Salmonella vaccine materials and methods
a technology of salmonella and vaccine materials, applied in the field of genetically engineered salmonella, can solve the problems of large doses, poor to moderate protection of vaccines, and most economic damag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
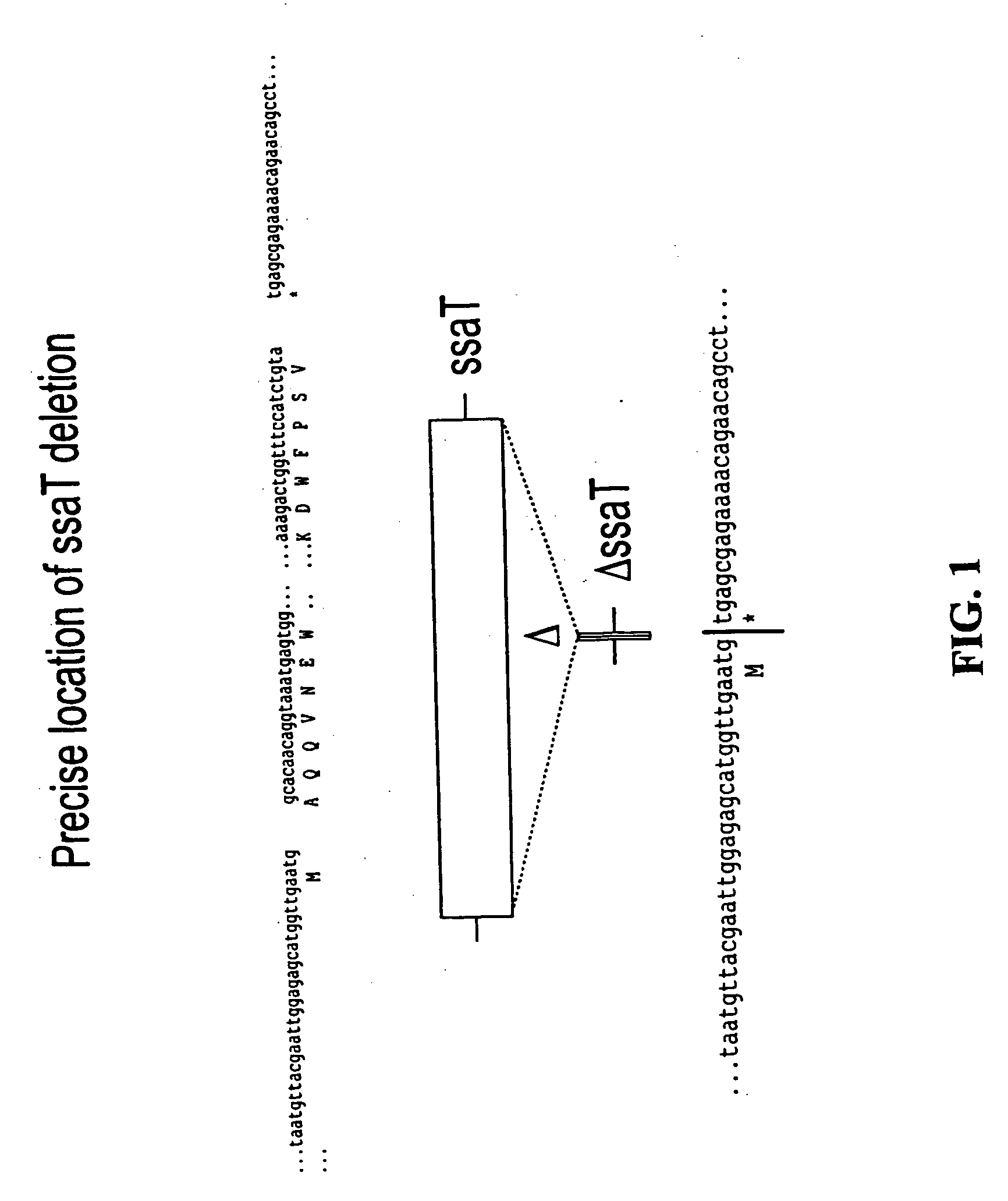
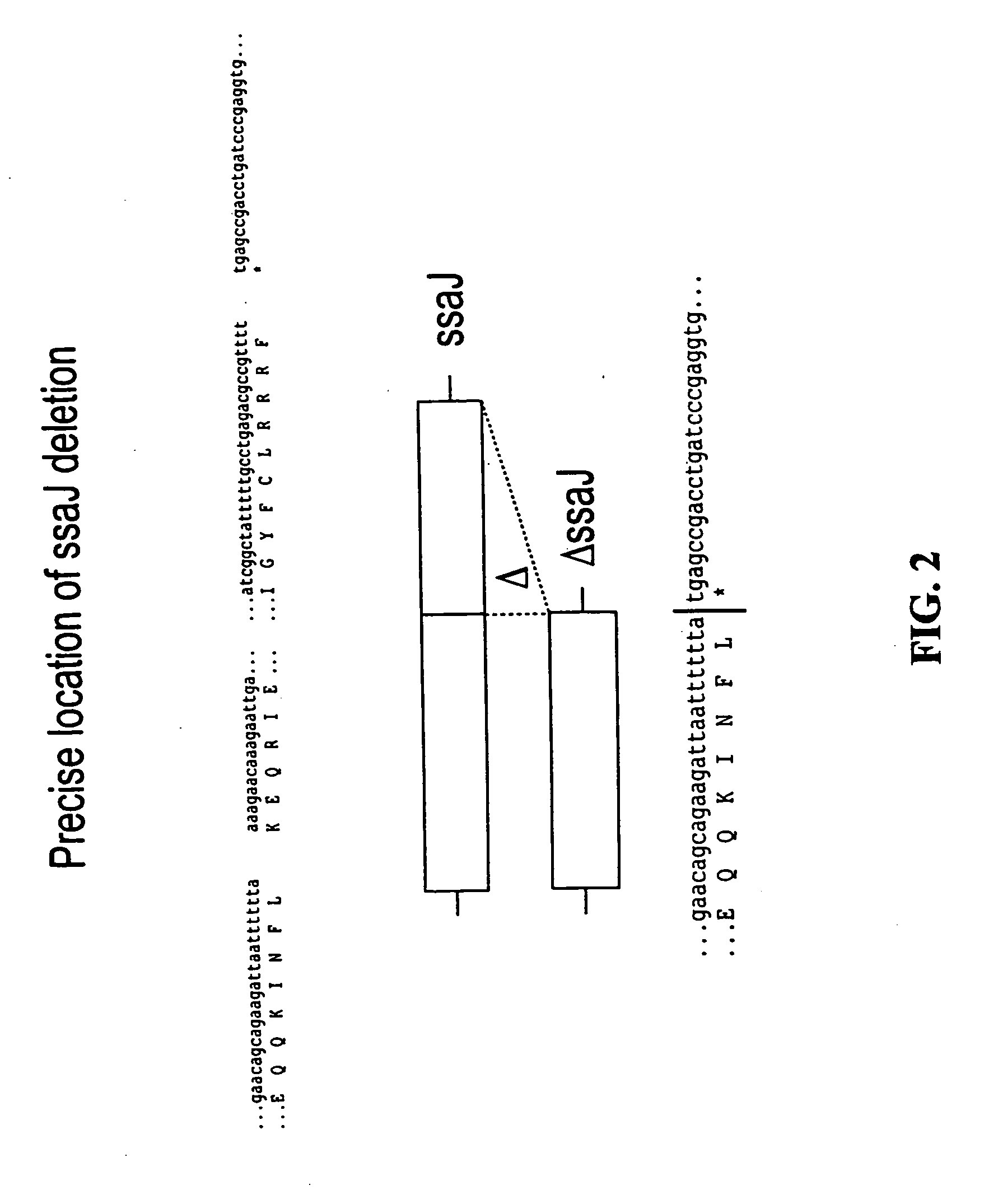
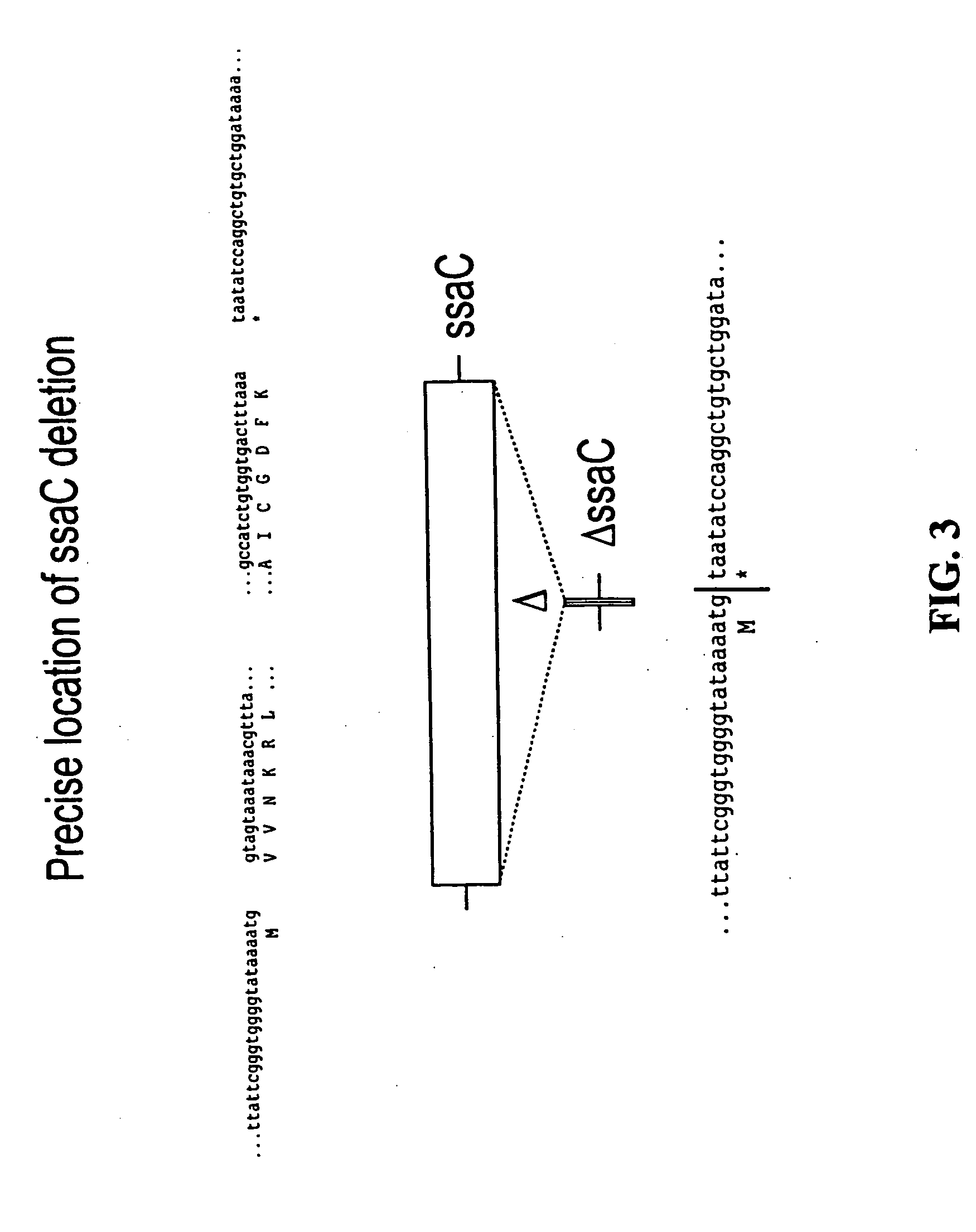
Construction of Salmonella Mutants Containing Single and Double Deletions of Selected Genes: ssaT, ssaJ, ssaC, rfaK and glnA
A. Construction of pCVD442::Δgene Plasmids
[0055] For each of the S. typhimurium and S. dublin ssaT, ssaJ, ssaC, rfaK and glnA genes, positive selection suicide vectors based on the plasmid pCVD442 [Donnenberg and Kaper, Infect Immun 59:4310-17 (1991)] were constricted that contained a portion of the 5′ and 3′ chromosomal regions flanking each gene but with substantial internal deletions (typically >95%) within the gene itself. Gene splicing by overlap extension (“gene SOEing” [Horton et al., Biotechniques 8:528-535 (1990)]) was used to generate DNA fragments which were complementary to the gene to be deleted, but which lacked the majority of the internal nucleotide sequence. The plasmids containing these internally deleted genes were designated pCVD442::ΔssaT, pCVD442::ΔssaJ, pCVD442::ΔssaC, pCVD442::ΔrfaK, and pCVD442::ΔglnA, respectively. These vectors we...
example 2
Safety and Efficacy of Single and Double Deletion SPI2 Mutants
A. Efficacy of a S. choleraesuis ΔssaC Mutant as a Vaccine in Swine
(Trial No. 704-7923-I-MJK-96-008)
[0074] The safety and efficacy of a live attenuated S. choleraesuis ΔssaC mutant as a vaccine was determined in swine (3-4 week old pigs). The pigs (8 pigs per group) were vaccinated either orally via the drinking water or intramuscularly (IM), at a dose of about ˜1×109 CFUs / pig. For oral vaccination, 10 ml of the lab grown culture of the vaccine (grown generally as described in Example 2.D. below) was diluted 1:4 in ddH2O. 40 ml of this mixture was further diluted by adding 960 ml of sterile ddH2O; 100 ml of this final mixture was given to pigs orally via a waterer (pigs drank approximately 50 ml of water, giving a final vaccine dose of ˜2.6×108 per pig). For the IM vaccination, 27 ml of the 1:4 diluted vaccine culture was added to 3 ml of sterile WFI; 2.5 ml of this mixture was administered intramuscularly to each a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com