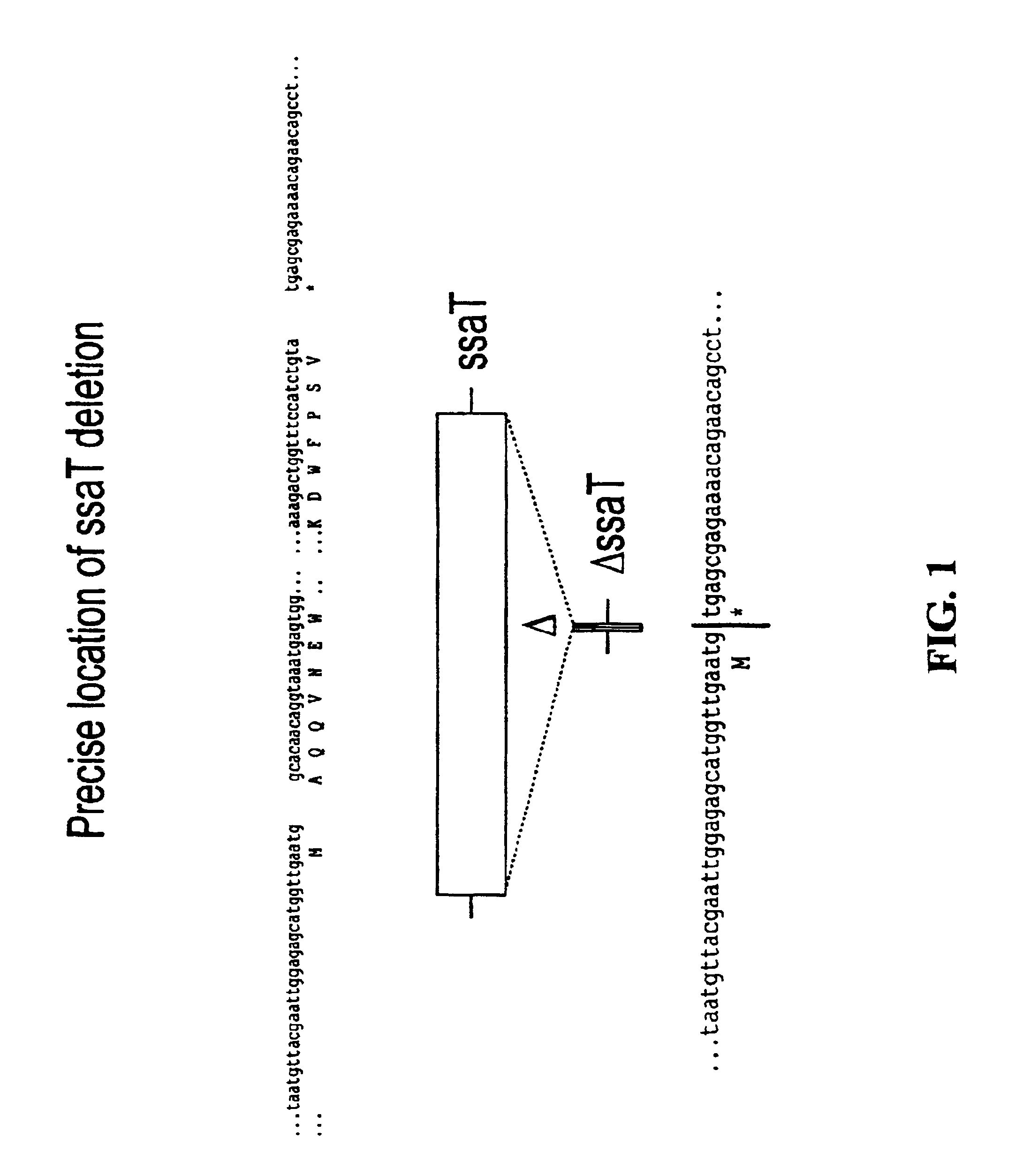
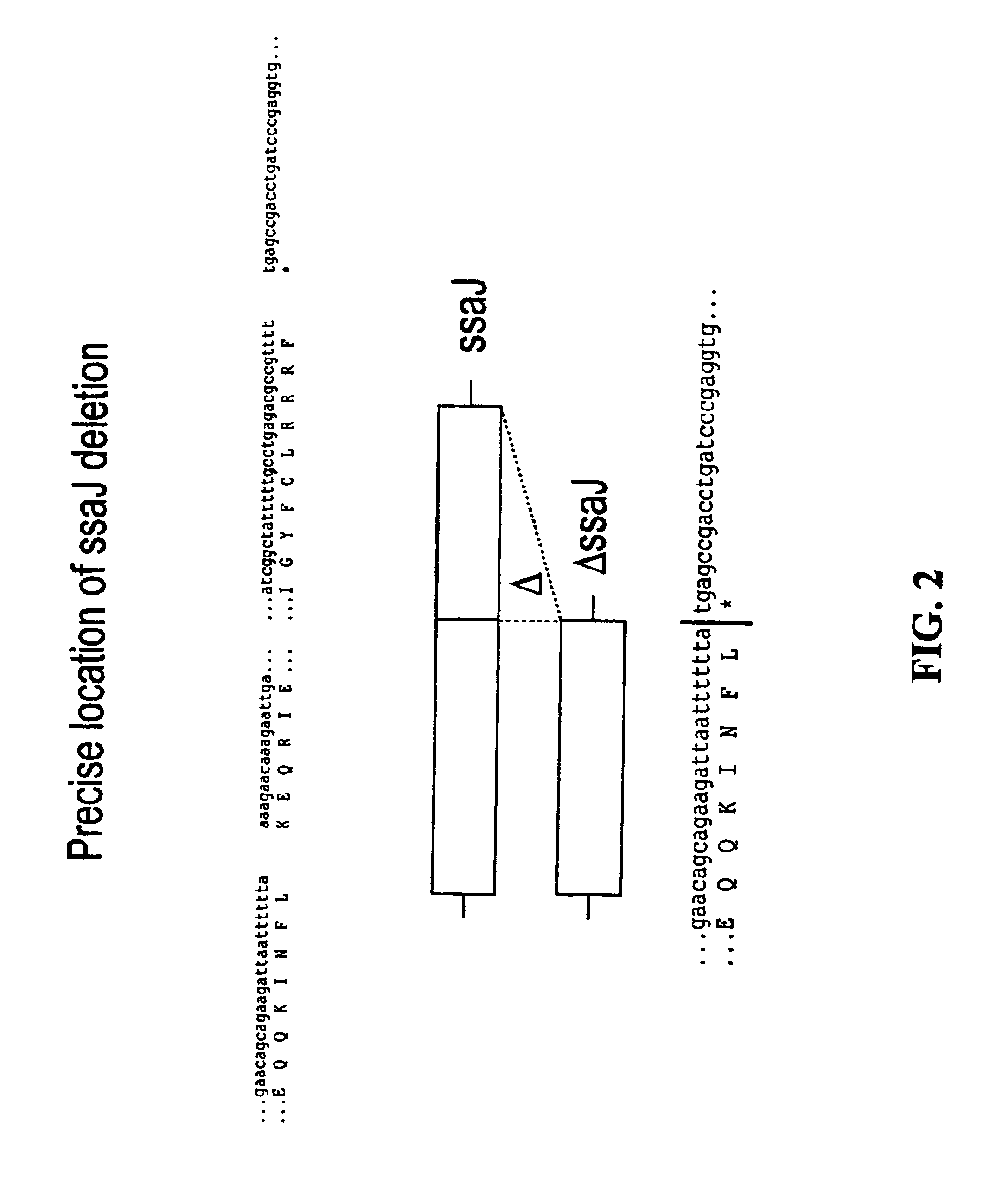
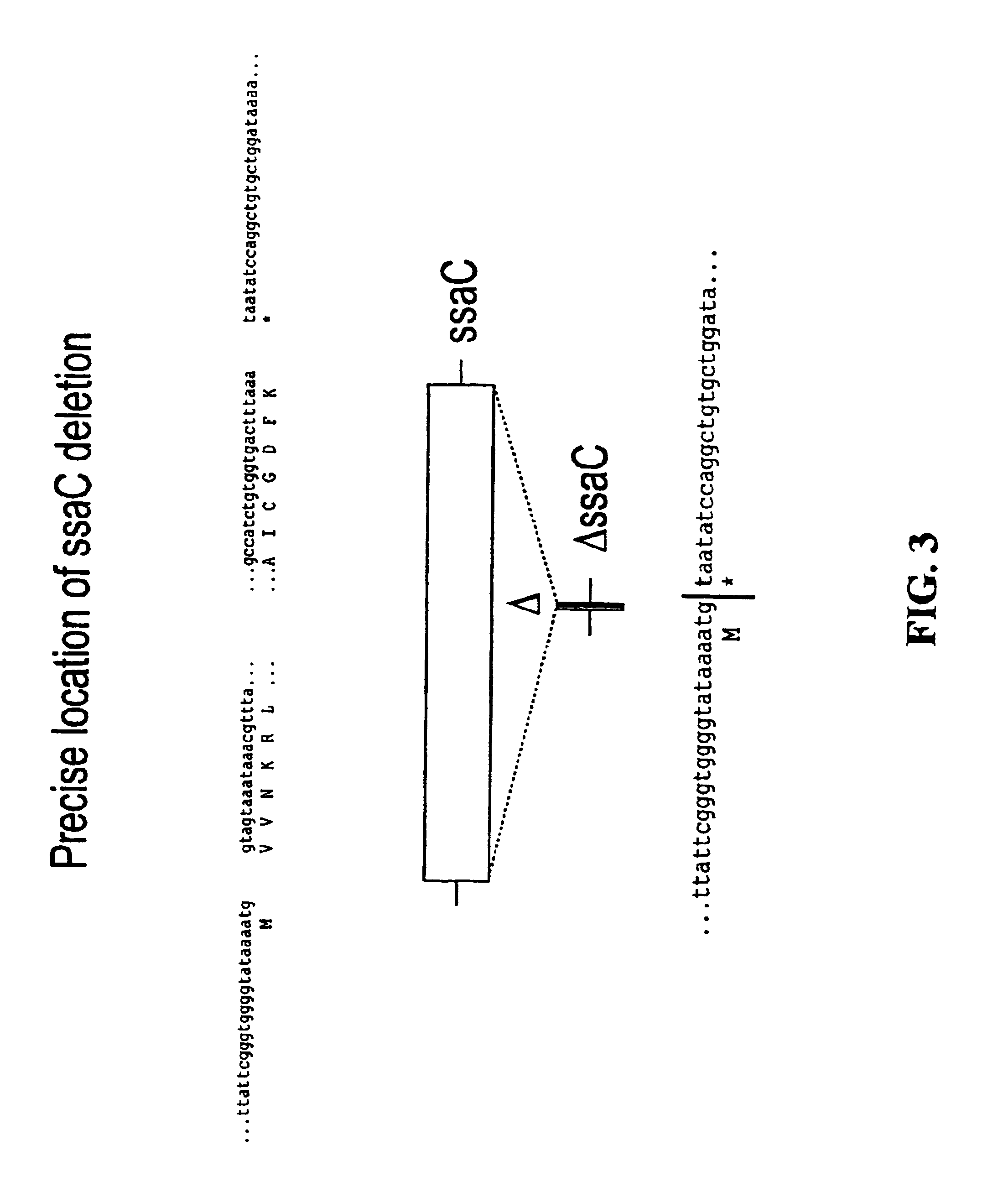
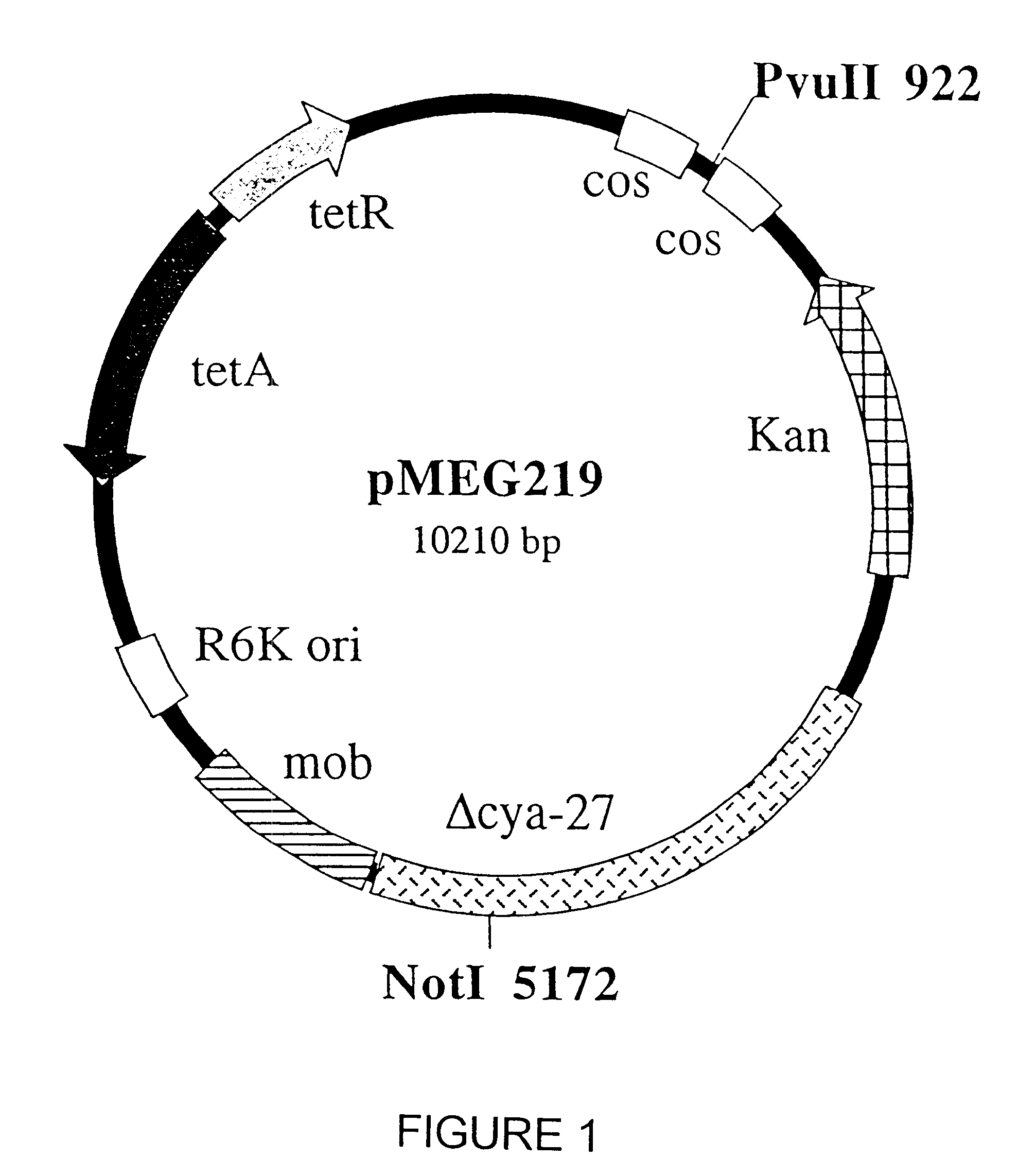
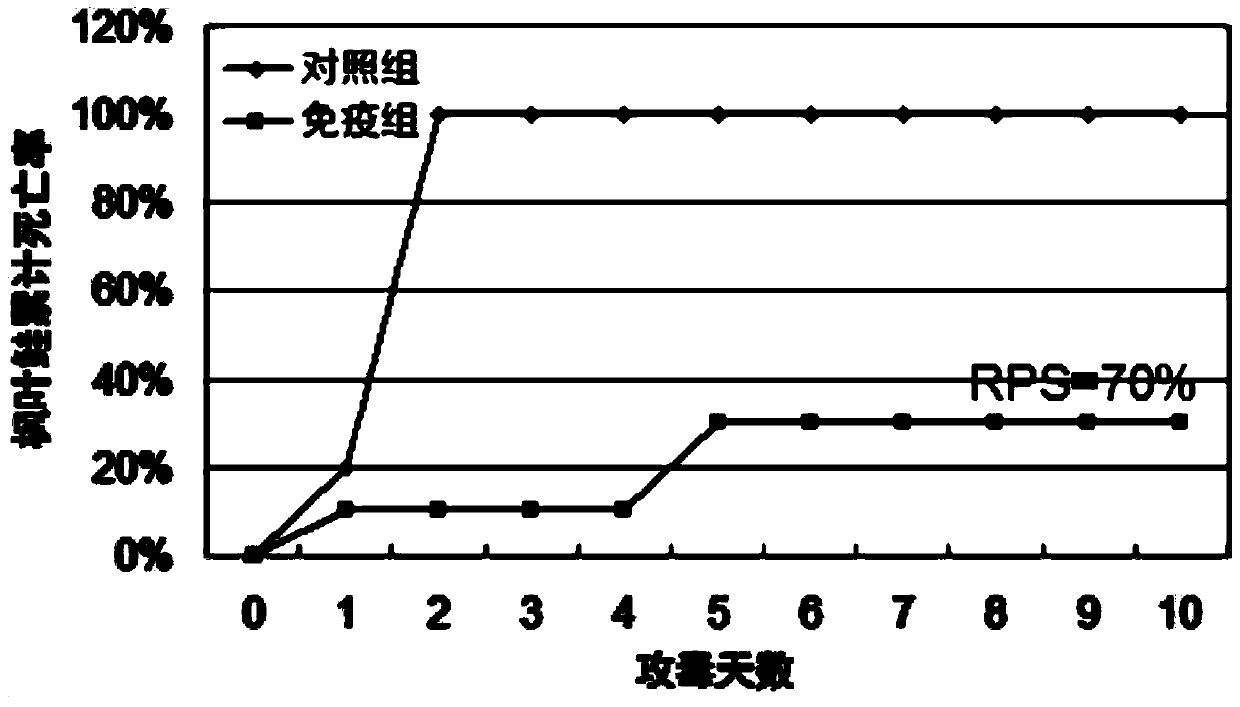
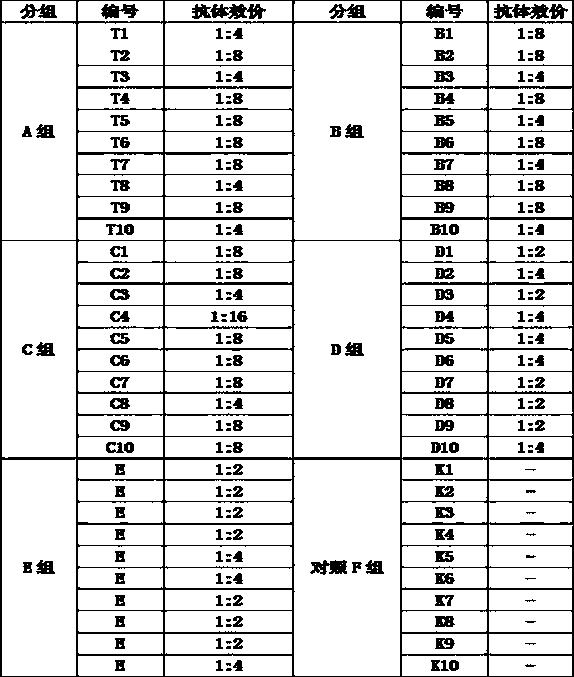
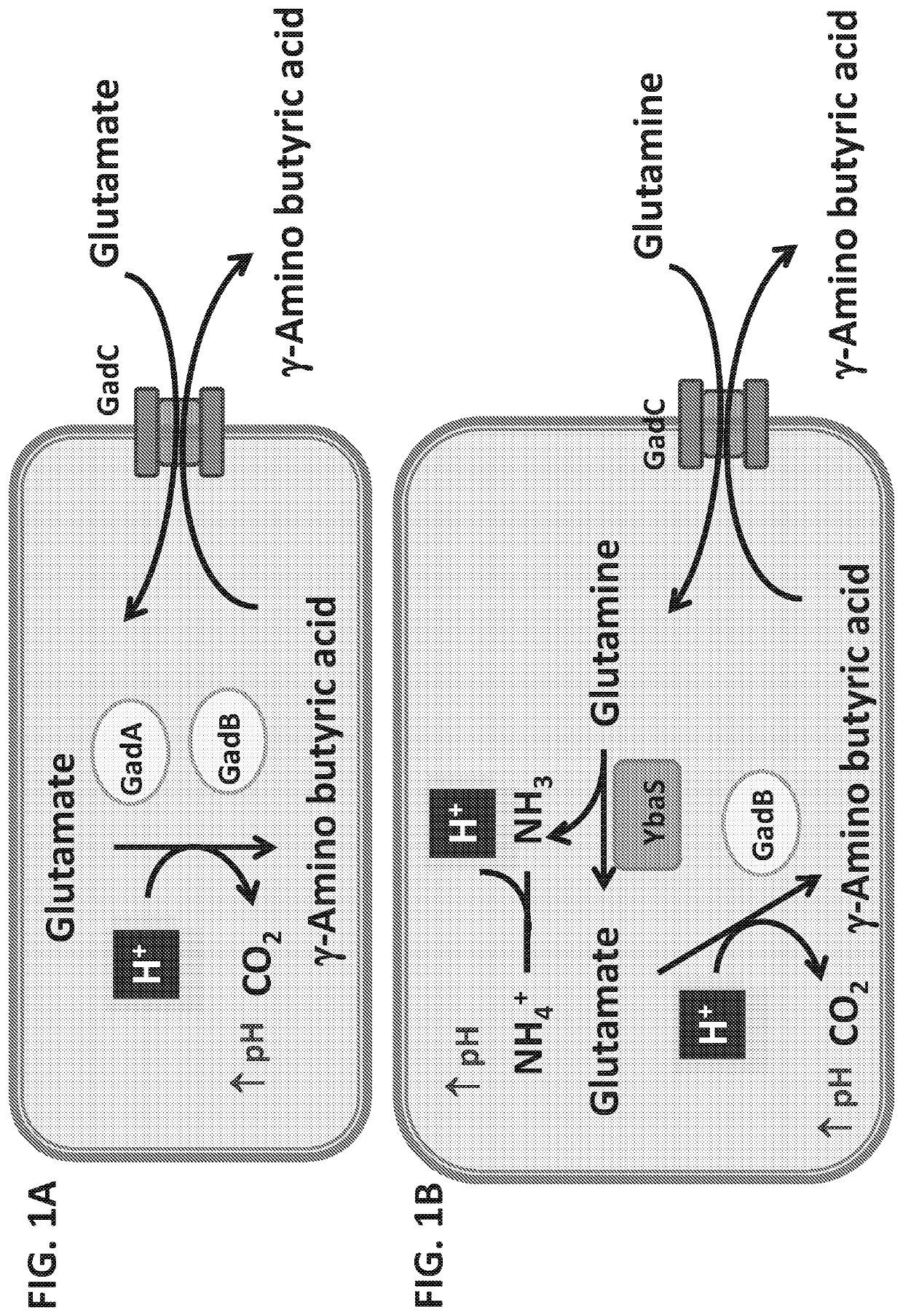
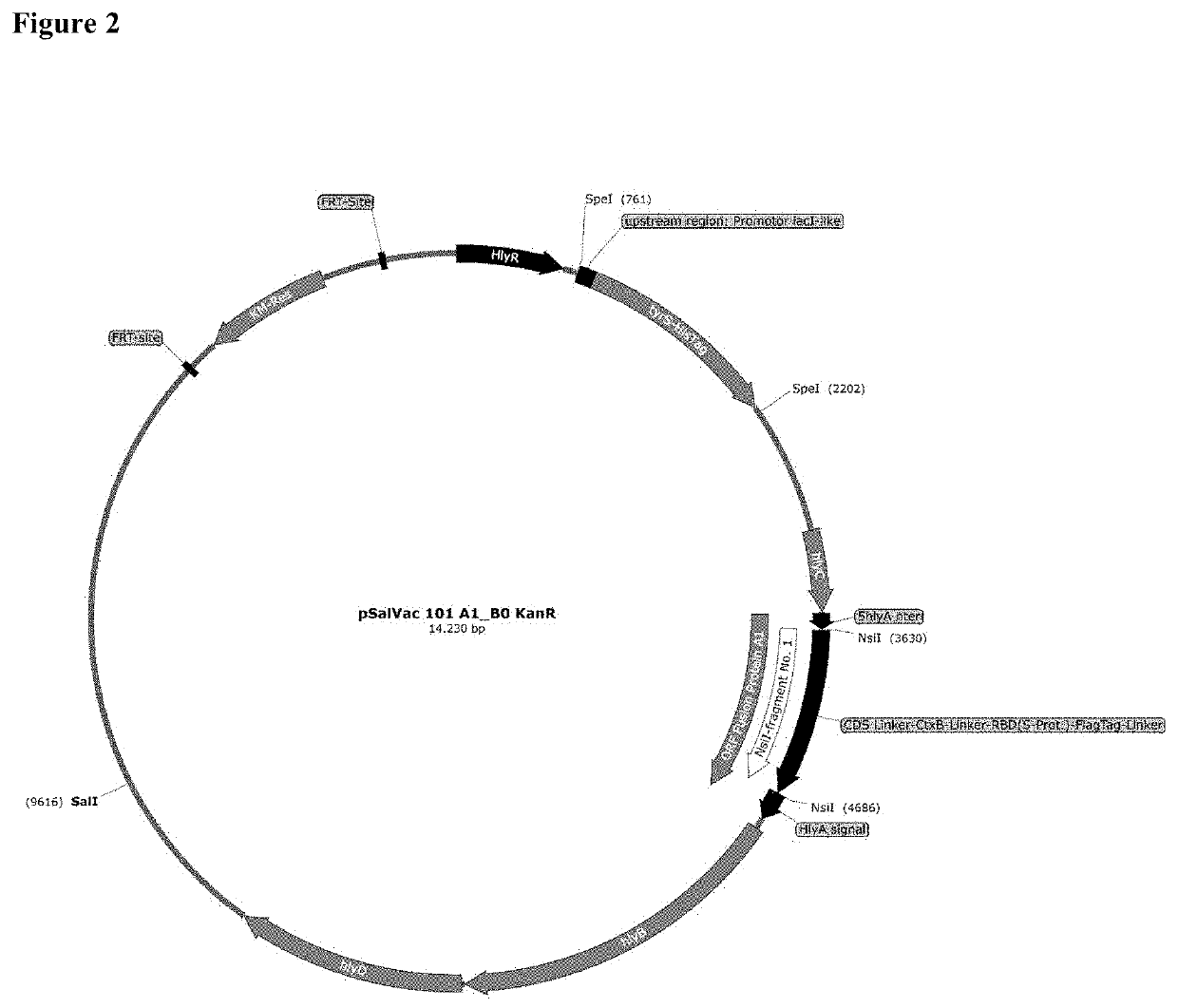
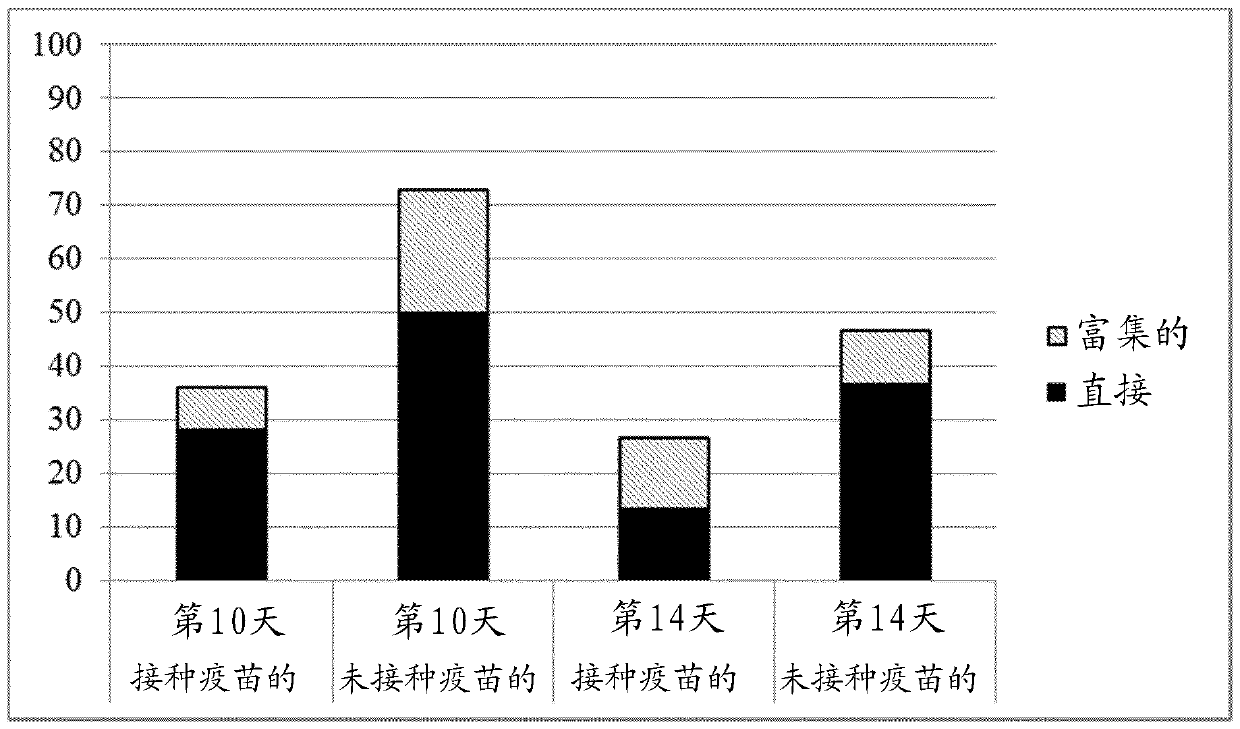
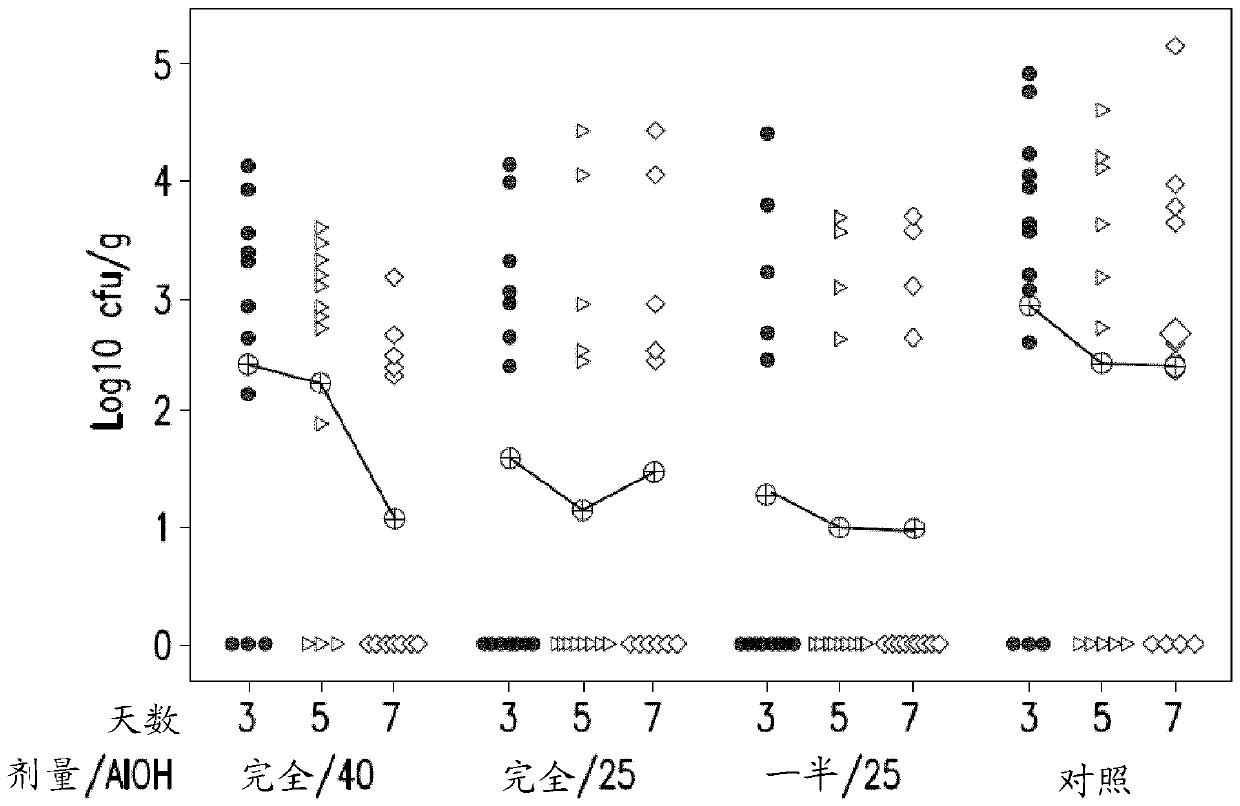
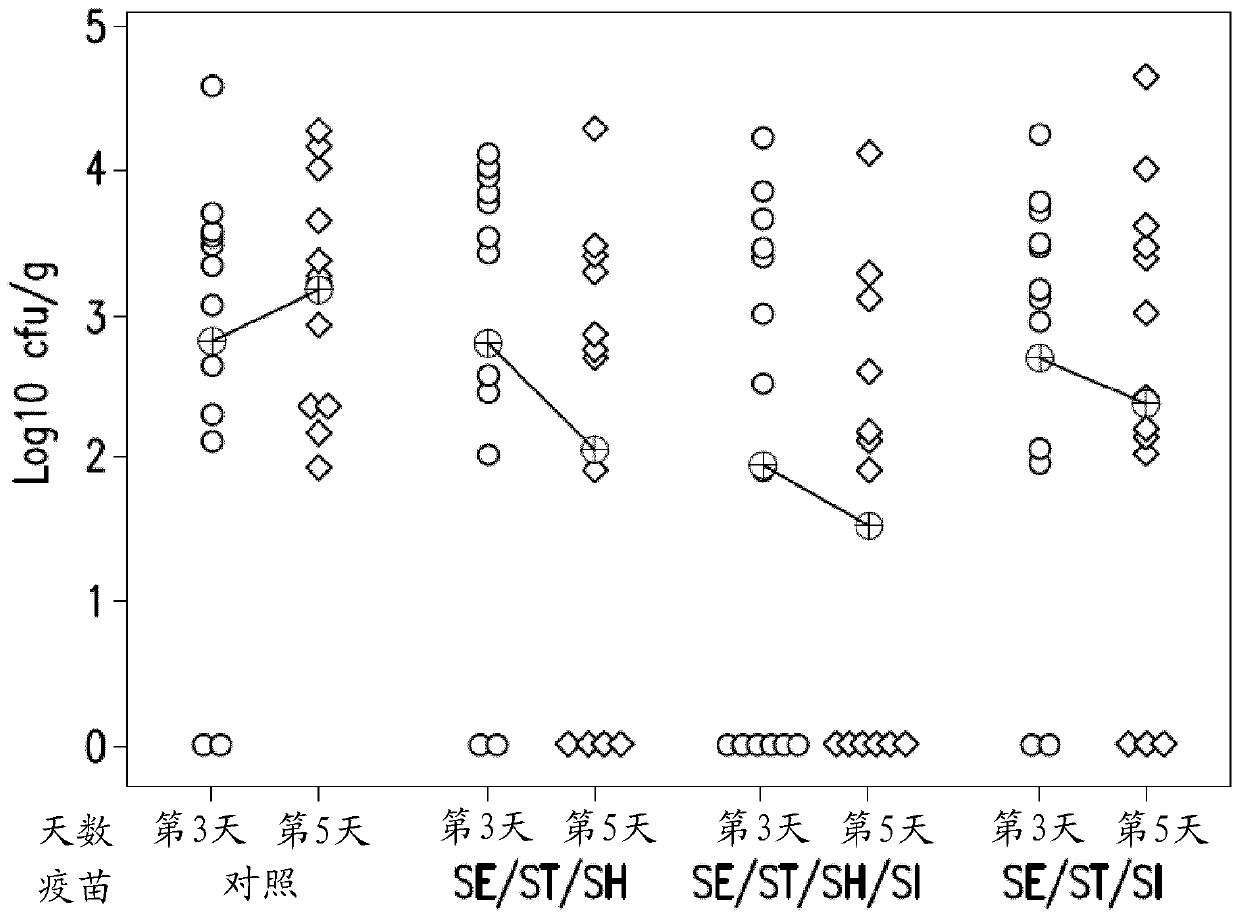
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Salmonella vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaccines or candidate vaccines used to prevent infection with salmonella; includes vaccines used to prevent typhoid or paratyphoid fever, and vaccines used to prevent nontyphoid salmonellosis.
Salmonella vaccine materials and methods
Attenuated mutant Salmonella bacteria containing inactivated virulence genes are provided for use in safe, efficacious vaccines.
Owner:PHARMACIA & UPJOHN CO
Live attenuated salmonella vaccines to control avian pathogens
A vaccine for protecting birds against infection by avian pathogenic gram negative microbes is disclosed. The vaccine is a recombinant Salmonella strain expressing O-antigen of an avian pathogenic gram negative microbe such as an E. coli strain that is pathogenic in poultry. The recombinant Salmonella strain also does not express Salmonella O-antigen. Methods of using the vaccine to immunize birds are also disclosed.
Owner:AVANT IMMUNOTHERAPEUTICS
Intranasal recombinant Salmonella vaccine encoding heterologous polysaccharide antigens
InactiveUS20050260225A1Promote robust immune responseReduce deliveryBacterial antigen ingredientsAgainst vector-borne diseasesHeterologousAntigen
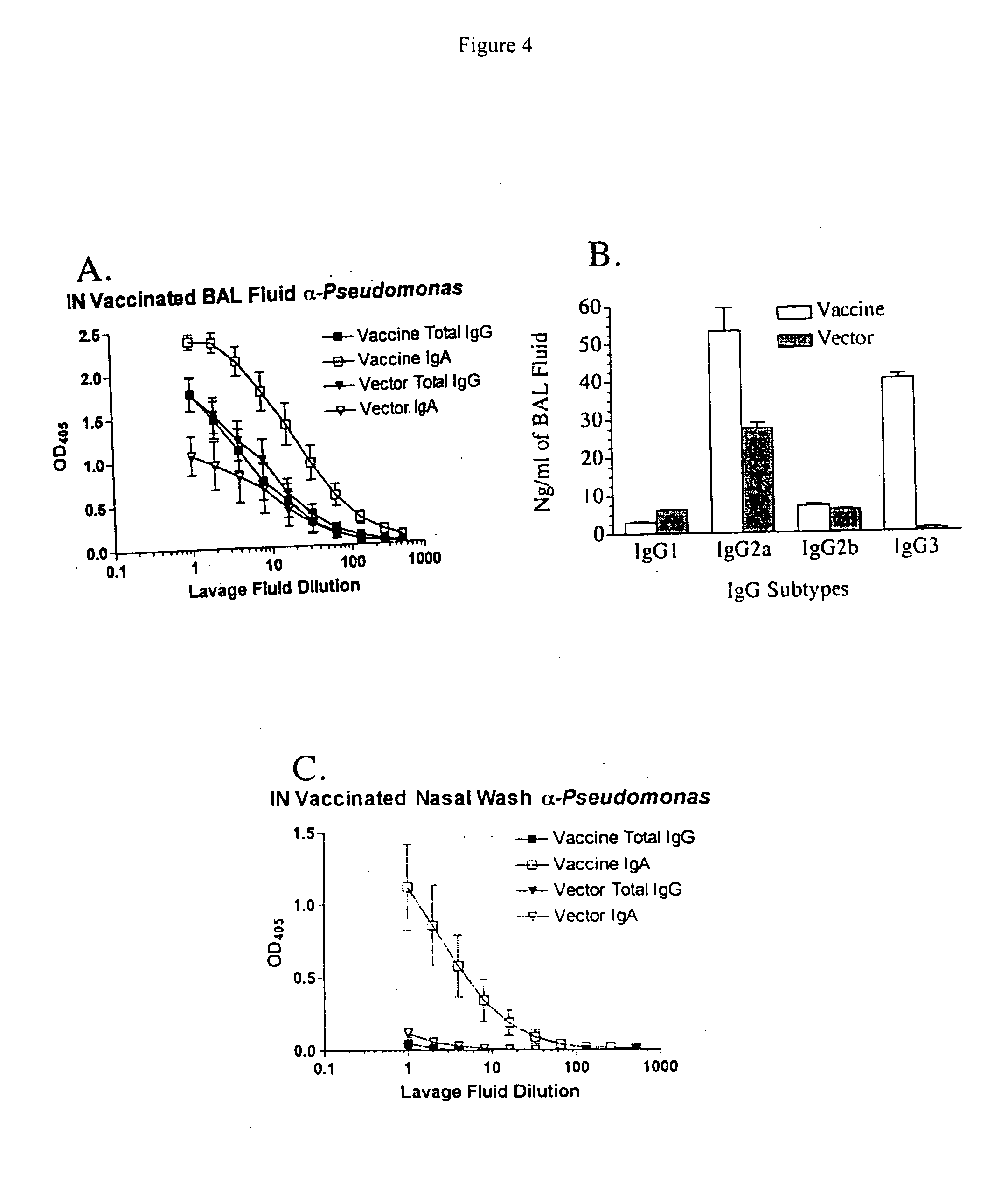
The invention relates of administration of an attenuated Salmonella strain expressing a lipopolysaccharide O antigen from a suitable pathogen, in particular Pseudomonas aeruginosa, and the use of the same as a vaccine to promote sterile immunity to the pathogen, e.g., P. aeruginosa, via intranasal vaccination. In one embodiment, the present invention is directed to a unique intranasal route of immunization for the delivery of relevant heterologous polysaccharide antigens via a live, attenuated Salmonella strain.
Owner:GOLDBERG JOANNA B +2
Salmonella vectored vaccines against Chlamydia and methods of use
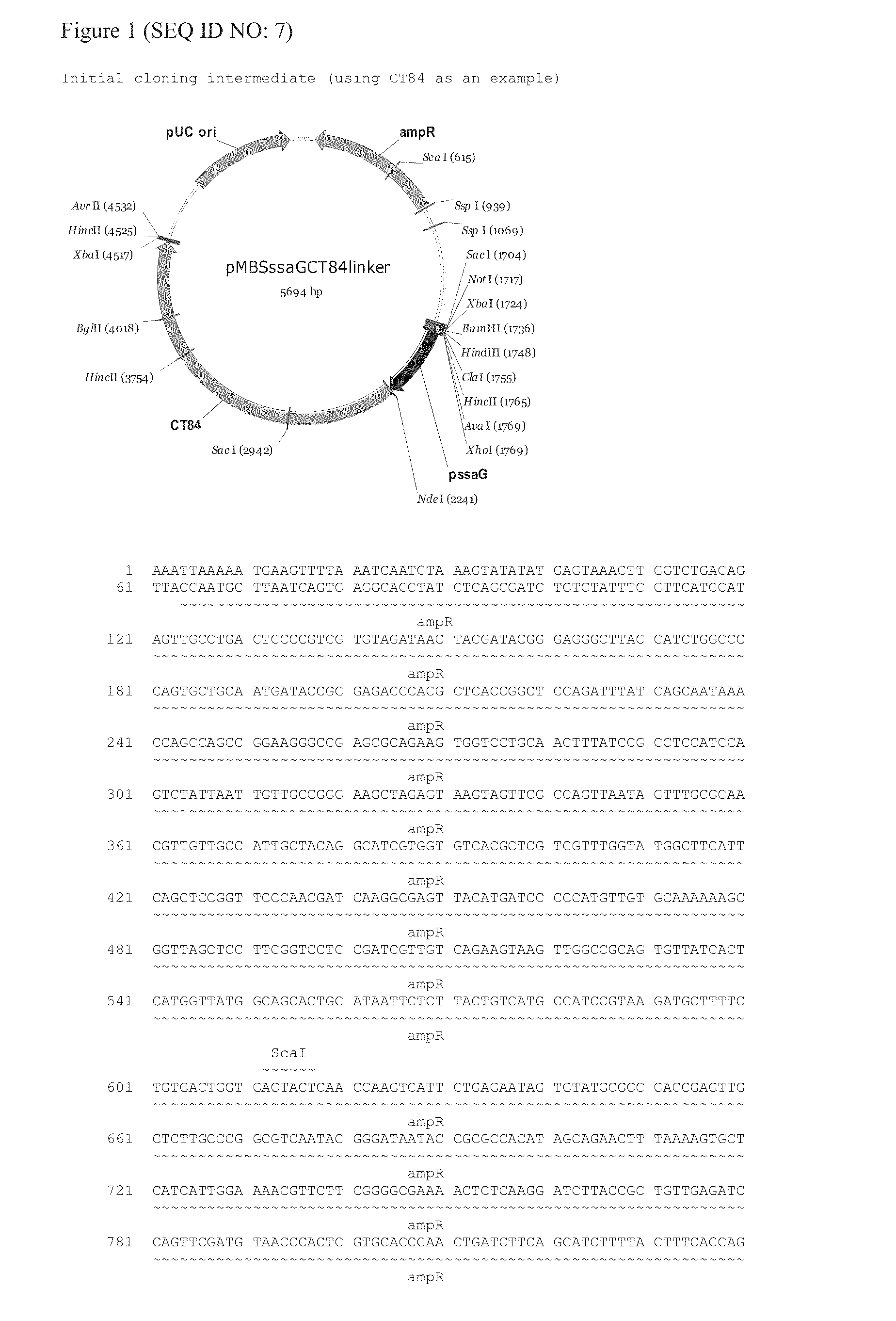
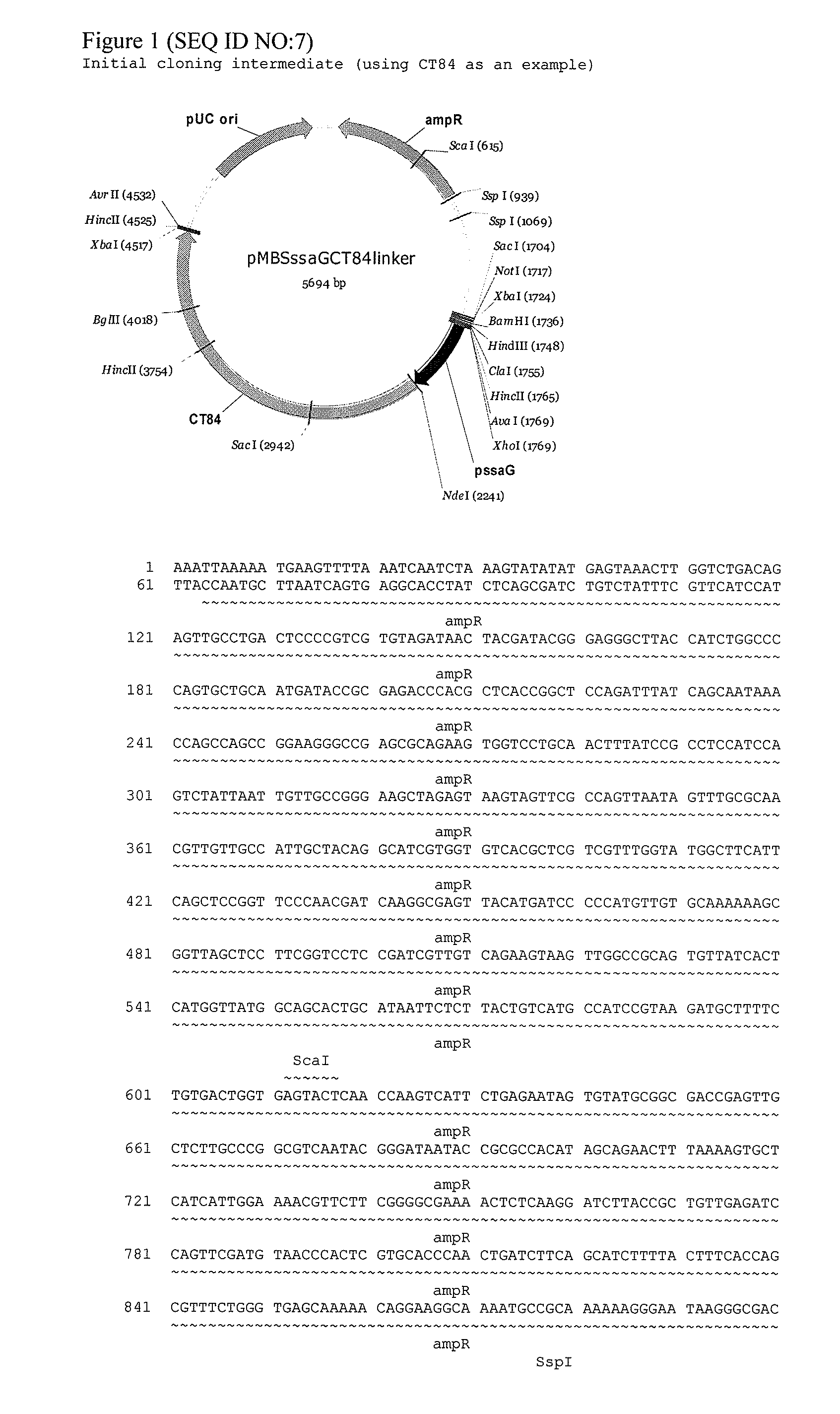
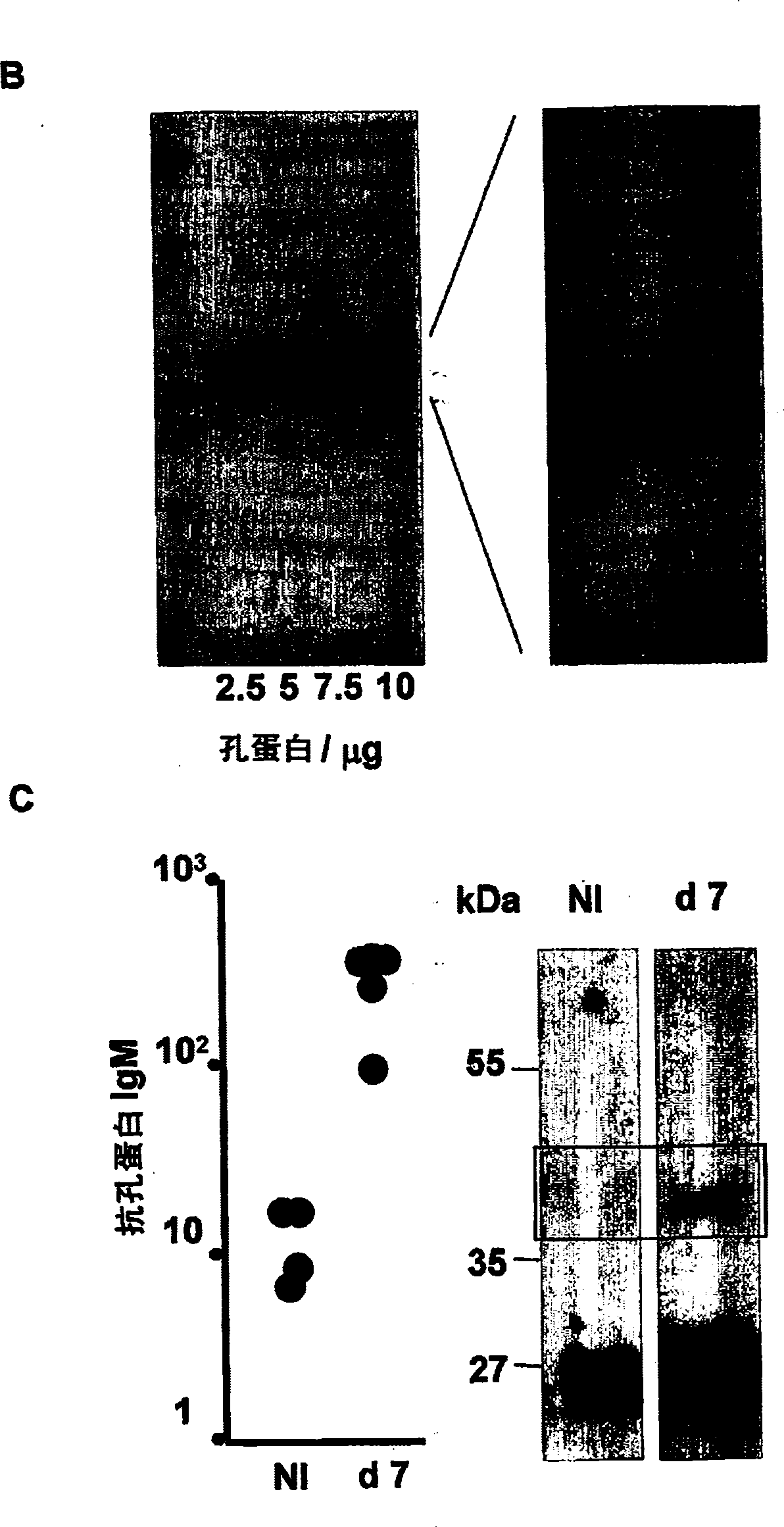
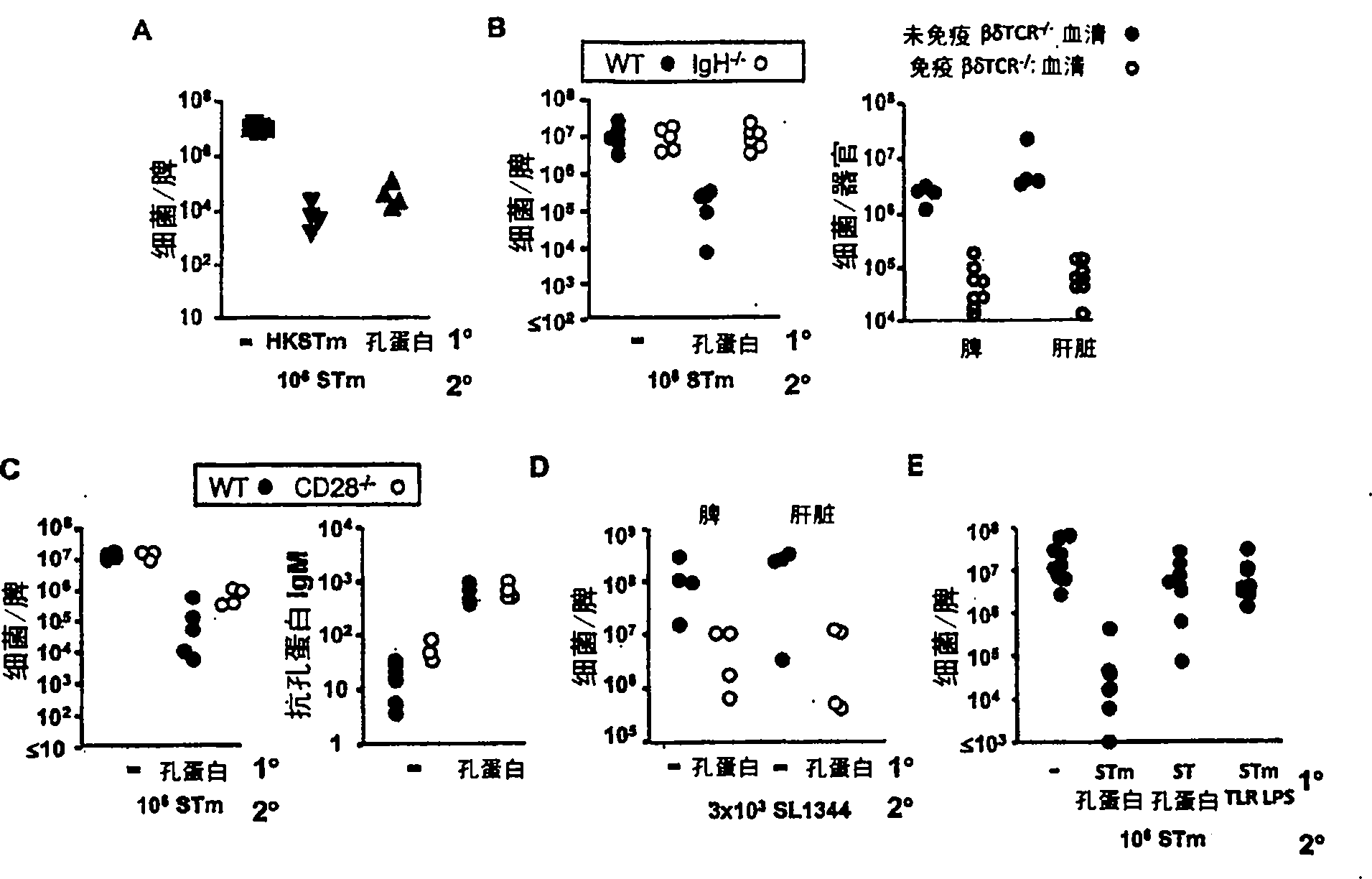
The invention provides an attenuated Salmonella vaccine vector comprising one or more heterologous polynucleotides that encode immunogenic Chlamydial peptides. In one embodiment, the attenuated Salmonella vaccine vector comprises aroC and ssaV attenuating mutations. The heterologous polynucleotides encoding the immunogenic Chlamydial peptides can be under the control of an inducible promoter such as a Salmonella ssaG promoter. In one embodiment of the invention, the immunogenic Chlamydial peptide is a PmpG peptide, for instance, a CT110, CT84 or CT40 peptide.
Owner:PROKARIUM
Cross-protective salmonella vaccines
The present invention relates to a method of protecting pigs against disease caused by infection by heterologous serotypes of Salmonella including but not limited to S. typhimurium comprising administering to the pigs a modified live vaccine incorporating S. cholerasuis.
Owner:INTERVET INT BV
Salmonella Vaccine
The present invention relates to the field of vaccination against Salmonella in animals, particularly avian animals. The present invention also encompasses kits and uses of Salmonella immunogenic compositions or vaccines. The present invention further relates to methods and compositions comprising at least one primo-administration of an attenuated immunogenic composition or vaccine, comprising a pharmaceutically or veterinarily acceptable excipient, diluent or vehicle and at least one attenuated Salmonella, administered to an avian animal before at least one boost-administration of an inactivated immunogenic composition or vaccine, comprising a pharmaceutically or veterinarily acceptable excipient, diluent or vehicle, and at least one inactivated Salmonella.
Owner:MERIAL INC
Salmonella abortus equi strain SMXJ-97 and application thereof in salmonella abortus equi vaccine
ActiveCN108220183AImprove securityNo danger of spreading poisonAntibacterial agentsBacteriaBacteroidesMalonate
The invention relates to a Salmonella abortus equi strain SMXJ-97 with the preservation number of CGMCC No.9047. The strain has 16S rRNA gene sequences in a sequence table 1. The stain is separated from abortion sick horses in Xinjiang, the bacterium takes the shapes of a straight rod, the size is 0.7-1.6 [mu]m*2.0-5 [mu]m, and the bacterium is a negative Gram bacterium. The strain is an aerobic facultative anaerobic bacterium and can grow on a common culture medium, the growth temperature is 25-40 DEG C, the most appropriate temperature is 37 DEG C, the pH growth range is 5-9, and the most appropriate pH value is 7.4-7.6. The strain can be adopted to ferment mannitol and decompose lysine, urea cannot be decomposed with the strain, and tryptophan, malonate, saligenin and sorbitol cannot beutilized with the strain. The strain can be used for preparing salmonella abortus equi inactivation vaccines. The vaccines prepared from the strain are high in disease specificity, low in cost, highin security and good in protection effect.
Owner:XINJIANG AGRI UNIV
Salmonella vectored vaccines against chlamydia and methods of use
ActiveUS20110268760A1Antibacterial agentsPolypeptide with localisation/targeting motifHeterologousNucleotide
The invention provides an attenuated Salmonella vaccine vector comprising one or more heterologous polynucleotides that encode immunogenic Chlamydial peptides. In one embodiment, the attenuated Salmonella vaccine vector comprises aroC and ssaV attenuating mutations. The heterologous polynucleotides encoding the immunogenic Chlamydial peptides can be under the control of an inducible promoter such as a Salmonella ssaG promoter. In one embodiment of the invention, the immunogenic Chlamydial peptide is a PmpG peptide, for instance, a CT110, CT84 or CT40 peptide.
Owner:PROKARIUM
Non-typhoidal salmonella vaccines
Owner:THE UNIV OF BIRMINGHAM
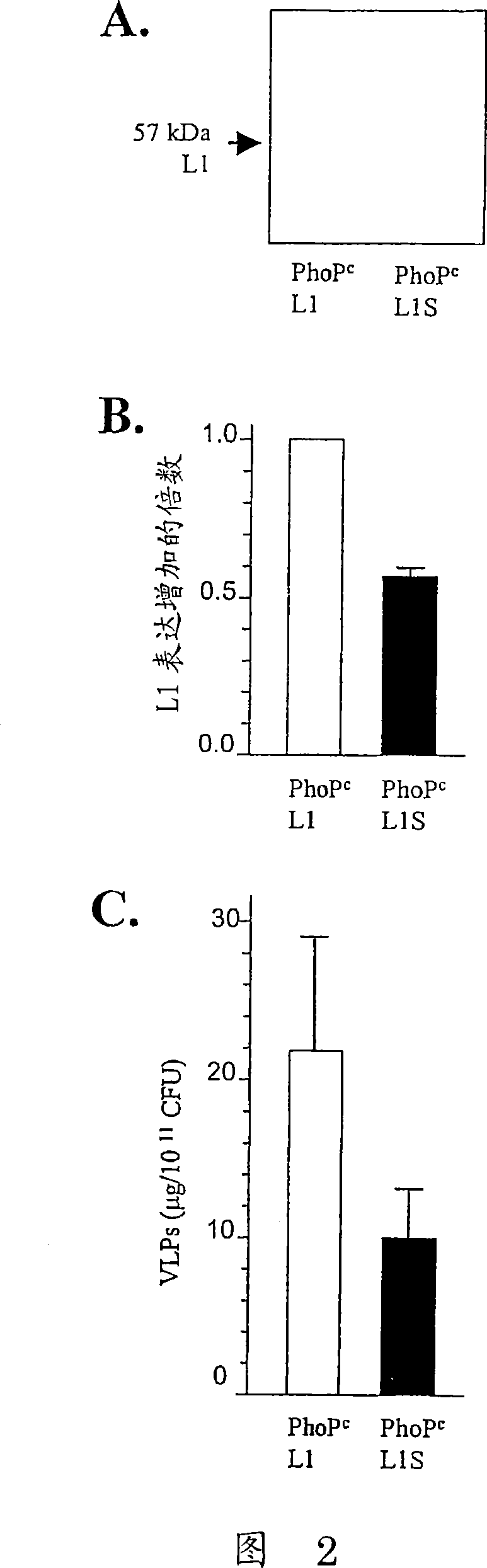
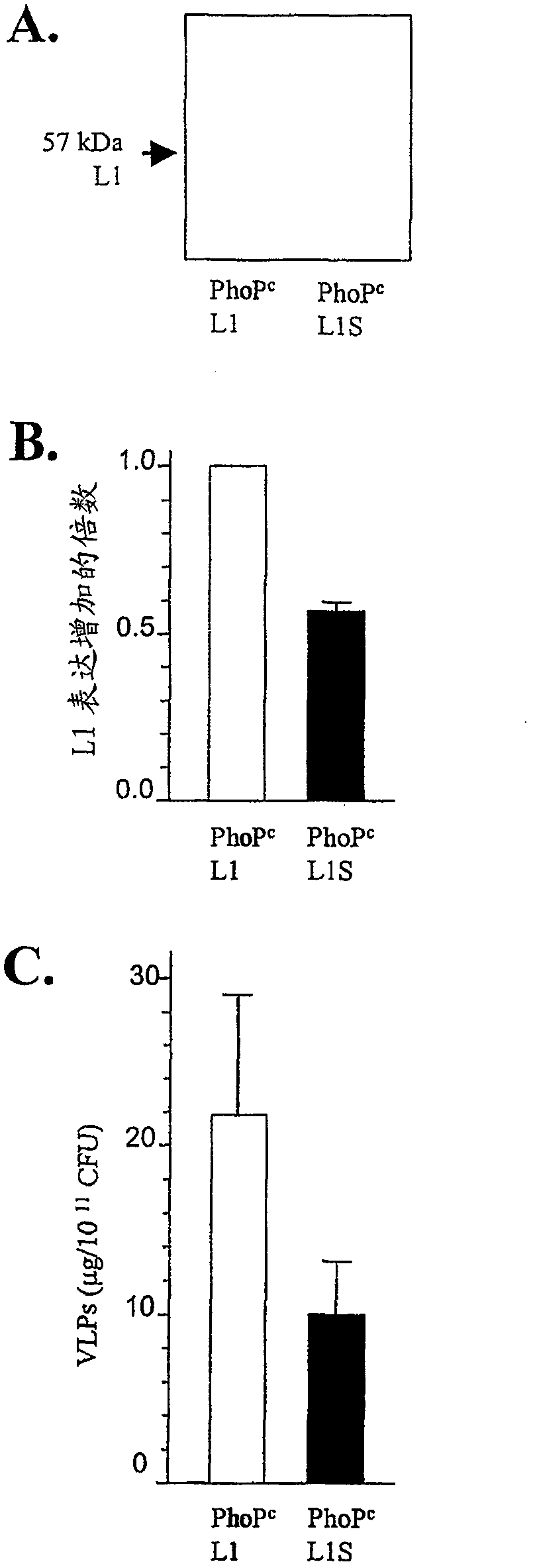
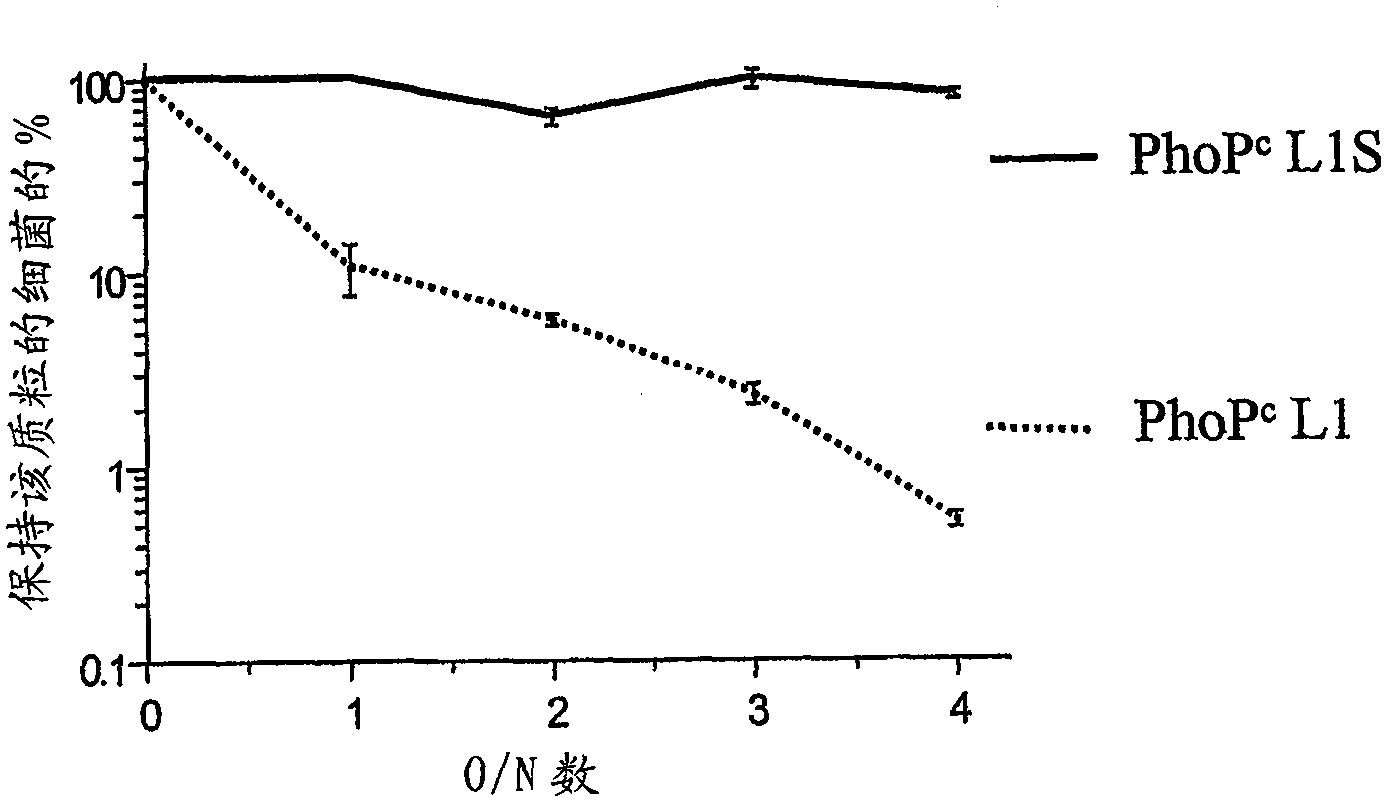
Codon-optimized HPV16LI for salmonella vaccine strains against human papillomavirus type 16
InactiveCN101115766AImproving immunogenicityImprove stabilityViral antigen ingredientsVirus peptidesAmpicillinKanamycin
The present invention relates to a novel nucleic acid sequence (HPV16 L1S) encoding antigenic HPV16 L1 protein as provided in SEQ ID NO: 1, wherein the sequence has atleast one modified codon for optimum stability of recombinant plasmid vector when transformed in the prokaryotic micro-organism for improved immunogenicity of the resulting prokaryotic micro-organism. The invention further relates to constructing recombinant vectors pFS14nsdHPV16L1 and pFS14nsdHPV16 kan L1S harboring SEQ ID NO: 1, wherein the former carries Ampicillin and the latter, Kanamycin as a selection marker. The invention also relates to an attenuated strain of a prokaryotic micro-organism transformed with nucleic acid encoding HPV16 (Human Papillomavirus) major capsid protein and expressing the corresponding protein. In addition the invention discloses a process of producing a vaccine based on prokaryotic micro-organism for the treatment of papillomavirus infection and associated risk of cancer.
Owner:INDIAN IMMUNOLOGICALS LIMITED
Live attenuated salmonella vaccine
InactiveUS20110052635A1Reduced remaining virulenceResidual virulenceAntibacterial agentsBacterial antigen ingredientsBacteroidesEscherichia coli
The present invention is related to double and triple attenuated mutant strains of a bacterium infecting veterinary species such as Salmonella enterica and / or (a pathogenic) Escherichia coli. The mutants of the invention contain at least one first genetic modification and at least one second genetic modification, said first modification in one or more motility genes, and said second modification in one or more genes involved in the survival or the proliferation of the pathogen in the host. The present invention further relates to live attenuated vaccines based on such mutants for preventing amongst others Salmonellosis and / or an infection by an E. coli pathogen in a veterinary species.
Owner:VRIJE UNIV BRUSSEL
Recombinant attenuated salmonella vaccine and pharmaceutical composition for treating solid tumors and application thereof
InactiveCN102526760AImprove securityWill not harmBacteria material medical ingredientsGenetic material ingredientsInterleukin IIHepatocyte growth factor
The invention provides a recombinant attenuated salmonella vaccine and pharmaceutical composition for treating solid tumors and application thereof. The invention relates to application of cytokines in the prevention and the targeted treatment of the solid tumors and use of genes with interleukin2-combined hepatocyte growth factor antagonists in the preparation of drugs for the prevention and the targeted treatment of the solid tumors. The invention further relates to a gene-based pharmaceutical composition containing the interleukin2-combined hepatocyte growth factor antagonists and a method for applying the interleukin2-combined hepatocyte growth factor antagonists to a needed subject for the prevention and the targeted treatment of the solid tumors. According to the recombinant attenuated salmonella vaccine and the pharmaceutical composition for treating the solid tumors and the application thereof, the recombinant attenuated salmonella vaccine and the pharmaceutical composition can be beneficial to the prevention and the targeted treatment of various solid tumors.
Owner:LANZHOU GENERAL HOSPITAL OF LANZHOU MILITARY REGION PLA
Salmonella vaccine
InactiveUS20080069843A1Intrinsically safeIncrease virulenceAntibacterial agentsBacterial antigen ingredientsMicroorganismMicrobial pathogenesis
The present invention relates to Salmonella bacteria. The invention also relates to methods of using Salmonella bacteria, including in vaccines based thereon that are useful for the prevention of microbial pathogenesis. Further, the invention relates to the use of such bacteria or the manufacture of such vaccines. The invention also relates to methods for the preparation of such vaccines.
Owner:INTERVET INT BV
Preparation method of anti prglet bacterial epidemic disease compound egg antibody medicinal preparation
A compound yolk antibody preparation for preventing bacterial disease of piglet, especially the bacteria diarrhea, is prepared through preparing salmonella vaccine and the genetically engineered vaccine of colibacillus K88, K99 and 9879, forced immunizing three times, collecting immunized egg, taking yolk, diluting with aseptic buffer liquid of phosphate, treating by hydroxypropylmethyl cellulose phthalate, filtering and spray drying.
Owner:SHANGHAI JIAO TONG UNIV
Method for purifying salmonella of laying hens
InactiveCN107517920AControl spreadImprove the degree of purificationGaseous substancesChemicalsDiseaseSalmonella Gallinarum
The invention relates to a method for purifying salmonella of laying hens. The purification method comprises the following steps that firstly, stock progenitor chickens are selected, and all antibody positive chickens are weeded out; secondly, parent chickens are bred, infectious disease sources are prevented in the breeding process, a conventional chicken salmonella vaccine is used for water drinking, finally, the bred parent chickens are detected, and all the antibody positive chickens are weeded out; thirdly, a chicken house needs to be thoroughly cleaned before hatching of commercial chickens, and the hatching environment needs to be disinfected strictly in the hatching process, wherein the hatching environment is disinfected once a week under the normal condition, disinfected twice a week in the case of diseases and not disinfected three days before and after immunization. The method has the advantages that the disease source of the salmonella of the laying hens can be effectively purified, and the purification degree of the laying hens can be greatly improved.
Owner:JIANGSU TIANCHENG SCI GRP
Salmonella vaccine materials and methods
InactiveUS20050260223A1Antibacterial agentsBacterial antigen ingredientsVirulent characteristicsBiology
Attenuated mutant Salmonella bacteria containing inactivated virulence genes are provided for use in safe efficacious vaccines.
Owner:PHARMACIA & UPJOHN CO
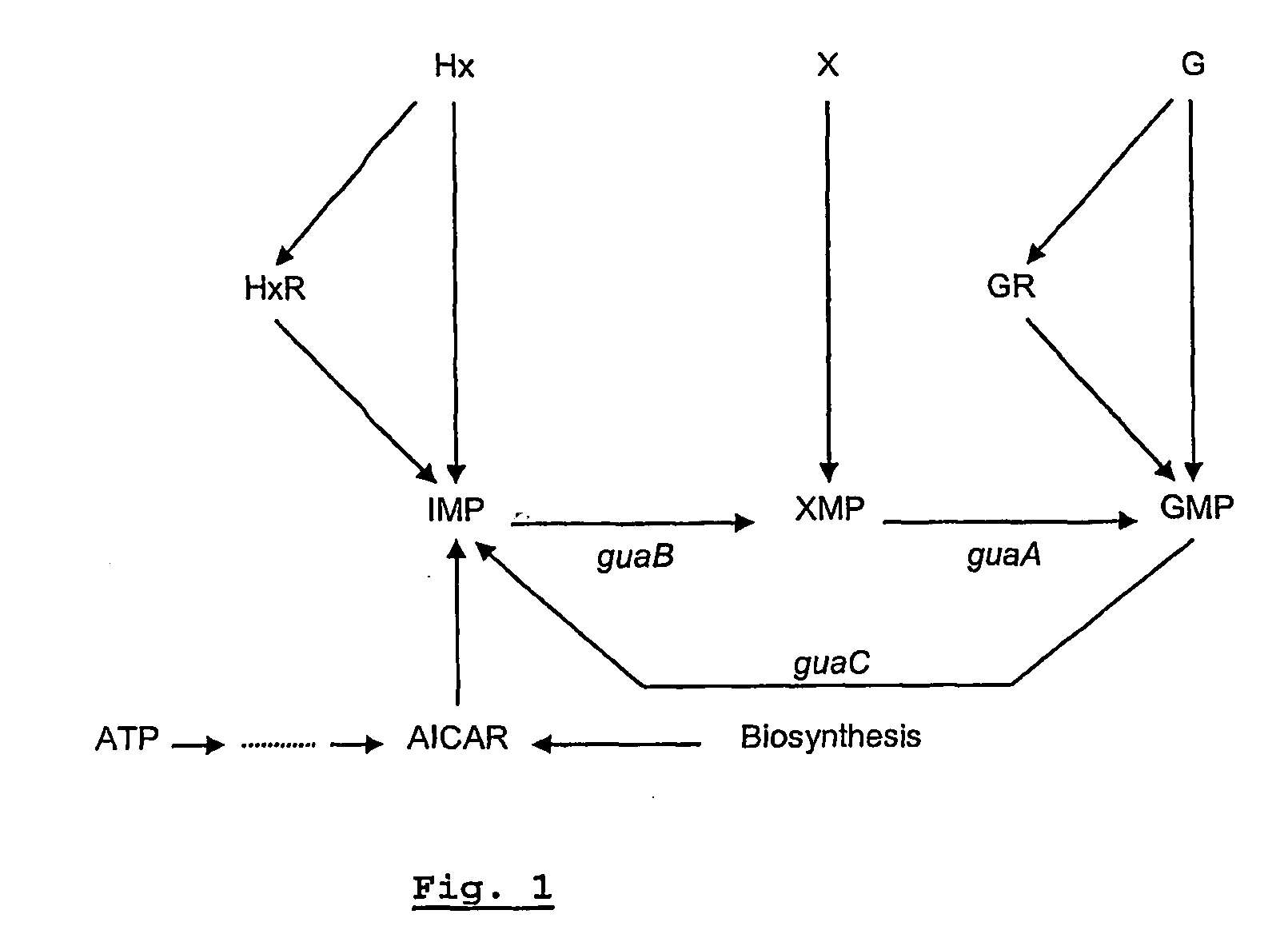
Live salmonella vaccine and methods to prevent fowl typhoid
InactiveUS20170049872A1PeptidesAntibody medical ingredientsVirulent characteristicsRecombinant vaccines
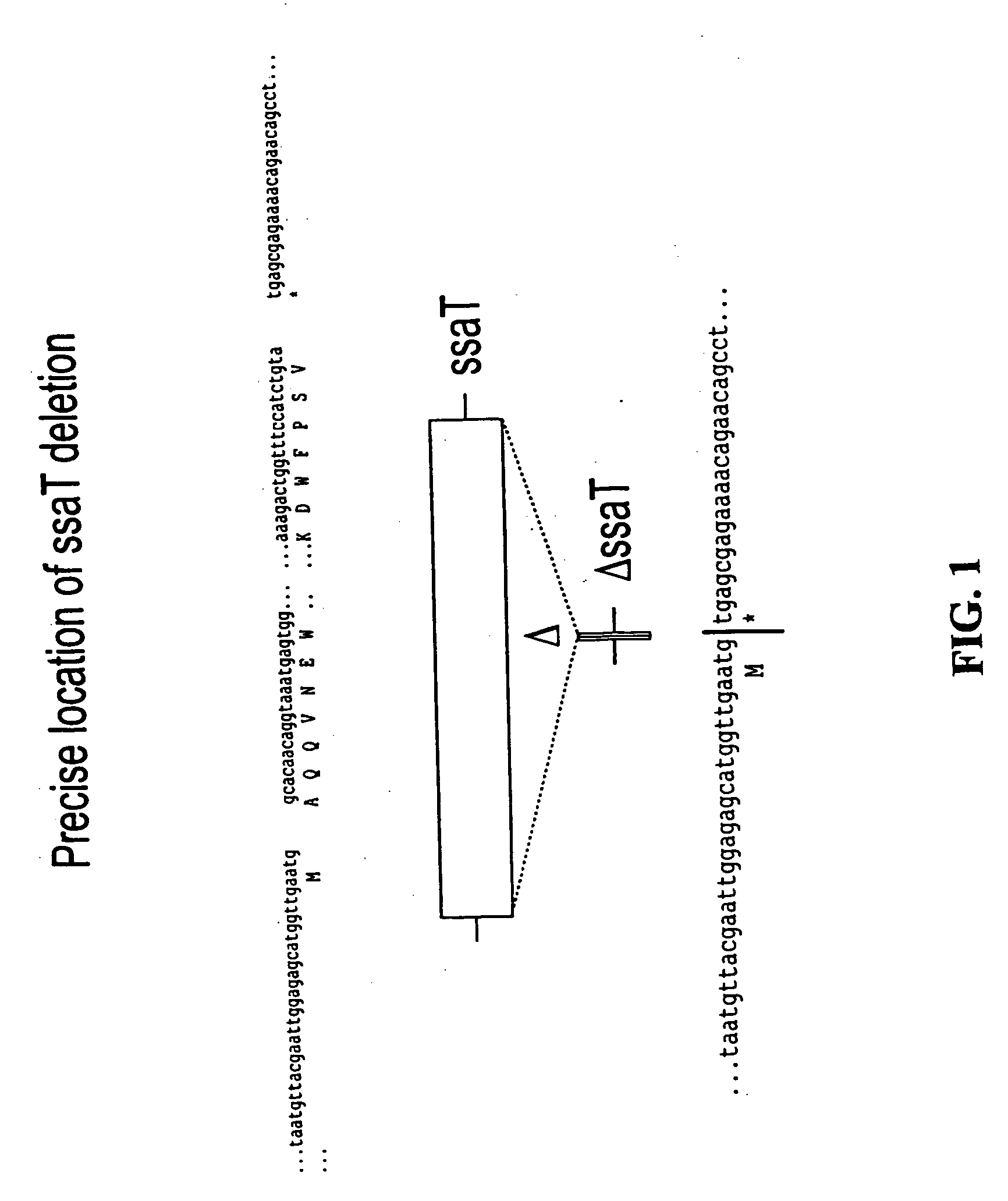
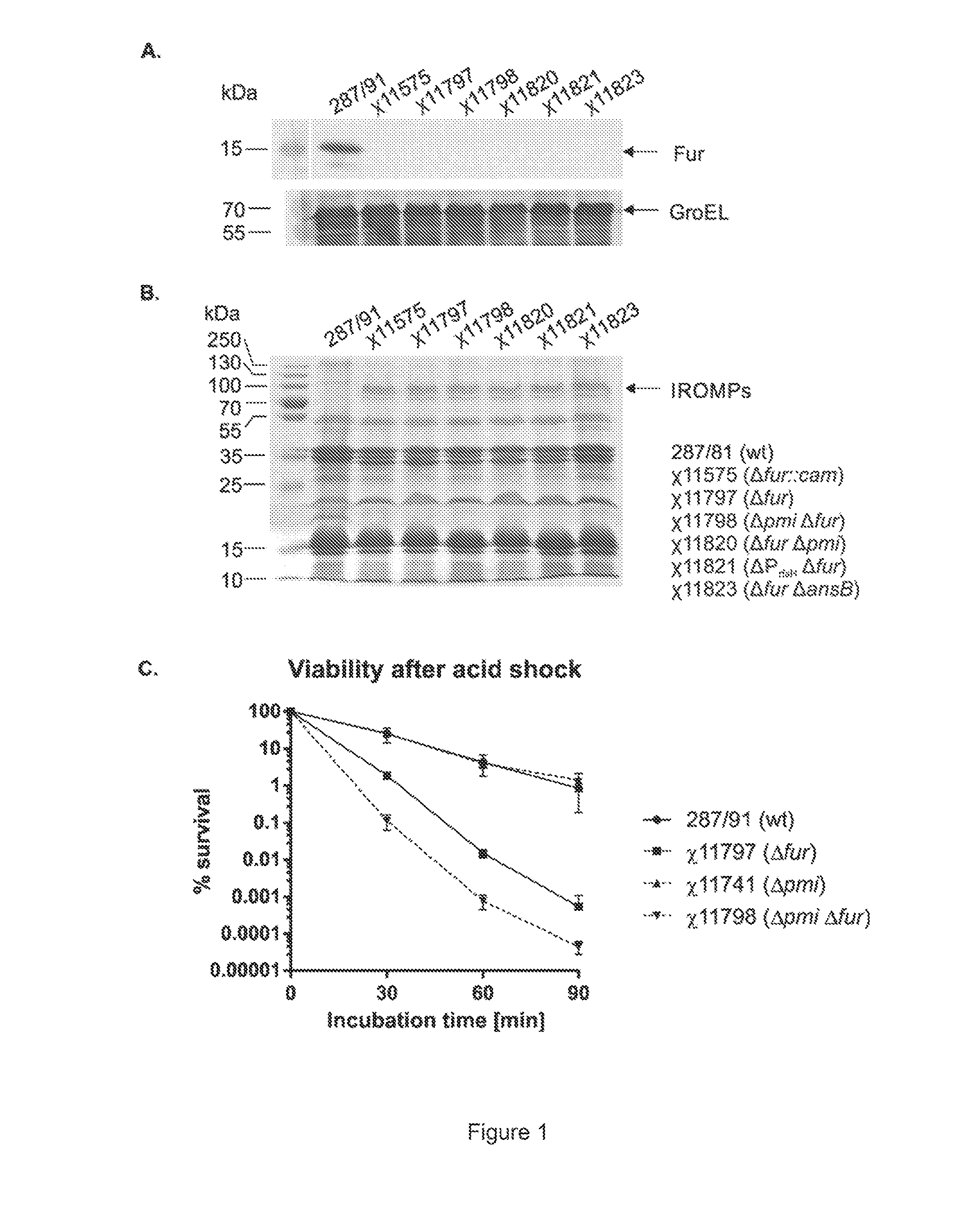
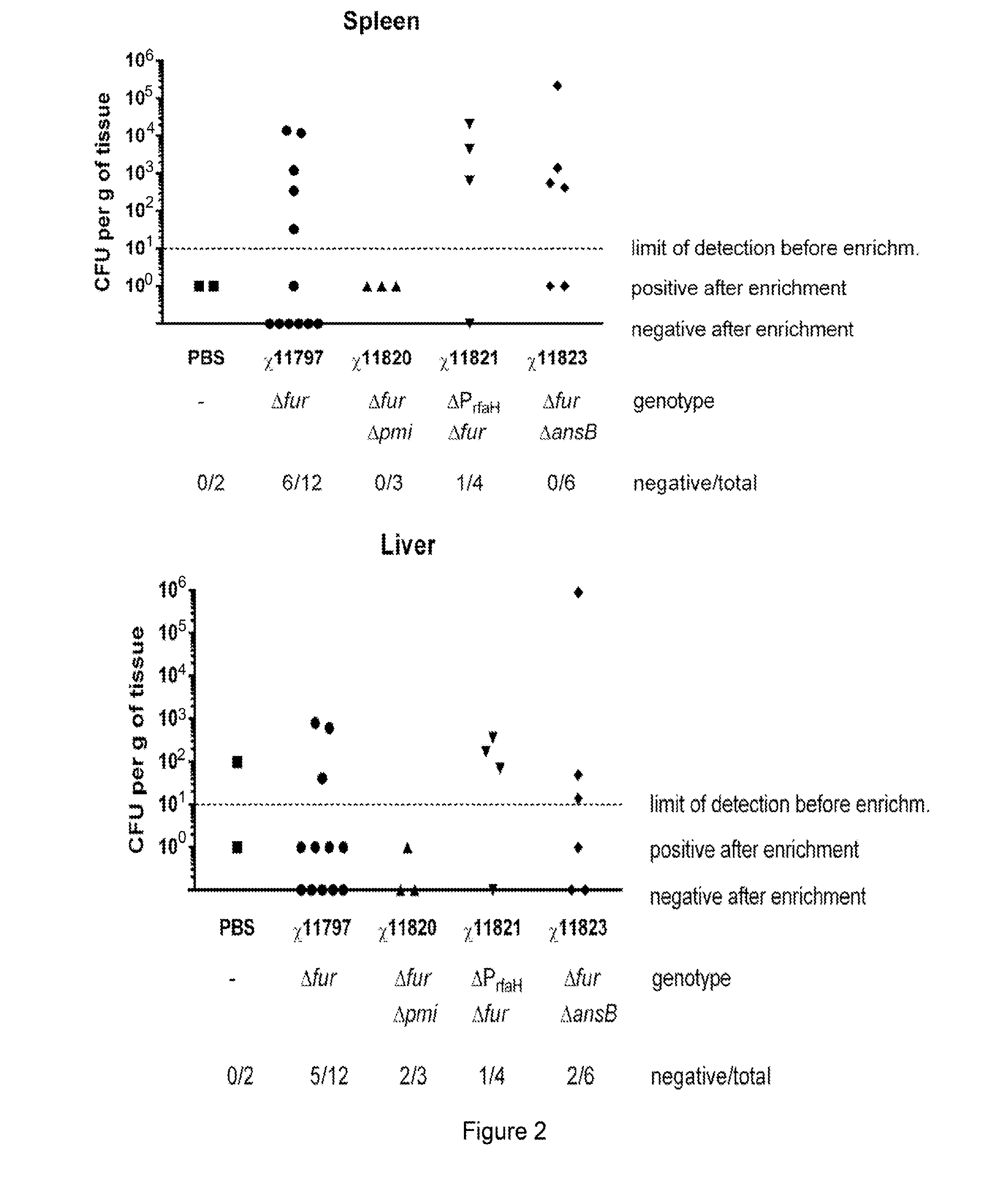
We constructed S. Gallinarum strains deleted for the global regulatory gene fur (FIG. 1) and evaluated their virulence and protective efficacy in Rhode Island Red chicks and Brown Leghorn layers. The fur deletion mutant was a virulent and, when delivered orally to chicks, elicited excellent protection against lethal S. Gallinarum challenge. We also examined the effect of a pmi mutant and a combination of fur deletions with mutations in the pmi and rfaH genes, which affect O-antigen synthesis, and ansB, whose product inhibits host T cell responses. The ΔAfur Δpmi and Δfur ΔansB double mutants were attenuated, but not protective when delivered orally to chicks. However, a Δpmi Δfur strain was substantially immunogenic when administrated intramuscularly. Altogether our results show that the fur gene is essential for virulence of S. Gallinarum and the fur mutant is effective as a live recombinant vaccine against fowl typhoid.
Owner:ARIZONA STATE UNIVERSITY
Salmonella vaccine
InactiveUS20130052230A1Reduce Salmonella colonizationLess detectableAntibacterial agentsWhole-cell/virus/DNA/RNA ingredientsVaccinationMammal
The present invention relates to novel Salmonella mutants, to a process for producing same and to vaccines containing same, wherein said Salmonella mutants are characterized in that they elicit a humoral response that can be distinguished from the humoral response elicited by the wild type strains. It is accordingly an object of the present invention to provide the use of said Salmonella mutants as serological marker strains in the vaccination of animals, in particular mammals and birds, more in particular cattle, poultry and pigs. The serological marker vaccine is of special value in animal farming and provides a (long-lasting) immunization against a wide range of Salmonella strains. The novel mutants are non-reverting and can be used for the efficient immunization of mammals and birds.
Owner:UNIV GENT
A kind of attenuated Salmonella vaccine of Haemophilus parasuis
ActiveCN103421731BGood immune protectionEasy to operateAntibacterial agentsBacterial antigen ingredientsAntigenHaemophilus
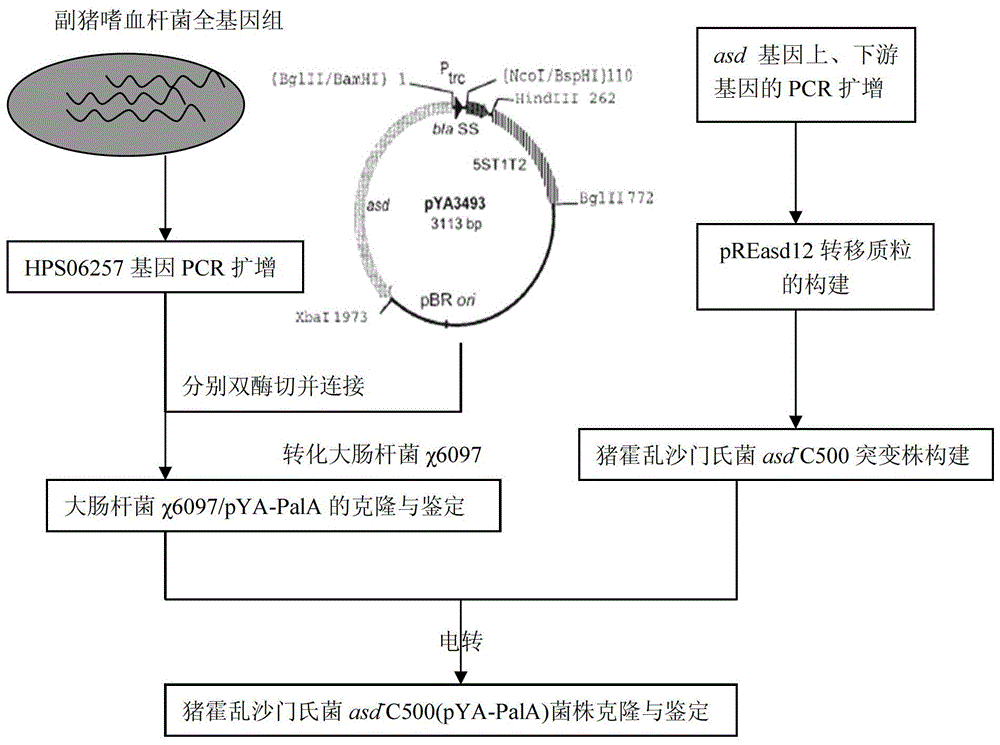
The invention belongs to the technical field of animal bacteriosis gene engineering vaccines, particularly relates to strain construction, vaccine preparation and application of a recombined haemophilus parasuis attenuated salmonella vaccine strain without resistance makers and used for expressing a surface antigen hps 06257 of haemophilus parasuis, and aims to obtain a recombinant salmonella choleraesuis asd-C500 / pYA-06257 (the preservation number is CCTCC NO: M2013054) without resistance makers and used for expressing the surface antigen HPS 06257 of haemophilus parasuis. The recombinant bacteria is lack of an asd gene on a salmonella choleraesuis genome, and contains a recombinant plasmid pYA-06257 which can express the asd gene and an outer membrane antigen hps 06257 gene of haemophilus parasuis on the strain. The invention further discloses a construction method of the recombinant strain asd-C500 / pYA-06257 and a corresponding preparation method of the haemophilus parasuis attenuated salmonella vaccine, as well as application to the preparation of the haemophilus parasuis attenuated salmonella vaccine.
Owner:HUAZHONG AGRI UNIV
A kind of inactivated vaccine of Aeromonas salmonicida and its application
ActiveCN106075419BImprove infection abilitySurface antigen retentionAntibacterial agentsAntibody medical ingredientsAntigenSpecific immunity
The invention belongs to the technical field of fish biological products and cultured fish disease prevention and control, and in particular relates to an aeromonas salmonicida inactivated vaccine and applications of the aeromonas salmonicida inactivated vaccine. The antigen of the inactivated vaccine is aeromonas salmonicida subsp.masoucida, and the bacterial strain is preserved in the China General Microbiological Culture Collection Center (CGMCC) on March 19, 2013, and is assigned with the accession number of CGMCC No.7335. The inactivated vaccine can effectively induce aquaculture fish to generate specific immune response, and provide effective protection, and a material for preparing the aeromonas salmonicida inactivated vaccine is low in cost and is safe to the environment and the host.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Chicken salmonella enteritidis inactivated vaccine and application thereof
PendingCN109663125AStrong targetingGood immune effectAntibacterial agentsBacteriaOil adjuvantImmune effects
The invention relates to the technical field of microorganisms, in particular to a poultry salmonella vaccine, and further relates to application of salmonella. The chicken salmonella enteritidis inactivated vaccine composition comprises chicken salmonella enteritidis liquid and an adjuvant, and the adjuvant is prepared from, by weight, 50 parts of a water-based oil adjuvant, 1.5-3.5 parts of Span-80, 1.5-2.5 parts of Tween-80, 0.1 part of small peptide and 1.5-2.5 parts of propolis. The enteritidis inactivated vaccine is high in pertinency, the immune effect on locally epidemic salmonella strains, the preparation process is easy to operate, the cost is low, storage is easy, and the chicken salmonella enteritidis inactivated vaccine is suitable for large-scale farms with laboratories.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Preparation method of anti prglet bacterial epidemic disease compound egg antibody medicinal preparation
A compound yolk antibody preparation for preventing bacterial disease of piglet, especially the bacteria diarrhea, is prepared through preparing salmonella vaccine and the genetically engineered vaccine of colibacillus K88, K99 and 9879, forced immunizing three times, collecting immunized egg, taking yolk, diluting with aseptic buffer liquid of phosphate, treating by hydroxypropylmethyl cellulose phthalate, filtering and spray drying.
Owner:SHANGHAI JIAO TONG UNIV
Live attenuated oral vaccine against shigellosis and typhoid fever
ActiveUS10695415B2Reduce morbidityReduce incidenceAntibacterial agentsBacterial antigen ingredientsSHIGELLOSESHeterologous
Disclosed is the attenuated Salmonella typhi vaccine Ty21a utilized as a vector for Shigella and / or enterotoxogenic E. coli genes stably integrated in the Ty21a chromosome. These genes include a heterologous Shigella sonnei O-antigen biosynthetic gene region that comprises the wzz gene and expresses Shigella sonnei form 1 O-antigen, as well as a heterologous acid resistance biosynthetic gene system comprising a YbaS gene, which enables increased stability of the Ty21a vector at pH 2.5 relative to Ty21a without the integrated acid resistance biosynthetic gene system.
Owner:PROTEIN POTENTIAL
Salmonella vaccine for the treatment of coronavirus
PendingUS20220047697A1Convenient treatmentImprove responseSsRNA viruses positive-senseCell receptors/surface-antigens/surface-determinantsAntigenAdjuvant
The present invention provides live-attenuated bacterium of the genus Salmonella comprising a recombinant plasmid encoding a fusion protein, wherein the fusion protein comprises a coronavirus antigen and an adjuvant peptide.
Owner:JULIUS MAXIMILIANS UNIV WURZBURG
Live attenuated salmonella vaccine
InactiveUS20100260794A1Antibacterial agentsBacterial antigen ingredientsBacteroidesAttenuated vaccine
The present invention relates to attenuated guaB deletion mutants of a bacterium infecting veterinary species, more in particular Salmonella enterica, to their use and production. The present invention further relates to live attenuated vaccines based on such mutants for preventing bacterial infections, and more in particular Salmonellosis, in a veterinary species, more in particular poultry.
Owner:VRIJE UNIV BRUSSEL
A Recombinant Attenuated Salmonella Enteritidis Vaccine
ActiveCN106591200BSimple production processReduce manufacturing costAntibacterial agentsBacteriaAdjuvantVaccine Production
The present invention provides an attenuated Salmonella Enteritidis vaccine, wherein the antigen is the recombinant Salmonella Enteritidis vaccine strain with the deposit number of CGMCC NO.13251. The vaccine provided by the invention is safe and reliable, and does not cause the risk of dispersing; the conventional injection of the vaccine will cause stress and the absorption effect of the vaccine is not good, but the vaccine can be immunized by oral administration, and the possibility of the immunogen being degraded before reaching the intestinal mucosa is avoided. ; This vaccine integrates the carrier and the adjuvant, which can not only induce the body to produce humoral immunity, but also produce cellular immunity and mucosal immunity, which can not only prevent and control IBDV, but also prevent and control Salmonella infection to a certain extent; The production process is simple and convenient, the production cost is extremely low, and no protein purification is required, and the vaccine bacteria only need to be expanded and lyophilized, and then oral immunization can save labor costs.
Owner:SHANDONG SINDER TECH +1
Salmonella vaccine for cross protection
The present invention discloses that Salmonella enterica serogroup C2-3 serotype cross-protects against Salmonella enterica serogroup C1 serotype and vice versa. Therefore, the present invention discloses the use of Salmonella enterica serogroup C2-3 serotype or Salmonella enterica serogroup C1 serotype for the preparation of a vaccine for administration to poultry to protect against diseases caused by Salmonella enterica serogroups Conditions of serotype C2‑3 and / or conditions resulting from Salmonella enterica serogroup C1 serotype.
Owner:INTERVET INT BV
Codon-optimized HPV16LI for salmonella vaccine strains against human papillomavirus type 16
InactiveCN101115766BImproving immunogenicityViral antigen ingredientsVirus peptidesAmpicillinHuman papillomavirus
The present invention relates to a novel nucleic acid sequence (HPV16 L1S) encoding antigenic HPV16 L1 protein as provided in SEQ ID NO: 1, wherein the said sequence has atleast one modified codon for optimum stability of recombinant plasmid vector when transformed in the prokaryotic micro-organism for improved immunogenicity of the resulting prokaryotic micro-organism. The invention further relates to constructing recombinant vectors pFS14nsdHPV16L1 and pFS14nsdHPV16 kan L1S harboring SEQ ID NO: 1, wherein the former carries Ampicillin and the latter, Kanamycin as a selection marker. The invention also relates to an attenuated strain of a prokaryotic micro-organism transformed with nucleic acid encoding HPV16 (Human Papillomavirus) major capsid protein and expressing the corresponding protein. In addition the invention discloses a process of producing a vaccine based on prokaryotic micro-organism for the treatment of papillomavirus infection and associated risk of cancer.
Owner:INDIAN IMMUNOLOGICALS LIMITED
A Salmonella abortus equine strain smxj-97 and its application in equine abortus salmonella vaccine
ActiveCN108220183BImprove securityNo danger of spreading poisonAntibacterial agentsBacteriaBiotechnologyStaining
The present invention relates to a strain of Salmonella abortus equine ( Salmonella abortus equi ) SMXJ‑97 CGMCC No.9047, which has the 16S rRNA gene sequence in Sequence Table 1; the strain was isolated from aborted horses in Xinjiang, and the bacteria were straight rod-shaped, with a size of 0.7~1.6μm×2.0~5μm, Gram Stain negative. It is an aerobic and facultative anaerobic bacterium, which can grow on ordinary culture medium. The growth temperature is from 25°C to 40°C, the optimum temperature is 37°C, the pH growth range is 5-9, and the optimum pH is 7.4-7.6. It can ferment mannitol, decompose lysine, but not urea, and cannot use tryptophan, malonate, salicin, and sorbitol. The strain can be used to prepare an inactivated vaccine against salmonellosis in equine abortion. The vaccine prepared by the bacterial strain has good pertinence, low cost, safety and good protection effect.
Owner:XINJIANG AGRI UNIV
A kind of preservation method and special protective agent of Salmonella choleraesuis vaccine strain
ActiveCN108018210BLower requirementSimple and fast operationBacteriaMicroorganism based processesBiotechnologyAnimals vaccines
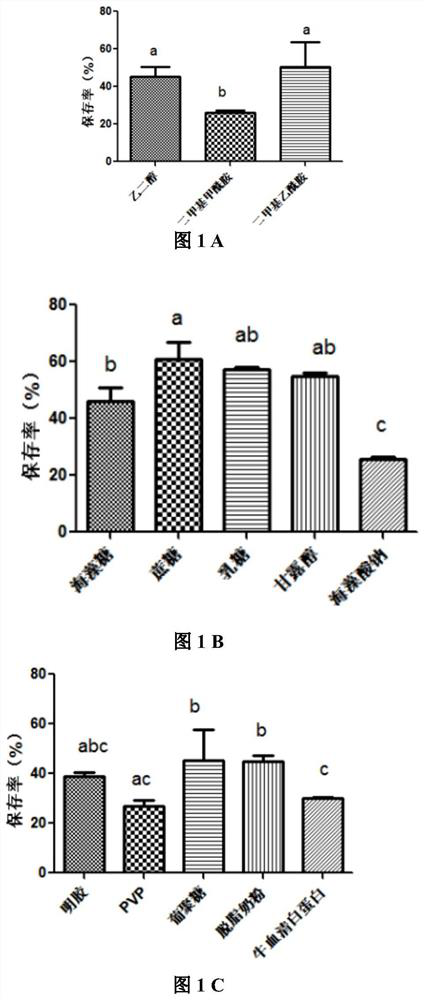
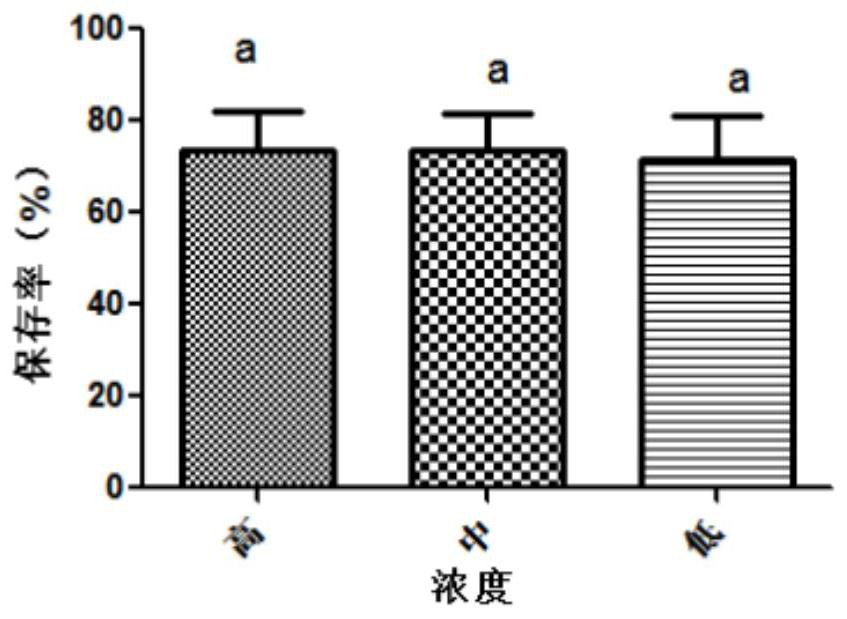
The invention belongs to the technical field of preparation of animal vaccine auxiliary preparations and relates to a method for preserving a salmonella choleraesuis vaccine strain and a special protective agent thereof. The method for preserving the salmonella choleraesuis vaccine strain deposited under liquid nitrogen conditions is obtained by screening and characterized in that a protective agent is added to a salmonella choleraesuis vaccine solution during the preservation of the salmonella choleraesuis vaccine strain. The protective agent is prepared from the following components in massratio: 2.5% of sucrose, 7% of glucan, 6% of dimethylacetamide, and 0.025% of methionine. The special protective agent is prepared from the following components in mass ratio: 2.5% of sucrose, 7% of glucan, 6% of dimethylacetamide, and 0.025% of methionine. The special protective agent has the effects of permeability, semi-permeability, non-permeability and anti-oxidation. A survival rate of different concentrations of vaccine bacteria at different preservation times under a selected protective agent formula has no significant difference, and the survival rate maintains 70% or higher.
Owner:HUAZHONG AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com