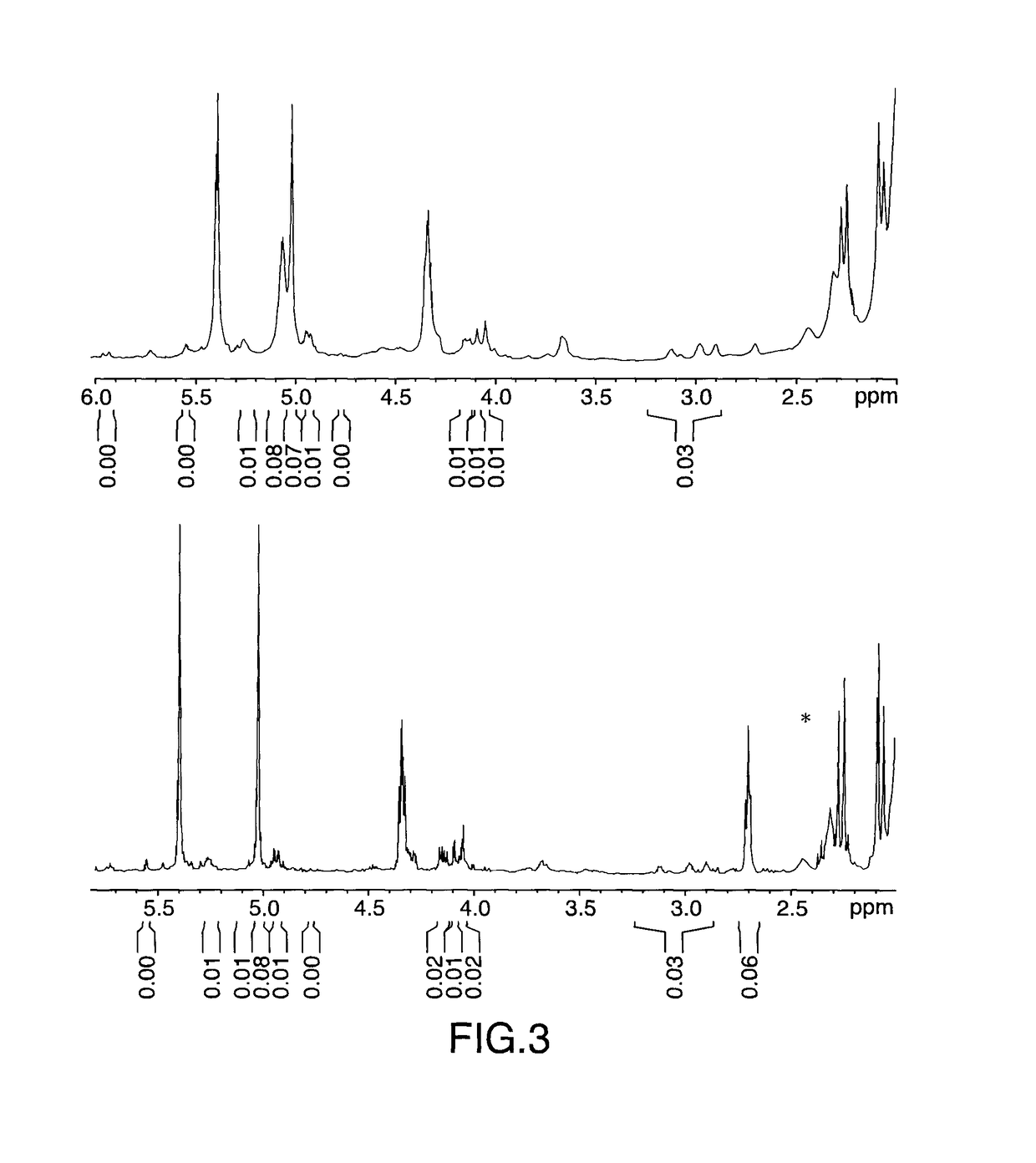
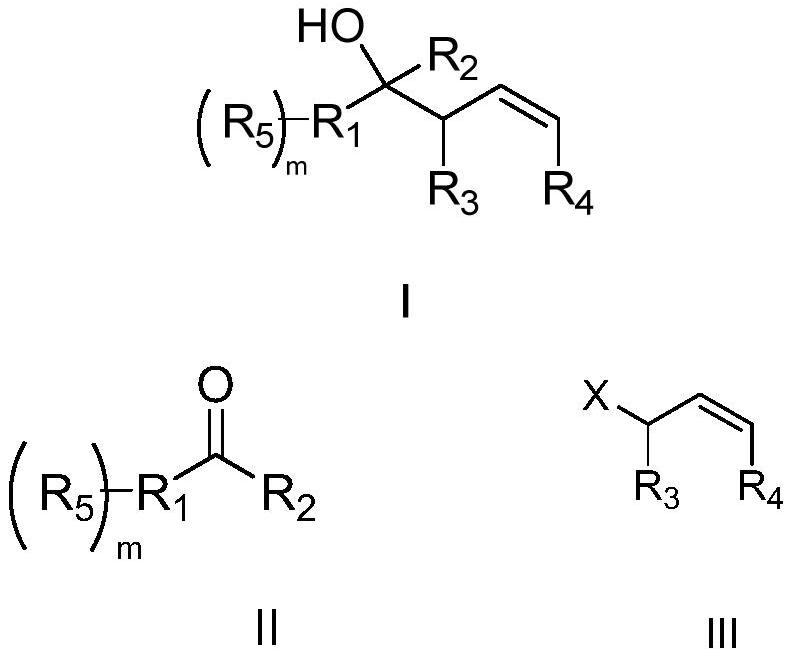
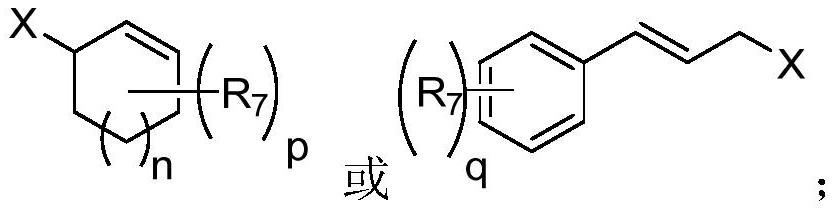
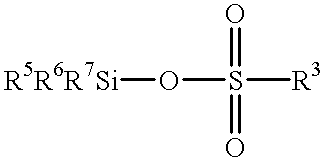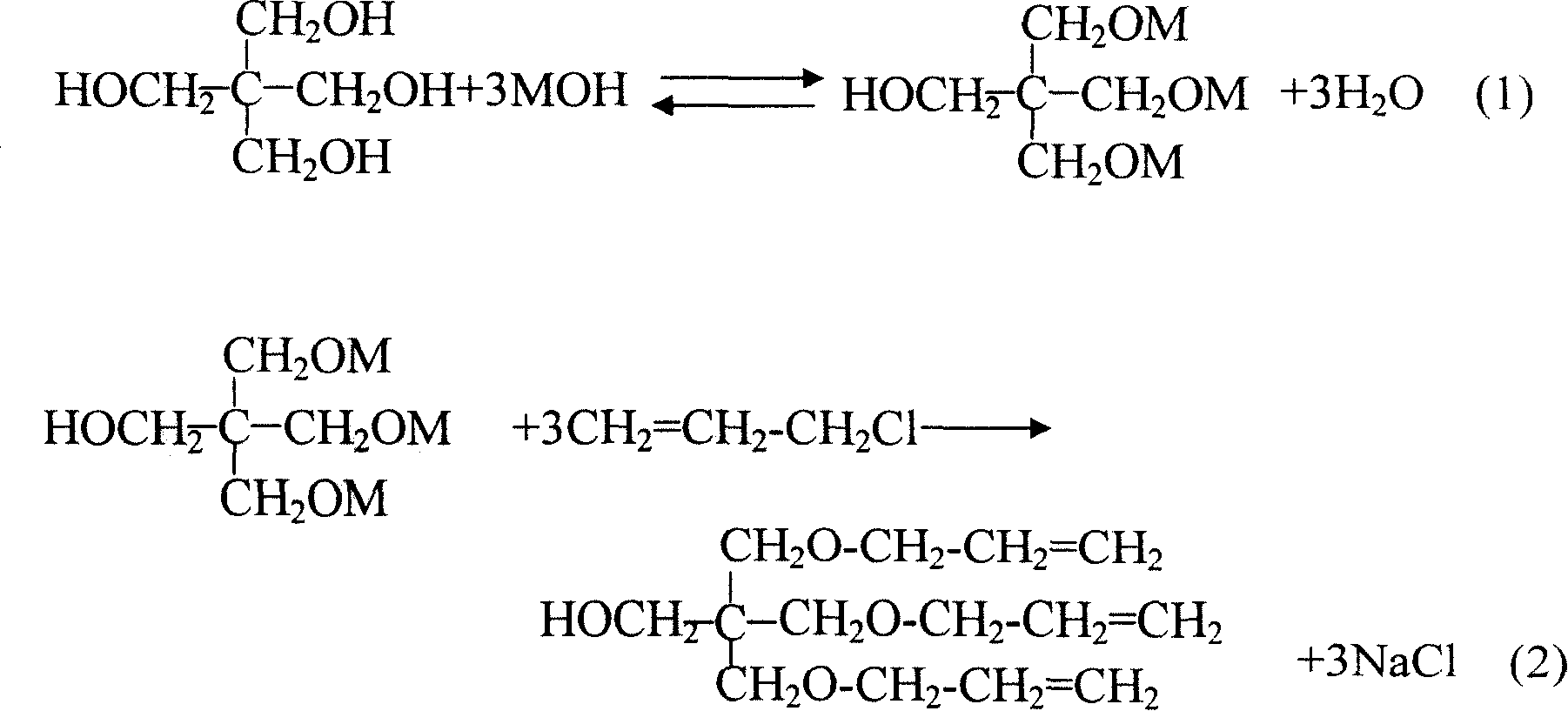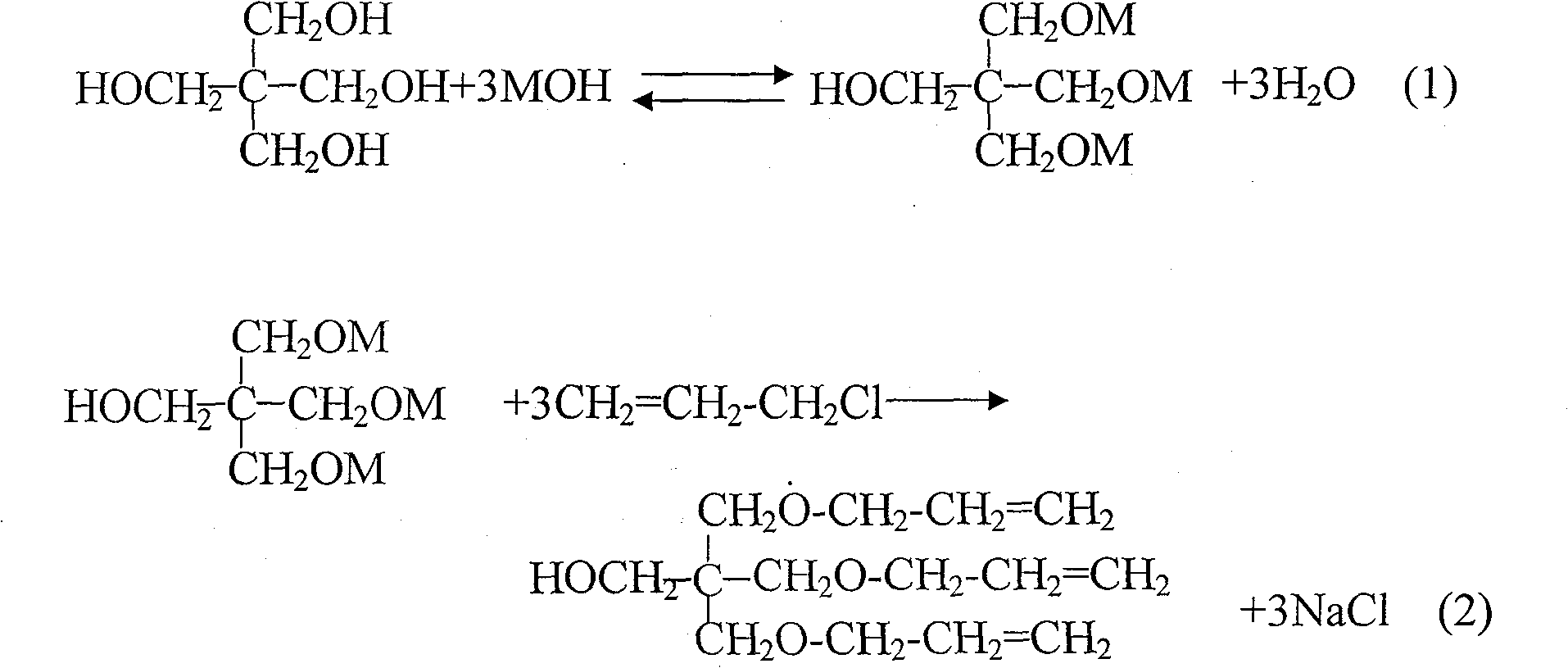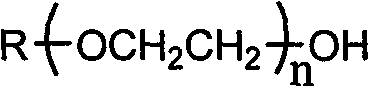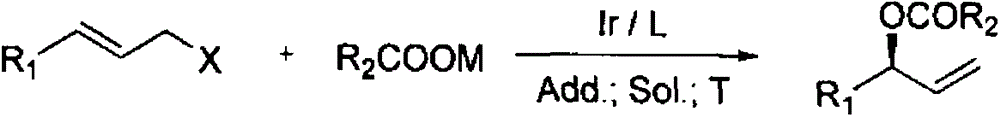Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Allyl halides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Allylic Halide. An allylic halide is an alkyl halide in whose molecule there are one or more halogen atoms on allylic carbons.
Partially or fully neutralized butyl ionomers in golf ball layers
ActiveUS20100009778A1Increasing resiliency and impact durabilityFibre treatmentSynthetic resin layered productsIonomerEngineering
A golf ball having a core, and at least one layer about the core, wherein the core or the layer is formed from partially or fully neutralized butyl ionomers or their blends to improve the resiliency and impact durability over a conventional butyl rubber. The core comprises a thermoplastic material, a thermoset material, or a rubber-based material, while at least one layer is formed from a composition comprising a partially or fully neutralized butyl rubber ionomer. The butyl rubber ionomer comprises repeating units derived from at least one isoolefin monomer, at least 0.5 mol % of repeating units derived from at least one multiolefin monomer, at least 0.5 mol % of repeating units derived from an allylic halide, and at least 0.5 mol % of an ionomeric moiety. The isoolefin comprises isobutylene, the multiolefin comprises isoprene and the allylic halide comprises a bromide.
Owner:ACUSHNET CO
Method for manufacturing silicon compound having substituents bonded to silicon atoms via Si-C bonds
InactiveUS6297340B1Strong position selectivityHigh activitySilicon organic compoundsSilyleneCarboxylic acid
A hydrosilylation reaction method that achieves high catalytic activity and stability, and improves the positional selectivity of the hydrosilylation reaction product. The method comprises reacting an unsaturated compound selected from the group consisting of aromatic vinyl compounds and allyl halides with a silicon compound having hydrosilyl groups described by formula HSiRn(Z)3-n, where n=0, 1, or 2, R is a hydrocarbon group and Z is selected from the group consisting of a silamino group, siloxy group, and siloxanoxy group in the presence of a carboxylic acid compound, a silyl ester of a sulfonic acid, and a platinum catalyst.
Owner:DOW CORNING ASIA LTD
Synthesis method of reactive hindered phenol oxidation-resistant polyolefin additive and application thereof
InactiveCN106633267ASynthetic reaction conditions are simpleSimple processOrganic compound preparationCarbonyl compound preparationPolymer sciencePolyolefin
The invention relates to a synthesis method of a reactive hindered phenol oxidation-resistant polyolefin additive and application thereof. The synthesis method comprises the following steps that a catalyst and 2,4 dyhydroxyl diphenylketone are dissolved in a solvent according to the molar ratio to 1-1.2 to 1, stirring reaction is performed at the temperature of 40-60 DEG C for 2-8 hours, then allyl halide is slowly and dropwise added, constant temperature reaction is performed at the temperature of 40-60 DEG C for 2-6 hours, standing and crystallization are performed, and a product is filtered to obtain the reactive hindered phenol oxidation-resistant polyolefin additive. The reactive hindered phenol oxidation-resistant polyolefin additive is applied to a polyolefin composite material, can improve the compatibility between the additive and polyolefin resin, has the advantages of extraction resistance, difficult migration, difficult volatilization and no environmental pollution, can keep a lasting oxidation-resistant effect, can capture peroxy radicals and can also capture alkyl free radicals, can play the oxidation-resistant effect simultaneously in the surface and interior of a polymer and is high in oxidation-resistant efficiency.
Owner:GUANGZHOU JET BIOFILTRATION CO LTD
Method for preparing bisphenol S allyl ether
InactiveCN102249960ASimple equipment requirementsEasy to controlOrganic chemistryOrganic compound preparationSimple Organic CompoundsEther
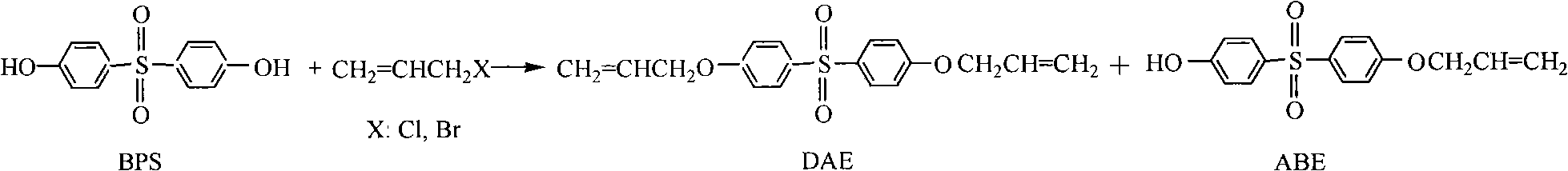
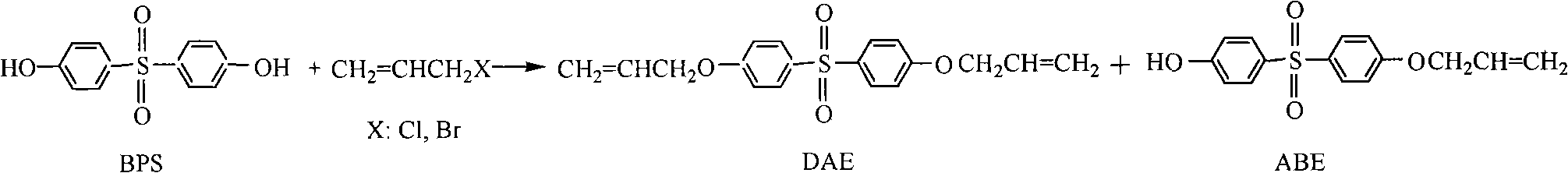
The invention belongs to the field of preparation of aromatic sulfones organic compounds, and in particular relates to a method for preparing bisphenol S allyl ether (including bisphenol S diallyl ether and bisphenol S monoallyl ether). The method provided by the invention comprises the following steps of: (1) carrying out an etherification reaction: heating and reflowing bisphenol S and allyl halides in an alkaline solution to react for 1-8 hours under the effect of a phase transferring catalyst; (2) recycling the catalyst; (3) separating diallyl ether (DAE) and allyl bisphenol ether (ABE); (4) refining the DAE; and (5) refining the ABE. The method disclosed by the invention has the advantages of simpleness in operation, convenience for recycling the catalyst and the solvent, environment friendliness, high product yield and purity and low manufacturing cost; and the method is suitable for the industrial production.
Owner:JILIN NORMAL UNIV
Partially or fully neutralized butyl ionomers in golf ball layers
ActiveUS8026304B2Increasing resiliency and impact durabilitySwimming aidsFibre treatmentIonomerEngineering
A golf ball having a core, and at least one layer about the core, wherein the core or the layer is formed from partially or fully neutralized butyl ionomers or their blends to improve the resiliency and impact durability over a conventional butyl rubber. The core comprises a thermoplastic material, a thermoset material, or a rubber-based material, while at least one layer is formed from a composition comprising a partially or fully neutralized butyl rubber ionomer. The butyl rubber ionomer comprises repeating units derived from at least one isoolefin monomer, at least 0.5 mol % of repeating units derived from at least one multiolefin monomer, at least 0.5 mol % of repeating units derived from an allylic halide, and at least 0.5 mol % of an ionomeric moiety. The isoolefin comprises isobutylene, the multiolefin comprises isoprene and the allylic halide comprises a bromide.
Owner:ACUSHNET CO
Method for preparing pentaerythrite allyl ether
InactiveCN101200413AHigh yieldReduce manufacturing costEther preparationPentaerythritolOrganic solvent
The present invention pertains to the technical field of organic synthesis, providing a method to prepare allyl pentaerythritol ether compound mainly composed of allyl pentaerythritol ether. The preparation method includes the following steps: (1) pentaerythritol, alkali metal hydroxide, water and inert organic solvent are mixed according to a proper ratio, heated and agitated during reaction; (2) a proper dosage of phase-transfer catalyst is added into the reactant mixture, allyl halide is added slowly and supplementary phase-transfer catalyst is added when the allyl halide is added, and then the mixture is agitated to end the etherification reaction; (3) after reaction, the salt, the unreacted alkali-metal hydroxide and the phase-transfer catalyst in the system are removed by water, the separated organic layer is distilled under normal pressure to remove the light component, and the desired product is achieved by vacuum distillation. The preparation method has the advantages of high yield of target products, low production cost, simple operation process, high security, and industrial production can be realized easily.
Owner:ZHUHAI FEIYANG NOVEL MATERIALS
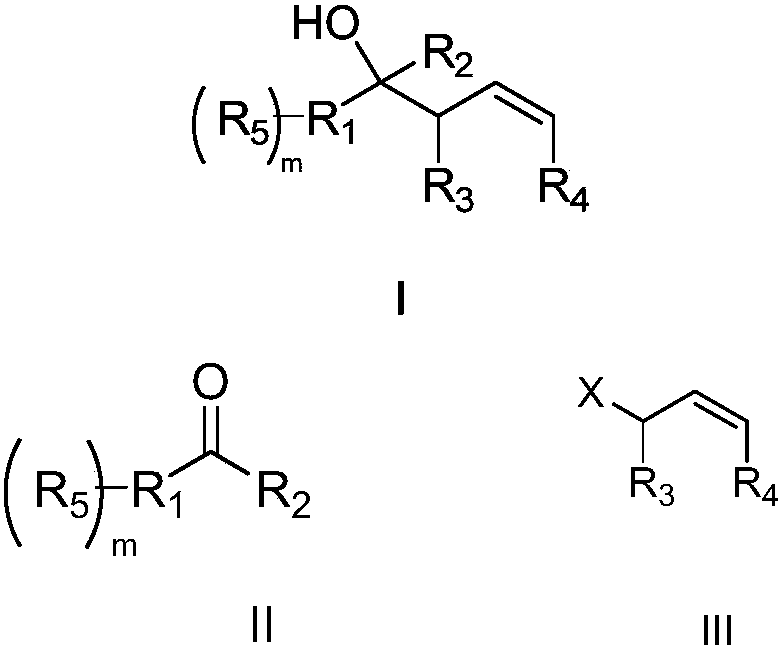
Preparation of homoallylic alcohols
InactiveCN101475445AAvoid anhydrous and anaerobic operationShort reaction timeOrganic compound preparationHydroxy compound preparationIndiumSilanes
The invention relates to a method for preparing high-allyl alcohol. The method comprises the steps of taking metallic indium as an accelerator, taking saturated dihalide and silane as activators and forming high-allyl alcohol through the reaction of aldehyde ketone compounds and allyl halide at a temperature between 10 and 50 DEG C. The method has the advantages of mild reaction conditions, simple post-treatment, high product yield, no need for operation with no water or oxygen, capability of realizing large-scale production and good industrial application prospects.
Owner:NORTHWEST NORMAL UNIVERSITY
Method for preparing pentaerythrite allyl ether
InactiveCN101200413BHigh yieldReduce manufacturing costEther preparationPentaerythritolOrganic solvent
Owner:ZHUHAI FEIYANG NOVEL MATERIALS
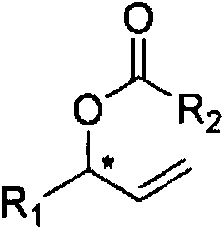
Chiral allyl ester compound and preparation method thereof
InactiveCN104402718AOvercoming productivityOvercoming selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIridiumOrganic synthesis
The invention relates to a chiral allyl ester compound and a preparation method thereof. The structural formula of the chiral allyl ester compound is represented in the description. The preparation method comprises the following steps: adding raw materials (carboxylate and allyl halide) into an organic solvent, then adding an iridium catalyst, which is prepared by reacting an iridium complex with a ligand, adding additives, controlling the reaction temperature in a range of 0 to 120 DEG C, carrying out reactions for 1 to 36 hours, and separating the reaction products so as to obtain the chiral allyl ester compound. In the prior art, when active allyl halide is taken as the substrate, the reaction yield is low and the enantioselectivity is bad, and the provided preparation method overcomes the problems mentioned above. Moreover the preparation method has the characteristics of high reaction yield, good region-selectivity, and high enantioselectivity, and thus is widely used in the organic synthesis methodology and natural product synthesis.
Owner:TONGJI UNIV
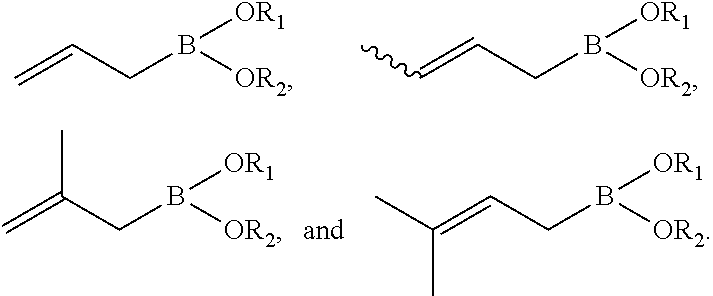
Synthesis of boronic esters and boronic acids using grignard reagents
Boronic esters and boronic acids are synthesized at ambient temperature in an ethereal solvent by the reaction of Grignard reagents with a boron-containing substrate. The boron-containing substrate may be a boronic ester such as pinacolborane, neopentylglycolborane, or a dialkylaminoborane compound such as diisopropylaminoborane. The Grignard reagents may be pre-formed or generated from an alkyl, alkenyl, aryl, arylalkyl, heteroaryl, vinyl, or allyl halide compound and Mg0. When the boron-containing substrate is a boronic ester, the reactions generally proceed at room temperature without added base in about 1 to 3 hours to form a boronic ester compound. When the boron-containing substrate is a dialkylaminoborane compound, the reactions generally proceed to completion at 0° C. in about 1 hour to form a boronic acid compound.
Owner:RGT UNIV OF CALIFORNIA
Method for preparing high allyl alcohol ester
InactiveCN101519351AEasy to operateShort reaction timePreparation from carboxylic acid halidesCarboxylic acid nitrile preparationIndiumAccelerant
The invention relates to a method for preparing high allyl alcohol ester. In the method, metal indium is used as an accelerant, and an aldehyde compound, an allyl halide and an acylating agent are mixed at one time to react at a temperature of between 5 and 30 DEG C to form the high allyl alcohol ester. The method adopts a three-composition one-pot serial reaction requiring mild conditions and short time, is simple in post treatment and high in yield, can realize large-scale production and has a good industrial application prospect.
Owner:NORTHWEST NORMAL UNIVERSITY
Processes for preparing epoxidized polymers
The present invention is directed to a process for preparing epoxidized polymers. The process comprises reacting an unsaturated polymer with hydrogen peroxide in the presence of a polymer support having a sulfonic acid group. The present invention is also directed to an epoxidized halogenated-polymer which comprises repeating units derived from at least one isoolefin monomer and repeating units derived from at least one diolefinic monomer, and one or more allylic halide groups and one or more oxirane functional groups in the polymer backbone.
Owner:ARLANXEO CANADA INC
Method for synthesizing allyl fatty alcohol-polyoxyethylene ether or allyl alkylphenol ethoxylate
InactiveCN102585194ASolve the separation problemHigh reaction conversion rateEther preparation by ester reactionsReaction temperatureAlkylphenol
The invention discloses a method for synthesizing allyl fatty alcohol-polyoxyethylene ether or allyl alkylphenol ethoxylate. The method comprises the following steps of: adding fatty alcohol-polyoxyethylene ether or alkylphenol ethoxylate and a solid alkali hydroxide into a stainless steel high pressure reactor, keeping the vacuum degree of the reactor to be 0.085 to 0.1MPa, rising the temperature of the reactor to 70 to 200 DEG C, performing reaction for 1 to 5 hours, stopping a vacuum system, continuing adding allyl halide dropwise into the reactor with continuously stirring, performing reaction under 30 to 100 DEG C and 0.1 to 0.5MPa for 1 to 8 hours, starting the vacuum system to remove residual allyl halide from a product, and discharging a raw product; and adding hot water into the raw product, uniformly mixing the hot water and the raw product, and standing and demixing the mixture to obtain the allyl fatty alcohol-polyoxyethylene ether or the allyl alkylphenol ethoxylate. The method has the advantages of no pollution, low cost and high conversion rate.
Owner:CHINA RES INST OF DAILY CHEM IND
Preparation method of N,N-diallyl aniline
InactiveCN102040526AEasy to operateShort reaction timeOrganic compound preparationAmino compound preparationAnilineSolvent
The invention relates to a preparation method of N,N-diallyl aniline, which comprises the following steps of: (1) sequentially adding allyl halide, an amine compound and alkali into a reaction solvent, reacting at the temperature of 20-70 DEG C, and obtaining a reaction liquid after complete reaction; (2) extracting the reaction solvent to obtain an extraction liquid; and (3) drying, concentrating and carrying out column chromatography separation on the extraction liquid to obtain the N,N-diallyl aniline. The preparation method has the advantages of mild condition, short reaction time, simple postprocessing and high yield, and a noble metal is not needed to be used as a catalyst in the method, therefore, the preparation method is a green synthesis technology conforming to an environment-friendly standard, can realize large-scale production and has better industry application prospect.
Owner:NORTHWEST NORMAL UNIVERSITY
Synthetic process of N-allyl-O- isobutyl thionocarbamate
ActiveCN105061276AReduce remaining amountReaction does not affectOrganic chemistryThiocarbamateChemical products
The invention relates to the field of synthesis of chemical products and production of beneficiation reagents, in particular to a synthetic process of N-allyl-O- isobutyl thionocarbamate. The synthetic process is characterized in that the use quantity of a catalyst in a reaction is about 0.5-4 mole percent while the general use quantity of the catalyst is between 0.1 mole percent to 1 mole percent; the production technology improves the appearance, the yield and the odor of an N-allyl-O- isobutyl-thiocarbamate product prepared with fatty alcohol; an initial reactant allyl isothiocyanate is prepared through a reaction of allyl halide and alkali metal or ammonium thiocyanate under the action of a phase transfer catalyst; then the fatty alcohol, the catalyst prepared in the process and trimethylchlorosilane are added for the reaction; a reaction mixture is heated to 100-120 DEG C, and heat preservation is performed for 3-6 h. When the reaction is ended, unreacted alcohol is pumped through vacuum, and trimethylchlorosilane serving as the synthetic catalyst has higher reactivity, so that the reaction conversion rate is increased.
Owner:SHENYANG YOUYAN MINERAL CHEM CO LTD
Process for the preparation of benzyl-metal compounds and process for the preparation of 4-phenyl-1-butenes by the use of the same
InactiveUS6024897AHigh yieldAvoid thermal decompositionLithium organic compoundsOrganic compound preparationTolueneBenzyl group
PCT No. PCT / JP97 / 01843 Sec. 371 Date Jan. 30, 1998 Sec. 102(e) Date Jan. 30, 1998 PCT Filed May 29, 1997 PCT Pub. No. WO97 / 45433 PCT Pub. Date Dec. 4, 1997There is disclosed a method of preparing benzyl metal compounds represented by general formula (2), comprising reacting a phenyl metal compound represented by general formula (1) with toluene, in the presence of a catalytic amount of amine. According to this method, benzyl metal compounds can be prepared at a high yield, and in an industrially advantageous manner. Further, there is also disclosed a method of preparing 4-phenyl-1-butenes, comprising reacting the benzyl metal compound obtained in the method, with an allyl halide. wherein M represents an alkali metal, wherein M has the same meaning as the above.
Owner:K I CHEM IND CO LTD
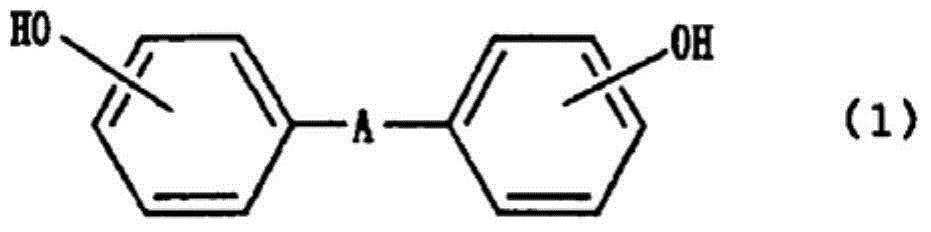
Method of consistently producing diallylbisphenols
ActiveCN104080761AAvoid lossEasy to manufactureOrganic chemistryOrganic compound preparationInorganic saltsSolvent
The purpose of the present invention is to provide a method of consistently producing high-quality diallylbisphenols with high yields from bisphenols. This method involves steps (1) through (3) below, and enables the consistent production of diallylbisphenols from bisphenols. (1) A step for reacting allyl halides and bisphenols or alkali metal salts thereof in a cellosolve solvent in the presence or absence of basic alkali metal salts, (2) a step for separating inorganic salt by-products from a reaction solution obtained in step (1), and (3) a step for heating and subjecting to a rearrangement reaction the reaction solution obtained in step (2).
Owner:KONISHI CHEM IND
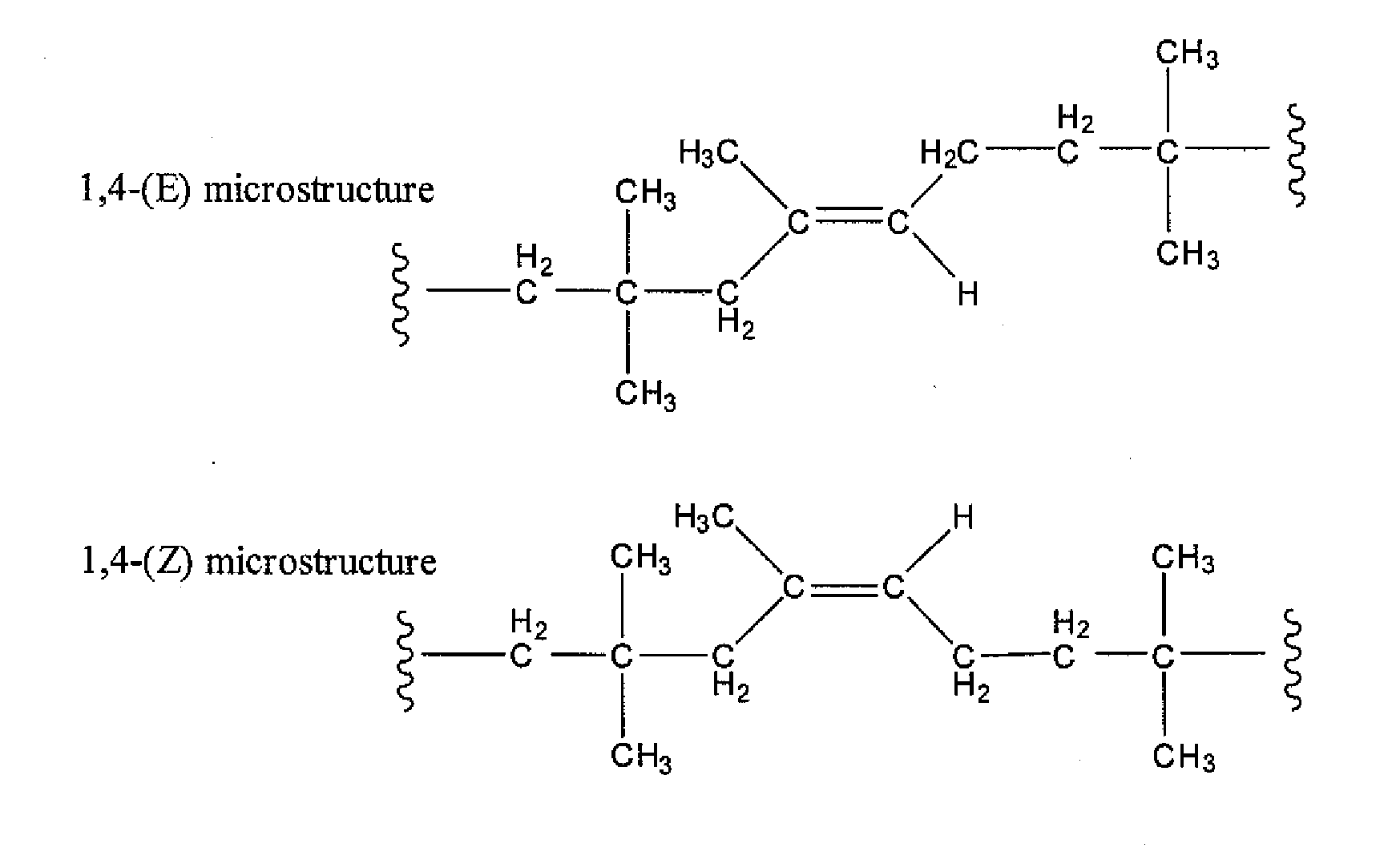
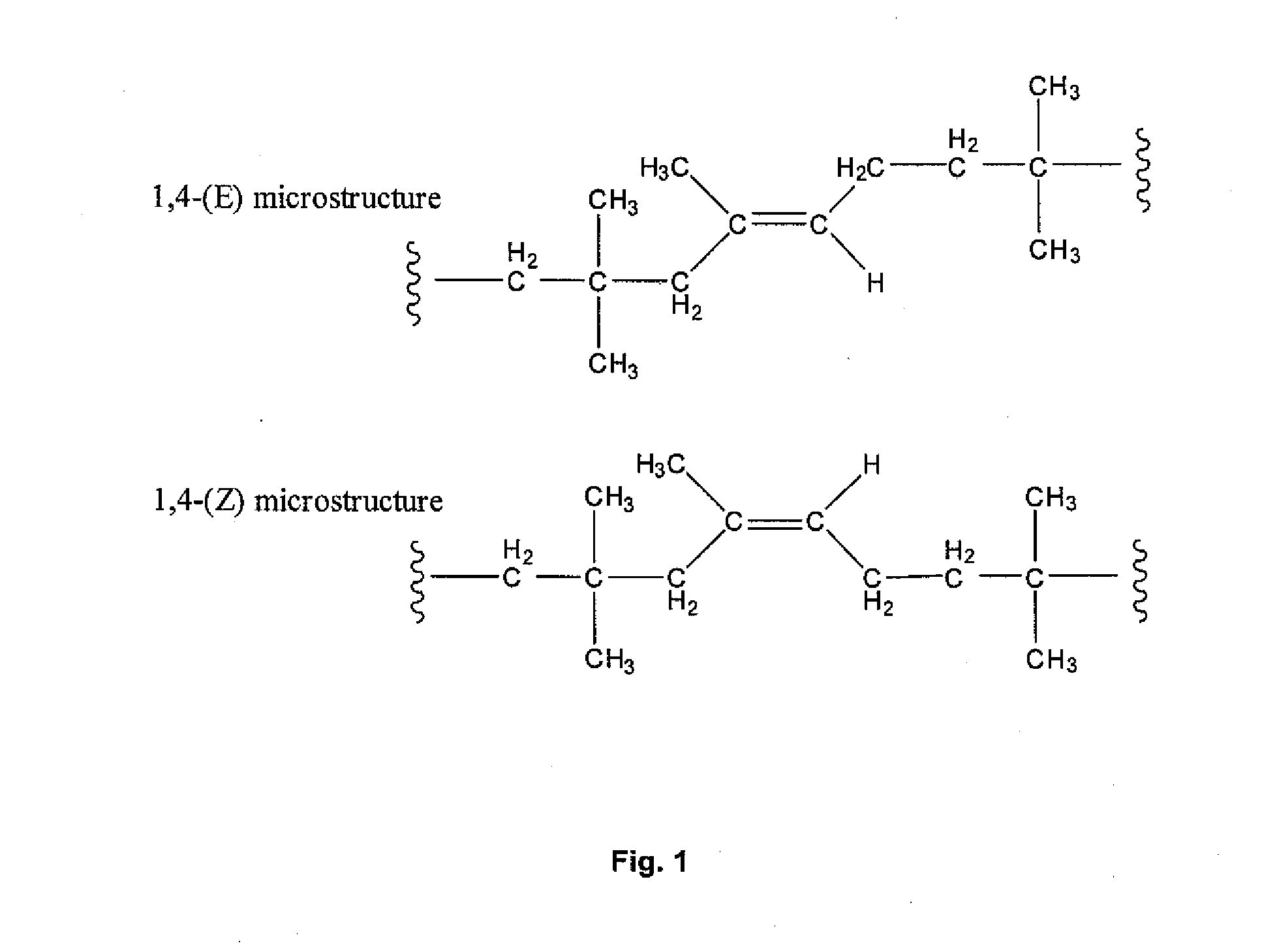
Halogenated Butyl Rubber Having Superior Reactivity
InactiveUS20120016091A1Organic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsPolymer scienceCarboxylic salt
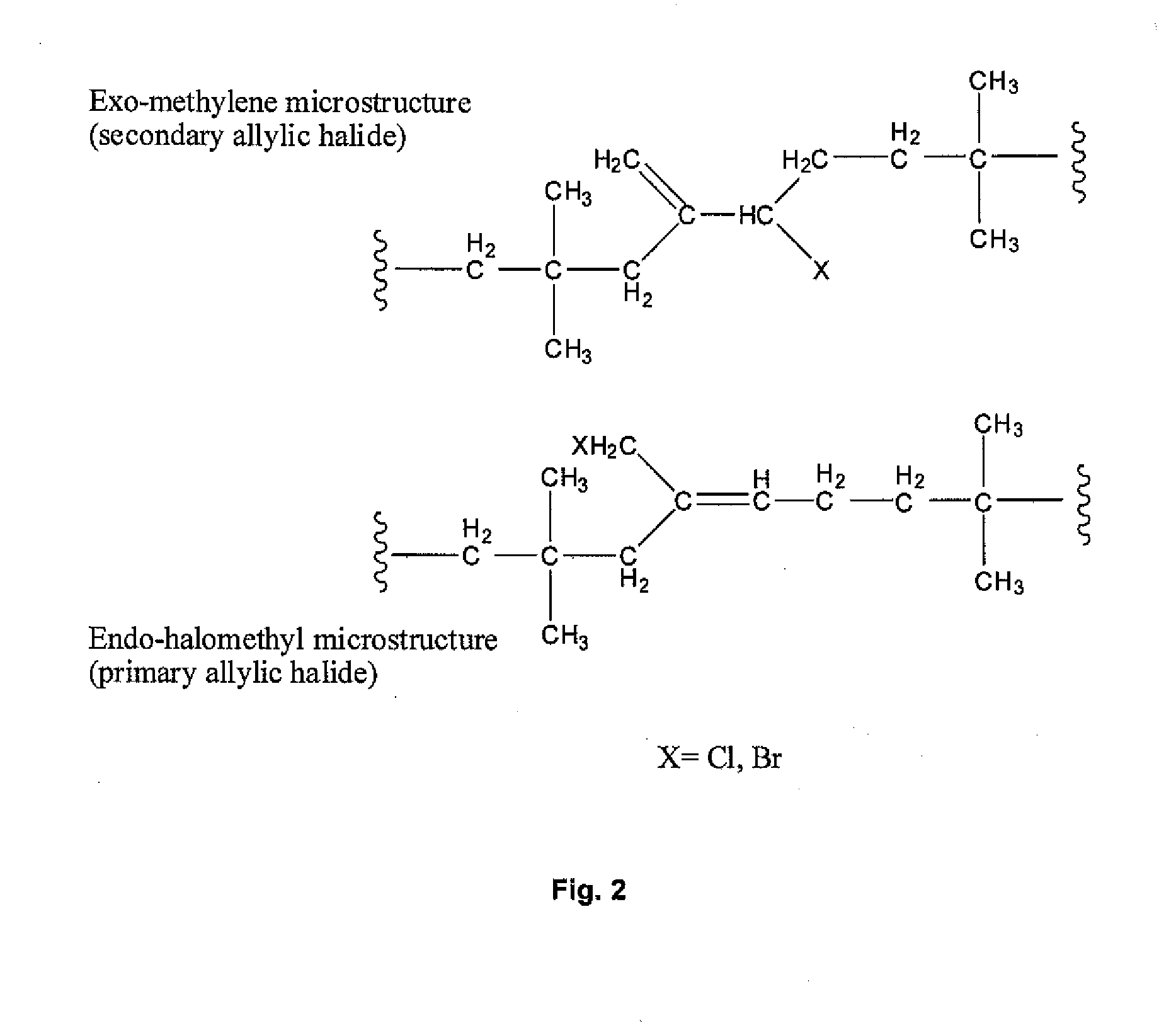
A process is described for isomerizing halogenated butyl rubber from a microstructure that is predominantly exo-methylene (secondary allylic halide) to one that is predominantly endo-halomethyl (primary allylic halide). Isomerized halobutyl rubber is a halobutyl rubber that is more reactive toward a wide range of nucleophiles, thereby supporting more efficient processes for producing a variety of butyl rubber derivatives. The process includes mixing halogenated butyl rubber and a catalytic amount of metal carboxylate and optionally heating to form isomerized halogenated butyl rubber, and may be conducted in the absence or presence of solvent.
Owner:QUEENS UNIV OF KINGSTON
Method for preparing high allyl alcohol compound
ActiveCN109627164ACarboxylic acid nitrile preparationOrganic compound preparationEnantio selectivityMetal
The invention relates to a method for preparing a high allyl alcohol compound. An effective metal-mediated highly diastereoselective allylation reaction of a carbonyl compound with allyl halide is mainly provided, a corresponding high allyl alcohol is obtained, a yield is good, and the compound has excellent diastereoselectivity and broad functional group tolerance.
Owner:盐城锦明药业有限公司 +2
Process for producing allyl ether
InactiveUS20050215830A1Increase production capacityHigh of raw materialOrganic compound preparationEther preparation from oxiranesPolyolReaction temperature
In the production of allyl ethers of polyol by reacting a straight-chain polyol compound with an allyl halide in the presence of an alkali metal hydroxide, wherein the straight-chain polyol compound has carbon atoms each having one hydroxyl group, the reaction between the straight-chain polyol compound and the allyl halide is proceeded in the presence of water having the amount dissolving the straight-chain polyol compound at a reaction temperature, then 14% by mol, based on total molar amount of a hydroxyl group contained in the straight-chain polyol compound, of the allyl halide is added to a reaction system before at least part of water is released out of the reaction system.
Owner:OSAKA SODA CO LTD
A kind of chiral allyl ester compound and preparation method thereof
InactiveCN104402718BVarious constructionsMultiple chiral centersOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIridiumOrganic synthesis
Owner:TONGJI UNIV
Processes for preparing epoxidized polymers
The present invention is directed to a process for preparing epoxidized polymers. The process comprises reacting an unsaturated polymer with hydrogen peroxide in the presence of a polymer support having a sulfonic acid group. The present invention is also directed to an epoxidized halogenated-polymer which comprises repeating units derived from at least one isoolefin monomer and repeating units derived from at least one diolefinic monomer, and one or more allylic halide groups and one or more oxirane functional groups in the polymer backbone.
Owner:ARLANXEO CANADA INC
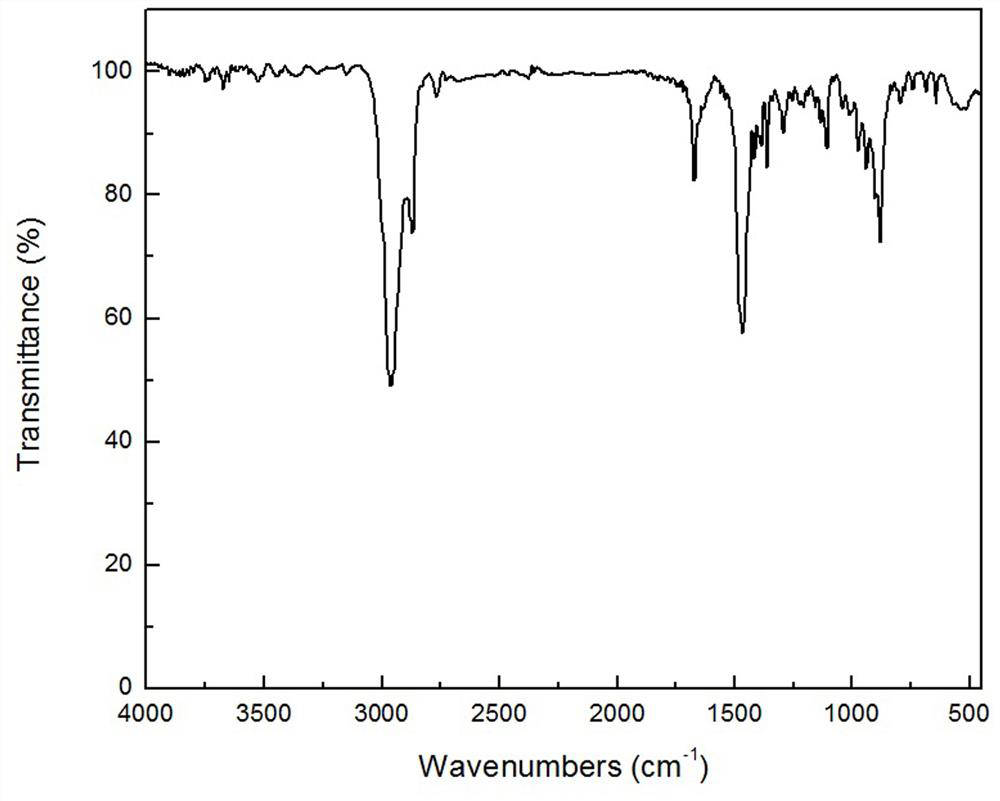
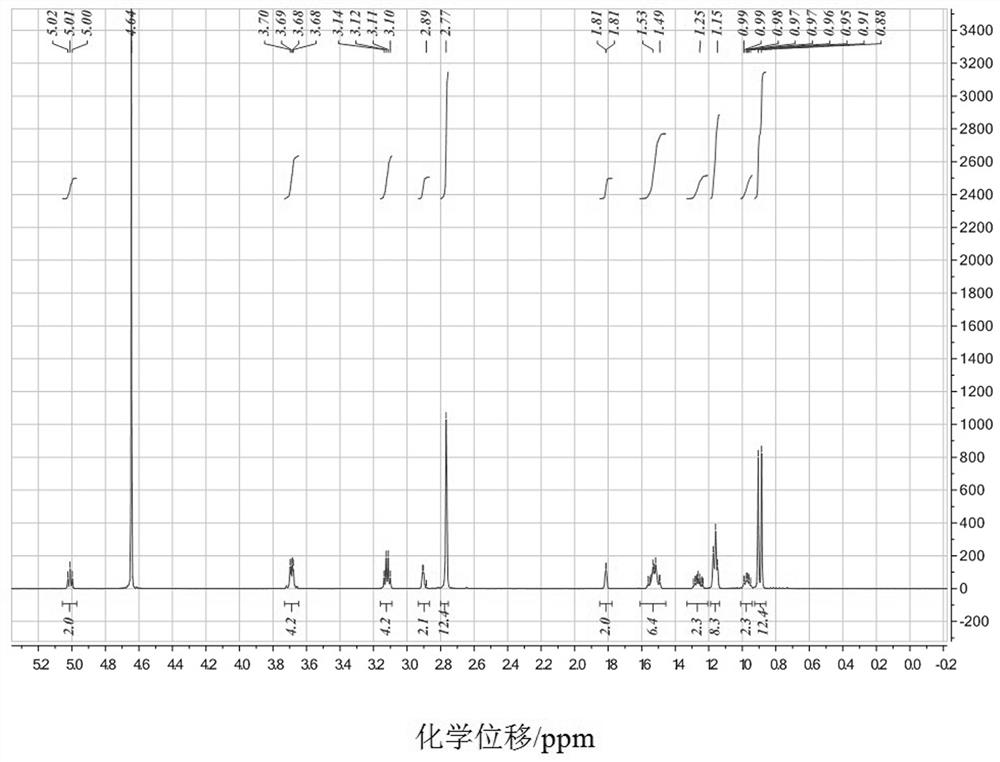
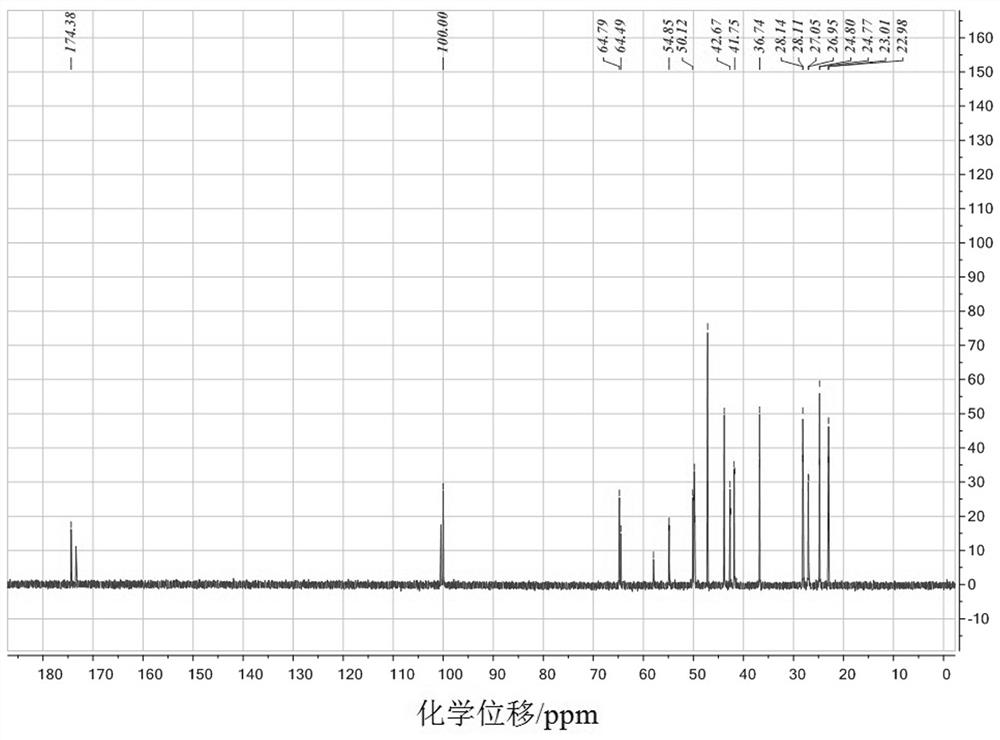
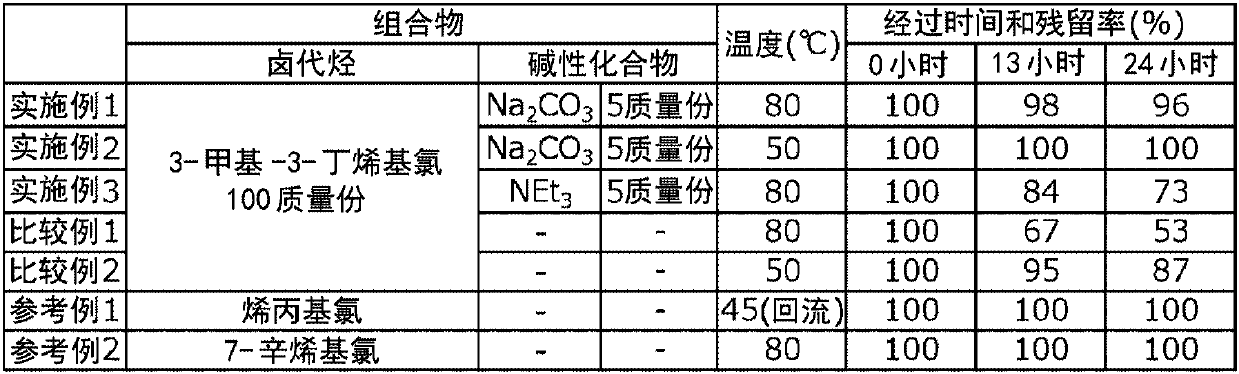
Homoallyl halide composition and method for storing homoallyl halide
ActiveUS10618860B2Safe and secure storageHalogenated hydrocarbon separation/purificationAlkaline earth metalPhysical chemistry
There is provided a composition containing one or more basic compounds selected from the group consisting of tertiary amines, nitrogen-containing heterocyclic aromatic compounds, alkali metal carbonate salts, alkaline earth metal carbonate salts and alkali metal hydrogencarbonate salts, and a homoallyl halide.
Owner:KURARAY CO LTD
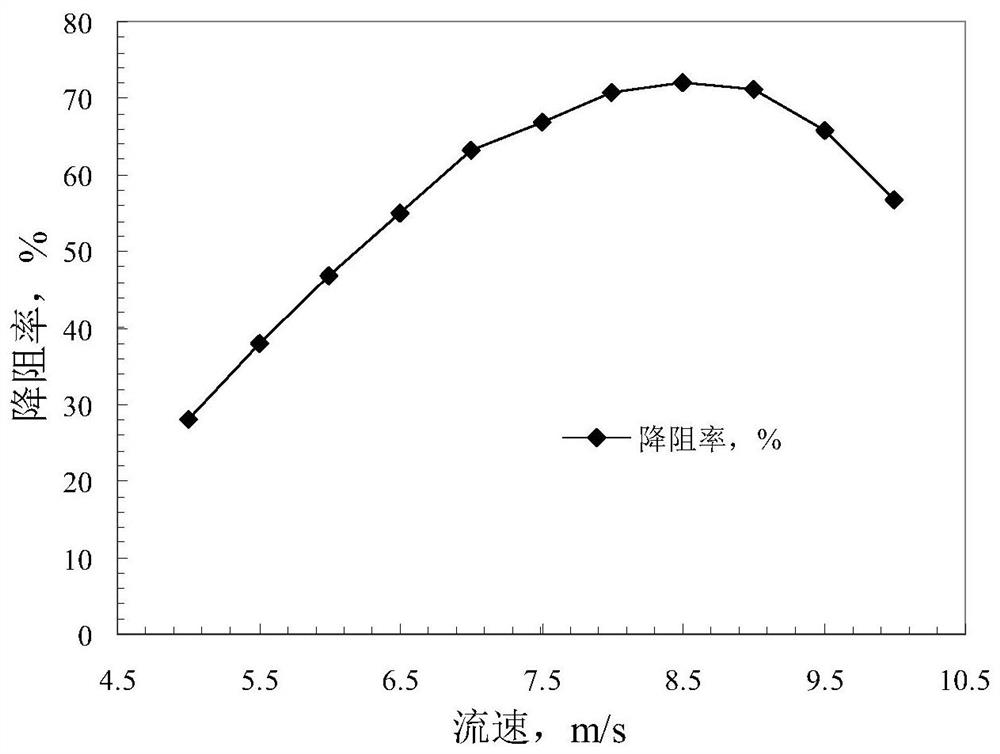
A kind of fracturing fluid and preparation method thereof
ActiveCN112852400BViscoelasticWon't hurtOrganic compound preparationDrilling compositionSulfonateActive agent
The invention discloses a fracturing fluid and a preparation method thereof, belonging to the technical field of fracturing. The fracturing fluid includes the following components in mass percentage: 0.5%-4% of surfactant, 0.15%-2.5% of counter ion salt, 0.01%-2% of clay stabilizer, and the balance is water; the surfactant is composed of allyl Prepared by the quaternization reaction of alkyl halides and alkyldimethyl tertiary amines; the counter ion salt is phenylsulfonate. The surfactant used in the fracturing fluid is prepared by quaternization reaction of allyl halide and alkyl dimethyl tertiary amine, the reaction raw materials are cheap and easy to obtain, the reaction conditions are mild, and the reaction process is simple and easy to operate. The formed surfactant is simply compounded with the counter ion salt, which can form a viscoelastic fluid with high resistance reduction rate, and its synergistic effect with clay stabilizer and water, the formed fracturing fluid system can be used to treat Oil and gas wells are fractured to achieve excellent stimulation effects.
Owner:PETROCHINA CO LTD
A kind of preparation method of homoallyl alcohol compound
ActiveCN109627164BCarboxylic acid nitrile preparationOrganic compound preparationAlcoholCombinatorial chemistry
The invention relates to a preparation method of homoallyl alcohol compounds, which mainly provides an effective metal-mediated highly diastereoselective allylation reaction of carbonyl compounds and allyl halides to obtain the corresponding Homoallylic alcohols in good yields with excellent diastereoselectivity and broad functional group tolerance.
Owner:盐城锦明药业有限公司 +2
A kind of n-allyl-o-isobutyl thiocarbamate synthetic technique
ActiveCN105061276BReduce remaining amountReaction does not affectOrganic chemistryThiocarbamateChemical products
The invention relates to the field of synthesis of chemical products and production of beneficiation reagents, in particular to a synthetic process of N-allyl-O- isobutyl thionocarbamate. The synthetic process is characterized in that the use quantity of a catalyst in a reaction is about 0.5-4 mole percent while the general use quantity of the catalyst is between 0.1 mole percent to 1 mole percent; the production technology improves the appearance, the yield and the odor of an N-allyl-O- isobutyl-thiocarbamate product prepared with fatty alcohol; an initial reactant allyl isothiocyanate is prepared through a reaction of allyl halide and alkali metal or ammonium thiocyanate under the action of a phase transfer catalyst; then the fatty alcohol, the catalyst prepared in the process and trimethylchlorosilane are added for the reaction; a reaction mixture is heated to 100-120 DEG C, and heat preservation is performed for 3-6 h. When the reaction is ended, unreacted alcohol is pumped through vacuum, and trimethylchlorosilane serving as the synthetic catalyst has higher reactivity, so that the reaction conversion rate is increased.
Owner:SHENYANG YOUYAN MINERAL CHEM CO LTD
Process for producing allyl ether
InactiveUS7157608B2Increase production capacityHigh of raw materialOrganic compound preparationEther preparation by ester reactionsPolyolReaction temperature
Owner:OSAKA SODA CO LTD
Gemini type quaternary ammonium salt surfactant containing natural terpene structure, synthesis method and application thereof
PendingCN111892506AGrowth inhibitionImprove surface activityBiocideOrganic compound preparationBiotechnologyActive agent
The invention belongs to the technical field of synthesis of forest fine chemicals, and particularly discloses a Gemini type quaternary ammonium salt surfactant containing a natural terpene structure,a synthesis method and application thereof. The surfactant has a natural terpene structure and an allyl quaternary ammonium functional group, and has a structure shown as formula (I), wherein R1 is ahydrophobic group and is allyl containing the natural terpene structure; R2 is a bridging group, and is ethylene group, 1, 3-propylidene or 1, 4-butylidene; and X is chlorine or bromine. According tothe synthesis method of the Gemini type quaternary ammonium salt surfactant containing the natural terpene structure, allyl halide containing the natural terpene structure and binary tertiary amine are used as the raw materials, and a target product is prepared through quaternization reaction. The surfactant has strong surface activity, good hydrophilicity and bacteriostatic activity, can inhibitthe growth of bacteria, fungi and algae, and can be used as a bactericide and an algicide.
Owner:GUANGXI UNIV FOR NATITIES
Preparation of homoallylic alcohols
InactiveCN101475445BAvoid anhydrous and anaerobic operationShort reaction timeOrganic compound preparationHydroxy compound preparationIndiumSilanes
The invention relates to a method for preparing high-allyl alcohol. The method comprises the steps of taking metallic indium as an accelerator, taking saturated dihalide and silane as activators and forming high-allyl alcohol through the reaction of aldehyde ketone compounds and allyl halide at a temperature between 10 and 50 DEG C. The method has the advantages of mild reaction conditions, simple post-treatment, high product yield, no need for operation with no water or oxygen, capability of realizing large-scale production and good industrial application prospects.
Owner:NORTHWEST NORMAL UNIVERSITY
Homoallyl halide composition and method for storing homoallyl halide
ActiveCN108026005ASafe and secure storageHalogenated hydrocarbon separation/purificationAlkaline earth metalNitrogen
A composition containing one or more types of basic compounds, selected from the group consisting of tertiary amines, nitrogen-containing heterocyclic aromatic compounds, alkali metal carbonates, alkaline earth metal carbonates, and alkali metal bicarbonates; and a homoallyl halide.
Owner:KURARAY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com