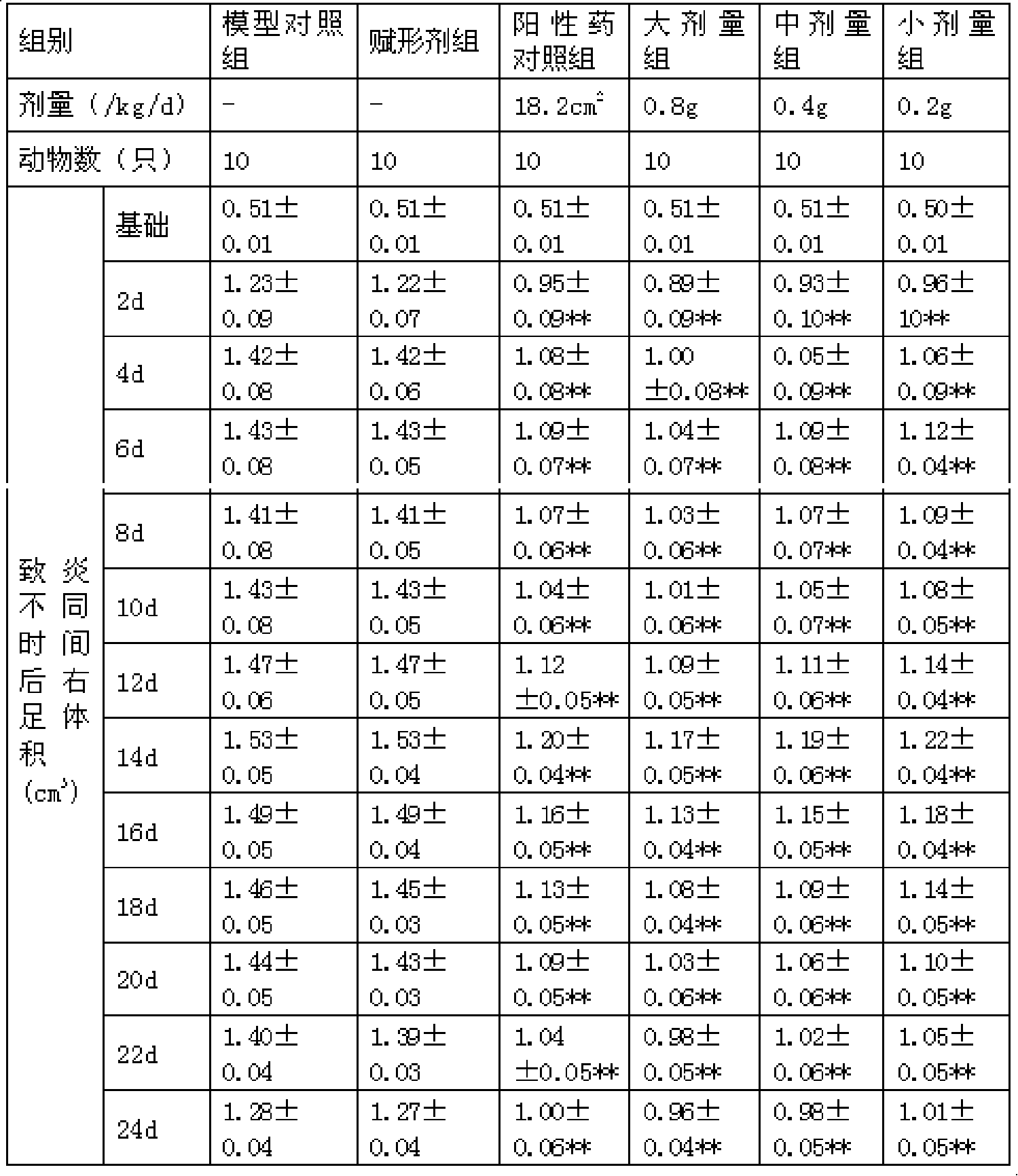
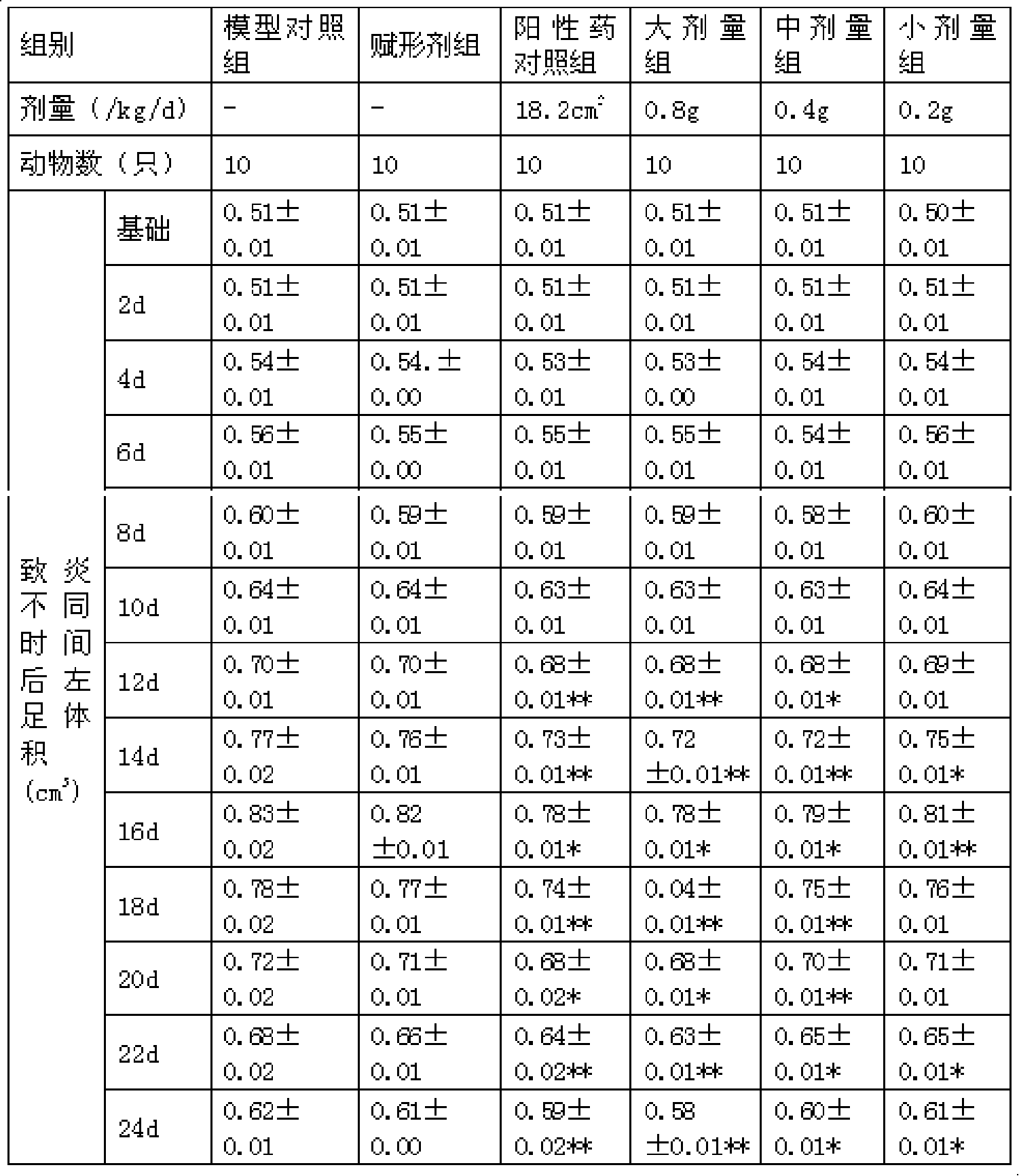
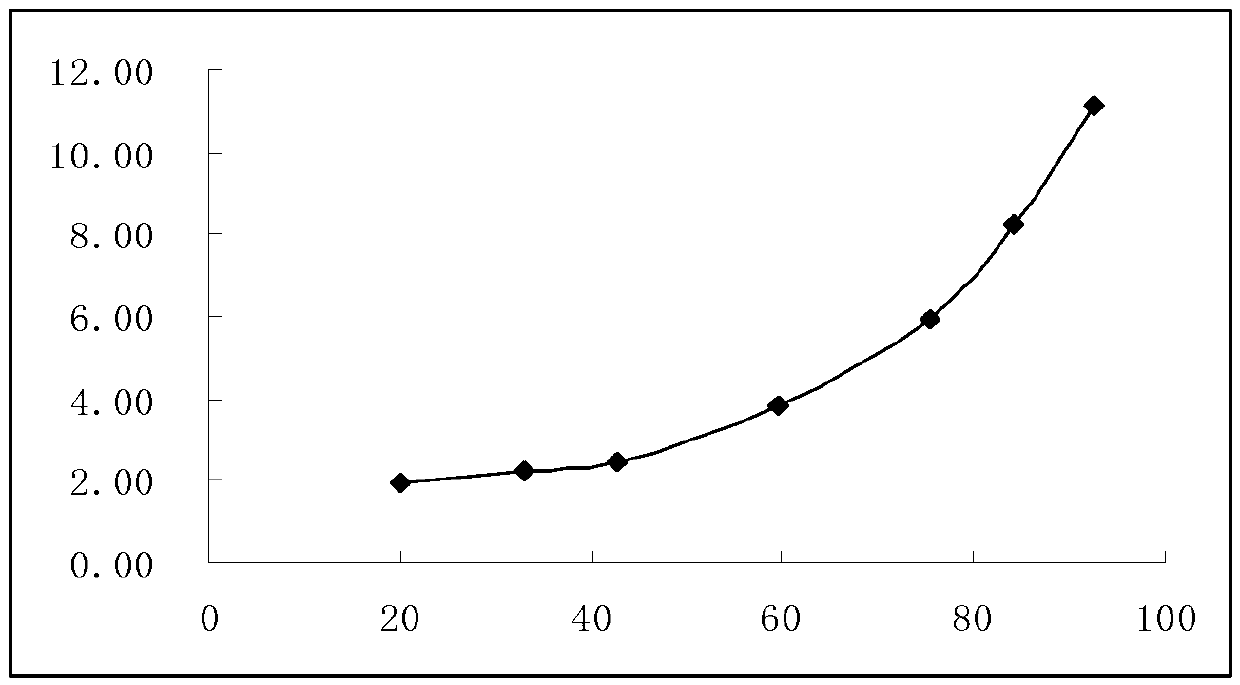
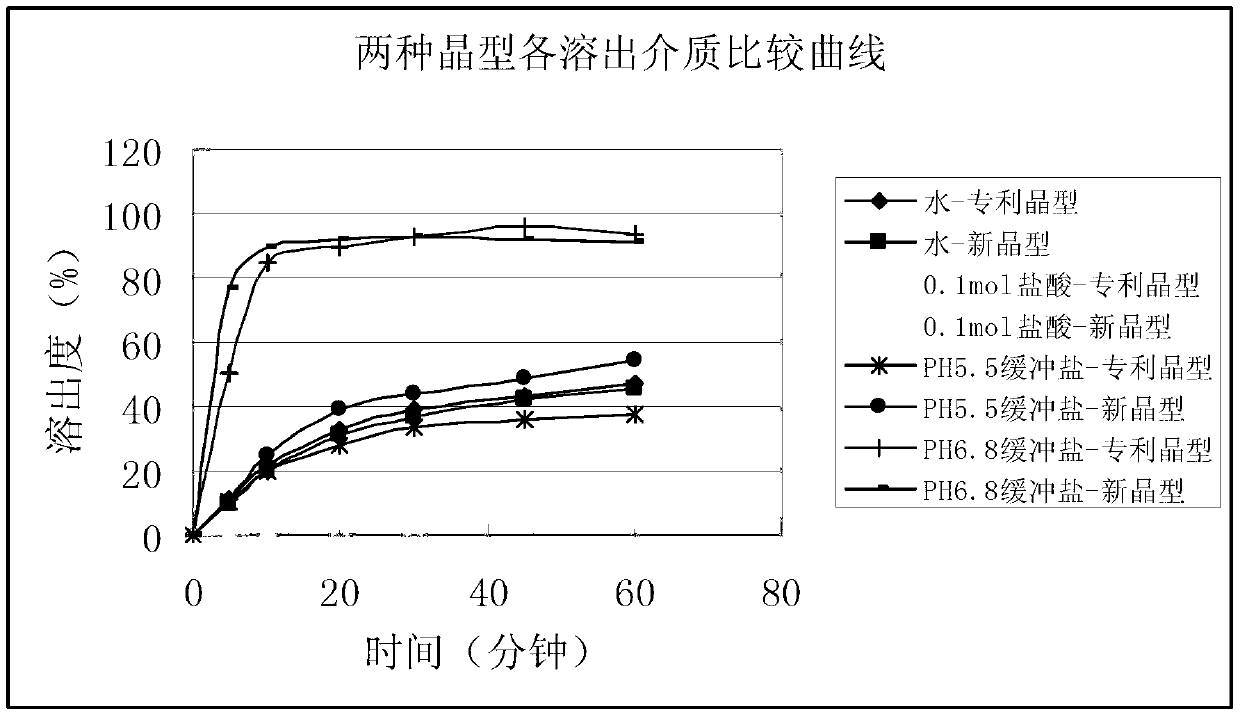
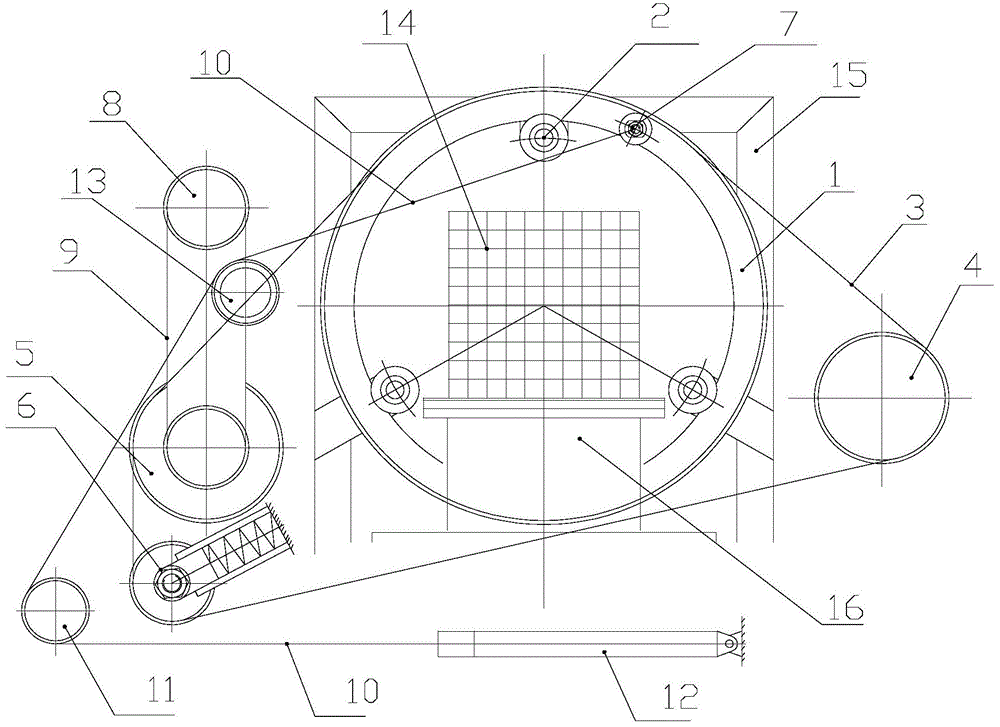
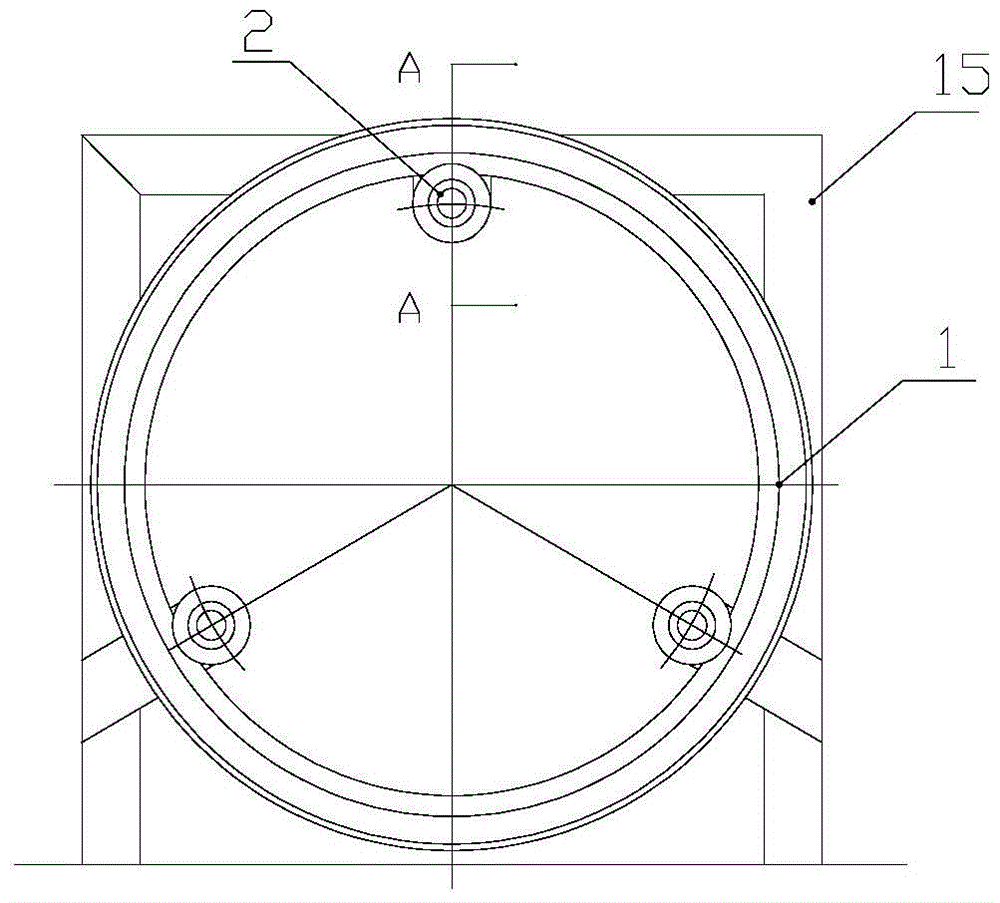
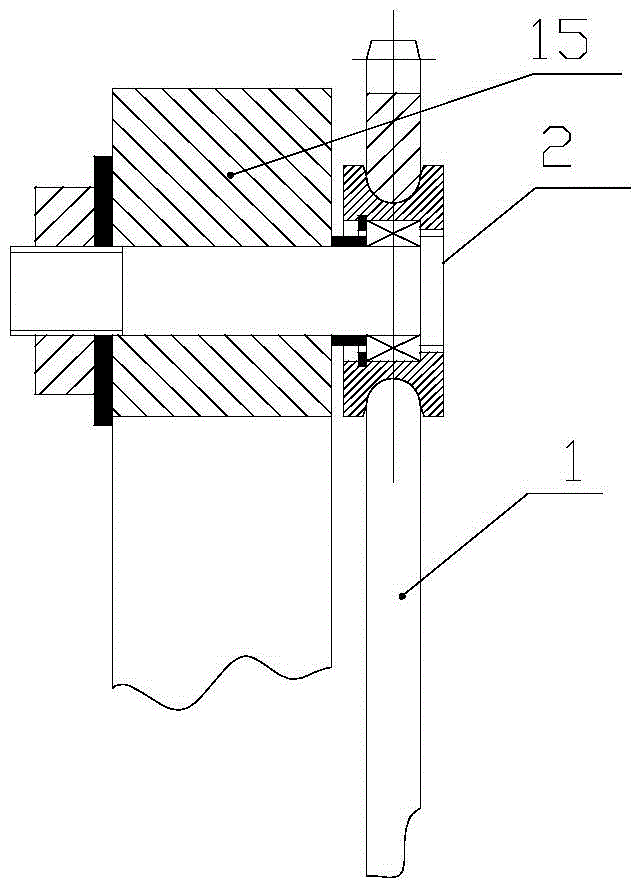
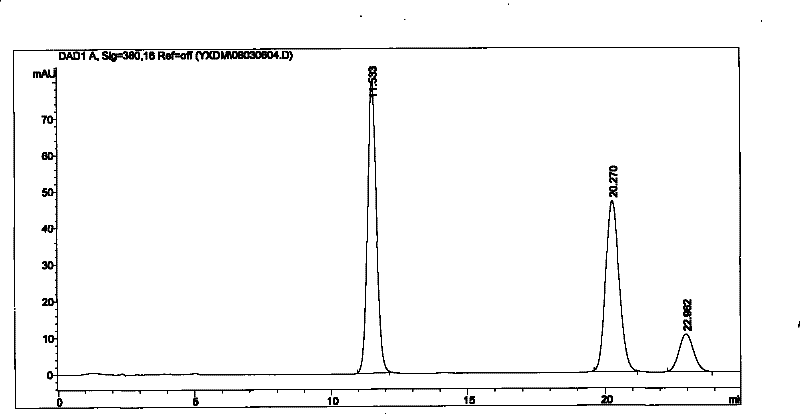
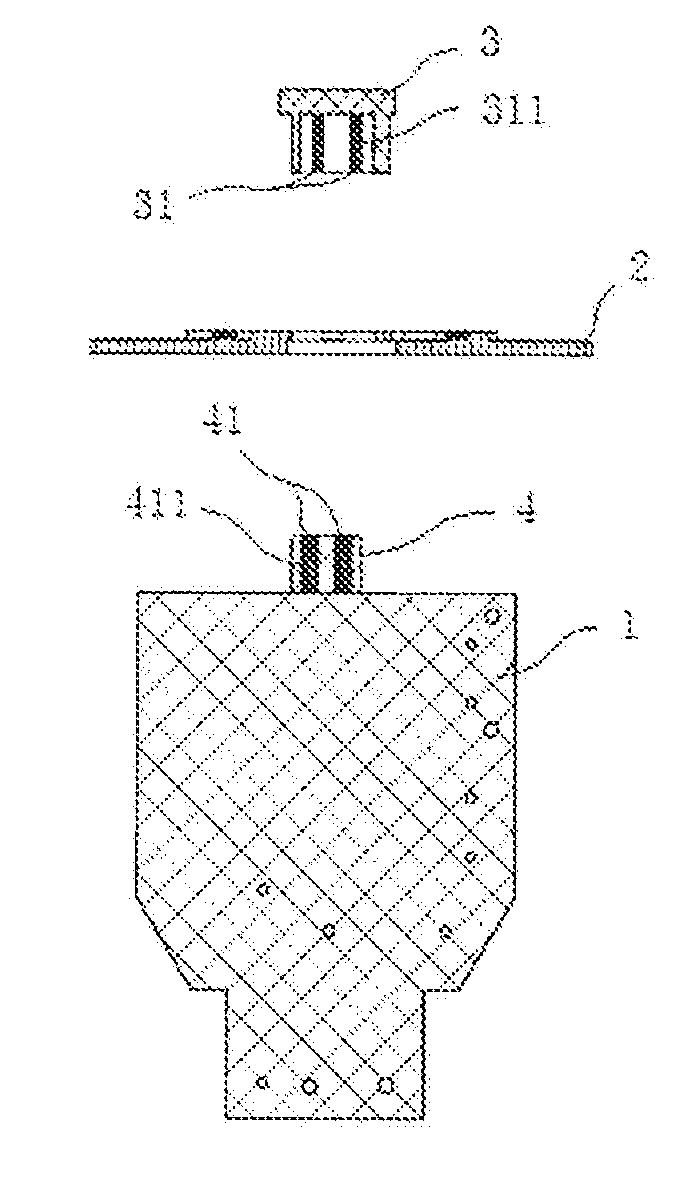
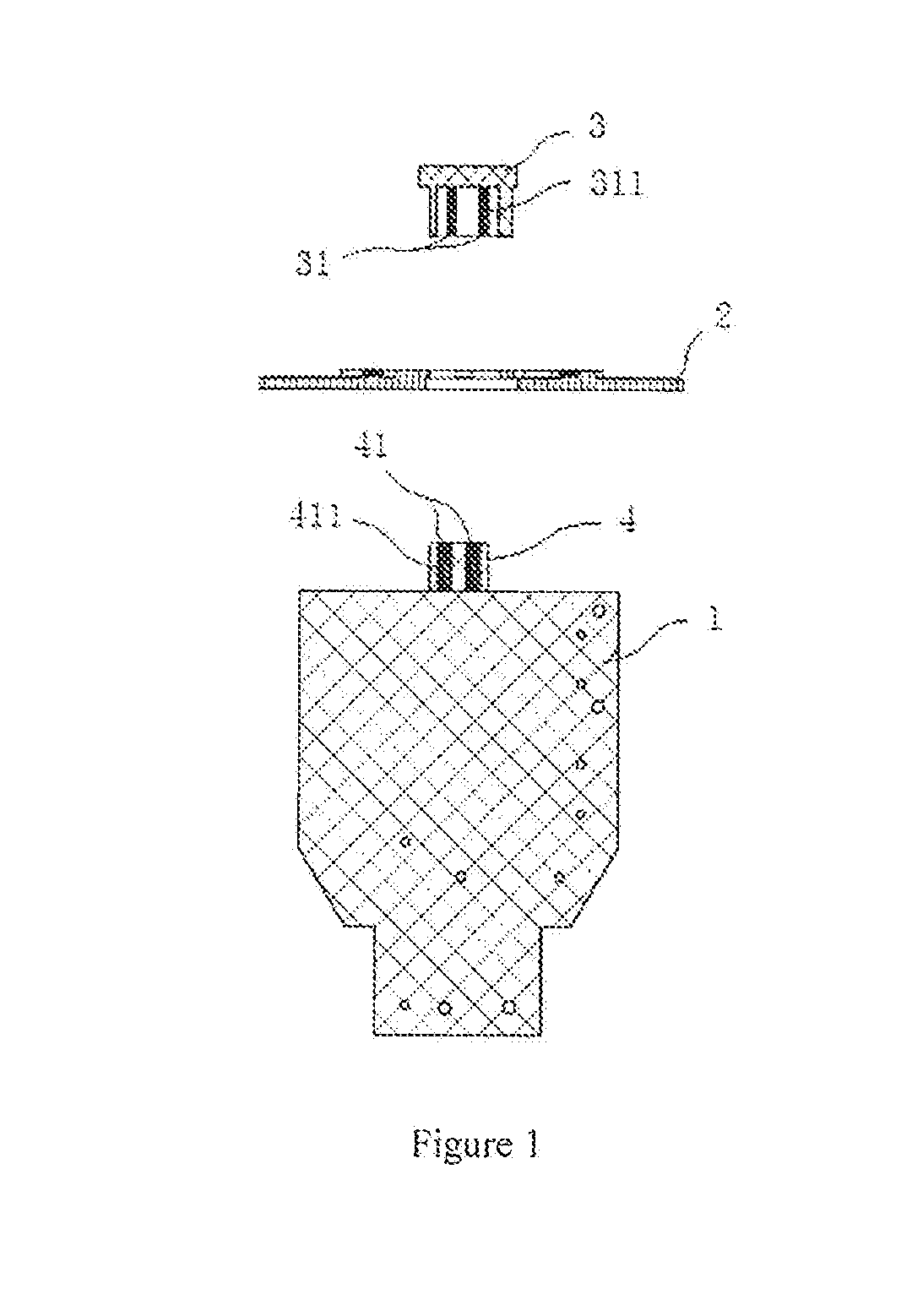
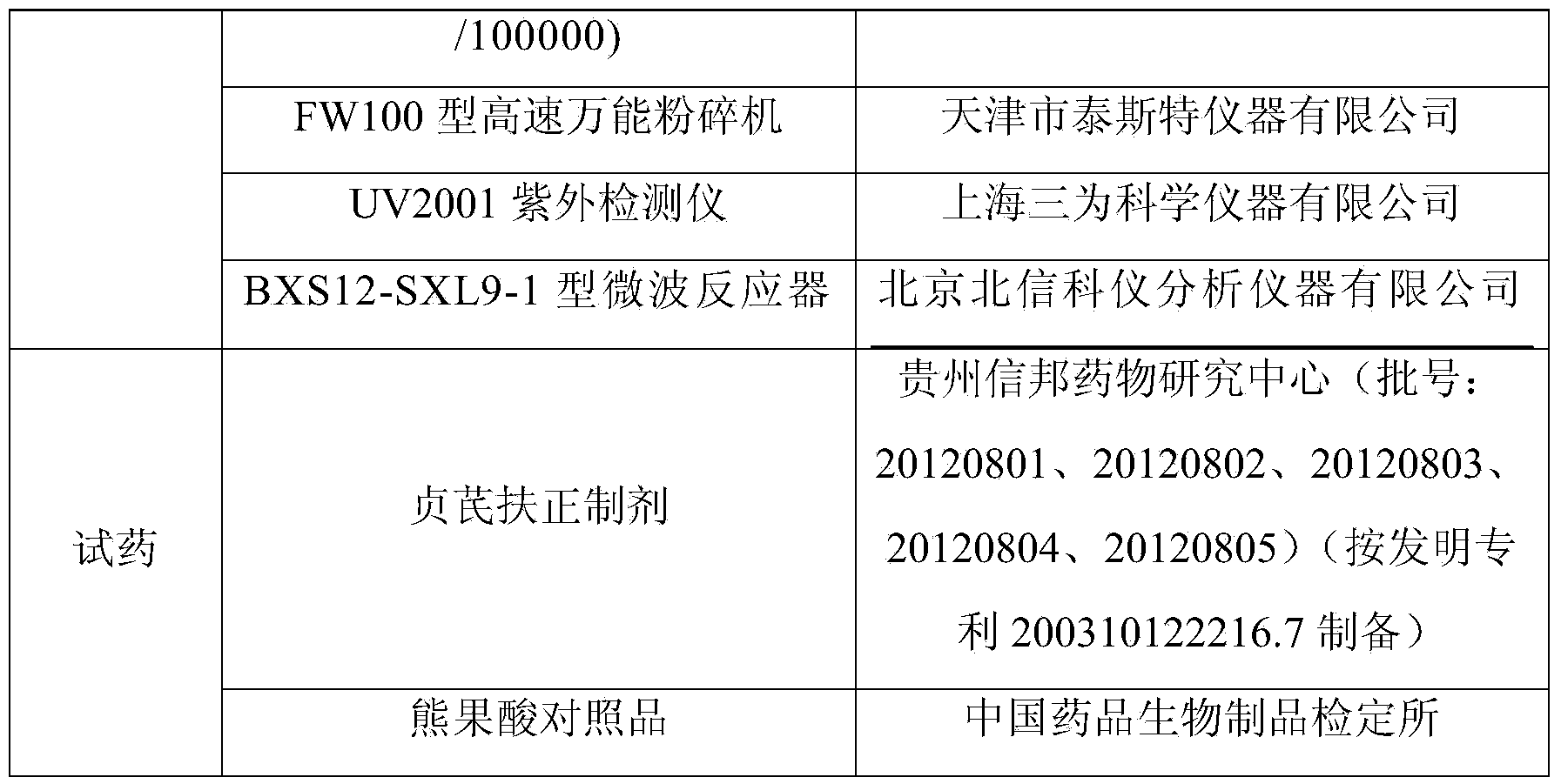
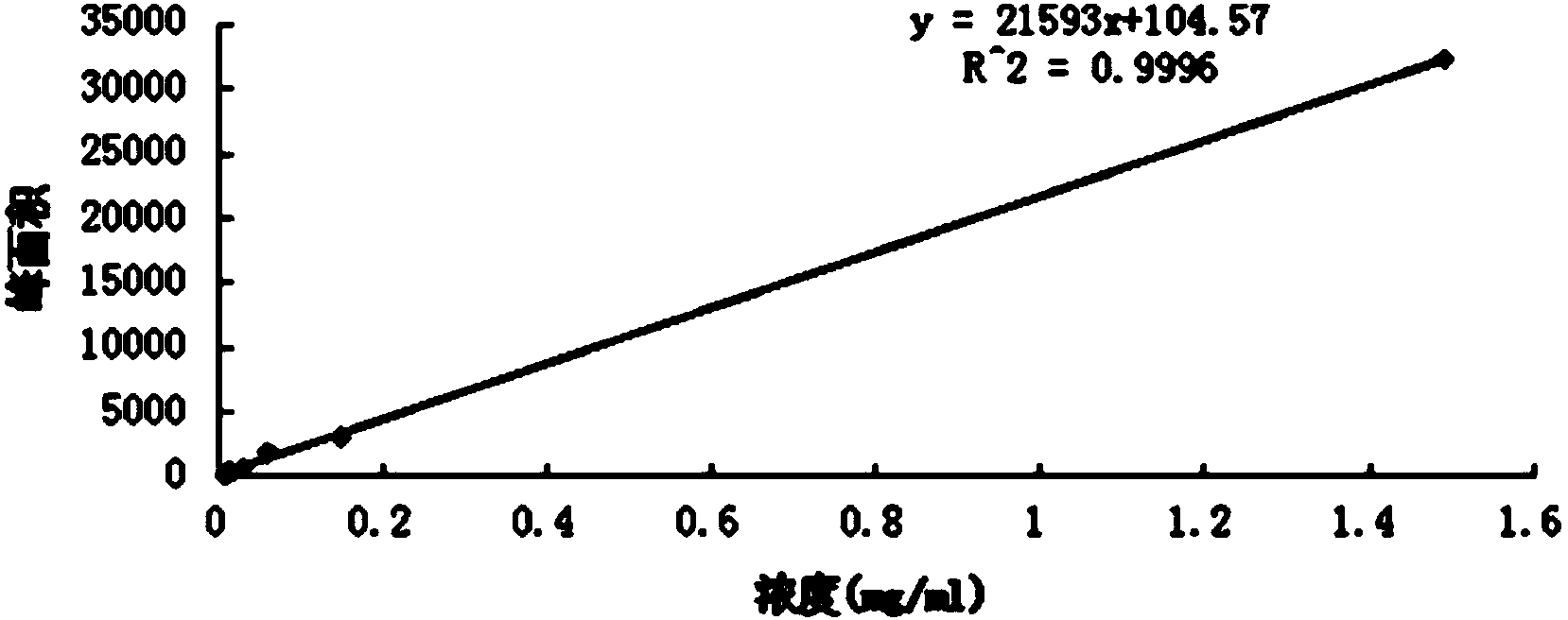Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35results about How to "Effective product quality" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for refining fasudil hydrochloride
The invention relates to a method for refining fasudil hydrochloride. The method comprises the following steps: 1, discoloring, namely adding active carbon into fasudil after resin adsorption, fully mixing the active carbon and the fasudil, filtering the mixture, removing the active carbon, and collecting the filtered solution for later use; 2, washing, namely adding purified water into the discolored solution, fully mixing the solution, separating the solution to remove water phase, and collecting the oil phase for later use; 3, acidifying and salifying, namely adding the purified water intothe oil phase, adjusting pH value of the solution by hydrochloric acid to fully acidify and salify the oil phase, and collecting the water phase for later use; and 4, crystallization, namely, adding n-butyl alcohol into the water phase, fully mixing the solution, then decompressing and azeotropic-crystallizing the solution, duly replenishing the n-butyl alcohol to keep the consistency of the solution, stopping crystallization till the moisture content in the mother solution is reduced to a certain value, and filtering and drying the crystals to obtain a refined fasudil hydrochloride product.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Preparation containing herba violae, rhizoma cyperi and herba leonuri and preparation method and detection method thereof
InactiveCN103028065AReduce the extraction rateHigh precisionComponent separationCapsule deliveryClinical efficacyViola yedoensis
The invention discloses a preparation containing herba violae, rhizoma cyperi and herba leonuri and a preparation method and a detection method of the preparation. The preparation is prepared by the following raw materials in parts by weight: 500-700 parts of perfoliote knotweed herb, 500-700 parts of herba violae, 500-700 parts of red rhizoma smilacis glabrae, 400-600 parts of cortex phellodendri, 300-500 parts of herba leonuri, 300-500 parts of caulis sargentodoxae, 300-500 parts of radix sophorae flavescentis and 200-400 parts of rhizoma cyperi. Compared with the prior art, the medicine provided by the invention is few in components, rigorous in structure and exact in efficacy, and corresponds to a method of diagnosis and treatment based on syndrome differentiation treatment of traditional Chinese medicines. The provided preparation method not only reduces paste extraction rate of the traditional Chinese medicine extraction, improves contents of effective ingredients, guarantees stability of efficacy, solves the problem of poor stability and strong hygroscopicity in a preparation process, and also greatly reduces the production cost. The provided detection method is strong in specificity and accurate in detection result, and can effectively control quality of the medicine; and therefore, the clinical efficacy of the medicine is ensured.
Owner:GUIZHOU NORMAL UNIVERSITY
Method for determining specnuezhenide content in Zhenqifuzheng preparation
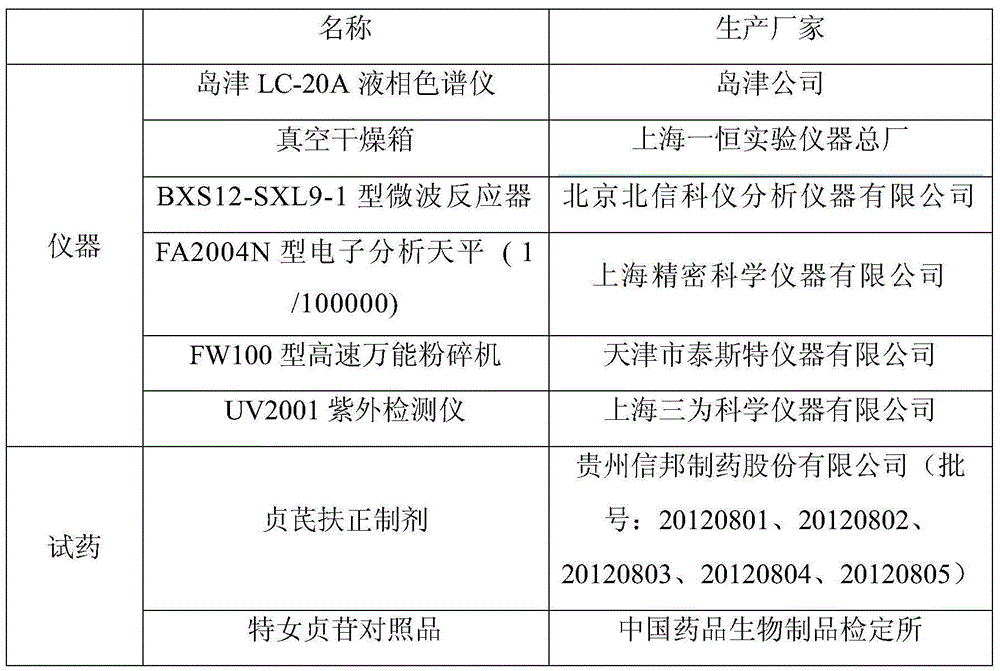
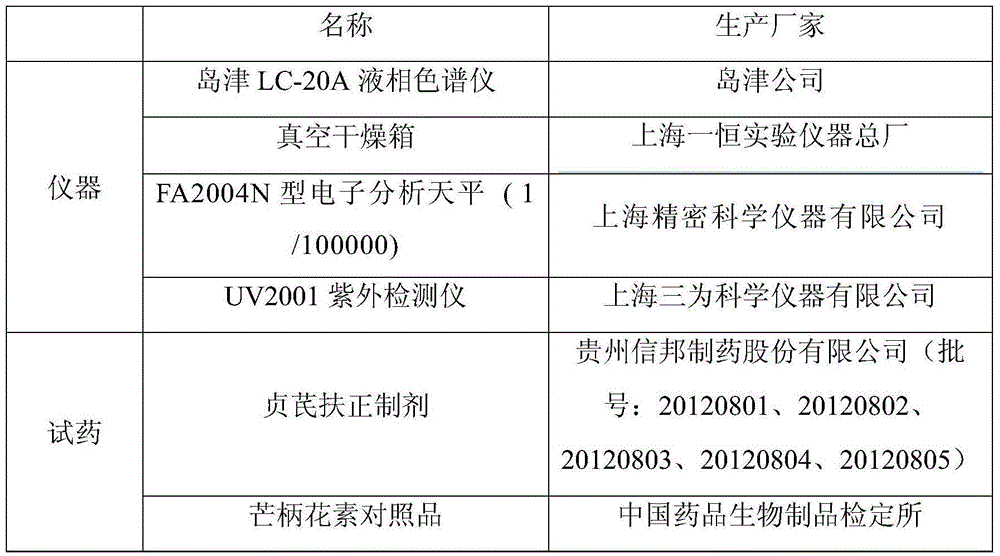
The invention discloses a method for determining specnuezhenide content in a Zhenqifuzheng preparation. Taking specnuezhenide standard as contrast, the method can determine the specnuezhenide content in the Zhenqifuzheng preparation by high-performance liquid chromatography by selecting a solution containing methanol and a mixed solution with a ratio of (20-50):(80-50) or a mixed solution containing acetonitrile and the mixed solution with a ratio of (5 to 35):(95-65), wherein the mixed solution consists of water, 0.01% to 5% of phosphoric acid water solution, 0.01% to 5% of glacial acetic acid, 0.01% to 0.5% of methanoic acid water solution. Compared with the prior art, the method provided by the invention has the advantages of high specificity, high precision, high repeatability, high yield, high stability, and accurate result by adopting high-performance liquid chromatography, and can effectively control the preparation quality, thereby ensuring the stability of product quality as well as clinical safety and effectiveness.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Process for preparing water-soluble ginkgo biloba extract used for injection
ActiveCN101596221AQuality improvementUniform qualityAntipyreticAnalgesicsGlycoside formationWater soluble
The invention discloses a process for preparing a water-soluble ginkgo biloba extract used for injection, which comprises the following steps: preparing a medicinal gelatin aqueous solution of 0.1-0.3 percent; dissolving the water-soluble ginkgo biloba extract by the gelatin aqueous solution; ultrafiltering and drying the dissolved ginkgo biloba extract in vacuum to prepare total ginkgo terpene lactones and a total ginkgo flavone-glycoides extract; and generally mixing the components according to respective contents so as to prepare a finished product. In the water-soluble ginkgo biloba extract, the content ratio of total flavonol glycosides to the total ginkgo terpene lactones is 4:1, thus the water-soluble ginkgo biloba extract can be used as a water-soluble ginkgo injection raw material, thereby enlarging the range of application and ensuring the safety, the effectiveness, the stability and the controllability of product quality.
Owner:SHANGHAI SINE PROMOD PHARMA
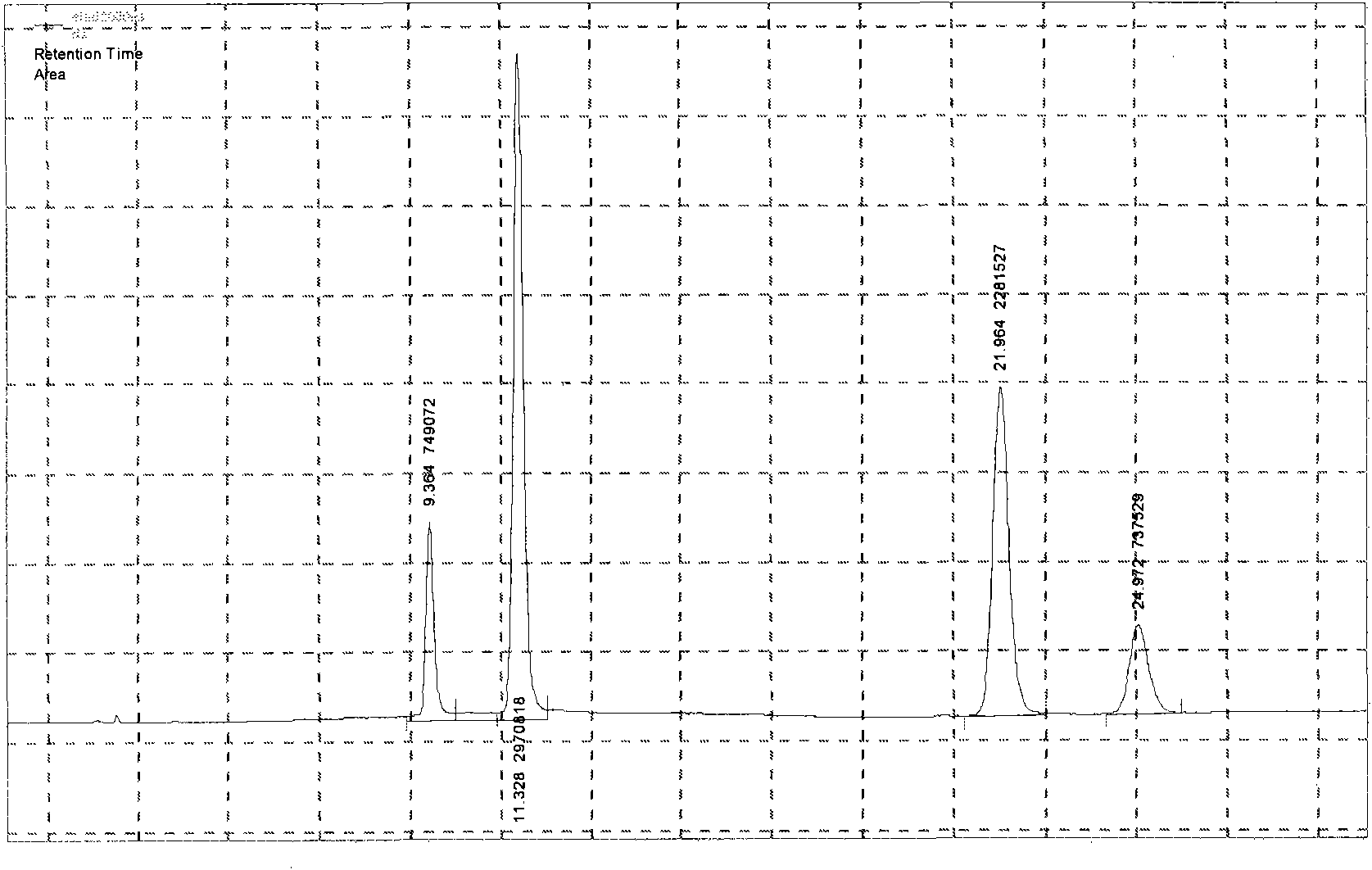
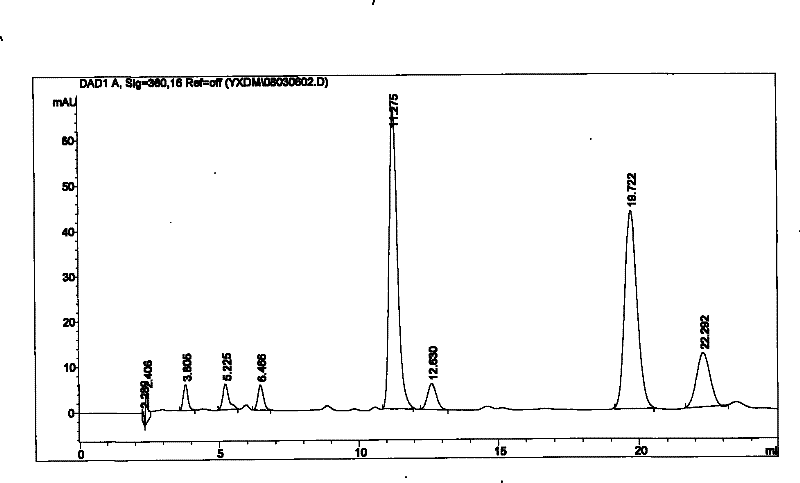
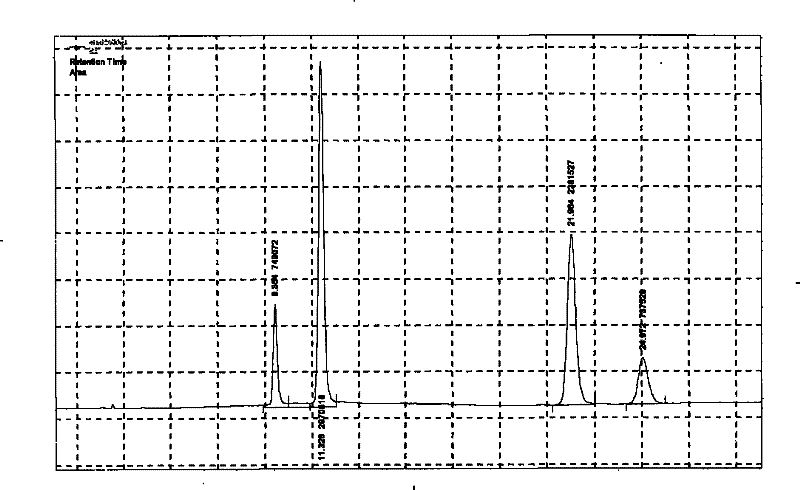
Method for measuring content of ginsenoside in heart-benefiting preparation
The invention discloses a method for measuring the content of ginsenoside in a heart-benefiting preparation. The method is a high performance liquid chromatography method for gradient elution by taking ginsenoside Rg1, Re, Rb1 and Rc contrasts as contrasts, methyl alcohol as a flowing phase and a 0.02-2% phosphoric acid aqueous solution as a flowing phase B. The method can be used for quickly and accurately measuring the contents of ginsenoside Rg1, Re, Rb1 and Rc components in the heart benefiting preparation in a high-repetitiveness and high-recycling-rate manner.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Method for measuring content of formononetin in Zhenqifuzheng preparation
The invention discloses a method for measuring content of formononetin in a Zhenqifuzheng preparation. The method is a high-performance liquid chromatography with a formononetin reference substance used as a contrast and the ratio of methyl alcohol to water / a 0.01%-5% phosphoric acid aqueous solution / a 0.01%-5% glacial acetic acid / a 0.01%-0.5% formic acid aqueous solution of (50-70):(50-30) or the ratio of acetonitrile to the water / the 0.01%-5% phosphoric acid aqueous solution / the 0.01%-5% glacial acetic acid / the 0.01%-0.5% formic acid aqueous solution of (20-50):(80-50) used as a mobile phase. The method is high in specificity, high in accuracy, good in repeatability, high in recovery rate, high in stability, accurate in measurement result and capable of achieving the purpose of effective control of the drug quality and ensuring the stability of the product quality and the safety and the effectiveness of clinical medication.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
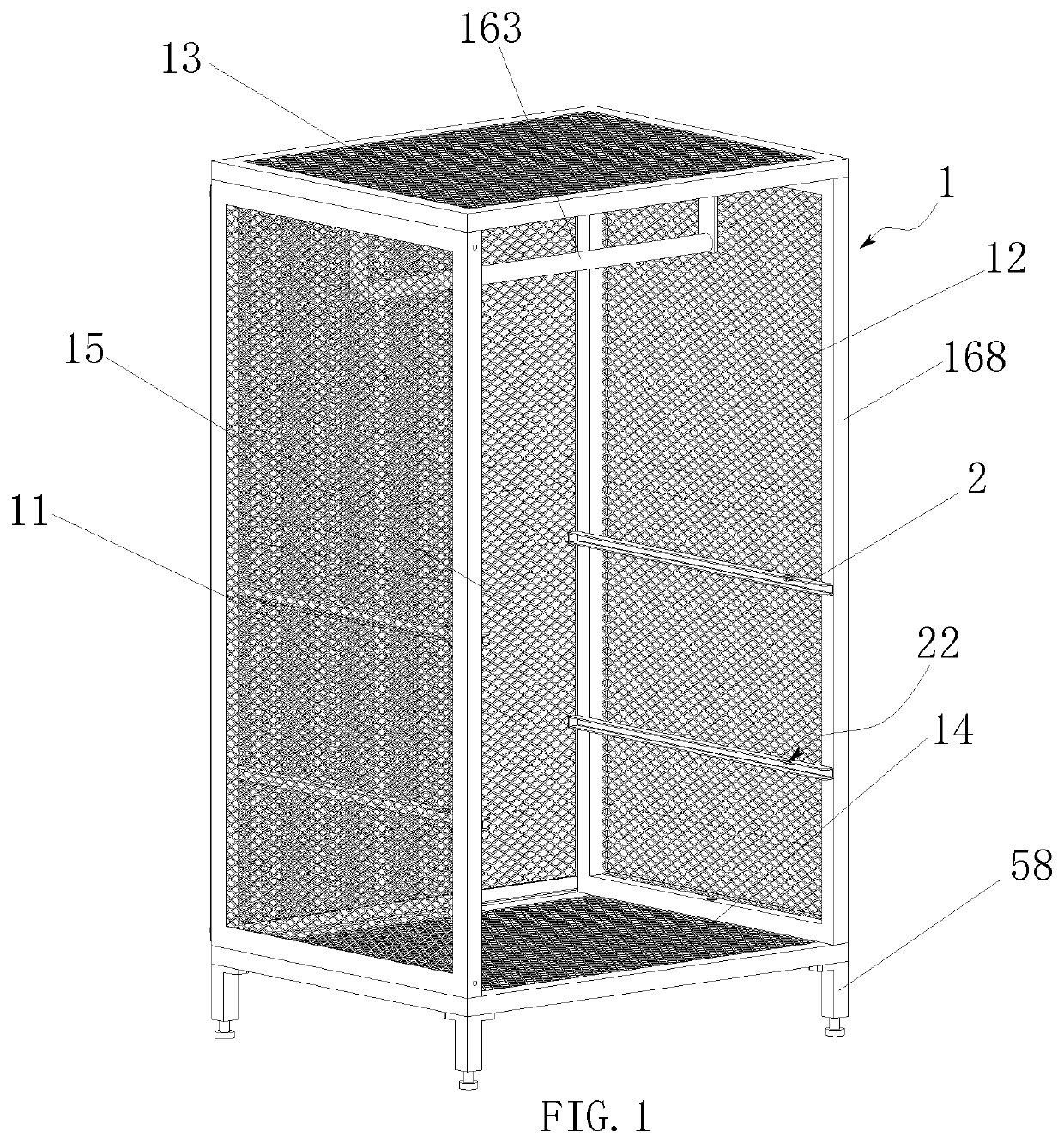
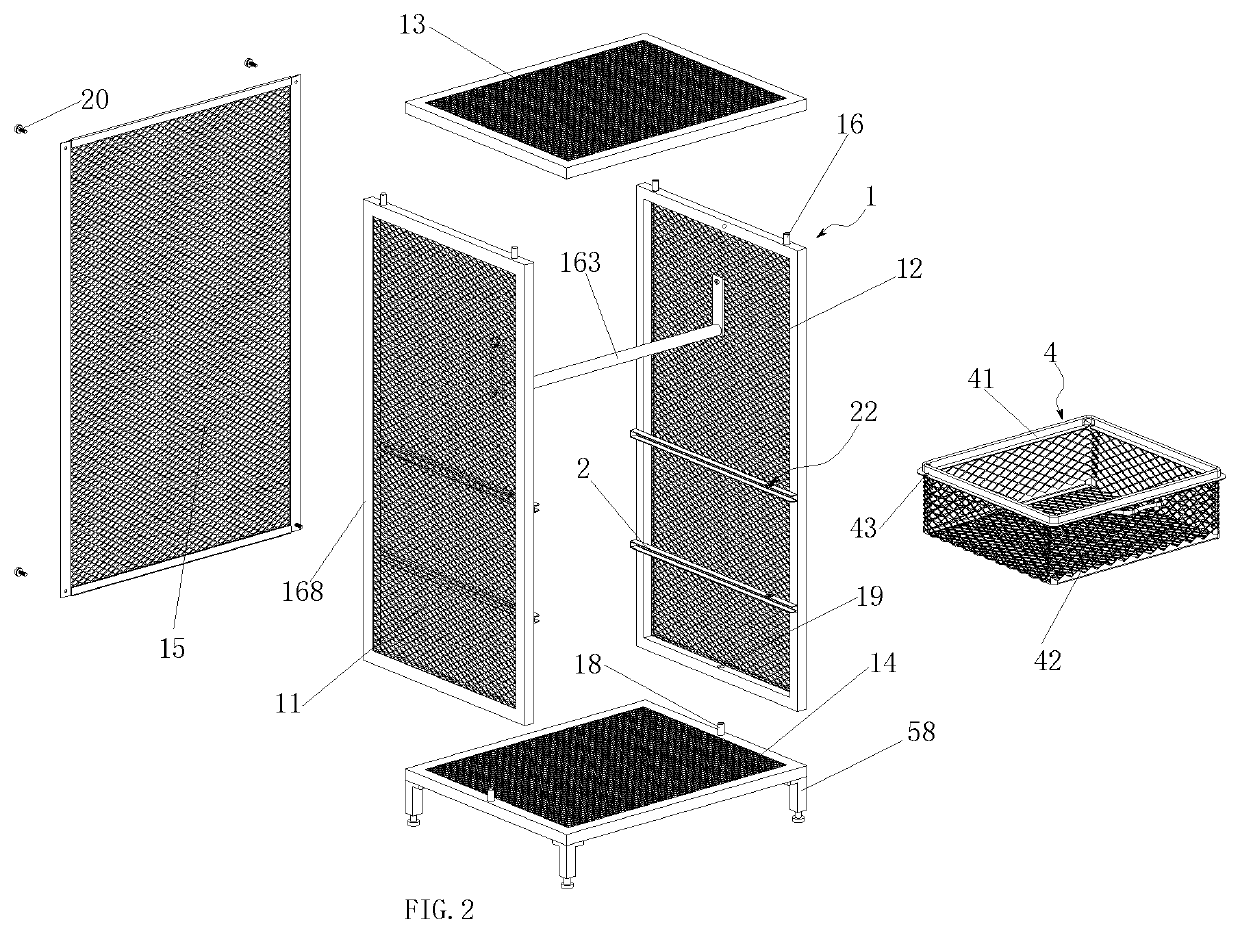
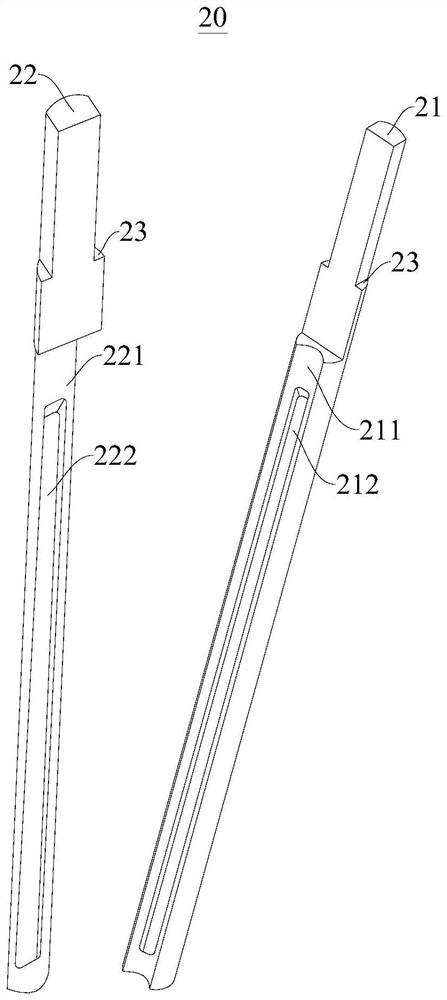
Multifunctional breathable storage cabinet
ActiveUS10674817B1Reduce transportationSimple structureFolding cabinetsSectional furnitureStructural engineeringMechanical engineering
A multifunctional breathable storage cabinet, including a frame body having a left frame and a right frame, and at least one set of sliding rails correspondingly mounted on the left frame and the right frame; a storage laminate or a storage basket capable of sliding along the sliding rail is mounted on the sliding rail; one inward end of the sliding rail is provided with a baffle, and the inner wall of one outward end of the sliding rail is provided with an elastic limiting device; the storage basket includes a sliding connecting piece capable of being inserted in the sliding rail and sliding along the sliding rail and a metal mesh storage basket body connected with the sliding connecting piece, and the sliding connecting piece is provided with a limiting protrusion capable of being in contact with the elastic limiting device to limit the storage basket.
Owner:CAO XIN
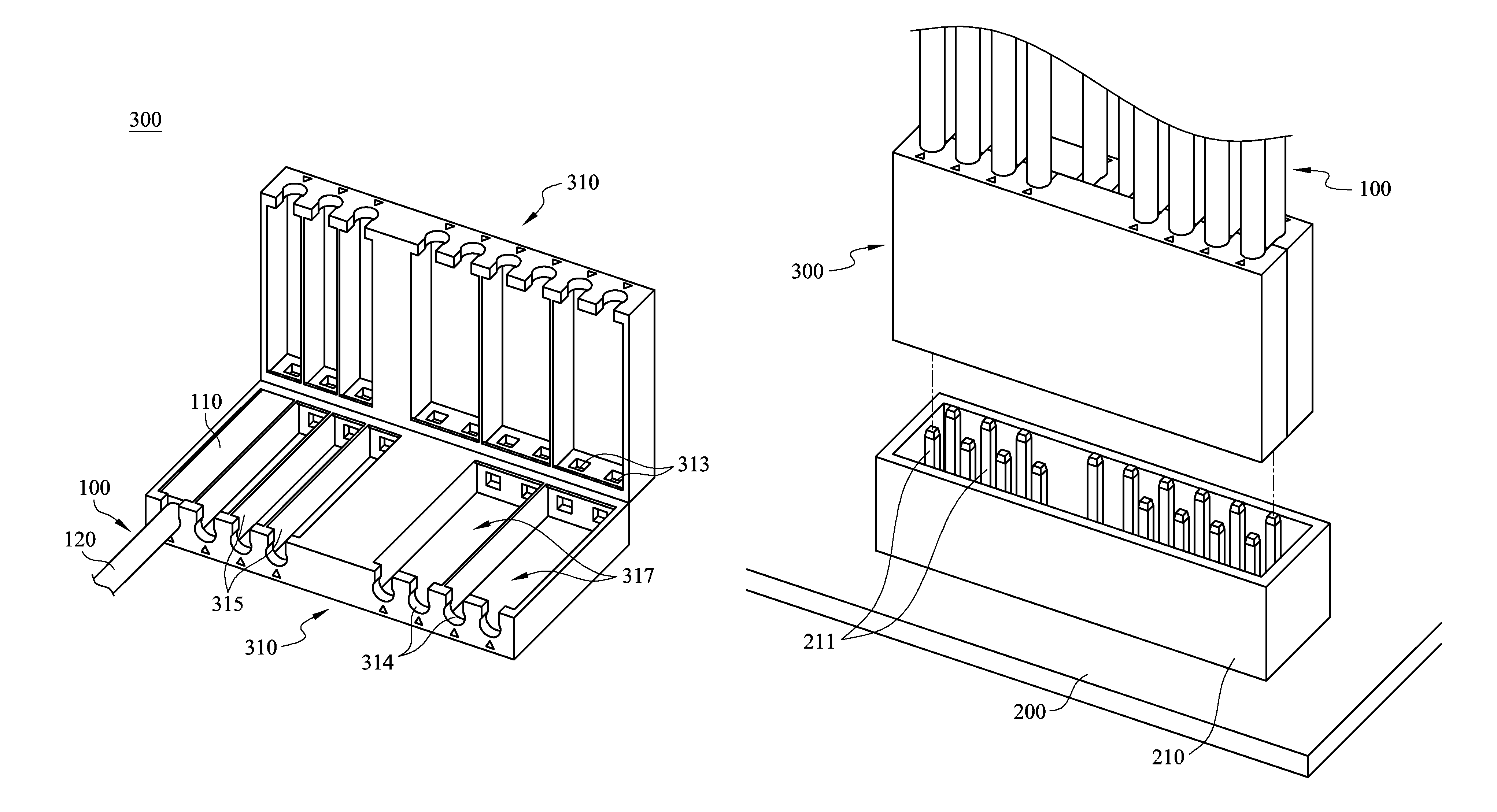
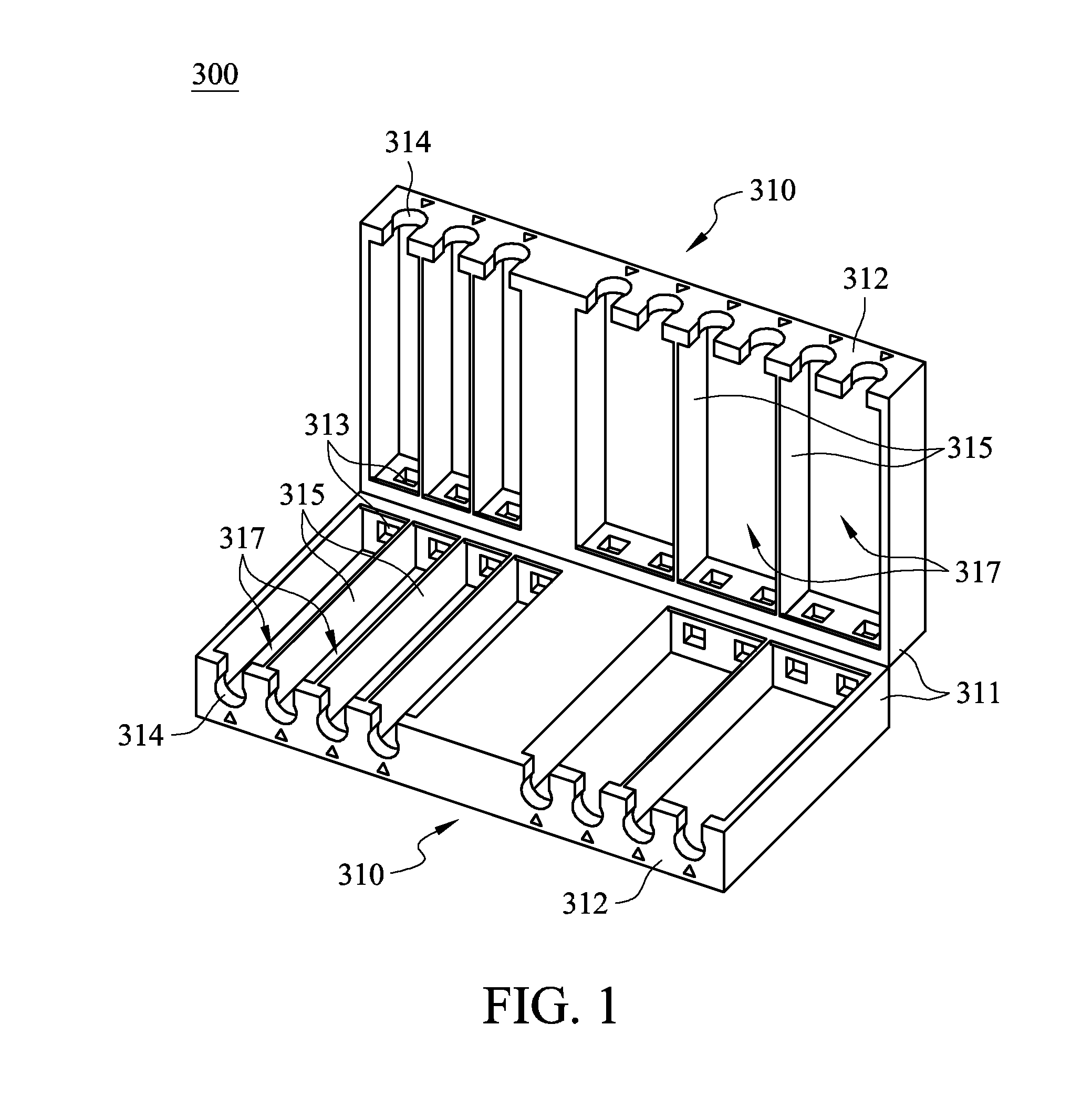
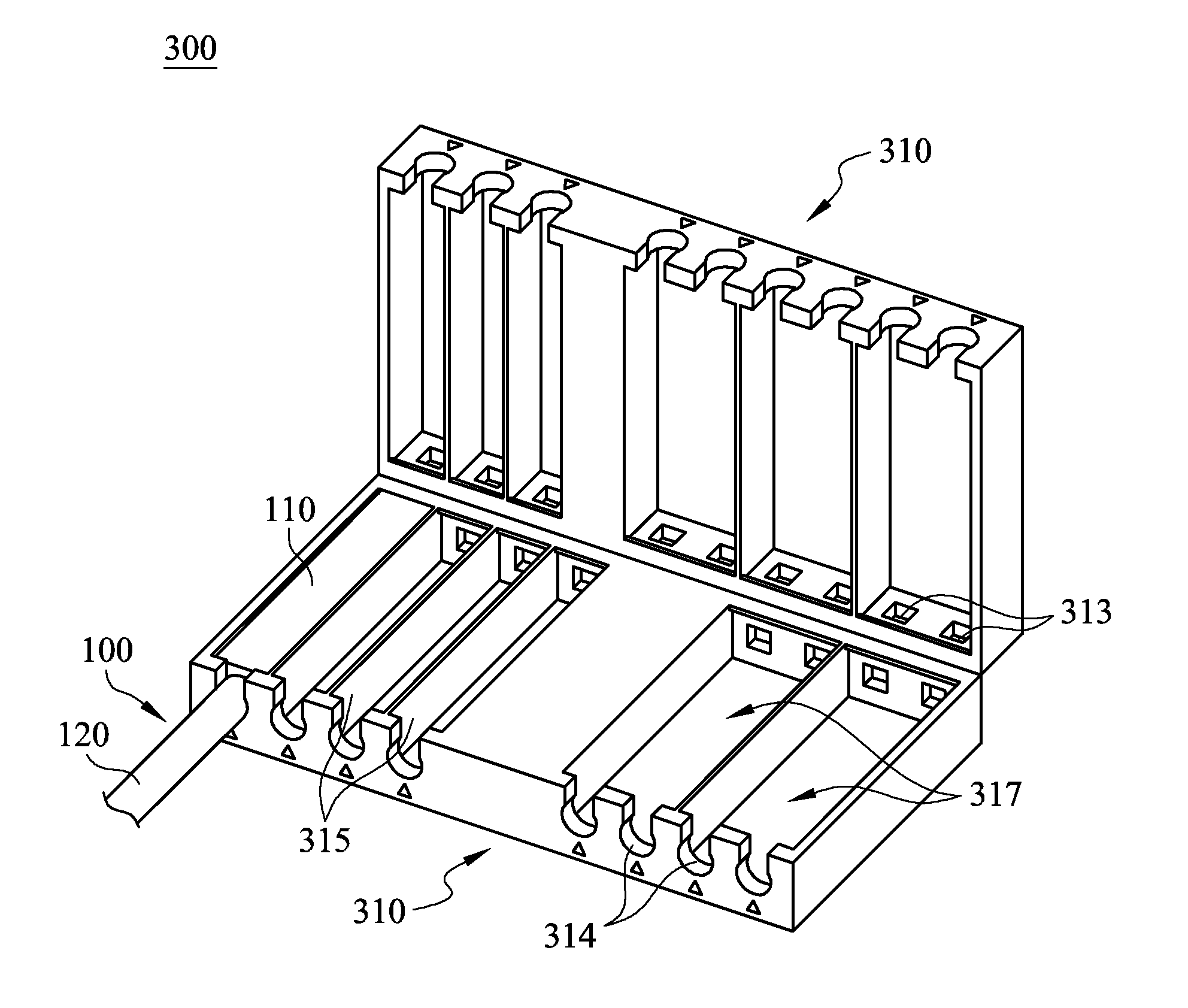
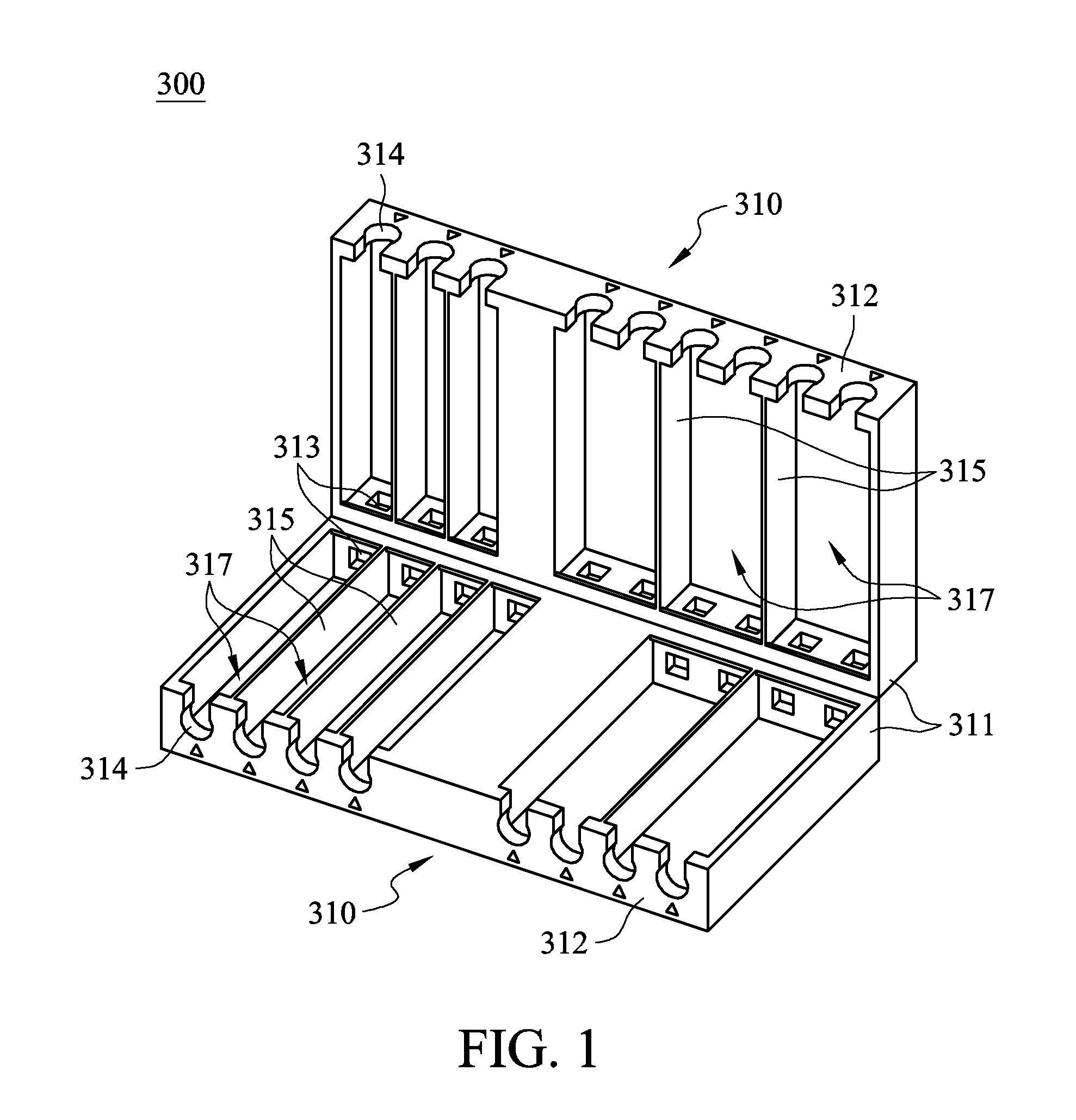
Cable management apparatus
ActiveUS9022805B2Complex processEffective product qualityElectrically conductive connectionsElectric discharge tubesEngineeringCable management
A cable management apparatus includes a half housing and a plurality of partitions. The half housing has a first side surface and a second side surface opposite to each other. The partitions are disposed in the half housing to divide the interior of the half housing into a plurality of compartments, and each compartment extends to the two faces of the half housing. A plurality of signal cables are respectively disposed into the compartments, so that the signal cables are able to be disposed in the electronic slots through the half housing and that the signal cables are electronically connected to the corresponding pins respectively.
Owner:GIGA BYTE TECH CO LTD
Method for measuring content of calycosin-7-glucoside in Zhenqifuzheng preparation
ActiveCN103592384AEffective quality controlHigh precisionComponent separationPhosphoric acidGlucoside
The invention discloses a method for measuring content of calycosin-7-glucoside in a Zhenqifuzheng preparation. The method is a high-performance liquid chromatography with a calycosin-7-glucoside reference substance used as a contrast and the ratio of methyl alcohol to water / a 0.01%-5% phosphoric acid aqueous solution / a 0.01%-5% glacial acetic acid / a 0.01%-0.5% formic acid aqueous solution of (30-70):(70-30) or the ratio of acetonitrile to the water / the 0.01%-5% phosphoric acid aqueous solution / the 0.01%-5% glacial acetic acid / the 0.01%-0.5% formic acid aqueous solution of (20-50):(80-50) used as a mobile phase. The method is high in specificity, high in accuracy, good in repeatability, high in recovery rate, high in stability, accurate in measurement result and capable of achieving the purpose of effective control of the drug quality and ensuring the stability of the product quality and the safety and the effectiveness of clinical medication.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
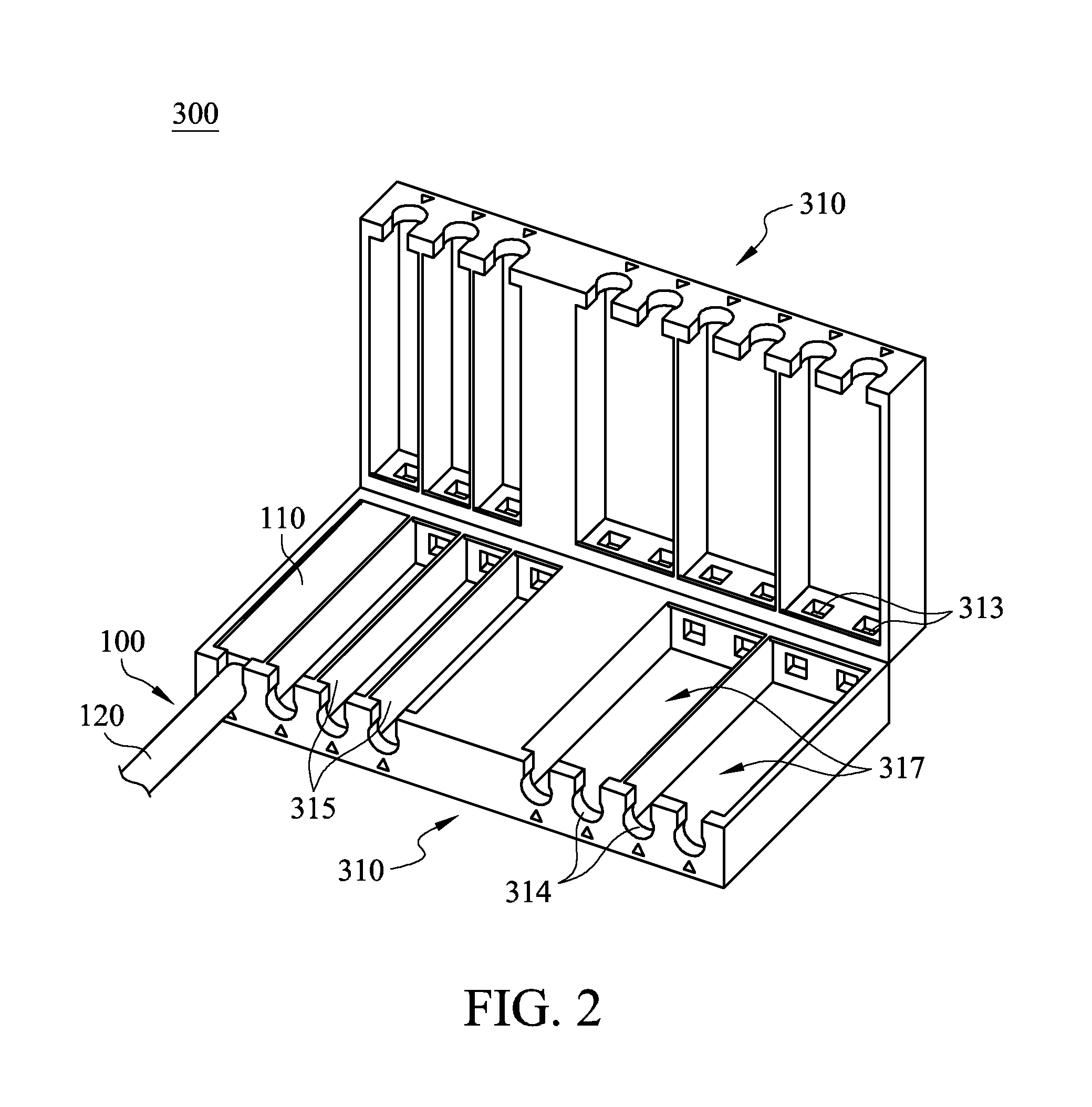
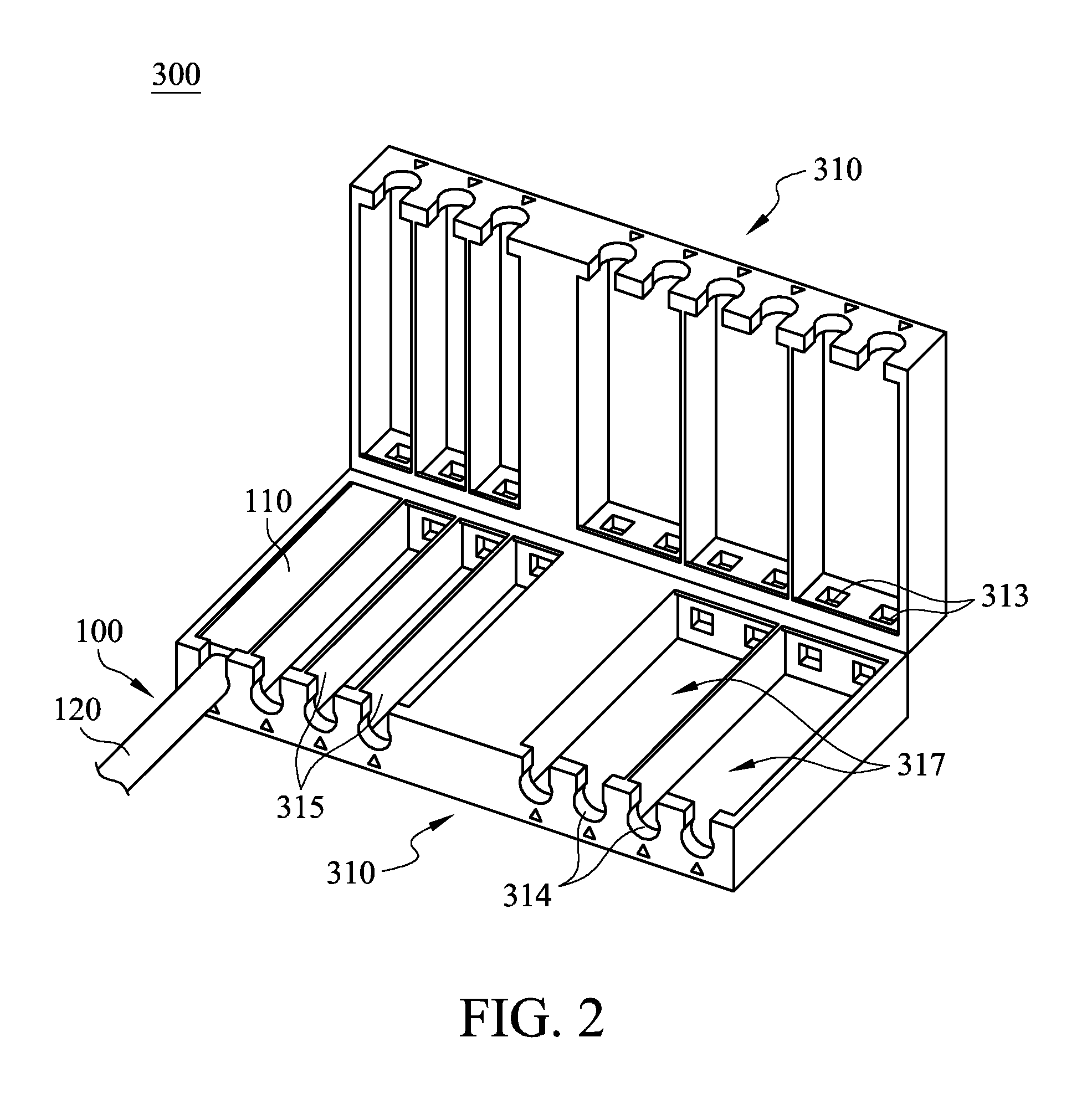
Cable management apparatus
ActiveUS20140187083A1Easy and fast assemblyQuick and easy positioningElectrically conductive connectionsCoupling device detailsElectricityCable management
A cable management apparatus includes a housing and a plurality of partitions. The housing has a first side surface and a second side surface opposite to each other. The partitions are disposed in the housing to divide the interior of the housing into a plurality of compartments, and each compartment extends to the two faces of the housing. A plurality of signal cables are respectively disposed into the compartments, so that the signal cables are able to be disposed in the electronic slots through the housing and that the signal cables are electronically connected to the corresponding pins respectively.
Owner:GIGA BYTE TECH CO LTD
Content measurement method for verbascoside in Weixuening preparation
InactiveCN110794057AEffective quality controlHigh precisionComponent separationO-Phosphoric AcidFluid phase
The invention discloses a content measurement method for verbascoside in Weixuening preparation. The content measurement method is a high performance liquid chromatography method taking a verbascosidereference substance as contrast, methyl alcohol as a mobile phase A and 0.02-2% phosphoric acid solution as a mobile phase B to elute in a gradient way. By the content measurement method, rapid, accurate, high-reproducibility and high-recycling rate measurement on content of the verbascoside in the Weixuening preparation can be achieved.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Method for measuring content of oleanolic acid in glossy privet fruit and astragalus membranaceus healthy energy-strengthening preparation
InactiveCN103558303AEffective quality controlHigh precisionComponent separationLength waveRepeatability
The invention discloses a method for measuring the content of oleanolic acid in a glossy privet fruit and astragalus membranaceus healthy energy-strengthening preparation. The method takes an oleanolic acid reference substance as a reference and can be used for measuring the content of the oleanolic acid in the glossy privet fruit and astragalus membranaceus healthy energy-strengthening preparation at the wavelength being 200-220nm by using a high-performance liquid chromatography. The method is high in specificity and precision, good in repeatability, high in recovery rate and stability and accurate in measurement result, and achieves the purpose of effectively controlling the quality of drugs so as to ensure the stable product quality and the safe and effective clinical treatment.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
An externally used traditional Chinese medicine preparation for treating rheumatism and rheumatoid as well as its preparation method
The invention provides Chinese medicine preparation for external use for treating rheumatism and rheumatoid disease and the preparation method thereof. The preparation is prepared by using large leaf gentian roots, Chinese clematis, gum mastic, myrrh, momordica cochinchinensis(Lour.) spreng, castor seeds, and sesame oil. The gum mastic and the myrrh are pulverized into fine powder, the large leaf gentian roots are boiled by adding water, and plaster is obtained after filter liquor is decompressed and concentrated. The Chinese clematis is soaked in the sesame oil, the momordica cochinchinensis(Lour.) spreng and the castor seeds are soaked in the obtained medical oil, and then beaten to be slurry, beeswax is added into the obtained medical oil for melting, then the fine powder, the plaster, the slurry and the medical oil are mixed evenly, and various preparation formulations are produced according to conventional preparation craft. Compared with the prior art, the invention uses the common Chinese medicines as the raw materials, the used raw materials are in a small variety, the product quality is easy to be controlled, the effective components of the raw material medicines are obtained after extraction, to be more easily absorbed and to exert the drug efficacy, thereby providing a medicine for external use for treating rheumatism and rheumatoid disease with low administration cost and good curative effect.
Owner:GUIZHOU XINYI MEDICINE
Febuxostat tablets and preparation method and detection method thereof
ActiveCN102988326ASimple preparation processThe preparation process is feasibleOrganic active ingredientsComponent separationDiseaseClinical efficacy
The invention provides Febuxostat tablets and a preparation method and a detection method thereof. The tablets are prepared from 40 to 120 parts of Febuxostat, 95 to 165 parts of microcrystalline cellulose, 20 to 60 parts of lactose, 10 to 27 parts of croscarmellose sodium and 1 to 3 parts of magnesium stearate according to parts by weight. Aiming at the shortcomings of the prior art, the formula and the preparation process of the Febuxostat tablets are optimized, so that the curative effects of the Febuxostat tablets for treating diseases such as hyperuricemia and gout are more remarkable, and the systematic, complete and effective component identification and content determination methods are established to effectively control the quality of the medicine, thereby ensuring the clinical curative effect.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Content determination method for radix ophiopogonis total saponins in Yixinshu preparation
InactiveCN109298093AEffective quality controlHigh precisionComponent separationAlcoholPhosphoric acid
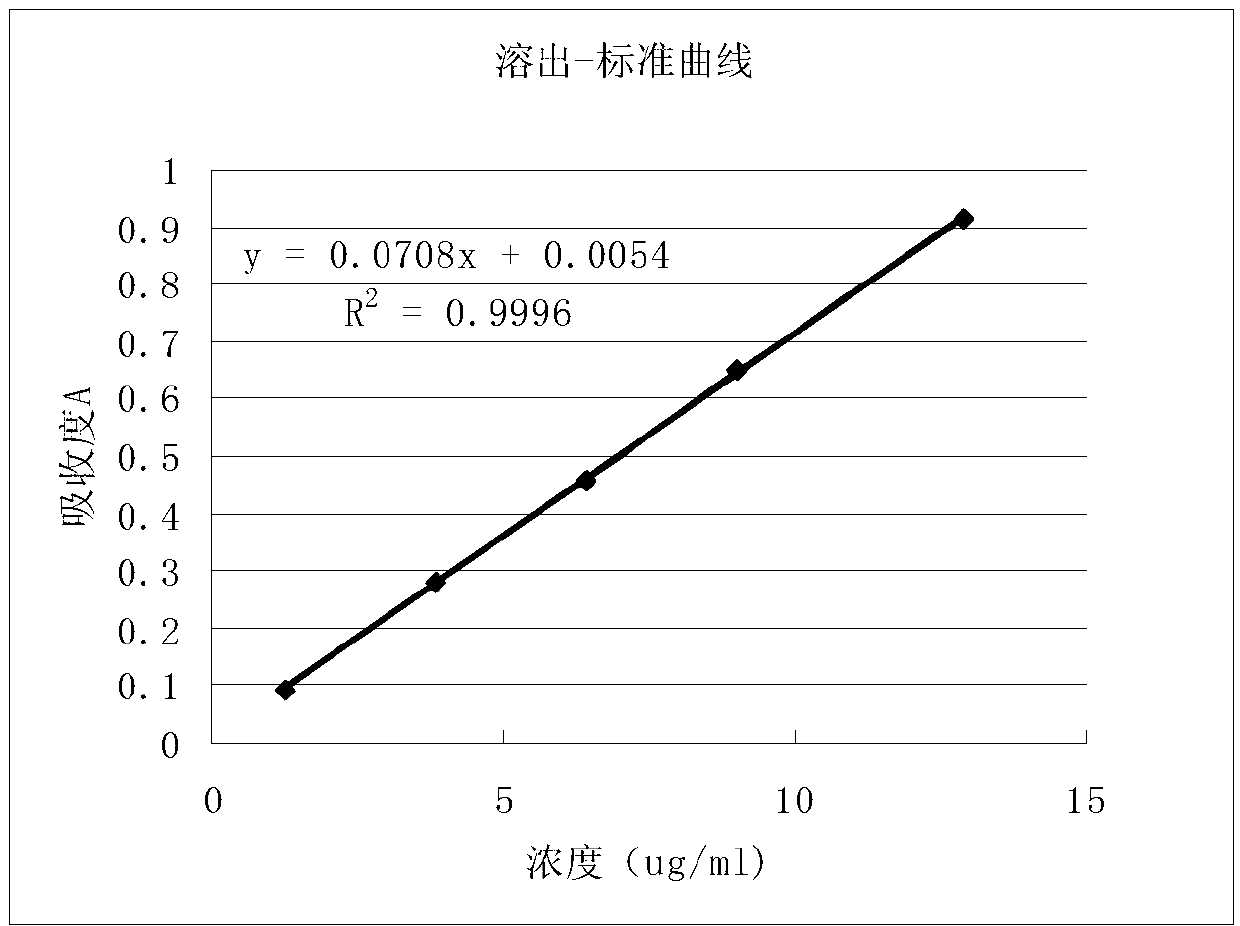
The invention relates to a content determination method for radix ophiopogonis total saponins in Yixinshu traditional Chinese medicine preparation. The content determination method comprises the stepsthat high performance liquid chromatography is adopted to determine, the radix ophiopogonis total saponins contrasts with ruscogenin (C27H4204) as a reference substance, methyl alcohol is taken as mobile phase A, and 0.02% to 2% phosphoric acid aqueous solution is taken as mobile phase B gradient elute to determine by adopting the high performance liquid chromatography. The content determinationmethod has high specificity, high accuracy, good repeatability, high recovery, high stability and accurate measuring results, the aim that the drug quality is effectively controlled is achieved, and the stability of the product quality and the safety and effectivity of clinical medication are guaranteed.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Method for determining content of schisandrin in Yixinshu traditional Chinese medicine preparation
InactiveCN103592387AEffective quality controlHigh precisionComponent separationSchisandrinTraditional medicine
The invention discloses a method for determining the content of schisandrin in a Yixinshu traditional Chinese medicine preparation. A schisandrin reference substance is taken as a contrast, methanol and water in a ratio of (65-75):(35-25) are taken as a mobile phase, and the content of the schisandrin in the Yixinshu traditional Chinese medicine preparation is determined with a high performance liquid chromatography. The method for determining the content of the schisandrin in the Yixinshu traditional Chinese medicine preparation with the high performance liquid chromatography is high in specificity, high in precision, good in repeatability, high in recovery rate, high in stability and accurate in measurement result, so that the medicine quality is effectively controlled, the stability of the product quality as well as the safety and effectiveness of clinical medication are guaranteed.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
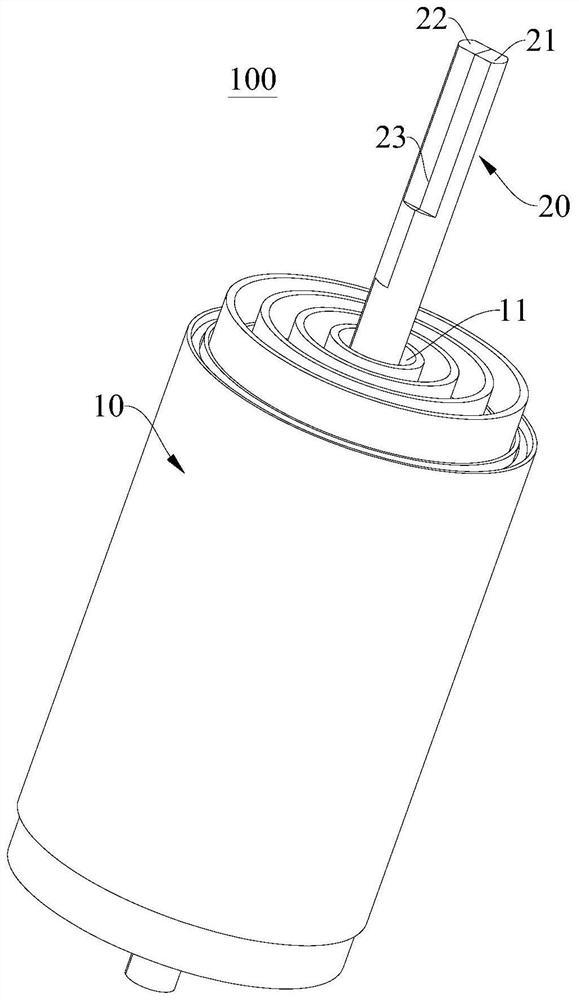
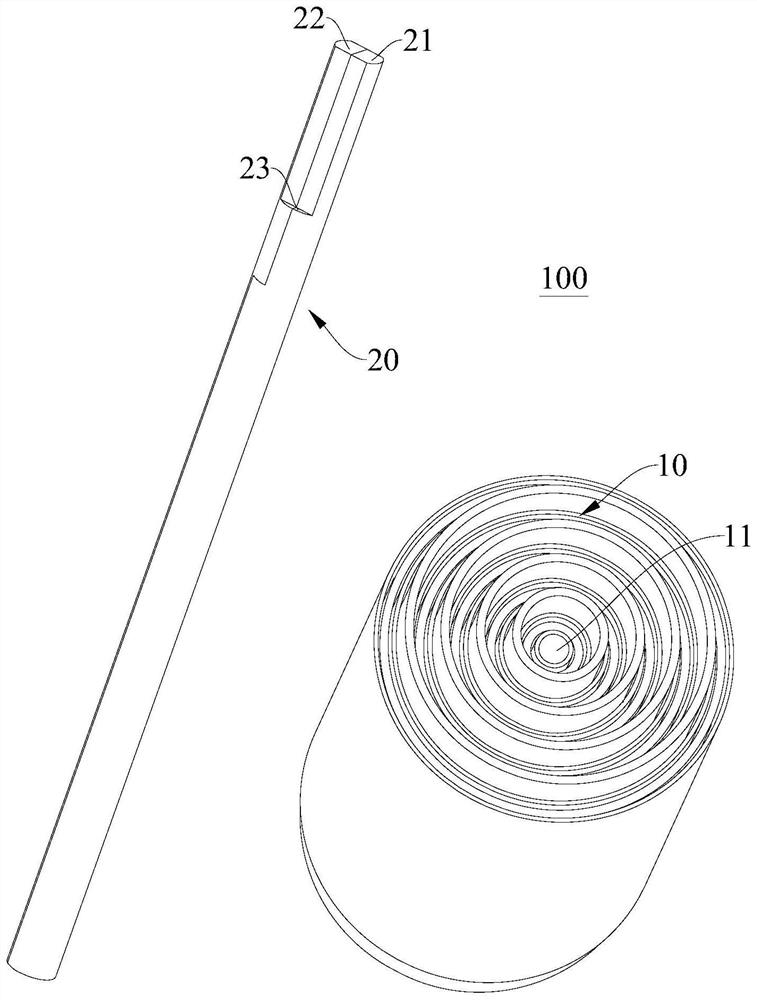
Winding device and method for winding winding core
ActiveCN114583237AQuality improvementEffective product qualityAssembling battery machinesFinal product manufactureStructural engineeringMechanical engineering
The invention discloses a winding device and a method for winding a winding core. The winding device comprises a winding main body; the winding needle is inserted into the winding main body and rotates under the driving of the winding main body; and the heating piece is arranged on the winding needle or the winding main body so as to heat the diaphragm through the winding needle. After winding is completed, the heating piece is used for heating, the clamped diaphragm can be melted, two layers or multiple layers of diaphragms in the winding core are bonded together, a center hole without diaphragm blocking and a new diaphragm layer are formed in the winding core, product production and manufacturing are facilitated, the consistency quality is good, and after the diaphragms in the winding core are melted and bonded, the diaphragm in the winding core can not be blocked by the diaphragm. And a central hole with certain strength is formed by cooling and can be used as a vent hole when the battery cell is actually used, so that the cost is saved, and the product quality of the battery cell is effectively improved.
Owner:XIAMEN HAICHEN NEW ENERGY TECH CO LTD
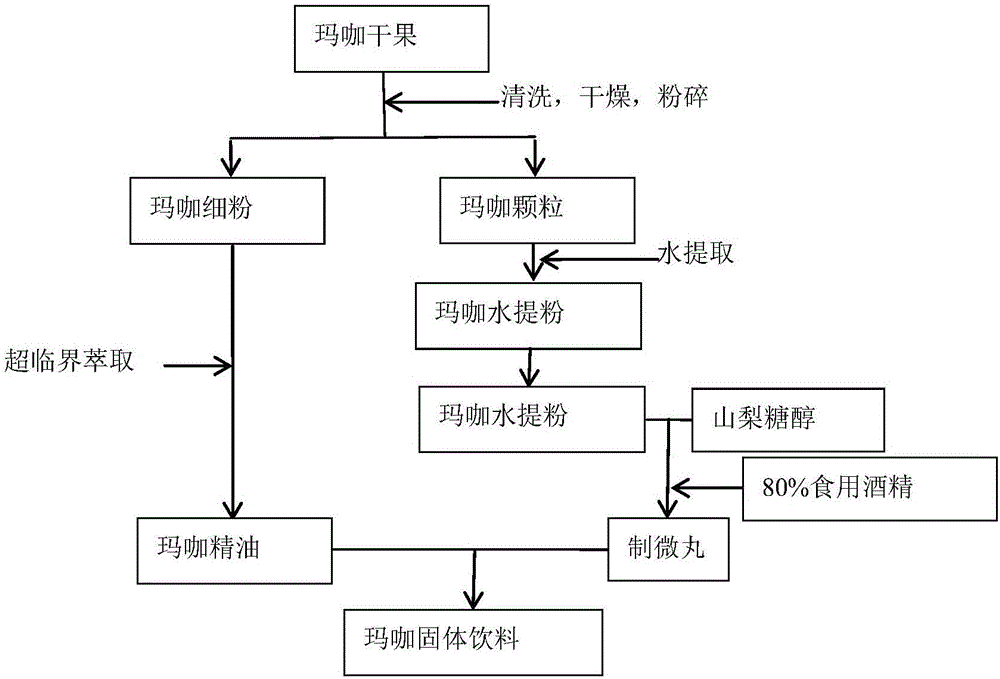
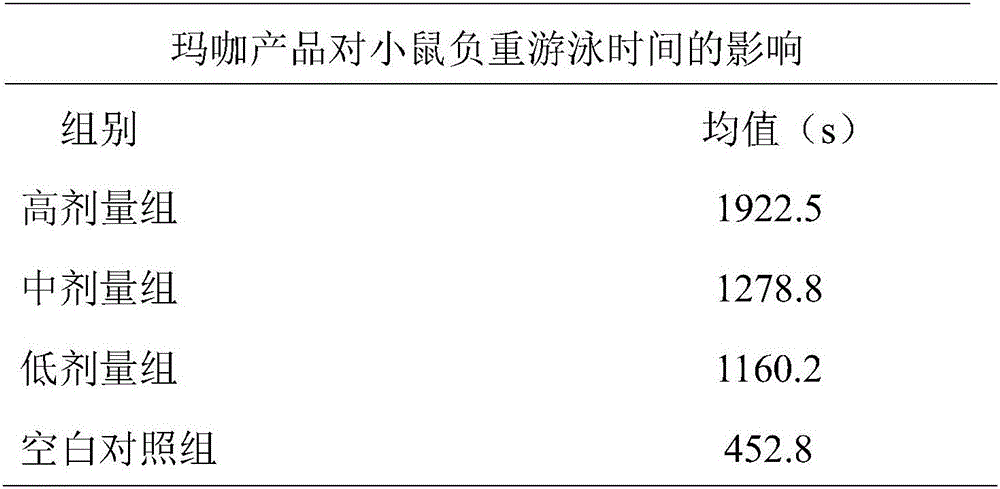
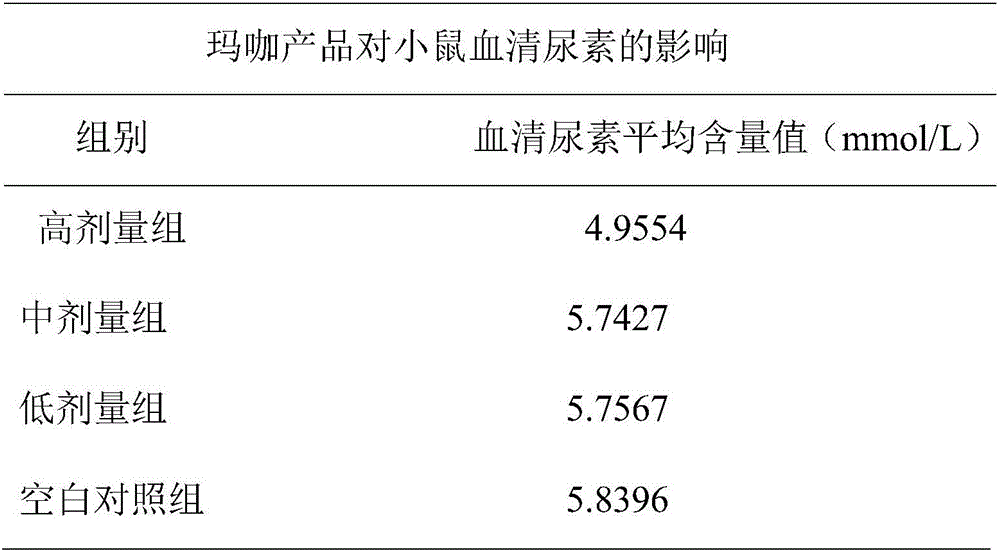
Maca solid drink and preparing method thereof
InactiveCN106619772AFully combinedCompatibility is simpleDispersion deliveryAntinoxious agentsChemistryEssence oil
The invention discloses a maca solid drink and a preparing method thereof. The drink is prepared from, by mass, maca aqueous extract powder which makes total protein content 4.5-7.0%, 4.0-7.0% of maca essential oil which makes the mass percentage content of macamides in the solid drink 0.1-0.5%, and the balance auxiliary materials. The solid drink particles are 20-50-mesh pellets. During preparation, the powdery auxiliary materials are evenly mixed with the maca aqueous extract powder, 80-85% of edible alcohol is used as a binder, the mixed powder is made into pellets, and after low-temperature drying, the maca essential oil is evenly sprayed into the pellets so that the maca solid drink is obtained. The maca solid drink is simple in compatibility, safe in preparation and free of pollution. The maca aqueous extract and oil-soluble effective constituents are fully combined, product quality and effectiveness are guaranteed, and fatigue resistance can be effectively achieved.
Owner:广东普利生生物科技开发有限公司
A kind of soft state billet cutting method and its cutting device
ActiveCN104325489BControl exothermic reactionsRealize effective cutting in one passMetal working apparatusEngineeringSprocket
The invention discloses a soft blank cutting method and a soft blank cutting device. The method comprises the following steps: a linear steel wire cutter moves to realize soft blank cutting; in a movable cutting process of the steel wire cutter, an inclined angle being more than zero is formed between the steel wire cutter and the horizontal plane; the steel wire cutter moves along the tension direction of the steel wire cutter when doing vertical undercutting movement; after the steel wire cutter finishes cutting, the steel wire cutter does resetting movement on a vertical undercutting face by bypassing a soft blank; the cutting device comprises a steel wire cutter driving device and a steel wire constant tension device, wherein the steel wire cutter driving device is composed of a chain wheel ring and a dynamic tracking and guiding device. According to the soft blank cutting method and the soft blank cutting device, the cutting process of the deformable linear steel wire cutter can be controlled by coordinating cutting movement and matching equipment, so that the one-way effective cutting of reciprocated cutting movement in a continuous conveying process of a strip-shaped blank is realized and the cutting requirements that the deformation of a cut section of an extruded and molded soft mud blank is small are met; the effective service life of a steel wire type cutter without blades is prolonged.
Owner:CHENGDU DONGFANG KWH ENVIRONMENTAL PROTECTION CATALYSTS
Process for preparing water-soluble ginkgo biloba extract used for injection
ActiveCN101596221BMeet the requirementsGood water solubilityAntipyreticAnalgesicsGlycosideWater soluble
The invention discloses a process for preparing a water-soluble ginkgo biloba extract used for injection, which comprises the following steps: preparing a medicinal gelatin aqueous solution of 0.1-0.3 percent; dissolving the water-soluble ginkgo biloba extract by the gelatin aqueous solution; ultrafiltering and drying the dissolved ginkgo biloba extract in vacuum to prepare total ginkgo terpene lactones and a total ginkgo flavone-glycoides extract; and generally mixing the components according to respective contents so as to prepare a finished product. In the water-soluble ginkgo biloba extract, the content ratio of total flavonol glycosides to the total ginkgo terpene lactones is 4:1, thus the water-soluble ginkgo biloba extract can be used as a water-soluble ginkgo injection raw material, thereby enlarging the range of application and ensuring the safety, the effectiveness, the stability and the controllability of product quality.
Owner:SHANGHAI SINE PROMOD PHARMA
A method for determining the content of ginsenosides in Yixinshu preparation
The invention discloses a method for measuring the content of ginsenoside in a heart-benefiting preparation. The method is a high performance liquid chromatography method for gradient elution by taking ginsenoside Rg1, Re, Rb1 and Rc contrasts as contrasts, methyl alcohol as a flowing phase and a 0.02-2% phosphoric acid aqueous solution as a flowing phase B. The method can be used for quickly and accurately measuring the contents of ginsenoside Rg1, Re, Rb1 and Rc components in the heart benefiting preparation in a high-repetitiveness and high-recycling-rate manner.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Method for measuring content of gentiopicroside in arthralgia-treating pill
InactiveCN110794058AEffective quality controlHigh precisionComponent separationOther chemical processesAcetic acidUse medication
The invention discloses a method for measuring content of gentiopicroside in an arthralgia-treating pill. The method is a high performance liquid chromatography method which takes a gentiopicroside reference substance as a reference and takes methanol, water, 0.01-5% phosphoric acid aqueous solution, 0.01-5% glacial acetic acid and 0.01-0.5% formic acid aqueous solution in a ratio of (50-70): (50-30) or acetonitrile, water, 0.01-5% phosphoric acid aqueous solution, 0.01-5% glacial acetic acid and 0.01-0.5% formic acid aqueous solution in a ratio of (20-50): (80-50) as a mobile phase. The method disclosed by the invention is high in specificity, high in precision, high in repeatability, high in recovery rate, high in stability and accurate in measurement result, the aim of effectively controlling the quality of medicines is achieved, and the stability of the product quality and the safety and effectiveness of clinical medication are ensured.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Quality detection method of five-flavor manna medicine bath preparation
ActiveCN102707007BRaise quality standardsQuality is easy to controlComponent separationJuniperus formosanaSilica gel
The invention discloses a quality detection method of a five-flavor manna medicine bath preparation. The five-flavor manna medicine bath preparation is made from raw materials of Juniperus formosana, ephedra, rhododendron anthopogonoide, myricaria and artemisia sieversiana according to a conventional method of pharmaceutics. On the basis of the primary standard, the thin layer chromatography of ephedra and rhododendron anthopogonoide is revised, the thin layer chromatography of Juniperus formosana and artemisia sieversiana is added. Under the simple and convenient condition of mobile phase, octyl silane bonding silica gel or phenyl bonding silica gel is used as filler, and simultaneously, the contents of ephedrine hydrochloride and pseudoephedrine hydrochloride in the ephedra are detected. The invention also provides a method for measuring the content of hyperoside in five-flavor manna preparation rhododendron anthopogonoide, thus ensuring the safety, effectiveness and controllability of product quality. By the quality detection method, the quality standard of the existing five-flavor manna medicine bath preparation is improved correspondingly.
Owner:JINKE TIBETAN MEDICINE QINGHAI PROV
Connection structure of plug-in power supply and light source plate
ActiveUS20180198224A1Connection process be simpleReliable connectionCoupling contact membersElectronic circuitEngineering
The present invention provides a connection structure of a plug-in power supply and a light source plate, and it relates to the field of electronic circuit connection. The connection structure of the plug-in power supply, and the light source plate comprises: a card slot, which is soldered on the light source plate; a card, which is matched with the card slot and soldered on the power supply; metal contacts, which is arranged in the card slot; pre-printed circuits connected to the metal contacts and arranged on the card; the power supply is connected to the light source plate through the matching of the card slot and the card, which facilitates the disassembly and assembly and improves the production efficiency.
Owner:NINGBO YAMAO OPTOELECTRONICS CO LTD
Method for measuring content of atractylis in joint arthralgia-relieving pill
InactiveCN110794055AEffective quality controlHigh precisionComponent separationO-Phosphoric AcidJoint arthralgia
The invention discloses a method for measuring content of atractylis in a joint arthralgia-relieving pill. The method is a high performance liquid chromatography method which takes an atractylis reference substance as a reference and takes methanol, water, 0.01-5% phosphoric acid aqueous solution, 0.01-5% glacial acetic acid and 0.01-0.5% formic acid aqueous solution in a ratio of (50-70): (50-30)or acetonitrile, water, 0.01-5% phosphoric acid aqueous solution, 0.01-5% glacial acetic acid and 0.01-0.5% formic acid aqueous solution in a ratio of (20-50): (80-50) as a mobile phase. The method disclosed by the invention is high in specificity, high in precision, high in repeatability, high in recovery rate, high in stability and accurate in measurement result, the aim of effectively controlling the quality of medicines is achieved, and the stability of the product quality and the safety and effectiveness of clinical medication are ensured.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Detection method of compound danshen dripping pills
ActiveCN102119961BQuality improvementImprove product qualityHydroxy compound active ingredientsComponent separationSalvia miltiorrhizaChromatographic fingerprint
The invention relates to a detection method of compound danshen dripping pills, comprising the following contents of: observation of characters, discrimination of contents, inspection of contents, comparison of finger prints and assaying of contained components. The detection method provided by the invention comprises a discriminating method of the medicinal component panax notoginseng, the discriminating method adopts thin-layer chromatography, the reference materials adopt a panax notoginseng reference medicinal material, a ginsenoside Rg1 reference substance, a ginsenoside Re reference substance, a ginsenoside Rb1 reference substance and a panax notoginseng saponin R1 reference substance. The detection method provided by the invention also comprises a discriminating method and an assaying method of the medicinal component danshen, wherein the discriminating method adopts a sub-2 mu m liquid chromatography technology to perform chromatographic fingerprint discrimination, and adopts sodium danshensu as a reference material; and the assaying method adopts a sub-2 mu m liquid chromatography technology to perform assaying on the components such as danshensu, panax notoginseng saponin R1, ginsenoside Rg1 and ginsenoside Rb1 in the danshen.
Owner:TIANJIN TASLY PHARMA CO LTD
Determination method of privetin in Zhenqi Fuzheng preparation
ActiveCN103592391BEffective quality controlHigh precisionComponent separationPhosphoric acidSpecnuezhenide
The invention discloses a method for determining specnuezhenide content in a Zhenqifuzheng preparation. Taking specnuezhenide standard as contrast, the method can determine the specnuezhenide content in the Zhenqifuzheng preparation by high-performance liquid chromatography by selecting a solution containing methanol and a mixed solution with a ratio of (20-50):(80-50) or a mixed solution containing acetonitrile and the mixed solution with a ratio of (5 to 35):(95-65), wherein the mixed solution consists of water, 0.01% to 5% of phosphoric acid water solution, 0.01% to 5% of glacial acetic acid, 0.01% to 0.5% of methanoic acid water solution. Compared with the prior art, the method provided by the invention has the advantages of high specificity, high precision, high repeatability, high yield, high stability, and accurate result by adopting high-performance liquid chromatography, and can effectively control the preparation quality, thereby ensuring the stability of product quality as well as clinical safety and effectiveness.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
A method for determining the content of formononetin in Zhenqi Fuzheng preparation
ActiveCN103592385BEffective quality controlHigh precisionComponent separationPhosphoric acidMethanol
The invention discloses a method for measuring content of formononetin in a Zhenqifuzheng preparation. The method is a high-performance liquid chromatography with a formononetin reference substance used as a contrast and the ratio of methyl alcohol to water / a 0.01%-5% phosphoric acid aqueous solution / a 0.01%-5% glacial acetic acid / a 0.01%-0.5% formic acid aqueous solution of (50-70):(50-30) or the ratio of acetonitrile to the water / the 0.01%-5% phosphoric acid aqueous solution / the 0.01%-5% glacial acetic acid / the 0.01%-0.5% formic acid aqueous solution of (20-50):(80-50) used as a mobile phase. The method is high in specificity, high in accuracy, good in repeatability, high in recovery rate, high in stability, accurate in measurement result and capable of achieving the purpose of effective control of the drug quality and ensuring the stability of the product quality and the safety and the effectiveness of clinical medication.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
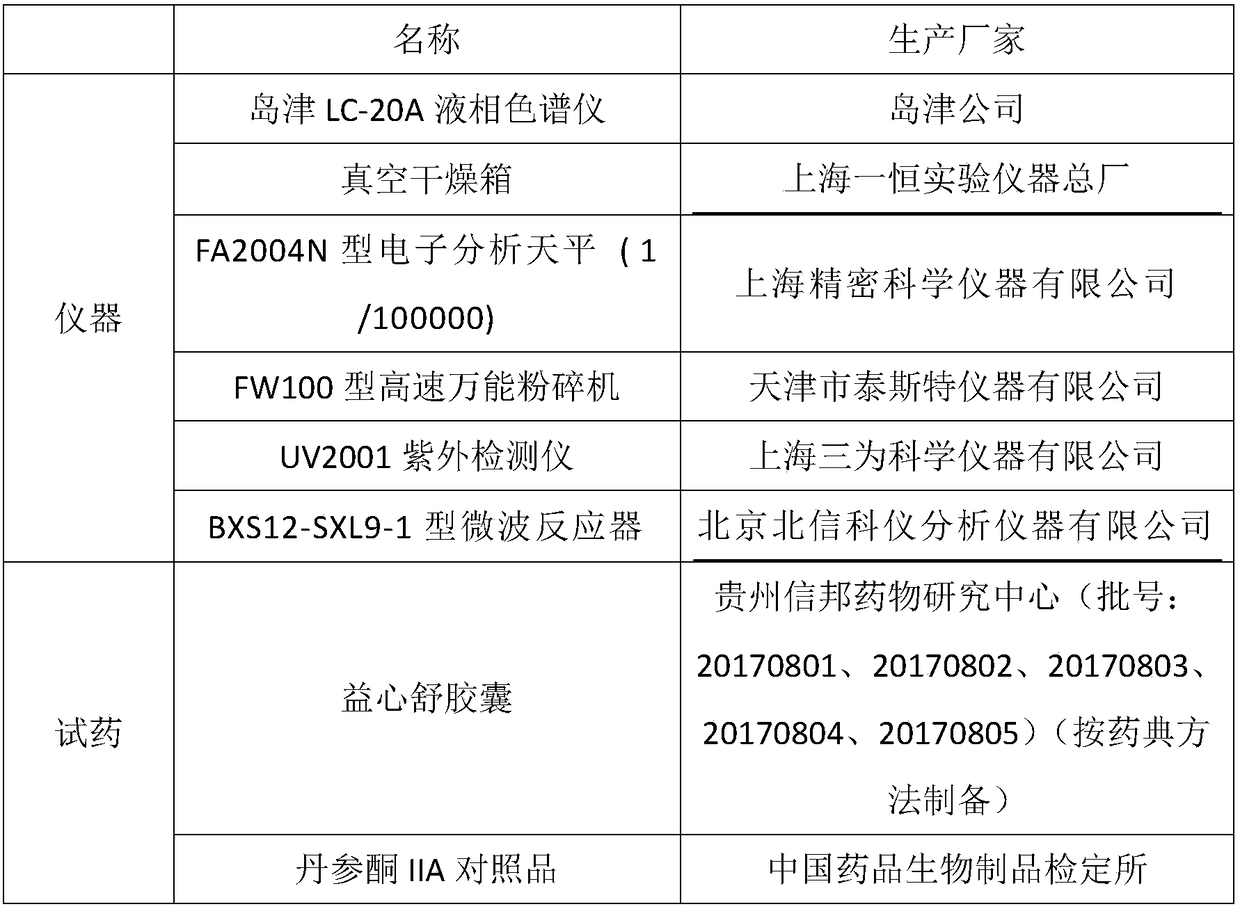
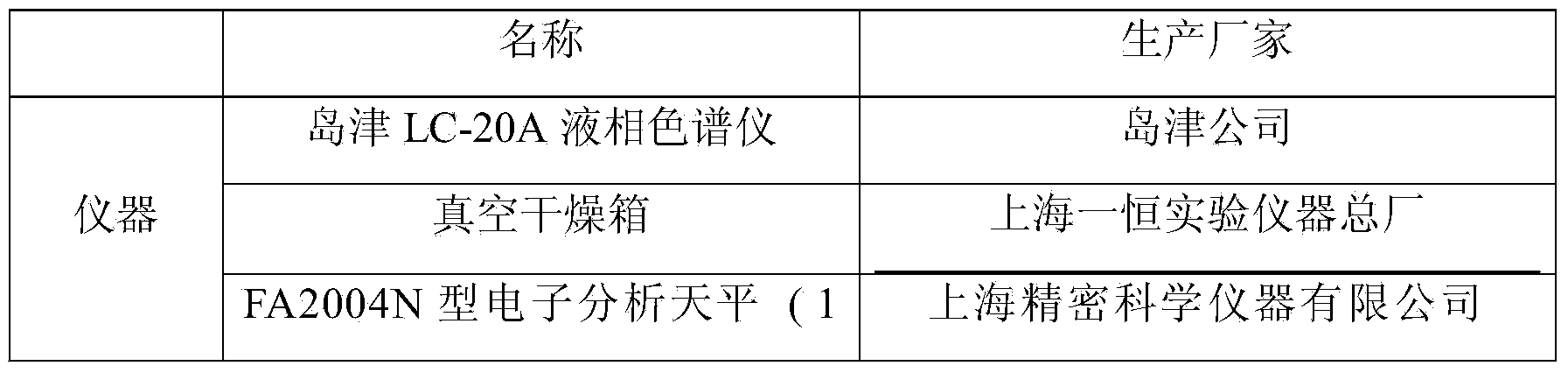
Content detection method for tanshinone IIA in Yixinshu traditional Chinese medicine preparation
The invention relates to a content detection method for tanshinone IIA in Yixinshu traditional Chinese medicine preparation. A high performance liquid chromatography method is adopted for measurement,a tanshinone IIA reference substance is taken as a reference, and the high performance liquid chromatography method is adopted to measure the contents of tanshinone IIA in the Yixinshu traditional Chinese medicine preparation on the wave length of 250-300nm. Compared with the prior art, the method measures the contents of astragaloside in the Yixinshu traditional Chinese medicine preparation through the high performance liquid chromatography method. The measurement method has the advantages of being high in specificity, good in repeatability, high in recovery rate, high in stability and accurate in measurement results, a purpose of effectively controlling medicine quality is achieved, product quality is guaranteed to be stable, and clinical medicine utilization is guaranteed to be safe and effective.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Method for measuring content of ursolic acid in glossy privet fruit and astragalus membranaceus healthy energy-strengthening preparation
InactiveCN103558304AEffective quality controlHigh precisionComponent separationBiotechnologyFluid phase
The invention discloses a method for measuring the content of ursolic acid in a glossy privet fruit and astragalus membranaceus healthy energy-strengthening preparation. The method takes an ursolic acid reference substance as a reference and can be used for measuring the content of the ursolic acid in the glossy privet fruit and astragalus membranaceus healthy energy-strengthening preparation at the wavelength being 200-220nm by using a high-performance liquid chromatography. The method is high in specificity and precision, good in repeatability, high in recovery rate and stability and accurate in measurement result, achieves the purpose of effectively controlling the quality of drugs so as to ensure the stable product quality and the safe and effective clinical treatment.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com