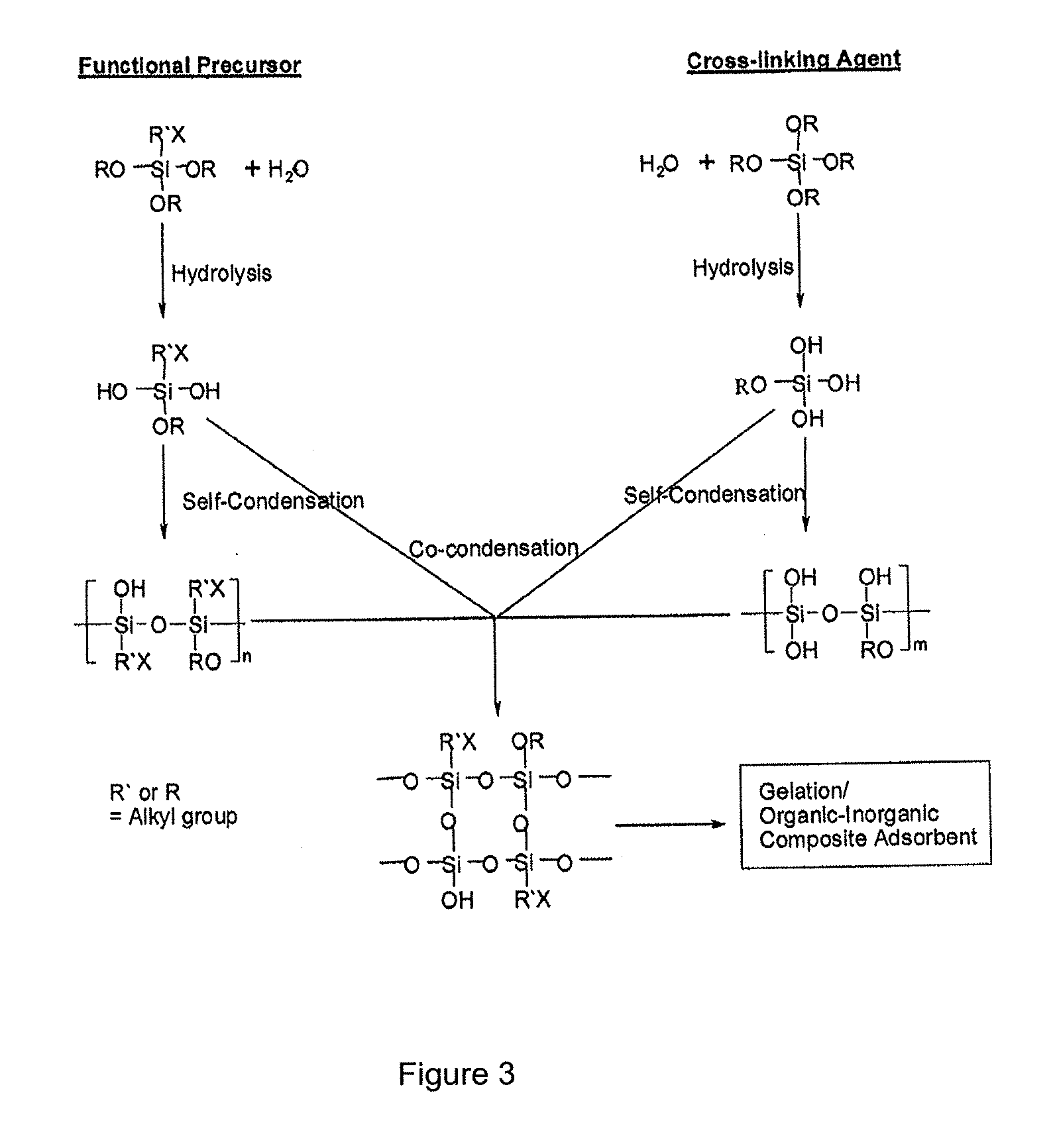
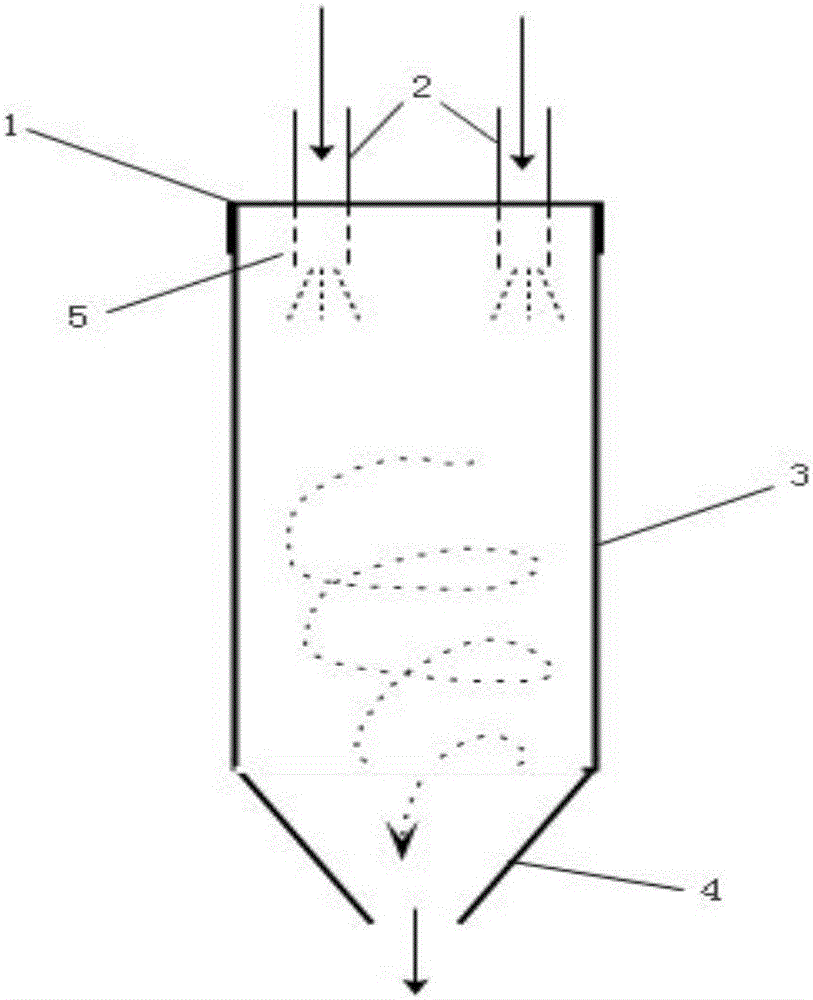
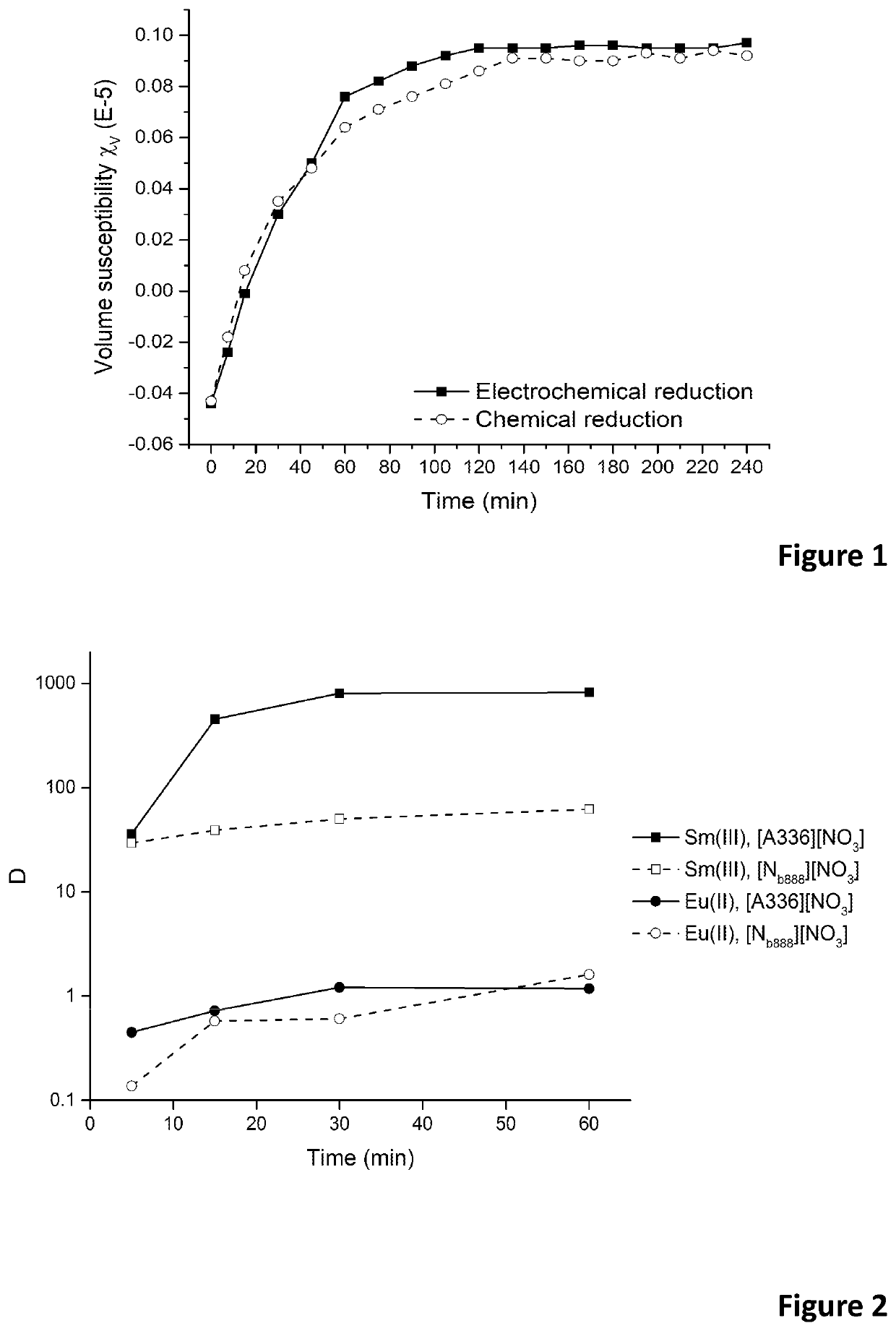
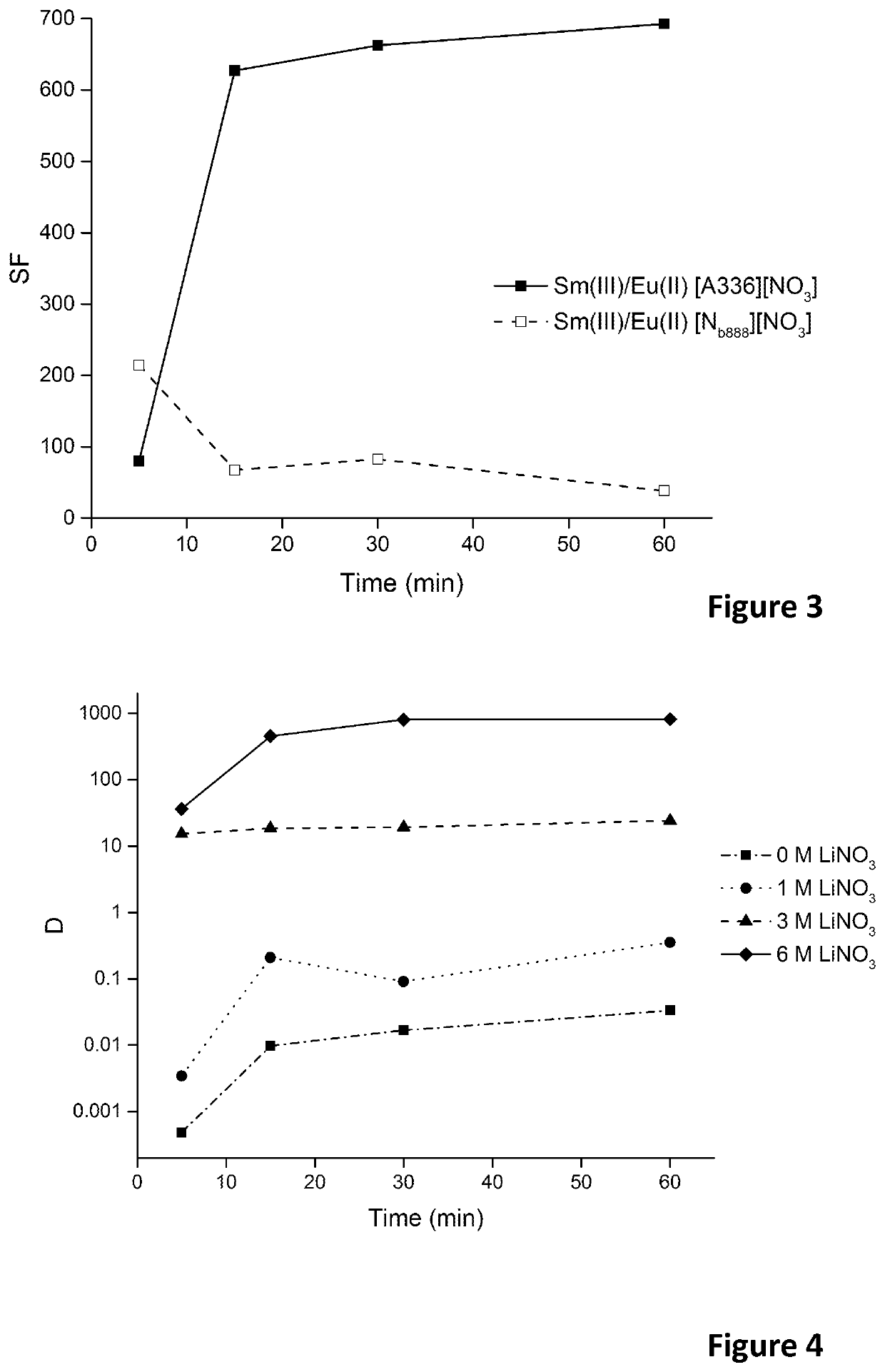
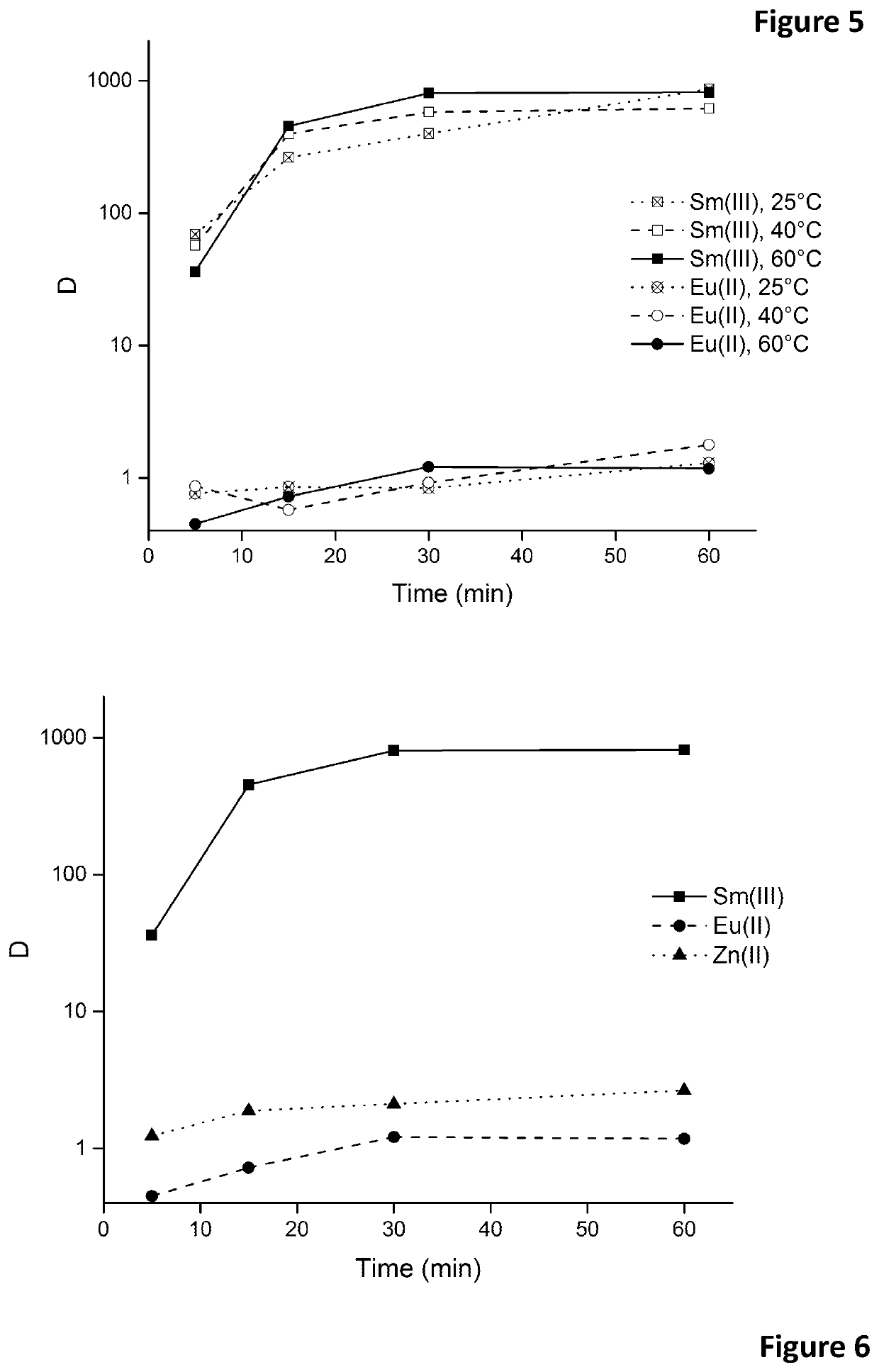
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36results about "Rare earth metal nitrates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for recovering rare earth, aluminum and silicon from rare earth-containing aluminum and silicon wastes
ActiveCN106319218AReduce dosageEfficient separationRare earth metal oxides/hydroxidesRare earth metal chloridesAluminum IonAluminium hydroxide
The invention provides a method for recovering rare earth, aluminum and silicon from rare earth-containing aluminum and silicon wastes. The method comprises the following steps: 1, carrying out acid dipping on the rare earth-containing aluminum and silicon wastes by using an aqueous inorganic acid solution to obtain silicon-rich residues and an acid dipping solution containing rare earth ions and aluminum ions; 2, adding an alkaline substance to the acid dipping solution containing rare earth ions and aluminum ions to control the pH value of the acid dipping solution to be 3.5-5.2, and carrying out solid-liquid separation to obtain an aluminum hydroxide-containing precipitate and a rare earth-containing filtrate; and 3, reacting the aluminum hydroxide-containing precipitate with sodium hydroxide to obtain a sodium metaaluminate solution and aluminum and silicon residues, and using the rare earth-containing filtrate to prepare a rare earth compound product. Aluminum and rare earth are dissolved in the acid, segmented alkaline transfer is carried out, the aluminum ions are precipitated to obtain aluminum hydroxide and the rare earth ions which are separated from the aluminum hydroxide, and excess sodium hydroxide is added to convert aluminum hydroxide into the sodium metaaluminate solution, so simultaneous and high-efficiency recycling of the rare earth and aluminum is realized, the use amount of sodium hydroxide is greatly reduced, and the recovery cost is reduced.
Owner:GRIREM ADVANCED MATERIALS CO LTD
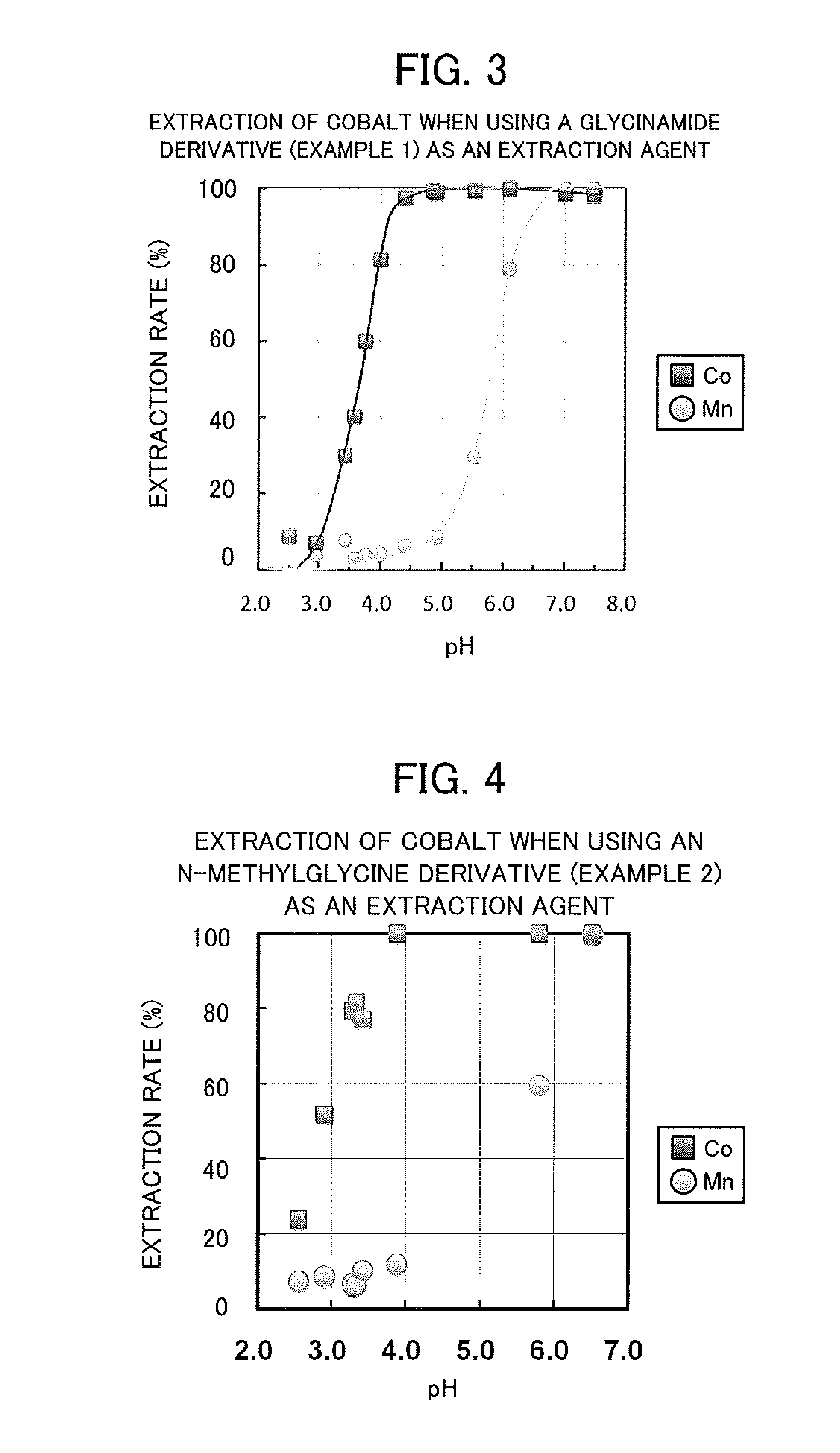
Valuable metal extraction agent and valuable metal extraction method using said extraction agent
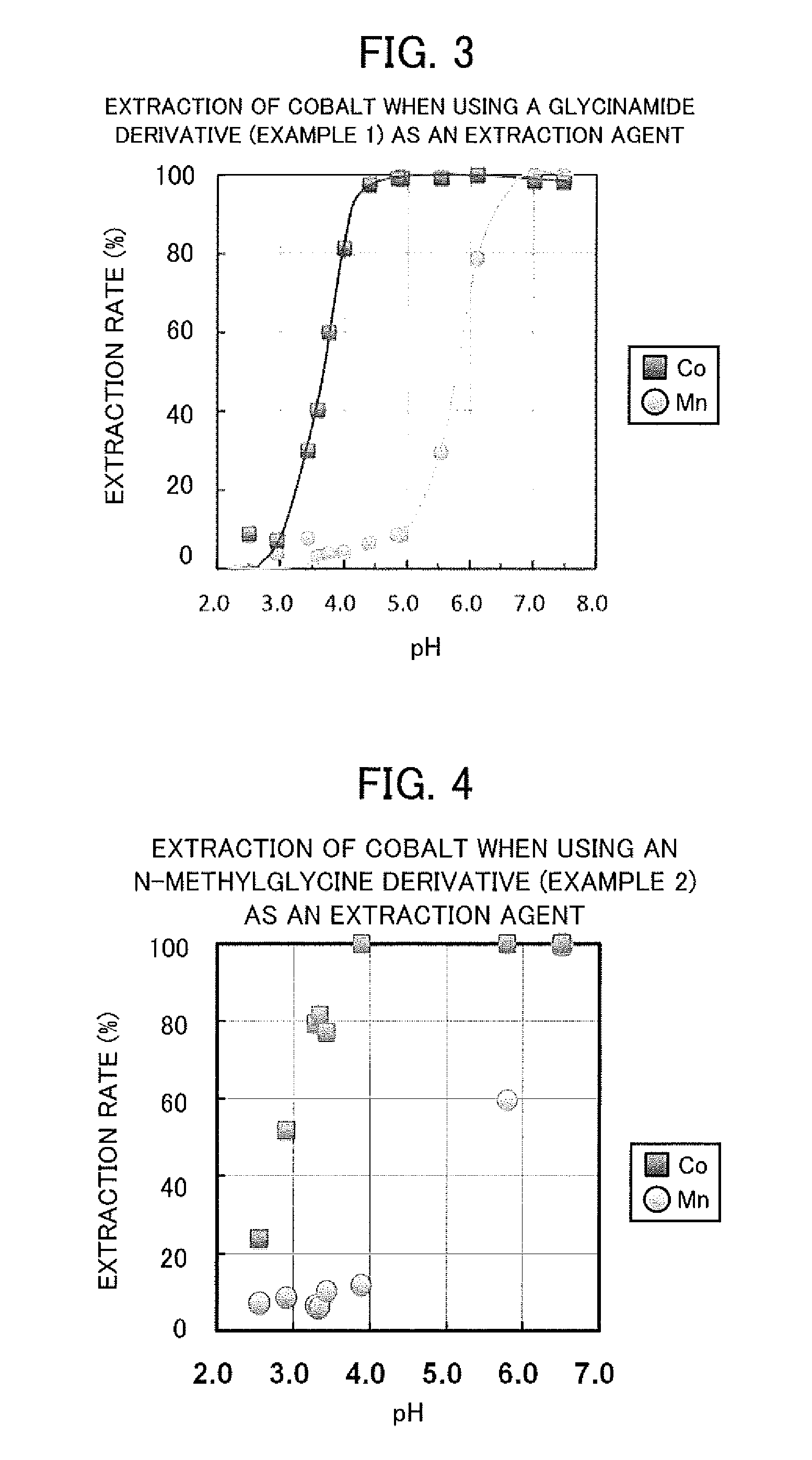
The objective of the present invention is to selectively extract light rare earth metals, and by extension, europium, from an acidic solution containing a plurality of types of rare earth metal. This valuable metal extraction agent is represented by the general formula. In the formula: R1 and R2 each indicate the same or different alkyl group; R3 indicates a hydrogen atom or an alkyl group; and R4 indicates a hydrogen atom or any given group other than an amino group bonded to the α carbon as an amino acid. Preferably, the general formula has a glycine unit, a histidine unit, a lysine unit, an aspartic acid unit, or an N-methylglycine unit. Preferably, when extracting europium using the extraction agent, the pH is adjusted into the range of 2.0-3.0 inclusive, and when selectively extracting light rare earth metals, the pH is adjusted to 1.7-2.7 inclusive.
Owner:KYUSHU UNIV +1
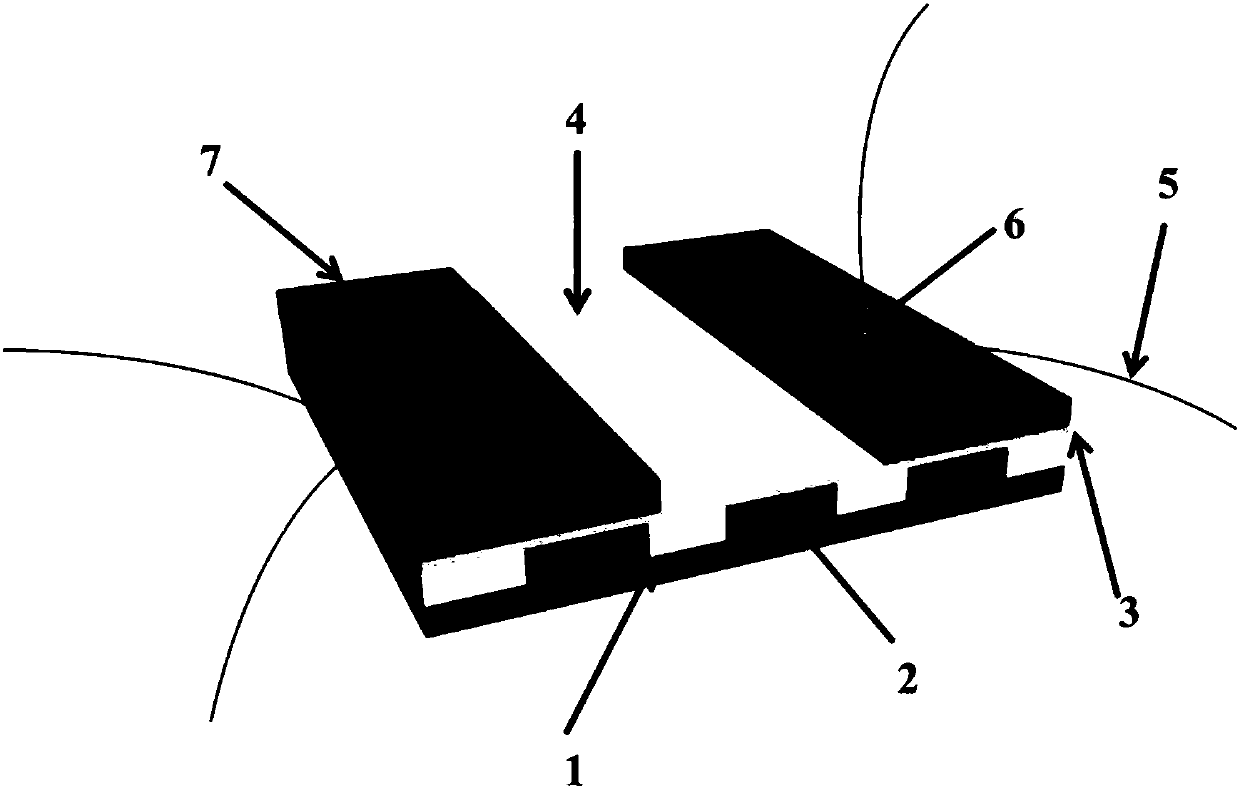
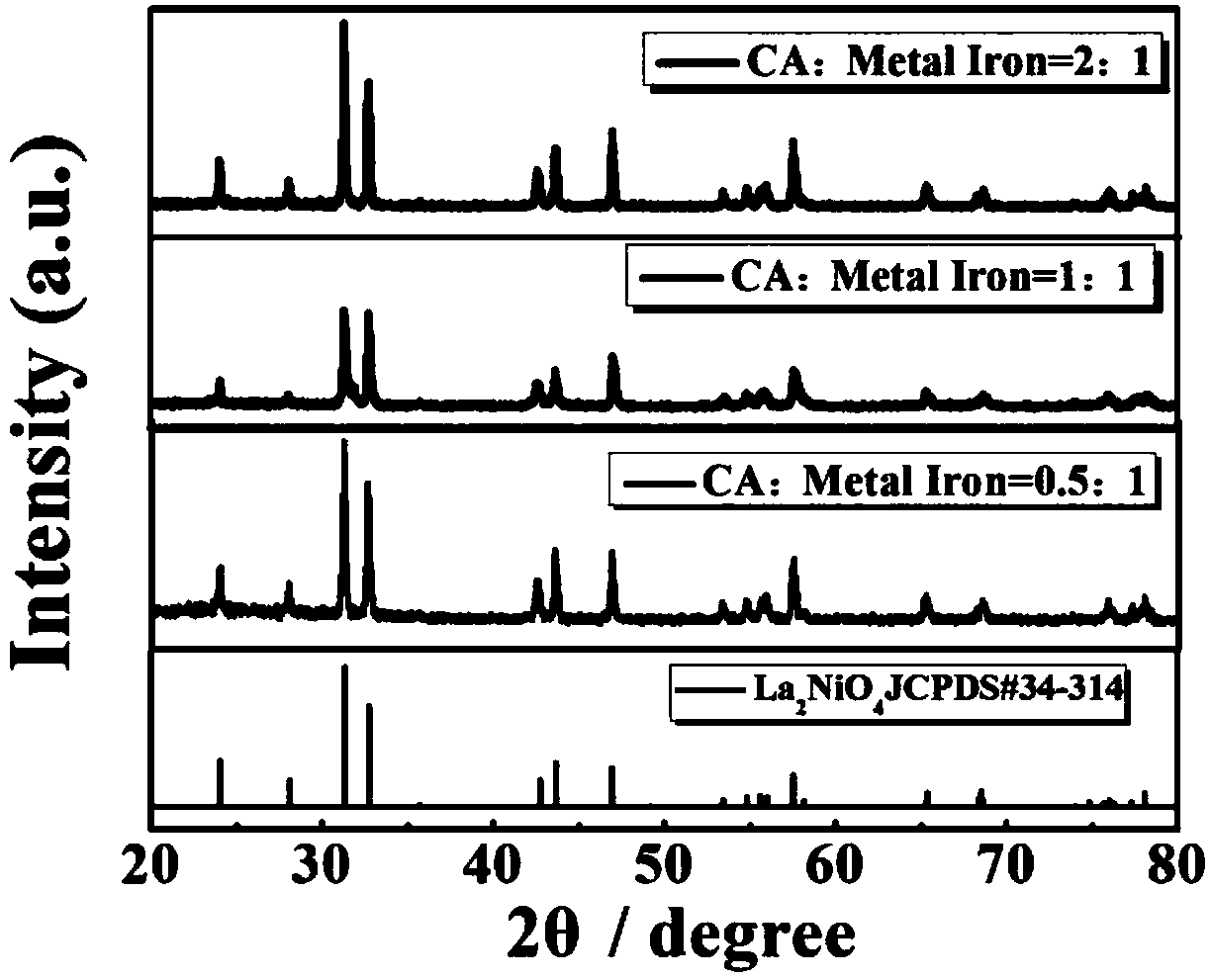
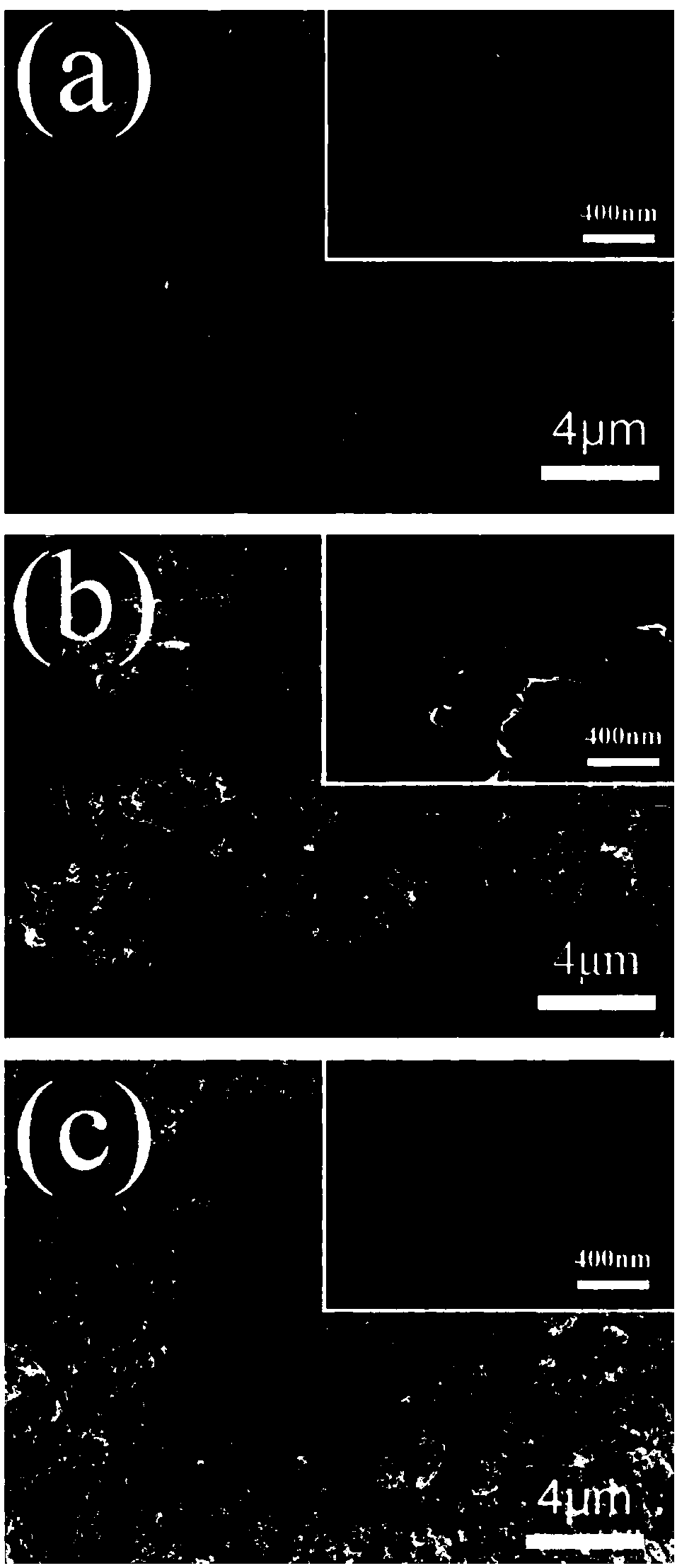
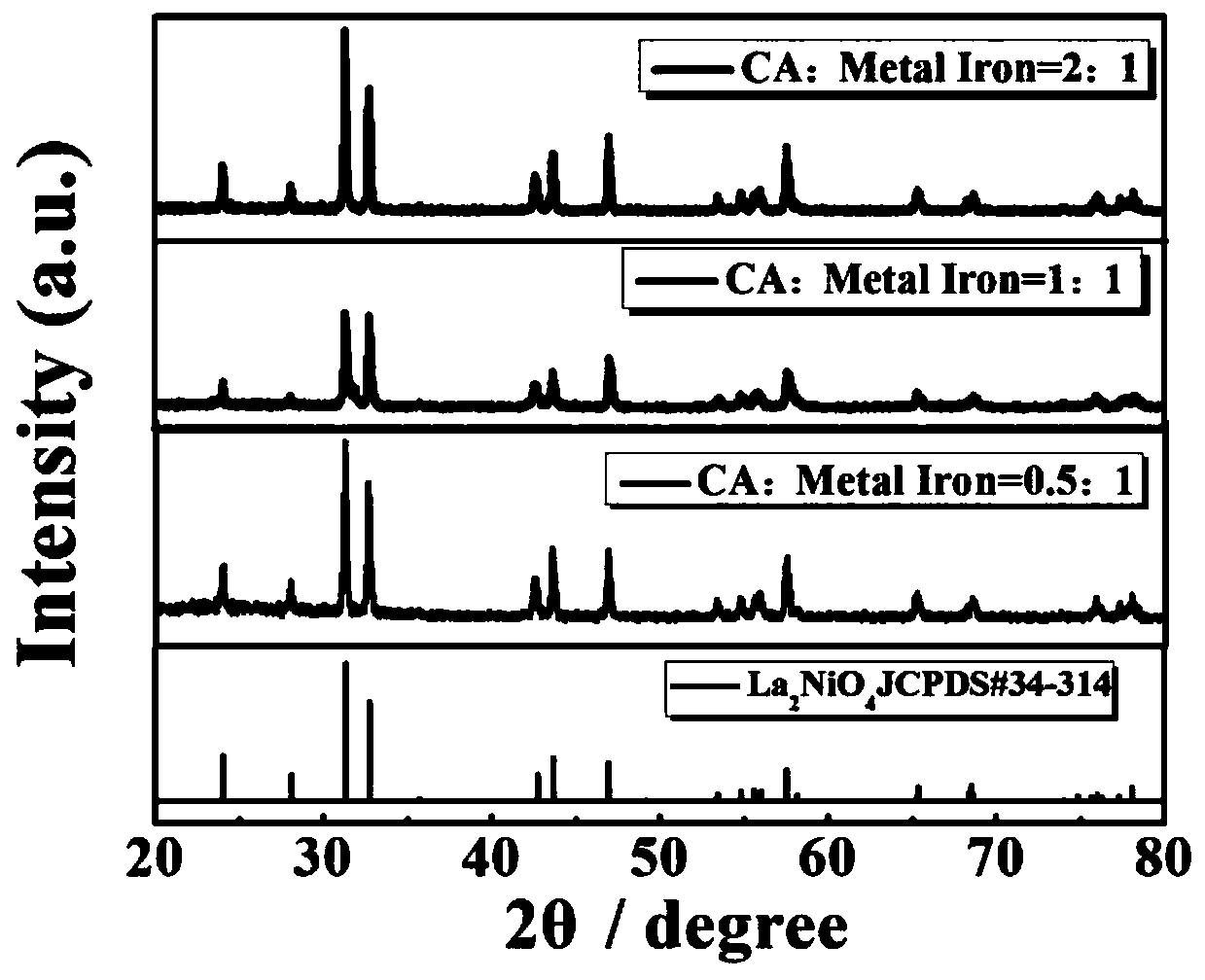
YSZ (yttria-stabilized zirconia)-based mixed potential type H2S sensor with La2NiO4 used as sensitive electrode and method for preparing YSZ-based mixed potential type H2S sensor
ActiveCN107655948AImprove thermal stabilityGood chemical stabilityRare earth metal nitratesMaterial electrochemical variablesToxic gasSulfur hydride
The invention discloses a YSZ (yttria-stabilized zirconia)-based mixed potential type hydrogen sulfide (H2S) sensor with La2NiO4 used as a sensitive electrode and a method for preparing the YSZ-basedmixed potential type hydrogen sulfide sensor, and belongs to the technical field of gas sensors. The YSZ-based mixed potential type hydrogen sulfide sensor is mainly used for detecting toxic gas H2S in industrial production and daily life. The YSZ-based mixed potential type hydrogen sulfide sensor sequentially comprises an Al2O3 ceramic plate with Pt heating electrodes, a YSZ-based plate, a Pt reference electrode and the La2NiO4 sensitive electrode. The reference electrode and the sensitive electrode are independently and symmetrically prepared at two ends of the upper surface of the YSZ-basedplate, and the lower surface of the YSZ-based plate is adhered with the Al2O3 ceramic plate with the Pt heating electrodes. The YSZ-based mixed potential type hydrogen sulfide sensor and the method have the advantages that the La2NiO4 with high electrochemical catalytic activity is used as the sensitive electrode, the quantities of citric acid and the like which are added in material synthesis procedures are changed, accordingly, the electrochemical catalytic activity of sensitive electrode materials can be enhanced, and effects of improving the sensitivity characteristics of the YSZ-based mixed potential type hydrogen sulfide sensor can be realized; a short temperature pulse is applied in sensor recovery procedure, accordingly, the recovery time of the YSZ-based mixed potential type hydrogen sulfide sensor can be shortened, and the purpose of enhancing the response and recovery characteristics of the YSZ-based mixed potential type hydrogen sulfide sensor can be achieved.
Owner:JILIN UNIV
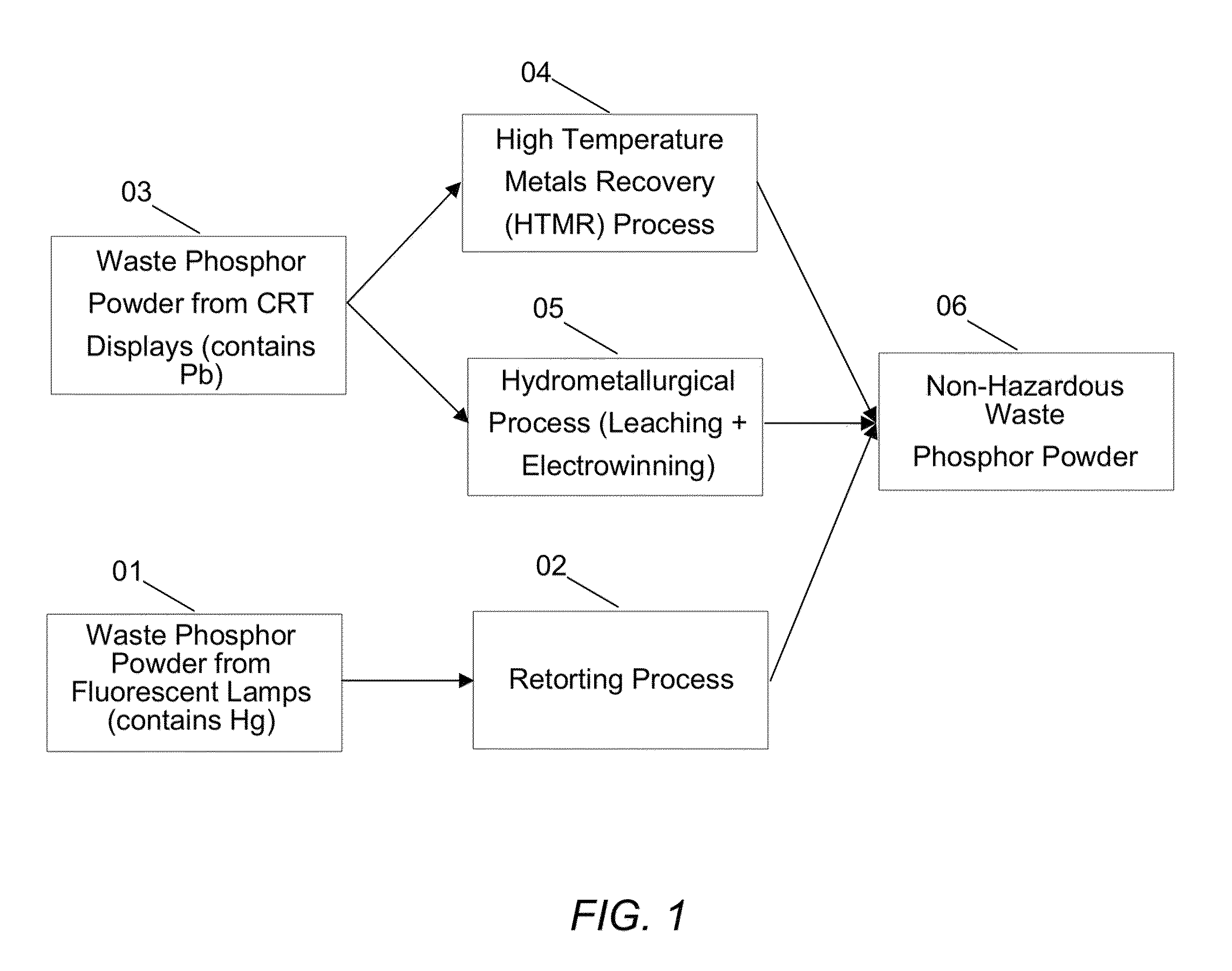
Rare earth recovery from fluorescent material and associated method
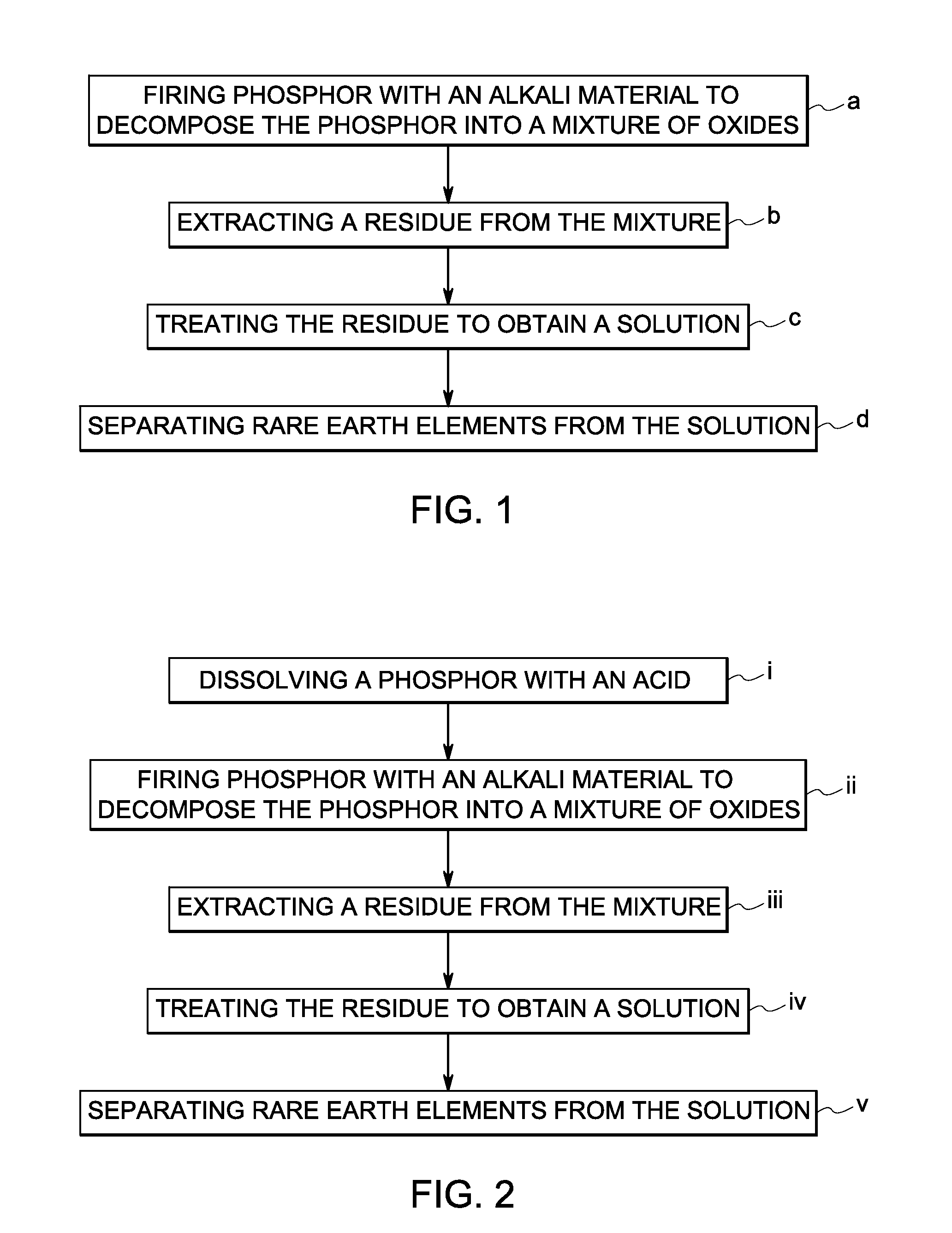
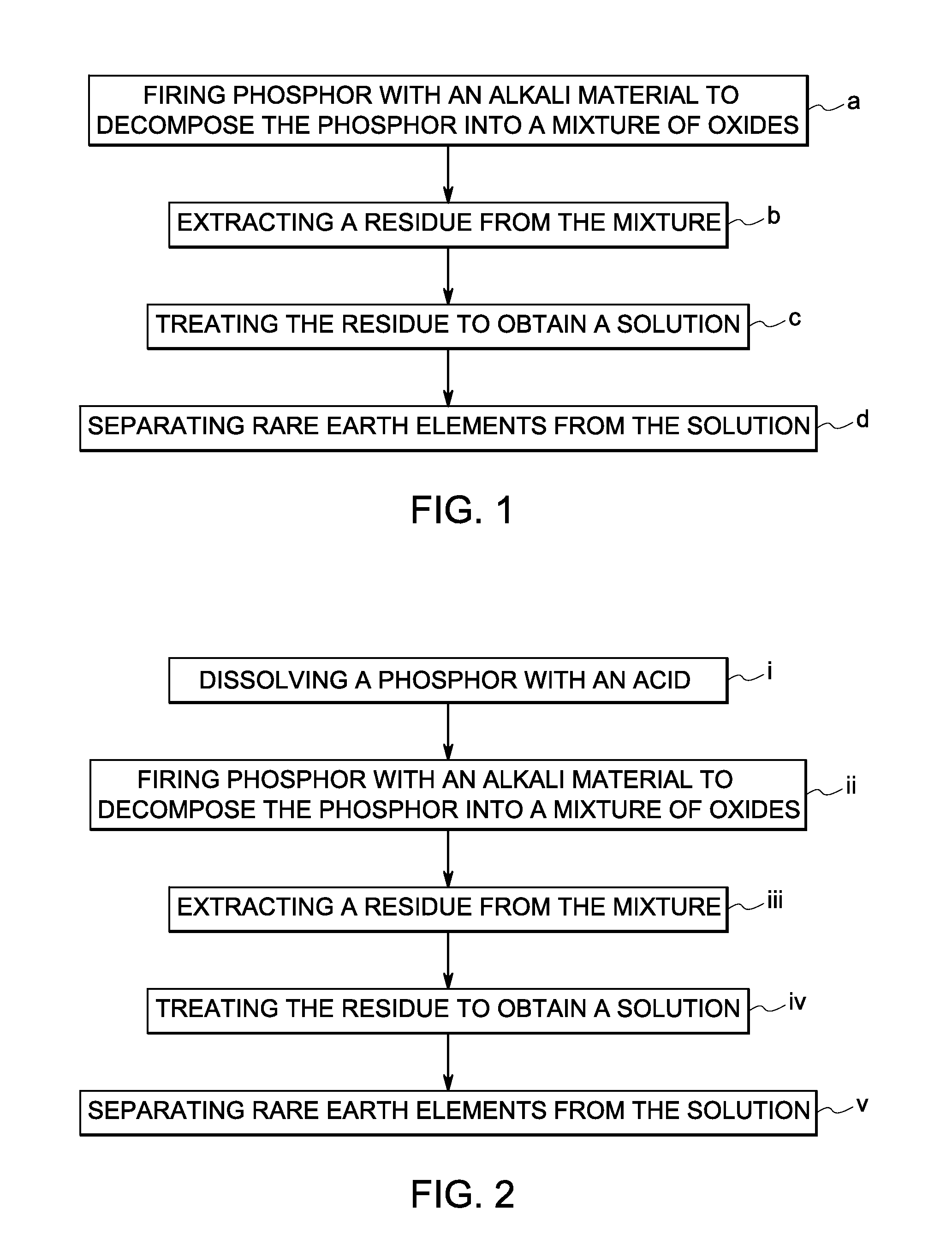
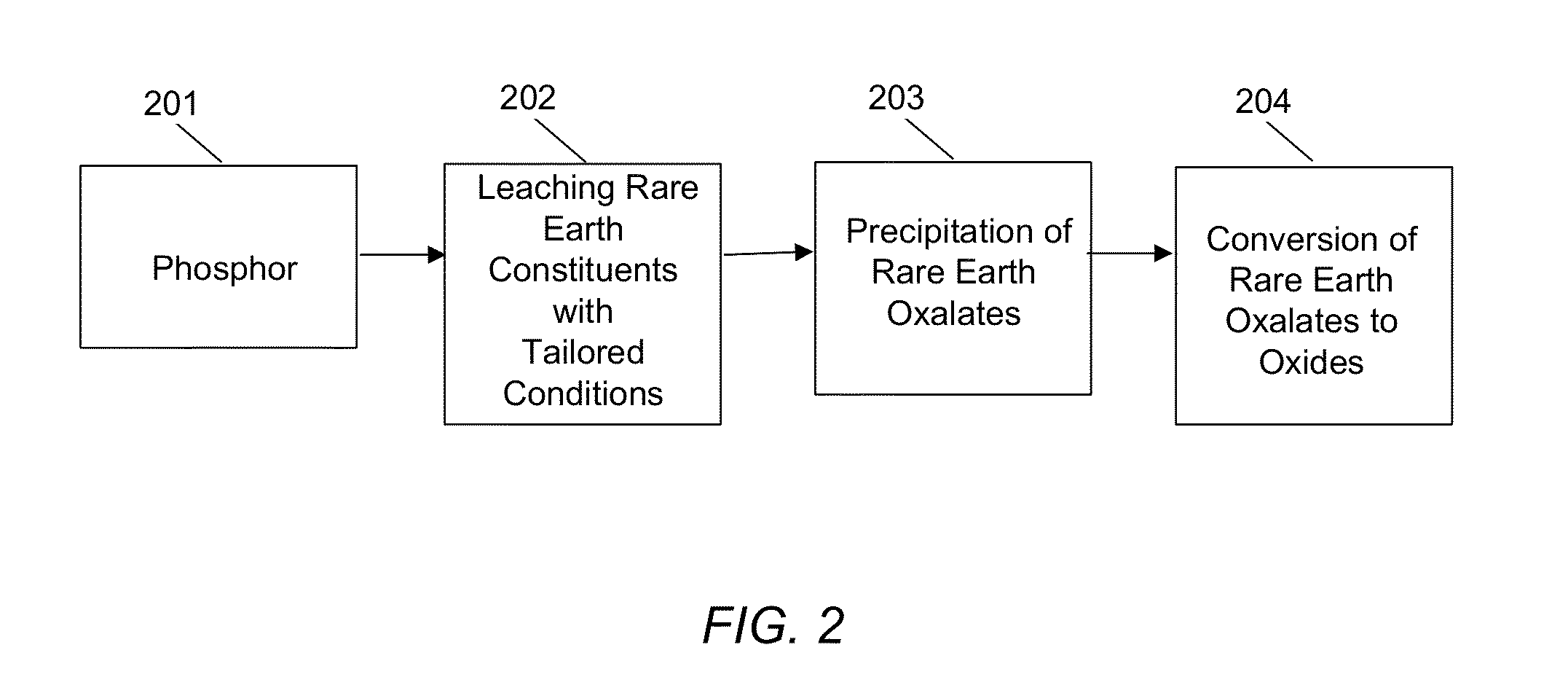
A method of recovering a rare earth constituent from a phosphor is presented. The method can include a number of steps (a) to (d). In step (a), the phosphor is fired with an alkali material under conditions sufficient to decompose the phosphor into a mixture of oxides. A residue containing rare earth oxides is extracted from the mixture in step (b). In step (c), the residue is treated to obtain a solution, which comprises rare earth constituents in salt form. Rare earth constituents are separated from the solution in step (d).
Owner:GENERAL ELECTRIC CO
Rare earth recovery from fluorescent material and associated method
A method of recovering a rare earth constituent from a phosphor is presented. The method can include a number of steps (a) to (d). In step (a), the phosphor is fired with an alkali material under conditions sufficient to decompose the phosphor into a mixture of oxides. A residue containing rare earth oxides is extracted from the mixture in step (b). In step (c), the residue is treated to obtain a solution, which comprises rare earth constituents in salt form. Rare earth constituents are separated from the solution in step (d).
Owner:GENERAL ELECTRIC CO
Rare Earth Elements Separation Using Phosphorus Based Adsorbent
ActiveUS20120100049A1Large capacityControlled distributionWater treatment parameter controlLanthanide oxides/hydroxidesRare-earth elementSorbent
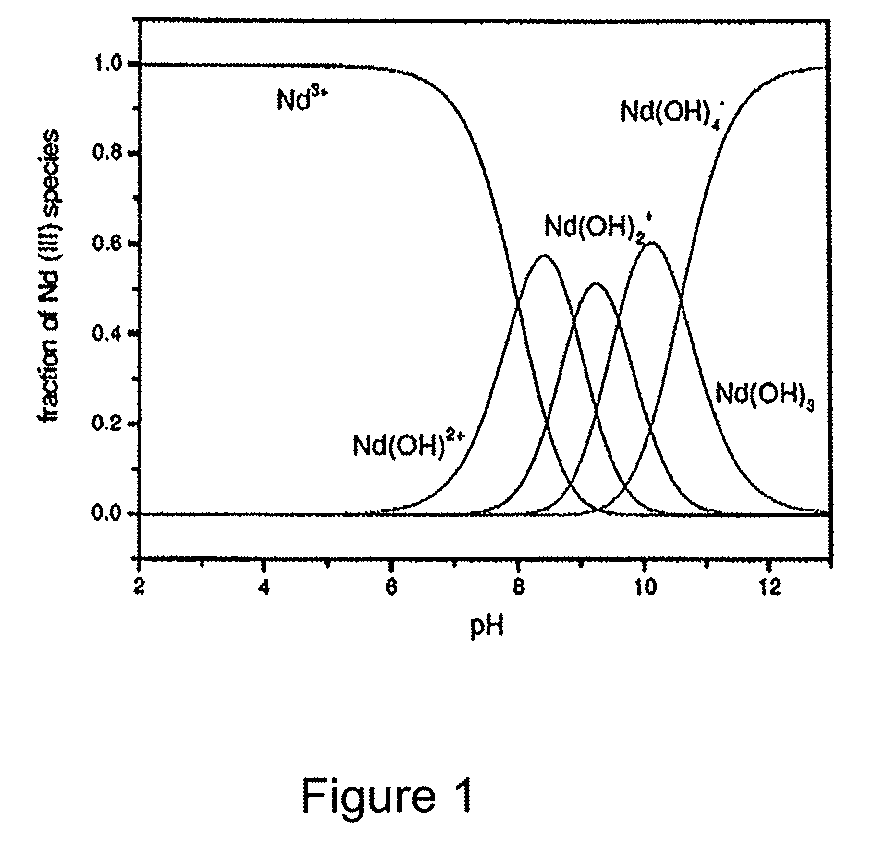
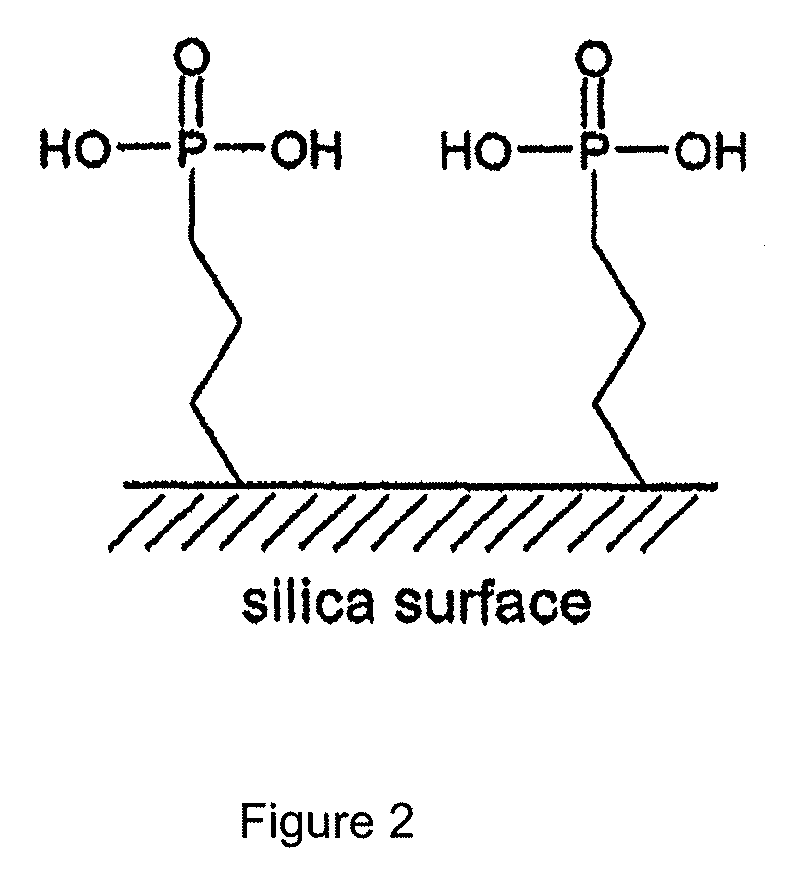
The present invention relates to methods for the separation of rare earth elements from aqueous solutions and, more particularly, to the separation of lanthanides (e.g., neodymium(III)) from aqueous solutions using an organo phosphorus functionalized adsorbent.
Owner:SYRACUSE UNIVERSITY
Cobalt extraction method
The objective of the present invention is to selectively extract cobalt from an acidic solution containing a high concentration of manganese. This cobalt extraction method extracts cobalt from an acidic solution containing manganese and cobalt by subjecting the acidic solution to solvent extraction by means of a valuable metal extraction agent comprising an amide derivative represented by general formula (I). The valuable metal extraction agent is represented by the general formula. In the formula: R1 and R2 each represent the same or different alkyl group; R3 represents a hydrogen atom or an alkyl group; and R4 represents a hydrogen atom or any given group aside from an amino group bonded to the α carbon as an amino acid. Preferably, the general formula has a glycine unit, a histidine unit, a lysine unit, an aspartic acid unit, or an N-methylglycine unit. Preferably, the pH of the acidic solution is 3.5-5.5 inclusive.
Owner:KYUSHU UNIV +1
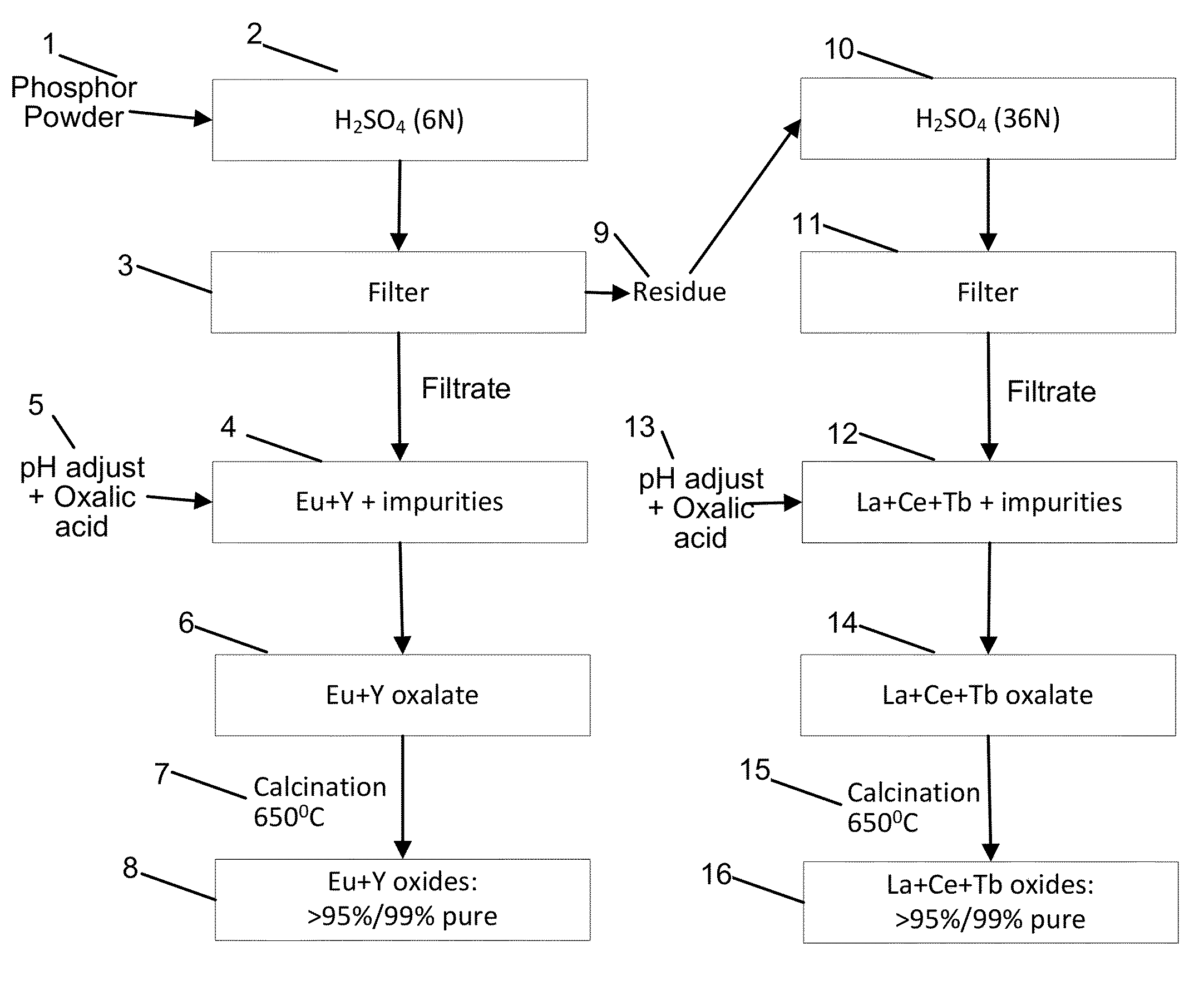
Rare earth recovery from phosphor
InactiveUS20130156660A1Efficient methodEfficient and effectivePhysical/chemical process catalystsRare earth metal chloridesPhosphorRare earth
A method is described to produce high purity rare earth oxides of the elements La, Ce, Tb, Eu and Y from phosphor, such as waste phosphor powders originating in various consumer products. One approach involves leaching the powder in two stages and converting to two groups of relatively high purity mixed rare earth oxides. The first group containing Eu and Y is initially separated by solvent extraction. Once separated, Eu is purified using Zn reduction with custom apparatus. Y is purified by running another solvent extraction process using tricaprylmethylammonium chloride. Ce is separated from the second group of oxides, containing La, Ce and Tb by using solvent extraction. Subsequently, La and Tb are separated from each other and converted to pure oxides by using solvent extraction processes. A one-stage leaching process, wherein all rare earths get leached into the solution and subsequently processed, is also described.
Owner:REENEWAL
Shaded Zirconia Ceramic Material and Machinable Sintered Ceramic Bodies Made Therefrom
ActiveUS20180237345A1Improve flexural strengthUniform colorImpression capsRare earth metal nitratesMachiningZirconia ceramic
Shaded, zirconia ceramic materials are disclosed that are suitable for use in dental applications. Ceramic bodies are made from a zirconia-containing ceramic material and a coloring composition comprising a terbium (Tb)-containing component and a chromium (Cr)-containing component as a coloring agent. The pre-shaded ceramic body is machinable into a dental restoration either as a bisque body or sintered body. A pre-shaded machinable sintered ceramic body may obviate the need for further processing steps, such as shading or sintering, and may be suitable for use in chair-side machining applications, such as in a dentist's office, significantly reducing the time to create a custom finished product.
Owner:JAMES R GLIDEWELL DENTAL CERAMICS
Rare earth recovery from phosphor
InactiveUS8524176B2Efficient and effectiveRare earth metal chloridesPhysical/chemical process catalystsPhosphorRare earth
A method is described to produce high purity rare earth oxides of the elements La, Ce, Tb, Eu and Y from phosphor, such as waste phosphor powders originating in various consumer products. One approach involves leaching the powder in two stages and converting to two groups of relatively high purity mixed rare earth oxides. The first group containing Eu and Y is initially separated by solvent extraction. Once separated, Eu is purified using Zn reduction with custom apparatus. Y is purified by running another solvent extraction process using tricaprylmethylammonium chloride. Ce is separated from the second group of oxides, containing La, Ce and Tb by using solvent extraction. Subsequently, La and Tb are separated from each other and converted to pure oxides by using solvent extraction processes. A one-stage leaching process, wherein all rare earths get leached into the solution and subsequently processed, is also described.
Owner:REENEWAL
Organic-inorganic hybrid nanoflower and preparation method thereof
ActiveCN110669755AImprove catalytic performanceImprove stabilityRare earth metal nitratesOxidoreductasesHorse-radishChemical compound
The invention provides an organic-inorganic hybrid nanoflower and a preparation method thereof, which relate to the technical field of enzyme immobilization. The organic-inorganic hybrid nanoflower provided by the invention is a flower-like immobilized enzyme formed by self-assembly compounding by taking a rare earth layered compound as an inorganic carrier and taking a biological enzyme as an organic component; wherein the rare earth layered compound is Ln2 (OH) 5NO3.nH2O, Ln is one or more of La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb and Y, and n is 1.1-2.5; wherein the biological enzyme is one or more of alpha-amylase, horse radish peroxidase and laccase. According to the invention, the rare earth layered compound is used as an inorganic carrier to load the biological enzyme and form the flower-like immobilized enzyme for the first time, and compared with free enzyme, the obtained immobilized enzyme has better stability and catalytic performance; the preparation method is mild in condition, simple in process and short in consumed time.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
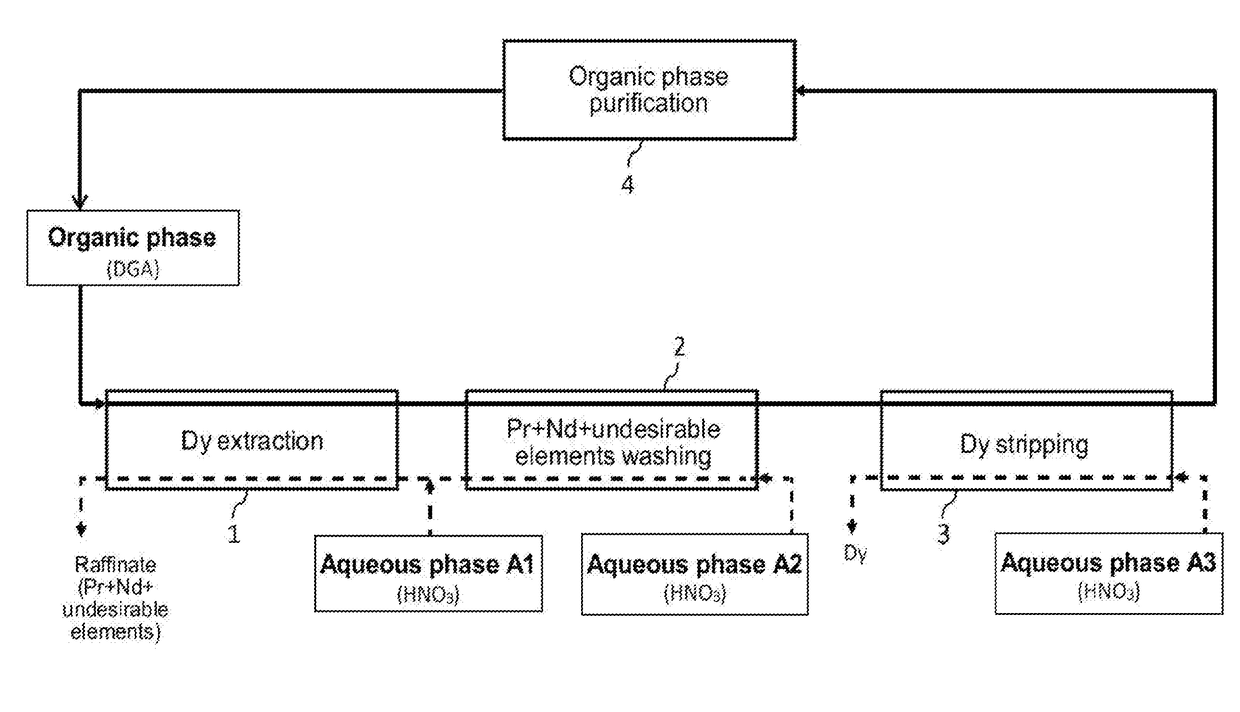
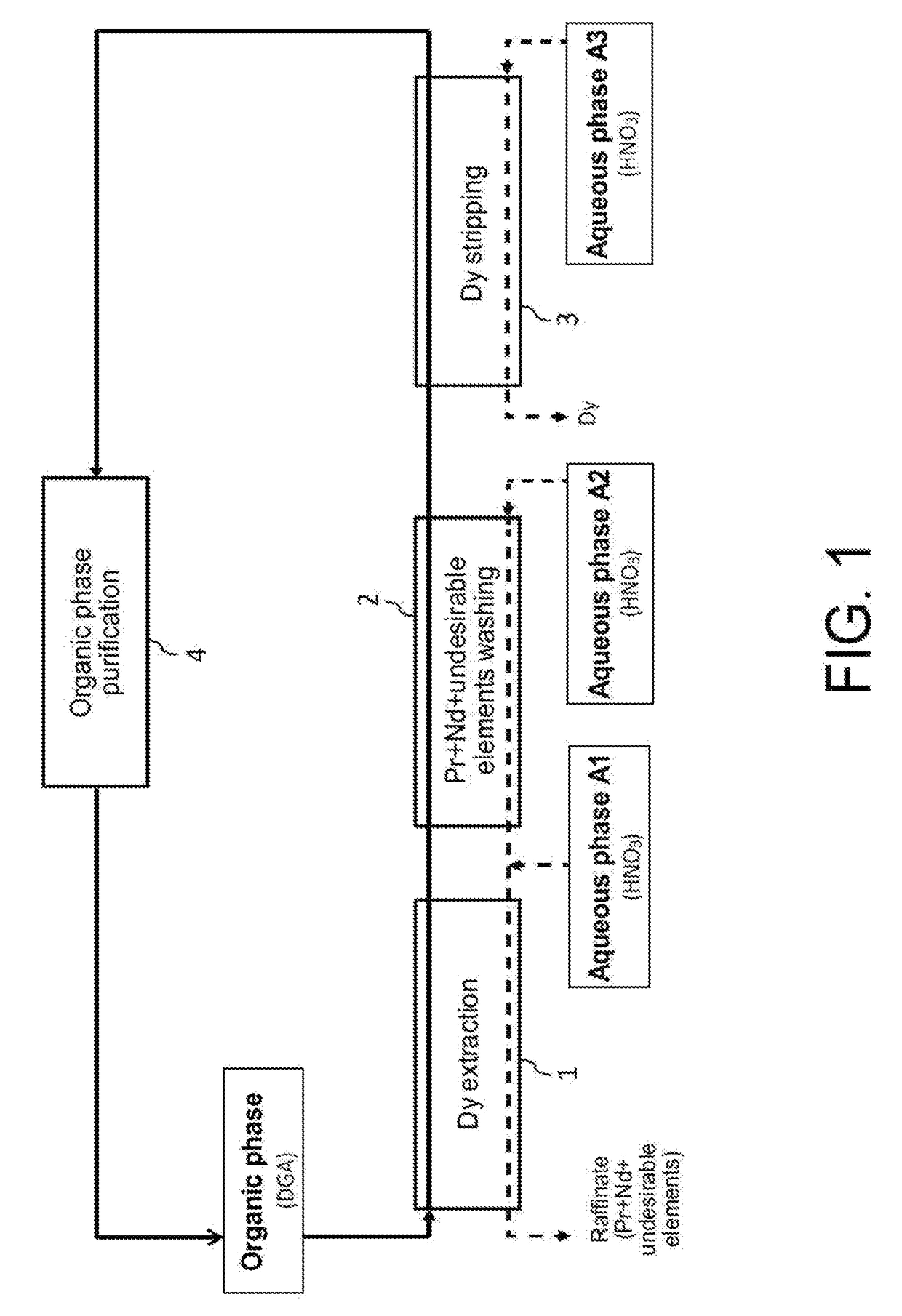
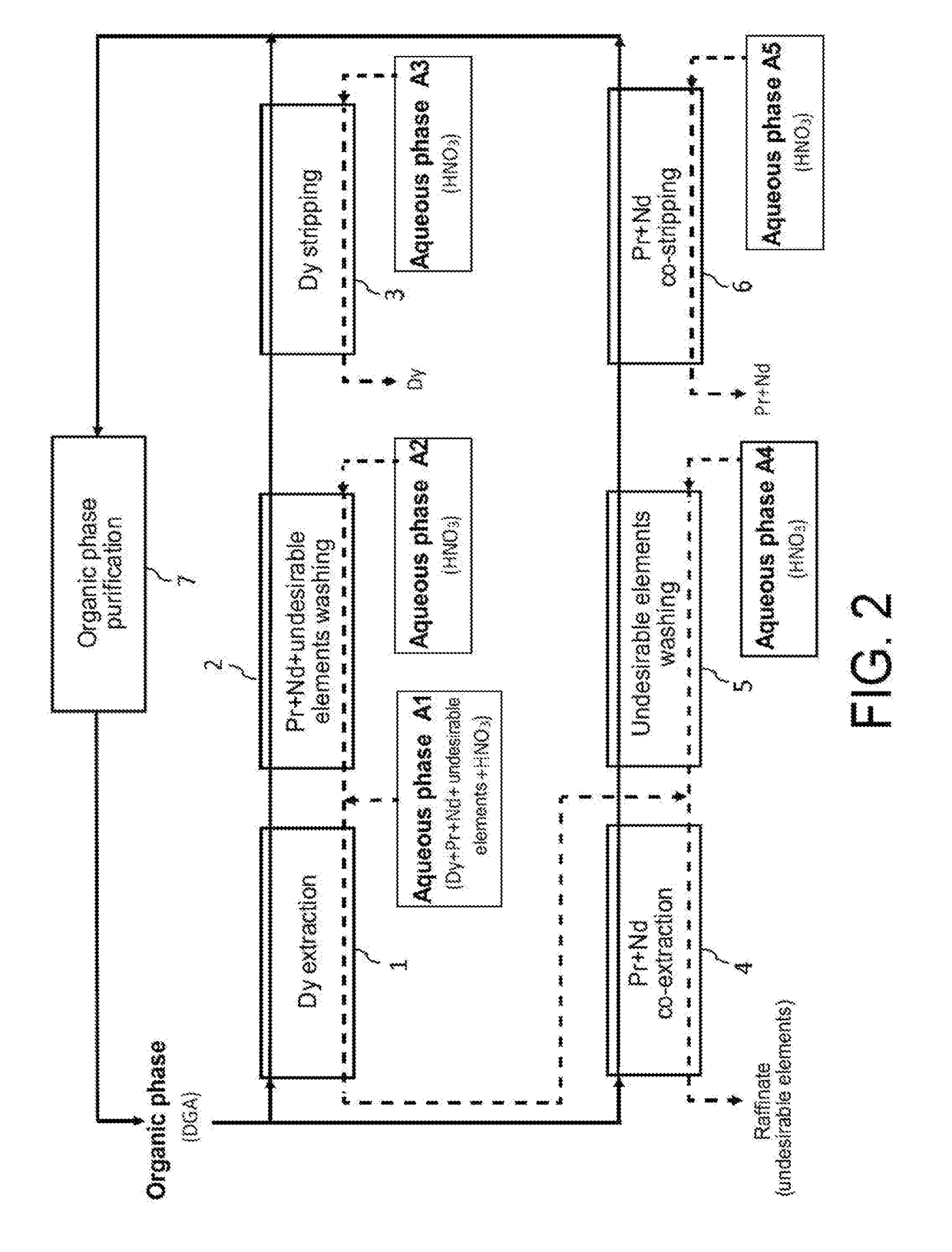
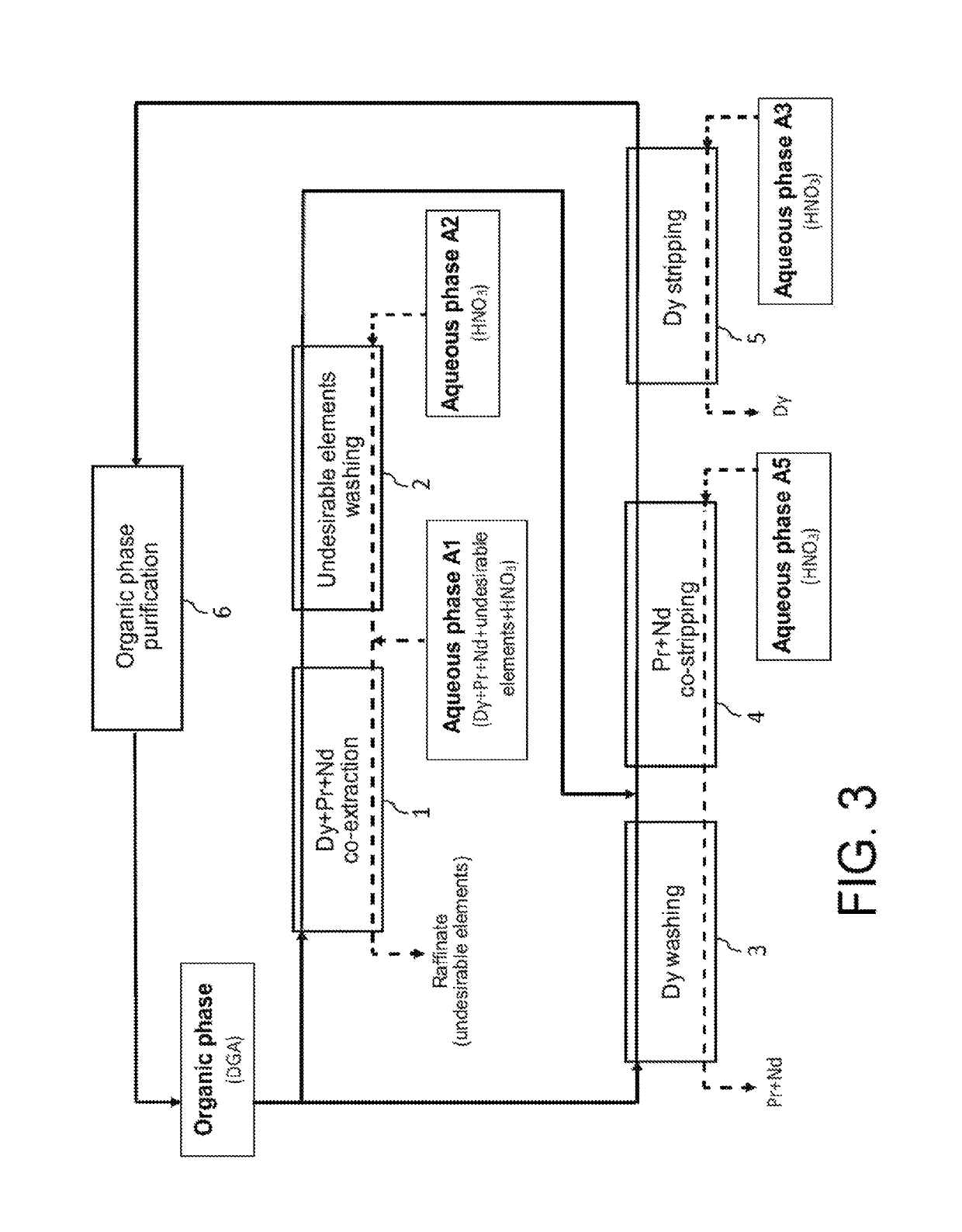
Processes for selective recovery of rare earth metals present in acidic aqueous phases resulting from the treatment of spent or scrapped permanent magnets
ActiveUS20170291827A1Rare earth metal nitratesRare earth metal compounds preparation/treatmentRare earthDysprosium
The invention relates to a hydrometallurgical process which makes it possible to selectively recover at least one “heavy” rare earth metal, i.e. a rare earth metal with an atomic number at least equal to 62, that is in an acidic aqueous phase resulting from the treatment of spent or scrapped permanent magnets. It also relates to a hydrometallurgical process which makes it possible to selectively recover, on the one hand, at least one heavy rare earth metal present in an acidic aqueous phase resulting from the treatment of spent or scrapped permanent magnets and, on the other hand, at least one “light” rare earth metal, i.e. a rare earth metal with an atomic number at most equal to 61, that is also in this acidic aqueous phase. The invention has in particular an application in the recycling of rare earth metals present in spent or scrapped permanent magnets of the type Neodymium-Iron-Boron (or NdFeB) and, in particular, dysprosium, praseodymium and neodymium, and also in the recycling of samarium present in spent or scrapped permanent magnets of the type samarium-cobalt (or SmCo).
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Shaded zirconia ceramic material and machinable sintered ceramic bodies made therefrom
ActiveUS10479729B2Shorten the timeUse in some applicationImpression capsRare earth metal nitratesTooth enamelMachining
Shaded, zirconia ceramic materials are disclosed that are suitable for use in dental applications. Ceramic bodies are made from a zirconia-containing ceramic material and a coloring composition comprising a terbium (Tb)-containing component and a chromium (Cr)-containing component as a coloring agent. The pre-shaded ceramic body is machinable into a dental restoration either as a bisque body or sintered body. A pre-shaded machinable sintered ceramic body may obviate the need for further processing steps, such as shading or sintering, and may be suitable for use in chair-side machining applications, such as in a dentist's office, significantly reducing the time to create a custom finished product.
Owner:JAMES R GLIDEWELL DENTAL CERAMICS
Fluid extraction of metal and/or metalloid
InactiveCN1082825CSelectiveCost-effective methodRare earth metal nitratesContaminated soil reclamationPhosphateLanthanide
A method of extracting metalloid and metal species from a solid or liquid material by exposing the material to a supercritical fluid solvent containing a chelating agent is described. The chelating agent forms chelates that are soluble in the supercritical fluid to allow removal of the species from the material. In preferred embodiments, the extraction solvent is supercritical carbon dioxide and the chelating agent is a fluorinated beta -diketone. In especially preferred embodiments the extraction solvent is supercritical carbon dioxide, and the chelating agent comprises a fluorinated beta -diketone and a trialkyl phosphate, or a fluorinated beta -diketone and a trialkylphosphine oxide. Although a trialkyl phosphate can extract lanthanides and actinides from acidic solutions, a binary mixture comprising a fluorinated beta -diketone and a trialkyl phosphate or a trialkylphosphine oxide tends to enhance the extraction efficiencies for actinides and lanthanides. The method provides an environmentally benign process for removing contaminants from industrial waste without using acids or biologically harmful solvents. The method is particularly useful for extracting actinides and lanthanides from acidic solutions. The chelate and supercritical fluid can be regenerated, and the contaminant species recovered, to provide an economic, efficient process.
Owner:IDAHO RESARCH FOUNDATION INC
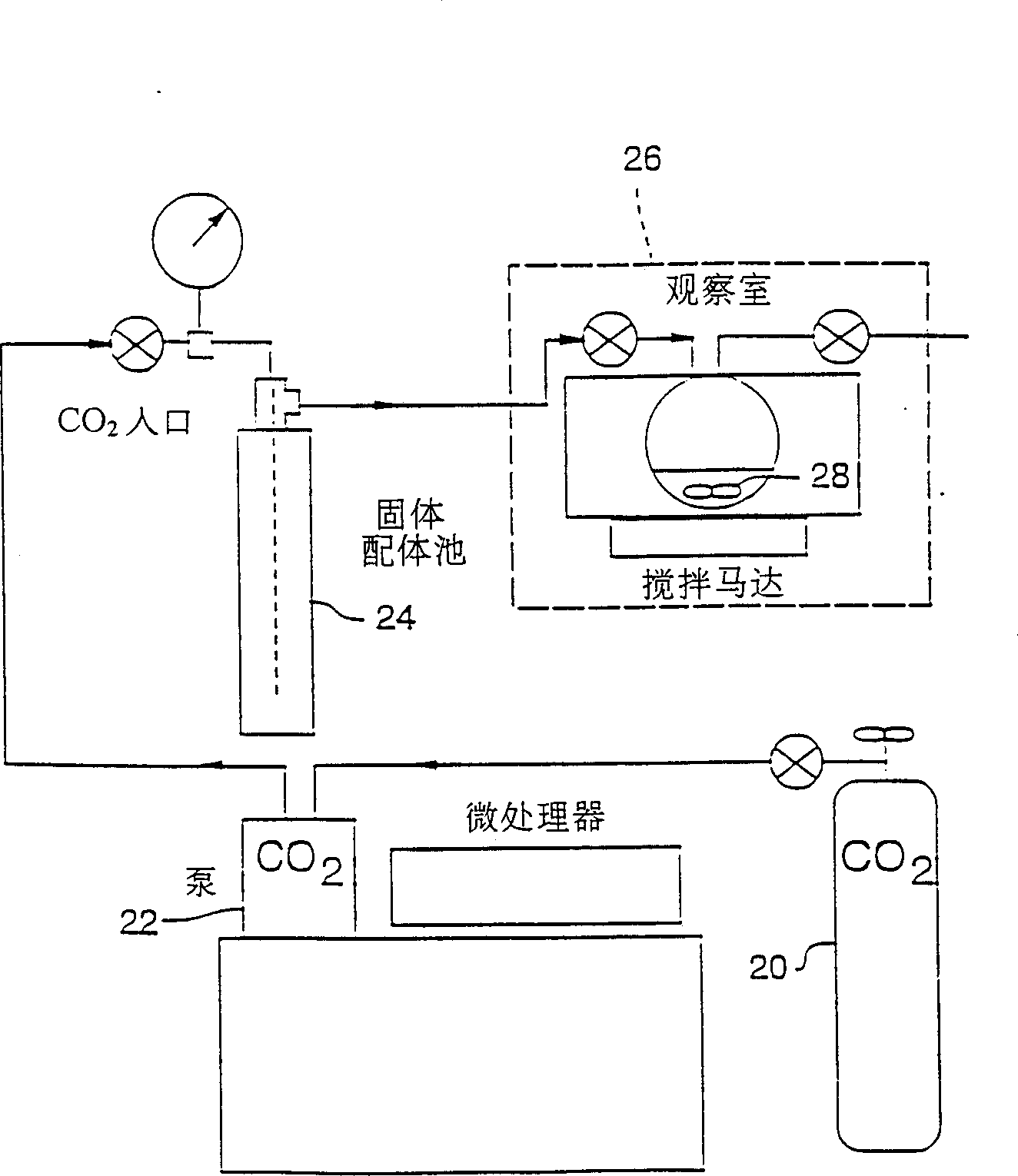
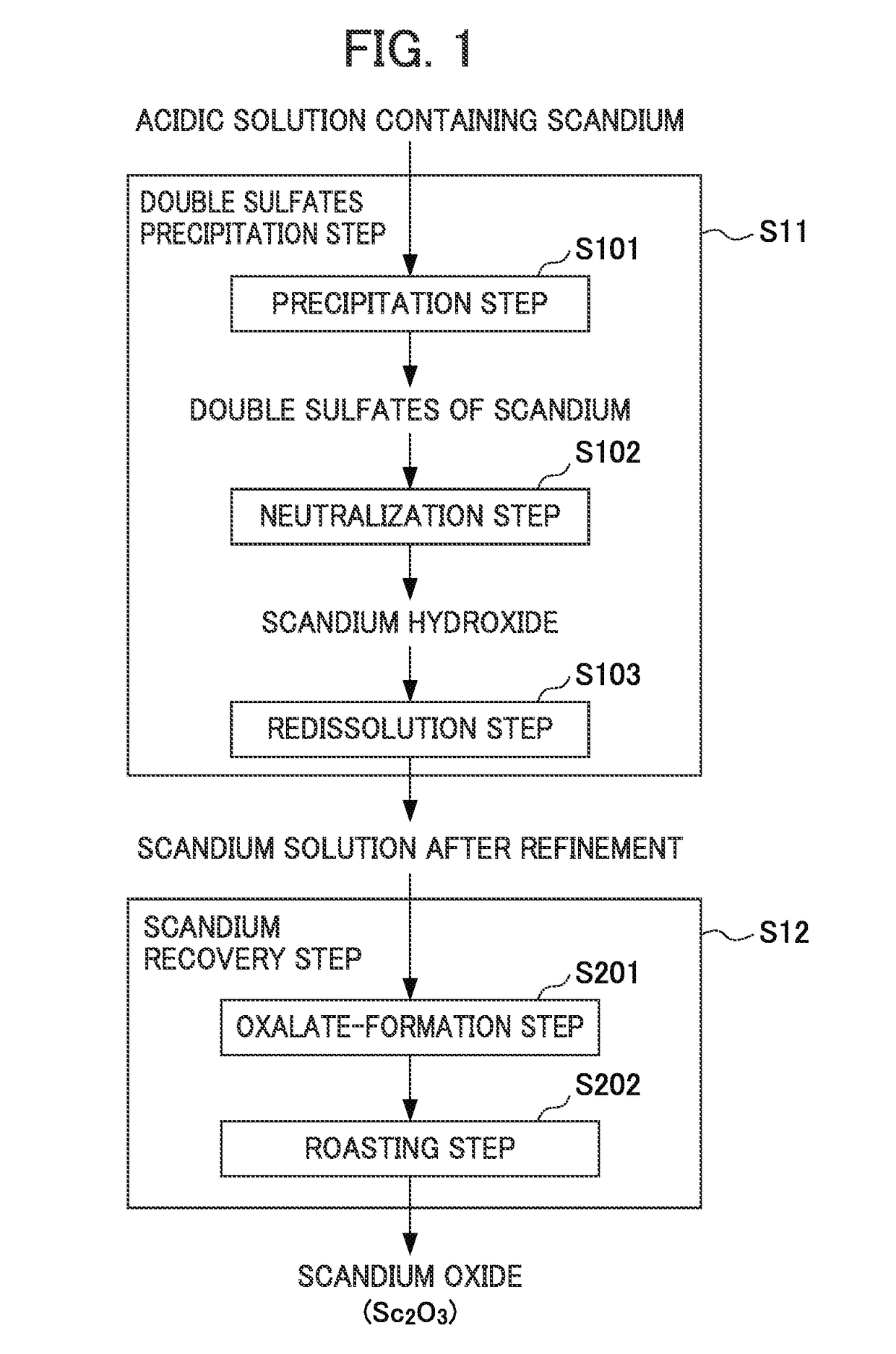
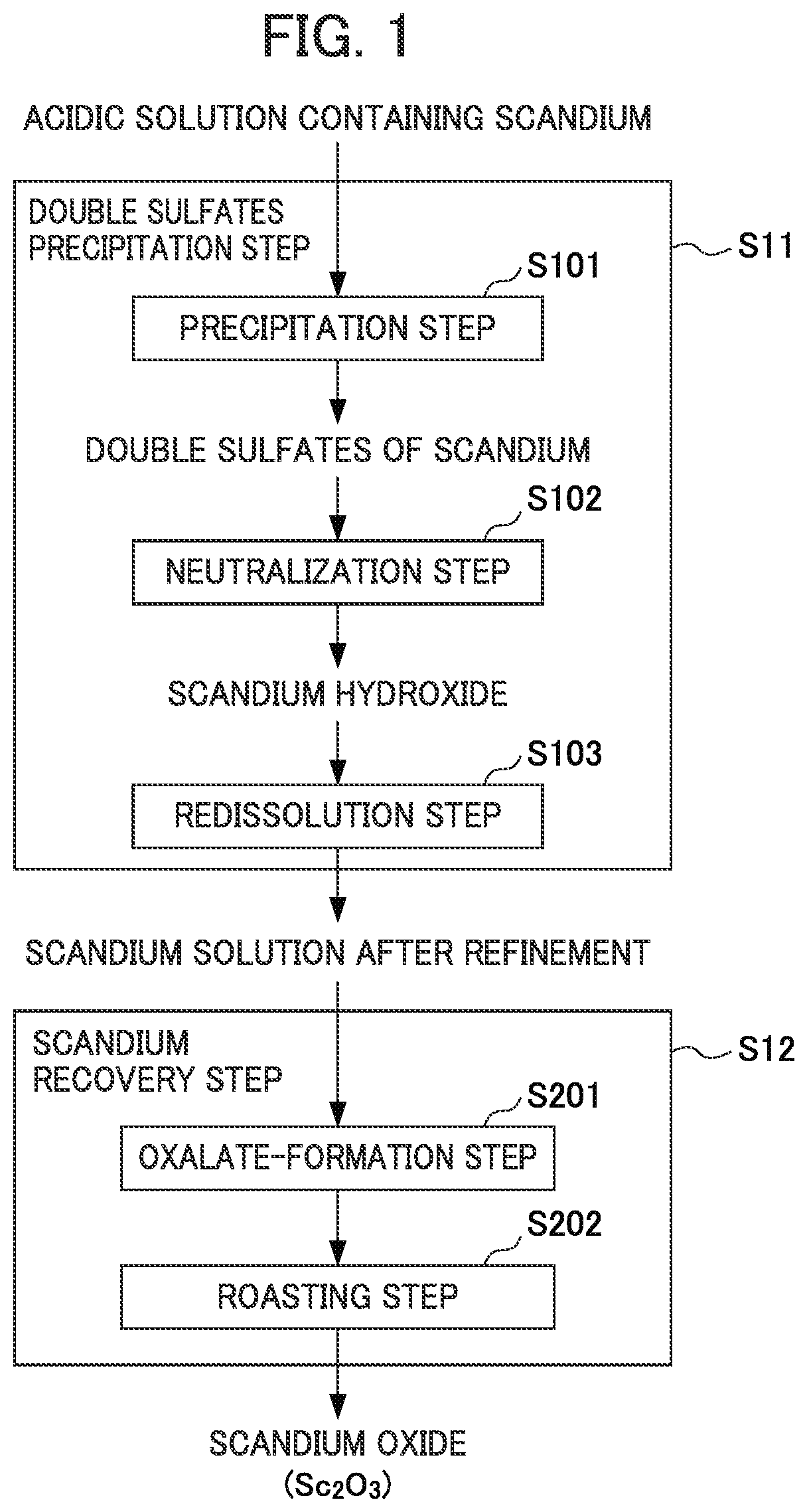
Method for recovering scandium
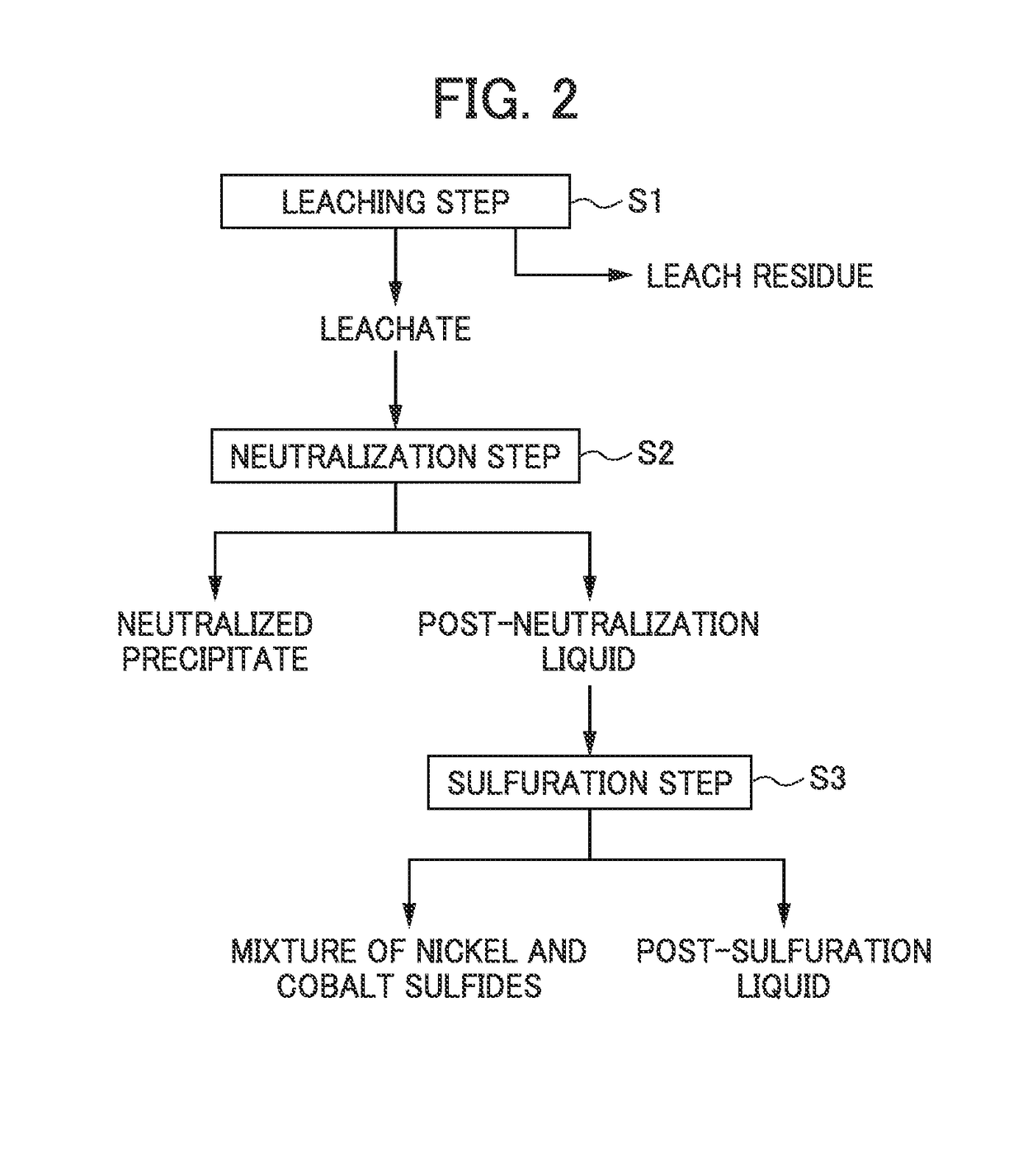
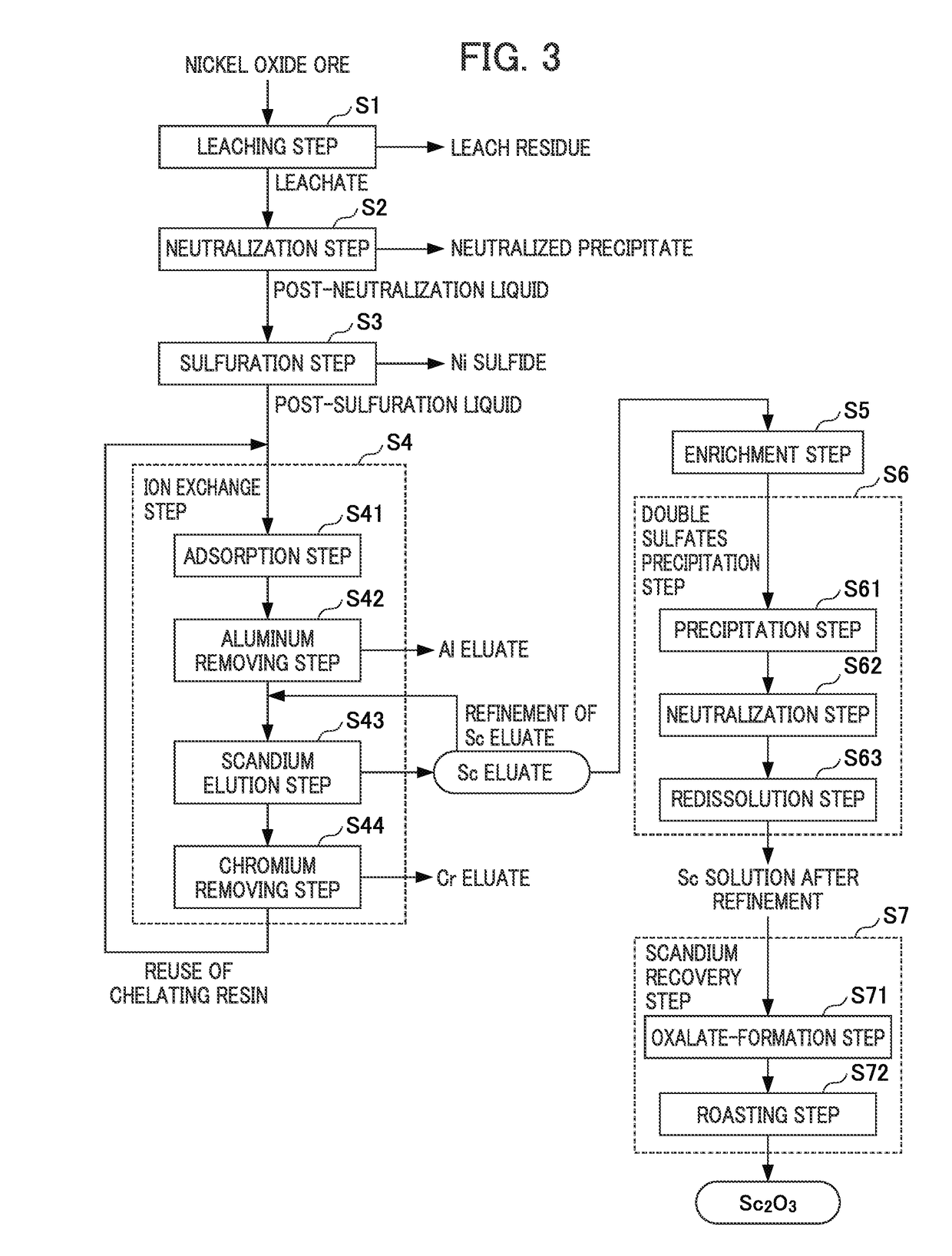
ActiveUS20180087128A1Recovered conveniently and efficientlyRare earth metal sulfatesRare earth metal chloridesSulfateDissolution
According to this method for recovering scandium, an acidic solution containing scandium is used and a scandium dissolution liquid after purification is obtained by a double sulfate precipitation step, and scandium is recovered from the obtained scandium dissolution liquid, as follows: [A] A precipitation step wherein sodium sulfate is added into the acidic solution containing scandium, so that a precipitate of a scandium double sulfate is obtained; [B] A neutralization step wherein pure water is added to the precipitate of a scandium double sulfate obtained in the precipitation step to dissolve the precipitate of a scandium double sulfate therein, and scandium hydroxide is obtained by adding a neutralizing agent into the obtained dissolution liquid; and [C] A re-dissolution step wherein an acid is added to the scandium hydroxide obtained in the neutralization step, so that a scandium dissolution after purification, in which the scandium hydroxide is dissolved, is obtained.
Owner:SUMITOMO METAL MINING CO LTD
Rare earth elements separation using phosphorus based adsorbent
ActiveUS8721893B2SelectivityReduce waste accumulationWater treatment parameter controlLanthanide oxides/hydroxidesRare-earth elementSorbent
The present invention relates to methods for the separation of rare earth elements from aqueous solutions and, more particularly, to the separation of lanthanides (e.g., neodymium(III)) from aqueous solutions using an organo phosphorus functionalized adsorbent.
Owner:SYRACUSE UNIVERSITY
Method and system for supercritical fluid extraction of metal
A method for supercritical fluid extraction of metal from a source, the method comprising: providing a reactor chamber; providing a source comprising a target metal; optionally, providing a chelatingagent; providing a solvent; adding the source comprising the target metal, the chelating agent and the solvent into the reactor chamber; adjusting the temperature and pressure in the reactor chamber so that the solvent is heated and compressed above its critical temperature and pressure; optionally, providing mechanical agitation to the reactor chamber; and recovering a chelate comprising the target metal.
Owner:THE GOVERNING COUNCIL OF THE UNIV OF TORONTO
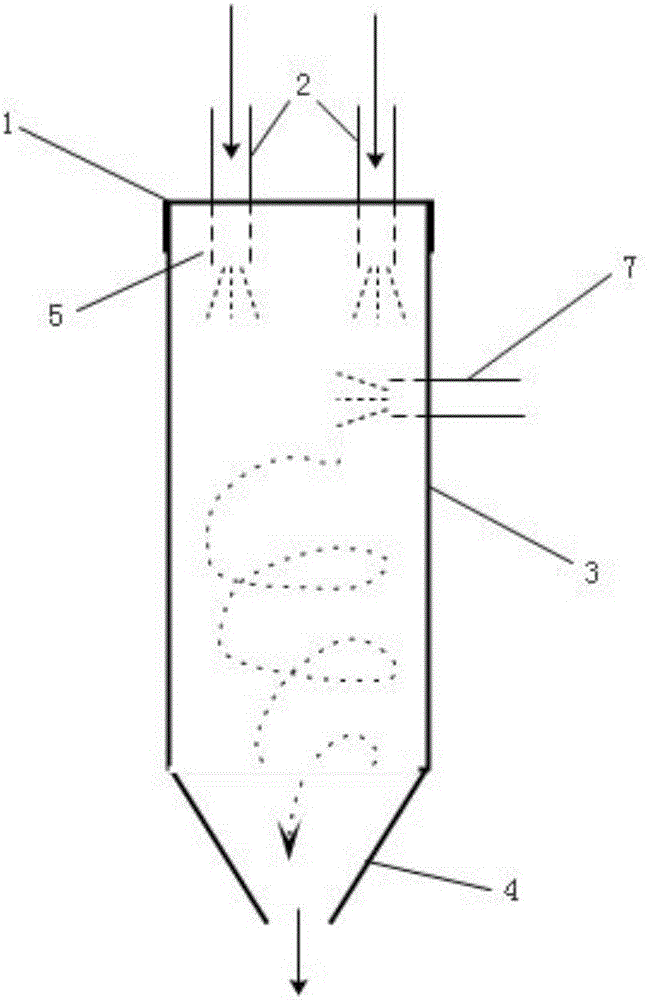
Reaction device and method for preparing superfine rare earth compound through same
InactiveCN106362657AReduce volumeEasy to useRare earth metal sulfatesRare earth metal chloridesRare earthEngineering
Owner:QIANDONG RARE EARTH GRP
Method and system for supercritical fluid extraction of metal
PendingUS20210265678A1Rare earth metal nitratesLithium compoundsPhysical chemistryMetal Chelating Agents
A method for supercritical fluid extraction of metal from a source, the method comprising: providing a reactor chamber; providing a source comprising a target metal; optionally, providing a chelating agent; providing a solvent; adding the source comprising the target metal, the chelating agent and the solvent into the reactor chamber; adjusting the temperature and pressure in the reactor chamber so that the solvent is heated and compressed above its critical temperature and pressure; optionally, providing mechanical agitation to the reactor chamber; recovering a chelate comprising the target metal.
Owner:THE GOVERNINIG COUNCIL OF THE UNIV OF TORANTO
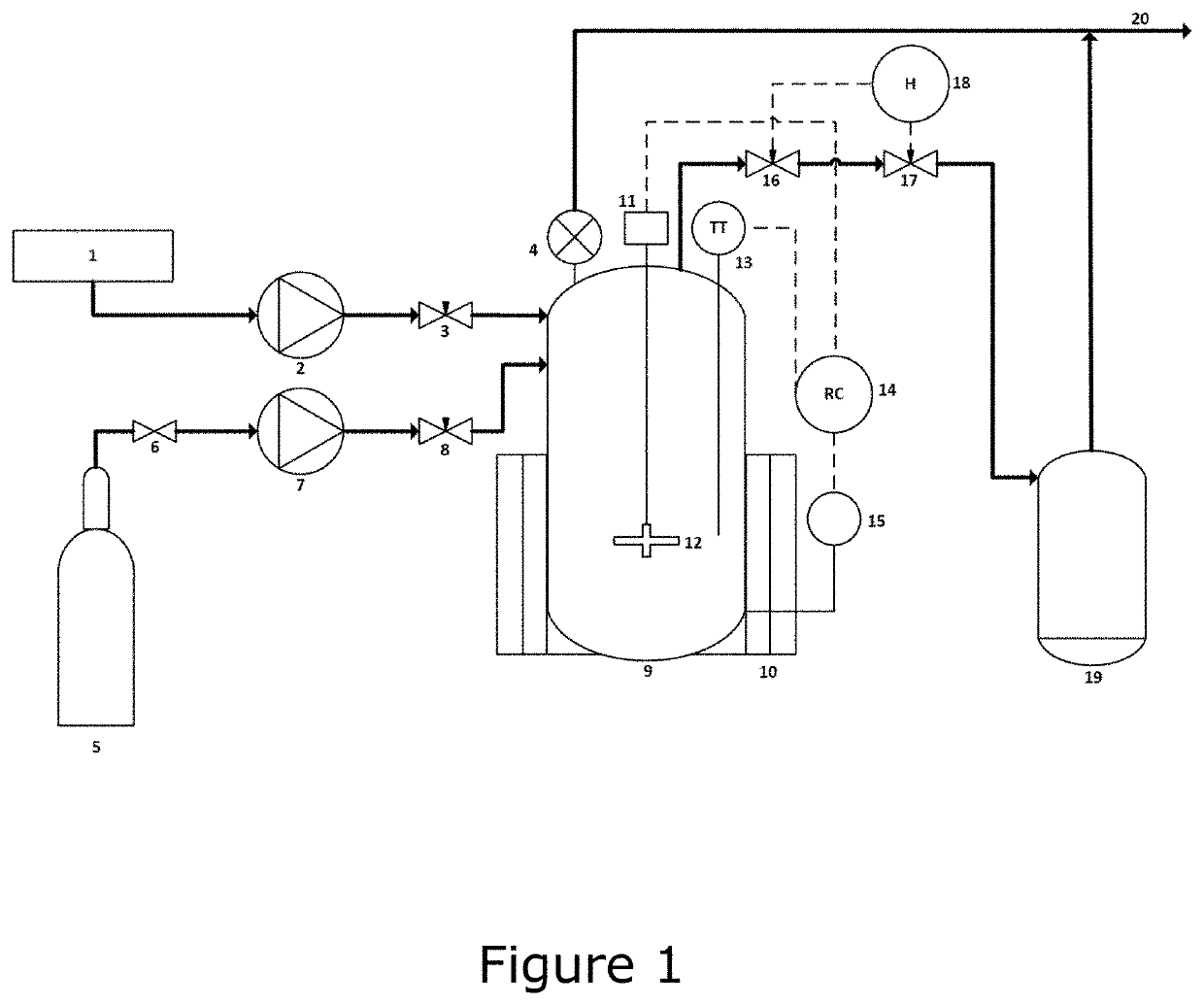
Rare earth solution preparation process capable of quickly and efficiently removing acid
ActiveCN111392761AQuick removalImprove efficiencyRare earth metal sulfatesRare earth metal chloridesPhysical chemistryStrong acids
The invention discloses a rare earth solution preparation process capable of quickly and efficiently removing acid. The preparation process comprises the following steps: drying and crushing, grinding, dissolving and stirring, stabilizing the solution, carrying out microwave digestion and carrying out double-layer acid removal. According to the rare earth solution preparation process capable of rapidly and efficiently removing acid, rare earth mineral powder is prepared through the processes of drying, crushing and grinding, after the rare earth mineral powder is dissolved through strong acidand stabilized through a metallic soap stabilizer, part of dissolved sample acid is removed through sulfuric acid or perchloric acid by heating, and then residual acid removing is conducted through atemperature-controlled acid removing instrument; with application of the process, the sample dissolving acid in the rare earth solution can be quickly removed, the residue of the sample dissolving acid is effectively reduced, the time of the preparation process flow is shortened, the acid driving efficiency and the preparation efficiency of the rare earth solution are improved and the process is worthy of popularization.
Owner:中稀(宜兴)稀土新材料有限公司
Removal of europium impurities from samarium-153 in nitrate media using ionic liquids
InactiveUS20210070628A1Easy to useMore efficiencyCation exchanger materialsSolvent extractionNitrate anionNitrate salts
A process of isolating samarium from a hydrophilic composition comprises nitrate ions, europium and samarium, by reducing europium(III) to europium(II) in this hydrophilic composition, and by extracting the samarium with a water-immiscible organic phase comprising an ionic liquid comprising nitrate anions.
Owner:SCK CEN STUDIECENTRUM VOOR KERNENERGIE CENT DETUDE DE LENERGIE NUCLEAIRE
Method for recovering rare earth elements from a solid mixture containing a halophosphate and a compound of one or more rare earth elements
InactiveCN102395529AHigh recovery rateRare earth metal oxides/hydroxidesRare earth metal chloridesAcid etchingRare-earth element
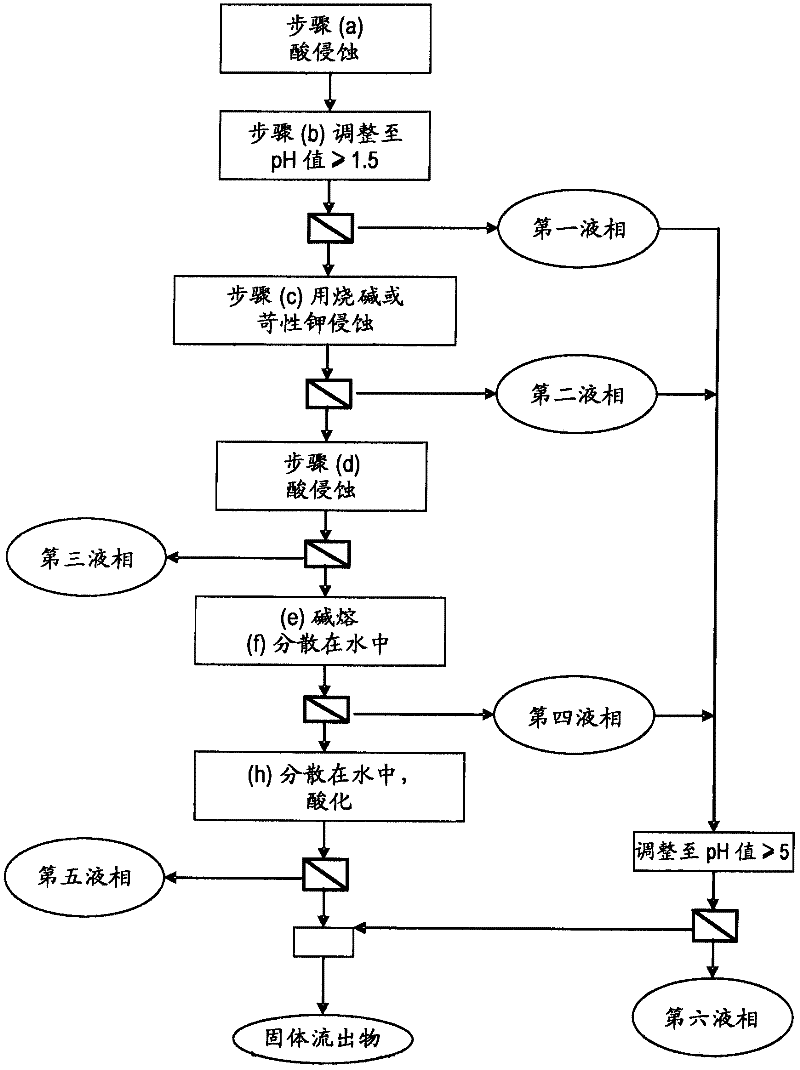
The invention relates to a method for recovering rare earth elements from a solid mixture containing a halophosphate and at least one compound of one or more rare earth elements, including: (a) acid etching the mixture; (b) adding a base to bring the pH back up to a value of at least 1,5; (c) etching the solid from step (b) with a solution of soda or potash; (d) acid etching the solid from step (c) until a pH of less than 7 is obtained, resulting in a solid phase and a liquid phase including at least one rare earth salt, and separating the solid phase from the liquid phase.
Owner:RHODIA OPERATIONS SAS
Preparation method of ammonium paratungstate composite powder comprising rare earth
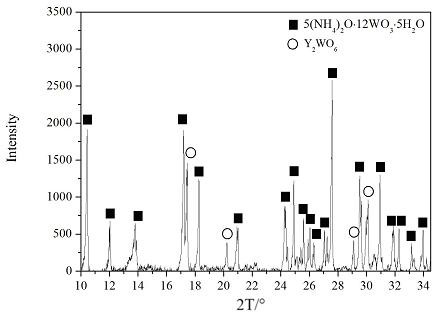
ActiveCN109368701AGood dispersionImprove uniformityRare earth metal nitratesTungsten compoundsRare-earth elementProduction line
The invention relates to a preparation method of ammonium paratungstate composite powder comprising rare earth. The preparation method comprises an alkaline method or an acidic method for obtaining ammonium tungstate solution as well as the impurity removal and evaporation crystallization of the ammonium tungstate solution. The method adds the rare-earth element into the pure ammonium tungstate solution in a form of rare-earth salt solution to obtain the ammonium paratungstate / rare-earth salt composite powder with the rare-earth element being sufficiently and uniformly distributed. The preparation method comprises the following steps: (1) preparing the ammonium tungstate solution; (2) preparing a uniform and stable rare-earth salt solution; (3) preparing a precursor composite solution; and(4) evaporating crystallizing, and obtaining the ammonium paratungstate composite powder comprising the rare earth. The rare-earth salt solution is directly added in the intermediate product ammoniumtungstate solution for preparing the APT, and then the ammonium paratungstate / rare-earth salt composite powder is obtained by virtue of evaporation crystallization, and the mass production of the ammonium paratungstate / rare-earth salt composite powder can be realized by slightly modifying the existing industrialized APT production line.
Owner:INST OF APPLIED PHYSICS JIANGXI ACADEMY OF SCI
A kind of preparation method of ammonium paratungstate composite powder containing rare earth
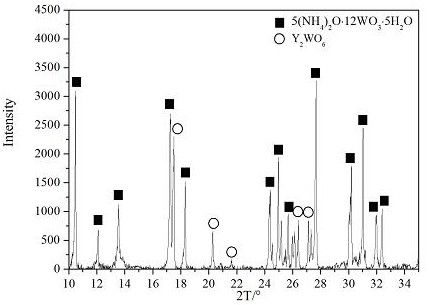
ActiveCN109368701BGood dispersionImprove uniformityRare earth metal chloridesRare earth metal nitratesRare-earth elementAmmonium paratungstate
A method for preparing a rare earth-containing ammonium paratungstate composite powder, which includes alkali treatment or acid treatment to obtain an ammonium tungstate solution, impurity removal treatment of the ammonium tungstate solution, and evaporative crystallization, and the method adds rare earth elements in the form of a rare earth salt solution. into a pure ammonium tungstate solution to obtain an ammonium paratungstate / rare earth salt composite powder in which rare earth elements are fully and uniformly distributed. The method steps of the invention include: (1) preparing an ammonium tungstate solution; (2) preparing a homogeneous and stable rare earth salt solution; (3) preparing a precursor composite solution; (4) evaporating and crystallizing to obtain a rare earth-containing ammonium paratungstate composite powder. In the invention, the rare earth salt solution is directly added to the ammonium tungstate solution of the intermediate product for preparing APT, and then the ammonium paratungstate / rare earth salt composite powder is obtained by evaporation and crystallization, and the ammonium paratungstate / rare earth salt can be realized by slightly modifying the existing industrialized APT production line. Mass production of composite powders.
Owner:INST OF APPLIED PHYSICS JIANGXI ACADEMY OF SCI
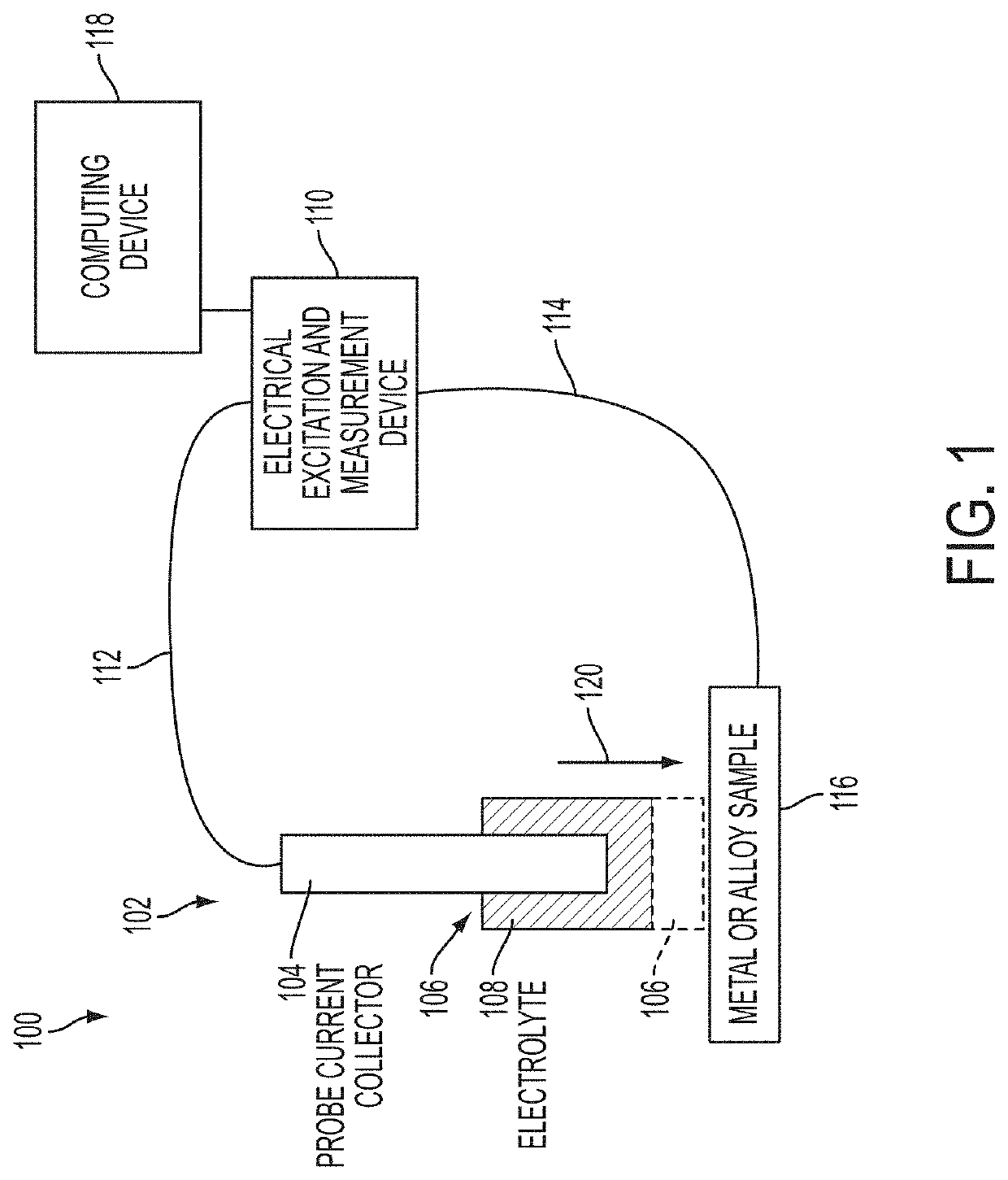
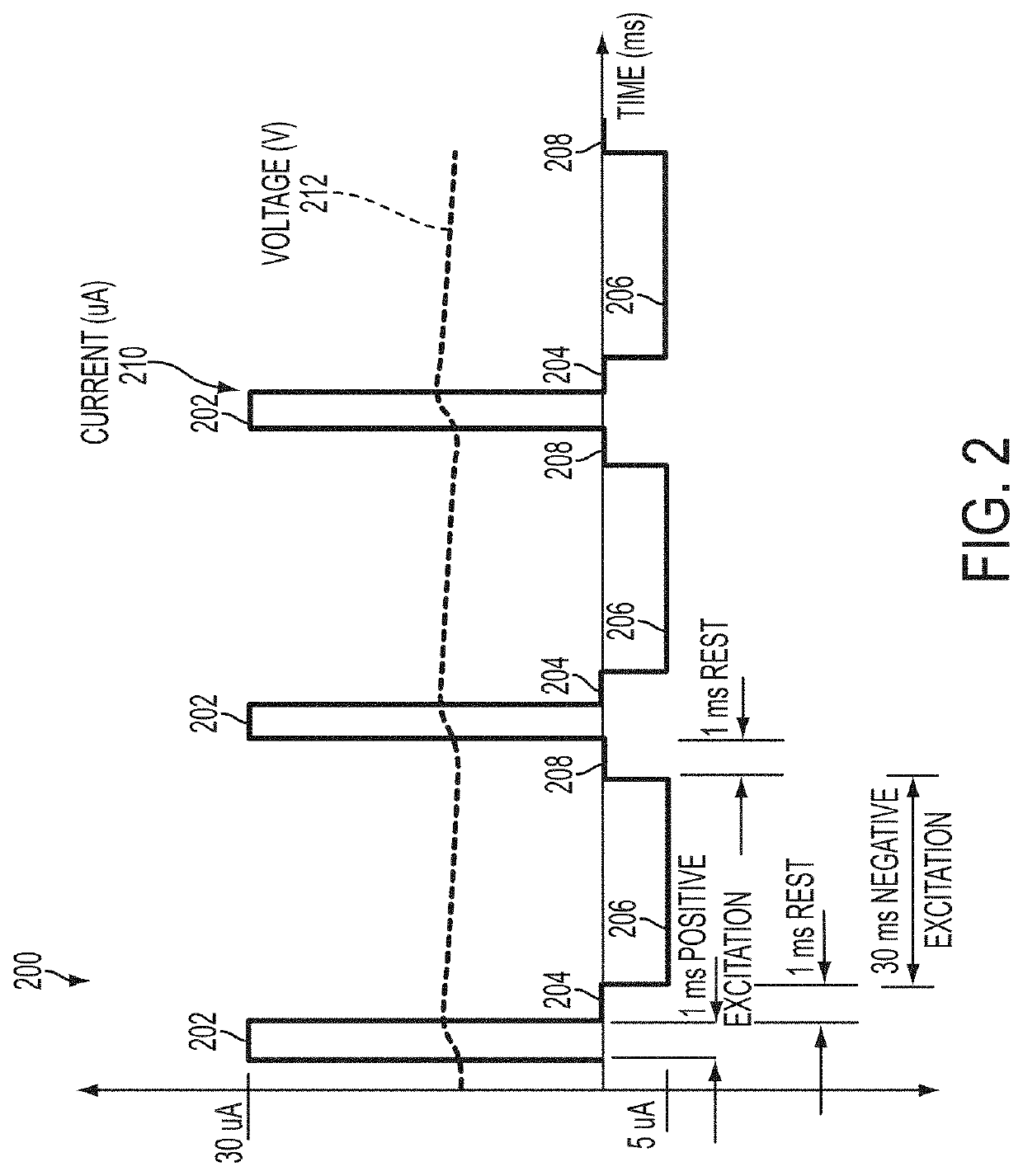
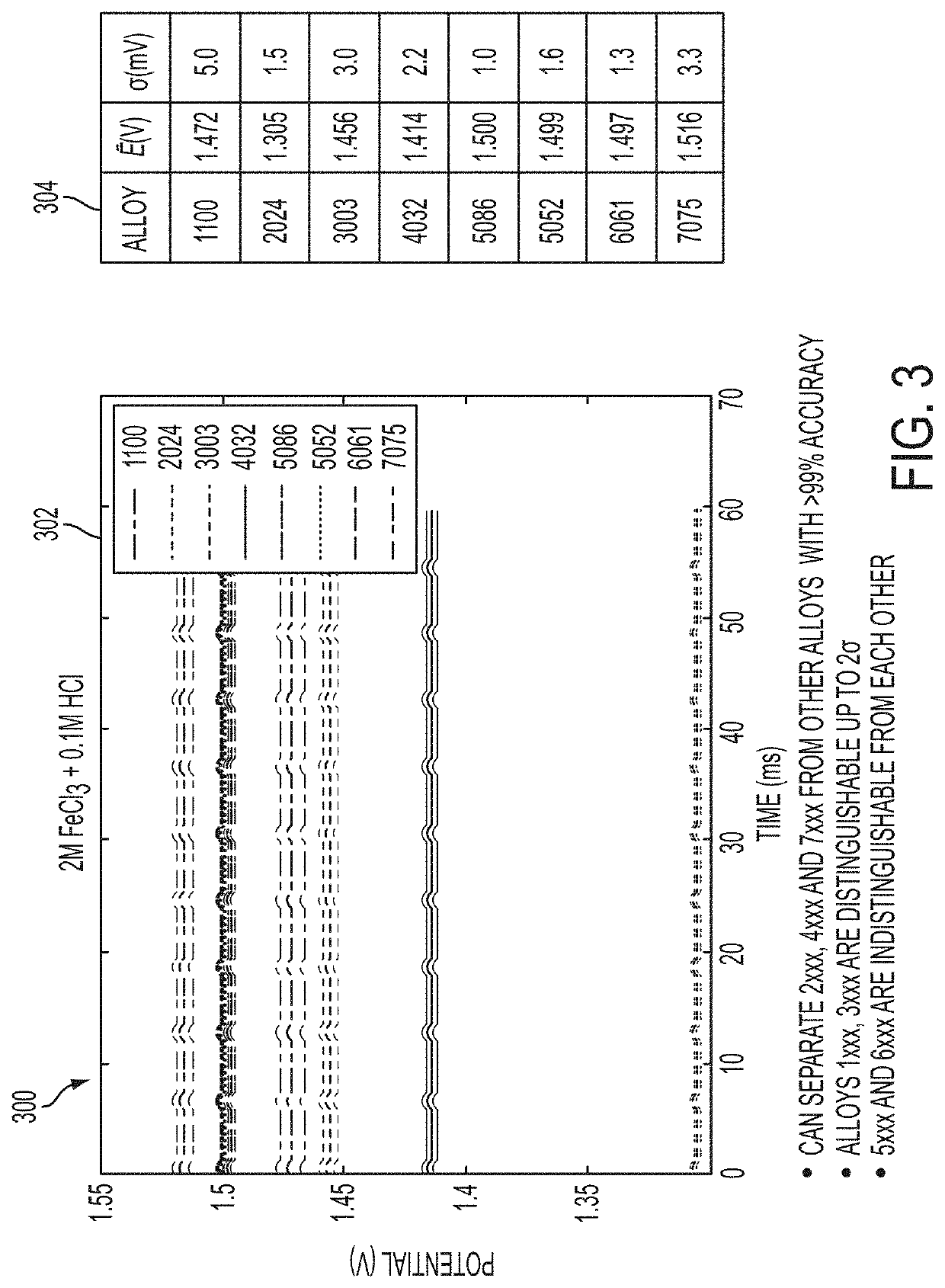
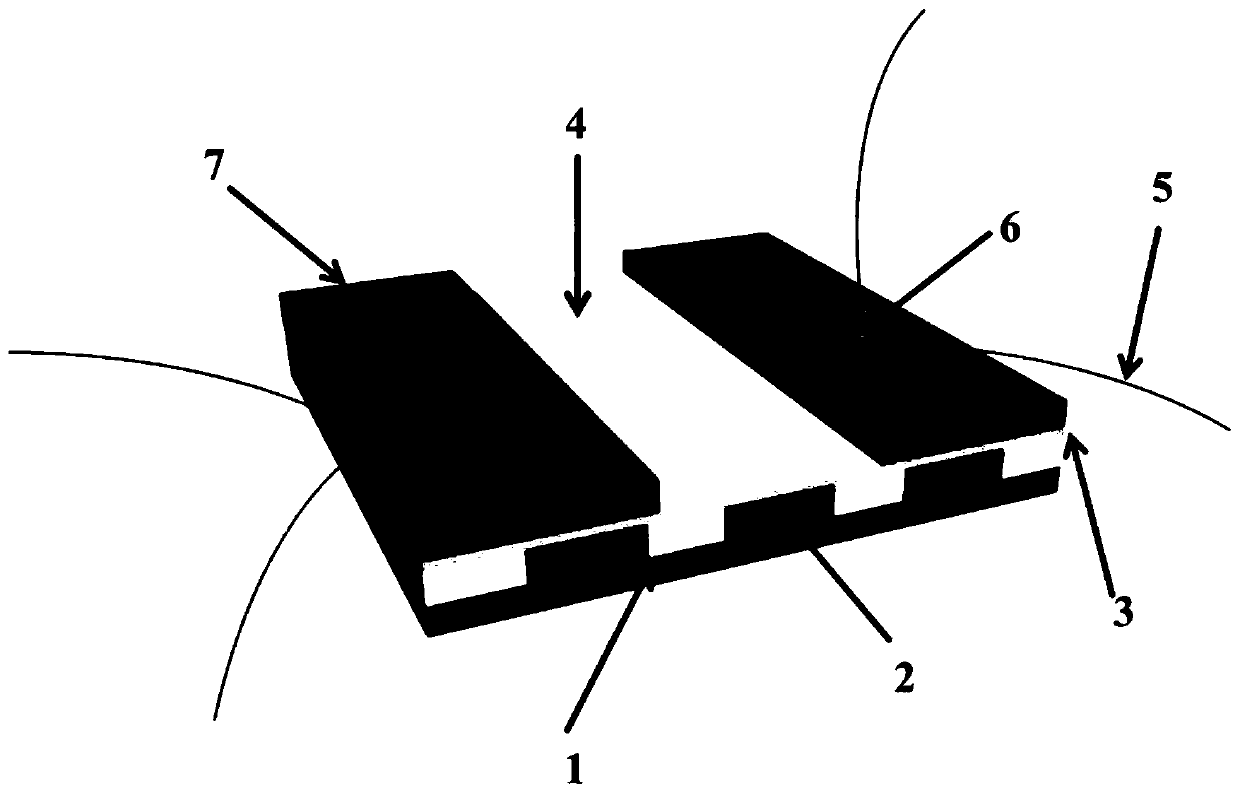
Alloy identification device
ActiveUS10794858B2Rare earth metal nitratesMaterial electrochemical variablesElectrolytic agentMetal alloy
An electrochemical metal alloy identification device employing electrolytes to measure and identify different potentials of alloys is presented. This includes physical structure, disposables, electrical systems, control circuitry, and algorithms to identify alloys.
Owner:XEROX CORP
Method for recovering scandium
ActiveUS10570480B2Recovered conveniently and efficientlyRare earth metal sulfatesRare earth metal chloridesSulfatePhysical chemistry
The invention provides a method for recovering scandium from an acidic solution containing scandium. The method having [a] a precipitation step wherein sodium sulfate is added into the acidic solution containing scandium to obtain a precipitate of a scandium double sulfate; [b] a neutralization step wherein pure water is added to the precipitate of a scandium double sulfate to dissolve the precipitate of a scandium double sulfate therein, and scandium hydroxide is obtained by adding a neutralizing agent into the dissolution liquid; and [c] a re-dissolution step wherein an acid is added to the scandium hydroxide obtained in the neutralization step, so that a scandium dissolution after purification, in which the scandium hydroxide is dissolved, is obtained.
Owner:SUMITOMO METAL MINING CO LTD
Anion conductor and layered metal hydroxide
InactiveCN106104886AImprove heat resistanceExcellent physical strengthFuel and secondary cellsRare earth metal nitratesChemistryPhysical strength
The present invention provides a novel anion conductor which comprises a layered metal hydroxide and can be used as an alkaline electrolyte film for use in a fuel cell or the like. An anion conductor characterized by comprising a molded product of a layered metal hydroxide represented by formula (1): [Mx(OH)y(A)([alpha]x-y) / z-nH2O] (wherein M represents a metal that can serve as a bivalent or trivalent cation; [alpha] represents the number of valency of the metal M, A represents an atom or an atomic group that can serve as an anion, and z represents the number of valency of the anion A, wherein, when ([alpha]x-y) / z is 2 or greater, A's may be different types of anions which can serve as anions having the same valencies as each other, or may be anions having different valencies from each other; and n represents the average number of molecules of interlayer water contained per one repeating unit). The anion conductor according to the present invention is composed of an inorganic material, and therefore has excellent heat resistance and physical strength and can be operated for a longer period at a higher temperature compared with the conventional ones when used as an anion conductor for a fuel cell, an air cell or the like.
Owner:TOKUYAMA CORP
Method for recovering rare earth, aluminum and silicon from rare earth-containing aluminum-silicon waste
ActiveCN106319218BReduce dosageEfficient separationRare earth metal oxides/hydroxidesRare earth metal chloridesAluminum IonSlag
The invention provides a method for recovering rare earth, aluminum and silicon from rare earth-containing aluminum and silicon waste. The method comprises: S1, acid leaching the rare earth-containing aluminum-silicon waste with an aqueous inorganic acid solution to obtain silicon-rich slag and an acid leaching solution containing rare earth and aluminum ions; S2, adding Alkaline substances and control the pH value of the pickling solution to 3.5 to 5.2, solid-liquid separation to obtain aluminum hydroxide-containing precipitates and rare earth-containing filtrates; S3, use aluminum hydroxide-containing precipitates to react with sodium hydroxide to obtain metaaluminic acid Sodium solution and aluminum silicon slag, and use the filtrate containing rare earth to prepare rare earth compound products. In the above method, aluminum and rare earth are dissolved with acid, and then by segmental alkali conversion, aluminum ions are precipitated to obtain aluminum hydroxide and rare earth ions are separated, and then excessive sodium hydroxide is added to convert aluminum hydroxide into sodium metaaluminate solution to realize Rare earth and aluminum are efficiently recycled at the same time, and the amount of sodium hydroxide is greatly reduced, which reduces the cost of recycling.
Owner:GRIREM ADVANCED MATERIALS CO LTD
Processes for selective recovery of rare earth metals present in acidic aqueous phases resulting from the treatment of spent or scrapped permanent magnets
The invention relates to a hydrometallurgical process which makes it possible to selectively recover at least one “heavy” rare earth metal, i.e. a rare earth metal with an atomic number at least equal to 62, that is in an acidic aqueous phase resulting from the treatment of spent or scrapped permanent magnets. It also relates to a hydrometallurgical process which makes it possible to selectively recover, on the one hand, at least one heavy rare earth metal present in an acidic aqueous phase resulting from the treatment of spent or scrapped permanent magnets and, on the other hand, at least one “light” rare earth metal, i.e. a rare earth metal with an atomic number at most equal to 61, that is also in this acidic aqueous phase. The invention has in particular an application in the recycling of rare earth metals present in spent or scrapped permanent magnets of the type Neodymium-Iron-Boron (or NdFeB) and, in particular, dysprosium, praseodymium and neodymium, and also in the recycling of samarium present in spent or scrapped permanent magnets of the type samarium-cobalt (or SmCo).
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
a kind of la 2 nio 4 ysz-based hybrid potential type h for sensitive electrodes 2 s sensor and its preparation method
ActiveCN107655948BImprove thermal stabilityGood chemical stabilityRare earth metal nitratesMaterial electrochemical variablesToxic gasMaterial synthesis
The invention discloses a YSZ (yttria-stabilized zirconia)-based mixed potential type hydrogen sulfide (H2S) sensor with La2NiO4 used as a sensitive electrode and a method for preparing the YSZ-basedmixed potential type hydrogen sulfide sensor, and belongs to the technical field of gas sensors. The YSZ-based mixed potential type hydrogen sulfide sensor is mainly used for detecting toxic gas H2S in industrial production and daily life. The YSZ-based mixed potential type hydrogen sulfide sensor sequentially comprises an Al2O3 ceramic plate with Pt heating electrodes, a YSZ-based plate, a Pt reference electrode and the La2NiO4 sensitive electrode. The reference electrode and the sensitive electrode are independently and symmetrically prepared at two ends of the upper surface of the YSZ-basedplate, and the lower surface of the YSZ-based plate is adhered with the Al2O3 ceramic plate with the Pt heating electrodes. The YSZ-based mixed potential type hydrogen sulfide sensor and the method have the advantages that the La2NiO4 with high electrochemical catalytic activity is used as the sensitive electrode, the quantities of citric acid and the like which are added in material synthesis procedures are changed, accordingly, the electrochemical catalytic activity of sensitive electrode materials can be enhanced, and effects of improving the sensitivity characteristics of the YSZ-based mixed potential type hydrogen sulfide sensor can be realized; a short temperature pulse is applied in sensor recovery procedure, accordingly, the recovery time of the YSZ-based mixed potential type hydrogen sulfide sensor can be shortened, and the purpose of enhancing the response and recovery characteristics of the YSZ-based mixed potential type hydrogen sulfide sensor can be achieved.
Owner:JILIN UNIV
Popular searches
Process efficiency improvement Alkali-metal aluminates/aluminium-oxide/aluminium-hydroxide preparation Waste accumulators reclaiming Battery recycling Magnesium nitrates Calcium/strontium/barium nitrates Phosphorus compounds Luminescent compositions Electronic waste recycling Tube/lamp material recovery
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com