Gemini type quaternary ammonium salt surfactant containing natural terpene structure, synthesis method and application thereof
A technology of surfactants and synthetic methods, applied in the fields of application, chemical instruments and methods, botany equipment and methods, etc., can solve the problem of insufficient research on biological activity and application performance, limited variety and quantity, and restrictions on popularization and application, etc. problems, to achieve the effect of good application prospects, strong surface activity, and good hydrophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A kind of synthetic method containing natural terpene structure Gemini type quaternary ammonium salt surfactant, its synthetic method comprises the steps:
[0031] In a 50 ml round bottom flask, 3.68 g (20 mmol) ω-chloromethyl camphene and 1.16 g (10 mmol) tetramethylethylenediamine were added, and heated to reflux at 85 °C for 6 h with magnetic stirring. After the reaction was completed, cool to room temperature, add 10 ml of ethyl acetate, and stir thoroughly to precipitate the product, which was collected by decantation and filtration to obtain a gray crude product. The crude product was recrystallized and purified with ethyl acetate as a solvent, and after vacuum drying at 45 °C for 4 h, the off-white N 1 ,N 2 -Bis(camphenylmethyl)-N 1 ,N 1 ,N 2 ,N 2 - 3.50 g of tetramethylethane-1,2-diammonium chloride (Ia), yield 72.2%.
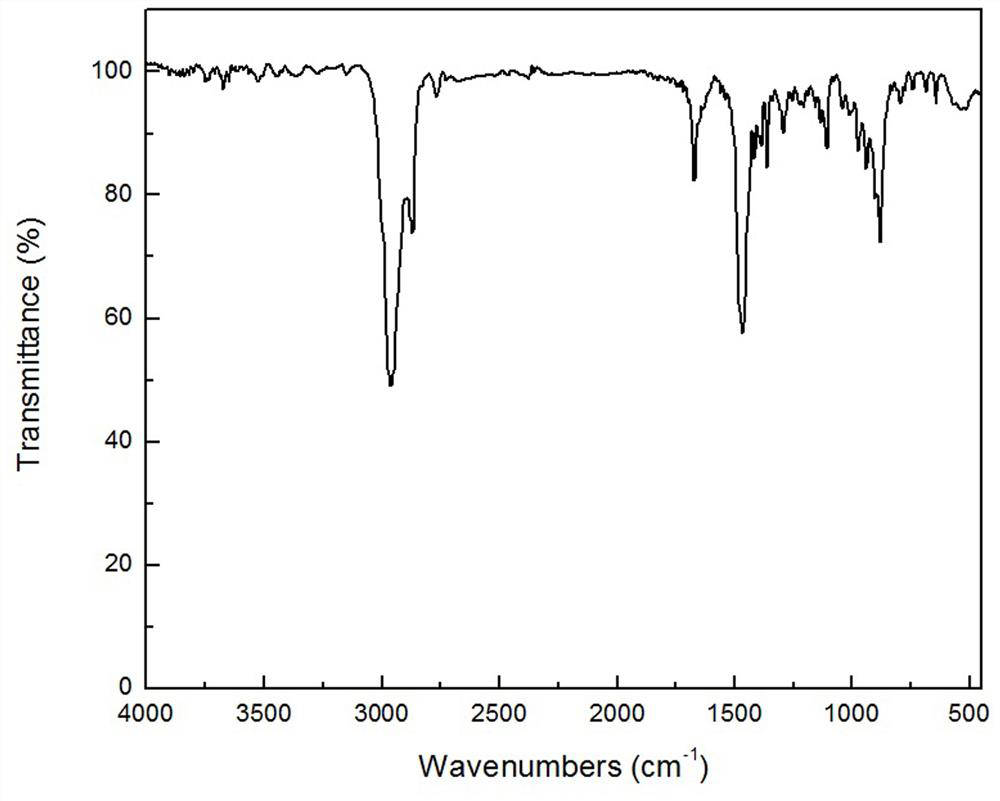
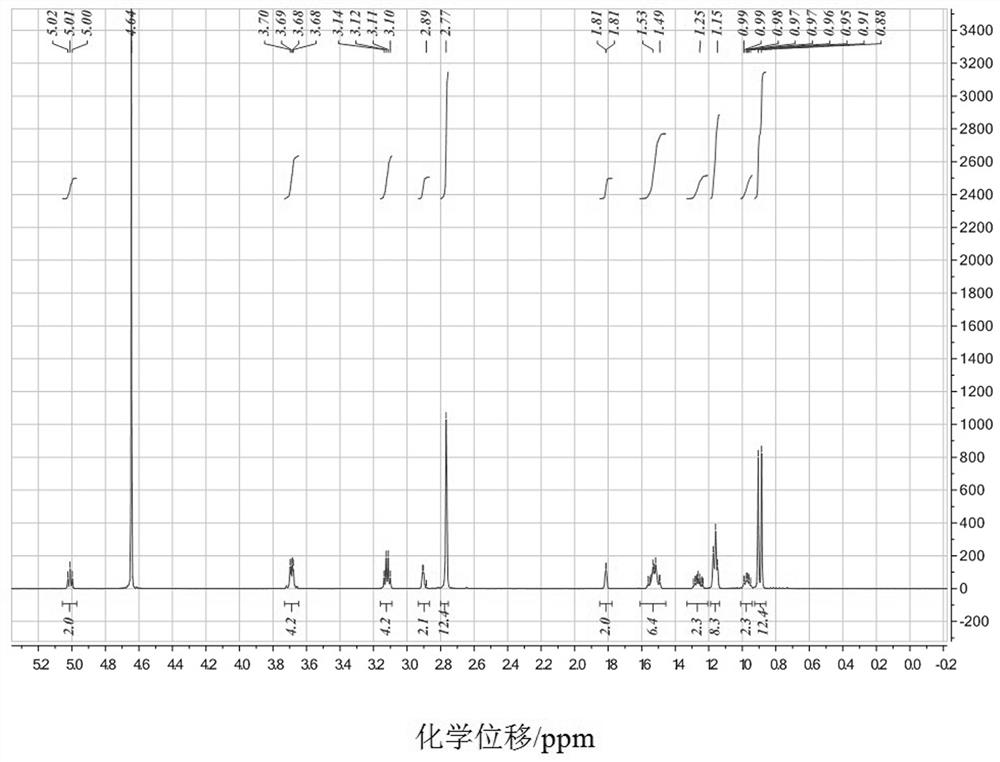
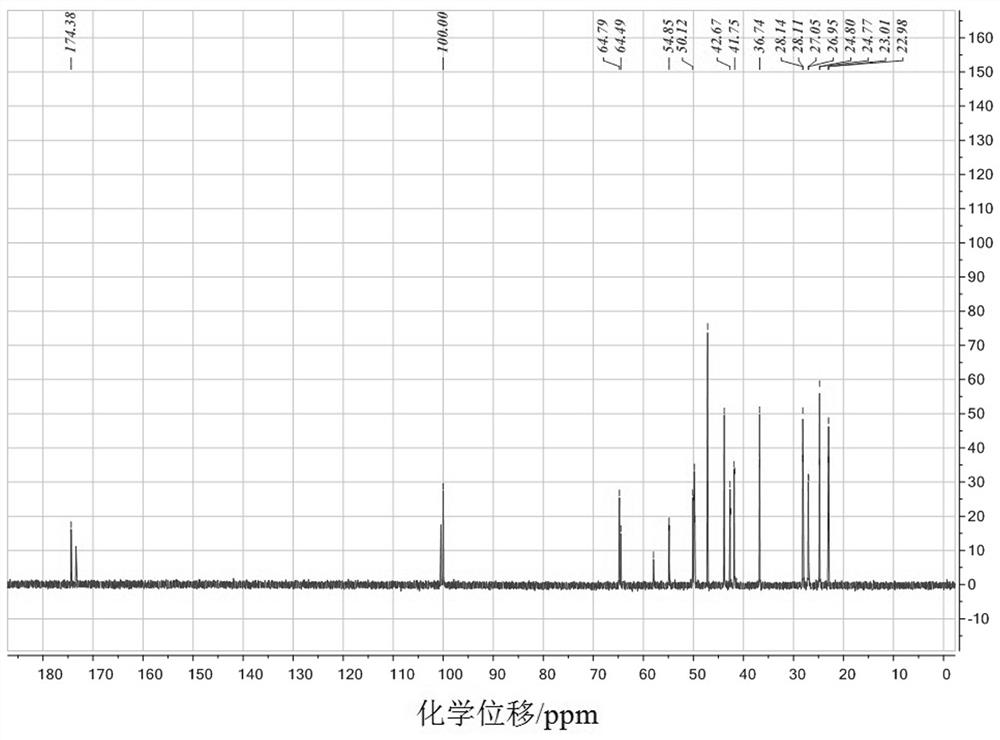
[0032] FT-IR of compound Ia, 1 H-NMR and 13 C-NMR spectroscopy data and attribution are as follows:
[0033] FT-IR υ / cm -1 : 2960, ...
Embodiment 2
[0037] Contain the synthetic method of natural terpene structure Gemini type quaternary ammonium salt surfactant, its synthetic method comprises the steps:
[0038] In a 50 ml round bottom flask, 5.29 g (21 mmol) ω-chloromethyl longifolene and 1.16 g (10 mmol) tetramethylethylenediamine were added, and heated to reflux at 85°C for 6 h with magnetic stirring. After the reaction was completed, cool to room temperature, add 10 ml of ethyl acetate, stir well, and let it stand still to precipitate the product, which was collected by decantation and filtration to obtain a gray crude product. The crude product was purified by recrystallization using ethyl acetate as a solvent, and after vacuum drying at 45 °C for 4 h, the off-white solid N 1 ,N 2 -Bis(longifenylmethyl)-N 1 ,N 1 ,N 2 ,N 2 - Tetramethylethane-1,2-diammonium chloride (Ib) 4.35g, yield 70.0%.
Embodiment 3
[0040] Contain the synthetic method of natural terpene structure Gemini type quaternary ammonium salt surfactant, its synthetic method comprises the steps:
[0041] In a 50 ml round bottom flask, add 3.68 g (20 mmol) ω-chloromethyl camphene and 1.30 g (10 mmol) N,N,N,N-tetramethyl-1,3-propanediamine, in Magnetic stirring was carried out at 90°C and heated to reflux for 5.5 hours. After the reaction was completed, cool to room temperature, add 10 ml of acetone, and stir thoroughly to precipitate the product, which was collected by decantation and filtration to obtain a gray crude product. The crude product was purified by recrystallization using acetone as a solvent, and after vacuum drying at 45 °C for 4 h, the off-white N 1 ,N 3 -Bis(camphenylmethyl)-N 1 ,N 1 ,N 3 ,N 3 - Tetramethylpropane-1,3-diammonium chloride (Ic) 3.75g, yield 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com