Content detection method for tanshinone IIA in Yixinshu traditional Chinese medicine preparation
A technology of traditional Chinese medicine preparation and tanshinone, which is applied in the field of traditional Chinese medicine and can solve problems such as not being able to fully reflect product quality status and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
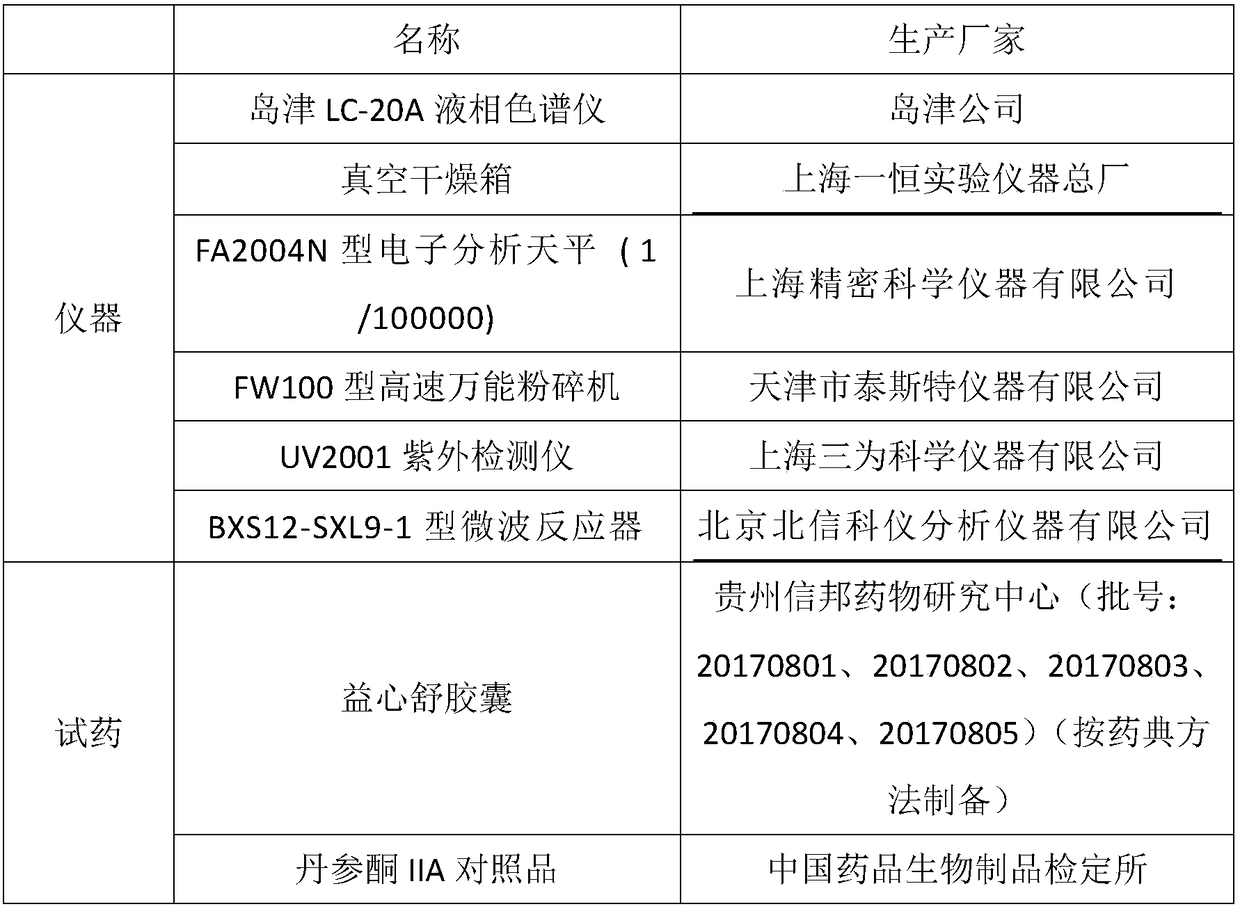
[0070] Determination of Tanshinone IIA in Yixinshu Capsules (prepared according to Pharmacopoeia method):
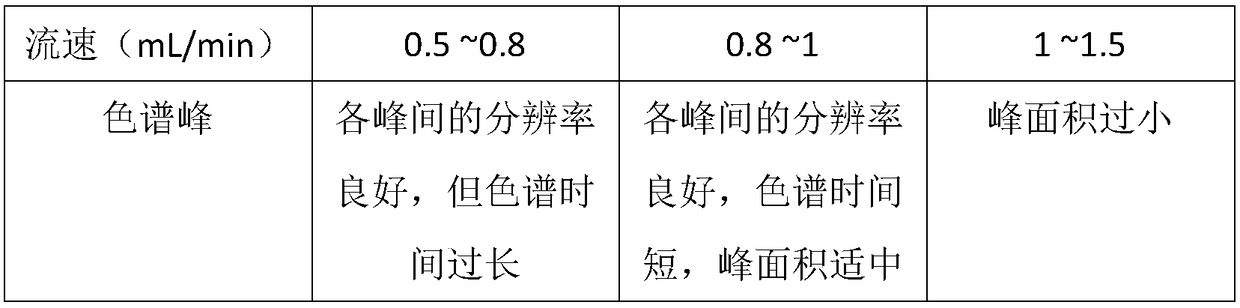
[0071] (1) Chromatographic conditions and system suitability test: octadecylsilane bonded silica gel is used as filler; acetonitrile: methanol: 0.5% ammonium acetate = 67:12:21 is used as mobile phase; flow rate is 0.8-1.0mL min -1 ;The detection wavelength is 270nm; the column temperature is 25°C; the number of theoretical plates should not be less than 2000 based on the peak of tanshinone IIA;
[0072] (2) Preparation of reference substance solution: accurately weigh an appropriate amount of tanshinone IIA reference substance, add methanol to dissolve, and make 1 mg / mL tanshinone IIA reference substance solution;
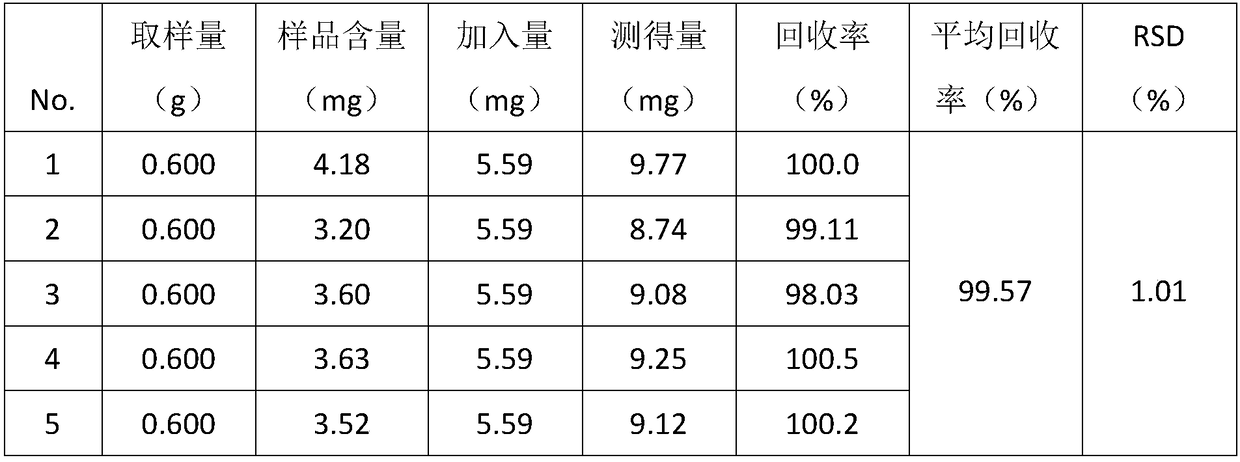
[0073] (3) Preparation of the test solution: Accurately weigh 14g of the contents of Yixinshu Capsules, accurately add 100mL of methanol, weigh the weight, ultrasonically extract for 40min, make up the weight with methanol, and filter; take the subsequent fi...
Embodiment 2
[0076] Determination of Tanshinone IIA in Yixinshu Tablets (prepared according to Pharmacopoeia method):
[0077] (1) Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as filler; use 0.04mol / L sodium dihydrogen phosphate: acetonitrile = 20:80 as mobile phase; flow rate 0.8~1.0mL min -1 ;The detection wavelength is 270nm; the column temperature is 25°C; the number of theoretical plates should not be less than 2000 based on the peak of tanshinone IIA;
[0078] (2) Preparation of reference substance solution: accurately weigh an appropriate amount of tanshinone IIA reference substance, add methanol to dissolve, and make 1 mg / mL tanshinone IIA reference substance solution;
[0079] (3) Preparation of the test solution: Accurately weigh 14g of Yixinshu tablet, accurately add 100mL of methanol, weigh, ultrasonically extract for 60min, make up the weight with methanol, filter; take the subsequent filtrate, which is the test solution ; (4) ...
Embodiment 3
[0081] Determination of Tanshinone IIA in Yixinshu Capsules (prepared according to the method described in Pharmacopoeia 2015 Edition):
[0082] (1) Chromatographic conditions and system suitability test: octadecylsilane bonded silica gel is used as filler; acetonitrile: methanol: 0.01% ammonium acetate = 70:15:15 is used as mobile phase; flow rate is 1.5mL min -1 ;The detection wavelength is 270nm; the column temperature is 40°C; the number of theoretical plates should not be less than 2000 based on the peak of tanshinone IIA;
[0083] (2) Preparation of reference substance solution: accurately weigh an appropriate amount of tanshinone IIA reference substance, add methanol to dissolve, and make 1 mg / mL tanshinone IIA reference substance solution;
[0084] (3) Preparation of the test solution: Accurately weigh 14g of the contents of Yixinshu Capsules, accurately add 100mL of methanol, weigh, ultrasonically extract for 30min, make up the weight with methanol, and filter; take t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com