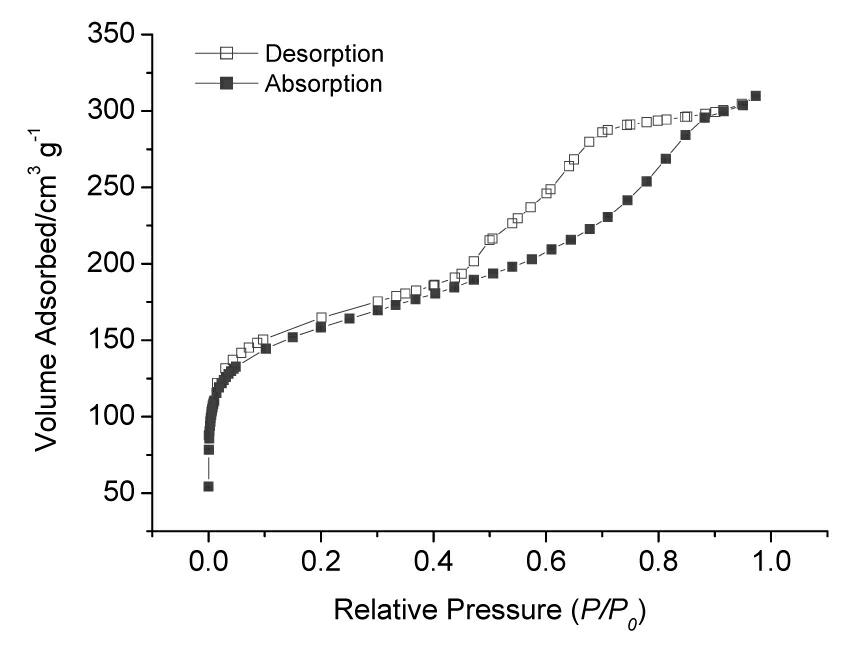
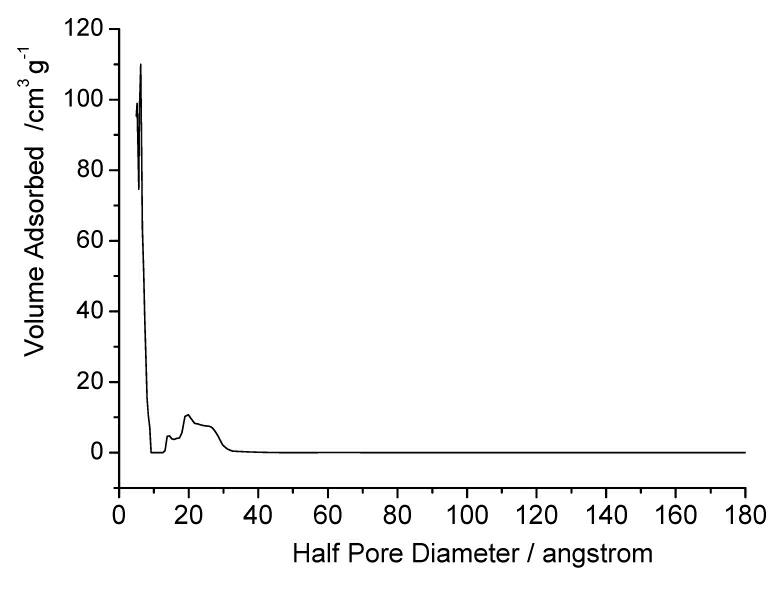
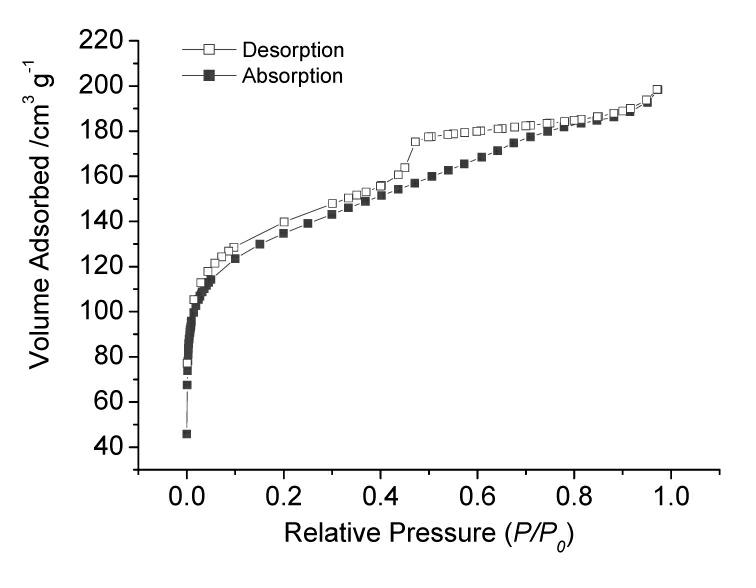
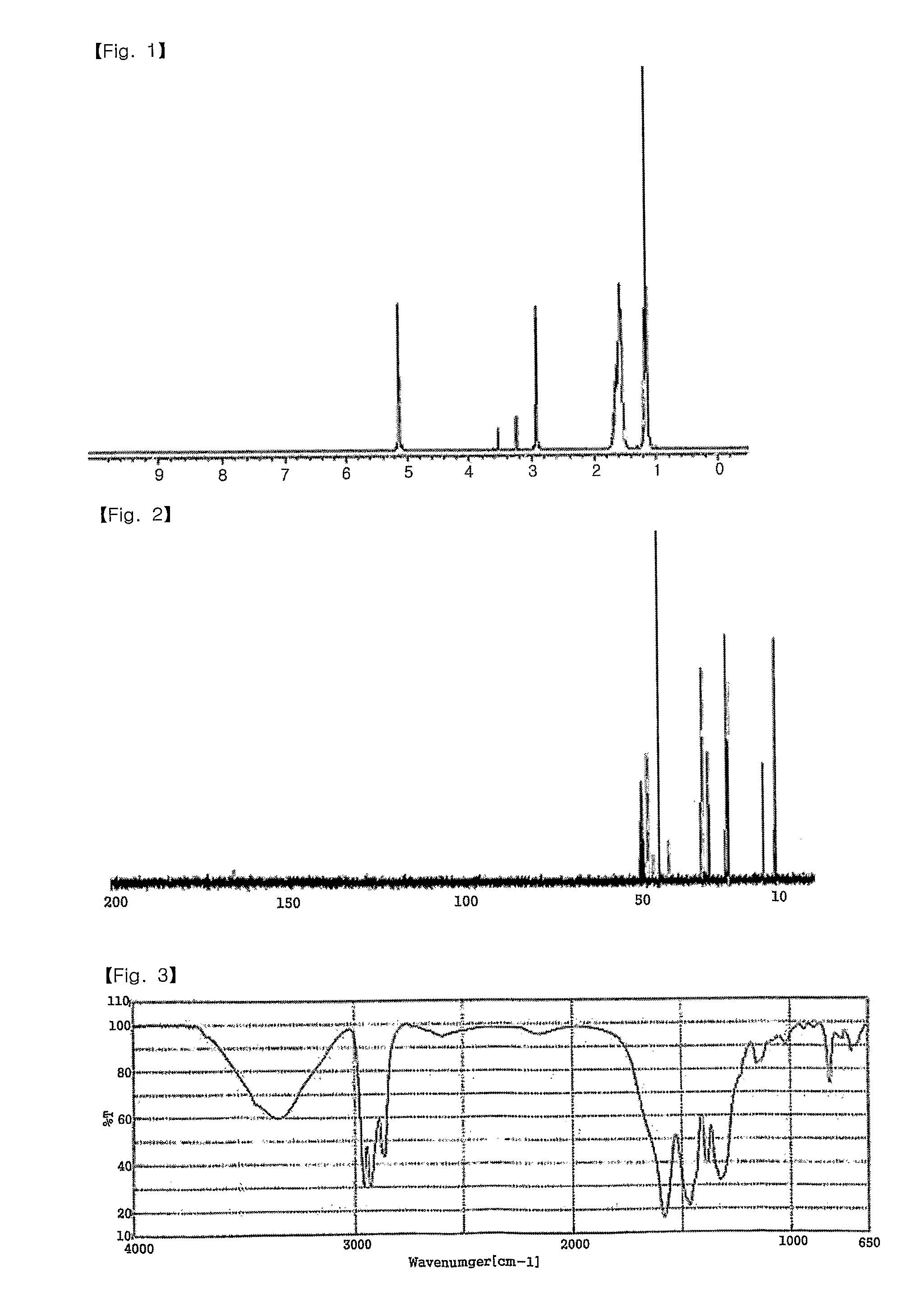
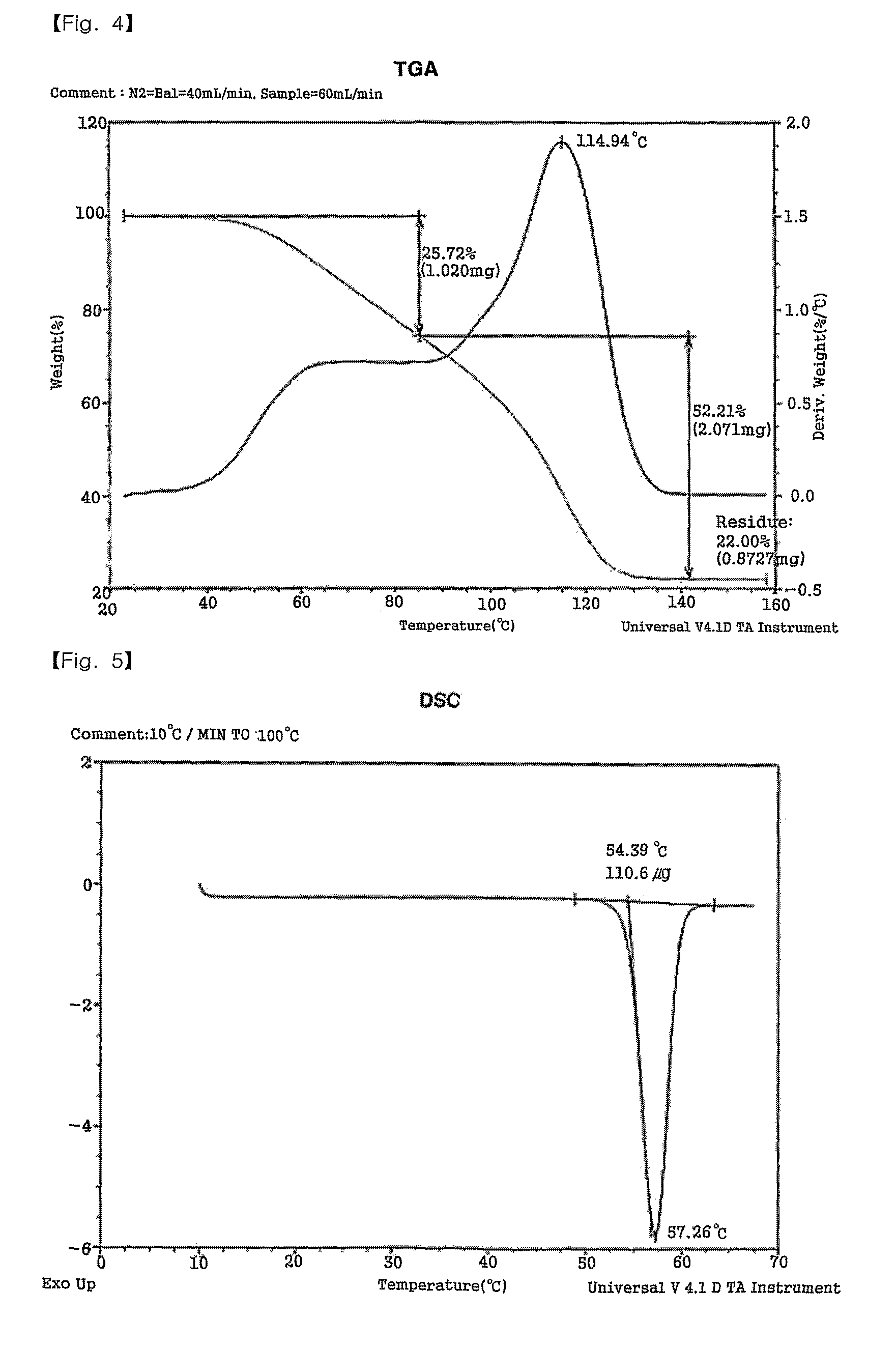
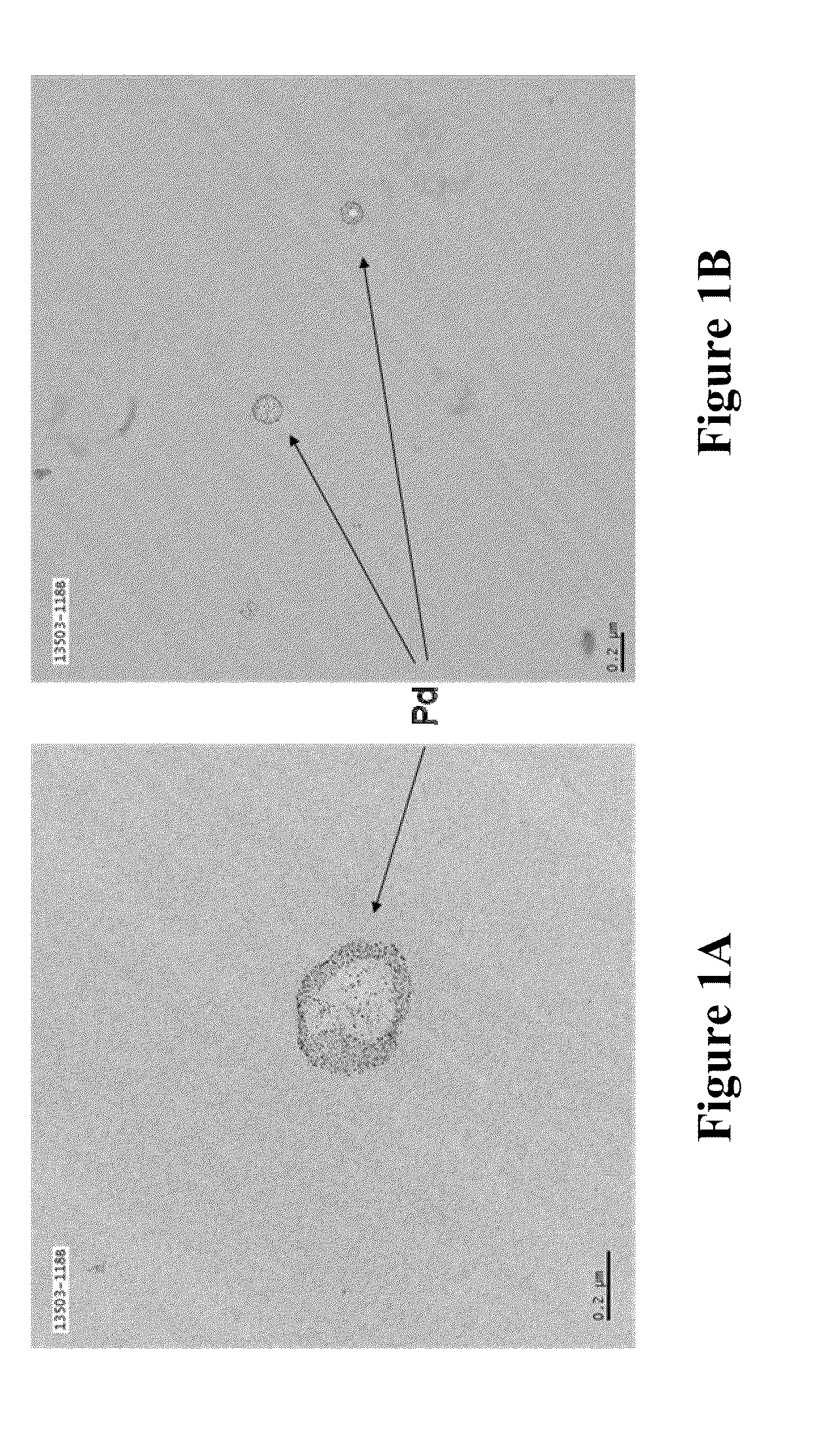
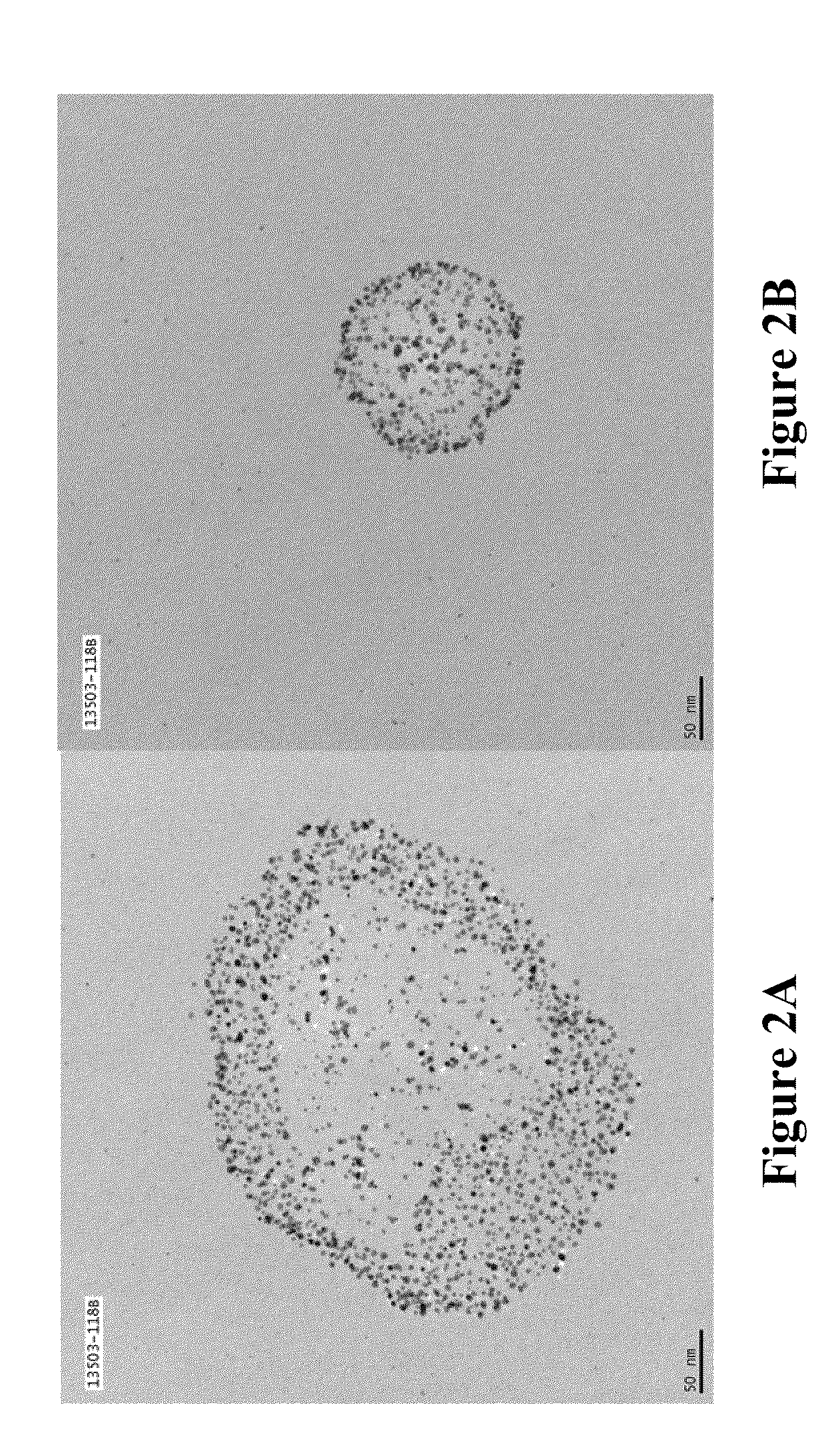
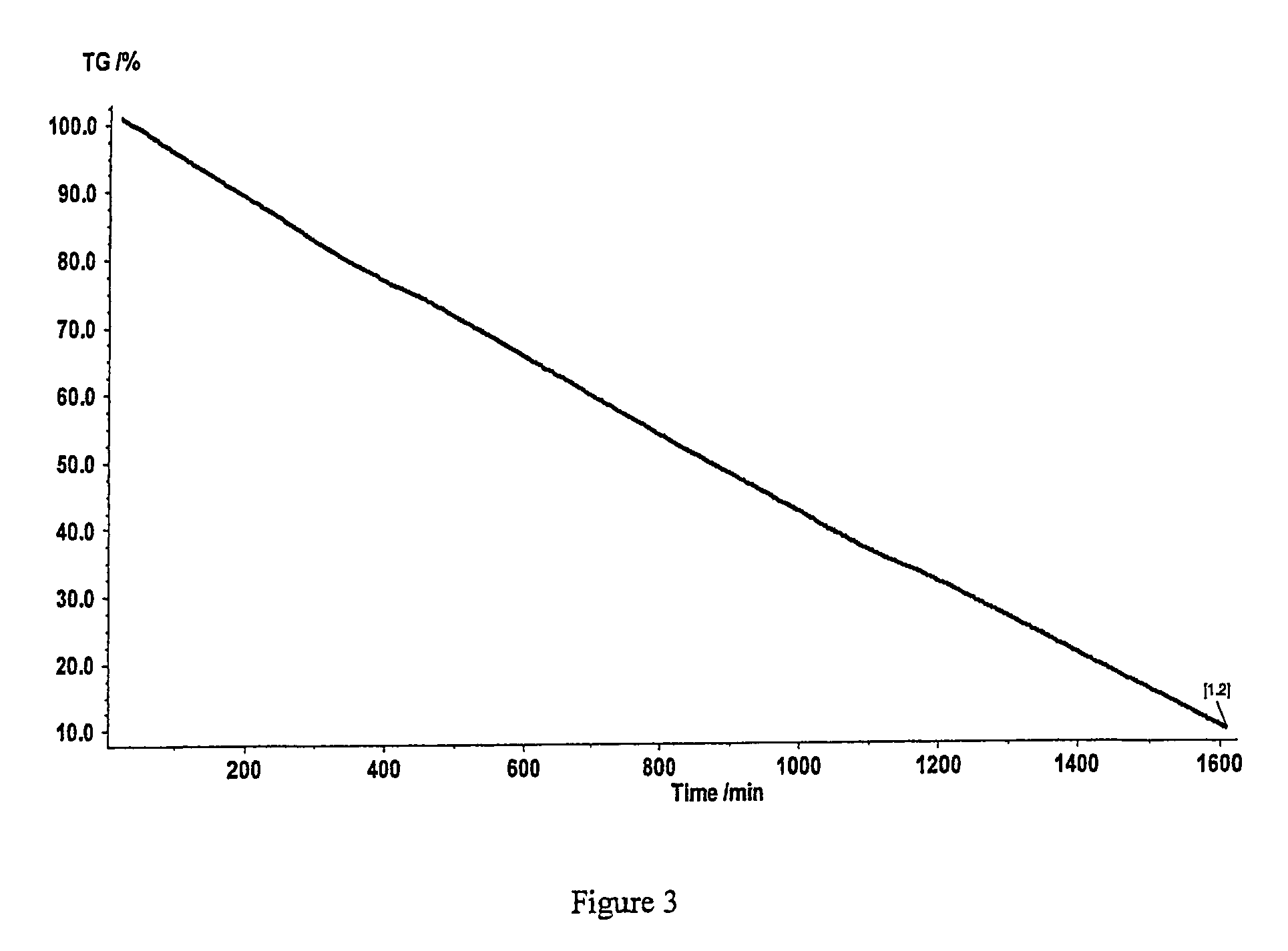
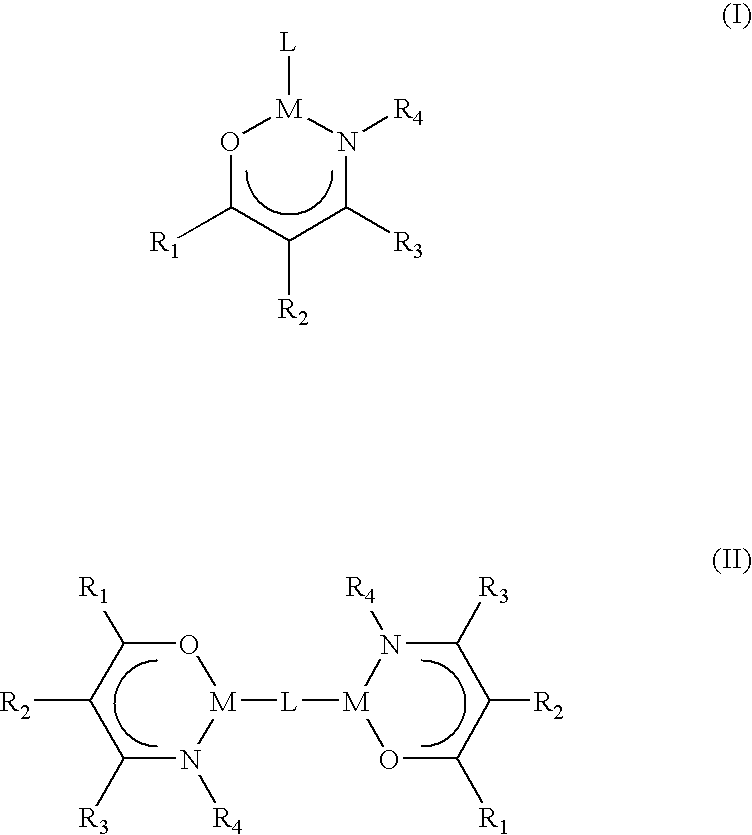
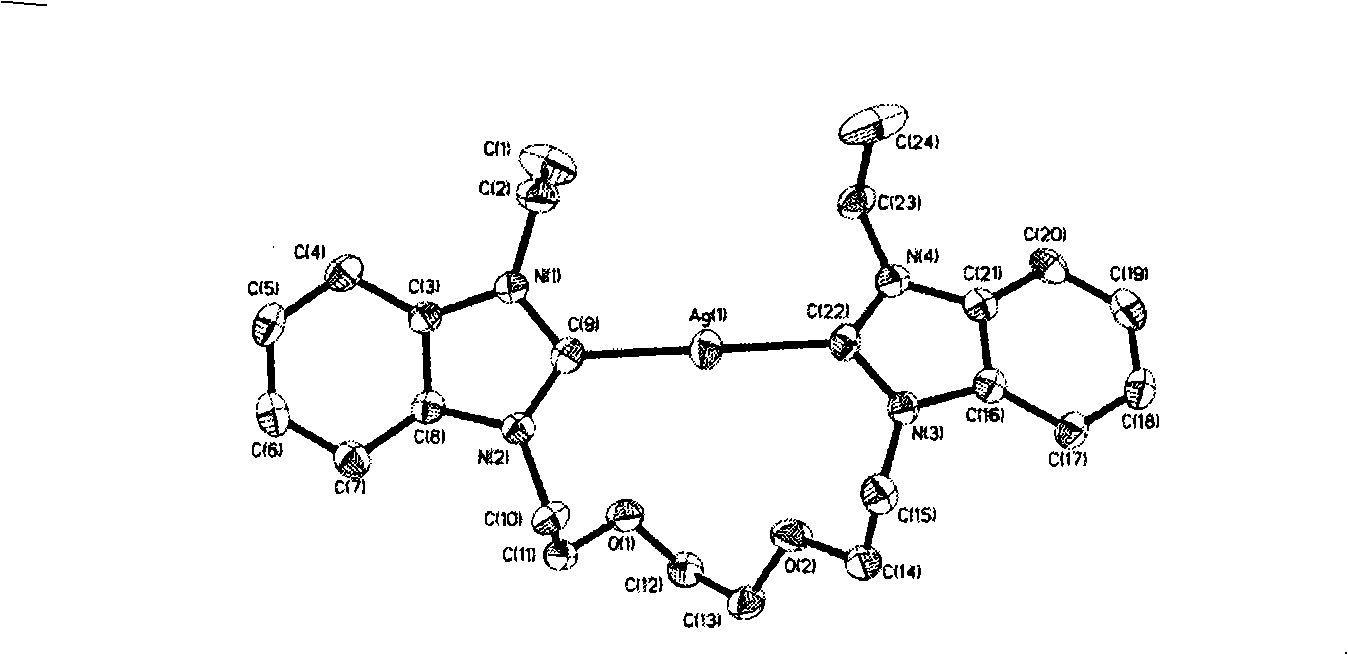
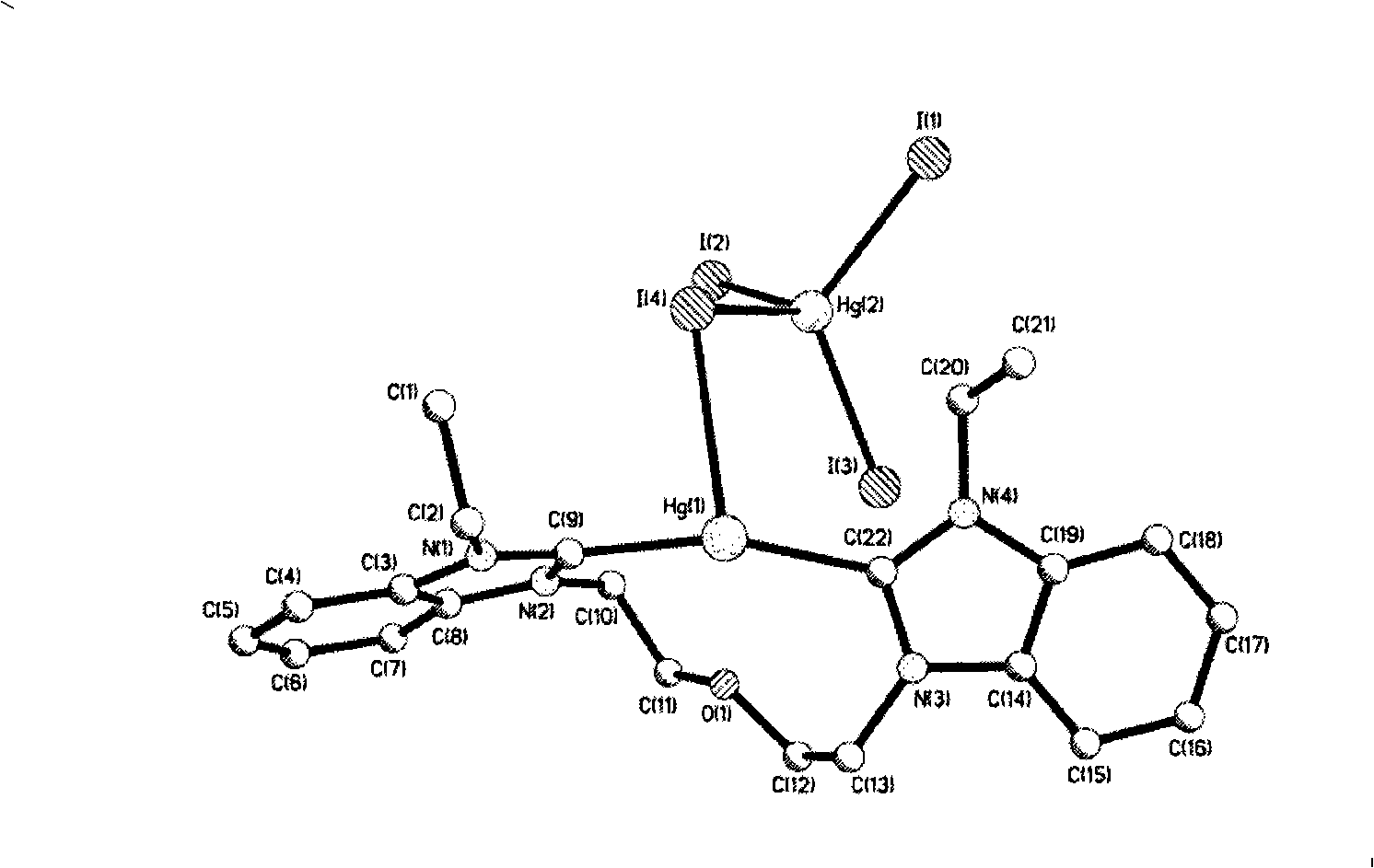
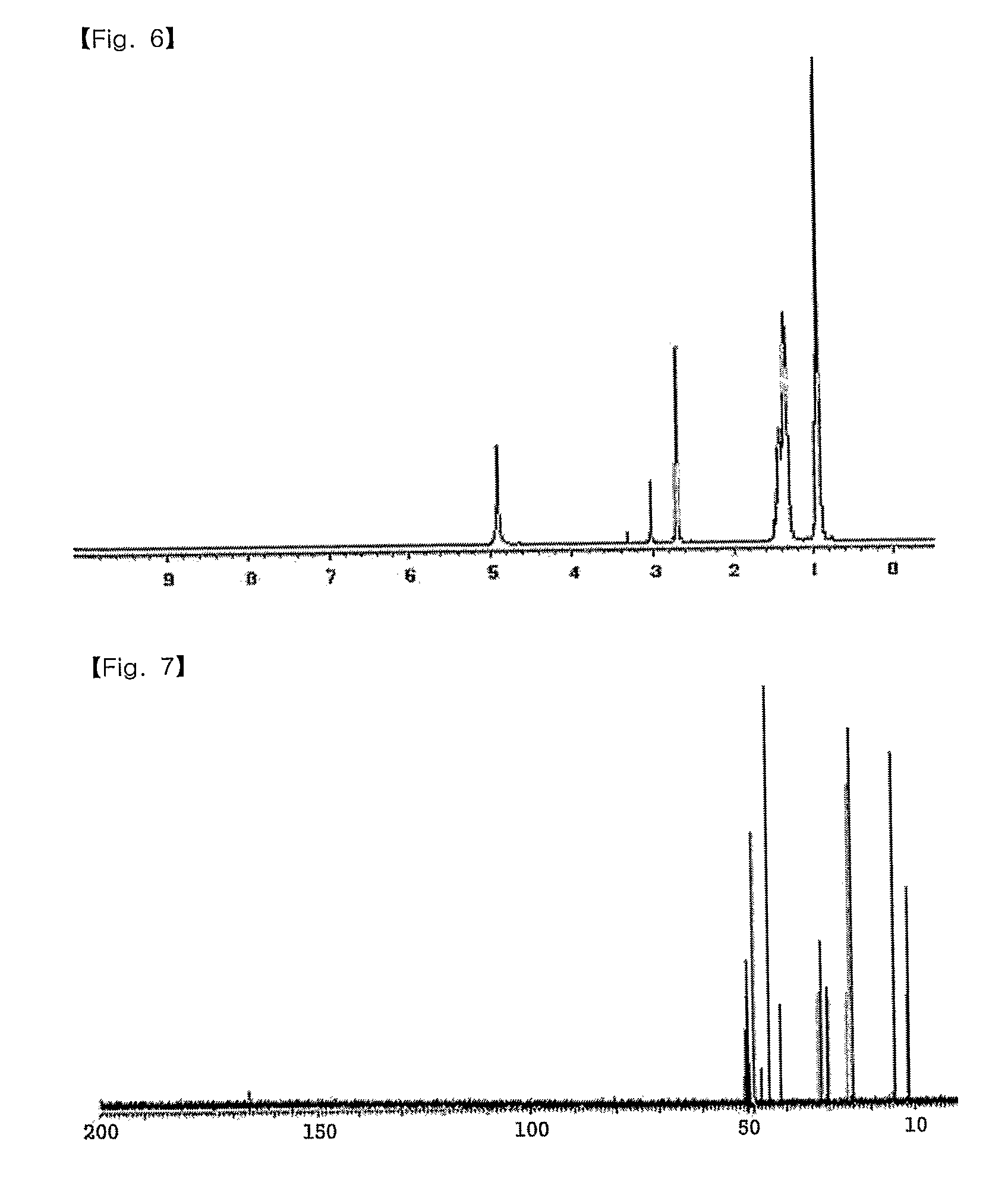
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
314results about "Silver organic compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
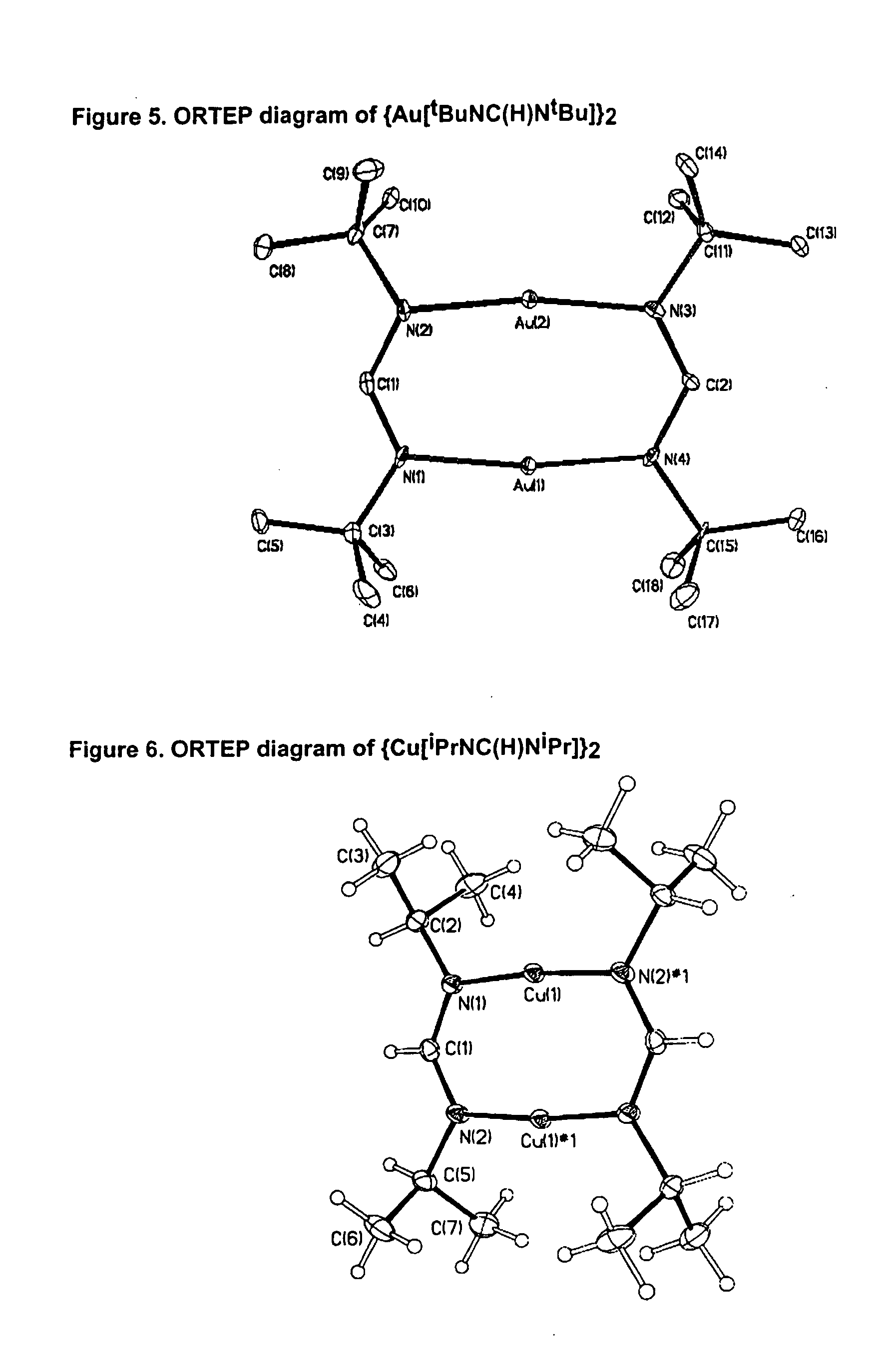
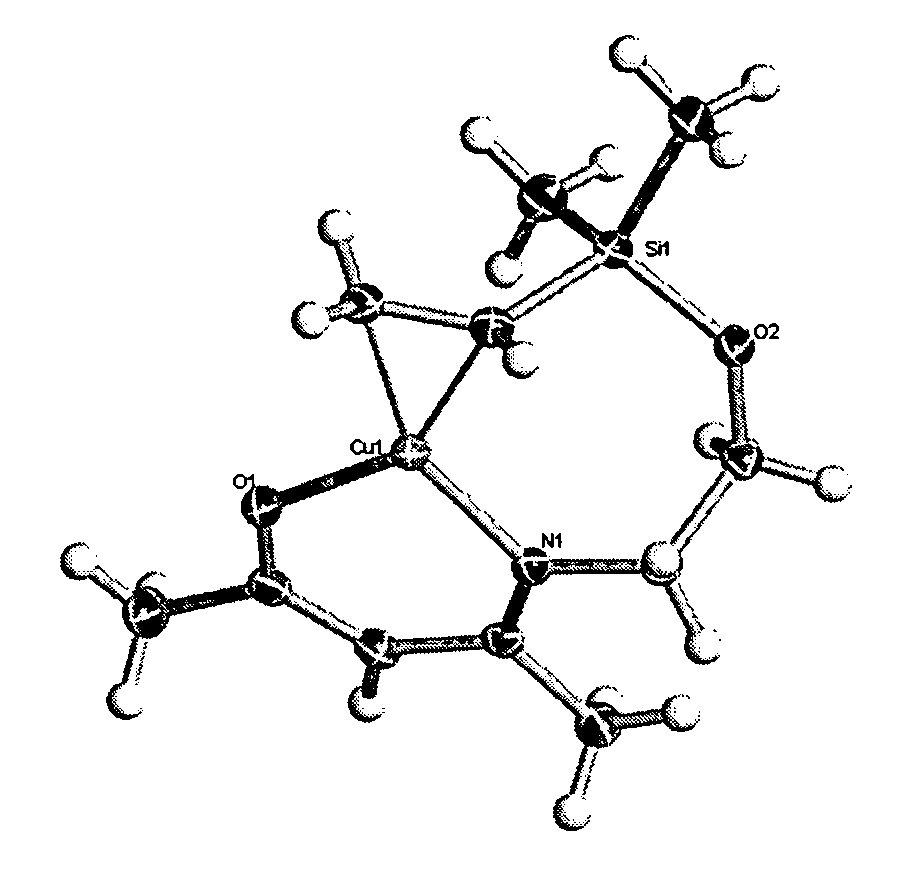
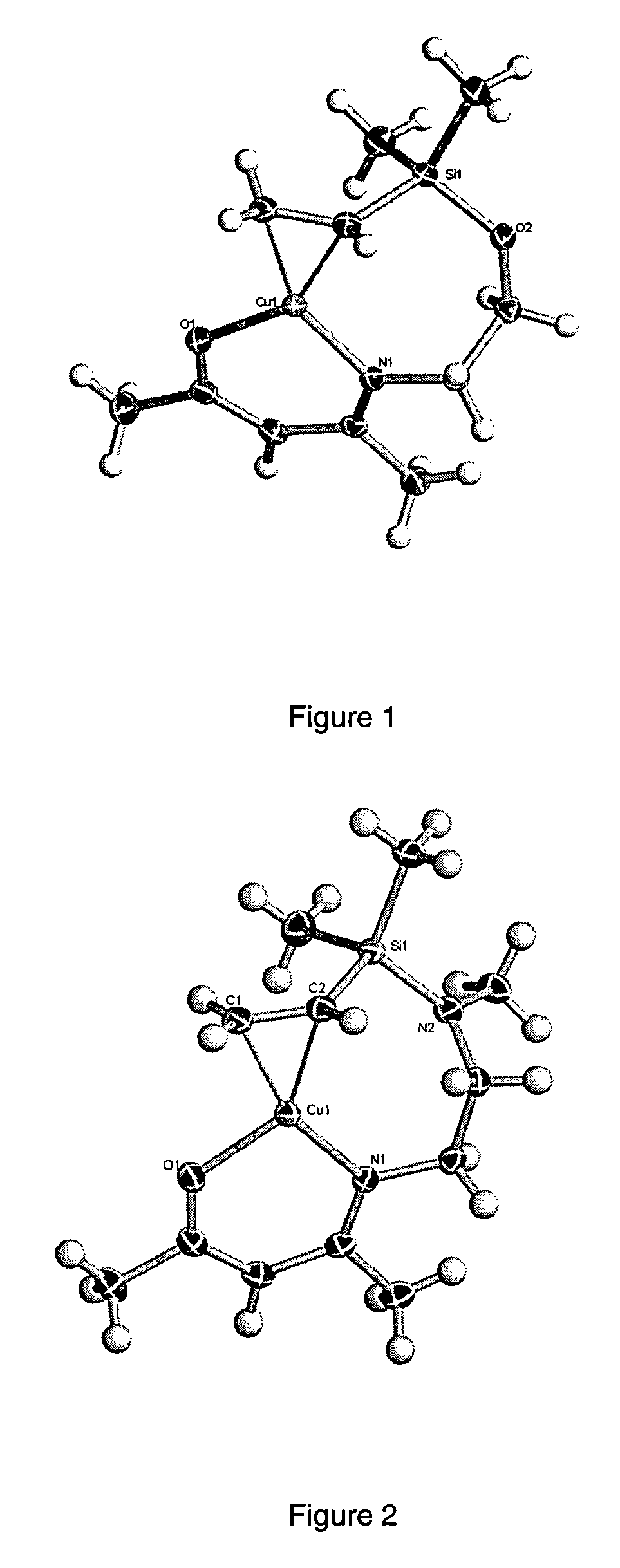
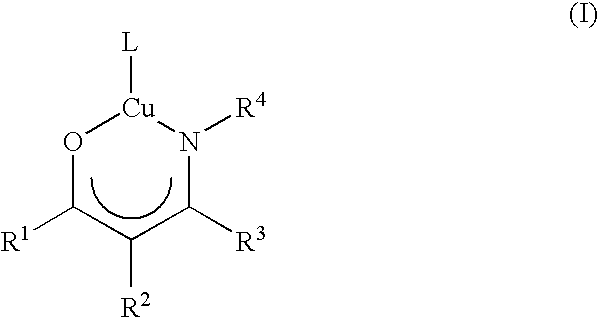
Atomic layer deposition using metal amidinates
ActiveUS20090291208A1Good step coverageImprove conductivityGroup 8/9/10/18 element organic compoundsCopper organic compoundsHydrogenWater vapor
Metal films are deposited with uniform thickness and excellent step coverage. Copper metal films were deposited on heated substrates by the reaction of alternating doses of copper(I) NN′-diispropylacetamidinate vapor and hydrogen gas. Cobalt metal films were deposited on heated substrates b the reaction of alternating doses of cobalt(II) bis(N,N′-diispropylacetamidinate) vapor and hydrogen gas. Nitrides and oxides of these metals can be formed by replacing the hydrogen with ammonia or water vapor, respectively. The films have very uniform thickness and excellent step coverage in narrow holes. Suitable applications include electrical interconnects in microelectronics and magnetoresistant layers in magnetic information storage devices.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Volatile noble metal organometallic complexes
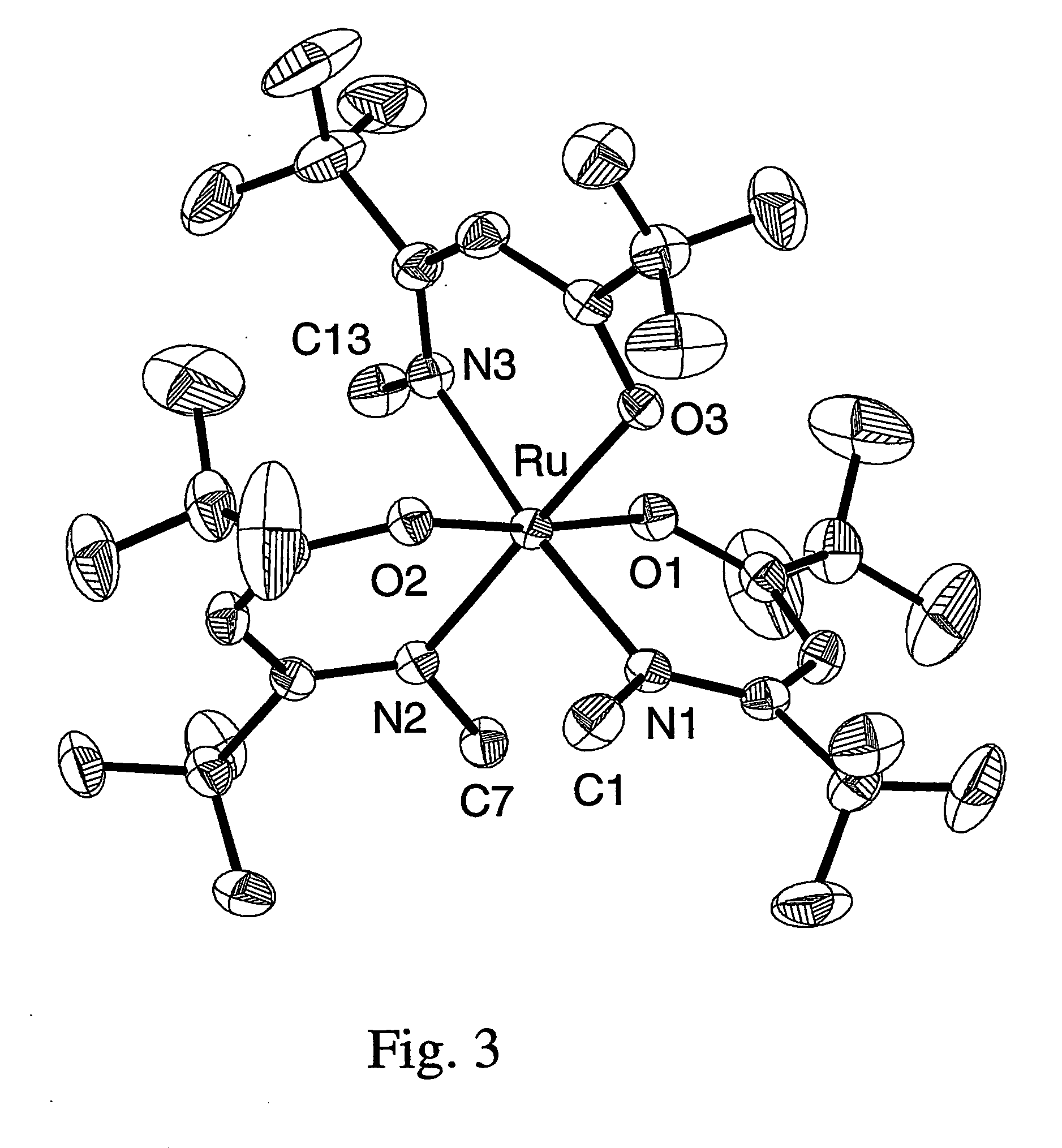
InactiveUS20050033075A1Reduce Van der Waals interactionBoiling and sublimation temperatureFurnaces without endless coreRuthenium organic compoundsIridiumIodide
A series of noble metal organometallic complexes of the general formula (I): MLaXb(FBC)c, wherein M is a noble metal such as iridium, ruthenium or osmium, and L is a neutral ligand such as carbonyl, alkene or diene; X is an anionic ligand such as chloride, bromide, iodide and trifluoroacetate group; and FBC is a fluorinated bidentate chelate ligand such as beta diketonate, beta-ketoiminate, amino-alcoholate and amino-alcoholate ligand, wherein a is an integer of from zero (0) to three (3), b is an integer of from zero (0) to one (1) and c is an 10 integer of from one (1) to three (3). The resulting noble metal complexes possess enhanced volatility and thermal stability characteristics, and are suitable for chemical vapor deposition(CVD) applications. The corresponding noble metal complex is formed by treatment of the FBC ligand with a less volatile metal halide. Also disclosed are CVD methods for using the noble metal complexes as source reagents for deposition of noble metal-containing films such as Ir, Ru and Os, or even metal oxide film materials IrO2, OsO2 and RuO2.
Owner:NATIONAL TSING HUA UNIVERSITY +1
Process for preparation of silver nanoparticles, and the compositions of silver ink containing the same
ActiveUS20100189901A1Small sizeGood physical propertiesMaterial nanotechnologyNanostructure manufactureSilver inkAmmonium carbamate
The present invention relates to a process for preparation of silver complex compound and the compositions of silver ink containing the same. The present invention includes a) preparing silver complex compound with special structure by reacting silver compound with at least one or two mixtures selected from ammonium carbamate compound, ammonium carbonate compound or ammonium bicarbonate compound and b) preparing silver nano particles by reacting the silver complex compound with reducer or reducing or pyrolyzing the silver complex compound by applying heat thereto. The preparing method according to the present invention can prepare the silver nano practical with various shapes through a simple preparation process, improve the selectivity of the size of the silver complex compound, fire the silver complex compound even when it is fired at a low temperature of 150° C. or less during a short time, provide the ink compositions capable of forming the coating or the fine pattern showing the high conductivity while facilitating the thickness control of the coating, and provide the ink compositions capable of being applied to the reflective film material, the electromagnetic wave shield, and the antimicrobial agent, etc.
Owner:INKTEC CO LTD
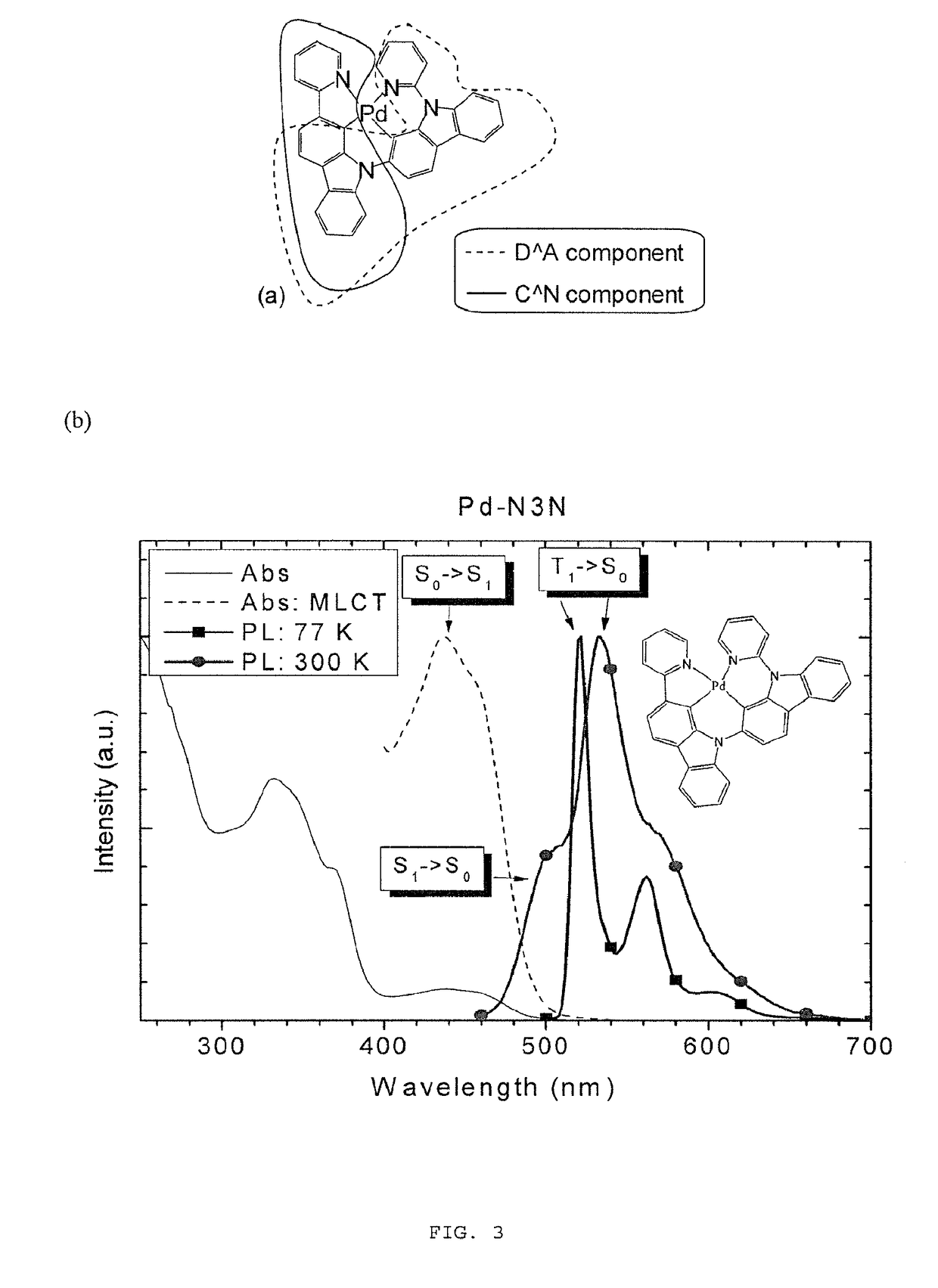
Metal complexes, methods, and uses thereof
Metal complexes that exhibit multiple radiative decay mechanisms, together with methods for the preparation and use thereof.
Owner:ARIZONA STATE UNIVERSITY
Class of volatile compounds for the deposition of thin films of metals and metal compounds
The invention provides an organometallic complex, containing oxygen free organic ligands, for the deposition of a metal, preferably copper, silver or gold, and preferably by way of chemical vapor deposition. The organometallic complex having the formula [(Do)nMLx]k where M is a metal preferably selected from the group consisting of Cu, Ag and Au; Do is selected from the group comprising ethers, phosphines, olefins, sulfides, pyridines, carbonyl, hydroxyl, cyclopentadiene, benzene derivatives, allyls, alkyls, amines, polyamines, aniline derivatives, cyclooctadiene and combinations thereof; n is an integer having a value from 0 to 4; k is an integer having a value from 1 to 4; x is an integer having a value from 1 to 4; and L is an amidinate ligand of the formula R1—NC(R2)N—R3 where R1, R2 and R3 are selected from the group consisting of alkyls, allyls, aryls, heteroaryls, hydrogen, non-metals and metalloids; and where R1, R2 and R3 are different or the same.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
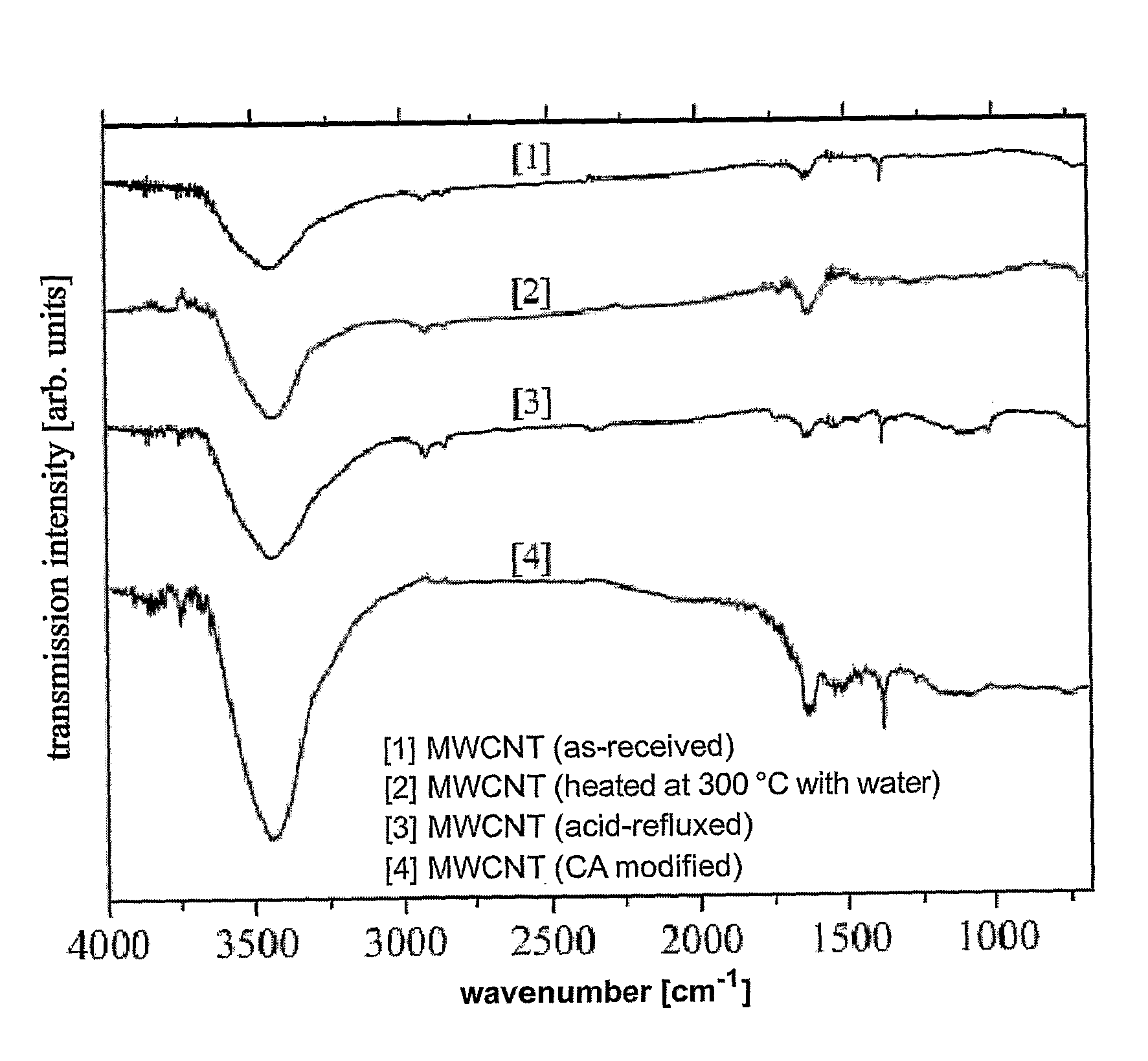
Method of functionalizing a carbon material
InactiveUS20110053050A1Material nanotechnologyGroup 8/9/10/18 element organic compoundsCarboxylic acidMaterials science
The present invention relates to a method of functionalizing a carbon material. A carbon material is contacted with a carboxylic acid, whereby a mixture is formed. The mixture is heated for a suitable period of time at a temperature below the thermal decomposition temperature of the carbon material.
Owner:AGENCY FOR SCI TECH & RES
Coordination polymer material with multistage pore passage structure and preparation method thereof
InactiveCN102161671ALarge hole sizeEasy to prepareCopper organic compoundsNickel organic compoundsSelf-assemblyChemical engineering
The invention provides a coordination polymer material with a multistage pore passage structure and a preparation method thereof. The coordination polymer material with a multistage pore passage structure is internally provided with the multistage pore passage structure, the multistage pore passage structure is a microporous and / or mesoporous and / or macroporous multistage pore passage structure which is formed by the self assembly between the metal ion and the organic ligand, the apertures of the micropores are less than or equal to 2nm, the apertures of the mesopores are from 2nm to 50nm, and the apertures of the macropores are more than 50nm. The coordination polymer material with a multistage pore passage structure is large in specific surface, the large-size organic ligand does not need to be synthesized, and the coordination polymer material with larger porous sizes can be obtained without the template agent and the pore assisting agent, so that the invention is simple in preparation method, and low in cost.
Owner:SUN YAT SEN UNIV
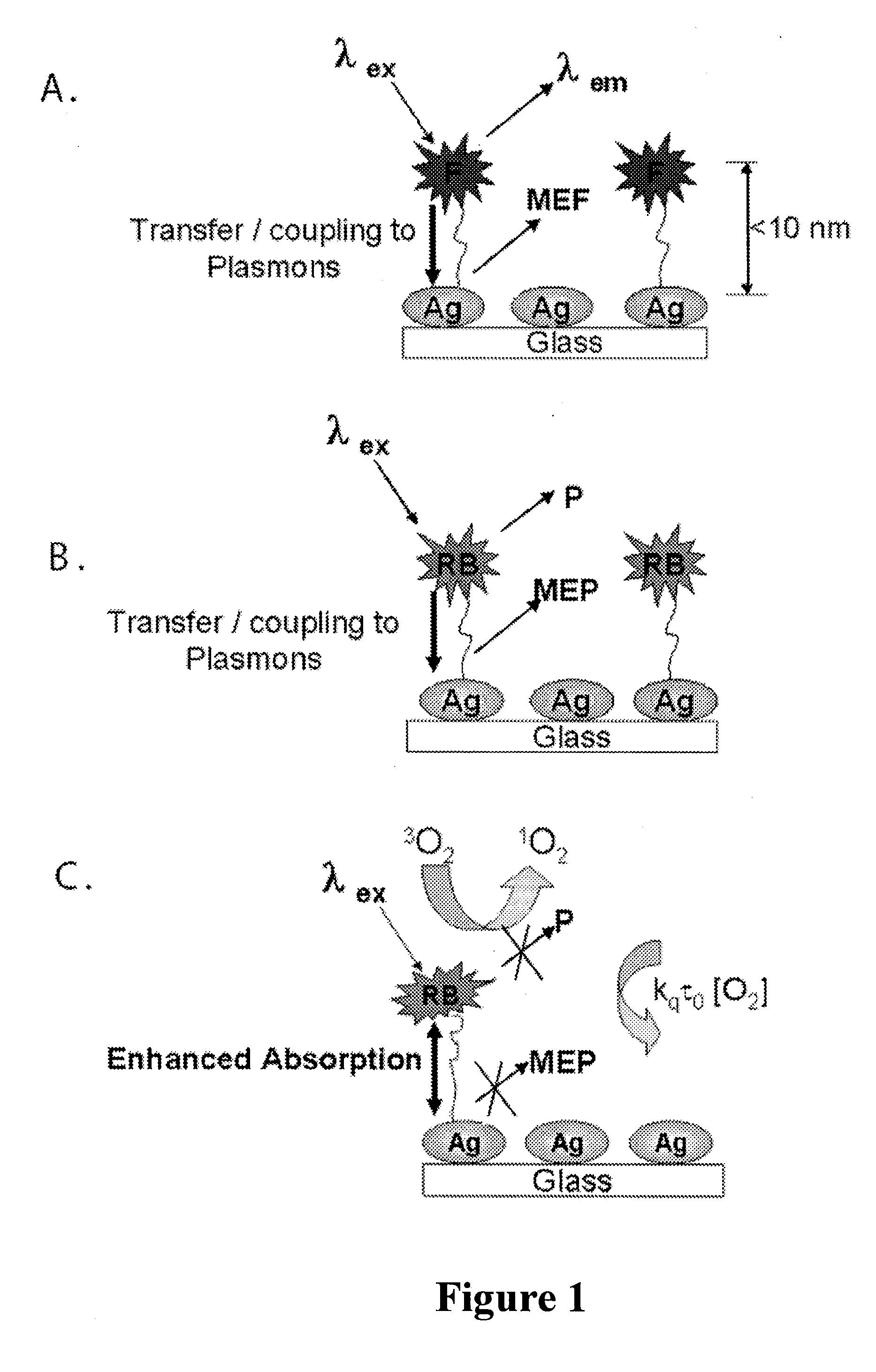
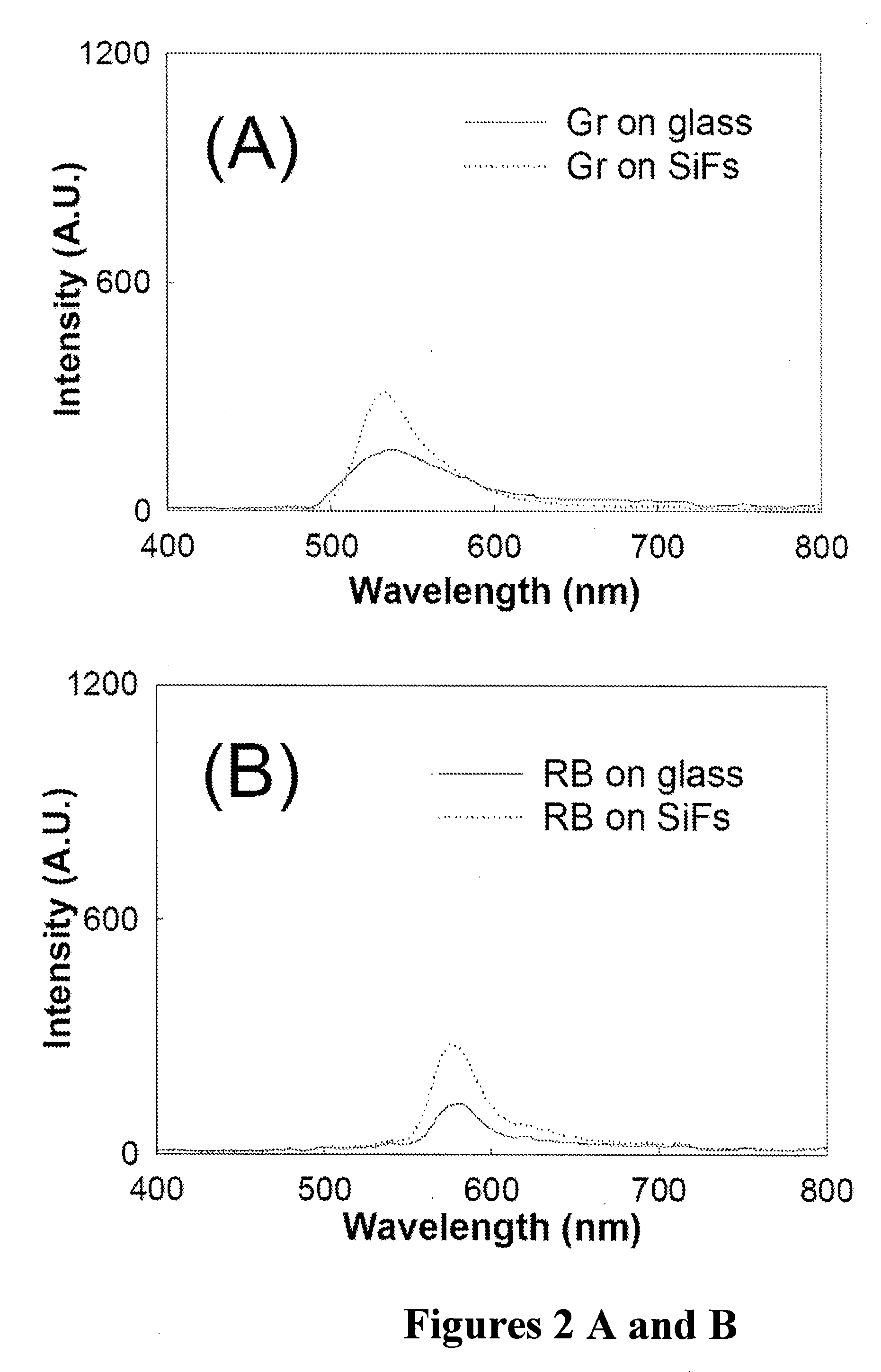
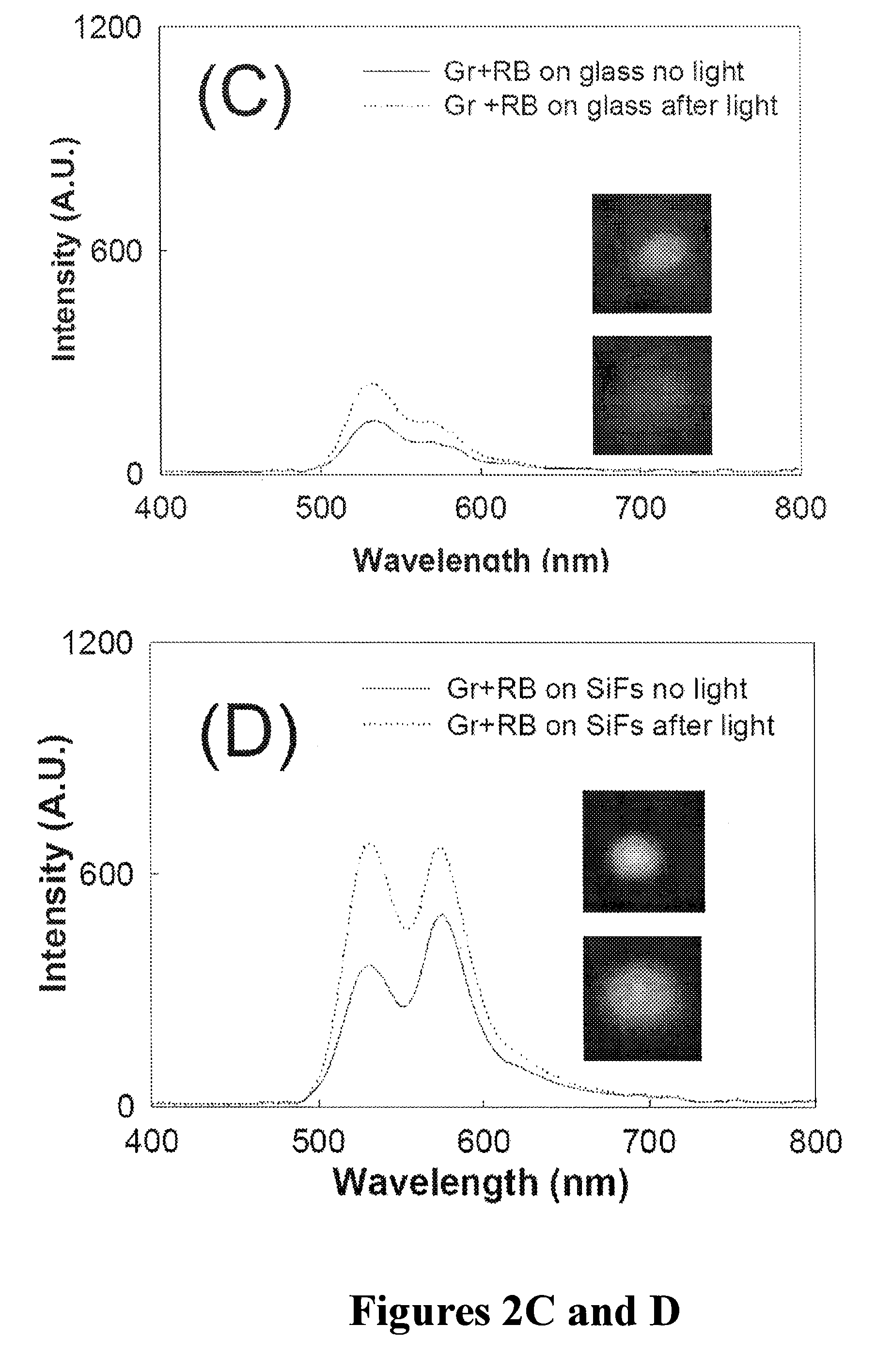
Plasmonic engineering of singlet oxygen and/or superoxide generation
ActiveUS20080215122A1Increase triplet yieldIncreased oxygen generationBiocideGroup 8/9/10/18 element organic compoundsEnergy absorptionSuperoxide
The present invention provides for a method to increase the triplet yield of a photosensitizer by the coupling to metal surface plasmons which leads to increased singlet oxygen generation by electric field enhancement or enhanced energy absorption of the photosensitizer. The extent of singlet oxygen enhancement can be tuned for applications in singlet oxygen based clinical therapy by modifying plasmon coupling parameters, such as metallic nanoparticle size and shape, photosensitizer / metallic nanoparticle distance, and the excitation wavelength of the coupling photosensitizer.
Owner:UNIV OF MARYLAND BALTIMORE COUNTY
Metal Complexes, Methods, and Uses Thereof
Metal complexes that exhibit multiple radiative decay mechanisms, together with methods for the preparation and use thereof.
Owner:ARIZONA STATE UNIVERSITY
Organic silver complexes, their preparation methods and their methods for forming thin layers
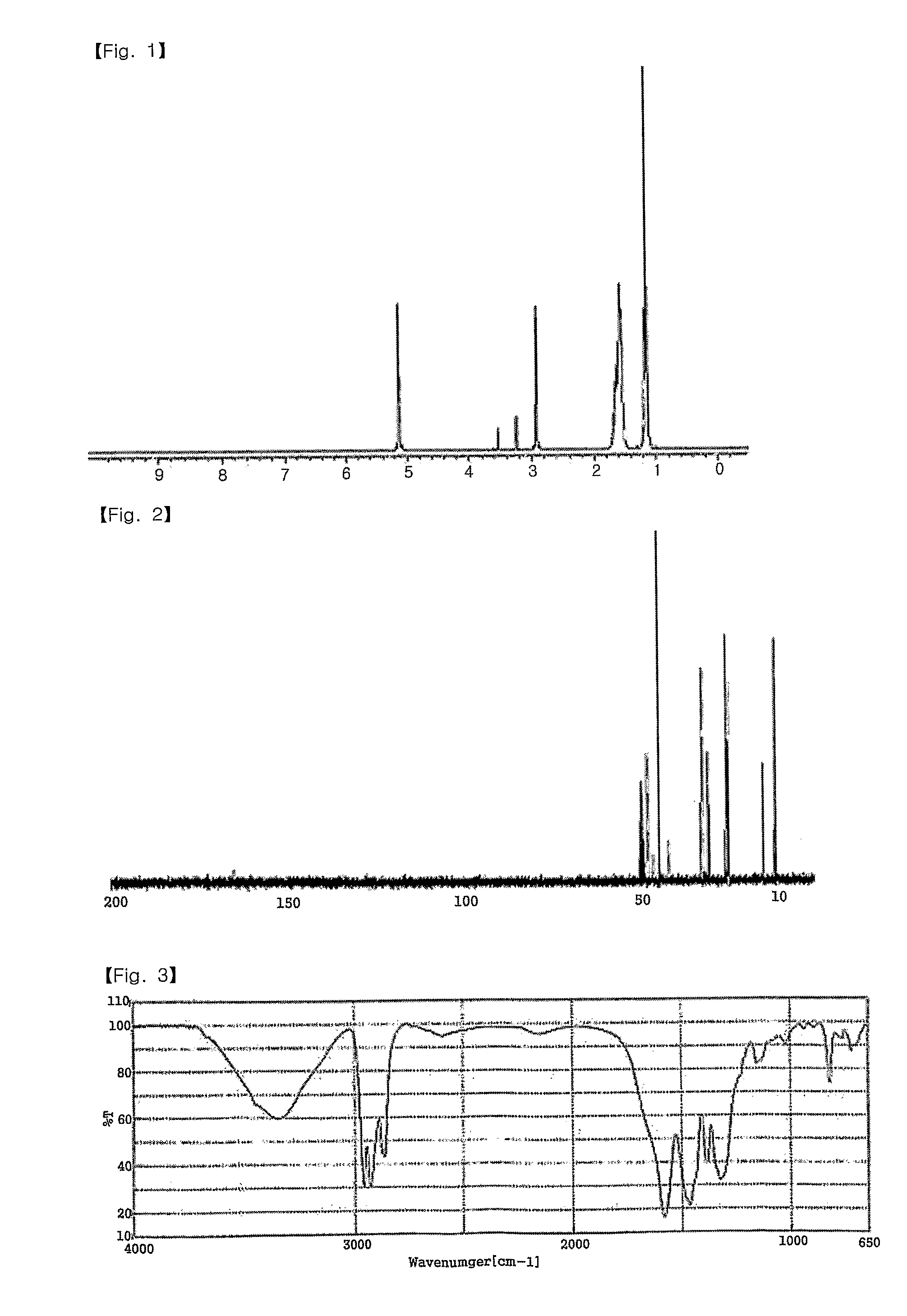
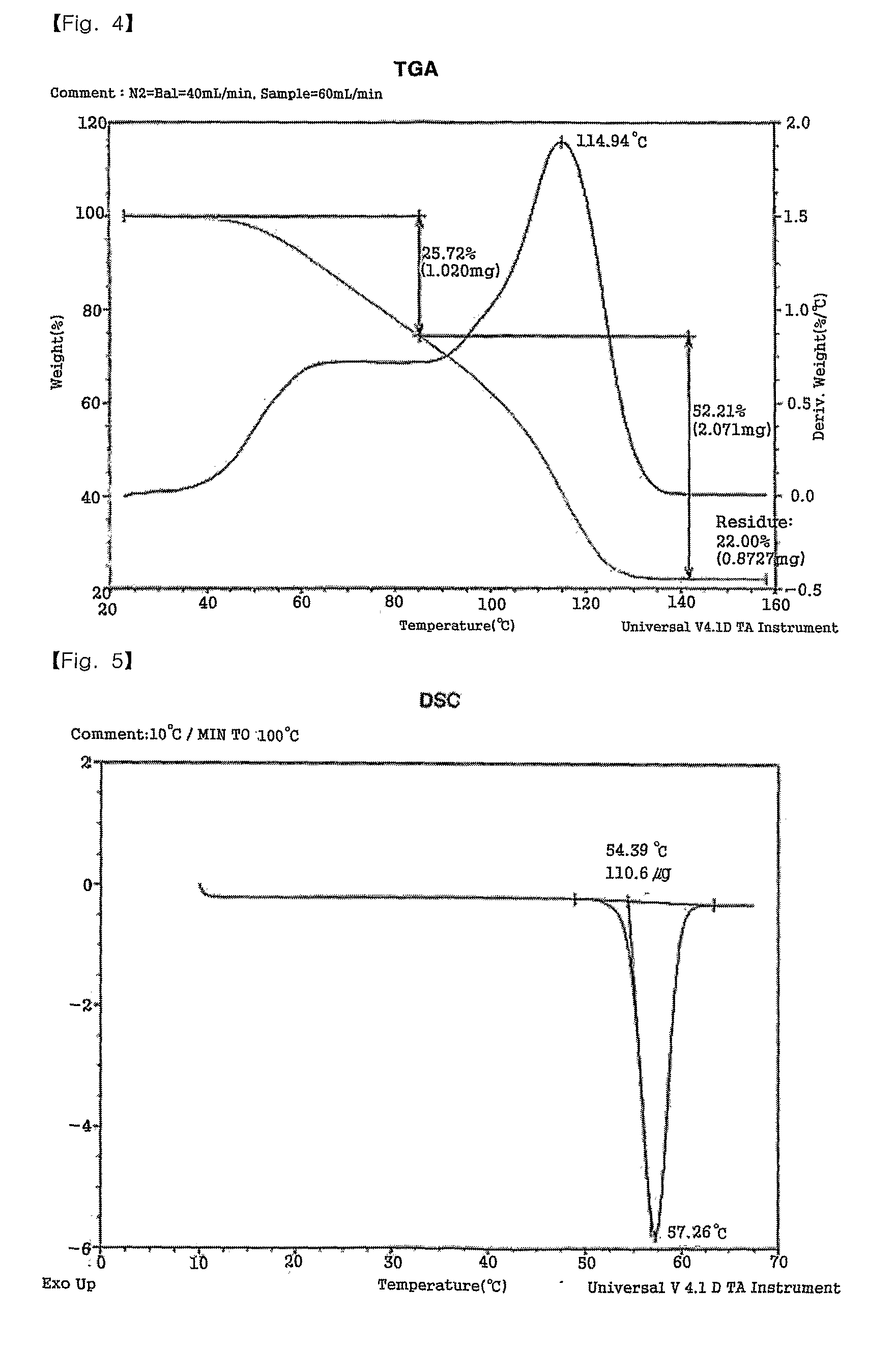
ActiveUS8226755B2Superior stability and solubilityEasy to prepareGroup 1/11 organic compounds without C-metal linkagesRadiation applicationsThin layerAmmonium carbonate
Owner:INKTEC CO LTD
Soluble metal salts for use as conductivity promoters
ActiveUS20090215940A1Improve conductivityNon-macromolecular adhesive additivesConductive materialHydrocarbonMetal salts
The present invention provides conductivity promoters, and in particular soluble conductivity promoters that contain a hydrocarbon moiety or a siloxane moiety and a metal. The present invention also provides methods of making soluble conductivity promoters and adhesive compositions containing the conductivity promoters of the invention.
Owner:DESIGNER MOLECULES
Volatile metal beta-ketoiminate and metal beta-diiminate complexes
ActiveUS7205422B2Group 1/11 organic compounds without C-metal linkagesCopper organic compoundsIndiumGas phase
Metal ketoiminate or diiminate complexes, containing copper, silver, gold, cobalt, ruthenium, rhodium, platinum, palladium, nickel, osmium, or indium, and methods for making and using same are described herein. In certain embodiments, the metal complexes described herein may be used as precursors to deposit metal and metal-containing films on a substrate through, for example, atomic layer deposition or chemical vapor deposition conditions.
Owner:VERSUM MATERIALS US LLC
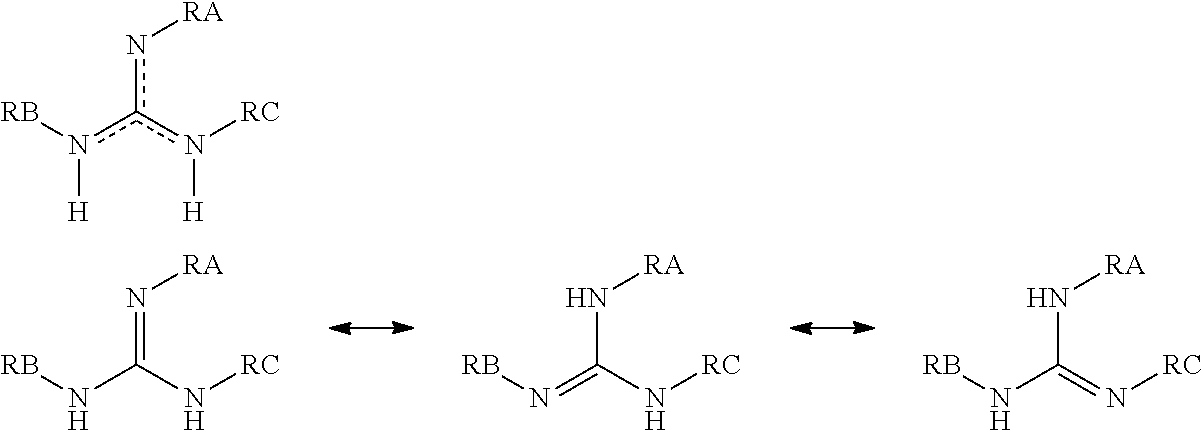
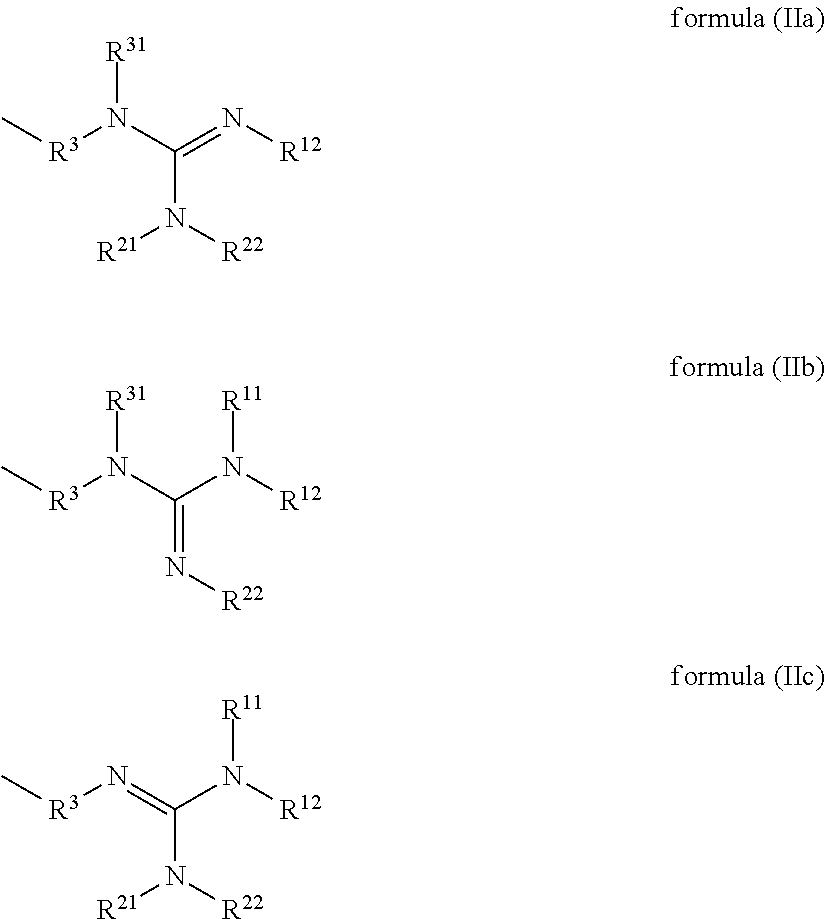
Compounds having guanidine groups and containing semi-organic silicon groups
This invention relates to compounds having guanidine groups and containing semi-organic silicon groups, their use for the curing of compounds containing alkoxysilyl groups, compositions comprising the curing catalysts of the invention, and use of the compositions as adhesives and sealants and also as coating materials.
Owner:EVONIK OPERATIONS GMBH
Carboxylic acid stabilized silver nanoparticles and process for producing same
ActiveUS20100090179A1Group 1/11 organic compounds without C-metal linkagesConductive materialOrganic solventCarboxylic acid
Processes for producing carboxylic acid-stabilized silver nanoparticles are disclosed. The processes comprise (a) forming a suspension of silver salt particles in a carboxylic acid; (b) forming a solution of an organohydrazine and a first organic solvent; (c) heating the suspension; (d) adding the solution to the suspension to form a mixture; and (e) reacting the mixture to form carboxylic acid-stabilized silver nanoparticles.
Owner:XEROX CORP
Antibacterial medical equipment and method for producing the same
ActiveUS20120064132A1Excellent in sufficient antibacterial activityImprove compatibilityAntibacterial agentsBiocidePhosphatePhosphoric acid
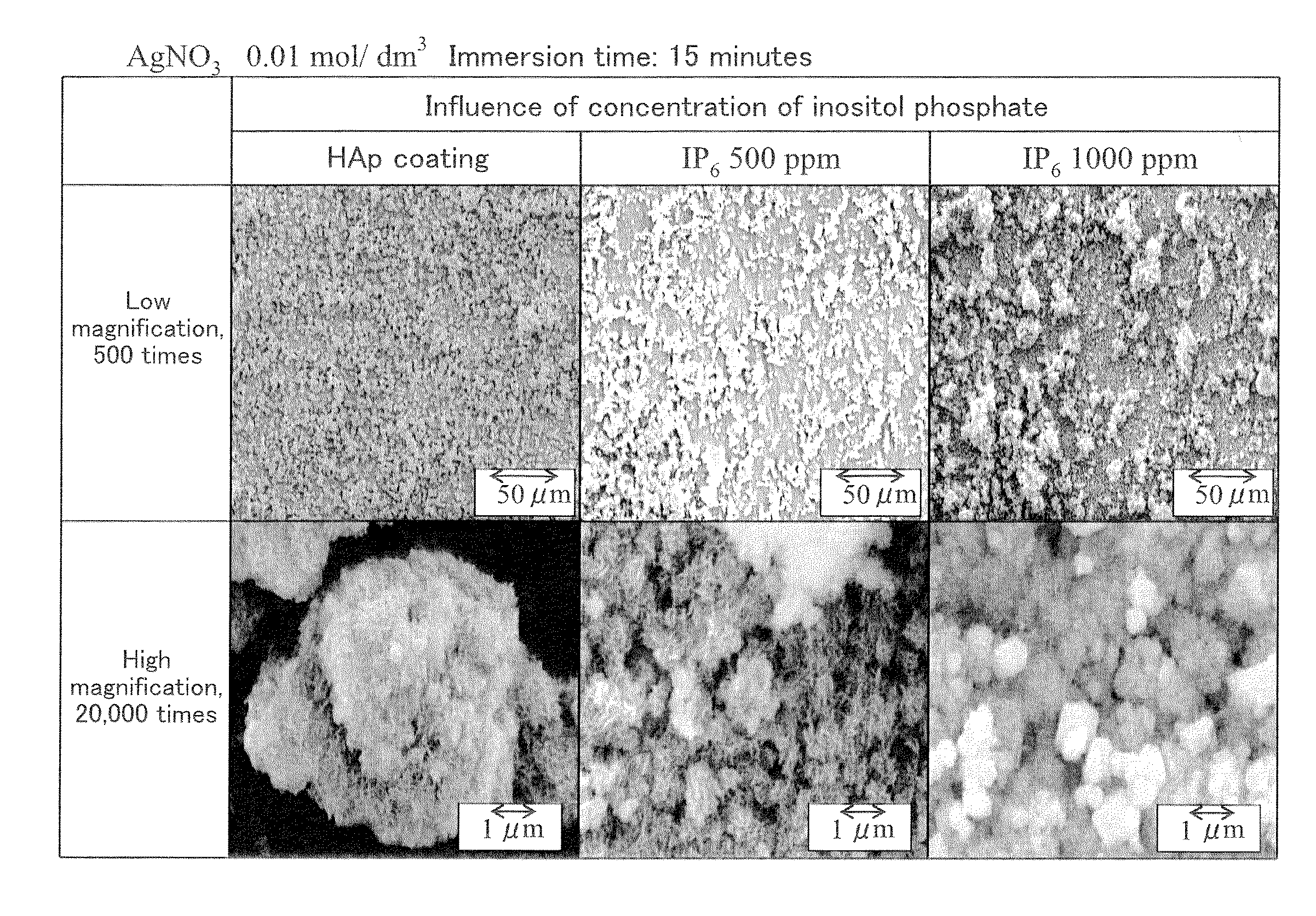
An object of the present invention is to provide an antibacterial medical equipment which has sufficient antibacterial activity in vivo and is excellent in compatibility with living tissues, and also can maintain antibacterial activity over a long period and has high safety.An antibacterial medical equipment characterized in that inositol phosphate is bonded to a Ca compound of a medical equipment whose surface is at least coated with a layer of the Ca compound, or a medical equipment comprising the Ca compound. The antibacterial medical equipment as described above, wherein silver ions are bonded to the inositol phosphate. A method for producing an antibacterial medical equipment, which comprises bringing a medical equipment whose surface is at least coated with a layer of a Ca compound, or a medical equipment comprising a Ca compound into contact with an aqueous solution of inositol phosphate to obtain an antibacterial medical equipment in which inositol phosphate is bonded to the Ca compound. The method for producing an antibacterial medical equipment, wherein inositol phosphate is bonded to the Ca compound and then the Ca compound is brought into contact with an aqueous solution containing silver ions to obtain an antibacterial medical equipment in which silver ions are bonded to the inositol phosphate.
Owner:MEIJI UNIV +1
Compositions for Forming Reflecting Layer Having Organic Silver Complexes, and Method for Preparing Reflecting Layer Using Same
ActiveUS20090220696A1Promote illuminance and adhesive forceRisk of pollutionGroup 1/11 organic compounds without C-metal linkagesNickel organic compoundsSilver coatingMetal
The present invention relates to compositions for forming reflecting layer having organic silver complexes, and method for preparing reflecting layer using the same. More specifically, it relates to compositions for forming reflecting layer including silver complexes that have distinct structures and the method for preparing reflecting layer, where primary coating is applied to promote the adhesive force to materials such as plastic, ceramic, metal, etc. and then a high-reflecting layer is formed by using the silver coating fluid, followed by transparent coating for protection.
Owner:INKTEC CO LTD
Metal complexes of N-heterocyclic carbenes as radiopharmaceuticals and antibiotics
A method for inhibiting microbial growth comprises administering an effective amount of a silver complex of a N-heterocyclic amine. A method for treating cancer cells or a method for imaging one or more tissues of a patient includes administering an effective amount of a complex of a N-heterocyclic amine and a radioactive metal. A method for treating urinary tract infections utilizing silver complexes of N-heterocyclic carbenes. N-heterocyclic carbenes of the present invention may be represented by the formula wherein Z is a heterocyclic group, and R1 and R2 are, independently or in combination, hydrogen or a C1-C12 organic group selected from the group consisting of alkyl, cycloalkyl, alkenyl, cycloalkenyl, alkynyl, aryl, arylalkyl, alkylaryl, heterocyclic, and alkoxy groups and substituted derivatives thereof.
Owner:THE UNIVERSITY OF AKRON
Metal precursor and metal precursor ink using the same
ActiveUS20150336878A1High metal contentInduce intramolecularOrganic compound preparationCopper organic compoundsSolubilityMetallurgy
Provided are a metal precursor containing an oxime group, which is represented by general formula 1, and a metal precursor ink containing same. The metal precursor ink according to the present invention enhance metal content, induce intramolecular and / or intermolecular complexation, thereby enabling low temperature sintering with excellent solubility and stability. The metal precursor ink according to the present invention can be used to form a metal wire with a desired shape. Therefore, the metal precursor ink can find applications in the field of printed electronics, particularly various electrodes, such as mesh type transparent electrodes.
Owner:PESOLVE
Volatile metal beta-ketoiminate and metal beta-diiminate complexes
ActiveUS20060145142A1Group 1/11 organic compounds without C-metal linkagesCopper organic compoundsIndiumGas phase
Metal ketoiminate or diiminate complexes, containing copper, silver, gold, cobalt, ruthenium, rhodium, platinum, palladium, nickel, osmium, or indium, and methods for making and using same are described herein. In certain embodiments, the metal complexes described herein may be used as precursors to deposit metal and metal-containing films on a substrate through, for example, atomic layer deposition or chemical vapor deposition conditions.
Owner:VERSUM MATERIALS US LLC
Volatile metal beta-ketoiminate complexes
ActiveUS7034169B1Silicon organic compoundsGroup 1/11 organic compounds without C-metal linkagesIndiumGas phase
Metal complexes, containing copper, silver, gold, cobalt, ruthenium, rhodium, platinum, palladium, nickel, osmium, and / or indium, and methods for making and using same are described herein. In certain embodiments, the metal complexes described herein may be used as precursors to deposit metal or metal-containing films on a substrate through, for example, atomic layer deposition or chemical vapor deposition conditions.
Owner:VERSUM MATERIALS US LLC
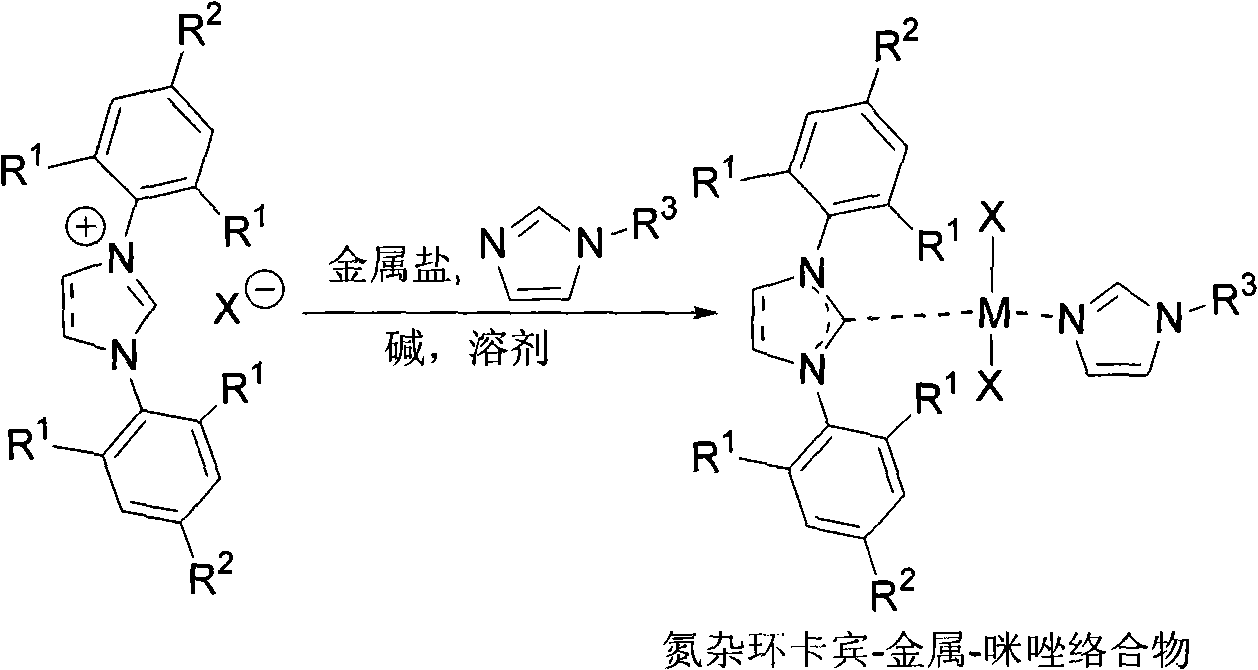
N-heterocyclic dicarbene metal complex connected with ether chain, method for preparing same and use
InactiveCN101307065AAdjustable structureEasy to manufactureCopper organic compoundsSilver organic compoundsOrganic solventNitrogen
The invention discloses a cyclic N-heterocyclic bis-carbene metal complex linked by ether and a method for preparing the same. The method comprises the steps of: adding imidazole or benzimidazolium salt linked by the ether and a metallic compound into a reactor according to the mol ratio of 0.5 to 3-2 to 5mol, under the protection of inert gas; and reacting for 12 to 24 hours at a temperature of between 0 and 100 DEG C, after dissolving the mixture by a dewatered high-purity organic solvent; filtering and naturally volatilizing the reacted mixture and solvent to produce a carbene metal complex. The cyclic N-heterocyclic bis-carbene metal complex linked by the ether, which is prepared with the method, is mainly used for preparing fluorescent materials.
Owner:TIANJIN NORMAL UNIVERSITY
Composition and process for treating acne
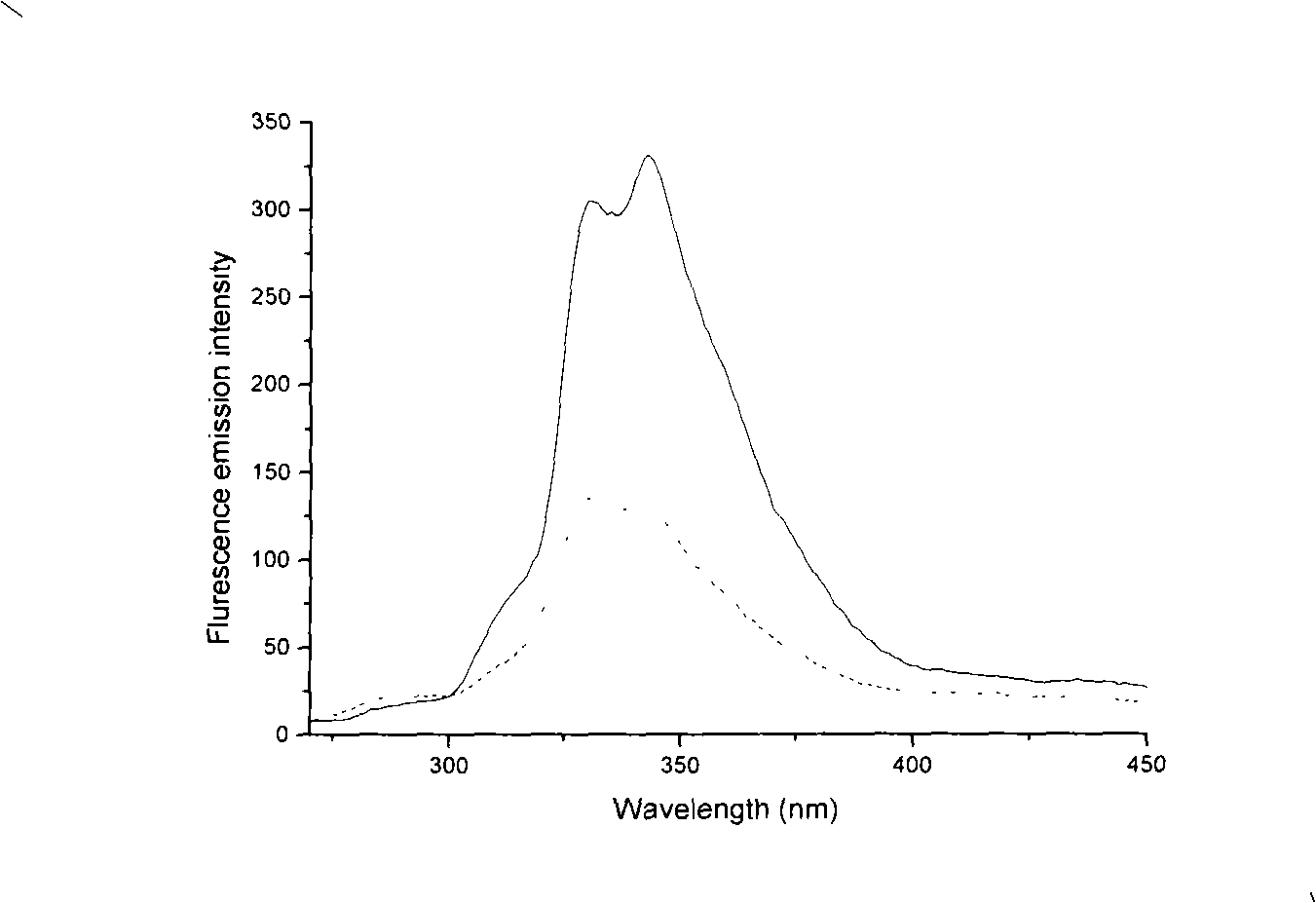
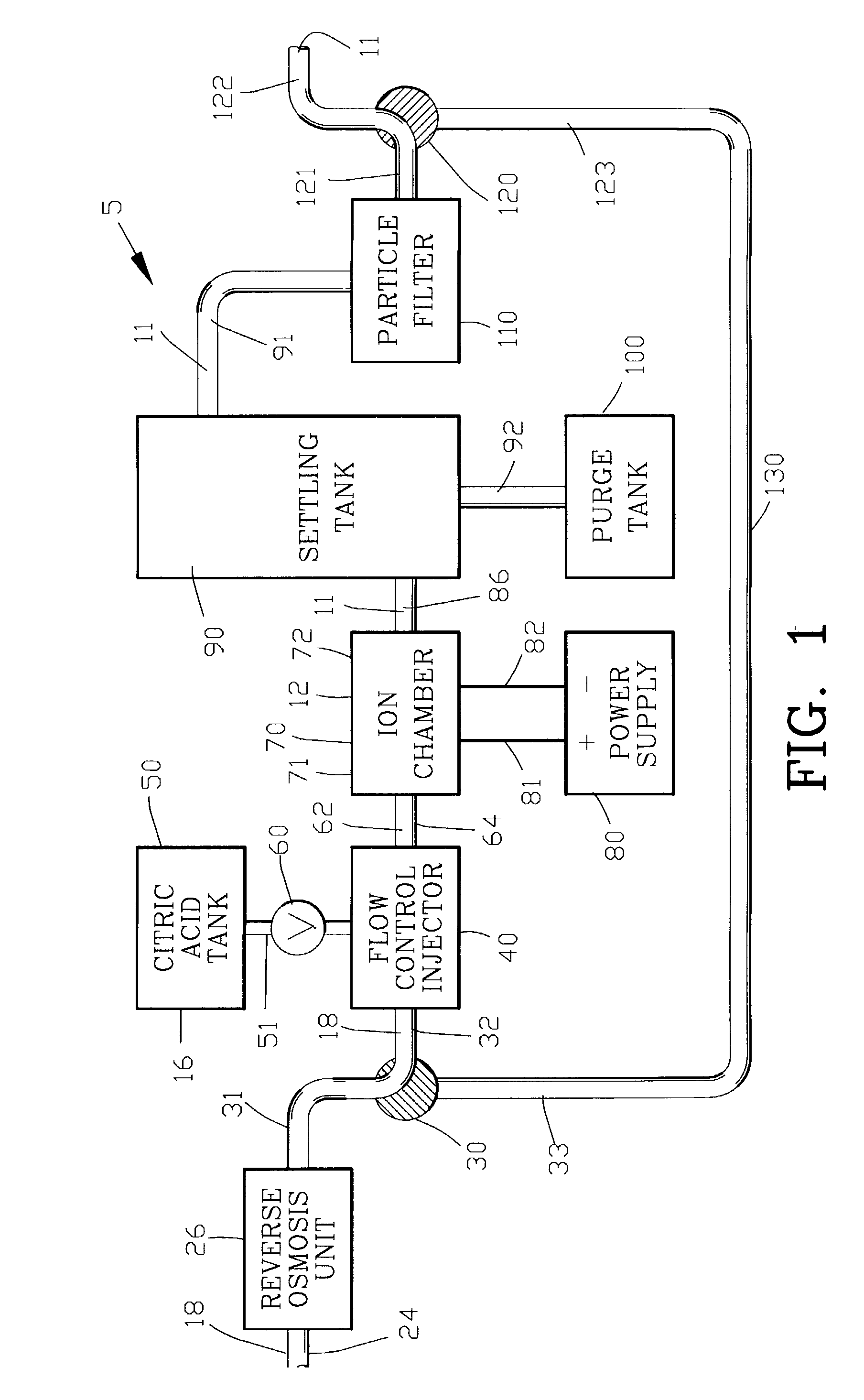
A composition and process is disclosed for treating an acne infection with a composition of electrolytically generated silver citrate. The composition of electrolytically generated silver citrate may be applied topically to the acne infection in an aqueous solution or may be incorporated within a cosmetic product., The acne composition may be combined with a composition component such as a cosmetic, a lotion, an emulsion, a gel or a soap.
Owner:INNOVATIVE MEDICAL SERVICES
Organic Silver Complexes, Their Preparation Methods and Their Methods for Forming Thin Layers
ActiveUS20090120800A1Superior stability and solubilityEasy to prepareGroup 1/11 organic compounds without C-metal linkagesRadiation applicationsThin layerAmmonium carbamate
Owner:INKTEC CO LTD
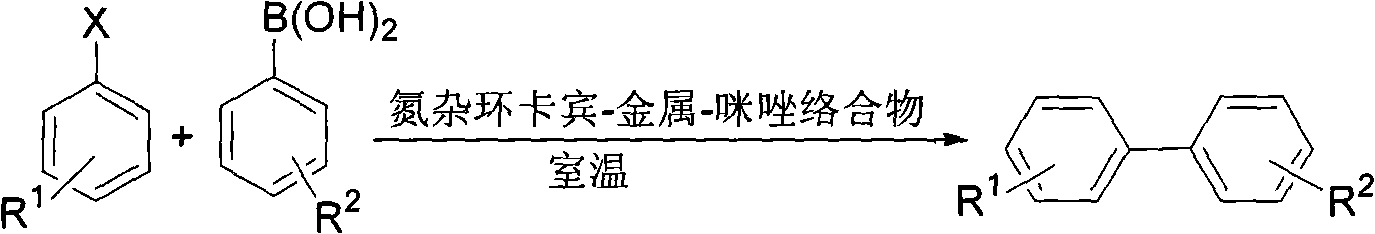
Suzuki-Miyaura coupling reaction of catalyzing aryl chloride by N-heterocyclic carbine-palladium-imidazole complex at room temperature under condition of water phase
InactiveCN102153592AEasy to manufactureLow priceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsBoronic acidCarbene
The invention provides a reaction method which is as follows: starting from simple and available imidazole salts, corresponding N-heterocyclic carbine-metal-imidazole complexes are synthesized; and then a Suzuki-Miyaura coupling reaction of aryl or heteroaryl boronic acid and aryl chloride by utilizing the complexes at room temperature under the condition of a water phase is carried out. The centers of metals related in the metal salts in the invention can be iron, copper, silver, nickel, palladium, cobalt, rhodium, ruthenium and the like. A catalyst used in the invention is easy to prepare, can be quantitatively synthesized and is stable to air and moisture. By using the catalyst, the corresponding coupling reaction is achieved under the condition that catalytic amount is only 0.01%, and yield is excellent. Compared with other systems reported in documents, the catalytic system is cheap in price, reaction is easy to operate and can be smoothly carried out at room temperature, and the requirement on reaction conditions is extremely low, thus the catalytic system has a good industrial application prospect.
Owner:WENZHOU UNIVERSITY
Nitrogen analogs of copper II beta-diketonates as source reagents for semiconductor processing
InactiveUS20030097013A1Furnaces without endless coreGroup 8/9/10/18 element organic compoundsNitrogenSolvent
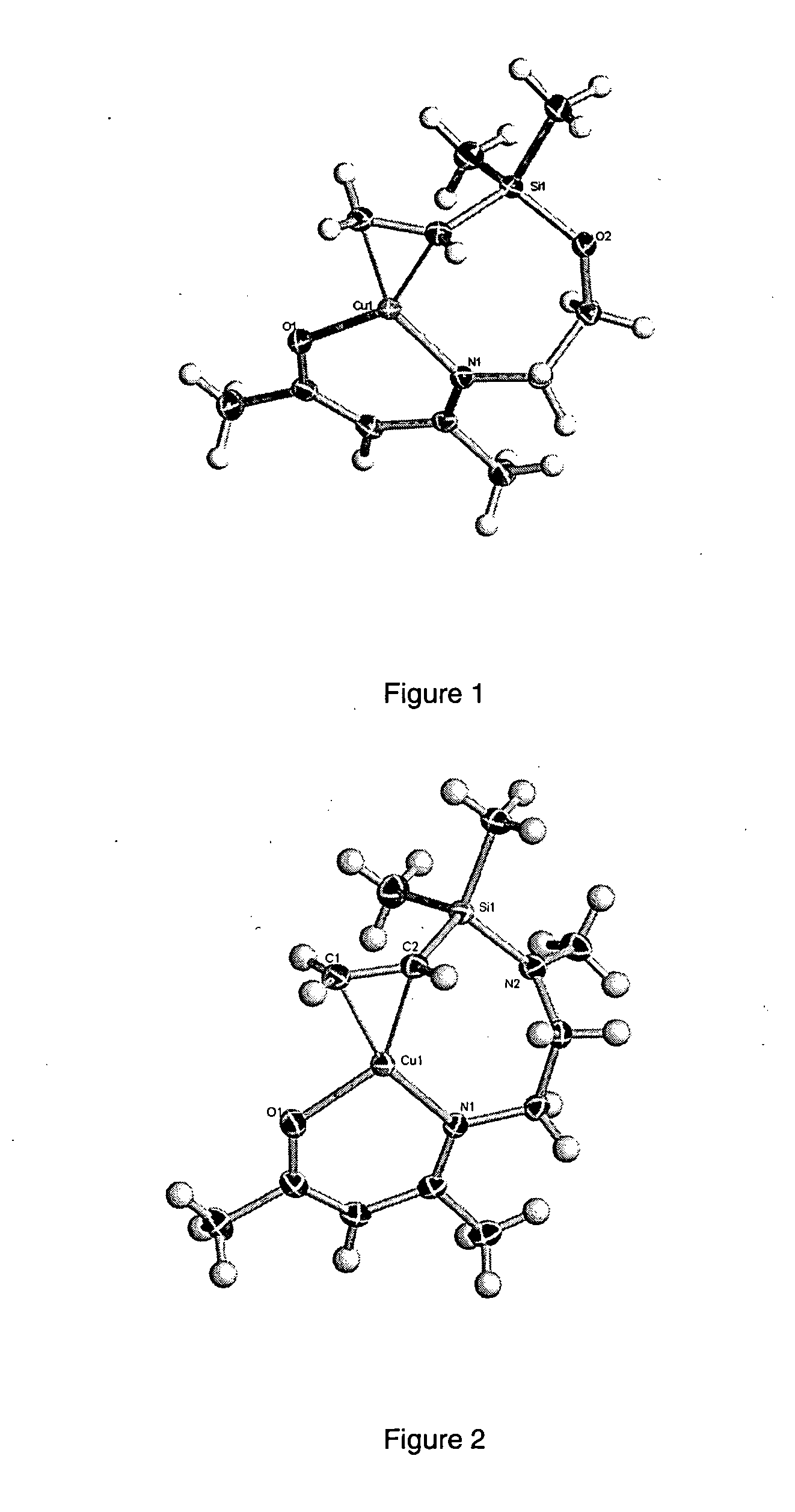
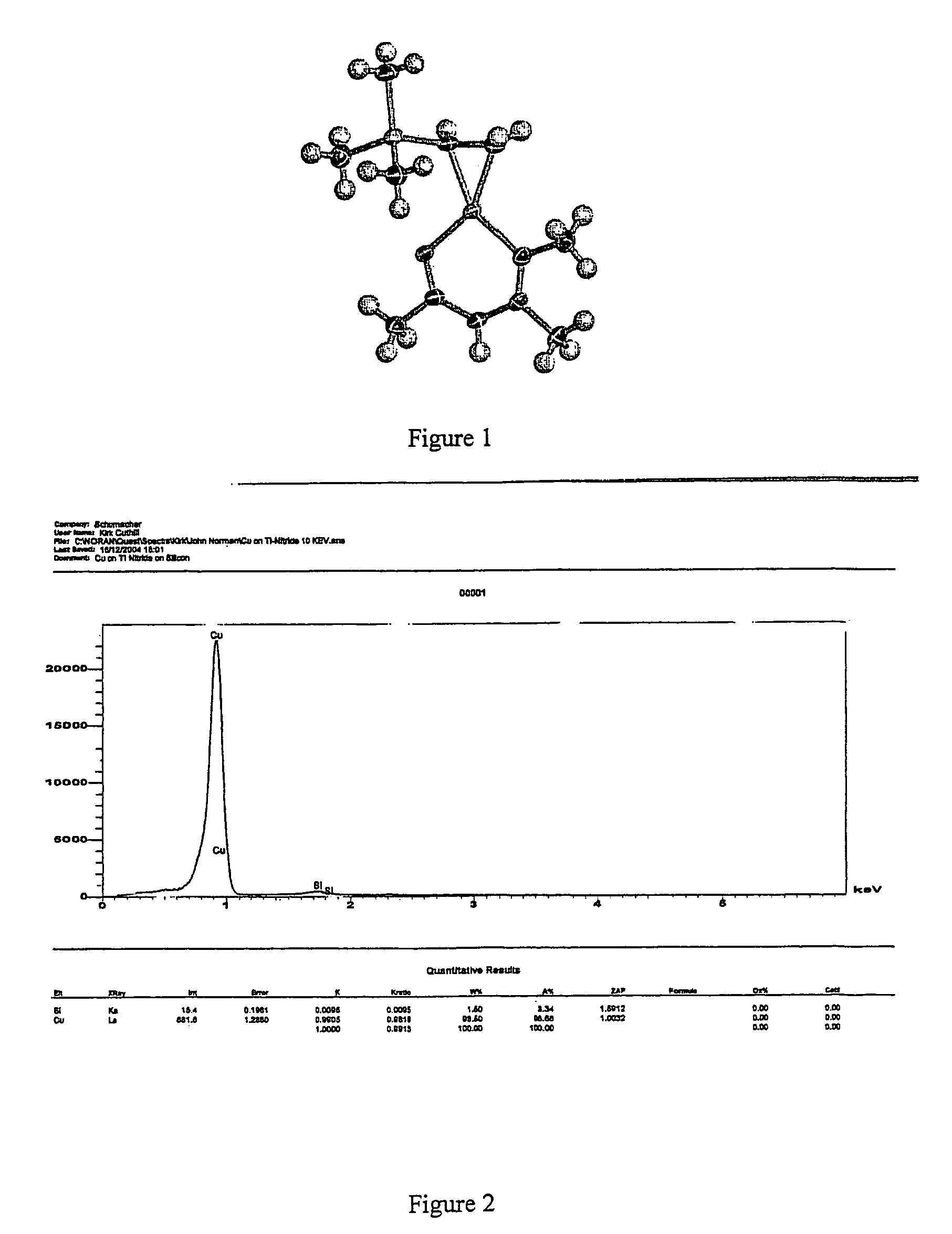
Nitrogen containing analogs of Copper II beta-diketonates which analogs are more stable source reagents for copper deposition when substantially free of solvents of excess ligands. The nitrogen containing analogs replace -O- with -N(R'')-wherein R'' is an alkyl group having from one to four carbon atoms. Replacement of each -O- is preferred although replacement of one -O- per cyclic ring is sufficient to improve stability of the copper source reagents. The source reagent can be purified by sublimation to remove solvents and excess ligands prior to semiconductor processing.
Owner:APPLIED MATERIALS INC
Compounds having guanidine groups and containing semi-organic silicon groups
This invention relates to compounds having guanidine groups and containing semi-organic silicon groups, their use for the curing of compounds containing alkoxysilyl groups, compositions comprising the curing catalysts of the invention, and use of the compositions as adhesives and sealants and also as coating materials.
Owner:EVONIK OPERATIONS GMBH
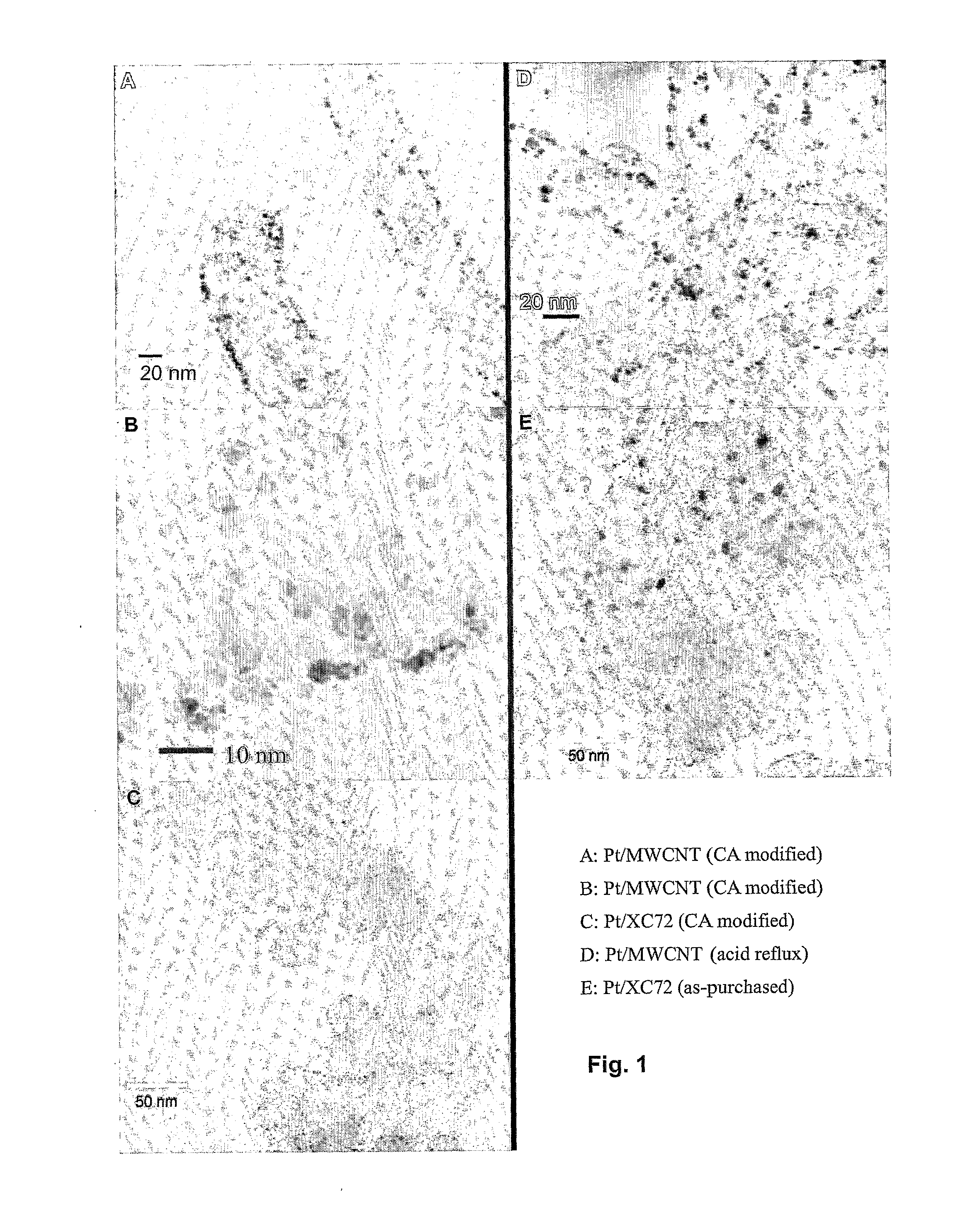
Conductive carbon nanotube-metal composite ink
An electrically conductive carbon nanotube-metal composite ink may include a carbon nanotube-metal composite in which metal nanoparticles are bound to a surface of a carbon nanotube by chemical self-assembly. The electrically conductive carbon nanotube-metal composite ink may have higher electrical conductivity than a commonly used metal nanoparticles-based conductive ink, and may also be used in deformable electronic devices that are flexible and stretchable, as well as commonly used electronic devices, due to the bending and stretching properties of the carbon nanotube itself.
Owner:SAMSUNG ELECTRONICS CO LTD
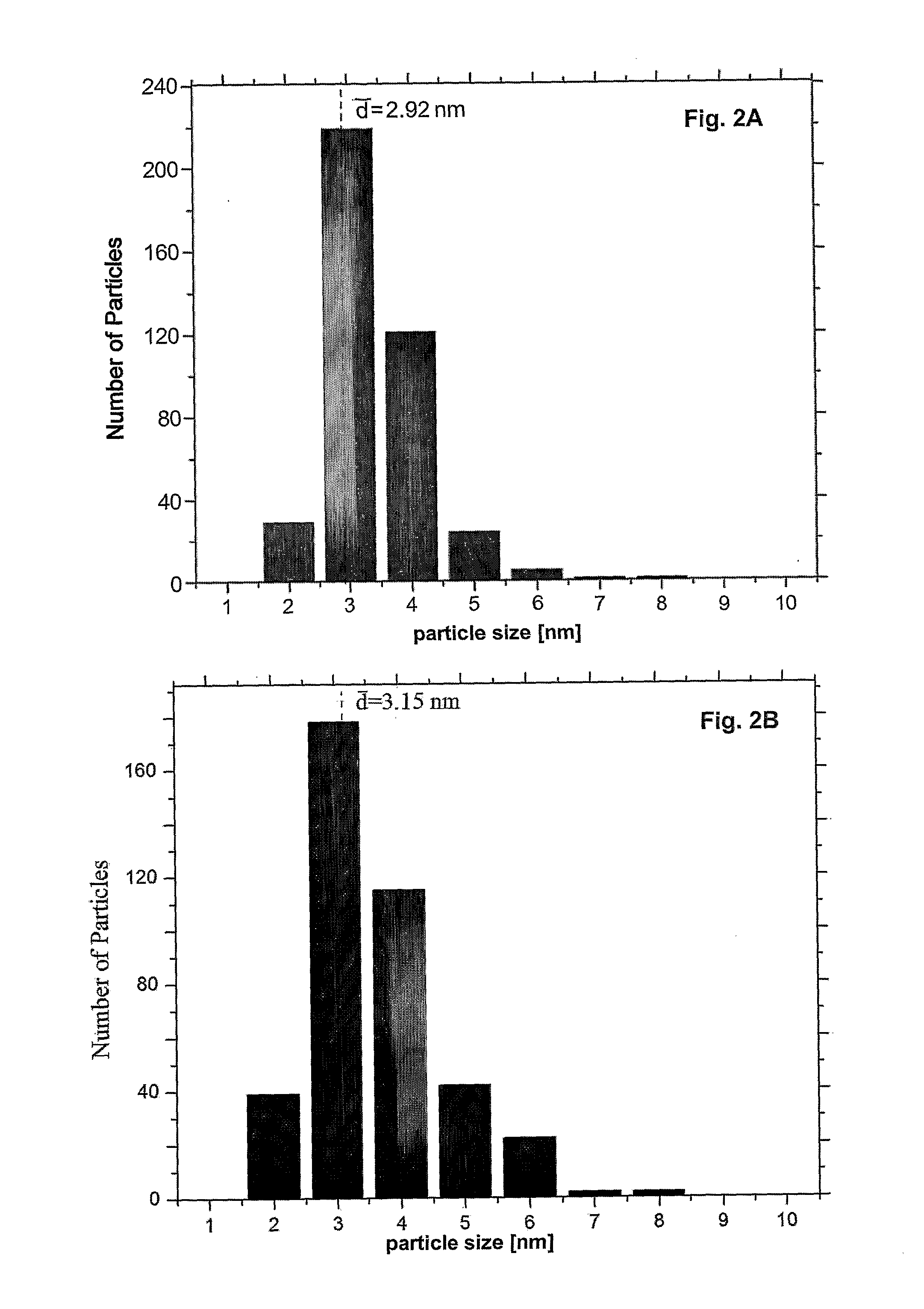
Methods for producing carboxylic acid stabilized silver nanoparticles
ActiveUS20100040863A1Low costGood environmental stabilityMaterial nanotechnologyLayered productsCarboxylic acidMaterials science
Processes for producing carboxylic acid-stabilized silver nanoparticles are disclosed. A reaction mixture comprising a silver salt, a carboxylic acid, and a tertiary amine is heated to form carboxylic acid-stabilized silver nanoparticles.
Owner:XEROX CORP
Metal complex using plumbagin as ligand, synthesizing method and usage thereof
InactiveCN101037447AGood antitumor activityHigh medicinal valueCopper organic compoundsNickel organic compoundsSynthesis methodsMetal chelate
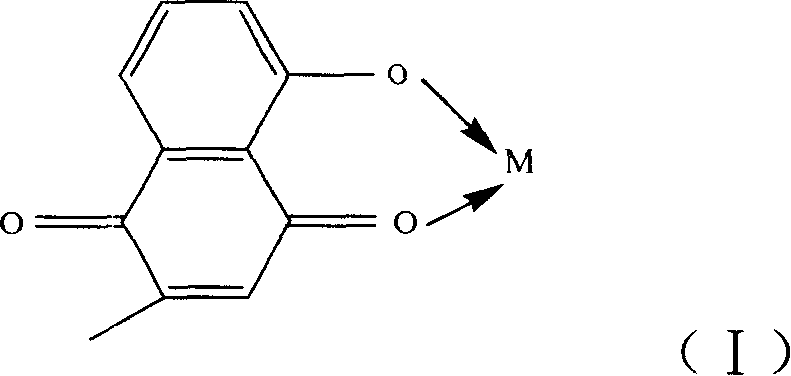
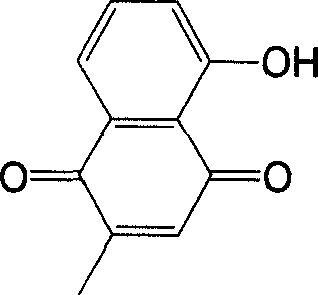
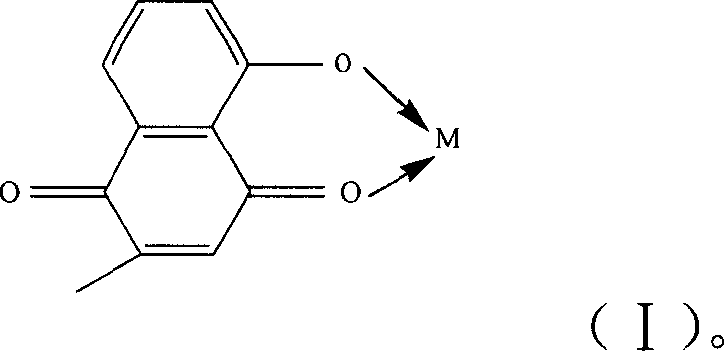
The invention discloses a metal chelate which takes plumbagin as ligand, and the synthesis method and application thereof. The metal chelate is represented by the right formula, wherein, M represents the metal ion able to accept lone pair electrons can be main group matel ion, or transition metal ion, or rare earth metal ion such as Ca(II), Mg(II), Sn(IV), Cu(II), Co(II), Ni(II), Zn(II), Fe(II), Mn(II), Ag(I), Mo(VI), Pd(II), Ru(III), W(VI), Pt(II), Y(III), La(III), Nd(III), Sm(III), Eu(III), Er(III). The invention synthesizes a series of metal chelate which takes plumbagin as ligand and reacts with the metal salt. The produced chelate has an inhibition to a plurality of tumor line. The result indicates a plurality of chelate having a more remarkable anti-tumor activity than the plumbagin and have a higher medicine value and can be used for the preparation of different anti-tumor medicine.
Owner:GUANGXI NORMAL UNIV
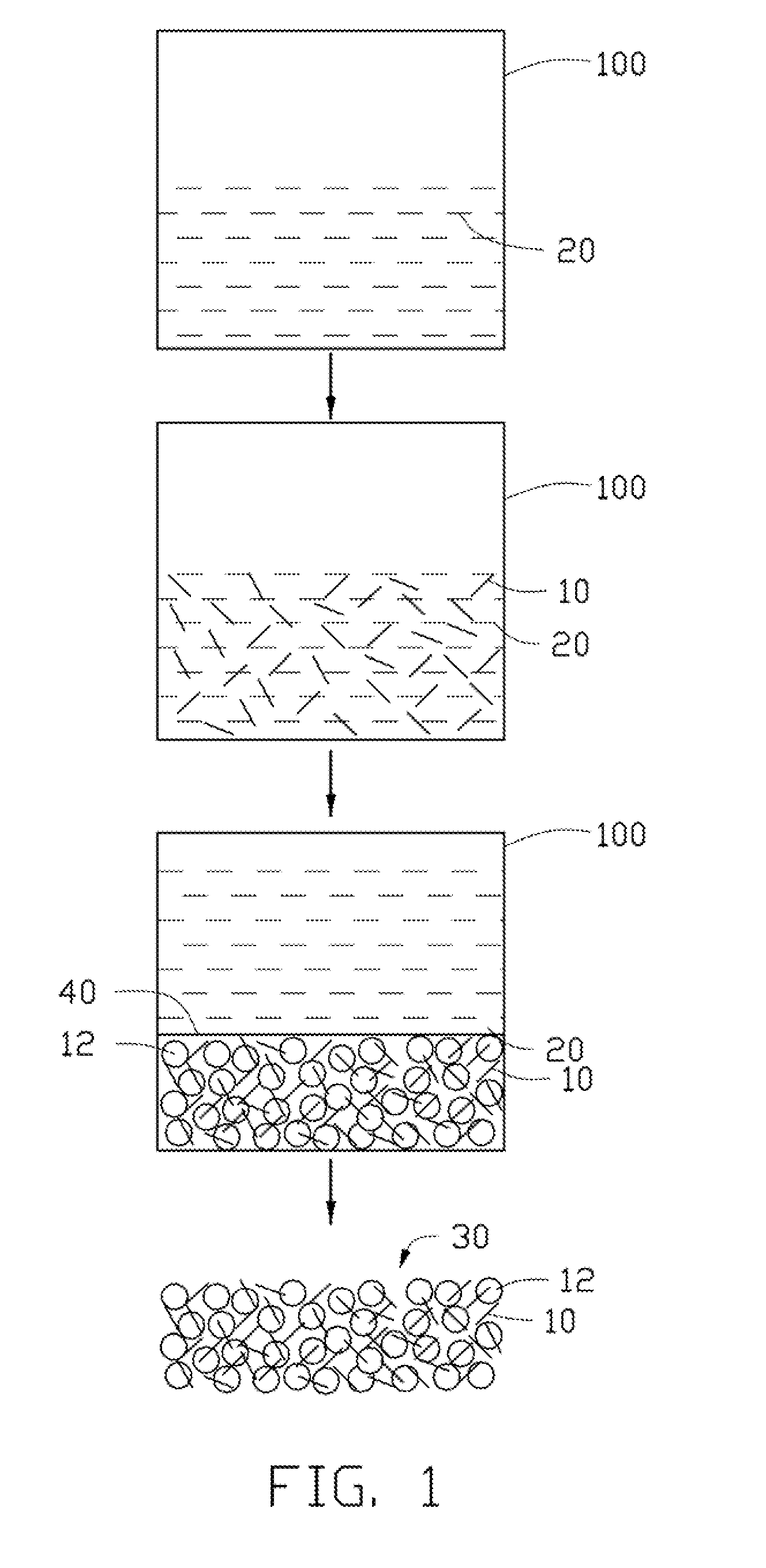
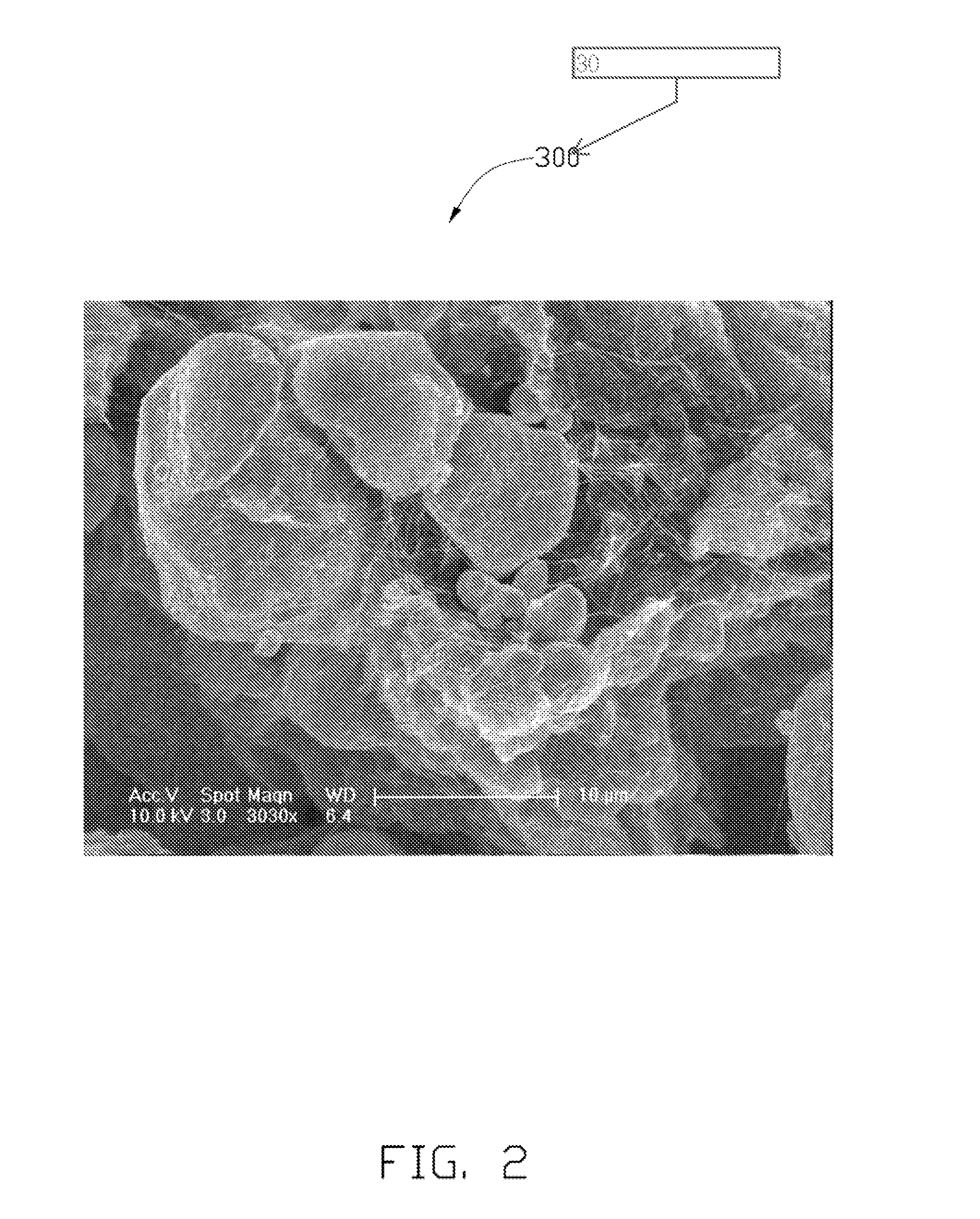
Method for making carbon nanotube metal composite
ActiveUS20110180968A1Preparation from carboxylic acid saltsOrganic compound preparationCarbon nanotubeSolvent
A method for making a carbon nanotube metal composite includes the following steps. A number of carbon nanotubes is dispersed in a solvent to obtain a suspension. Metal powder is added into the suspension, and then the suspension agitated. The suspension containing the metal powder is allowed to stand for a while. The solvent is reduced to obtain a mixture of the number of carbon nanotubes and the metal powder.
Owner:TSINGHUA UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com