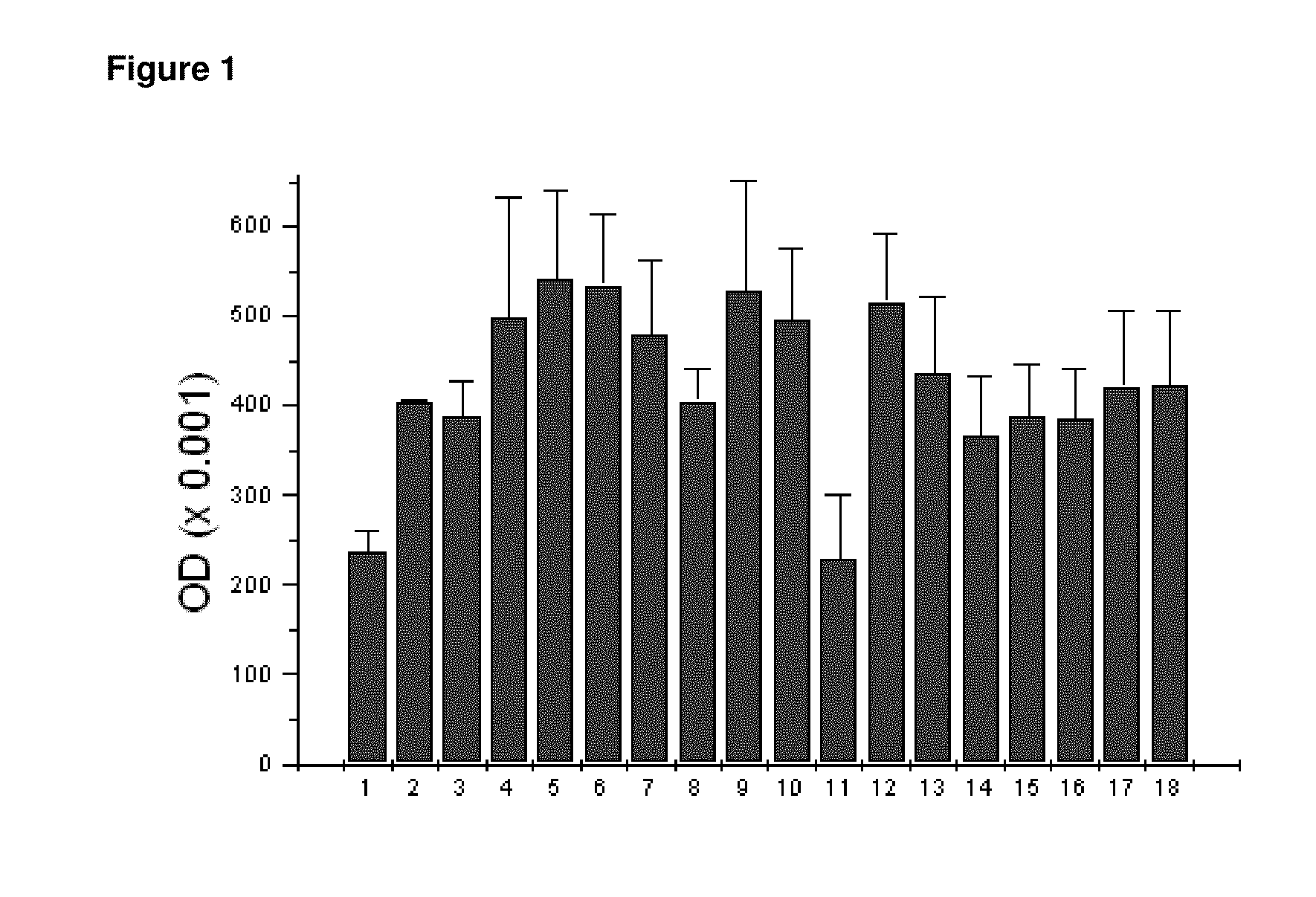
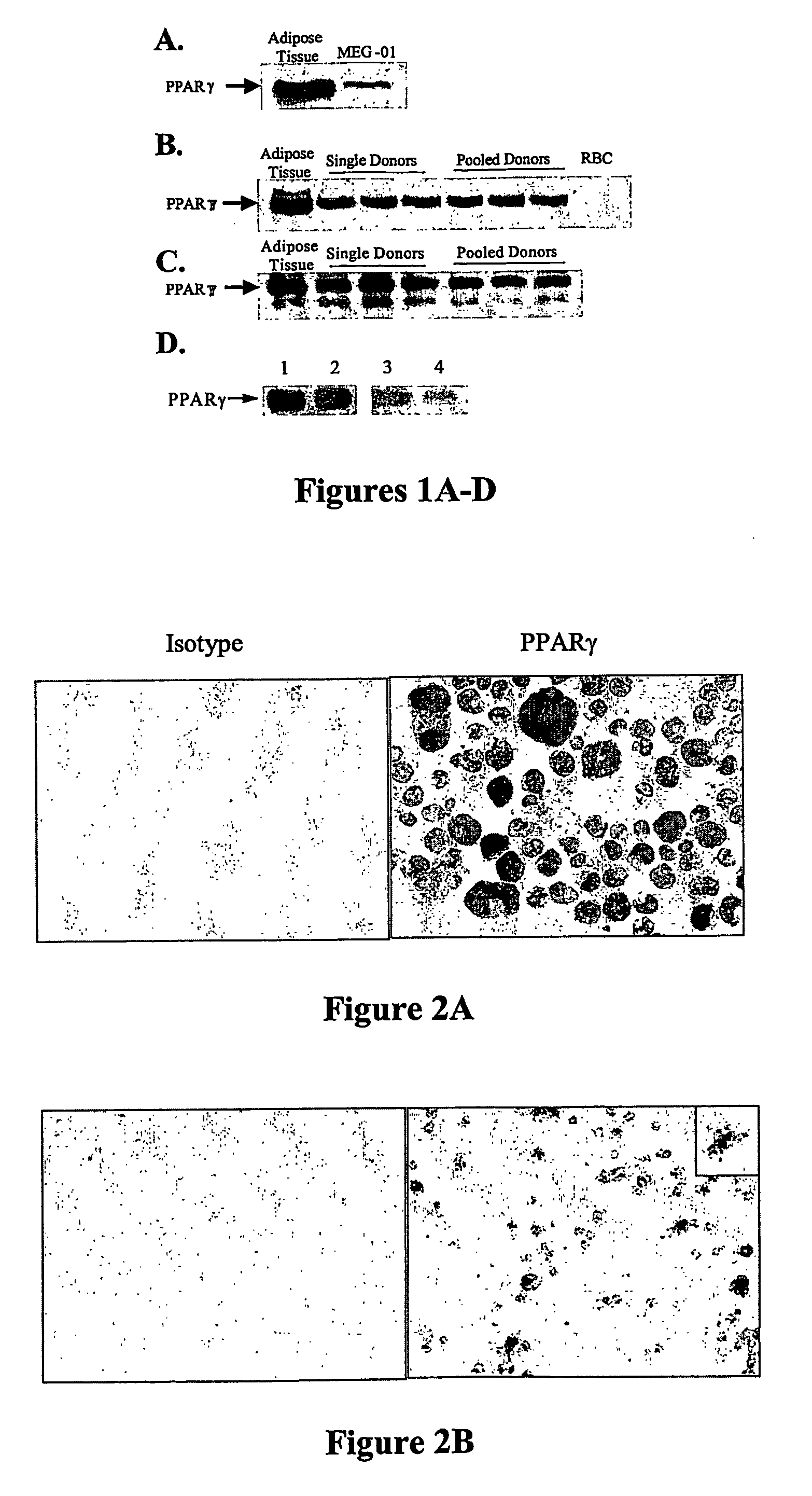
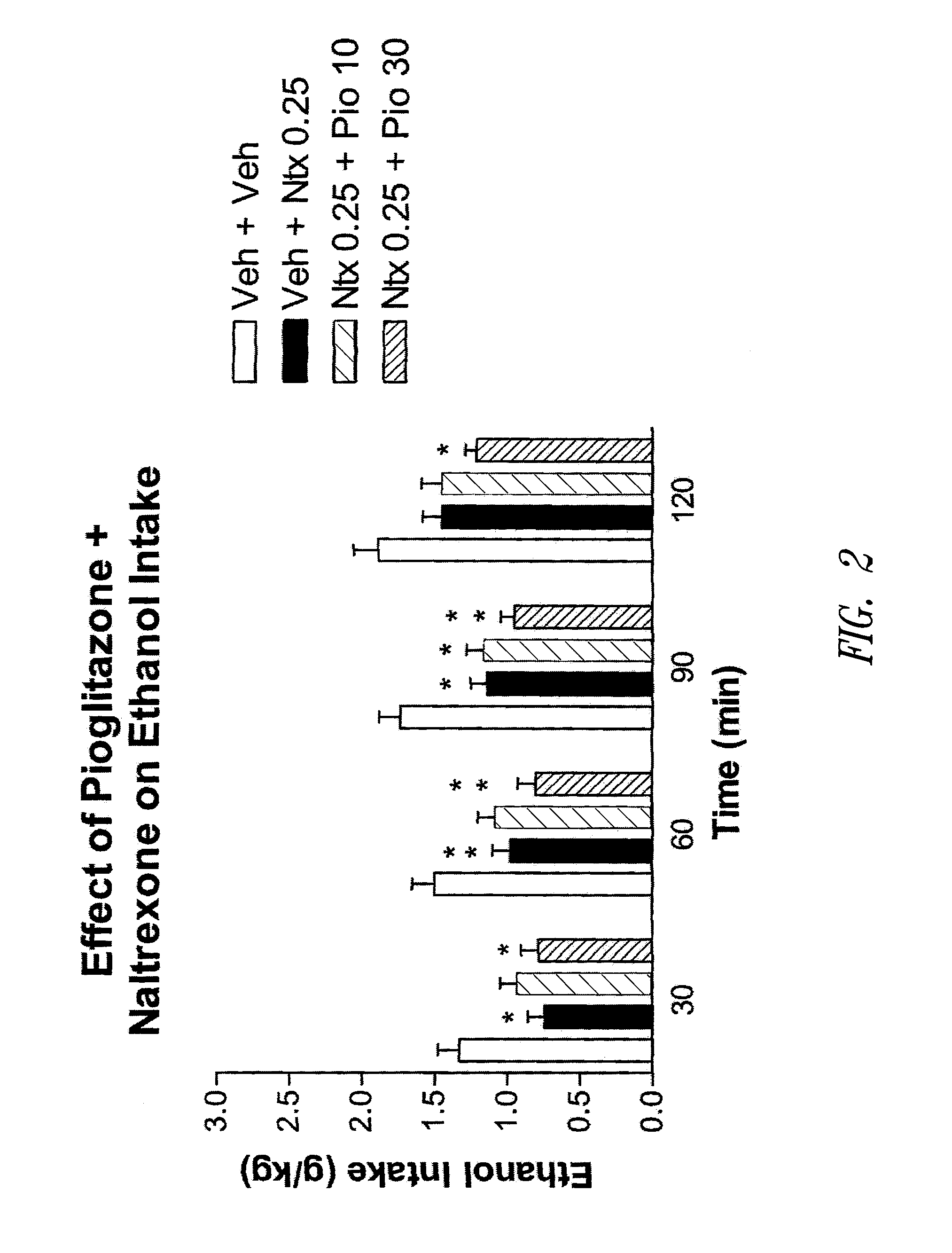
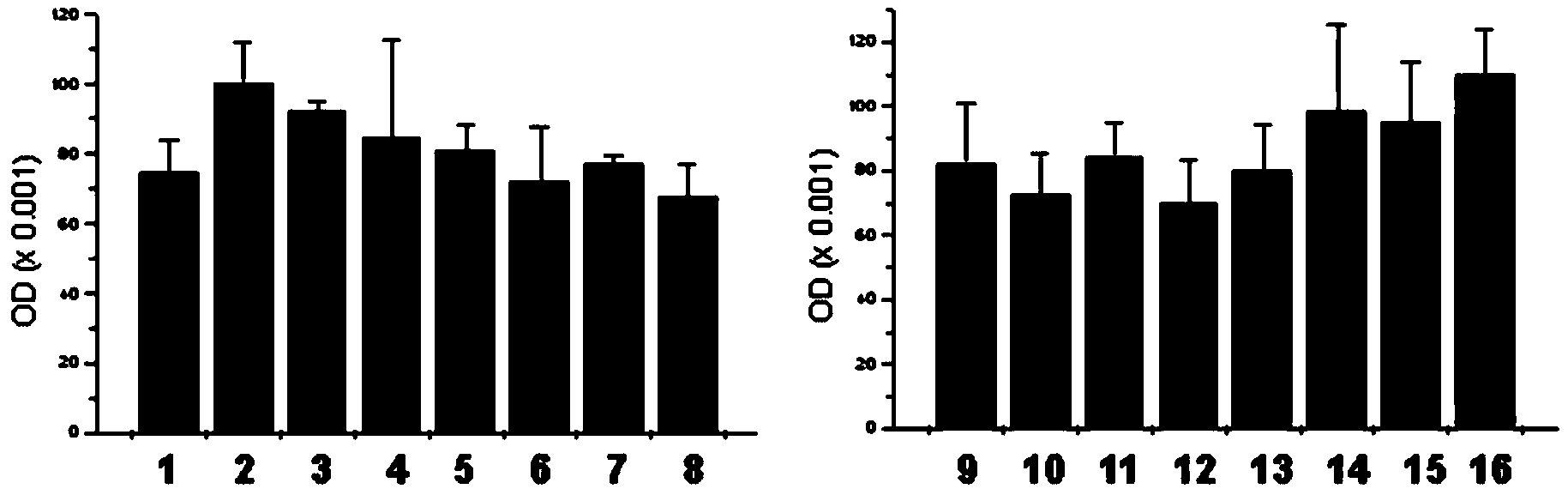
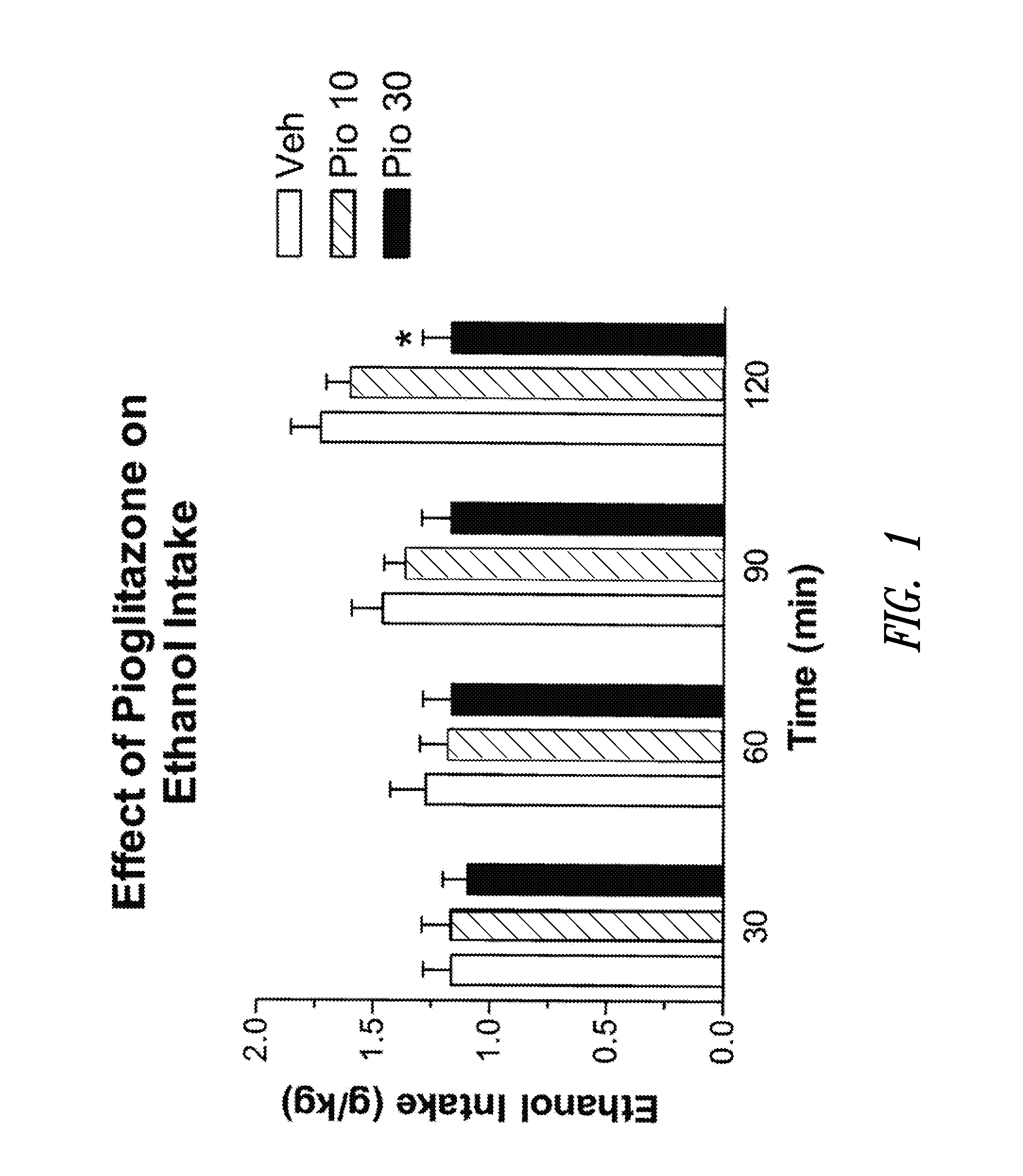
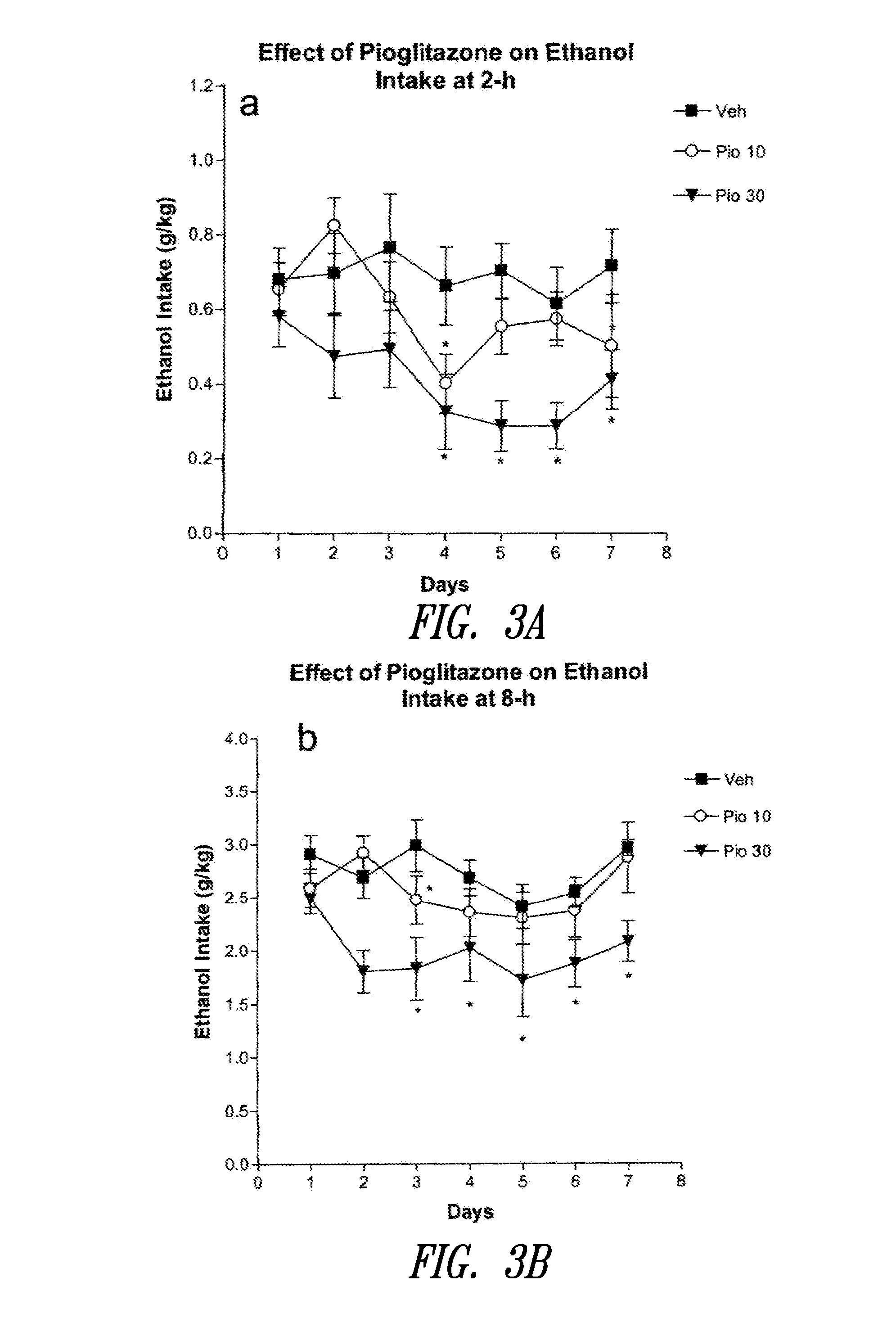
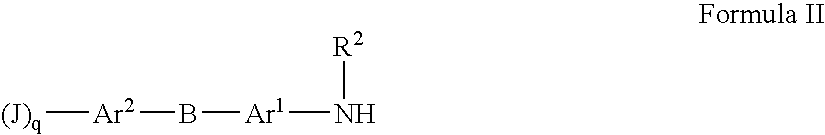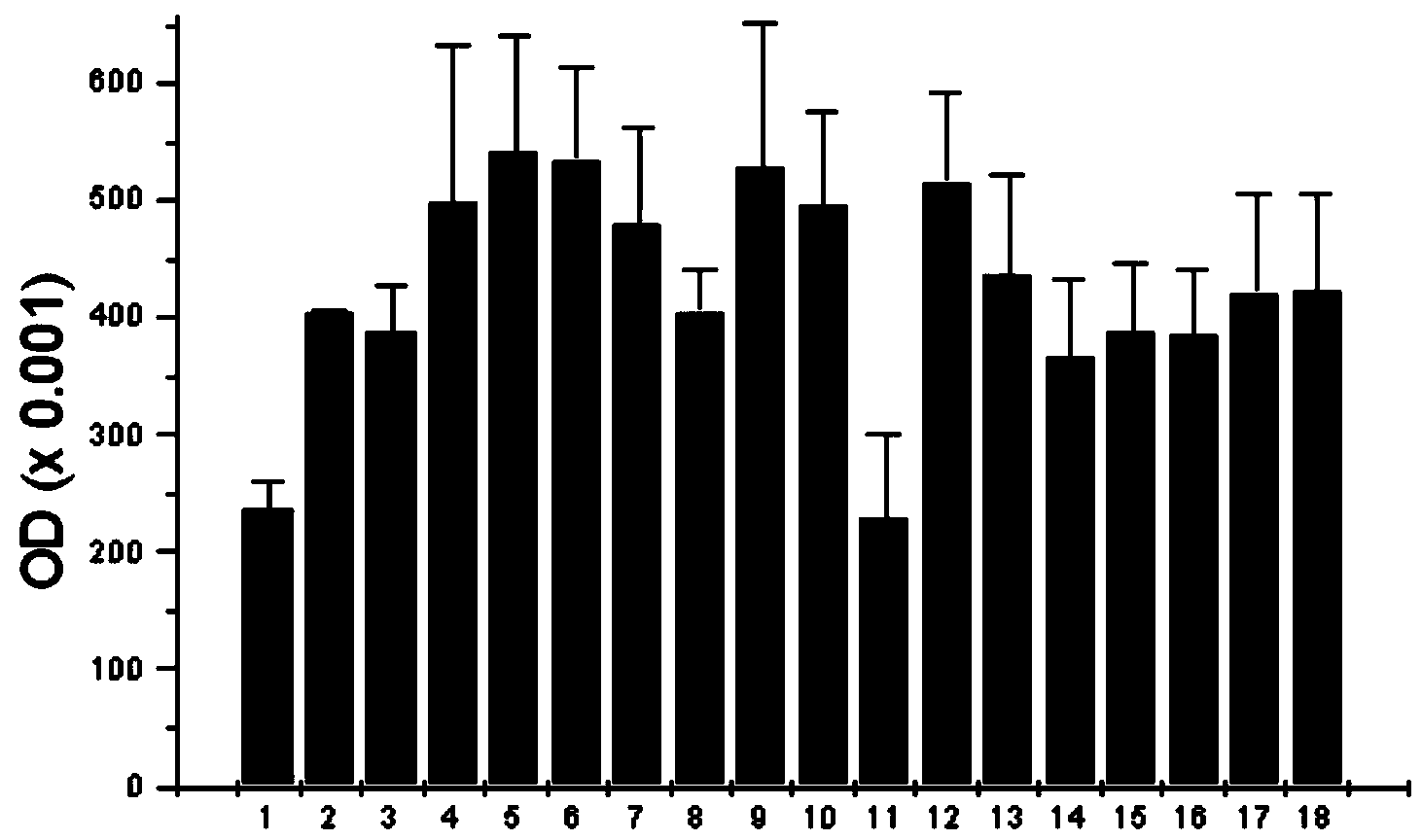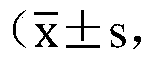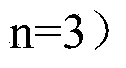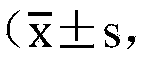Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
55 results about "Peroxisome proliferator-activated receptor gamma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Peroxisome proliferator-activated receptor gamma (PPAR-γ or PPARG), also known as the glitazone receptor, or NR1C3 (nuclear receptor subfamily 1, group C, member 3) is a type II nuclear receptor that in humans is encoded by the PPARG gene.
Composition and method of treating facial skin defect
InactiveUS20090291986A1Increase differentiationReduce appearance problemsCosmetic preparationsBiocideFacial skinAgonist
This invention relates to a subcutaneous deliverable composition containing an agonist of the peroxisome proliferator-activated receptor-gamma, and a method for treating facial skin defects in a mammalian subject using the subcutaneous deliverable composition.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Regulatory t cells and methods of making and using same
InactiveUS20090136470A1Inhibiting IL-6-driven induction of Th-1Adjust balanceBiocideSenses disorderRegulatory T cellPeroxisome
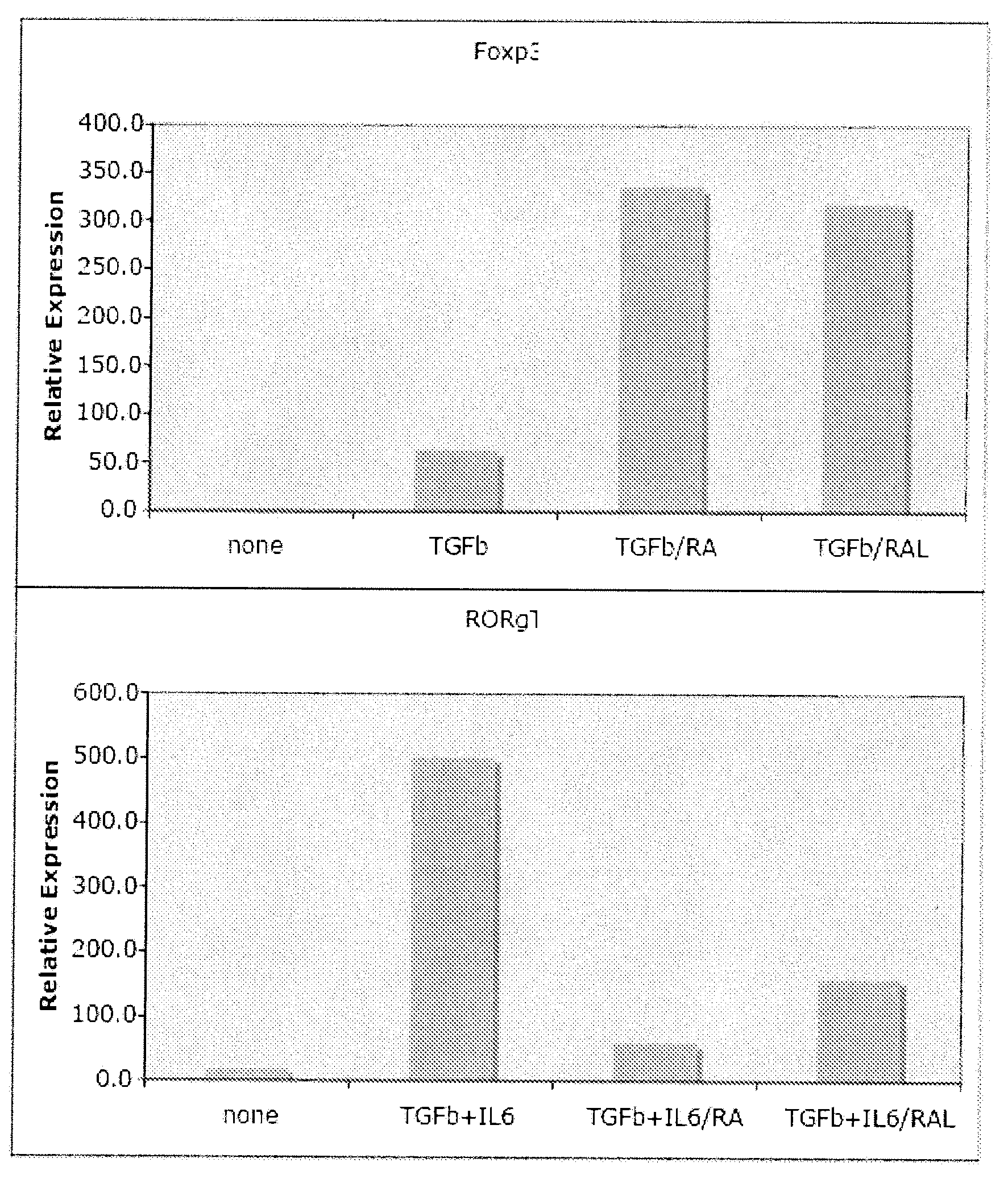
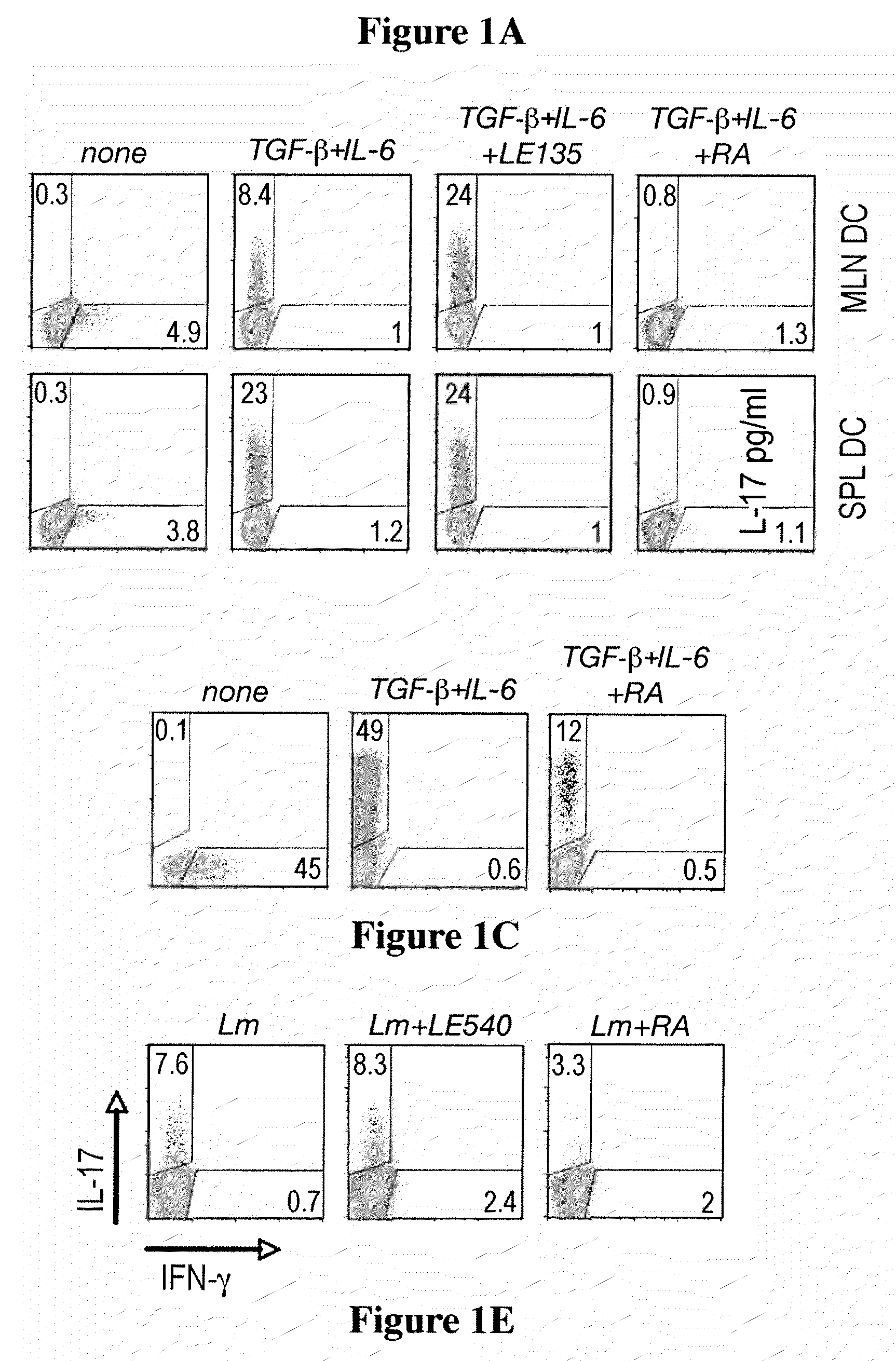
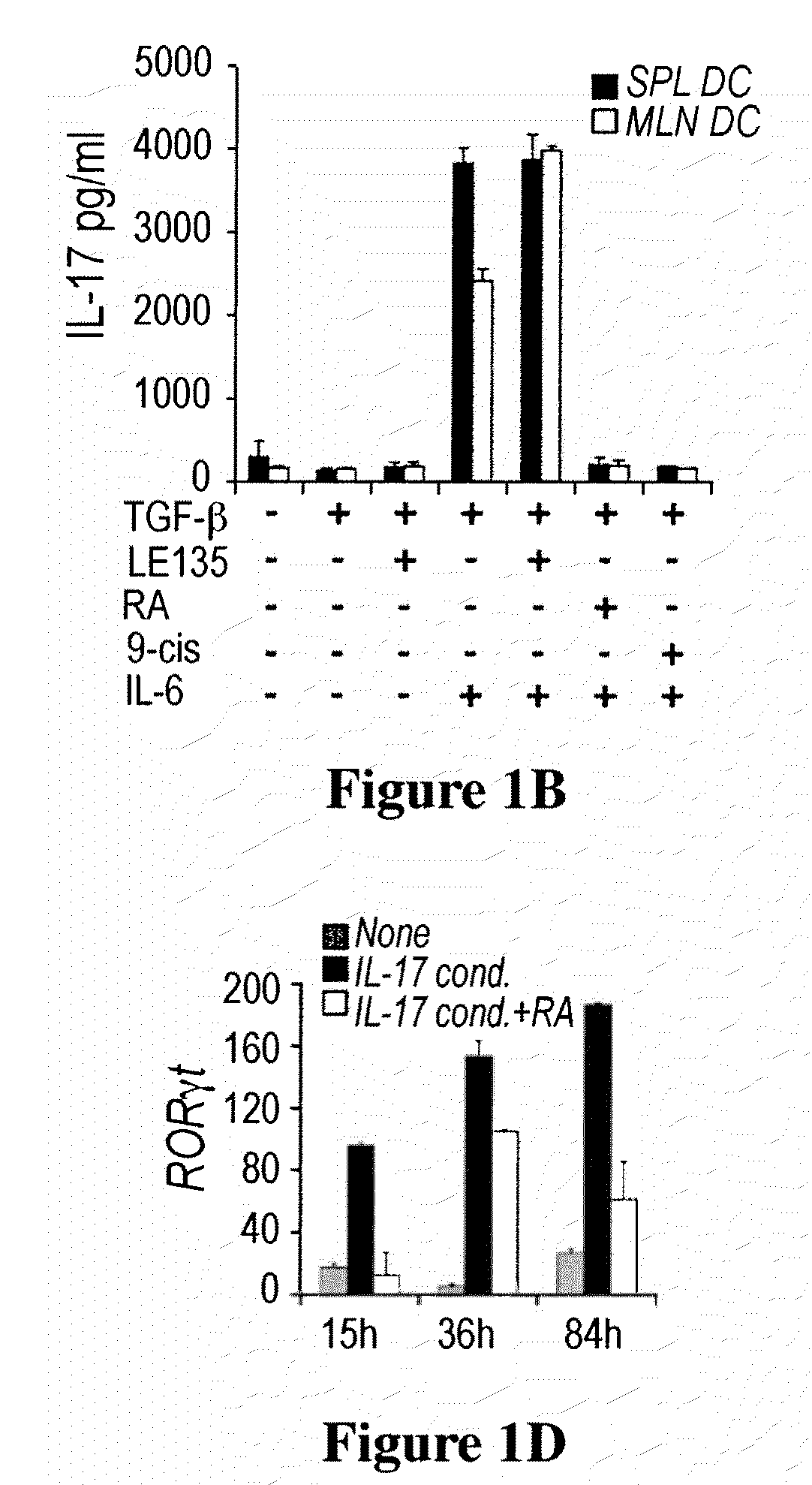
Methods of stimulating or increasing differentiation to regulatory T cells, cultures of regulatory T cells and methods of reducing or decreasing an immune response, inflammation or an inflammatory response, among other things, are provided. Methods include, among other things, contacting blood cells or T cells with an amount of TGF-beta or a TGF-beta analogue and a retinoic acid receptor agonist, or an amount of a retinoid X receptor (RXR) or peroxisome proliferator activated receptor-gamma (PPARgamma) agonist, sufficient to stimulate or increase differentiation to regulatory T cells. Cultures of regulatory T cells include T cells that express a marker associated with regulatory T cells, such as cultures in which regulatory T cells represent, for example, 30% or more of the total number of cells in the culture.
Owner:LA JOLLA INST FOR ALLERGY & IMMUNOLOGY
Peroxisome proliferator-acitvated receptor gamma ligand eluting medical device
Implantable medical devices having an anti-restenotic coatings are disclosed. Specifically, implantable medical devices having coatings of peroxisome proliferator-activated receptor gamma (PPAR.gamma.) agonists are disclosed. The anti-restenotic PPAR.gamma. ligands include thiazolidinedione compounds including ciglitazone. The anti-restenotic medial devices include stents, catheters, micro-particles, probes and vascular grafts. The medical devices can be coated using any method known in the art including compounding the thiazolidinedione with a biocompatible polymer prior to applying the coating. Moreover, medical devices composed entirely of biocompatible polymer-thiazolidinedione blends are disclosed. Additionally, medical devices having a coating comprising at least one thiazolidinedione in combination with at least one additional therapeutic agent are also disclosed. Furthermore, related methods of using and making the anti-restenotic implantable devices are also disclosed.
Owner:MEDTRONIC AVE
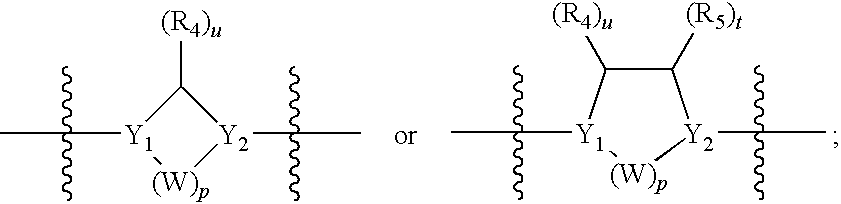
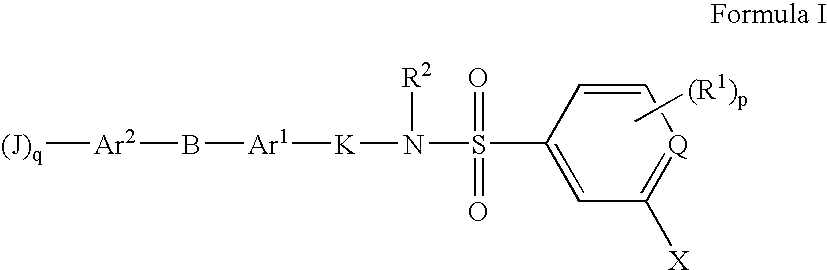
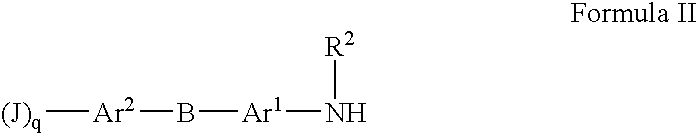
Sulfonyl-Substituted Aryl Compounds as Modulators of Peroxisome Proliferator Activated Receptors
InactiveUS20090143396A1Useful PPAR-modulating activityOrganic active ingredientsOrganic chemistryArylDisease
Compounds as modulators of peroxisome proliferator activated receptors, pharmaceutical compositions comprising the same, and methods of treating disease using the same are disclosed.
Owner:MALECHA JAMES W +3
Dermatological pharmaceutical composition for the treatment of skin inflammation diseases, such as dermatitis, atopic dermatitis, vitiligo, alopecia areata, acne, psoriasis, pruritus or combinations of same
InactiveUS20110129546A1Improve antioxidant capacityGood anti-inflammatory effectBiocideAntimycoticsDiseaseIsoflavones
The invention relates to a dermatological pharmaceutical composition for the treatment of skin inflammation diseases, such as dermatitis, atopic dermatitis, vitiligo, alopecia areata, acne, psoriasis and pruritus. The invention comprises a base anti-inflammatory agent, such as indometacin; one or more optional active ingredients selected alternatively from among at least a corticoid and an antibiotic; and a combination of topical antioxidants used to potentiate the anti-inflammatory effect, selected from among green tea, lipoic acid, curcumin, ascorbyl palmitate, Coenzyme Q10, resveratrol, Pycnogenol™, L-camosine, taurine, vitamin E, vitamin C, papaya extract, isoflavones, manganese, lycopene and quercetin. At least one of the topical antioxidants is a peroxisome proliferator-activated receptor-gamma (PPAR-γ) activator. The invention also includes at least one antioxidant substance with an antiproliferative effect on keratonocytes, e.g. manganese, and at least one substance that blocks tumour necrosis factor-alpha (TNF-α) or other cytokines that provoke the acute phase of the inflammatory reaction, also with an antiproliferative effect, e.g. pentoxifylline.
Owner:UMBERT MILL IGNACIO
Compositions and methods for prophylaxis and treatment of addictions
ActiveUS20090048232A1Reduce the possibilityMany symptomBiocideNervous disorderAmphetamine useReceptor antagonist
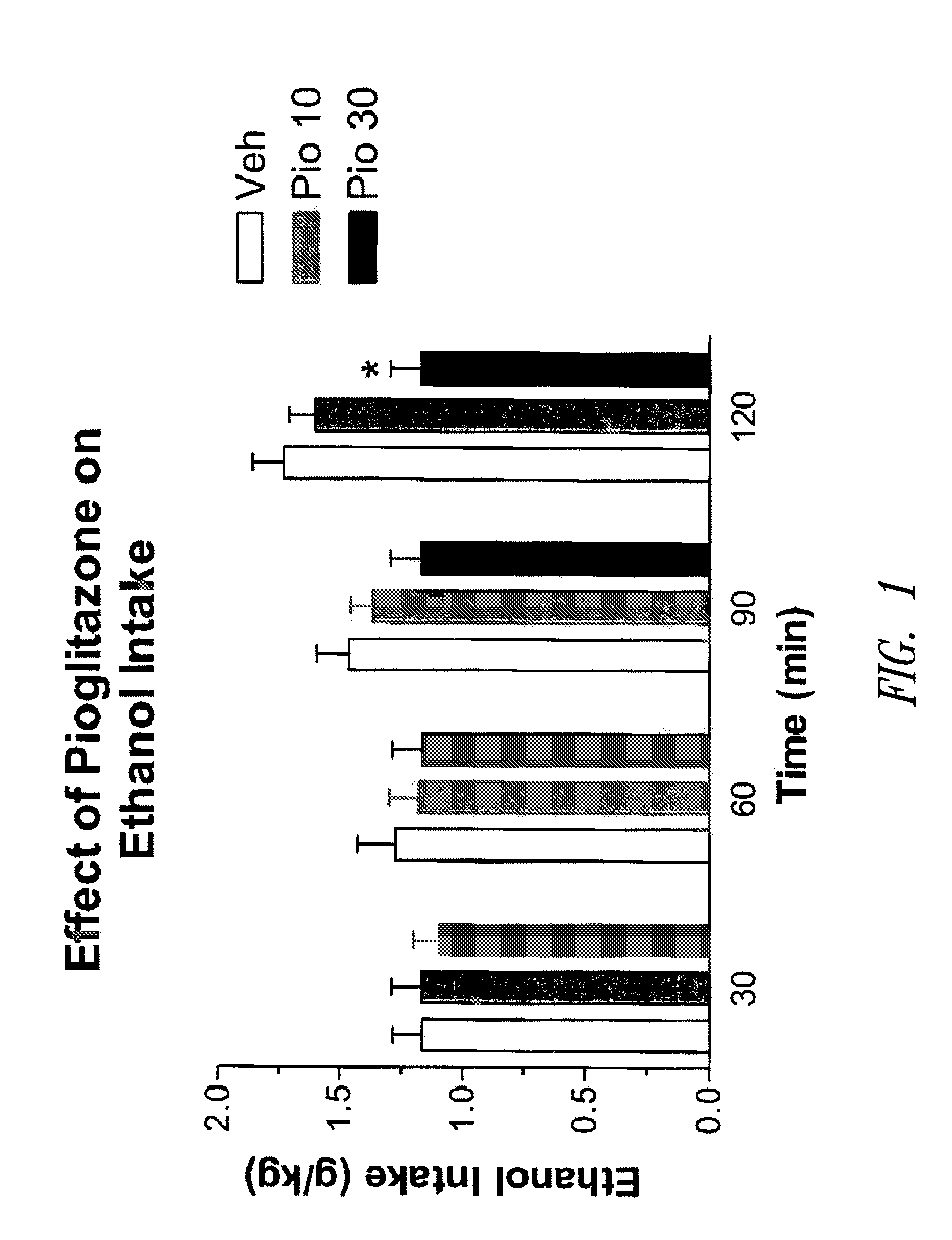
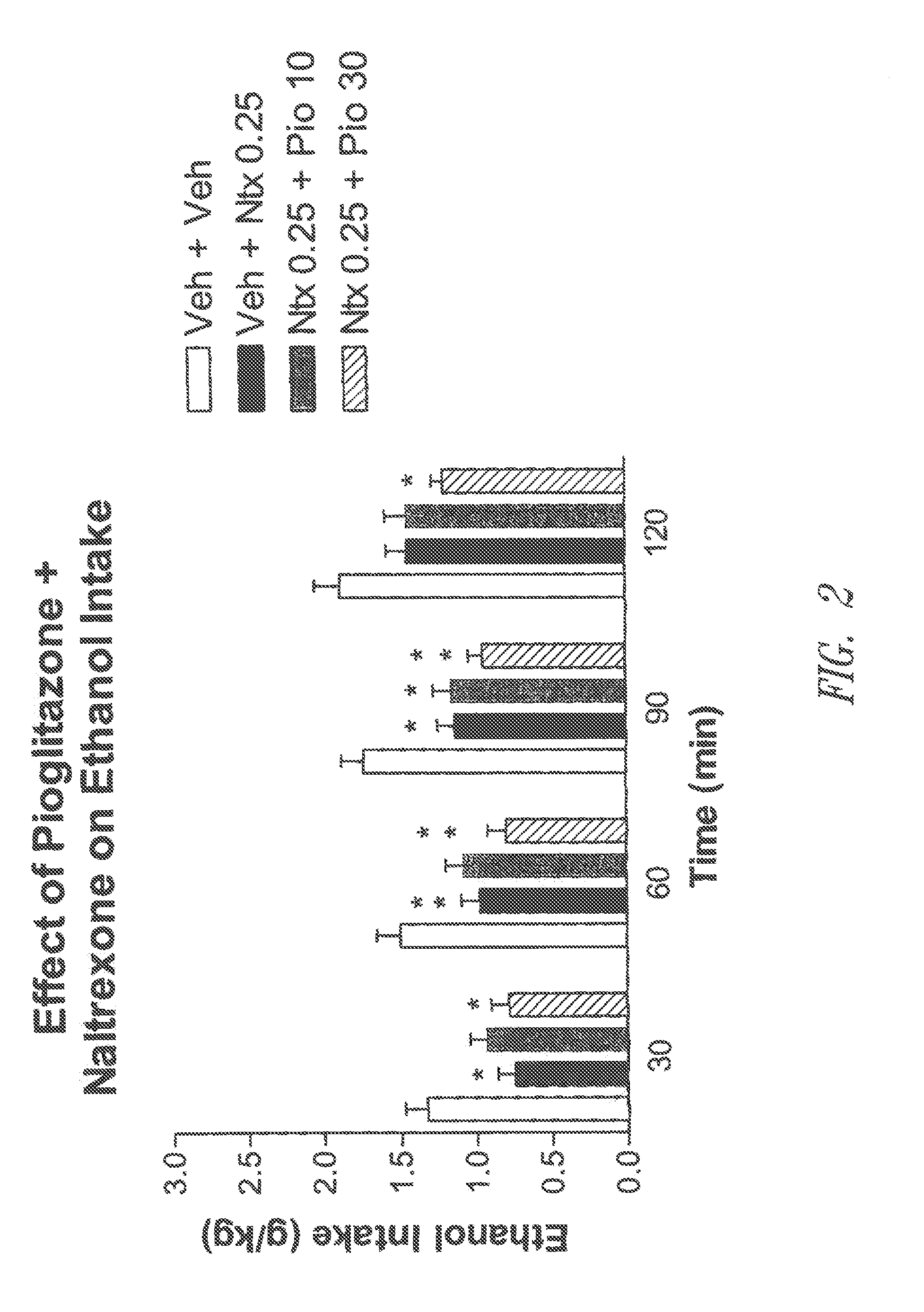
The present invention relates to methods of treating or preventing addiction and relapse use of addictive agents, and treating or preventing addictive or compulsive behaviour and relapse practice of an addictive behaviour or compulsion, by administering a peroxisome proliferator-activated receptor gamma (PPARγ) agonist, alone or in combination with another therapeutic agent, such as, for example, an opioid receptor antagonist or an antidepressant. The present invention also includes pharmaceutical compositions for treating or preventing addiction or relapse that include a PPARγ agonist and one or more other therapeutic agents, as well as unit dosage forms of such pharmaceutical compositions, which contain a dosage effective in treating or preventing addiction or relapse. The methods and compositions of the invention are useful in the treatment or prevention of addiction to any agent, including alcohol, nicotine, marijuana, cocaine, and amphetamines, as well as compulsive and addictive behaviours, including pathological gambling and pathological overeating.
Owner:OMEROS CORP
Use of a plant extract or plant juice
InactiveUS20080131534A1High activityPPARγactivity is increasedBiocideMetabolism disorderRed CloverReceptor for activated C kinase 1
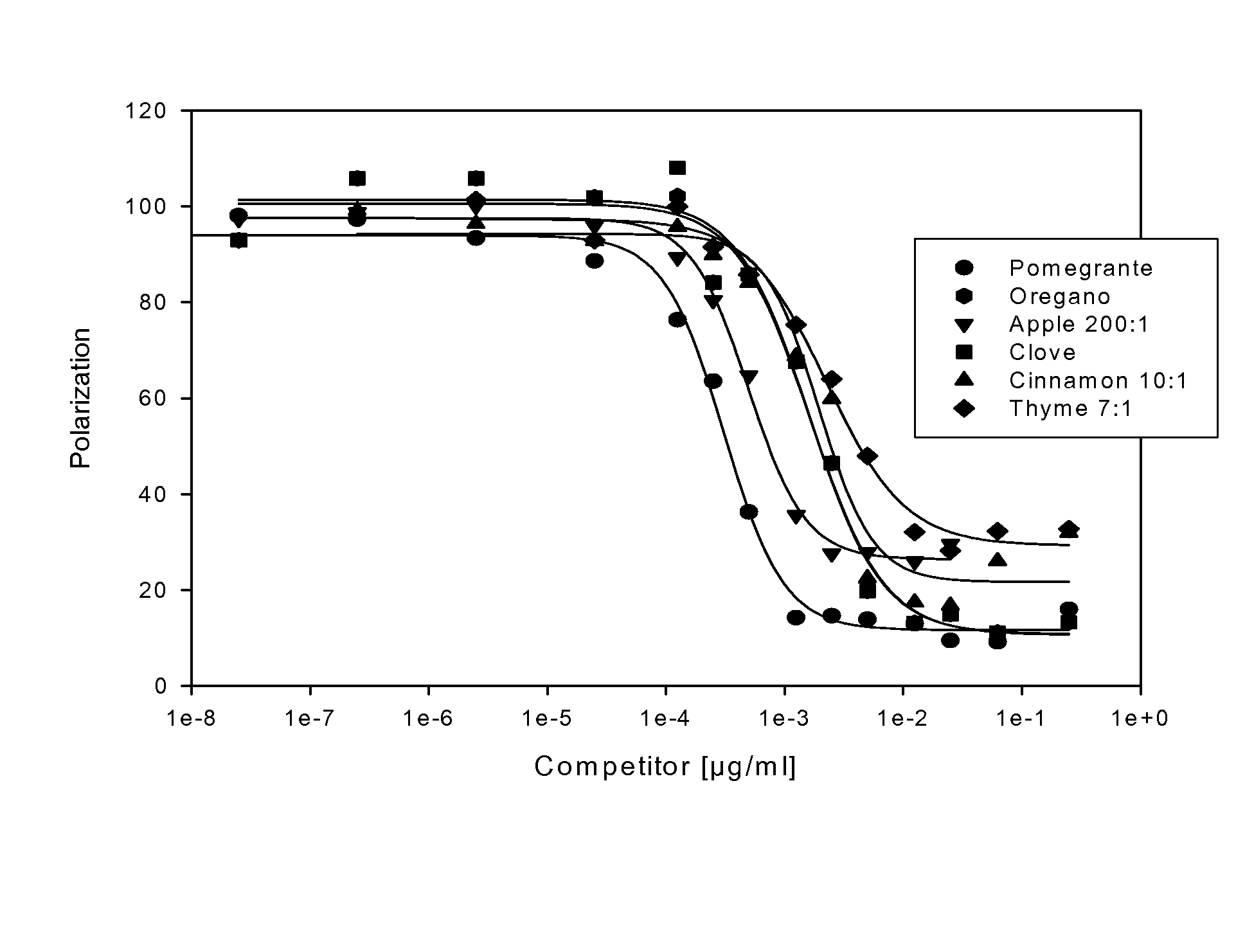
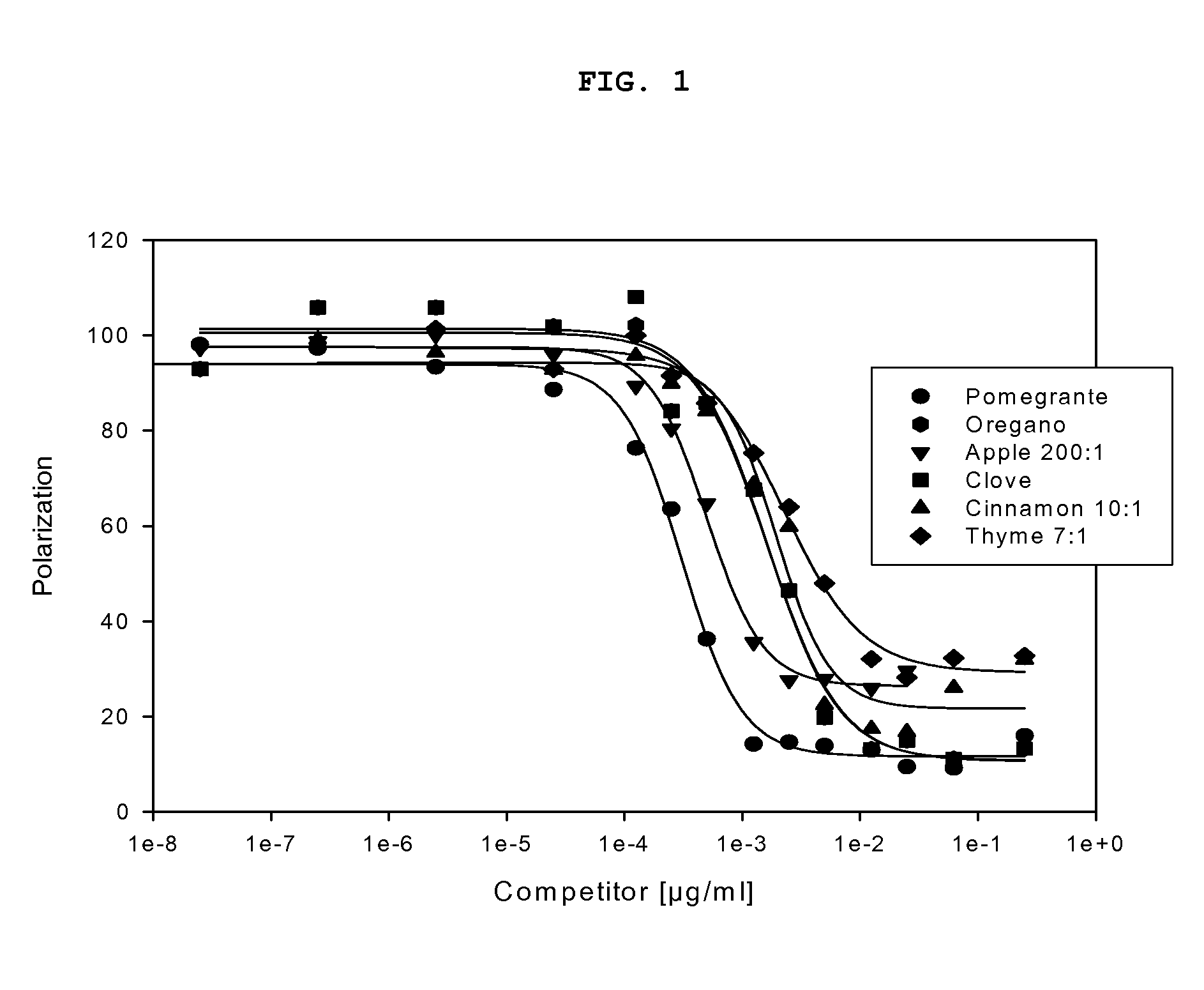
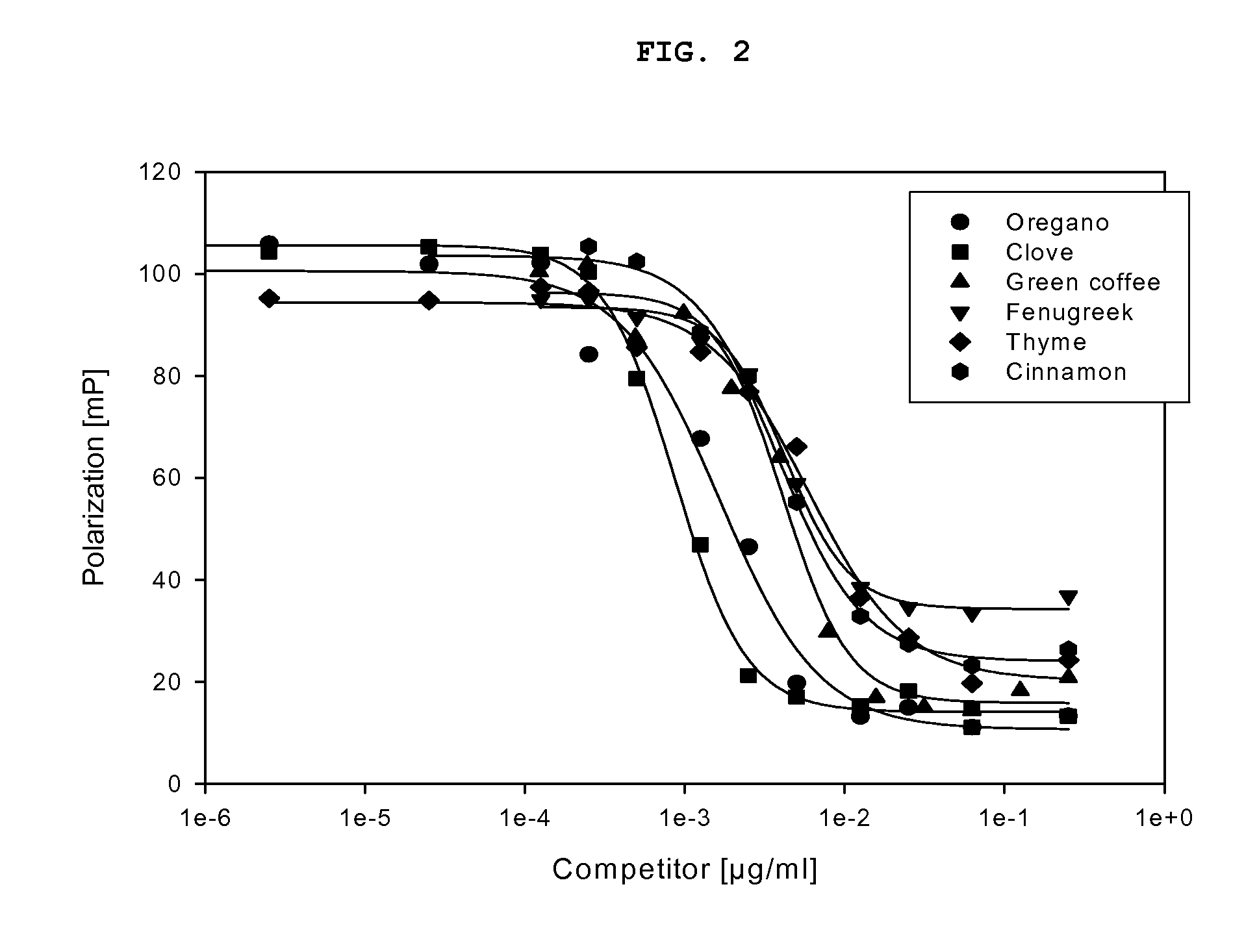
The present invention provides methods for increasing peroxisome proliferator-activated receptor-gamma (PPARγ) activity and / or endothelial nitric oxide synthase (eNOS) activity in a subject by administering to the subject a plant extract or plant juice from thyme, oregano, clove, nutmeg, red clover, bay leaves, red onion or grapes.
Owner:SONN & PARTNER PATENTANWALTE
Substituted Heteroaryl- and Phenylsulfamoyl Compounds
InactiveUS20060229363A1Negative energy balanceExcessive accumulation of triglycerides in liver tissue is prevented or alleviatedBiocideNervous disorderArylTriglyceride
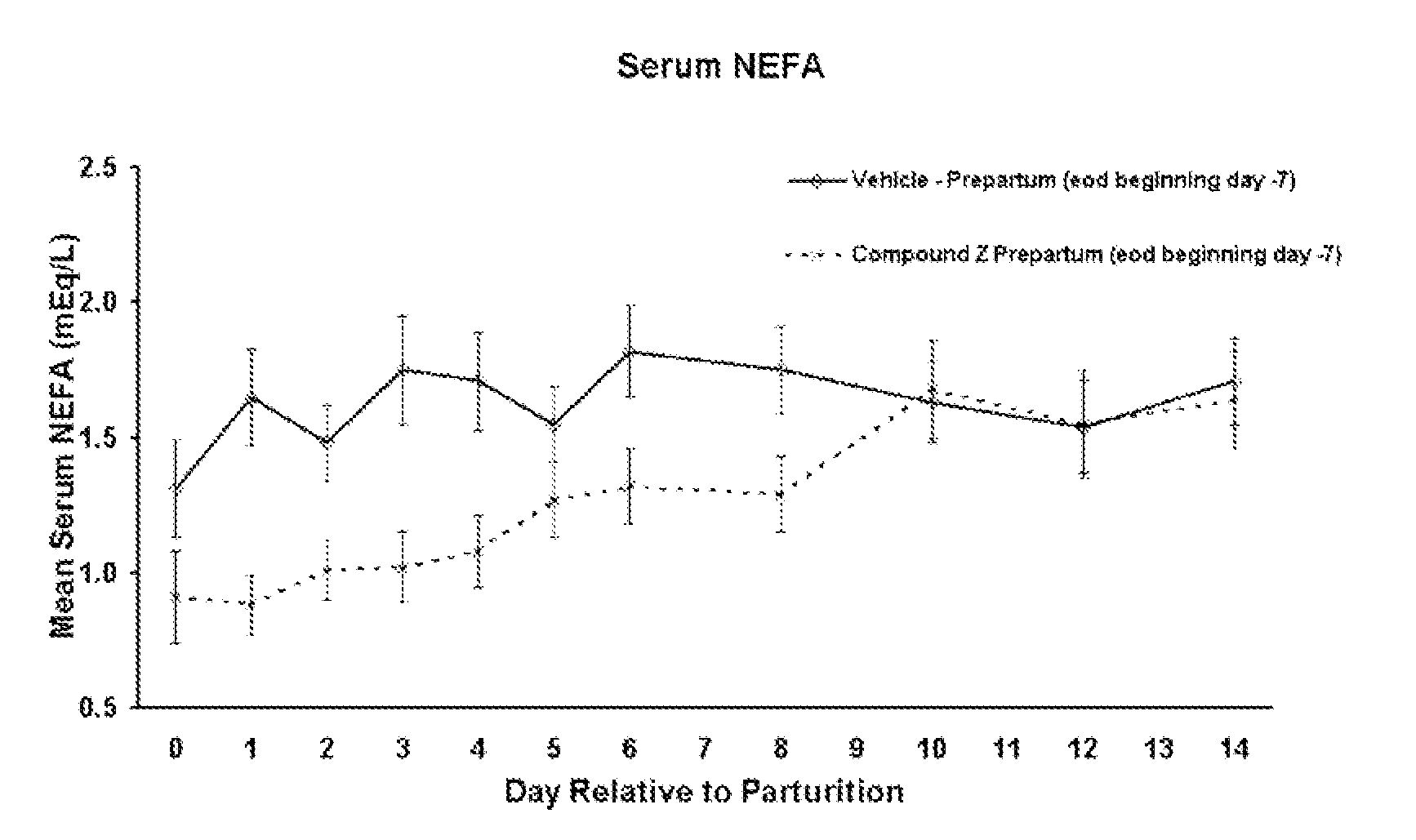
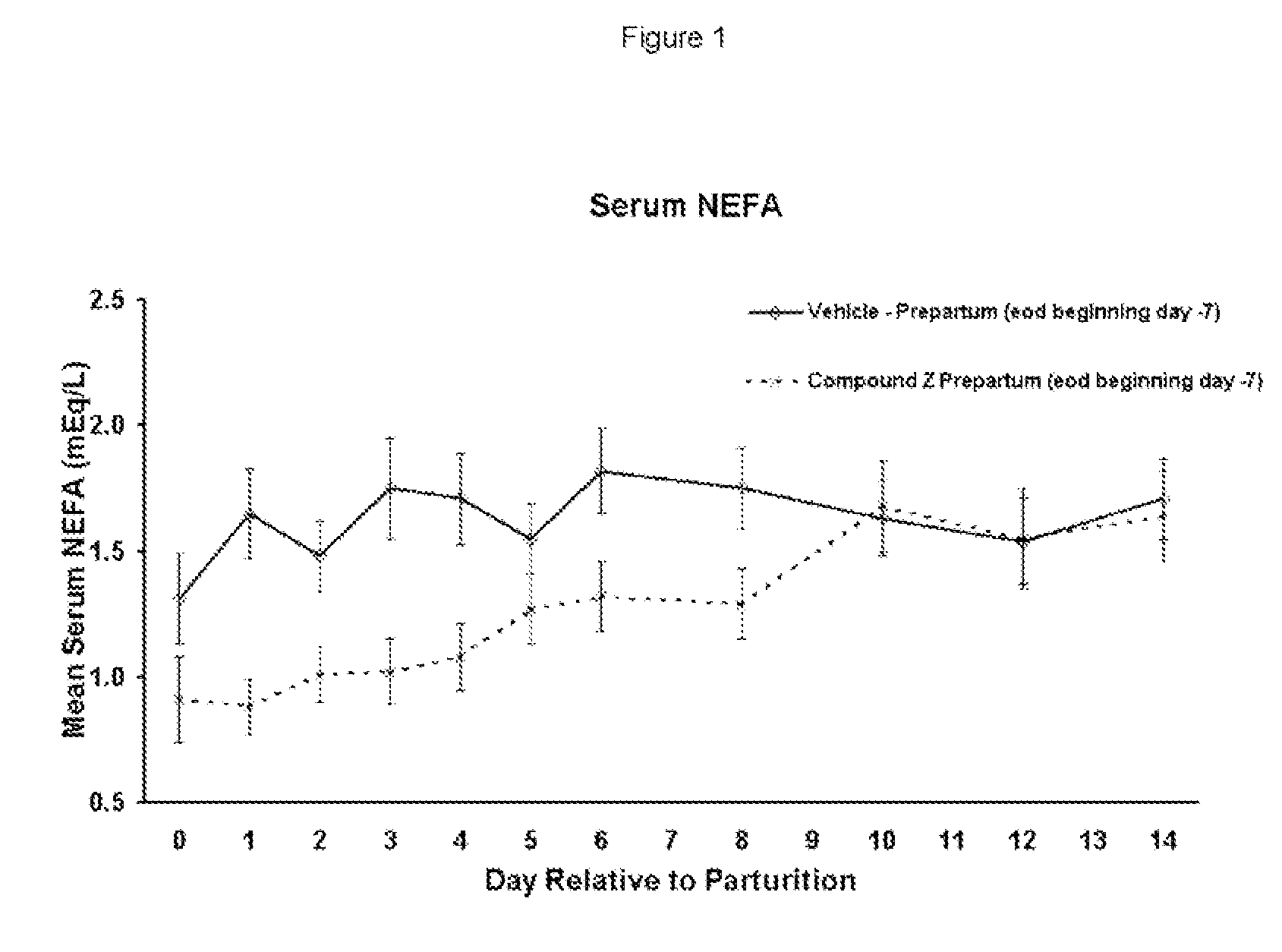
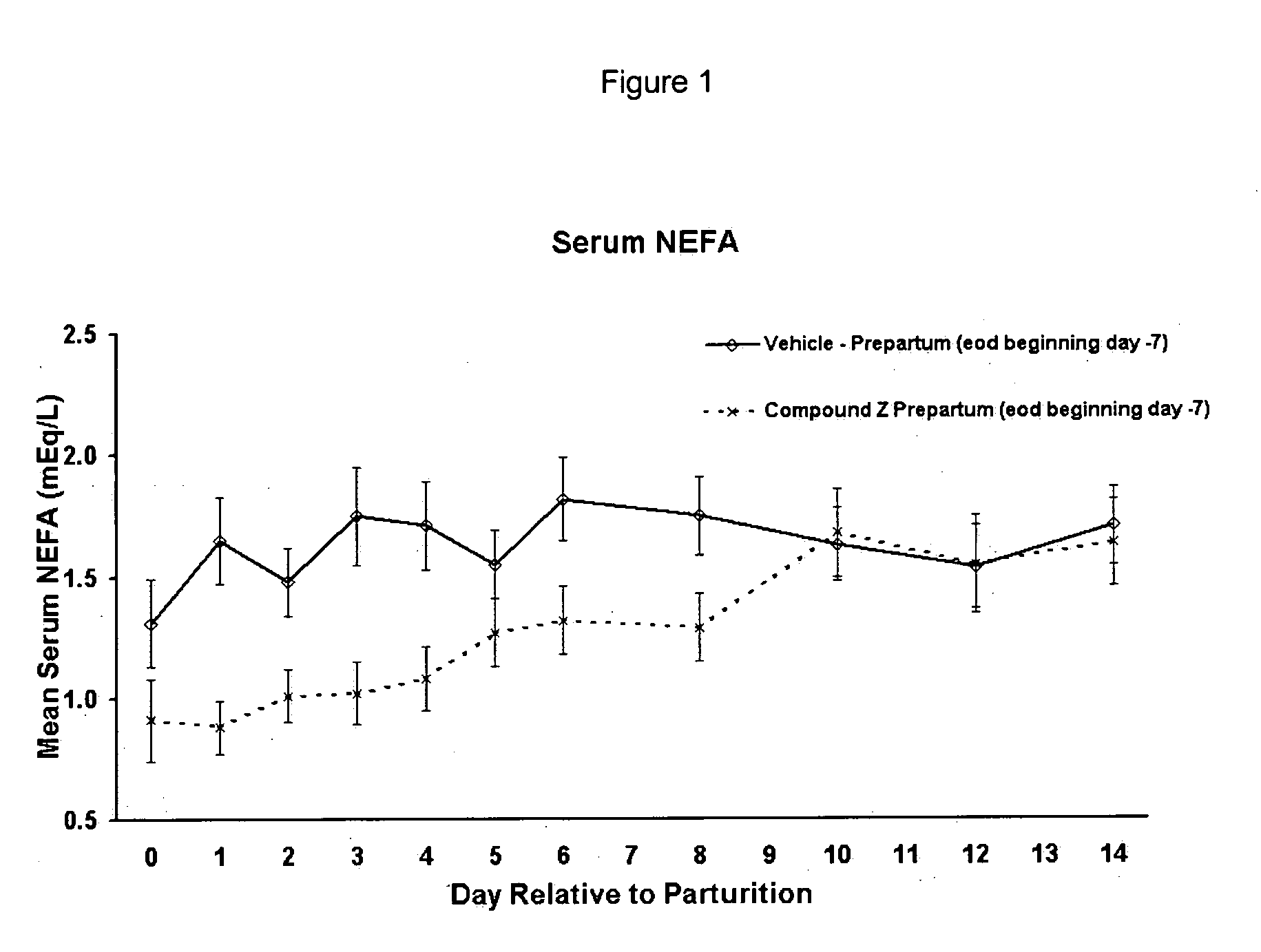
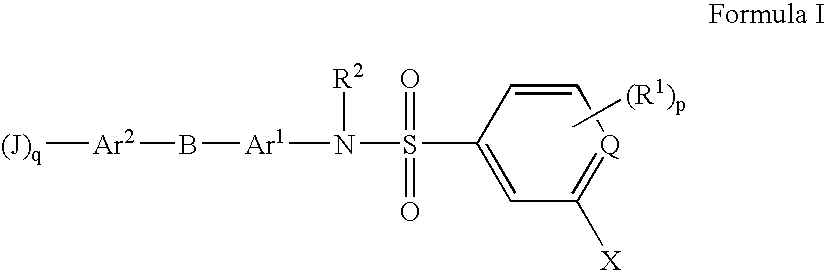
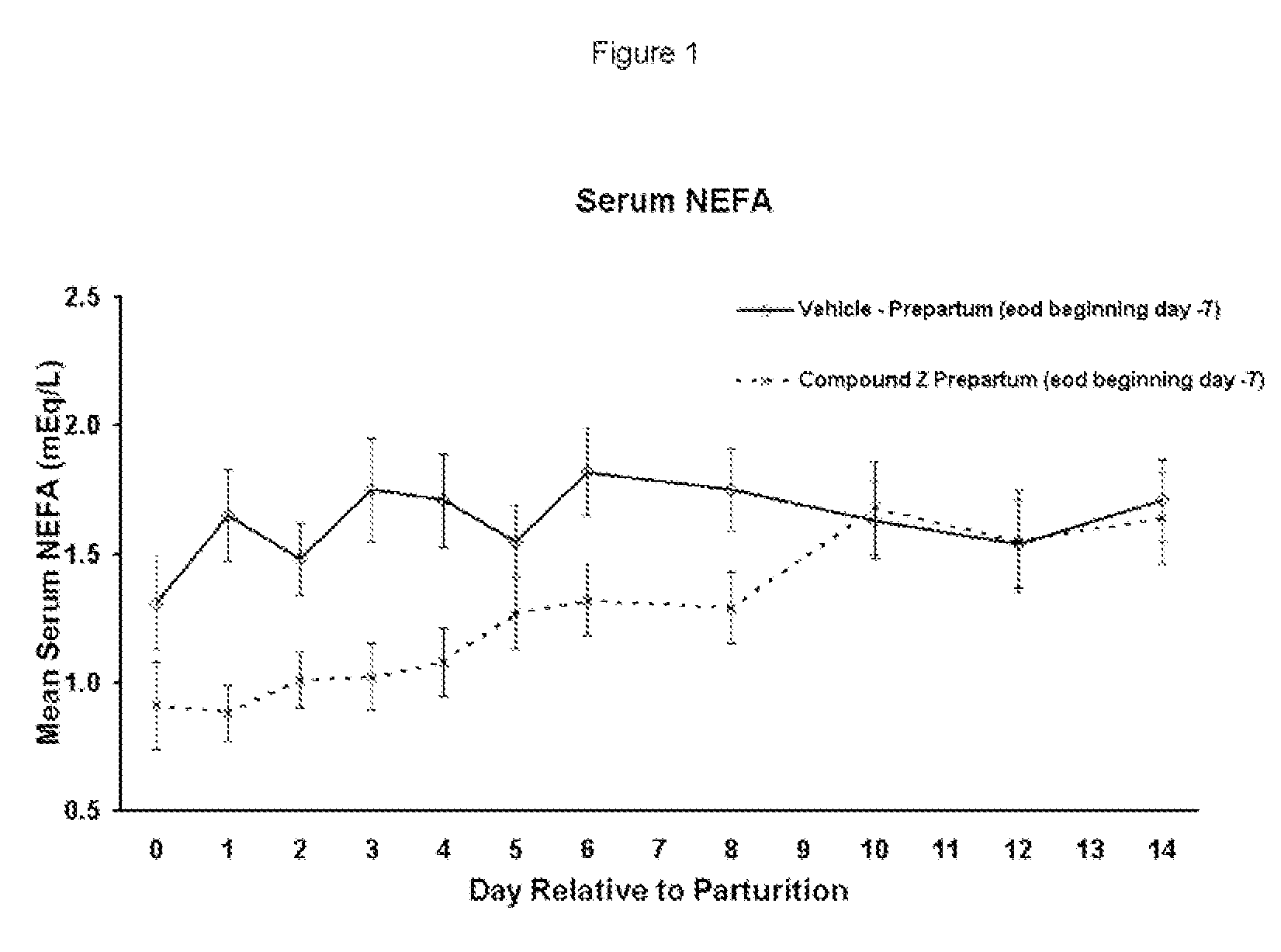
The present invention is directed at substituted heteroaryl and phenylsulfamoyl compounds, pharmaceutical compositions containing such compounds and the use of such compounds as peroxisome proliferator activator receptor (PPAR) agonists. PPAR alpha activators, pharmaceutical compositions containing such compounds and the use of such compounds to elevate certain plasma lipid levels, including high density lipoprotein-cholesterol and to lower certain other plasma lipid levels such as LDL-cholesterol and triglycerides and accordingly to treat diseases which are exacerbated by low levels of HDL cholesterol and / or high levels of LDL-cholesterol and triglycerides such as atherosclerosis and cardiovascular diseases, in mammals, including humans. The compounds are also useful for the treatment of negative energy balance (NEB) and associated diseases in ruminants.
Owner:HAMANAKA ERNEST
Composition and method of treating facial skin defect
InactiveUS20090291066A1Increase differentiationReduce appearance problemsCosmetic preparationsBiocideFacial skinAgonist
This invention relates to a subcutaneous deliverable composition containing an agonist of the peroxisome proliferator-activated receptor-gamma, and a method for treating facial skin defects in a mammalian subject using the subcutaneous deliverable composition.
Owner:PAPPAS APOSTOLOS +2
Substituted heteroaryl- and phenylsulfamoyl compounds
InactiveUS20050228015A1Increase ruminant milk quality milkIncrease milk milk yieldBiocideCosmetic preparationsTriglyceridePeroxisome proliferator
The present invention is directed at substituted heteroaryl- and phenylsulfamoyl compounds, pharmaceutical compositions containing such compounds and the use of such compounds as peroxisome proliferator activator receptor (PPAR) agonists. PPAR alpha activators, pharmaceutical compositions containing such compounds and the use of such compounds to elevate certain plasma lipid levels, including high density lipoprotein-cholesterol and to lower certain other plasma lipid levels, such as LDL-cholesterol and triglycerides and accordingly to treat diseases which are exacerbated by low levels of HDL cholesterol and / or high levels of LDL-cholesterol and triglycerides, such as atherosclerosis and cardiovascular diseases, in mammals, including humans. The compounds are also useful for the treatment of negative energy balance (NEB) and associated diseases in ruminants.
Owner:PFIZER INC +1
Compositions, Kits, and Methods for Identification, Assessment, Prevention, and Therapy of Metabolic Disorders
ActiveUS20130019326A1Reduce the amount of solutionHigh activityPeptide/protein ingredientsLibrary screeningPhosphorylationBiological activation
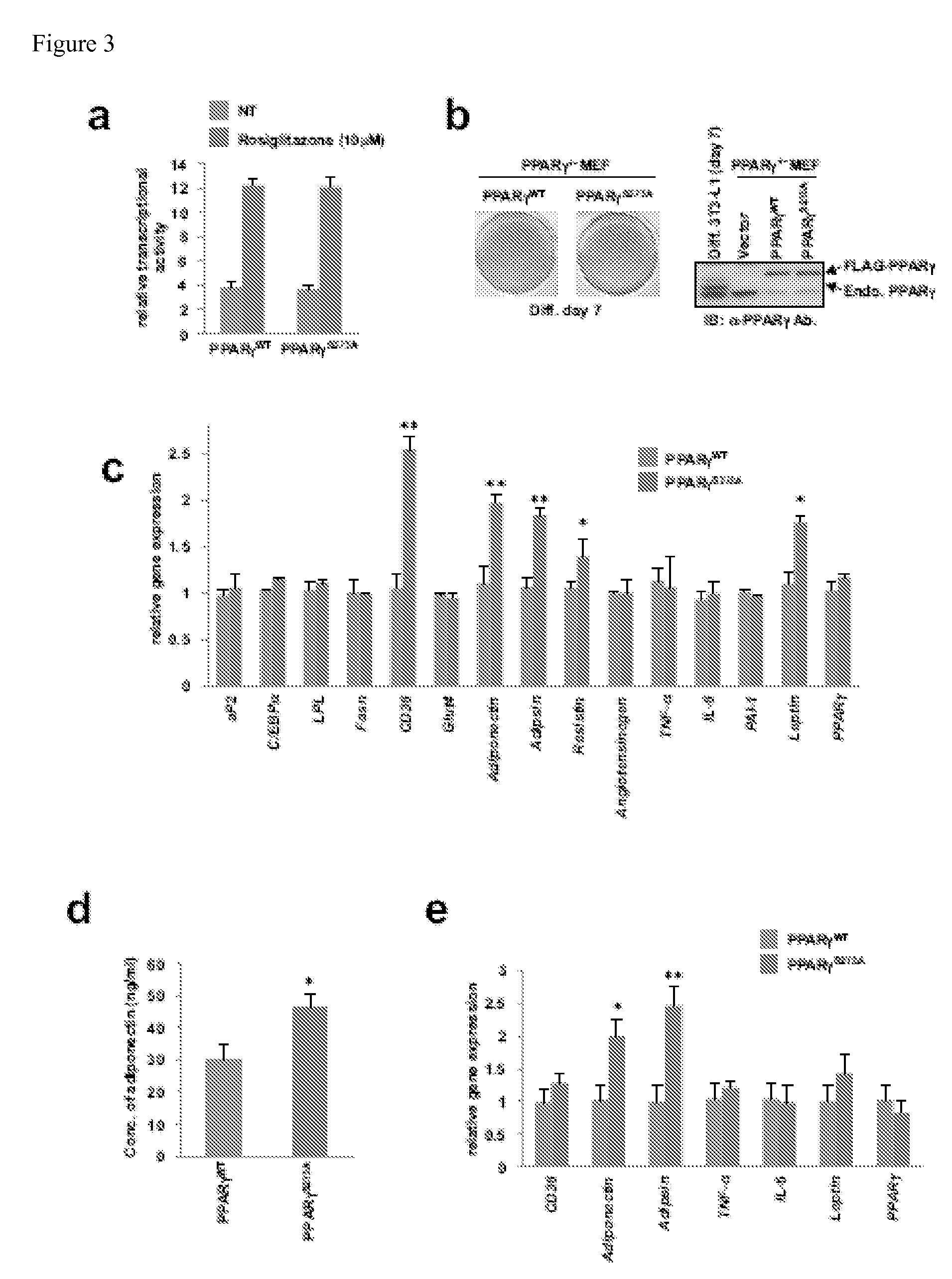
The invention provides methods and compositions for selectively promoting anti-metabolic disorder activity over classical PPAR gamma activation through modulation of PPAR gamma phosphorylation (e.g., Ser-273 phosphorylation of murine peroxisome proliferator activated receptor gamma (PPAR gamma) 2 or a corresponding serine residue in a murine PPAR gamma 2 homolog, including a human). Also provided are methods for preventing, treating, or predictiving responsiveness of therapies for metabolic disorders in a subject through selective inhibition of such PPAR gamma phosphorylation. Further provided are methods for identifying compounds that are capable of modulating such PPAR gamma phosphorylation.
Owner:DANA FARBER CANCER INST INC
Dermatological pharmaceutical composition for the treatment of skin inflammation diseases, such as dermatitis, atopic dermatitis, vitiligo, alopecia areata, acne, psoriasis, pruritus or combinations of same
The invention relates to a dermatological pharmaceutical composition for the treatment of skin inflammation diseases, such as dermatitis, atopic dermatitis, vitiligo, alopecia areata, acne, psoriasis and pruritus. The invention comprises a basic anti-inflammatory agent, such as indometacin; one or more optional active principles selected alternatively from among at least a corticoid and an antibiotic; and a combination of topical antioxidants used to potentiate the anti-inflammatory effect, selected from among green tea, lipoic acid, curcumin, ascorbyl palmitate, Coenzyme Q10, resveratrol, Pycnogenol TM , L-camosine, taurine, vitamin E, vitamin C, papaya extract, isoflavones, manganese, lycopene and quercetin. At least one of the topical antioxidants is a peroxisome proliferator-activated receptor-gamma (PPAR- gamma) activator. The invention also includes at least one antioxidant substance with an antiproliferative effect on keratonocytes, e.g. manganese, and at least one substance that blocks tumour necrosis factor-alpha (TNF- alpha) or other cytokines that provoke the acute phase of the inflammatory reaction, also with an antiproliferative effect, e.g. pentoxifylline.
Owner:伊格纳西奥安贝尔特米列特
Agents derived from holoptelea integrifolia and their compositions for the control of metabolic syndrome and associated diseases
ActiveUS20120231095A1BiocideHydroxy compound active ingredientsDiseaseAdipose Differentiation-Related Protein
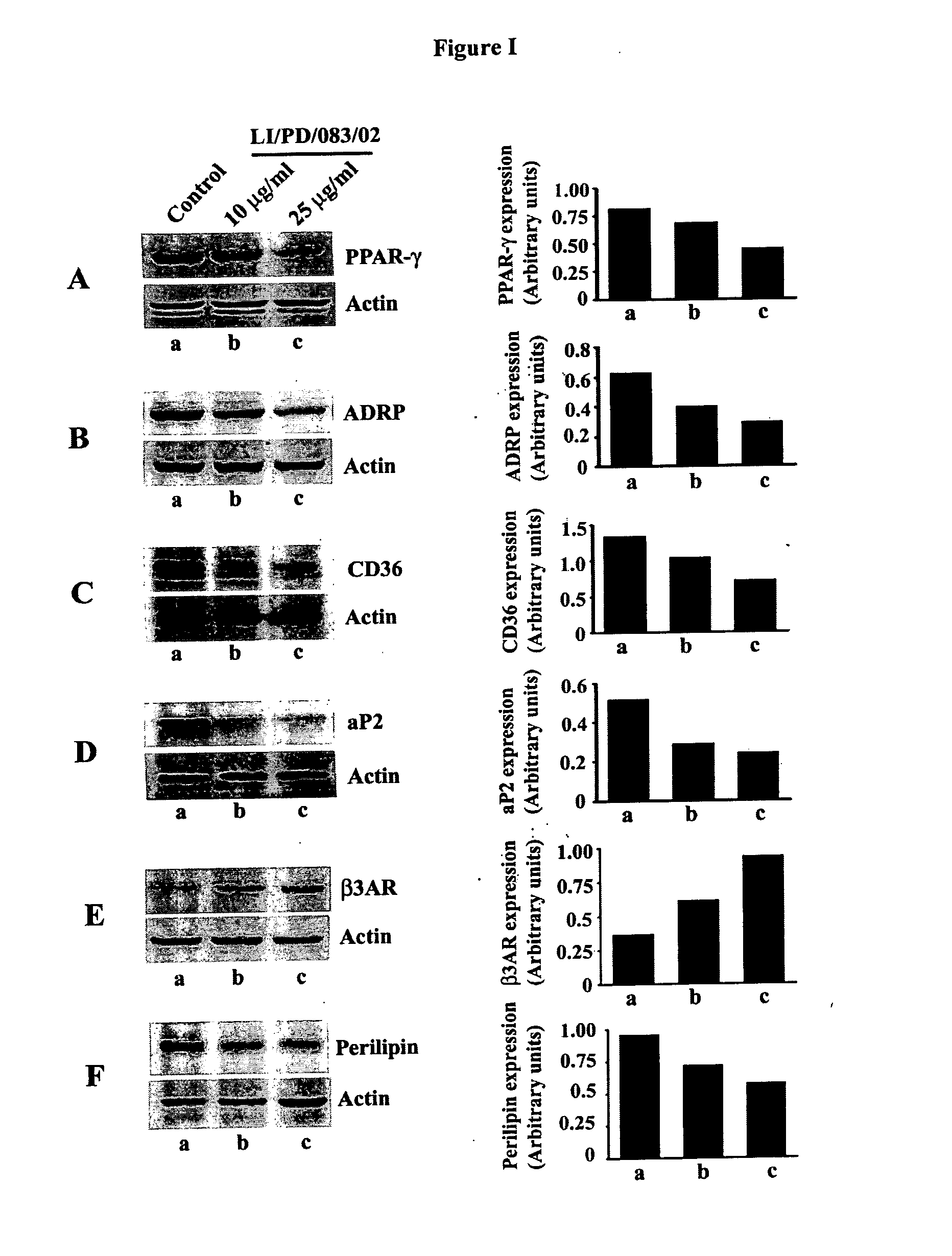
The invention discloses phytochemical agents derived from Holoptelea integrifolia and novel composition(s) comprising at least one component selected from the extract(s), fraction(s) and active compound(s) for the protection and alleviation of Metabolic Syndrome, insulin resistance, endothelial dysfunction, chronic kidney disease, atherosclerosis, diabetes and other disease conditions associated with metabolic syndrome. The invention also discloses the amelioration of certain biomarker molecules such as Peroxisome proliferator-activated receptor gamma (PPAR-γ), Adipose Differentiation Related Protein (ADRP), CD36, Adipocyte Fatty-acid-Binding Protein (aP2 / FABP-4 / A-FABP), beta-3 Adrenergic Receptor (β3AR), Leptin, Perilipin and Adiponectin by using the phytochemical components derived from Holoptelea integrifolia.
Owner:LAILA NUTRACEUTICALS
Substituted Heteroaryl- and Phenylsulfamoyl Compounds
InactiveUS20060258723A1Negative energy balanceExcessive accumulation of triglycerides in liver tissue is prevented or alleviatedBiocideNervous disorderMammalBlood plasma
The present invention is directed at substituted heteroaryl and phenylsulfamoyl compounds, pharmaceutical compositions containing such compounds and the use of such compounds as peroxisome proliferator actuator receptor (PPAR) agonists. PPAR alpha activators, pharmaceutical compositions containing such compounds and the use of such compounds to elevate certain plasma lipid levels, including high density lipoprotein-cholesterol and to lower certain other plasma lipid levels, such as LDL-cholesterol and triglycerides and accordingly to treat diseases which are exacerbated by low levels of HDL cholesterol and / or high levels of LDL-cholesterol and triglycerides, such as atherosclerosis and cardiovascular diseases, in mammals, including humans. The compounds are also useful for the treatment of negative energy balance (NEB) and associated diseases in ruminants.
Owner:PFIZER INC
RNA interference carrier used for osteoporosis and rat mesenchymal stem cells
InactiveCN101555487AGood experimental platformGenetic material ingredientsSkeletal disorderEukaryotic plasmidsA-DNA
The invention relates to a RNA interference carrier used for osteoporosis and mesenchymal stem cells. The RNA interference carrier is constructed by introducing a DNA fragment which uses peroxisome proliferator-activated receptor Gamma (PPAR Gamma) as an action target and can express short chain RNA (shRNA) so as to build a slow virus expression carrier in a framework plasmid expressed based on the short chain RNA of slow virus; the constructed RNA interference carrier can express a special short chain RNA aiming at the PPAR Gamma, thereby reducing the expression of the PPAR Gamma. The invention also provides rat mesenchymal stem cells transfected by the RNA interference carrier. The invention not only provides a new way for preparing the medicine for treating the osteoporosis, but also provides a better experiment platform for the development of other stem cell-origin cell therapy and tissue engineering products.
Owner:GUANGDONG PHARMA UNIV
Composition comprising peroxisome proliferator-activated receptor-gamma
InactiveUS20130331332A1Increase volumeGood effectCosmetic preparationsPeptide/protein ingredientsDermatologyPeroxisome proliferators
An injectable composition comprising peroxisome proliferator-activated receptor-gamma for subcutaneous administration and a method for improving imperfections of the skin, wherein the injectable composition is subcutaneously administered at the area of skin imperfections comprising the steps: a) identifying an area of skin imperfections, b) administering a safe and cosmetically effective amount of the composition subcutaneously to the area of skin imperfections.
Owner:MERZ PHARMA GMBH & CO KGAA
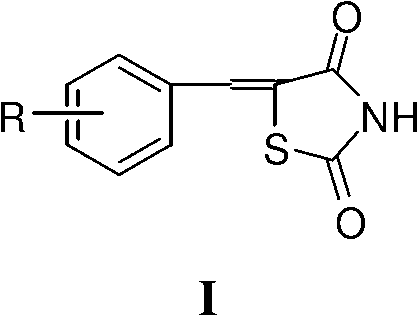
Application of 5- aryl (heterocycle) methylenethiazolidine-2,4-dione in preparation of PPAR (Peroxisome Proliferator Activated Receptor) agonist
InactiveCN102727489APossesses PPAR agonistic activityAgonistic activityOrganic active ingredientsAntimycoticsDiabetes mellitusAryl
The invention provides application of 5-arylmethylenethiazolidine-2,4-dione shown as the general formula I and 5-heterocyclemethylenethiazolidine-2,4-dione shown as the general formula II in preparation of a PPAR (Peroxisome Proliferator Activated Receptor) agonist, wherein 5-arylmethylenethiazolidine-2,4-dione and 5-heterocyclemethylene-thiazolidine-2,4-dione both has PPAR agonistic activity, the relative agonist ratio of a part of compounds to pioglitazone which is an existing PPARgamma agonist is higher than 100% and can be 239.77% at most, and therefore the part of compounds can be developed into high-efficiency low-toxicity antidiabetic drug possibly or can be used as antidiabetic leading molecules to be applied to the further structural optimization.
Owner:SOUTHWEST UNIVERSITY
Use of peroxisome proliferator-activated receptor gamma (ppary) and/or retinoic acid receptor (rxr) agonists to inhibit platelet functions
InactiveUS20070135382A1Inhibits platelet aggregationEffectively control levelBiocideElcosanoid active ingredientsWhole blood productThrombus
Methods of inhibiting mammalian platelet release of CD40 ligand, thromboxanes, or prostaglandin E2, or surface expression of CD40 ligand that involve contacting mammalian platelets with an effective amount of a PPARγ agonist, an RXR agonist, or a combination thereof. As a consequence of inhibiting CD40 ligand and thromboxane release, the present invention allows for inhibition of thrombus fon-nation by (or clotting activities of) activated platelets, as well as treating or preventing CD40 ligand-mediated conditions and / or thromboxane-mediated conditions. Use of PPARγ agonist, RXR agonist, and / or inducers of PPARγ agonist in preparing a stored blood product, and for diagnostic testing of patient samples is also disclosed.
Owner:UNIVERSITY OF ROCHESTER
Compositions and methods for prophylaxis and treatment of addictions
InactiveUS20100234413A1Many symptomReduce oneBiocideNervous disorderAmphetamine useCompulsive behaviour
The present invention relates to methods of treating or preventing addiction and relapse use of addictive agents, and treating or preventing addictive or compulsive behaviour and relapse practice of an addictive behaviour or compulsion, by administering a peroxisome proliferator-activated receptor gamma (PPARγ) agonist, alone or in combination with another therapeutic agent, such as, for example, an opioid receptor antagonist or an antidepressant, or an addictive agent, such as, for example, an opioid agonist. The present invention also includes pharmaceutical compositions for treating or preventing addiction or relapse that include a PPARγ agonist and one or more other therapeutic or addictive agents, as well as unit dosage forms of such pharmaceutical compositions, which contain a dosage effective in treating or preventing addiction or relapse. The methods and compositions of the invention are useful in the treatment or prevention of addiction to any agent, including alcohol, nicotine, marijuana, cocaine, and amphetamines, as well as compulsive and addictive behaviours, including pathological gambling and pathological overeating.
Owner:OMEROS CORP
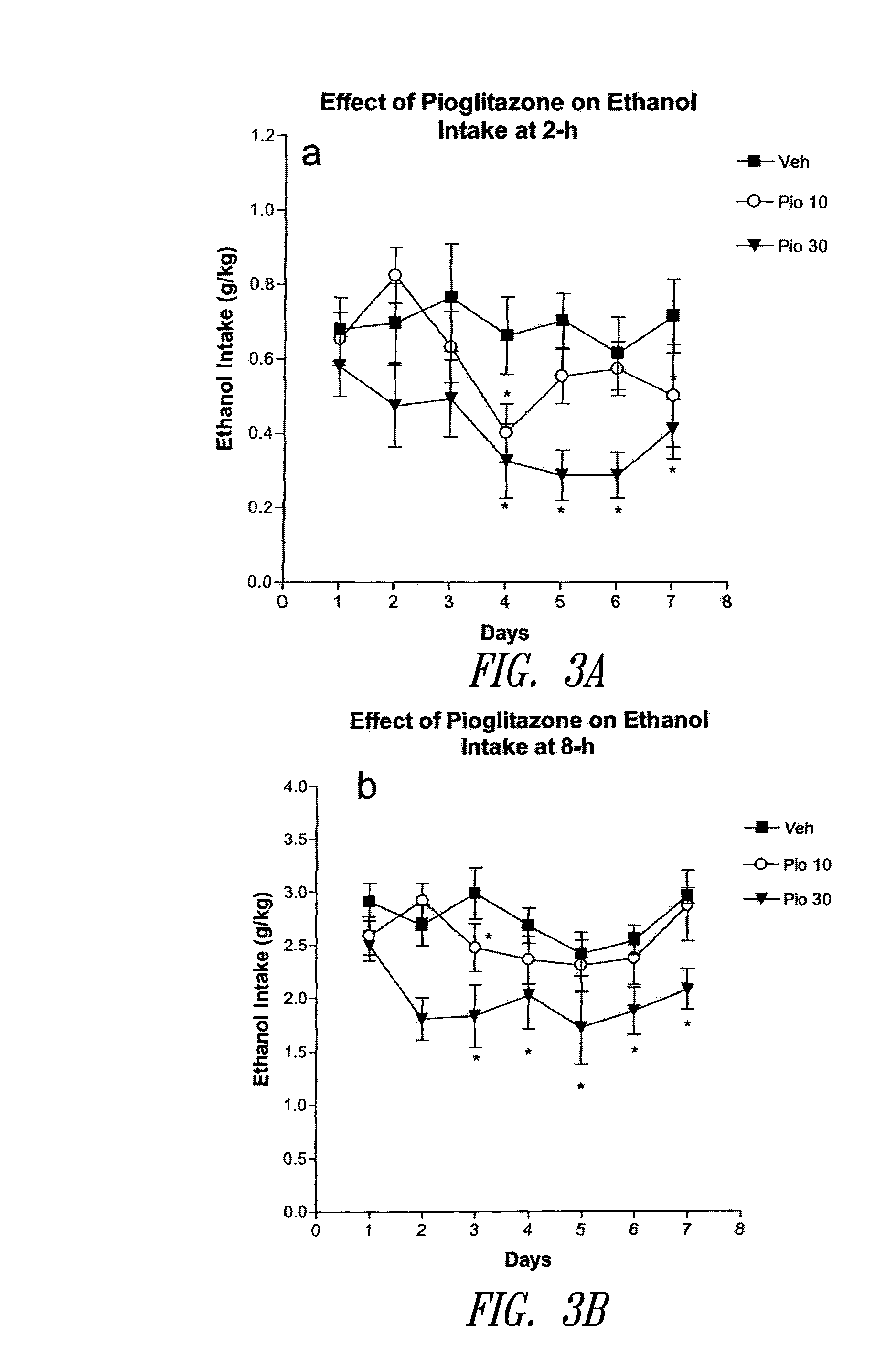
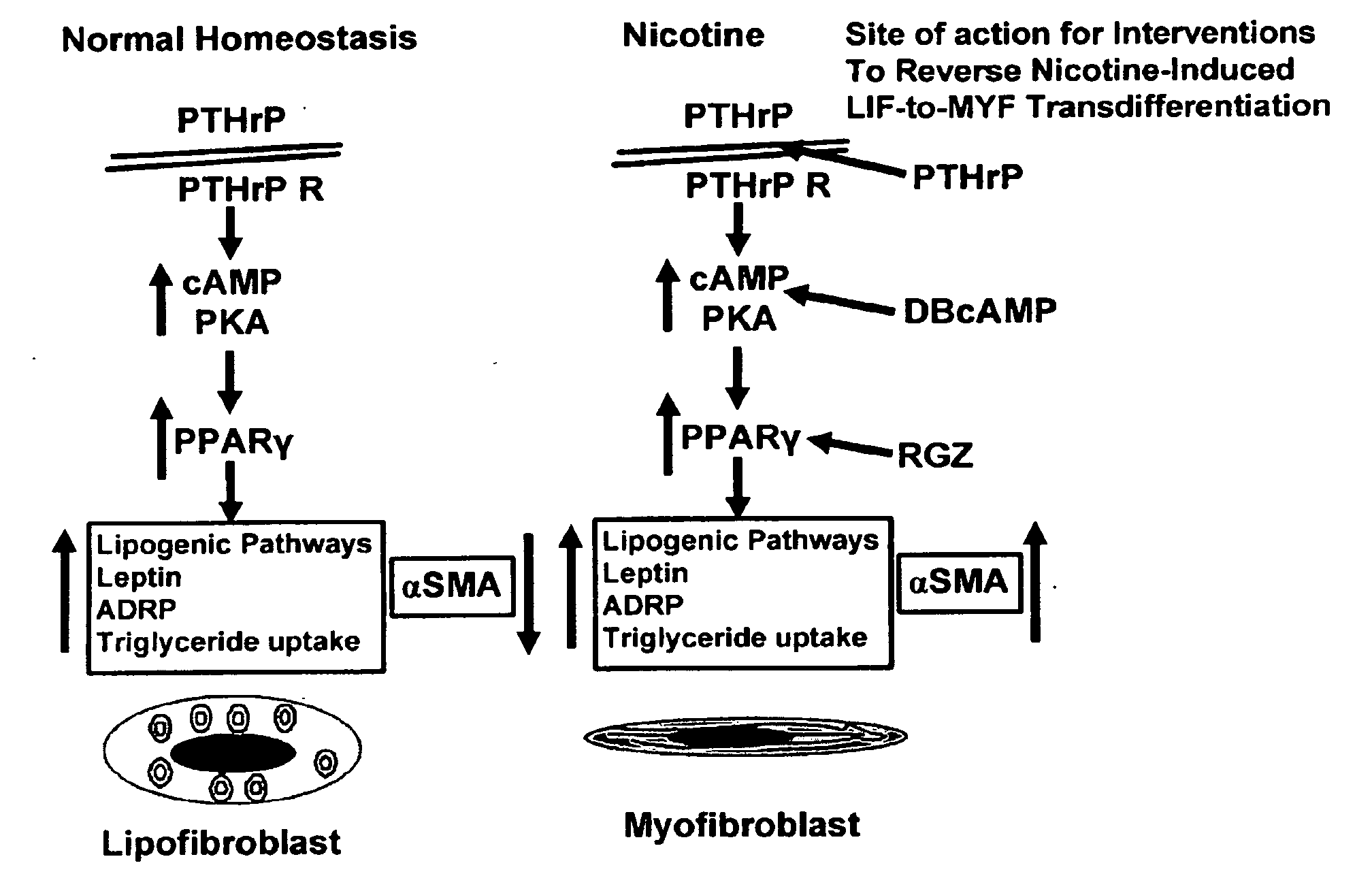
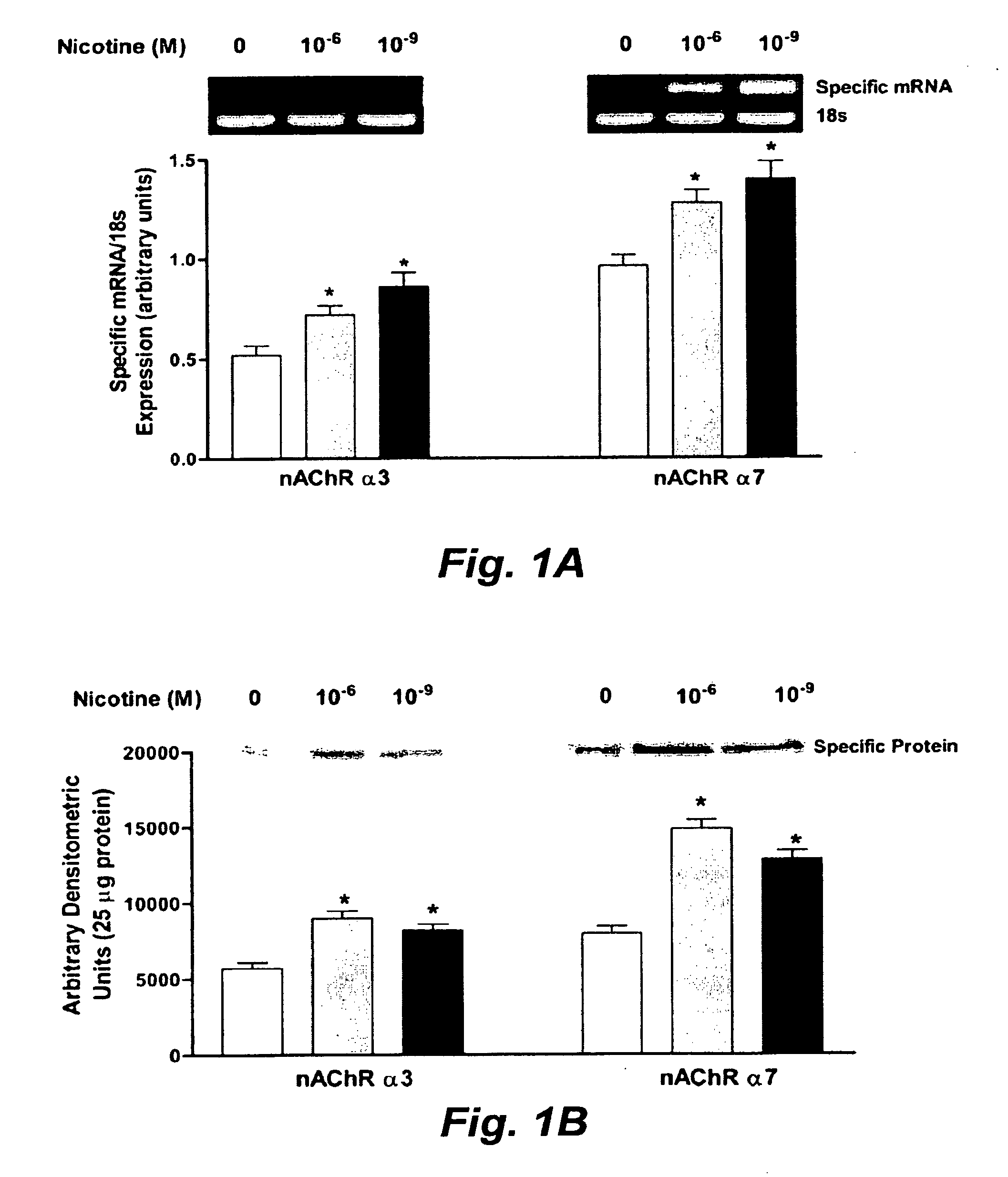
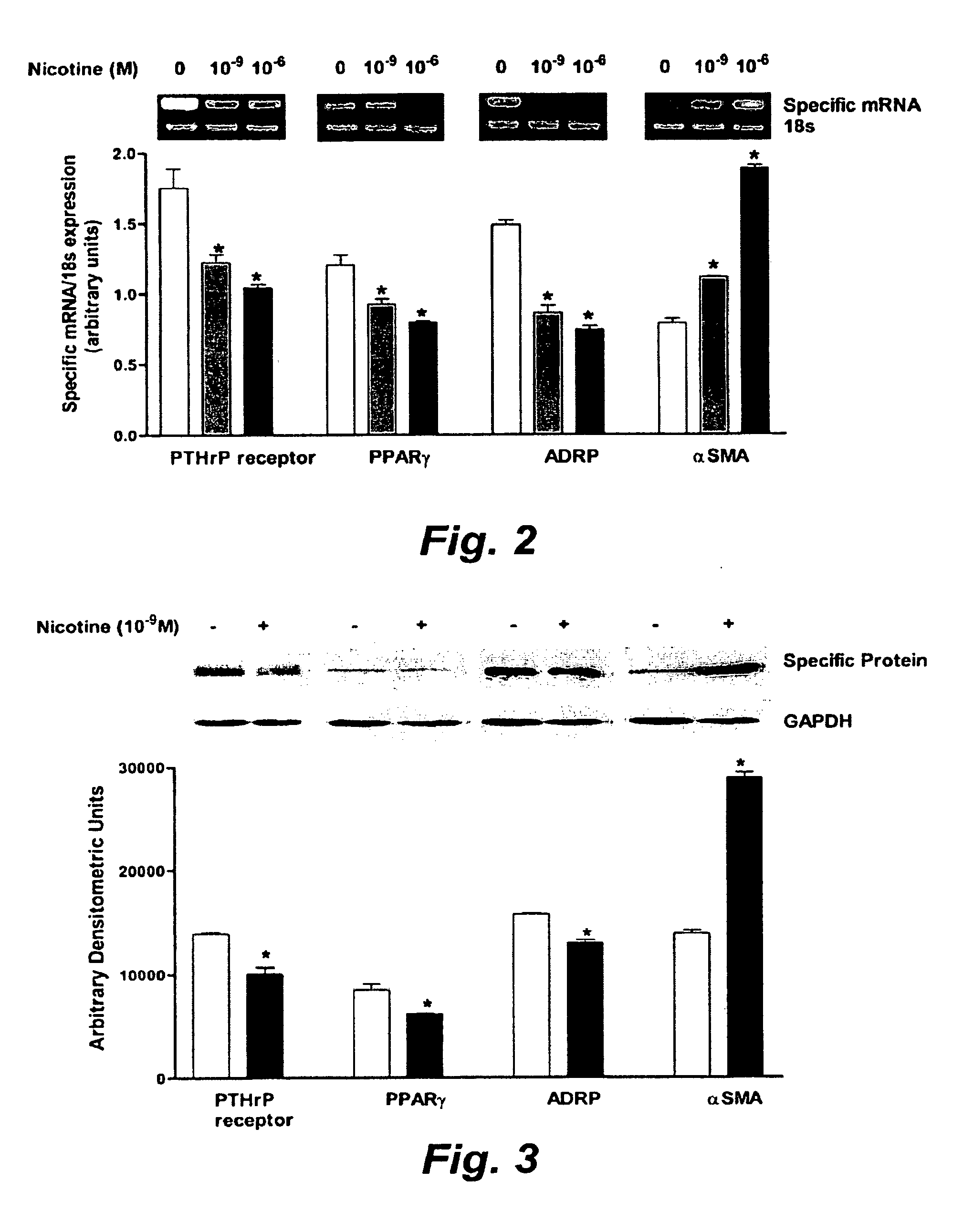
Treatment for nicotine-induced lung disease using peroxisome proliferator-activated receptor gamma agonists
ActiveUS20100010056A1Inhibiting and repairing deleterious effectOrganic active ingredientsBiocideGerm layerInjury cause
This invention provides pertains to the discovery that that nicotine interrupts molecular signaling between endodermal and mesodermal cells of the lung alveolus. Treatment of the lung with specific molecular agents (e.g., PPAR gamma agonists) can prevent and / or reverse the injury caused by nicotine.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
Composition comprising peroxisome proliferator-activated receptor-gamma
InactiveCN103442676AGood regenerating adipose tissueCosmetic preparationsAntipyreticDermatologyPeroxisome proliferator-activated receptor gamma
The invention relates to an injectable composition comprising peroxisome proliferator-activated receptor-gamma used for subcutaneous administration, and a method for improving skin imperfections. The injectable composition is subcutaneously administered at an area of the skin imperfections by steps of a) identifying the area of the skin imperfections, and b) administering a safe and cosmetically effective amount of the composition subcutaneously to the area of the skin imperfections by subcutaneous administration or subdermal administration.
Owner:MERZ PHARMA GMBH & CO KGAA
Compositions and Methods for Prophylaxis and Treatment of Addictions
The present invention relates to methods of treating or preventing addiction and relapse use of addictive agents, and treating or preventing addictive or compulsive behaviour and relapse practice of an addictive behaviour or compulsion, by administering a peroxisome proliferator-activated receptor gamma (PPARγ) agonist, alone or in combination with another therapeutic agent, such as, for example, an opioid receptor antagonist or an antidepressant, or an addictive agent, such as, for example, an opioid agonist. The present invention also includes pharmaceutical compositions for treating or preventing addiction or relapse that include a PPARγ agonist and one or more other therapeutic or addictive agents, as well as unit dosage forms of such pharmaceutical compositions, which contain a dosage effective in treating or preventing addiction or relapse. The methods and compositions of the invention are useful in the treatment or prevention of addiction to any agent, including alcohol, nicotine, marijuana, cocaine, and amphetamines, as well as compulsive and addictive behaviours, including pathological gambling and pathological overeating.
Owner:OMEROS CORP
Exercise and diet program
A method of individualized weight management for a subject includes obtaining a biological sample from the subject; detecting the presence or absence of polymorphisms associated with at least seven genes comprising fatty acid-binding protein 2 (FABP2), peroxisome proliferator-activated receptor gamma (PPARG), beta-2-adrenergic receptor (ADRB2), beta-3-adrenergic receptor (ADRB3), angiotensin-converting enzyme (ACE), alpha-actinin-3 (ACTN3), and proton-linked monocarboxylate transporter (MCT1) in the biological sample to obtain genotype pattern data for the subject; wherein the polymorphisms are indicative of at least one nutritional trait and at least one fitness trait and preparing a nutritional and fitness program based on the subject's genotype pattern data; wherein the fitness program comprises sequences of resistance, cardio, and excess post-exercise oxygen consumption (EPOC) training routines.
Owner:ALDO CONSULTING & PROJECT MANAGEMENT
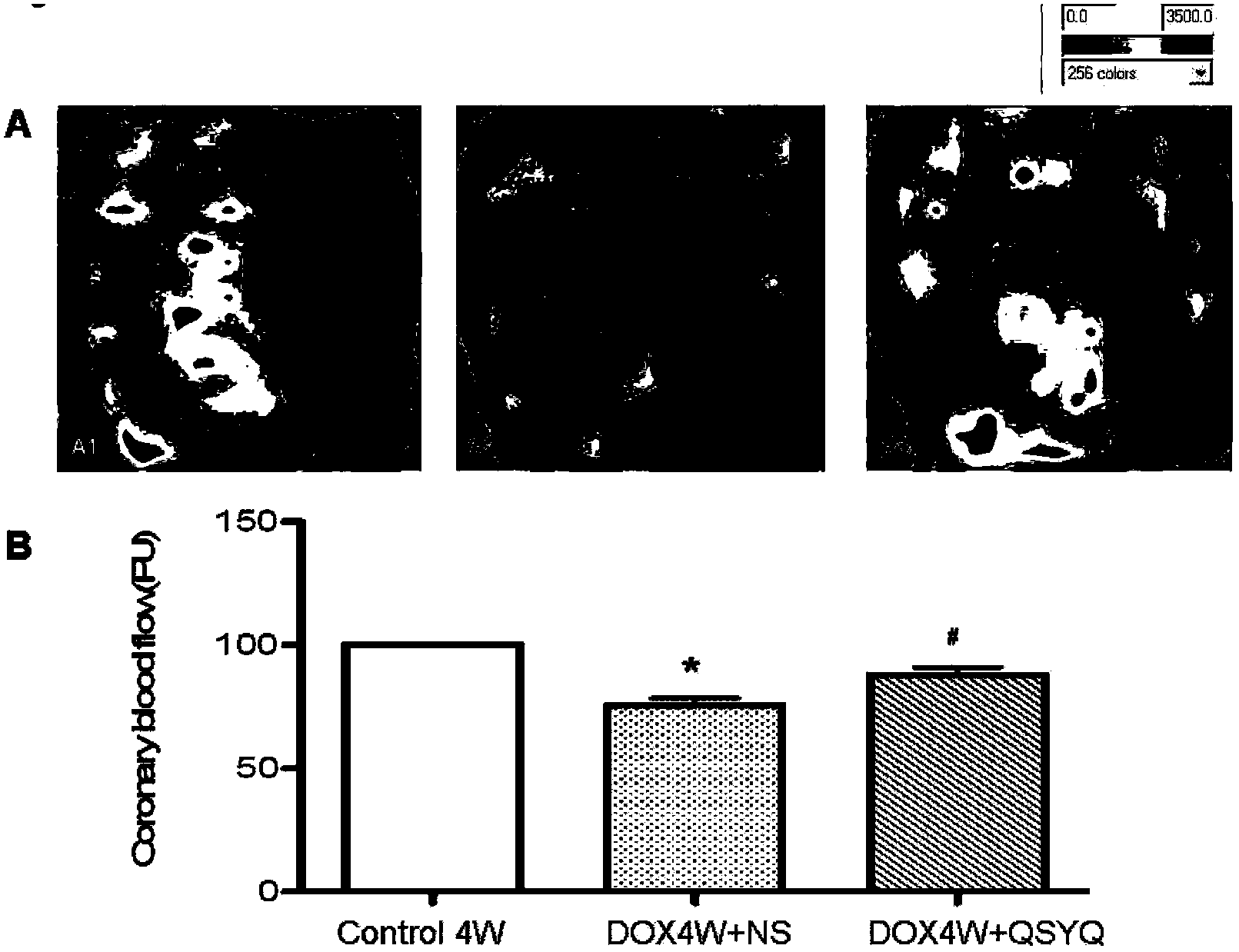
Application of astragalus-salviae miltiorrhizae qi-benefiting dropping pills in resistance to anti-cancer medicine cardiotoxicity
InactiveCN103520270AImprove perfusion flowPromote oxidationPill deliveryCardiovascular disorderCardiac muscleCardiotoxicity
The invention discloses an application of astragalus-salviae miltiorrhizae qi-benefiting dropping pills in the resistance to anti-cancer medicine cardiotoxicity. The astragalus-salviae miltiorrhizae qi-benefiting dropping pills can be used for improving the myocardium loss and the cardiac function damage caused by anti-cancer medicines, the degradation of rat cardiac muscle ATP (Adenosine Triphosphate) caused by doxorubicin, the reduction of peroxisome proliferator-activated receptor alpha and the reduction of peroxisome proliferator-activated receptor gamma coactivator 1 alpha, alleviating the eccentric hypertrophy of myocardium and the myocardial damage, increasing the blood flow volume of heart surface and improving the cardiacfunction.
Owner:TIANJIN TASLY PHARMA CO LTD
Use of Pl3K Inhibitors for the Treatment of Obesity, Steatosis and Ageing
ActiveUS20140154232A1Good for weight lossReduced adiposityOrganic chemistryPeptide/protein ingredientsDiseaseBrown adipose cell
The first aspect of the invention relates to a phosphoinositide 3-kinase inhibitor for use in the treatment or prevention of a disease or condition associated with the expression of peroxisome proliferator-activated receptor gamma coactivator 1-α (Pgd1α) and / or uncoupling protein 1 (Thermogenin / Ucp1) in brown adipocytes. The disease or condition may be positive energy imbalance-associated, for example, obesity, an obesity-associated disease or condition, steatosis and biological aging (performance aging). Another aspect of the invention provides the use of a phosphoinositide 3-kinase inhibitor for promoting weight loss in an individual.
Owner:FUNDACION CENT NACIONAL DE INVESTIGACIONES ONCOLOGICAS CARLOS III
Method for distinguishing activities of complete agonist, partial agonist and antagonist of peroxisome proliferator-activated receptor gamma
ActiveCN110426512AReduce usageReduce workloadMolecular designPeptide preparation methodsPharmacophoreReceptor for activated C kinase 1
The invention discloses a method for distinguishing the activities of a complete agonist, a partial agonist and an antagonist of a peroxisome proliferator-activated receptor gamma. The method comprises: modeling of the receptor and a ligand; molecular docking; simulation of conventional molecular dynamics; simulation of well-tempered meta-dynamic molecular dynamics; and construction of a pharmacophore. The method disclosed by the invention can quickly distinguish the activities of the PPARs gamma of different structural compounds, so that use of chemical drugs and cells in a conventional toxicity experiment process in a laboratory is greatly reduced, the workload in the laboratory is relieved, and expenses on the laboratory are saved; therefore, the activities of the PPARs gamma of the compounds are distinguished before QSAR (quantitative structure-activity relationship) modeling, and a QSAR model result is closer to an actual model; and a traditional QSAR is used more widely.
Owner:NANJING UNIV
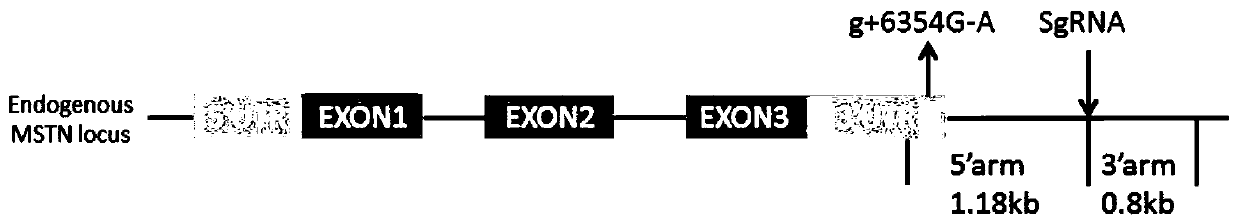
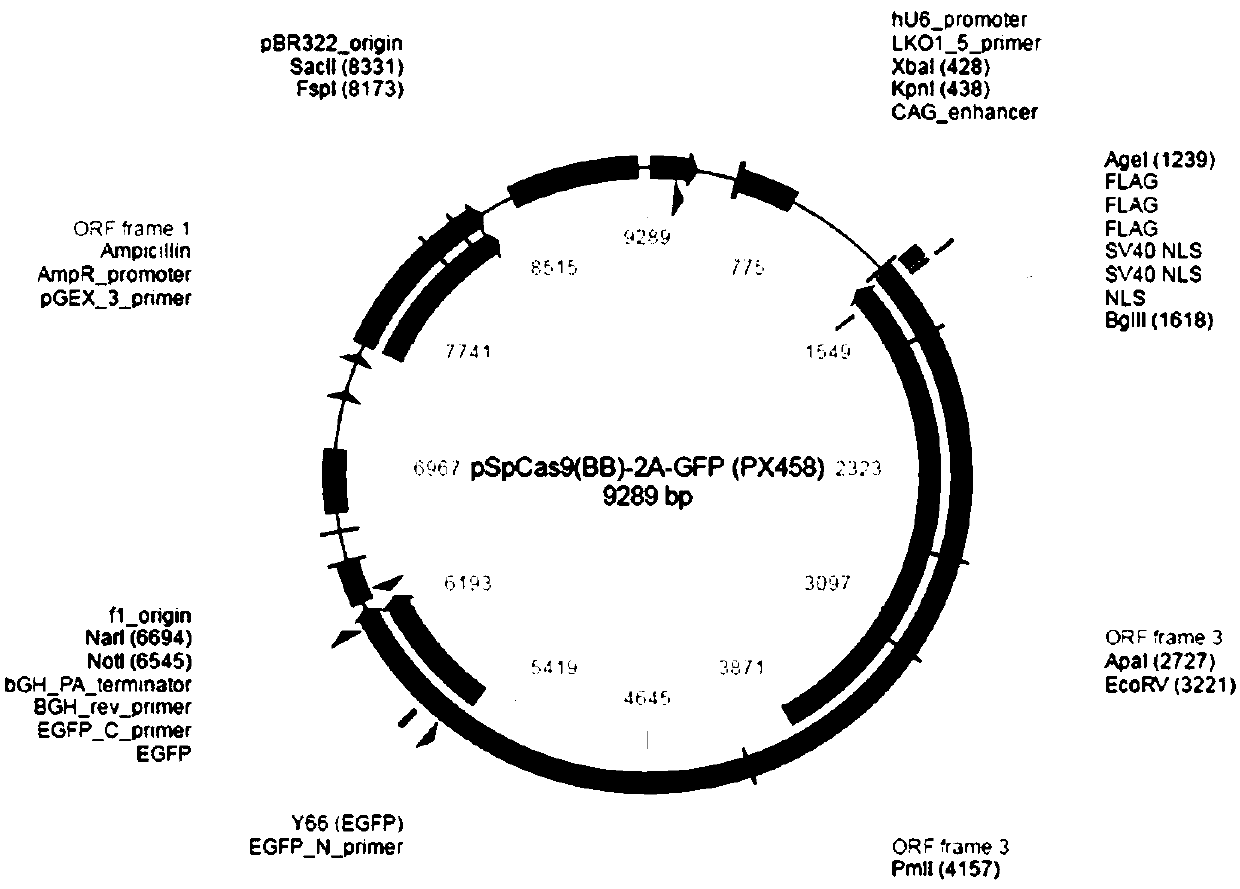
Vector for carrying out site-specific mutagenesis on MSTN (Myostatin) and site-specific integration on PPARgamma (Peroxisome Proliferator-activated Receptor gamma) at same time
ActiveCN109679998ARealize simultaneous editingAchieving the goal of simultaneous editingGenetically modified cellsStable introduction of DNAMyostatinNuclear transfer
The invention discloses a vector for carrying out site-specific mutagenesis on MSTN (Myostatin) and site-specific integration on PPARgamma (Peroxisome Proliferator-activated Receptor gamma) at the same time, and recombinant cells. The vector comprises a Cas9 cutting expression vector and a targeting vector. According to the vector disclosed by the invention, a recombinant cell strain can be constructed by carrying out co-transfection on host cells by the Cas9 cutting expression vector and the targeting vector, the recombinant cell strain can be used as nuclear transfer donor cells for producing transgenic cloned embryo, function exertion of the MSTN can be inhibited, and meanwhile, specific expression of the PPARgamma in skeletal muscle cells can be realized, so that support is provided for quickly and efficiently developing high-quality transgenic beef new products.
Owner:NORTHWEST A & F UNIV
Plasmid-type adenovirus vector pAd-PPARGC1A and construction method thereof
InactiveCN102766653AEnsure biosecurityEfficient expressionFermentationVector-based foreign material introductionLiposomeInverted Terminal Repeat
The invention relates to a plasmid-type adenovirus vector pAd-PPARGC1A (peroxisome proliferator-activated receptor gamma coactivator 1 alpha) and a construction method thereof, and belongs to the technical field of gene engineering. The construction method is as below: inserting a Qinchuan cattle PPARGC1A gene under a CMV promoter of a pAdTrack-CMV shuttle plasmid to construct a recombinant shuttle plasmid pAdTrack-CMV-PPARGC1A; linearizing by PmeI and transforming E.coli BJ5183 competent bacteria containing pAdEasy-l adenovirus backbone plasmids; carrying out homologous recombination on the pAdTrack-CMV-PPARGC1A and the pAdEasy-1 in bacteria, by using an efficient homologous recombination system of an E.coli BJ5183 endogenous Cre recombinase; naming a correct recombinant adenovirus plasmid after pAd-PPARGC1A; linearizing the pAd-PPARGC1A by Pac I and exposing inverted terminal repeats (ITR); and transfecting HEK-293A cells by the liposome to produce recombinant virus particles. The present invention has advantages of ensuring biological safety of the recombinant adenovirus vector, promoting efficient expression of the gene and benefiting expression of the gene for subsequent detection.
Owner:NORTHWEST A & F UNIV
Diabetic retinopathy (DR) metabolic memory detection reagent and application thereof
InactiveCN104212908AEarly diagnosisRapid diagnosisMicrobiological testing/measurementExperimental proofDiabetes retinopathy
The invention belongs to the field of biotechnology and medical science and particularly relates to a diabetic retinopathy (DR) metabolic memory detection reagent and application thereof. According to experimental proof, methylation of locus (+214) in a peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1-alpha) gene is an early diagnosis mark of DR metabolic memory. On the basis of the study, the detection reagent is provided for detecting the diagnosis mark, and a corresponding detection kit is prepared. According to the provided methylation detection reagent, whether methylation of a detected object of the PGC1-alpha gene locus +214 occurs or not and methylation degree can be qualitatively or quantitatively detected, thereby, whether DR metabolic memory and illness risks of the detected object exist or not can be predicted, and the clinical application value is excellent.
Owner:陈有信
Medicaments
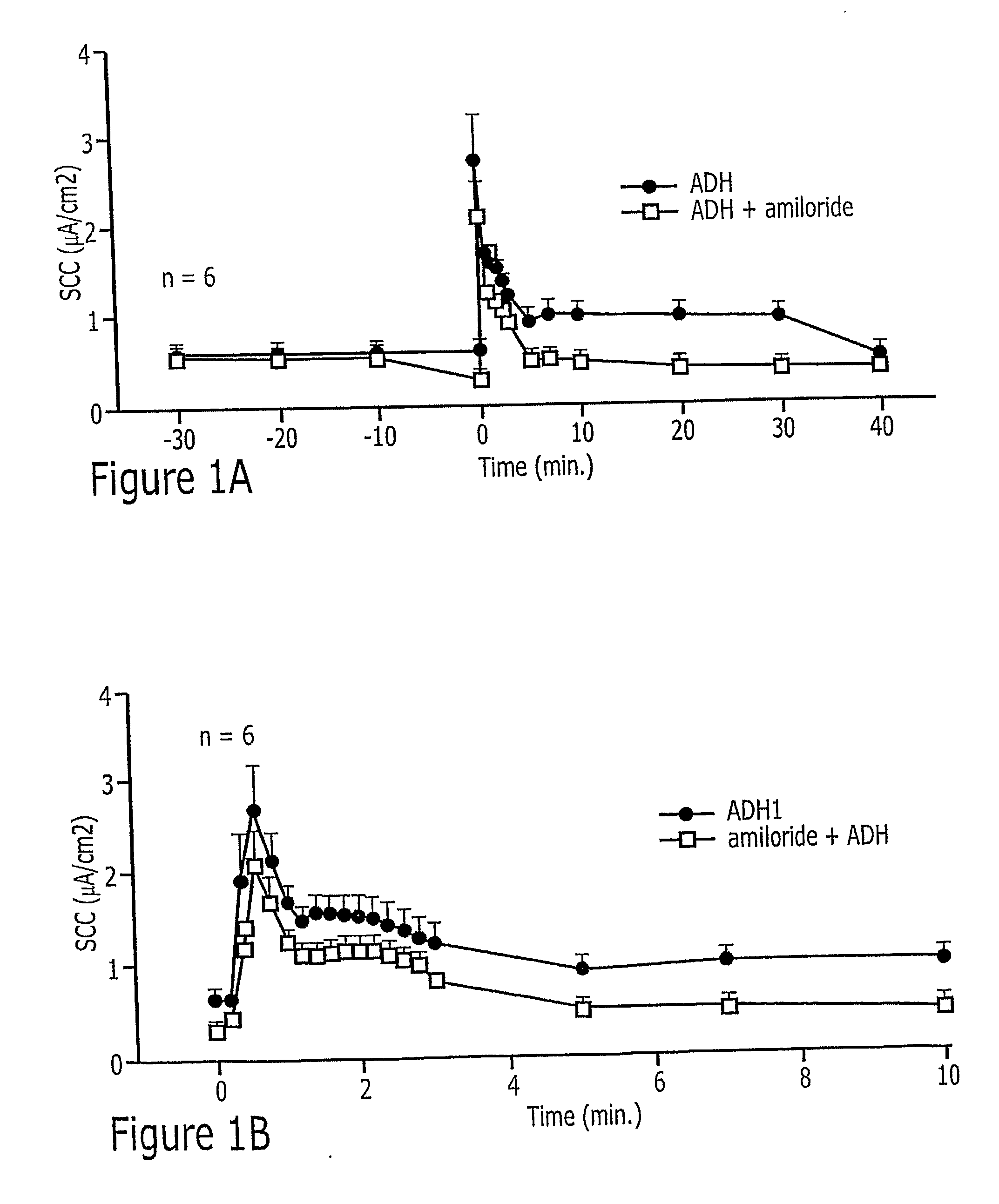
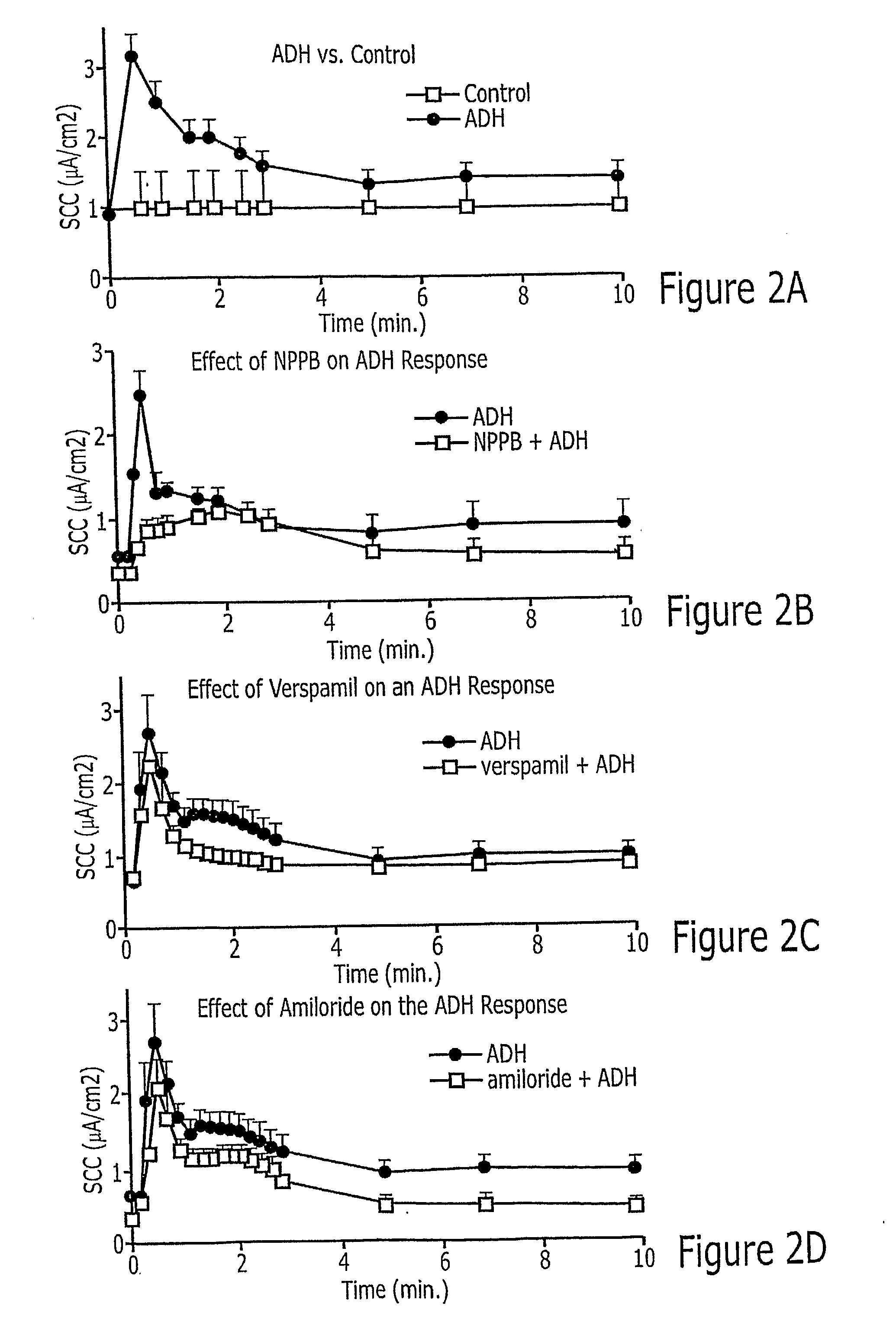
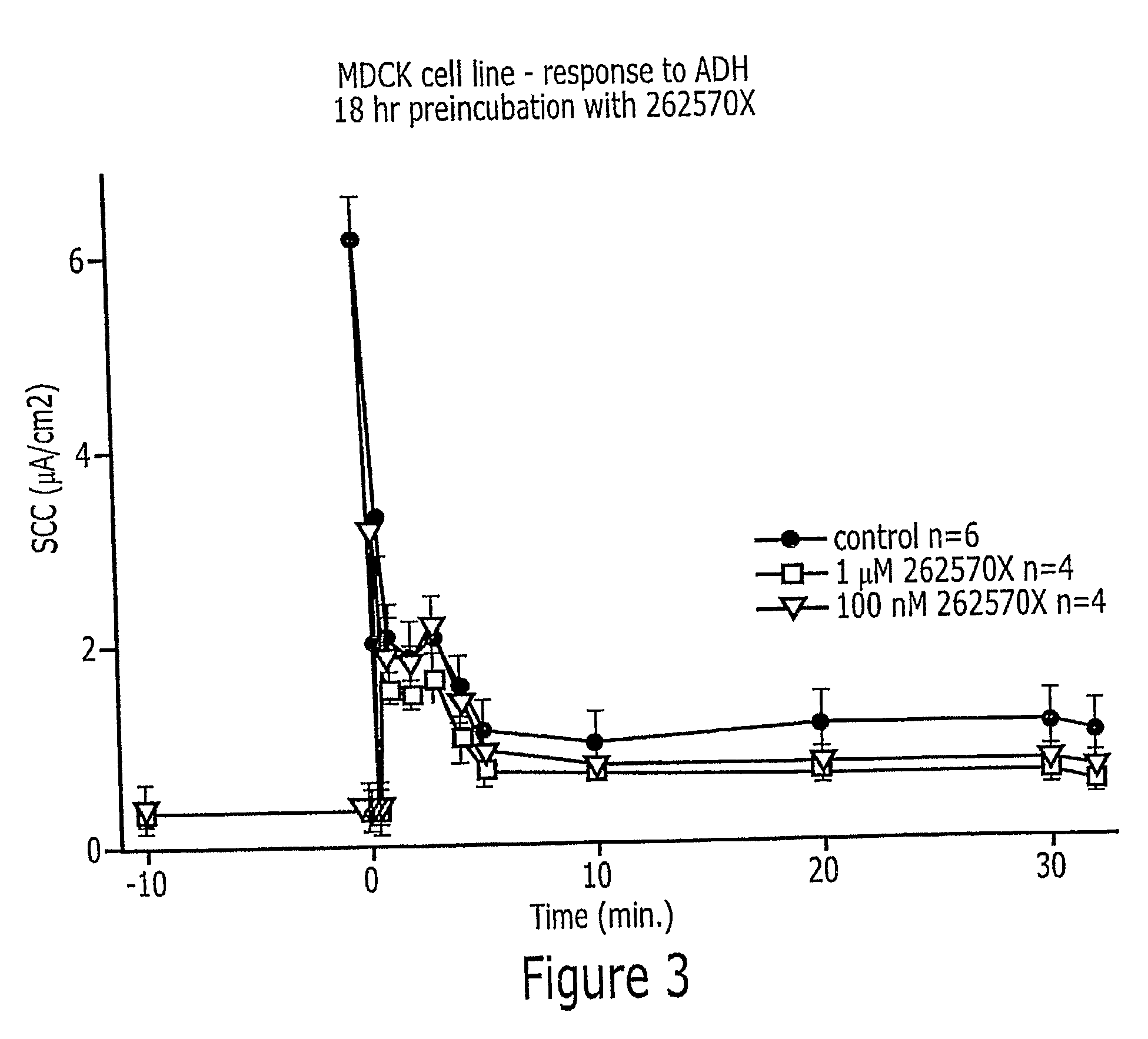
InactiveUS20080113996A1BiocideUrinary disorderNephrosisAutosomal Recessive Polycystic Kidney Disease
Methods of prevention or treatment of renal diseases or conditions associated with abnormal ion flux, in particular autosomal dominant polycystic kidney disease, with a modulator of human peroxisome proliferator activated receptor gamma.
Owner:GLAXO SMITHKLINE LLC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com