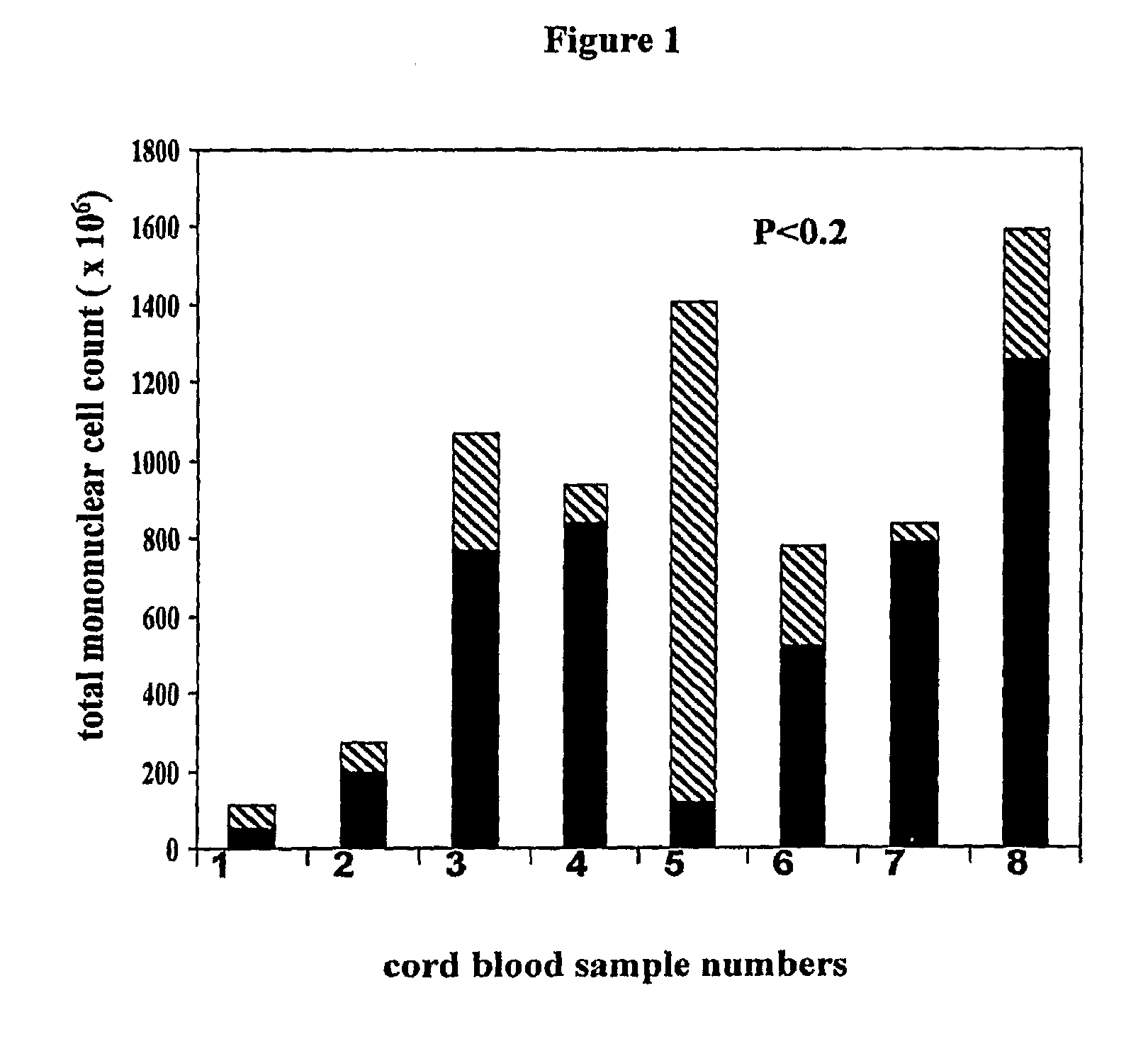
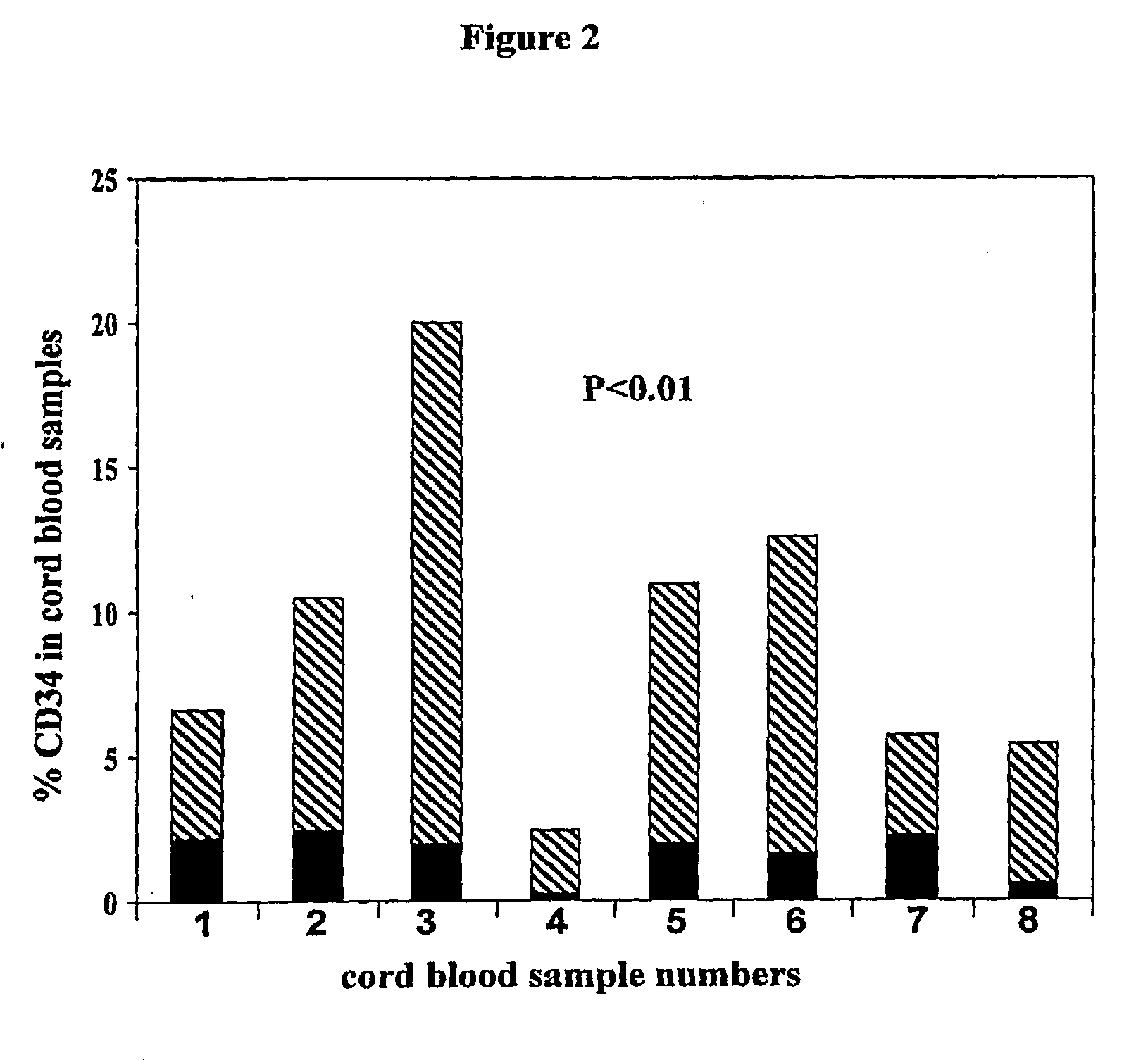
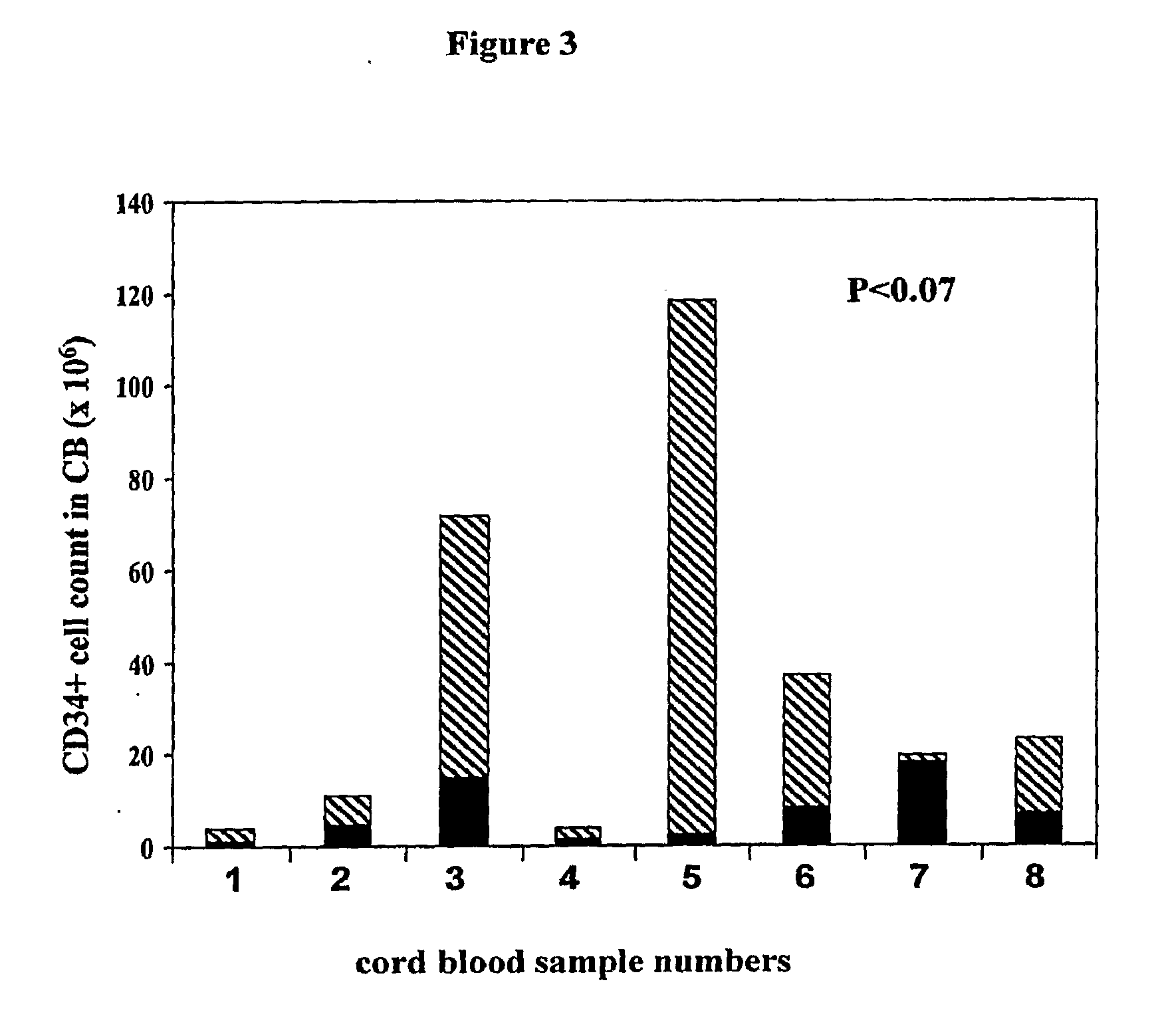
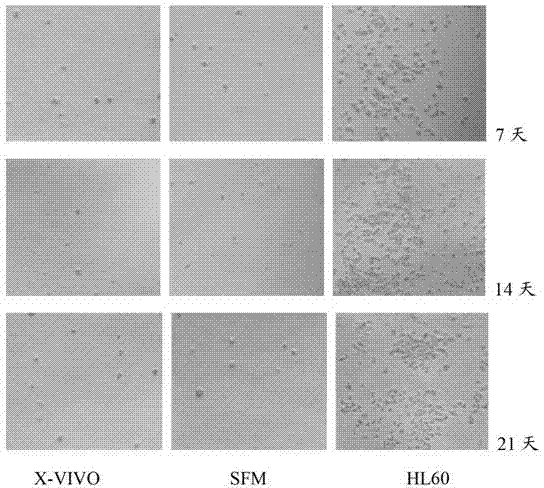
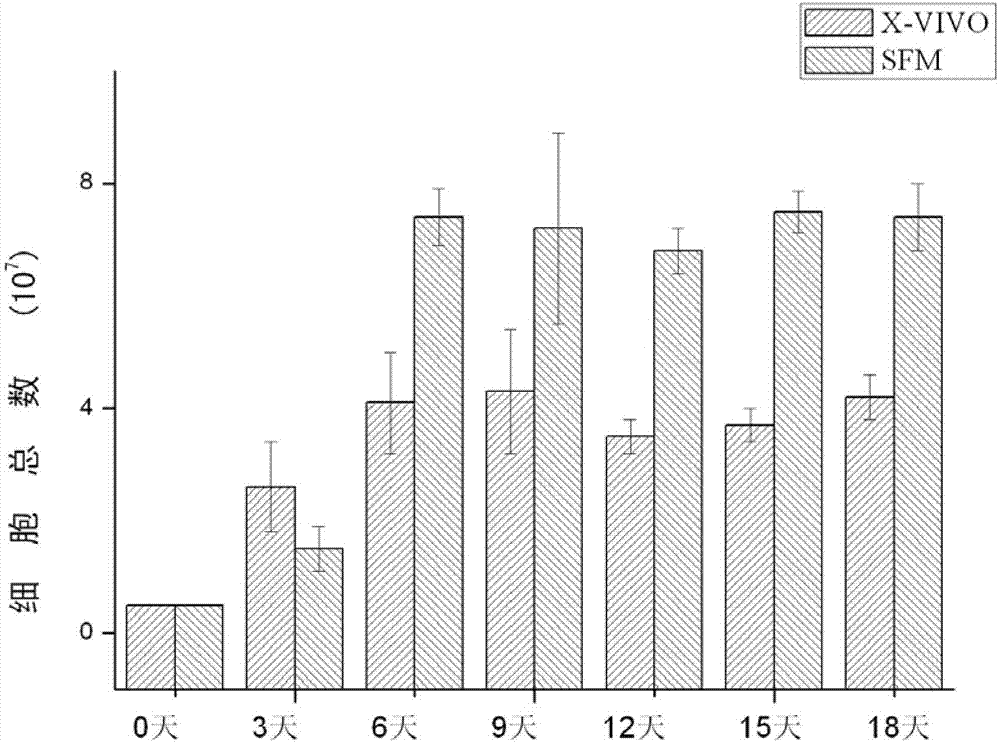
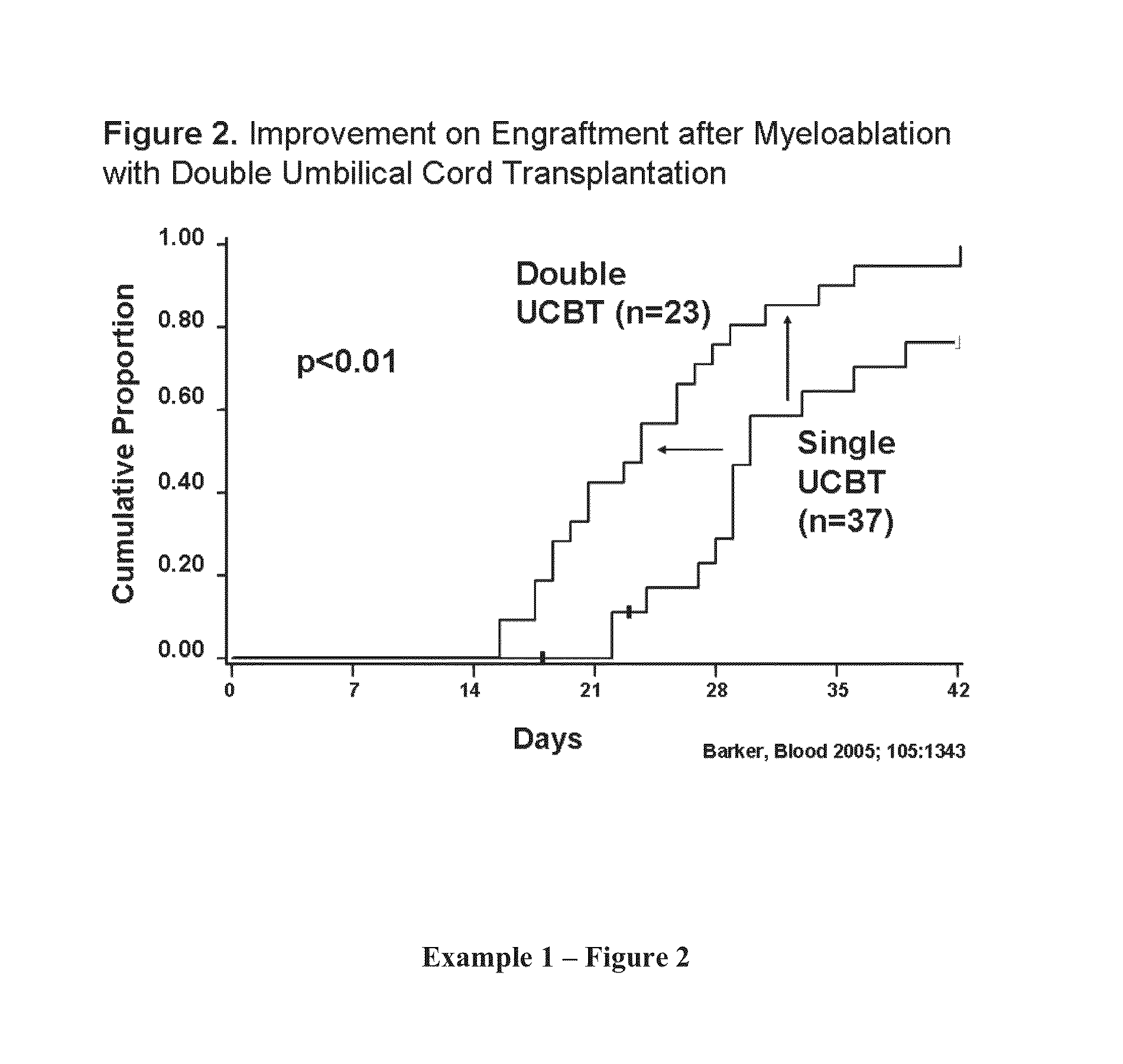
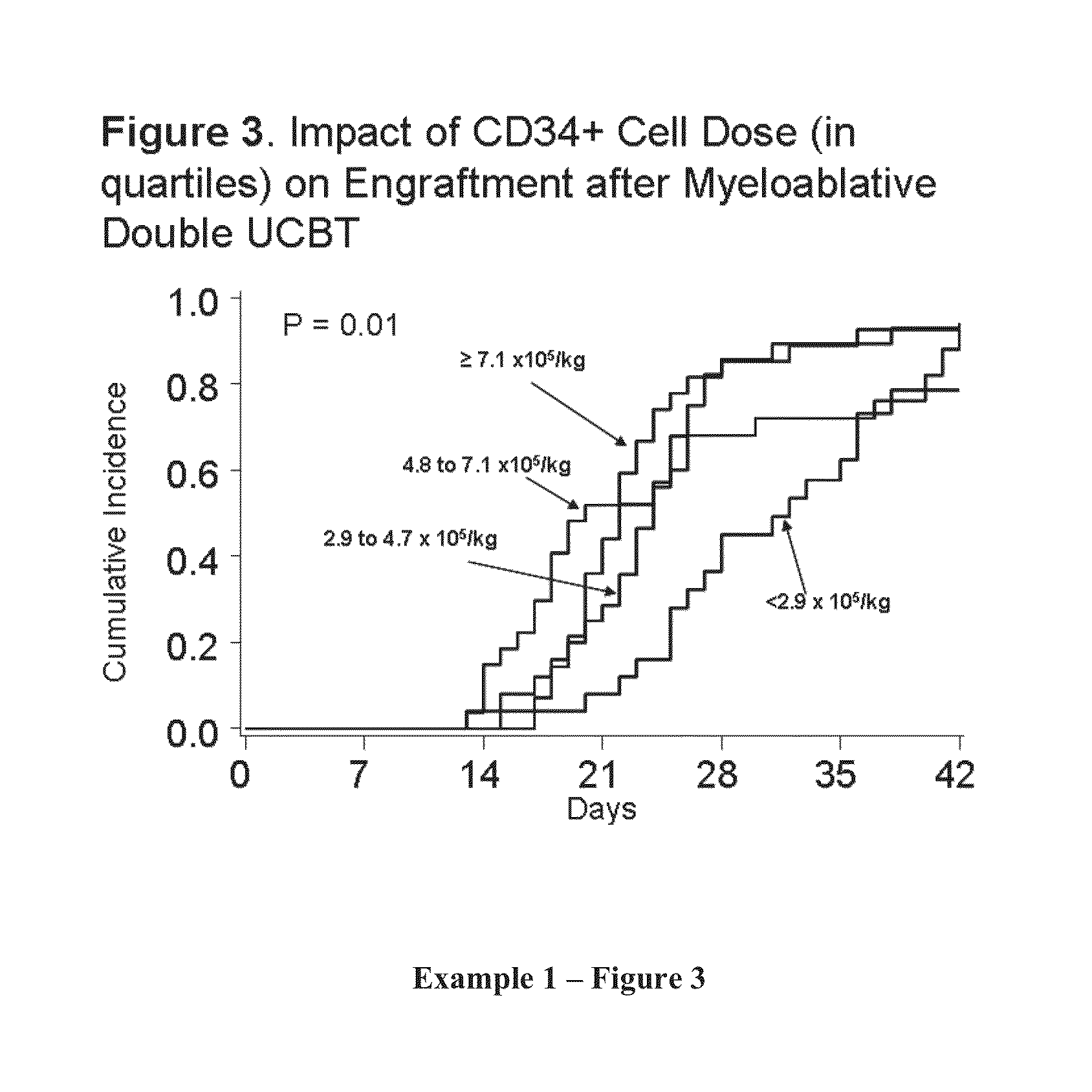
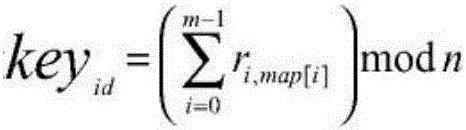Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
108 results about "Umbilical Cord Blood Stem Cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A stem cell obtained from the umbilical cord.
Use of umbilical cord blood to treat individuals having a disease, disorder or condition
The present invention provides methods of using cord blood and cord blood-derived stem cells in high doses to treat various conditions, diseases and disorders. The high-dose cord blood and cord blood-derived stem cells have a multitude of uses and applications, including but not limited to, therapeutic uses for transplantation and treatment and prevention of disease, and diagnostic and research uses. In particular, the cord blood or cord blood-derived stem cells are delivered in high doses, e.g., at least 3 billion nucleated cells per treatment, where treatment may comprise a single or multiple infusions. The invention also provides for the use of cord blood or cord blood-derived stem cells from multiple donors without the need for HLA typing.
Owner:CELULARITY INC
Stem cell culture medium and application thereof and stem cell cultivation method
ActiveCN103060264AHigh speedRapid expansionCulture processCell culture mediaMesenchymeCell culture media
The invention discloses a stem cell culture medium, an application of the stem cell culture medium and a stem cell cultivation method. Blood serum does not exist in the stem cell culture medium. The stem cell culture medium comprises amino acid, vitamin, salt, lipoid, cytokines and egg white polypeptide. The stem cell culture medium is suitable for fast cultivating stem cells which are tissue sources of human and mammal, and comprises but not is limited by fat mesenchyme stem cells, mesenchymal stem cells and umbilical cord blood stem cells. The culture medium enables increasing speed of the cells to be improved by 3-5 times, and differential potentials of the cells can not be affected. Compared with an ordinary stem cell culture medium, stem cells from different sources can be fast expanded, passage number is prolonged, and proficiency properties of the stem cells can be well kept.
Owner:苏州博棠再生医学科技有限公司
Treatment of premature birth complications
InactiveUS20090136471A1Promote formationMany formatsAntibacterial agentsOrganic active ingredientsPremature thelarcheCord blood stem cell
The present invention provides methods of treating one or more complications of premature birth suffered by premature infants, comprising administering to the premature infant umbilical cord blood stem cells and, optionally, placental stem cells. The present invention also provides methods of combining and administering, and compositions comprising, umbilical cord blood stem cells, particularly autologous cord blood cells, and placental stem cells for the treatment of premature infants.
Owner:CELULARITY INC
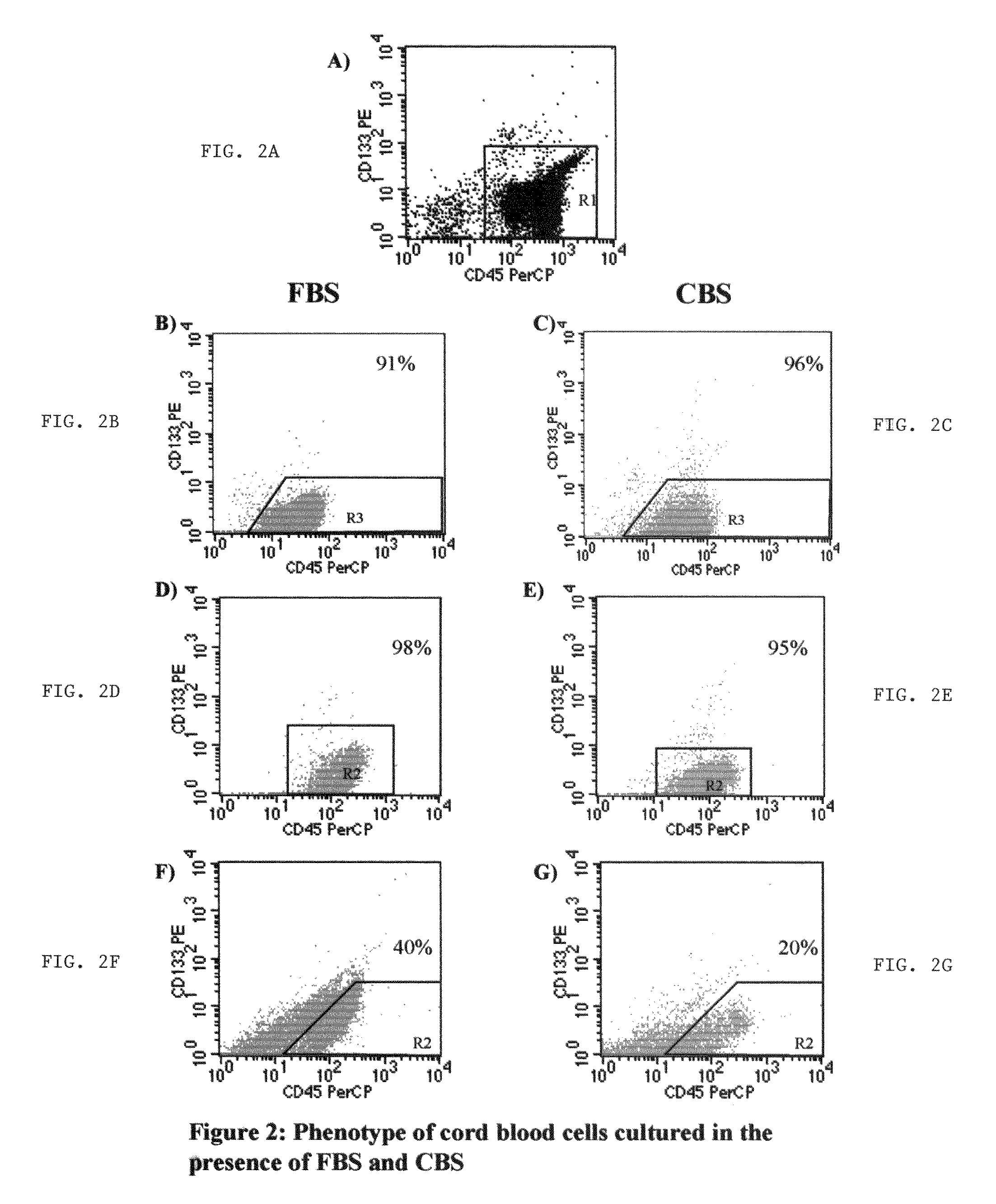
Growth of neural precursor cells using umbilical cord blood serum and a process for the preparation thereof for therapeutic purposes
InactiveUS20040203142A1Promote growthArtificial cell constructsBlood/immune system cellsCord blood stem cellCell culture media
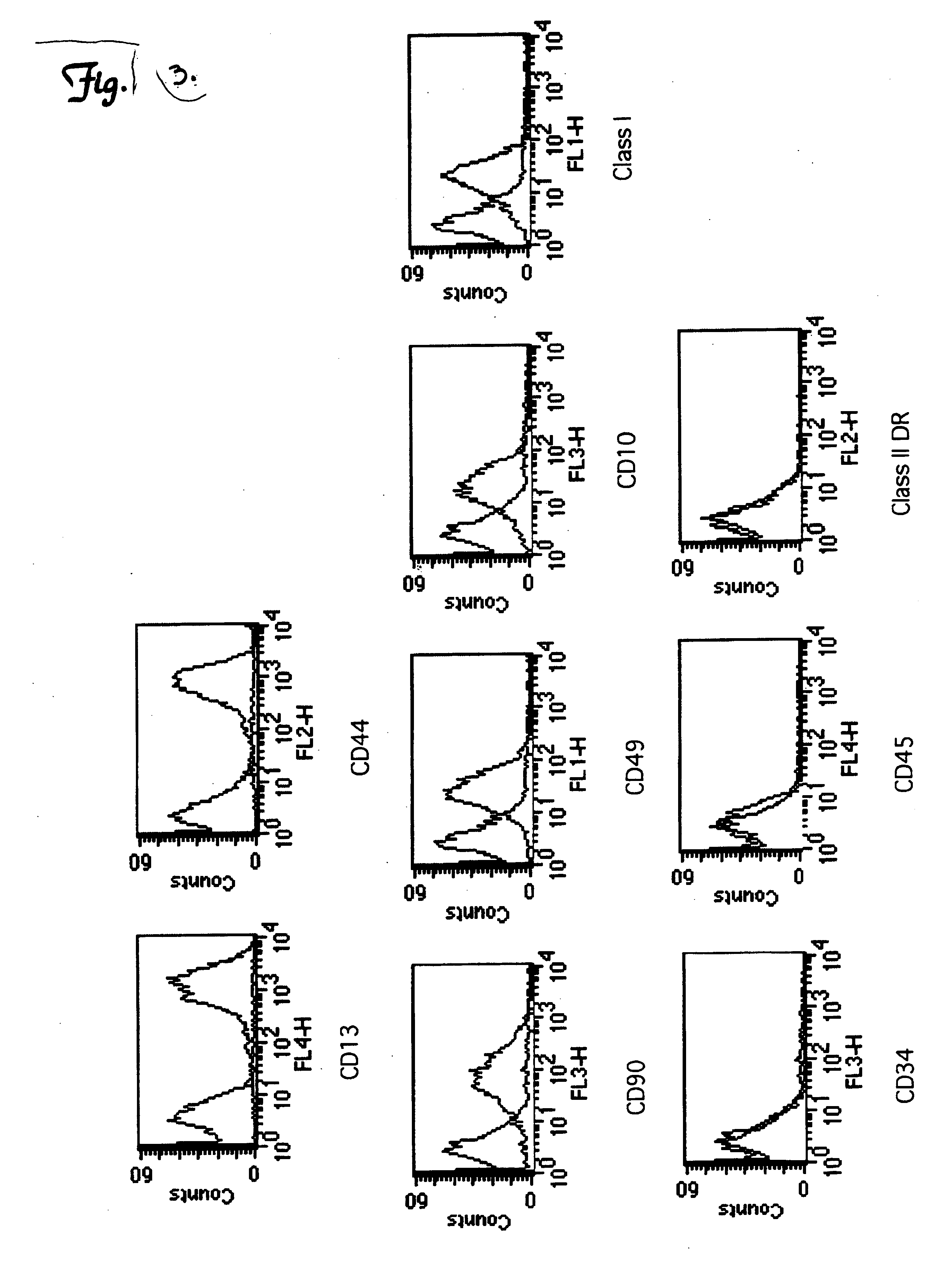
This invention is concerned with stem cells derived from umbilical cord blood serum and a method for growing human embryonic stem cells and adult cells comprising sera separated from clotted umbilical cord blood, including growing and differentiating cord blood stem cells into neural precursors, comprising transdifferentiating CD34+ stem cells from mononuclear cells derived from umbilical cord blood to neural precursors. The stem cells obtained from the umbilical cord include pluripotent stem and progenitor cell population of mononuclear cells, and separating pluripotent stem and progenitor cell population of mononuclear cells obtained from the umbilical cord blood. A magnetic cell separator is used to separate out cells which contain a CD marker and then expanding the cells in a growth medium containing retinoic acid and one or more growth factors BDNF, GDNF, NGF and FGF as a differentiating agent. The invention is also concerned with the transplantation and repair of nerve damage, stokes, spinal injury, Parkinson's and Alzheimer's, prepared in accordance with the aforesaid method and a media for culturing umbilical cord blood stem calls consisting essentially of cord blood stem cells derived from umbilical cord blood serum.
Owner:RELIANCE LIFE SCI PYT
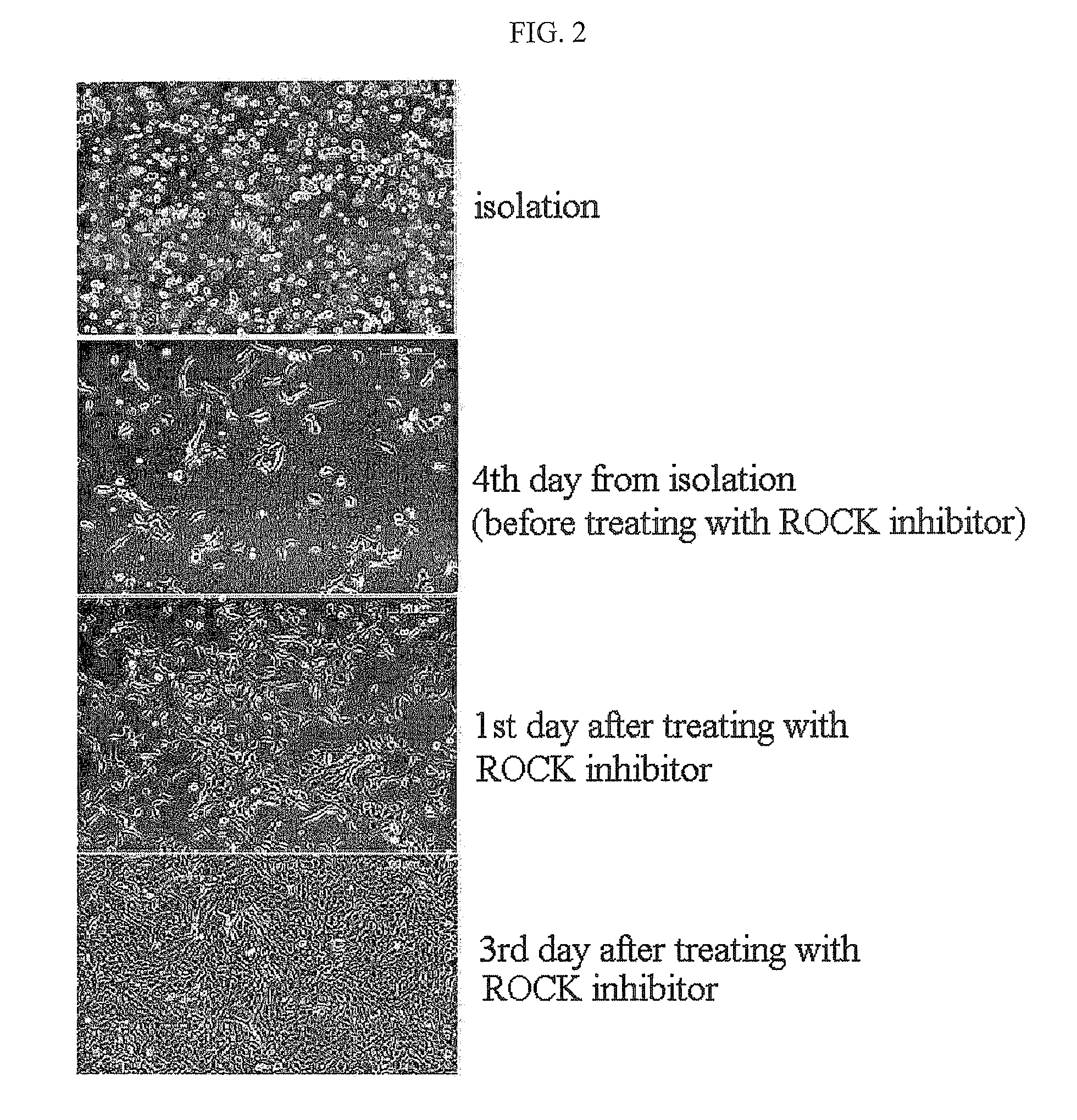
Method for isolating and culturing adult stem cells derived from human amniotic epithelium
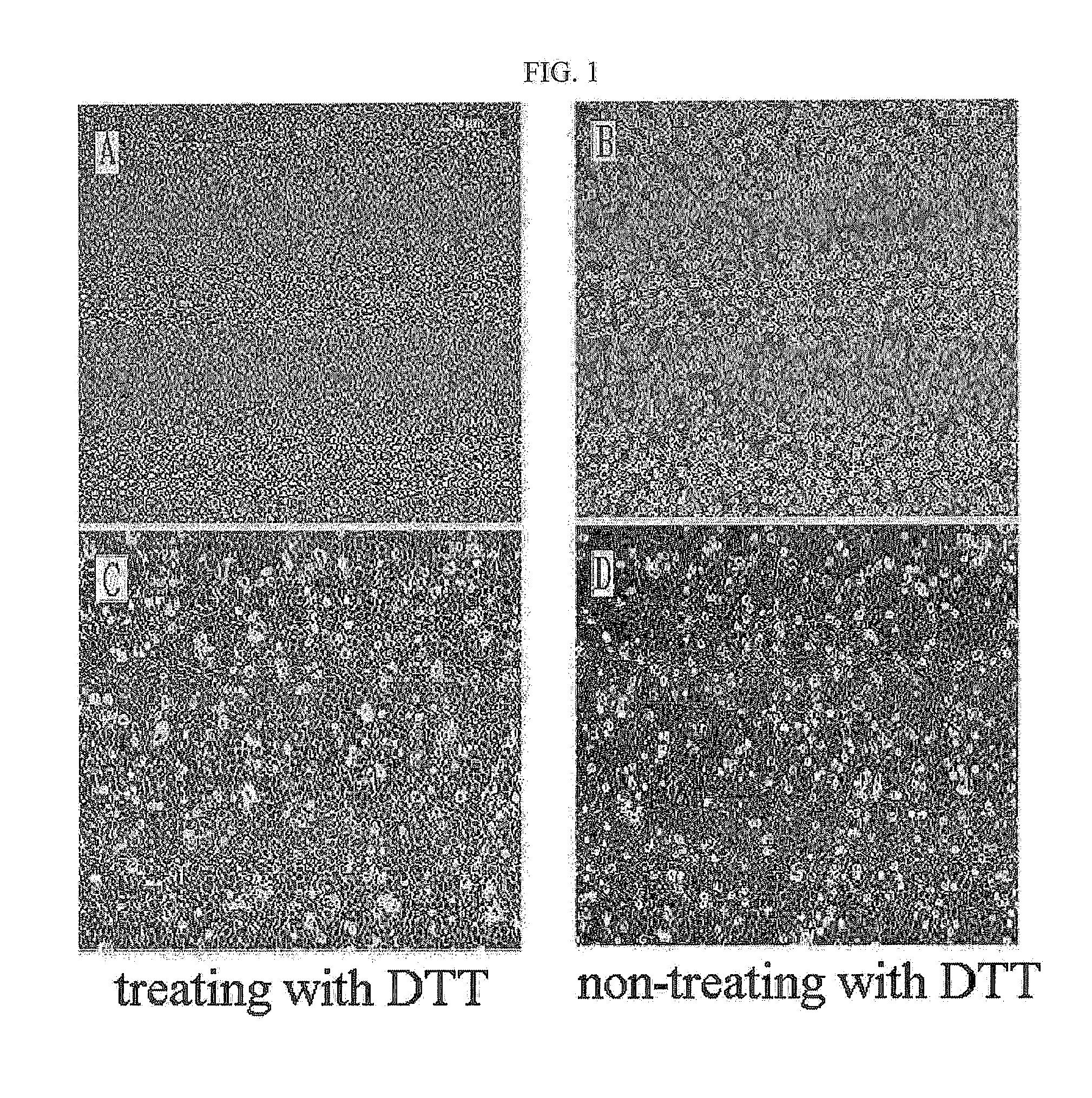
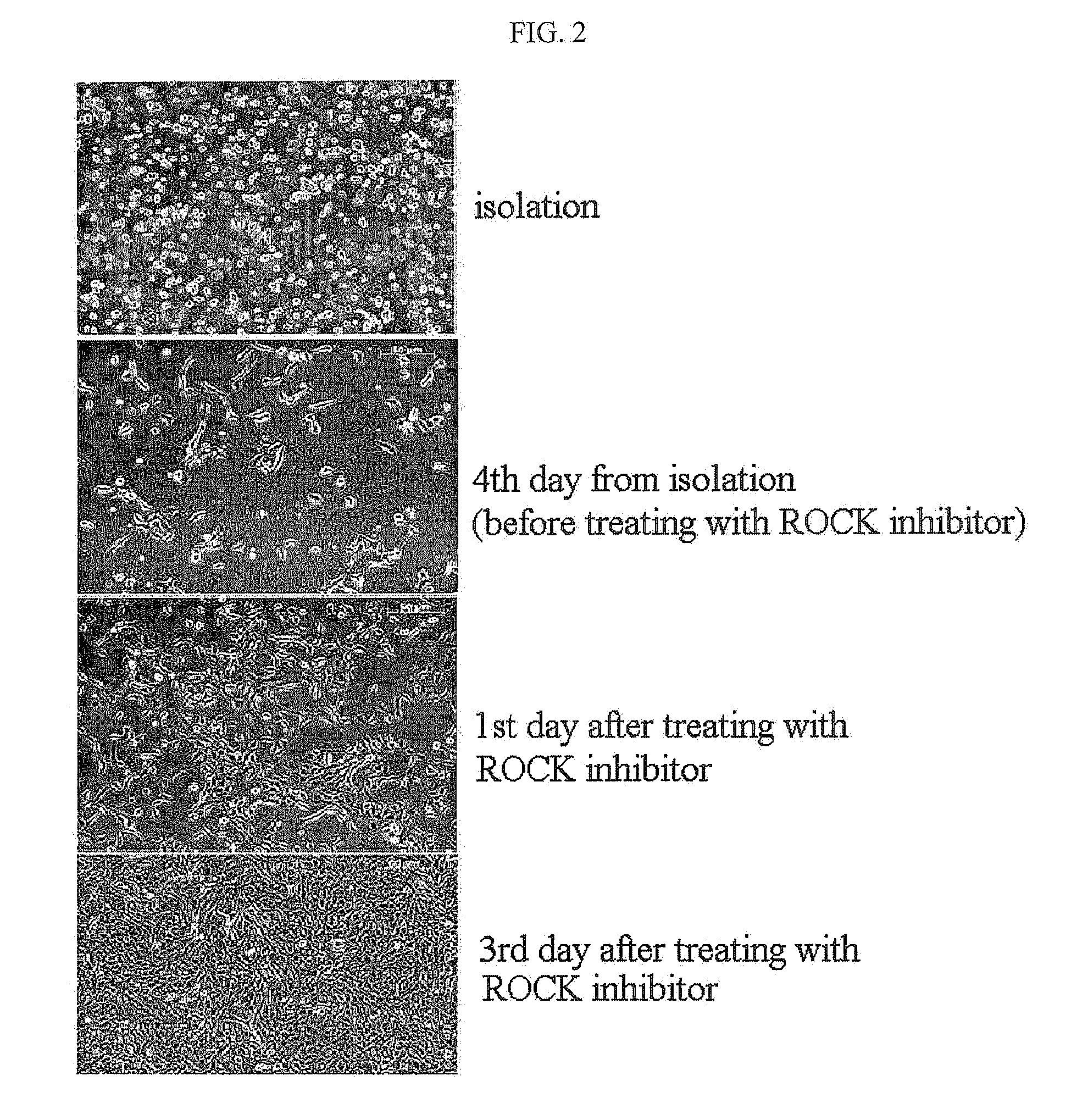
The present invention relates to a method for isolating and culturing adult stem cells derived from human amniotic membrane in high yield, and more particularly to a method for obtaining a large amount of adult stem cells, the method comprising obtaining amniotic epithelial cells from human amniotic tissue in high yield by treatment with dithiothreitol (DTT) and a low concentration of trypsin and culturing the amniotic epithelial cells in a medium containing a Rho-associated kinase inhibitor. The human amniotic epithelial cell-derived stem cells are easily extracted compared to existing therapeutic stem cells such as umbilical cord blood stem cells and bone marrow stem cells, the yield and proliferation thereof are significantly increased by DTT treatment, the addition of the ROCK inhibitor or the replacement of medium. Thus, the method can be used to efficiently prepare adult stem cells.
Owner:RNL BIO
Marrow umbilical cord blood stem cell in vitro separating kit and application method thereof
InactiveCN101144070ASimple methodConvenient treatmentArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsAntigenFicoll
The present invention relates to a reagent kit of the isolation of a bone marrow umbilical cord blood stem cell in vitro and the application method thereof. The present invention is characterized in that Lin antibody red blood cell precipitant cell preserving solution, and sodium iothalamate-polysucrose 400 # or HISTOPAQUE1077 or Ficoll are contained in the reagent kit, and each reagent solution is preserved dividually. The present invention adopts the method which combines a negative collection method and a density method, thus the surface of the stem cell which is collected and abstracted has no any label, the cells which are isolated and abstracted are Lin antigenic negative cells, the method is simple, and the present invention can be directly used to isolate and abstract the stem cells clinically. The reagent kit of the present invention can operate the industrialized production, to realize the promotion of the isolation and purification technology of the human hematopoietic stem cell, leading the doctor to conveniently cure the patient with the stem cells like the medicine usage.
Owner:NINGXIA ZHONGLIANDA BIOPHYSICS
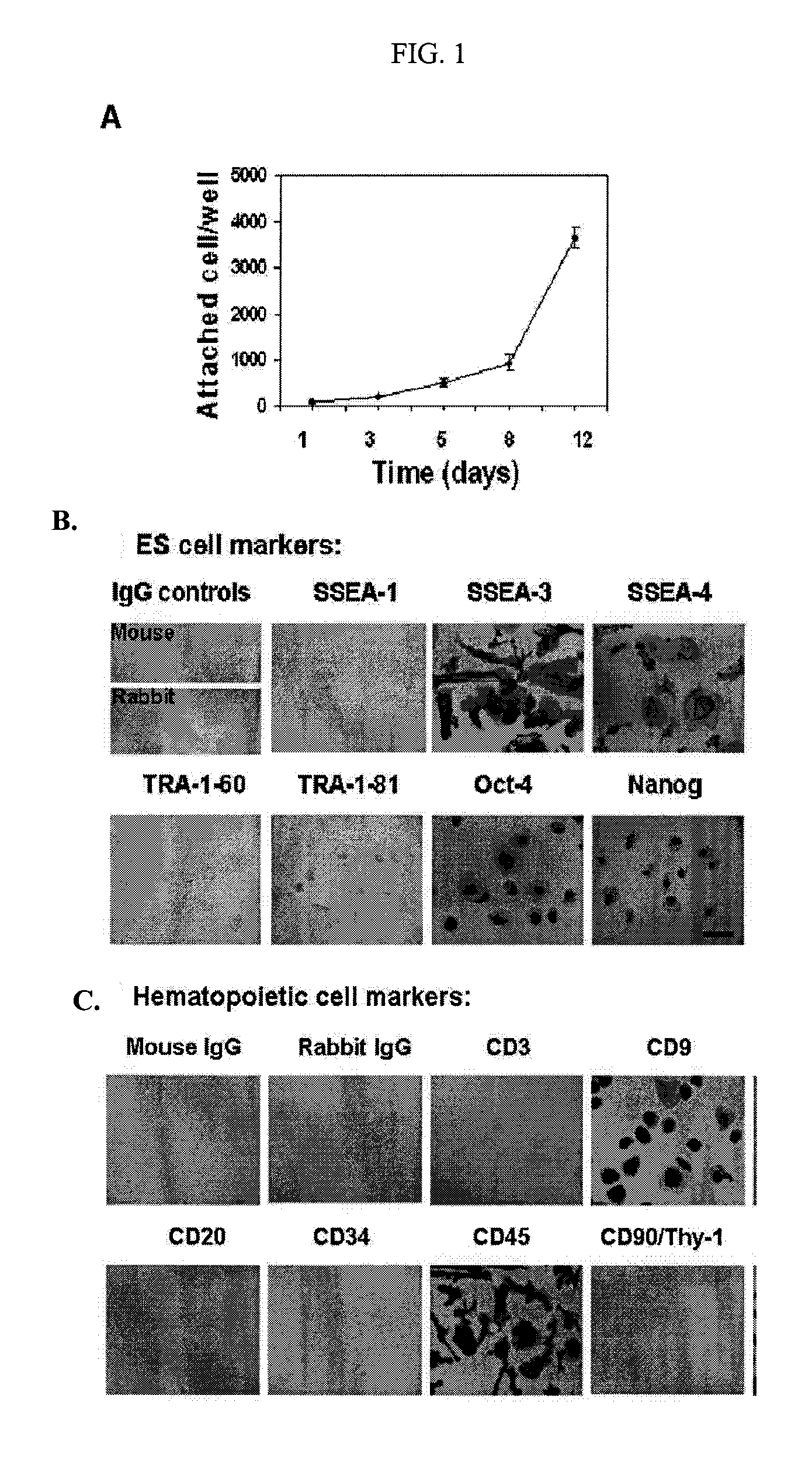
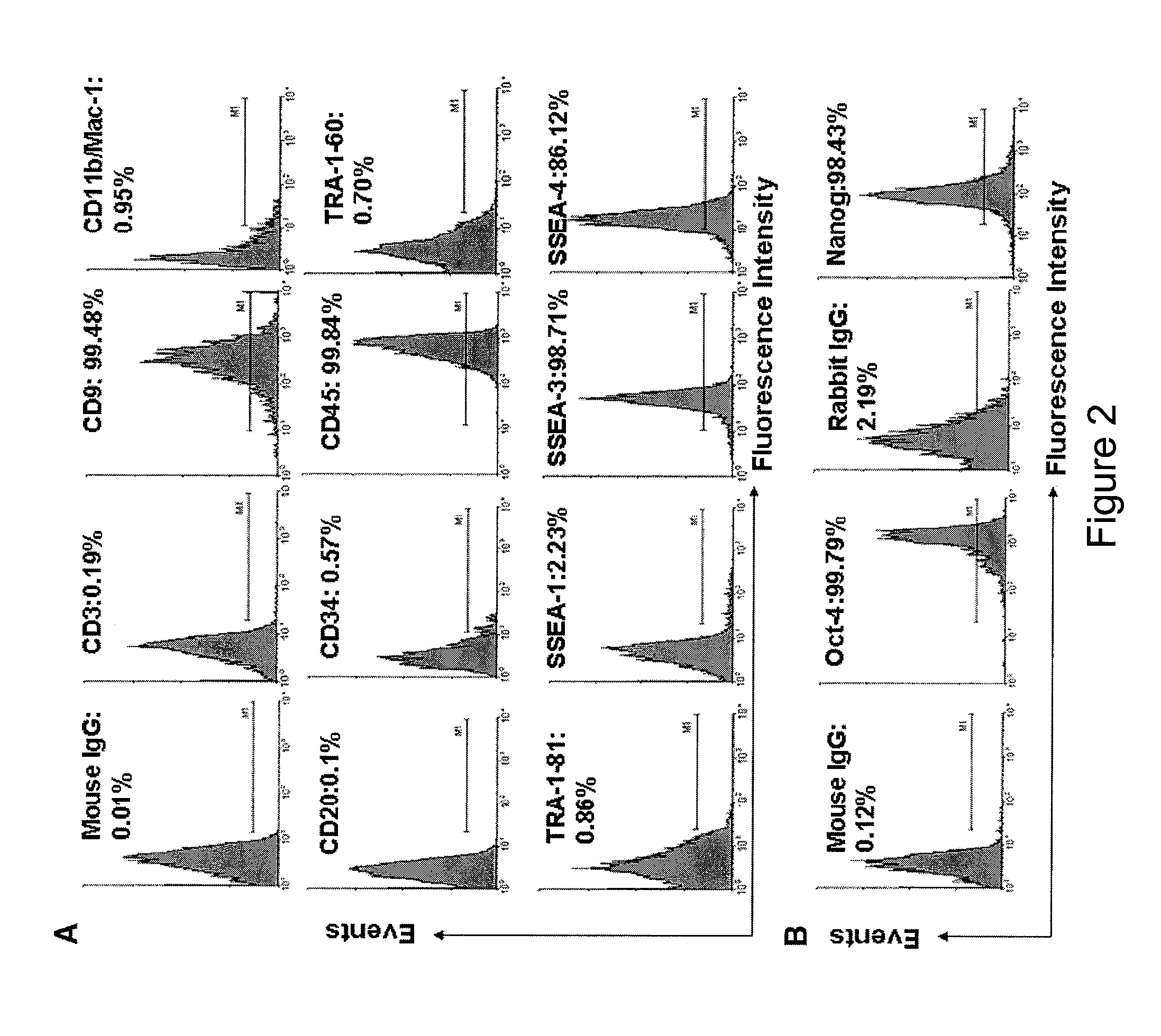
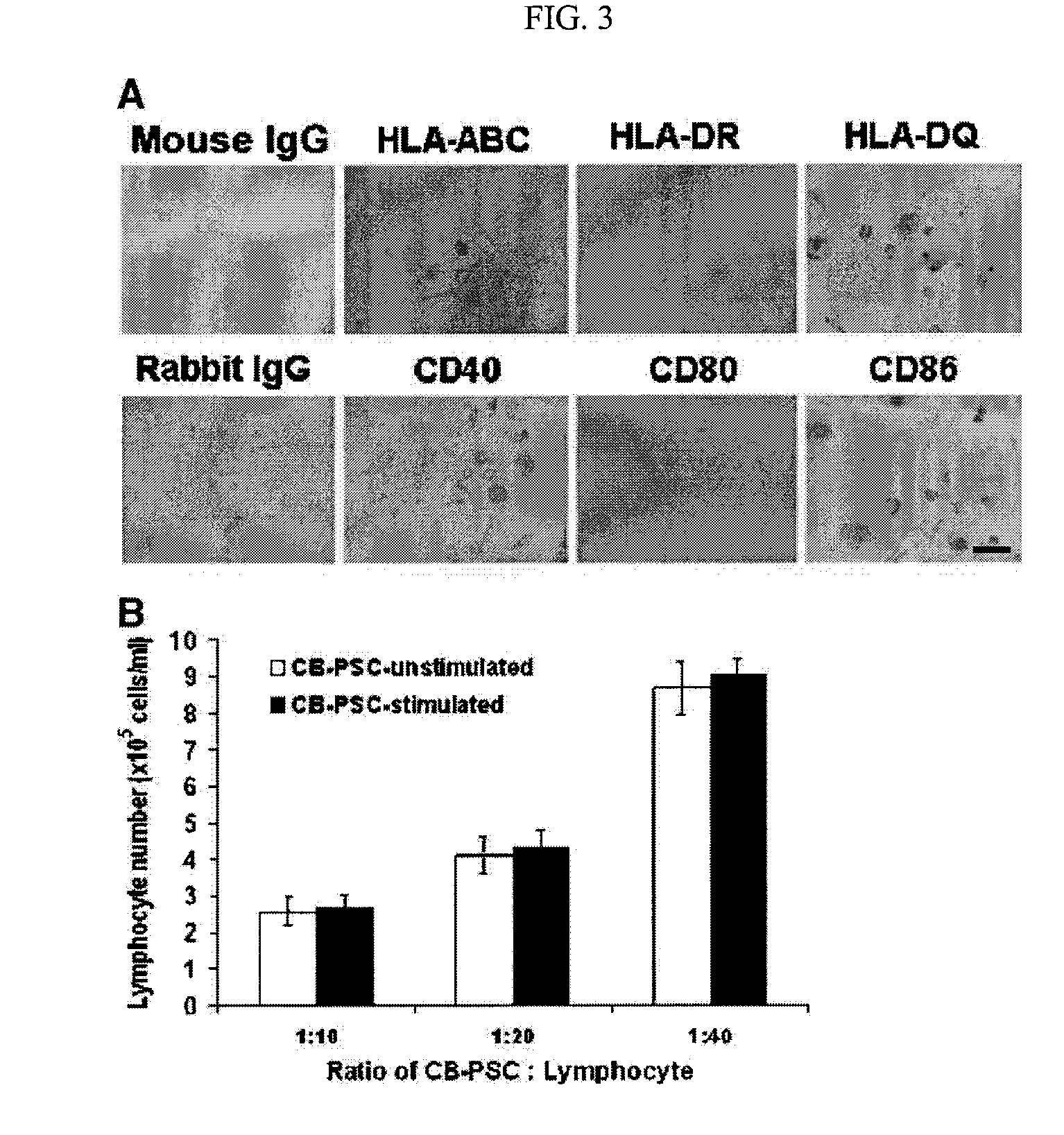
Isolated Embryonic-Like Stem Cells Derived From Human Umbilical Cord Blood
ActiveUS20090175832A1Low immunogenicityAbility to proliferateBiocideMetabolism disorderHematopoietic cellAutoimmune condition
The present invention is related generally to embryonic-like stem cells isolated from human umbilical cord blood, designated herein as cord blood-stem cells (CB-SC's), which display the characteristics of embryonic stem cells and hematopoietic cells. These cells have the capability of proliferation and are able to differentiate to multiple types of cells. In addition, the CB-SC display low immunogenicity and immune regulation. These cells are, therefore, suitable for use in stem cell-based therapies for the treatment of diseases such as Parkinson's disease, diabetes, spinal cord damage, multiple sclerosis, cardiovascular disease, stroke and birth defects, and for preventing, treating and / or reducing an autoimmune disease in a mammalian subject.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
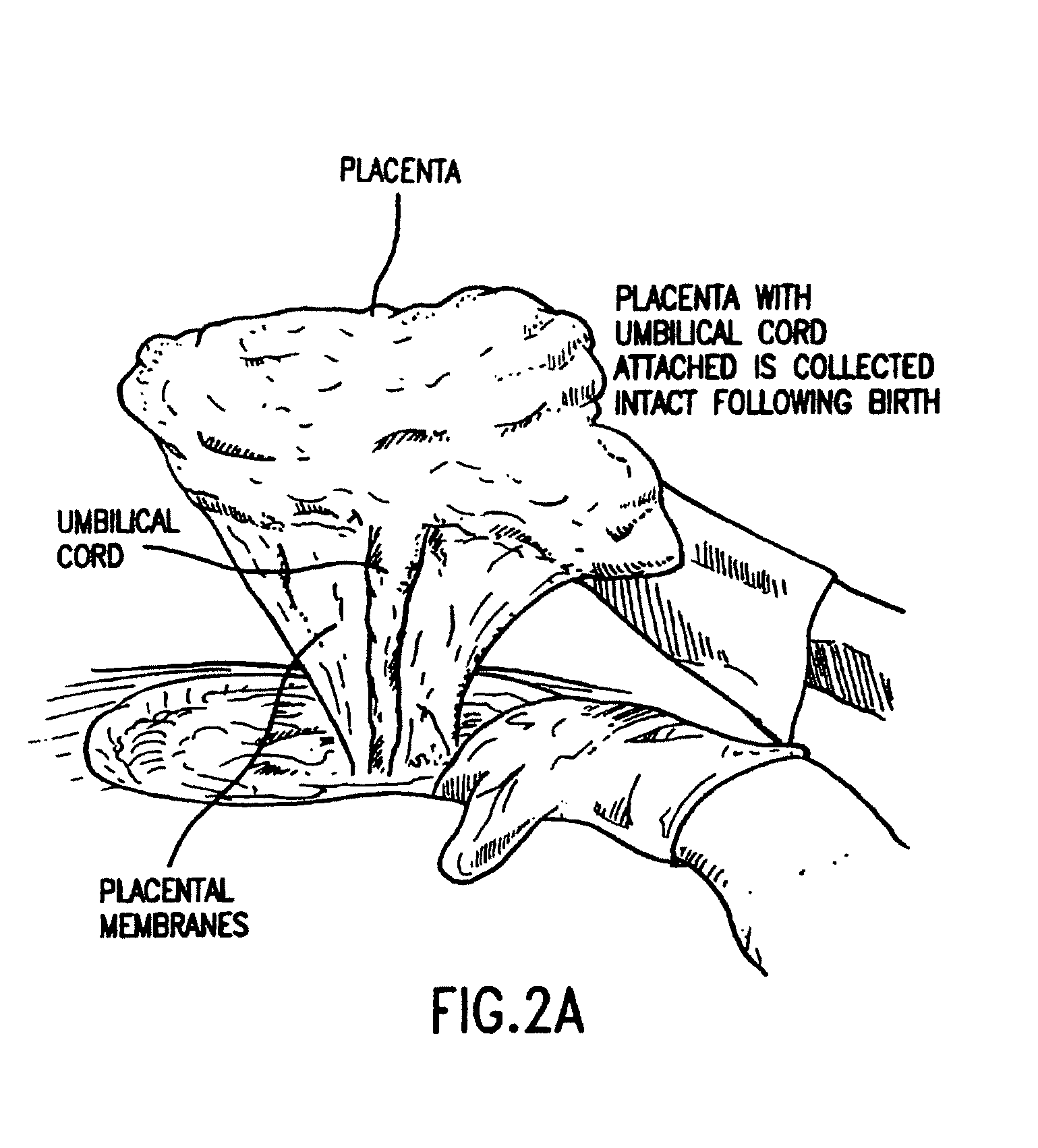
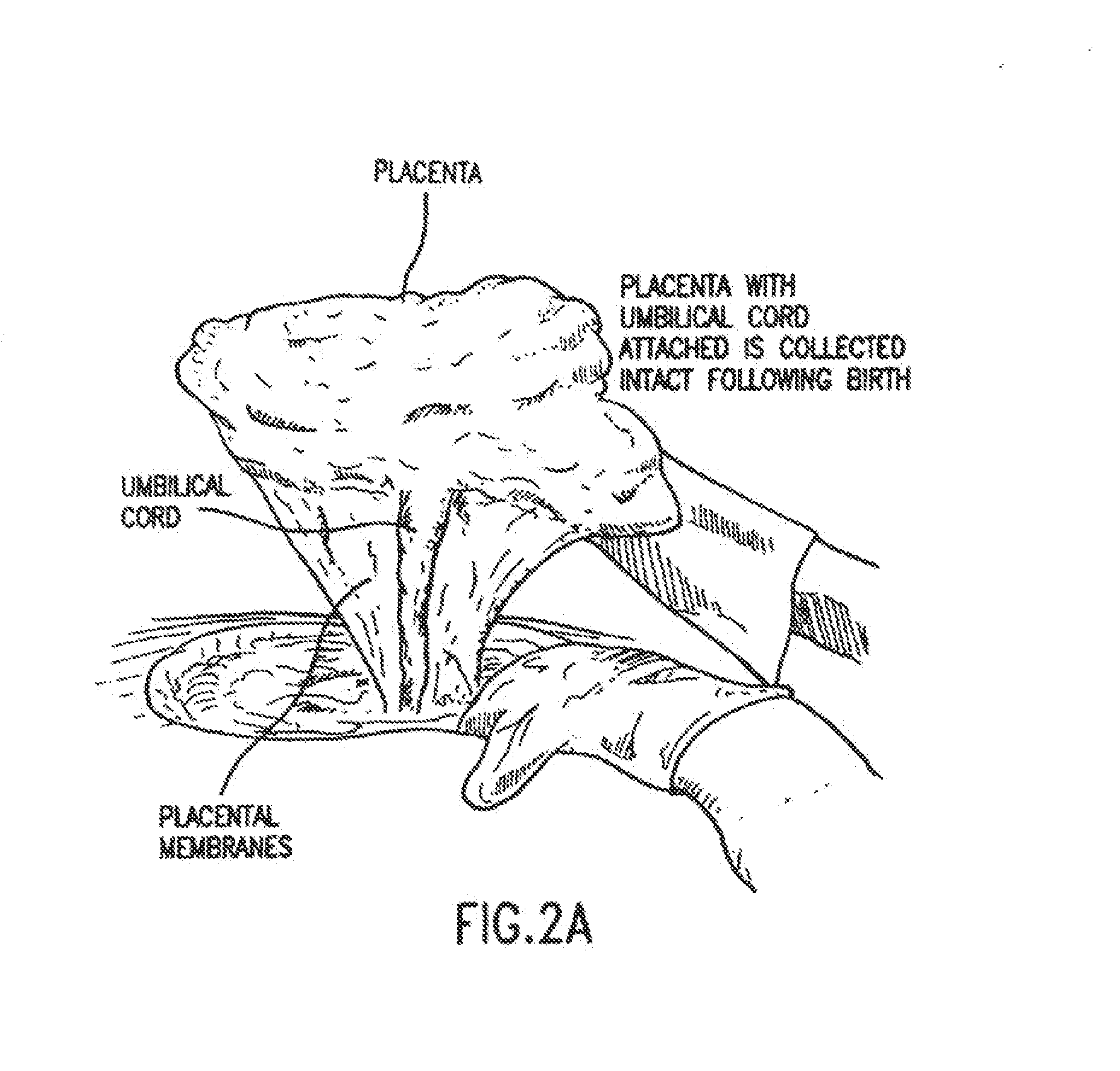
Methods for collecting and using placenta cord blood stem cells
ActiveUS20090123437A1Raise countBiocideMammal material medical ingredientsPeristaltic pumpCord blood stem cell
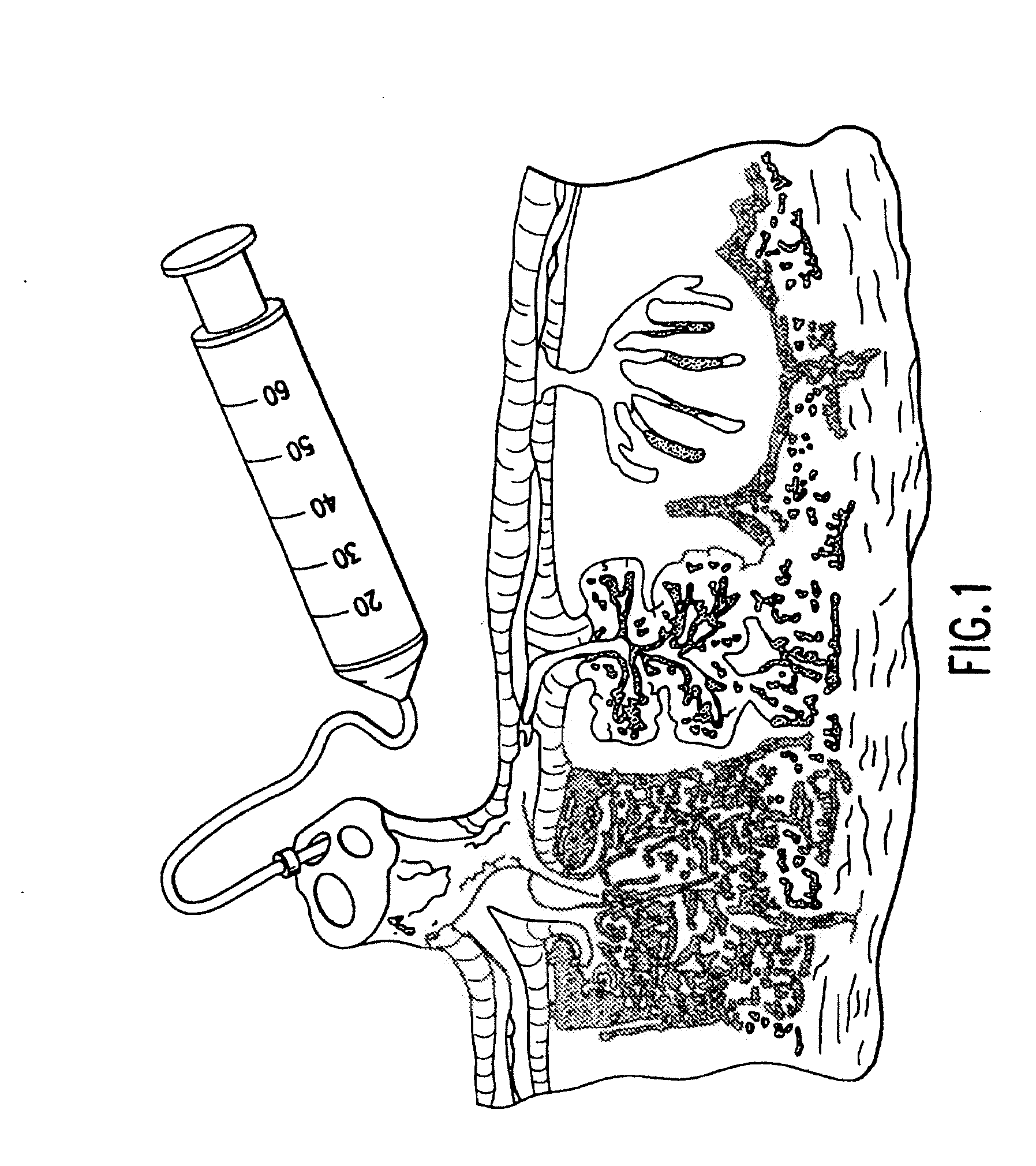
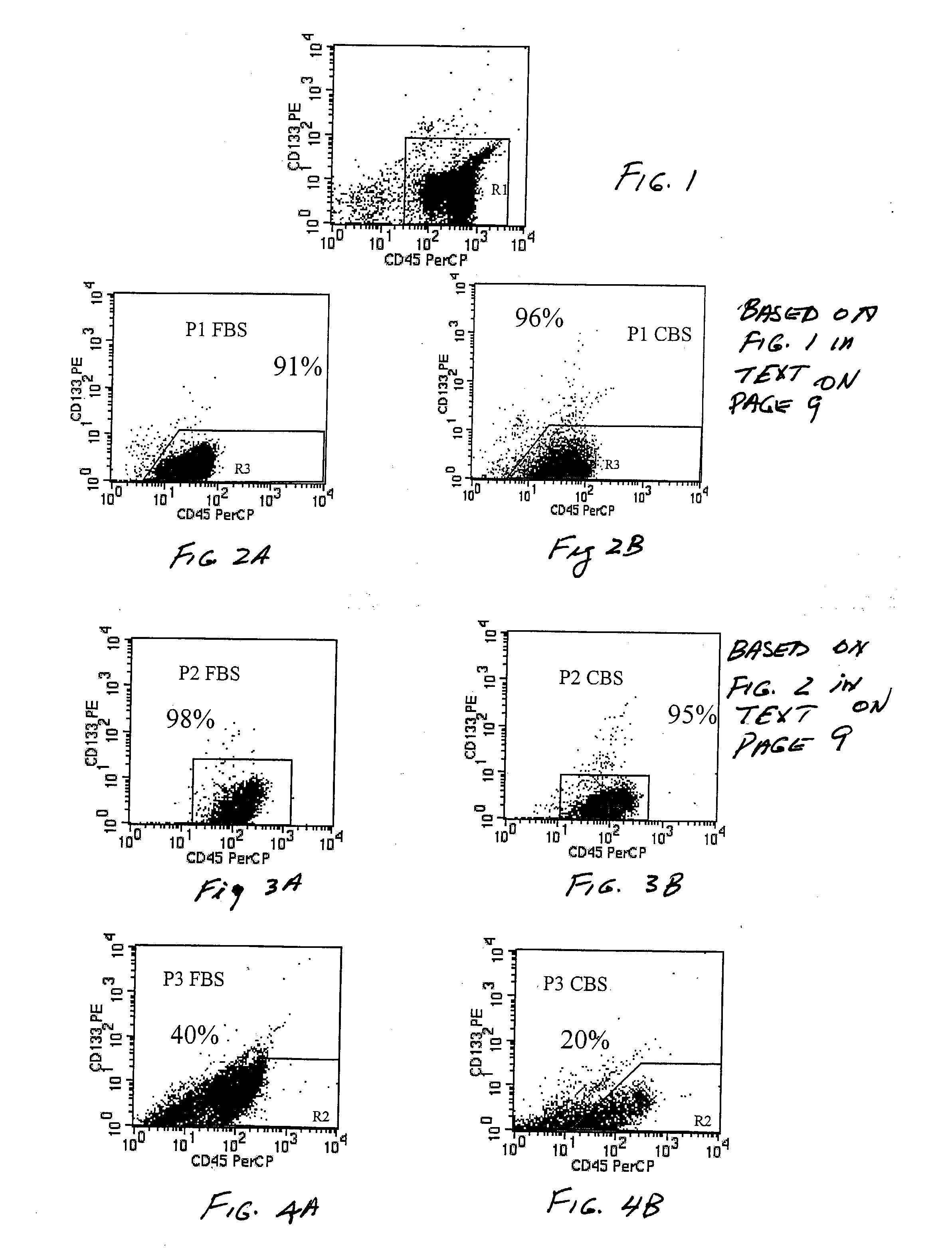
An innovative method of collecting cord blood stem cells from an isolated mammalian non-exsanguinated or partially exsanguinated placenta by placental perfusion is described and also an easy method for safe long duration cold storage of the placenta. Placental perfusion can include perfusing the isolated placenta with a pulsatile flow of perfusion solution, for example, using a pulsatile or peristaltic pump or device. The stem cells can then be isolated from the perfusate. Significantly increased amounts of CD133+ stem cells can be collected from the perfusate. The perfusion solution can include an anticoagulant. The isolated mammalian placenta need not be treated with an anticoagulant prior to perfusing. The isolated placenta can be free from an anticoagulant prior to perfusing.
Owner:CELULARITY INC
Medical dressing with bioactivity and preparation method of medical dressing
InactiveCN106139230AAvoid immune rejectionImprove mechanical propertiesAbsorbent padsBandagesDiseaseFreeze-drying
The invention relates to a medical dressing with bioactivity and a preparation method of the medical dressing and belongs to the technical field of biomedical engineering and material science. The medical dressing with the bioactivity is used for covering skin defect wounds caused by burning, scalding, operation, wounds and diseases and inducing wound repair and is prepared as follows: one or more of embryonic stem cells, umbilical cord blood stem cells, amniotic fluid stem cells, peripheral blood stem cells and bone mesenchymal stem cells of humans or animals are cultured in vitro, stem cell growth factors secreted in the logarithmic growth phase are collected and mixed in proportion with one or more of I type collagen, III type collagen, chitosan, hyaluronic acid, chondroitin sulfate and sodium alginate, thin-layer sponge is taken as an inner layer, a porous high-polymer material film outer layer prepared from a mixture formed by one or more of PLA (polylactic acid), PGA (polyglycolic acid), PLGA (poly(lactic-co-glycolic acid)) and PCL (polycaprolactone) is attached, the product is frozen-dried and cut, and the medical dressing with the bioactivity is formed and used for treating skin defect wounds caused by burning, scalding, operation, wounds and diseases and inducing wound repairing.
Owner:南京天其美生物技术有限公司
Stem cell culture medium and its applications as well as a stem cell culture method
ActiveUS20140178987A1Increase proliferation speedMaintain pluripotencyCulture processArtificial cell constructsPotential wellMammalian tissue
The present invention discloses a stem cell culture medium and its applications as well as a stem cell culture method. The said stem cell culture medium contains no serum. The said stem cell culture medium contains amino acids, vitamins, salts, lipids, cytokines and protein polypeptides. The said stem cell culture medium is suitable for rapid culture of stem cells derived from human and mammalian tissues, including but not limited to, adipose mesenchymal stem cells, bone marrow mesenchymal stem cells and umbilical cord blood stem cells. The said culture medium can increase the proliferation speed of the stem cells 3-5 times, without any affects on their differentiation potentials. Comparing the said stem cell culture medium to a routine culture medium, the said culture medium is not only able to proliferate stem cells derived from different sources more rapidly and achieve more proliferation generations, but also keep their differentiation potentials well.
Owner:BIOTOWNTEK CO LTD
Non-animal-source serum-free culture medium for umbilical cord blood stem cells
ActiveCN102827810AAvoid instabilityClear natureBlood/immune system cellsCell phenotypeLipid formation
The invention relates to the field of biology, and discloses a non-animal-source serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, phytohaemagglutinin (PHA), lipid, amino acid, vitamins, trace elements, interleukin-3(IL-3), stem cell factor, (SCF), Fit3-L, IL-6 and granulocyte colony-stimulating factor (G-CSF). The non-animal source serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation, avoids the doped animal components and unstability of batches, and the results of cultured umbilical cord blood stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the non-animal-source serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
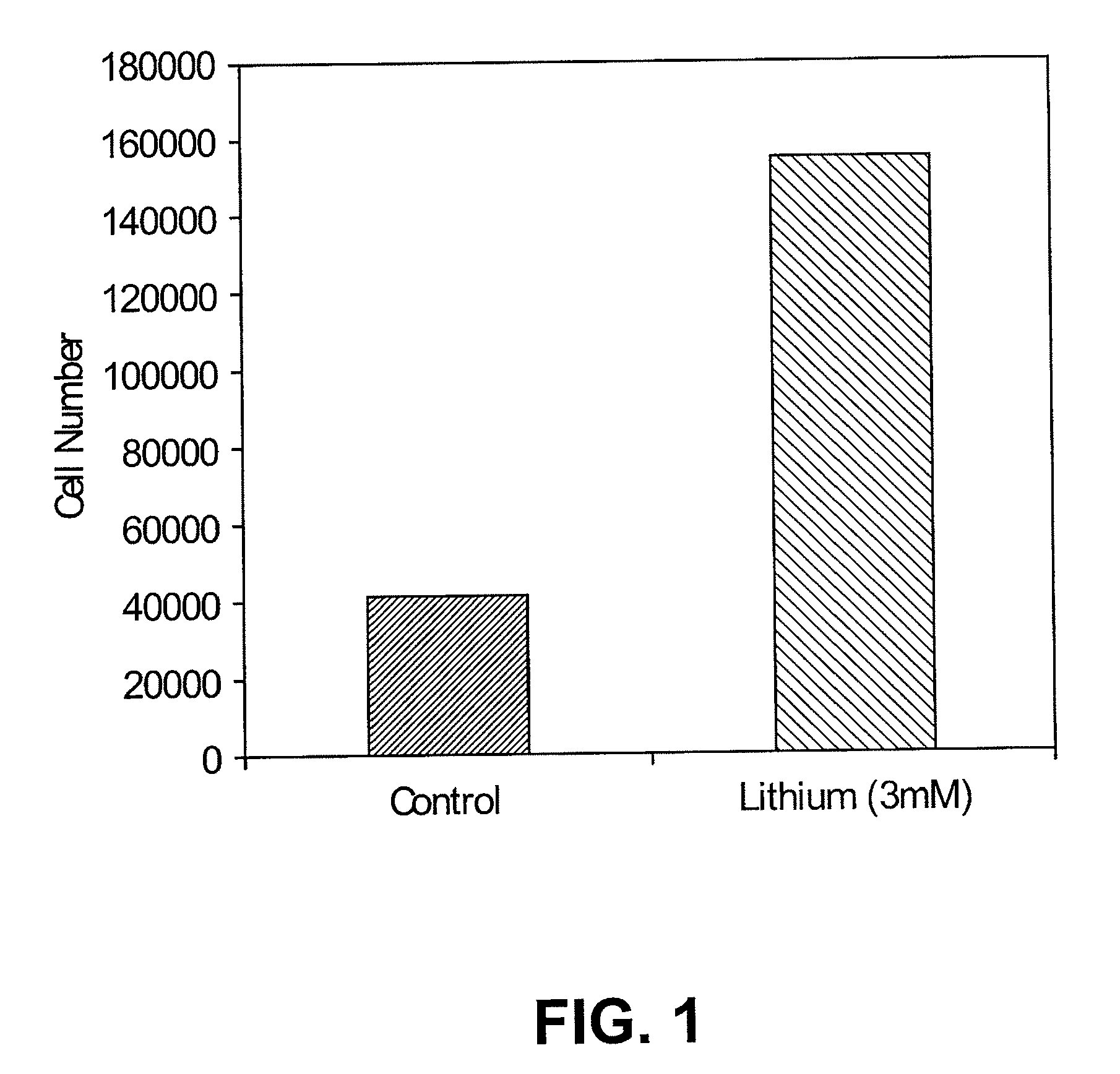
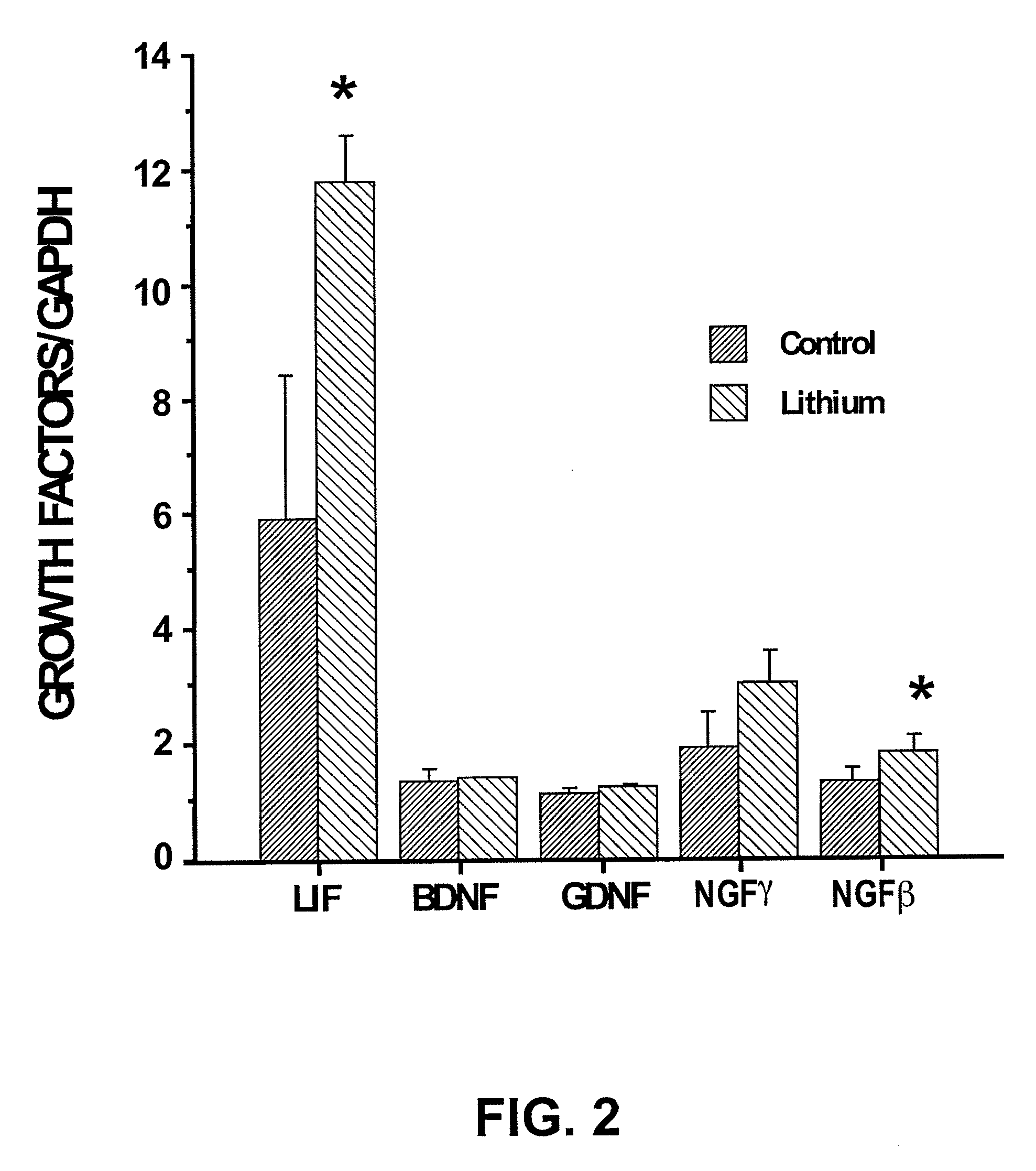
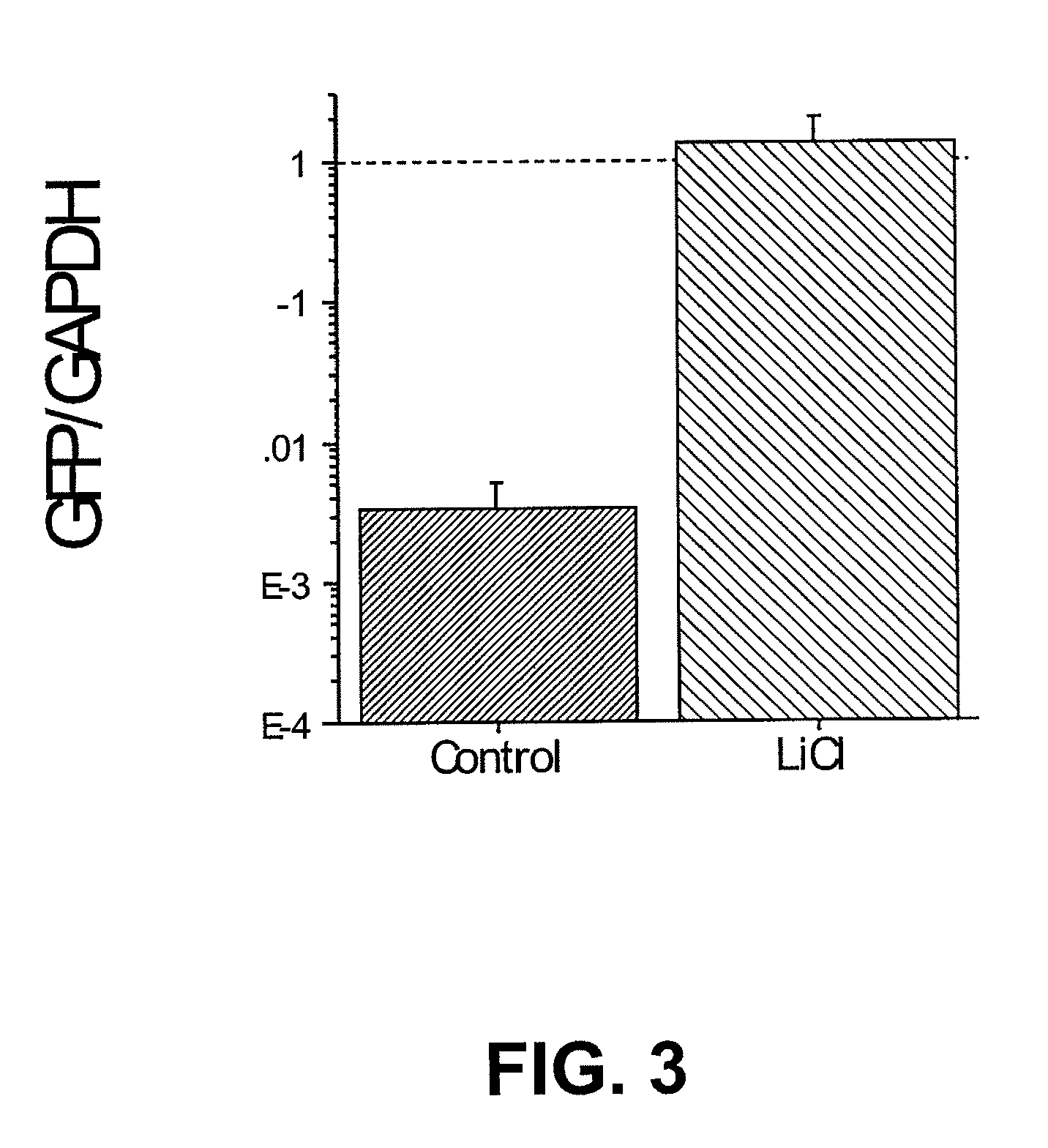
Lithium stimulation of cord blood stem cell proliferation and growth factor production
ActiveUS20100189696A1Stimulating growth factor productionEnhancing survival and growthBiocideNervous disorderLithiumCord blood stem cell
The present invention provides methods for expanding human umbilical cord blood stem cells and methods for stimulating growth factor production by cord blood stem cells using an in vitro cell culture system comprising a lithium salt. The present invention also provides in vivo methods for enhancing the survival and growth of transplanted cord blood stem cells by treating the cells with a lithium salt prior to transplantation. In vivo methods for reducing rejection of transplanted cord blood stem cells by administering a lithium salt after transplantation are also provided.
Owner:RUTGERS THE STATE UNIV
Treatment of premature birth complications
InactiveUS20130259845A1Promote formationMany formatsAntibacterial agentsHeavy metal active ingredientsPremature thelarcheCord blood stem cell
The present invention provides methods of treating one or more complications of premature birth suffered by premature infants, comprising administering to the premature infant umbilical cord blood stem cells and, optionally, placental stem cells. The present invention also provides methods of combining and administering, and compositions comprising, umbilical cord blood stem cells, particularly autologous cord blood cells, and placental stem cells for the treatment of premature infants.
Owner:CELULARITY INC
Stem cell essence and preparation method thereof
ActiveCN104546639AFully activated differentiationIncrease elasticityCosmetic preparationsToilet preparationsCuticlePhysiology
The invention relates to an anti-aging skin care product, and in particular discloses a stem cell essence and a preparation method thereof. The stem cell essence is prepared by mixing the following raw materials in percentage by weight: 28-32% of a stem cell extract, 3-7% of glycerol, 0.2-0.8% of lactic acid, 0.7-1.3% of trehalose, 0.2-0.5% of vitamin A acetate, 0.1-0.3% of vitamin E acetate, 0.1-0.3% of 4',6,7-trihydroxyisoflavone, 0.5-0.7% of titanium dioxide, 0.01-0.02% of xanthan gum, 0.02-0.03% of triethanolamine, 2-4% of isopropyl myristate, 0.8-1% of propylene glycol, 0.02-0.07% of sodium hyaluronate, 0.8-1.2% of an antiseptic and the balance of de-ionized water, wherein the stem cell extract is an extract of sheep umbilical cord blood stem cells. The stem cell essence provided by the invention, after being absorbed by the epidermis, can penetrate to the corium layer and the subcutaneous tissue, so as to comprehensively activate the differentiation of stem cells in skin and rapidly replace old cells, so that collagen secretion is enhanced and skin elasticity is improved; the stem cell essence can be used for promoting the synthesis activation of protein and enhancing the softness of the skin; and the stem cell essence can be also used for stimulating the muscular layer to generate fibroblast and skin protein so as to enhance skin toughness and elasticity.
Owner:丰泽康生物医药(深圳)有限公司
Growth of neural precursor cells using umbilical cord blood serum and a process for the preparation for therapeutic purposes
InactiveUS20080124701A1Dead animal preservationArtificial cell constructsCord blood stem cellTherapeutic intent
This invention is concerned with stem cells derived from umbilical cord blood serum and a method for growing human embryonic stem cells and adult cells comprising sera separated from clotted umbilical cord blood, including growing and differentiating cord blood stem cells into neural precursors, comprising transdifferentiating CD34+, CD45= and CD133+ stem cells from mononuclear cells derived from umbilical cord blood to neural precursors. The stem cells obtained from the umbilical cord include pluripotent stem and progenitor cell population of mononuclear cells, and separating pluripotent stem and progenitor cell population of mononuclear cells obtained from the umbilical cord blood. A magnetic cell separator is used to separate out cells which contain a CD marker and then expanding the cells in a medium containing retinoic acid as a differentiating agent supplemented with one or more growth factors BDNF, GDNF, NGF and FGF in presence of cord blood serum. The invention is also concerned with the transplantation and repair of nerve damage, strokes, spinal injury, Parkinson's and Alzheimer's, prepared with a media for culturing umbilical cord blood stem cells in umbilical cord serum.
Owner:RELIANCE LIFE SCI PVT
Production of typed human cells, tissues and organs
InactiveUS20020100065A1High yieldNew breed animal cellsArtificial cell constructsCord blood stem cellMammal
A method of obtaining a high yield of differentiated human cells and organs includes the steps of providing typed human bone marrow or cord blood stem cells, providing pre-immune non-human mammalian fetuses, implanting the cells into the fetuses, permitting the fetuses to grow for a sufficient time to produce differentiated cells in hybrid organs, and harvesting the differentiated cells from the mammals. A method is presented to produce hybrid functioning human-animal solid organs for clinical transplantations. The method includes obtaining bone marrow mononuclear cells (BMNC) from the patient, obtaining enriched populations of HSC from the BMNC, and transplanting the enriched cells into Preimmune fetal sheep or pigs intraperitoneally to produce functioning donor (patient)-animal hybrid organs. A method is also presented in which enriched HSC isolated from pre-HLA typed normal human fetal liver / bone marrow, cord blood, or bone marrow will be transplanted into preimmune fetal sheep or pigs in order to create functioning human-animal hybrid organs that can be transplanted into compatible patients. Methods are also presented to obtain high yield of different types (e.g. hepatocytes) of donor (patient or HLA-typed normal donors) cells from the human-animal hybrid organs that can be used either for transplant into patients and / or treatment of the patient. Also disclosed is a method of producing purified human proteins that includes providing a non-human, pre-immune mammal into which human bone marrow or cord blood cells has been implanted into the mammal at the pre-immune state, obtaining blood from the non-human mammal, and isolating the human proteins from the mammalian blood.
Owner:ZANJANI ESMAIL D
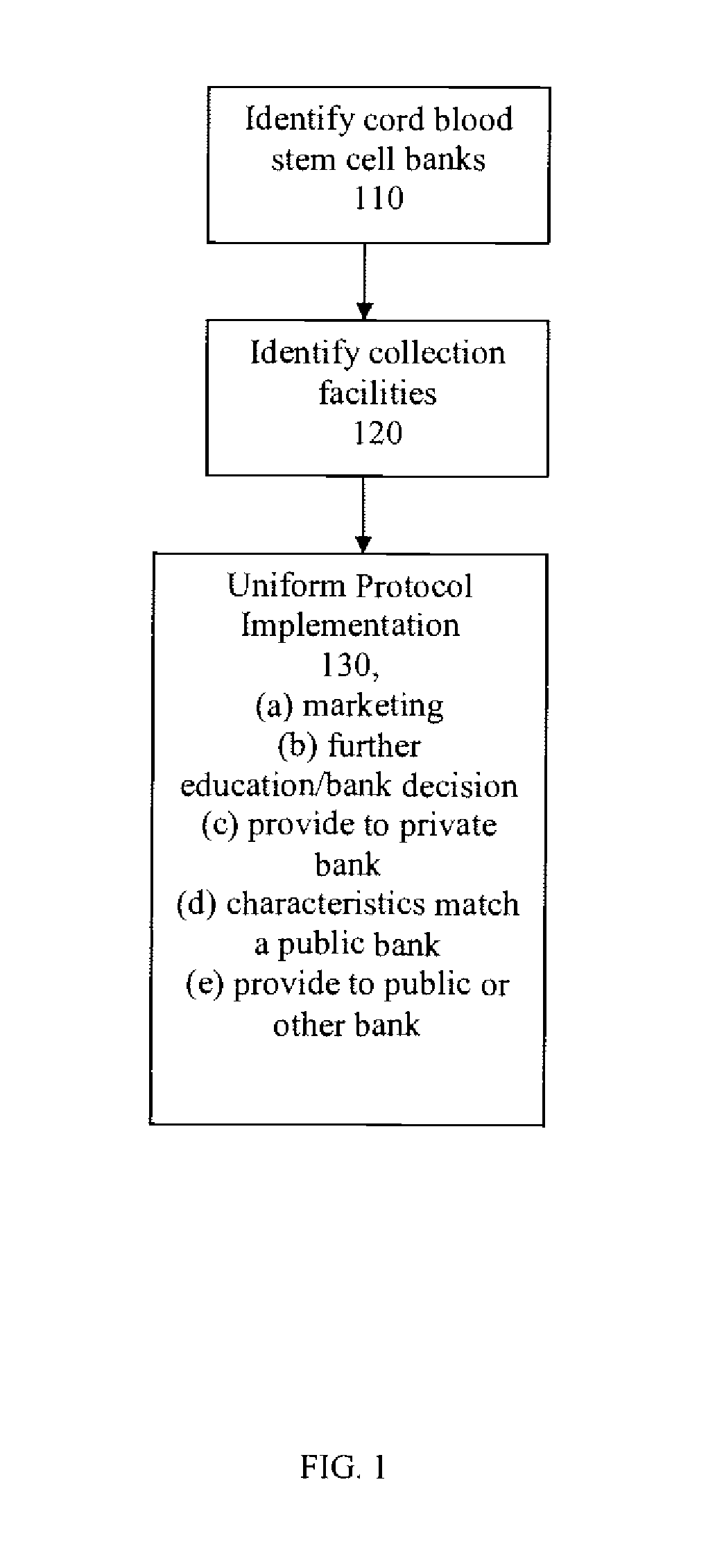
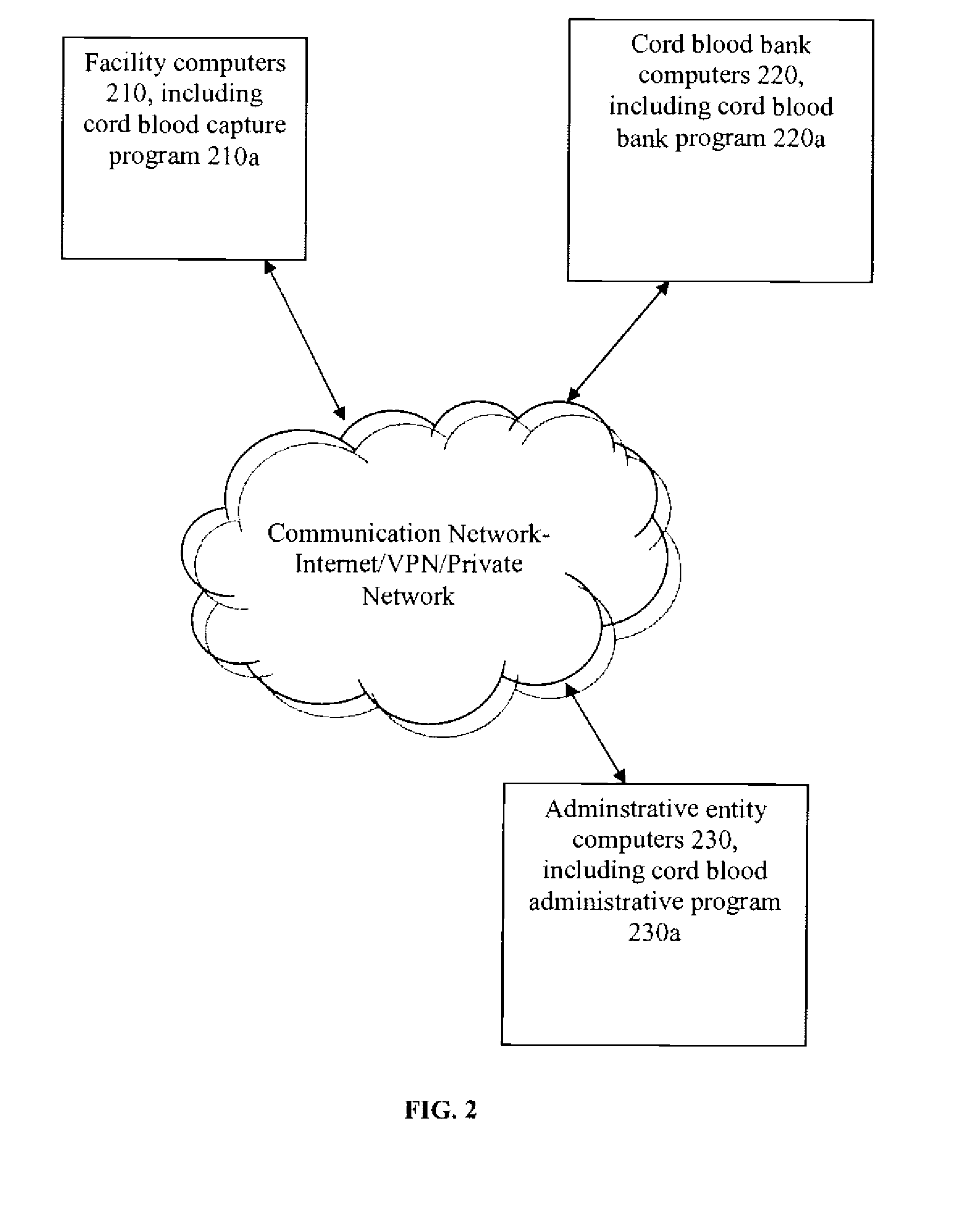
Method for the Collection And Distribution of Cord Blood Stem Cells
InactiveUS20080189045A1Increase the number ofData processing applicationsComputer-assisted medical data acquisitionCord blood stem cellUmbilical Cord Blood Stem Cell
The present invention provides a method for the collection and distribution of cord blood stem cells, particularly in order to increase the number of usable cord blood stem cells that are collected overall. By using a single collection and distribution entity that applies a uniform protocol to obtain cord blood stem cell samples at each of a plurality of different collection facilities, a greater number of samples for both private and public cord blood stem cell banks can be obtained.
Owner:CBR SYST
Composition for Treating Baldness with Stem Cell Derived From Umbilical Cord Blood
InactiveUS20070298017A1Easy to produceSolve immune rejectionBiocideUnknown materialsTherapeutic TechniqueUmbilical Cord Blood Stem Cell
Provided is a therapeutic technique for treating baldness, using an umbilical cord blood-derived stem cell. Transplantation of a composition for treating baldness into a bald area of a patient can make great contributions to treatment for baldness, wherein the composition comprises stem cells isolated and cultured from umbilical cord blood in which 6 HLA (Human Leukocyte Antigen) are identical with a patient, or one or two HLA are not identical with the patient.
Owner:HAN HOON
Use of umbilical cord blood stem cells to treat ischemic event
ActiveUS8309070B2Ameliorate or mitiReduce harmBiocideNervous disorderUmbilical Cord Blood Stem CellImmunology
The invention relates generally to methods for isolation and culture of umbilical cord blood stem cells, cells isolated by the methods, and therapeutic uses for those cells.
Owner:RGT UNIV OF MINNESOTA
Isolating method for umbilical cord blood-derived pluripotent stem cells expressing znf281
ActiveUS20120021509A1Treatment be applyNervous disorderSkeletal disorderInduced pluripotent stem cellUmbilical Cord Blood Stem Cell
The present invention relates to a method for isolating pluripotent / multipotent stem cells derived from umbilical cord blood, characterized by culturing monocytes isolated from umbilical cord blood in a culture vessel containing fibronectin and then harvesting stem cells from the culture, the umbilical cord blood-derived pluripotent / multipotent stem cells isolated thereby; and a cell therapeutic agent containing the pluripotent / multipotent stem cells derived from umbilical cord blood or cells differentiated therefrom. The present invention also relates to a novel culture media for stem cells, a culture method for stem cells which is characterized by culturing and proliferating stem cells in the culture media, and a method for increasing stemness of stem cells which is characterized by a sphere culture or a three-dimensional culture of stem cells.
Owner:SEOUL NAT UNIV R&DB FOUND
Growth of neural precursor cells using umbilical cord blood serum and a process for the preparation for therapeutic purposes
InactiveUS7897388B2Dead animal preservationArtificial cell constructsPluripotential stem cellCord blood stem cell
This invention is concerned with stem cells derived from umbilical cord blood serum and a method for growing human embryonic stem cells and adult cells comprising sera separated from clotted umbilical cord blood, including growing and differentiating cord blood stem cells into neural precursors, comprising transdifferentiating CD34+, CD45+ and CD133+ stem cells from mononuclear cells derived from umbilical cord blood to neural precursors. The stem cells obtained from the umbilical cord include pluripotent stem and progenitor cell population of mononuclear cells, and separating pluripotent stem and progenitor cell population of mononuclear cells obtained from the umbilical cord blood. A magnetic cell separator is used to separate out cells which contain a CD marker and then expanding the cells in a medium containing retinoic acid as a differentiating agent supplemented with one or more growth factors BDNF, GDNF, NGF and FGF in presence of cord blood serum. The invention is also concerned with the transplantation and repair of nerve damage, strokes, spinal injury, Parkinson's and Alzheimer's, prepared with a media for culturing umbilical cord blood stem cells in umbilical cord serum.
Owner:RELIANCE LIFE SCI PVT
Methods for collecting and using placenta cord blood stem cell
ActiveUS8329468B2Raise countBiocideMammal material medical ingredientsObstetricsCord blood stem cell
An innovative method of collecting cord blood stem cells from an isolated mammalian non-exsanguinated or partially exsanguinated placenta by placental perfusion is described and also an easy method for safe long duration cold storage of the placenta. Placental perfusion can include perfusing the isolated placenta with a pulsatile flow of perfusion solution, for example, using a pulsatile or peristaltic pump or device. The stem cells can then be isolated from the perfusate. Significantly increased amounts of CD133+ stem cells can be collected from the perfusate. The perfusion solution can include an anticoagulant. The isolated mammalian placenta need not be treated with an anticoagulant prior to perfusing. The isolated placenta can be free from an anticoagulant prior to perfusing.
Owner:CELULARITY INC
Method for intrathecal administration of autologous stem cells in premature infants
ActiveUS20100226896A1Improve pathophysiologyReduce severityBiocideMammal material medical ingredientsPremature thelarcheCord blood stem cell
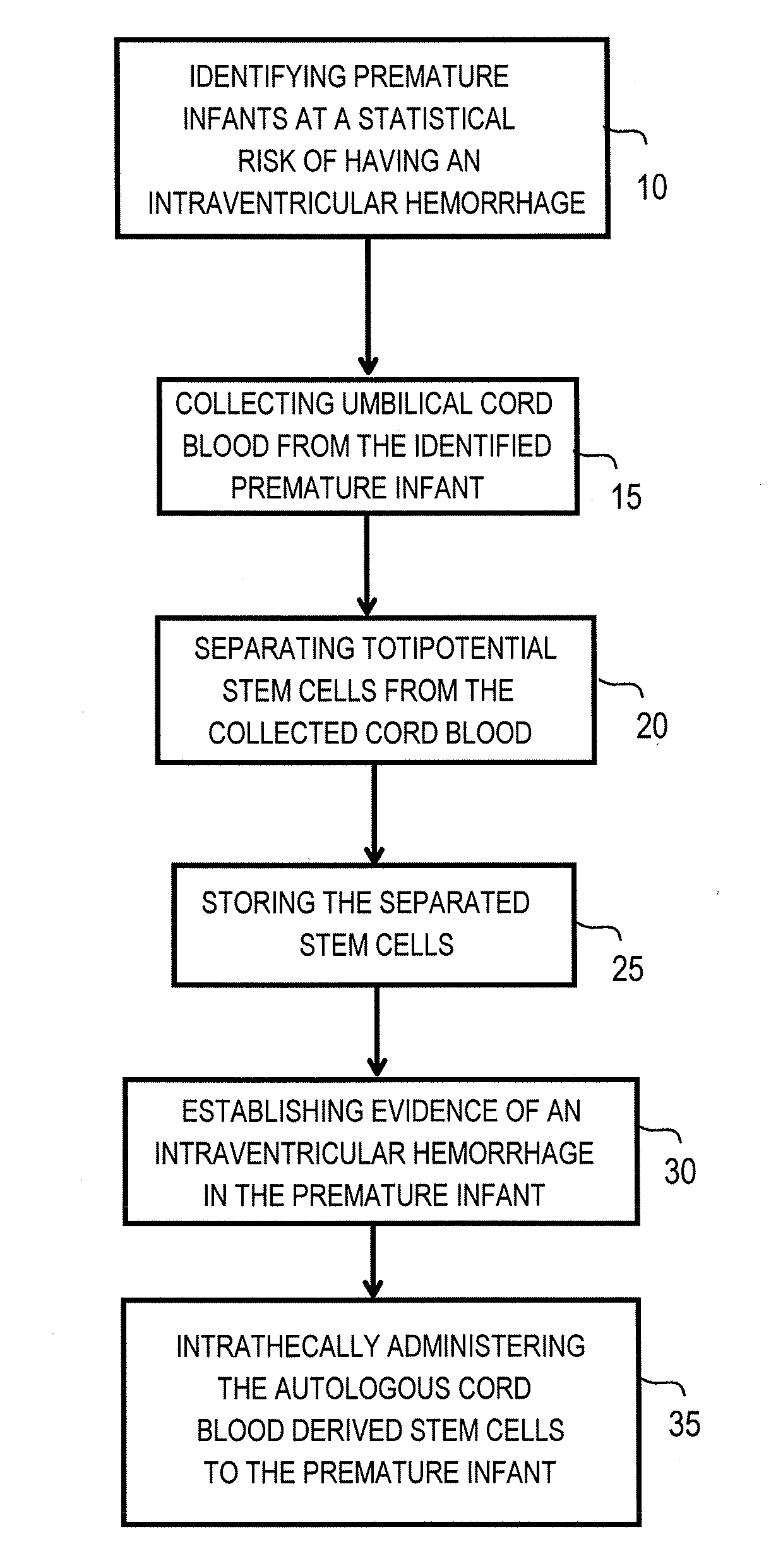
The invention provides a method of treating a premature infant at a statistical risk of sustaining an intraventricular hemorrhage. The method, or therapeutic protocol, can include at least one or more of the following steps: identifying premature infants at a statistical risk of having an intraventricular hemorrhage, collecting umbilical cord blood from the identified premature infant, separating totipotential stem cells (e.g., having the ability to proliferate and differentiate as neural stem cells) from the collected cord blood, storing the separated stem cells, establishing evidence of an intraventricular hemorrhage (preferably prior to Grade III / IV if possible) in the premature infant, and intrathecally administering the autologous cord blood derived stem cells to the premature infant.
Owner:DRACKER ROBERT A
Umbilical cord blood stem cell injection solution with anti-aging effect and preparation method thereof
InactiveCN105816481ANo negative impactQuality controlledAntinoxious agentsPharmaceutical delivery mechanismHuman bodyTissue repair
The invention discloses an umbilical cord blood stem cell injection solution with an anti-aging effect and a preparation method thereof. The umbilical cord blood stem cell injection solution contains the following components by volume or concentration: 5X10<5> to 10X10<5> umbilical cord blood stem cells per ml, 2wt% of human albumin, 10-20v% of umbilical cord blood autologous plasma and 78-88v% of a compound electrolyte solution. According to the umbilical cord blood stem cell injection solution, the used stem cells are blood related stem cells and have the characteristics of amplification and directional differentiation; the cells return to an injured part when receiving an injured part signal; the cells are subjected to amplification and induced differentiation at the injured part and the stem cells of the injured part are promoted to recover functions, thereby quickly repairing and replacing injured cells and tissues; and cell factors, growth factors and tissue related factors are secreted quickly in time, thereby improving blood circulation of a human body, metabolic function, tissue repair capability and immunity, and further achieving the purpose of aging resistance.
Owner:SHANGHAI HUAYAN MEDICINE TECH CO LTD
Use of umbilical cord blood stem cells to treat ischemic event
ActiveUS20070172465A1Reduce harmAmeliorate or mitiBiocideNervous disorderUmbilical Cord Blood Stem CellImmunology
The invention relates generally to methods for isolation and culture of umbilical cord blood stem cells, cells isolated by the methods, and therapeutic uses for those cells.
Owner:RGT UNIV OF MINNESOTA
Kit for separating human umbilical cord blood stem cells and its using method
The invention relates to a kit for separating human umbilical cord blood stem cells and its using method. The kit is composed of a solution A, a solution B and a solution C, wherein, the solution A is added with appropriate amounts of puerarin and 4-hydroxycinnamic acid phenethyl ester. The invention also relates to a human umbilical cord blood stem cell treatment liquid, which contains the human umbilical cord blood stem cells prepared by the kit of the invention. The kit provided in the invention can rapidly and efficiently separate stem cells from umbilical cord blood, and is in favor of popularizing stem cell treatment technologies.
Owner:葛龙海
Cord Blood Stem Cell Cryopreservation Box
ActiveCN108207933BAvoid affecting the freezing effectShorten the timeDead animal preservationClose relativesCord blood stem cell
The invention relates to an umbilical cord blood stem cell cryopreservation box, relates to the field of medical instruments and mainly solves the technical problem that the stem cell freezing effectis affected due to the fact that longer time is consumed when a collection bag on a lower layer in an existing cryopreservation box is taken out. According to the technical scheme, the umbilical cordblood stem cell cryopreservation box comprises a box body, collection bag accommodating mechanisms and position adjusting mechanisms; the box body comprises a box cover and an access port located on one side surface, and the box cover can be opened and closed relative to the access port; each collection bag accommodating mechanism comprises a support plate and a press plate which are arranged in parallel, and the press plate is located above the support plate; each press plate can move relative to the corresponding support plate, an accommodating gap used for accommodating a collection bag isformed between each support plate and the corresponding press plate when the press plate moves to the first position, and a gap port of the accommodating gap is opposite to the access port; each pressplate applies pressure to each collection bag when moving to the second position; the position adjusting mechanisms are matched with the box cover, so that the press plates are located in the first position when the box cover is opened and located in the second position when the box cover is closed.
Owner:广州市流式生物科技有限公司
Compositions and methods for cxcr4 signaling and umbilical cord blood stem cell engraftment
ActiveUS20130236425A1Improve responsivenessEnhancing homingBiocideMammal material medical ingredientsProgenitorNormal protein
The present invention provides for enhancing engraftment by co-infusing at least two partially HLA matched umbilical cord blood (“UCB”) units. The invention further provides for positive C3a mediated priming on responsiveness to doses of SDF-1 and C3a induced incorporation of CXCR4 in membranes in HSC and progenitors. The invention further provides for enhancing the homing of UCB HSC and progenitors via the SDF-1 / CXCR4 pathway and that C3a and LL-37 are useful for this method. It is also disclosed herein that fragments of C3a (e.g., des-Arg) are effective in the methods of the invention, including enhancing homing of HSPCs to BM. The invention further encompasses the disclosure herein of NFAT1 regulation post-transcriptionally by both mir-184 and IFN-γ. The present invention further provides for measuring and using differences between UCB and adult CD4+ / 45RA+ T-cells as a means of defining strategies to enhance optimal allogeneic stem cell transplantation outcomes. The present invention further provides methods for maintaining IL-2 production in the absence of NFAT1 normal protein levels.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND +1
Umbilical cord blood stem cell management system
ActiveCN106203549AGuaranteed storageConvenient and efficient entryCo-operative working arrangementsUmbilical Cord Blood Stem CellPediatrics
The invention discloses an umbilical cord blood stem cell management system. The system comprises a remote server, a terminal controller, an input apparatus, a client tracking updating module, an umbilical cord blood collection preparation module, a work station management module, a maneuvering module, an umbilical cord blood collection module, an umbilical cord blood analysis collection module, an umbilical cord blood bank management module and an integration query module. Information logging and modification can be conveniently and high-efficiently performed. Client umbilical cord blood can be ensured to be collected at first time and umbilical cord blood warehousing storage can be guaranteed. In the invention, through the umbilical cord blood collection preparation module, the work station management module, the umbilical cord blood collection module, the umbilical cord blood analysis collection module and the umbilical cord blood bank management module, a detection step and a process are optimized; detection of collected umbilical cord blood is rapid, high-efficient and timely; detection cost is greatly reduced and resource wastes are avoided.
Owner:GUANGDONG CARDIOVASCULAR INSITITUTE +1
Method for isolating and culturing adult stem cells derived from human amniotic epithelium
The present invention relates to a method for isolating and culturing adult stem cells derived from human amniotic membrane in high yield, and more particularly to a method for obtaining a large amount of adult stem cells, the method comprising obtaining amniotic epithelial cells from human amniotic tissue in high yield by treatment with dithiothreitol (DTT) and a low concentration of trypsin and culturing the amniotic epithelial cells in a medium containing a Rho-associated kinase inhibitor. The human amniotic epithelial cell-derived stem cells are easily extracted compared to existing therapeutic stem cells such as umbilical cord blood stem cells and bone marrow stem cells, the yield and proliferation thereof are significantly increased by DTT treatment, the addition of the ROCK inhibitor or the replacement of medium. Thus, the method can be used to efficiently prepare adult stem cells.
Owner:RNL BIO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com