Salmonella vaccine for cross protection
A technology of Salmonella and Salmonella enterica, applied in vaccines, veterinary vaccines, bacteria, etc., can solve the problem of not inhibiting the reactivity of monoclonal antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
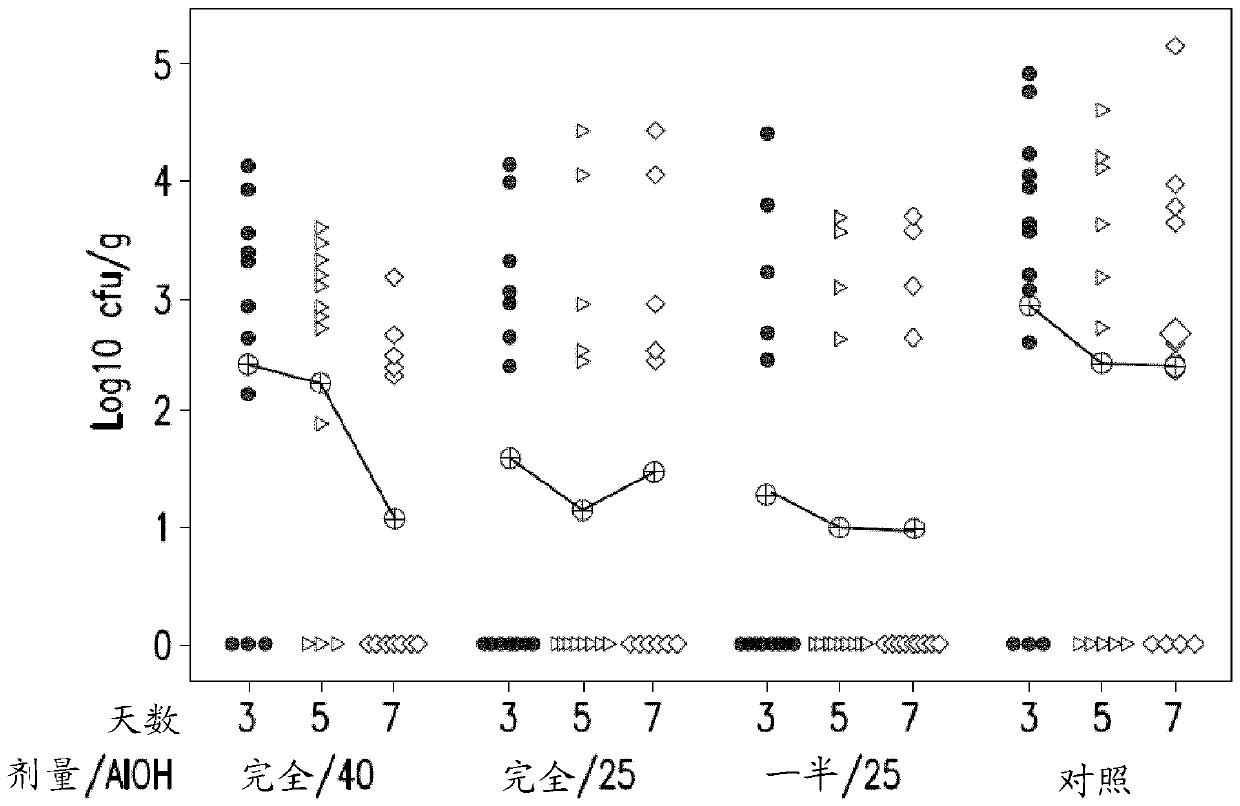
[0077] Comparison of vaccines containing different Salmonella serotypes
[0078] Preparations containing Salmonella hadar alone or with inactivated ( S. Hadar; SH) and / or Salmonella infantis ( S. Infantis; SI) serotype combined, inactivated Salmonella enteritidis ( S. Enteritidis; SE) and Salmonella typhimurium ( S. Typhimurium; ST) (NB: S. Enteritidis and S. Typhimurium serotypes are the same as those in SALENVAC T) serotypes. All serotypes were grown in iron-limited media and formalin inactivated prior to use. The vaccine formulations used in the study are listed in Table 1.
[0079] Table 1: Vaccine Compositions
[0080]
[0081] SE / ST / SH / SI Quadrivalent Vaccine 1 (Quad 1) contains an additional amount of adjuvant, as previous studies with similar vaccines containing high cell numbers have shown that 25% aluminum hydroxide content is 9 cells / ml of vaccine may not be sufficient. SE / ST / SH / SI Quad 2 (Quad 2) contained 25% adjuvant to investigate adjuvant conce...
Embodiment 2
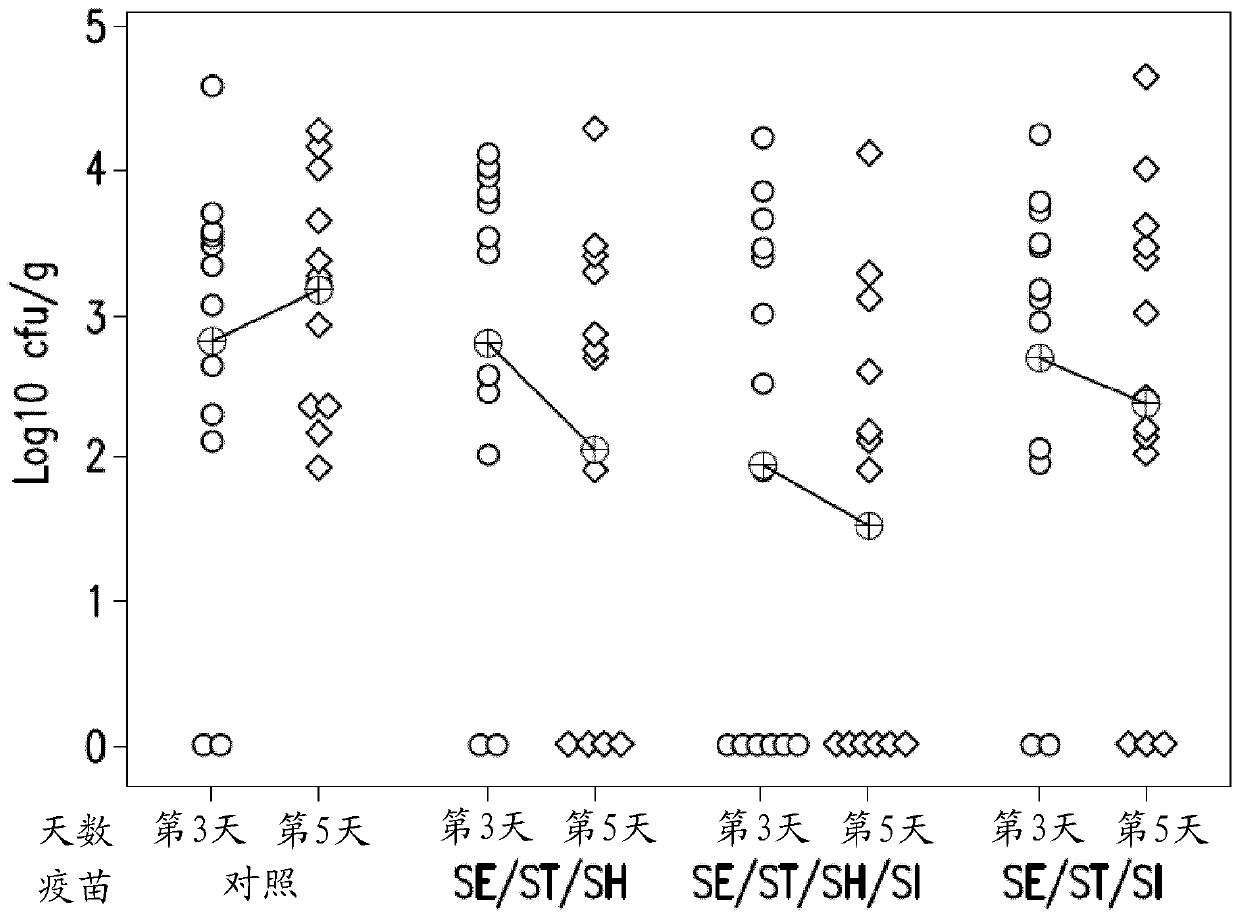
[0118] Passive protection in broiler chickens challenged with S. hadar or S. infantis at 4 days of age
[0119] Parental birds were vaccinated with the S. enteritidis + S. typhimurium + S. infantis (SE / ST / SI) combination vaccine with 25% Rehydrogel™, or were optionally not vaccinated as a control. Four-day-old broiler chickens that had hatched from eggs of vaccinated or non-vaccinated hens were challenged with S. hadar or S. infantis to test passive protection against these two different serotypes.
[0120] Recovery of challenge serotypes from study birds was high. No differences were seen between vaccinated and control groups with respect to challenge shedding, monitored by cloacal swabs or in cecal contents post mortem. However, when invasion of both liver and spleen was considered, fewer positive birds were present in the vaccinated group than in the control group. It was found that this was sampled 10 days after the attack and using 10 2 CFU Hadar Salmonella or 10 3...
Embodiment 3
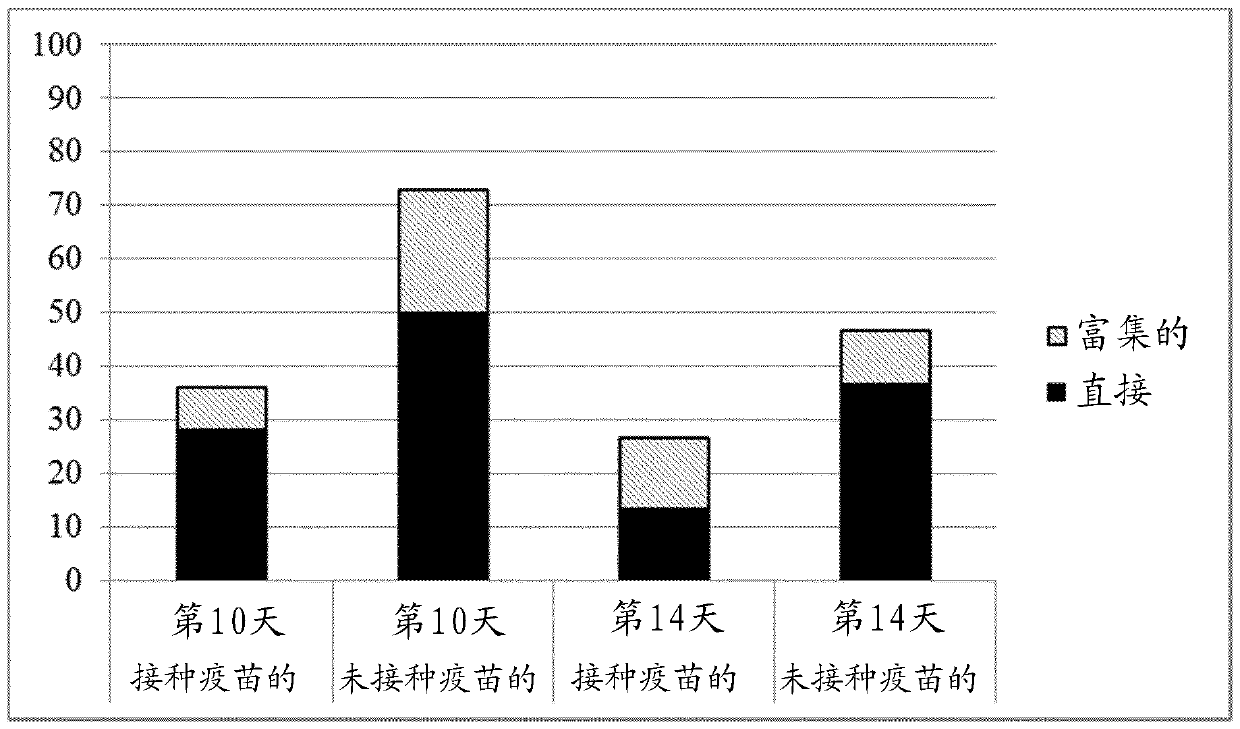
[0139] Efficacy test of a trivalent vaccine against Salmonella hadar challenge in laying hens at 14 weeks of age
[0140] summarize
[0141] Six-week-old SPF origin laying chickens were immunized with a trivalent vaccine containing equal numbers of formalin-killed cells of S. grow under restricted conditions. The second vaccine dose was given at 10 weeks of age. Four weeks after the second vaccination, birds and unvaccinated cohorts were challenged with S. Hadar by the oral route, and shedding of the challenge strain was monitored by cloacal swab examination. Dissemination of challenge bacteria to guts was determined by postmortem examination of the birds at 10 and 14 days post-challenge.
[0142] When individual bird shedding levels were compared to controls, significantly fewer challenge organisms were shed by the vaccinated group (p=0.001).
[0143] The proportion of positive spleen samples from control birds was significantly higher than in vaccinated groups (p = 0.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com