Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
90 results about "ACE Inhibitor Fetopathy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
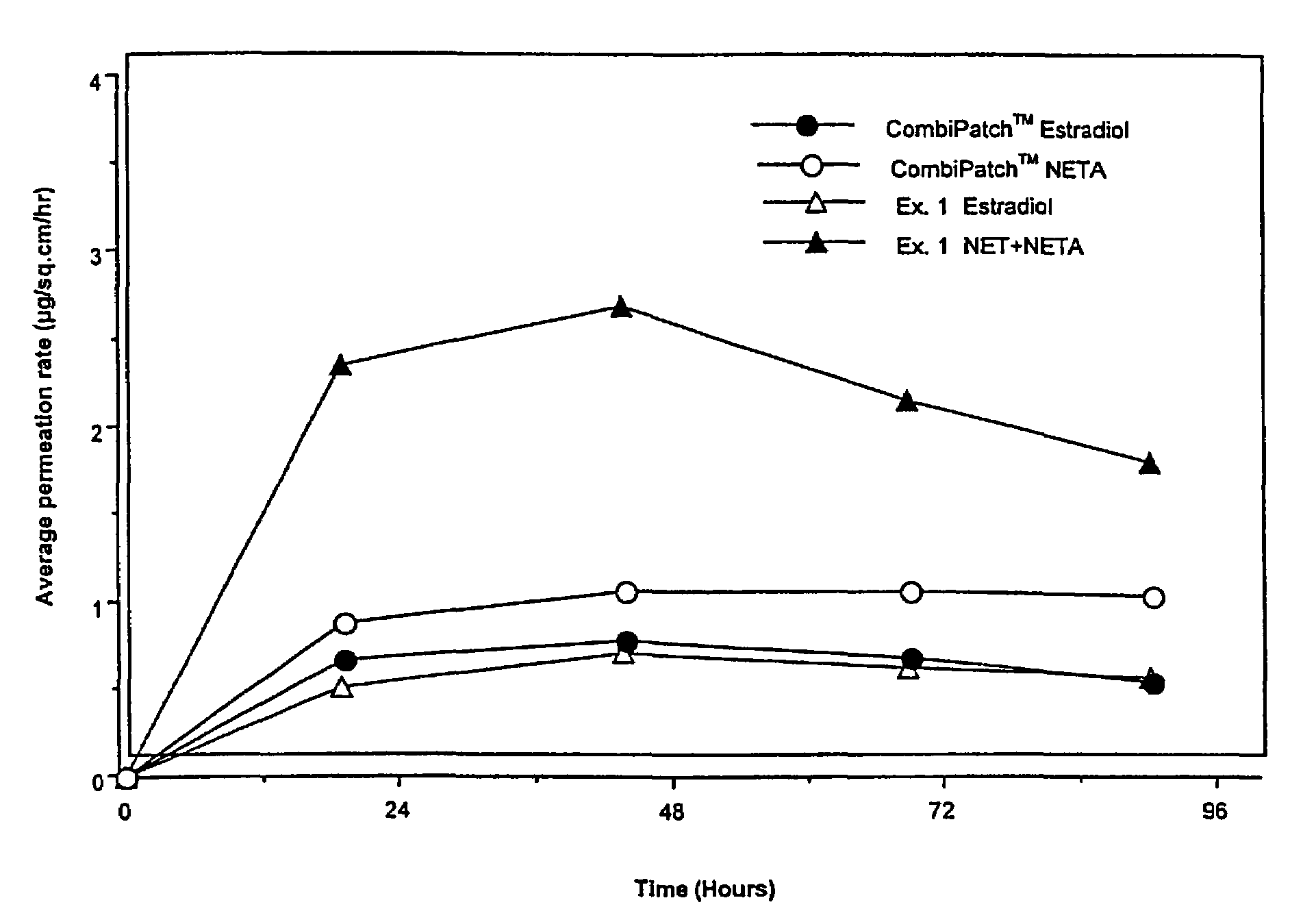
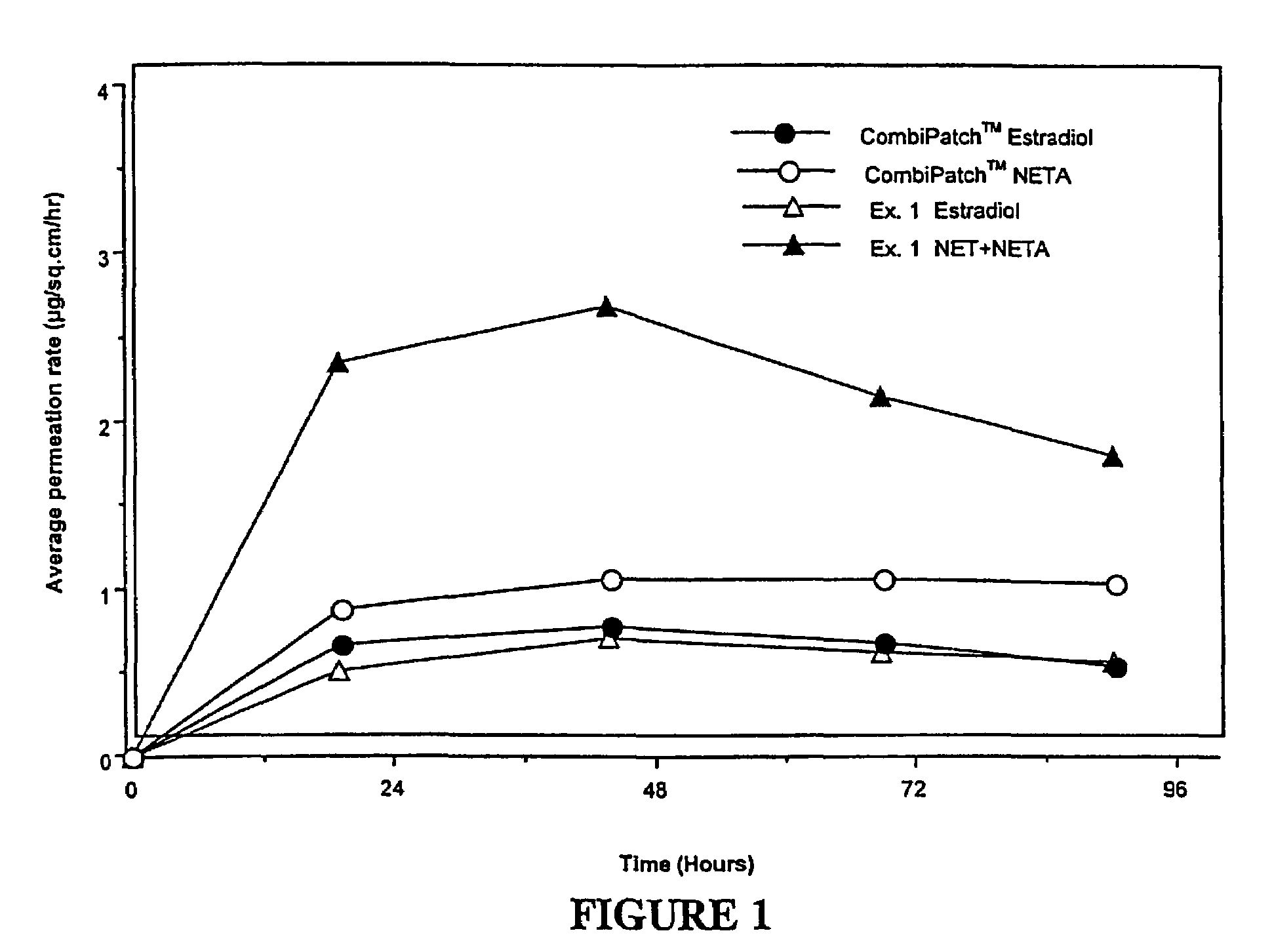
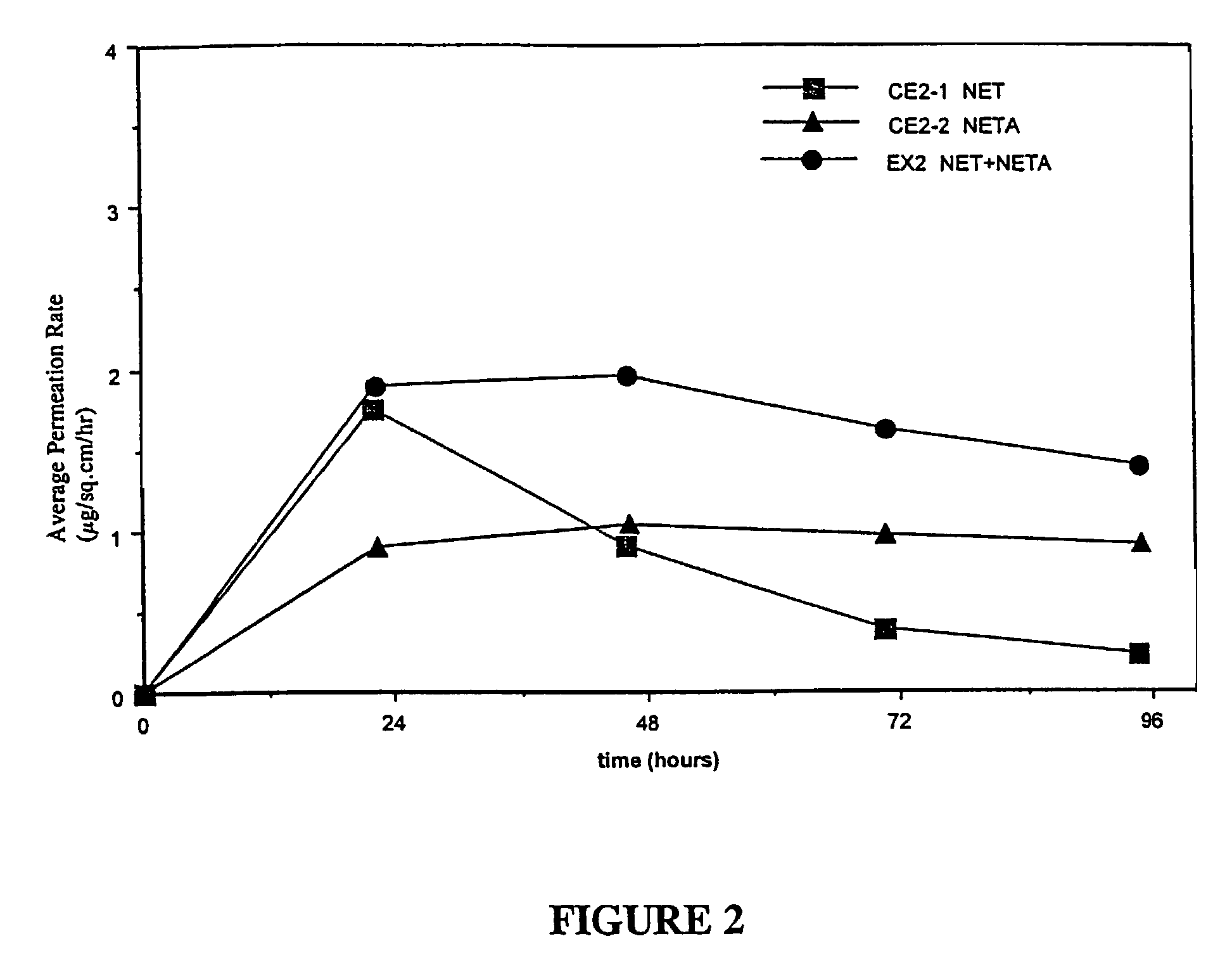
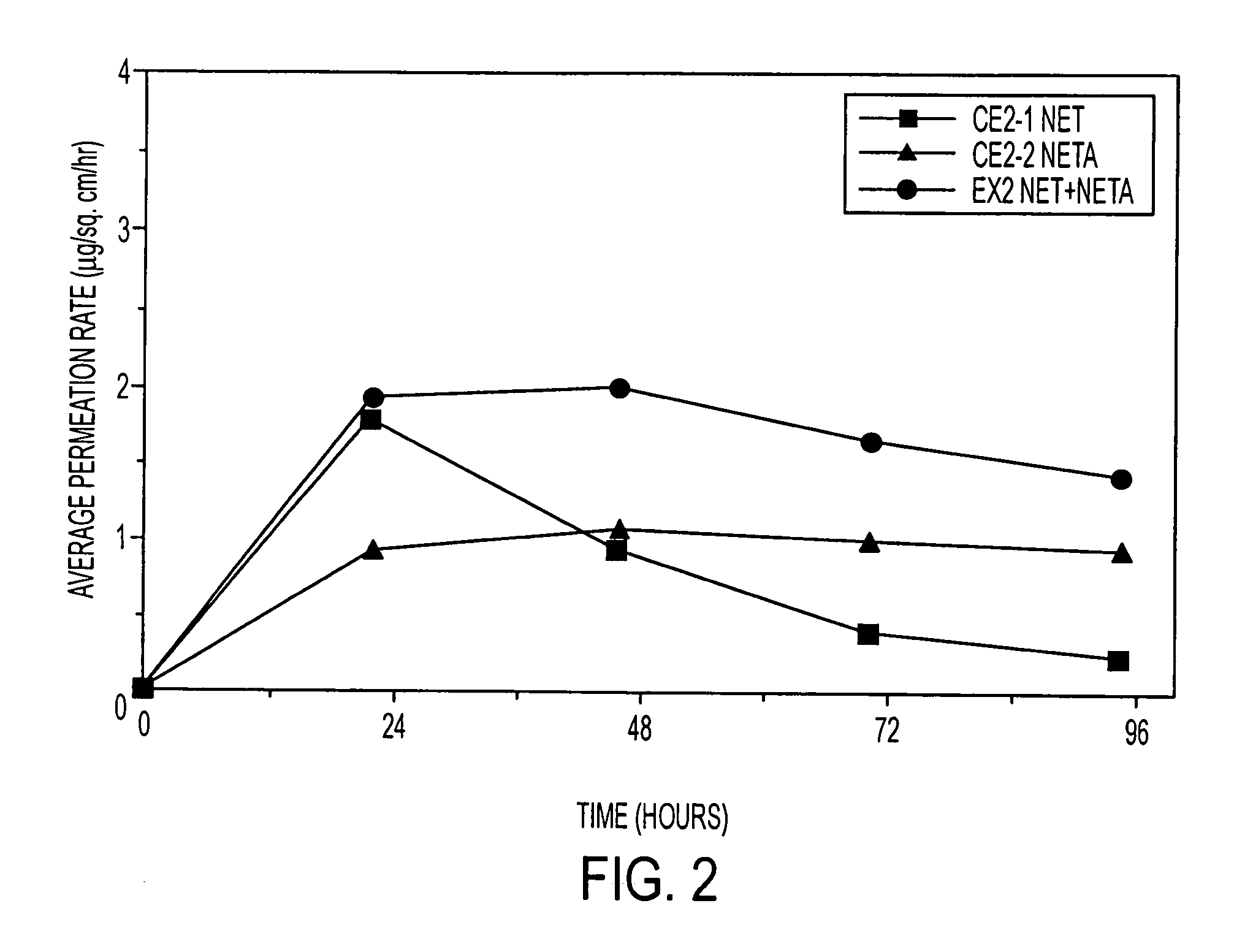
Enhanced drug delivery in transdermal systems
InactiveUS7456159B2Convenient amountGood synergyOrganic active ingredientsBiocideActive agentEthyl ester
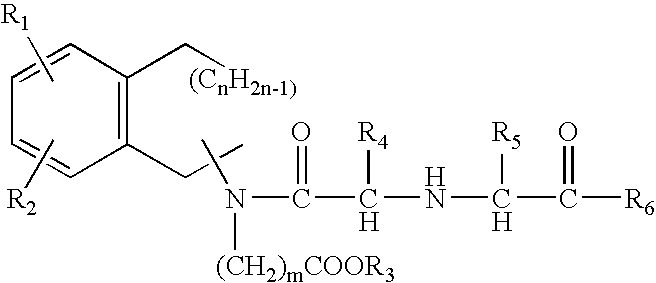
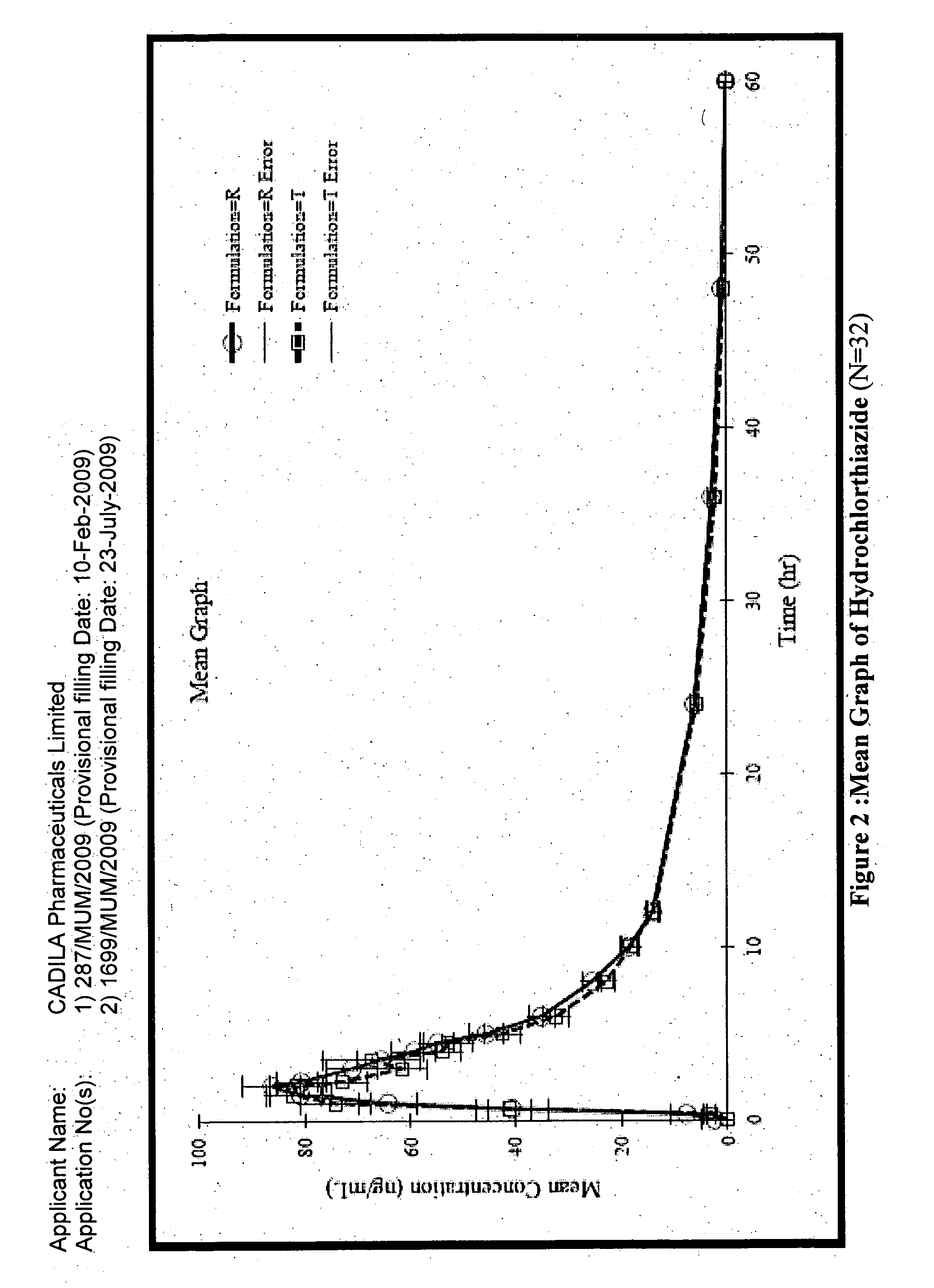
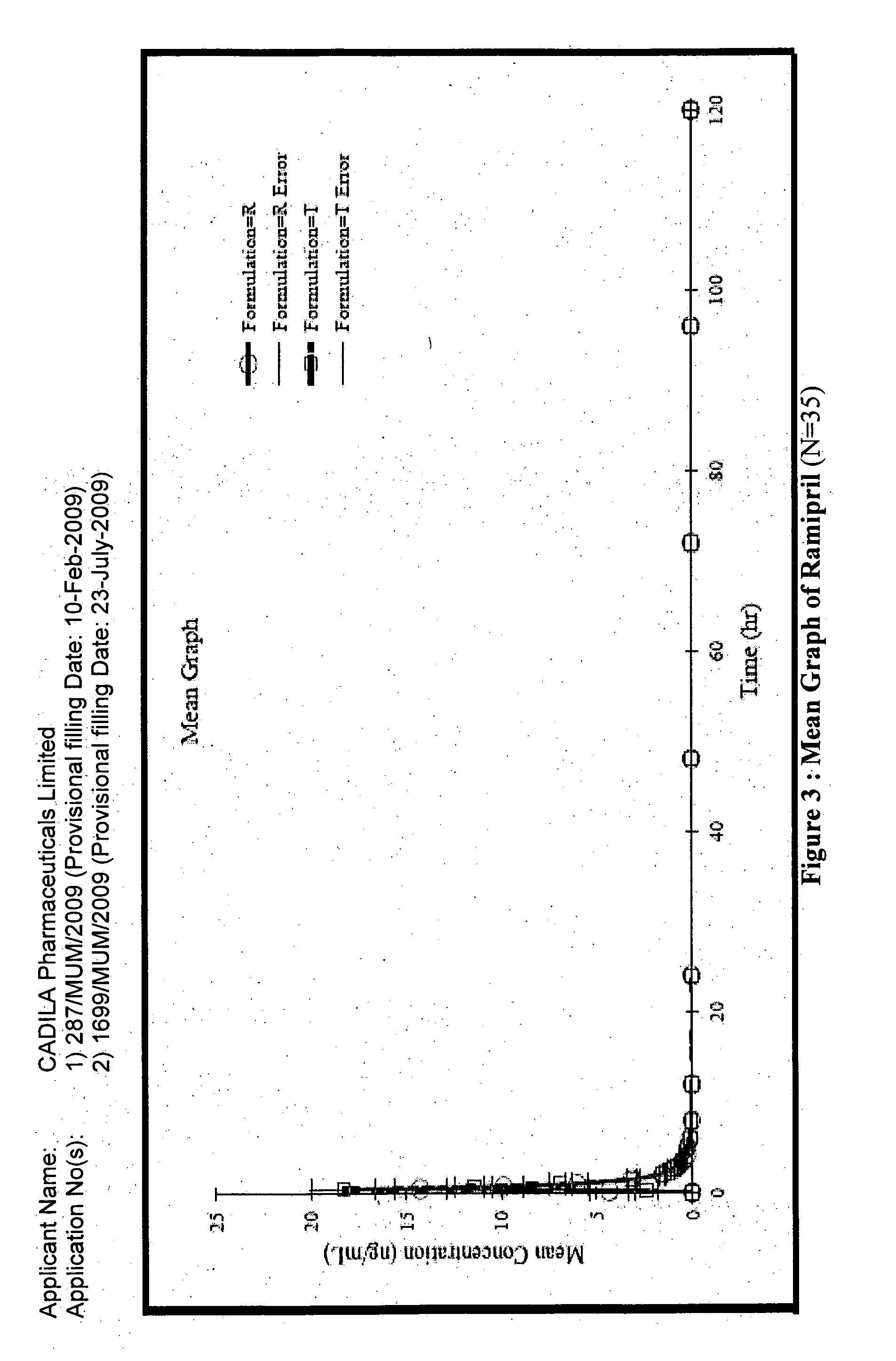
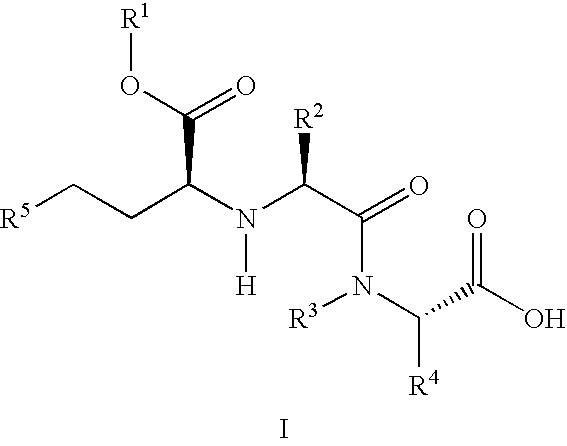
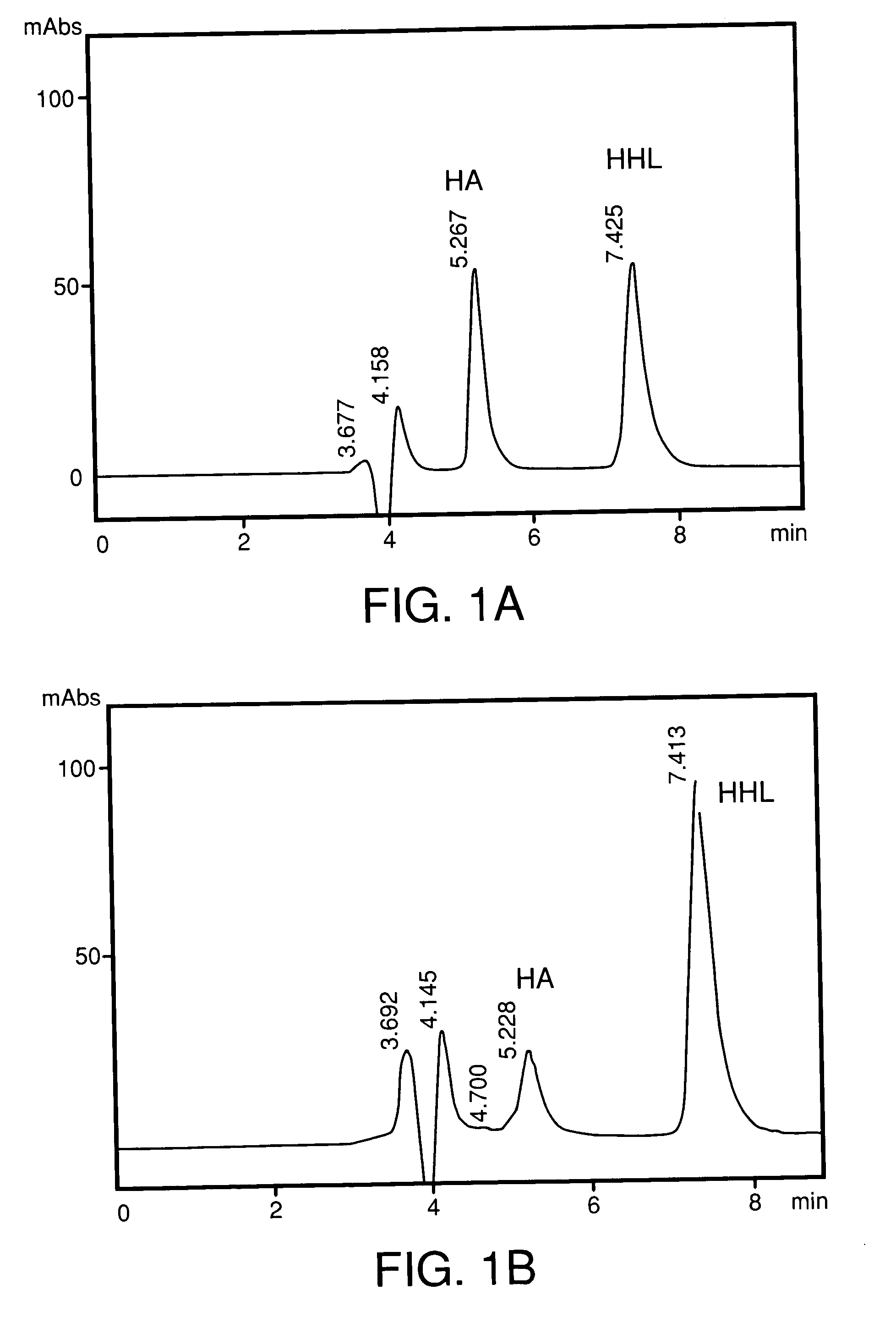
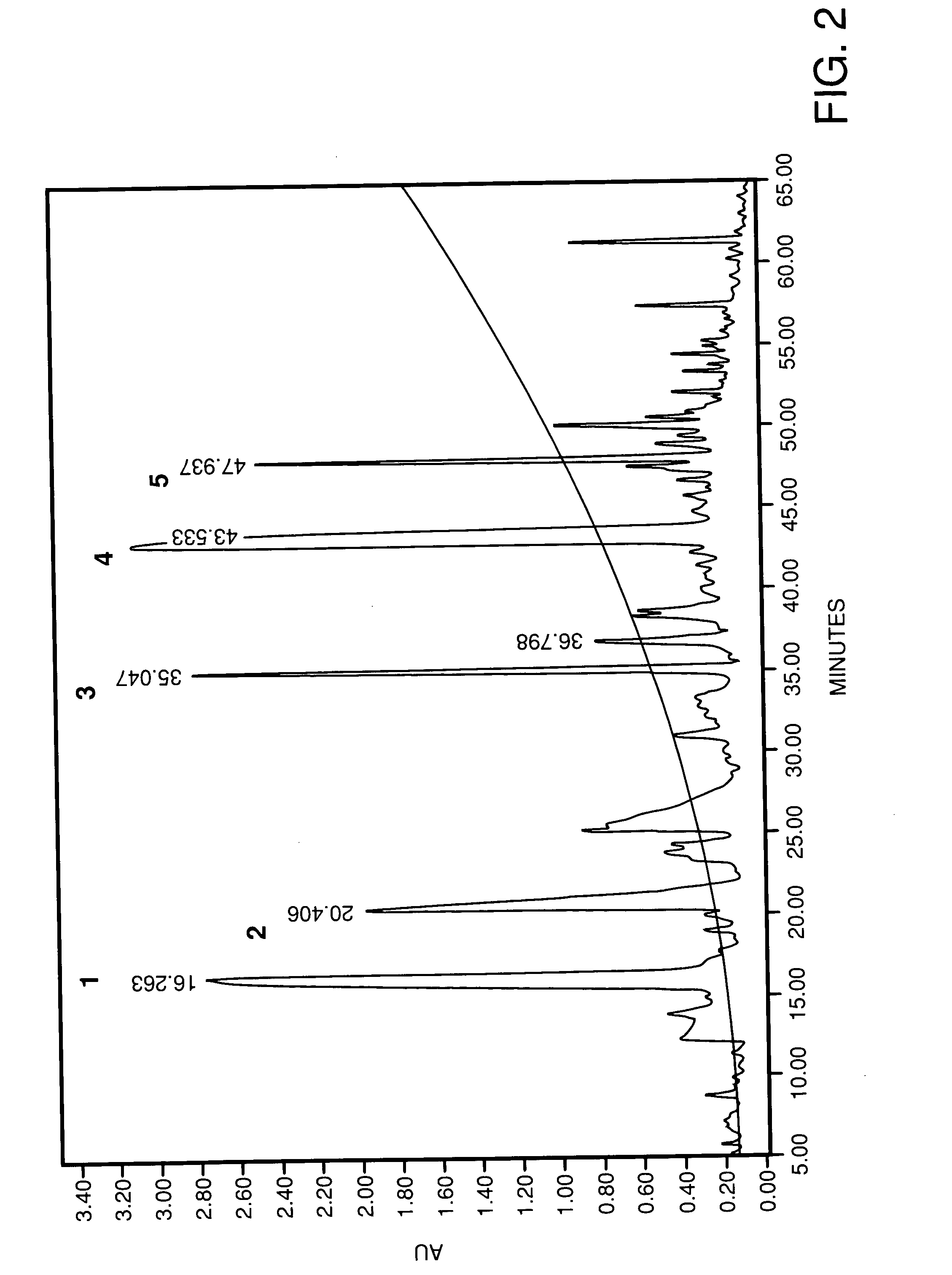
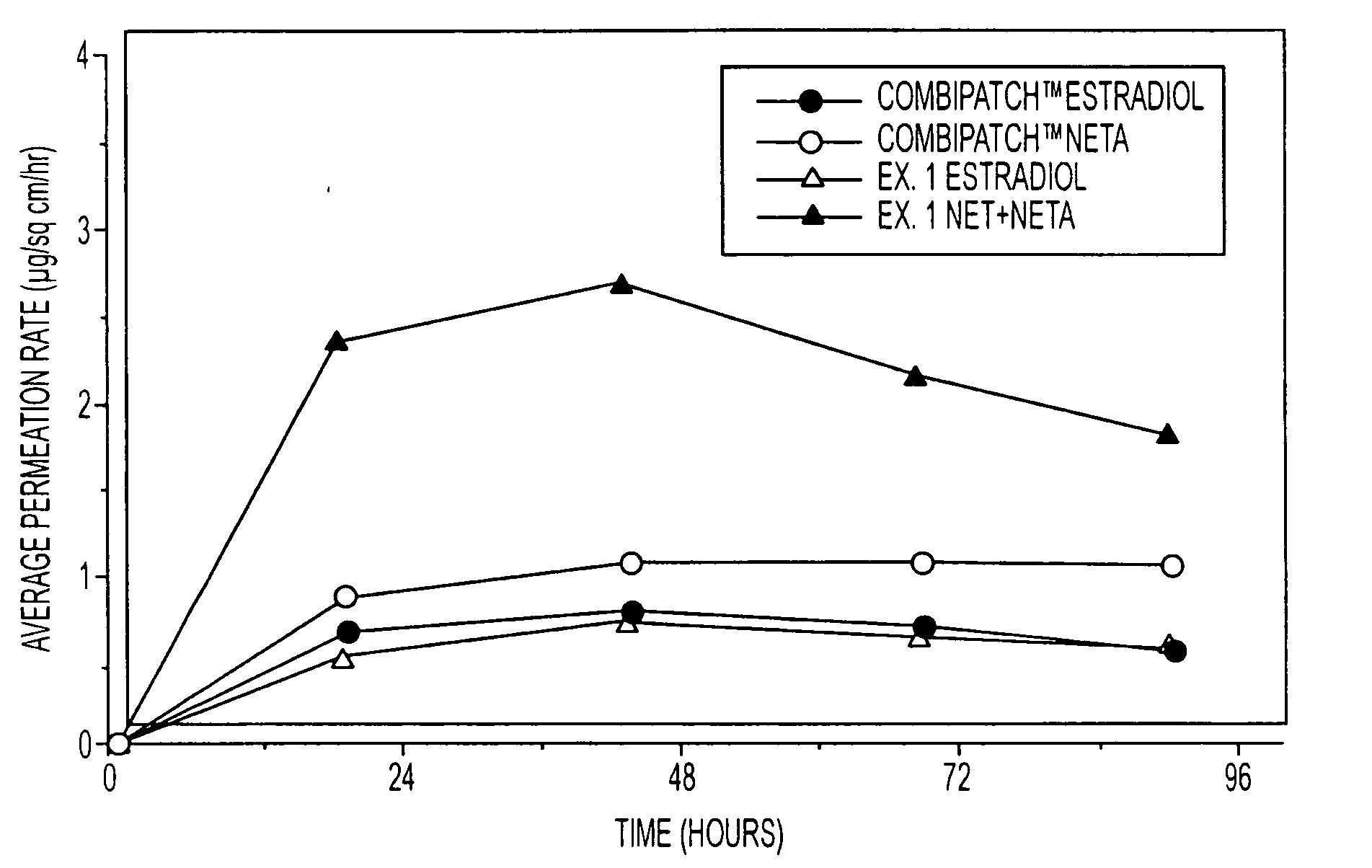
A composition for transdermal administration resulting from an admixture includes: a therapeutically effective amount of a drug that includes a parent drug and a prodrug; and and a pharmaceutically acceptable carrier, wherein the parent drug and prodrug are individually present in an amount sufficient for a pharmacological effect. In a preferred embodiment, the admixture includes: a therapeutically effective amount of a pharmaceutically active agent that includes a corresponding steroid and a steroid derivative; and a carrier for the pharmaceutically active agent. The steroid and the corresponding steroid derivative are present in a weight ratio of 10:1 to 1:10 steroid: corresponding steroid derivative. In a preferred embodiment ratio is 6:1 to 1:6. In a preferred embodiment, the corresponding steroid derivative is a steroid ester. In another preferred embodiment, the carrier is a polymer that includes a pressure-sensitive adhesive. In another preferred embodiment, the parent drug is an ACE inhibitor such as ramipril and the prodrug is an ACE inhibitor prodrug such as ramipril ethyl and / or methyl ester.
Owner:NOVEN PHARMA
Combination Therapies Employing Ace Inhibitors and Uses Thereof for the Treatment of Diabetic Disorders
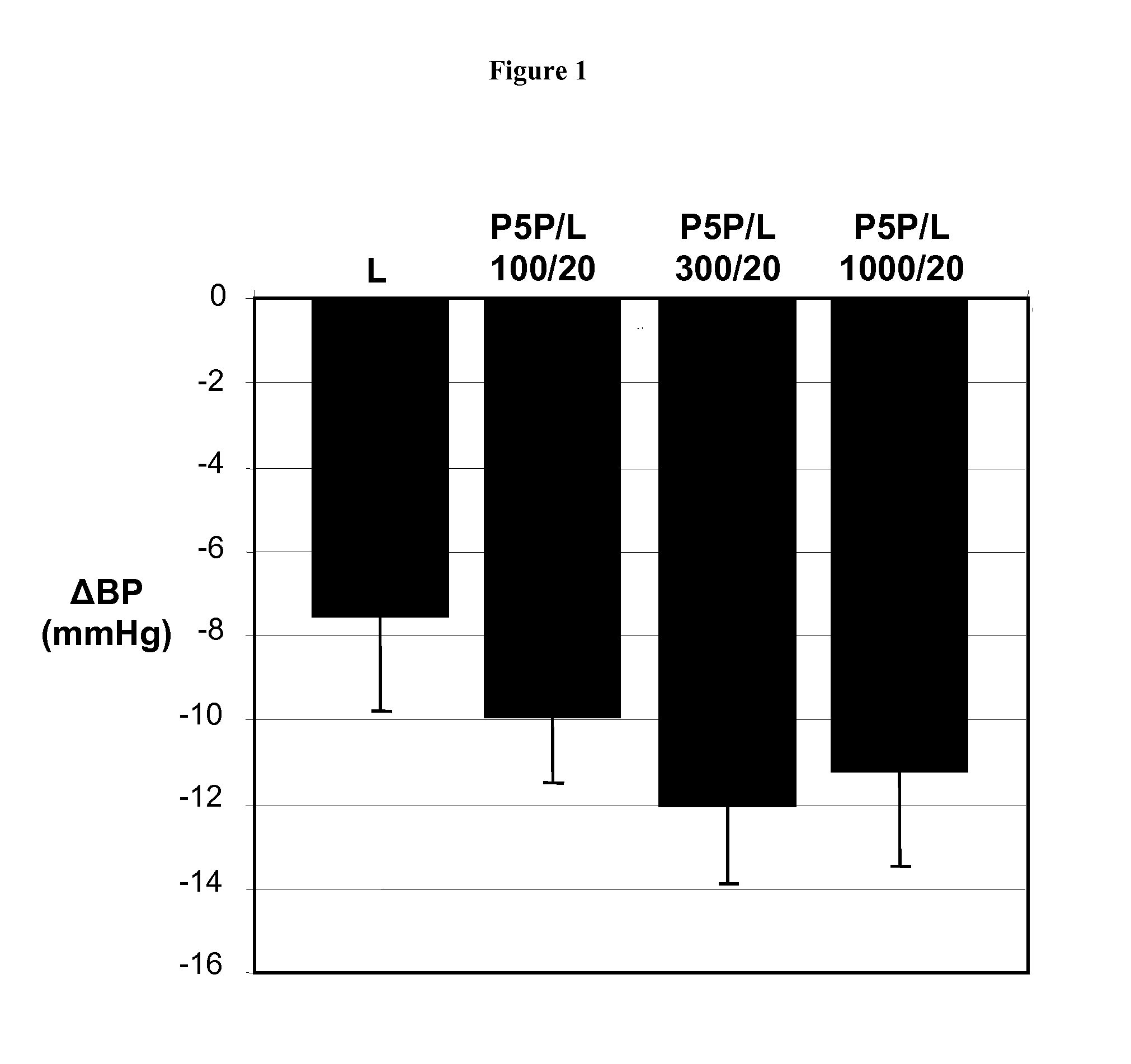
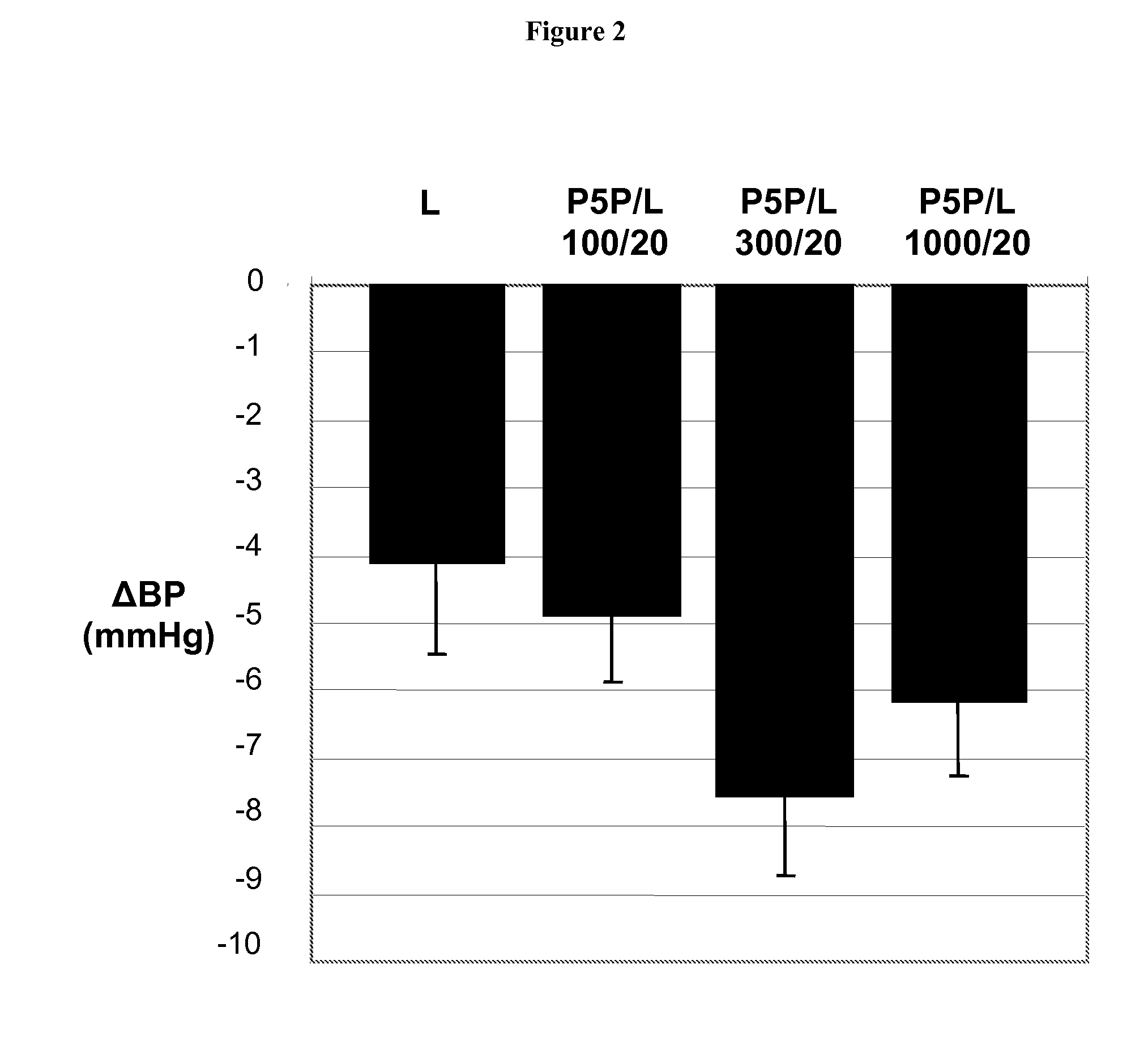
InactiveUS20080139511A1Enhance metabolismIncrease insulin sensitivityOrganic active ingredientsBiocideDiabetes mellitusVitamin B6 synthesis
Owner:MEDICURE INT INC
Cosmetic and pharmaceutical compositions comprising ace inhibitors and/or angiotensin ii receptor antagonists
InactiveUS20090137556A1Reduced gene expressionIncrease gene expressionAntibacterial agentsBiocideDermatological disordersACE Inhibitor Fetopathy
In one aspect, the present invention relates to use of an ACE inhibitor and / or angiotensin II receptor antagonist for the preparation of a medicament for the treatment of a dermatological disorder, particularly by topical application of said ACE inhibitor and / or angiotensin II receptor antagonist. The present invention also provides cosmetic methods for improving and / or maintaining the skin tone of an individual suffering from, or at risk of suffering from, a dermatological disorder, said method comprising contacting the skin of said individual with an ACE inhibitor and / or angiotensin II receptor antagonist.
Owner:ACE APS
Stable pharmaceutical compositions containing an ACE inhibitor
InactiveUS6869963B2Extended shelf lifeMinimize impactBiocidePill deliveryAlkaline earth metalInstability
A stable pharmaceutical composition comprising about 1 wt. % to about 80 wt. % of an ACE inhibitor or a pharmaceutical acceptable salt thereof, about 1 wt. % to about 70 wt. % of an alkali or alkaline earth metal carbonate, and about 1 wt. % to about 80 wt. % of hydroxypropyl cellulose, wherein the ACE inhibitor is selected from the group consisting of quinapril, enalapril, spirapril, ramipril, perindopril, indolapril, lisinopril, alacepril, trandolapril, benazapril, libenzapril, delapril, cilazapril and combinations thereof; wherein the formation of an internal cyclization product, and / or ester hydrolysis product, and / or oxidation product, has been reduced or eliminated, and the weight percents are based on the total weight of the pharmaceutical composition. The stabilized pharmaceutical compositions of the invention exhibit a number of advantages as follows: (i) the ACE inhibitor or a pharmaceutical acceptable salt thereof present in the compositions is preserved from degradation; (ii) the compositions exhibit extended shelf-life under normal storage conditions; (iii) the effect of moisture on the compositions is minimized; (iv) the compositions exhibit minimal, if any, discoloration over a significant period of time; and (v) the compositions exhibit minimal, if any, instability when employed in the presence of colorants.
Owner:SANDOZ AG
Conjoint administration of morphogens and ACE inhibitors in treatment of chronic renal failure
InactiveUS20050272649A1Preventing delaying needReducing necessary frequencyBiocidePeptide/protein ingredientsRenal disorderMorphine
The present invention provides reagents and methods for the treatment, and pharmaceuticals for use in the prevention and / or treatment, of chronic renal failure and other renal disorders in subjects (particularly mammalian subjects) renal replacement therapy. The methods involve the conjoint administration of ACE (Angiotensin-Converting Enzyme) inhibitors or Angiotensin II Receptor Antagonists (AIIRAs) with one or more OP / BMP family of proteins (morphogens, or inducers of morphogens, or agonists of the corresponding morphogen receptors, etc.). The invention also provides methods for implantation of renal cells induced with the conjoint administration of ACE inhibitors or AIIRAs with those morphogens.
Owner:BARNES JEWISH HOSPITAL +1
Co-formulations or kits of bioactive agents
Provided, among other things, is a formulation or kit comprising: (a) a pharmaceutically effective dosage of one or more a glucose-level-controlling bioactive agents selected from an α-glucodase inhibitor, sulfonylurea, meglitinide, thiazolidinediones, biguanide, insulin, dual PPARα / γ agonist, PPARγ agonist or insulin secretagogue; and (b) a pharmaceutically effective dosage of (i) one or more of an antihypertensive bioactive agent selected from an ACE inhibitor, calcium channel blocker, beta blocker, angiotension II receptor antagonist or diuretic, or (ii) one or more of an anti-dyslipidemia bioactive agent selected from a HMG-CoA reductase inhibitor, bile acid sequestrant, fibric acid derivative, sterol, cholesterol absorption inhibitor, MTP inhibitor or nicotinic acid derivative; wherein: in the case of (i) a combination of a first bioactive agent of group (a) that is metformin with a second bioactive agent of group (b), or (ii) a combination of a first bioactive agent of group (a) that is a thiazolidinedione or dual PPARα / γ agonist with an angiotension II receptor antagonist, one or more of the following applies: (I) one of the first bioactive agent or the second bioactive agent is formulated for sustained release, and the other is formulated for immediate release, each formulated for once-a-day dosing; or (II) the co-formulation or kit comprises (A) a biguanide and a thiazolidinedione and (B) one or more group (b) bioactive agents.
Owner:ABEILLE PHARMA
Antihypertensive therapy
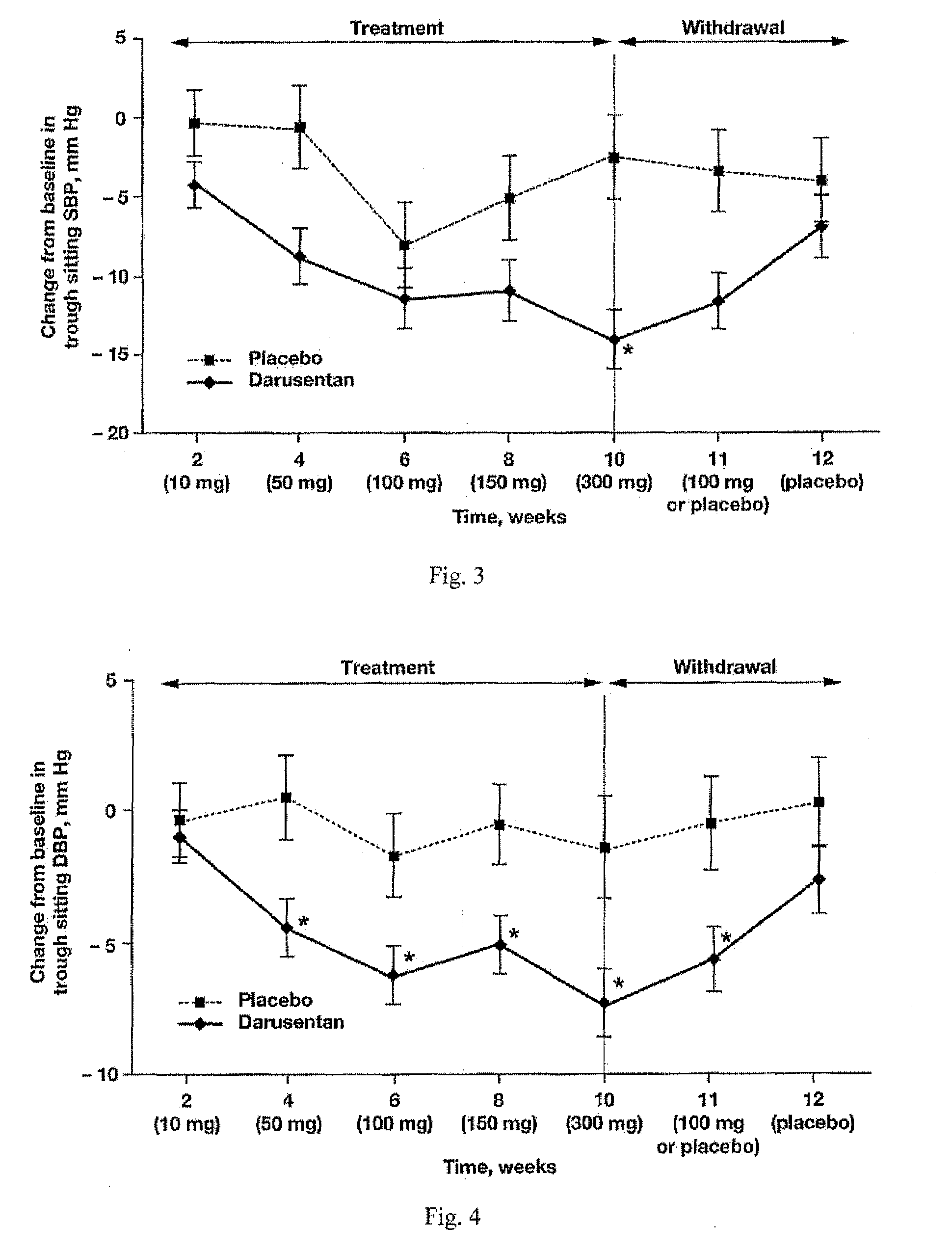
InactiveUS20090221549A1Beneficial effect on renal functionLower blood pressureBiocideAnimal repellantsAmbulatoryAnti hypertensive treatment
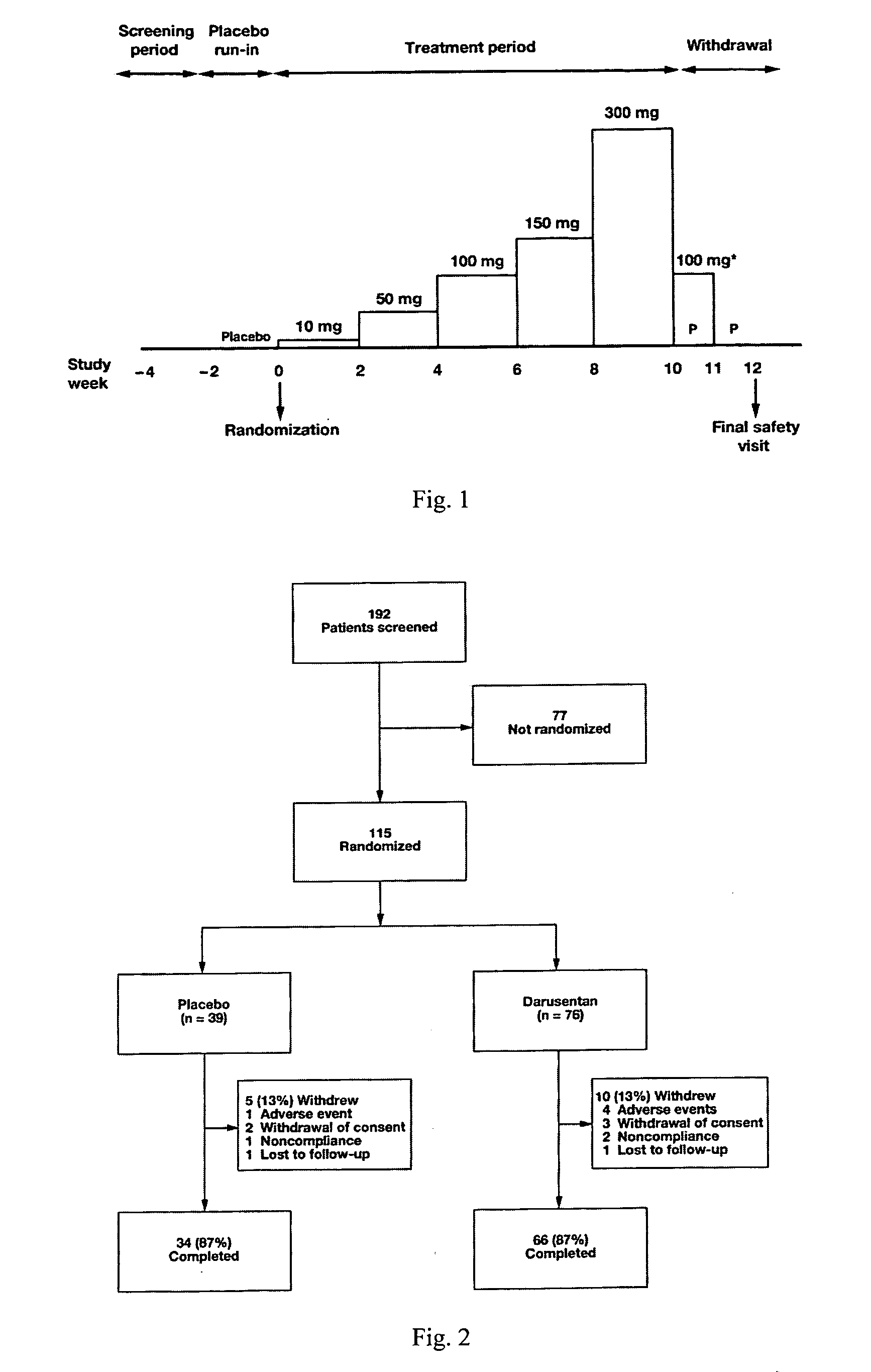
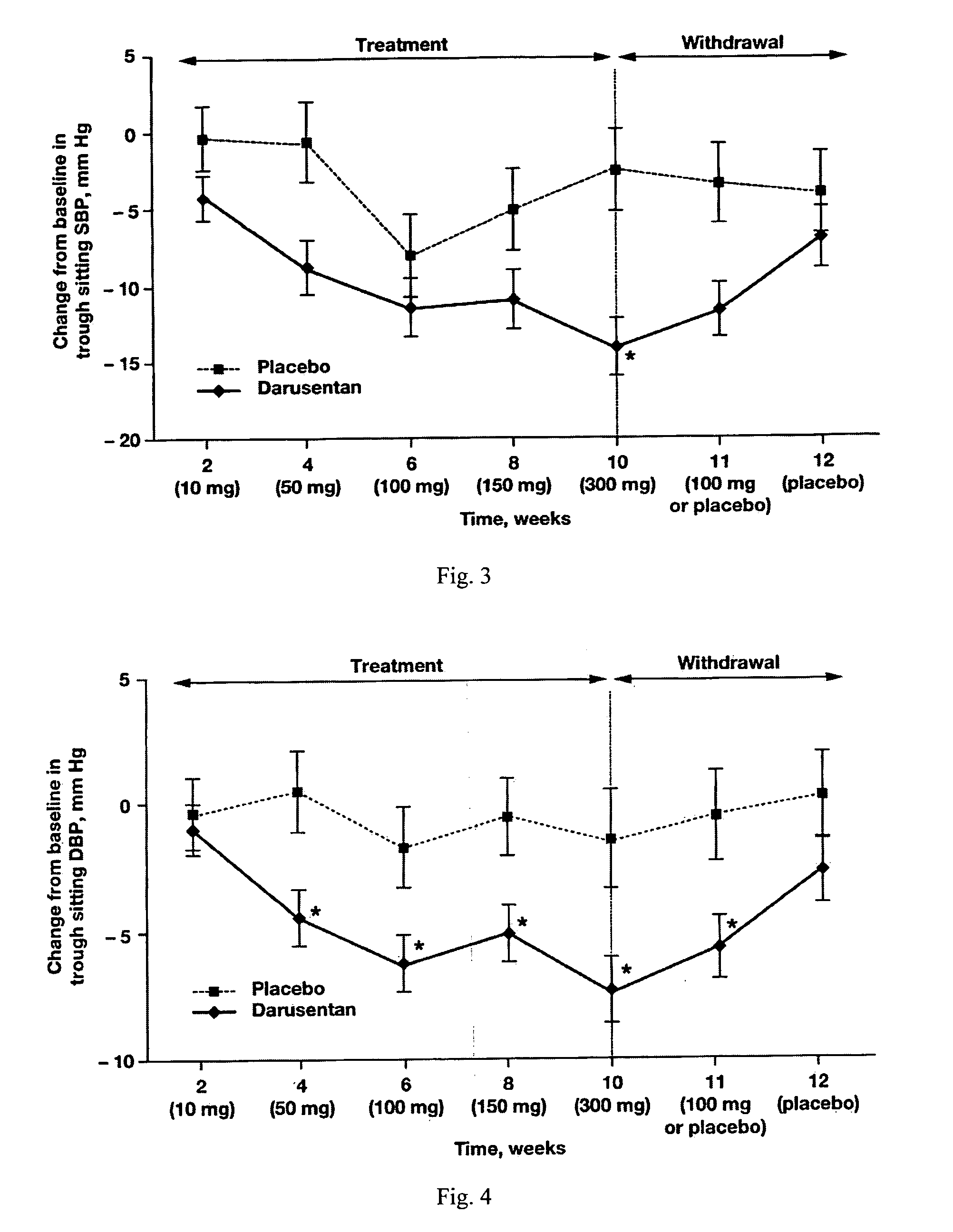
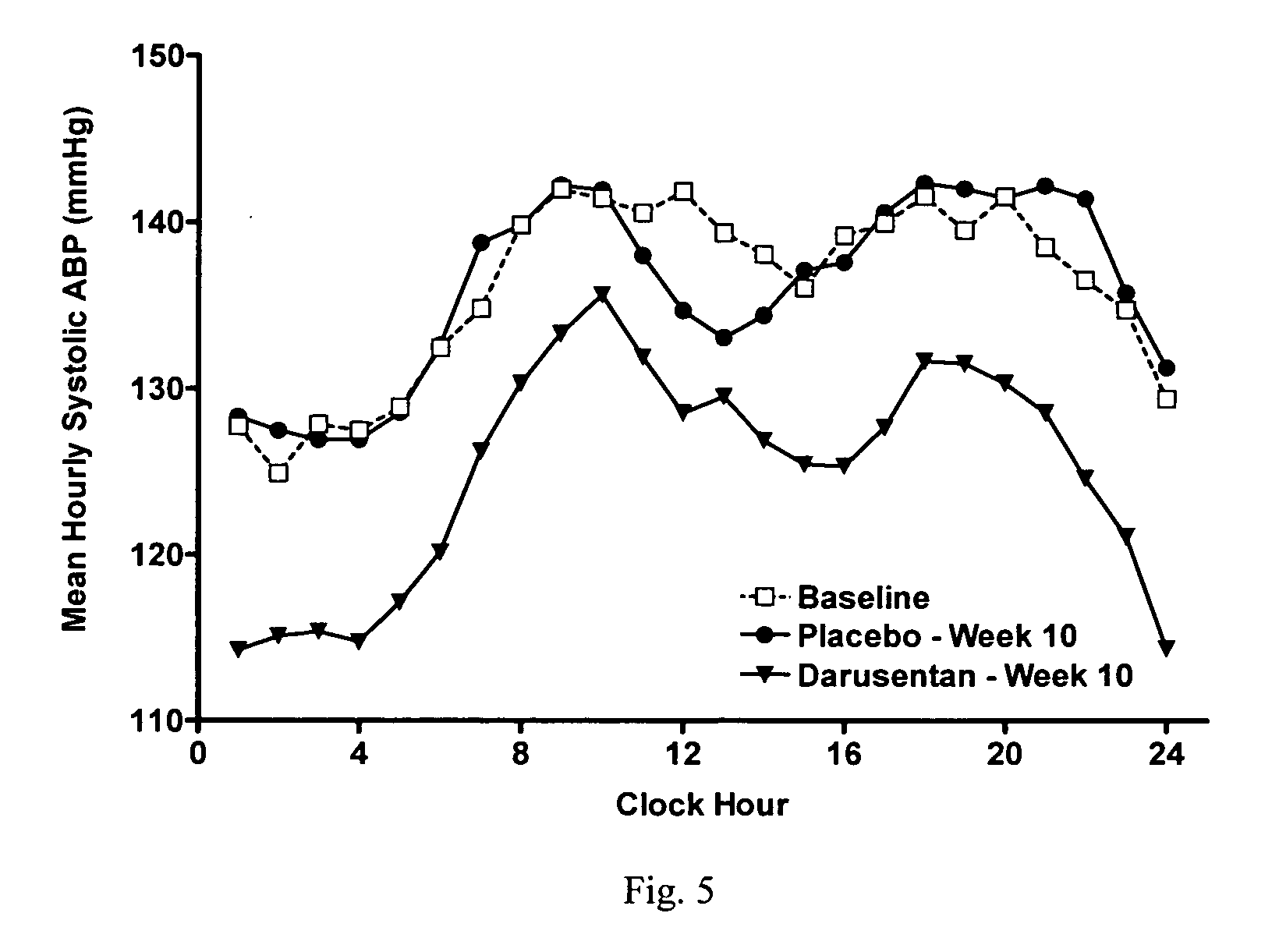
A new use of darusentan is provided in preparation of a pharmaceutical composition for lowering blood pressure in a patient exhibiting resistance to a baseline antihypertensive therapy with one or more drugs. The composition comprises darusentan in an amount providing a therapeutically effective daily dose; wherein (a) the composition is orally deliverable and / or (b) the daily dose of darusentan is effective to provide a reduction of at least about 3 mmHg in one or more blood pressure parameters selected from trough sitting systolic, trough sitting diastolic, 24-hour ambulatory systolic, 24-hour ambulatory diastolic, maximum diurnal systolic and maximum diurnal diastolic blood pressures. Further provided is a new use of darusentan in preparation of a pharmaceutical composition for lowering blood pressure in a patient exhibiting resistance to a baseline antihypertensive therapy, wherein the composition is administered adjunctively with at least one diuretic and at least one antihypertensive drug selected from ACE inhibitors, angiotensin II receptor blockers, beta-adrenergic receptor blockers and calcium channel blockers.
Owner:ABBOTT GMBH & CO KG
Stabilization of quinapril using magnesium oxide
InactiveUS20060106057A1Minimizes cyclization degradationOptimized formulaBiocideInorganic non-active ingredientsQuinaprilACE Inhibitor Fetopathy
The present invention is directed to ACE inhibitor-containing compositions stabilized by the presence of magnesium oxide. Preferably, the ACE inhibitor, quinapril, is protected from certain forms of degradation when prepared in a pharmaceutical composition consisting essentially of magnesium oxide as the stabilizing agent. The presence of magnesium oxide also lends itself to favorable processing conditions during the manufacture of ACE inhibitor-containing compositions, especially processing by wet granulation.
Owner:WARNER LAMBERT CO LLC
Stable formulations of ace inhibitors, and methods for preparation thereof
InactiveUS20080221156A1Easy to produceBiocidePeptide/protein ingredientsTime efficientIndustrial scale
The present invention provides stable formulations of ACE inhibitors, especially enalapril maleate, that can be manufactured in a time efficient, cost effective manner. Such formulations can be prepared simply and on a large industrial scale. The present invention also provides methods for the preparation of stable formulations of ACE inhibitors, especially enalapril maleate.
Owner:MUTUAL PHARMA CO INC
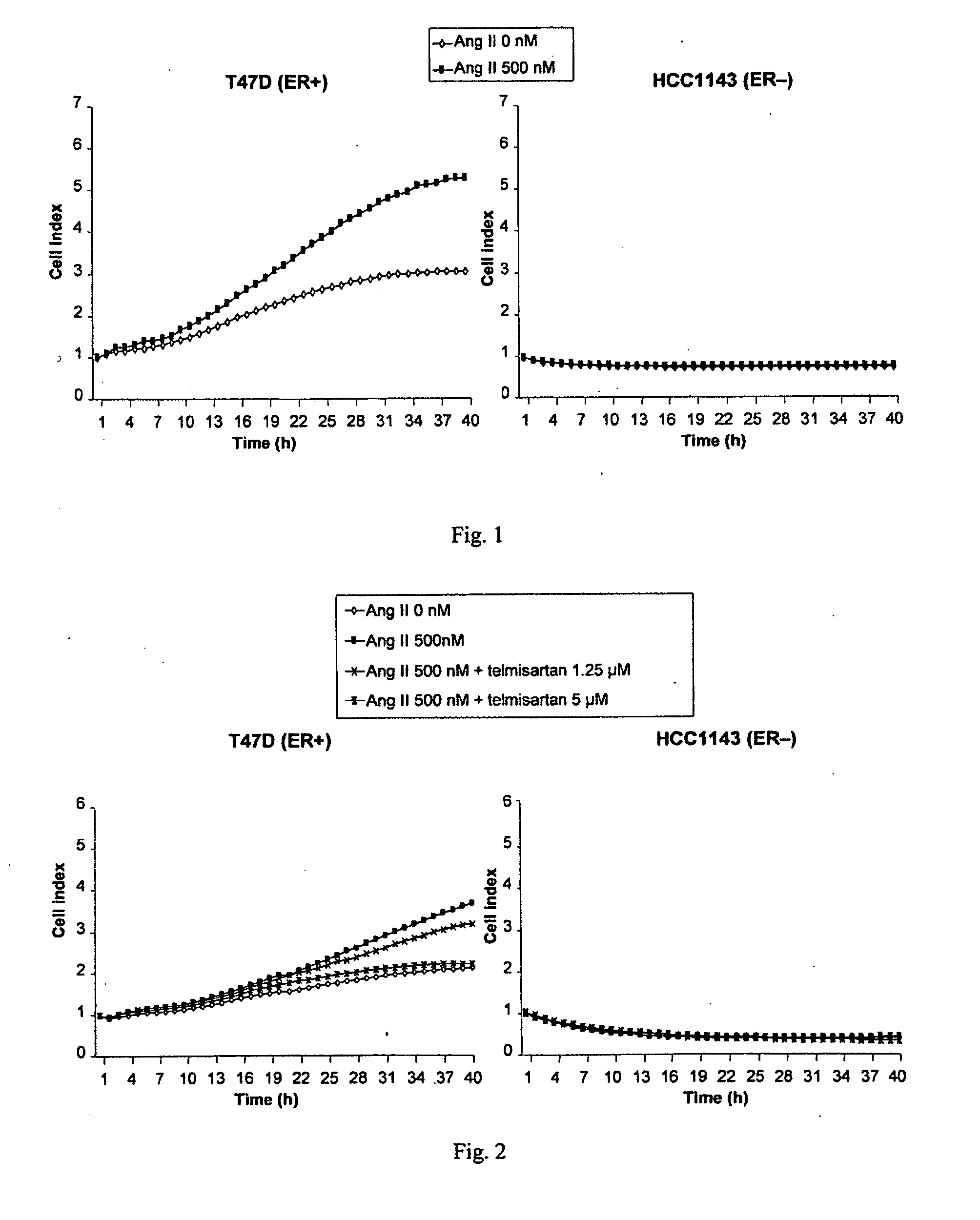
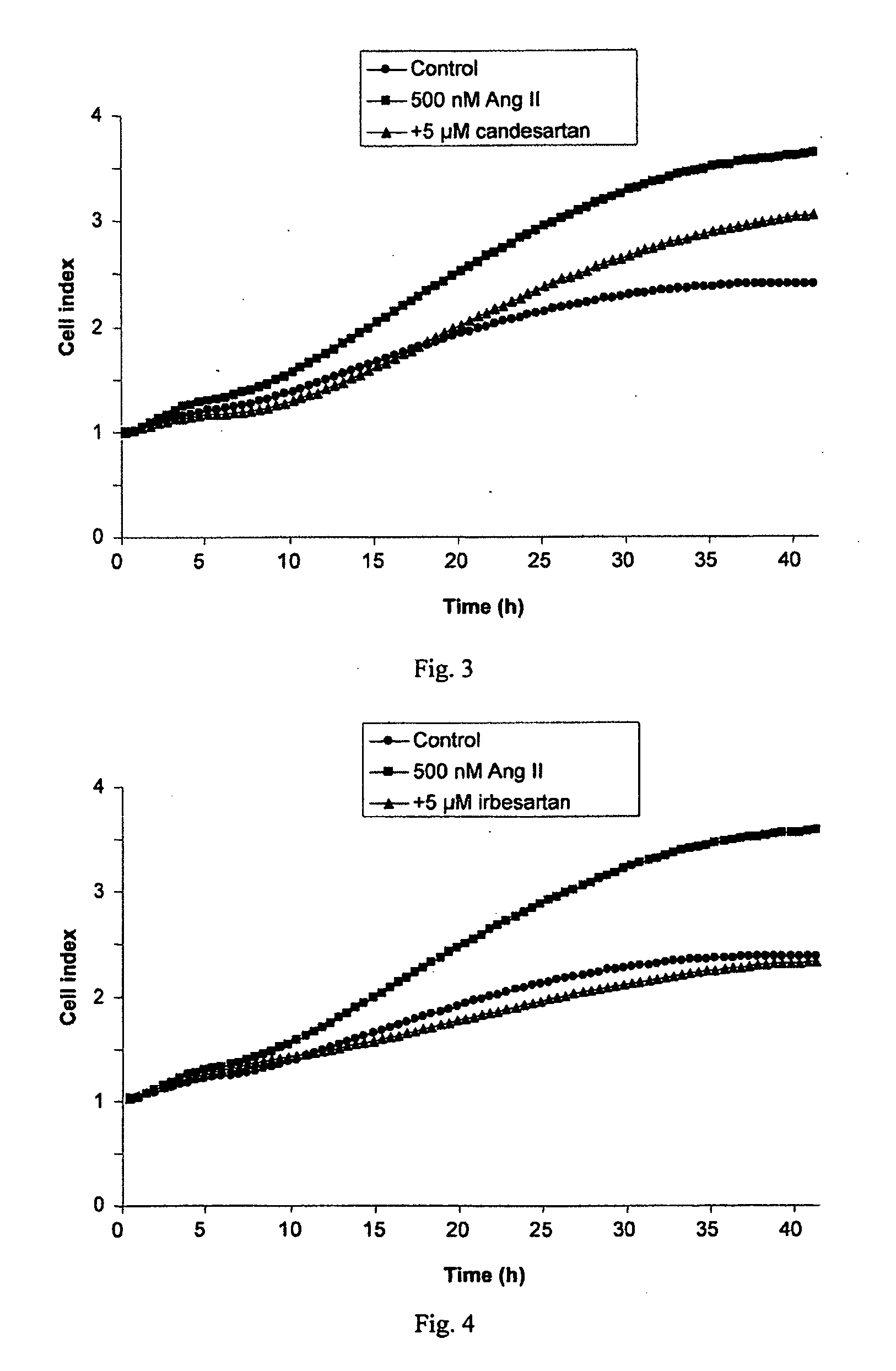
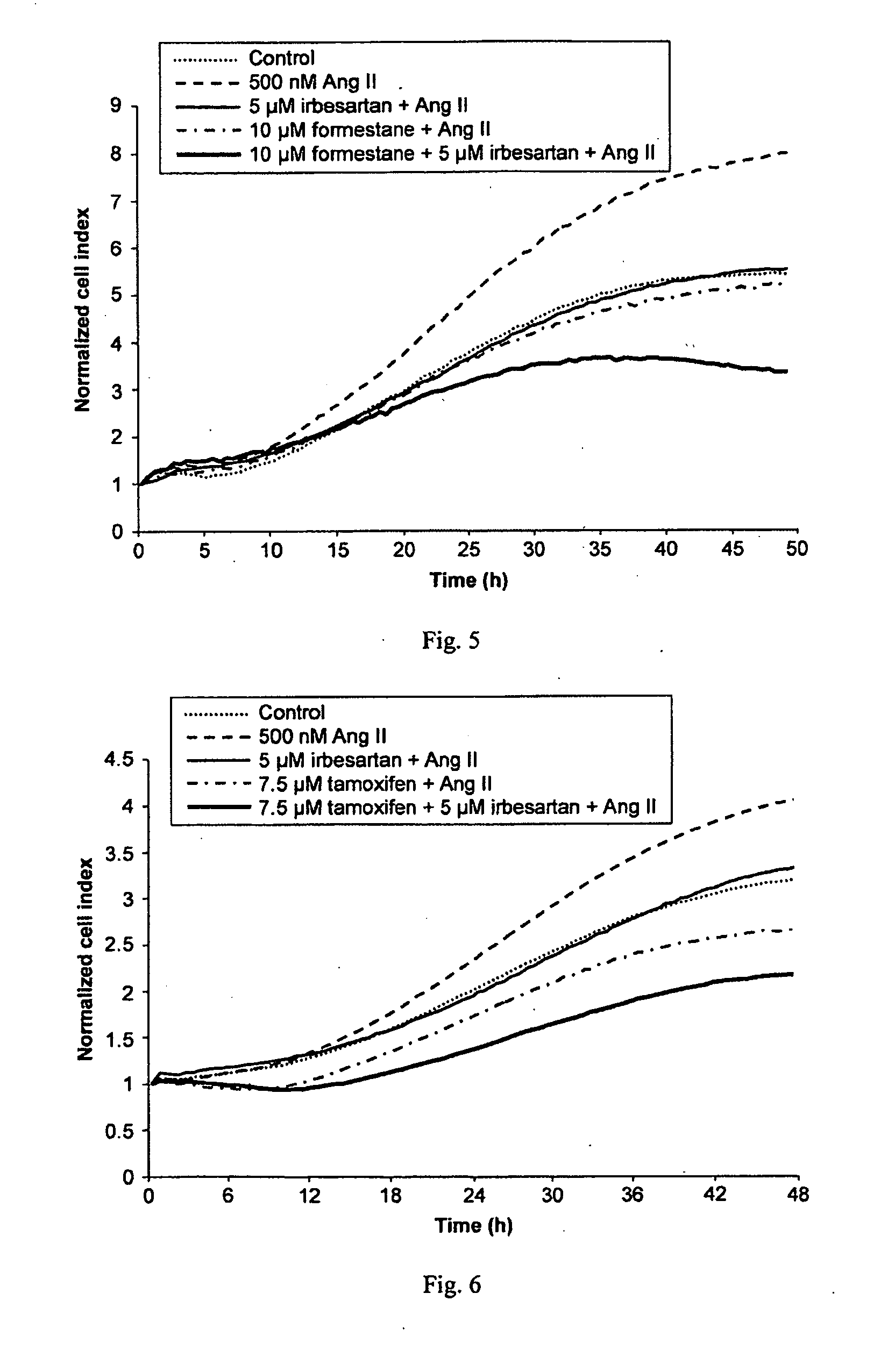
Methods for breast cancer screening and treatment
InactiveUS20100029734A1Decreasing Ang II-induced cell proliferationPromote growthBiocideDisease diagnosisBreast cancer screeningACE Inhibitor Fetopathy
A method for selecting a breast cancer patient for therapy with an agent that reduces production of angiotensin II, for example an ACE inhibitor or renin inhibitor, comprises (a) determining whether the cancer comprises a tumor that is estrogen receptor positive (ER+) and (b) selecting the patient for such therapy only if the cancer is determined to comprise an ER+ tumor. A method for treating breast cancer in a patient further comprises (c) administering to the patient, if so selected, an agent that reduces production of angiotensin II, for example an ACE inhibitor or renin inhibitor. A method for treating a breast tumor in a patient having SERM-resistant ER+ breast cancer comprises administering to the patient an agent that reduces production of angiotensin II, for example an ACE inhibitor or renin inhibitor. A therapeutic combination useful in treatment of a breast tumor comprises an agent that reduces production of angiotensin II, for example an ACE inhibitor or renin inhibitor, and a second agent that comprises (a) an aromatase inhibitor or (b) an estrogen receptor modulator or antagonist.
Owner:ORE PHARMA
Method for treating resistant hypertension
A method is provided for lowering blood pressure in a patient having clinically diagnosed resistant hypertension. The method comprises administering darusentan to the patient adjunctively with a baseline antihypertensive regimen that comprises administration of at least one diuretic and at least two antihypertensive drugs selected from at least two of (a) ACE inhibitors and angiotensin II receptor blockers, (b) beta-adrenergic receptor blockers and (c) calcium channel blockers. The darusentan is orally administered at a dose and frequency effective, in combination with the baseline regimen, to provide a reduction of at least about 3 mmHg in one or more blood pressure parameters selected from trough sitting systolic, trough sitting diastolic, 24-hour ambulatory systolic, 24-hour ambulatory diastolic, maximum diurnal systolic and maximum diurnal diastolic blood pressures.
Owner:MYOGEN INC +1
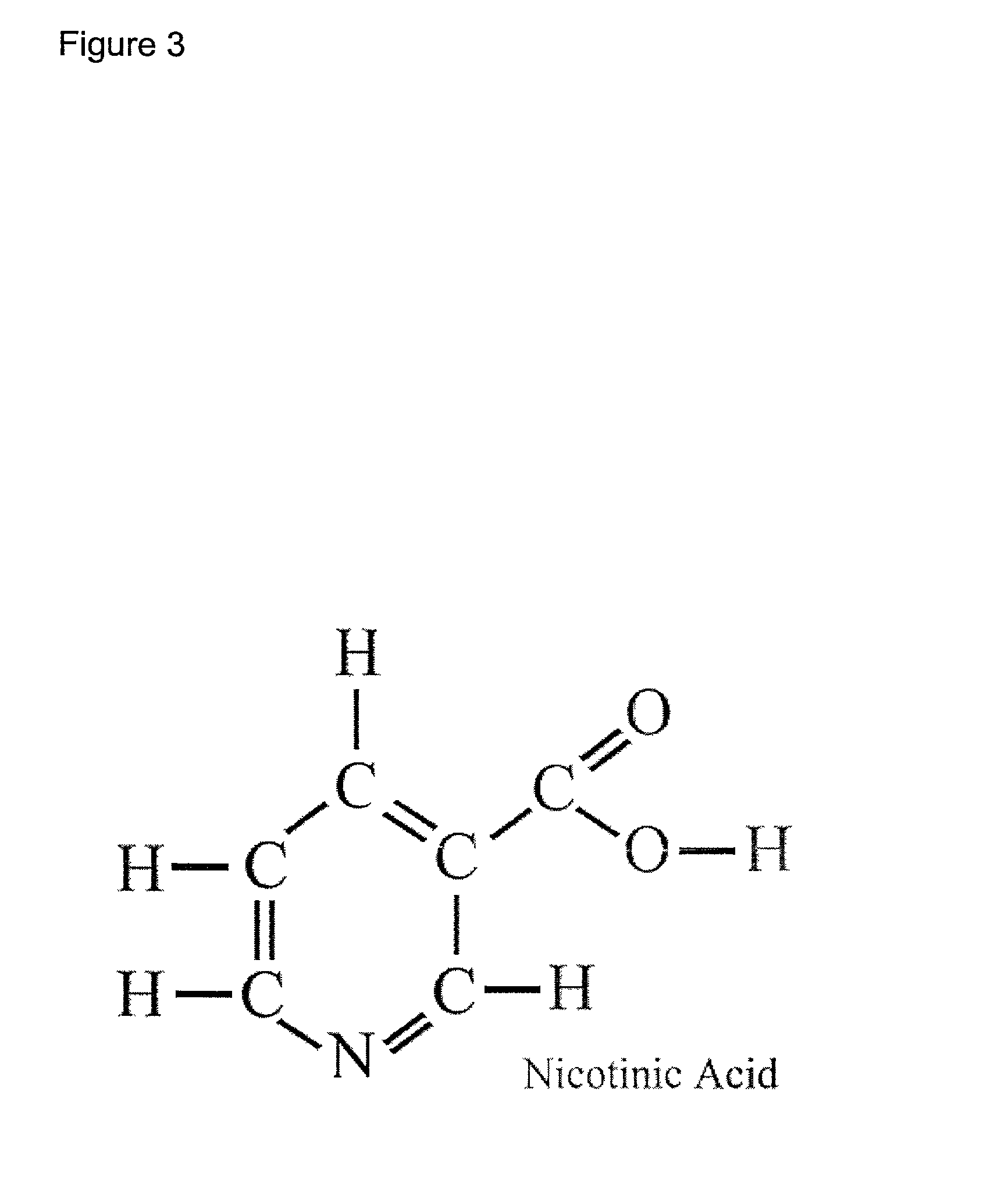
B complex vitamin compositions that protect against cellular damage caused by ultraviolet light
InactiveUS7018623B2Reduces UV damageReduce riskBiocideCosmetic preparationsVitamin K2Immune depression
The present invention relates generally to the use of vitamin B12 (cobalamin or cyanocobalamin) alone or in combination with other photoprotective agents, including specifically other vitamins such as vitamin B9 (folic acid or folate) and vitamin B3 (niacin or niacinamide), or any chemical derivative of these vitamins and their salts, as a filter to protect cells against the damaging effects of ultraviolet (UV) light. The invention is, in one aspect, a method of reducing the rate of UV damage to cells exposed to a UV light source, by treating the cells with the vitamin composition, either alone or in combination with other photoprotective agents. Other aspects of the invention are compositions comprising effective amounts of vitamin B12 alone or in combination with other photoprotective agents including vitamin B9 and vitamin B3 and a pharmaceutically-acceptable carrier, that are useful in protecting cells, particularly skin cells, against the burning, genotoxic (mutagenic and carcinogenic), immunosuppressive and photoaging effects of UV light, especially sunlight. The invention has application as a UV light filter in oral preparations including tablets and drinks, topical creams, lotions, sprays, wipes and cosmetics. The invention also has application as a medicinal treatment for dermatological conditions caused by exposure to sunlight, such as actinic keratoses, photodermatitis, photo-induced (discoid) lupus erythematosus and the photosensitizing effects of a variety of drugs used commonly in clinical practice (e.g. certain antihistamines, ACE inhibitors, and antibiotics such as tetracycline).
Owner:BARCLAY BARRY J
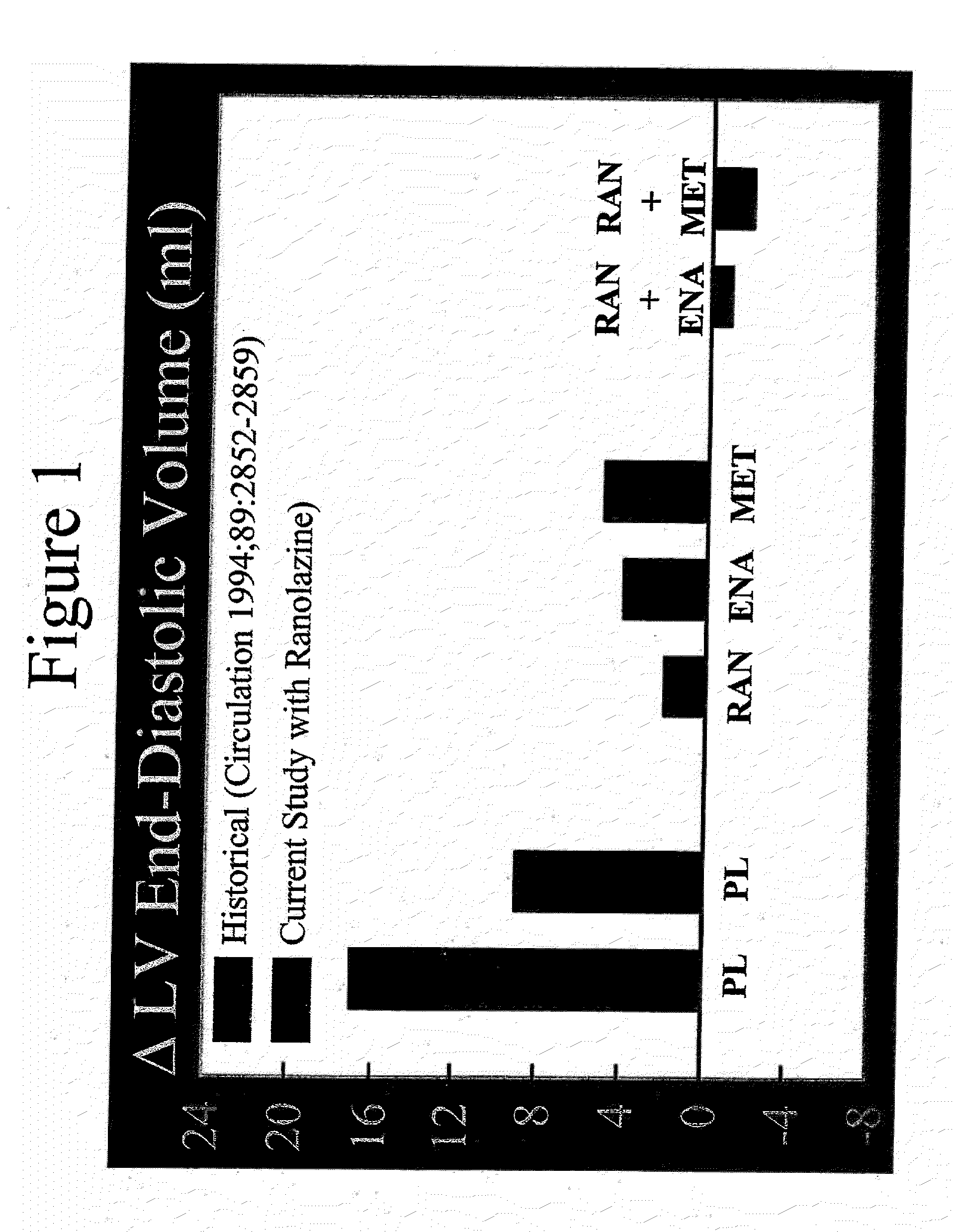
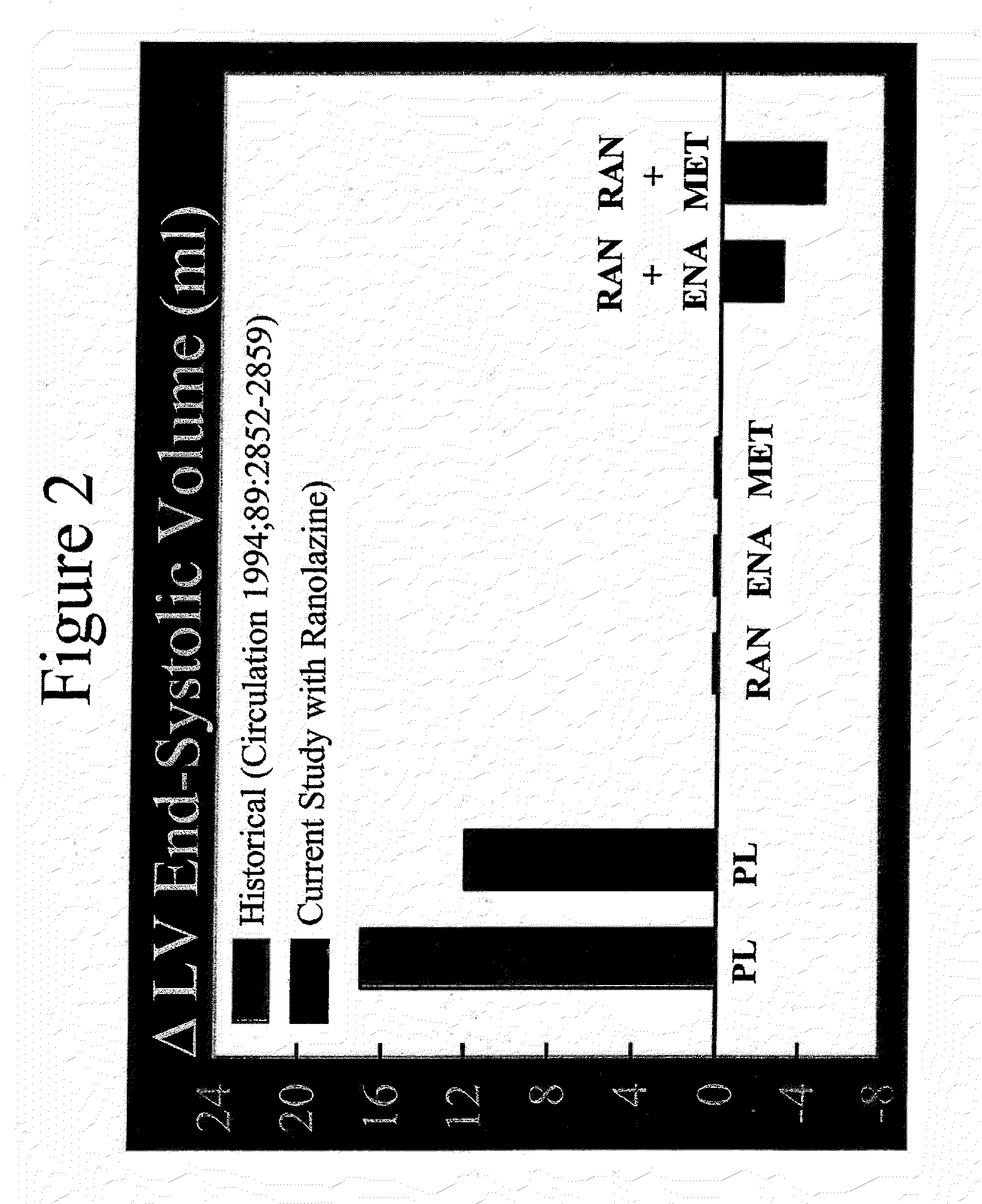
Method of reversing left ventricular remodeling
InactiveUS20090176772A1Improve actionReduce releaseCardiovascular disorderHeterocyclic compound active ingredientsBeta blockerLeft ventricular size
The present invention relates to method of reversing left ventricle remodeling by combined administration of therapeutically effective amounts ranolazine and at least one co-remodeling agent, which may be an ACE inhibitor, an ARB, or a beta-blocker. The method finds utility in the treatment of heart failure. This invention also relates to pharmaceutical formulations that are suitable for such combined administration.
Owner:GILEAD SCI INC
Use of ace inhibitors and/or angiostensin ii receptor antagonists for the improving and/or maintaining the skin tone and for the treatment of skin ageing
InactiveUS20090143458A1Reduced gene expressionIncrease gene expressionCosmetic preparationsBiocideNK1 receptor antagonistDepressant
The present invention relates to use of an ACE inhibitor and / or angiotensin II receptor antagonist of the preparation of a medicament for the treatment of skin ageing or wrinkling. Furthermore, the present invention relates to use of an ACE inhibitor and / or angiotensin II receptor antagonist for the preparation of a cosmetic composition.
Owner:ACE APS
ACE inhibitor formulation
InactiveUS20070009591A1Facilitated releaseWood working apparatusPill deliveryAdditive ingredientMoexipril Hydrochloride
A process for preparing pharmaceutical micro-tablets comprises (a) preparing a tableting mix that comprises an ACE inhibitor, for example moexipril hydrochloride, and excipient ingredients that comprise one or more lubricants; and (b) compressing the tableting mix in a tablet press, to form micro-tablets having an average uncoated weight of about 1 to about 40 mg; wherein the process employs means for promoting release of the mirco-tablets from the tablet press, said release promoting means being unnecessary for otherwise similar standard tablets having an average uncoated weight greater than about 50 mg. A plurality of micro-tablets thus prepared, collectively comprising a therapeutically effective amount of the ACE inhibitor, can be filled into a pharmaceutically acceptable capsule shell to provide a dosage form.
Owner:UCB MFG
Stable pharmaceutical compositions of calcium channel blocker and an ACE inhibitor
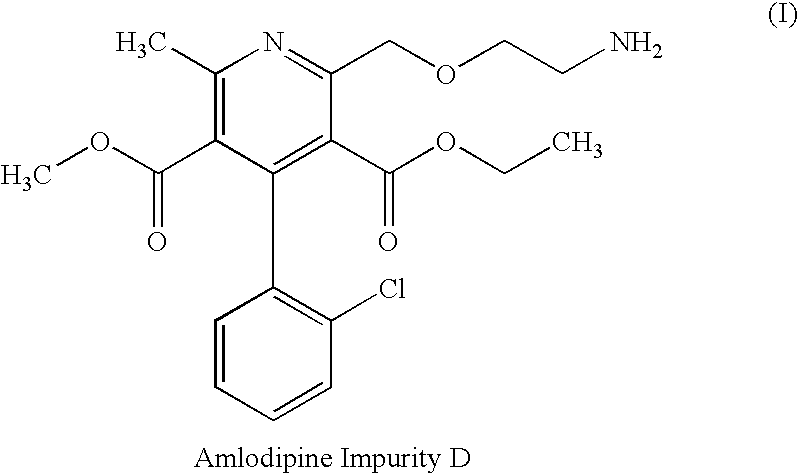
The present invention relates to a stable pharmaceutical composition of a combination of amlodipine and an ACE inhibitor; wherein the two active ingredients are not physically separated and the composition has a pH of more than 6.0. It also relates to a process for preparation, and a method for using such a composition.
Owner:TORRENT PHARMA LTD
Stable Pharmaceutical Composition Comprising an Ace Inhibitor
InactiveUS20080038342A1Minimize degradationStable pharmaceutical compositionBiocidePill deliveryStress inducedCoronary heart disease
The present invention relates to a stable pharmaceutical composition comprising an ACE inhibitor or a pharmaceutically acceptable salt or derivative thereof. In particular, the invention relates to a pharmaceutical composition, which comprises an ACE inhibitor, or a pharmaceutically acceptable salt or a derivative thereof, and a C16-C28 glyceride. ACE inhibitors useful in the present invention are susceptible to heat and / or mechanical stress-induced degradation. Preferred ACE inhibitors are ramipril, trandolapril, quinapril and pharmaceutically acceptable salts and derivatives thereof. The composition of the present invention may be for use as a medicament for the treatment or prevention of a cardiovascular disease, a coronary heart disease, a cerebrovascular disease, a peripheral vascular disease, arrhythmia, hypertension, cardiac failure, cardiovascular death, myocardial infraction, stroke or angina. The present invention further relates to a method of preparing the pharmaceutical composition of the present invention. The present invention also relates to a method of providing a stable pharmaceutical composition comprising an ACE inhibitor, or a pharmaceutically acceptable salt or derivative thereof, by incorporating a C16-C28 glyceride into the composition. The present invention further relates to a use of C16-C28 glyceride to provide a stable pharmaceutical composition comprising an ACE inhibitor or a pharmaceutically acceptable salt or derivative thereof.
Owner:NICHE GENERICS
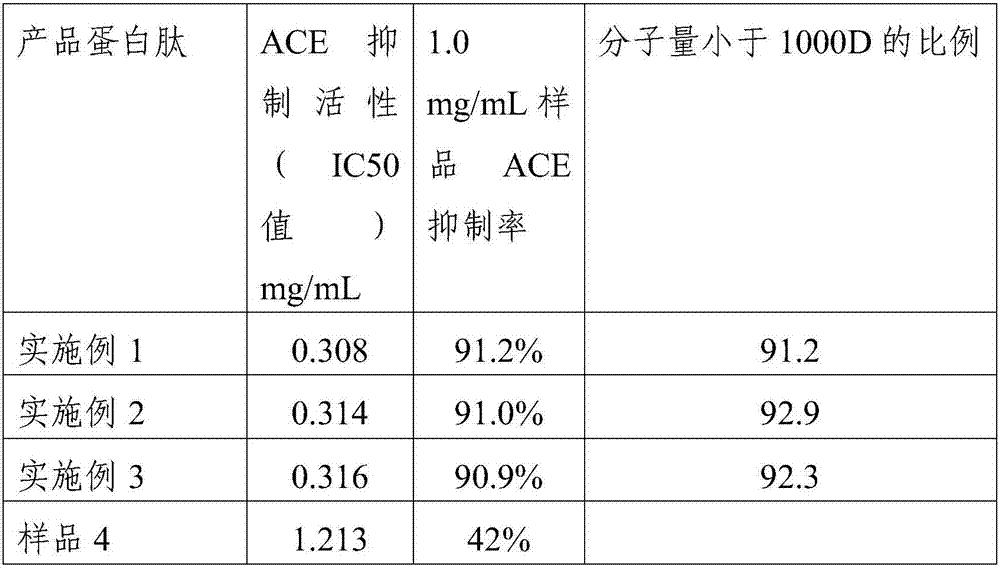
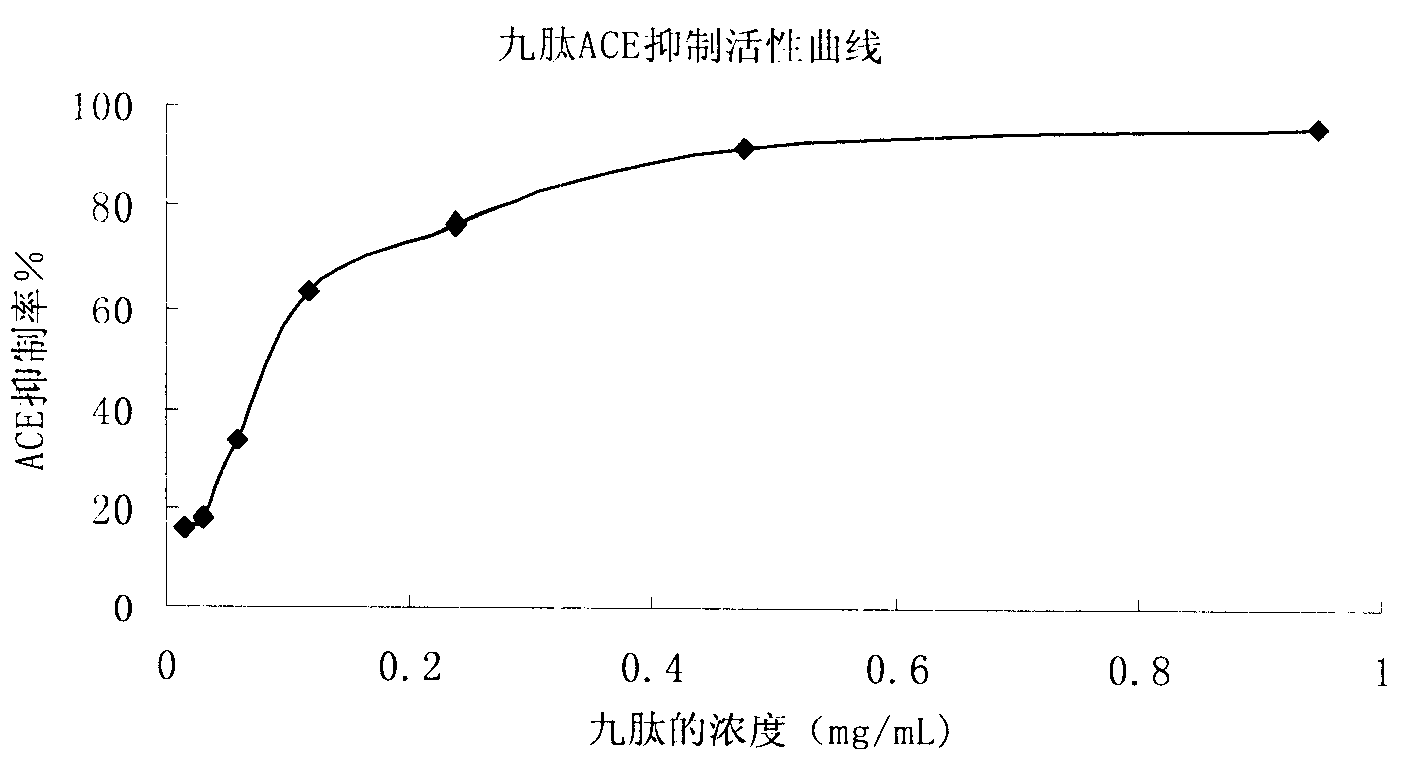
Yak bone protein peptide with ACE inhibiting function and preparation method and application thereof
ActiveCN107082807AReasonable useEfficient use ofConnective tissue peptidesPeptide/protein ingredientsIc50 valuesEnzymatic hydrolysis
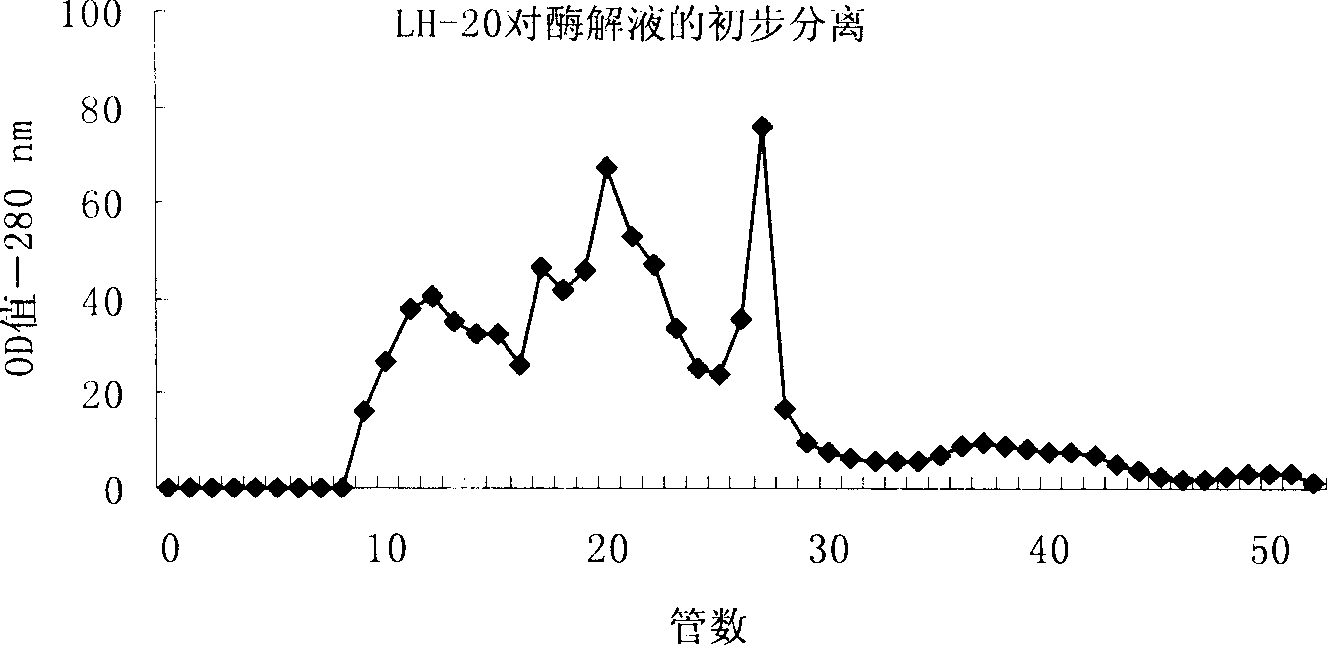
The invention provides a yak bone protein peptide with an ACE inhibiting function and a preparation method and application thereof. The yak bone protein peptide is prepared from enzymatically hydrolyzed yak bone protein by multiple-step enzymatic hydrolysis by compound protease, and the compound protease consists of alkali protease, papain, trypsin and flavourzyme. The yak bone protein peptide provided by the invention has an excellent ACE inhibiting function, the ACE inhibiting activity (IC50 value) is smaller than 0.32mg / mL, and the yak bone protein peptide can be used as an ACE inhibitor to be applied to special foods and nutritional foods. The preparation method of the yak bone protein peptide provided by the invention is simple, the whole processing course is not added with acid or alkali for regulating the pH value, and the product maintains good functional characteristics and is easier to realize industrialized production.
Owner:ANHUI GUOTAI BIOTECHNOLOGY CO LTD
Pharmaceutical composition comprising a sodium hydrogen exchange inhibitor and an angiotensin converting enzyme inhibitor
InactiveUS6844361B2Avoid failurePreventing age-related disordersBiocideAnimal repellantsDiseaseHydrogen
This invention is directed to a pharmaceutical composition comprising the sodium-hydrogen exchanger (NHE) inhibitor cariporide and an angiotensin converting enzyme (ACE) inhibitor which exhibits unexpectedly efficacious properties for preventing heart failure and other age-related organ dysfunctions, age-related disorders and for prolonging life, and to methods of preventing heart failure and other age-related organ dysfunctions, age-related disorders and for prolonging life comprising administering pharmaceutically effective amounts of the sodium-hydrogen exchange inhibitor cariporide and an ACE inhibitor.
Owner:SANOFI AVENTIS DEUT GMBH
Stable formulations of ace inhibitors and methods for preparation thereof
Stabilized pharmaceutical solid composition of ACE inhibitor comprising an ACE inhibitor and a selective dosage formulation thereof comprising of meglumine. The ACE inhibitor selectively combined with a dosage form including essentially the meglumine is surprisingly found to avoid the degradation of ACE inhibitor by such dosage forms especially the commonly used pharmaceutical excepients. In particular, the presence of the meglumine in the dosage form for the active along with the active ACE inhibitor surprisingly avoid the degradation of the ACE inhibitor due to a) cyclization via internal nucleophilic attack to form substituted diketopiperazines, b) hydrolysis of the side chain ester group, and c) oxidation to form products having often unwanted coloration.
Owner:LUPIN LTD
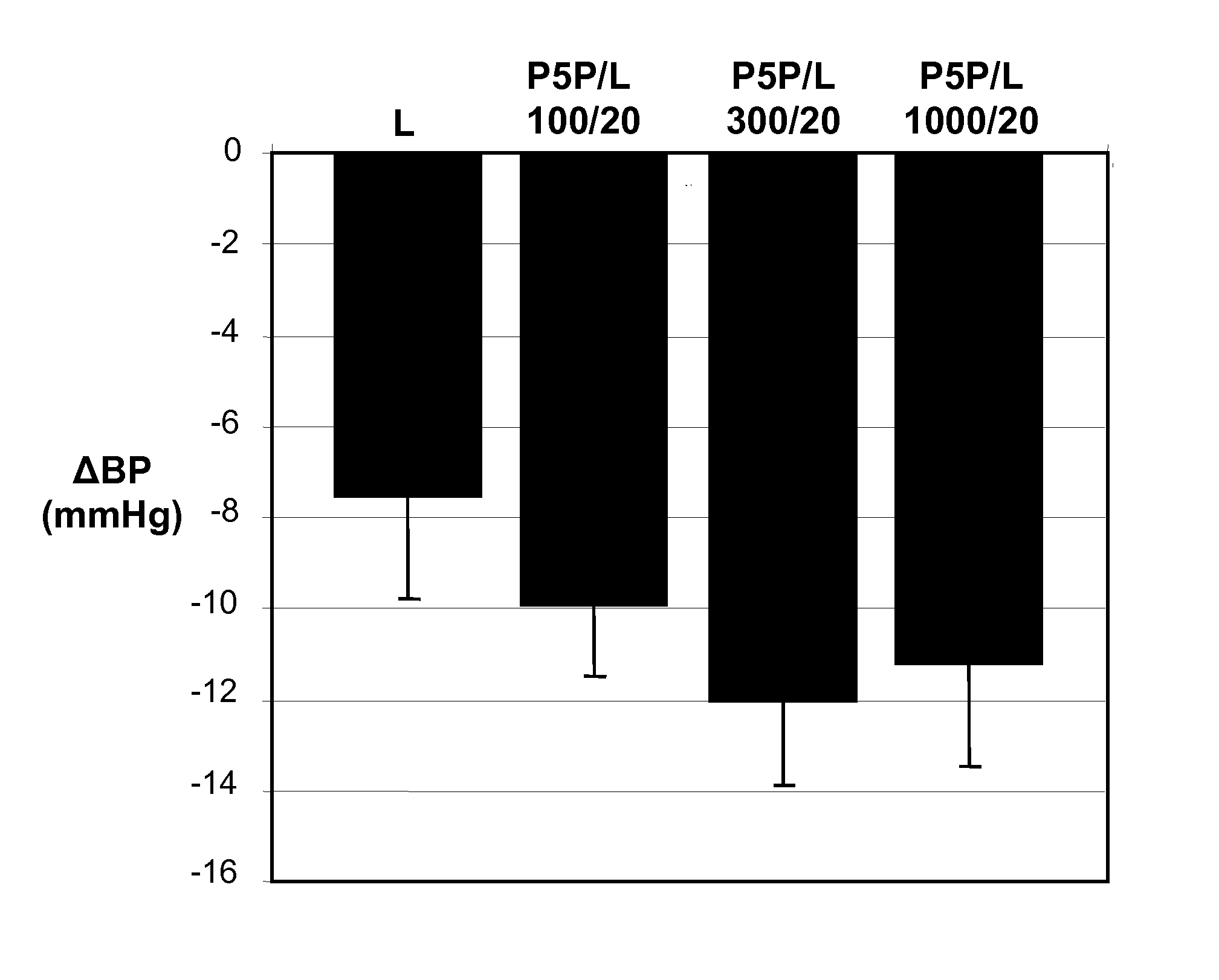
Stable pharmaceutical composition for atherosclerosis
ActiveUS20120027849A1Good antihypertensive effectGood blood pressure effectBiocideAntipyreticLipid formationLipid lowering drug
The present invention relates to a stable solid oral pharmaceutical multi-component composition comprising combination of blood pressure lowering drugs with lipid lowering agent / s and optionally a platelet aggregation inhibitor in a single dosage form. The blood pressure lowering agents are selected from β-adrenergic receptor blocking agent, ACE inhibitor and diuretic. The lipid lowering agent is selected from HMG Co-enzyme-A reductase inhibitor. The pharmaceutical composition made as per present invention a) overcomes any drug-drug interactions, b) exhibits pharmacokinetic and pharmacodynamic profile of individual therapeutic agent, c) has minimal side effects. The invention provides multi-component composition (MCC) to increase adherences to therapy. The MCC as per present invention provides compositions that maintain activity of all active ingredients without significant increase in adverse event profile. The present invention further relates to a method of preparing the said pharmaceutical composition.
Owner:CADILA PHARMA
Active peptide and application thereof
InactiveCN101210047AStrong blood pressure lowering effectPeptide/protein ingredientsAnimals/human peptidesOysterElevated blood
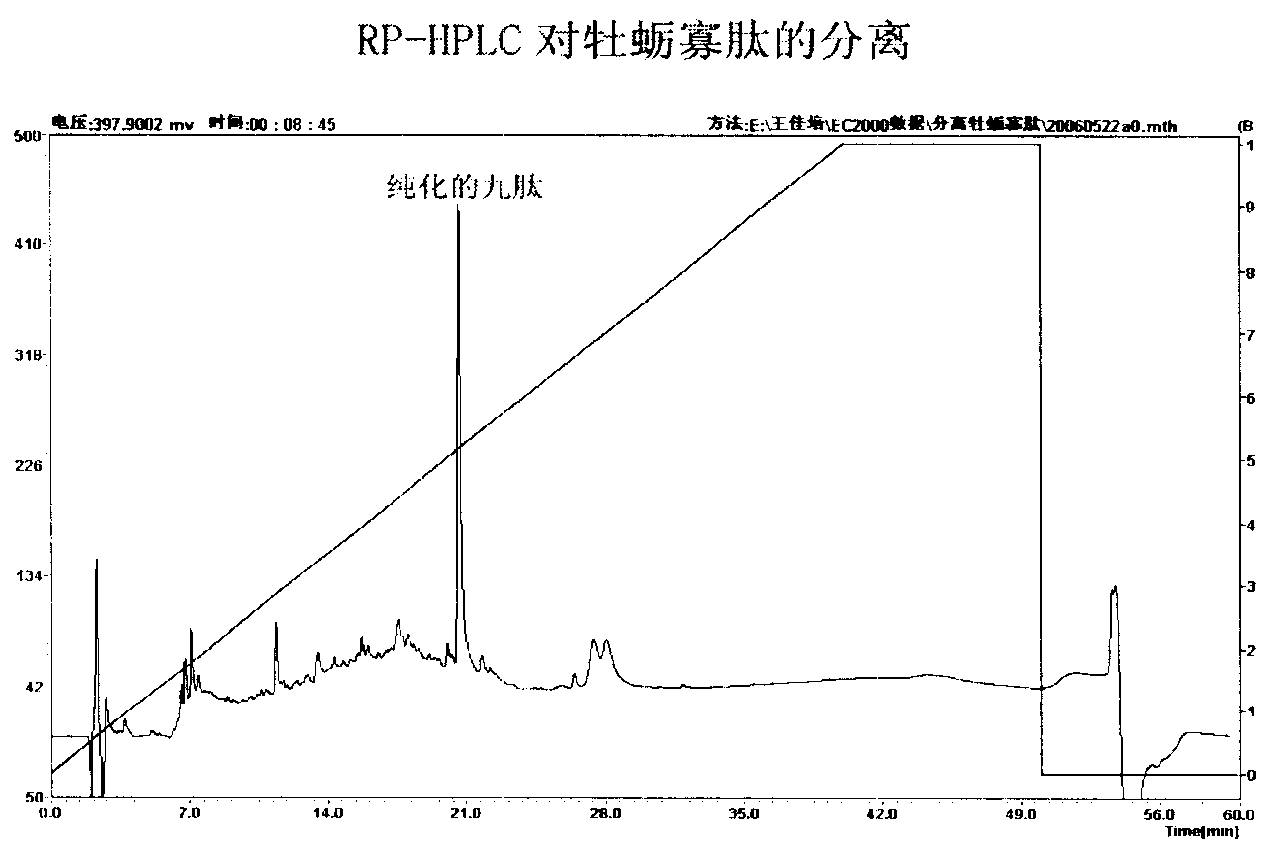
The invention relates to an angiotensin I converting enzyme inhibitor peptide and the analogue thereof or an ACE inhibitor formed from the salt thereof, in particular to an active single peptide separated from oyster protease decomposition product and application thereof; the active peptide is with amino acid sequence of sequence table No.1. The invention adopts enzyme catalysis technology to carry out enzymolysis on the oyster protease, takes advantage of biological molecule separation technology to carry out isolation and purification on the part with stronger ACE inhibition activity; Amino acid sequence analysis is carried out on the single peptide obtained after purification and the inhibition activity and stability of the single peptide against the ACE in vivo and in vitro are studied; the result shows that the single peptide has stronger effect of lowering blood pressure. Therefore, the active single peptide and the ramification thereof or the salt thereof can be used as long-term therapy medicine for patients with elevated blood pressure or can be used as food additive to be made into health foods.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Formulations of quinapril and related ace nhibitors
Stable formulations of ACE-inhibitors compounds such as quinapril can be produced with the use of excipients comprising a basic compound, preferably an alkali-oralkaline earth metal carbonate, and an insoluble alkaline-earth metal carbonate, and an insoluble alkaline-earth metal hydrogen phosphate. Tablets of such formulations have good storage stability, dissolution characteristics, and the formulations are suitable for use in drug combinations.
Owner:ACTAVIS GRP PTC EHF
Selective inhibition of cyclooxygenase 1 in the treatment of diabetic nephropathy
InactiveUS20050143356A1BiocideSalicyclic acid active ingredientsThromboxane ProductionCyclooxygenase
The present invention relates to the use of COX-1 selective inhibitors to treat diabetic nephropathy. In particular, COX-1 inhibitors may be combined with standard insulin replacment therapy, and / or combined with ACE inhbitor therapy. The therapy is, in a specific embodiment, designed to inhibit systemic COX-1 activity, reflected by inhibition of platelet-stimulated thromboxane production, while not inhibiting macrophage PGE2 production.
Owner:VANDERBILT UNIV
Enhanced drug delivery in transdermal systems
InactiveUS20080167280A1Convenient amountGood synergyBiocidePeptide/protein ingredientsActive agentEthyl ester
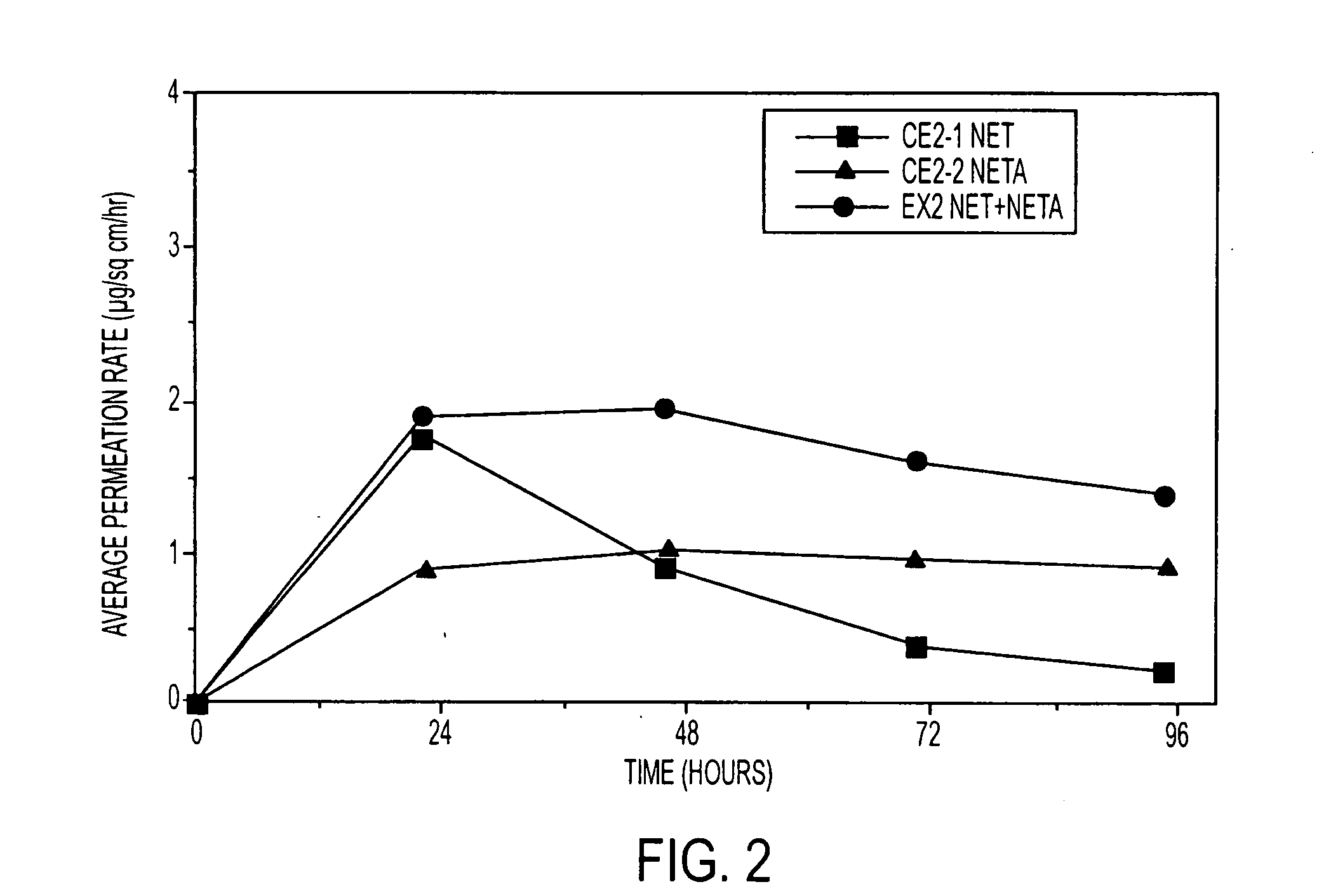
A composition for transdermal administration resulting from an admixture includes: a therapeutically effective amount of a drug that includes a parent drug and a prodrug; and a pharmaceutically acceptable carrier, wherein the parent drug and prodrug are individually present in an amount sufficient for a pharmacological effect. In a preferred embodiment, the admixture includes: a therapeutically effective amount of a pharmaceutically active agent that includes a corresponding steroid and a steroid derivative; and a carrier for the pharmaceutically active agent. The steroid and the corresponding steroid derivative are present in a weight ratio of 10:1 to 1:10 steroid:corresponding steroid derivative. In a preferred embodiment ratio is 6:1 to 1:6. In a preferred embodiment, the corresponding steroid derivative is a steroid ester. In another preferred embodiment, the carrier is a polymer that includes a pressure-sensitive adhesive. In another preferred embodiment, the parent drug is an ACE inhibitor such as ramipril and the prodrug is an ACE inhibitor prodrug such as ramipril ethyl and / or methyl ester.
Owner:NOVEN PHARMA
Process for the preparation of angiotensis converting enzyme (ACE) inhibitors and its use
InactiveUS20040191857A1Potent ACE inhibitory activityPeptide/protein ingredientsImmunoglobulinsSoy flourACE Inhibitor Fetopathy
A process for preparing Angiotensin Converting Enzyme (ACE) inhibitors from glycinin of soy flour and its use as ACE inhibitors.
Owner:COUNCIL OF SCI & IND RES
Enhanced drug delivery in transdermal systems
ActiveUS20080167365A1Convenient amountGood synergyBiocidePeptide/protein ingredientsActive agentEthyl ester
A composition for transdermal administration resulting from an admixture includes: a therapeutically effective amount of a drug that includes a parent drug and a prodrug; and a pharmaceutically acceptable carrier, wherein the parent drug and prodrug are individually present in an amount sufficient for a pharmacological effect. In a preferred embodiment, the admixture includes: a therapeutically effective amount of a pharmaceutically active agent that includes a corresponding steroid and a steroid derivative; and a carrier for the pharmaceutically active agent. The steroid and the corresponding steroid derivative are present in a weight ratio of 10:1 to 1:10 steroid: corresponding steroid derivative. In a preferred embodiment ratio is 6:1 to 1:6. In a preferred embodiment, the corresponding steroid derivative is a steroid ester. In another preferred embodiment, the carrier is a polymer that includes a pressure-sensitive adhesive. In another preferred embodiment, the parent drug is an ACE inhibitor such as ramipril and the prodrug is an ACE inhibitor prodrug such as ramipril ethyl and / or methyl ester.
Owner:NOVEN PHARMA
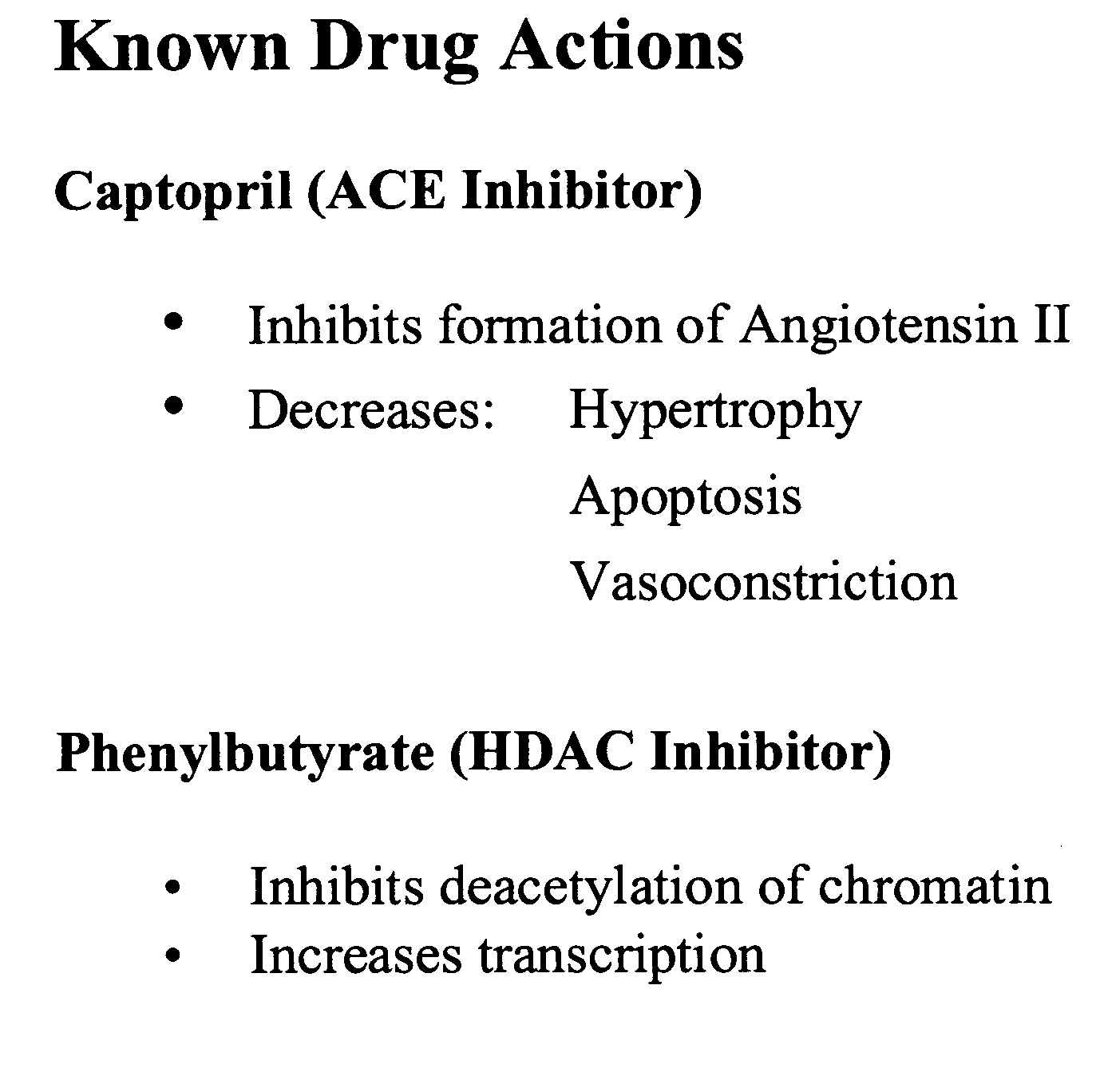
Method and composition for treating heart failure
A method and composition for treating, preventing or ameliorating heart failure, cardiac hypertrophy, and / or myocardial dysfunction includes administering a therapeutically effective amount of a HDAC inhibitor, such as phenylbutyrate, in combination with an ACE inhibitor, such as captopril.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Cottonseed protein polypeptide with high angiotensin converting enzyme (ACE) inhibition activity and preparation method of cottonseed protein polypeptide
InactiveCN107557422AIncrease blood pressure lowering activityPeptide preparation methodsFermentationACE Inhibitor FetopathyACE inhibitor
The invention aims at providing a method for improving the angiotensin converting enzyme (ACE) inhibition activity of cottonseed protein ACE inhibition peptide. The ACE inhibition peptide is preparedby taking cottonseed protein as a raw material and carrying out plastein reaction modification on the cottonseed protein ACE inhibition peptide; the ACE inhibition rate of the prepared product reaches90% or above, so that the activity of the cottonseed protein ACE inhibition peptide is improved; therefore, a new way is provided for the preparation of the natural efficient ACE inhibitor, and a basis is provided for the comprehensive development and utilization of cottonseed resource.
Owner:NORTHWEST UNIVERSITY FOR NATIONALITIES
Preparation method of N-carboxyalkyl dipeptide type angiotensin converting enzyme inhibitor
InactiveCN1429835ASimple and efficient processSuitable for industrialized mass productionDipeptidesCardiovascular disorderDipeptideDepressant
A process for preparing N-carboxyalkyl dipeptide type angiotensin converting enzyme (ACE) depressant (such as enalapril maleate, ramipril, etc) includes such steps as preparing N-carboxylic acid anhydride from bis (trichloromethyl) carbonate, and coupling with relative alpha-amino acid.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com