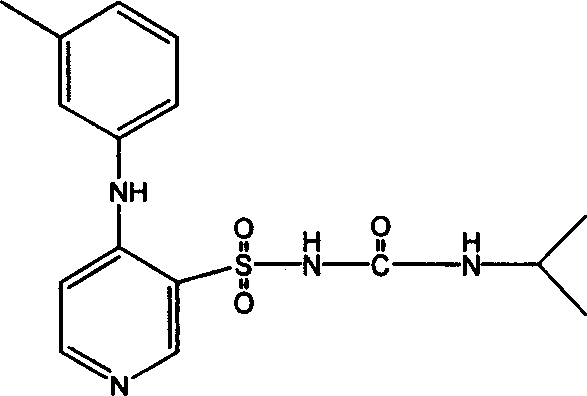
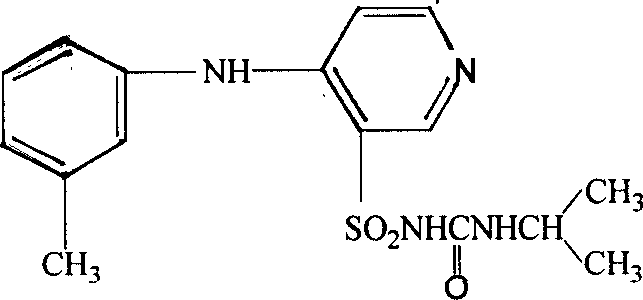
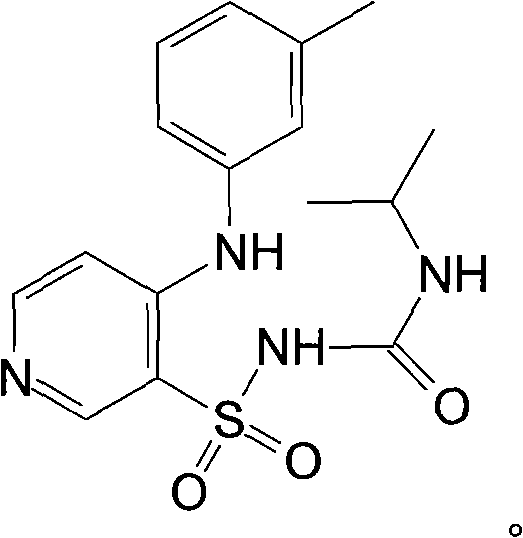
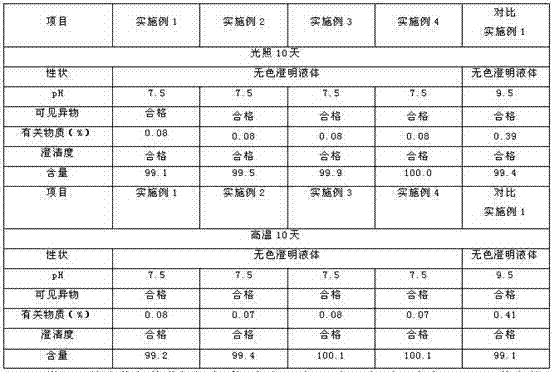
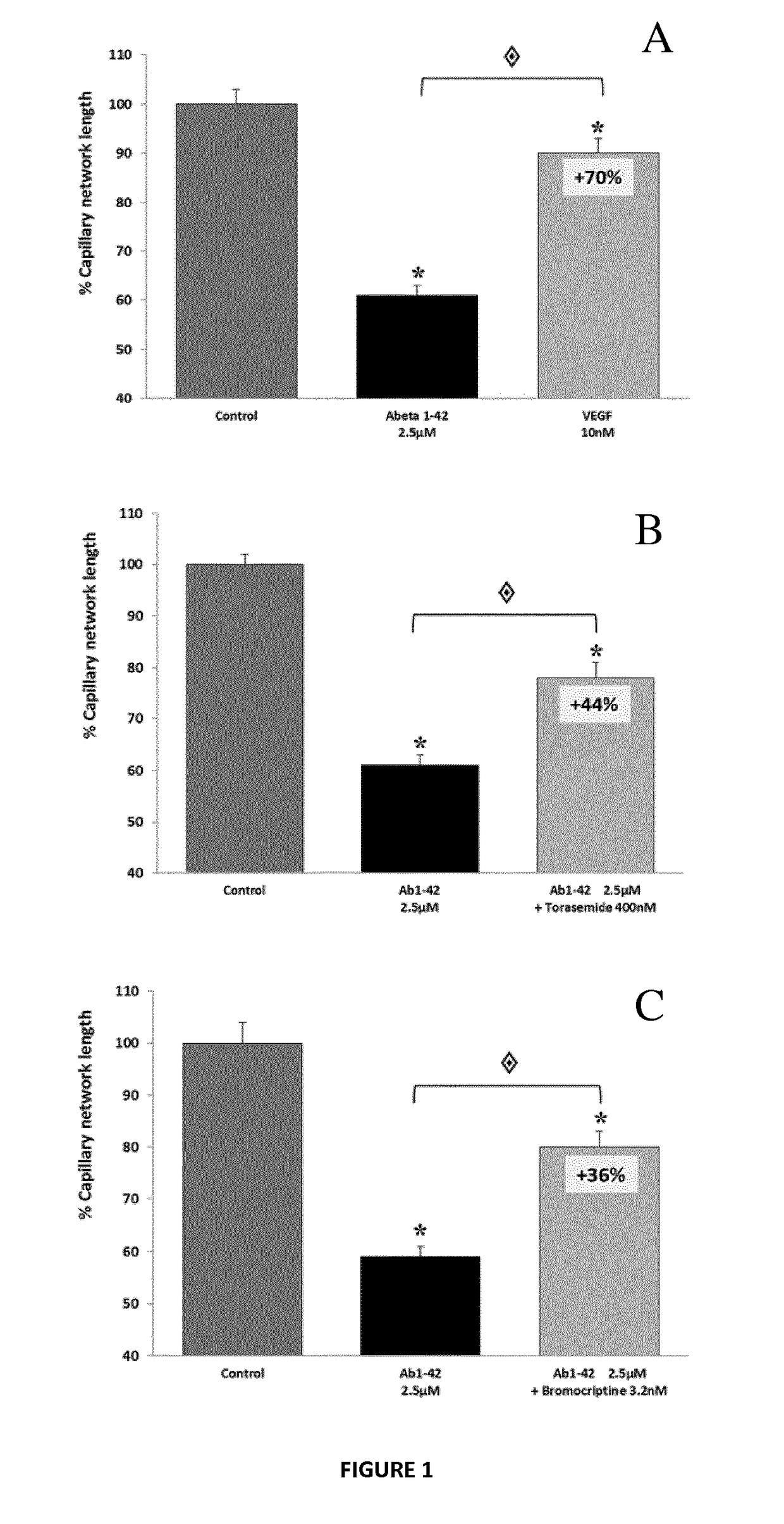
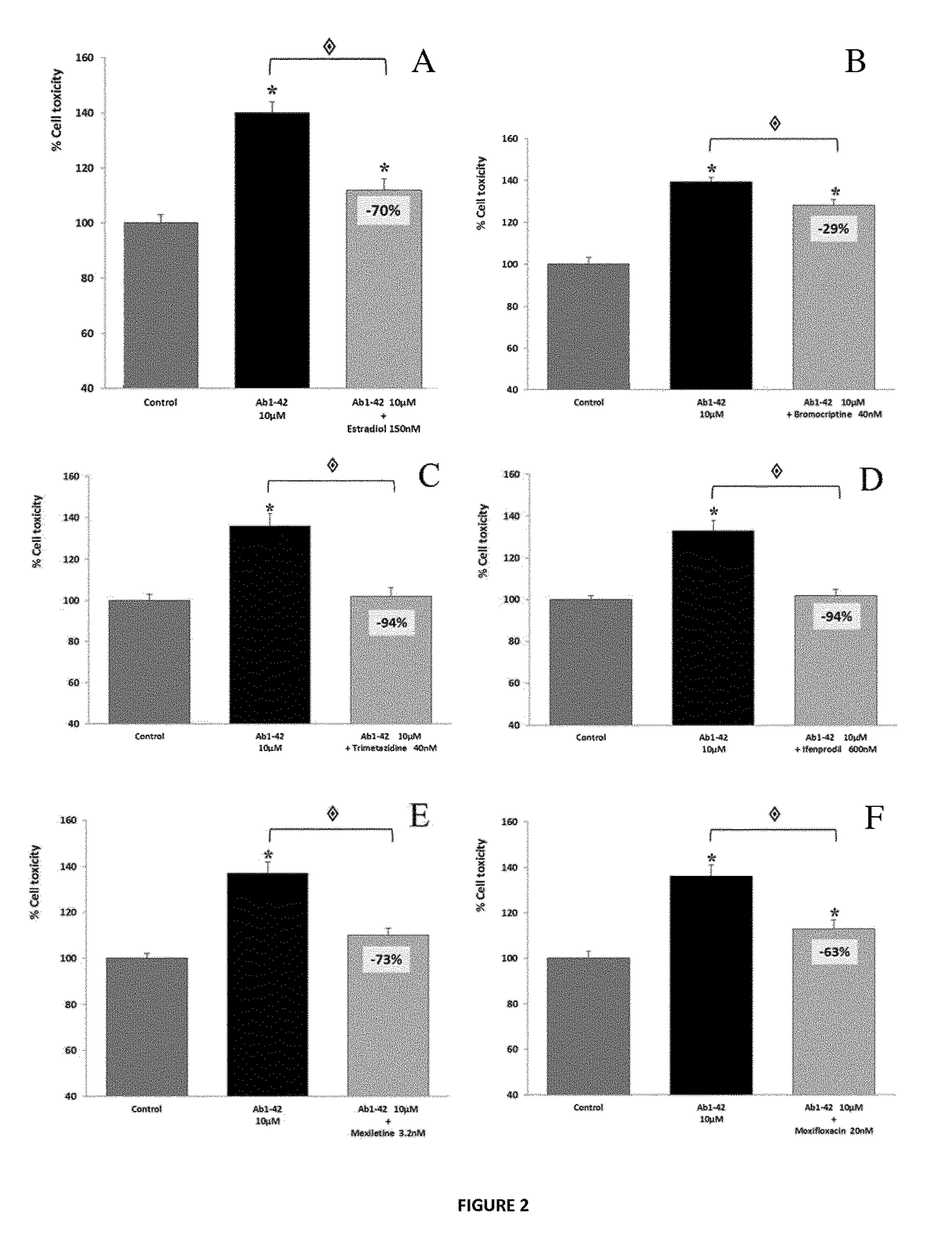
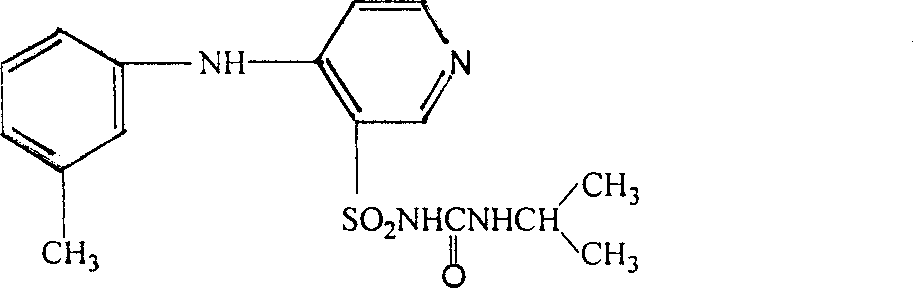
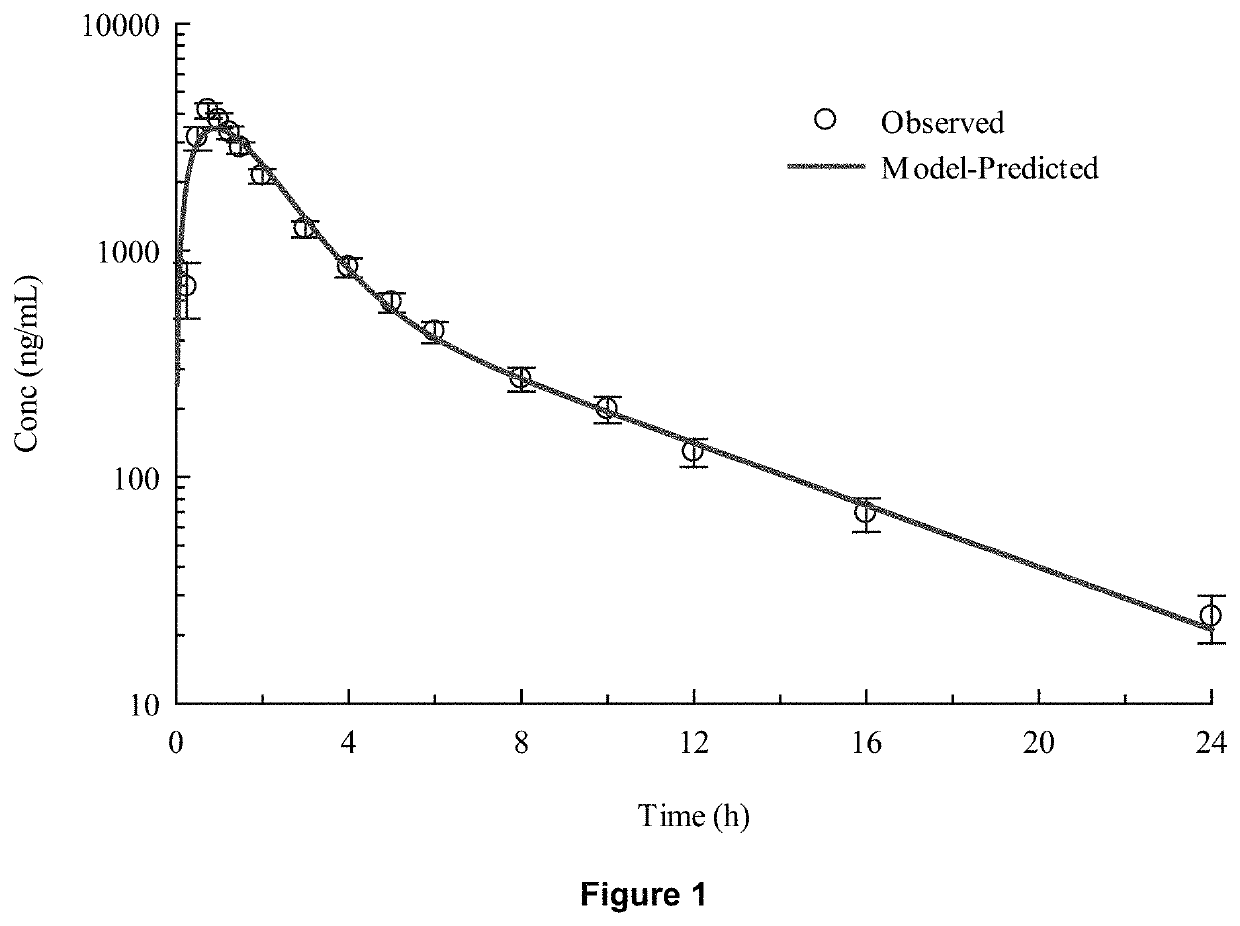
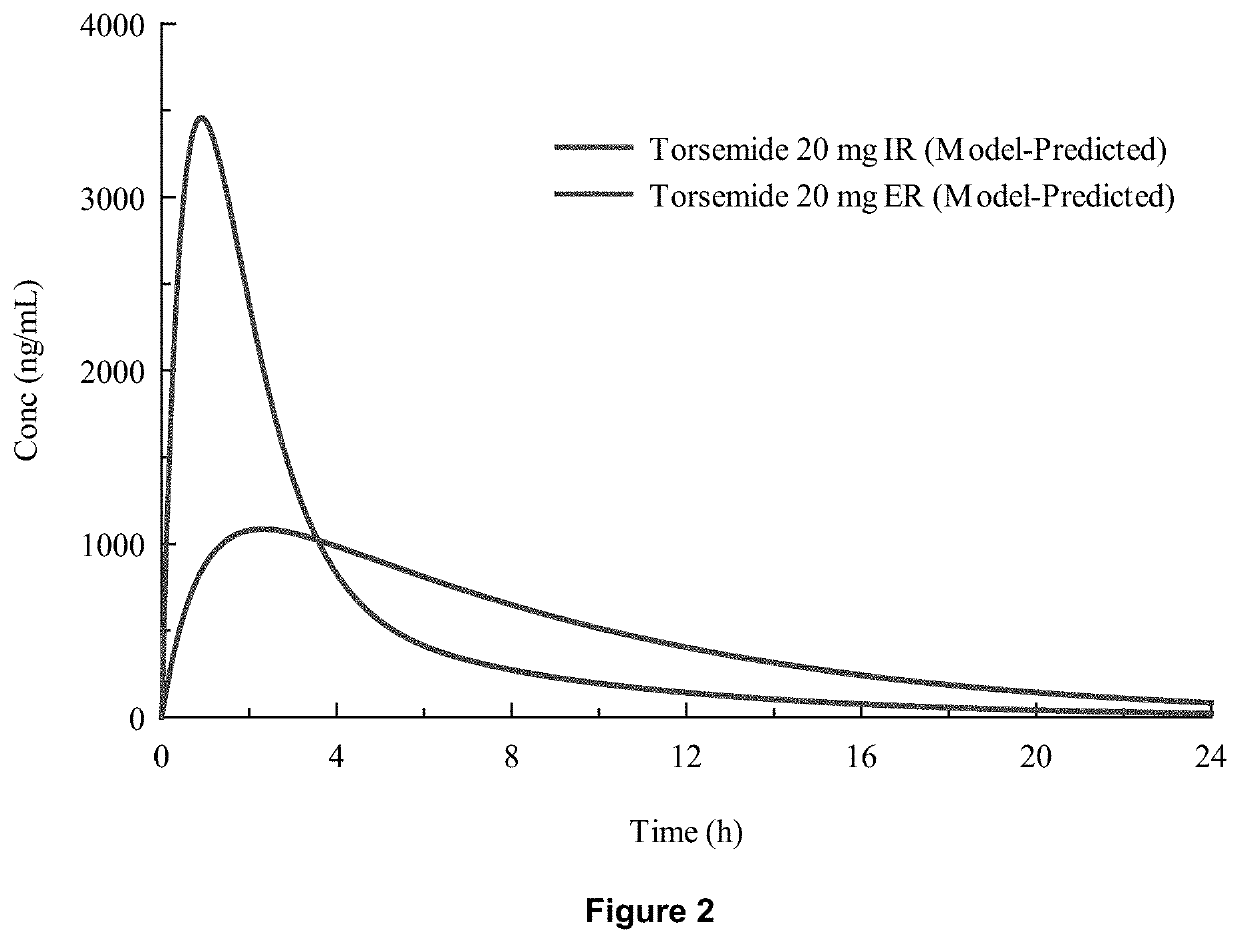
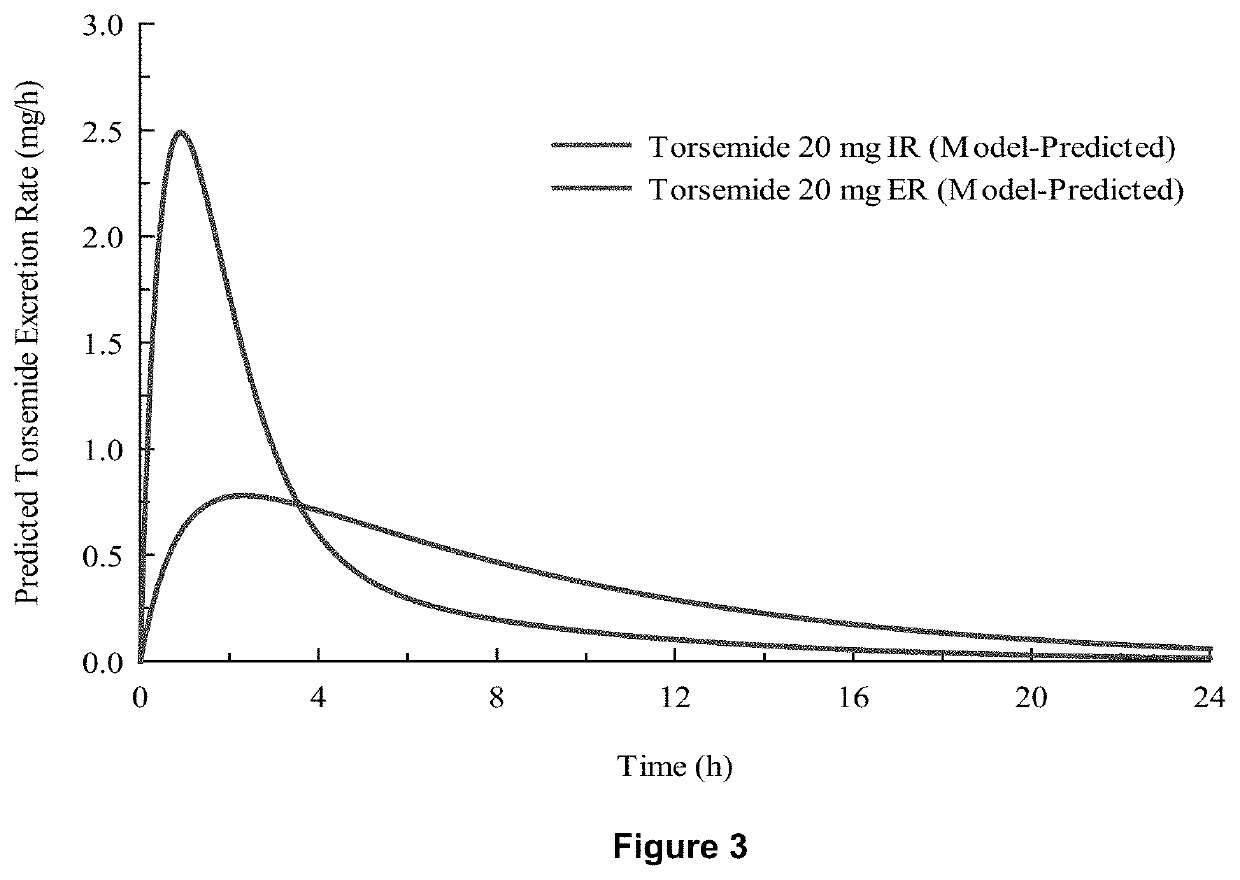
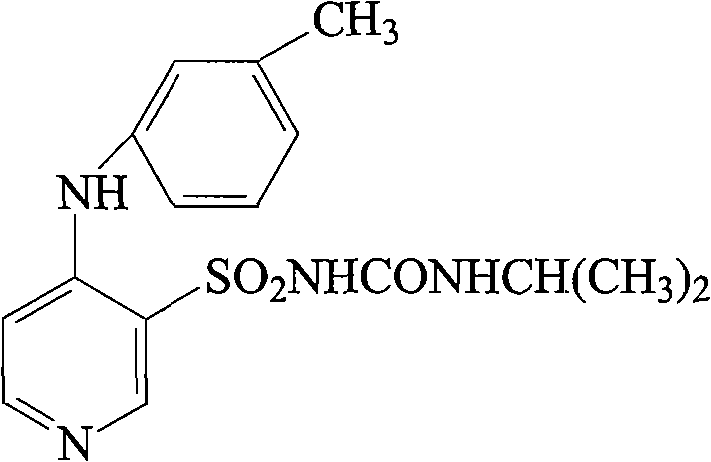
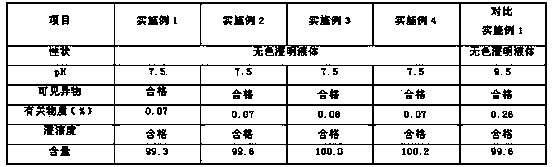
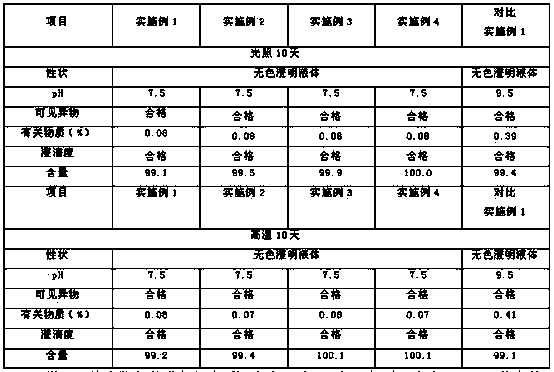
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
55 results about "Torsemide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Torsemide is used to reduce extra fluid in the body (edema) caused by conditions such as heart failure, liver disease, and kidney disease.
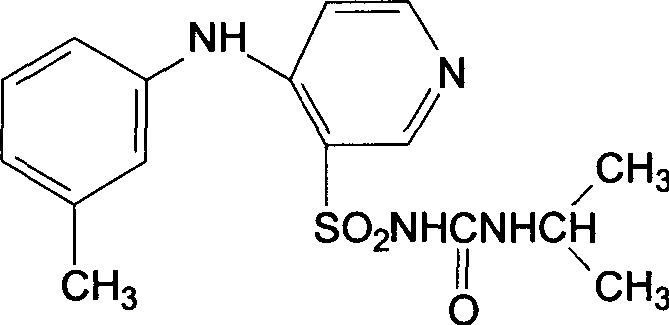
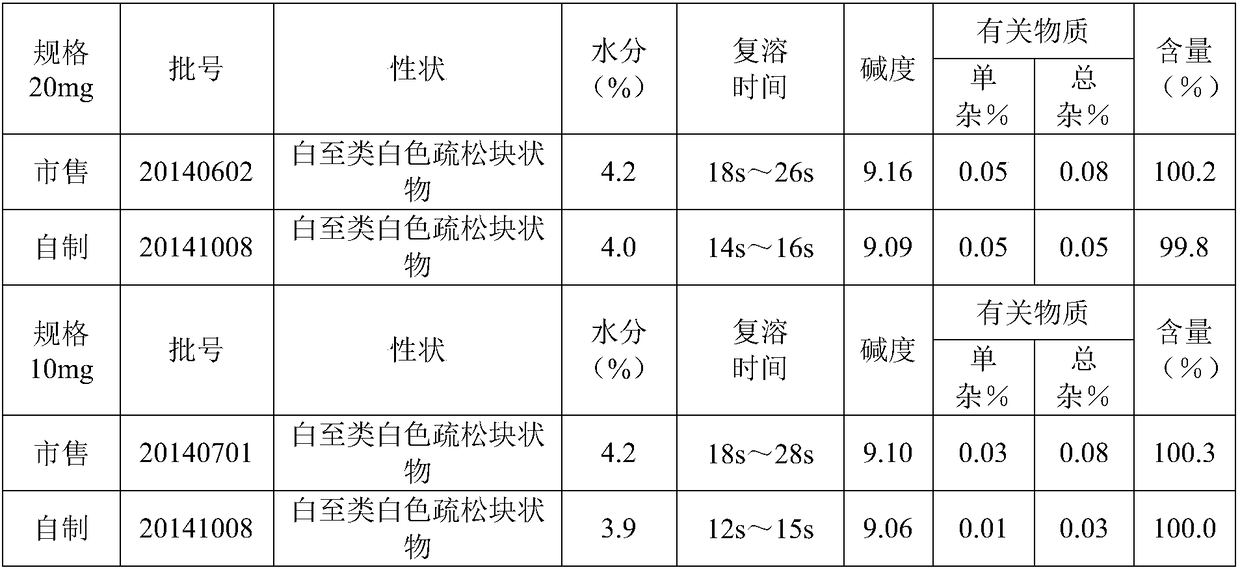
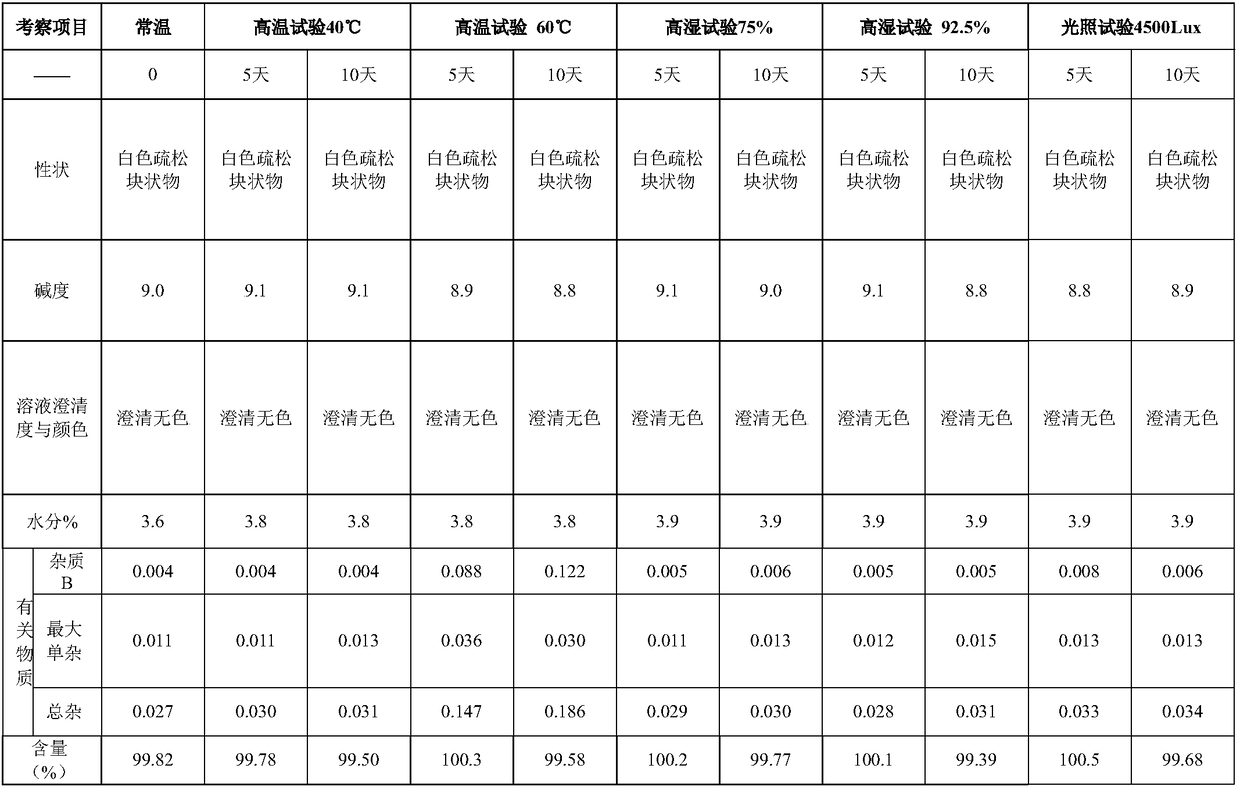
A stable torasemide injection and its preparation method
InactiveCN101007003AReduced dosing timeNo hemolyticPharmaceutical delivery mechanismPharmaceutical non-active ingredientsMaterial growthActivated carbon
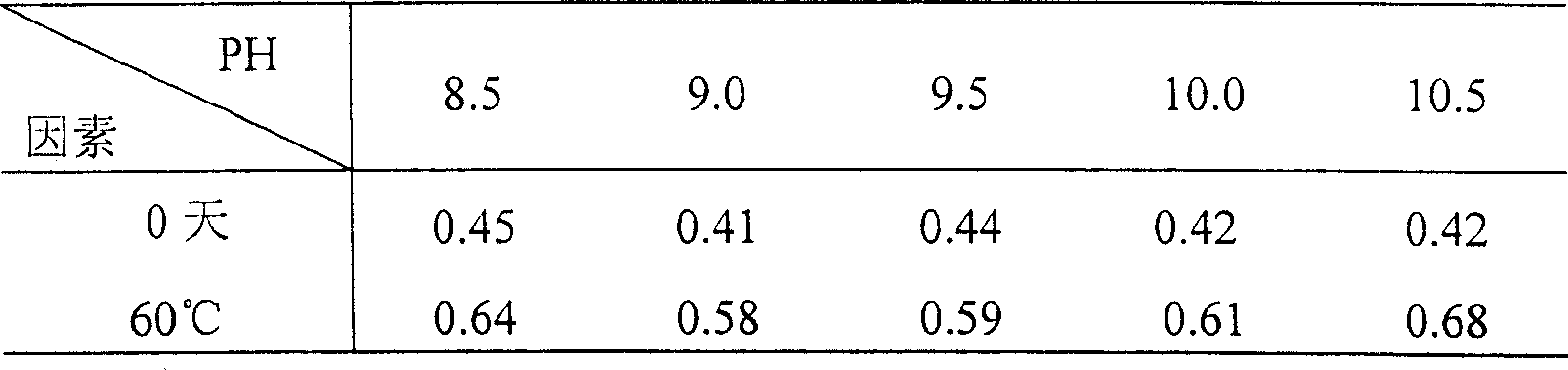
There is provided an injection with torasemide as the main active ingredient in the invention, the preparing method of the injection is also included in the invention. The stability of the injection in the invention has been greatly improved. There is solubilizing agent and stabilizer in the preparation, the pH value is 9.0 or larger. The preparing method includes the following steps: adding 1-50% (by weight) of solubilizing agent to certain amount of injecting water; adding torasemide to the water, after it is completely dissolved, adding 0.1-5% part of stabilizer; adding water to requested amount and stirring uniformly; adding activated char to adsorb for 30min at high temperature; decarbonizing and filtering; adding medically used alkali to regulate the ph value to 9.0 or larger; aseptically filtering; after the assay is approved, packaging, degerming and stocking. The advantages of the invention include: high stability, short preparing time, low cost, no haemolyticus, blood vessel stimulus and allerfic response, low growing speed of relative substances.
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD +1
Torasemide freeze-drying preparation and prepn. method
A freeze-dried composition of torasemide features that it contains sodium hydrogen carbonate for higher solubility. Its preparing process is also disclosed.
Owner:南京海辰药业股份有限公司
Method for purification of torasemide and preparation of big crystal form
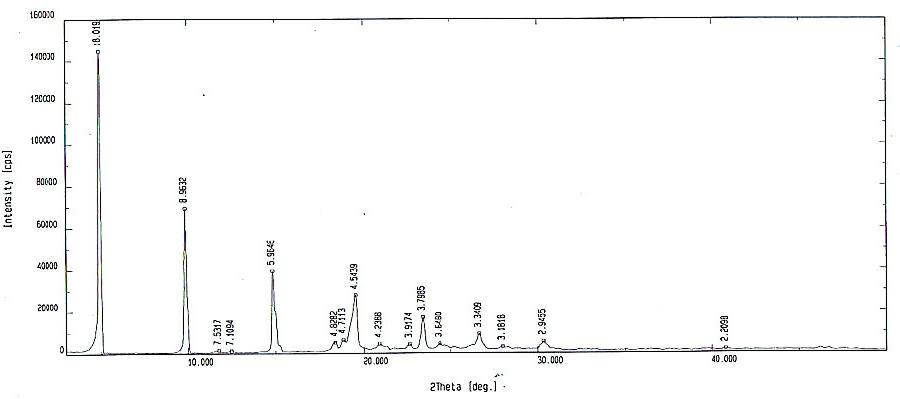
The invention discloses a method for purification of torasemide and preparation of big crystal form, which is characterized in that Torasemide crude is dissolved in acidulous solvent to raise the temperature to 85-100 DEG C; the mixture is stirred for dissolved clarification and is decolorized and filtered; the temperature of filtrate is slowly lowered to 5-30 DEG C for crystallization; crystal is obtained by filtering and separating; and then the obtained product is dried at the temperature of 50-70 DEG C to obtain purified Torasemide crystal. The invention has the advantage of simple processing method and can obtain the crystal of which the purity is 99% and average grain diameter is 50-350 mu m by dissolving, crystallizing and purifying.
Owner:HUBEI BIOCAUSE HEILEN PHARM CO LTD
Novel torasemide oral medicine composition
ActiveCN101632665AMature technologyEasy to operatePharmaceutical product form changePill deliveryOral medicineGelatin
The invention relates to a pharmaceutical composition containing a torasemide odor masking composition and a preparation method thereof. The invention is characterized in that flavoring agent in the torasemide odor masking composition is selected from gelatin, mannite and aspartame; and the pharmaceutical composition exists in the form of powder or grains, can lead to good preparation stability and covers the bitterness, has good mouth taste, and is fast to beak down and absorb and convenient to carry.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Torasemide compound and new preparation method thereof
The invention provides a torasemide compound and a new preparation method thereof, and the method comprises the following steps: (1) processing the torasemide raw material by alkali metals or alkaline earth metal alkane oxides with the presence of an appropriate solvent or solvent mixture under an alkaline and heating condition; (2) adjusting the pH value by an appropriate acid; (3) adsorbing the torasemide by strong basic ion exchange resin and eluting the product; (4) adjusting the pH value by an appropriate acid to obtain a three-stage purified torasemide. The preparation method of torasemide improves the product quality of the preparation, reduces the toxic and side effects, is simple and easy to operate, and is applicable to large-scale industrial production.
Owner:HAINAN MEIDA PHARMA
Torasemide pharmaceutical composition with stabilization and safety for injection
ActiveCN102512360ASolve discolorationQuality assurancePharmaceutical delivery mechanismPharmaceutical non-active ingredientsDiseaseTherapeutic effect
The invention relates to a torasemide pharmaceutical composition with stabilization and safety for injection and its preparation method, especially relates to the torasemide pharmaceutical composition used for patients with requirement of rapid diuresis or congestive cardiac failure without oral diuresis, cirrhosis ascites, oedema caused by kidney diseases, the torasemide pharmaceutical composition is an injection preferably. The pharmaceutical composition is composed of active component torasemide, ethanol and polysorbate 80. The torasemide pharmaceutical composition for injection possesses stable pH value and low impurity content, and is capable of effectively raising the treatment effect of the products, minimizing the side reaction, raising the yield, reducing the cost and creating higher benefit.
Owner:TIANJIN HANKANG PHARMA BIOTECH
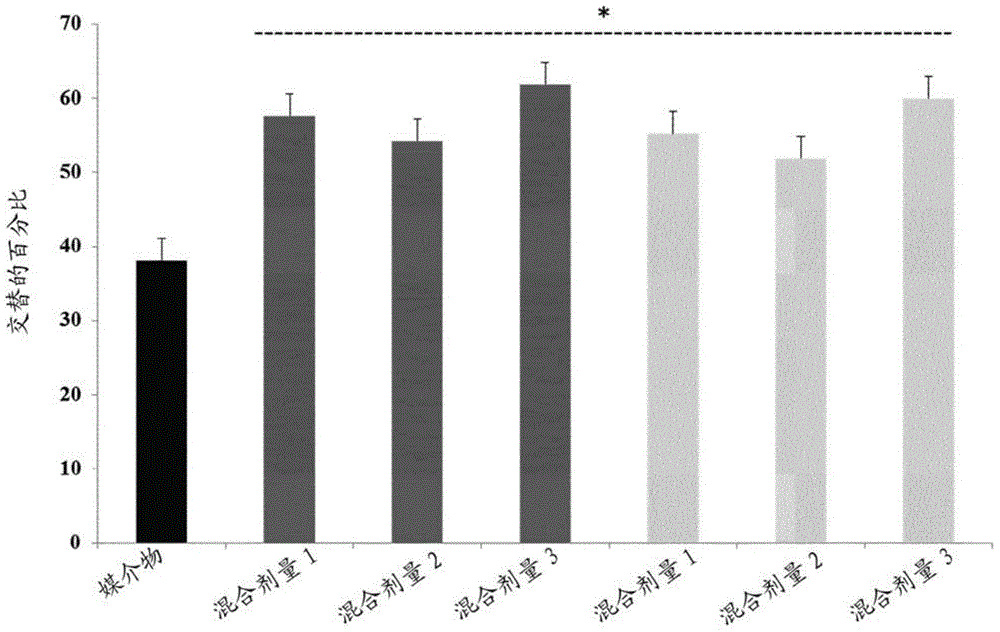
Composition comprising torasemide and baclofen for treating neurological disorders
ActiveUS9931326B2Sulfonylurea active ingredientsAnhydride/acid/halide active ingredientsAlcoholismsHuntingtons chorea
The present invention relates to compositions and methods for the treatment of neurological disorders related to glutamate excitotoxicity and Amyloid β toxicity. More specifically, the present invention relates to novel combinatorial therapies of multiple sclerosis, Alzheimer's disease, Alzheimer's disease-related disorders, amyotrophic lateral sclerosis, Parkinson's disease, Huntington's disease, neuropathic pain, alcoholic neuropathy, alcoholism or alcohol withdrawal, or spinal cord injury.
Owner:PHARNEXT
Torasemide freeze-drying preparation and prepration method
A freeze-dried composition of torasemide features that it contains sodium hydrogen carbonate for higher solubility. Its preparing process is also disclosed.
Owner:南京海辰药业股份有限公司
Disperese torasemide tablet and its prepn and application
The present invention relates to one kind of disperse torasemide tablet including torasemide, disintegrating agent and diluent. The disperse torasemide tablet features the disintegrating agent comprising sodium carboxymethyl starch and hydroxypropyl cellulose, and diluent of microcrystalline cellulose. The disperse torasemide tablet has fast disintegration, high dispersing homogeneity, high leaching degree and high stability, and is used in treating heart failure, dropsy and cerebral edema caused by nephrosis and hepatopathy, hypertension, etc.
Owner:周卓和
Method for optimizing freeze-drying curve of torasemide freeze-drying preparation for injection
InactiveCN106692078AReduce addOptimizing the freeze-drying profilePowder deliveryPharmaceutical non-active ingredientsFreeze-dryingAquatic product
The invention discloses a method for optimizing a freeze-drying curve of a torasemide freeze-drying preparation for injection. According to the technical scheme, the characters of dried frozen aquatic products are improved by selecting reasonable pre-freezing and sublimating temperatures. The quality standards meet various indexes specified in National Drug Standards. The method disclosed by the invention has the advantages of stable characters of the dried frozen aquatic products, small auxiliary material addition amount, short freeze-drying time and the like.
Owner:青岛华迈士药业有限公司
A torasemide for injection and preparation process thereof
ActiveCN109200022AQuality improvementStable storagePowder deliveryPharmaceutical non-active ingredientsSodium bicarbonateMANNITOL/SORBITOL
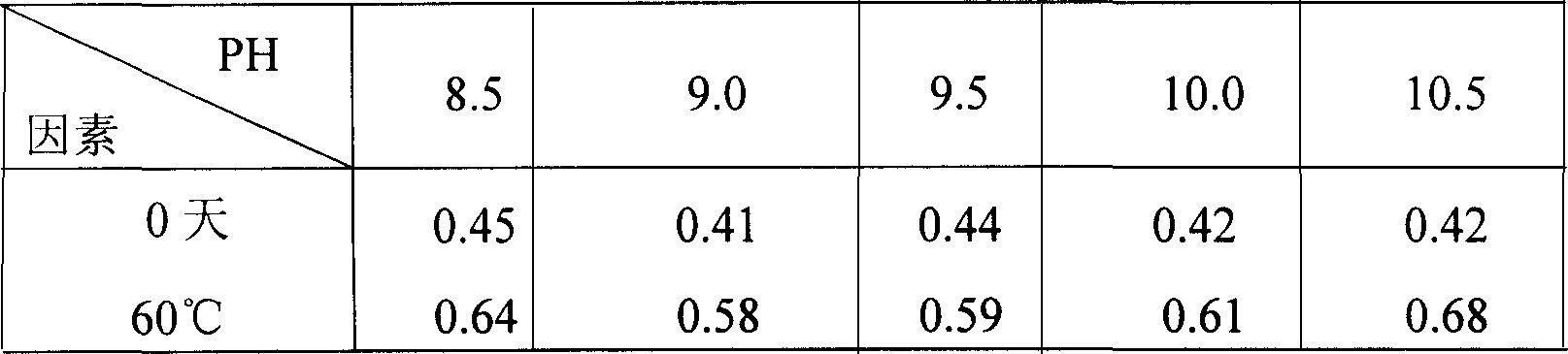
The invention relates to a torasemide freeze-dried powder injection and a preparation process thereof. The freeze-dried powder injection comprises 1 part by weight of torasemide, 1 part by weight of sodium bicarbonate, 8 to 17 parts by weight of mannitol, an appropriate amount of a pH regulator, and the pH value of the freeze-dried composition aqueous solution is adjusted to be 8.5 to 9.5. The torasemide freeze-dried powder injection prepare by that method according to the prescription of the invention has the advantages of short redissolution time, low moisture content, good appearance and high stability, and is very suitable for clinical use.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES +3
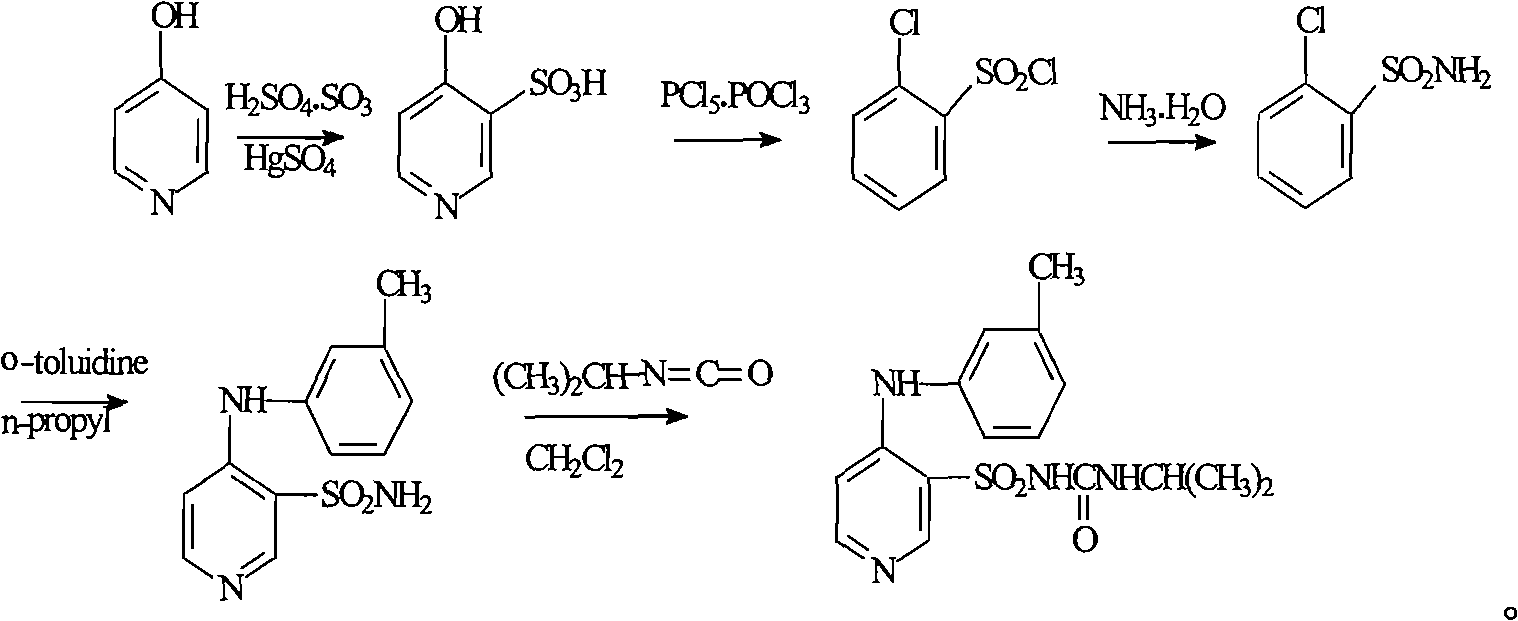
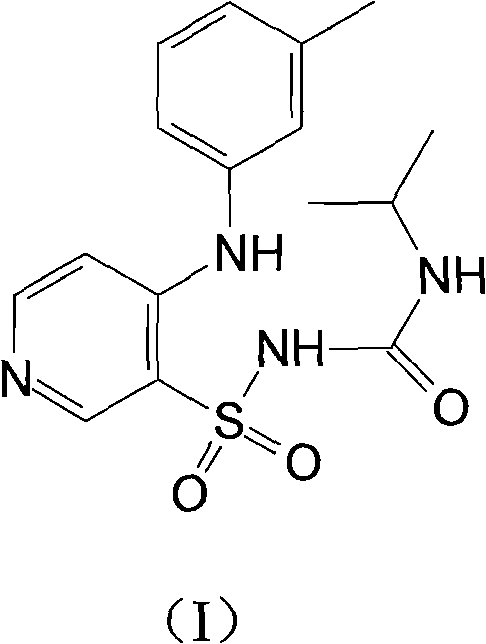
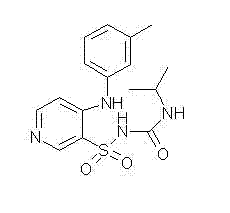
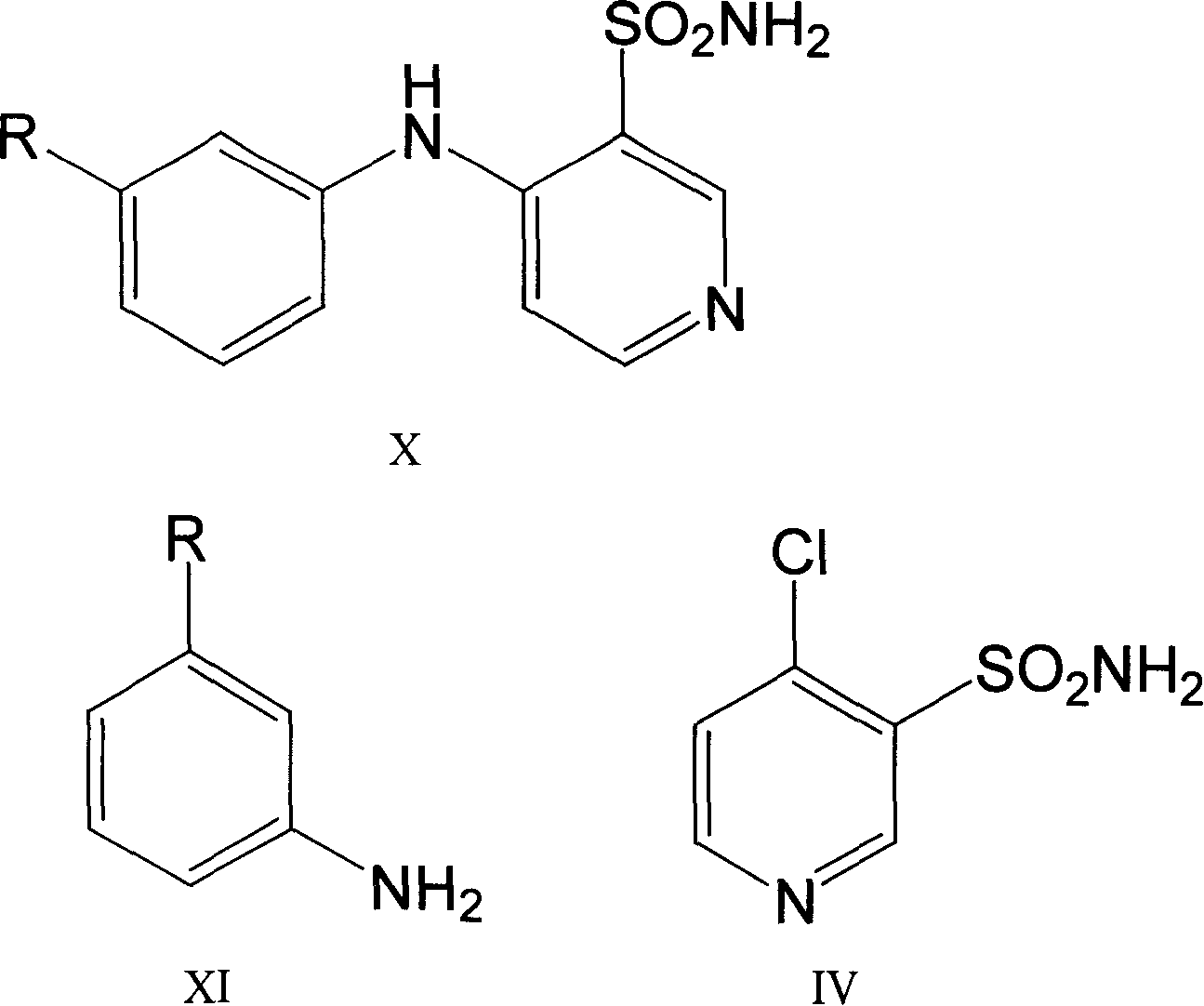
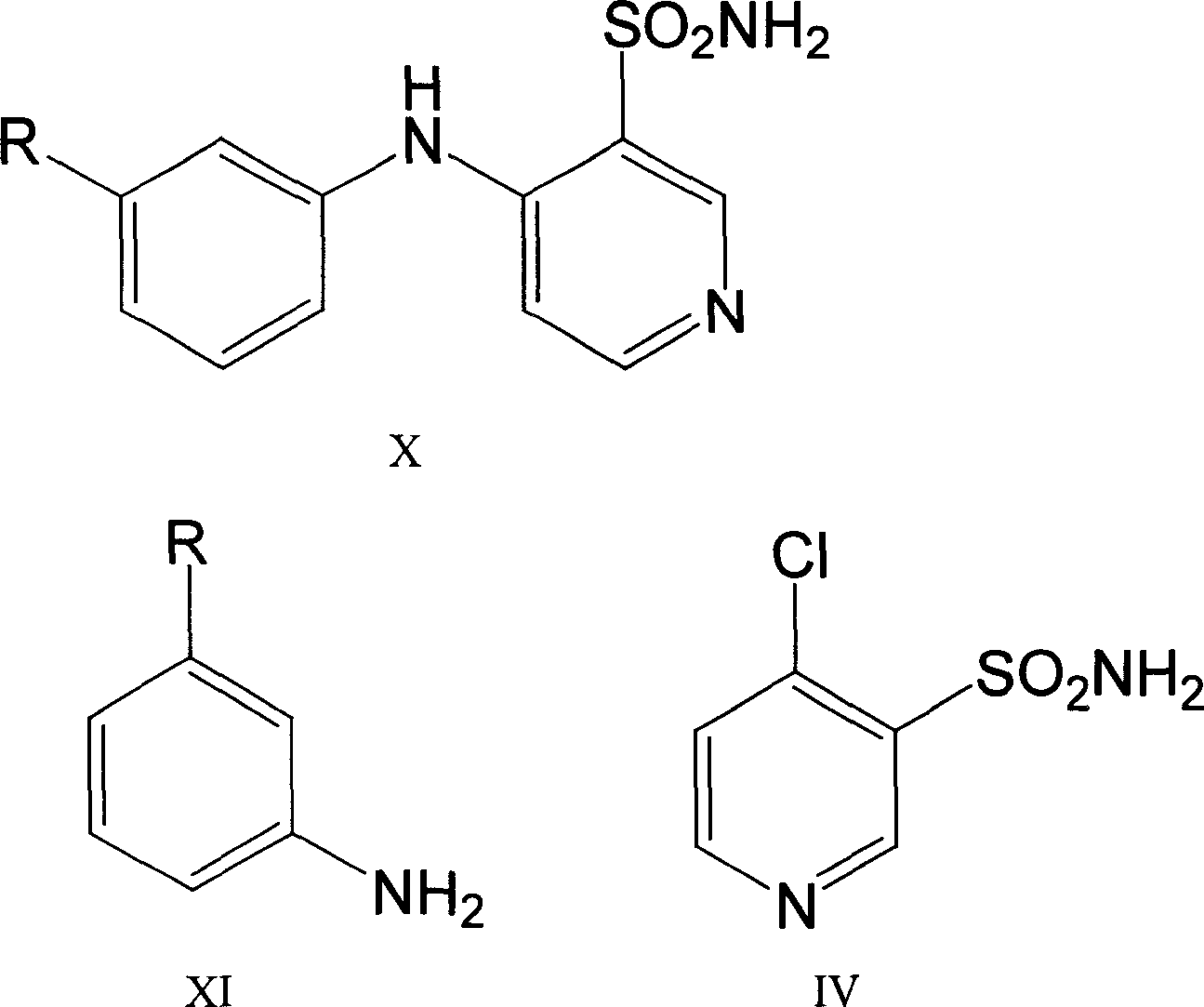
Method for preparing torasemide and derivative thereof
InactiveCN104744355AMild reaction conditionsSimple and fast operationOrganic chemistryDrugs synthesisSulfamide
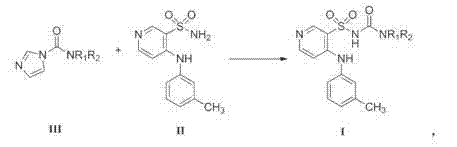
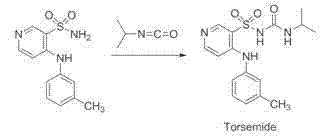
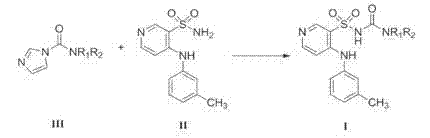
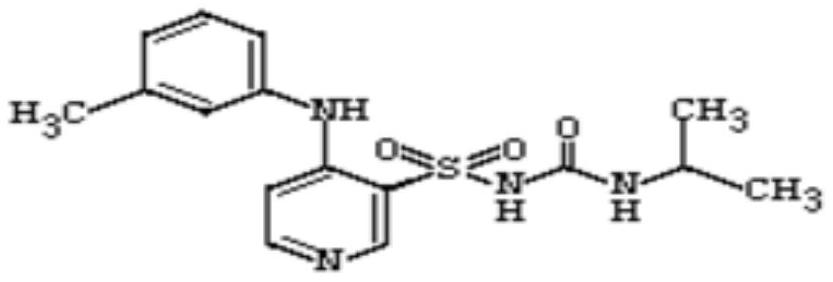
The invention belongs to the field of medicine synthesis and provides a method for preparing torasemide and a derivative thereof. The method comprises the following steps: by taking N-alkyl-1H-imidazole-1-formamide as the raw material, reacting with 4-(m-toluene amino) pyridine-3-sulfamide to obtain the torasemide and the derivative thereof. The method for preparing the torasemide and the derivative thereof has the advantages of low toxin, low pollution, high safety, short production period and simple operation and is suitable for industrial production.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
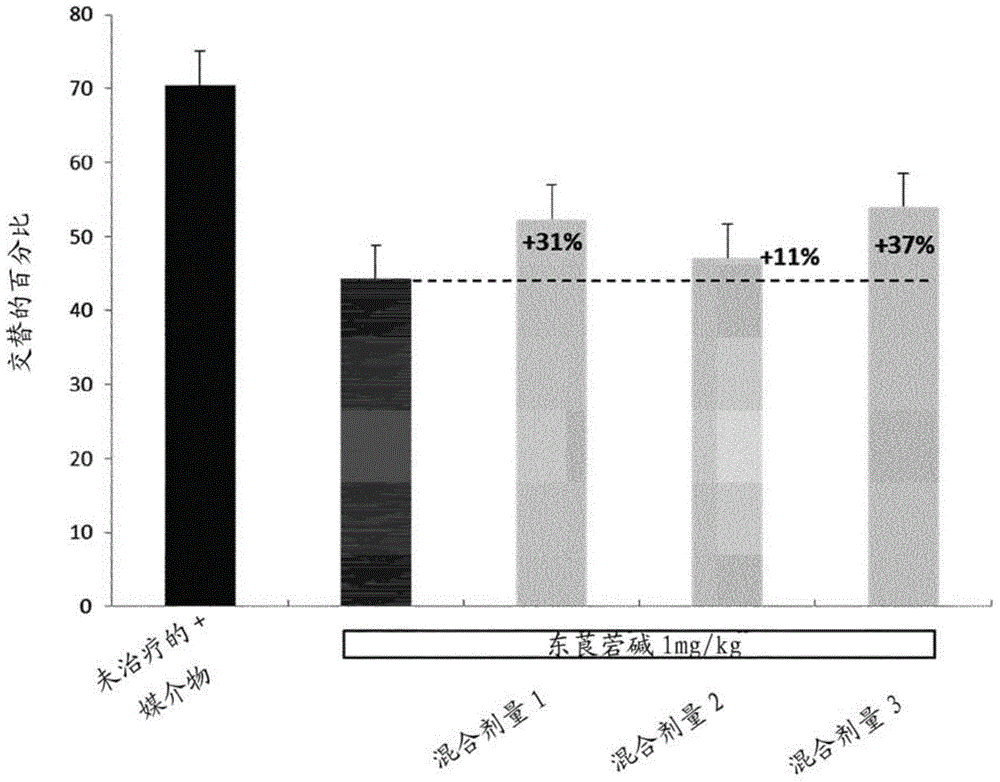
Methods of treatment comprising torsemide
Disclosed herein are treatments for diseases such as sleep apnea, hyperuricemia, autistic spectrum disorder (ASD) and other brain disorders using controlled-release (CR, e.g., extended-release (ER) or prolonged-release (PR)) oral dosage formulations comprising an effective amount of Torsemide.
Owner:SARFEZ PHARMA INC
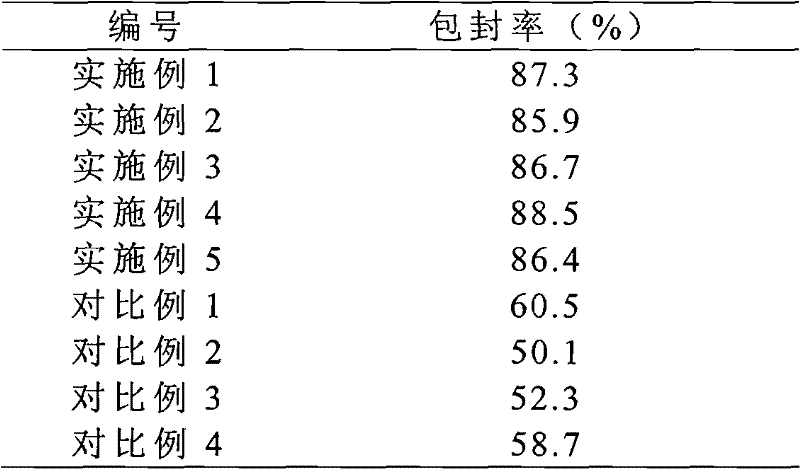
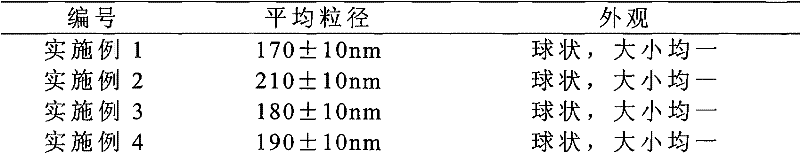
Solid preparation of Torasemide liposome
InactiveCN102078300AImprove qualityImprove stabilityPharmaceutical non-active ingredientsPill deliverySide effectBioavailability
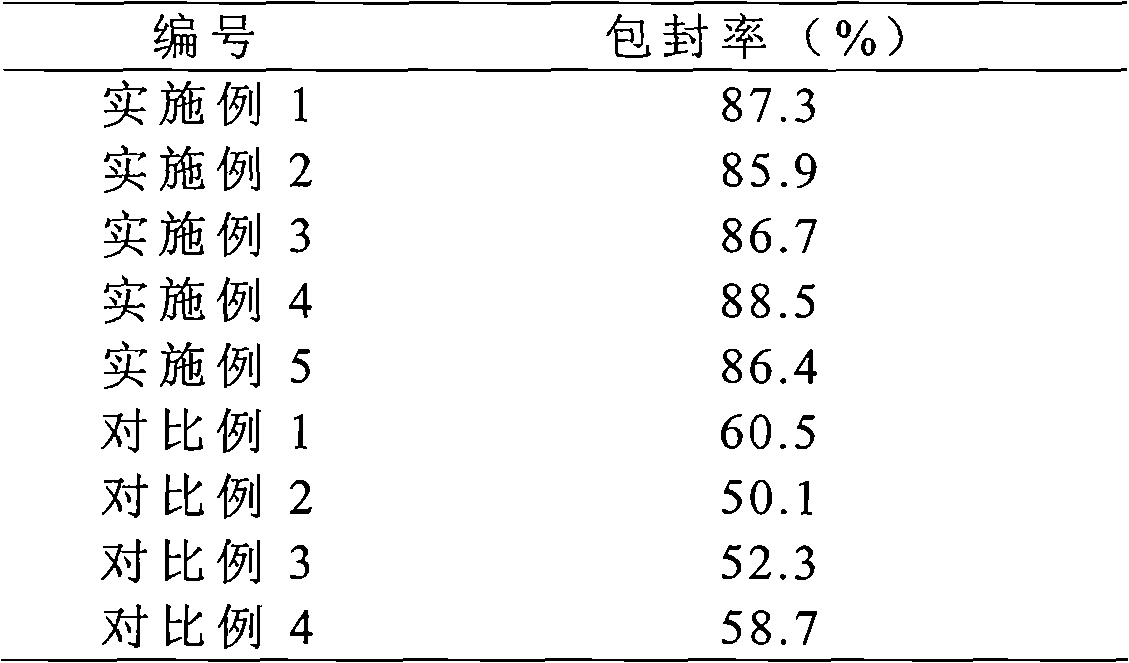
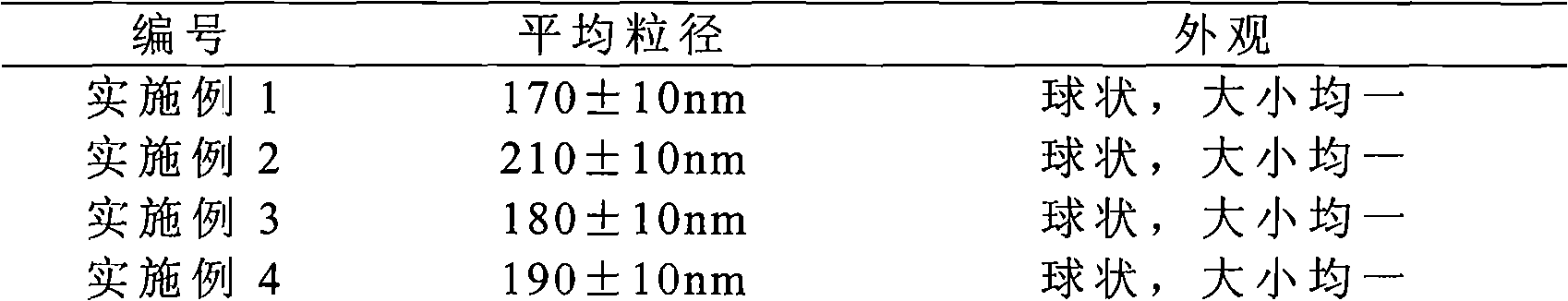
The invention provides a solid preparation of Torasemide liposome, which is prepared from the following raw materials and auxiliaries in parts by weight: 10 parts of Torasemide, 40 to 100 parts of soybean lecithin, 5 to 40 parts of cholesterin, 4 to 20 parts of sodium glycocholate, 60 to 200 parts of sorbitol and 5 to 20 parts of soyasterol. The solid preparation provided by the invention has high dissolubility, high stability, simple preparation method, high envelope rate, uniform particle size and long remaining time in body, thus the quality of preparation product is enhanced, the bioavailability is raised and the toxic and side effects are reduced.
Owner:HAINAN MEIDA PHARMA
Method for qualitatively and quantitatively detecting torasemide illegally added in food and application
PendingCN113514584ARapid qualitative confirmationHigh sensitivityComponent separationFluid phaseFood safety
Owner:辽宁省药品检验检测院
Use of a torasemide-based veterinary composition for low-dose administering
InactiveUS20170105979A1Maintain beneficial effectReduce harmful effectsMetabolism disorderSulfonylurea active ingredientsVeterinary pharmaceuticalsCardiac failures
The present invention relates to a veterinary pharmaceutical composition containing torasemide to be used for treating cardiac failure. The torasemide is administered in a daily dose of 0.02 mg / kg to 0.1 mg / kg during long-term treatment.
Owner:VIRB AC SA
Multi-use torasemide composition
PendingCN111741745ADispersion deliverySulfonylurea active ingredientsOrganic solventCombinatorial chemistry
Owner:威隆股份公司
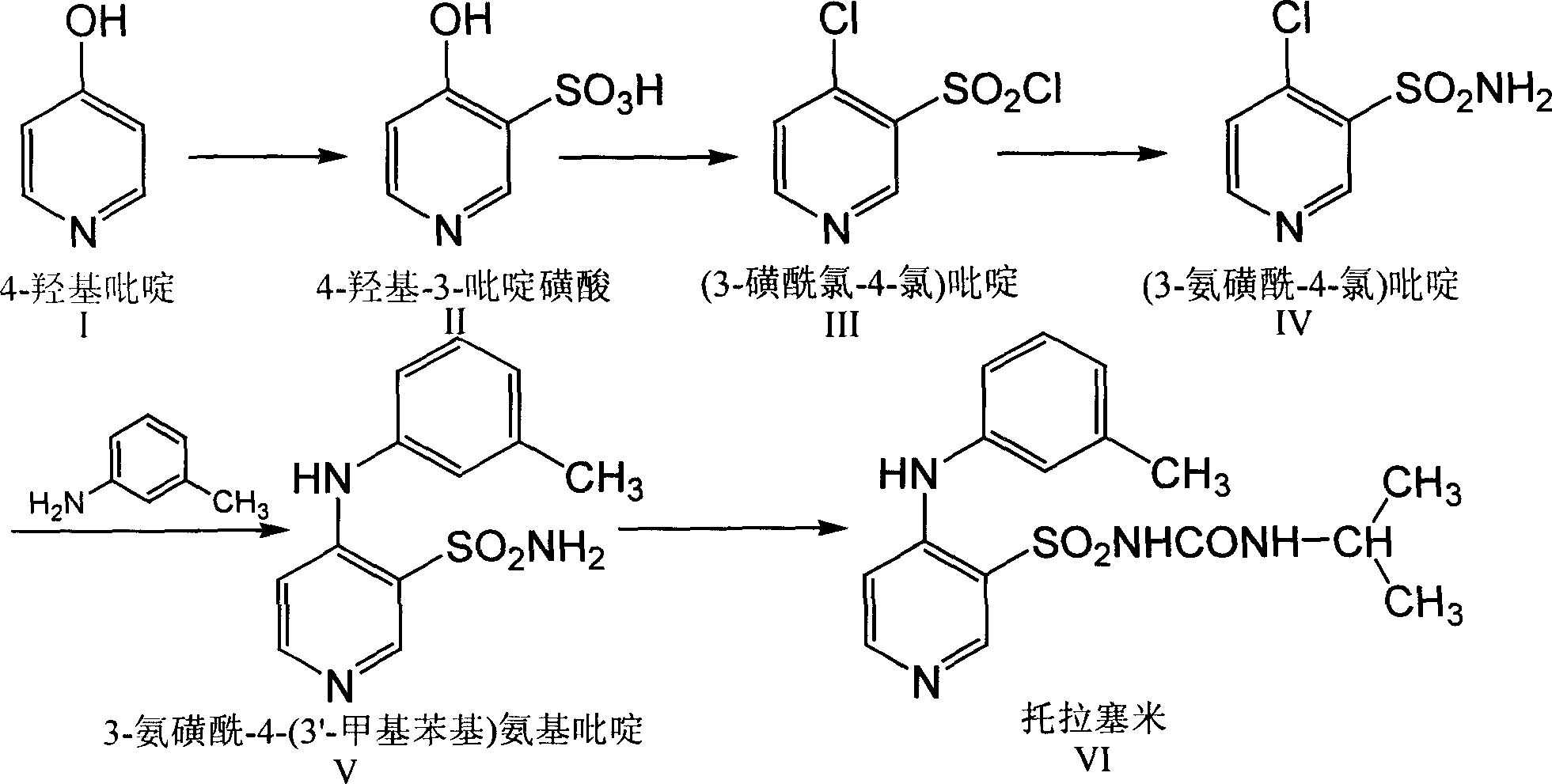
Process for preparing Torasemide intermediate and its analogue
The invention relates to a process for preparing torasemide intermediate compound or the like, which comprises selecting right solvent, and carrying reaction at relative mild temperature. The process requires no copper powder, thus the difficulty of purification can be avoided, and large scale production process can be realized.
Owner:南京海辰药业股份有限公司
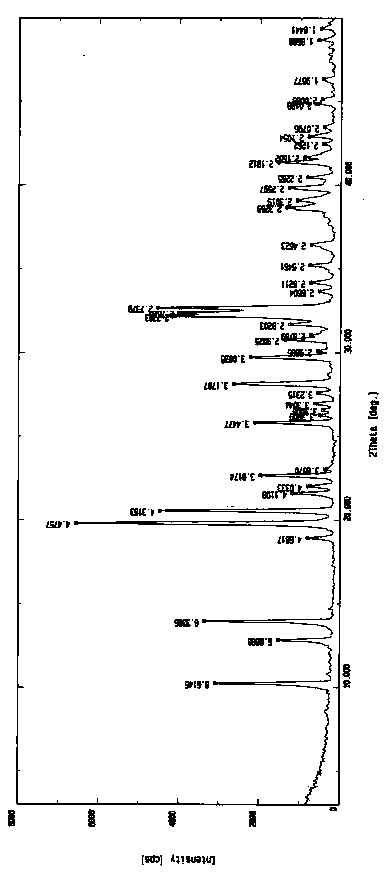
High-purity torasemide compound
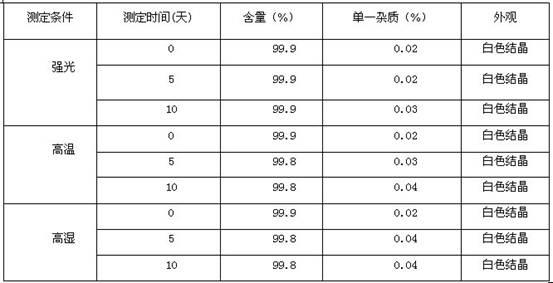
The invention belongs to the technical field of medicines, and particularly relates to a torasemide compound. The torasemide compound obtained in the invention has the advantages of small relevant substances, high purity, good stability and unobvious moisture absorption and weight increment even under the condition of high humidity.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Torasemide tablet
InactiveCN106038500AGood quality and stabilitySimple manufacturing methodDigestive systemPharmaceutical non-active ingredientsSolubilitySide effect
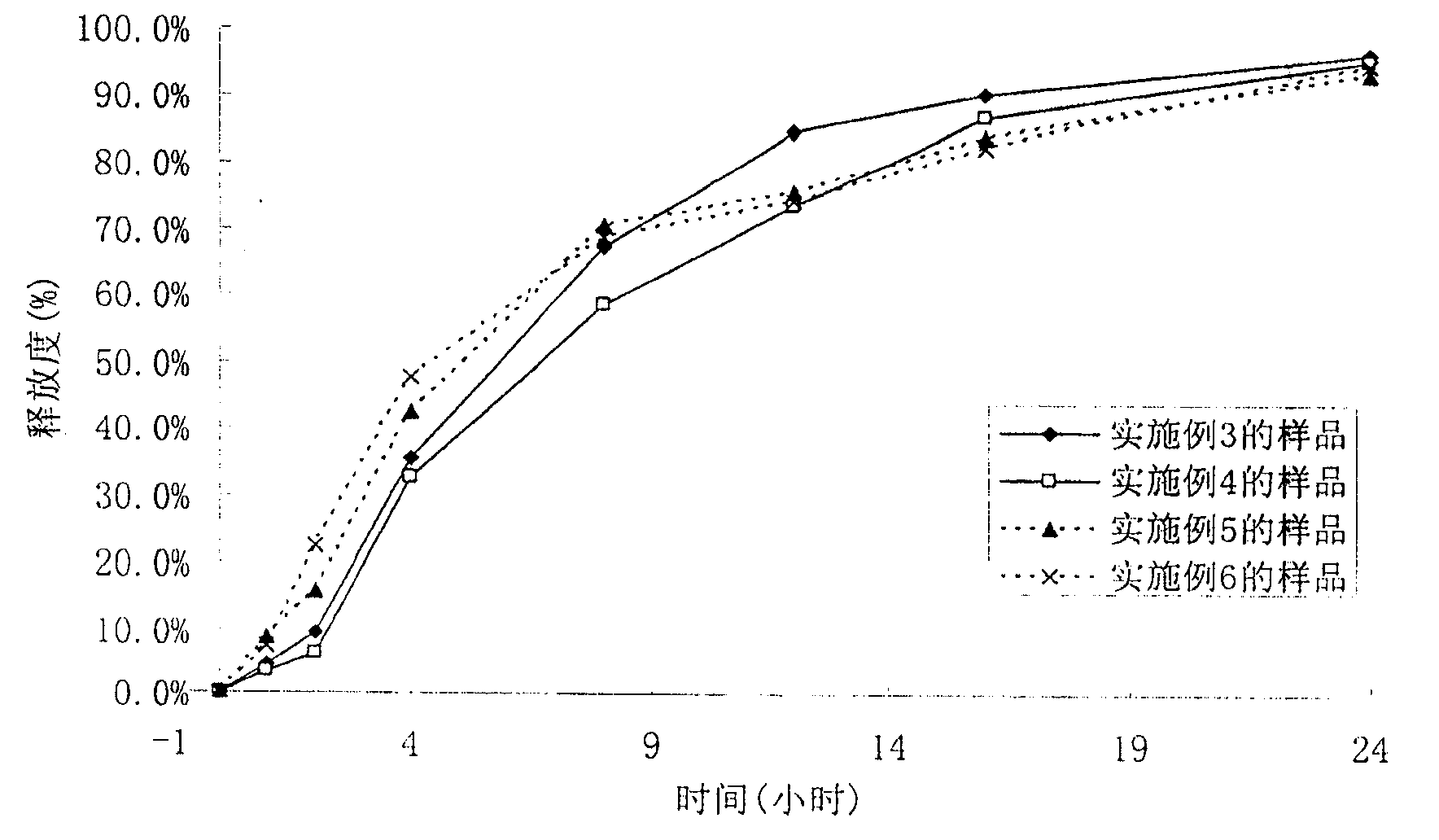
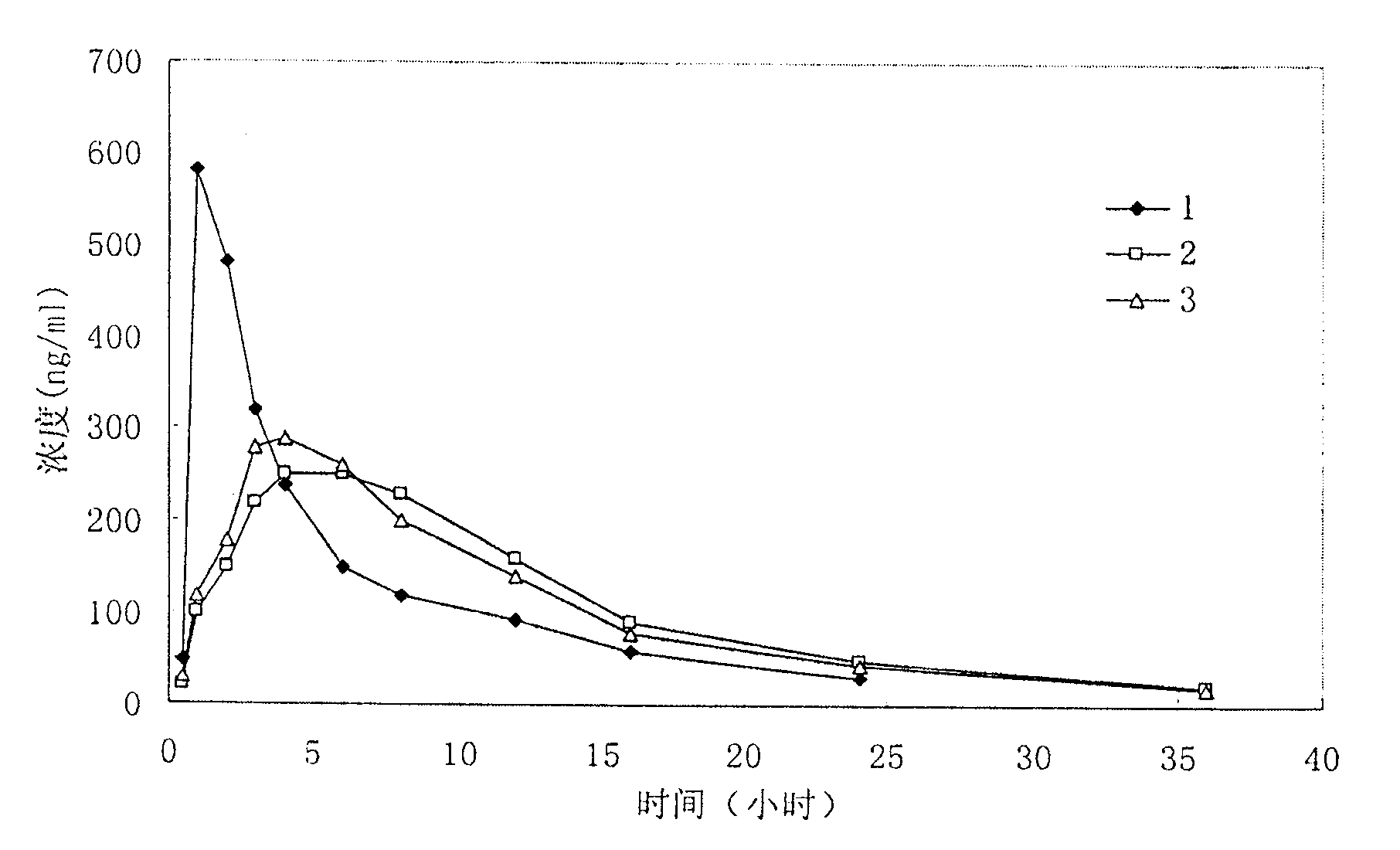
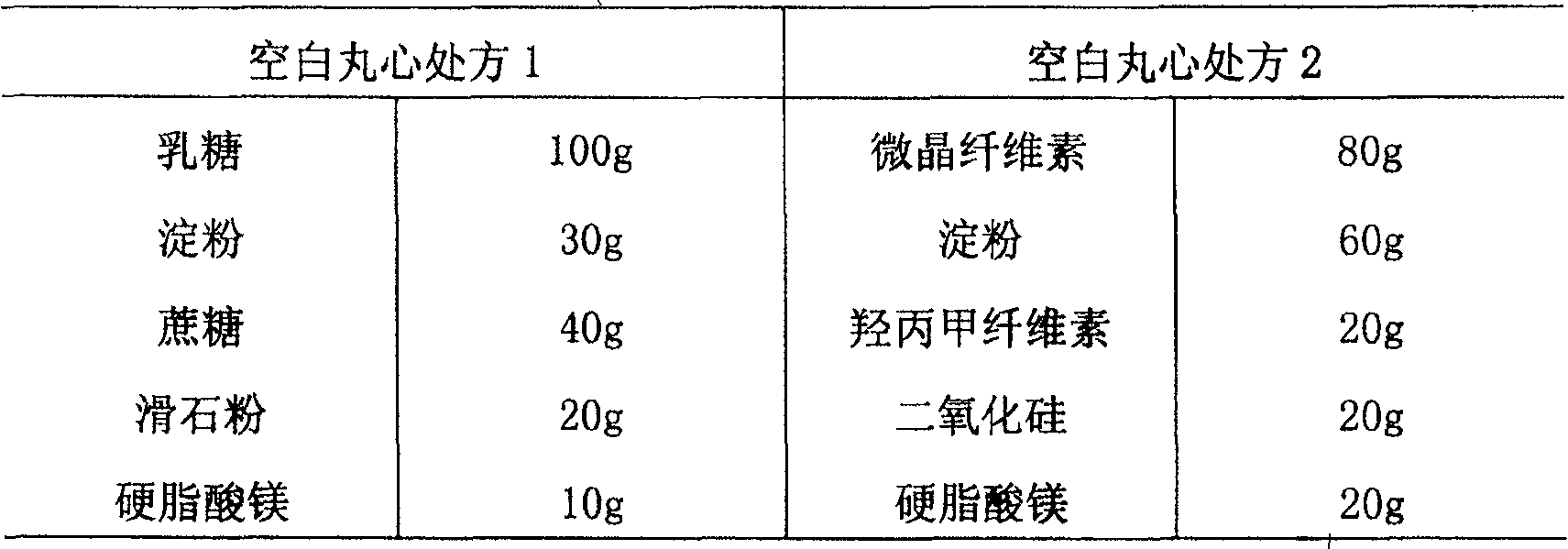
The divisional application of the invention provides torasemide with a novel crystal form and with an application number of 201610363797.0, and discloses a torasemide tablet. The torasemide tablet is a preparation mainly prepared from the torasemide with the novel crystal form. According to the crystal form, the aspects of the stability, dissolution rate, water solubility and the like of the preparation are greatly improved. The torasemide tablet can play a role of slow release without a retardant, so that the use of the retardant is reduced, toxic and side effects are reduced, and the preparation is very applicable to clinical application. According to the torasemide tablet, the zero-level release, smooth dissolution and stable decompression are realized, the awkwardness of urgent urination of a patient is avoided, the toxic and side effects are reduced, and the torasemide tablet can be adequately absorbed after being orally taken. The torasemide tablet is good in stability and medication compliance, simple in production process, relatively low in cost and easy to industrialize.
Owner:NANJING ZENKOM PHARMA
Slow release tablet comprising toraesmide active ingredient
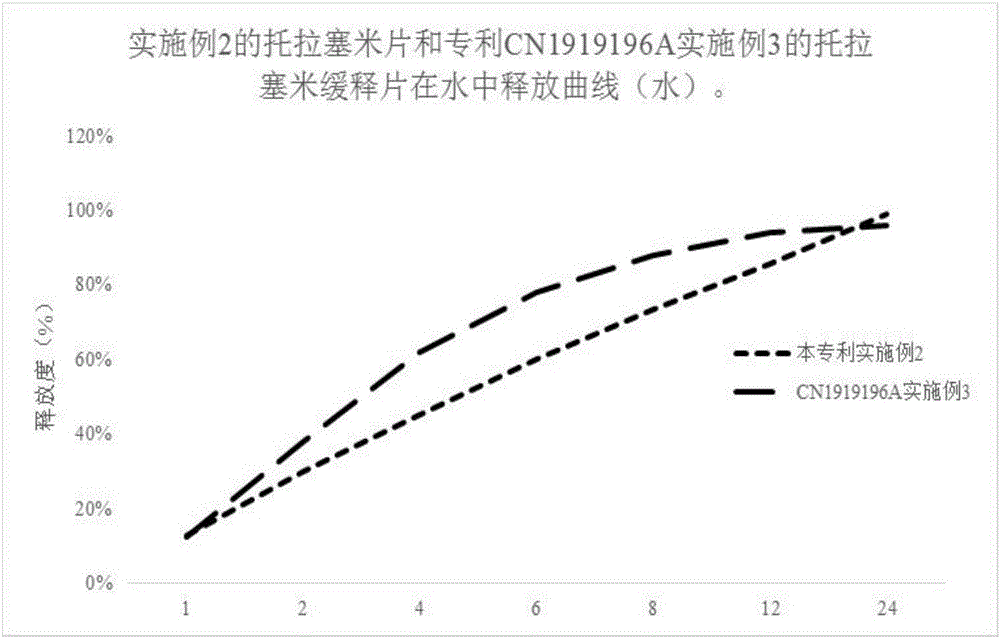
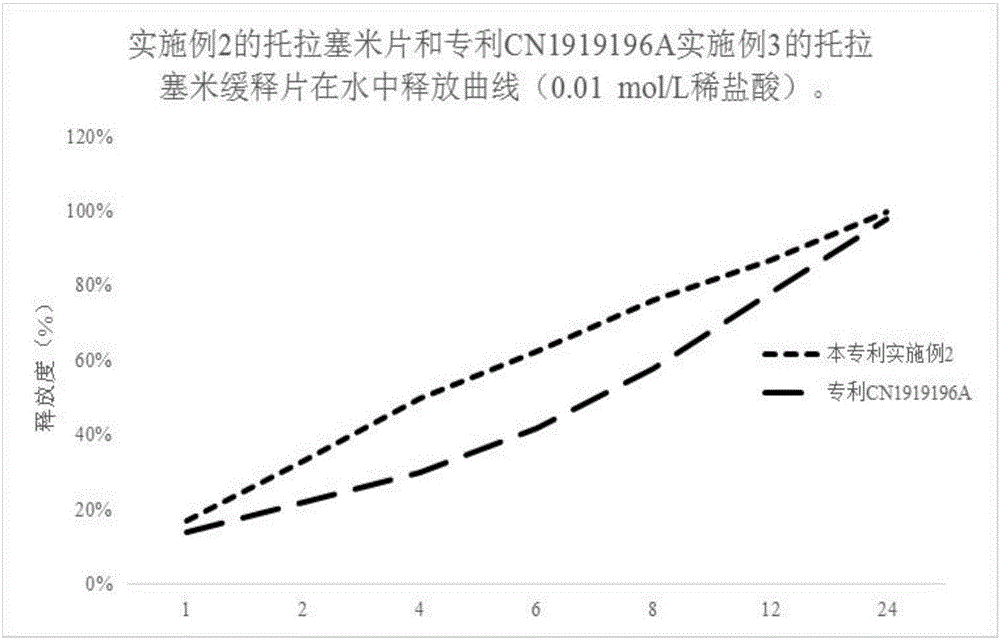
InactiveCN1919196AGood reproducibilityImprove consistencyPharmaceutical delivery mechanismBlood disorderMedicineBULK ACTIVE INGREDIENT
The invention discloses a slow release tablet containing active ingredient of torasemide, which comprises torasemide, slow release material and other auxiliary materials by the weight ratio of 1 : 0.2-20 : 10-200, preferably 1 : 0.5-10 : 20-100. The prepared slow release tablet is suitable for patients of hypertension.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
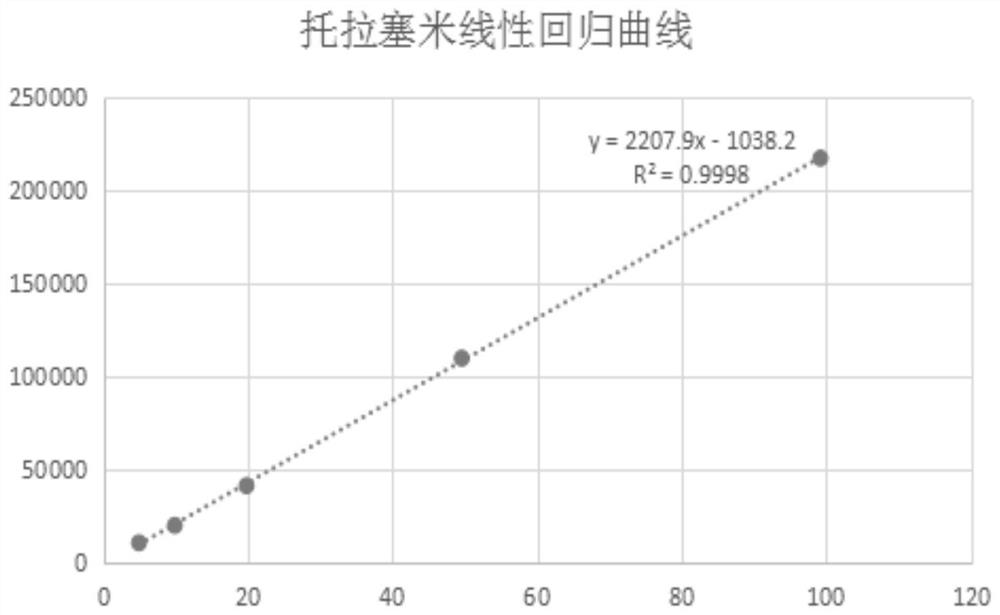
Indirect determination method for impurity content in torasemide injection
PendingCN114487155AEffective control generationLower control costsComponent separationPolyethylene glycolPhysical chemistry
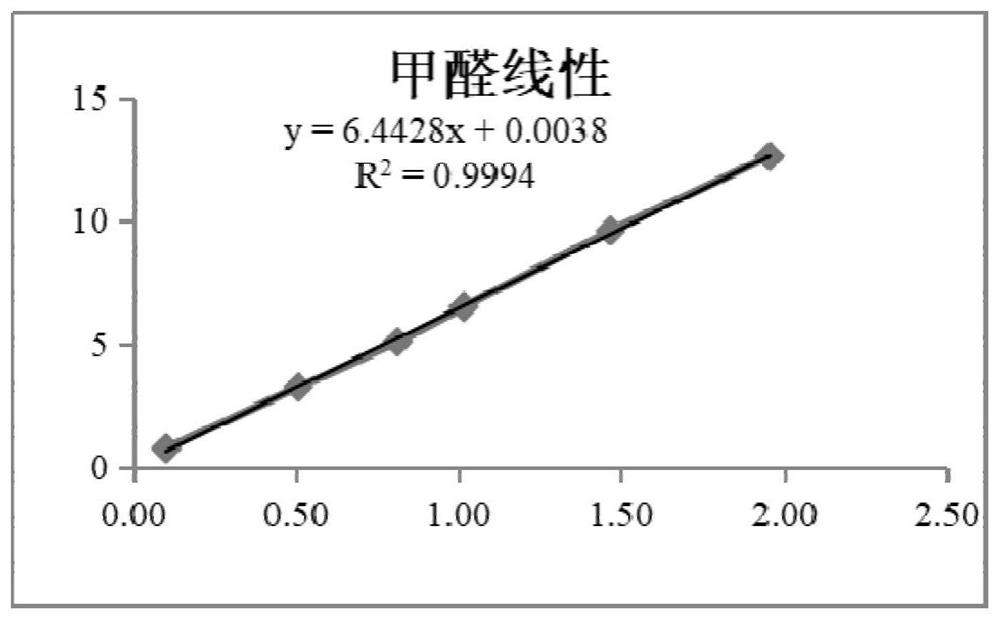
The invention provides an indirect determination method for impurity content in a torasemide injection, which is characterized in that the amount of impurity F is determined by directly determining the reduction amount of polyethylene glycol 400 and the content of formaldehyde in the torasemide injection through headspace gas chromatography. Through analysis, generation of PEG400 and formaldehyde in the technological process is effectively monitored in the production of the torasemide injection. According to the method, the content of formaldehyde in the polyethylene glycol 400 and torasemide injection is detected by headspace gas chromatography; the headspace gas chromatography is adopted, derivatization is not needed, a quartz capillary column, temperature programming, nitrogen serving as carrier gas and a flame ionization detector are used, and the method is easy to operate, high in specificity, high in sensitivity, low in control cost and capable of being used for quantitative analysis.
Owner:江苏睿实生物科技有限公司
Novel medicine application of torasemide
InactiveCN106176743AHeterocyclic compound active ingredientsCardiovascular disorderMyocarditisHypertrophic cardiomegaly
The invention discloses a novel medicine application of torasemide. The torasemide can inhibit cardiomegaly caused by high blood pressure, heart failure, myocardial fibrosis, myocarditis and the like. A small dosage of torasemide or injection can obviously improve cardiomegaly.
Owner:NANJING ZENKOM PHARMA
Low-impurity and high-stability torasemide injection and preparation method
ActiveCN112168776AReduce the risk of security incidentsGeneration of controlInorganic non-active ingredientsPharmaceutical delivery mechanismActivated carbonPolythylene glycol
The invention relates to a low-impurity and high-stability torasemide injection and a preparation method. The low-impurity and high-stability torasemide injection is prepared from the following components of torasemide, polyethylene glycol 400, tromethamine, sodium hydroxide and water for injection with the weight ratio of (20-80):(100-2000):(0.5-10):(1-10):(5000-15000), and the preparation methodcomprises the following steps: (1) measuring water for injection and controlling water temperature at 30-65 DEG C; (2) sequentially adding polyethylene glycol 400, tromethamine and sodium hydroxide,and uniformly stirring; (3) adding torasemide, and stirring; (4) adjusting the pH value to 8.5-9.5 by using a sodium hydroxide solution, and adding water to a constant volume; and (5) filtering to obtain the torasemide injection. The preparation method aims to shorten the batching time by optimizing the feeding sequence; an activated carbon adsorption procedure is not adopted, so that the drug safety risk is reduced; and medium-low temperature is adopted in all procedures, and generation of degradation impurities is controlled.
Owner:KAMP PHARMA
Slow release drop pills comprising toraesmide active ingredient and method for preparing same
InactiveCN100548297CAvoid burst phenomenonImprove securityUrinary disorderGranular deliverySustained release pelletsPlasticizer
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
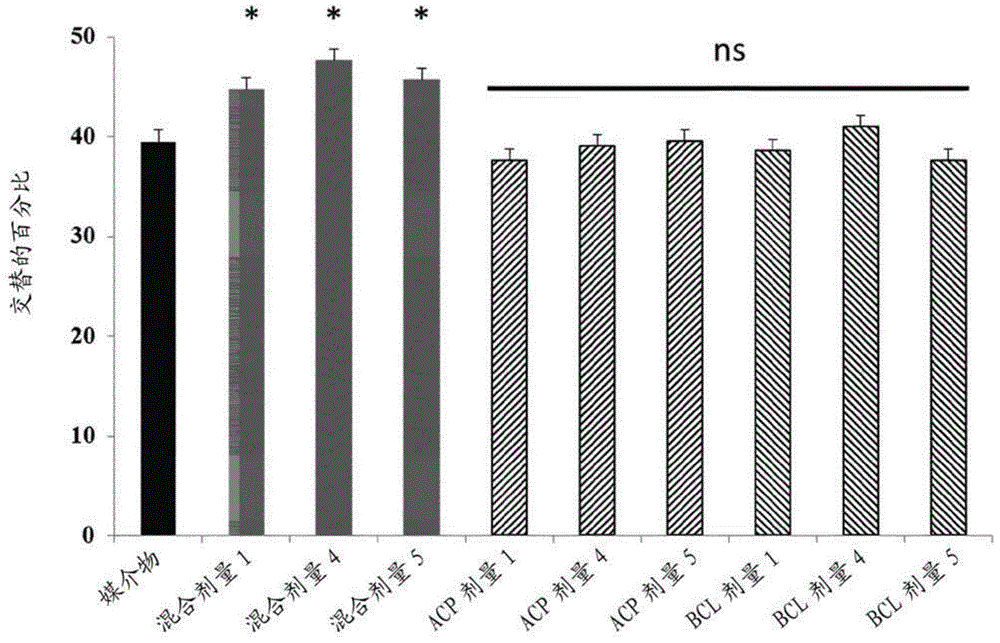
Nootropic compositions for improving memory performance
ActiveCN104884053AEnhance memoryEasy to learnNervous disorderPeptide/protein ingredientsAcamprosateSulfisoxazole
The invention relates to compositions and methods for improving memory and related functions as alertness, attention, concentration, learning, and language processing. More particularly, the invention relates to compositions comprising at least two drugs selected from cinacalcet, baclofen, acamprosate, mexiletine, sulfisoxazole and torasemide useful to enhance memory and related functions in healthy subjects or subjects suffering from conditions or disorders having a negative impact on their memory.
Owner:PHARNEXT
Torasemide compound
The invention belongs to the technical field of medicine, and specifically relates to a torasemide compound and a preparation method thereof. The provided novel torasemide crystal form has the advantages of high purity (the maximal impurity content is less than 0.05%), and good stability. Moreover, the preparation method has a good repeatability, and the purity and crystal form can be well repeated when the preparation method is amplified to a pilot scale. The invention further relates to an application of the novel torasemide crystal form in preparation of drugs for treating medium and heavy edema caused by heart failure, kidney failure, cirrhosis ascites, encephaledema, and the like.
Owner:TIANJIN HANRUI PHARMA
Torasemide pharmaceutical composition with stabilization and safety for injection
ActiveCN102512360BSolve discolorationQuality assurancePharmaceutical delivery mechanismPharmaceutical non-active ingredientsDiseaseTherapeutic effect
The invention relates to a torasemide pharmaceutical composition with stabilization and safety for injection and its preparation method, especially relates to the torasemide pharmaceutical composition used for patients with requirement of rapid diuresis or congestive cardiac failure without oral diuresis, cirrhosis ascites, oedema caused by kidney diseases, the torasemide pharmaceutical composition is an injection preferably. The pharmaceutical composition is composed of active component torasemide, ethanol and polysorbate 80. The torasemide pharmaceutical composition for injection possesses stable pH value and low impurity content, and is capable of effectively raising the treatment effect of the products, minimizing the side reaction, raising the yield, reducing the cost and creating higher benefit.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Solid preparation of Torasemide liposome
InactiveCN102078300BWell mixedImprove solubilityPill deliveryPharmaceutical non-active ingredientsSide effectBioavailability
The invention provides a solid preparation of Torasemide liposome, which is prepared from the following raw materials and auxiliaries in parts by weight: 10 parts of Torasemide, 40 to 100 parts of soybean lecithin, 5 to 40 parts of cholesterin, 4 to 20 parts of sodium glycocholate, 60 to 200 parts of sorbitol and 5 to 20 parts of soyasterol. The solid preparation provided by the invention has high dissolubility, high stability, simple preparation method, high envelope rate, uniform particle size and long remaining time in body, thus the quality of preparation product is enhanced, the bioavailability is raised and the toxic and side effects are reduced.
Owner:HAINAN MEIDA PHARMA
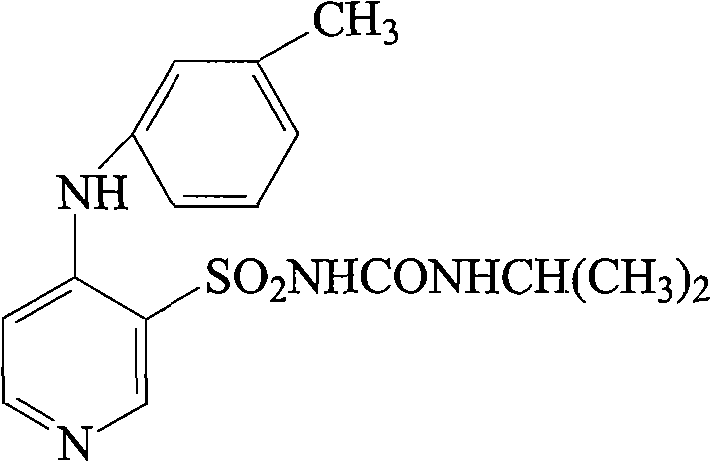
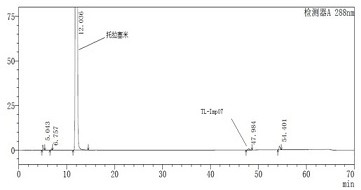
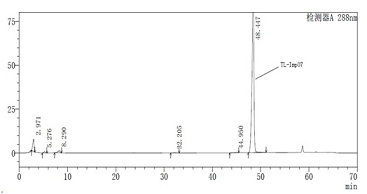
Novel torasemide impurity and preparation method thereof
The invention relates to a novel torasemide impurity and a preparation method thereof. The invention relates to a novel torasemide impurity, namely TL-Imp07: 4-m-toluene amino-N-[(4-m-toluene aminopyridine-3-yl) sulfonyl] pyridine-3-sulfonamide. The preparation method of the TL-Imp07 comprises the steps of carrying out condensation and ammoniation reactions on 4-chloro-3-pyridine sulfonamide. The impurity is novel in structure, is reported for the first time and has great significance on quality control of torasemide crude drugs and preparations. Meanwhile, the preparation method has the advantages of being simple, convenient to operate, high in product purity and the like and can be applied to research of impurity reference substances of torasemide.
Owner:南京恒正药物研究院有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com