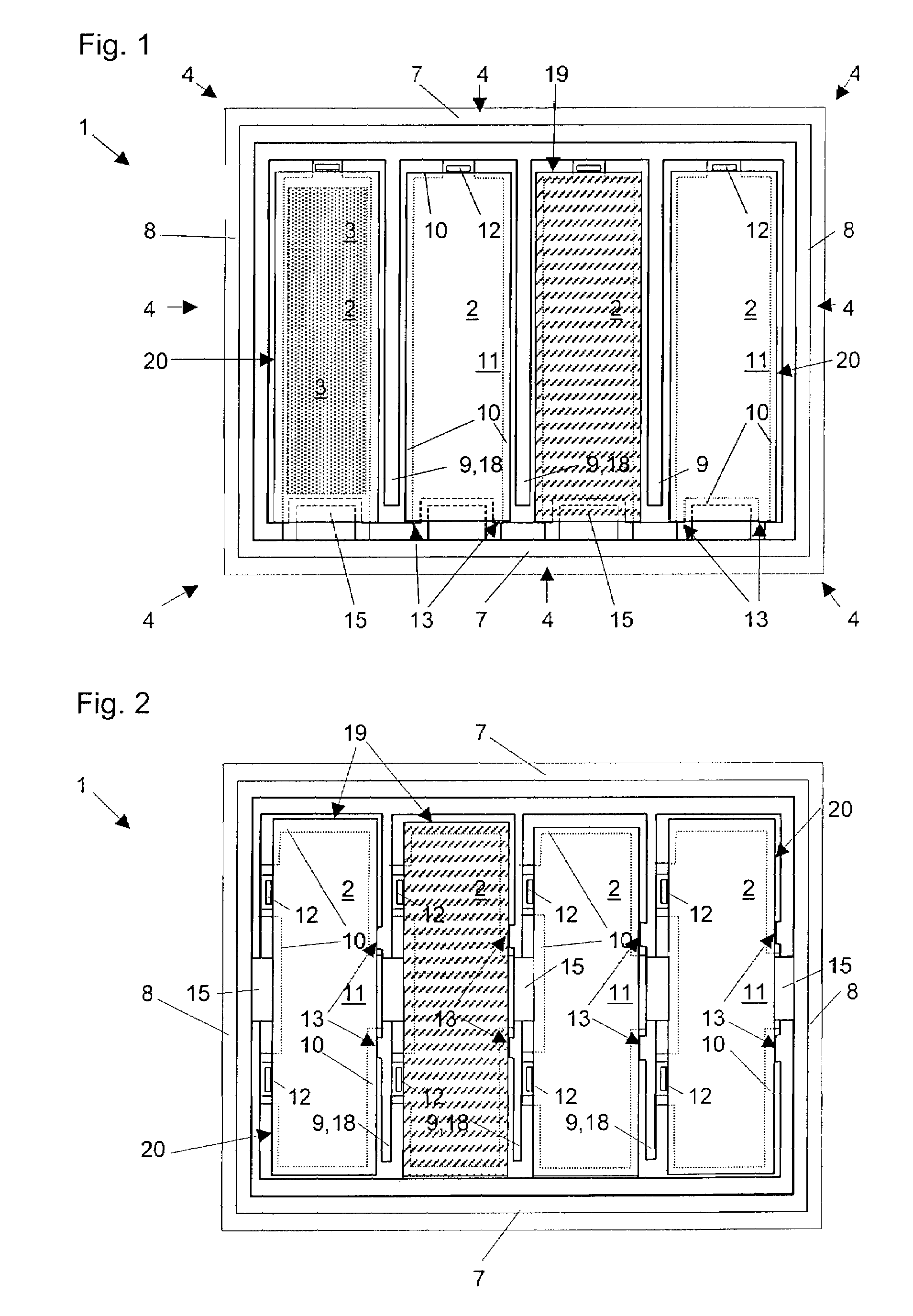
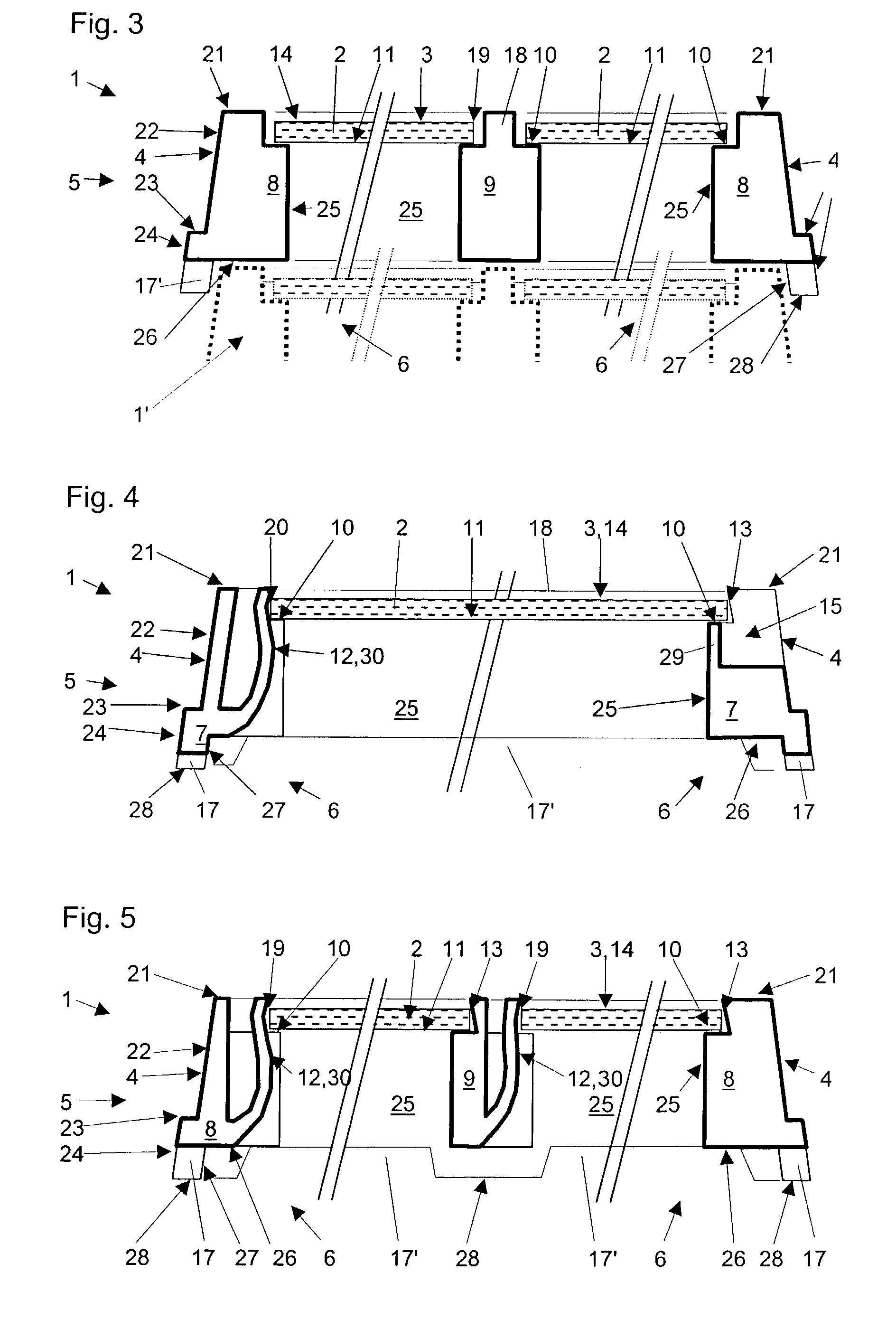
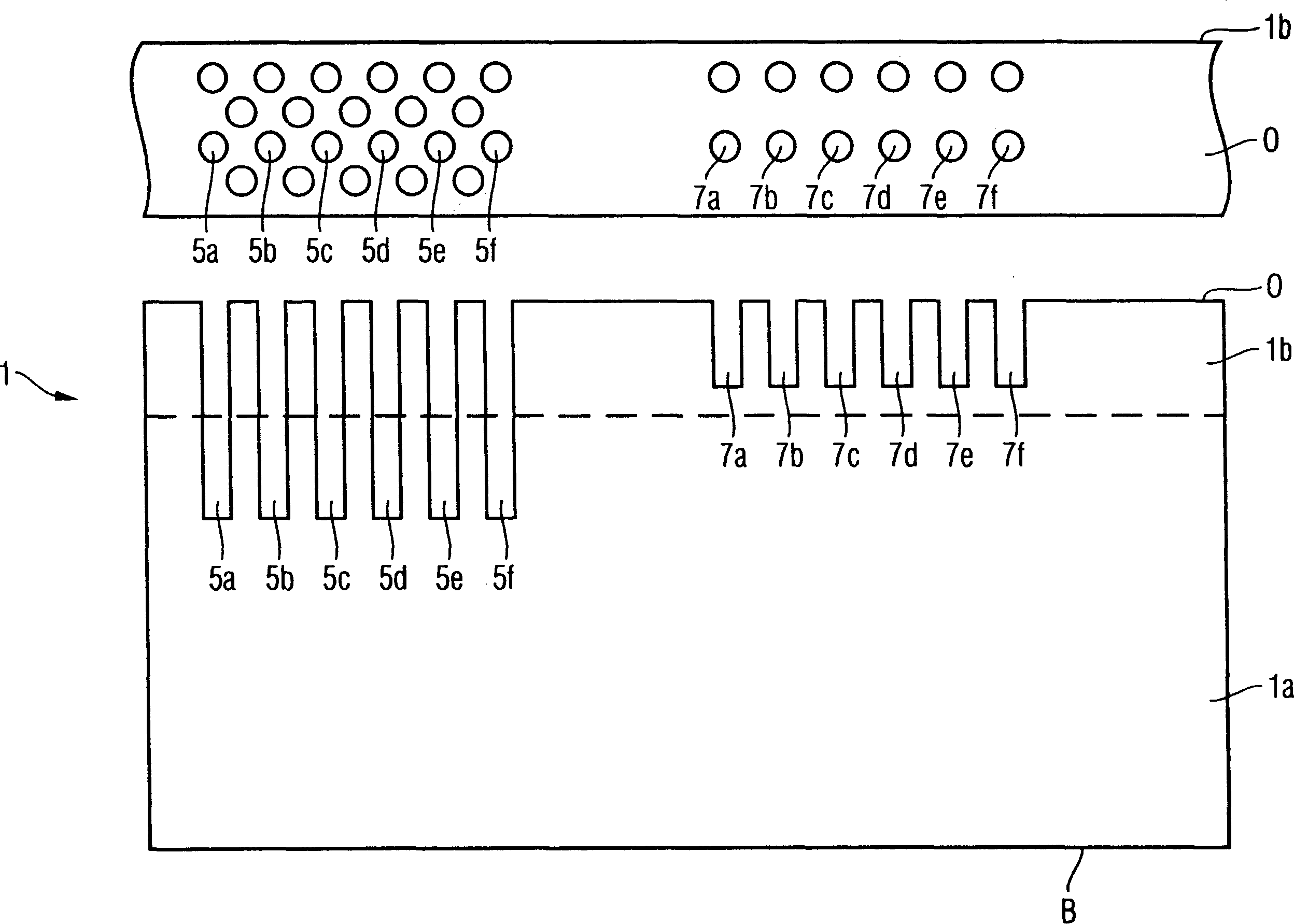
The invention provides a method of manufacturing a semiconductor structure having a wafer through-contact that can be achieved easily and safely. The method of manufacturing a semiconductor structure having a wafer through-contact comprises: a step of providing a semiconductor wafer (1) having a bulk region (1a) and an active region (1b); a step of forming multiple contact trenches (5a-5f), which extend from the top surface (0) of the active region (1b) to the bulk region (1a), in the semiconductor wafer (1); a step of forming a first dielectric isolation layer (8) on the sidewalls and the bottoms of the contact trenches (5a-5f); a step of providing a first conductive filler (10) in the multiple contact trenches (5a-5f); a step of forming a via (V) that is arranged in the semiconductor wafer (1), extends from the backside (B) of the bulk region (1a) to the multiple contact trenches (5a-5f), and exposes the conductive filler (10); a step of forming a second dielectric isolation layer (15) on the sidewall of the via (V); and a step of providing a second conductive filler (20) in the via (V) which comes into contact with the exposed conductive filler (10).
Owner:QIMONDA
Opthalmologic therapy system and method for processing a portion of a processing volume of a transparent material by application of focused radiation
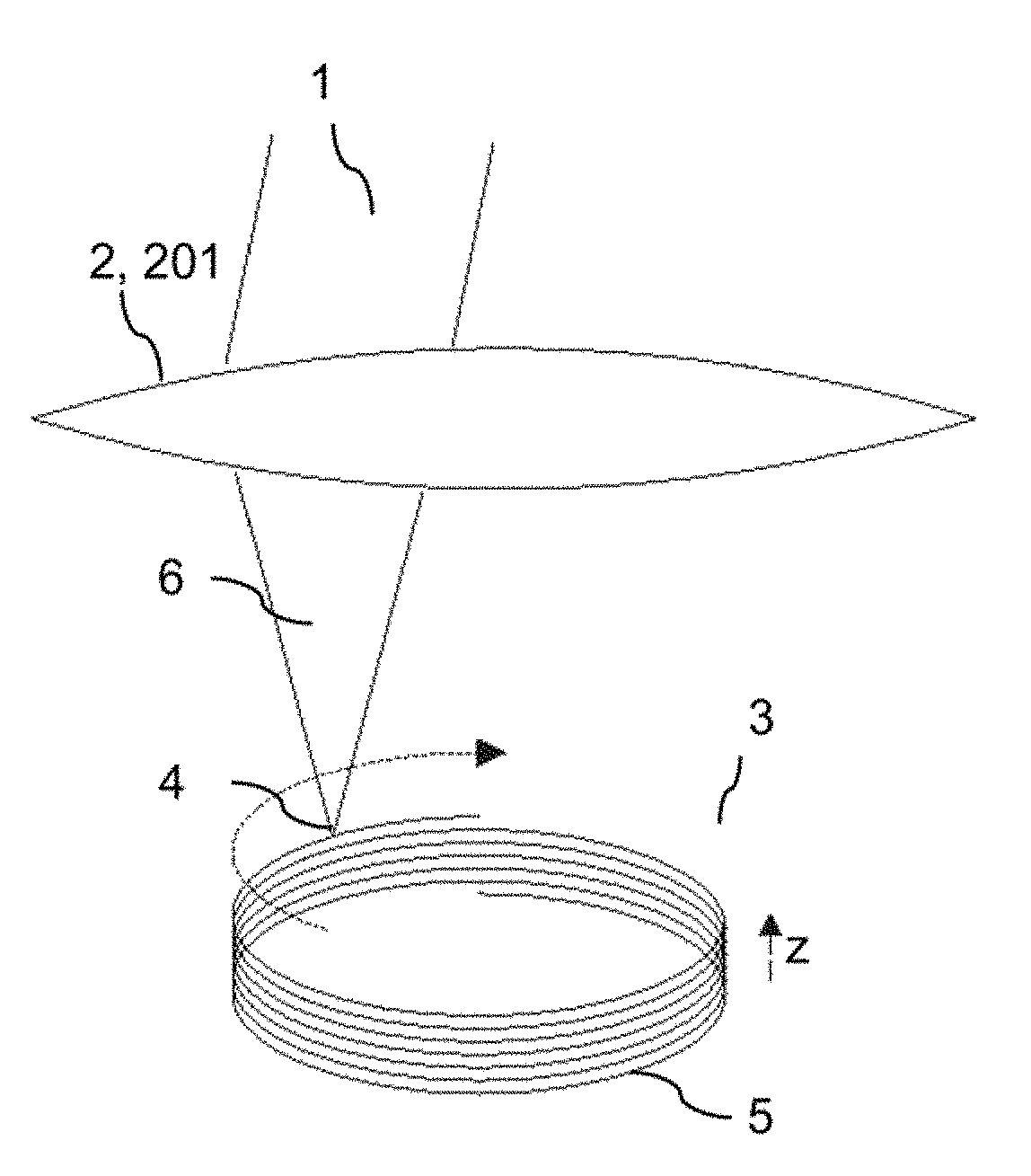
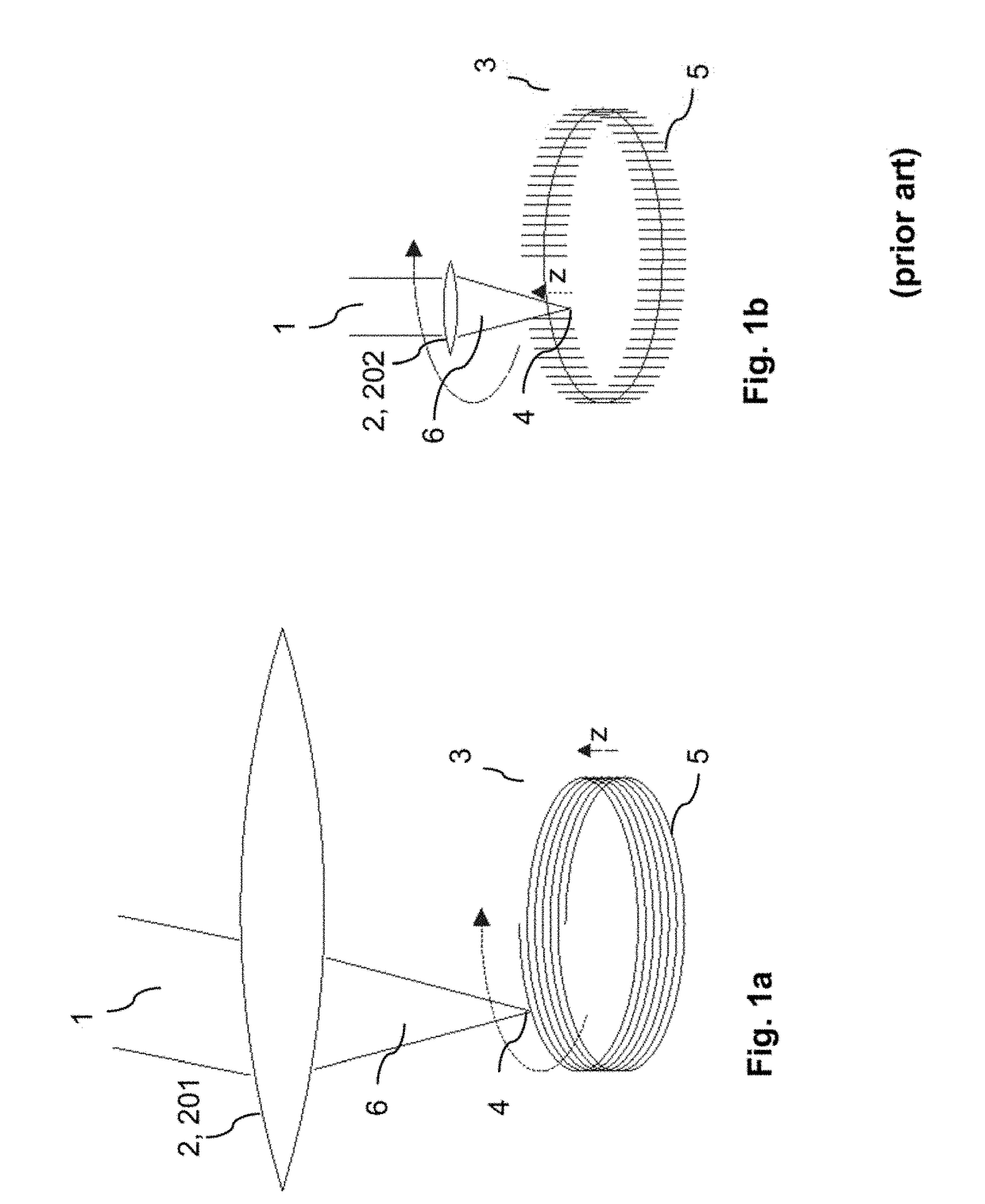
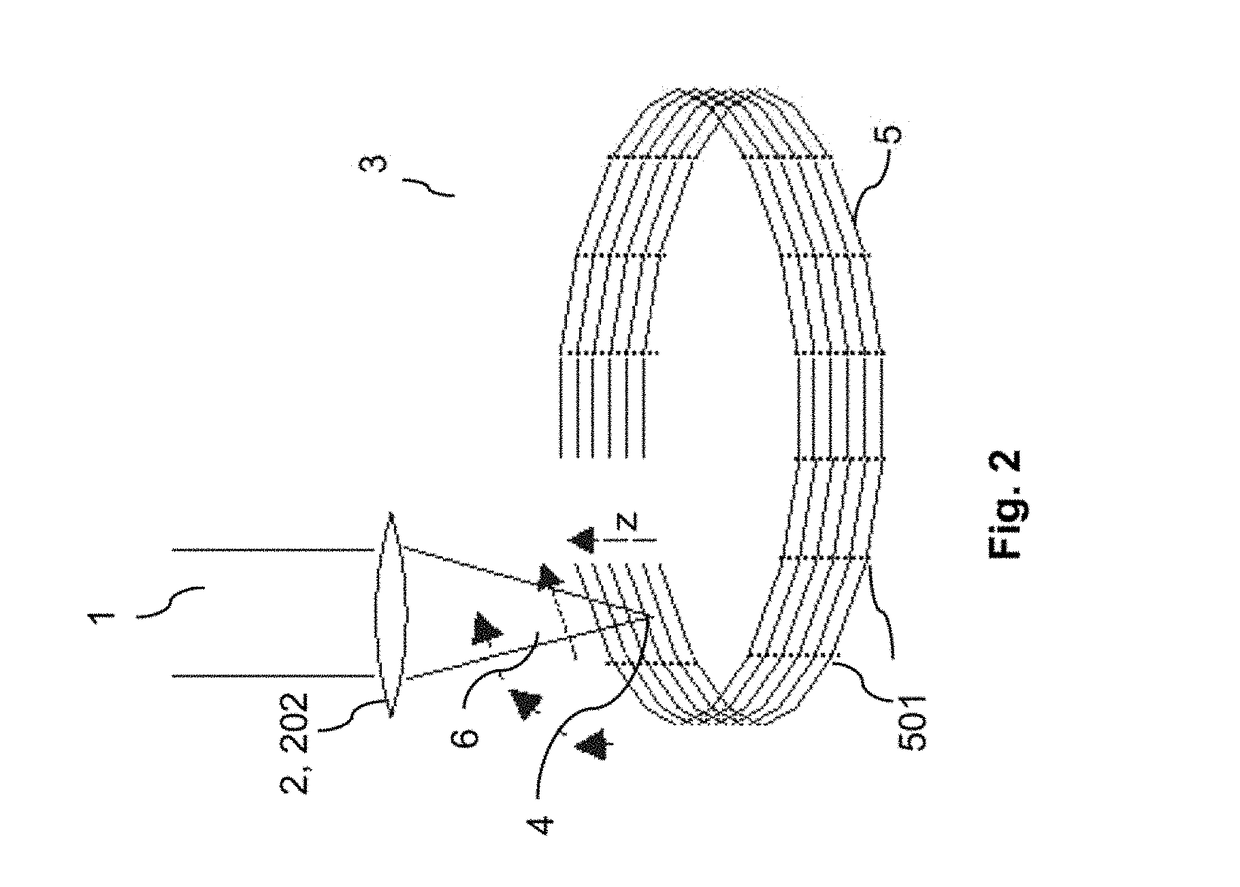
ActiveUS20180193196A1Quality improvementGreat possible flexibilityLaser surgeryComputational physicsFast scanning
A system for processing a portion in a processing volume of a transparent material by application of focused radiation including a device for generating and an optical system for focusing radiation, with a device for changing the position of the focus of the radiation and a control device. This system performs a slow scanning movement of the focus and an independent fast scanning movement of the focus which section can be moved by the slow scanning movement in the entire processing volume in an arbitrary direction; as well as by a system into which a scan pattern is encoded, with scanning movement including at least one lateral base component in the x- and / or y-direction, which is superimposed by components with synchronous change-of-direction-movements in the z-direction and in x-direction and / or y-direction. The invention also includes corresponding methods, a control program product and a planning unit.
Owner:CARL ZEISS MEDITEC AG
Method for releasing the pressure in a fuel system in a crash
InactiveCN104582992AAutomatic pressure reliefMinimize the risk of damagePedestrian/occupant safety arrangementElectric propulsion mountingInternal pressureOn board
It is proposed a method for controlling the depressurization of a fuel system mounted on board of a vehicle equipped with a vehicle impact sensor (14, 15), the method comprising the steps of: - detecting, using the vehicle impact sensor, whether an impact on the vehicle is present; and - when the impact on the vehicle is detected, using a controller (9, 10) for generating at least one signal for controlling the opening of at least one valve disposed in the fuel system, so as to release the internal pressure of the fuel system.
Owner:PLASTIC OMNIUM ADVANCED INNOVATION & RES SA
Mobile Washer Unit
InactiveUS20120234938A1Minimize the risk of damageMinimize of wearHand carts with one axisLiquid spraying apparatusMechanical engineeringEngineering
A mobile pressure washing system is provided. The system comprises a frame with hinged elements for transitioning the frame between an upright position of use and a compact position for secure transport and storage of the system. System features further include a removable and replaceable tank adapted to fit on a portion of the frame, storage features, and pressure washing features such as a motor and pump.
Owner:KARCHER NORTH AMERICA INC
Frozen soil layer natural gas hydrate horizontal branch well pattern mining system and method
InactiveCN109736769AReasonable designImprove production efficiency and economic benefitsFluid removalDirectional drillingDecompositionPlacer mining
The invention discloses a frozen soil layer natural gas hydrate horizontal branch well pattern mining system and method. The system comprises a main drilling well, a production area, a production well, an area multi-branch well, a production area boundary, a filling body, a decomposition enhancement area, a main drilling well horizontal section, a main drilling well vertical section, a branch well, a decomposition circle and an arch structure. The multi-branch well pattern has the beneficial effects that the multi-branch well pattern can improve the pressure sensitivity of formation fluid andprovide more decomposition free surfaces for natural gas hydrates; after mining is finished, grouting operation is carried out to fill cavities and through cracks formed after mining, the rock stratumarch structure is enhanced, potential environment damage risks are reduced, and the division of production areas and the succession order between the production areas in the long-term production process are planned.
Owner:CHINA UNIV OF MINING & TECH
Photovoltaic mounting rail connector with drop-down connection to first photovoltaic module and slide-in connection to second photovoltaic module
ActiveUS20170063007A1Shorten the timeSave energyPhotovoltaic supportsCoupling device connectionsElectrical and Electronics engineeringPhotovoltaics
A connector for attaching first and second photovoltaic modules to a mounting rail, with a lower body portion that rotates to lock into a mounting rail groove and an upper body portion with a hook that is lowered towards the lower body portion to grasp onto the first photovoltaic module and a key that receives the second photovoltaic module slidably-connected thereon.
Owner:TESLA INC
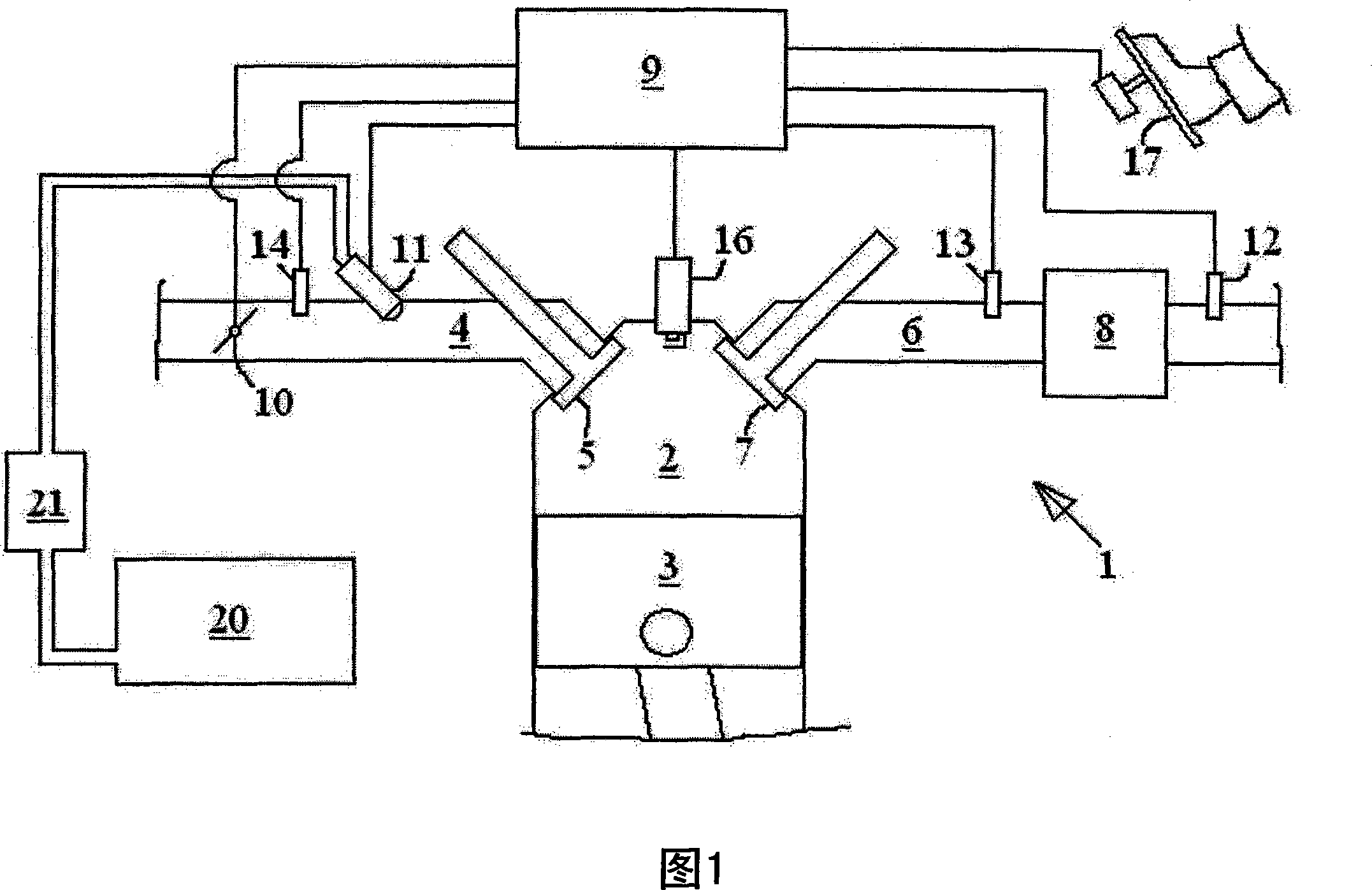
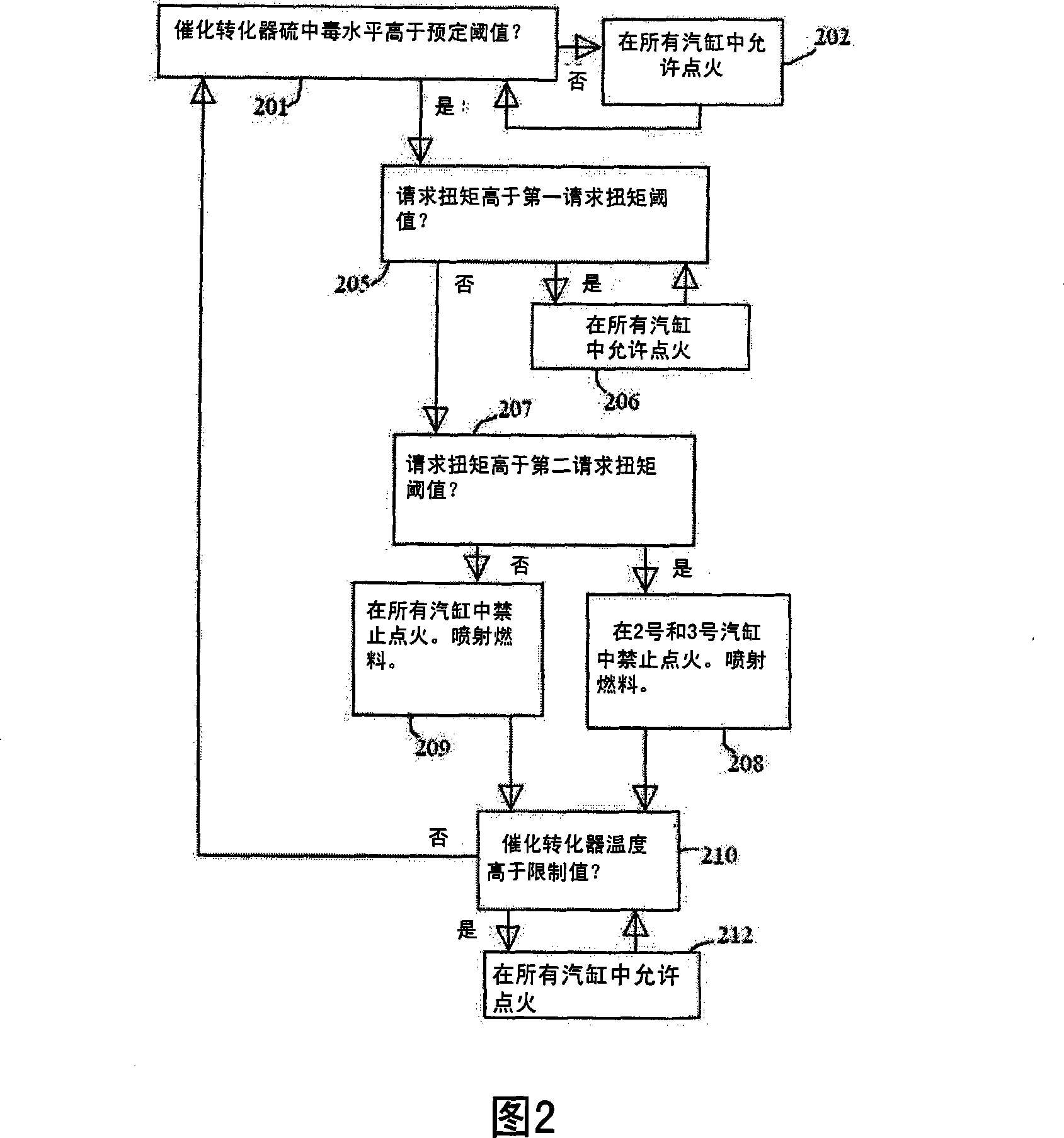
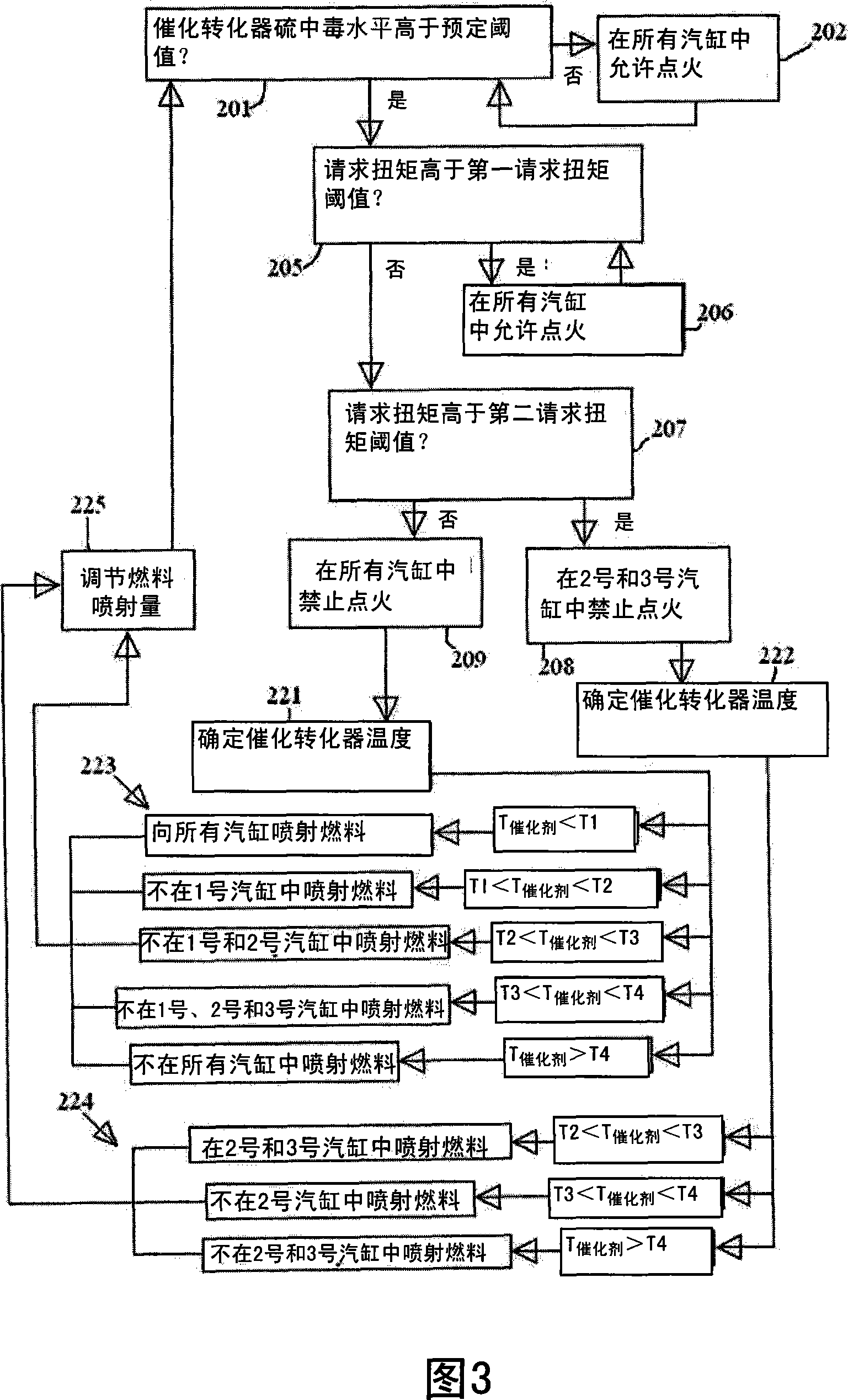
Exhaust gas treatment device regeneration inhibiting fuel combustion in engine cylinder
ActiveCN101205826AMinimize the risk of damageEfficient regenerationElectrical controlInternal combustion piston enginesCombustionExternal combustion engine
A method for an exhaust gas treatment device (8) in an engine system, and an engine system comprising an internal combustion engine with at least one cylinder (2) are presented. The engine system further comprises fuel injection means (11) and an exhaust gas treatment device (8). The method comprises performing a combustion inhibition exhaust gas treatment device regeneration (208, 209, 223, 224,225), comprising inhibiting combustion in at least one of the cylinders (2), and controlling the fuel injection means (11) so that fuel is allowed into at least one of the cylinders (2) in which combustion is inhibited.
Owner:VOLVO CAR CORP
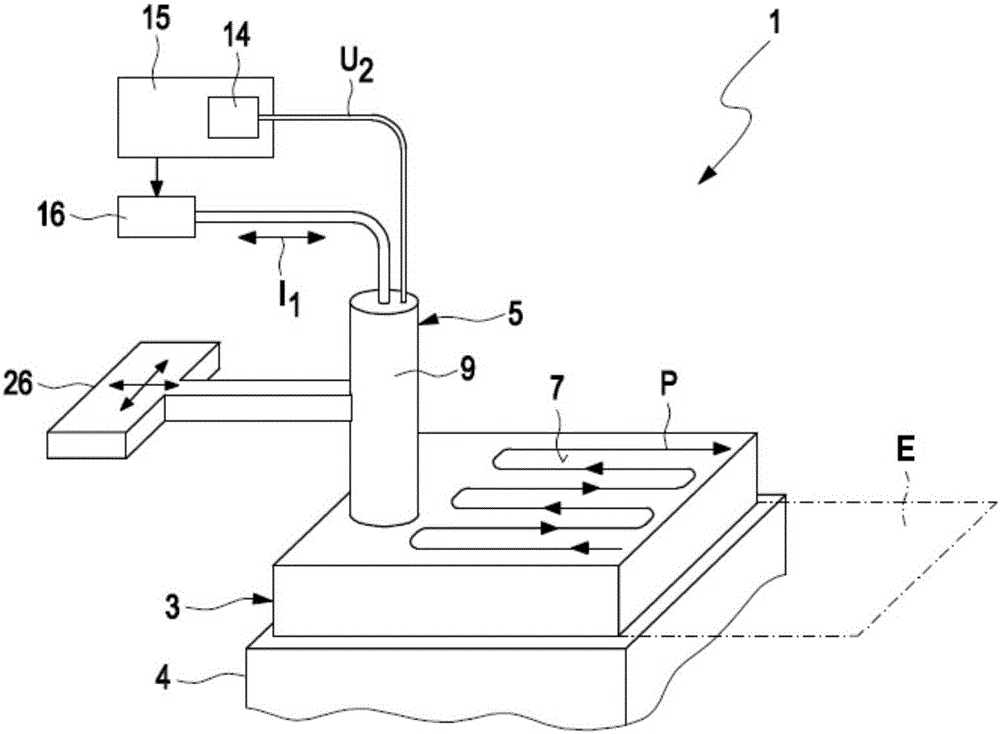
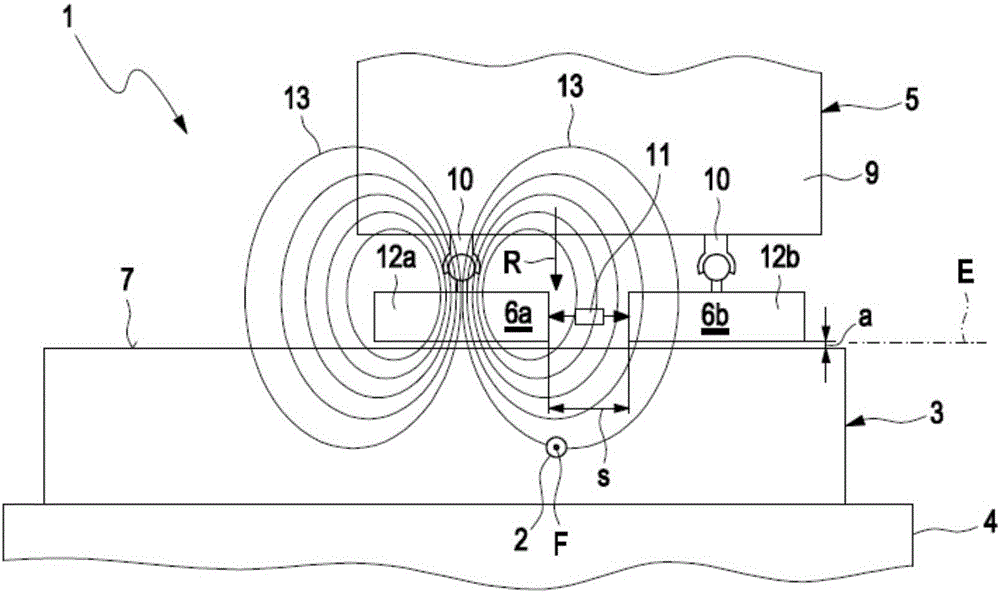
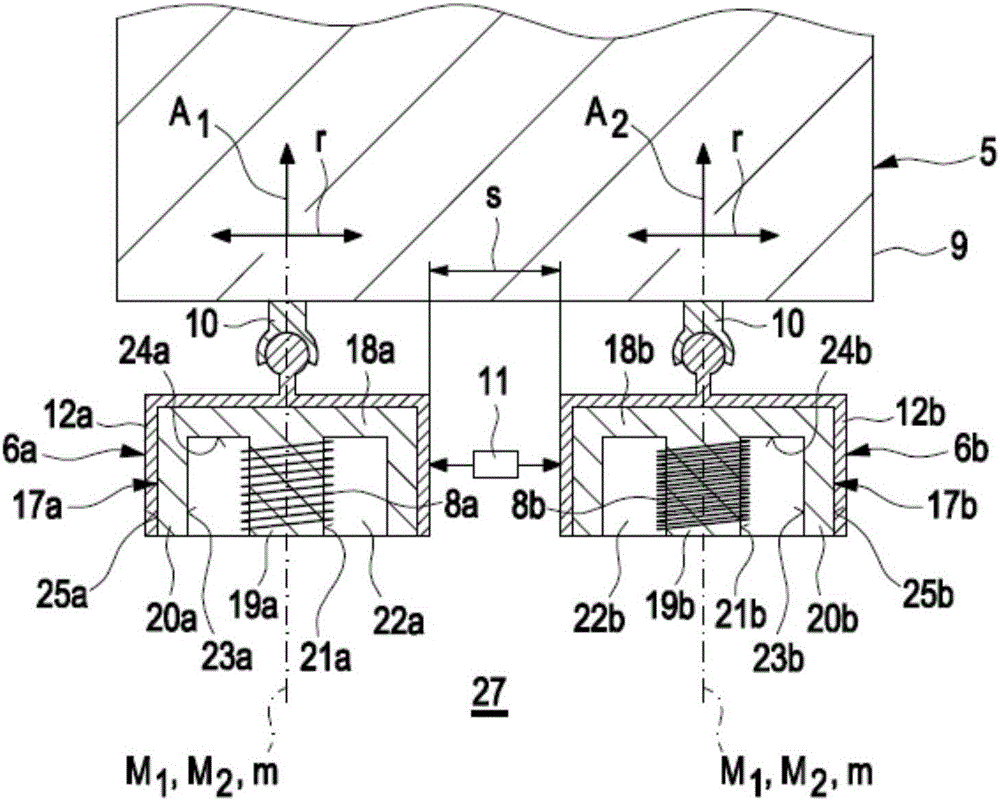
Method for detecting defects in a piston for an internal combustion engine
InactiveCN105911136AEasy to moveMinimize the risk of damageInternal-combustion engine testingMaterial magnetic variablesElectricityEngineering
A method for detecting defects in a piston for an internal combustion engine may include a) providing the piston (3) in a measurement arrangement (1) that includes a measurement probe (5) having a first electrical coil element (6a) for generating an electromagnetic alternating field and a second electrical coil element for detecting an electromagnetic alternating field. The method may also include b) moving the measurement probe (5) over a piston surface (7) of the piston, and providing an electrical alternating current (I1) in the first coil element (6a) to generate an electromagnetic alternating field (13) that interacts with a material of the piston in the region under the piston surface (7). The method may further include evaluating an electrical alternating voltage (U2) induced in the second coil element (6b) by the electromagnetic alternating field (18) after interaction with the material of the piston (3).
Owner:MAHLE INT GMBH
Device and method for forming a stack of sheets on a delivery surface
ActiveUS20050248079A1Prevent movementMinimize the risk of damagePile receiversArticle deliverySheet material
Owner:OCE TECH
Apparatus for locking a device to a cycle footrest
InactiveUS20060112745A1Conveniently unlockSafely securedBicycle locksAnti-theft cycle devicesLocking mechanismEngineering
A cycle footrest assembly including a footrest and a locking device fixedly mounted to the footrest. The locking device preferably includes a body, a locking mechanism, and a movable locking arm rotatably coupled to the body wherein the movable locking arm is operable by manipulating the locking mechanism.
Owner:COLLIER JAMES W
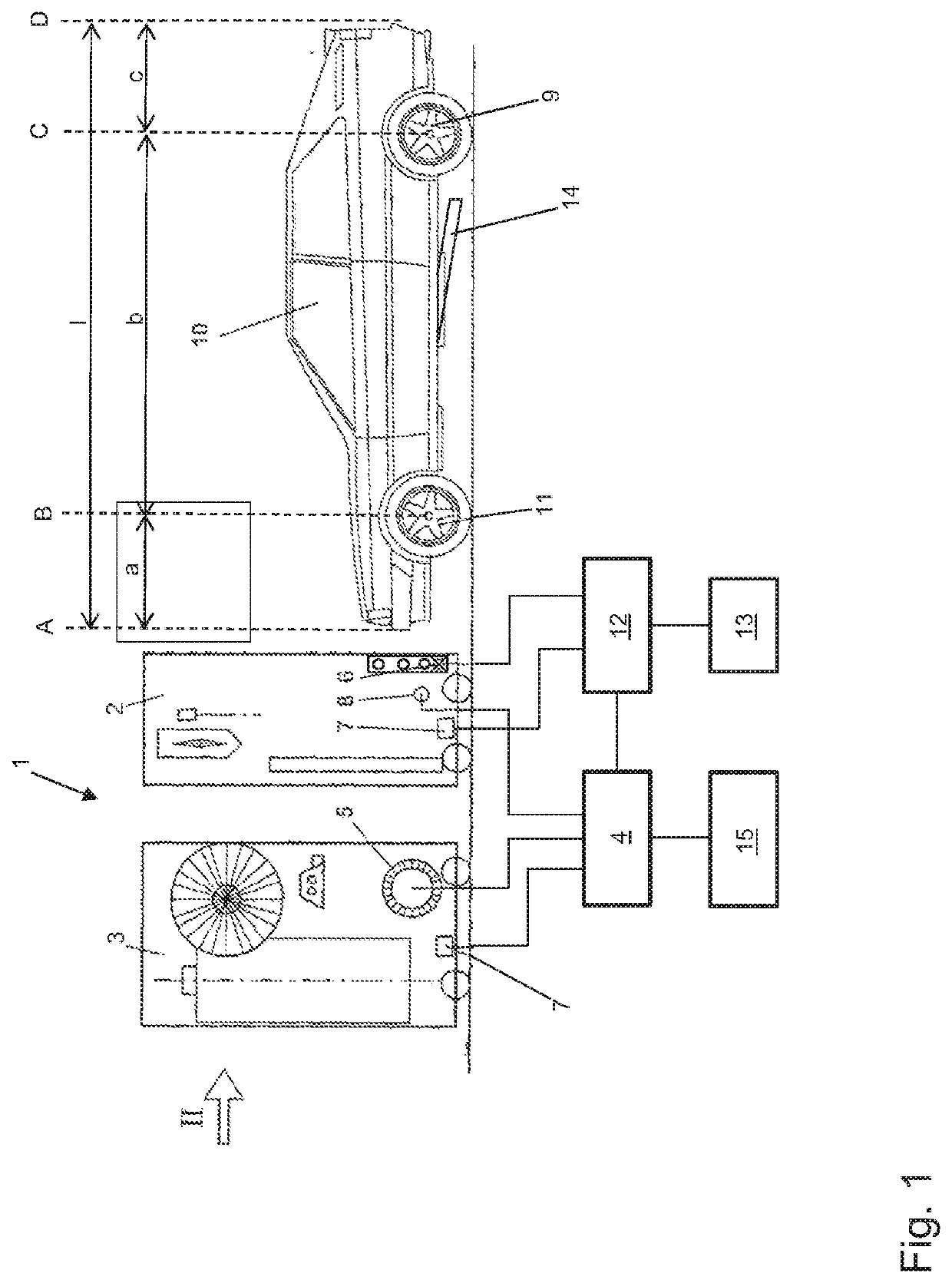
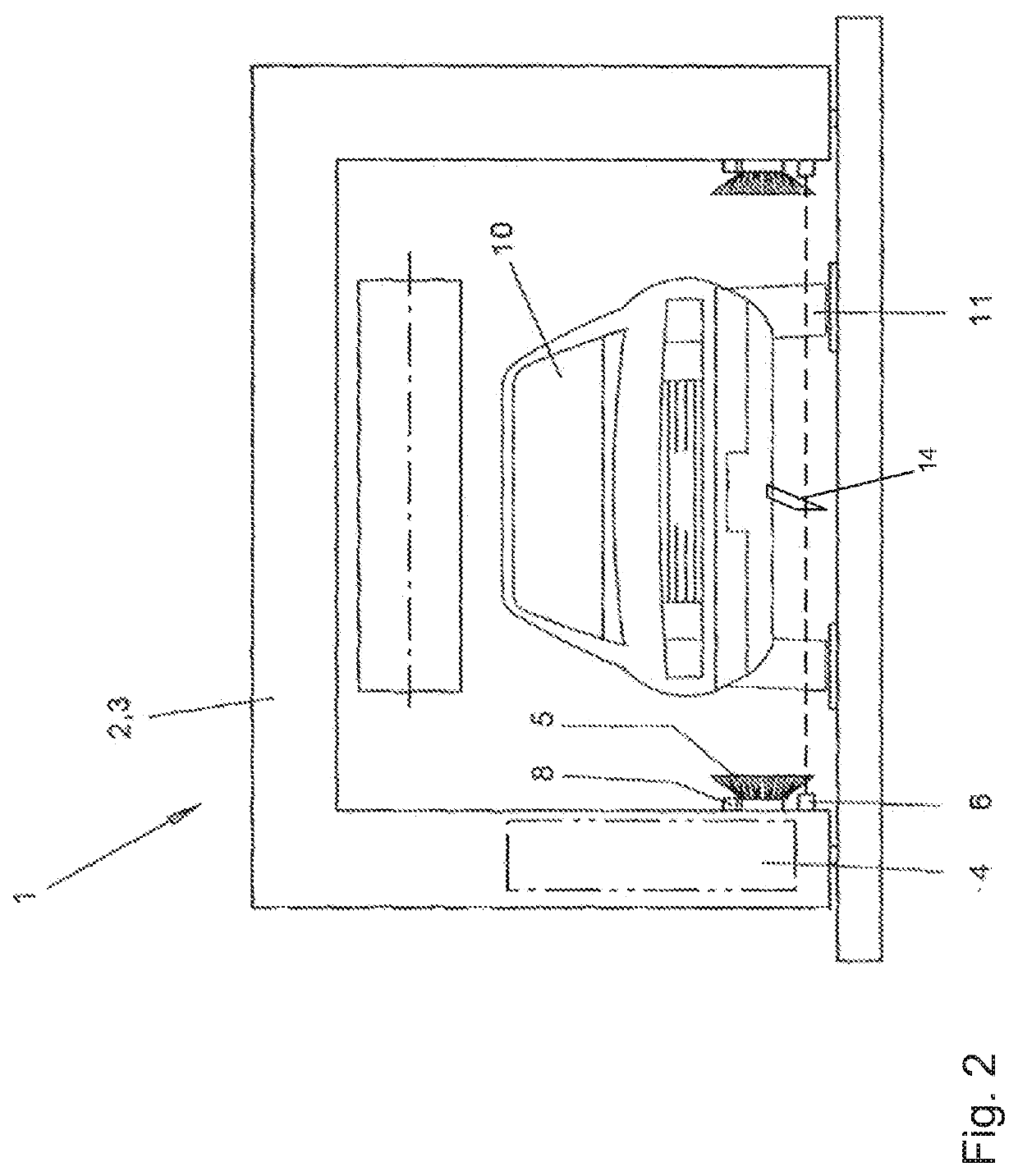
Method for operating a vehicle washing system, and vehicle washing system
ActiveUS11173880B2Minimize the risk of damageMinimize damageCleaning apparatus for vehicle exteriorsCleaning using toolsControl theoryMechanical engineering
A vehicle washing system and a method for operating such a vehicle washing system in which a vehicle is scanned in a longitudinal direction by a sensor device. Measurement data are captured so that position data for the positions of vehicle wheels are determined based on an evaluation of the measurement data. The determined position data of the positions of the wheels are subjected to a confidence check, so that a confidence measure is determined. If the determined confidence measure is greater than or equal to a confidence threshold value, a treatment of the wheels is carried out in a first mode at the positions associated with the position data, and, if the determined confidence measure is smaller than the confidence threshold value, a treatment of the wheels is not carried out, or is carried out in a second mode at the positions associated with the position data.
Owner:WASHTEC HLDG
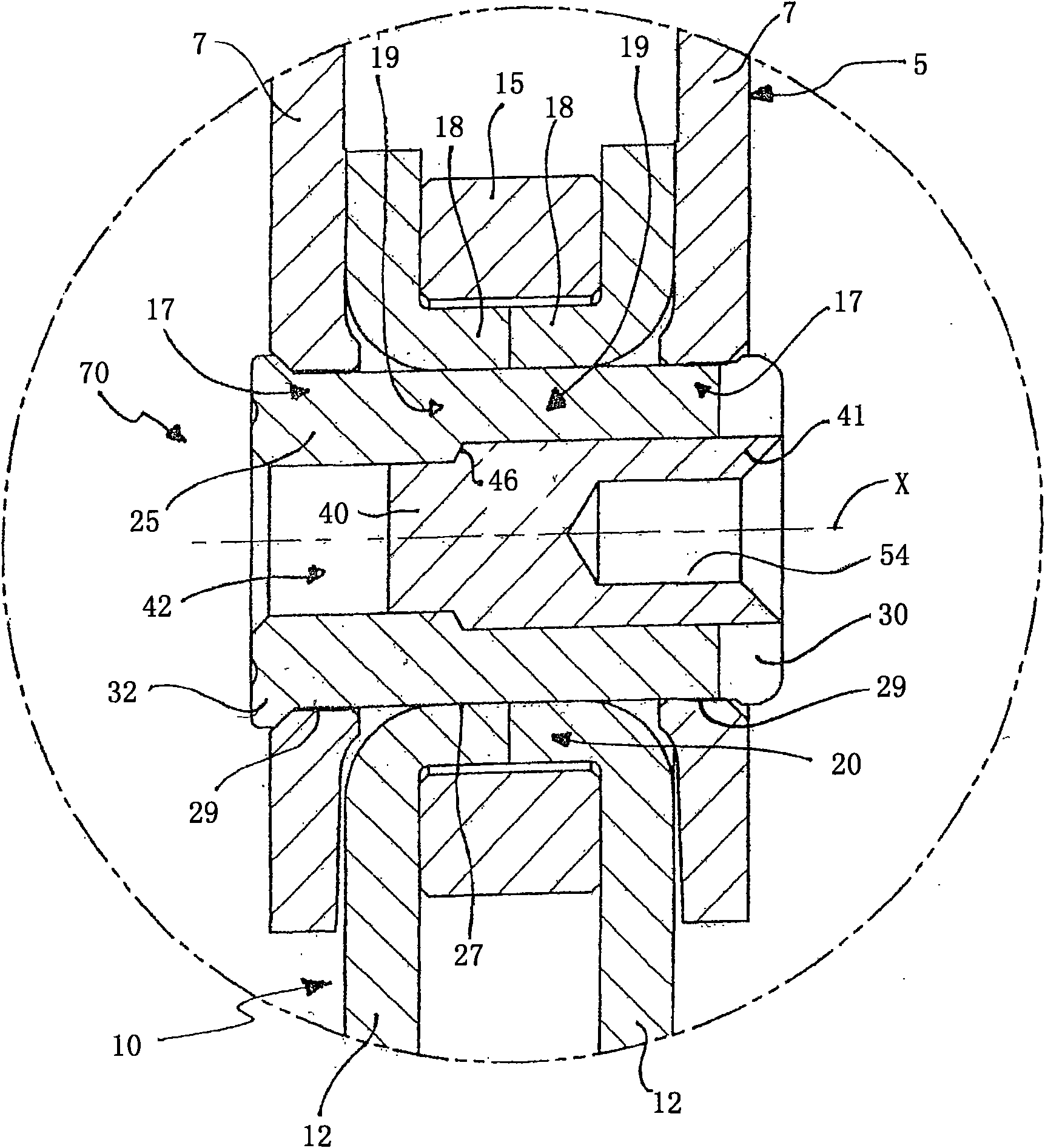
Method for producing a tapered roller assembly, tapered roller assembly and tapered roller bearing
Tapered roller bearings have in many design forms an inner ring, an outer ring and a plurality of tapered rollers which are arranged to roll between the inner ring and outer ring. It is the object ofthe present invention to propose a method for producing a tapered roller assembly which can be mounted in a particularly simple manner. For this purpose, a method for producing a tapered roller assembly (1) having an inner ring (3) and a roller ring (12) is proposed, wherein the method comprises the following steps: a) providing the inner ring (3), wherein the inner ring (3) has a flanged mountingrim (10) which is produced within a preliminary flanging stage by flanging over an edge region (6) of the inner ring (3); b) mounting the roller ring (12) on the inner ring (3), wherein the roller ring (12) is pushed over the flanged mounting rim (10) onto the inner ring (3); c) flanging over the flanged mounting rim (10) into a flanged rim (15), wherein the flanged rim (15), as a retaining rim,captively retains the roller ring (12); and d) heat-treating the flanged mounting rim (10) before step b) or step c), wherein a hardness of the flanged mounting rim (10) is reduced by comparison witha hardness of a raceway (5) of the inner ring (3). Also proposed is a tapered roller bearing (20) formed with such a tapered roller assembly (1).
Owner:SCHAEFFLER TECH AG & CO KG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com