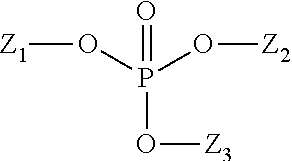
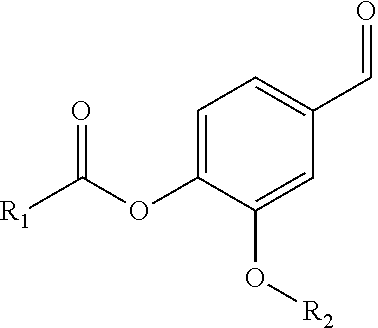
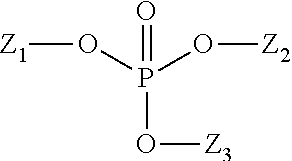
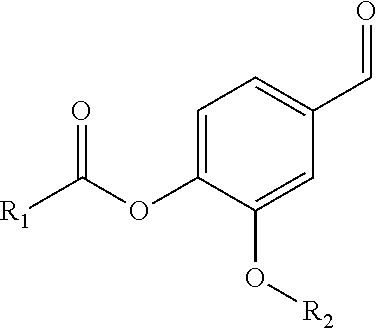
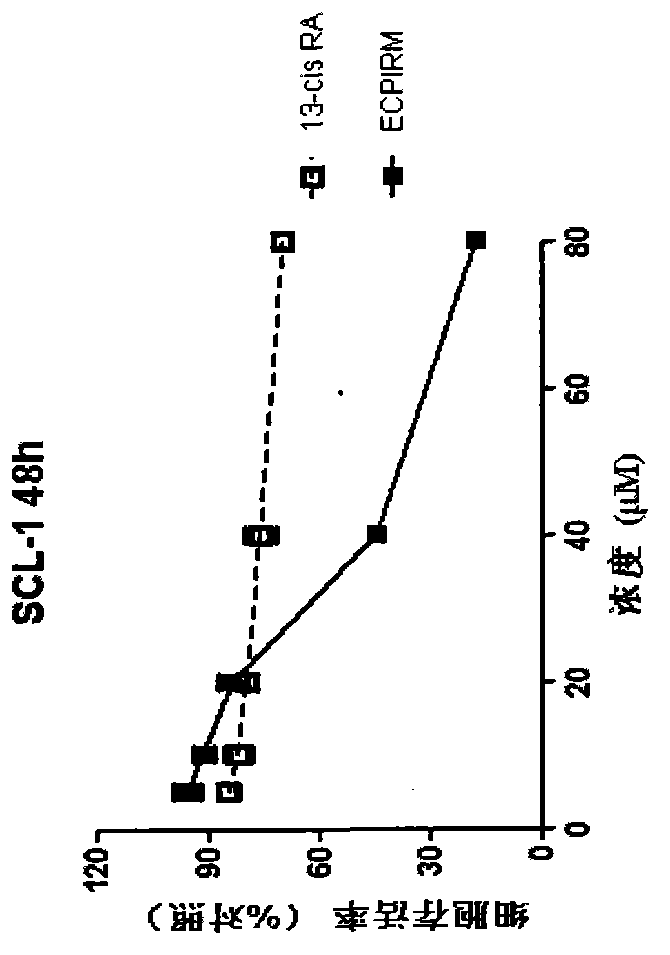
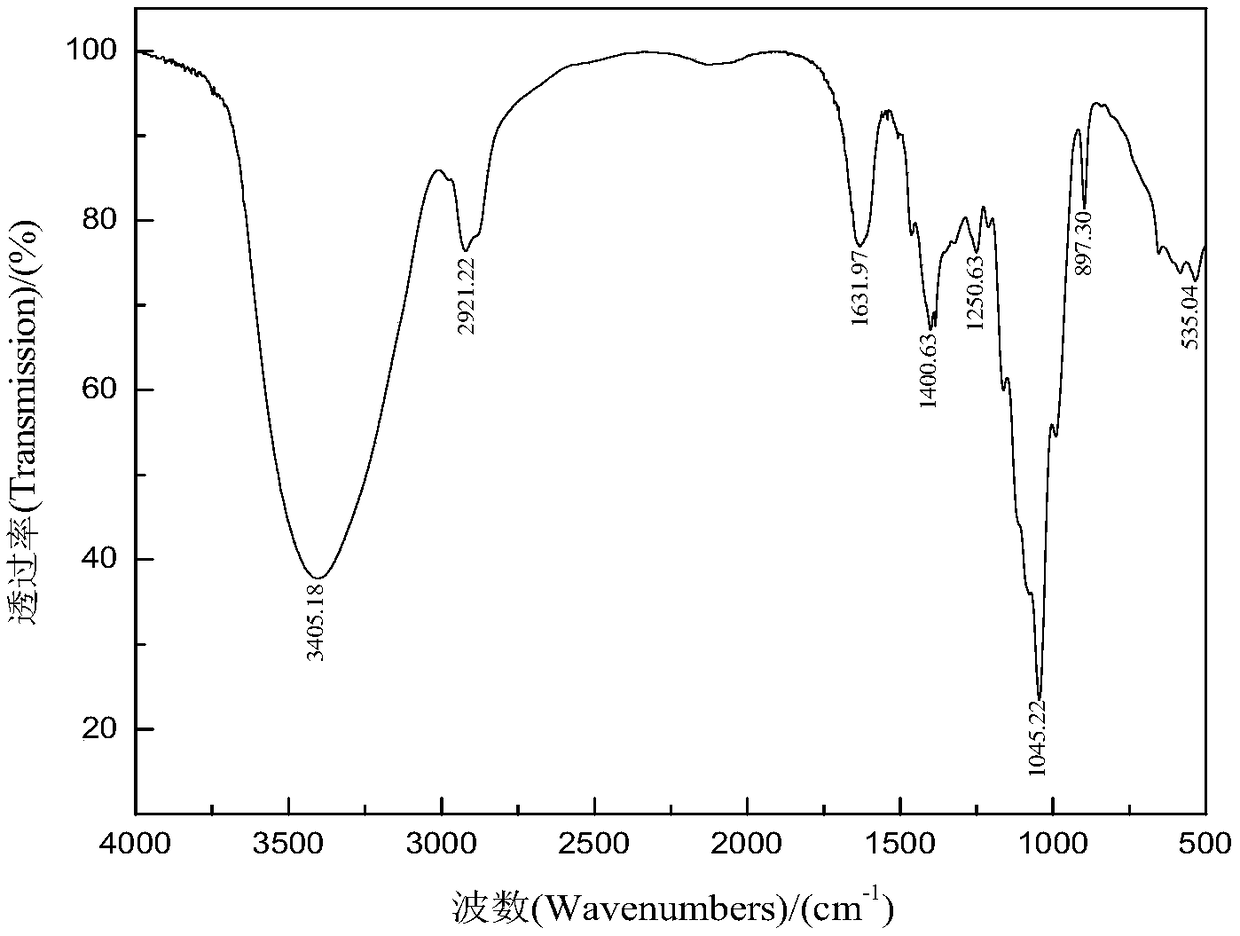
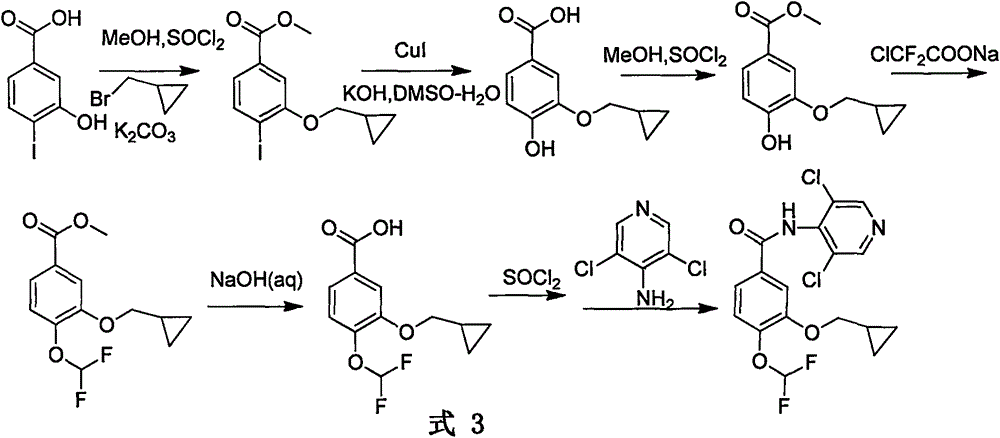
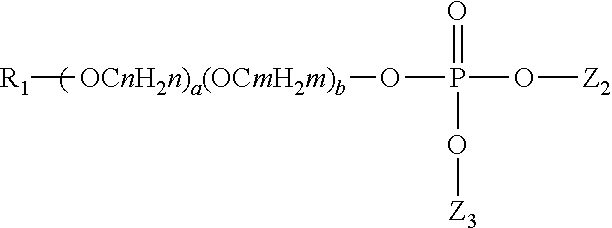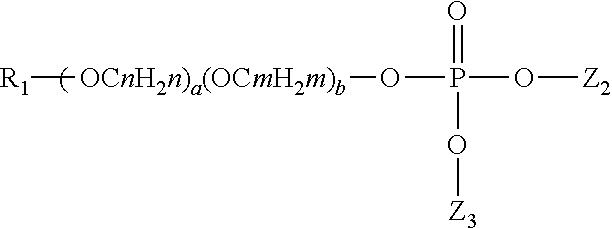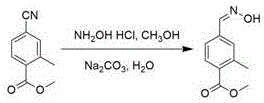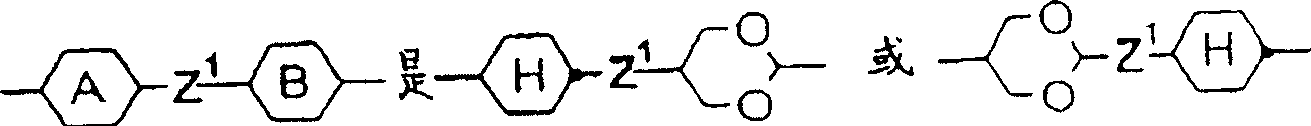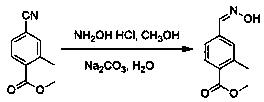Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Benzoate derivative" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oral Care Compositions With Improved Flavor
Oral care compositions having improved taste, said compositions comprising: a carrier material; from about 0.001 to about 10%, by weight of the composition, of an oral care component selected from metal salts, antimicrobial agents, bad breath reduction agents, bleaching agents, surfactants, or a combination thereof; and from about 0.0001 to about 1%, by weight of the composition, of a TRPA1 agonist selected from vanillin esters; benzoate esters; hydroxybenzoate derivatives; methoxy benzoate derivatives; hydroxybutanedioate derivatives; benzamidobenzoate derivatives; methylpropanoate derivatives; phenyl acetate derivatives; hex-3-enoate derivatives; 2-(furan-2-ylmethylsulfanyl)-3-methylpyrazine; phenylmethoxymethylbenzene; (2R)-2-azaniumyl-3-[(2R)-2-azaniumyl-3-oxido-3-oxopropyl]disulfanylpropanoate; (3E)-2-hydroxy-4,8-dimethylnona-3,7-dienal; (2R)-2-azaniumyl-3-[(2S)-2-azaniumyl-3-oxido-3-oxopropyl]disulfanylpropanoate; (3Z)-3-butylidene-2-benzofuran-1-one; 3-methyl-N-(3-methylbutyl)butan-1-imine; 2-(furan-2-ylmethyldisulfanylmethyl)furan; and combinations thereof. Uses thereof and methods of improving the taste of an oral care composition.
Owner:THE PROCTER & GAMBLE COMPANY
Indoleamine2,3-dioxygenase inhibitor, as well as preparation method and application thereof
The invention relates to an indoleamine2,3-dioxygenase inhibitor having a structure as shown in a formula (I), as well as a preparation method and application thereof. The IDO inhibitor is (Z)-N'-hydroxyl-N-benzoate derivative, has high inhibiting activity for IDO, can be used for effectively inhibiting the IDO activity and inhibiting immunosuppression of patients, can be widely applied to treatment or prevention of cancer or tumors, virus infection, depression, neural degeneration disease, trauma, age related cataract, organ transplant rejection or autoimmune disease, and is expected to be developed into a new generation of immunosuppressors.
Owner:SHANGHAI HANSOH BIOMEDICAL +1
Internal and External Donor Compounds for Olefin Polymerization Catalysts III
ActiveUS20110213106A1Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationSolid componentElectron donor
The present invention relates to solid catalyst components comprising titanium, magnesium, halogen and an internal electron donor compound containing at least one 1,8-naphthyl diester compound. The 1,8-naphthyl diester compounds include naphthalene-1,8-diyl dicycloalkanecarboxylate derivatives, dicycloalkenecarboxylate derivatives, 8-(cycloalkanecarbonyloxy)naphthalene-1-yl benzoate derivatives, and 8-(cycloalkenecarbonyloxy)naphthalene-1-yl benzoate derivatives. The present invention further relates to catalyst systems containing the catalyst solid components, organoaluminum compounds, and organosilicon compounds. The present invention also relates to methods of making the solid catalyst components and the catalyst systems, and methods of polymerizing or copolymerizing alpha-olefins using the catalyst systems.
Owner:BRASKEM AMERICA +1
Internal and external donor compounds for olefin polymerization catalysts ii
InactiveUS20110207901A1Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationHalogenElectron donor
The present invention relates to catalyst systems containing solid catalyst components comprising titanium, magnesium, halogen and an internal electron donor compound having at least one ether group and at least one ketone group; organoaluminum compounds and alkyl benzoate derivatives as external electron donors. The present invention also relates to methods of making the catalyst systems, and methods of polymerizing or copolymerizing alpha-olefins using the catalyst systems.
Owner:WR GRACE & CO CONN
Internal and external donor compounds for olefin polymerization catalysts III
ActiveUS8318626B2Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationSolid componentElectron donor
The present invention relates to solid catalyst components comprising titanium, magnesium, halogen and an internal electron donor compound containing at least one 1,8-naphthyl diester compound. The 1,8-naphthyl diester compounds include naphthalene-1,8-diyl dicycloalkanecarboxylate derivatives, dicycloalkenecarboxylate derivatives, 8-(cycloalkanecarbonyloxy)naphthalene-1-yl benzoate derivatives, and 8-(cycloalkenecarbonyloxy)naphthalene-1-yl benzoate derivatives. The present invention further relates to catalyst systems containing the catalyst solid components, organoaluminum compounds, and organosilicon compounds. The present invention also relates to methods of making the solid catalyst components and the catalyst systems, and methods of polymerizing or copolymerizing alpha-olefins using the catalyst systems.
Owner:BRASKEM AMERICA +1
Internal and external donor compounds for olefin polymerization catalysts
InactiveUS8211819B2Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationElectron donorAlpha-olefin
The present invention relates to catalyst systems containing solid catalyst components comprising titanium, magnesium, halogen and a 1,8-naphthyl diaryloate internal electron donor compound; organoaluminum compounds and alkyl benzoate derivatives as external electron donors. The present invention also relates to methods of making the catalyst systems, and methods of polymerizing or copolymerizing alpha-olefins using the catalyst systems.
Owner:WR GRACE & CO
Benzoate derivatives, preparation method and application
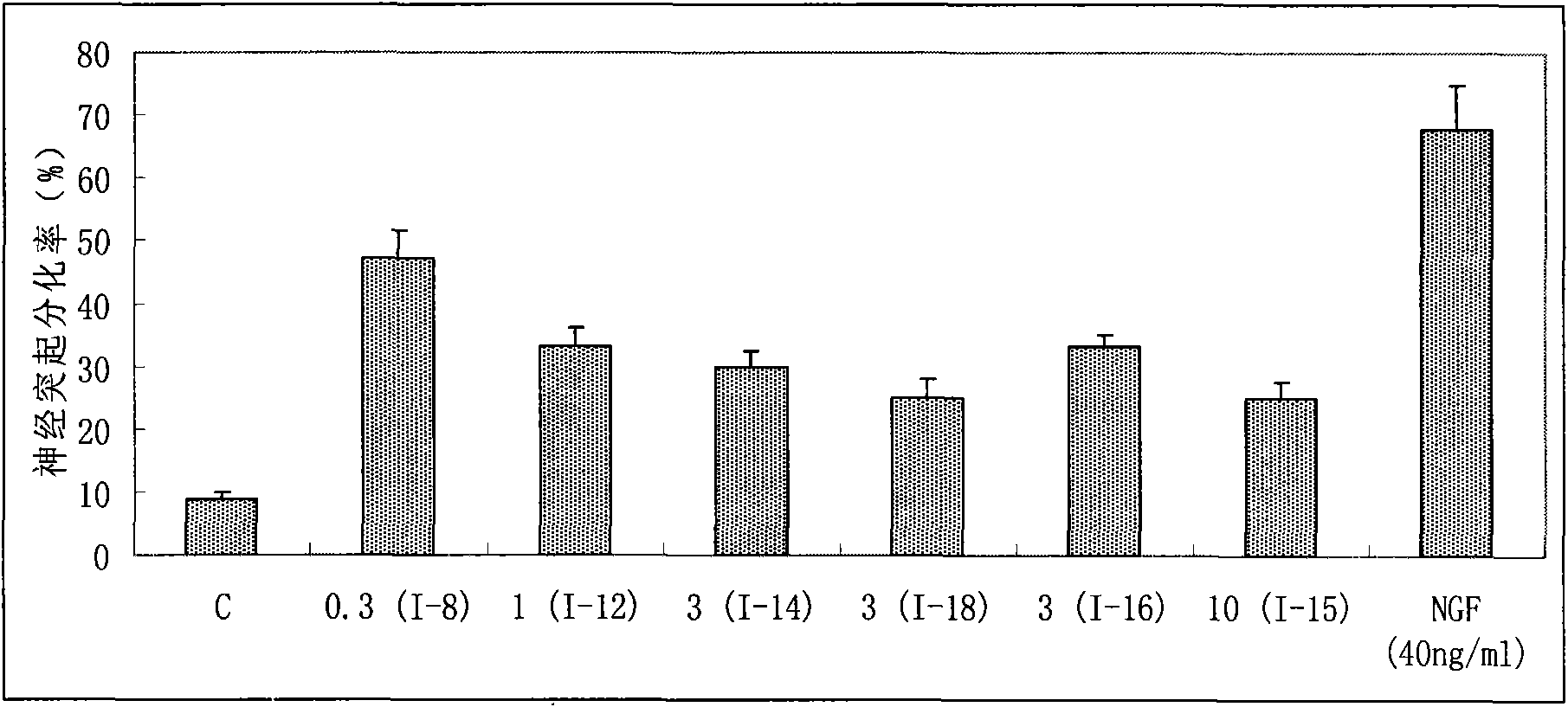
ActiveCN101817761AEnhance memoryReasonable designNervous disorderOrganic compound preparationBenzoic acidNeuro-degenerative disease
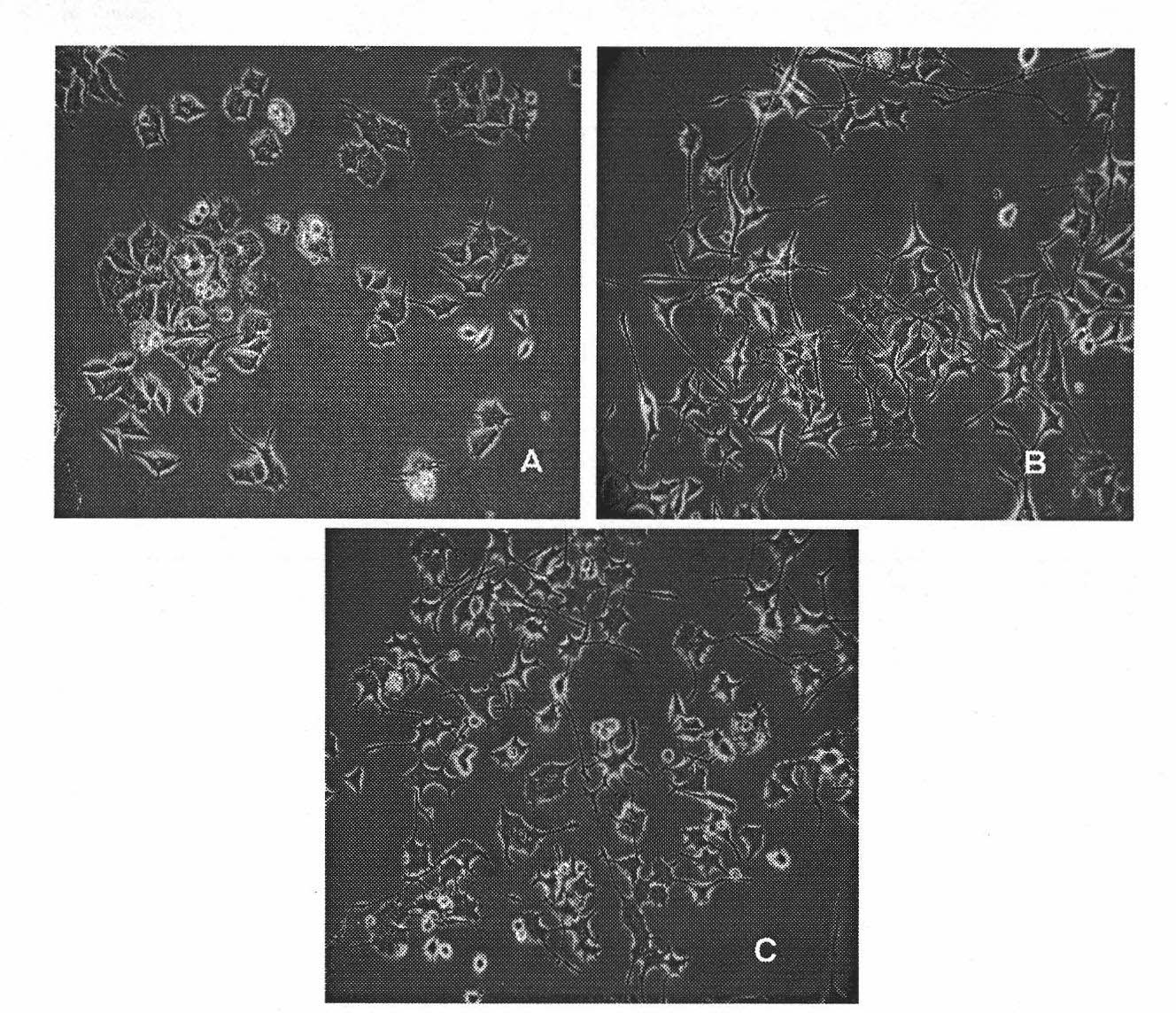
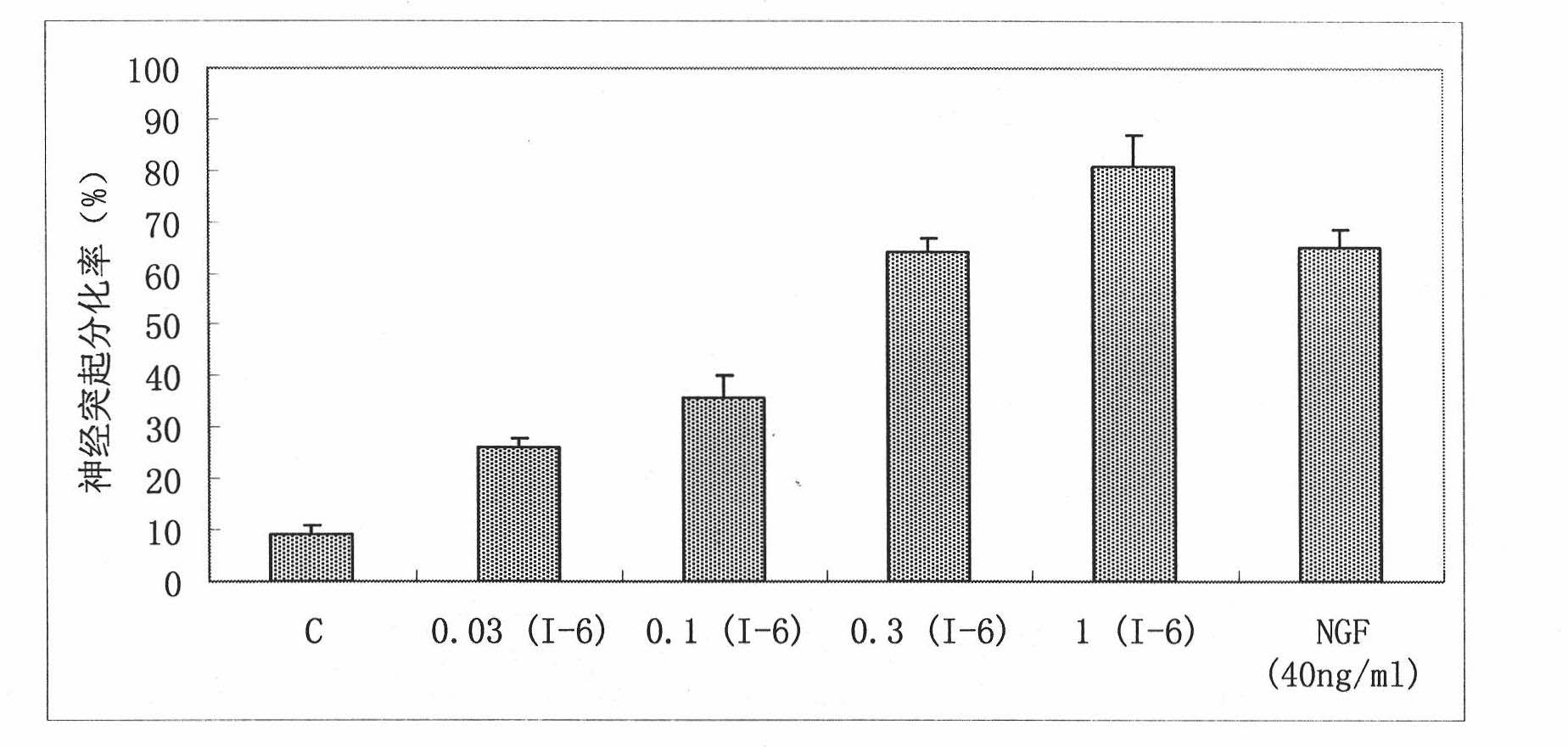
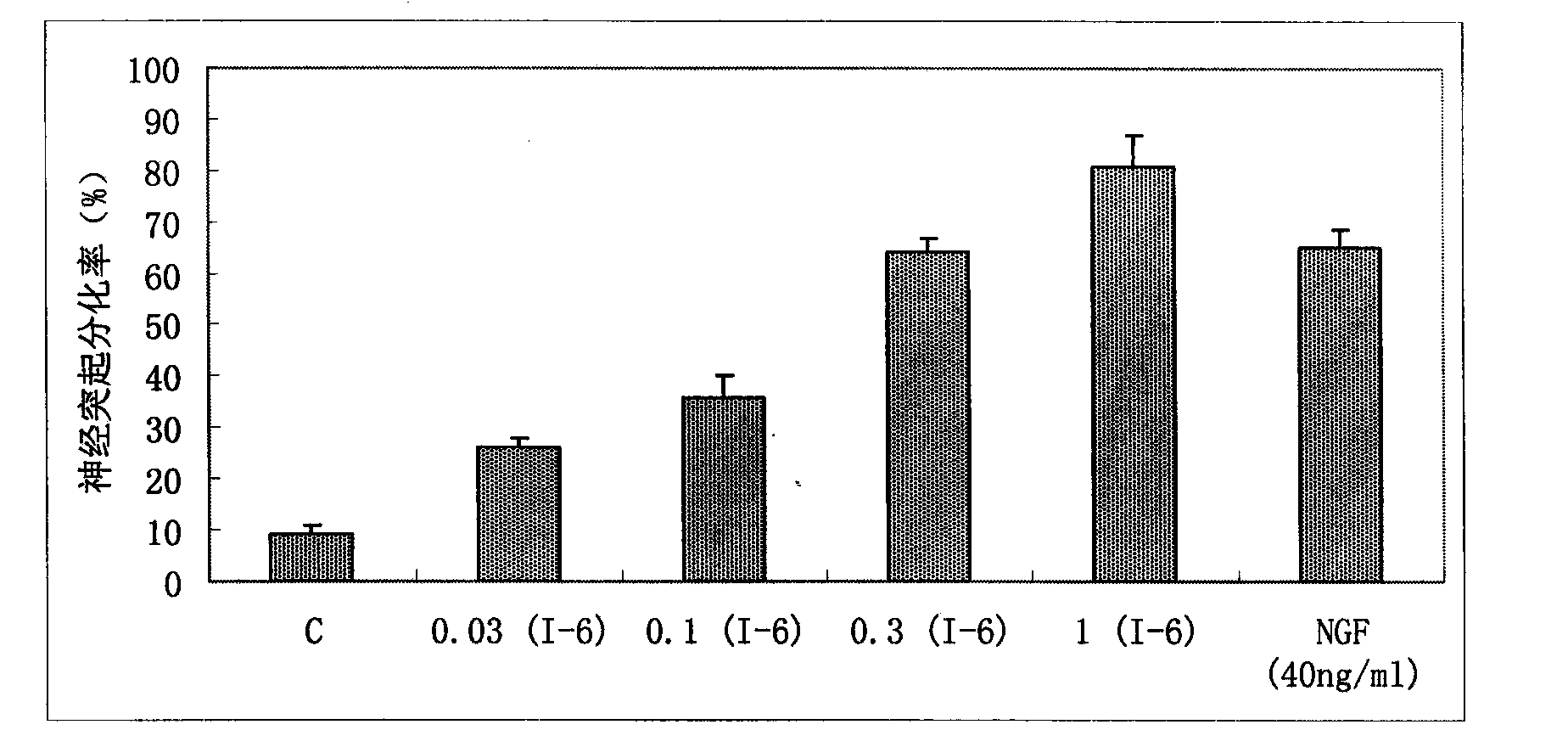
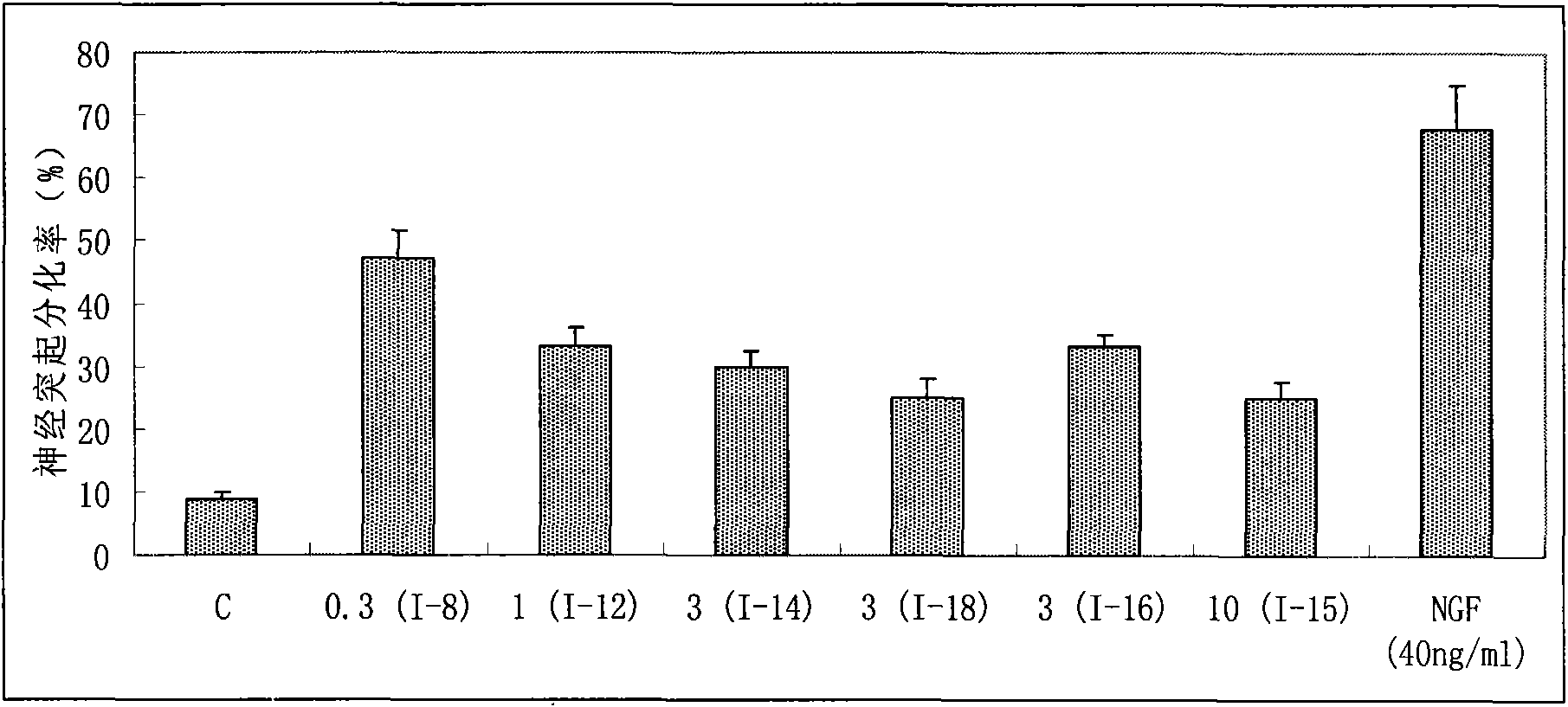
The invention provides benzoate derivatives. A series of benzoate derivatives are synthesized through a chemical method, comprising ester compounds, (sulfur) ether compounds and amide compounds, wherein most synthesized compounds have new chemical structures, and proved through in vitro cell activity experiments, the synthesized benzoate derivatives have obvious activities similar to nerve growth factors (NGF). Proved through an in vitro animal experiment, 2,3-dyhydroxy benzoic acid tetradecyl ester which is a new synthesized compound has the effect of enhancing the memory of senile mice, thus the benzoate derivative can be applied to preparing medicaments for preventing and treating senile dementia neurodegenerative diseases, particularly to preparing medicaments for treating the neurodegenerative diseases such as the Alzheimer's diseases (AD), and the like. The invention opens up a new medical application of the benzoate derivatives, has reasonable preparation method and simple and convenient operation and provides new treatment medicaments for preventing and treating the neurodegenerative diseases such as the senile dementia, and the like.
Owner:杭州常青藤医药科技有限公司
Internal and external donor compounds for olefin polymerization catalysts
InactiveUS20110152481A1Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationHalogenElectron donor
The present invention relates to catalyst systems containing solid catalyst components comprising titanium, magnesium, halogen and a 1,8-naphthyl diaryloate internal electron donor compound; organoaluminum compounds and alkyl benzoate derivatives as external electron donors. The present invention also relates to methods of making the catalyst systems, and methods of polymerizing or copolymerizing alpha-olefins using the catalyst systems.
Owner:WR GRACE & CO CONN
Internal and external donor compounds for olefin polymerization catalysts II
InactiveUS8569195B2Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationBenzoic acidPolymer science
Owner:WR GRACE & CO CONN
Oral care compositions with improved flavor
Owner:PROCTER & GAMBLE CO
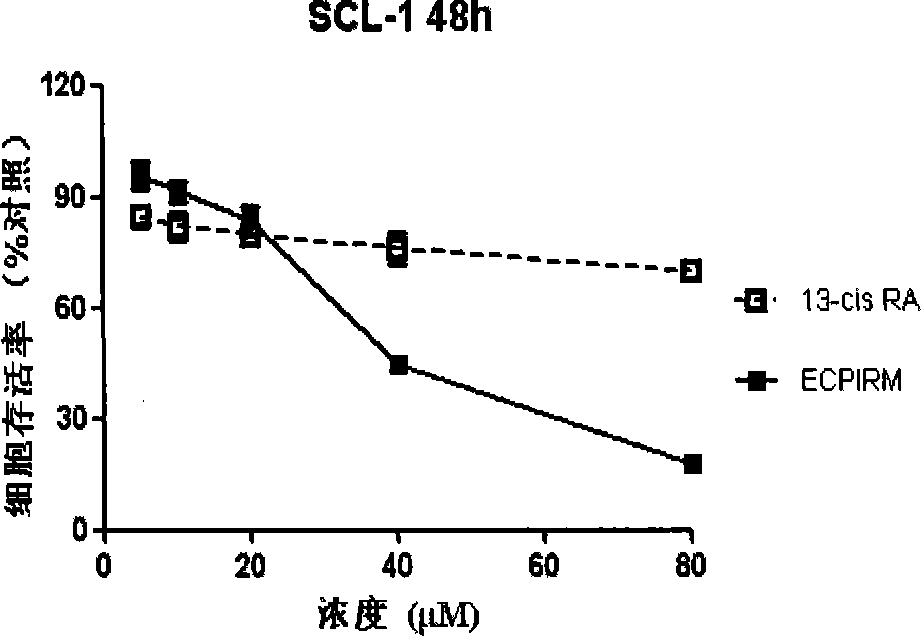
Isotretinoin amido derivative, preparation method thereof and applications thereof
ActiveCN103319365BIncrease medication optionsOvercome the technical difficulty of greatly reducing the acylation reaction activityOrganic active ingredientsOrganic compound preparationBenzoic acidDisease
The invention discloses an isotretinoin amido alkyl benzoate derivative, a preparation method thereof and applications thereof. The derivative has stronger inhibition and differentiation regulating effects than isotretinoin on psoriasis, acne and epithelial cell tumors including, but not limited to skin squamous epithelial cell carcinoma, stomach cancer, lung cancer, and cervical cancer; the derivative has less influence on normal tissue cells, has a certain targeting effect on inhibiting proliferating cells, and has very less side effects than isotretinoin; and the derivative has wide promising prophylaxis and treatment applications such as cornification abnormality diseases and cell abnormal proliferation including tumors, psoriasis, acne, and other cornification abnormality dermatopathy.
Owner:CHUGOKU IGAKU KAGAKUIN HIFUBIYOU KENKYUSHO
Method for synthesizing dual-esterified 3,4,5-trihydroxybenzoxy bagasse xylan benzoate
The invention discloses a method for synthesizing dual-esterified 3,4,5-trihydroxybenzoxy bagasse xylan benzoate. The method comprises the steps: subjecting 3,4,5-triacetyl benzoyl chloride, which isproduced through subjecting 3,4,5-trihydroxybenzoic acid to acetylation and acyl chlorination and serves as an esterifying agent, to an esterification reaction with bagasse xylan in a N,N-dimethylformamide solvent, so as to synthesize bagasse xylan 3,4,5-trihydroxy benzoate; then, carrying out a second-step esterification reaction by taking benzoic acid as an esterifying agent and triethylamine asa catalyst, and synthesizing a dual-esterified 3,4,5-trihydroxybenzoxy bagasse xylan benzoate derivative in a dichloromethane solvent. According to the method, on the basis of unique bioactivity of xylan esterified derivatives, through introducing two kinds of active groups, the product has the advantages that the bioactivity of xylan is improved, and meanwhile, the range of application of the xylan derivatives in the fields of medicine, biology, functional materials and the like is widened.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
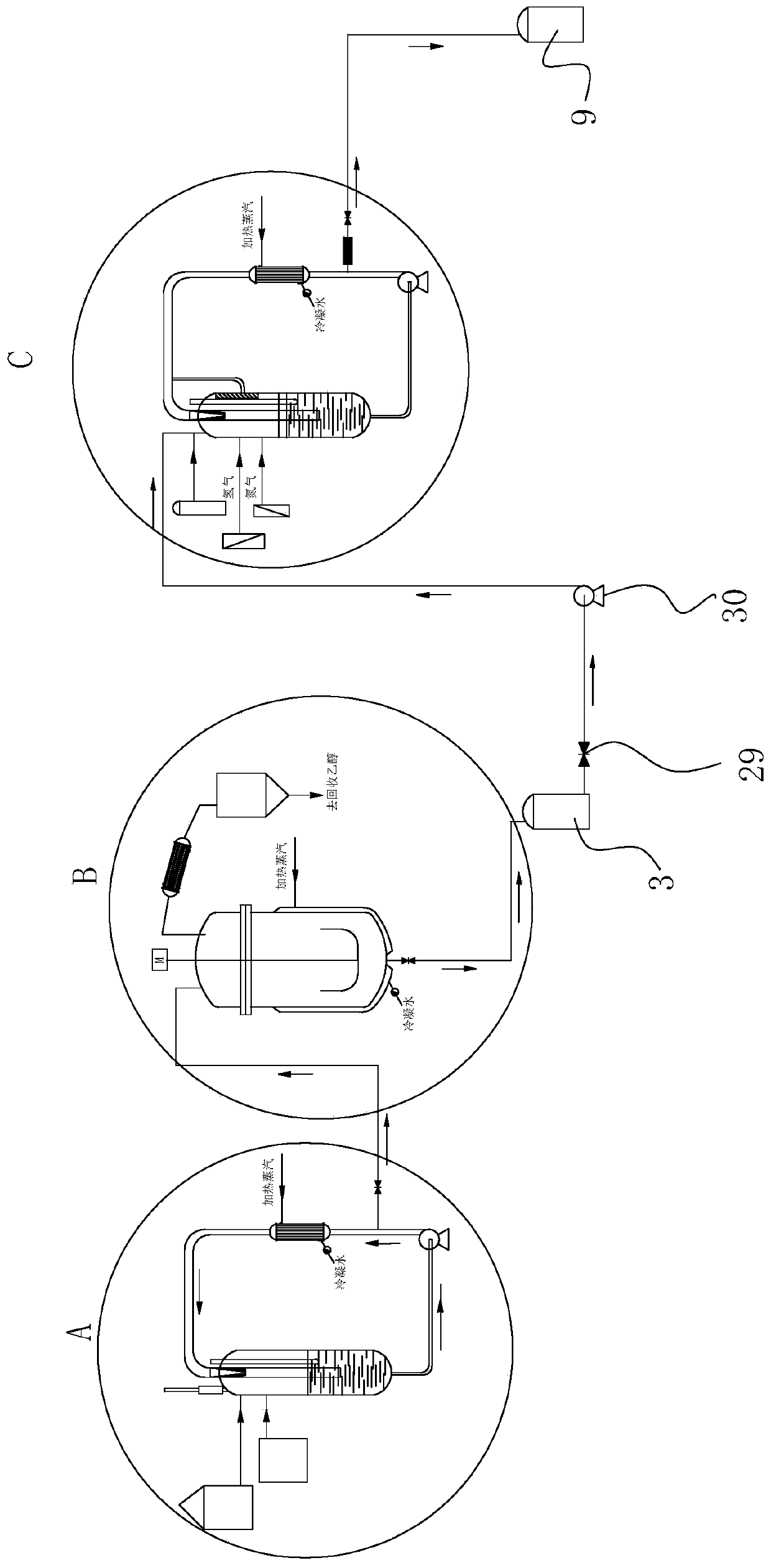
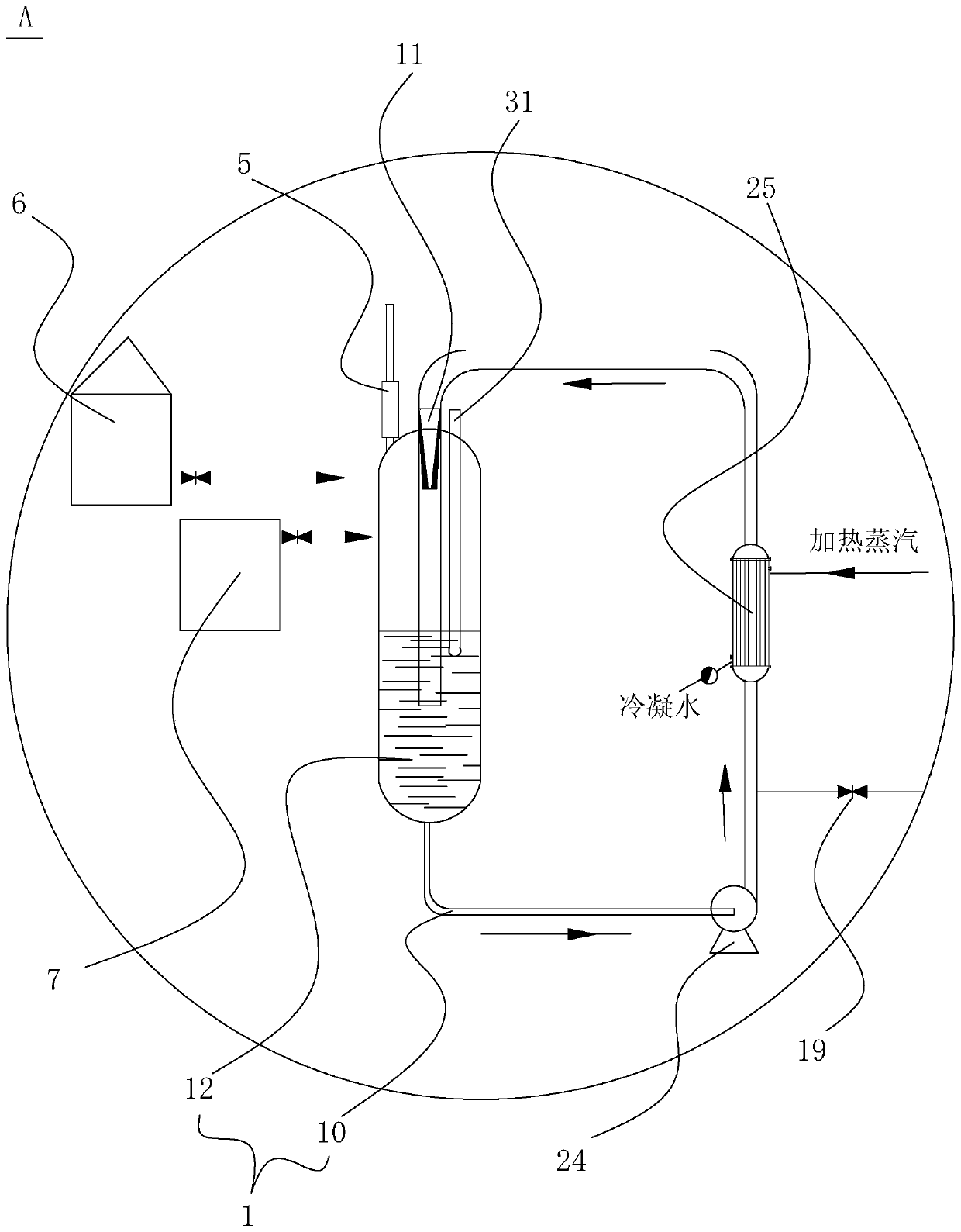
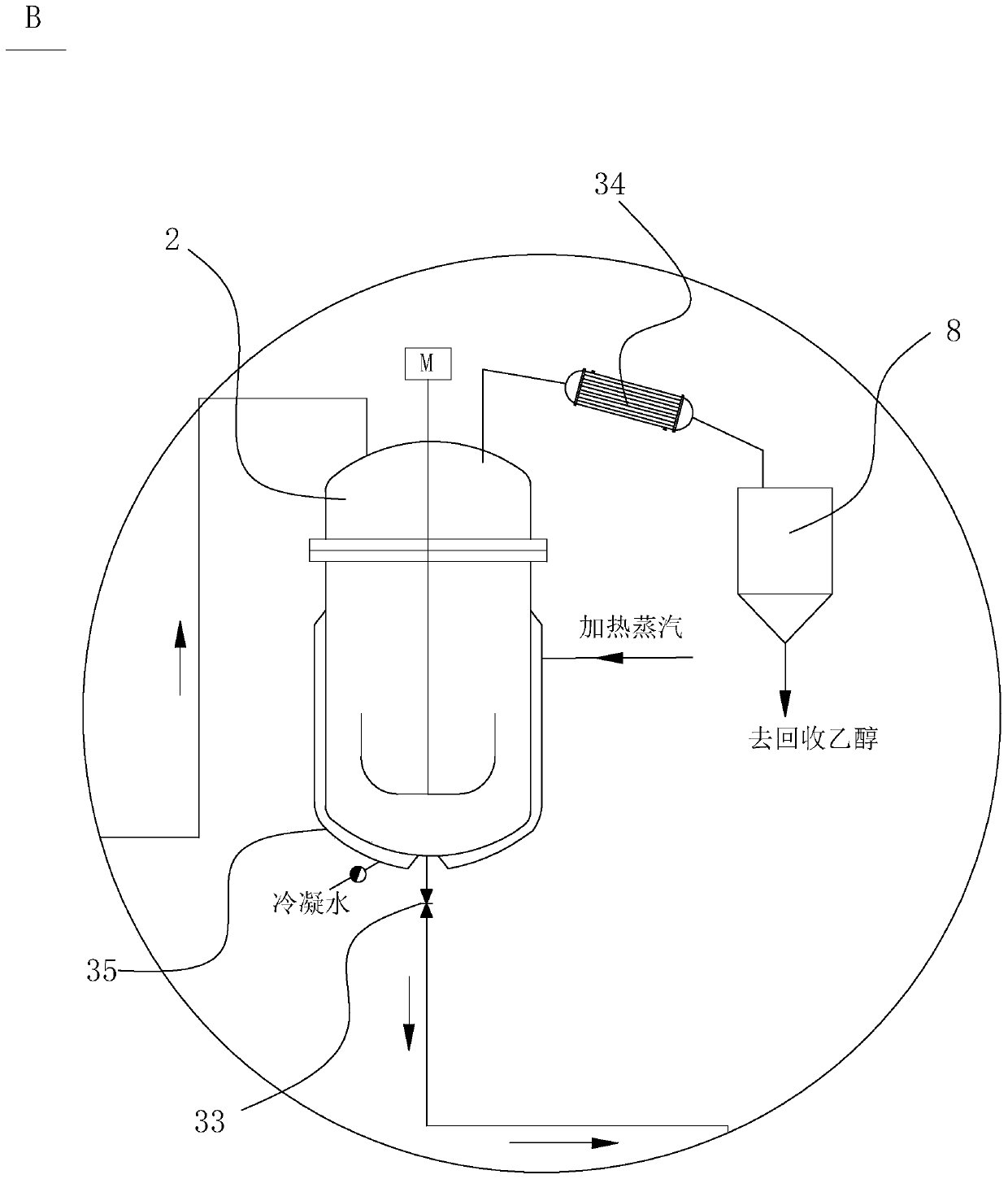
Process for continuously producing an aminobenzoate derivative, and synthesis system thereof
ActiveCN110642733AIncrease productivityHigh degree of automationOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidPtru catalyst
The invention provides a process for continuously producing an aminobenzoate derivative, and a synthesis system thereof. The process comprises: putting a nitrobenzoic acid derivative, ethanol and a first catalyst into an esterification reaction device, and reacting for 1-1.5 h at a temperature of 70-75 DEG C; supplementing a nitrobenzoic acid derivatives, ethanol and a first catalyst into the esterification reaction device; making the liquid in the esterification reaction device flow into a dehydration esterification kettle; heating to azeotrope the ethanol-water in the dehydration esterification kettle, evaporating the azeotrope out of the dehydration esterification kettle, collecting the azeotrope, and discharging the liquid in the dehydration esterification kettle into a middle liquid storage groove; and making the liquid in the middle liquid storage groove flow into a hydrogenation reaction device, and carrying out a hydrogenation reaction under the action of a second catalyst at areaction temperature of 100-120 DEG C to obtain the product, wherein the pressure is kept at 0.6-1 Mpa. According to the invention, the process has advantages of continuous production and the like.
Owner:ZHEJIANG UNIV OF TECH
Automotive compounds featuring low surface tack
The present invention relates to a polymer composition comprising a heterophasic propylene copolymer, a mineral filler and a light stabilizers comprising a fatty acid derivative and a benzoate derivative. The polymer composition according to the present invention is applicable for automotive articles whereupon the undesired effect of stickiness is reduced significantly compared to automotive articles comprising conventional light stabilizers.
Owner:BOREALIS AG
Method for preparing drug Roflumilast for treating chronic obstructive pulmonary disease
InactiveCN106632015AReduce usageQuantity is easy to controlOrganic chemistryBenzoic acidObstructive Pulmonary Diseases
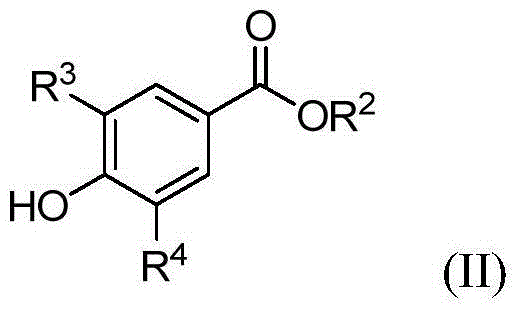
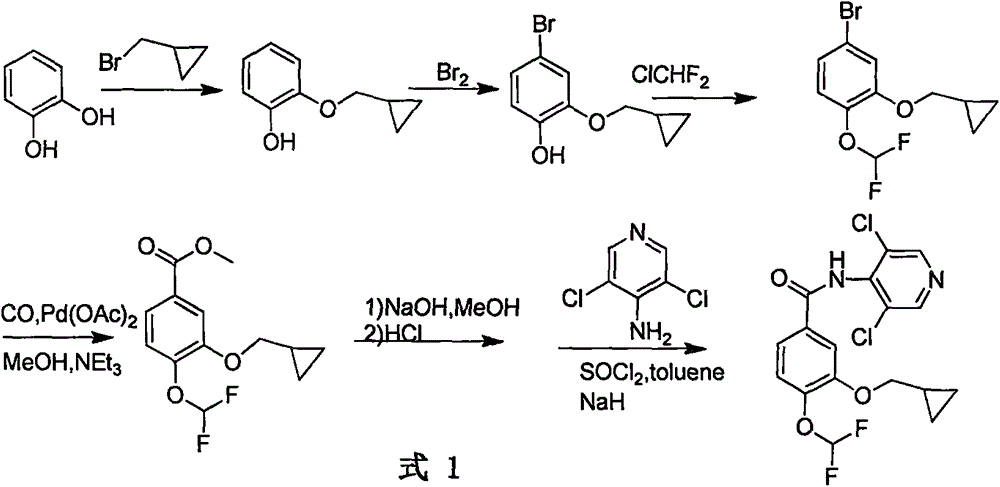
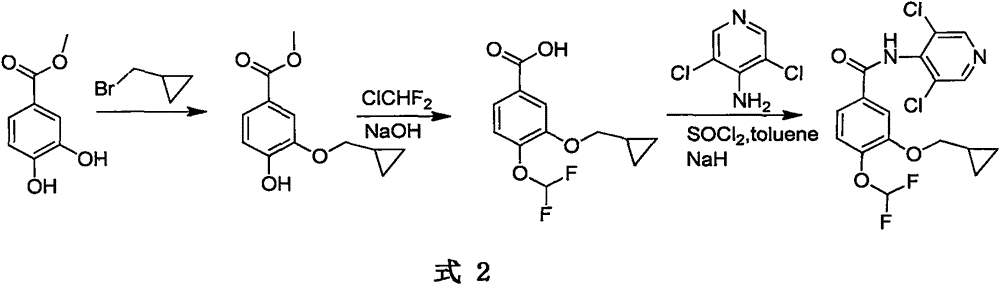
The invention relates to a method for preparing a drug Roflumilast for treating the chronic obstructive pulmonary disease. The Chinese chemical name of the drug Roflumilast is 3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl)-4-(difluoromethoxy)benzamide. According to the method, in a process route, a dihydroxyl-substituted benzoate derivative, which can be extensively obtained, is adopted as a starting raw material, so that the consumption of noble metal catalysts is avoided, the synthesis process route is shortened, and the production cost is reduced greatly; through introducing difluoromethoxy by replacing a gaseous reagent with a solid difluoromethyl etherification reagent, which is safe, efficient, cheap and readily available, side reactions are reduced, and the selectivity of the reaction is improved; and the target product is obtained efficiently through condensing carboxyl groups and amino groups.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Benzoate derivatives, preparation method and application
ActiveCN101817761BEnhance memoryReasonable designNervous disorderOrganic compound preparationToxic reactionSenile dementia
The uses of benzoate and its derivatives for anti-aging of brain, and for preventing and treating the neurodegenerative diseases such as the senile dementia and the like are provided in the present invention. A series of benzoate and its derivatives are synthesized through a chemical method in the present invention. Proved through cell activity experiments in vitro, the synthesized benzoate and its derivatives have distinguished activities similar to nerve growth factors. Proved through an animal experiment, benzoate and its derivatives do not induce toxic reaction during long term oral or celiac administration, they are able to cross the blood-brain barrier and promote the regeneration of cerebral neuron.
Owner:杭州常青藤医药科技有限公司
Isotretinoin amido derivative, preparation method thereof and applications thereof
ActiveCN103319365AEnhanced inhibitory effectEasy to adjustOrganic active ingredientsOrganic compound preparationDiseaseSide effect
The invention discloses an isotretinoin amido alkyl benzoate derivative, a preparation method thereof and applications thereof. The derivative has stronger inhibition and differentiation regulating effects than isotretinoin on psoriasis, acne and epithelial cell tumors including, but not limited to skin squamous epithelial cell carcinoma, stomach cancer, lung cancer, and cervical cancer; the derivative has less influence on normal tissue cells, has a certain targeting effect on inhibiting proliferating cells, and has very less side effects than isotretinoin; and the derivative has wide promising prophylaxis and treatment applications such as cornification abnormality diseases and cell abnormal proliferation including tumors, psoriasis, acne, and other cornification abnormality dermatopathy.
Owner:CHUGOKU IGAKU KAGAKUIN HIFUBIYOU KENKYUSHO
Chemical synthesis method for 2,3,4,5-tetrahydro-1H-2-benzazepin-1-one derivatives
InactiveCN102796044AThere is no defect of isomerizationHigh yieldOrganic chemistryBenzoic acidChemical synthesis
The invention discloses a chemical synthesis method for 2,3,4,5-tetrahydro-1H-2-benzazepin-1-one derivatives. The synthesis method of the invention includes: reacting a substituted ortho-halo benzoic acid as a raw material with an alcohol to obtain corresponding substituted acid ester; reacting with acrylonitrile to obtain a 2-(2'-cyano-alkenyl)benzoate derivative intermediate; and conducting a ring-closure reaction in the presence of alkoxide to obtain the 2,3,4,5-tetrahydro-1H-2-benzazepin-1-one derivative. The method has the advantages of fewer reaction steps and high yield.
Owner:NANTONG UNIVERSITY
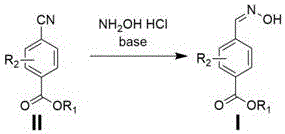
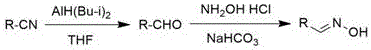
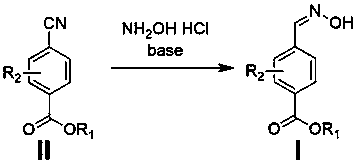
4-formaldoxime benzoate derivative preparation method
ActiveCN106631885AReduce manufacturing costMild reaction conditionsOximes preparationAlcohol4-cyanobenzoate
The invention provides a 4-formaldoxime benzoate derivative preparation method. The method mainly includes steps: dissolving 4-cyanobenzoate derivatives and hydroxylamine hydrochloride into an alcohol solvent, adding an appropriate amount of alkali, and performing reflux reaction to obtain a target product 4-formaldoxime benzoate derivative. According to the method, cyano compounds are adopted to directly obtain oxime compounds through one-step reaction. The method has advantages of mild reaction conditions, simplicity in operation, low cost, high product yield, high purity and the like and is suitable for large-scale production.
Owner:荆门医药工业技术研究院 +1
Process for the preparation of zafirlukast
InactiveUS20040186300A1Improve purification effectHigh yieldOrganic chemistryChemical recyclingBenzoic acidAlkyl transfer
The present invention provides a novel process for the preparation of alkyl (1-alkylindol-3-ylmethyl)benzoate derivatives which process comprises the steps of: (a) reacting of an alkyl (halomethyl)benzoate with excess of an indole, said indole being unsubstituted at positions 1-, 2- and 3-, under conditions promoting alkylation at the 3-position of the indole to yield a mixture comprising alkyl (indol-3-ylmethyl)benzoate and unreacted starting indole, (b) treating the mixture obtained in step (a) with base to yield a mixture, comprising the salt of (indol-3-ylmethyl)benzoic acid and the unreacted indole, (c) recovering the unreacted indole from the mixture obtained in step (b), and recycling the indole as starting material to step (a), (d) isolating the salt of (indol-3-ylmethyl)benzoic acid and / or acidifying the salt to form (indol-3-ylmethyl)benzoic acid, (e) reacting the (indol-3-ylmethyl)benzoic acid or it's salt with alkylating agent in the presence of base to form the desired alkyl (1-alkylindol-3-ylmethyl)benzoate. The above process affords also the preparation of the anti-asthmatic leukotriene antagonist zafirlukast. In such case, methyl 3-methoxy-4-(1-methyl-5-nitroindol-3-ylmethyl)benzoate [a]is formed in step (e) of the process and this compound is subsequently converted into zafirlukast by known methods.
Owner:FINETECH LAB
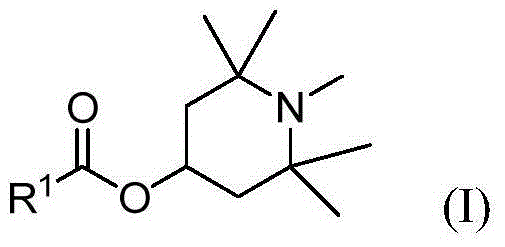
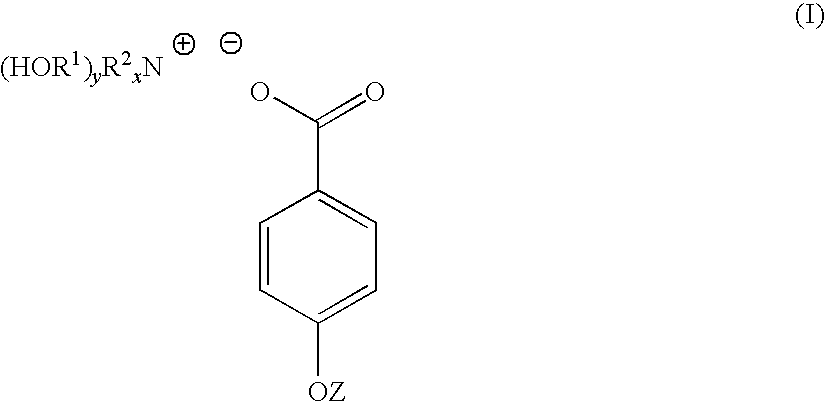
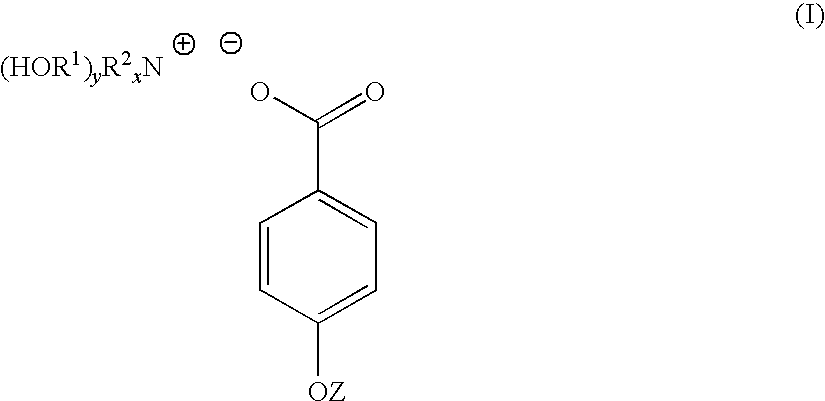
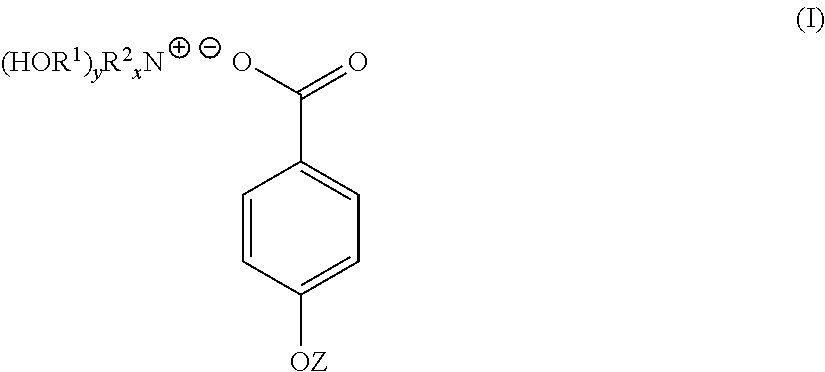
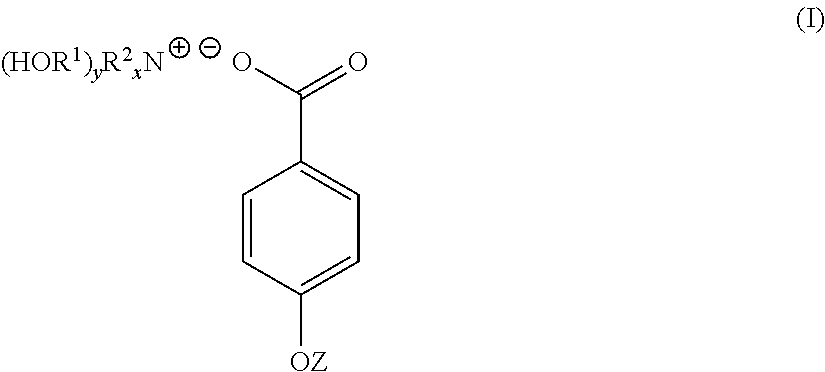
4-hydroxy benzoate derivatives for use in the treatment of infection, inflammation or pain
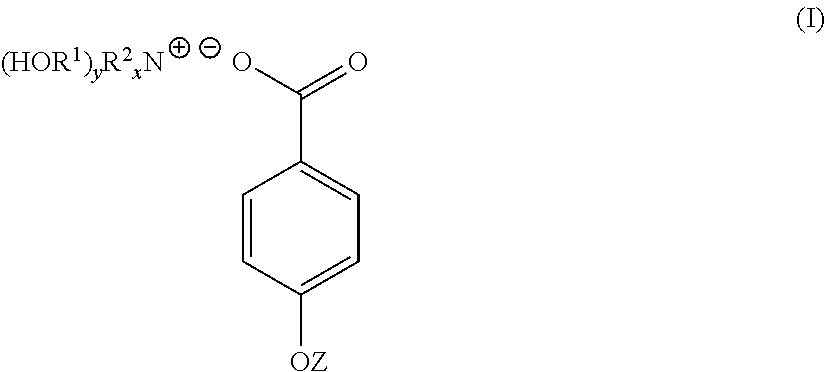
A compound of the general formula (I) is provided for use in the topical treatment of infection, inflammation and / or pain: wherein R1 independently represents a methylene group, an ethylene group or a straight or branched chain C3 to C6 alkylene group; R2 independently represents a hydrogen atom, a methyl group, an ethyl group or a straight or branched chain C3 to C20 alkyl group; x represents 0 or an integer from 1 to 4 and y represents 0 or an integer from 1 to 4, wherein the sum of x and y is 4; and Z represents a hydrogen atom or (HOR1)yR2XN+; compositions including the compound; use of the compound in the manufacture of a medicament; and methods of medical treatment including the topical application of the compound.
Owner:JORDAN ROY ARLINGTON
Preparation and plant growth-regulating activity of water-oil soluble o-(4-chloro) benzylthio-benzoate derivatives
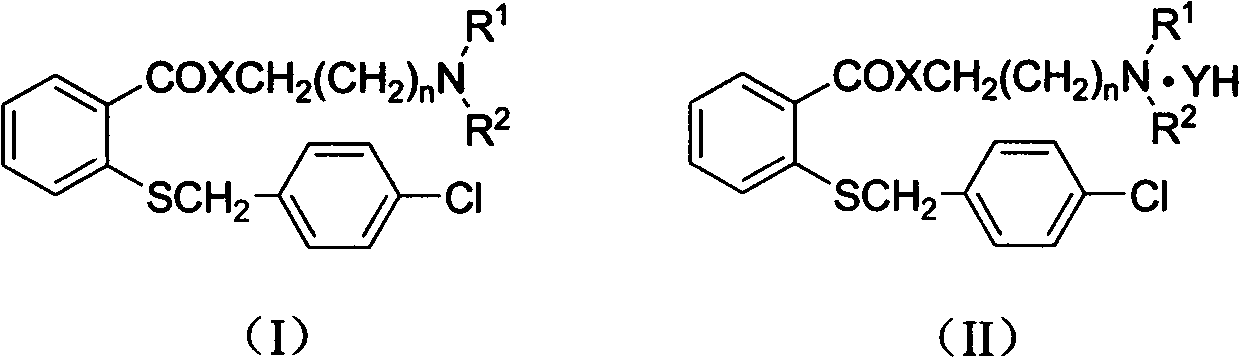
InactiveCN102731354AGood water solubilityGood oil solubilityBiocidePlant growth regulatorsSalicylic acidP-Toluenesulfonic acid
The invention provides water-oil soluble o-(4-chloro) benzylthio-benzoate derivatives with plant growth-regulating activity and salts thereof. The derivatives are shown as a general formula (I, II), wherein X is an oxygen atom, a nitrogen atom or a sulfur atom; n is 0, 1, 2, 3, 4, 5 and so on or (CH2)n represents alkyl carrying a branched chain; Y is Cl<->, Br<->, F<->, I<->, AcO<->, acetylsalicylic acid radical, nitric acid radical, salicylic acid radical, p-toluenesulfonic acid radical, bisulfate radical or other negative ions; R<1> is alkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, alkenyl of 1-6 carbon atoms, or aryl; R<2> is alkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, alkenyl of 1-6 atoms, or aryl; or R<1> and R<2> are selected from the structure in the specification.
Owner:NANKAI UNIV
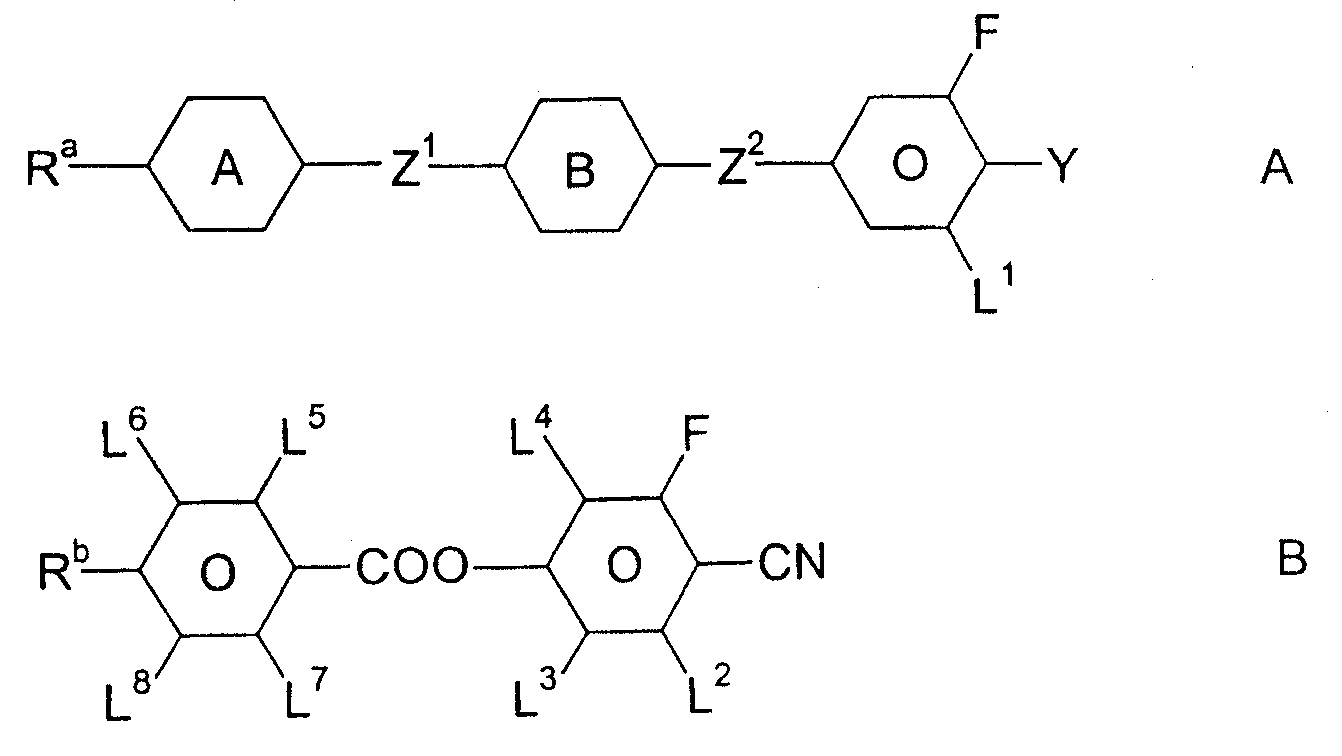
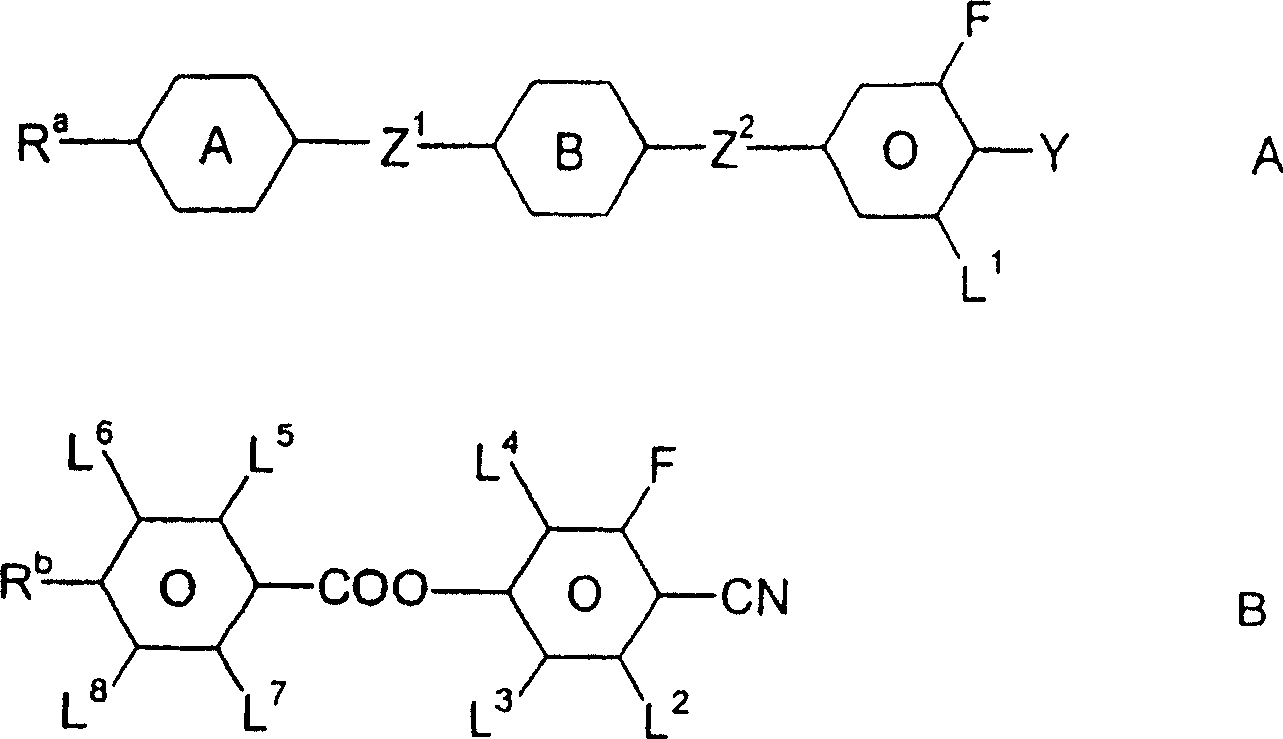
Liquid crystal medium
InactiveCN100513518CLow viscosityLower threshold voltageLiquid crystal compositionsThin material handlingLiquid crystallineAngle of incidence
A liquid crystalline medium containing (A) compound(s) with three 1,4-linked 6-membered rings in which one of the terminal rings is a fluorinated phenyl group and (B) 4-cyano-3-fluorophenyl benzoate derivative(s). A liquid crystalline (LC) medium (I) containing compound(s) of formula (A) and compound(s) of formula (B). Ra, Rb = H, 1-12C alkyl (optionally substituted with one CN or CF3 group or at least one halogen, and optionally with CH2 group(s) replaced by -O-, -S-, cyclobutane-1,3-diyl, -CH=CH-, -Cequivalent toC-, -CO-, -COO-, -OCO- or -OCOO-), -(A)-Z1-(B)-, -Cyc-Z1-Diox- or -Diox-Z1-Cyc-; (A), (B) = rings A, B as above; Cyc = trans-1,4-cyclohexylene; Diox = 1,3-dioxan-5,2-diyl; Z1, Z2 = -CH2CH2-, -(CH2)4-, -CH2-, -(CH2)3- or a single bond; L1-L8 = H or F; Y = F, Cl, SF5, NCS, OCN, SCN or a mono- or poly-halogenated 1-5C alkyl, alkoxy, alkenyl or alkenyloxy group. Independent claims are also included for (1) (1) electro-optical LC displays containing (I) (2) (2) TN or STN LC displays with two base plates forming a cell, a nematic LC mixture with positive dielectric anisotropy (DELTAe) in the cell, electrode layers with orientation layers on the inside of the base plates, an angle of incidence of 0-30 degrees between the base plates and the long axis of the molecules on their surface and a twist angle of 22.5-600 degrees from orientation layer to orientation layer in the cell, in which the nematic LC mixture comprises (a) 15-75 wt% of an LC component A consisting of compound(s) with a DELTAe of more than +1.5, (b) 25-85 wt% of an LC component B consisting of compound(s) with a DELTAe between -1.5 and +1.5, (c) 0-20 wt% of an LC component D consisting of compound(s) with a DELTAe of below -1.5 and (d) optionally an optically active component C in amounts such that the ratio between layer thickness (plate spacing) and natural pitch of the chiral nematic LC mixture = 0.2-1.3, and in which component A contains compound(s) of formula (A) and compound(s) of formula (B)
Owner:MERCK PATENT GMBH
2-(isoxazole-5-yl) phenyl-3, 4-dihydroxy-benzoate and derivative thereof, synthetic method and application of 2-(isoxazole-5-yl) phenyl-3, 4-dihydroxy-benzoate and derivative thereof
PendingCN114751870APrevent proliferationThe synthetic route is simpleOrganic chemistryAntineoplastic agentsBenzoic acidHydroxybenzoates
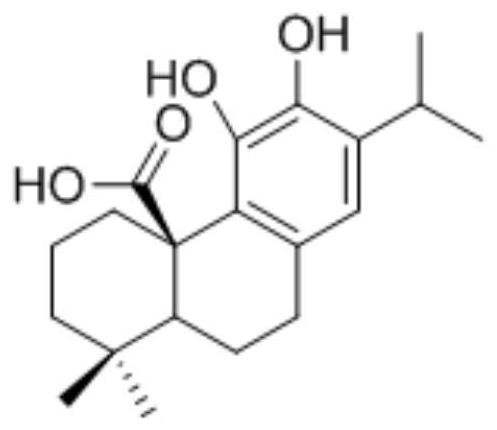
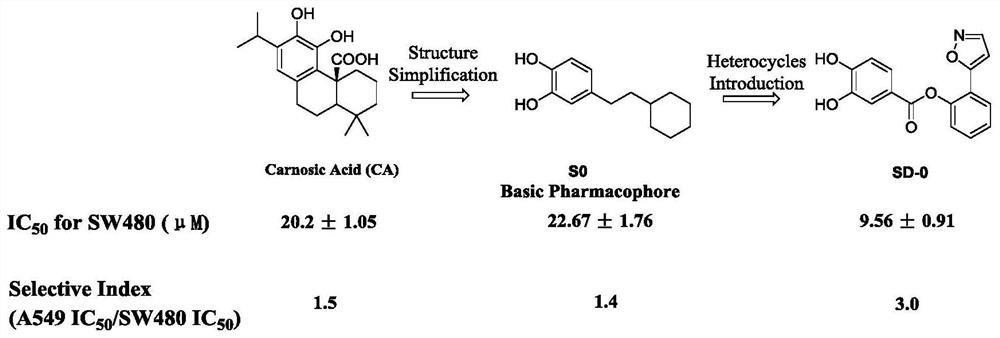
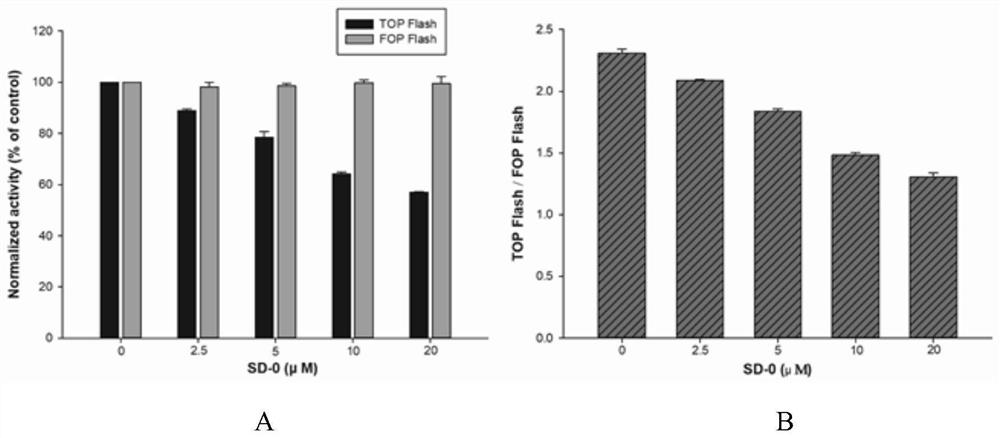
The invention belongs to the field of medicinal chemistry, and particularly relates to 2-(isoxazole-5-yl) phenyl-3, 4-dihydroxy benzoate and a derivative thereof as well as a synthesis method and application of the 2-(isoxazole-5-yl) phenyl-3, 4-dihydroxy benzoate. 2-(isoxazole-5-yl) phenyl-3, 4-dihydroxy benzoate is designed and synthesized, and research shows that SD-0 does not affect the intracellular total beta-catenin content, and meanwhile, beta-catenin / BCL9PPI is affected, so that beta-catenin nuclear transfer is reduced, and colorectal cancer cell proliferation caused by Wnt / beta-catenin abnormal expression is inhibited; a series of 2-(isoxazole-5-yl) phenyl-3, 4-dihydroxy benzoate derivatives are synthesized, and meanwhile, the synthetic route for preparing the compound is simple, the reaction condition is mild, and post-treatment is convenient.
Owner:GUIZHOU MEDICAL UNIV
Herbicidally active pyrimidinesalicylic acid compound, its preparation method and its use as herbicide
ActiveCN104302629BImprove herbicidal activityHas a broad-spectrum effectBiocideOrganic chemistryBenzoic acidBenzoate derivative
A pyrimidine salicylate compound with herbicidal activity, its preparation method and its use as a herbicide, the compound is 2,6-bis((4,6-dimethoxypyrimidinyl-2-yl)oxy base) imino benzoate derivatives, which can effectively control weeds such as barnyardgrass, stephenia, bermudagrass, water amaranth, clove Polygonum, knotweed, etc., have a broad-spectrum effect, and are environmentally friendly and low-toxic. human safety.
Owner:常州瑞平化工有限公司
4-hydroxy benzoate derivatives for use in the treatment of infection, inflammation or pain
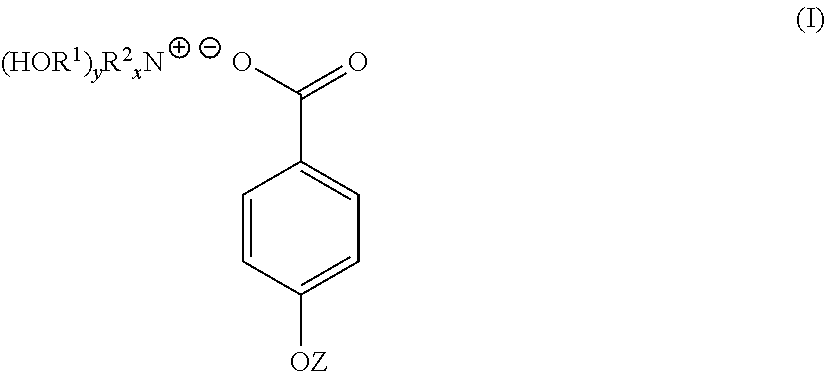
A compound of the general formula (I) is provided for use in the topical treatment of infection, inflammation and / or pain: wherein R1 independently represents a methylene group, an ethylene group or a straight or branched chain C3 to C6 alkylene group; R2 independently represents a hydrogen atom, a methyl group, an ethyl group or a straight or branched chain C3 to C20 alkyl group; x represents 0 or an integer from 1 to 4 and y represents 0 or an integer from 1 to 4, wherein the sum of x and y is 4; and Z represents a hydrogen atom or (HOR1)yR2xN+; compositions including the compound; use of the compound in the manufacture of a medicament; and methods of medical treatment including the topical application of the compound.
Owner:JORDAN ROY ARLINGTON
4-hydroxy benzoate derivatives for use in the treatment of infection, inflammation or pain
A compound of the general formula (I) is provided for use in the topical treatment of infection, inflammation and / or pain: wherein R1 independently represents a methylene group, an ethylene group or a straight or branched chain C3 to C6 alkylene group; R2 independently represents a hydrogen atom, a methyl group, an ethyl group or a straight or branched chain C3 to C20 alkyl group; x represents 0 or an integer from 1 to 4 and y represents 0 or an integer from 1 to 4, wherein the sum of x and y is 4; and Z represents a hydrogen atom or (HOR1)yR2XN+; compositions including the compound; use of the compound in the manufacture of a medicament; and methods of medical treatment including the topical application of the compound.
Owner:JORDAN ROY ARLINGTON
A method for preparing 4-formaldoxime benzoate derivatives
ActiveCN106631885BReduce manufacturing costMild reaction conditionsOximes preparationAlcohol4-cyanobenzoate
Owner:荆门医药工业技术研究院 +1
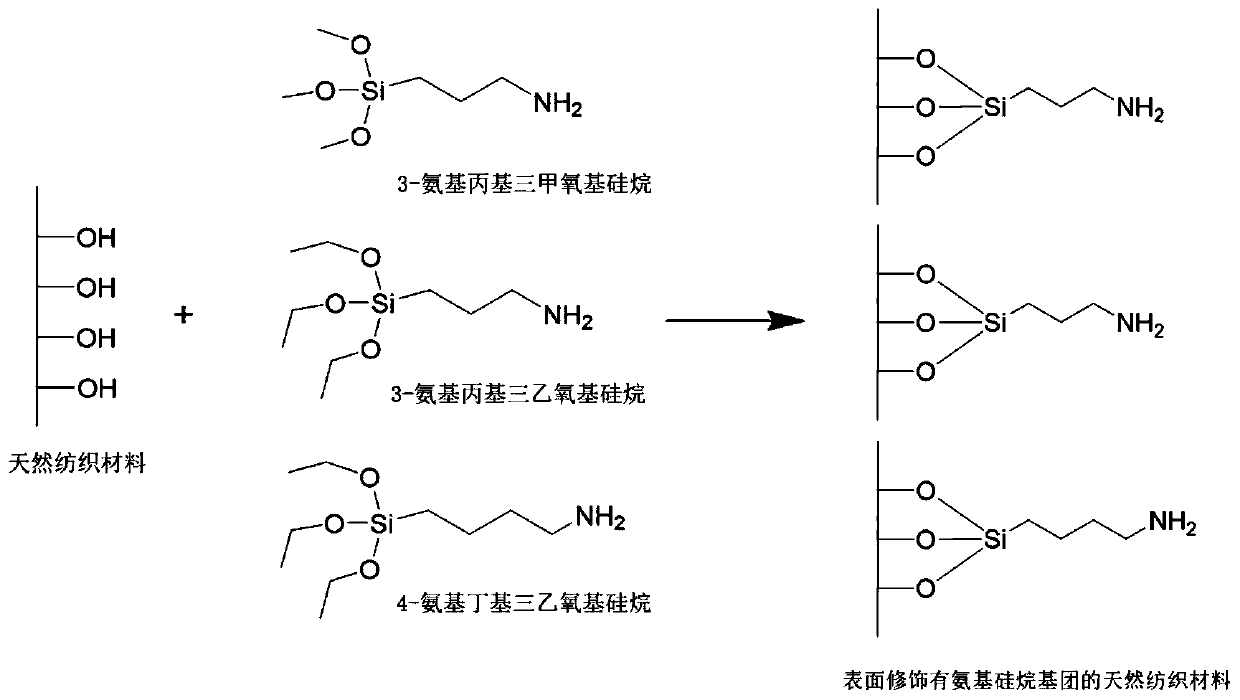
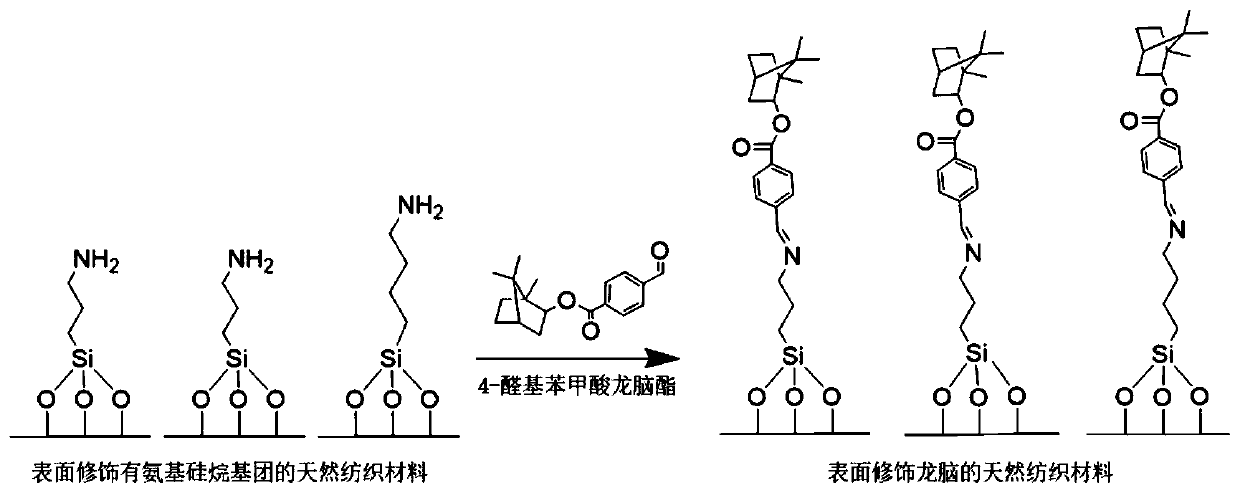
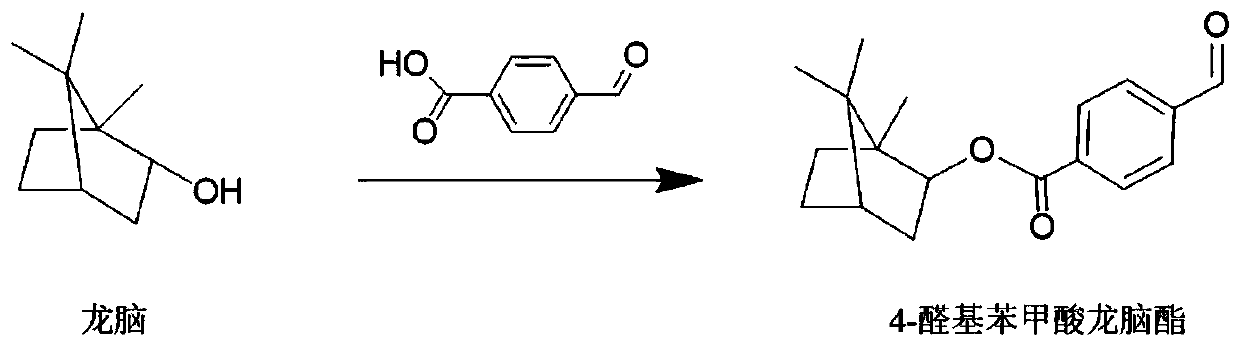
Antibacterial natural textile material with surface modified borneol and its preparation method and application
ActiveCN110306340BInhibit or prevent adhesionGood anti-adhesion effectBiochemical fibre treatmentVegetal fibresBiotechnologyBenzoic acid
The present invention relates to a borneol surface-modified antimicrobial natural textile material, using a new antimicrobial strategy of surface stereochemistry, obtained by means of a natural textile material coupled to aminosiloxane and a borneol 4-aldehyde benzoate derivative, reaction conditions of a preparation process being mild, and the preparation technique being simple. The antimicrobial natural textile material is a non-release type antimicrobial textile material, and will not release bactericide to kill a microorganism, instead affecting microorganism adhesion by means of a material surface stereochemical structure, which can ensure safety of use, will not stimulate and sensitize skin, and will not harm natural skin flora; in addition, the present material has good anti-adhesion functionality for both bacteria and fungi, capable of effectively inhibiting bacterial and fungal adhesion in the long term, and the structure is stable and washable. The present antimicrobial natural textile material is a safe, stable and environmental new antimicrobial natural textile product, which may be wide used in the medical, hygiene, environmental and clothing industries.
Owner:BEIJING UNIV OF CHEM TECH
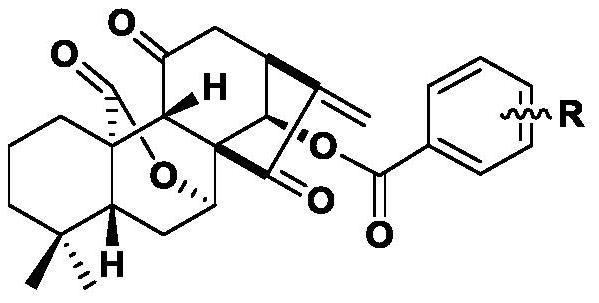
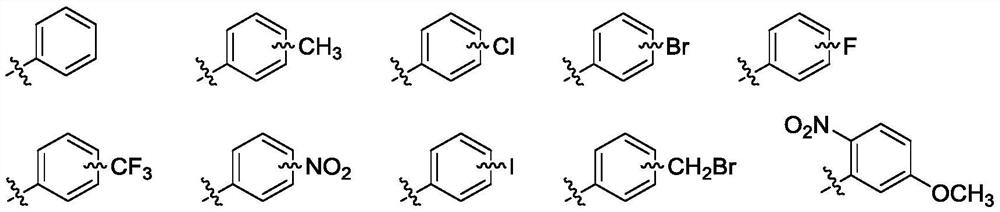
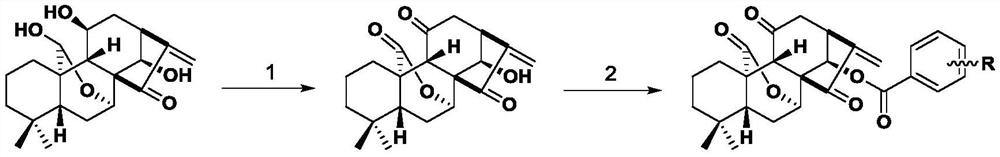
11,20-Dicarbonyl Jiyuan Rubescensin A 14-O-benzoate derivative and its preparation method and use
ActiveCN113004241BImprove stabilityImprove anti-tumor effectOrganic chemistryAntineoplastic agentsBenzoic acidDisease
The invention relates to the field of natural products and medicinal chemistry, and discloses 11,20-dicarbonyl Jiyuan oridonin A 14-O-benzoate derivative and a preparation method and application thereof. Its preparation method: taking Jiyuan Rubescensine A (JOA) as a starting material, oxidizing it to obtain 11,20-dicarbonyl Jiyuan Rubescensine A, and then without destroying its active center α-methylene group. Under the premise of cyclopentanone, benzoic acid or substituted benzoic acid and its 14-OH undergo esterification to obtain a series of 11,20-dicarbonyl Jiyuan Rubescensine A 14-O-benzoate derivatives. The compounds have good anti-tumor activity and can be used for preparing anti-cancer drugs and treating diseases such as esophageal cancer, gastric cancer, liver cancer, breast cancer, pancreatic cancer and the like. Its general structural formula is as follows:
Owner:ZHENGZHOU UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com