Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
192results about "Lithium sulfates/sulfites" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clean production process of plateau sulfate type boron-lithium salt lake brine
InactiveCN102910652AHigh purityReduce the ratio of magnesium to lithiumChemical industryAlkali metal halide purificationHydration reactionSylvinite
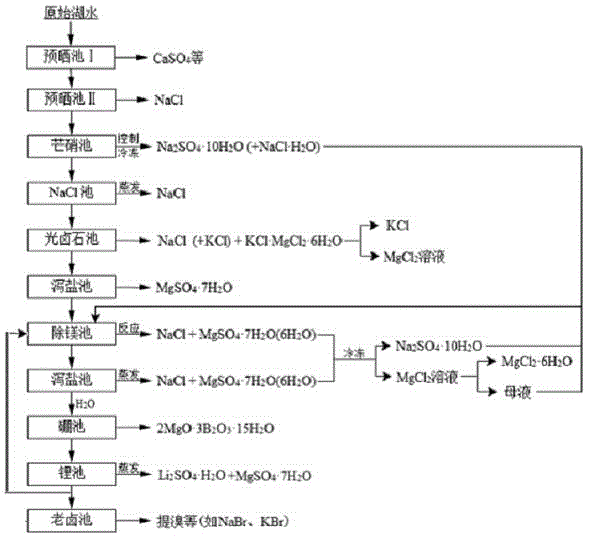
The invention relates to a clean production process of plateau sulfate type boron-lithium salt lake brine. The process comprises the following steps of: (1) arranging a pre-airing pond, a mirabilite pond, a NaCl pond, a carnallite pond, an epsom salt pond I, a magnesium removing pond, an epsom salt pond II, a boron pond, a lithium pond and an old brine pond; (2) controlling the sodium ion concentration in plateau sulfate type boron-lithium salt lake brine, precipitating mirabilite out in winter to obtain brine A, naturally evaporating the brine A, and salting out to obtain brine B; (3) naturally evaporating the brine B, and precipitating sylvine and carnallite out in sequence to obtain brine C; (4) naturally evaporating the brine C, precipitating an epsom salt out, and performing solid-liquid separation to obtain brine D and a solid A; (5) blending the brine D with mirabilite, removing magnesium to obtain brine E, and naturally evaporating brine E to obtain brine F and a solid B; (6) performing a hydration reaction on brine F, naturally evaporating, and precipitating reservoir water / inderite and brine G out; and (7) evaporating brine G or refrigerating for precipitating lithium sulfate, and processing the lithium sulfate into a corresponding product. The process has the advantages of comprehensive utilization of natural energy, saving in energy and environment friendliness.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI +1
Production process of lithium hydroxide monohydrate
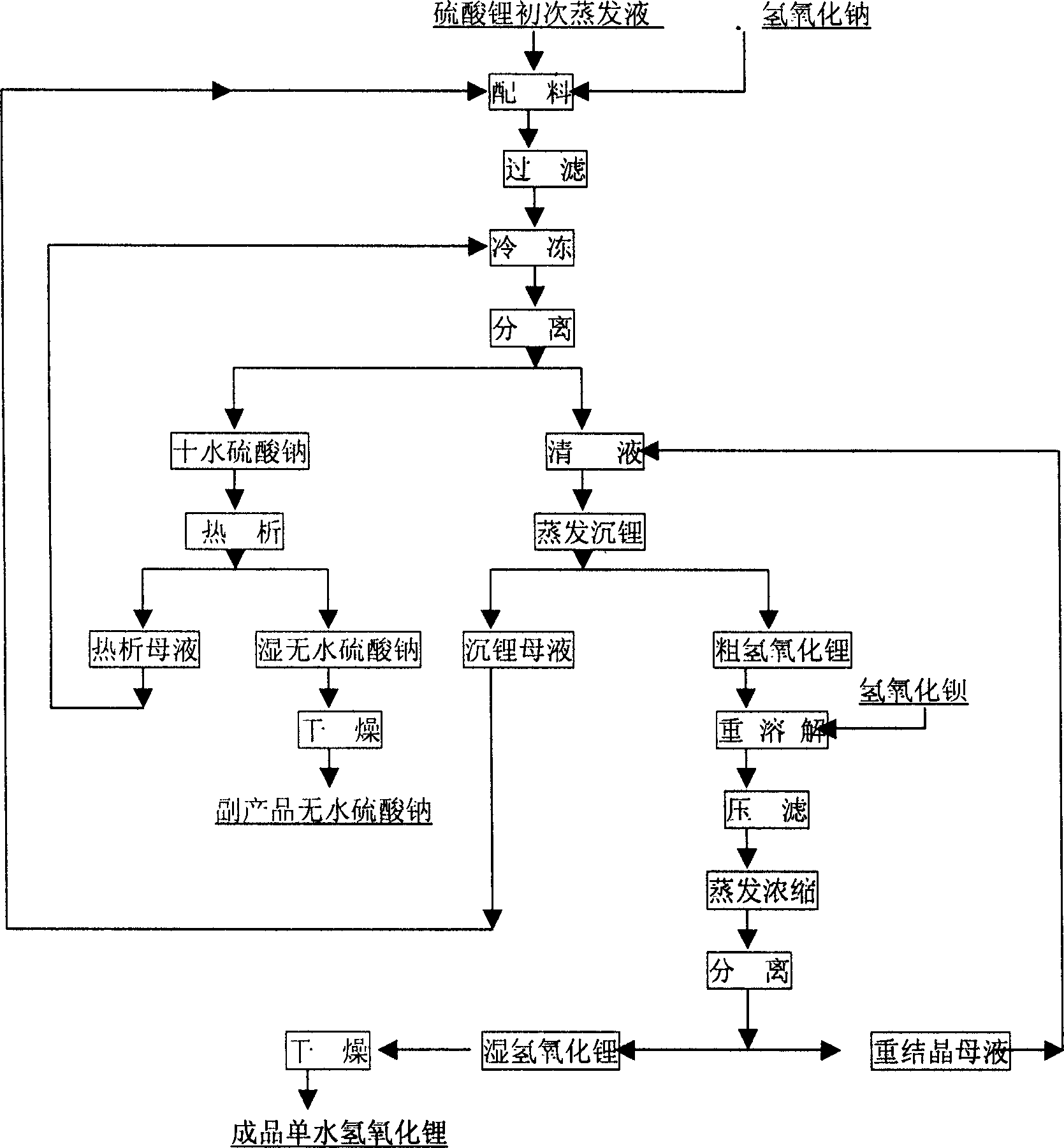
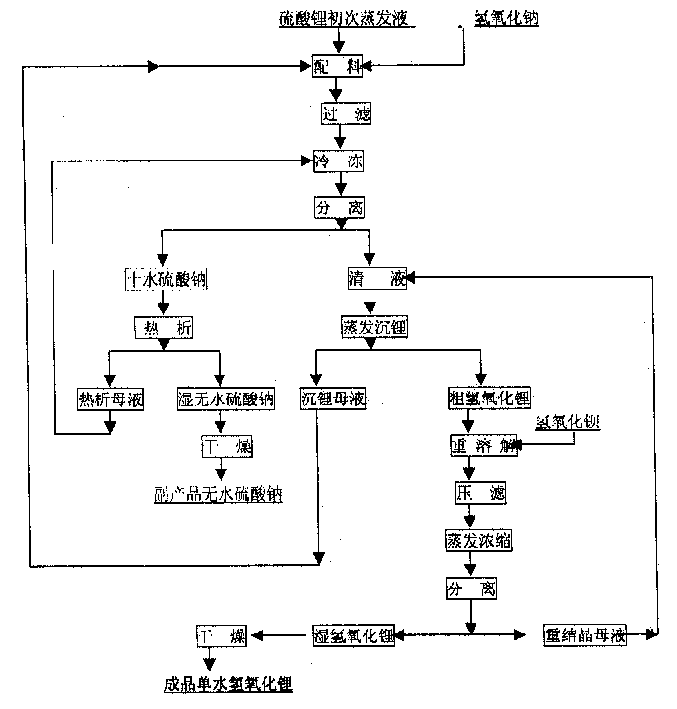
InactiveCN1486931AShort process routeHigh yieldSulfate/bisulfate preparationLithium oxides/hydroxidesSolubilityStrontium hydroxide octahydrate
In the production process of lithium hydroxide monohydrate, lithium sulfate solution and caustic soda are made to produce metathetic reaction to form mixture solution of sodium sulfate and lithium hydroxide, and sodium sulfate and lithium hydroxide monohydrate are then separated by means of the obvious difference in low temperature solubility. The production process includes the following steps: adding sodium hydroxide into lithium sulfate solution obtained through serial production steps to obtain mixture solution of sodium sulfate and lithium hydroxide; cooling to minus 10 deg.c to 5 deg.c for the crystallization and separation of sodium sulfate; heating to concentrate the separated clear liquid; crystallization and separation to obtain coarse lithium hydroxide monohydrate product; water dissolving coarse lithium hydroxide monohydrate, adding barium hydroxide to form insoluble barium sulfate, filtering, concentrating filtrate, crystallizing to separate wet lithium hydroxide monohydrate; and drying.
Owner:JIANGSU RONGHUI GENERAL LITHIUM IND CO LTD
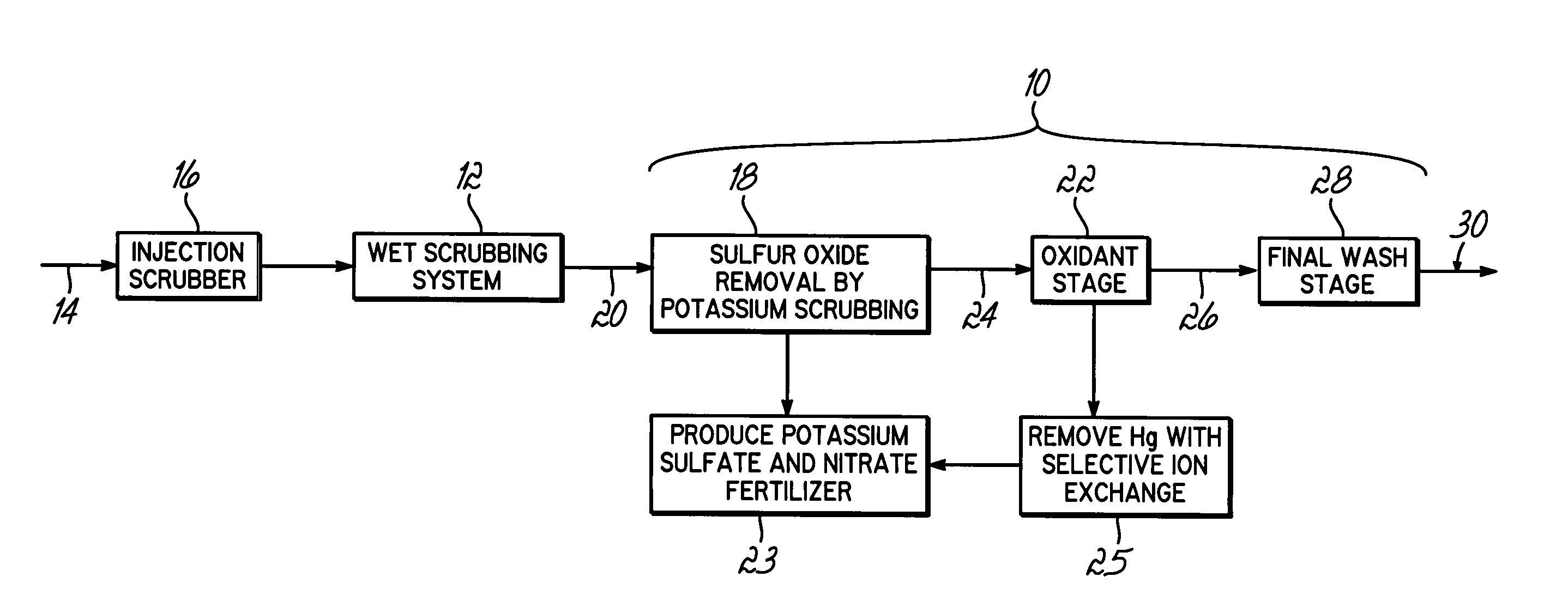
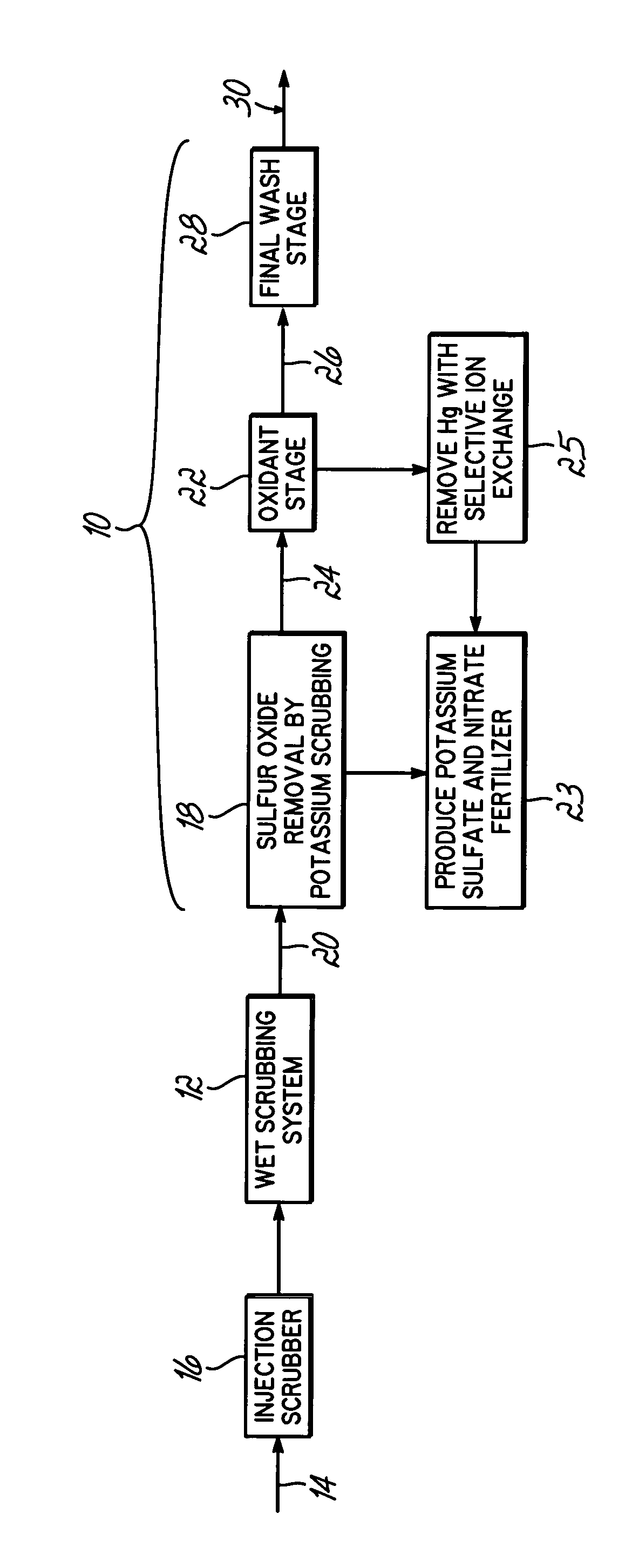
Method for removing sulfur dioxide, mercury, and nitrogen oxides from a gas stream
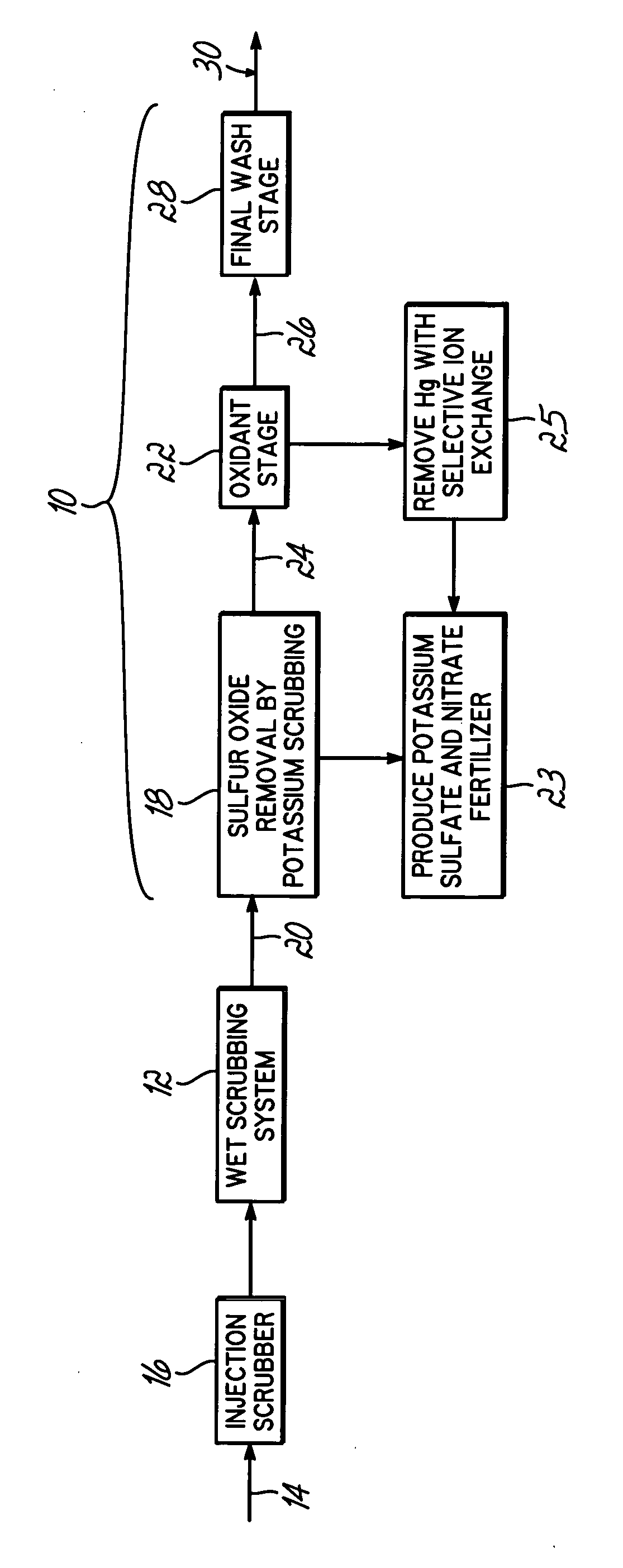
InactiveUS20060239877A1Low costImprove performanceAmmonium nitratesNitrogen compoundsSorbentPotassium
Methods for scrubbing gas streams to remove acid gases including sulfur dioxide, mercury-containing substances, and / or nitrogen oxides from the gas stream. The gas stream is contacted with a potassium-based sorbent effective for removing at least a portion of the acid gases. The partially cleaned gas stream is then contacted with an oxidant effective to remove at least a portion of the nitrogen oxides and / or mercury-containing substances after partially removing the acid gas substance.
Owner:ENVIROSOLV ENERGY
Method for removing sulfur dioxide, mercury, and nitrogen oxides from a gas stream
InactiveUS7514053B2Improve performanceAvoid pollutionAmmonium nitratesNitrogen compoundsNitrogen oxidesSorbent
Methods for scrubbing gas streams to remove acid gases including sulfur dioxide, mercury-containing substances, and / or nitrogen oxides from the gas stream. The gas stream is contacted with a potassium-based sorbent effective for removing at least a portion of the acid gases. The partially cleaned gas stream is then contacted with an oxidant effective to remove at least a portion of the nitrogen oxides and / or mercury-containing substances after partially removing the acid gas substance.
Owner:ENVIROSOLV ENERGY
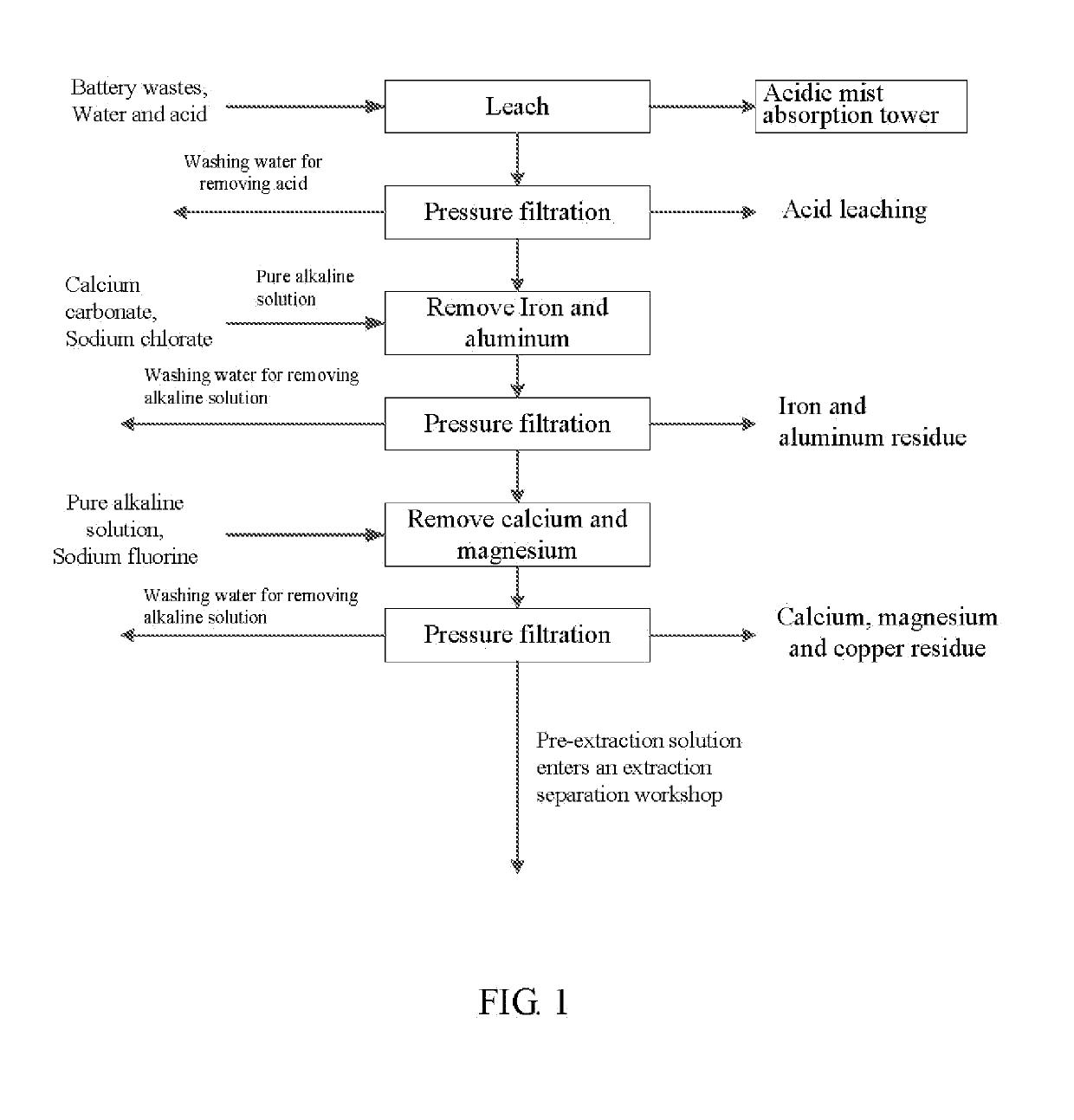
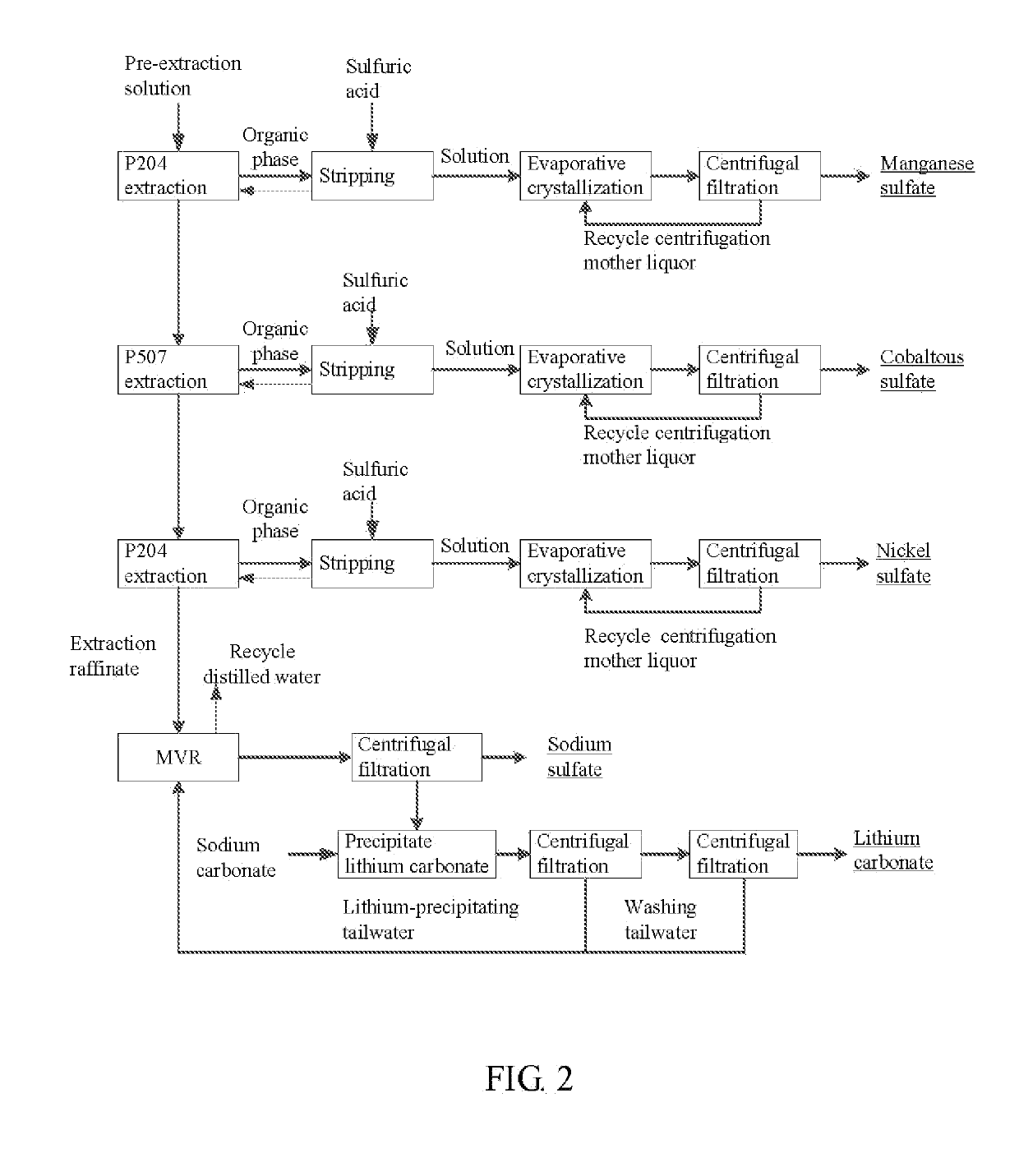
Method for preparing nickel/manganese/lithium/cobalt sulfate and tricobalt tetraoxide from battery wastes
ActiveUS20190152797A1Reduce productionHigh puritySolvent extractionCobalt sulfatesManganeseCobalt Sulfate
A method for preparing nickel / manganese / lithium / cobalt sulfate and tricobalt tetraoxide from battery wastes adopts the following process: dissolving battery wastes with acid, removing iron and aluminum, removing calcium, magnesium and copper, carrying extraction separation, and carrying out evaporative crystallization to prepare nickel sulfate, manganese sulfate, lithium sulfate, cobalt sulfate or / and tricobalt tetraoxide. By using the method, multiple metal elements, such as nickel, manganese, lithium and cobalt, can be simultaneously recovered from the battery wastes, the recovered products are high in purity and can reach battery grade, battery-grade tricobalt tetraoxide can also be directly produced. The method is simple in process, low in, energy consumption and free in exhaust gas pollution, and can realize zero release of wastewater.
Owner:HUNAN JINYUAN NEW MATERIALS CO LTD
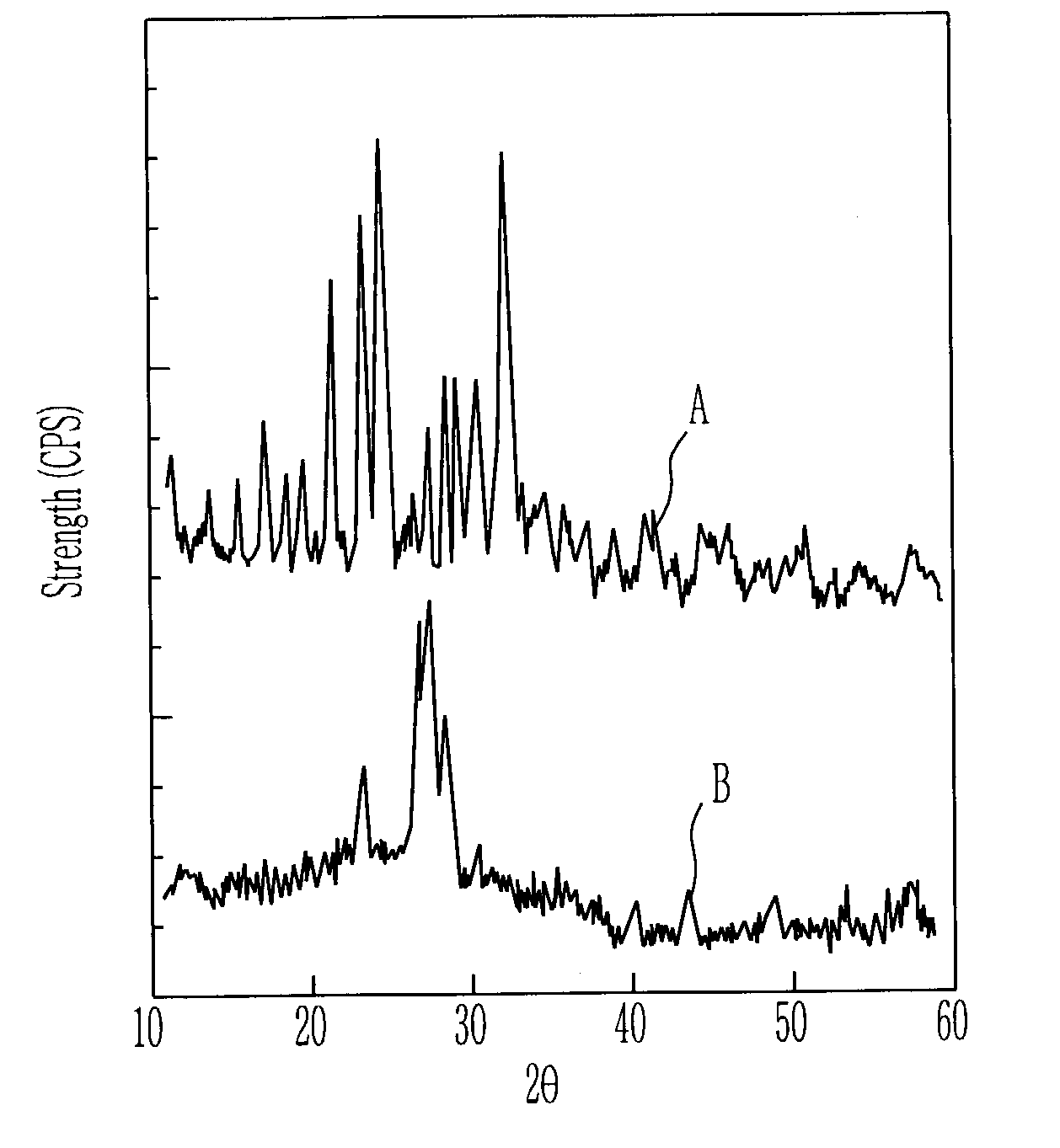
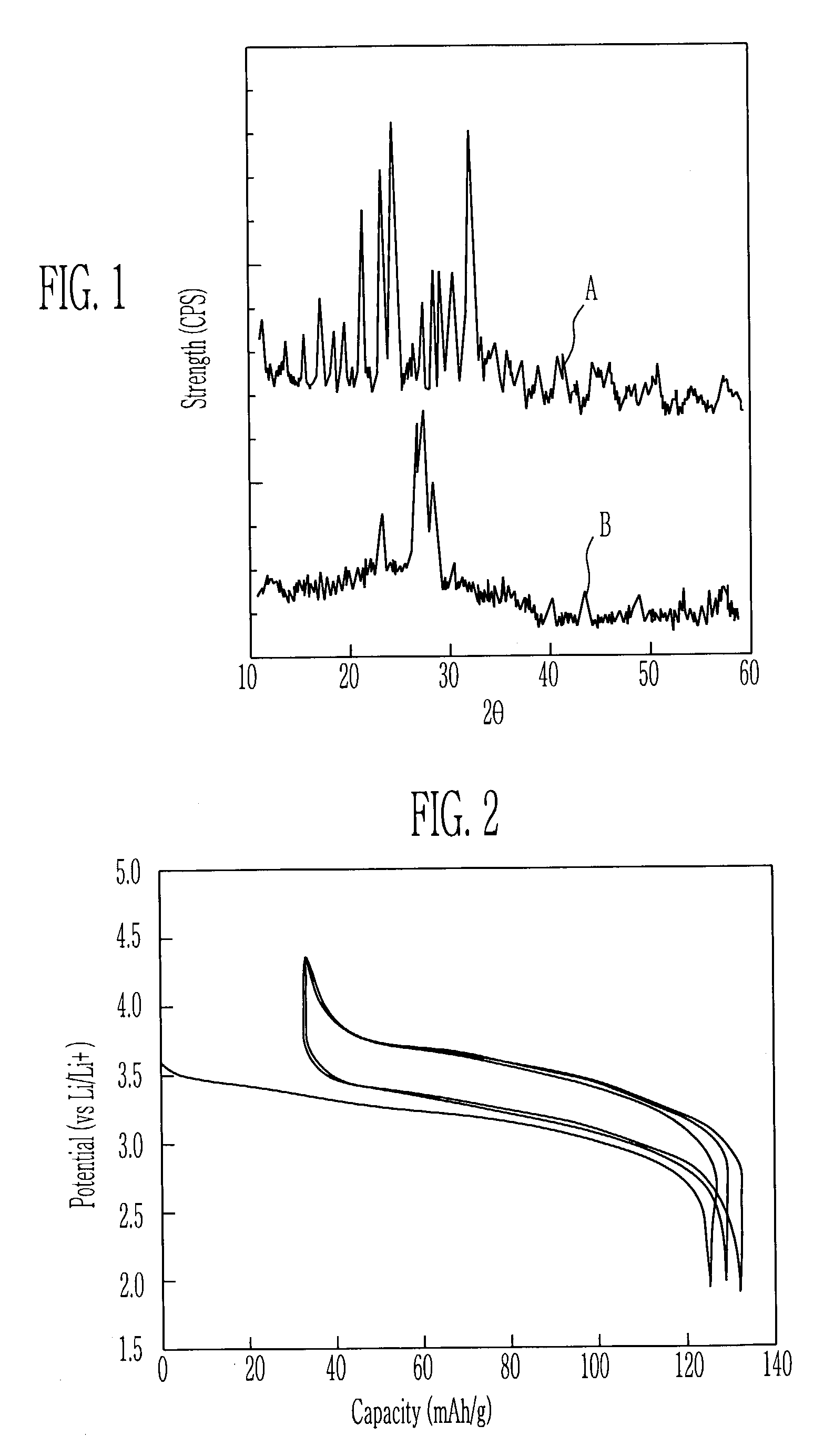
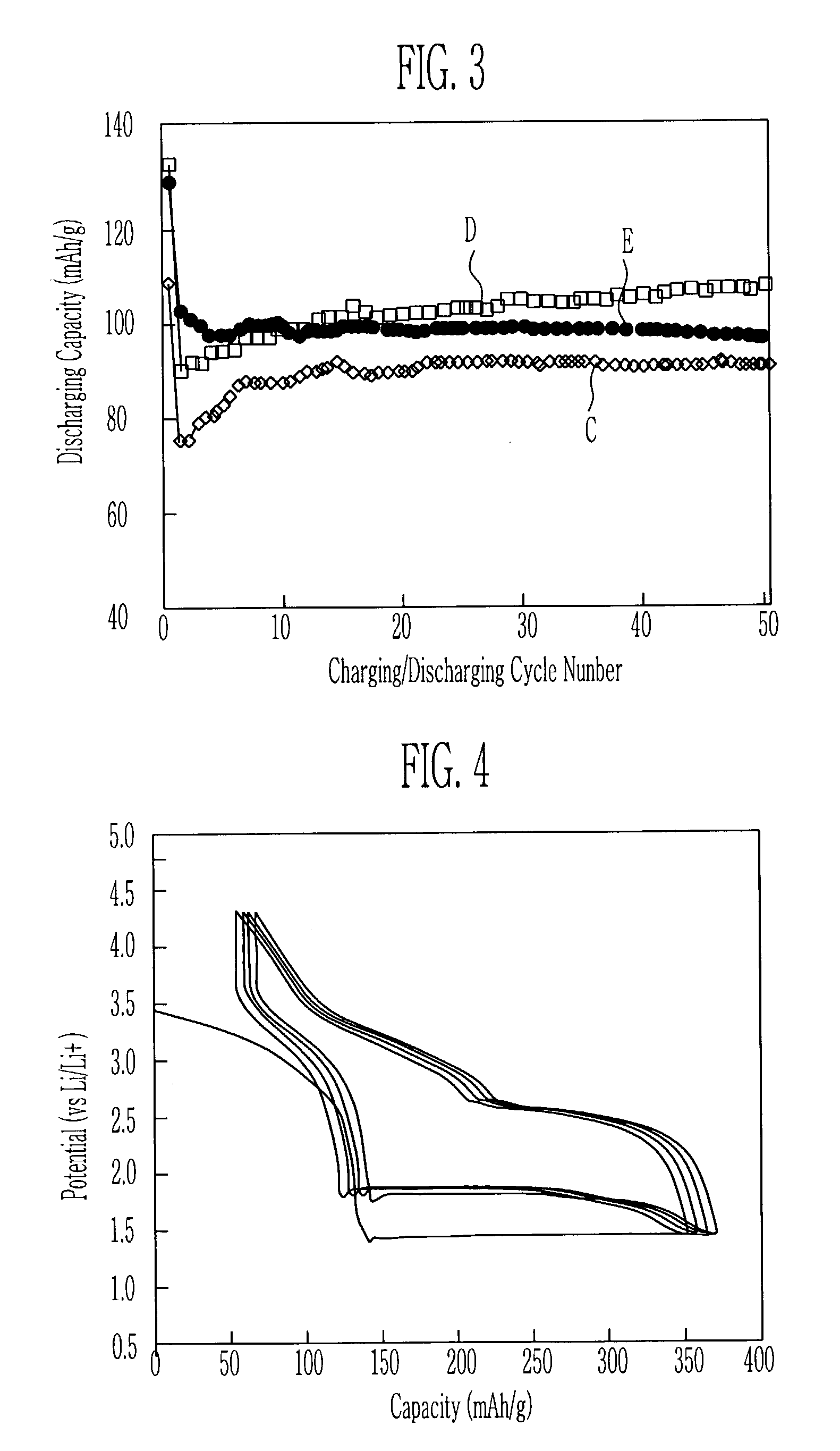
Cathode active material for lithium secondary cell and method for manufacturing the same
ActiveUS6986968B2Good electrochemical propertiesEasy to getPhosphatesSulfur compoundsChemical treatmentLithium
A cathode active material for a lithium secondary cell used in a cellular phone is disclosed. The cathode active material for the lithium secondary cell and the method the same having a high capacity and a long lifetime, different from LiCoO2 and LiMn2O4, Li(Ni, Co)O2, and V-system oxide that has been researched as the active material for substituting LiCoO2 are provided. The cathode active material for the lithium secondary cell in the next formula 1 is obtained by heating or chemically treating diadochite [Fe2(PO4)(SO4)(OH).6H2O] that is the mineral containing PO43−, SO42−, and OH−.LiaFebMc(PO4)x(SO4)y(OH)z (1)In the formula, M is at least one element selected from a radical consisting of Mg, Ti, Cr, Mn, Co, Ni, Cu, Zn, Al, and Si, with 0≦a, c≦0.5, 1≦b≦2, 0.5≦x, y, z≦1.5.
Owner:ELECTRONICS & TELECOMM RES INST
Process for recovery of sulphate of potash
ActiveUS7041268B2Eliminate needMaximize recoveryChemical industrySulfate preparationPhysical chemistryCarnallite
The present invention is directed to a novel integrated process for the recovery of sulphate of potash (SOP) from sulphate rich bittern. The process requires bittern and lime as raw materials. Kainite type mixed salt is obtained by fractional crystallization of the bittern, and is converted to schoenite which is subsequently reacted with muriate of potash (MOP) for its conversion to SOP. End liquor from kainite to schoenite conversion (SEL) is desulphated and supplemented with MgCl2 using end bittern generated in the process of making carnallite. Decomposed carnallite liquor produced is reacted with hydrated lime for preparing CaCl2 solution and high purity Mg(OH)2 having low boron content. It is shown that the liquid streams containing potash are recycled in the process, and the recovery of potash in the form of SOP is quantitative.
Owner:COUNCIL OF SCI & IND RES
Process for recovery of sulphate of potash
ActiveUS20050220698A1Minimize effluent generationEnhance potash recoveryEnergy inputSulfate preparationDecompositionCarnallite
A novel integrated process for the recovery of sulphate of potash (SOP) from sulphate rich bittern is disclosed. The process requires only bittern and lime as raw materials. Kainite type mixed salt is obtained by fractional crystallization of the bittern, Kainite is converted to schoenite with simultaneous removal of NaCl by processing it with water and end liquor obtained from reaction of schoenite with MOP for its conversion to SOP. The end liquor from kainite to schoenite conversion (SEL) is used for the recovery of MOP. SEL is desulphated and supplemented with MgCl2 using end bittern generated in the process of making carnallite. The carnallite is decomposed to get crude potash which in turn processed to get MOP. The carnallite decomposed liquor produced in the decomposition of carnallite is reacted with hydrated lime for preparing CaCl2 solution and high purity Mg(OH)2 having low boron content, The CaCl2 solution is used for desulphatation of SEL producing high purity gypsum as a byproduct. It is shown that the liquid steams containing potash are recycled in the process, the recovery of potash in the form of SOP is quantitative.
Owner:COUNCIL OF SCI & IND RES
Lithium ion battery and positive electrode material thereof
InactiveCN108075111AReduce residual lithium on the surfaceLarge specific surface areaSecondary cellsPositive electrodesPhysical chemistryLithium-ion battery
The invention discloses a lithium ion battery and a positive electrode material thereof. The general chemical formula of the positive electrode material of the lithium ion battery is LiNixM[1-x]O2, wherein x is greater than or equal to 0.5 but smaller than 1; the M is one or more of Co, Mn, Al, Mg, Ti and Zr; the specific surface area of the positive electrode material of the lithium ion battery is 0.2 to 0.6m<2> / g; the surface lithium residue quantity is 200 to 1000 ppm. Compared with the prior art, the positive electrode material of the lithium ion battery is prepared through solid phase reaction; the surface lithium residue quantity of the material can be obviously reduced; the specific surface area increase of the positive electrode material of the lithium ion battery in the reaction process can also be avoided. The circulation stability of the positive electrode material of the lithium ion battery is good; the preparation method is simple; the implementation is easy; the production cost is low; good application prospects are realized. The invention also discloses the lithium ion battery.
Owner:CONTEMPORARY AMPEREX TECH CO
Method for preparing lithium salt ore from plateau sulfate type salt lake brine
ActiveCN103204523ANo pollution in the processReduce manufacturing costChemical industryLithium sulfates/sulfitesHigh magnesiumThenardite
The invention discloses a method for preparing lithium salt ore from plateau sulfate type salt lake brine. The method comprises the following steps of: evaporating sulfate type salt lake brine to be in the sodium chloride saturated state, freezing in winter to separate out mirabilite, and carrying out solid-liquid separation when the content of the sulfate ion in the brine is controlled to be 1g / L to 7g / L; evaporating the brine from which the mirabilite is separated out, so as to separate sodium chloride; evaporating the brine from which the sodium chloride is separated out, so as to separate out sylvine, carnallite and epsomite, carrying out solid-liquid separation when the lithium ion concentration in the brine is controlled to be greater than or equal to 6g / L, wherein the brine subjected to solid-liquid separation is the brine with high magnesium chloride content; mixing the brine with high magnesium chloride content with the mirabilite to react so as to separate out sodium salt and magnesium salt, and carrying out solid-liquid separation when the magnesium-lithium ratio in the solution is controlled to be less than or equal to 8:1, so as to obtain boron-lithium-rich brine; and reacting the boron-lithium-rich brine with water to separate out boron rock, carrying out solid-liquid separation so as to obtain lithium-rich brine; and introducing the lithium-rich brine into a lithium salt pool, and evaporating so as to separate out the lithium salt ore.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI +1
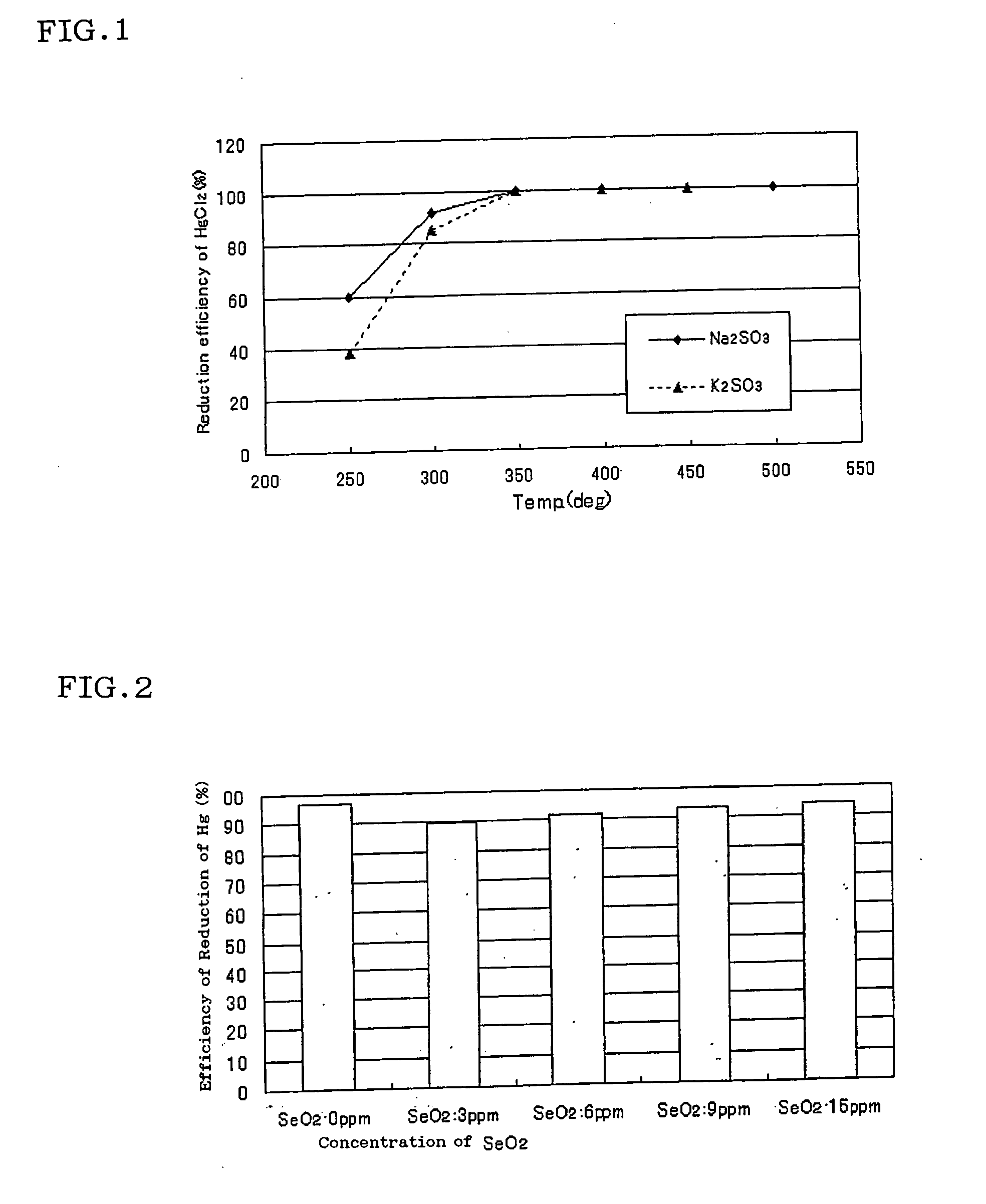
Catalyst for reducing mercury, a mercury conversion unit, and an apparatus for measuring total mercury in combustion exhaust gas by using the same
InactiveUS20070232488A1Reduce functionImprove accuracyCalcium/strontium/barium carbonatesUsing liquid separation agentCombustionPhosphate
The present invention relates to a catalyst for reducing mercury, which comprises a reagent comprising any of the sulfites of potassium, sodium, calcium and magnesium, or any of the phosphates thereof, or a combination of them, as a main reagent of a catalyst component. And the present invention relates to the catalyst for reducing mercury, wherein the catalyst component is mixed with a different salt as an agent for inhibiting crystallization of the catalyst component.
Owner:HORIBA LTD
Methods for synthesis of graphene derivatives and functional materials from asphaltenes
ActiveUS20160039678A1Low costFrom normal temperature solutionsOrganic chemistryElectrophilic aromatic substitutionGraphene derivatives
Embodiments described are directed to methods for the functionalization of asphaltene materials and to compositions made from functionalized asphaltenes. Disclosed is a method for synthesizing graphene derivatives, such as 2D single crystalline carbon allotropes of graphene and functional materials, such as sulfonic acid and its derivatives. Also disclosed is a method for the transformation of asphaltene into a source of graphene derivatives and functional materials, such as, 0D, 1D, 2D and combinations of 0D and 1D by utilizing chemical substitution reaction mechanism, such as, electrophilic aromatic substitution, nucleophilic aromatic substitution and Sandmeyer mechanism. Also disclosed are novel graphene materials comprising: acetylenic linkage and hydrogenated graphene. These novel materials, which may be produced by these methods, include, e.g.: 2D single crystalline carbon allotropes of graphene with asymmetric unit formulas C7H6N2O4, C6H4N2O4, C7H7O3S− H3O+, C7H7O3SH+, and a 2D single crystal with asymmetric unit formula (Na6O16S4)n.
Owner:TANIMOLA OLANREWAJU W
Method for purifying lithium sulfate crude ore
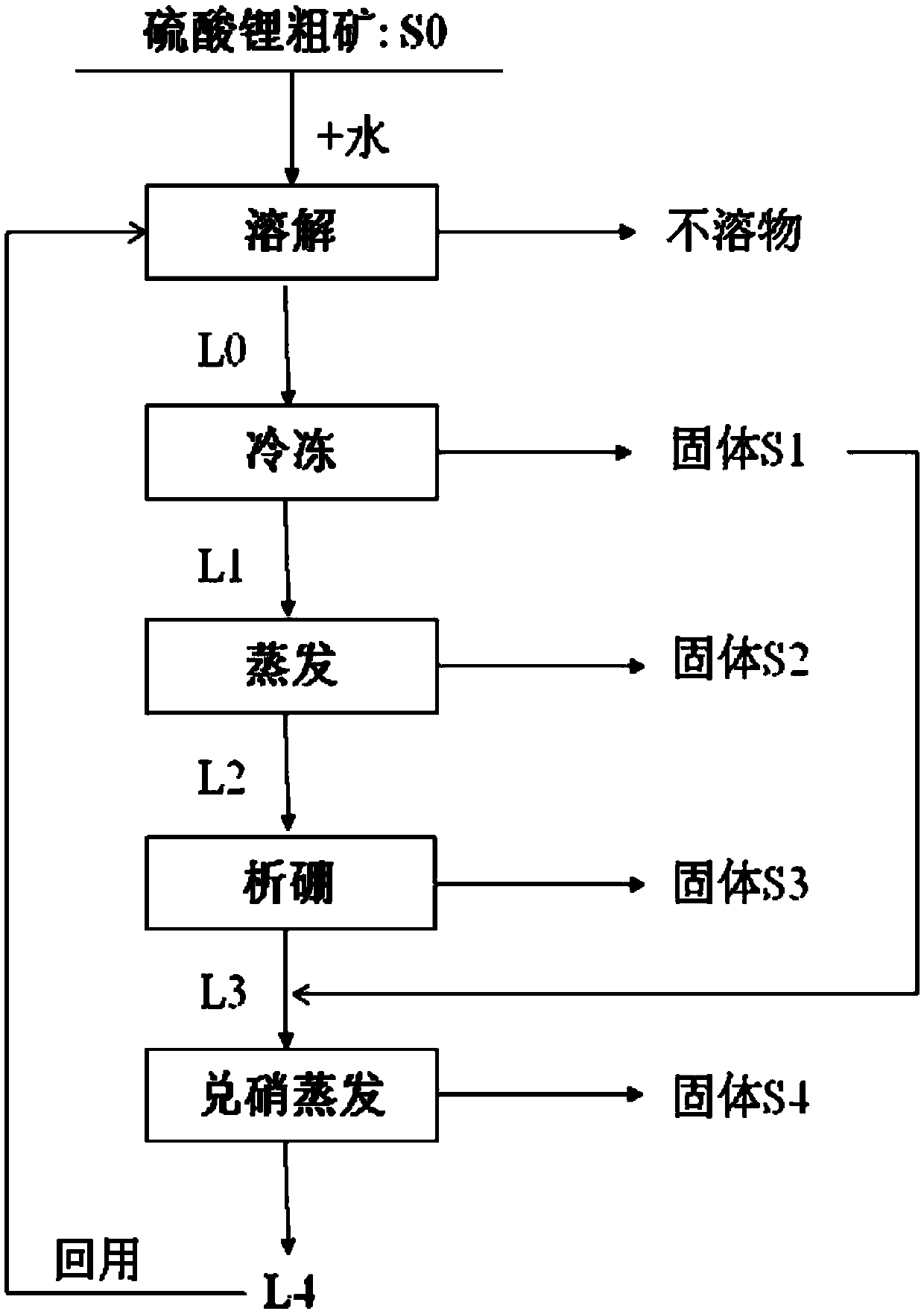
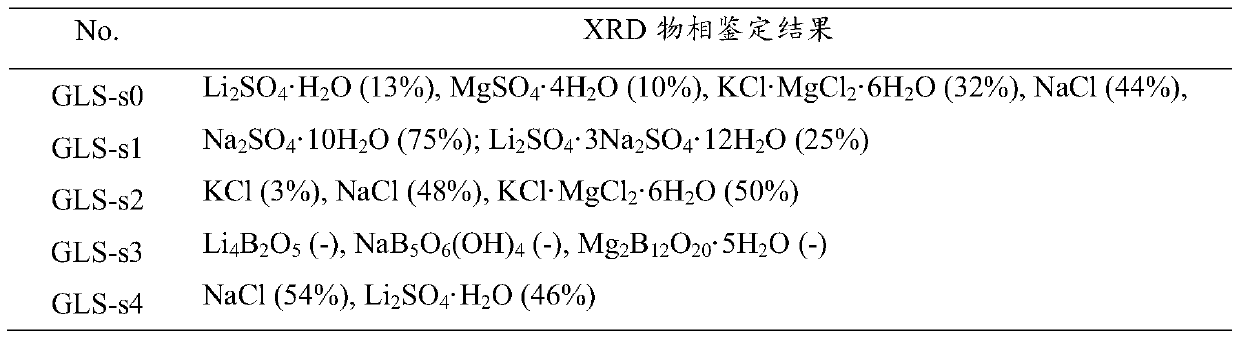
ActiveCN105502440AHigh yieldSimple process routeMagnesium chloridesAlkali metal chloridesLithium sulphateMirabilite
The invention provides a method for purifying lithium sulfate crude ore. The method comprises the following steps: step I, mixing the lithium sulfate crude ore S0 with excessive water to ensure that soluble ingredients in the lithium sulfate crude ore are just dissolved completely, and carrying out solid-liquid separation to obtain a solution L0; step II, freezing the solution L0 to separate out mirabilite, and carrying out solid-liquid separation to obtain a solution L1 and a solid S1; step III, evaporating the solution L1 to separate out a solid phase, and carrying out solid-liquid separation to obtain a solution L2 and a solid S2; step IV, allowing the solution L2 to stand still in a sealed condition for 7 to 50 days at 0 to 40 DEG C to separate out borate, and carrying out solid-liquid separation to obtain a solution L3; step V, mixing the solid S1 obtained in the freezing process in the step II with the solution L3, and evaporating the mixture at 0 to 40 DEG C to separate lithium sulfate concentrate.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Roasting system and roasting method for lithium-ore acidification by using externally-heated type rotary furnace
ActiveCN102874850AAvoid emissionsReduce the amount of smokeChemical industryLithium sulfates/sulfitesMetallic lithiumReaction temperature
The invention discloses a roasting system and a roasting method for lithium-ore acidification by using an externally-heated type rotary furnace, which comprises the following steps of: generating acidifying grog through acidifying roasting after mixing beta lithium ores and sulfuric acid by using the externally-heated type rotary furnace as the core equipment of acidifying roasting reaction, and sending the acidifying grog which is generated after acidifying roasting into a subsequent working procedure after cooling the acidifying grog, wherein the sulfuric acid for acidifying roasting is used after the concentration of an acid liquor after tail gas is washed is adjusted through fuming sulfuric acid and can also be directly prepared from concentrated sulfuric acid or fuming acid or by mixing the two acids; the tail gas which is generated in the process of acidifying roasting is discharged with reaching standards after acid washing and alkali washing, and most of the acidic gas in the tail gas returns to the roasting system along with the washing acid to be reutilized during acid washing. The invention overcomes the defects of large tail-gas quantity, serious pollution and the like of a traditional internally-heated type acidifying-roasting process and provides a good method for the extraction of metallic lithium in the lithium ores, the self-heating of the reaction is fully utilized, energy does not need to be additionally consumed after the self reaction temperature is reached, and the energy-saving effect is remarkable.
Owner:西安三瑞超鼎新能源科技有限公司
Processing of manganous sulphate/dithionate liquors derived from manganese resource material
ActiveUS8460631B2Rubidium/caesium/francium compoundsProcess efficiency improvementManganese sulphateDithionate
The process concerns hydrometallurgical processing of manganese sulphate and manganese dithionate containing liquors and recovery of water therefrom. Sodium sulphate and / or sodium dithionate containing liquors are derived from manganese sulphate and manganese dithionate containing liquids, which are then cooled to produce crystals of sodium sulphate decahydrate and sodium dithionate dehydrate. The sodium sulphate decahydrate and sodium dithionate dehydrate crystals are then heated to a temperature sufficient to decompose the sodium sulphate decahydrate crystals to form anhydrous sodium sulphate crystals, sodium dithionate hydrate crystals and water, after which water is removed from the sodium sulphate and sodium dithionate hydrate crystal. The sodium sulphate and sodium dithionate dehydrate crystals are then heated to form anhydrous sodium sulphate, sulfur dioxide and water or steam. The anhydrous sodium sulphate is then separated from the sulfur dioxide and water.
Owner:AMERICAN MANGANESE
Methods of making cesium salts and other alkali metal salts
A method of making a cesium salt is described and involves reacting a cesium sulfate containing solution with lime to form 1) a solution containing at least cesium hydroxide and 2) a residue comprising calcium sulfate. The method further involves removing the residue from the solution and converting the cesium hydroxide that is present in the solution to at least one type of cesium salt. The present invention further relates to uses of the cesium salt as well as methods of making cesium hydroxide using lime. Also, methods of making alkali metal salts and alkali metal hydroxides are also described.
Owner:CABOT SPECIALTY FLUIDS
Alkali/Transition Metal Halo-And Hydroxy-Phosphates And Related Electrode Active Materials
InactiveUS20100276632A1Increase capacityImprove cycle performancePhosphatesFluoride preparationHalogenPhosphate
The invention provides a novel polyanion-based electrode active material for use in a secondary or rechargeable electrochemical cell, wherein the electrode active material is represented by the general formula AaMb(SO4)2Zd.
Owner:VALENCE TECH INC
Recovery of Li values from sodium saturate brine
InactiveUS8309043B2Efficient and high throughputOther chemical processesCrystallization separationHydrated aluminaAqueous solution
The present invention provides a process for recovering Li values from a sodium saturated brine. The process includes recovering Li values from a sodium saturated brine which contains LiX. The process includes concentrating the sodium saturated brine to at least 9000 mg / l LiX, passing the concentrated brine through a bed of polycrystalline hydrated alumina pellets until the pellets are loaded with LiX from the concentrated brine, displacing brine held-up in the bed by using concentrated NaX, unloading LiX from the pellets by flowing through the bed an aqueous solution of LiX which is not saturated, displacing the LiX from the bed using concentrated NaX, and repeating the steps at least one additional time to provide the Li values.
Owner:FMC CORP
Recovery of li values from sodium saturate brine
InactiveUS20120141342A1Efficient and high throughput processEfficient and high throughputOther chemical processesEvaporator accessoriesHydrated aluminaAqueous solution
The present invention provides a process for recovering Li values from a sodium saturated brine. The process includes recovering Li values from a sodium saturated brine which contains LiX. The process includes concentrating the sodium saturated brine to at least 9000 mg / l LiX, passing the concentrated brine through a bed of polycrystalline hydrated alumina pellets until the pellets are loaded with LiX from the concentrated brine, displacing brine held-up in the bed by using concentrated NaX, unloading LiX from the pellets by flowing through the bed an aqueous solution of LiX which is not saturated, displacing the LiX from the bed using concentrated NaX, and repeating the steps at least one additional time to provide the Li values.
Owner:FMC CORP
Methods of processing solutions of potassium sulfate and magnesium sulfate, methods of producing potassium sulfate, and related systems
ActiveUS20140072507A1Economic value maximizationIncrease productionOther chemical processesDouble sulfate preparationPotassium sulfateAqueous solution
Methods of processing an aqueous solution comprising potassium sulfate and magnesium sulfate include crystallizing K2SO4, crystallizing recycle crystals, and mixing at least a portion of the recycle crystals with the aqueous solution. Systems for processing potassium sulfate and magnesium sulfate include a first crystallizer and a second crystallizer in fluid communication with the second mix tank. The second crystallizer is structured and adapted to precipitate recycle crystals from the concentrated liquor to form a potassium-depleted recycle brine. The recycle crystals precipitated in the second crystallizer have a composition suitable to be recycled to the first crystallizer to increase the production of SOP.
Owner:INTERCONTINENTAL POTASH CORP USA
Lithium ion battery and positive electrode material thereof
InactiveUS20180145324A1Avoid increase of specific surface areaSlow capacity fading rateSecondary cellsPositive electrodesLithium-ion batteryBattery cell
The present invention provides a lithium ion battery and positive electrode material thereof. The positive electrode material includes a high nickel material having a chemical formula of LiNixM1-xO2 and a coating layer, wherein 0.5≤X<1, M is selected from at least one of Co, Mn, Al, Mg, Ti and Zr, a specific surface area of the positive electrode material is 0.2 to 0.6 m2 / g, and a residual lithium content on a surface of the positive electrode material is 200 to 1000 ppm. Compared with the prior art, the positive electrode material for lithium ion battery of the present invention is prepared by solid phase reaction, which not only can significantly reduce the residual lithium content on the surface of the positive electrode material, but also can avoid increase of the specific surface area of the positive electrode material for lithium ion battery.
Owner:CONTEMPORARY AMPEREX TECH CO
Methods of making cesium salts and other alkali metal salts
A method of making a cesium salt is described and involves reacting a cesium sulfate containing solution with lime to form 1) a solution containing at least cesium hydroxide and 2) a residue comprising calcium sulfate. The method further involves removing the residue from the solution and converting the cesium hydroxide that is present in the solution to at least one type of cesium salt. The present invention further relates to uses of the cesium salt as well as methods of making cesium hydroxide using lime. Also, methods of making alkali metal salts and alkali metal hydroxides are also described.
Owner:CABOT SPECIALTY FLUIDS
Method for purifying lithium sulfide
InactiveUS20070196739A1Minimizes impurity-induced deteriorationImprove long-term stabilitySolid electrolytesSolid electrolyte cellsOrganic solventLithium hydroxide
A method of purifying lithium sulfide wherein lithium sulfide obtained by reacting lithium hydroxide with hydrogen sulfide in an aprotic organic solvent is washed with an organic solvent at a temperature of 100° C. or higher. Impurities contained in lithium sulfide can be reduced by the method of purification.
Owner:IDEMITSU KOSAN CO LTD
Preparing method of iPS cell and medium for preparing iPS cell
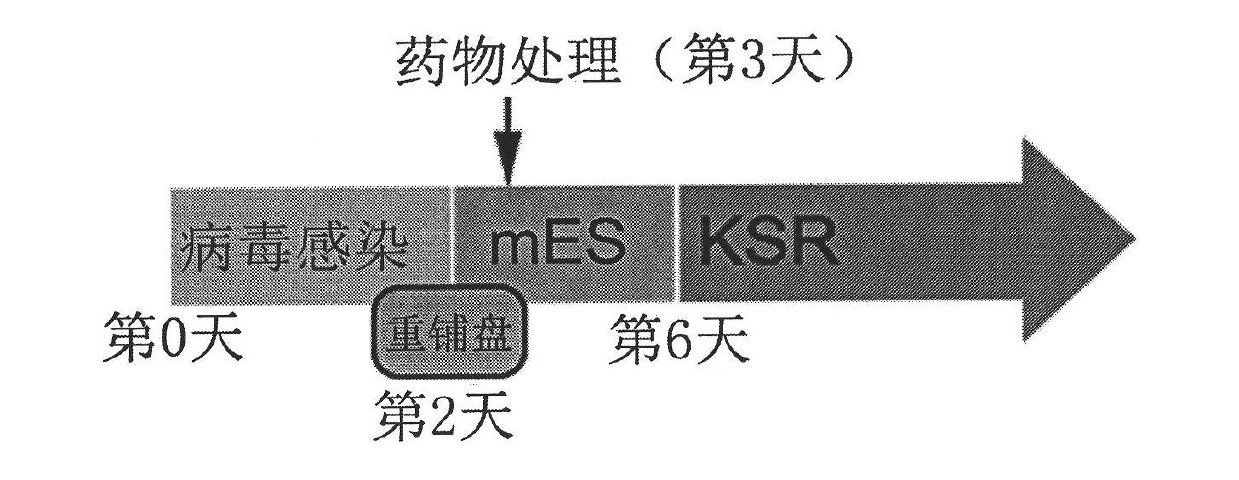
InactiveCN102559587AImprove versatilityAchieve germline transmissionGenetically modified cellsCulture processLithiumSex factors
The invention provides a preparing method of an iPS cell, which includes the following steps: step 1, introducing one or many stem cell pluripotent sex factors into a somatic cell; step 2, adopting the medium added with lithium to cultivate the somatic cell introduced with the stem cell pluripotent sex factors in the step 1; and step 3, identifying the cloning of the iPS cell. Furthermore, the invention also provides a medium for preparing the iPS cell, wherein the cell further includes lithium. The lithium-contained medium is applied to efficiently generating the iPS cell. In the invention, the lithium can improve the generation efficiency of the mouse iPS cell to be 5 to 60 times. The efficient iPS cell inducing method is highly significant in improving the iPS technical safety and applying the iPS cell to the field of regeneration medicine.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Methods of forming an alkali metal salt
ActiveUS20060239900A1Avoid timeAvoid effortOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkali metalMetal
A method of making an alkali metal salt is described and involves (1) reacting at least one alkali metal formate with an least one acid to form an alkali metal salt in the presence of formate ions and (2) substantially removing the formate ions from the alkali metal salt formed in step (1).
Owner:CABOT SPECIALTY FLUIDS
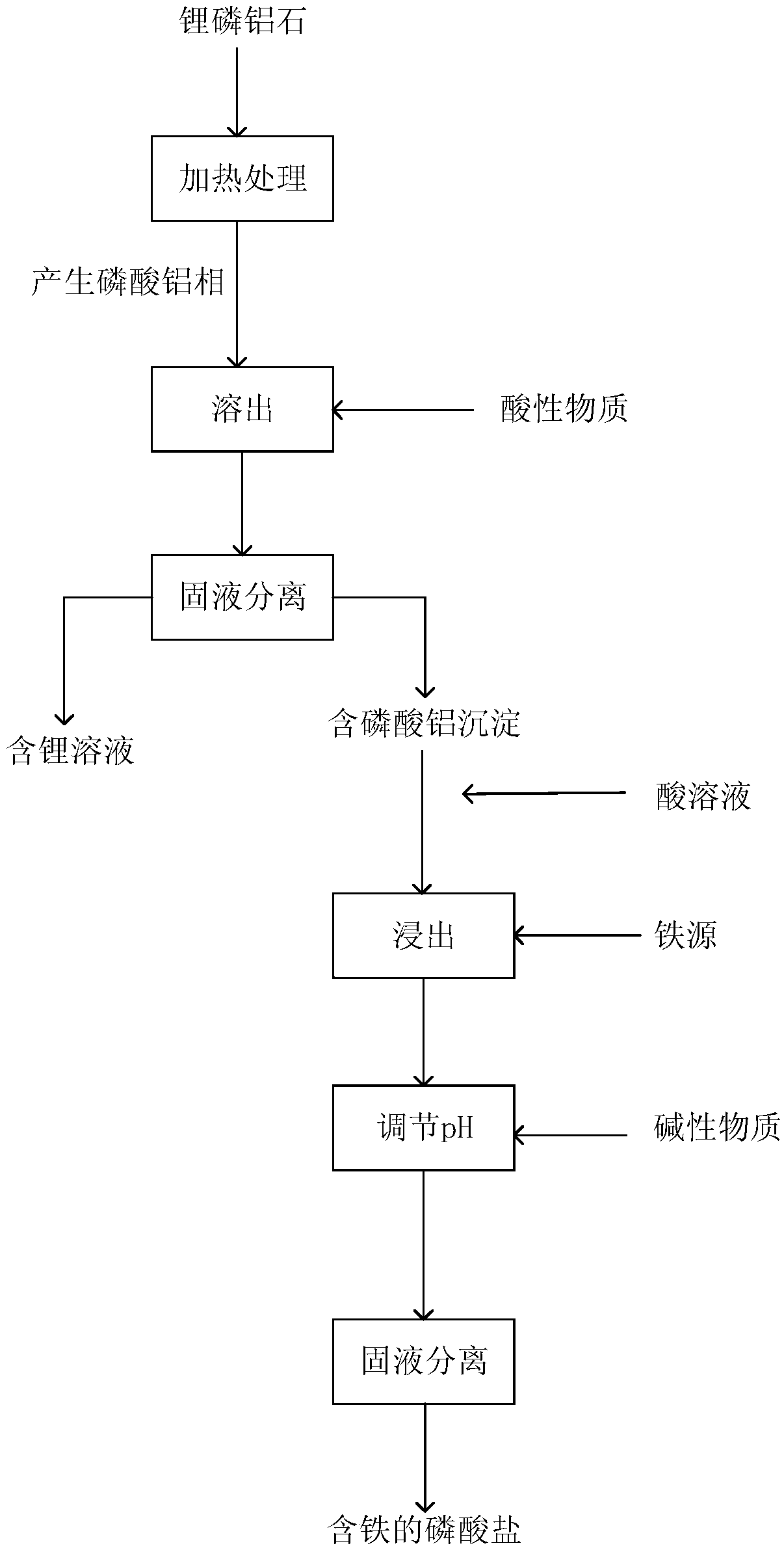
Method for extracting lithium from amblygonite and preparing iron-containing phosphate
ActiveCN108862227AHigh purityEasy to operateLithium halidesPhosphorus compoundsHydrogenLithium iron phosphate
The invention provides a method for extracting lithium from amblygonite and preparing iron-containing phosphate. The method comprises the following steps of (1) treating the amblygonite to dissolve out the lithium, so as to obtain a dissolved solution; (2) separating solid and liquid of the dissolved solution in step (1), so as to obtain a lithium-containing solution and an aluminum phosphate-containing precipitate; (3) extracting the aluminum phosphate-containing precipitate in step (2) by an acid solution, adding an iron source into the extracted solution, adding an alkaline matter to regulate the pH (potential of hydrogen) value, and separating solid and liquid, so as to obtain the iron-containing phosphate. The method has the advantages that the operation is simple, the process is short, the cost is low, and the extracting rate of lithium element can reach 93% or above; the purity of the lithium-containing product is high, and the purity of ferric phosphate and / or ferrous phosphatecan reach 95% by mass or above; the prepared ferric phosphate and / or ferrous phosphate can be used for further synthesizing the lithium iron phosphate.
Owner:SHENZHEN DYNANONIC
Lithium sulfur battery
ActiveUS20170331150A1Improved lifespan characteristicImprove securityCell electrodesFinal product manufactureConductive polymerLithium–sulfur battery
The present disclosure relates to a lithium sulfur battery, and the battery includes a cathode and an anode arranged facing each other; a separator interposed between the cathode and the anode; and an electrolyte, and further includes at least one or more membranes of a lithium ion conductive polymer membrane positioned between the cathode and the separator and having a sulfonic acid group (—SO3H), and a metal oxide membrane positioned between the anode and the separator, and therefore, an electrode active material loss is reduced, an improved lifespan characteristic is obtained by blocking the spread of lithium polysulfide to the anode, and in addition thereto, enhanced safety is obtained by suppressing a dendrite growth in the anode.
Owner:LG ENERGY SOLUTION LTD
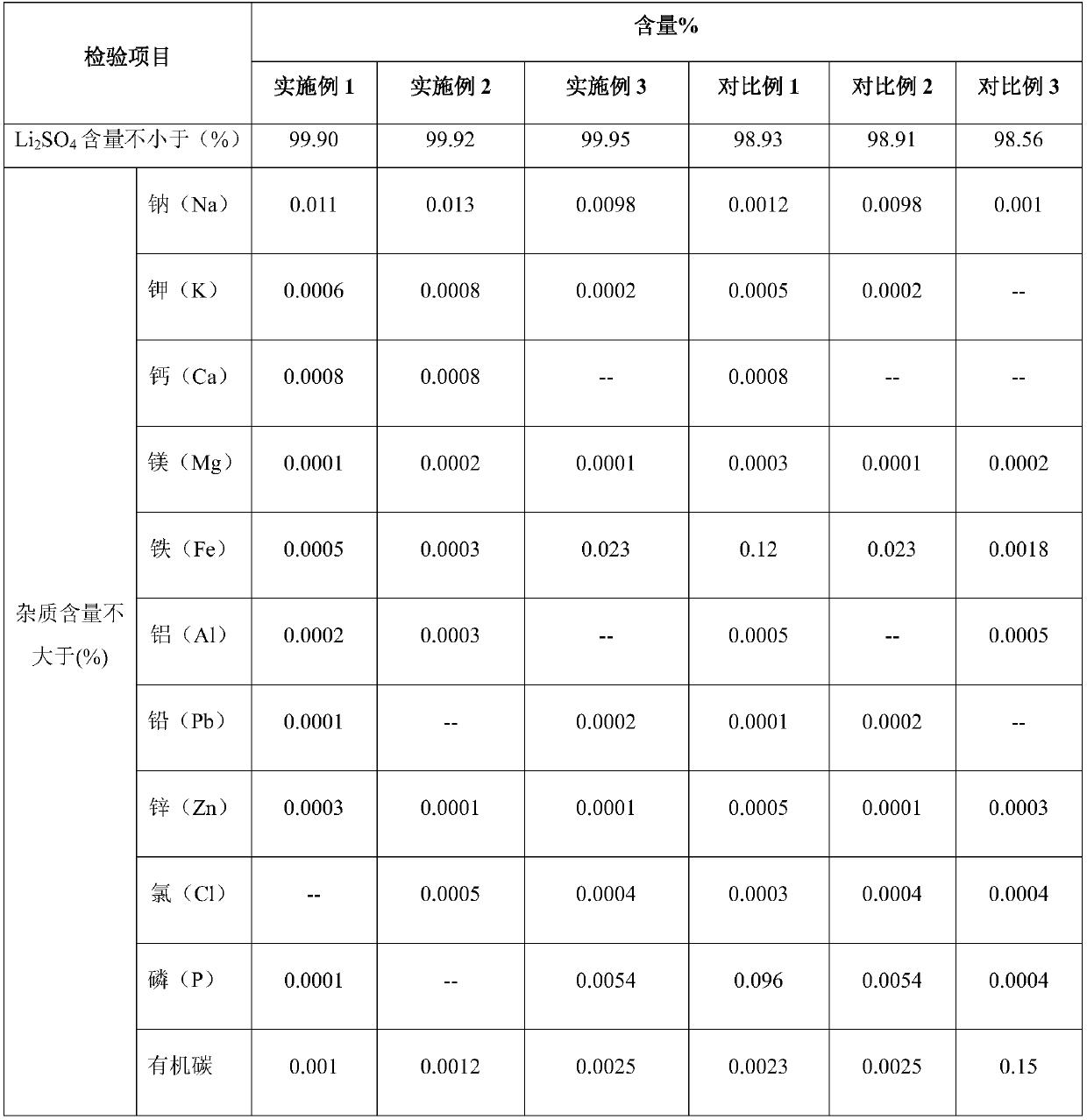
Method for preparing high-purity lithium sulfate from waste liquid recovered from hydrothermal method based lithium iron phosphate production
The invention belongs to the technical field of lithium iron phosphate waste liquid recovery and provides a method for preparing high-purity lithium sulfate from waste liquid recovered from hydrothermal method based lithium iron phosphate production. The method includes: 1) boiling waste liquid generated in a hydrothermal method based lithium iron phosphate production process, and adding sodium hydroxide to adjust pH; 2) adding hydrogen peroxide, adding an adsorbent after reaction, and filtering after reaction; 3) subjecting filtrate to evaporation concentration, centrifugal separation and drying to obtain high-purity anhydrous lithium sulfate; 4) stirring and washing filter residues with dilute sulfuric acid, and filtering to use as an adsorbent for cyclic adsorption. By hydrogen peroxide, residual organics in waste liquid is oxidized and decomposed or converted into easily-adsorbable organics, and the adsorbent is used for adsorption to remove the organics; after oxidization, adsorption and decontamination, solution is crystallized to obtain high-purity lithium sulfate, and the filter residues subjected to dilute sulfuric acid washing, stirring and filtering serve as the adsorbent for cyclic adsorption. Purity of the high-purity lithium sulfate prepared according to the method reaches 99.90%.
Owner:TIANQI LITHIUM CORP
Aluminium polychlorosulphates, process for their preparation and use thereof
Aluminum polychlorosulphates having the general formula (I)whereM represents an alkali metall, m, n, p represent the number of moles per mole of aluminum, so that1.74<=l<=2.25,0.01<=n<=0.17,0.32<=p<=1.49, andl+m+2n=p+3,their use as coagulation and flocculation agents, and their preparation process by reaction, at room temperature, of an alkali metal basic compound, such as Na2CO3, NaHCO3, NaOH, K2CO3, KHCO3 and KOH, and an alkali metal sulphate or sulphuric acid with an aluminum polychloride or polychlorosulphate having the general formula(I')where1.1<=1'<=1.44,n'<=0.10,p'<p(p of formula (I)), andl'+m'+2n'=p'+3.
Owner:POZZOLI BERNARDO
Conversion of sodium bromide to anhydrous hydrobromic acid and sodium bisulfate
InactiveUS20050135990A1Hydrogen bromideChlorine/hydrogen-chloride purificationSodium bisulfateSodium bromide
Process for the conversion of sodium bromide to anhydrous hydrobromic acid and sodium bisulfate, said process with the following sequential steps: reaction of sodium bromide and sulfuric acid in a solution of water to produce hydrobromic acid and sodium bisulfate wherein the conversion of sodium bromide is greater than about 99%; adsorption of iron bromide onto a solid adsorbent; separation of hydrobromic acid and water from the sodium bisulfate; separation and drying of hydrobromic acid; and solidification of the sodium bisulfate into a flaked or granular form.
Owner:THE PROCTER & GAMBLE COMPANY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com