Isotretinoin amido derivative, preparation method thereof and applications thereof
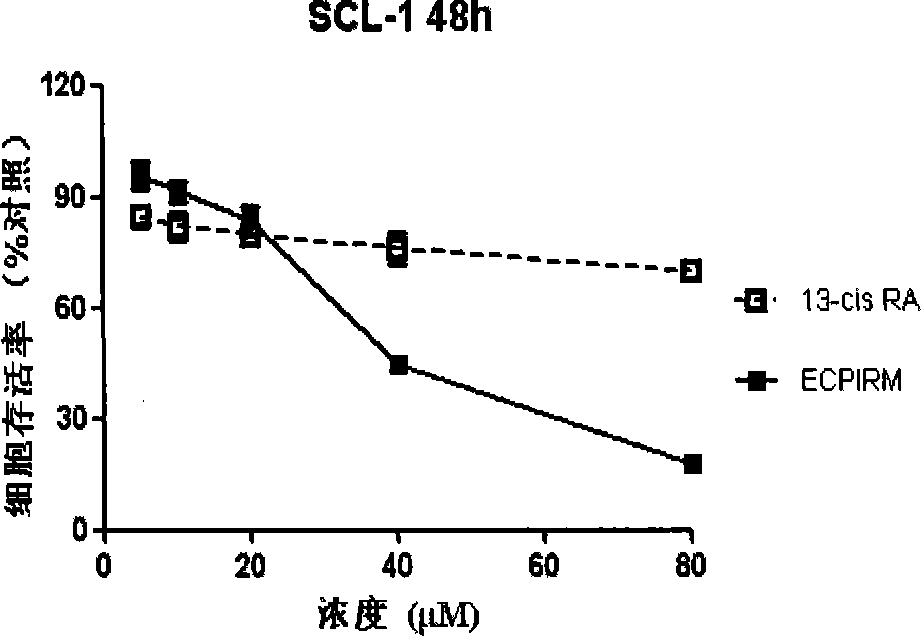
A technology of isotretinoin and its derivatives, which is applied in the field of biomedicine, can solve problems such as the activation ability of retinoic acid nuclear receptors and its side effects, the reduction of electron cloud density, and the reduction of amino nucleophilicity. Minimal adverse reactions, increased activity, reduced acylation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the synthesis of p-(isotretinoin amido) methyl benzoate
[0054] In a 250ml three-necked flask, add 4-dimethylaminopyridine (DMAP) (0.1g, 0.8mmol), p-toluenesulfonic acid (0.14g, 0.8mmol), add CH 2 Cl 2 110ml, then added isotretinoin (2.4g, 8mmol), methyl p-aminobenzoate (1.21g, 8.0mmol), stirred at room temperature for 10min, then added DCC (1.82g, 8.8mmol), stirred at 10°C for about 4 hours, stop the reaction. The above reactions were carried out under the protection of nitrogen and away from light. The above reaction mixture was placed at 0-5°C for about 12 hours, taken out and filtered, and the filtrate was rotary evaporated to obtain a crude product, which was separated by column chromatography (200-300 mesh silica gel, eluent: ethyl acetate:petroleum ether 1:15 ( V / V) ~ 1:10 (V / V)), gradient elution, and the resulting product was recrystallized from ethanol to obtain the reaction product (3.02g). The molecular mass of the reaction product is 433....
Embodiment 2
[0055] Embodiment 2: the synthesis of p-(isotretinoin amido) ethyl benzoate
[0056] In a 250ml three-necked flask, add 4-dimethylaminopyridine (DMAP) (0.1g, 0.8mmol), p-toluenesulfonic acid (0.14g, 0.8mmol), add CH 2 Cl 2 110ml, then added isotretinoin (2.4g, 8mmol), ethyl p-aminobenzoate (1.4g, 8.4mmol), stirred at room temperature for about 15min, then added DCC (1.87g, 9.1mmol), 40°C Stir and reflux for about 3 hours to stop the reaction. The above reactions were carried out under the protection of nitrogen and away from light. The above reaction mixture was placed at 0-5°C for about 12 hours, taken out and filtered, and the filtrate was rotary evaporated to obtain a crude product, which was separated by column chromatography (200-300 mesh silica gel, eluent: ethyl acetate:petroleum ether 1:20 ( V / V)), and the resulting product was recrystallized from ethanol to obtain a reaction product (3.58 g). The molecular mass of reaction product is 447.62, proton nuclear magneti...
Embodiment 3
[0057] Embodiment 3: the synthesis of p-(isotretinoin amido) propyl benzoate
[0058] In a 250ml three-necked flask, add 4-dimethylaminopyridine (DMAP) (0.12g, 0.96mmol), p-toluenesulfonic acid (0.17g, 0.96mmol), add CH 2 Cl 2 110ml, then added isotretinoin (2.4g, 8mmol), p-propyl aminobenzoate (1.72g, 9.6mmol), stirred at room temperature for 15min, then added DCC (2.15g, 10.4mmol), stirred at 30°C for about After 5 hours, the reaction stopped. The above reactions were carried out under the protection of nitrogen and away from light. The above reaction mixture was placed at 0-5°C for about 12 hours, taken out and filtered, and the filtrate was rotary evaporated to obtain a crude product, which was separated by column chromatography (200-300 mesh silica gel, eluent: ethyl acetate:petroleum ether 1:20 ( V / V)), and the resulting product was recrystallized from ethanol to obtain a reaction product (2.9 g). The molecular mass of the reaction product is 461.65, and the proton n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com