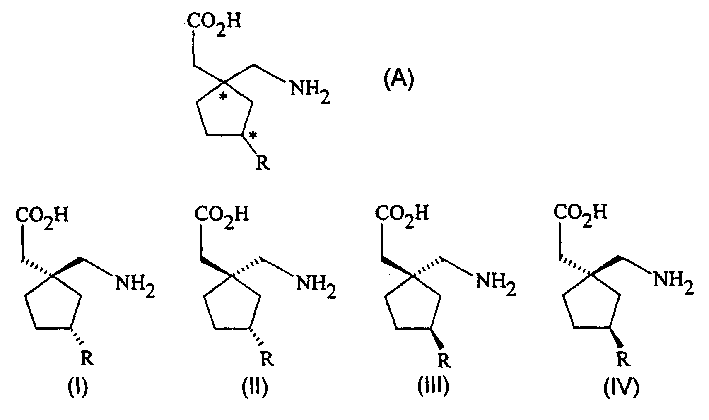
Stereoselective synthesis of cyclic amino acids
A kind of cycloalkyl, cyanoacetate technology, applied in Summary of the invention
In the field of 000, it is possible to solve problems such as being difficult to find stereoisomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0363] Example 1 (i) NCCH 2 CO 2 Et, NH 4 OAc, AcOH, toluene, reflux; (ii) PhCH 2 MgCl, THF, -78°C; (iii) KOH, ethylene glycol, 160°C; (iv) (S)-(-)-α-methylbenzylamine, EtOAc, 0°C; (v) HCl(aq ); (vi) (PhO) 2 P(O)N 3 ,Et 3 N, toluene, reflux; (vii) MeOH, toluene, reflux; (viii) RuCl 3 , NaIO 4 , CCl 4 , MeCN, H 2 O; (ix) 6N HCl, 1,4-dioxane. (E and Z)-cyano-((R)-3-methyl-1,1-cyclopentylene)-ethyl acetate
[0364](R)-(+)-3-Methylcyclopentanone (5g, 51.0mmol), ethyl cyanoacetate (5.42mL, 51.0mmol), ammonium acetate (0.4g, 5.1mmol) and glacial acetic acid (0.58 mL, 10.2 mmol) was refluxed in toluene (30 mL) using a Dean-Stark trap. After 6 hours, the mixture was cooled and diluted with ethyl acetate (100 mL), washed with water (3 x 80 mL) and brine then dried (MgSO 4 ). The solvent was evaporated under reduced pressure. The residue was chromatographed (silica gel, heptane / ethyl acetate, 9:1) to give 8.87 g (90%) of (E and Z)-cyano-((R)-3-methy...
Embodiment 2
[0372] Example 2 (i)(CH 3 ) 3 SiCHN 2 , MeOH, toluene; (ii) RuCl 3 , NaIO 4 , CCl 4 , MeCN, H 2 O; (iii) (PhO) 2 P(O)N 3 ,Et 3 N, toluene, reflux; (iv) MeOH, toluene, reflux; (v) 6N HCl, 1,4-dioxane. ((1S,3R)-1-Benzyl-3-methyl-cyclopentyl)-acetic acid methyl ester
[0373] Trimethylsilyldiazomethane (31.5mL of 2M hexane solution, 63mmol) was added dropwise to the stirred ((1S,3R)-1-benzyl-3-methyl- Cyclopentyl)-acetic acid (10 g, 43 mmol) in toluene (80 mL) and methanol (20 mL) and the mixture was then warmed to room temperature. The mixture was stirred for 1 hour and then the solvent was evaporated under reduced pressure. The residue was added to ethyl acetate (50 mL), washed with saturated sodium bicarbonate solution, dilute hydrochloric acid, dried (MgSO 4 ) and the solvent was distilled off in vacuo to give 10.6 g (100%) of ((1S,3R)-1-benzyl-3-methyl-cyclopentyl)-methyl acetate; Rf (heptane-ethyl acetate, 9: 1) 0.40; IR film (cm ...
Embodiment 3
[0378] Example 3 (i)a) Oxalyl chloride, DMF, CH 2 Cl 2 ; b) t-BuOH, (i-Pr) 2 Net, CH 2 Cl 2 ; (ii) RuCl 3 , NaIO 4 , CCl 4 , MeCN, H 2 O; (iii) (CH 3 ) 3 SiCHN 2 , MeOH, toluene; (iv) CF 3 CO 2 H, CH 2 Cl 2 ;(v)(PhO) 2 P(O)N 3,Et 3 N, toluene, reflux; (vi) MeOH, toluene, reflux; (vii) 6N HCl, 1,4-dioxane. ((1S,3R)-1-Benzyl-3-methyl-cyclopentyl)-tert-butyl acetate
[0379] Oxalyl chloride (4.14 mL, 47 mmol) was added dropwise to a stirred solution of ((1S,3R)-1-benzyl-3-methyl-cyclopentyl)-acetic acid (10 g, 43 mmol) under argon atmosphere at room temperature. in dichloromethane solution. The reaction mixture was cooled to 5°C, dimethylformamide (1 mL) was carefully added, the mixture was allowed to warm to room temperature and stirring was continued for 2 hours. The solvent was evaporated in vacuo and the residue was diluted with dichloromethane (60 mL). To the reaction mixture was carefully added 1,1-dimethylethanol (15 mL) foll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com