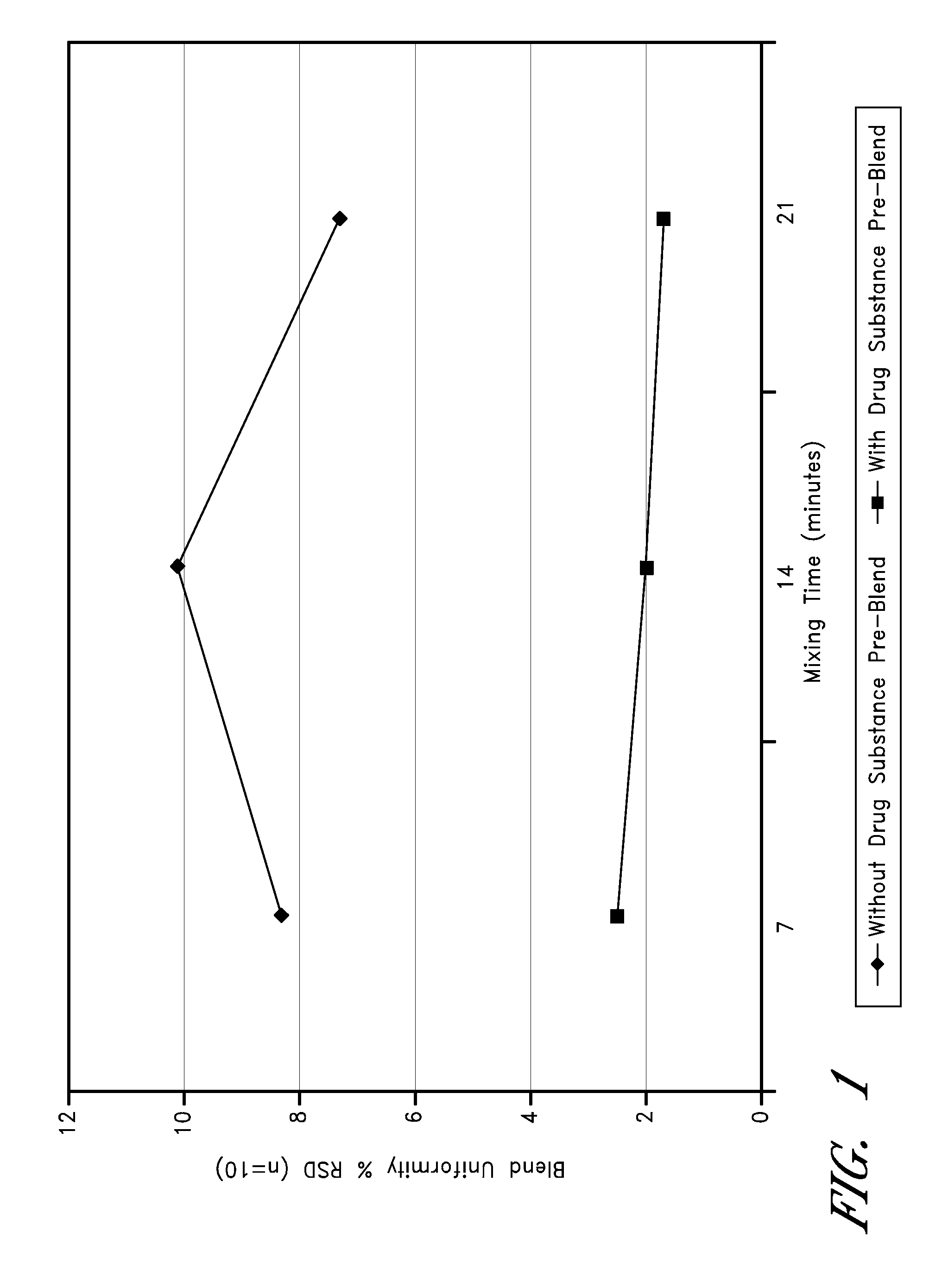
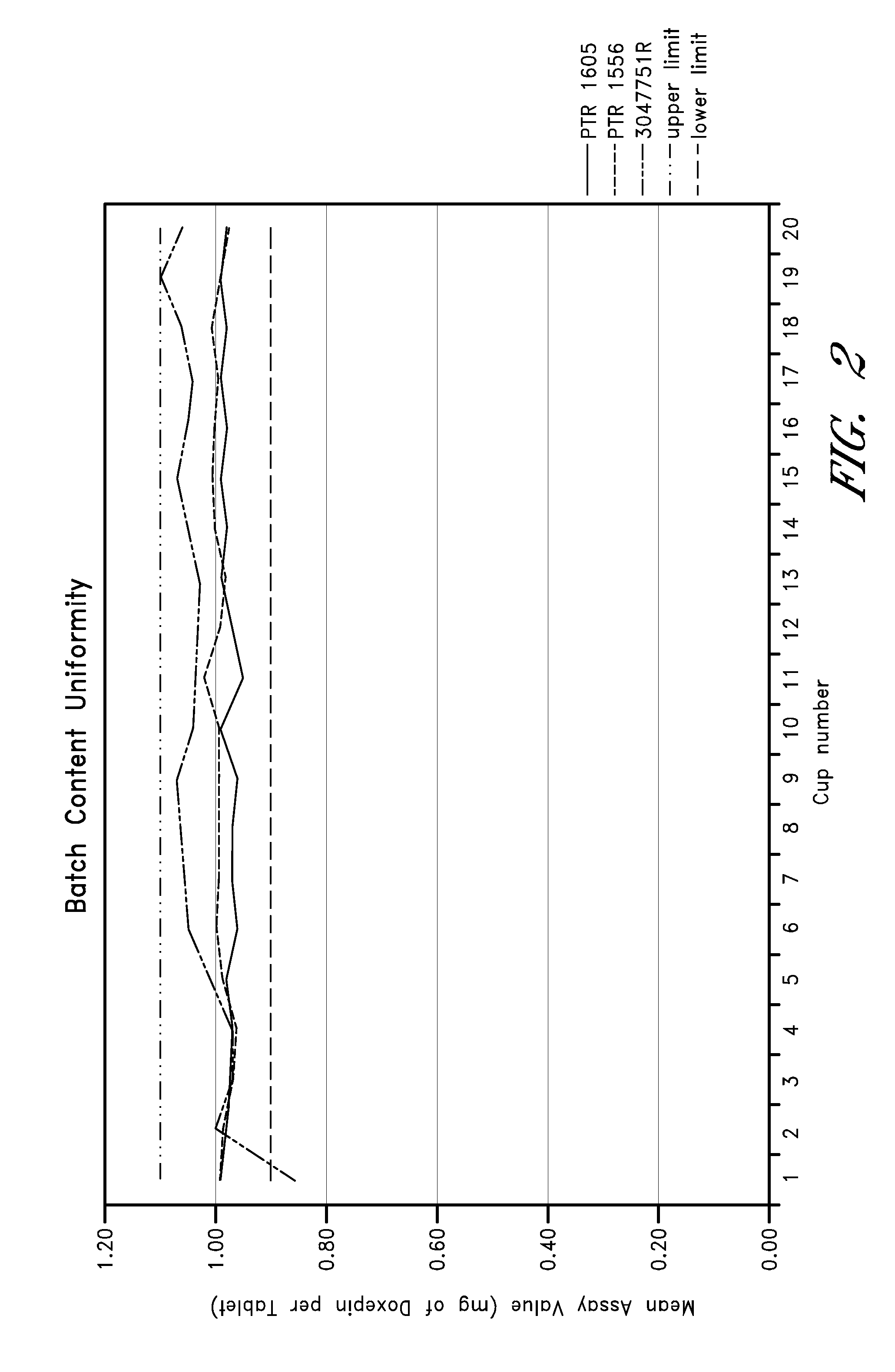
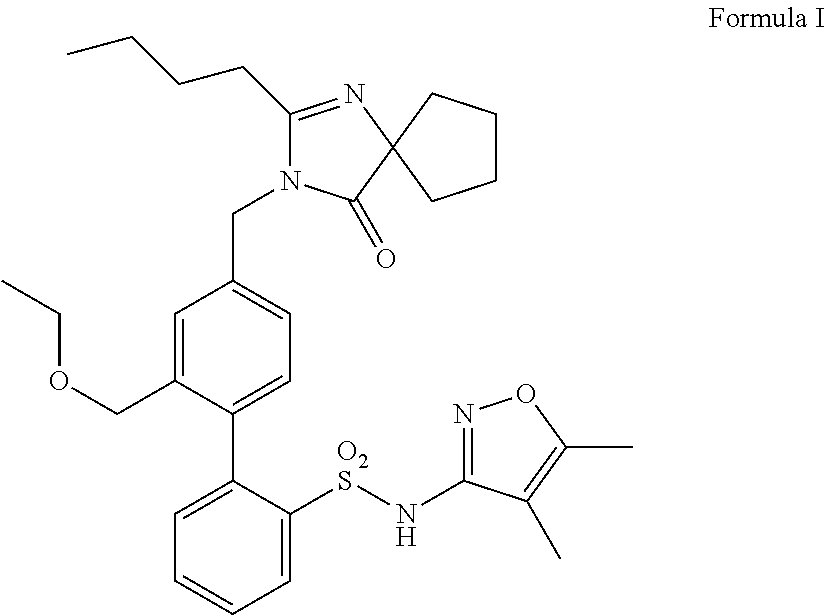
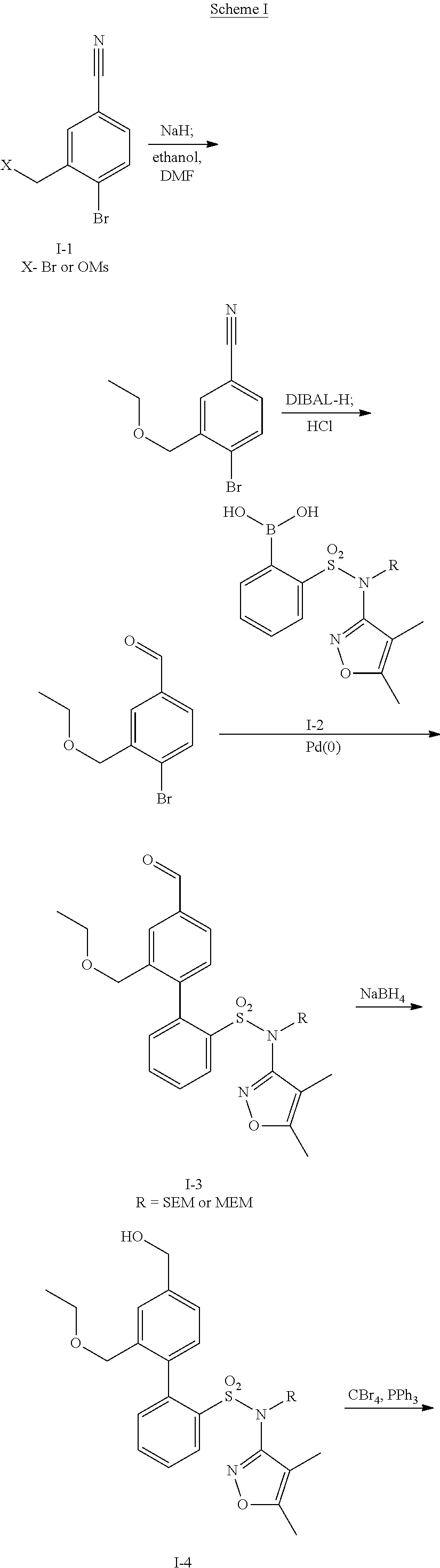
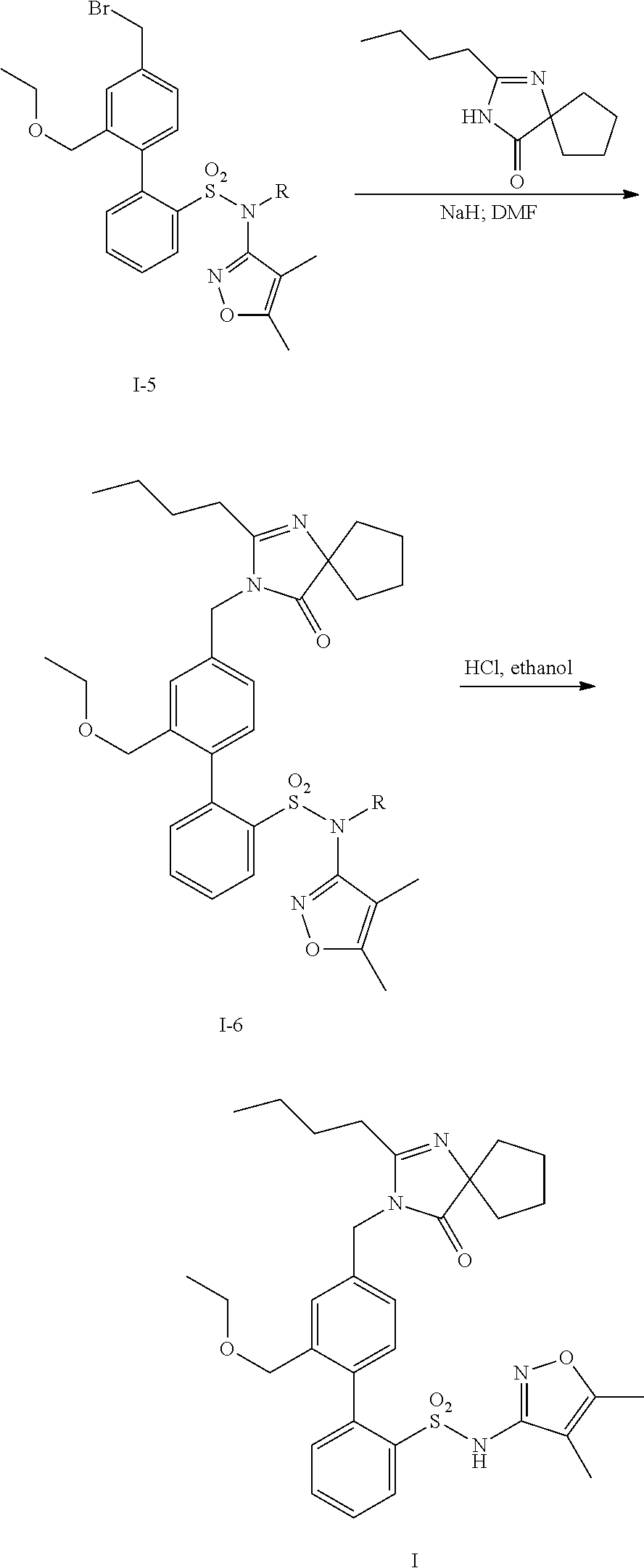
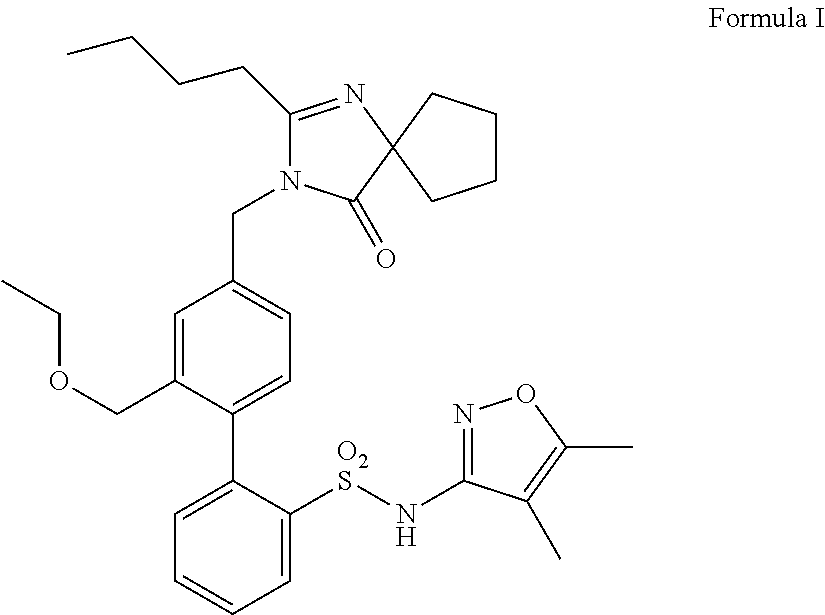
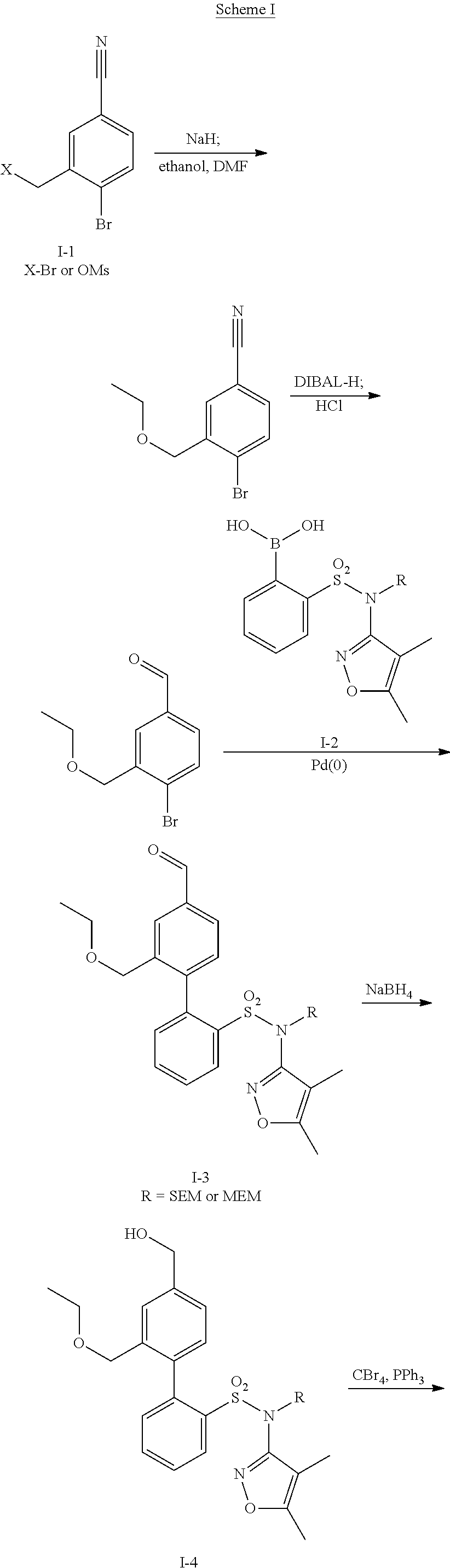
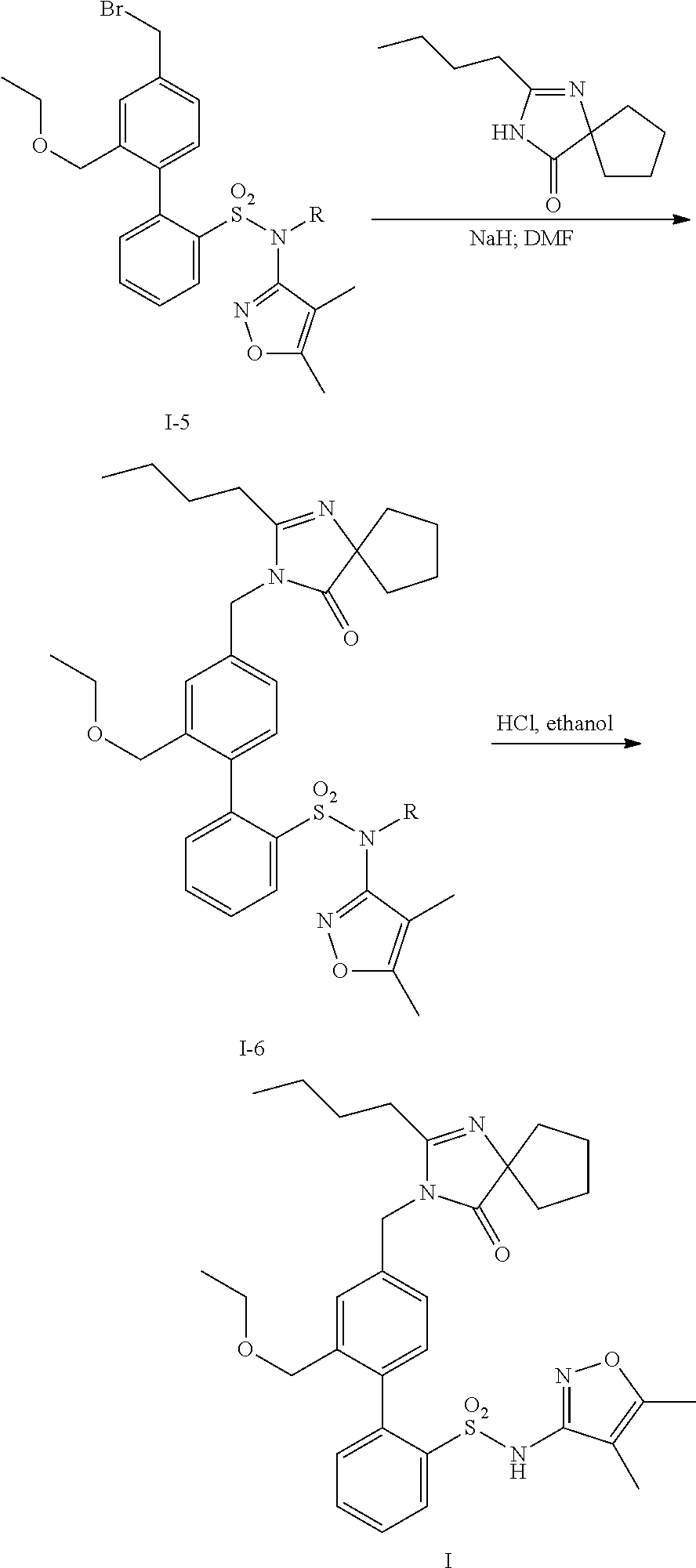
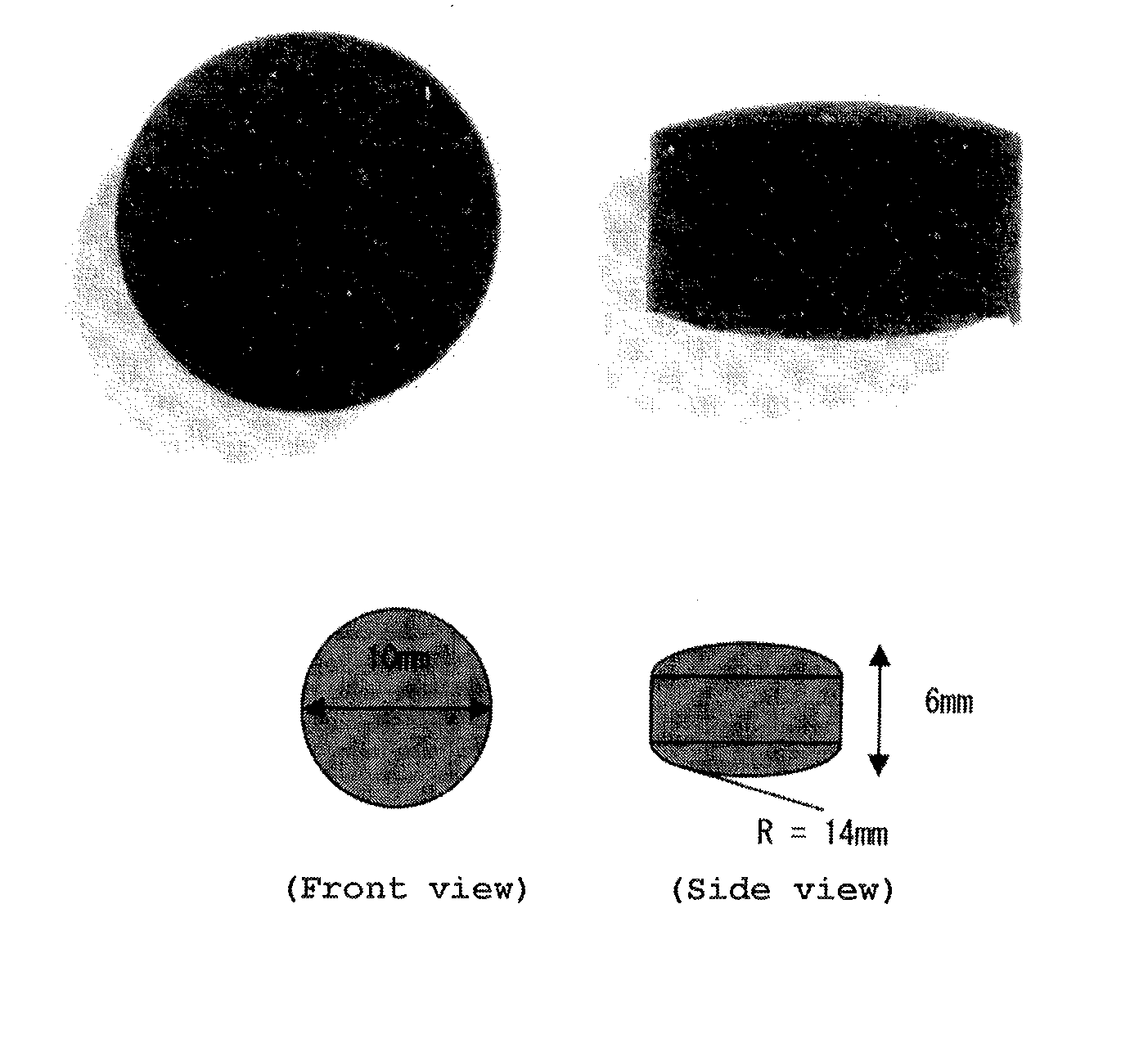
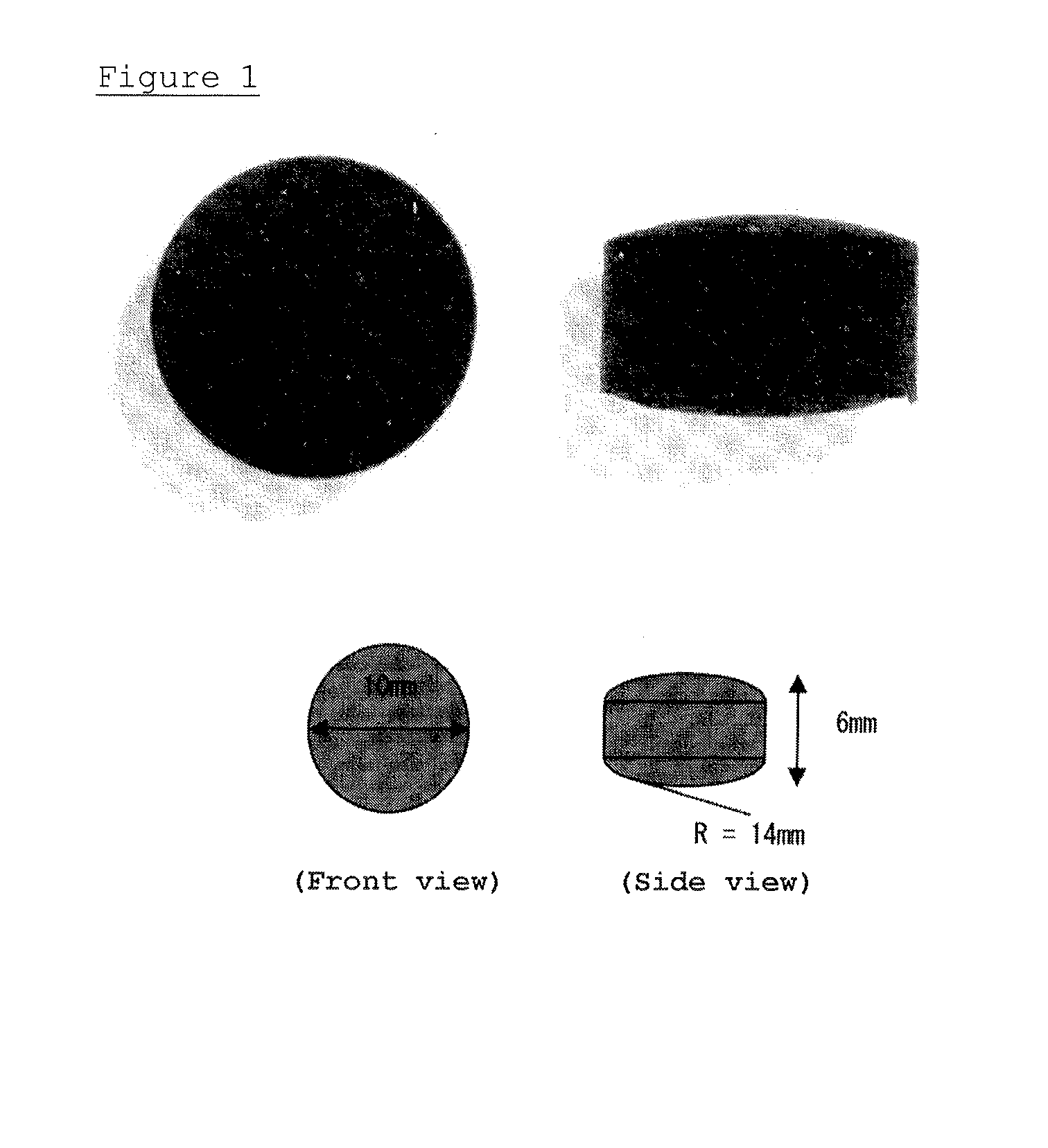
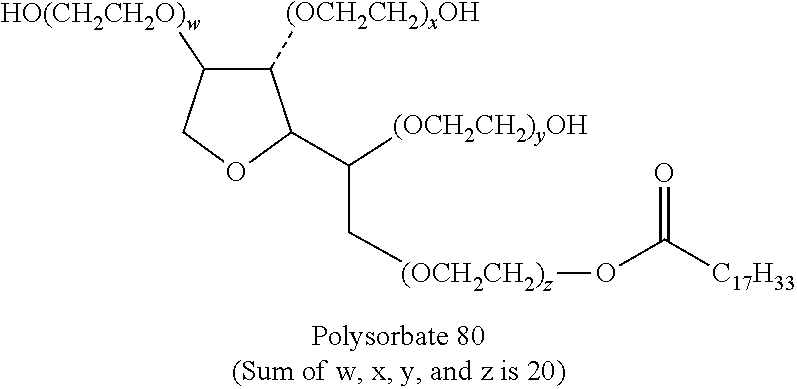
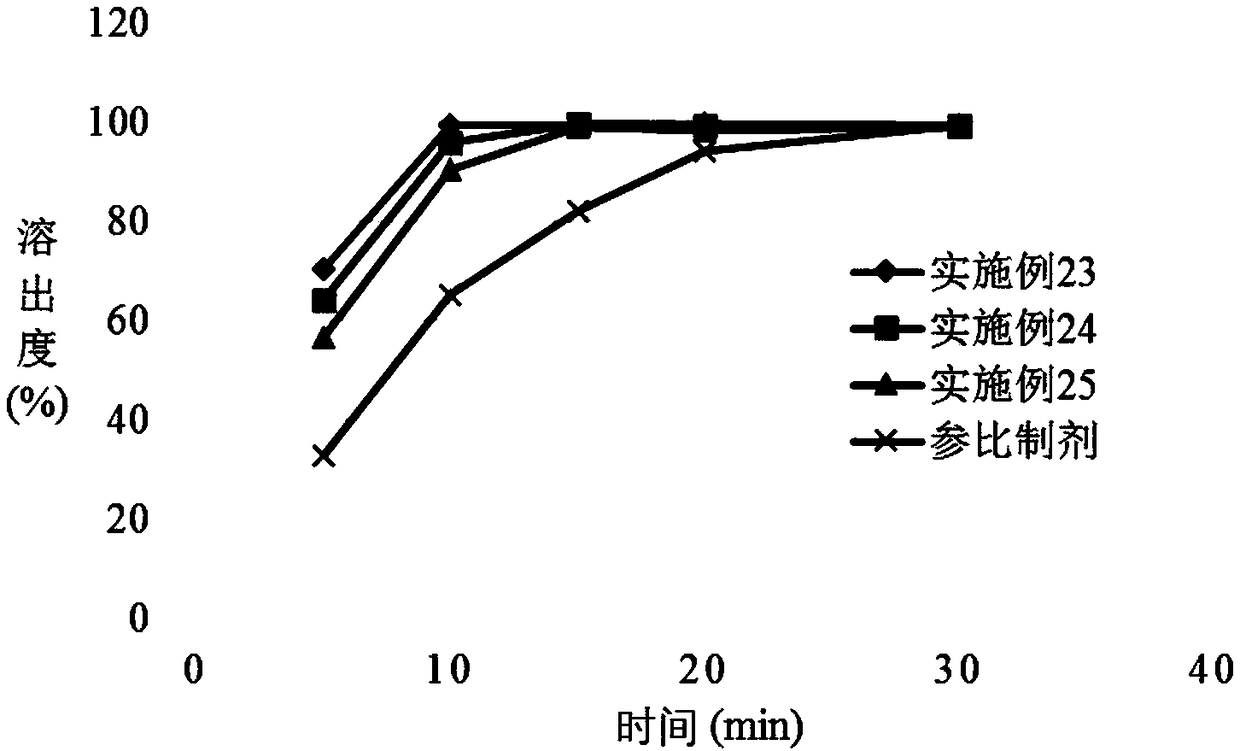
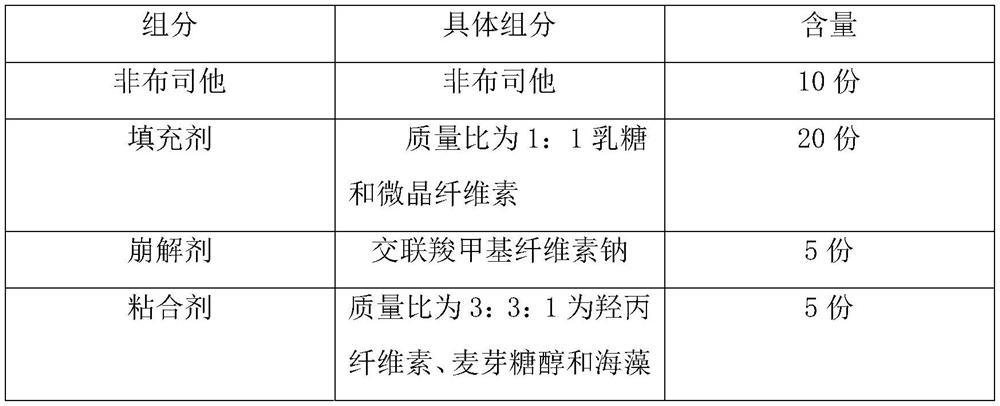
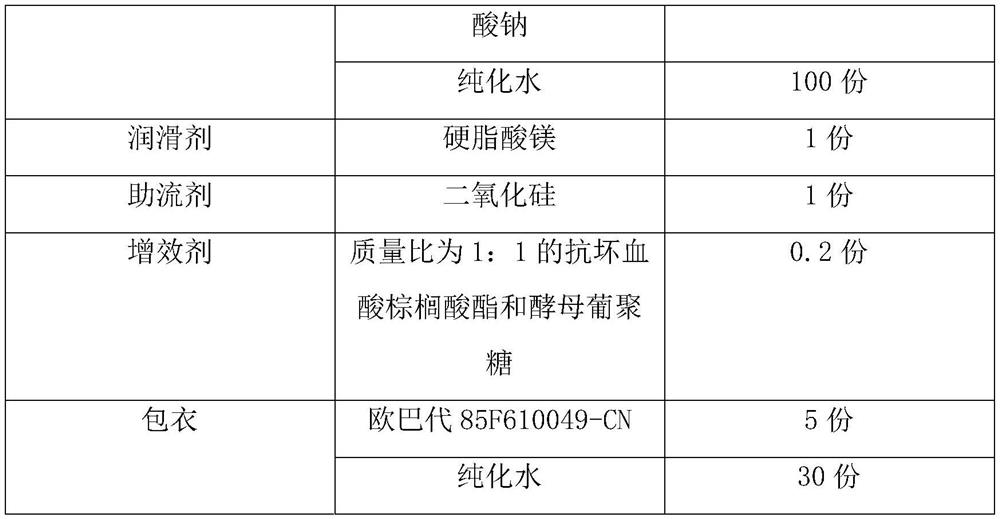
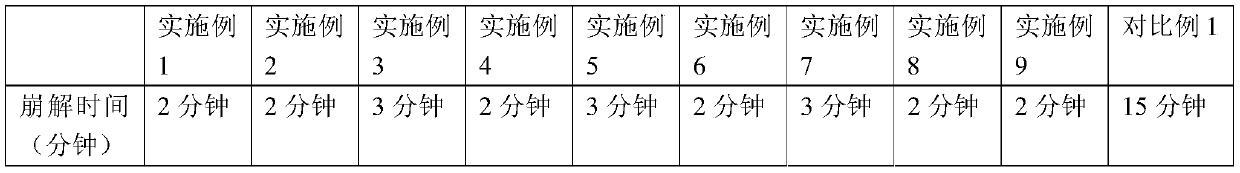
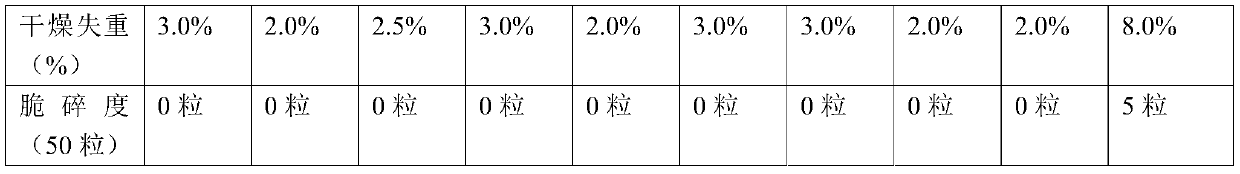
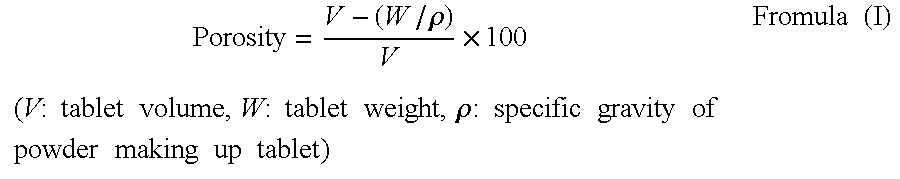Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40results about How to "Improves friability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Quick-disintegrating tablet in buccal cavity and manufacturing method thereof
InactiveUS6872405B2Improves friabilityHigh strengthPharmaceutical non-active ingredientsDrageesPharmaceutical SubstancesOrganic chemistry
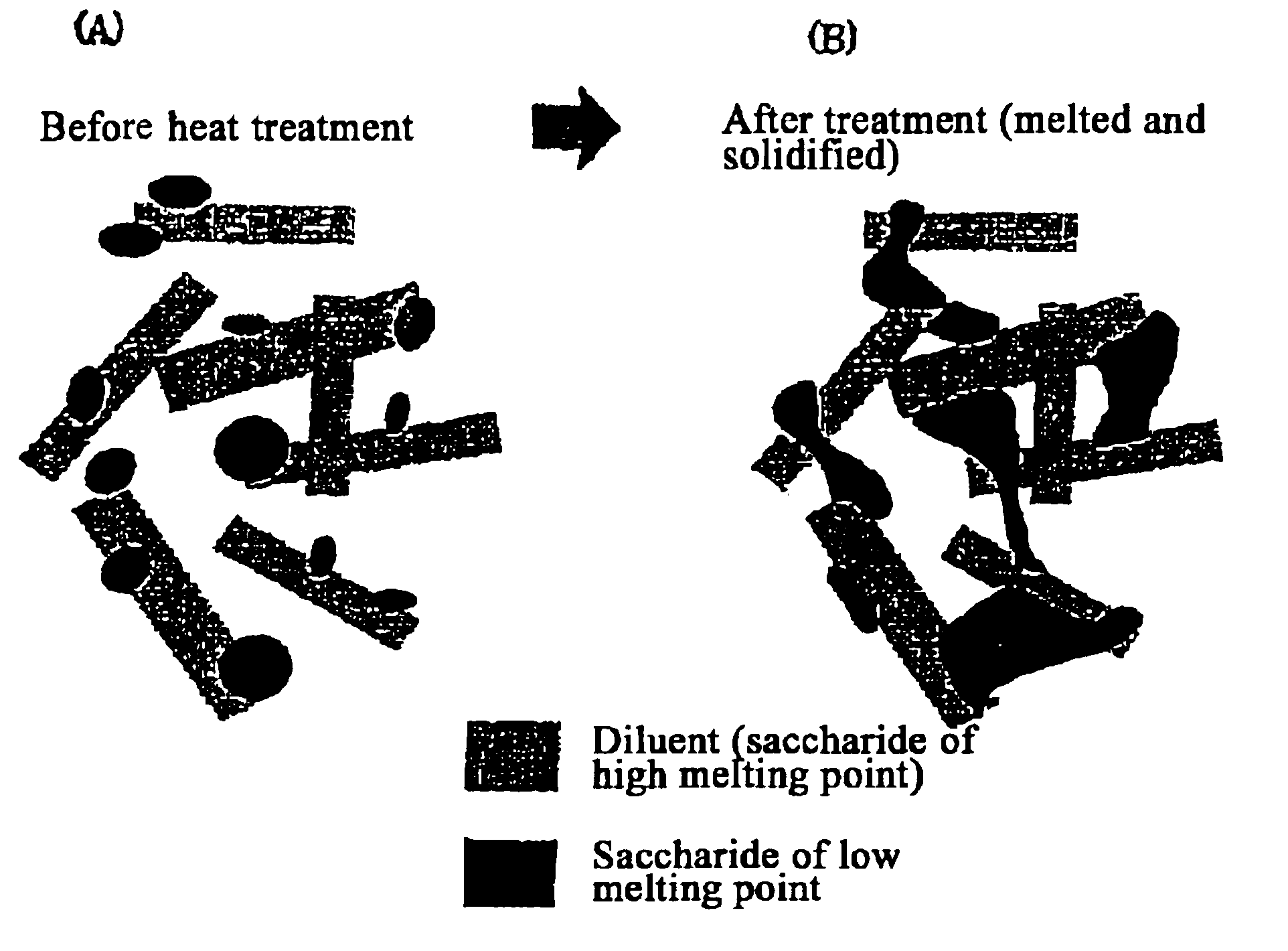
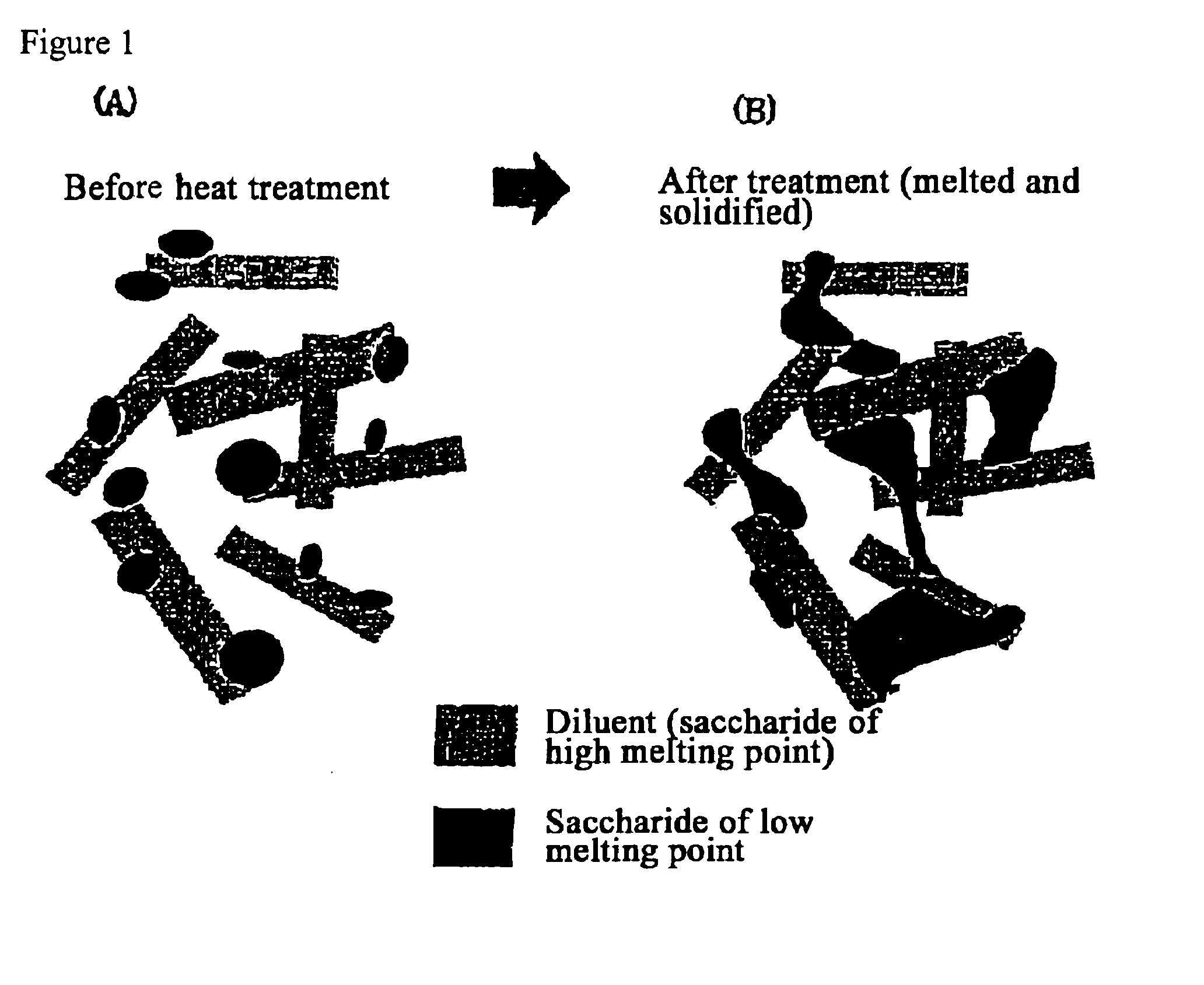
The present invention relates to a quick-disintegrating tablet in the buccal cavity comprising a drug, a diluent, and a saccharide with a relatively lower melting point than the drug and the diluent, which is obtained by uniformly mixing the saccharide with a low melting point in the tablet so that a bridge will be formed between said drug and / or said diluent particles by the product of melting and then solidification of this saccharide with a low melting point. Moreover, the present invention relates to a method of manufacturing a quick-disintegrating tablet in the buccal cavity comprising a drug, a diluent and a saccharide with a relatively lower melting point than the drug and the diluent, which comprises (a) the process whereby tablet starting materials including a drug, a diluent, and a saccharide with a relatively lower melting point than the drug and the diluent are molded under the low pressure necessary for retaining the shape of a tablet, (b) the process whereby the molded product obtained in process (a) is heated to at least the temperature at which this saccharide with a low melting point will melt, and (c) the process whereby the molded product obtained in process (b) is cooled to at least the temperature at which the molten saccharide with a low melting point solidifies. The present invention presents a quick-disintegrating tablet in the buccal cavity that can be used for practical purposes in that it has almost the same properties as conventional oral pharmaceutical tablets, that is, it has sufficient tablet strength that it can be used with automatic unit dosing machines, and it is produced by conventional tableting machines, and a manufacturing method thereof. Moreover, the present invention presents a quick-disintegrating tablet in the buccal cavity which, in comparison to conventional quick-disintegrating tablets in the buccal cavity, has increased tablet strength and an improved friability without prolonging the disintegration time in the buccal cavity, and a manufacturing method thereof.
Owner:ASTELLAS PHARMA INC
Low-dose doxepin formulations and methods of making and using the same
InactiveUS20090074862A1Dissolve fastEasy to swallowOrganic active ingredientsBiocideMedicinePharmaceutical formulation
The invention disclosed herein generally relates to low-dose oral doxepin pharmaceutical formulations and the use of these formulations to promote sleep.
Owner:SOMAXON PHARMA
Oral formulations of diphenylsulfonamide endothelin and angiotensin ii receptor agonists to treat elevated blood pressure and diabetic nephropathy
InactiveUS20140142149A1Improves friabilityEasy to compressBiocideSenses disorderEndothelin receptor antagonistAgonist
Methods of administering and pharmaceutical compositions of a biphenyl sulfonamide compound which is a dual angiotensin and endothelin receptor antagonist are disclosed for treating diseases.
Owner:LIGAND PHARMA INC
Pharmaceutical composition with sodium lauryl sulfate as an extra-granular absorption/compression enhancer and the process to make the same
InactiveUS20050051922A1Improve compressibilityImprove hardnessOrganic active ingredientsWood working apparatusDosage formMedicine
A process for preparing a pharmaceutical dosage form or core wherein an absorption / compression agent is introduced into the formulation extra-granularly, and a pharmaceutical tablet prepared by said process.
Owner:ANDRX LABS
Cellulose powder
ActiveUS20070028801A1Not limitedHigh hardnessSugar productsPharmaceutical non-active ingredientsPolymer sciencePolyethylene glycol
A cellulose powder which has an average degree of polymerization of 150 to 450, an average particle diameter of 30 to 250 μm, an apparent specific volume exceeding 7 cm3 / g, and a retention of polyethylene glycol having a molecular weight of 400 to 190% or higher.
Owner:ASAHI KASEI CHEM CORP
Oral formulations of diphenylsulfonamide endothelin and angiotensin ii receptor agonists to treat elevated blood pressure and diabetic nephropathy
ActiveUS20150164865A1Lower systolic blood pressureLower diastolic blood pressureBiocideSenses disorderDiseaseAngiotensin II receptor type 1
Methods of administering and pharmaceutical compositions of a biphenyl sulfonamide compound which is a dual angiotensin and endothelin receptor antagonist are disclosed for treating diseases.
Owner:LIGAND PHARMA INC
Cellulose powder
ActiveUS7514552B2High hardnessImproves friabilitySugar productsPharmaceutical non-active ingredientsPolymer sciencePolyethylene glycol
A cellulose powder which has an average degree of polymerization of 150 to 450, an average particle diameter of 30 to 250 μm, an apparent specific volume exceeding 7 cm3 / g, and a retention of polyethylene glycol having a molecular weight of 400 to 190% or higher.
Owner:ASAHI KASEI CHEM CORP
Method for reducing slag in biomass combustion
ActiveUS20120312206A1Improve brittlenessReduce fouling rateSolid fuel pretreatmentSolid fuelsPtru catalystCapital equipment
Biomass is quickly becoming an important feedstock for energy generation in power plants. Due to their composition and nature, certain biomass fuels contribute to slagging, fouling, and corrosion. This invention provides a novel method of reducing or suppressing slag deposition and / or cleaning deposited slag in energy production processes in which potassium-containing solid fuels are combusted. Besides acting as a slag suppressant, further advantages of this invention are that the additive has no detrimental side-effects on capital equipment, increases slag friability, decreases slag fouling rate, reduces heat transfer corrosion as well as increasing the lifetime of the selective catalytic reduction catalyst.
Owner:ECOLAB USA INC
Tablet-formed pharmaceutical composition for oral administration and method for producing same
InactiveUS20130344147A1Improved feel of ingestionLower the volumeBiocideNervous disorderSolubilityBULK ACTIVE INGREDIENT
The object of the present invention is to provide an adsorbent for oral administration, which can: obviate sandy texture of granules-formed adsorbent for oral administration, reduce the volume administered compared to encapsulated adsorbent for oral administration, and be easy to take.The object of the present invention is solved by a tablet-formed pharmaceutical composition consisting of 65% or more by weight of particulate substance as an active ingredient and one or more additives, characterized in that:(a) the particulate substance does not exhibit water solubility and swelling property in water, has a distortion rate of 2% or less when subjected to a pressure of 2 MPa, and has a fracture strength of 5 MPa or more,(b) the tablet-formed pharmaceutical composition comprises one or more particle-formulating additives wherein the amount of the particle-formulating additives is 1% or more by weight in total with respect to the weight of the tablet-formed pharmaceutical composition,and a thin layer of the particle-formulating additive can be formed when 0.5 mL of solution or dispersion liquid of the particle-formulating additive of 1% by weight is dropped on a plane surface of fluorine resin and heat-dried.
Owner:KUREHA KAGAKU KOGYO KK
Plant colonic-coated hollow capsule
InactiveCN102526750AReduce heavy metal contentSimple processCapsule deliveryMacromolecular non-active ingredientsMedicinePlasticizer
The invention discloses a plant colonic-coated hollow capsule, which is prepared by treating 80-95 percent of zein powder and 5-20 percent of a plasticizer serving as raw materials in a more than or equal to 80 percent ethanol solution or a more than or equal to 70 percent acetone solution with a known process and equipment. The quality of the capsule is accordant with the specifications in the colonic-coated hollow capsule standard of the Chinese Pharmacopoeia of the version 2010, the capsule is not disintegrated in an enteric process, is disintegrated on a colonic part within one hour, and has three years of shelf life.
Owner:ANHUI HUANGSHAN CAPSULE CO LTD
Toughening forming auxiliary for preparing hard capsule taking starch as matrix and application
ActiveCN103520729AImproves friabilityAvoid disintegration difficultiesPharmaceutical non-active ingredientsCapsule deliverySolubilityCarrageenan
The invention relates to a toughening forming auxiliary of a hard capsule taking starch as a matrix for food, healthcare products, medicines and the like, and a starch hard capsule for the food, healthcare products, medicines and the like, prepared from the toughening forming auxiliary of a hard capsule taking starch as a matrix in combination with a starch matrix. The toughening forming auxiliary for preparing a hard capsule taking starch as a matrix consists of 5-50wt% of carrageenan and 50-95wt% of ghatti gum. According to the toughening forming auxiliary provided by the invention, the toughening forming auxiliary is added into the starch matrix, so that the glue-dip forming is assisted by the gelation effect of the carrageenan, and a final capsule shell is toughened by the net structure generated in gelation and a natural polysaccharide carbohydrate chain structure in the ghatti gum; meanwhile, the disintegration of the hard capsule can be effectively promoted by the good solubility and emulsifiability of the ghatti gum, thus the prepared starch hard capsule has excellent flexibility and can be quickly disintegrated.
Owner:北京崇尚科技开发有限公司 +1
Tablets with improved friability
ActiveUS20140315719A1Improve brittlenessImprove performanceCosmetic preparationsBiocideSolubilityActive agent
Tablets are prepared with friability reducing agents to yield tablets that are more resistant to breakage or crumbling, but with satisfactory hardness. The friability reducing agents include low molecular weight polyethylene glycol as well as similar agents exhibiting at least three percent (3%) hydroxide moieties and a water solubility of at least eighty percent (80%) (w / w %) in room temperature water. The tablets may comprise an active agent and excipient of almost any type, and about 0.1-about 0.5% by weight friability reducing agent. They exhibit a hardness of at least eighty percent (80%) of the same tablet prepared without the friability reducing agent.
Owner:TOWER LAB
Method for preparing voglibose particles
InactiveCN108635332ASimple production processShorten production timeOrganic active ingredientsMetabolism disorderAdhesiveDiluent
The invention provides a method for preparing voglibose particles. The particles are prepared through a one-step prilling process,the friability is low,and the roundness is good. Voglibose can be dissolved out fast,the content uniformity is good,and the voglibose particles are suitable for being further prepared into capsules,tables (including dispersible tablets and orally disintegrating tablets)and granules. According to the preparation idea,the voglibose is added into a polar solvent containing a water-soluble adhesive,and the mixture is sprayed into a one-step pelletizer containing a diluent and a disintegrating agent in a top spraying prilling mode. The preparation method is easy to operate,the labor intensity is reduced,and the preparation method is suitable for large-scale production.
Owner:SHANDONG XINHUA PHARMA CO LTD
Tablets having improved tabletting characteristics and process for producing the same
InactiveUS20050089557A1Satisfy the tasteExcellent in compressing characteristicWood working apparatusPharmaceutical non-active ingredientsMedicineWater soluble
It is intended to provide tablets which have a favorable texture in taking and excellent compressing characteristics, friability and mechanical strength. Tablets which are produced by blending a powdery or granular mixture comprising (1) an effective amount of a pharmacologically active substance and (2) 50% by weight or more of a water-soluble filler based on the powdery or granular mixture with (3) a compressing characteristic improving agent containing moisture at least in an amount corresponding to the equilibrated moisture content at 25° C. under a humidity of 12% and having an average particle size of 100 μm or less, at a ratio of from 1 to 50% by weight based on the above mixture, and then compressing the resultant mixture.
Owner:OTSUKA PHARM CO LTD
Process for the preparation of robust formulations of valacyclovir hydrochloride tablets
InactiveUS20060147519A1Improves friabilityHigh hardnessBiocidePill deliveryPharmacologyValacyclovir Hydrochloride
The invention relates to robust formulations of valacyclovir hydrochloride tablets. A robust tablet includes a hydrated form of valacyclovir hydrochloride having a water of hydration content of more than approximately 3% w / w and a particle size of less than approximately 355 μm.
Owner:RANBAXY LAB LTD
Lipoic acid tablets with few types and small dose of accessories and preparation method thereof
The invention provides lipoic acid tablets with few types and small dose of accessories and a preparation method thereof. The preparation method comprises the steps of weighing filler and an adhesive, and preparing accessory tablets by a conventional tabletting process; putting the prepared accessory tablets into an efficient coating pan; weighing lipoic acid and sieving; spraying the sieved lipoic acid powder to coat the accessory tablets layer by layer by a spray gun by taking a sodium carboxymethylcellulose solution as a spray; drying and airing in a thermostatic chamber to obtain the lipoic acid tablets. The content of main drug is high, and the compliance is improved; by adopting few types and small dose of accessories, the adverse effect of the accessories is reduced, and the cost of raw materials is also reduced; the temperature of the operation process is strictly controlled, and the sticking phenomenon generated in lipoic acid tabletting is avoided by pressing the accessory tablets not containing lipoic acid; by adopting the spraying-coating process, color spots are avoided; moreover, the content uniformity, friability, chroma uniformity and stability of the lipoic acid tablets are all remarkably improved.
Owner:南京海鲸药业股份有限公司
Method for preparing hippophae rhamnoides seed micro powder capsules
InactiveCN101843311ADelay the aging of the bodyPromote growth and developmentFood shapingFood preparationNutritional compositionHippophae rhamnoides
The invention relates to a method for preparing hippophae rhamnoides seed micro powder capsules. The method for preparing the hippophae rhamnoides seed micro powder capsules comprises the following steps of: performing vacuum drying on hippophae rhamnoides seeds; adding konjac glucomannan-acrylic graft copolymer (KAC) which serves as an anticaking agent into the hippophae rhamnoides seeds; performing low-temperature superfine pulverization on the mixture; and encapsulating the mixture, and performing irradiation sterilization at the temperature of 600 DEG C. The method has the advantages of making full use of the hippophae rhamnoides seeds, generating no by-products, keeping the primitivity of the hippophae rhamnoides seeds, avoiding destruction to heat-sensitive and unstable active ingredients, reserving the effective active ingredients and the original various nutritional ingredients in the hippophae rhamnoides seeds to the utmost extent, and being favorable for absorption. An adopted anti-caking agent can solve the problems that the superfine powder is aggregated easily and is difficult to disperse due to superfine particle sizes, and if the added content of the anti-caking agent is less, the anti-caking performance is higher so that the purity of the capsule hippophae rhamnoides seed powder reaches over 99.6 percent. Besides, the method for preparing the hippophae rhamnoides seed micro powder capsules is simple, has less equipment investments, a low production cost and no three wastes outputs, is suitable for application in hippophae rhamnoides producing areas with underdeveloped economy, and is environment-friendly industrialized technology.
Owner:ENZYME ENG INST SHAANXI PROVINCE ACAD OF SCI
Plant hollow capsule and preparation method thereof
ActiveCN109846850AFast disintegrationSpeed up disintegrationPharmaceutical non-active ingredientsCapsule deliveryPlasticizerMoisture absorption
The invention provides a plant hollow capsule and a preparation method thereof, and belongs to the technical field of hollow capsule production. The plant hollow capsule is prepared from modified hydroxypropyl methylcellulose, gel, coagulant aid, plasticizer, surfactant and the balance of water, wherein the modified hydroxypropyl methylcellulose is prepared by adding a proper amount of microcrystalline cellulose into hydroxypropyl methylcellulose and performing uniform mixing. The plant hollow capsule has the beneficial effects that the capsule as certain moisturizing property besides the molding performance of a hydroxypropyl methylcellulose gel solution can be kept, moisture loss due to dryness and moisture absorption can be avoided in the storing process, shrinkage or expansion of a capsule shell can be avoided, occurrence of powder leakage can be effectively avoided, and the disintegrating speed of a capsule can be accelerated; the ductility and plasticization of the capsule can beimproved by properly adding the plasticizer, uniform wall thickness of the capsule can be guaranteed by combining with the gel; the disintegrating speed can be accelerated by properly adding the surfactant with the microcrystalline cellulose; and the friability can be effectively improved by properly adding the gel and the coagulant aid with the plasticizer.
Owner:CHONGQING HENGSHENG MEDICINAL CAPSULE
Quick dissolve compositions and tablets based thereon
InactiveUS20120082729A1Easy to processEliminate needBiocidePowder deliveryInorganic saltsMicrosphere
Owner:VALEANT INT BERMUDA
Hydroxypropyl methyl cellulose plant empty capsule
ActiveCN107296801AFast disintegrationLow loss on dryingPharmaceutical non-active ingredientsCapsule deliveryAlcoholOrganic solvent
The invention discloses a hydroxypropyl methyl cellulose plant empty capsule which is prepared through the following method: dissolving hydroxypropyl methyl cellulose in a mixed solvent of an organic solvent and water, preparing a hydroxypropyl methyl cellulose solution with the mass percentage of 10 to 33 percent, and making capsules by using the hydroxypropyl methyl cellulose solution, wherein the organic solvent is ethyl alcohol and / or acetone. The hydroxypropyl methyl cellulose plant empty capsule has the advantages that the capsule is disintegrated fast, is not brittle and has extremely low weight loss on drying; furthermore, according to the preparation method of the capsule, no heating is needed, so that energy consumption is reduced; no gelatinizer is added, so that influence on disintegration due to the gelatinizer is avoided; the capsule is cured and molded only through volatilization of the organic solvent, so that the hydroxypropyl methyl cellulose plant empty capsule is suitable for filling of certain medicines having special requirements.
Owner:ANHUI HUANGSHAN CAPSULE CO LTD
Chitosan tablets and preparation method thereof
ActiveCN105496982AHigh hardnessImproves friabilityOrganic active ingredientsMetabolism disorderPolythylene glycolMaterials science
The invention belongs to the technical field of pharmaceutical preparations and particularly relates to chitosan tablets and a preparation method thereof. The chitosan tablets comprise tablet cores and coating layers, wherein the tablet cores comprise components in parts by weight as follows: 70-76 parts of chitosan, 3-6 parts of crospovidone, 7-12 parts of calcium hydrogen phosphate, 8-12 parts of microcrystalline cellulose, 1-3 parts of aerosil and 4-6 parts of sodium carboxymethyl cellulose; the coating layers are prepared from raw materials in parts by weight as follows: 4-6 parts of zein, 7-9 parts of hydroxypropyl methoxyl cellulose, 3-5 parts of polyethylene glycol and 80-84 parts of ethyl alcohol with the volume fraction of 30%-40%. The chitosan tablets have bright and clean appearance and are stable in physical and chemical properties and long in shelf life, and the disintegration time, hardness, friability and the like agree with related regulations of the Chinese pharmacopoeia.
Owner:GUANGDONG PHARMA UNIV
Acerola cherry tabletted candies and preparation method thereof
PendingCN113973964AImprove fluidity and compressibilityImproves hardness and friabilityConfectionerySweetmeatsSucroseMagnesium stearate
The present invention relates to acerola cherry tabletted candies and a preparation method thereof. The candies consist of cherry powder, an excipient, a sweetening agent, an adhesive and a lubricant; the acerola cherry tabletted candies specifically comprise 25-33.35% of acerola cherry powder, 28.6-29.3% of sorbitol, 15-25% of microcrystalline cellulose, 1-8% of D-mannitol, 0.1-1% of stevioside, 10.65-20% of blueberry powder, 0.01-0.05% of sucralose and 0.3-1% of magnesium stearate. The tabletted candies are obtained by adopting the technologies of sieving, weighing, premixing, total mixing and direct pressing. The acerola cherry tabletted candies are simple in preparation process, stable in hardness, friability and taste, have blueberry flavor, are moderate in sourness and sweetness and convenient to eat, and have the beneficial effects of enhancing immunity, improving skin state, whitening, regulating intestinal flora and the like.
Owner:富诺健康股份有限公司
Lipoic acid tablets with few types and small dose of accessories and preparation method thereof
The invention provides lipoic acid tablets with few types and small dose of accessories and a preparation method thereof. The preparation method comprises the steps of weighing filler and an adhesive, and preparing accessory tablets by a conventional tabletting process; putting the prepared accessory tablets into an efficient coating pan; weighing lipoic acid and sieving; spraying the sieved lipoic acid powder to coat the accessory tablets layer by layer by a spray gun by taking a sodium carboxymethylcellulose solution as a spray; drying and airing in a thermostatic chamber to obtain the lipoic acid tablets. The content of main drug is high, and the compliance is improved; by adopting few types and small dose of accessories, the adverse effect of the accessories is reduced, and the cost of raw materials is also reduced; the temperature of the operation process is strictly controlled, and the sticking phenomenon generated in lipoic acid tabletting is avoided by pressing the accessory tablets not containing lipoic acid; by adopting the spraying-coating process, color spots are avoided; moreover, the content uniformity, friability, chroma uniformity and stability of the lipoic acid tablets are all remarkably improved.
Owner:南京海鲸药业股份有限公司
Toughening and molding aids for the preparation of starch-based hard capsules and their use
ActiveCN103520729BImproves friabilityAvoid disintegration difficultiesPharmaceutical non-active ingredientsCapsule deliverySolubilityHard Capsule
The invention relates to a toughening forming auxiliary of a hard capsule taking starch as a matrix for food, healthcare products, medicines and the like, and a starch hard capsule for the food, healthcare products, medicines and the like, prepared from the toughening forming auxiliary of a hard capsule taking starch as a matrix in combination with a starch matrix. The toughening forming auxiliary for preparing a hard capsule taking starch as a matrix consists of 5-50wt% of carrageenan and 50-95wt% of ghatti gum. According to the toughening forming auxiliary provided by the invention, the toughening forming auxiliary is added into the starch matrix, so that the glue-dip forming is assisted by the gelation effect of the carrageenan, and a final capsule shell is toughened by the net structure generated in gelation and a natural polysaccharide carbohydrate chain structure in the ghatti gum; meanwhile, the disintegration of the hard capsule can be effectively promoted by the good solubility and emulsifiability of the ghatti gum, thus the prepared starch hard capsule has excellent flexibility and can be quickly disintegrated.
Owner:北京崇尚科技开发有限公司 +1
Thyroid tablets produced by direct whole-powder tabletting and preparation process thereof
ActiveCN109464411AHigh densityLow densityPowder deliveryInorganic non-active ingredientsHydrogen phosphateMedicine
The invention belongs to the technical field of pharmaceutical preparations, and relates to thyroid tablets produced by direct whole-powder tabletting and a preparation process of the thyroid tabletsproduced by the direct whole-powder tabletting, in particular to synergetic application of calcium hydrogen phosphate-assisted optimization of powder properties of thyroid raw materials and freeze-drying assisted grinding technology. More than 98% of the particle sizes of thyroid mixed ground materials prepared by the invention are smaller than 250 microns, and the thyroid mixed ground materials are difficultly reaggregated, and uniformly mixed with other auxiliary materials; RSD of the T3 and T4 contents in thyroid powder is not greater than 4%; the angle of repose of the mixed powder is smaller than 35 degrees, so that requirements of powder direct tabletting are met; the content uniformity (A+2.2S) of T3 and T4 in the thyroid tablets is not greater than 20, each tablet is disintegratedwithin 15min, and the disintegration time limit difference is small (RSD is not greater than 5%). The thyroid tablets produced by the direct whole-powder tabletting and the preparation process of thethyroid tablets produced by the direct whole-powder tabletting disclosed by the invention effectively solve the problems that the thyroid raw materials are not easily ground, and not uniformly mixed with the auxiliary materials and the like, optimize the grinding effect of the thyroid raw materials, improve the powder properties of the thyroid raw materials, and greatly enhance the content uniformity of T3 and T4 in the thyroid tablets.
Owner:CHINA PHARM UNIV
A kind of thyroid tablet produced by full powder direct compression and its preparation process
ActiveCN109464411BHigh densityLow densityPowder deliveryInorganic non-active ingredientsMetallurgyFreeze-drying
Owner:CHINA PHARM UNIV
3D printed pharmaceutical composition containing levetiracetam
ActiveCN110772488BHigh API contentOverall small sizeOrganic active ingredientsNervous disorderCellulosePharmaceutical drug
The invention discloses a 3D printed pharmaceutical composition containing levetiracetam and a preparation method thereof. The pharmaceutical composition comprises 80%-96% levetiracetam, 2-10% disintegrant and 1.5%-5% carboxymethylcellulose sodium. The pharmaceutical composition of the present invention has high content of API, less excipients, maximum drug dosage, small volume and size of the unit composition, few surface defects of the preparation, easy oral administration, high hardness and low friability, It is beneficial to storage and transportation; the preparation method of the present invention adopts the technology of 3D extrusion printing (pressure extrusion printing), not only the prepared product has high drug loading capacity and small size, but also the process of the preparation method is simple and the production cost is low. The raw material utilization rate is high, and it is suitable for individualized production and individualized drug delivery.
Owner:ZHEJIANG JINGXIN PHARMA +1
A kind of febuxostat tablet and its preparation process
ActiveCN111419814BHigh hardnessImproves friabilityOrganic active ingredientsInorganic non-active ingredientsPharmaceutical formulationOrganic chemistry
The invention provides a febuxostat tablet and a preparation method thereof, and belongs to the technical field of pharmaceutical preparations. The tablet comprises 10-25 parts of febuxostat, 20-60 parts of a filler, 5-15 parts of a disintegrant, 5-10 parts of an adhesive, 1-5 parts of a lubricant, 1-3 parts of a glidant and 0.1-1 part of a synergist. In the implementation process, the specific parts of the filler and the adhesive are controlled, and the mass ratio of various components is controlled, so that the dissolution rate of the tablet is obviously improved. In the implementation process of the invention, the invention unexpectedly finds that when the mass ratio of the lubricant to the glidant is controlled to be (0.5-5):1, the smoothness of the tablet can be effectively improved,so that the obtained tablet has higher hardness and brittleness. The synergist is also added into the components disclosed by the invention, so that the stability of the tablet is obviously improved.
Owner:GUANGDONG YILI LUODING PHARMA
A kind of hydroxypropyl methylcellulose plant hollow capsule
ActiveCN107296801BFast disintegrationLow loss on dryingPharmaceutical non-active ingredientsCapsule deliveryCelluloseOrganic solvent
The invention discloses a hydroxypropyl methyl cellulose plant empty capsule which is prepared through the following method: dissolving hydroxypropyl methyl cellulose in a mixed solvent of an organic solvent and water, preparing a hydroxypropyl methyl cellulose solution with the mass percentage of 10 to 33 percent, and making capsules by using the hydroxypropyl methyl cellulose solution, wherein the organic solvent is ethyl alcohol and / or acetone. The hydroxypropyl methyl cellulose plant empty capsule has the advantages that the capsule is disintegrated fast, is not brittle and has extremely low weight loss on drying; furthermore, according to the preparation method of the capsule, no heating is needed, so that energy consumption is reduced; no gelatinizer is added, so that influence on disintegration due to the gelatinizer is avoided; the capsule is cured and molded only through volatilization of the organic solvent, so that the hydroxypropyl methyl cellulose plant empty capsule is suitable for filling of certain medicines having special requirements.
Owner:ANHUI HUANGSHAN CAPSULE CO LTD
Preparation process of radix notoginseng tablets
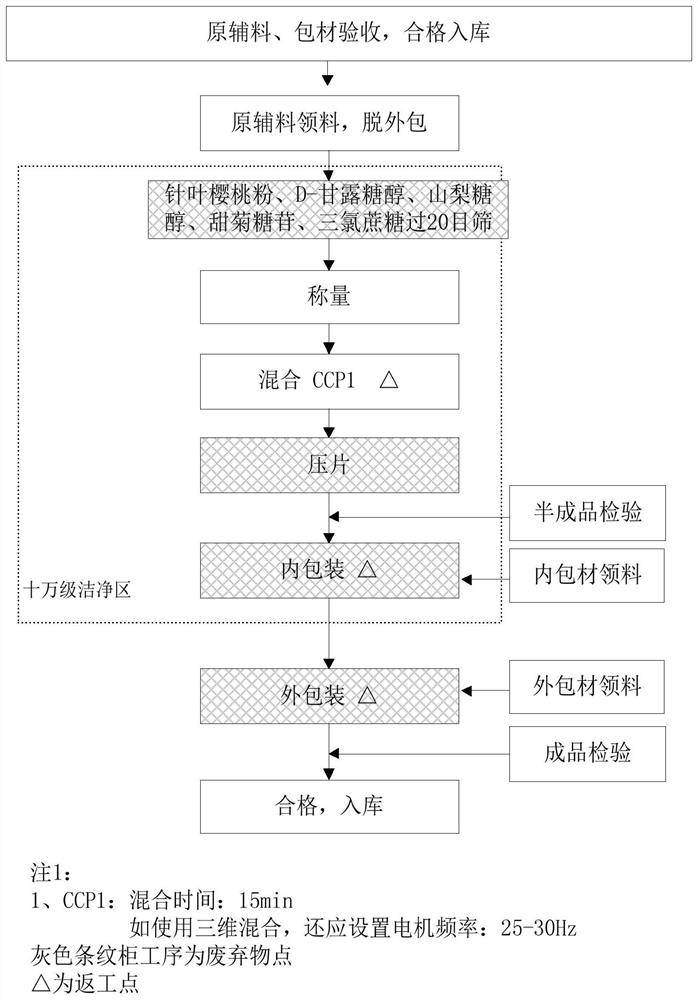
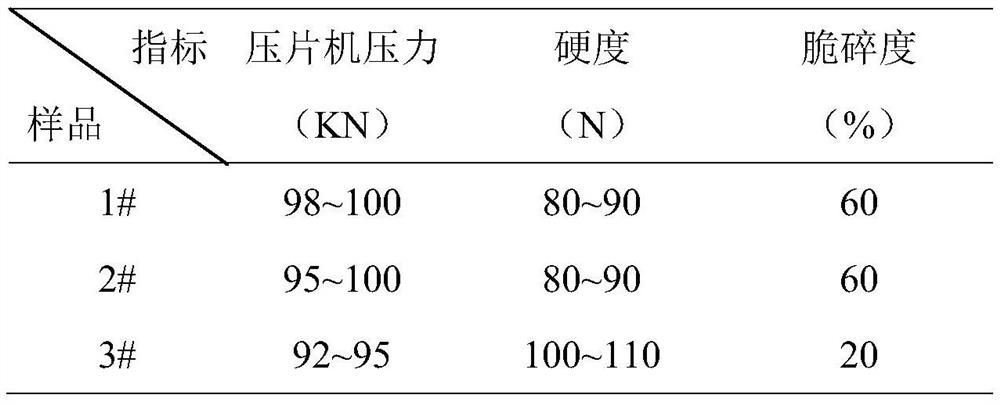
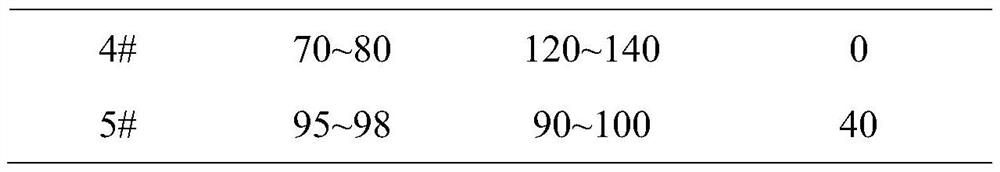
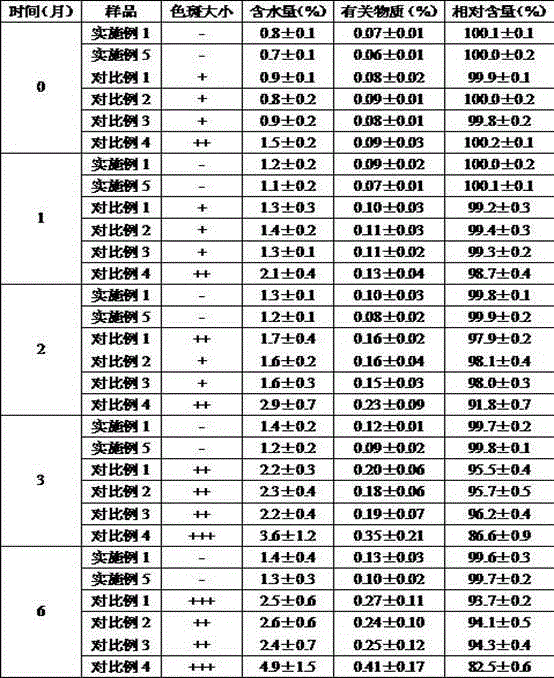
InactiveCN112402473AHigh hardnessImproves friabilityAntipyreticAnalgesicsPhysical chemistryMagnesium stearate
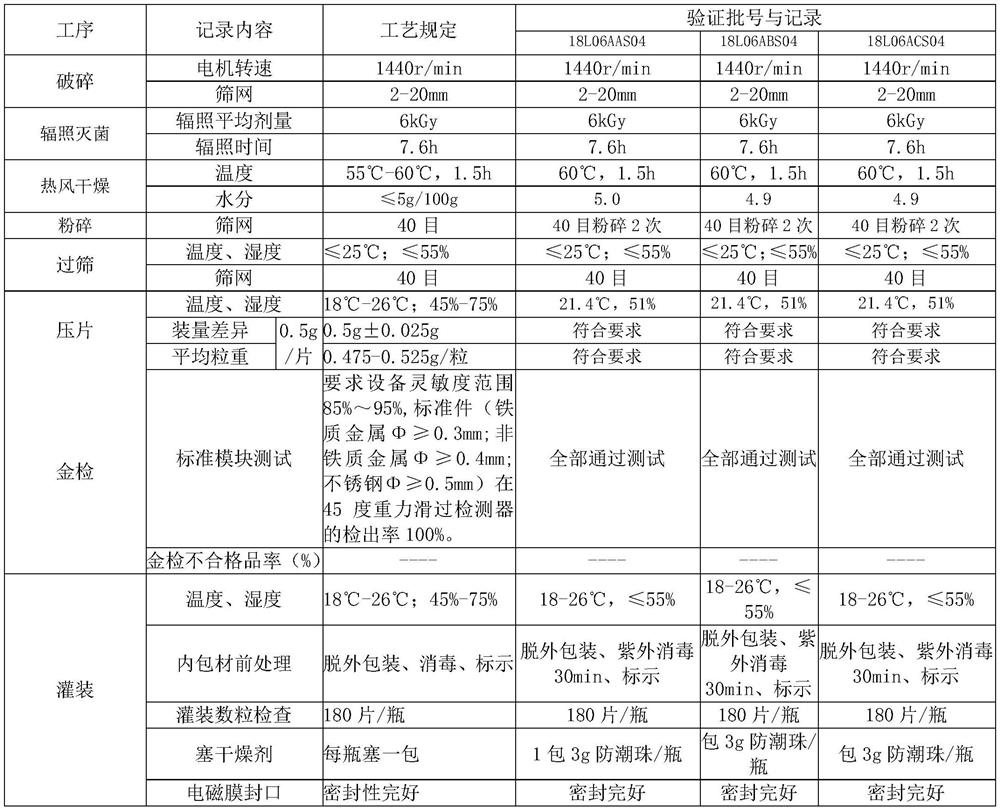
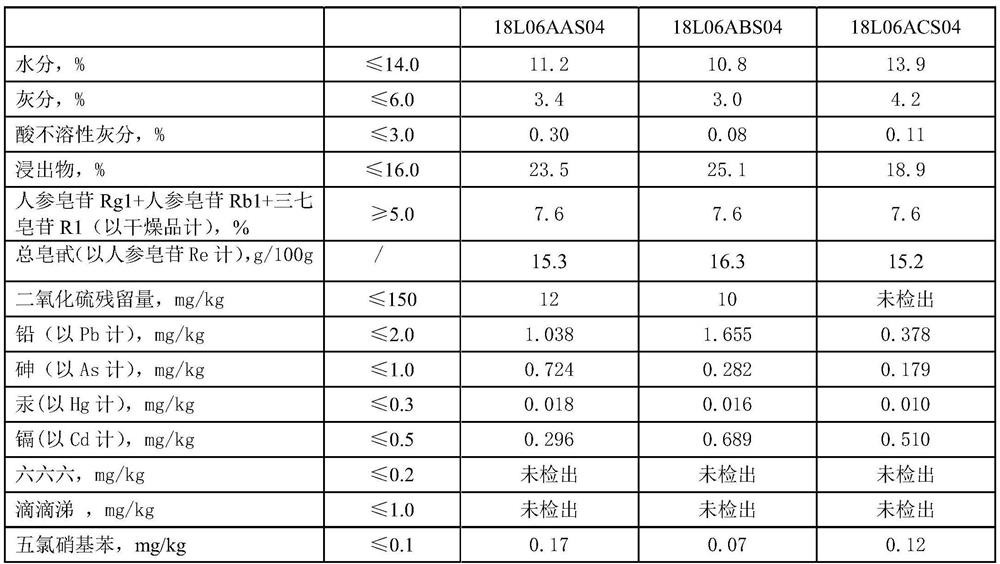
The invention relates to the field of preparation of radix notoginseng tablets, in particular to a preparation process of radix notoginseng tablets. The preparation process comprises the following steps: checking and crushing; irradiation sterilization; crushing and sieving; hot air drying; total mixing; tabletting; metal detection; and filling. Microcrystalline cellulose and magnesium stearate are added to 40-mesh radix notoginseng powder, so that the hardness, friability and disintegration time limit indexes of the radix notoginseng tablets are improved, the medicinal value of the radix notoginseng tablets is effectively exerted, and the practicability is high; and meanwhile, the preparation process of the radix notoginseng tablets is perfected, the production stability is good, the production efficiency is effectively improved and the yield is ideal.
Owner:孙红梅
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com