Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
70 results about "Candidal infections" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
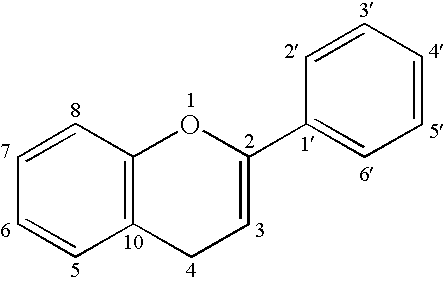
Candidiasis is a fungal infection due to any type of Candida (a type of yeast). When it affects the mouth, it is commonly called thrush. Signs and symptoms include white patches on the tongue or other areas of the mouth and throat.
Oral compositions and use thereof
InactiveUS20040057908A1Cosmetic preparationsToilet preparationsAphthous ulcerationsDental plaque formation
The present invention relates to an oral composition which includes an organoleptically suitable carrier and an amount of a terpenoid and a flavonoid, dispersed in the carrier, which is effective to prevent or treat dental caries, dental plaque formation, gingivitis, candidiasis, dental stomatitis, aphthous ulceration, or fungal infection. The invention also relates to various uses of oral compositions, containing a terpenoid, a flavonoid, or both, such uses include: inhibiting the activity of surface-bound glusosyltransferase; treating or inhibiting dental caries, gingivitis, candidiasis, denture stomatitis; inhibiting the accumulation of microorganisms on an oral surface; and / or treating or inhibiting aphthous ulcerations on an oral surface.
Owner:UNIV ESTADUAL DE CAMPINAS UNICAMP +1
Pharmaceutical compositions and methods to vaccinate against candidiasis
InactiveUS20030124134A1Treat prevent alleviateToxic reductionPeptide/protein ingredientsSnake antigen ingredientsHospitalized patientsVirulent characteristics
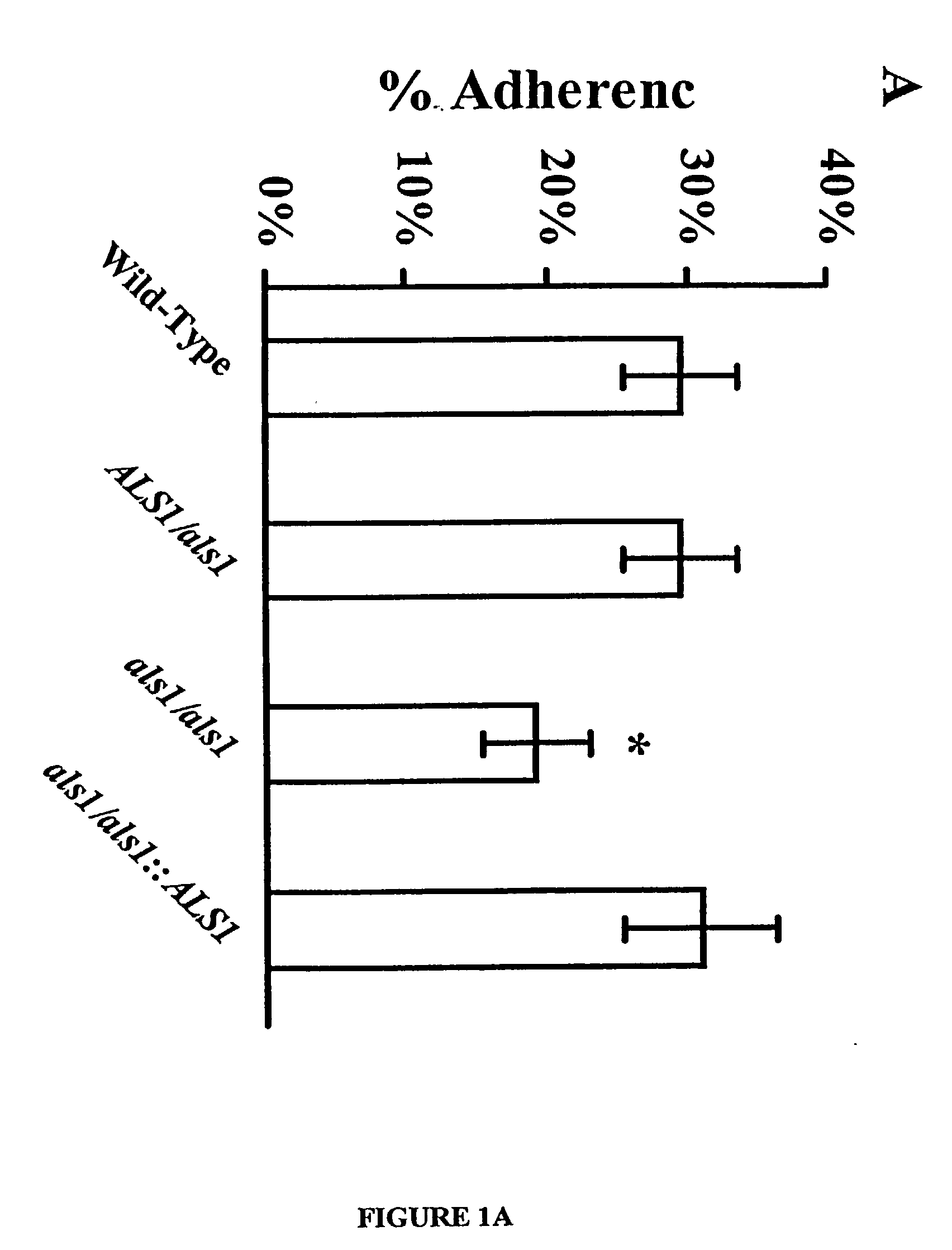
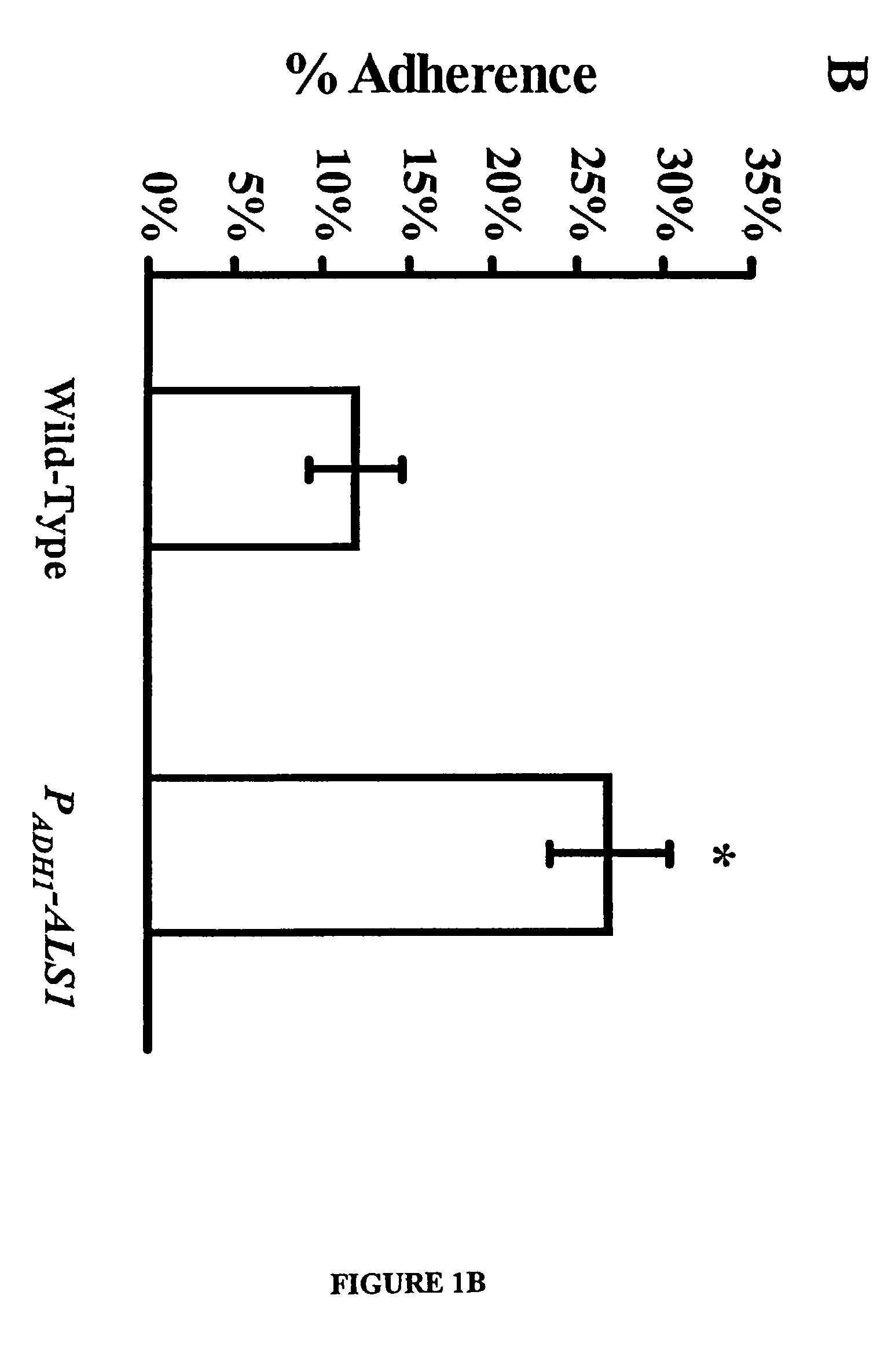
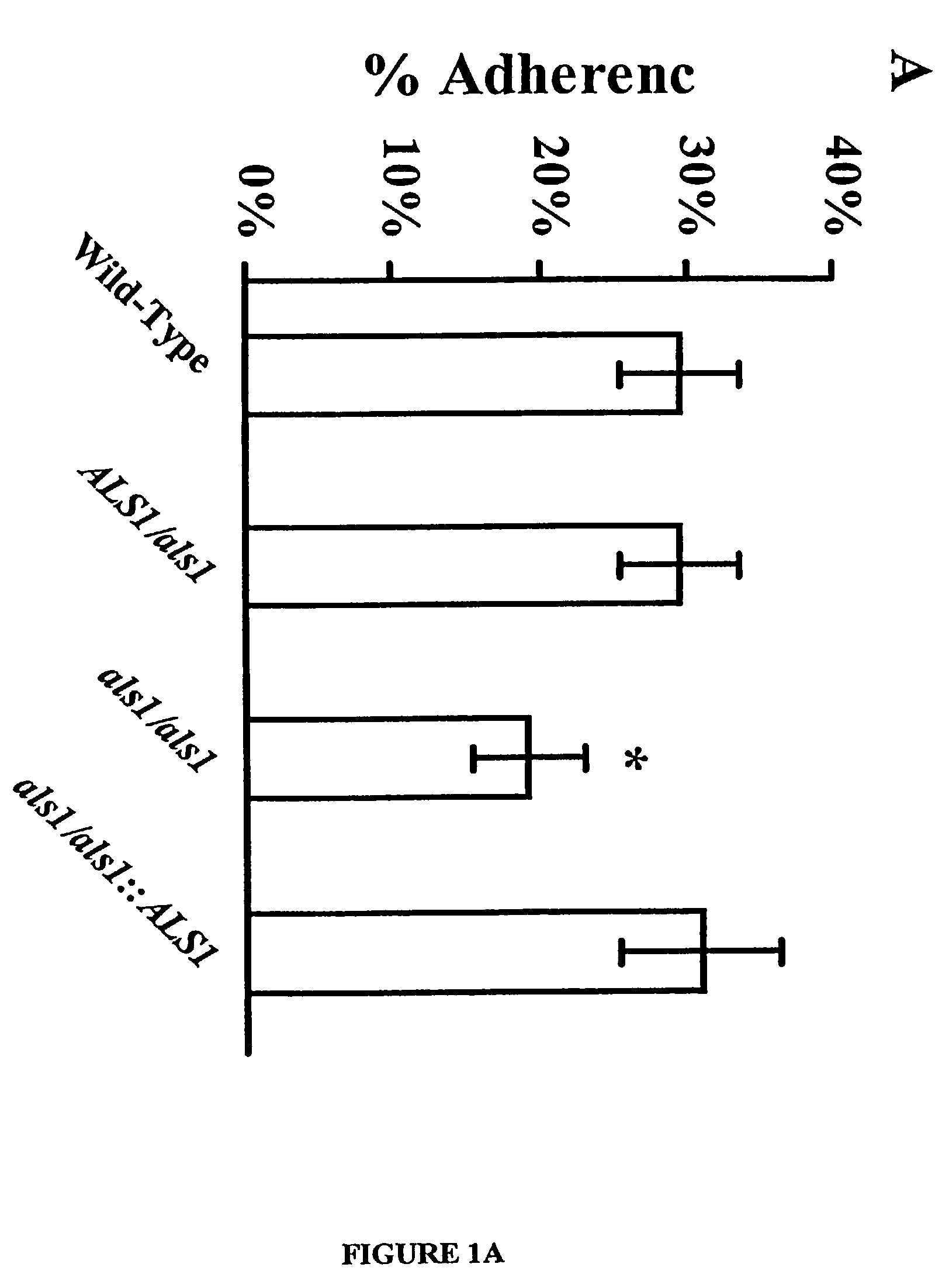
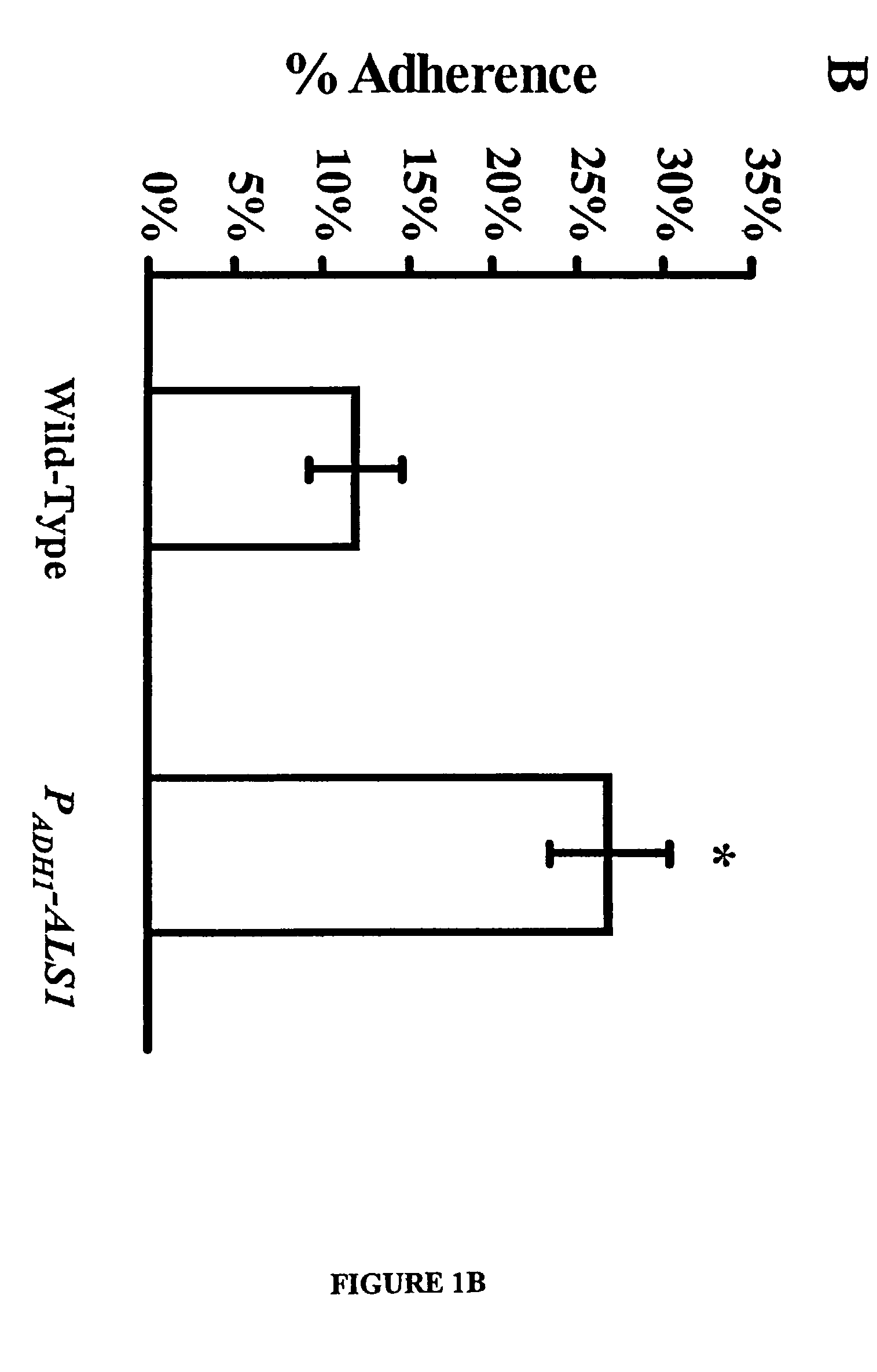
A Candida albicans bloodstream infections cause significant morbidity and mortality in hospitalized patients. Filament formation and adherence to host cells are critical virulence factors of C. albicans. Multiple filamentation regulatory pathways have been discovered, however the downstream effectors of these regulatory pathways remain unknown. The cell surface proteins in the ALS group are downstream effectors of the filamentation regulatory pathway. Particularly, Als1p mediates adherence to endothelial cells in vitro and is required for virulence. The blocking of adherence by the organism is described resulting from the use of a composition and method disclosed herein. Specifically, a pharmaceutical composition comprised of a gene, gene product, or specific antibody to the ALS gene family is administered as a vaccine to generate an immune response capable of blocking adherence of the organism.
Owner:LOS ANGELES BIOMEDICAL RES INST AT UCLA HARBOR MEDICAL CENT +1
Method and formulation for treating candidiasis using morinda citrifolia
InactiveUS7014873B2Good curative effectPrevent CandidiasisBiocideAnimal repellantsFruit juiceMonilinia laxa
The present invention features a novel use of processed ingredients from the Indian mulberry plant, and particularly a novel use of one or more processed Morinda citrifolia-based naturaceutical formulations comprising one or more of a processed Morinda citrifolia fruit juice, puree juice, oil or oil extract, dietary fiber, alcohol extract, etc., for inhibiting and preventing the overgrowth of Candida fungus and for treating Candidiasis and its associated symptoms.
Owner:TAHITIAN NONI INT INC
Method and formulation for treating Candidiasis using Morinda citrifolia
InactiveUS20060159788A1Good curative effectPrevent CandidiasisBiocideAntiinfectivesFruit juiceAlcohol
The present invention features a novel use of processed ingredients from the Indian mulberry plant, and particularly a novel use of one or more processed Morinda citrifolia-based naturaceutical formulations comprising one or more of a processed Morinda citrifolia fruit juice, puree juice, oil or oil extract, dietary fiber, alcohol extract, etc., for inhibiting and preventing the overgrowth of Candida fungus and for treating Candidiasis and its associated symptoms.
Owner:TAHITIAN NONI INT INC
Detection of secreted lipase proteins from Candida species
Methods and devices for the detection of proteins secreted by the pathogenic growth form of Candida species are disclosed. The disclosed devices may constitute a method for the diagnosis of acute or chronic infections, including candidiasis, caused by microorganisms of the Candida species, such as C. albicans, for example. The devices of the present invention incorporate antibodies specific to lipase proteins whose expression is differentiated upon the conversion of the Candida species from the commensal to the pathogenic form. The antibodies may be used in assays to allow the diagnosis of candidal infections and disease conditions. Either monoclonal antibodies or polyclonal antibodies may be used, and in the case of the monoclonals, the specific epitopes of the LIP protein may be detected as well as the LIP protein itself.
Owner:KIMBERLY-CLARK WORLDWIDE INC
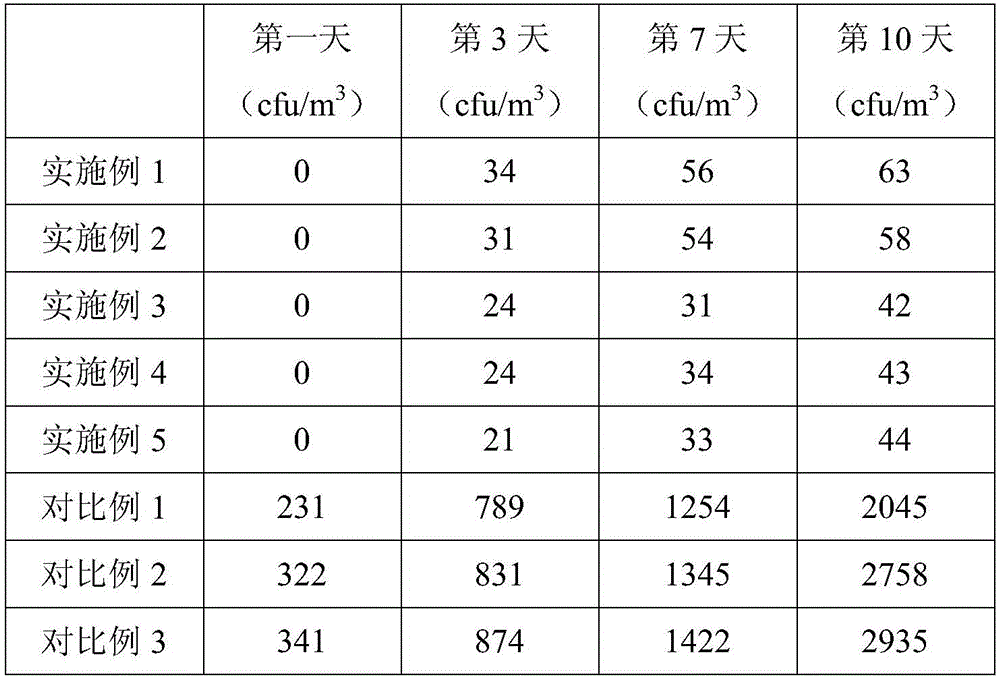
Chitosan antibacterial gel and preparation method thereof
ActiveCN103920072AGood curative effectConvenient careAntibacterial agentsHydroxy compound active ingredientsCelluloseGlycerol
The invention relates to the technical field of antibacterial gel, in particular to chitosan antibacterial gel and a preparation method thereof. Each liter of the chitosan antibacterial gel comprises the following raw materials by weight: 5-30g of modified chitosan, 2.5-10g of carbomer, 1-1.8g of alum, 2-40g of glycerol, 0.3-10g of lactic acid, 0.1-0.8g of menthol and 2-8g of sodium carboxymethylcellulose. The chitosan antibacterial gel which is good in curative effect, can be used for preventing relapse of inflammation and is harmless to human body is prepared by using the synergistic effect of modified chitosan serving as a major raw material as well as carbomer, alum, glycerol, lactic acid and sodium carboxymethylcellulose serving as auxiliary materials, and is suitable for preventing, treating and nursing various vaginal bacterial infections, trichomonas infections, mixed infections, vulvitis, leucorrhea, candidal infection as well as wound infections of cervix uteri, genital tract and mucous membrane. By using the chitosan antibacterial gel, wound healing after physical treatment of cervical erosion is promoted, and nursing of vaginal dryness as well as cross infections in sexual intercourse and the like is improved.
Owner:GUANGXI XINYE BIOLOGICAL TECH
Pharmaceutical compositions and methods to vaccinate against candidiasis
InactiveUS8541008B2Toxic reductionReduce adhesionPeptide/protein ingredientsSnake antigen ingredientsHospitalized patientsVirulent characteristics
A Candida albicans bloodstream infections cause significant morbidity and mortality in hospitalized patients. Filament formation and adherence to host cells are critical virulence factors of C. albicans. Multiple filamentation regulatory pathways have been discovered, however the downstream effectors of these regulatory pathways remain unknown. The cell surface proteins in the ALS group are downstream effectors of the filamentation regulatory pathway. Particularly, Als1p mediates adherence to endothelial cells in vitro and is required for virulence. The blocking of adherence by the organism is described resulting from the use of a composition and method disclosed herein. Specifically, a pharmaceutical composition comprised of a gene, gene product, or specific antibody to the ALS gene family is administered as a vaccine to generate an immune response capable of blocking adherence of the organism.
Owner:LOS ANGELES BIOMEDICAL RES INST AT UCLA HARBOR MEDICAL CENT +1
Detection of secreted aspartyl proteases from Candida species
InactiveUS20070134743A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDisease causeDisease injury
Methods and devices for the detection of proteins secreted by the hyphal growth form of Candida species are disclosed. The disclosed devices may constitute a method for the diagnosis of acute or chronic infections, including candidiasis, caused by microorganisms of the species Candida, such as C. albicans, for example. The devices of the present invention incorporate antibodies specific to secreted aspartyl protease proteins whose expression is upregulated upon the conversion of the Candida species from the commensal to the pathogenic form. The antibodies may be used in assays to allow the diagnosis of candidal infections and disease conditions. Either monoclonal antibodies or polyclonal antibodies may be used, and in the case of the monoclonals, the specific epitopes of the SAP protein may be detected as well as the SAP protein itself.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Method and formulation for treating candidiasis using morinda citrifolia
InactiveUS20030161901A1Good curative effectEnhance relief potentialBiocideUnknown materialsMonilinia laxaAlcohol
The present invention features a novel use of processed ingredients from the Indian mulberry plant, and particularly a novel use of one or more processed Morinda citrifolia-based naturaceutical formulations comprising one or more of a processed Morinda citrifolia fruit juice, puree juice, oil or oil extract, dietary fiber, alcohol extract, etc., for inhibiting and preventing the overgrowth of Candida fungus and for treating Candidiasis and its associated symptoms.
Owner:TAHITIAN NONI INT INC
Detection of secreted aspartyl proteases from Candida species
Methods and devices for the detection of proteins secreted by the hyphal growth form of Candida species are disclosed. The disclosed devices may constitute a method for the diagnosis of acute or chronic infections, including candidiasis, caused by microorganisms of the species Candida, such as C. albicans, for example. The devices of the present invention incorporate antibodies specific to secreted aspartyl protease proteins whose expression is upregulated upon the conversion of the Candida species from the commensal to the pathogenic form. The antibodies may be used in assays to allow the diagnosis of candidal infections and disease conditions. Either monoclonal antibodies or polyclonal antibodies may be used, and in the case of the monoclonals, the specific epitopes of the SAP protein may be detected as well as the SAP protein itself.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Aerosol inhalation device
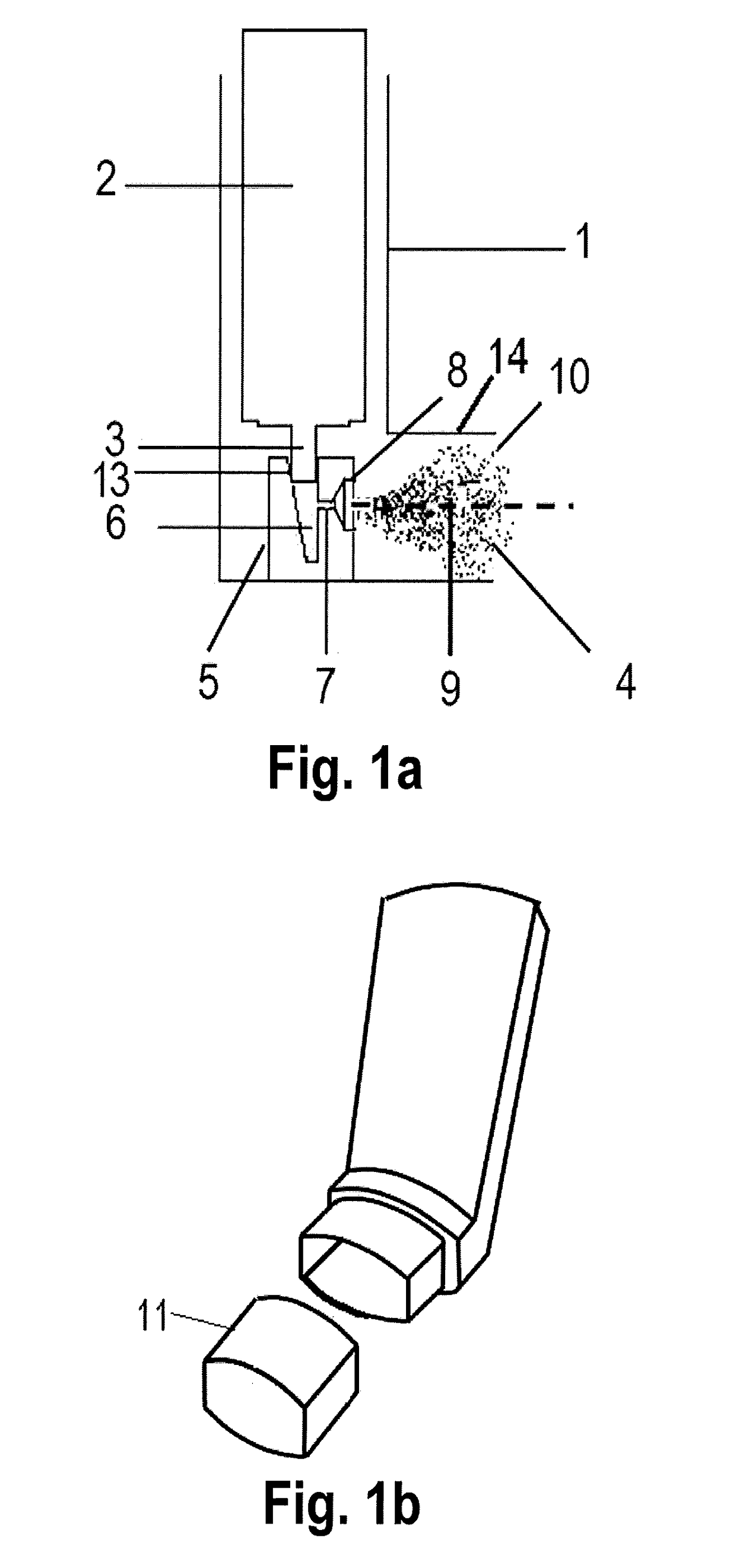
InactiveUS20140060531A1Eliminate requirementsReduce carryRespiratorsMedical devicesSide effectMedicine
Actuators for an aerosol inhalation device containing a housing adapted to receive an aerosol canister containing a pressurized medicament formulation, a mouthpiece portion through which the user inhales, a nozzle block and an orifice and a tubular element extending in the mouthpiece portion from the orifice aperture in a longitudinal axis substantially aligned with a longitudinal axis of the mouthpiece portion provide a significant reduction in the non-respirable, coarse fraction of the emitted aerosol medicament via inertial impaction and retention in the actuator than in the oro-pharynx, with consequent less associated side effects and oral candidiasis in the patient. In addition the presence of the tubular element has minimal, negligible impact on the fine particle dose and on the particle size distribution (PSD) of the delivered particles having aerodynamic diameter lower than 9 μm.
Owner:CHIESI FARM SPA
Method for biological catalytic preparing naprosyn
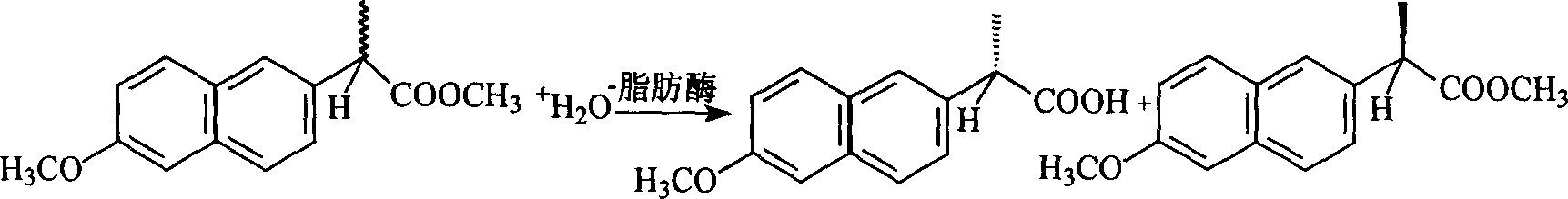
InactiveCN1584037AEasy to separateSolve the problem of slow responseFermentationMedicinal chemistryIonic liquid
A method for preparing optical pure naproxen by lipase chiral resoluted naproxen methoxycarbonyl in water-ionic liquid two-phase system is disclosed. It is carried out by taking lipase cylindrical candidiasis L-1754 as catalyst and taking racemic naproxen methoxycarbonyl as reactive substratum. It achieves three-dimensional selectivity and activity.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
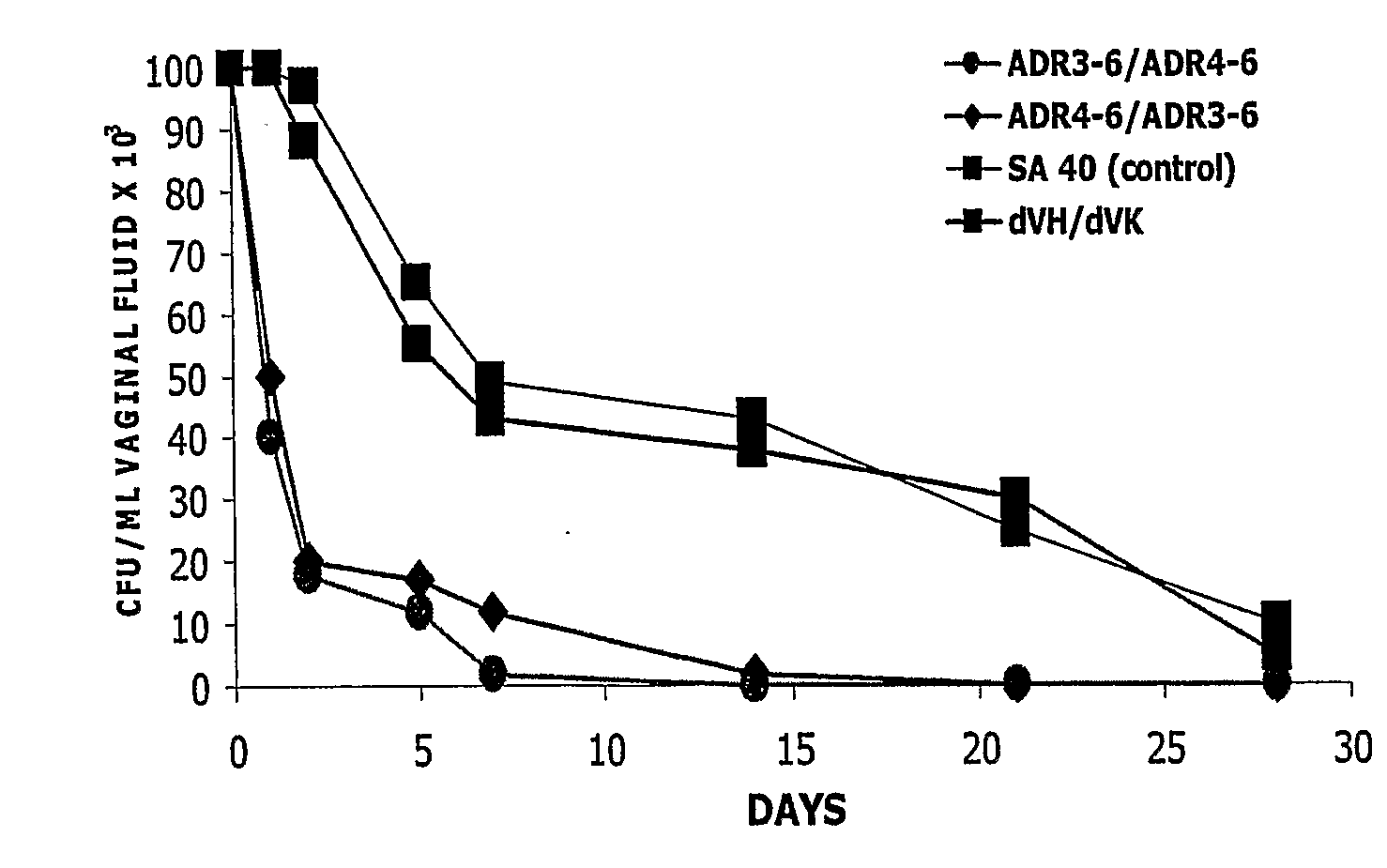
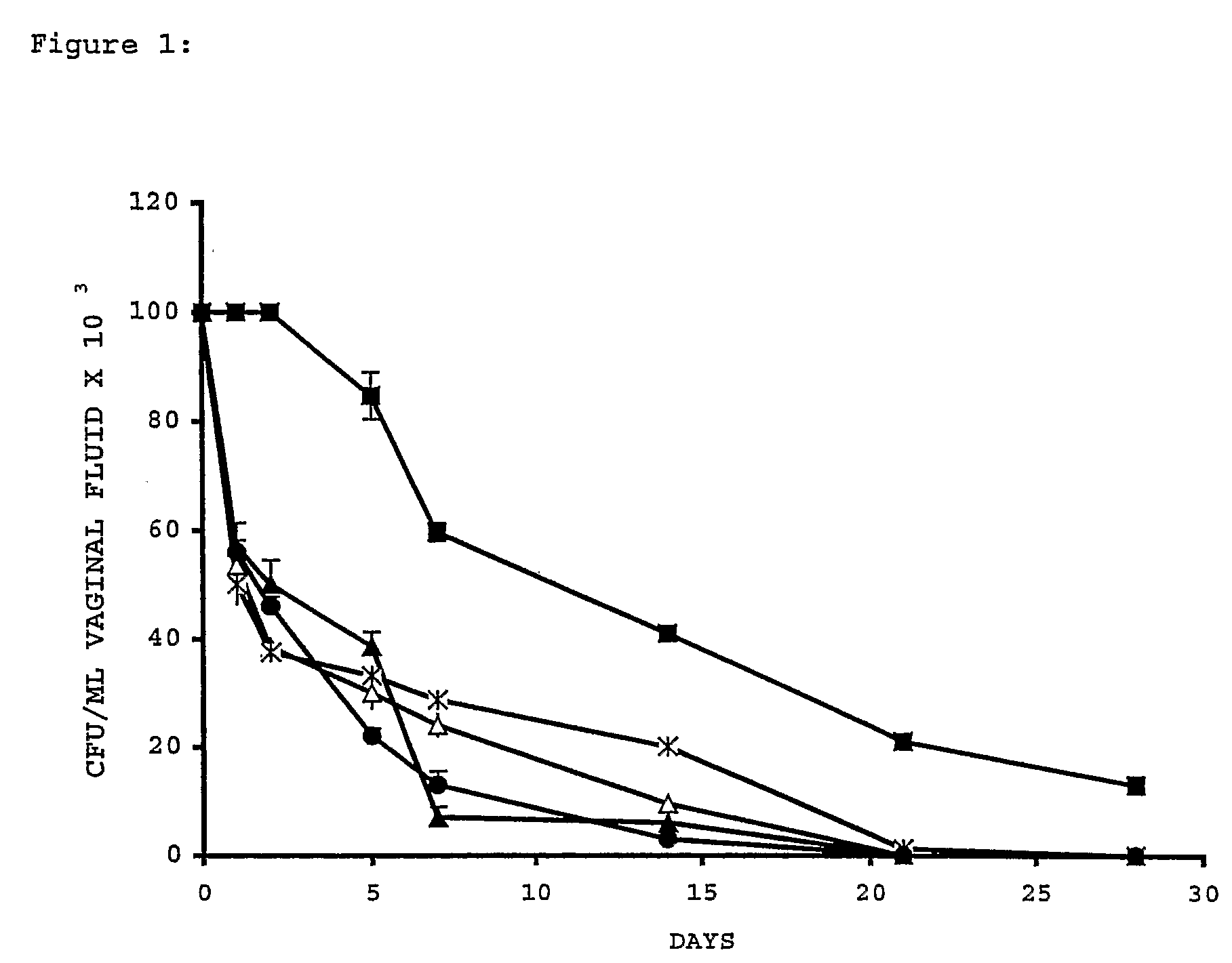
Method for preventing and/or treating vaginal and vulval infections
InactiveUS20060217443A1Without affecting Lactobacilli growthAvoid infectionBiocideAnimal repellantsGallic acid esterVulval Infection
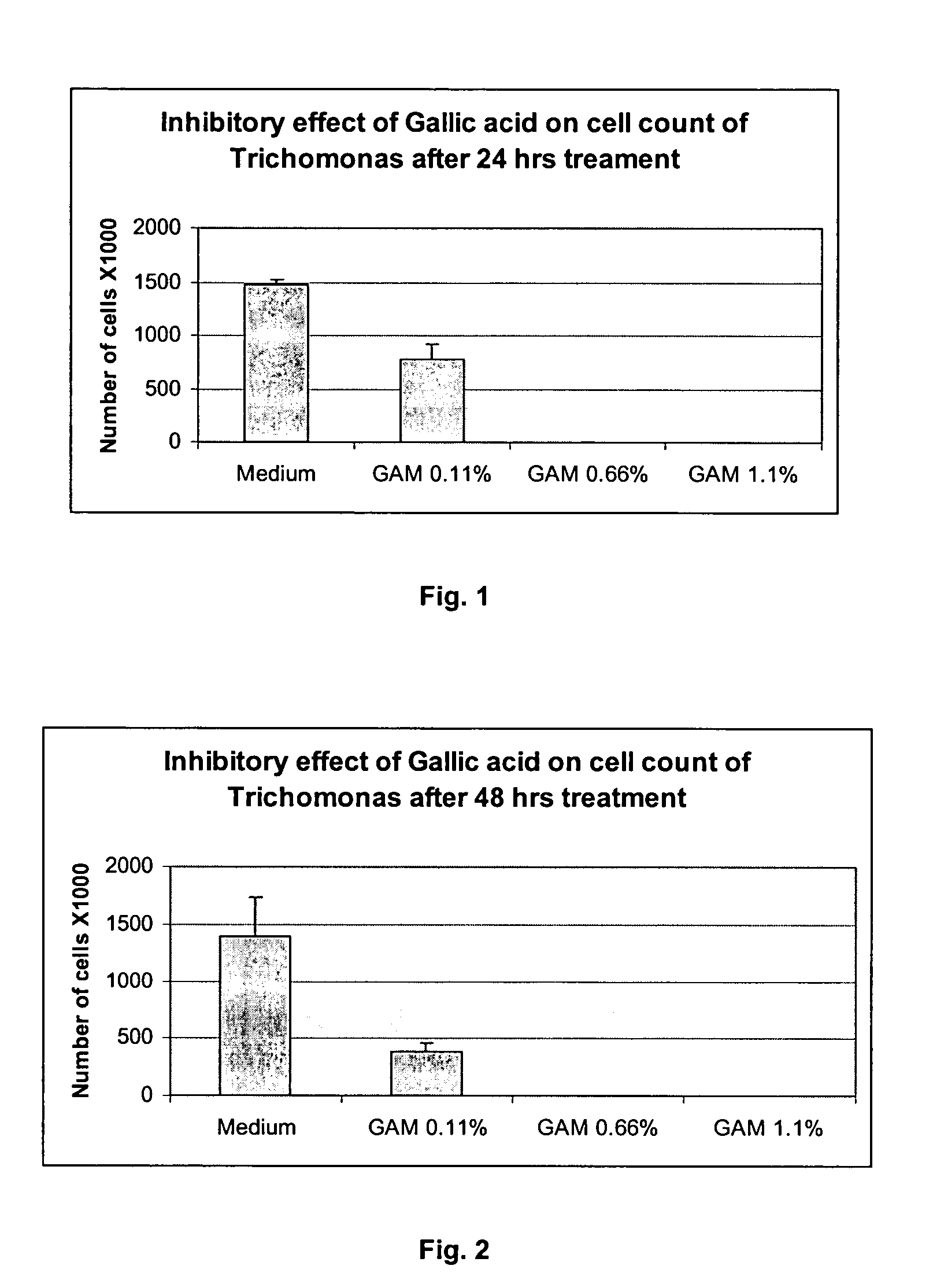
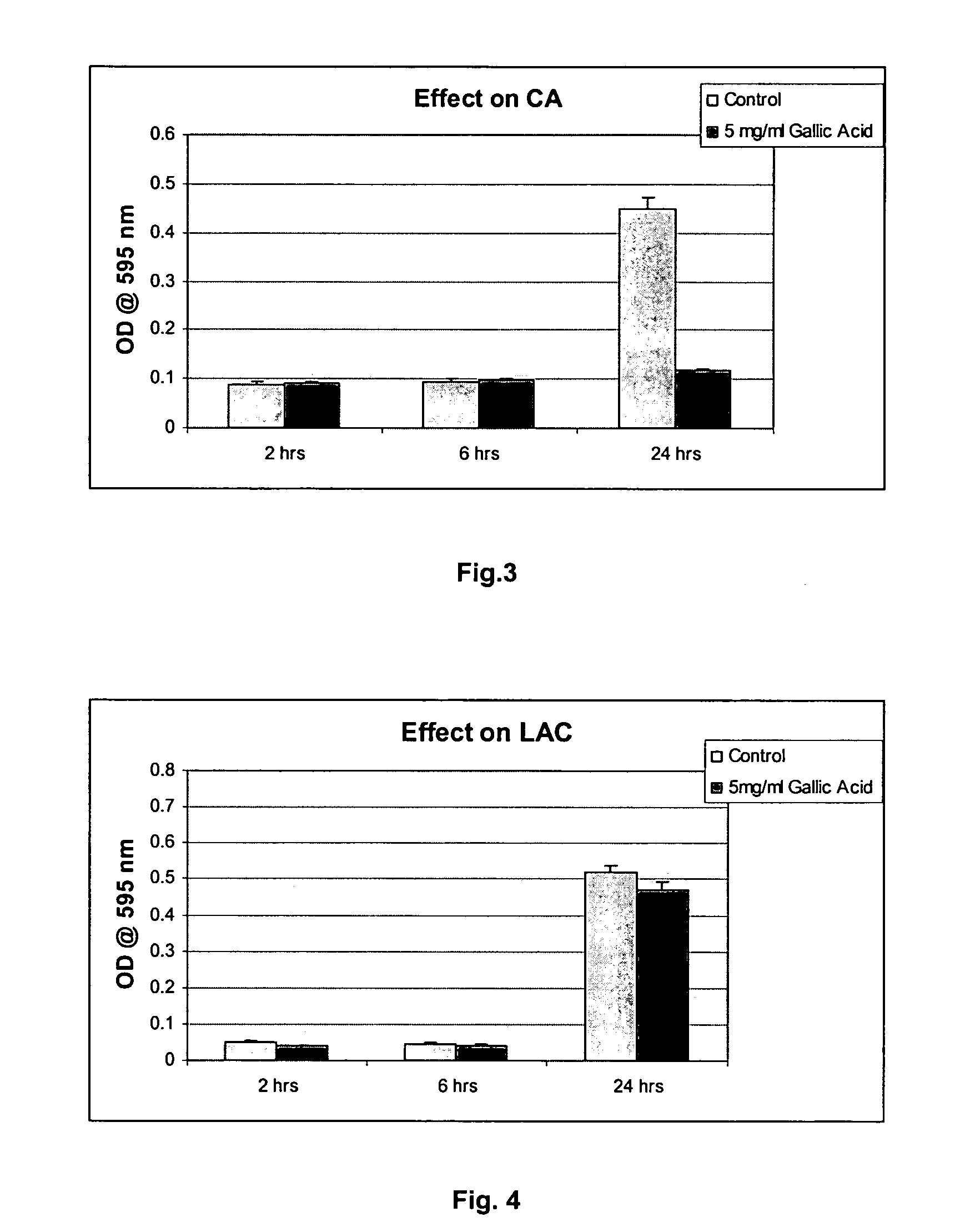
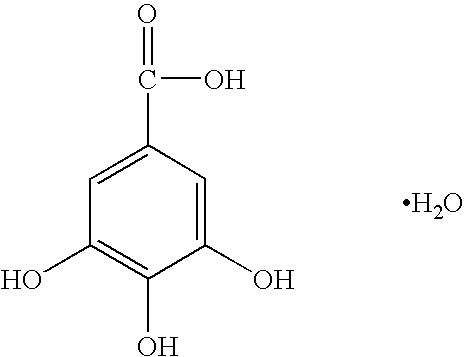
The invention provides a method for treating and / or preventing a wide scope of vaginal and vulval infections, such as those caused by bacteria, parasites or yeasts, by administering gallic acid to a subject in need of treatment. A composition containing gallic acid for treating vaginal infections is also disclosed. It has been found that gallic acid is capable of selectively inhibiting the growth of Trichomonas vaginalis, a parasite that causes trichomonas vaginitis; Gardnerella vaginalis, a bacterium that causes bacterial vaginosis; and Candida albicans, a yeast that causes candidiasis and vulvitis; while not inhibiting the growth of Lactobacillus acidophilus, the dominant bacteria in a healthy vaginal ecosystem. Gallic acid is safe and cost-effective, and can be used alone or incorporated into different vaginal health products to treat and / or prevent vaginal infections.
Owner:KIMBERLY-CLARK WORLDWIDE INC
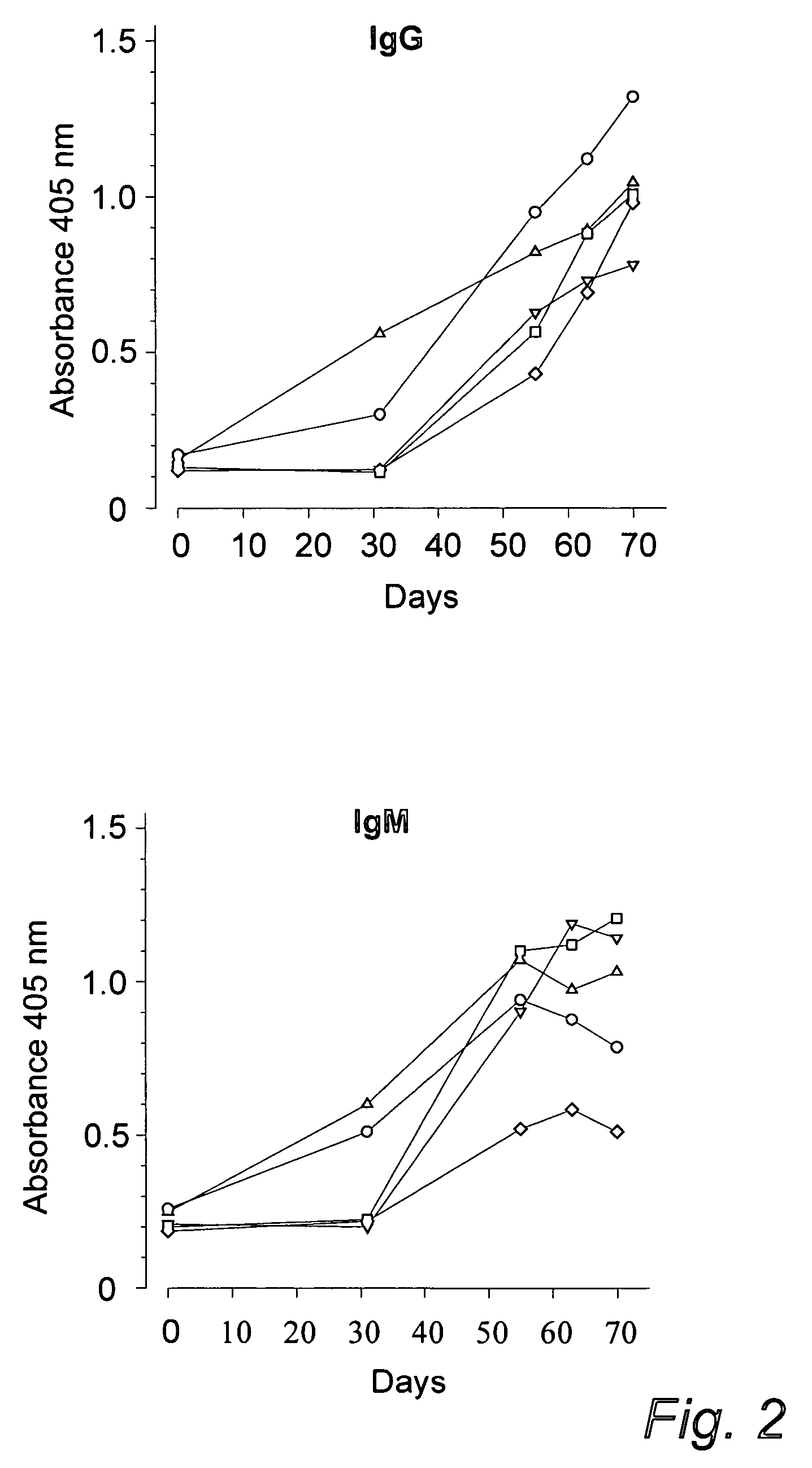
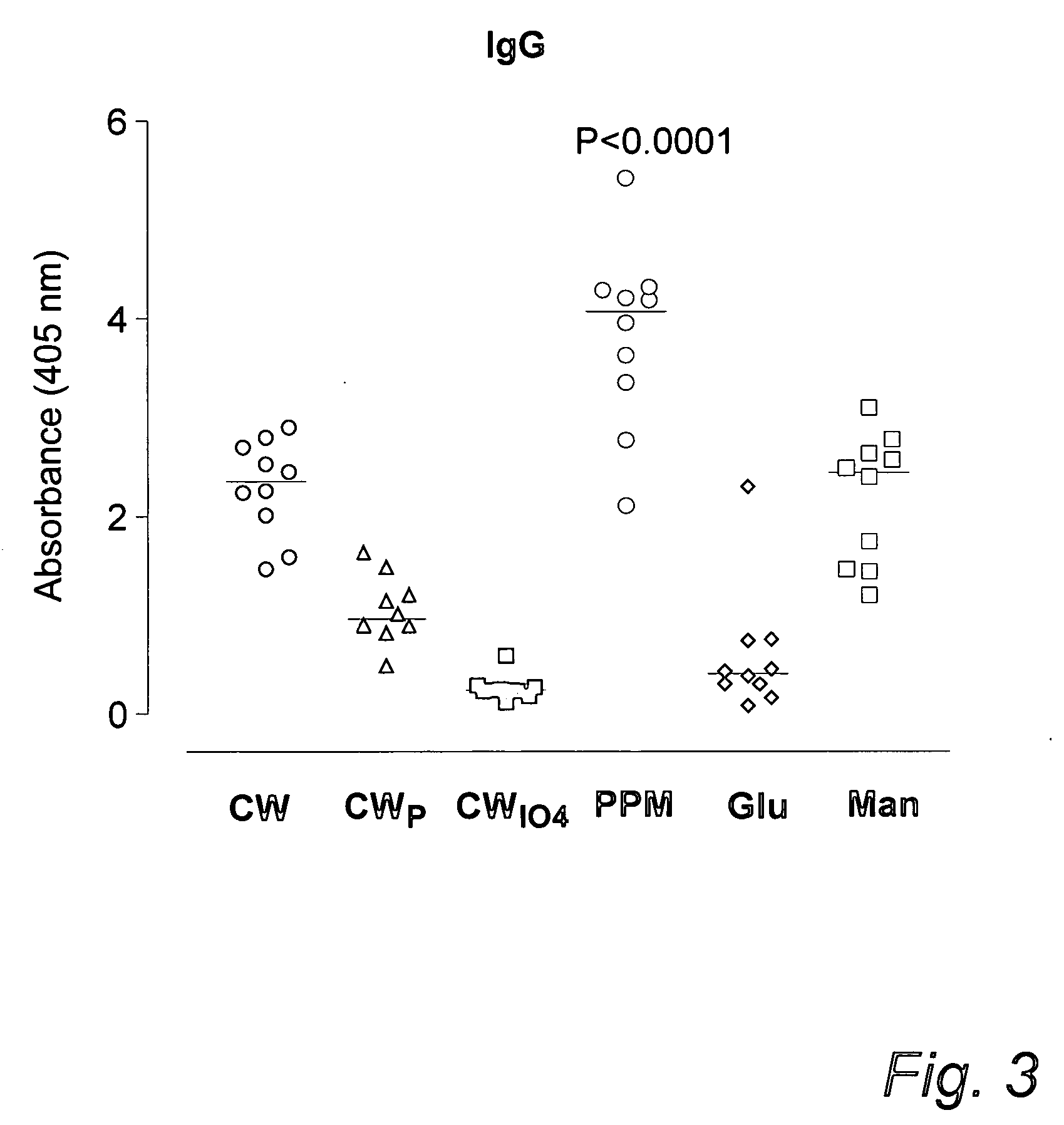
Diagnosis of candidiasis and candidemia or invasive candida infection
InactiveUS20060172353A1Improve serological testImmunoglobulins against fungi/algae/lichensBiological testingAntigenDiagnostic test
The use of an antibody to a C albicans cell wall antigen or to a solubilized phosphopeptidomannan (PPM) fraction of the cell wall of C albicans, or preferably a combination of an IgG2 antibody to a phosphopeptidomannan (PPM) fraction of the cell wall of C albicans, and an IgG1 or IgG3 antibody to a C albicans cell wall antigen in the diagnosis of candidiasis or invasive candidiasis is disclosed. Also diagnostic tests are disclosed.
Owner:FUNGEA +1
Anti-candidiasis albrican cream and preparation method thereof
InactiveCN101518608AReduce greasyModerate consistencyOrganic active ingredientsAntimycoticsMedicineFungal nail infection
The invention relates to Chinese and western medicament compound cream for preventing and treating onychomycosis infected by candidiasis albrican and a preparation method thereof. The cream is yellow cream formed by evenly dispersing mixture of a Chinese medicament extract and a western medicament in a substrate by use of the action of a mixed emulsifying agent. The cream prepared by the method has the advantages of even dispersed main medicament, even and smooth cream, proper viscosity, as long as easily coating on skin, mucous membrane and wound surface, and meeting the requirement of Chinese pharmacopoeia with controllable quality. Pharmacodynamic research proves that the cream has actions of preventing and treating the onychomycosis infected by Candida albicans, and has assistant treatment action on secondary inflammation because of mycotic infection, such as whitlow.
Owner:SICHUAN AGRI UNIV
Antibodies Against Candida Antigens
InactiveUS20080193450A1Improve standardizationA large amountAntimycoticsSnake antigen ingredientsVirulent characteristicsCANDIDA ANTIGEN
This invention relates to domain antibodies. In particular, it relates to domain antibodies (dAbs) that recognize virulence traits of Candida spp. and confer passive protection against candidiasis.
Owner:INST SUPERIORE DI SANITA
Application of streptococcus sanguis protein to preparation of drugs inhibiting candida albicans
The invention discloses application of streptococcus sanguis protein to preparation of drugs inhibiting candida albicans and relates to application of streptococcus sanguis protein on drugs. The problems that azole type and polyenoid type chemical synthesis drugs are large in toxic and side effects in treatment of candidal infection and drug-resistant strain bacteria can be generated easily are solved. The streptococcus sanguis mixed protein is used for preparing the drugs inhibiting the candida albicans. The streptococcus sanguis protein makes the cell morphology of the candida albicans change and the membrane permeability increased, the adhesive effect and the gemmation of the candida albicans are inhibited, and generation of hyphae is interdicted. According to the application, streptococcus sanguis extracts are used for funguses antagonism, and it is verified that the streptococcus sanguis extracts have a high inhibiting effect on funguses.
Owner:马晟利
Strain of lactobacillus pentosus as probiotic
ActiveUS20160193260A1High activityIncrease capacityAntibacterial agentsCosmetic preparationsBacterial vaginosisCandida famata
Provided is the new strain Lactobacillus pentosus CECT 7504 and compositions and products comprising said strain and uses in the prevention and / or treatment of candidiasis (oral, intestinal and vaginal) and of bacterial vaginosis.
Owner:GYNEA LAB
Pharmaceutical compositions and mentods for treatment of fungal infections
Pharmaceutical compositions and methods for treating skin or mucus fungal infections of feet, nails, groin, hands, tongue and other locations are disclosed herein. The pharmaceutical composition disclosed herein comprises at least one triazole agent. Methods of treating tinea pedis, tinea manuum, tinea unguium, tinea cruris, tinea corpora, tinea versicolor, candidiasis, aspergillosis, onychomycosis, coccidiodomycosis, cryptococcal meningitis, histoplasmosis, hoof thrush, hoof rot, tongue and mouth lesions, seborrheic dermatitis, and combinations thereof, are also presently disclosed.
Owner:UKRAINIAN INDEPENDENT INFORMATION AGENCY
Xiaomi Huayu Capsules
InactiveCN102266516AAchieve normal patencyPromote absorptionSexual disorderPlant ingredientsCervicitisMastitis
The Xiaomi Huayu Capsule of the present invention is a traditional Chinese medicine capsule preparation for treating inflammatory diseases such as vaginitis, cervicitis, ovariitis, and mastitis. If it is completely cured, when the body is weak and the vaginal environment is suitable, candida and trichomonas are very easy to multiply in large numbers, causing relapses, lingering and repeated, and even prolonged treatment. Traditional Chinese medicine believes that it belongs to the category of "itching of vulva" and "leukorrhagia". It is considered to be caused by deficiency of the spleen to produce dampness, dampness and heat betting, damp accumulation and insects, or exogenous damp pathogens, pathogenic poisons invading, and worms eating into the yin. The pathological properties are deficiency in origin and excess in superficiality, deficiency of the spleen as the foundation, and exogenous pathogenic infection as the standard. Treatment treats both symptoms and root causes. The "Xiaomi Huayu Capsule" of the present invention invigorates the spleen and removes dampness, regulates the whole body, relieves vaginal discharge and restrains dampness, cleans the genitals, kills insects and sterilizes, clears away heat and detoxifies. The present invention directly acts on the body, can obviously regulate the overall function, enhance immunity, resist the invasion of evil poison, improve microcirculation, and promote the absorption of inflammation, so the cure rate is high and the curative effect is good.
Owner:黄静波
Antifungal formulations
Owner:G & R PHARMA
Anti-candida compositions and uses thereof
ActiveUS10391134B2Avoid stickingInhibitionPharmaceutical delivery mechanismUnknown materialsCandida dubliniensisCandidatus
Owner:PHARMALP
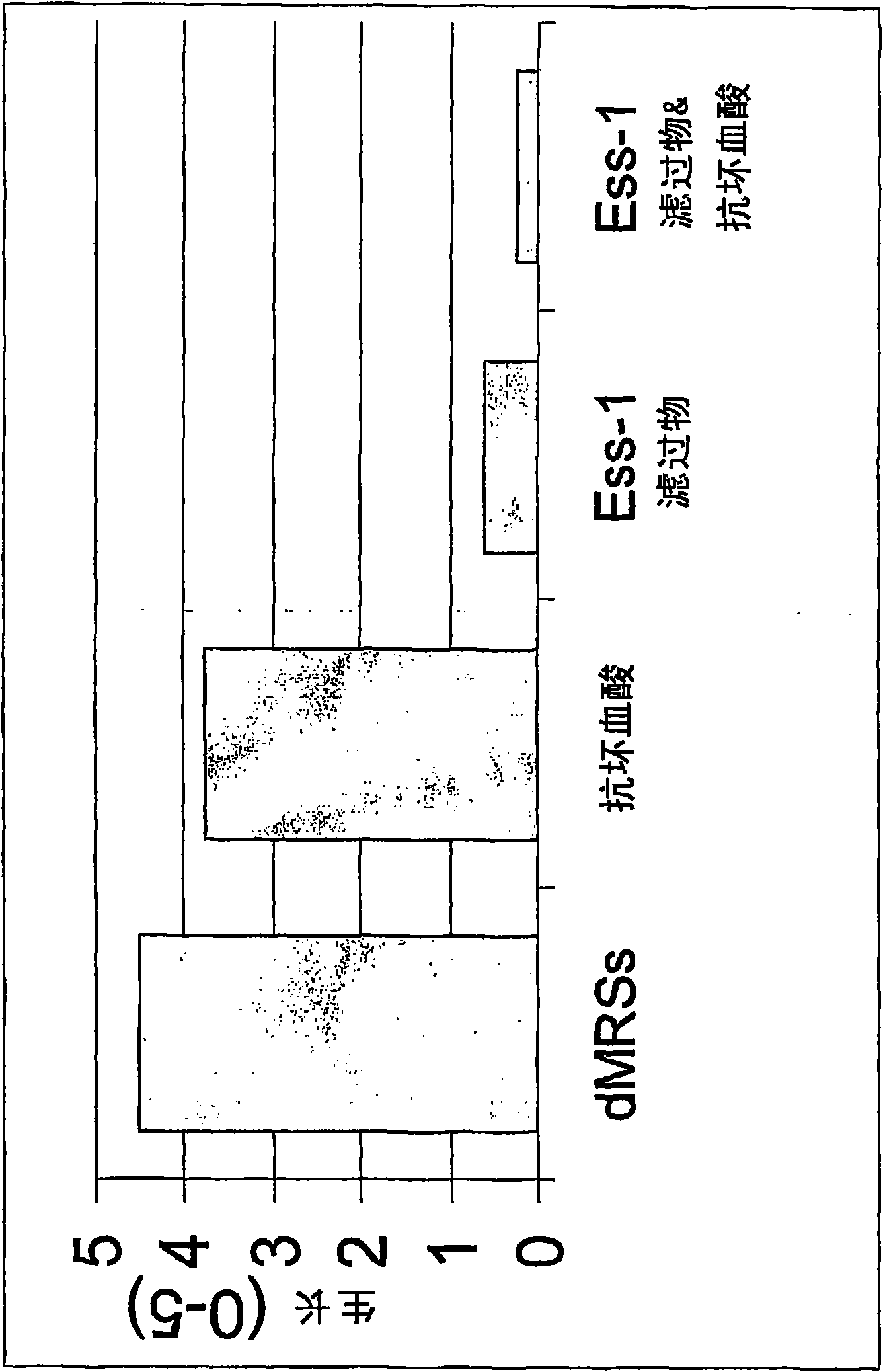
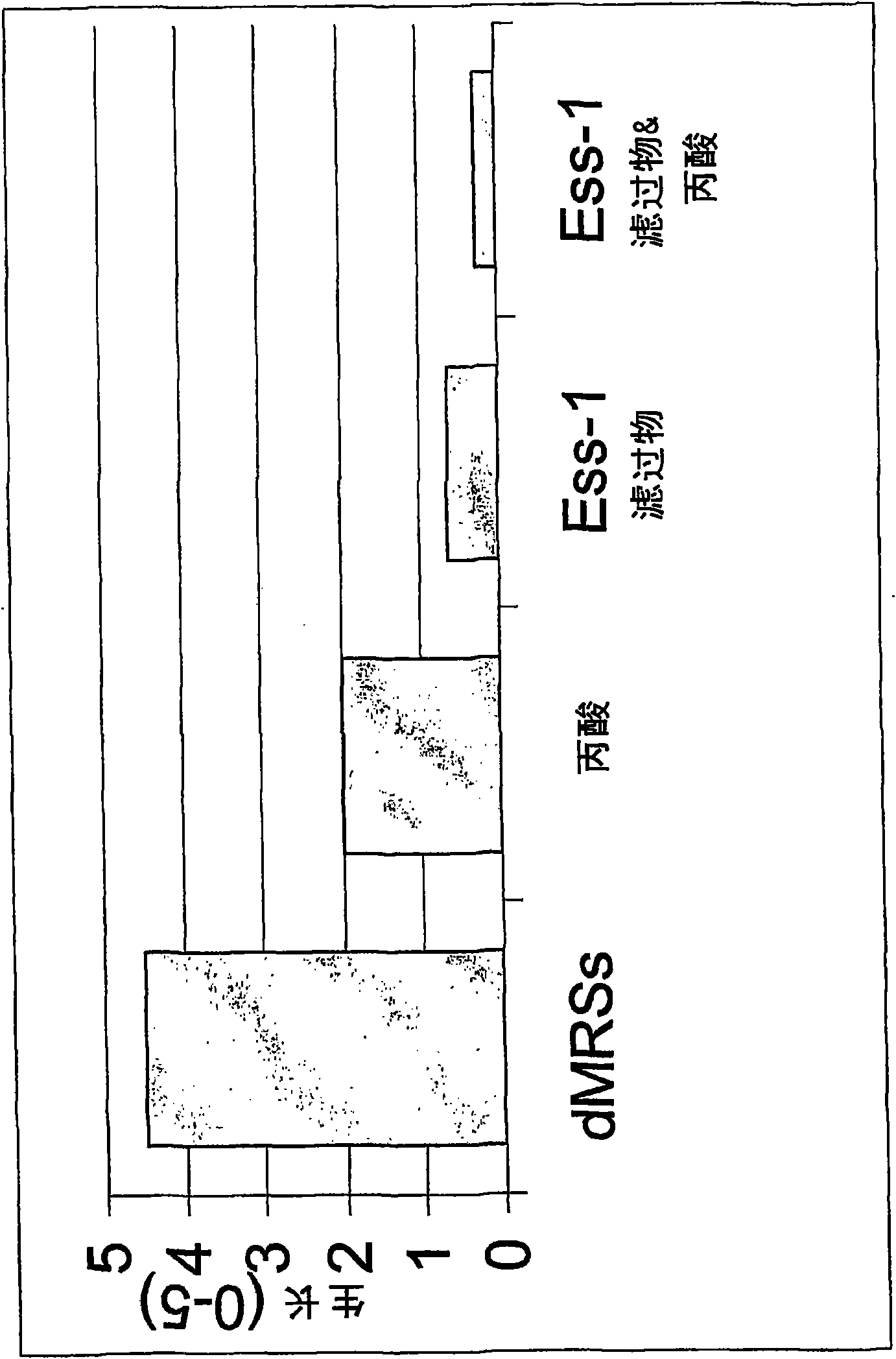
Lactobacillus fermentum ess-1, dsm17851 and its use for the treatment and/or prevention of candidiasis and urinary tracrinfections
InactiveCN101568638AOptimal growth inhibitionResolve Healing EffectsAntibacterial agentsAntimycoticsUpper urinary tract infectionLactobacillus fermentum
The present invention relates to a novel strain of Lactobacillus fermentum, denoted strain Ess-1, which has been deposited at Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH and has been assigned accession number DSM17851. Lactobacillus fermentum, strain Ess-1, has shown valuable properties in the inhibition of Candida. The invention therefore also relates to the medical use of Lactobacillus fermentum strain Ess-1, in particular for the treatment and / or prevention of candidasis and urinary tract infections. The invention also relates to pharmaceutical products and articles comprising this novel bacterial strain.
Owner:ESSITY HYGIENE & HEALTH AB
Application of jatrorrhizine for treating candida albicans infectious diseases
The invention discloses a novel application of jatrorrhizine for treating candida albicans infectious diseases. The jatrorrhizine can be extracted from plants of coptis roots, amur corktree barks, caulis mahoniae and the like. The study shows that half bacteriostasis concentration of the jatrorrhizine to candida albicans is 139+ / -21mg / L, and effective dose of the jatrorrhizine for enhancing body resistibility of a low immune function mouse to the candida albicans is 30mg / Kg. The jatrorrhizine and pharmaceutically acceptable salts, esters or sugars, excipients and the like are utilized for preparing medicines for treating diseases caused by the fact that the candida albicans infect human and livestock, and the jatrorrhizine can be applied to, but is not limited to, medicines for treating human skins, mucous membranes, internal organs or nervous centralis candidiasis, livestock upper digestive tract mycoses and the like. The jatrorrhizine and the pharmaceutically acceptable salts, esters or sugars and excipients and the like can be prepared to be injection, oral administration and external preparations and dosage forms thereof which are pharmaceutically acceptable such as injections, tablets, capsules, liniments, pastes, smearing agents, emulsions, nanoparticle emulsions, and the like.
Owner:XIAN YUYE PHARMA TECH CO LTD
Vaginal lotion based on female vaginal nursing
InactiveCN106729244AIncrease bactericidal functionEnhanced inhibitory effectAntibacterial agentsAntimycoticsLotionVagina
The invention discloses a vaginal lotion based on female vaginal nursing. The vaginal lotion is prepared from the following components: tea tree essential oil, radix sophorae flavescentis, cortex phellodendri, fructus cnidii, radix stemonae, cortex dictamni, herba senecionis scandentis and herba menthae. According to the vaginal lotion, the radix sophorae flavescentis, the cortex phellodendri, the fructus cnidii, the radix stemonae, the cortex dictamni and the herba senecionis scandentis, which have functions of clearing away heat and drying dampness, are combined; the components generate synergistic action; the effects of clearing away the heat and drying the dampness are effectively improved; meanwhile, according to the vaginal lotion, the tea tree essential oil which has an antibacterial function is added in a formula; not only can the sterilization function of the formula be improved, but also a better bacteriostatic effect is achieved; the vaginal lotion is particularly has a better inhibition effect on candidiasis albicans.
Owner:四川香龙丹医药科技有限公司
Intergenic rnas and methods for modulating gene expression and pathogenesis in candida
Intergenic non-coding RNA molecules that regulate the expression of HWP1 and ALS3 of Candida are provided as are methods of using the non-coding RNA molecules and complementary molecules thereof in modulating HWP1 or ALS3 expression; adherence, yeast-to-hyphal transition, or biofilm development of Candida; and preventing or treating candidiasis.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
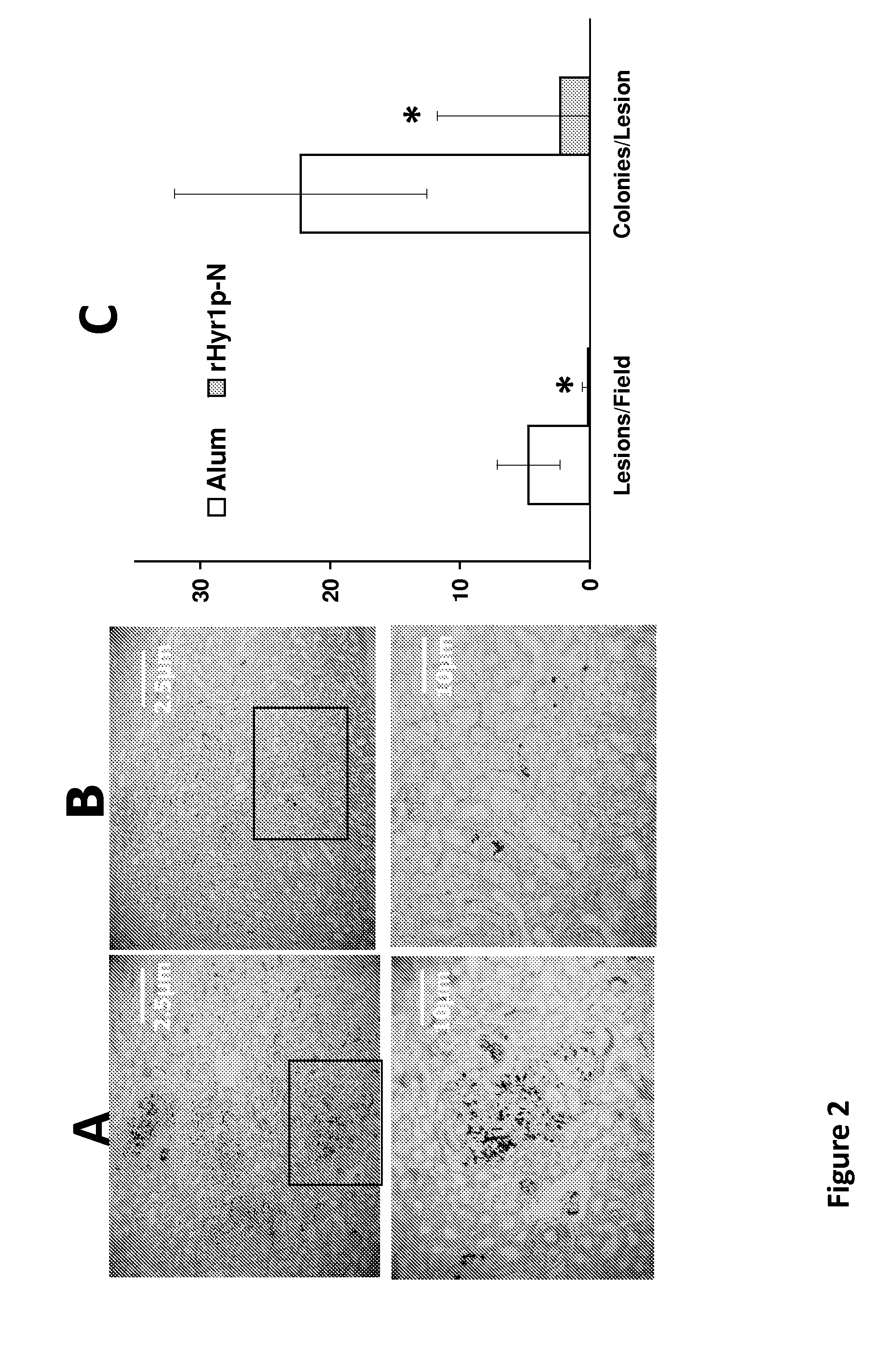
Hyr1-derived compositions and methods of treatment using same
ActiveUS20150191514A1Decreasing acquisitionGood antigenicityAntibacterial agentsAntimycoticsTreatment useAmino acid composition
The disclosure features isolated polypeptides of Hyr1. The disclosure further features vaccines and antibodies useful in treating or preventing candidiasis or Acinetobacter infections or both. Further disclosed are isolated polypeptides consisting of between 14 and 20 amino acids for vaccine preparation. The specific amino acid sequences of isolated polypeptides of Hyr1 are also disclosed.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
Medicaments for fungal infections
InactiveUS20090081196A1Reduce resistanceReduce capacityDead plant preservationDead animal preservationVirulent characteristicsCandida famata
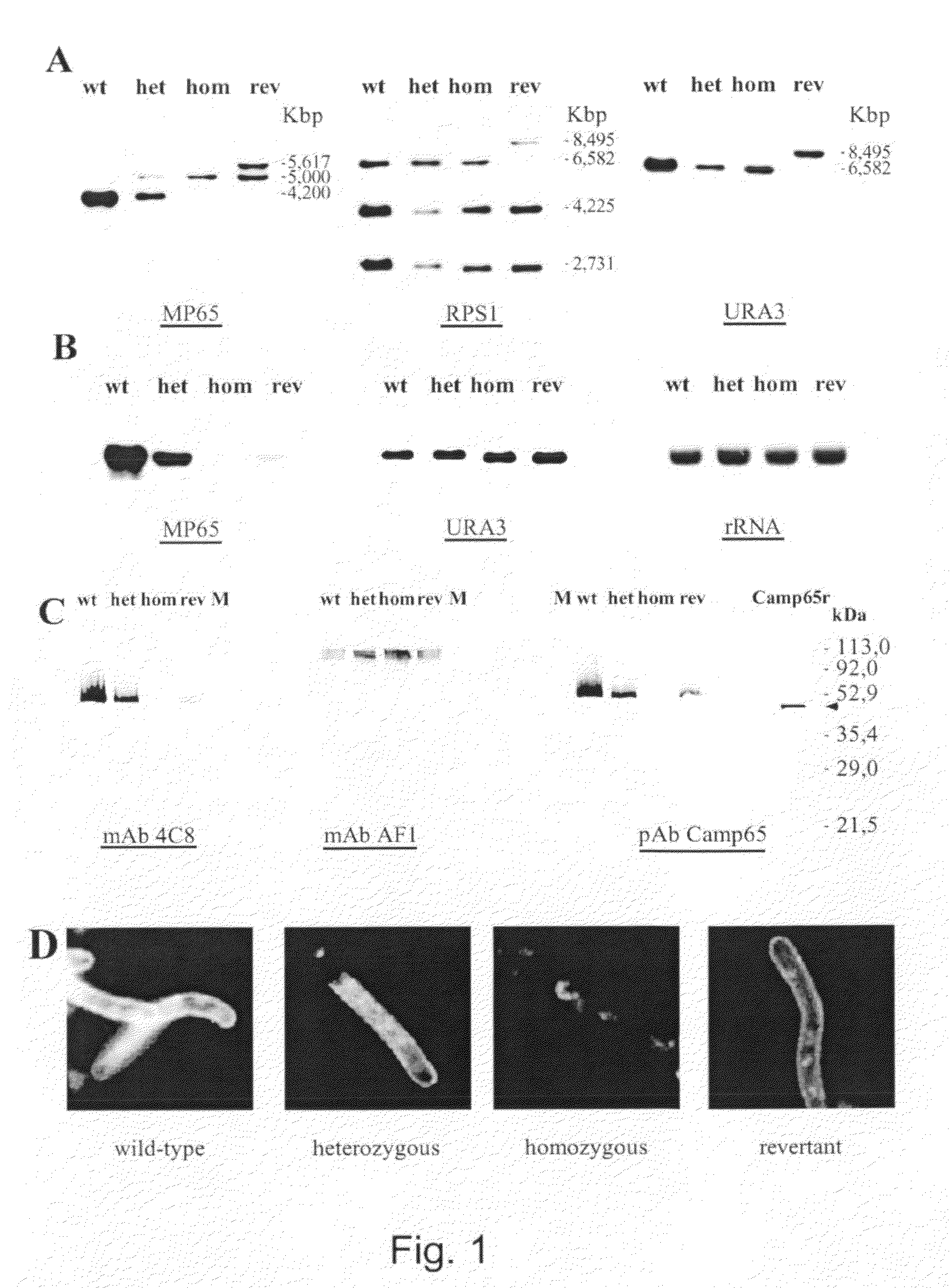
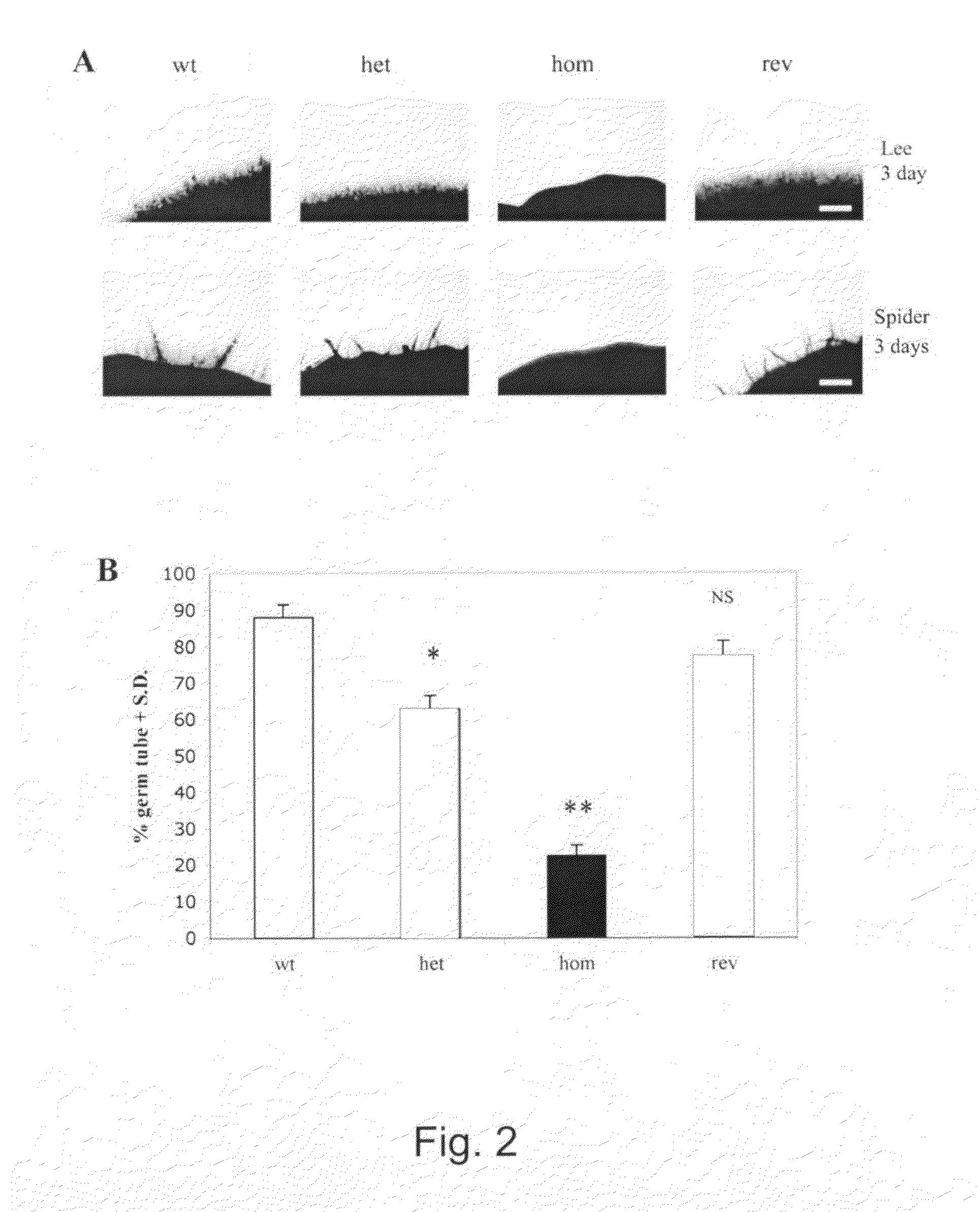
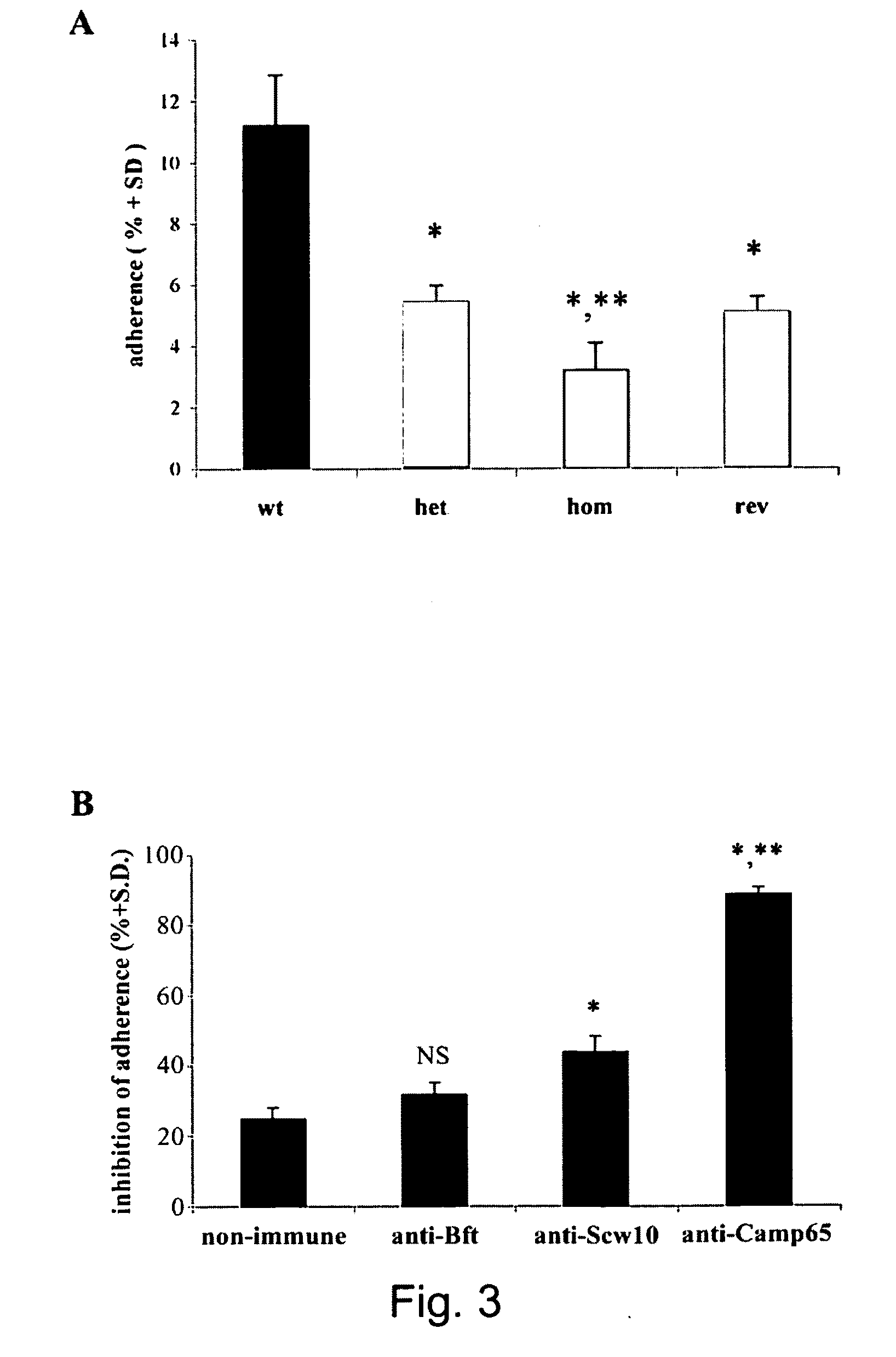
The protein CAMP65p of C. albicans has been found to be play a significant role in both adhesion and the production of hyphae, which are important factors in both virulence and resistance to clearing. This invention provides antibodies to CAMP65p useful for administration to patients for prophylaxis and treatment of candidal infections.
Owner:INST SUPERIORE DI SANITA
Aerosol inhalation device
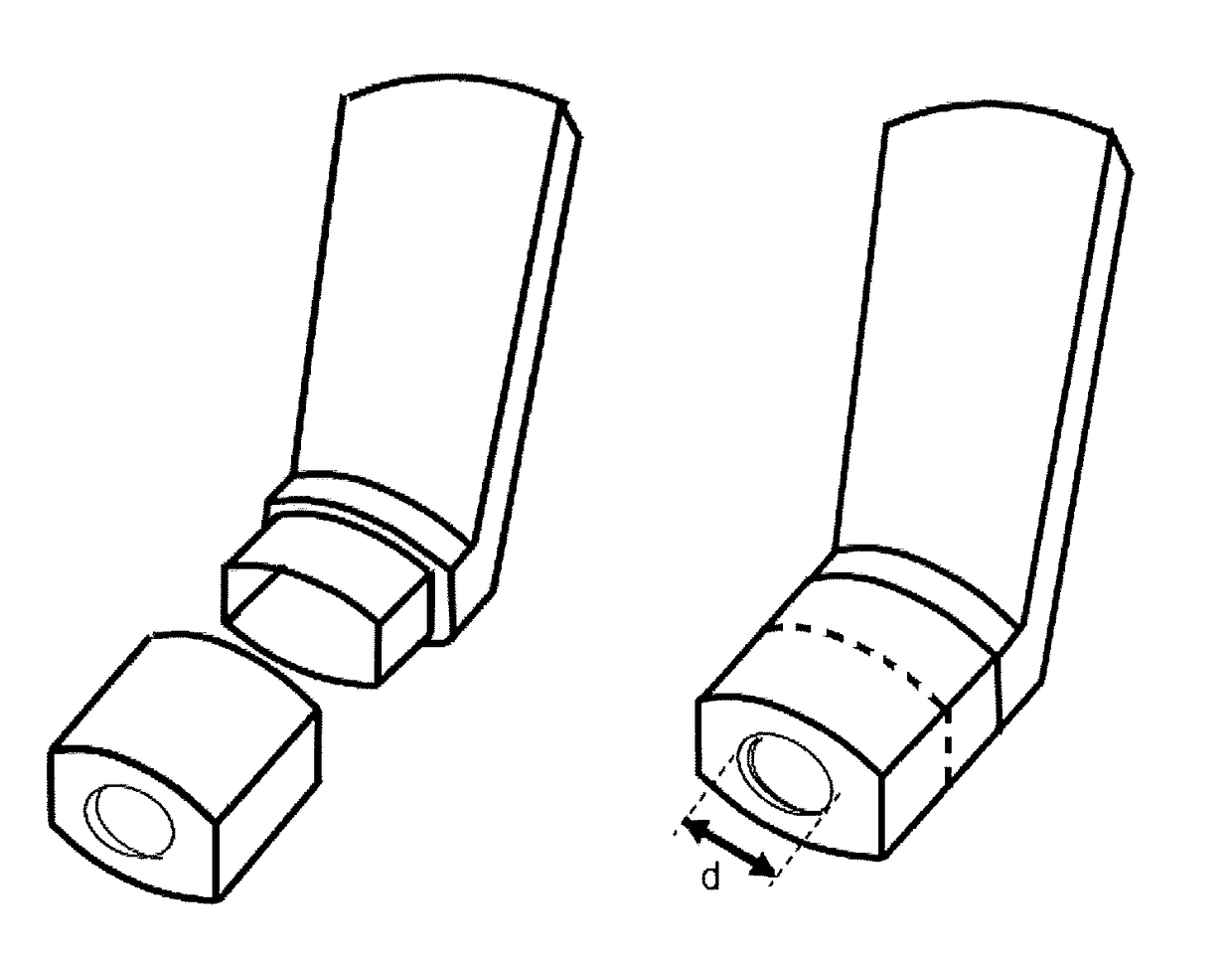
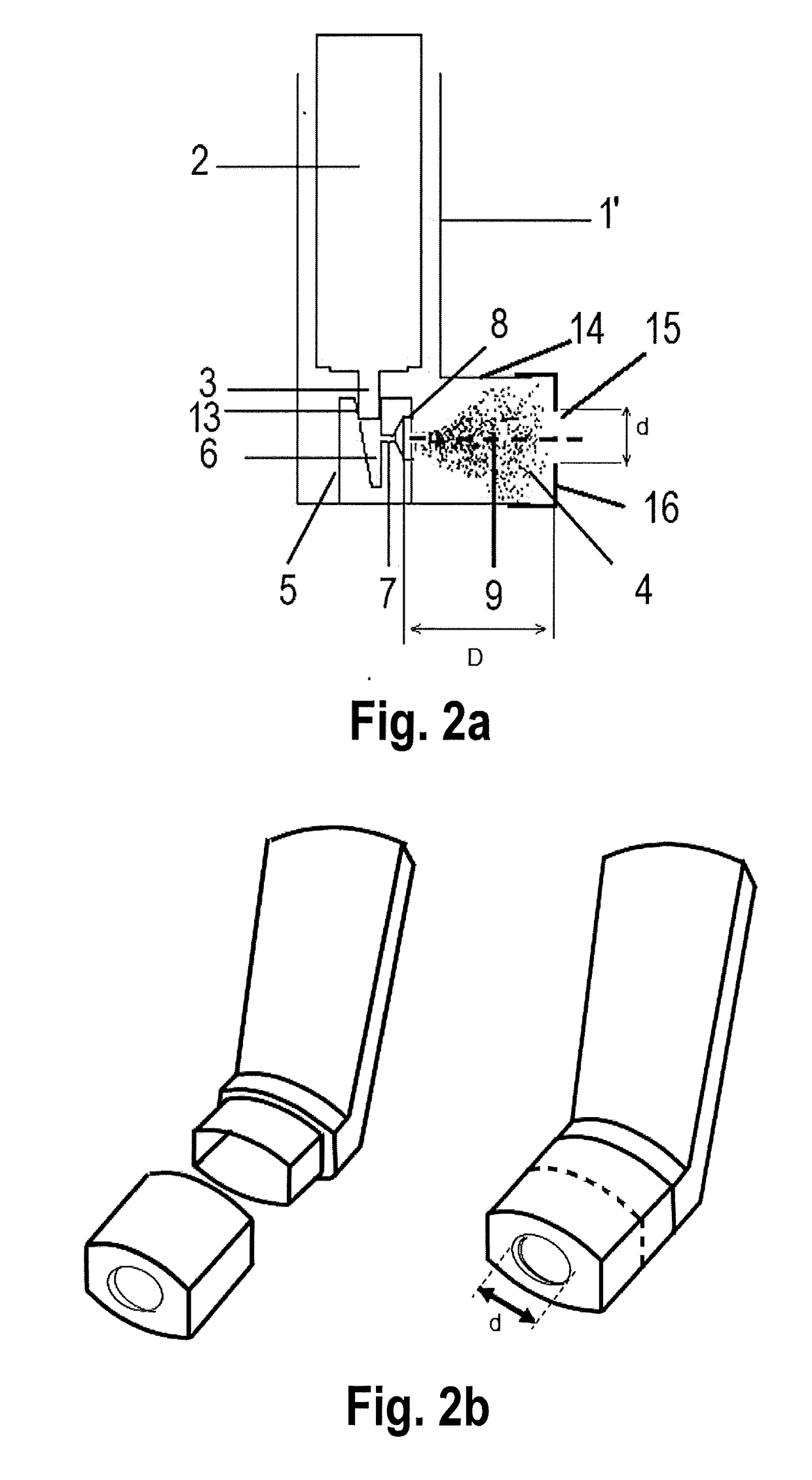
ActiveUS20170281885A1Patient compliance is goodLittle strengthMedical devicesInhalatorsSide effectMedicine
Actuators for a pressurised metered dose inhalers containing (a) a housing adapted to receive an aerosol canister containing a pressurised medicament formulation, (b) a nozzle block defining the receptacle for the aerosol canister valve stem, the expansion chamber or sump, the nozzle channel its external aperture, and (c) a mouthpiece portion comprising a central rounded opening of a defined width and distance from the nozzle aperture and a cylindrical recess, whose central axis is aligned with the central longitudinal axis of the mouthpiece portion and with the central longitudinal axis of the nozzle channel, provide a significant reduction in the non-respirable coarse fraction of the emitted aerosol medicament via inertial impaction and retention in the actuator rather than in the oro-pharynx, with consequent less associated side effects and oral candidiasis in the patient. In addition, the presence of the central rounded opening has minimal, negligible impact on the fine particle dose of the delivered aerosol particles.
Owner:CHIESI FARM SPA
Antifungal remedy formulation for external application
An antibacterial composition for external application, particularly, a remedial formulation for external use containing fluconazole is disclosed. The formulation comprises a base material, 0.1-5.0 percent by weight of fluconazole and 1.0-40.0 percent by weight of any one compound selected from a group consisting of fatty acid, fatty alcohol, higher fatty acid ester and lower alcohol based on weight of the base material. The composition according to the present invention has various advantages of a favorable absorption into a system through the skin of a human body and delivery of active material into horny layer of the skin, a reduced skin stimulation and an excellent stability. Therefore, the composition is efficiently useable in a medical treatment application for dermatomycosis-related infectious diseases including tinea pedis, tinea corporis, tinea cruris, tinea versicolor and / or candidiasis and the like.
Owner:BORYUNG PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com