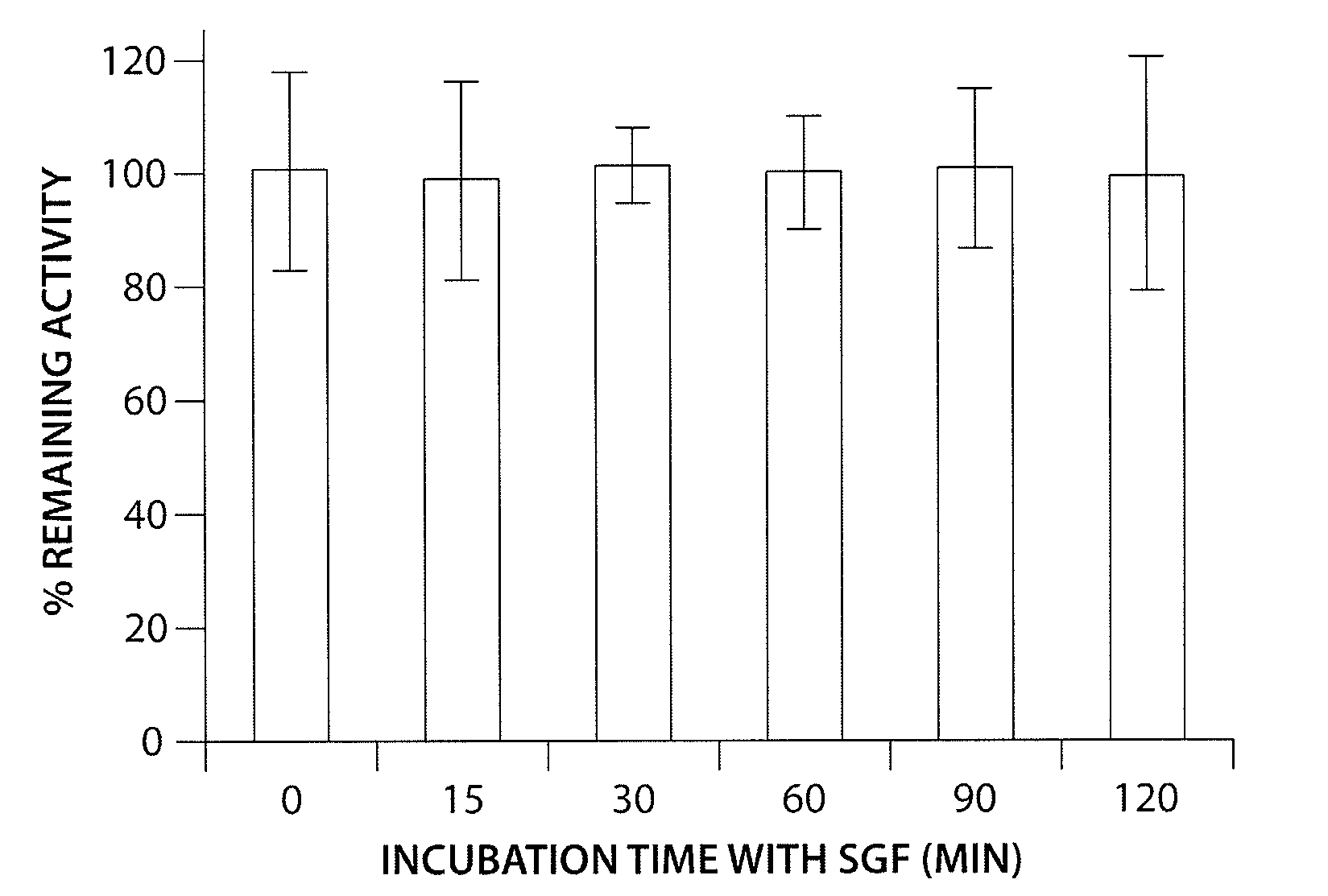
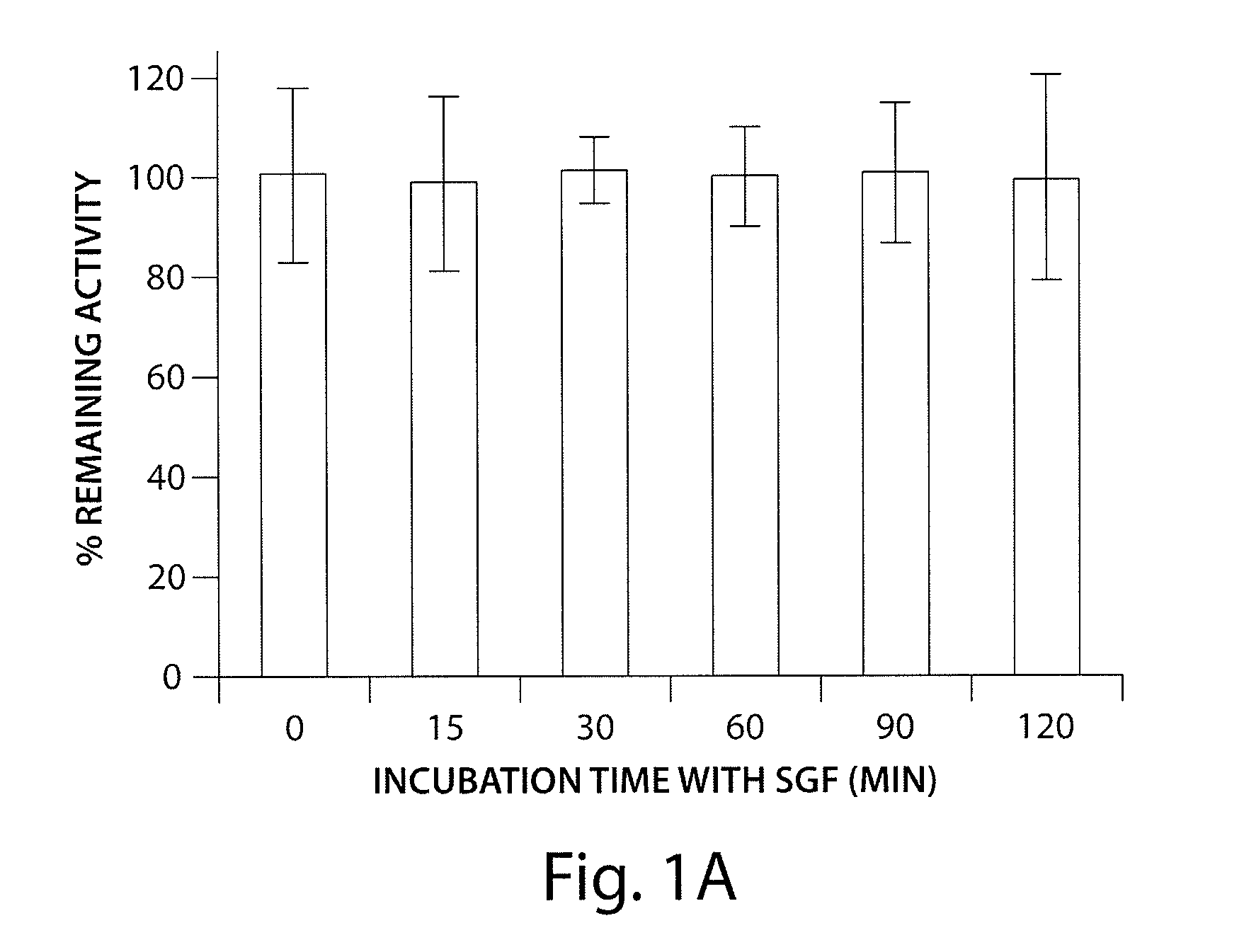
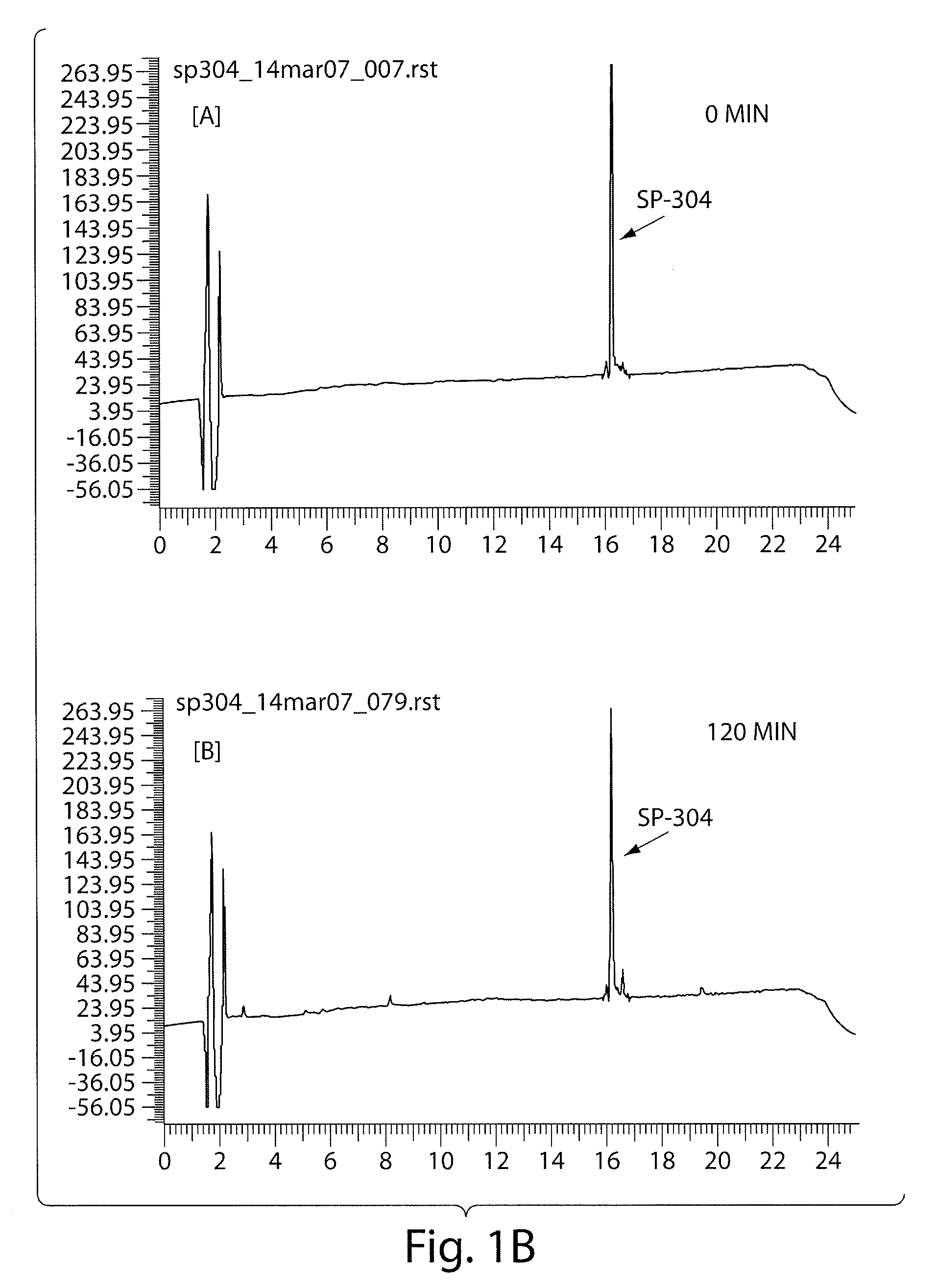
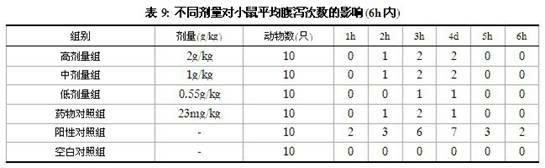
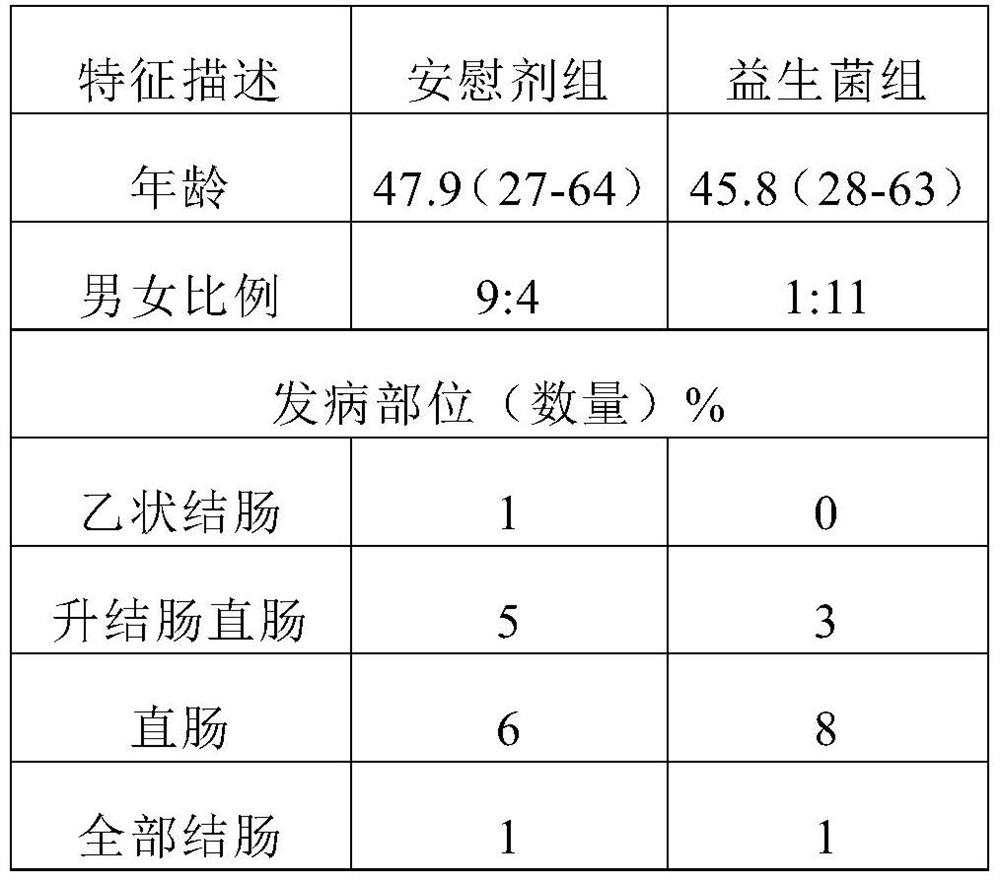
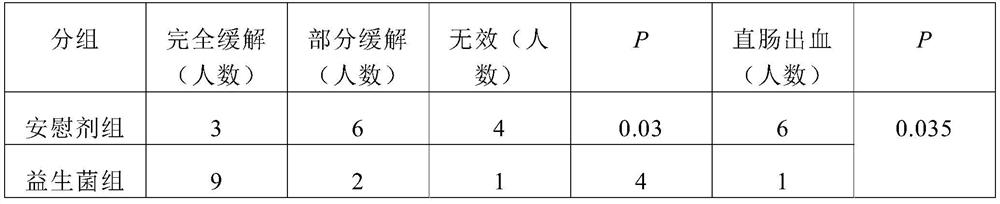
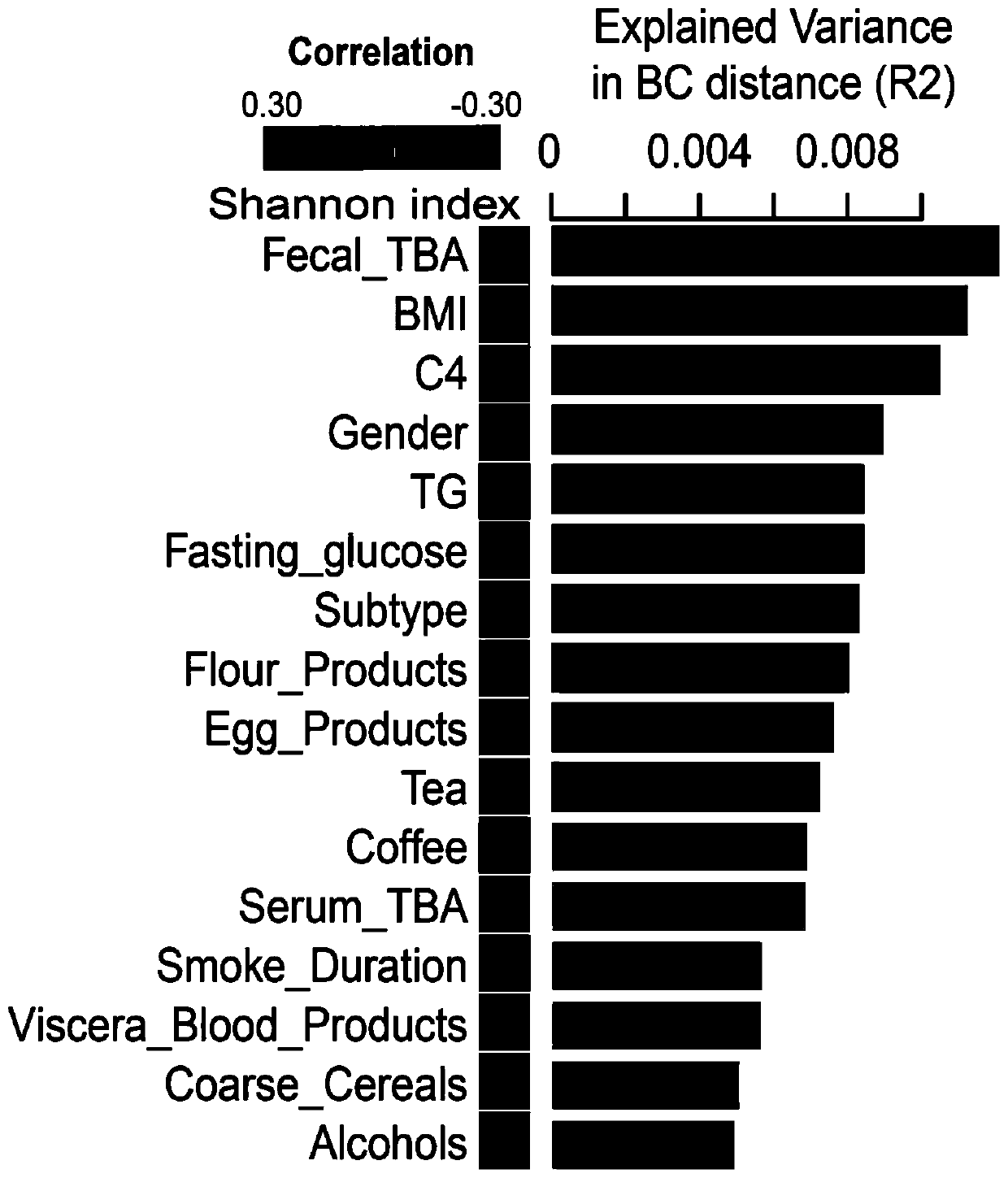
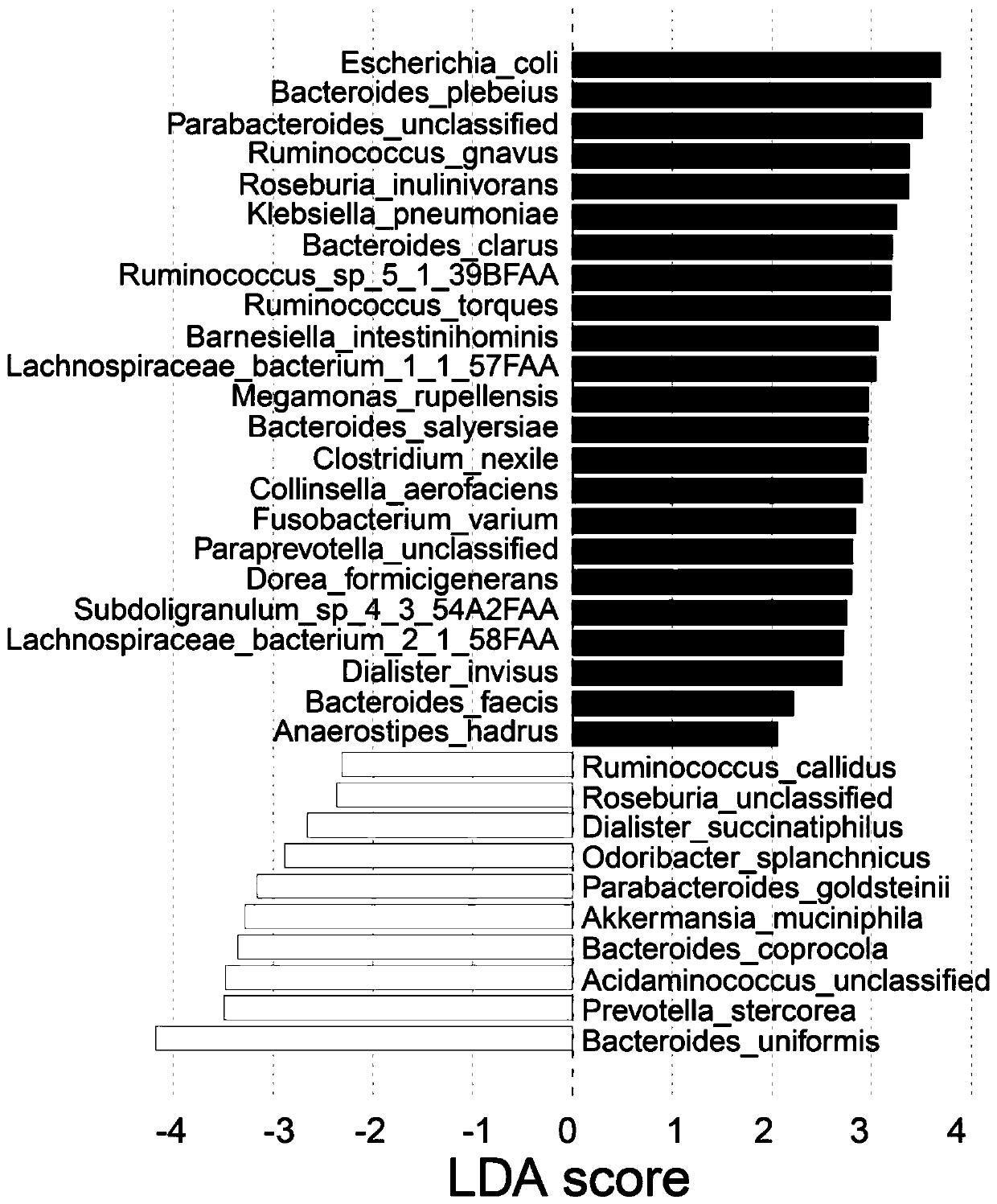
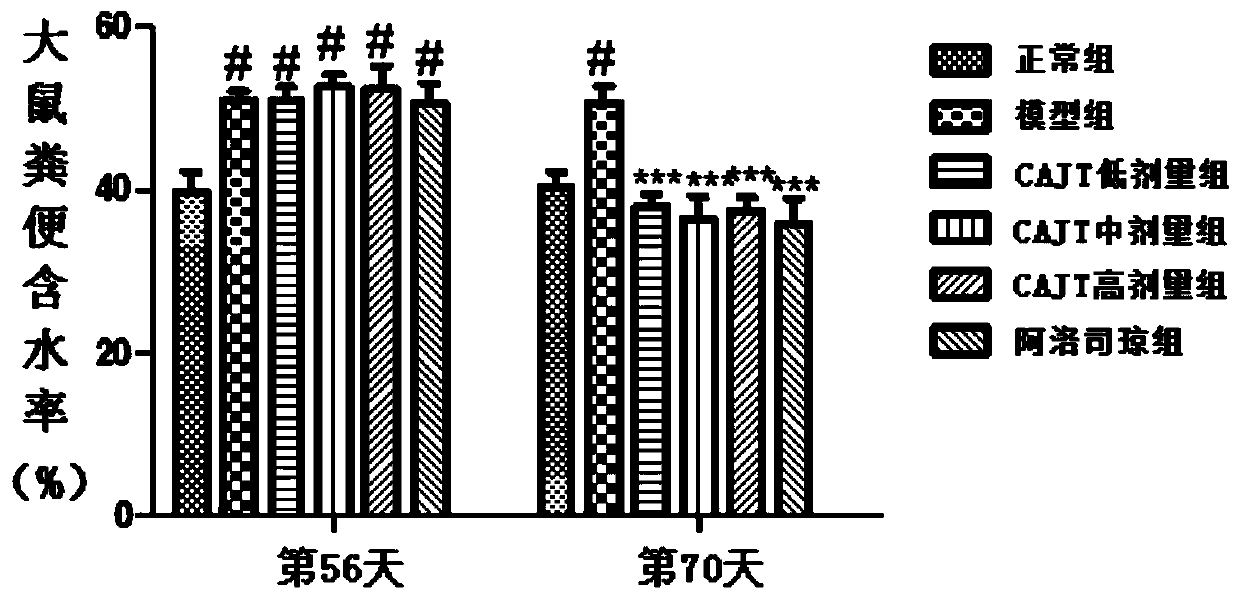
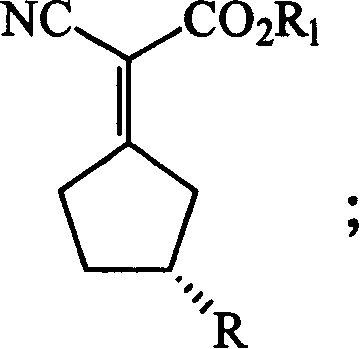
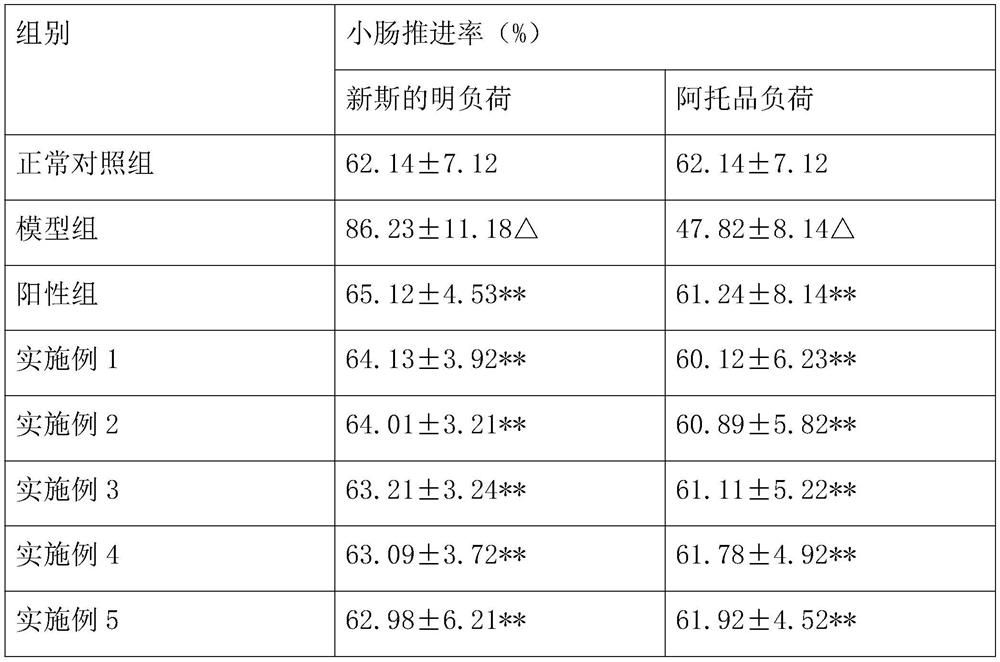
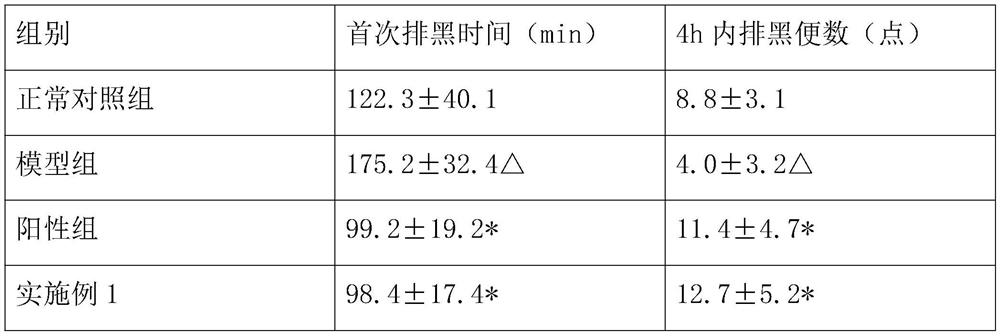
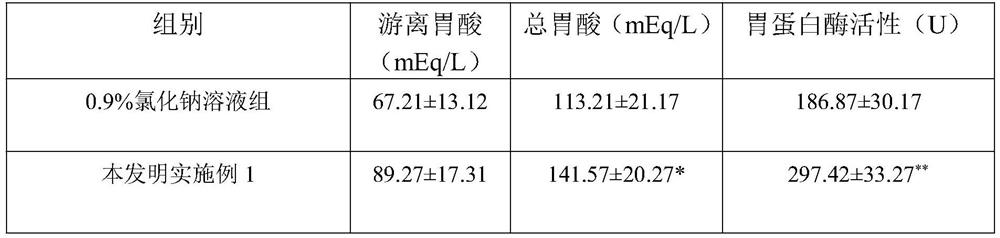
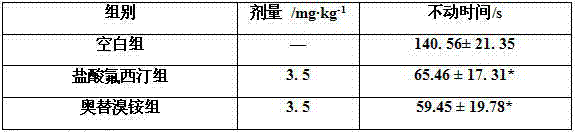
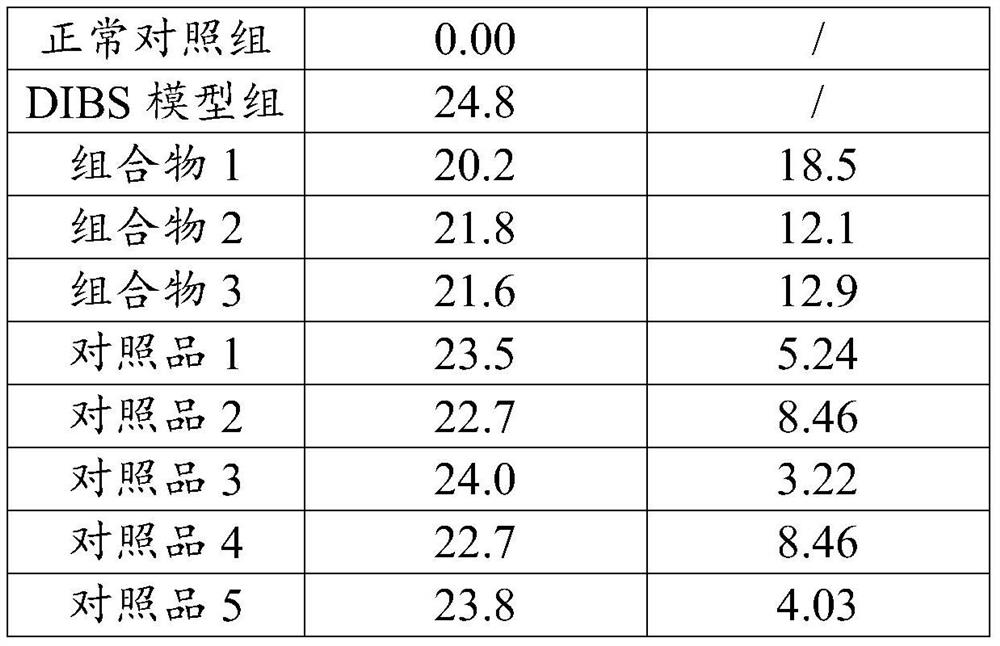
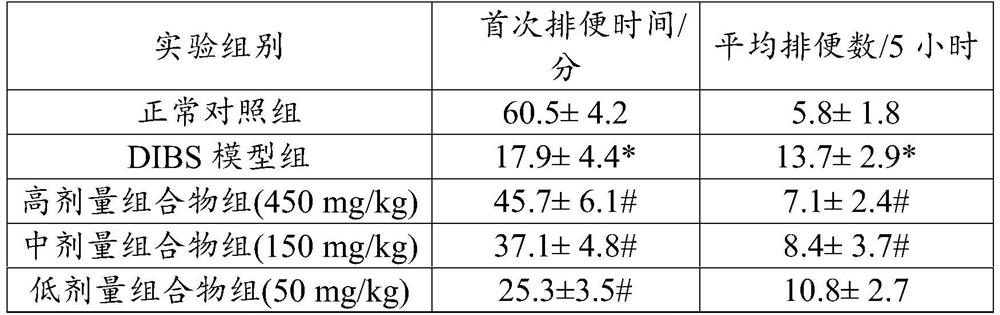
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Irritable colon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Irritable bowel syndrome is a functional disorder of the colon, meaning no structural, inflammatory or infectious abnormalities of the colon occur with the condition. Recurrent episodes of abdominal pain that subside with bowel movements are the hallmark symptom of irritable bowel syndrome.
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS20090048175A1Maintain good propertiesIncrease resistanceOrganic active ingredientsSenses disorderPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition, including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Traditional Chinese medicine composition for preventing and treating bacterial diarrhea of livestock and poultry and application thereof
InactiveCN102429967APromote growth and developmentGood treatment effectAntibacterial agentsAnthropod material medical ingredientsBiotechnologyChronic diarrhea
The invention discloses a traditional Chinese medicine composition for preventing and treating bacterial diarrhea of livestock and poultry, and belongs to the field of traditional Chinese medicines. The traditional Chinese medicine composition comprises the following components in percentage by weight: 18-19% of coptis root, 14-15% of nutgall, 14-15% of pomegranate rind, 14-15% of myrobalam, 22-23% of astragalus mongholicus, 7-8% of atractylis ovata and 7-8% of radix sileris. The traditional Chinese medicine composition for preventing and treating bacterial diarrhea of livestock and poultry disclosed by the invention has significant curative effects on treating colibacillosis, salmonellosis and other bacterial diarrhea as well as chronic enteritis, irritable colon and other chronic diarrhea of the livestock and poultry. At the same time, the traditional Chinese medicine composition disclosed by the invention has the advantages of low production cost, and small toxic or side effect.
Owner:GANSU AGRI UNIV
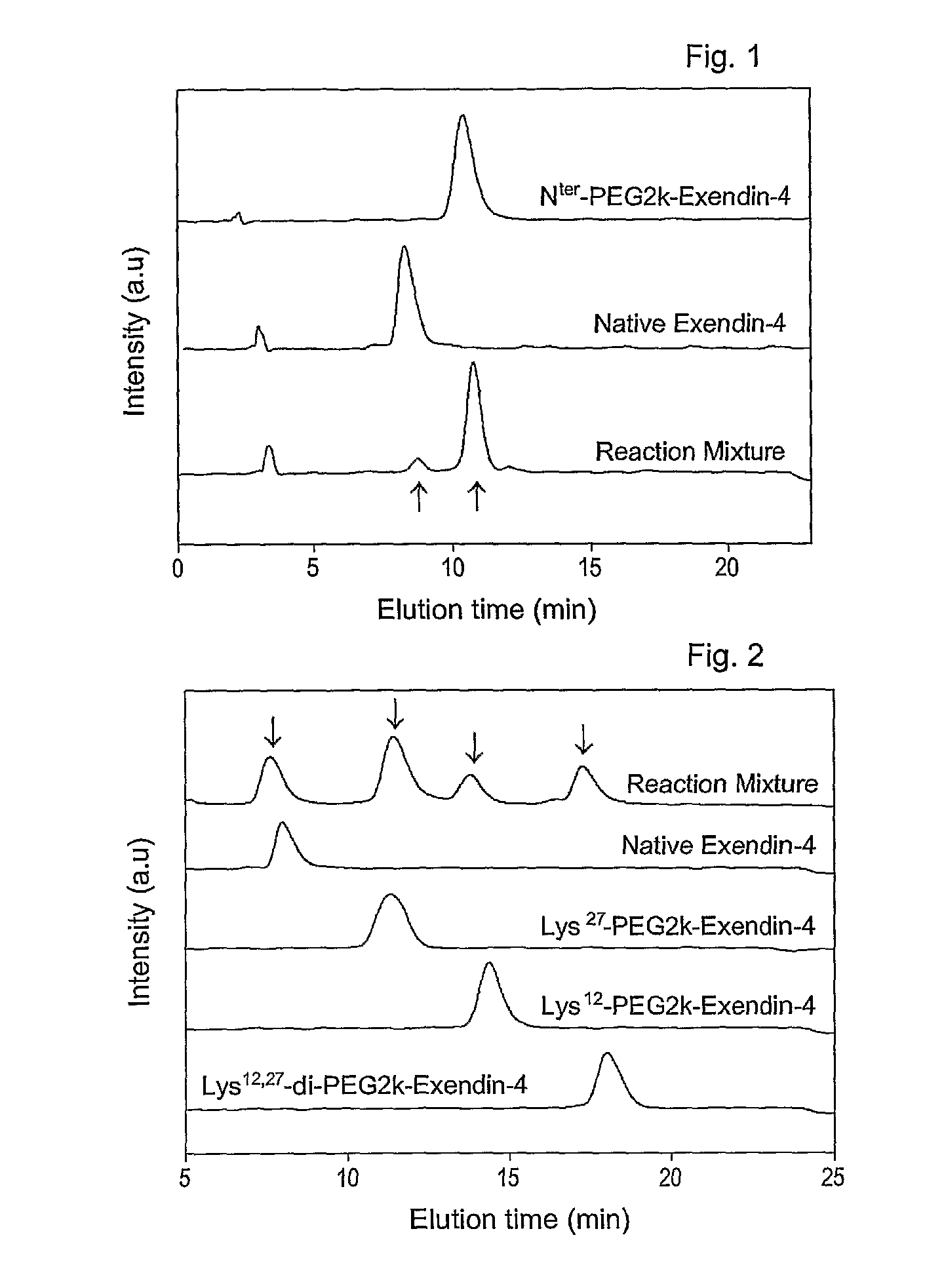
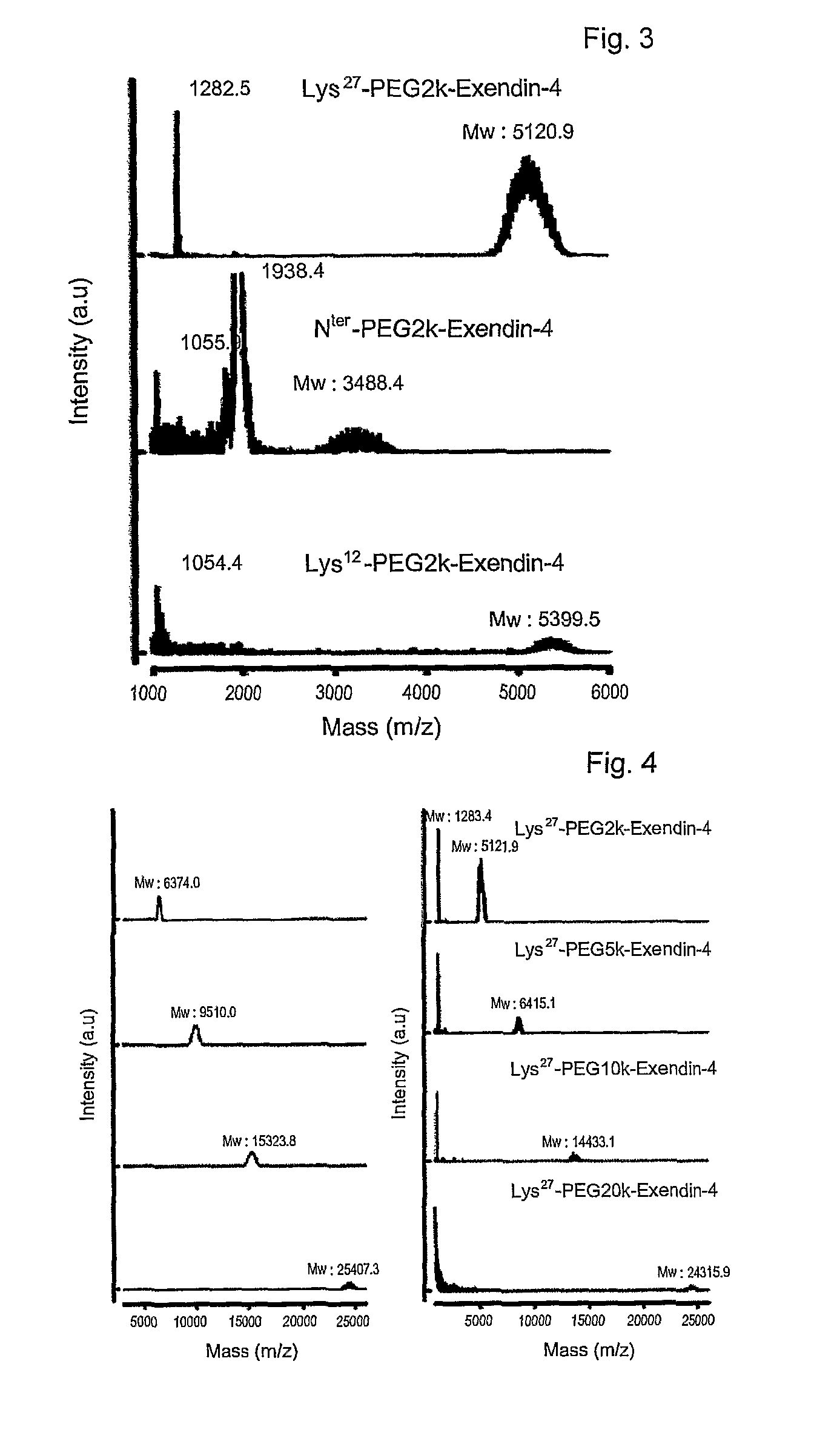
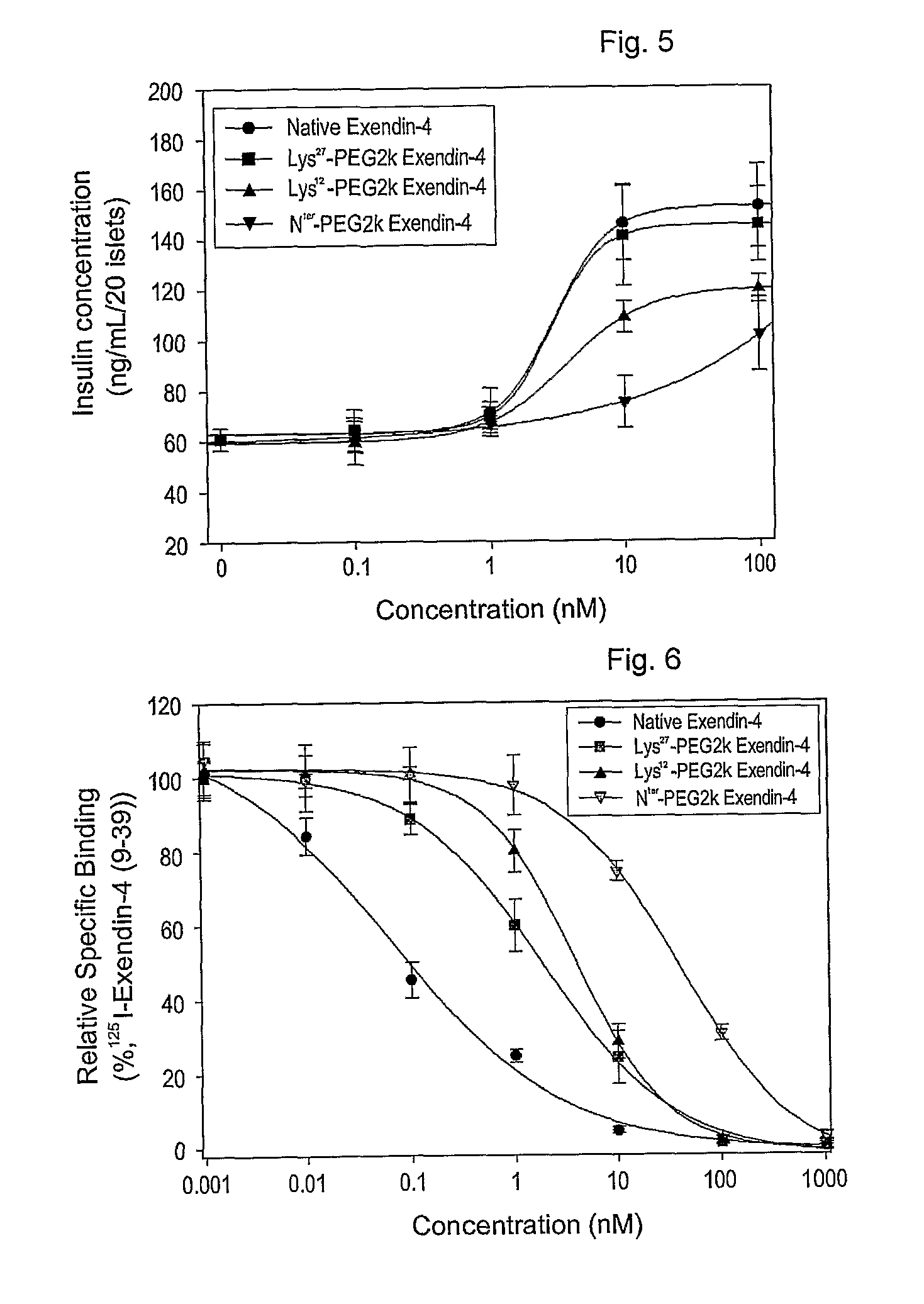
Mono modified exendin with polyethylene glycol or its derivatives and uses thereof
ActiveUS20100137558A1Extended half-lifeMinimize side effectsPeptide/protein ingredientsMetabolism disorderSide effectHalf-life
Disclosed herein are exendin singly modified with polyethylene glycole or a derivative thereof, a method for the preparation of the same, and uses thereof. Exendin modified at lysine (27) with polyethylene glycol shows biological activity similar to that of natural exendin, but is improved in half life. In addition, the modification position and the number of PEG or its derivative are restricted so as to minimize the side effects caused by a variety of combinations of such factors. The exendin is useful in the prevention and treatment of diseases caused by the over-secretion of insulin, or diseases caused due to a decrease in plasma glucose level, the inhibition of gastric or intestinal motility, the promotion of satiety, or the inhibition of food intake, especially diabetes, obesity and irritable colon syndrome.
Owner:D&D PHARMATECH INC
Trpm8 antagonists
ActiveUS20140371276A1High selectivityAdequate pharmacokinetic profileBiocideNervous disorderSelective antagonistNeurodegeneration
The invention relates to compounds acting as selective antagonists of Transient Receptor Potential cation channel subfamily M member 8 (TRPM8), and having formula (I). Said compounds are useful in the treatment of diseases associated with activity of TRPM8 such as pain, inflammation, ischaemia, neurodegeneration, stroke, psychiatric disorders, itch, irritable bowel diseases, cold induced and / or exhacerbated respiratory disorders and urological disorders.
Owner:DOMPE FARM SPA
Preparation and application of 'dingguiyou' soft capsule
InactiveCN101209285ANo inhibitionNo effect on sleep timeDigestive systemCapsule deliveryMedicineMedical prescription
The invention relates to a prescription which is mainly composed of clove and cinnamon, the indication of the prescription is irritable bowel syndrome, and the effective part of clove and cinnamon is volatile oil which is determined by screening of pharmacodynamics. The soft capsules which are prepared by the volatile oil have elegant appearance, convenient administration and stable quality; proved by pharmacodynamic tests, the soft capsules can meet the requirements of the clinical medication of irritable bowel syndrome.
Owner:牛锐
Intestine-moistening and bowel-relaxing composition, solid beverage and application
InactiveCN111972670AReduce morbidityReduce adhesionOrganic active ingredientsDigestive systemBiotechnologyIsomaltooligosaccharide
The invention discloses an intestine-moistening and bowel-relaxing composition. The intestine-moistening and bowel-relaxing composition comprises a probiotic compound and a prebiotic compound, whereinthe probiotic compound comprises the following components in parts by weight: 1-5 parts of lactobacillus acidophilus, 0.5-2 parts of bifidobacterium lactis, 0.5-4 parts of pediococcus acidilactici, 0.5-4 parts of bifidobacterium longum and 0.5-2 parts of lactobacillus plantarum; and the prebiotic compound comprises the following components in parts by weight: 3 to 40 parts of fructo-oligosaccharide, 2 to 30 parts of galacto-oligosaccharide, 0 to 30 parts of resistant dextrin, 0 to 20 parts of xylooligosaccharide, 0 to 30 parts of isomalto-oligosaccharide, 1 to 15 parts of inulin and 1 to 8 parts of stachyose. The invention also discloses an application of the composition and an intestine-moistening and bowel-relaxing solid beverage containing the composition. According to the composition,probiotics and prebiotics are combined, so that adhesion of pathogenic bacteria can be relieved, intestinal flora can be adjusted, and the incidence rate of irritable bowel comprehensive symptoms canbe remarkably reduced; and crowd food tasting experiments show that the composition has the effects of promoting defecation and relieving abdominal pain, abdominal distension and diarrhea symptoms.
Owner:GUANGDONG YUEWEI BIOLOGICAL TECH CO LTD
Preparation method of ramosetron derivatives and applications thereof
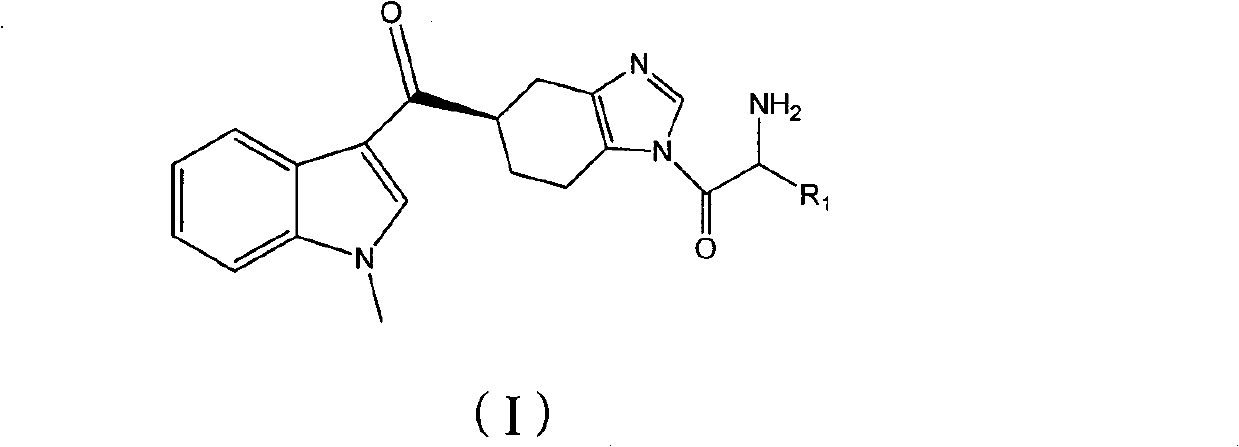
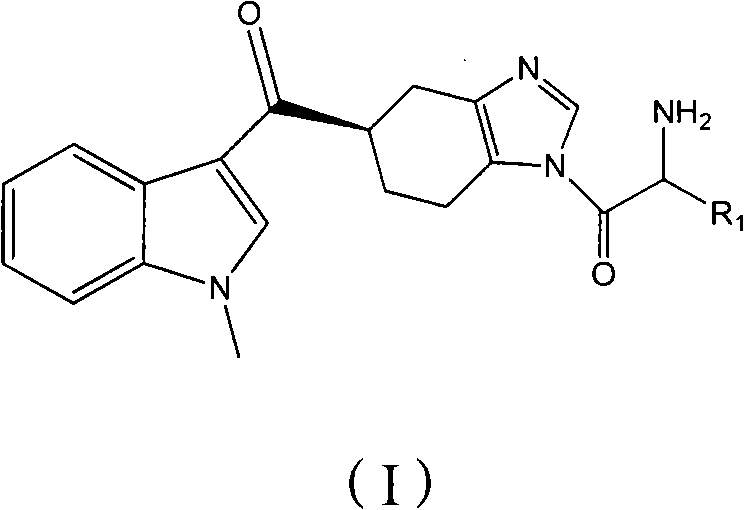
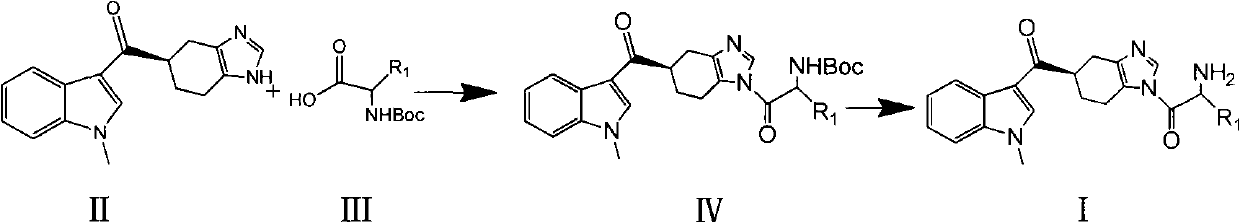
ActiveCN102101858AGood water solubilityEnhance pharmacological effectsOrganic active ingredientsOrganic chemistryMalignant Neoplasm TreatmentIrritable bowel syndrome
The invention relates to a preparation method of ramosetron derivatives and applications thereof. The invention provides a compound as expressed in the formula (I), a pharmaceutically acceptable salt thereof, an enantiomer or racemic mixture thereof, wherein, R1 is H, C1-C6 alkyl, C1-4 alkyloxys, aryls or substituted C1-C4 alkyl. The invention also provides a preparation method of the compound or the pharmaceutically acceptable salt thereof, the pharmaceutical composition containing the compound and the salt, and applications of the compound. The compound in the invention possesses functions of preventing and treating the digestive tract symptoms of nausea, vomit, etc. caused by anti-malignant tumor treatment, and preventing and treating irritable bowel syndrome. The compound can be used in preparing medicine for preventing and treating the digestive tract symptoms of nausea, vomit, etc. caused by anti-malignant tumor treatment, and irritable bowel syndrome.
Owner:天津康鸿医药科技发展有限公司
Mono modified exendin with polyethylene glycol or its derivatives and uses thereof
ActiveUS8420598B2Extended half-lifeMinimize side effectsPeptide/protein ingredientsMetabolism disorderDiseaseSide effect
Disclosed herein are exendin singly modified with polyethylene glycole or a derivative thereof, a method for the preparation of the same, and uses thereof. Exendin modified at lysine (27) with polyethylene glycol shows biological activity similar to that of natural exendin, but is improved in half life. In addition, the modification position and the number of PEG or its derivative are restricted so as to minimize the side effects caused by a variety of combinations of such factors. The exendin is useful in the prevention and treatment of diseases caused by the over-secretion of insulin, or diseases caused due to a decrease in plasma glucose level, the inhibition of gastric or intestinal motility, the promotion of satiety, or the inhibition of food intake, especially diabetes, obesity and irritable colon syndrome.
Owner:D&D PHARMATECH INC
Probiotic solid beverage for relieving or treating irritable bowel syndrome
InactiveCN111602761AEnhanced digestive functionGood for healthDigestive systemUnknown materialsProbiotic bacteriumGut flora
The invention discloses a probiotic solid beverage for relieving or treating irritable bowel syndrome. The product is prepared by mixing strains with excellent probiotic properties. A bacterial strainin the product comprises lactobacillus casei Zhang, bifidobacterium animalis subsp lactis V9, and lactobacillus plantarum P-8. The method comprises a step of carrying out correlation analysis on intestinal flora and activity index scores (IBSDAI) of patients of irritable bowel syndrome; the probiotic solid beverage can inhibit the growth of pathogenic bacteria by increasing the content of beneficial bacteria in intestinal tracts, the intestinal flora composition of patients is adjusted, and the effect of relieving or treating irritable bowel syndrome patients is achieved.
Owner:金华银河生物科技有限公司
Irritable bowel syndrome related flora marker and kit thereof
PendingCN110838365ASolve the difficult problem of auxiliary diagnosisImprove accuracyHealth-index calculationMicrobiological testing/measurementDisease riskIrritable colon
The invention discloses an irritable bowel syndrome related flora marker which can be used for developing a disease risk assessment system. According to the invention, an optimal set of 17 irritable bowel syndrome related flora markers is obtained, and a kit constructed based on the optimal set is used for diagnosing irritable bowel syndrome and can achieve higher accuracy.
Owner:康美华大基因技术有限公司
Medicament composition for treating spleen-insufficiency diarrhea type irritable bowel syndrome and preparation method thereof
InactiveCN103041081AEasy to operateReduce manufacturing costDigestive systemPlant ingredientsFormularyPrimula malacoides
The invention discloses a medicament composition for treating spleen-insufficiency diarrhea type irritable bowel syndrome and a preparation method thereof. The medicament composition comprises 20-40 parts of primula sikkmensis hook, 10-20 parts of red ginseng, 10-20 parts of bighead atractylodes rhizome, 10-20 parts of poria cocos, 5-15 parts of dried tangerine peel, 5-15 parts of pinellia ternate and 3-6 parts of licorice roots. The preparation method comprises the following steps of: weighing the raw materials in proportion; soaking all raw materials of which the weight is 3-5 times that of water into the water for 1-2 hours; after soaking, adding water of which the weight is 2-3 times that of the raw materials, decocting for 1-1.5 hours, and filtering to obtain a water decoction at a first time after the decoction is finished; adding water of which the weight is 5-8 times that of the raw materials, decocting for 1-1.5 hours and filtering to obtain a water decoction at a second time; and mixing the water decoction at the first time with the water decoction at the second time. The medicament composition is novel in formula, simple in composition and low in cost; the preparation is easy to operate, low in preparation cost and suitable for industrial production; and the medicament is convenient to orally take and carry, has no toxic and side effects and has a good clinical curative effect for patients suffering from the spleen-insufficiency diarrhea type irritable bowel syndrome.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Traditional Chinese medicine composition for treating irritable bowel syndrome and its preparation method
ActiveCN101147784ALow hygroscopicitySignificant effectDigestive systemPlant ingredientsMedicineCurative effect
The present invention discloses a Chinese medicine composition for effectively curing intestinal vulnerable syndrome. It is made up by using 8 Chinese medicinal materials of bighead atractylodes rhizome, white peony root, licorice, bupleurum root, corydalis tuber, Chinese yam and others through a certain preparation process.
Owner:HEHUANG PHARMA SHANGHAI
Traditional Chinese medicine composition for treating diarrhea-type irritable bowel syndrome
PendingCN110960594AGood analgesic effectImprove hypersensitivityAntipyreticComponent separationDiseaseFormulary
The invention discloses a traditional Chinese medicine composition for treating diarrhea-type irritable bowel syndrome. The traditional Chinese medicine composition comprises the following components,by weight: 15 to 25 parts of radix pseudostellariae, 5 to 15 parts of rhizoma atractylodis macrocephalae, 15 to 25 parts of poria cocos, 3 to 10 parts of honey-fried licorice root, 5 to 15 parts of the root of fangfeng, 10 to 20 parts of white paeony root, 3 to 10 parts of steamed dried tangerine or orange peel, 3 to 10 parts of the rhizome slices of Chinese goldthread and 5 to 15 parts of commonaucklandia root. The traditional Chinese medicine composition is proved to have an obvious analgesic effect on physical pain and chemical pain and can play an antidiarrheal role in inhibiting intestinal motility; the intestinal hypersensitivity state can be improved obviously; the moisture content of excrement can be reduced; the mucous membrane barrier function can be enhanced; and the intestinal flora can be improved. The traditional Chinese medicine formula which is safe, effective, free of drug dependence, mild and easy to take can be provided for diarrhea-type irritable bowel patients; and the traditional Chinese medicine formula has important medical values for improving the clinical cure rate of IBS-D, relieving clinical symptoms and standardizing and managing diseases.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU UNIV OF CHINESE MEDICINE
Treating irrtable bowel syndrome or disease
InactiveUS20030171432A1Reduce inflammationReduced responseBiocideDigestive systemCETYL MYRISTATEMedicine
The treatment of humans or other mammals for irritable bowel syndrome (IBS) or irritable bowel disease (IBD) using dosage forms or compositions that include cetyl myristate alone or (in admixture or serially) both cetyl myristate and cetyl palmitate.
Owner:LYPANOSYS PTE
Medicine for treating spleen-deficiency diarrhea type IBS (Irritable Bowel Syndrome) and manufacturing method thereof
The invention relates to a medicine for treating spleen-deficiency diarrhea type IBS (Irritable Bowel Syndrome), which uses Chinese medical herbs as the raw material, and a manufacturing method of the medicine. At present, when the spleen-deficiency diarrhea type IBS is treated by western medicine, no special therapy or drugs can be used, the symptomatic treatment manner is mainly adopted clinically, the untoward effects are various and side effects are larger after long-term use of western medicines, the possibility of relapse is high after drug withdrawal, and the long-term follow-up result is poor. According to the invention, Codonopsis pilosula, atractylodes macrocephala, poria cocos, radix paeoniae alba, Rhizoma Pinellinae Praeparata, Saposhnikovia divaricata, dried tangerine or orange peel, humifuse euphorbia herb, liquorice and cortex albiziae are weighed by proportion, and ground into powder, so as to prepare a conventional medicament for oral use. Traditional Chinese medicines are used for achieving the effect of treating both manifestation and root cause of disease, the total effective rate is 96%, radical treatment is implemented based on pathology; the effect is ideal, side effects are avoided, and pain of patients suffering from the spleen-deficiency diarrhea type IBS can be relieved.
Owner:李柏群
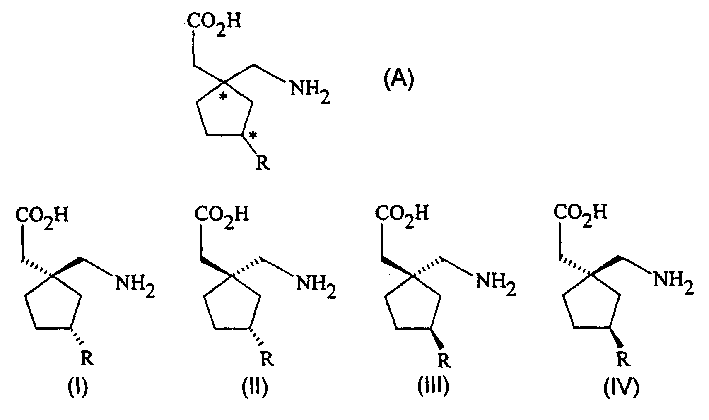
Method for the stereoselective synthesis of cyclic amino acids
The present invention relates to a method for stereospecifically synthesizing 3-substituted 5-membered ring isomers of formula (A). The final product is useful in the treatment of epilepsy, syncope, hypokinesia, cranial disorders, neurodegenerative disorders, depression, anxiety, panic, pain, neuropathological disorders, gastrointestinal disorders such as irritable bowel syndrome (IBS), Medications for inflammation especially arthritis, sleep disorders, PMS, and hot flashes. The present invention provides a method for stereoselectively synthesizing gabapentin (Neurontin) analogues of formulas (I), (II), (III) and (IV) and pharmaceutically acceptable salts thereof, wherein R is C1-C10 alkyl Or C3-C10 cycloalkyl.
Owner:WARNER LAMBERT CO LLC
Traditional Chinese medicine composition for treating gastrointestinal diseases and preparation method thereof
The invention provides a traditional Chinese medicine composition for treating gastrointestinal diseases. The traditional Chinese medicine composition is prepared from the following raw materials: ganoderma spores, red ganoderma lucidum spores, coriolus versicolor, cordyceps militaris sporocarp, hericium erinaceus, herba dendrobium officinale, crataegi fructus, santali albi lignum, nutgrass galingale rhizome, pakchoi seedling, galli gigerii endothelium corneum and amomi fructus rotundus. The traditional Chinese medicine composition can obviously improve spleen deficiency signs and has a rehabilitation effect; intestinal hyperfunction is obviously antagonized, intestinal function inhibition is improved, and the effect of regulating intestinal functions is achieved; an adjusting effect is achieved on gastrointestinal dysfunction; and the obvious effects of relieving pain, resisting fatigue, resisting anoxia and resisting stress are achieved. Research results show that the composition hasa significant improvement effect on functional gastrointestinal diseases or irritable bowel syndrome, is low in cost and easy to operate, and has a wide application prospect.
Owner:北京沃德中医药研究院
Health-care food with functions of tonifying spleen, eliminating dampness and regulating gastrointestinal tract as well as preparation method and application thereof
PendingCN113068833AThe combination ratio is scientific and reasonableImprove bowel functionConfectionerySweetmeatsVigna umbellataWolfiporia extensa
The invention discloses a health-care food with functions of tonifying spleen, clearing damp and regulating gastrointestinal tract and a preparation method thereof. The health-care food is prepared from 1-3 parts of ginseng, 1-3 parts of poria cocos, 1-3 parts of Chinese yam, 1-3 parts of white hyacinth bean, 1-3 parts of lotus seed, 2-6 parts of coix seed, 8-24 parts of fructus amomi, 5-15 parts of platycodon grandiflorum, 10-30 parts of liquorice, 10-30 parts of orange peel, 1-3 parts of gordon euryale seed, 1-3 parts of phaseolus calcaratus, 8-24 parts of ginger, 20-60 parts of agastache rugosus, 17-51 parts of purslane, 2-6 parts of endothelium corneum gigeriae galli, 2-6 parts of clove and 10-30 parts of rhizoma polygonati. According to the health-care product provided by the invention, raw materials are screened according to a theory of treatment based on syndrome differentiation, and all the raw materials are raw materials for medicine and food. Functional experiment results show that the health-care product has good functions of tonifying spleen, eliminating dampness, regulating gastrointestinal tracts and the like, also has an auxiliary conditioning effect on irritable bowel syndrome, is free of toxic and side effects, and can be conveniently prepared into health-care foods such as jelly, solid beverages, candies or paste and the like.
Owner:苏州雷允上健康养生有限公司
Composition for the treatment of digestive pathologies
InactiveCN1909919ARelieve painRelieve symptomsOrganic active ingredientsNervous disorderPathology diagnosisHyperalgesia
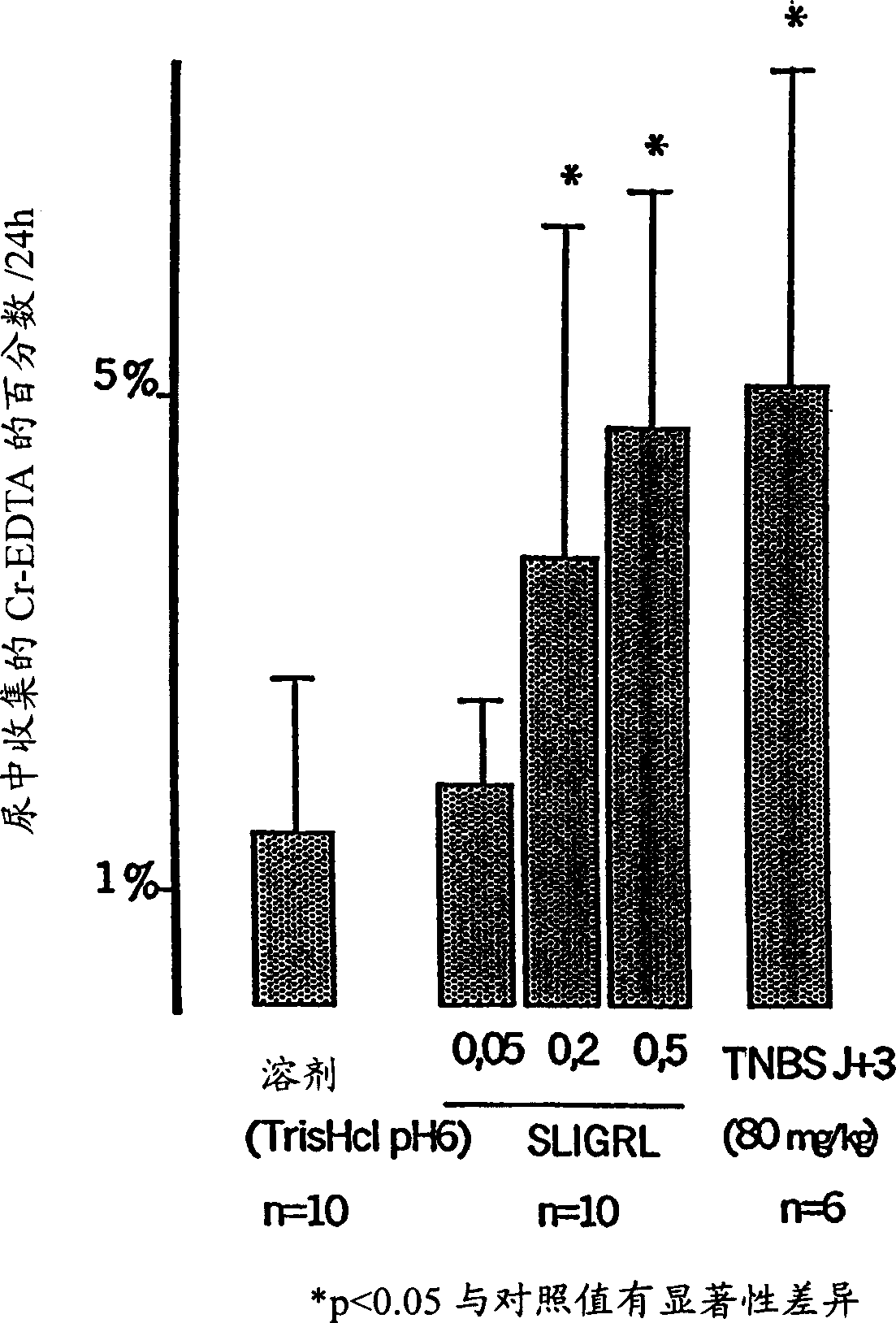
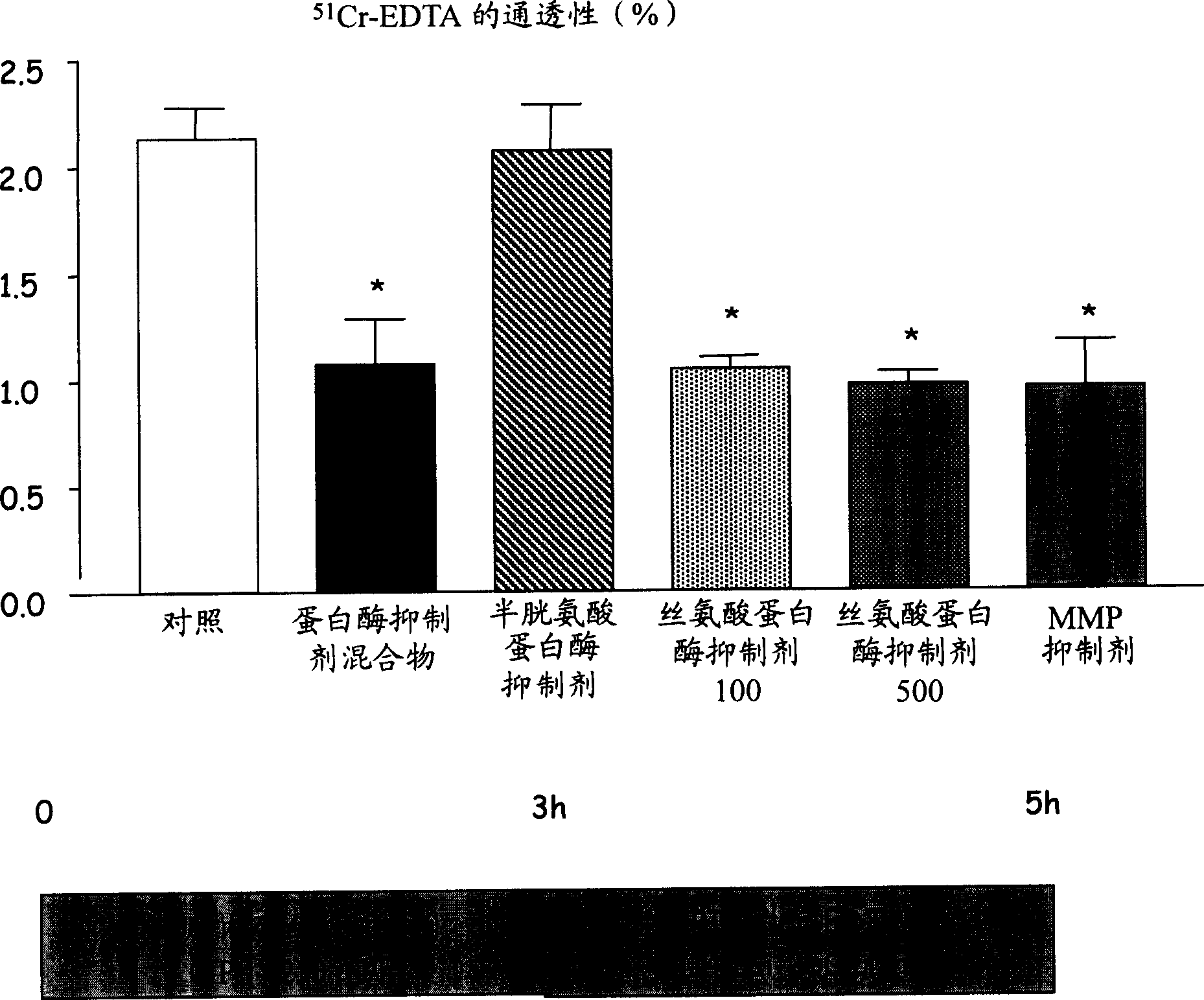
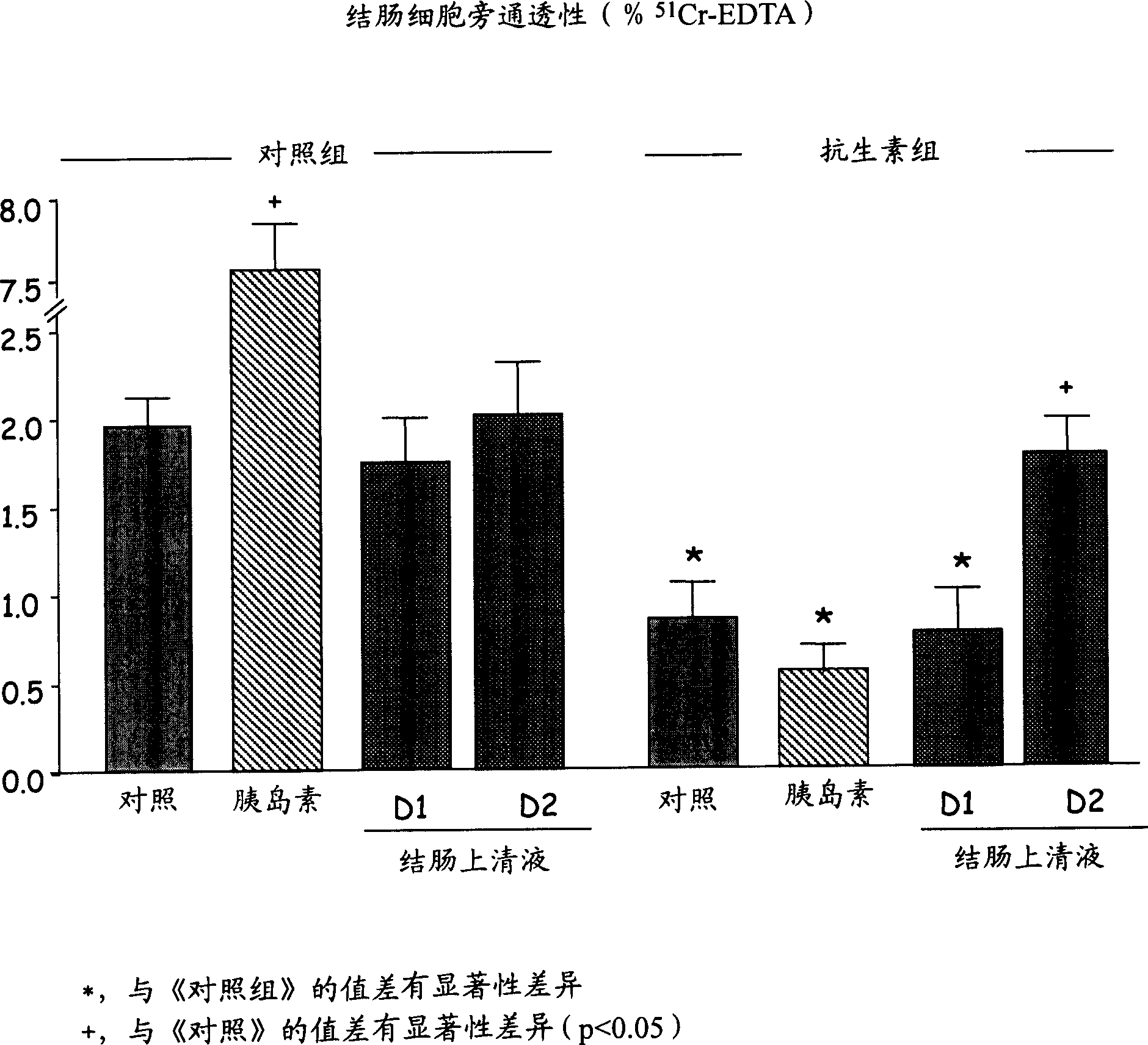
Use of at least one protease inhibitor (I) to prepare a composition for preventative or curative treatment of intestinal disorders characterized by hyperalgesia. - An INDEPENDENT CLAIM is also included for a pharmaceutical product (A) containing at least one (I) and at least one other agent (II), i.e. an anticholinergic, prokinetic or antidiarrhea agent; laxative; modifier of motility or of viscero-sensitivity, for combined use, separately or sequentially. - ACTIVITY - Analgesic; Antiinflammatory. - MECHANISM OF ACTION - (I) control (reduce) the paracellular permeability of the intestinal epithelium, by controlling the light junctions, and associated pain. Proteases, produced by bacteria in the colon, activate receptors in the membrane of epithelial cells that modulate opening of these junctions, where opening is responsible for hyperalgesia. Mice were perfused, through the colon, with 250 mu l / hour of a solution of protease inhibitors, labeled with 51-chromium-EDTA. After 5 hours, the colons were removed and the amount of bound radioactivity measured as an indicator of paracellular permeability. For a control, retention of radioactivity was about 2.1%; compare about 1.1% following perfusion with a cocktail of protease inhibitors (or with serine protease or matrix metalloprotease inhibitors).
Owner:RYTEK
Stereoselective synthesis of cyclic amino acids
The instant invention is a route to stereospecific 3-substituted 5-membered ring isomers of Formula (A). The final products are useful as agents in the treatment of epilepsy, faintness attacks, hypokinesia, cranial disorders, neurodegenerative disorders, depression, anxiety, panic, pain, neuropathological disorders, gastrointestinal disorders such as irritable bowel syndrome (IBS), inflammation especially arthritis, sleep disorders, premenstrual syndrome, and hot flashes. The invention provides novel routes to synthesize stereoselectively analogs of gabapentin (Neurontin3) of Formulas (I), (II), (III) and (IV) wherein R is C1-C10 alkyl or C3-C10 cycloalkyl and pharmaceutically acceptable salts thereof.
Owner:WARNER-LAMBERT CO
Acetyl mimic compounds for the inhibition of isoprenyl-s-cysteinyl methyltransferase
Among other things, the present invention provides novel compounds capable of effectively inhibiting inflammatory responses that are mediated by G-proteins or GPCRs in neutrophils, macrophages and platelets. In particular, compounds of the present invention act as inhibitors of edema, inhibitors of erythema and inhibitors of MPO (myeloperoxidase), pharmaceutical compositions containing the same compounds and the use thereof for the treatment of diseases that may benefit from edema, erythema and MPO inhibition, such as inflammation (acute or chronic), asthma, autoimmune diseases, and chronic obstructive pulmonary disease (COPD) (e.g., emphysema, chronic bronchitis and small airways disease, etc.), inflammatory responses of the immune system, skin diseases (e.g., reducing acute skin irritation for patients suffering from rosacea, atopic dermatitis, seborrheic dermatitis, psoriasis), irritable bowel syndrome (e.g., Chron's disease and ulcerative colitis, etc.), and central nervous system disorders (e.g., Parkinson's disease).
Owner:SIGNUM BIOSCIENCES INC
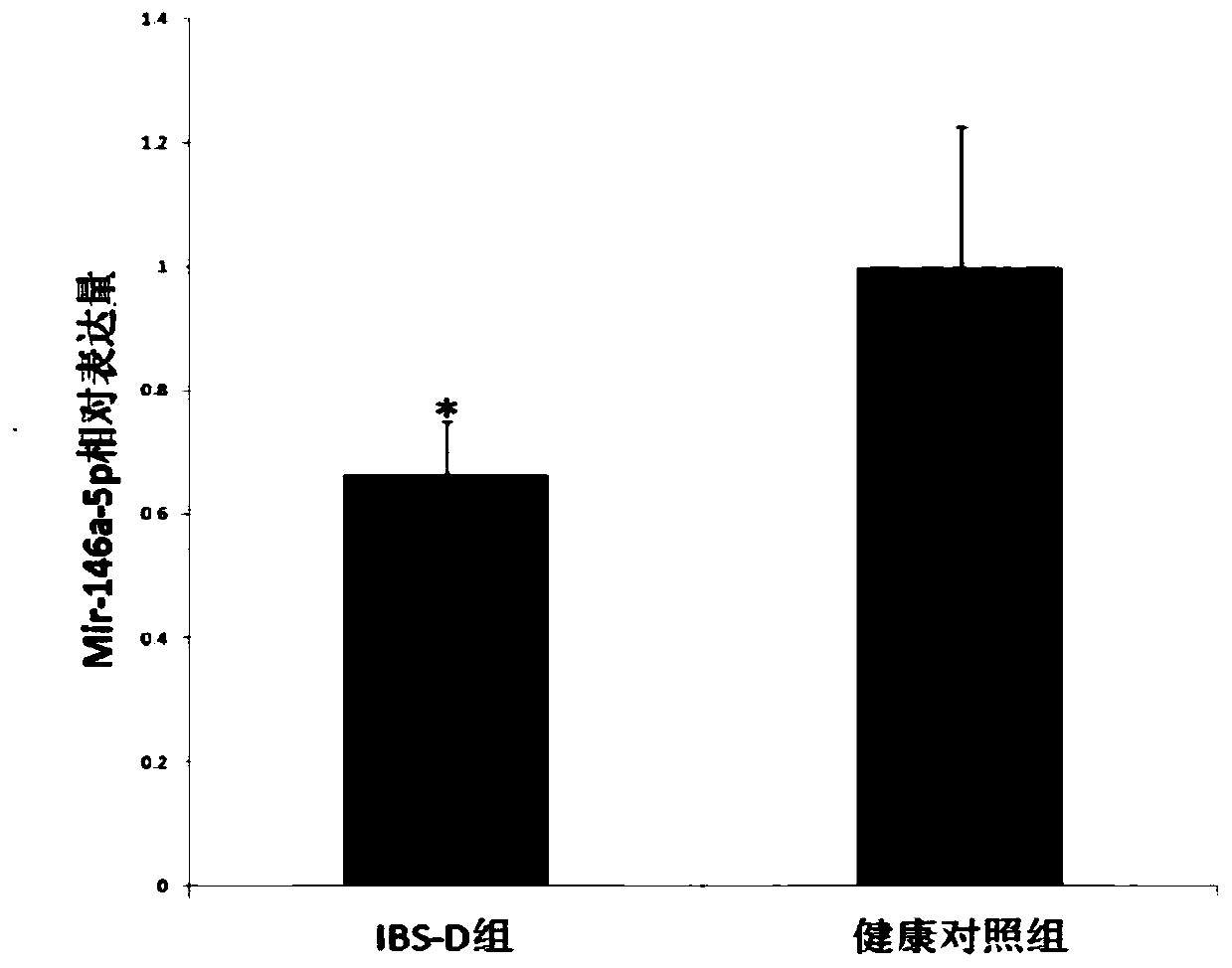
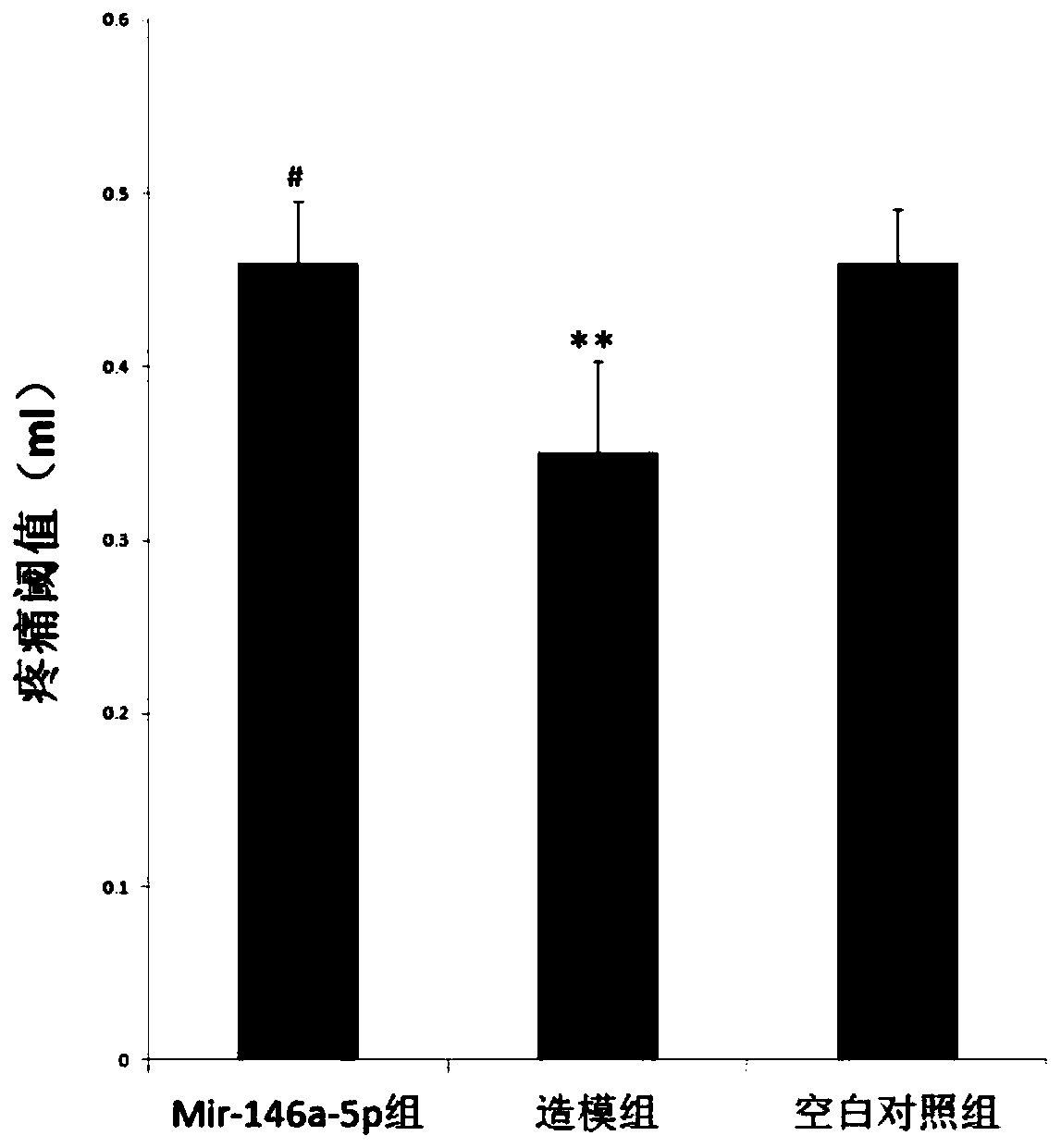
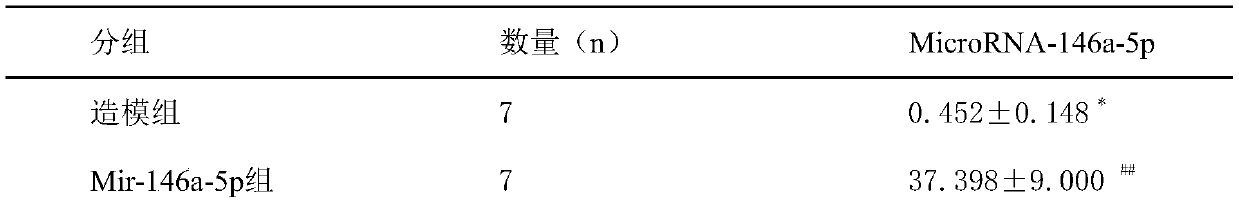
Application of mir-146a-5p in the treatment of visceral hypersensitivity in irritable bowel syndrome
ActiveCN107951898BReduce sensitivityOrganic active ingredientsGenetic material ingredientsIntraperitoneal routeDisease
The invention discloses application of mir-146a-5p to treating visceral hypersensitivity of irritable bowel syndrome. The mir-146a-5p is low in expression in peripheral serum of patients with visceralhypersensitivity of irritable bowel syndrome and intestinal mucosa of disease model mice with irritable bowel syndrome; meanwhile, after the disease model mice of irritable bowel syndrome receive intraperitoneal injection of hsa-mir-146a-5p agomir to achieve high expression of mir-146a-5p, the visceral sensitivity of the disease model mice is significantly reduced, so that the effectiveness of mir-146a-5p on reducing visceral hypersensitivity of the irritable bowel syndrome can be proved, and an effective way for treating visceral hypersensitivity of irritable bowel syndrome can be provided.
Owner:SHANDONG UNIV QILU HOSPITAL
Spiroindoline derivatives as gonadotropin-releasing hormone receptor antagonists
InactiveCN104169287AOrganic active ingredientsOrganic chemistryGonadotropin-releasing hormone receptorDisease
Owner:BAYER IP GMBH
Clostridium consortium compositions and methods of treating obesity, metabolic syndrome and irritable bowel diseases
PendingCN114258299AIncrease abundanceIncrease weightBacteriaMetabolism disorderIrritable bowelSmall intestine
Disclosed herein are compositions comprising clostridium consortia, and methods of treating obesity, metabolic syndrome, irritable bowel disease, and reducing weight gain and inhibiting lipid absorption in the small intestine by administering the compositions to a subject.
Owner:UNIV OF UTAH RES FOUND
Probiotic Strains for Use in Improving the Enteric Nervous System
ActiveUS20130195822A1Function increasePromote bowel movementsBiocideBacteriaLactobacillusNervous system
The invention relates to the use of lactic acid bacteria, for use in modifying the enteric nervous system and more particularly in treating and / or preventing intestinal disorders such as constipation and / or irritable bowel disease.
Owner:DANONE
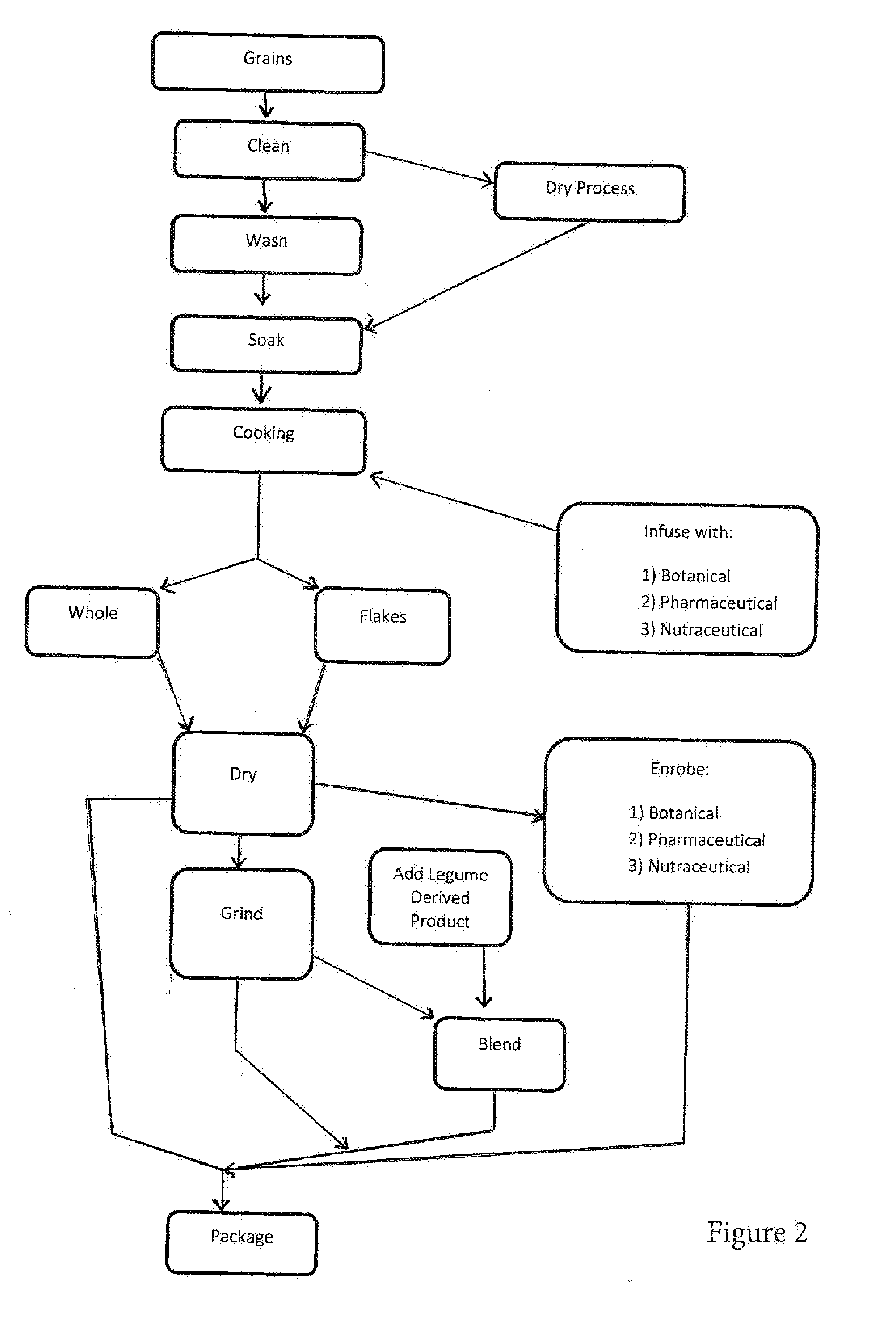
Legume/grain based food product with prebiotic/probiotic source
ActiveUS20160175368A1Reduce impactPrevent goodBiocidePharmaceutical delivery mechanismDiseaseReady to eat
A minimally processed ready-to-eat legume / grain product is provided containing a significant amount of probiotic fiber and a probiotic source or is consumed with a probiotic source for the prevention and / or treatment of inflammatory chronic conditions such as inflammatory, autoimmune chronic conditions such as Irritable Bowel Syndrome (IBS), digestive disorders such chronic constipation, gastric acid reflux, diabetes, heart disease, obesity, some type of cancer, malabsorptive disorders, eczema as well mental health disorders such as anxiety disorder. The present invention is suitable for men and woman of all ages.
Owner:MARK M STERNER
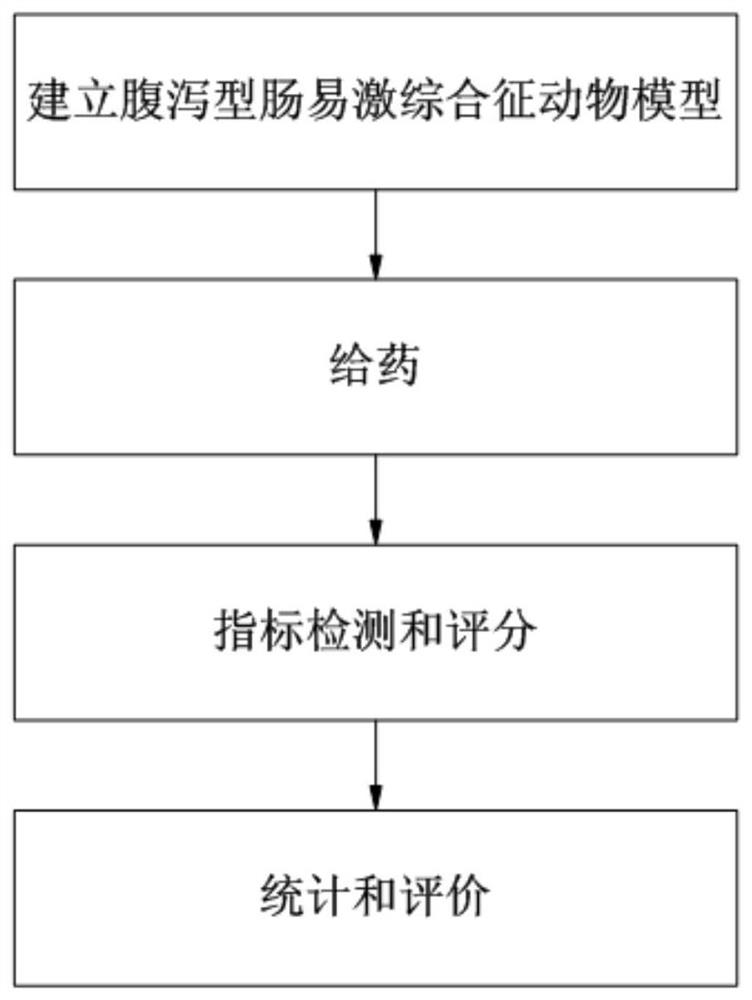
Method for evaluating curative effect of compound glutamine formula on diarrhea-predominant irritable bowel syndromes
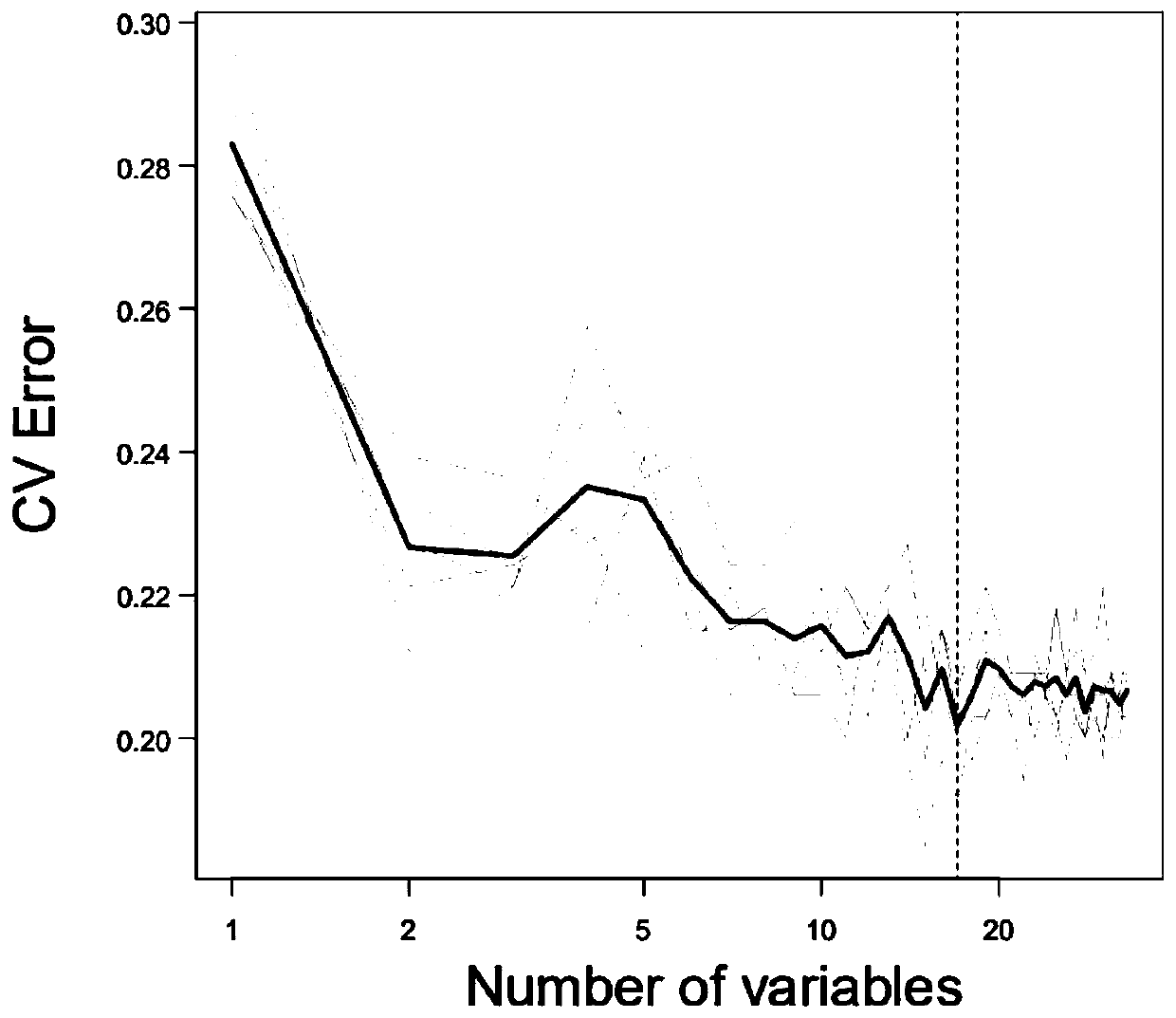
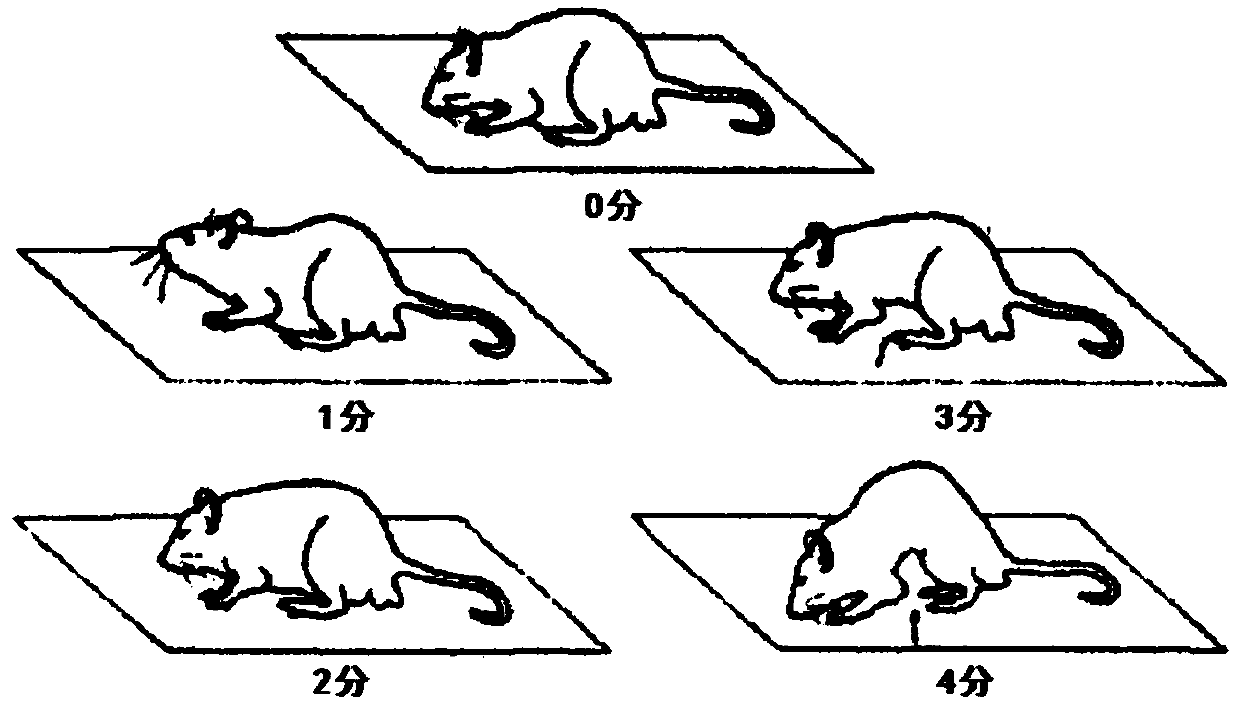
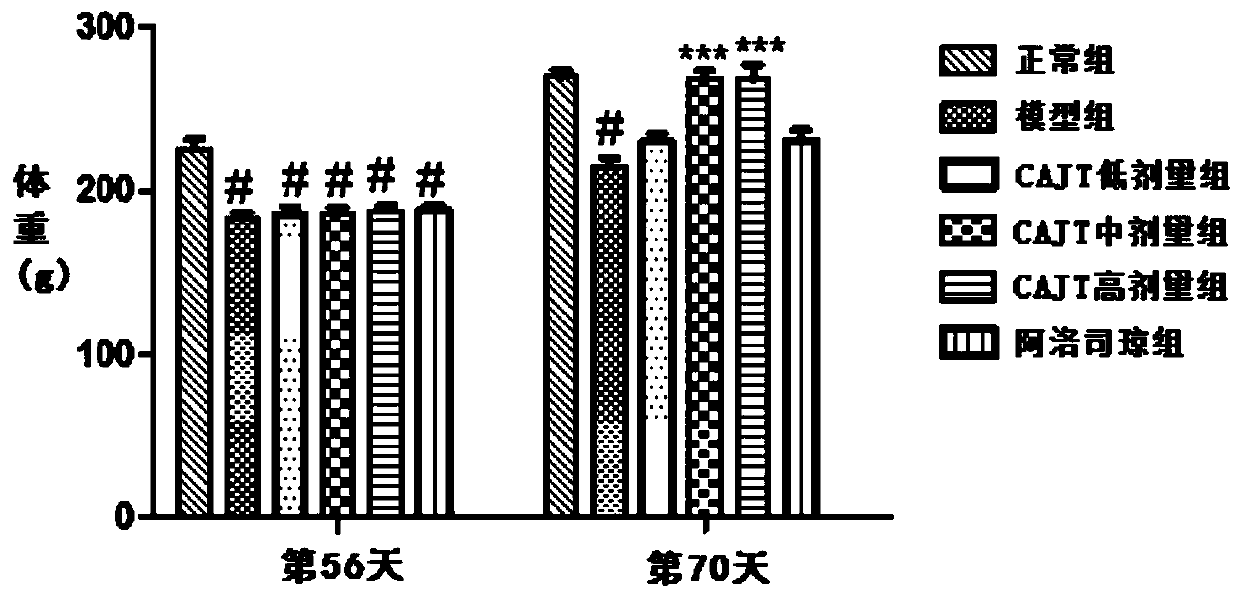
ActiveCN111905111AFully reflectFull evaluationCompounds screening/testingIrritable bowelIntestinal motility
The invention discloses a method for evaluating the curative effect of a compound glutamine formula on diarrhea-predominant irritable bowel syndromes. The method mainly comprises the following steps:I, establishing a diarrhea-predominant irritable bowel syndrome animal model; II, performing medicine application; III, performing index detection and scoring (including general clinical observation,excrement and urine detection, intestinal motility influence observation, visceral sensitivity evaluation and colon tissue pathological examination); and IV, performing counting and evaluation. With the adoption of the scheme, the compound glutamine formula with different dosage forms and different use doses can be accurately evaluated in the aspect of treating diarrhea-predominant irritable bowelsyndromes; and preposed basis and valuable guidance are provided for subsequent continuous optimization of dosage form rationality and dosage use of the compound glutamine formula, and further betterin-depth clinical treatment and research of the diarrhea-predominant irritable bowel syndromes and continuous clarification of IBS curative effects.
Owner:DIAO GRP CHENGDU PHARMA
Traditional Chinese medicine composition for preventing and treating bacterial diarrhea of livestock and poultry and application thereof
InactiveCN102429967BPromote growth and developmentGood treatment effectAntibacterial agentsAnthropod material medical ingredientsBiotechnologyChronic diarrhea
The invention discloses a traditional Chinese medicine composition for preventing and treating bacterial diarrhea of livestock and poultry, and belongs to the field of traditional Chinese medicines. The traditional Chinese medicine composition comprises the following components in percentage by weight: 18-19% of coptis root, 14-15% of nutgall, 14-15% of pomegranate rind, 14-15% of myrobalam, 22-23% of astragalus mongholicus, 7-8% of atractylis ovata and 7-8% of radix sileris. The traditional Chinese medicine composition for preventing and treating bacterial diarrhea of livestock and poultry disclosed by the invention has significant curative effects on treating colibacillosis, salmonellosis and other bacterial diarrhea as well as chronic enteritis, irritable colon and other chronic diarrhea of the livestock and poultry. At the same time, the traditional Chinese medicine composition disclosed by the invention has the advantages of low production cost, and small toxic or side effect.
Owner:GANSU AGRI UNIV
Pharmaceutical preparation and application thereof to preparation of drug for treating depressive disorder and enteritis
The invention relates to the technical field of chemical drugs, discloses a pharmaceutical preparation and application thereof to preparation of a drug for treating depressive disorder and enteritis and relates to an otilonium bromide drug and application thereof to preparation of a drug for treating depressive disorder, post-stroke depression, depressive episode, chronic enteritis, irritable bowel syndrome and intestinal spasm. The otilonium bromide drug provided by the invention is an otilonium bromide tablet, an otilonium bromide capsule, an otilonium bromide enteric-coated tablet and an otilonium bromide enteric capsule. According to the technology provided by the invention, the clinical application range of otilonium bromide is widened, and meanwhile, a novel medication choice and a novel clinical diagnosis and treatment scheme are provided for treating diseases such as depressive disorder, post-stroke depression, depressive episode, chronic enteritis, irritable bowel syndrome and intestinal spasm.
Owner:王秀芹
Plant composition capable of relieving diarrhea-type irritable bowel syndrome
ActiveCN113786460AImprove and relieve symptomsDigestive systemPlant ingredientsLophatherumIrritable colon
The invention discloses a plant composition capable of relieving diarrhea type irritable bowel syndrome, and belongs to the field of plant composition application. According to the plant composition, gynura procumbens is used as a main component, meanwhile, perilla frutescens, lophatherum gracile, platycodon grandiflorum and poria cocos are used as auxiliary components to jointly generate a synergistic effect, the symptoms of the diarrhea type irritable bowel syndrome can be remarkably improved and relieved, and the plant composition is safe and free of toxic and side effects. The invention also discloses a preparation method of the plant composition and a medicinal preparation which is prepared from the plant composition and can relieve diarrhea-type irritable bowel syndrome.
Owner:完美(广东)日用品有限公司 +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com