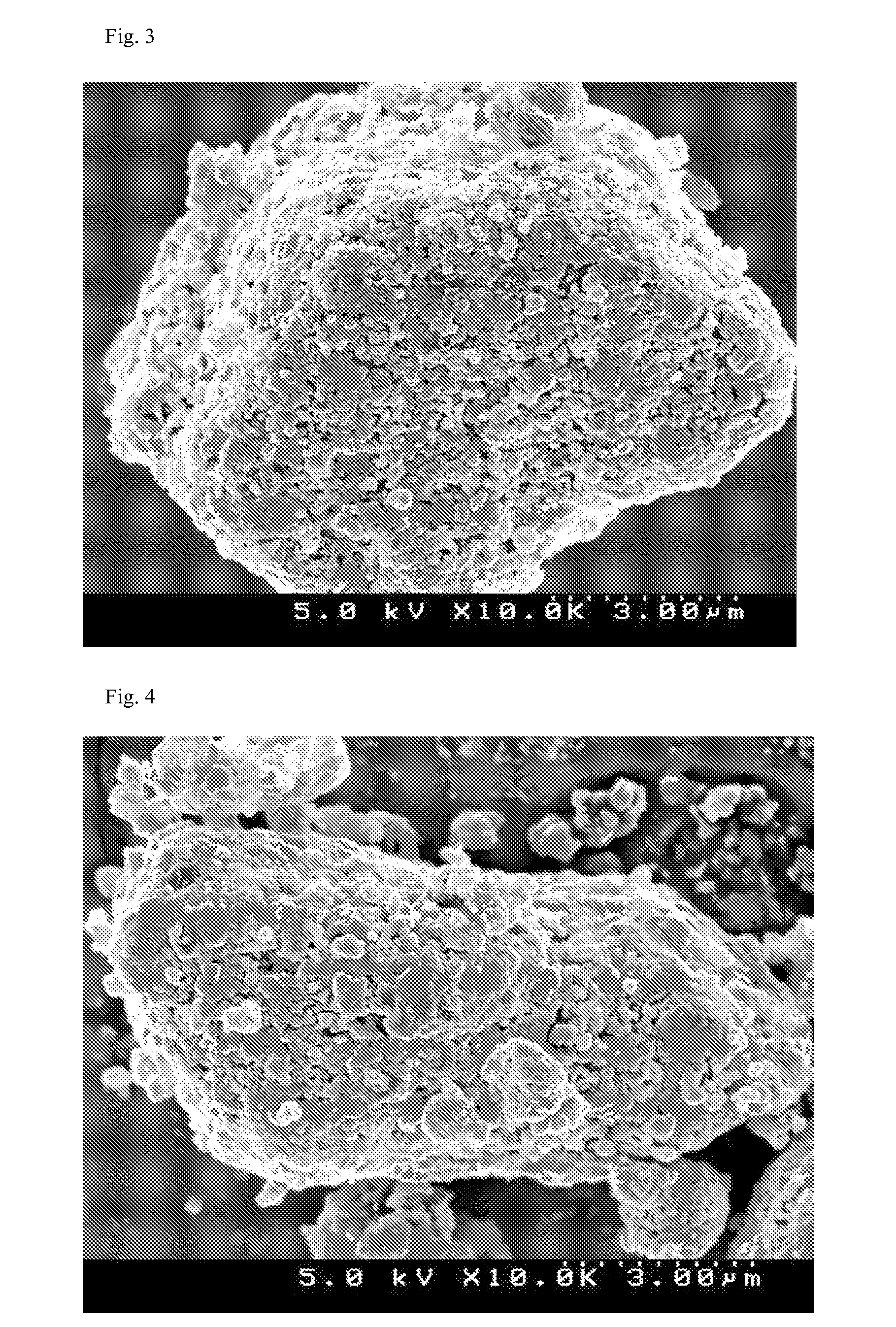
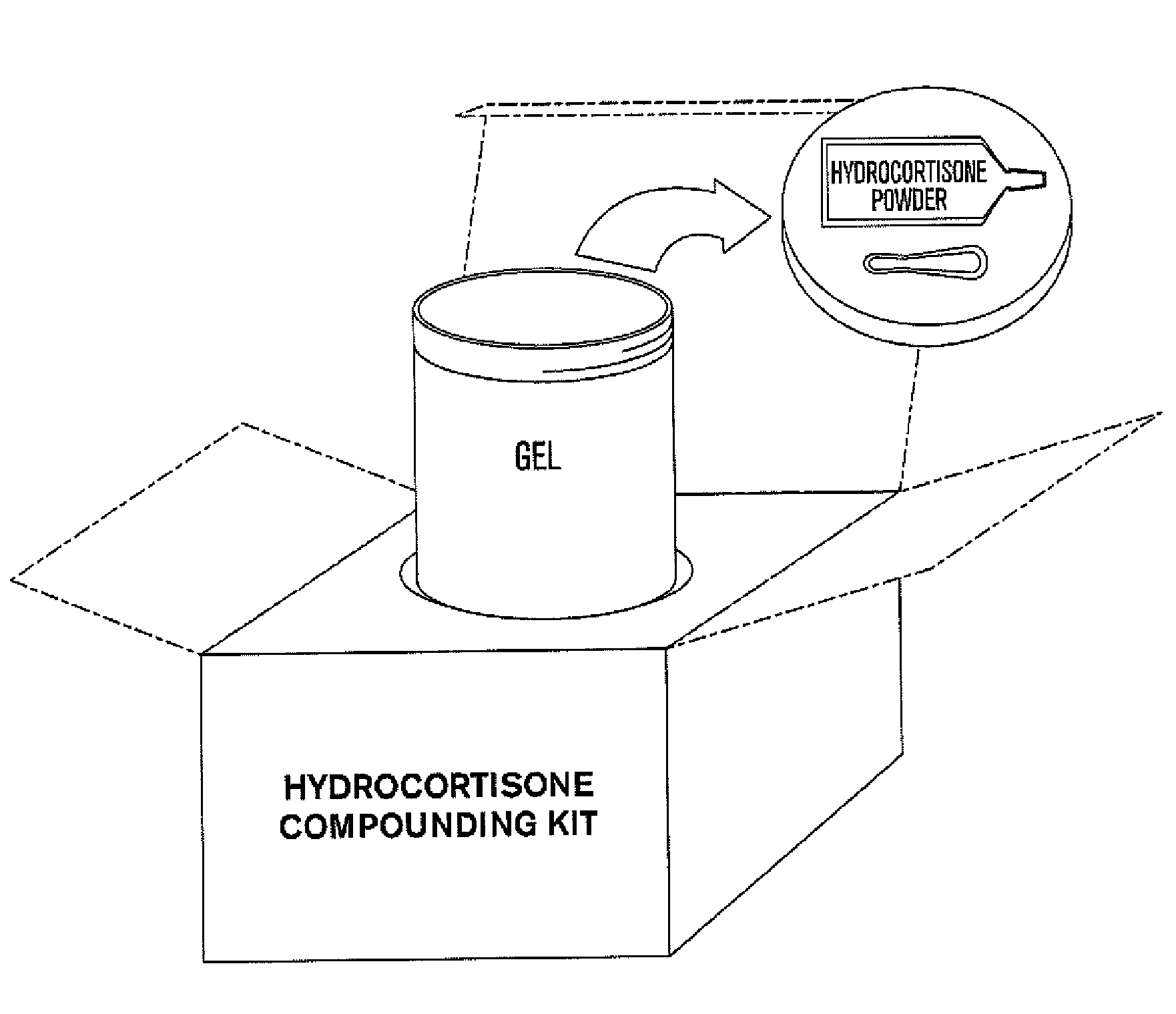
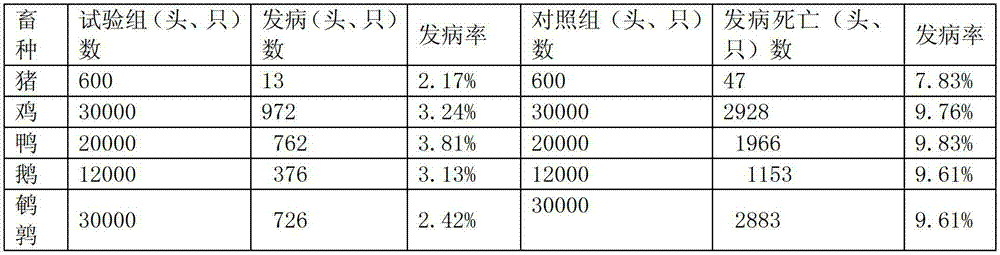
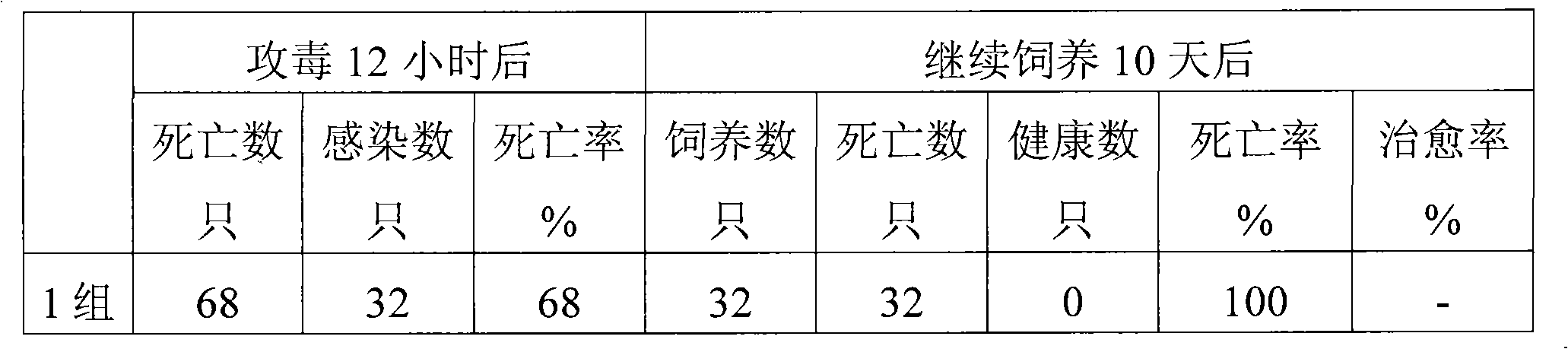
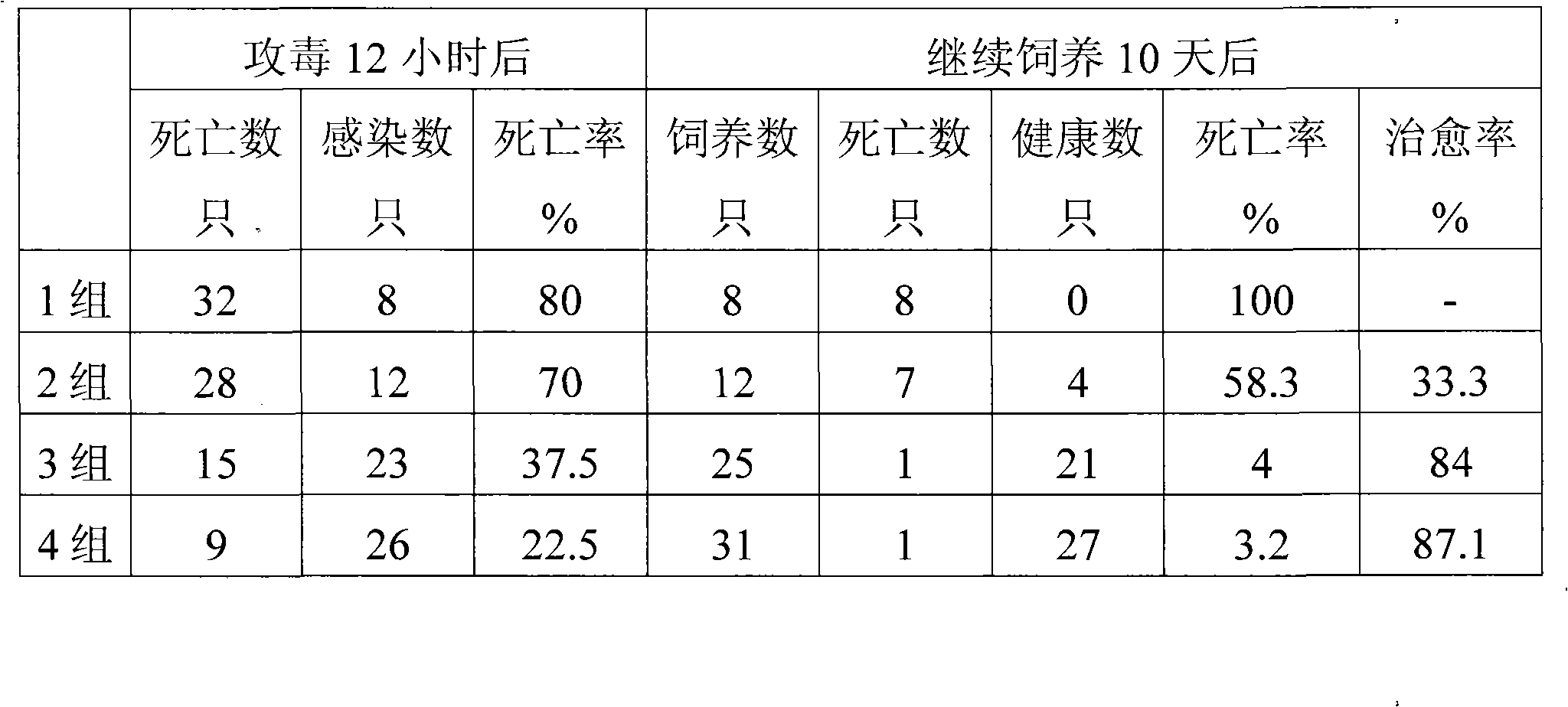
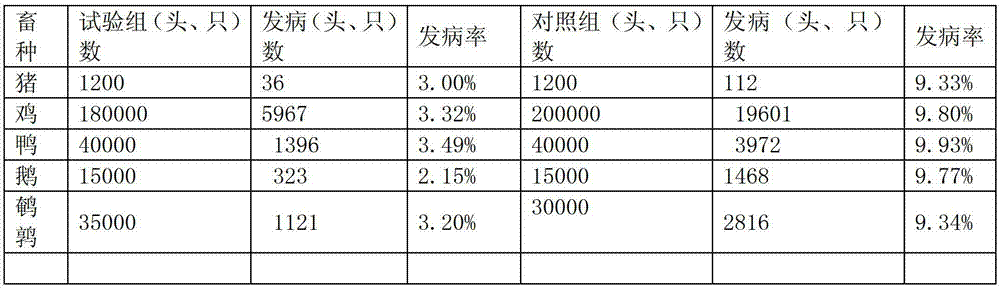
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
88 results about "Veterinary pharmaceuticals" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
DVM Pharmaceuticals Inc., is located at Miami, FL and the Animal Health Division of IVAX Corporation, devotes its energies to the research and development of innovative veterinary pharmaceutical products.
Pharmaceutical compositions for the stimulation of stem cells
InactiveUS20120301538A1Improve abilitiesOrganic active ingredientsPowder deliveryVeterinary pharmaceuticalsExcipient
The invention relates to a human or veterinary pharmaceutical composition (B) for the stimulation of stem cells, comprising at least two stem-cells-stimulating-agents and at least one pharmaceutically acceptable excipient.
Owner:CARDIO3 BIOSCI
Meloxicam in veterinary medicine
A veterinary pharmaceutical formulation containing meloxicam or a pharmacologically acceptable meloxicam salt of an organic or inorganic base and one or more vehicles having analgesic efficacy for the treatment of inflammatory painful diseases, particularly for the treatment of mild or moderate mastitis cases. The treatment leads to an effective long lasting reduction of a hypersensitive state associated with inflammatory pain in mild or moderate mastitis cases, particularly chronic states thereof.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
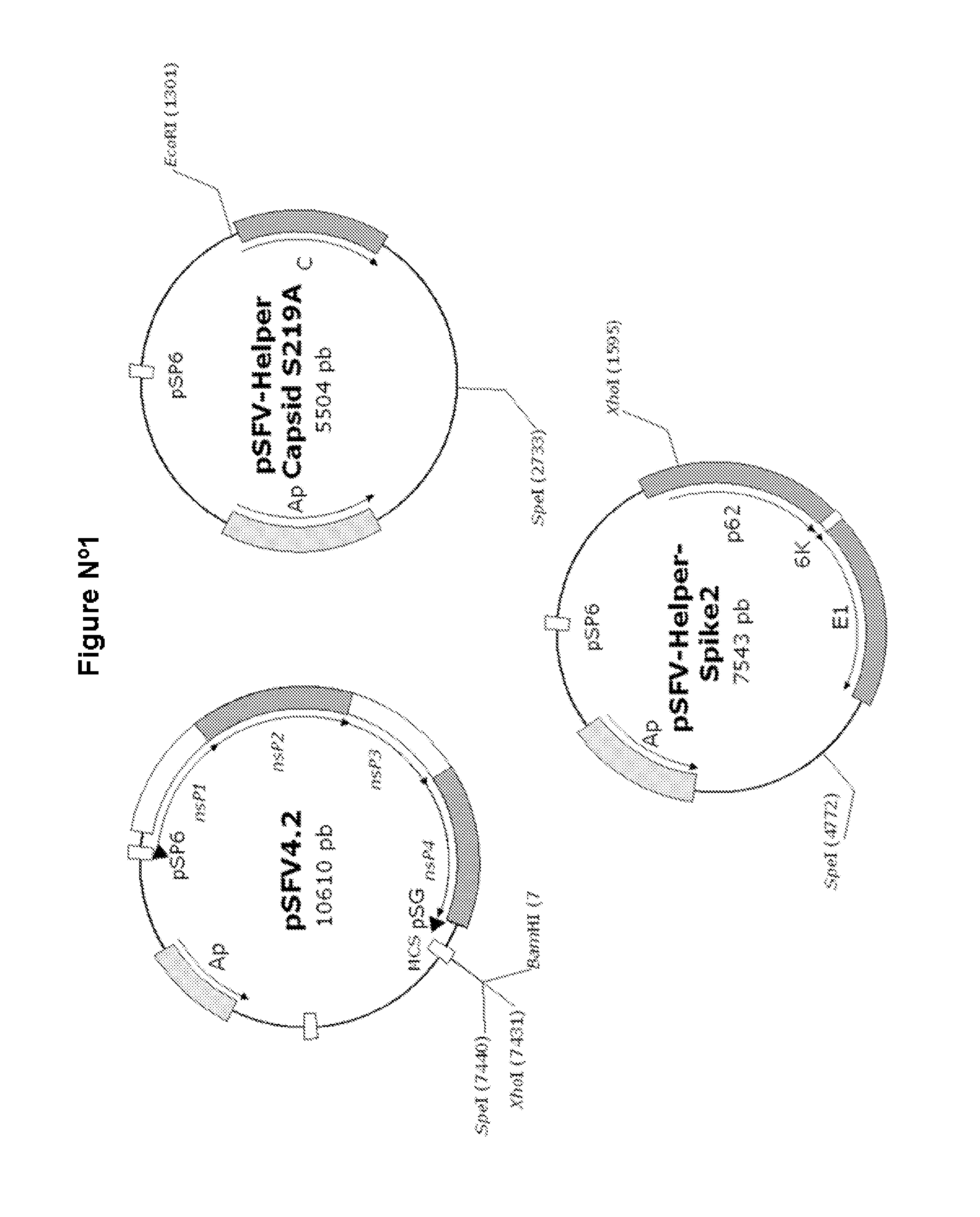
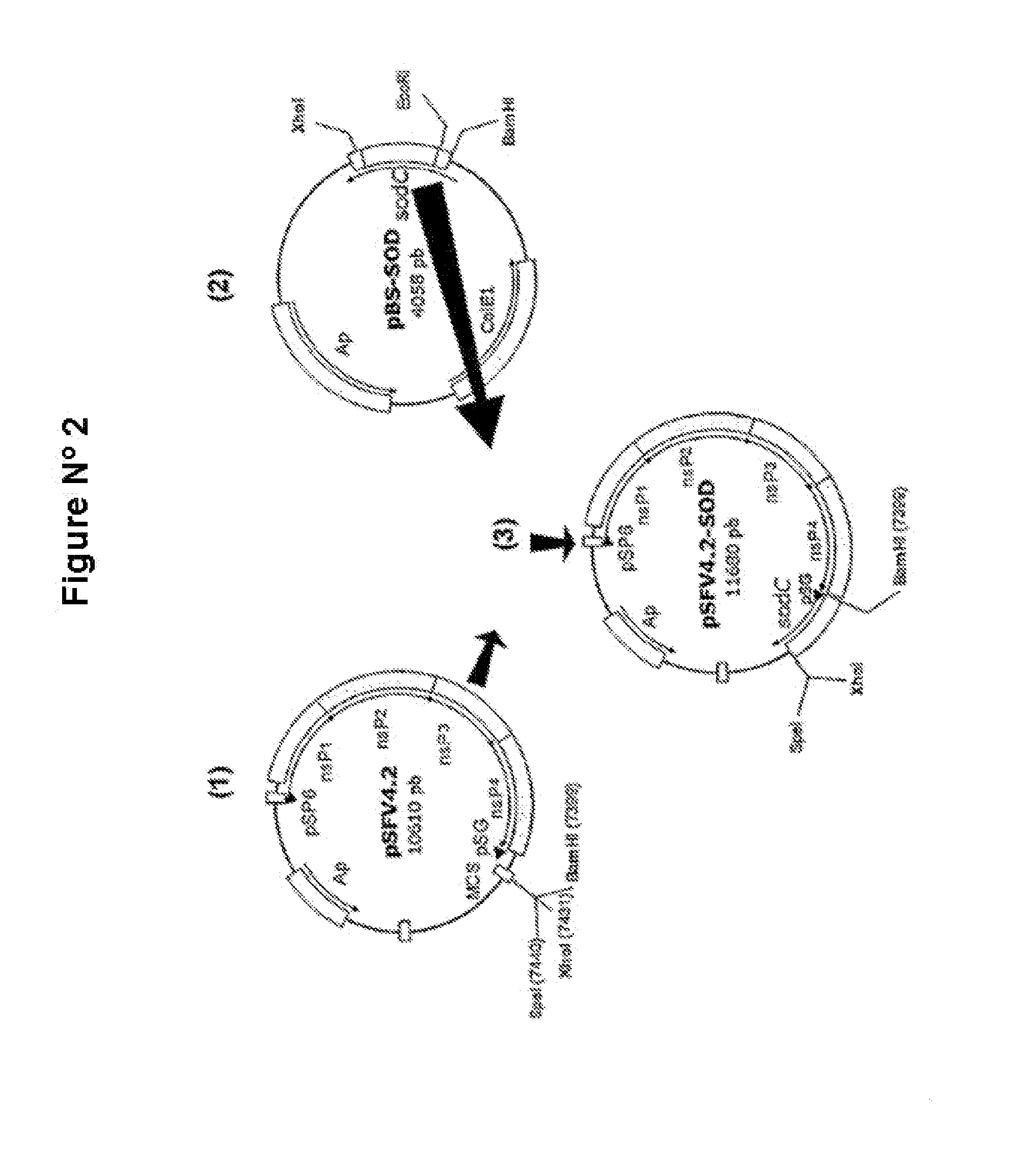
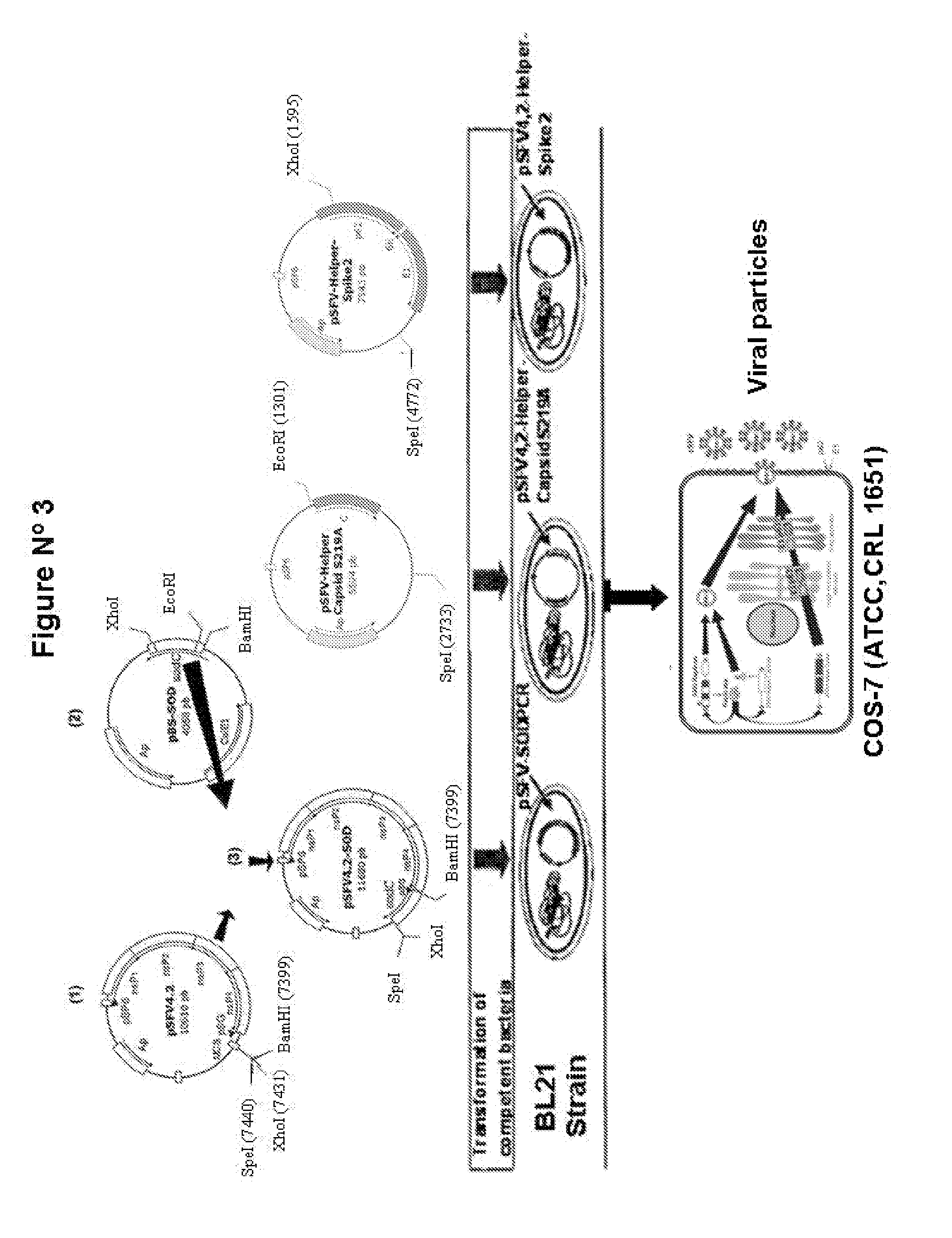
Veterinary pharmaceutical formulacion that comprises an RNA recombinant particle that encodes for a cu/zn superoxide dismutase protein of ruminant pathogenic bacteria and at least one RNA alphavirus belonging to the semliki forest virus family
InactiveUS20110200667A1Improve efficacyProtective efficacyAntibacterial agentsOrganic active ingredientsBrucella abortusZn superoxide dismutase
The technology is a veterinary pharmaceutical formulation of two vaccines, one from an RNA viral vector system constituted by an RNA recombinant particle that codifies for a Cu / Zn superoxide dismutase protein of Brucella abortus, and the other based on naked RNA constituted by a recombinant molecule of naked RNA that carries a sequence for the synthesis of at least one recombinant Cu / Zn superoxide dismutase protein of Brucella abortus and some Semliki Forest virus genes. An expression system based on the Semliki Forest virus and a use of this system, in addition to a method for the preparation of the pharmaceutical formulations.
Owner:UNIV DE CONCEPCION
Aerosol lotion formulations
Non-foaming aqueous emulsion compositions; methylparaben-based preservative systems for aqueous aerosol compositions having a pH at or above a pH where methylparaben begins to hydrolyze; cosmetic, household, and human and veterinary pharmaceutical products containing these compositions.
Owner:MSD CONSUMER CARE INC
Veterinary pharmaceutical composition
InactiveUS6322821B1Stimulates the cow's appetiteIncrease appetiteHeavy metal active ingredientsBiocideReflexRumen
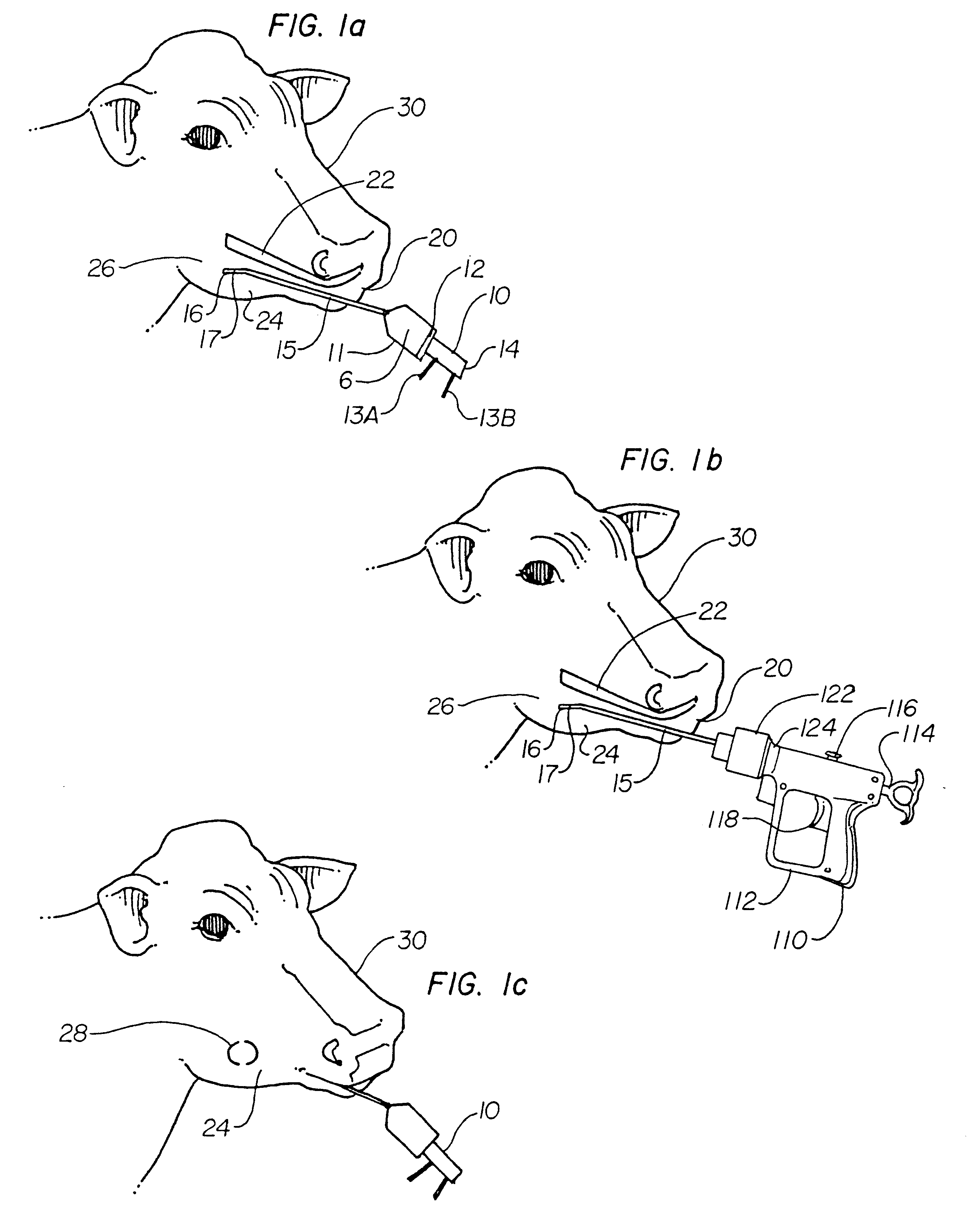
A composition for preventing and treating milk fever in freshening cows, and a method of administering the composition. The basic composition is a mixture of water, calcium chloride, propylene glycol, B vitamins and minerals. The calcium content is lower than conventional calcium treatments used for this purpose. The propylene glycol gives the cow an energy boost and sweetens the taste so the cow does not object to it as it does to conventional gels and liquids. The B vitamins stimulate the cow's appetite. The minerals replace minerals lost in the milk and also help the cow absorb calcium. The composition of the present invention is in a liquid form and is preferably administered using a 200-300 cc drench gun. The end of the dispensing tube of the drench gun is placed between the teeth and cheek of the cow near the receptors that stimulate the esophageal groove reflex. A feature near the end of the dispensing tube causes a bulge in the cow's cheek which indicates the position of the dispensing tube within the cow's mouth. The composition stimulates the receptors and closes the esophageal groove, thereby allowing the liquid into the omasum where calcium is absorbed quicker than in the rumen. The cow swallows the liquid mixture in a near normal manner which prevents the solution from causing aspiration pneumonia.
Owner:REGISTER JACK W
Opiopathies
The present invention provides novel methods for classifying, diagnosing and / or treating a group of human and veterinary ailments involving endogenous opioid concentrations. Also provided is a novel use for an existing class of compounds, the opioids, to treat opiopathic ailments, particularly paresis / paralysis, pseudo-atrophy and / or opiopathic pain, and in the manufacture of pharmaceutical and veterinary formulations therefor. The invention also relates to neuropathic, polyneuropathic, neurologic and neurogenic ailments typically characterized by paresis / paralysis. These ailments can involve an abnormal concentration of one or more endogenous opioids, or the blockade, underexpression or overexpression of one or more opioid receptors. In that regard, the invention encompasses therapeutic uses, methods and compositions employing opiates and / or their receptors. In particular, the invention relates to certain laboratory testing methods, clinical testing methods, research and development methods, business methods, methods of treatment, novel therapeutic uses, and human and veterinary pharmaceutical dosage forms, dosing regimens and formulations, especially those pertaining to opiopathy (particularly hypo-opiopathy).
Owner:BROOKS KORN HOWARD
Opiopathies
The present invention provides novel methods for classifying, diagnosing and / or treating a group of human and veterinary ailments involving endogenous opioid concentrations. Also provided is a novel use for an existing class of compounds, the opioids, to treat opiopathic ailments, particularly paresis / paralysis, pseudo-atrophy and / or opiopathic pain, and in the manufacture of pharmaceutical and veterinary formulations therefor. The invention also relates to neuropathic, polyneuropathic, neurologic and neurogenic ailments typically characterized by paresis / paralysis. These ailments can involve an abnormal concentration of one or more endogenous opioids, or the blockade, underexpression or overexpression of one or more opioid receptors. In that regard, the invention encompasses therapeutic uses, methods and compositions employing opiates and / or their receptors. In particular, the invention relates to certain laboratory testing methods, clinical testing methods, research and development methods, business methods, methods of treatment, novel therapeutic uses, and human and veterinary pharmaceutical dosage forms, dosing regimens and formulations, especially those pertaining to opiopathy (particularly hypo-opiopathy).
Owner:BROOKS KORN HOWARD
Pharmaceutical composition for treating livestock diarrhea and preparation method thereof
ActiveCN103893272AImprove antibacterial propertiesLower resistanceAntibacterial agentsClimate change adaptationMedicineVeterinary pharmaceuticals
The invention discloses a pharmaceutical composition for treating livestock diarrhea and a preparation method thereof, and belongs to the field of veterinary medicines. The method is characterized by comprising the following steps: proportioning sowthistle tasselflower herb, scandent hop, daghestan sweetclover herb, salvia chinensis, herba pteridis multifidae and hairy euphorbia according to a certain ratio; extracting and refining; adding auxiliary materials of the preparation to prepare corresponding preparation according to the conventional method. The pharmaceutical composition disclosed by the invention has a good effect when being applied to treatment of the livestock diarrhea.
Owner:QINGDAO AGRI UNIV
Carbohydrate crosslinked glycoprotein crystals
InactiveUS7087728B2Reduce controlled dissolution propertyIncreased insolubilityPeptide/protein ingredientsHydrolasesPersonal careBinding site
The present invention relates to the field of carbohydrate crosslinked glycoprotein crystals. Advantageously, such crosslinked glycoprotein crystals display stability to harsh environmental conditions, while maintaining the structural and functional integrity of the glycoprotein backbone. According to one embodiment, this invention relates to methods for concentrating proteins that have been modified by carbohydrates and for releasing their activity at controlled rates. This invention also provides methods for producing carbohydrate crosslinked glycoprotein crystals and methods for using them in pharmaceutical formulations, vaccines, immunotherapeutics, personal care compositions, including cosmetics, veterinary pharmaceutical compositions and vaccines, foods, feeds, diagnostics, cleaning agents, including detergents and decontamination formulations. The physical and chemical characteristics of carbohydrate crosslinked glycoprotein crystals render them particularly useful as sorbents for separations, such as chiral chromatography, or affinity chromatography—which are based on specific interactions between the active binding site of the glycoprotein component of the crystals and the substance or molecule of interest. Such characteristics also render carbohydrate crosslinked glycoprotein crystals useful as catalytic and binding components for the production of biosensing devices.
Owner:ALTHEA TECH
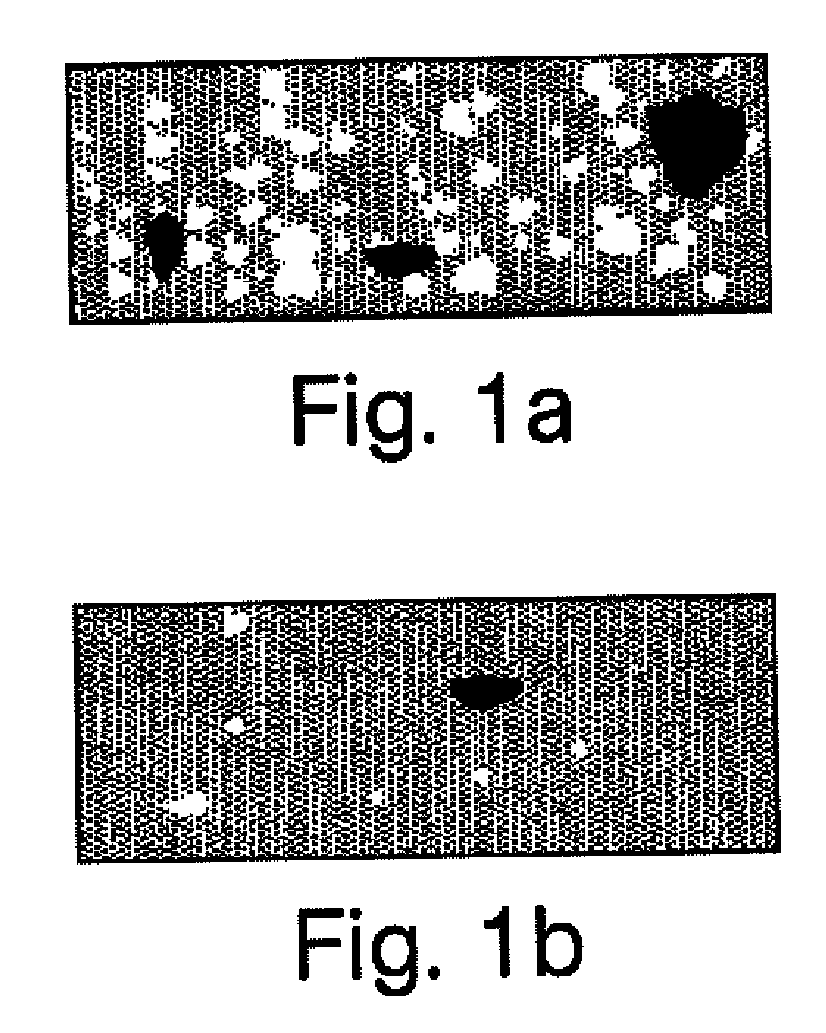
Compositions and kits for compounding pharmaceuticals
The invention provides compositions and methods for the convenient compounding of pharmaceuticals. Single and multiple unit of use kits are provided which contain all the necessary components required for preparing a compounded pharmaceutical. The kits of the invention include a first container having a first active agent and a second container having at least one second inactive agent. The kits of the invention are also useful for compounding veterinary pharmaceuticals.
Owner:CUTISPHARMA
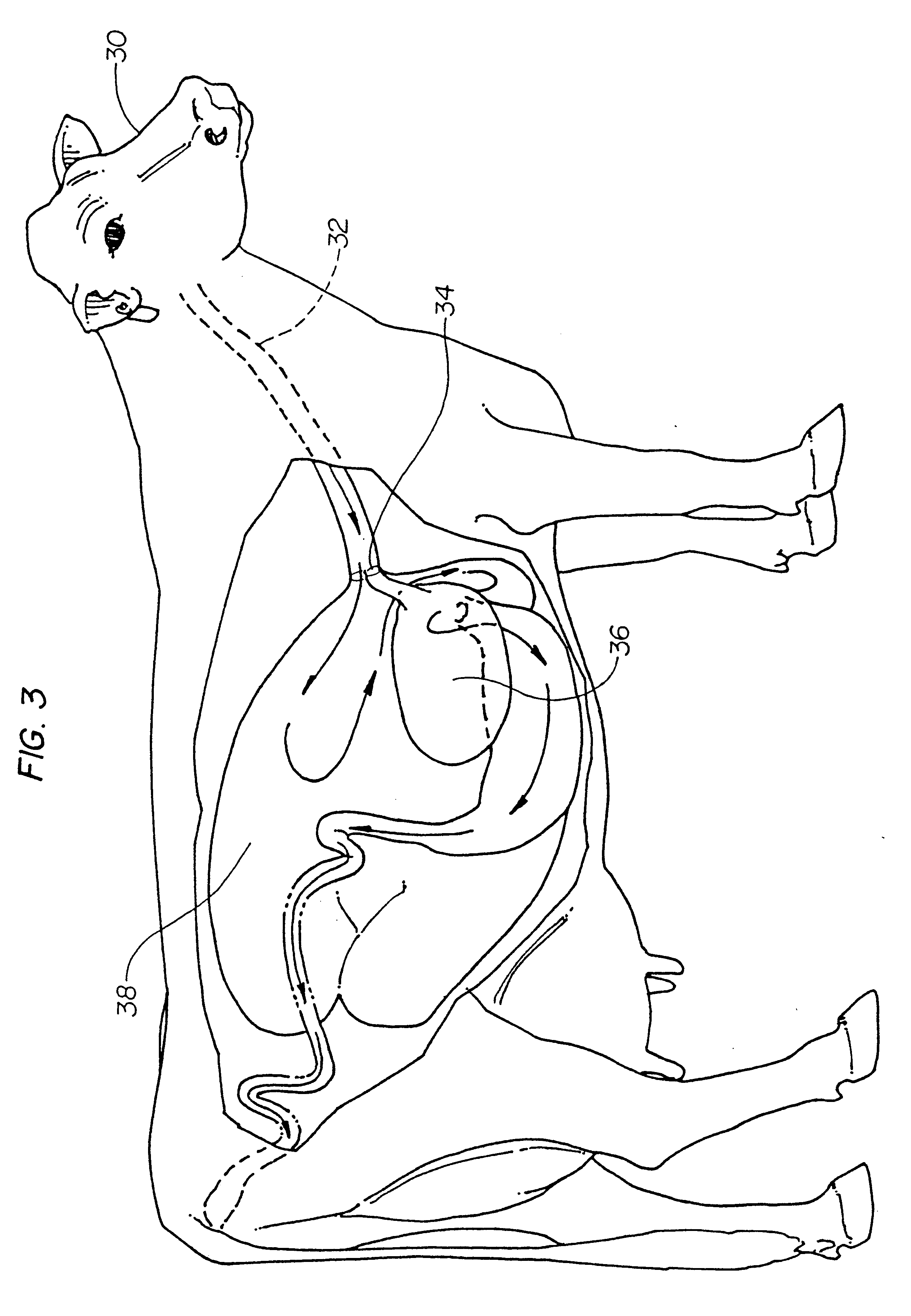
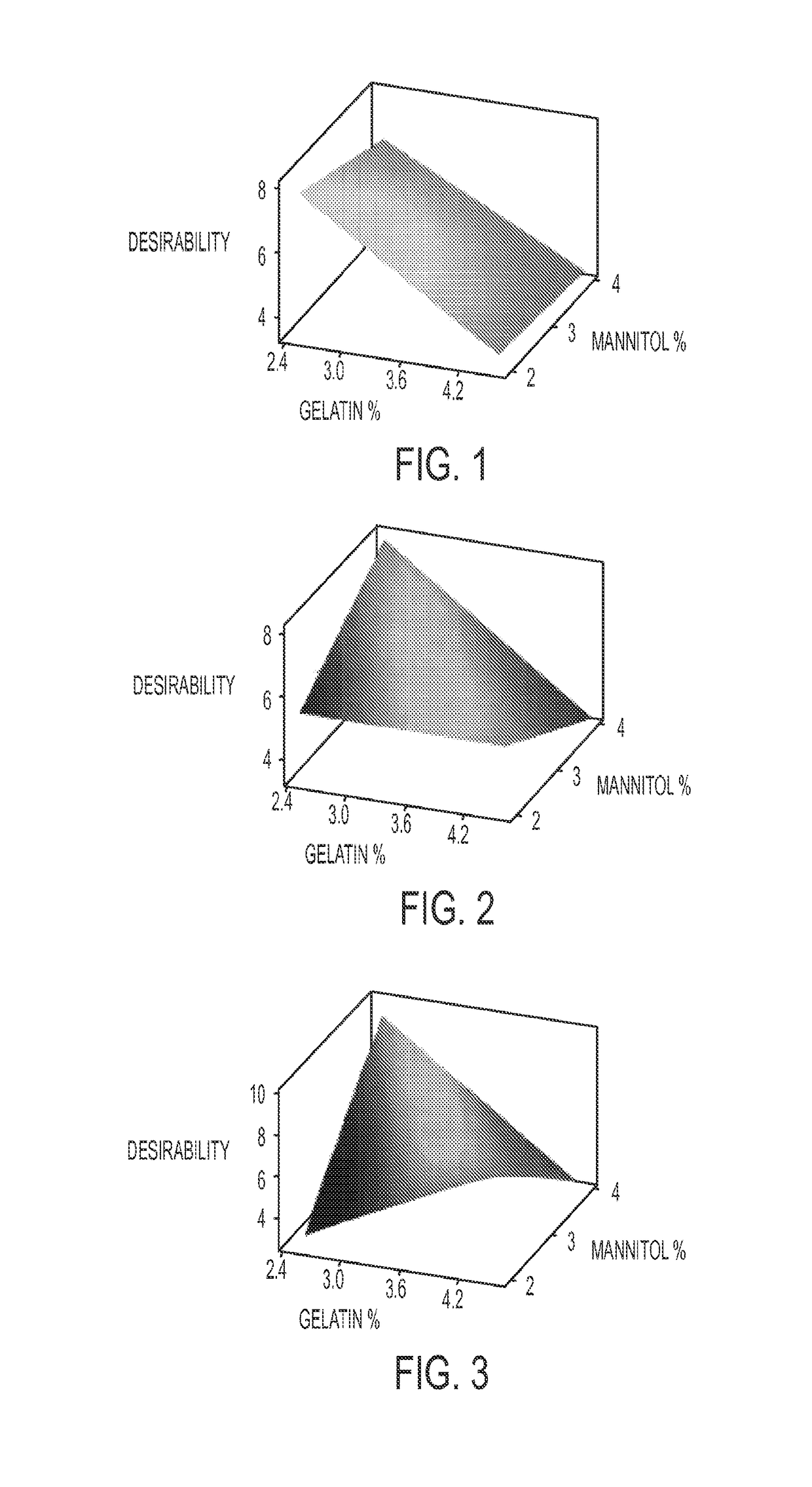
Pharmaceutical compositions for direct systemic introduction
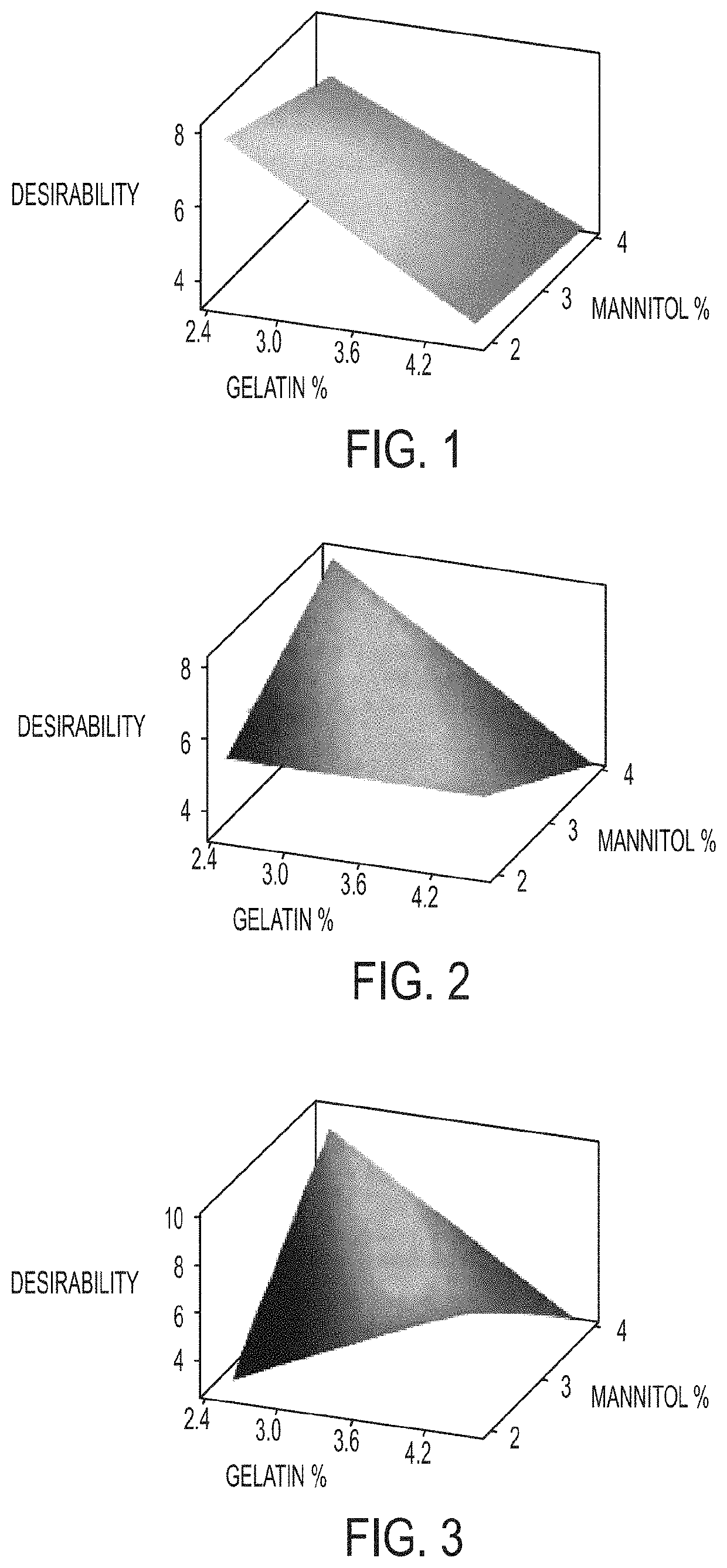
The invention relates to pharmaceutical compositions for direct systemic introduction, are also known as DSI pharmaceutical compositions, for used as human veterinary pharmaceutical compositions. In one embodiment, the invention relates to a pharmaceutical composition for direct system introduction comprising bovine gelatin, mannitol, an optional surfactant, an optional flavorant, and an active pharmaceutical ingredient. A DSI pharmaceutical composition of the invention has a disintegration time of 7 seconds or less in deionized water maintained at 37.0° C.±0.5° C. The invention also relates to a method of delivering an active pharmaceutical ingredient to an animal comprising the step of placing a DSI pharmaceutical composition of the invention into a mucosal cavity of an animal to be treated with the active pharmaceutical ingredient and to the corresponding methods of treatment.
Owner:RIDALL MARK
Pharmaceutical compositions for direct systemic introduction
The invention relates to pharmaceutical compositions for direct systemic introduction, are also known as DSI pharmaceutical compositions, for used as human veterinary pharmaceutical compositions. In one embodiment, the invention relates to a pharmaceutical composition for direct system introduction comprising bovine gelatin, mannitol, an optional surfactant, an optional flavorant, and an active pharmaceutical ingredient. A DSI pharmaceutical composition of the invention has a disintegration time of 7 seconds or less in deionized water maintained at 37.0° C.±0.5° C. The invention also relates to a method of delivering an active pharmaceutical ingredient to an animal comprising the step of placing a DSI pharmaceutical composition of the invention into a mucosal cavity of an animal to be treated with the active pharmaceutical ingredient and to the corresponding methods of treatment.
Owner:RIDALL MARK
Method for improving the aqueous solubility of poorly-soluble substances
ActiveUS9023398B2High cost performanceIncrease productivityPowder deliveryBiocideCalcium biphosphateSubstance use
Provided is a method for increasing the solubility of a poorly-soluble substance used in pharmaceutical products, veterinary pharmaceutical products, quasi-drugs, cosmetic products, food products, agricultural chemicals, and the like, without using large amounts of additives. This is a method for improving aqueous solubility, which comprises coating the surface of the particle of a poorly-soluble substance used in pharmaceutical products, veterinary pharmaceutical products, quasi-drugs, cosmetic products, food products, agricultural chemicals, and the like, with microparticles of a calcium compound such as calcium phosphate or calcium carbonate.
Owner:SANGI CO LTD
Compositions and kits for compounding pharmaceuticals
InactiveUS20090048349A1Dispersion deliveryNitro compound active ingredientsActive agentVeterinary pharmaceuticals
The invention provides compositions and methods for the convenient compounding of pharmaceuticals. Single and multiple unit of use kits are provided which contain all the necessary components required for preparing a compounded pharmaceutical. The kits of the invention include a first container having a first active agent and a second container having at least one second inactive agent. The kits of the invention are also useful for compounding veterinary pharmaceuticals.
Owner:CUTISPHARMA
Compositions comprising dehydrated micro-organisms, method for preparing same, and uses thereof
ActiveUS9259448B2Certain timeCertain stabilityVitamin food ingredientsYeast food ingredientsBiotechnologyMicroorganism
The invention relates to a composition comprising revivable dehydrated micro-organisms. The invention is characterized in that it further comprises particles at least 50% of which have a mean diameter greater than 250 μm. The invention is applicable, in particular, to human or veterinary pharmaceuticals, to dietetics or to food products.
Owner:VIRB AC SA
Pharmaceutical compositions for direct systemic introduction
The invention relates to pharmaceutical compositions for direct systemic introduction, are also known as DSI pharmaceutical compositions, for used as human veterinary pharmaceutical compositions. In one embodiment, the invention relates to a pharmaceutical composition for direct system introduction comprising bovine gelatin, mannitol, an optional surfactant, an optional flavorant, and an active pharmaceutical ingredient. A DSI pharmaceutical composition of the invention has a disintegration time of 7 seconds or less in deionized water maintained at 37.0° C.±0.5° C. The invention also relates to a method of delivering an active pharmaceutical ingredient to an animal comprising the step of placing a DSI pharmaceutical composition of the invention into a mucosal cavity of an animal to be treated with the active pharmaceutical ingredient and to the corresponding methods of treatment.
Owner:NEW MARKET PHARMA
Chinese herbal medicine feed additive for preventing and curing colibacillosis
The invention belongs to the technical field of veterinary medicines, and in particular relates to a Chinese herbal medicine feed additive for preventing and curing colibacillosis. The feed additive mainly comprises the following components in parts by weight: 10-20 parts of coptis, 10-20 parts of phellodendron bark, 15-20 parts of dandelion, 15-20 parts of red paeonia and 10-20 parts of cortex moutan radicis. The Chinese herbal medicine feed additive for preventing and curing colibacillosis disclosed by the invention has a prominent effect on preventing and curing colibacillosis, and is also effective to infection caused by gramnegativebacteria and diarrhoea caused by multiple reasons, and furthermore, the Chinese herbal medicine feed additive has antiviral efficacy, and can effectively avoid the problems of anti-milk and medicine residue and foodstuff safety.
Owner:JILIN UNIV
Oral liquid vaccine for preventing and controlling avian influenza of domestic animals and domestic birds and method of preparing the same
InactiveCN101279028AImprove immunityPrevent intrusionAntiviralsSolution deliveryInfection rateAloe arborescens
The invention relates to a veterinary pharmaceutical composition and a preparation method thereof. The veterinary pharmaceutical composition of the invention is a vaccine oral solution for the prevention and the treatment of bird flu of domestic animal and fowl, and is prepared by the raw materials according to the following parts by weight: 10 to 20 parts of forsythia suspensa, 10 to 25 parts of asarum, 5 to 20 parts of ilex pubescens, 15 to 30 parts of rhizoma anemarrhenae, 8 to 20 parts of wild chrysanthemum, 10 to 20 parts of polygalae, 10 to 25 parts of lobelia chinensis, 2 to 5 parts of snake galls, 10 to 25 parts of ox to gall, 40 to 60 parts of aloe, 25 to 40 parts of rock sugar, 5 to 15 parts of rimantadine and 3 to 10 parts of azithromycin. The oral solution of the invention can be directly used as daily feed for feeding domestic animals and fowl, which can effectively inhibit bird flu virus, reduce the infection rate of respiratory tract and lung and prevent domestic animal and fowl from being infected by bird flu.
Owner:辽宁天鹏生物科技股份有限公司
Veterinary pharmaceutical composition and method (alternatives) for the prophylaxis and treatment of diseases of the gastrointestinal tract and intoxications of diverse etiology in animals
InactiveUS20120196811A1Effective pharmaceuticalGood curative effectDigestive systemAnimal feeding stuffEtiologyDisease
A veterinary pharmaceutical composition for treating diseases of the gastrointestinal tract and intoxications of diverse etiology in animals comprises, as the active component, hydrolyzed lignin and a prebiotic from the following group: lactulose, fructooligo-saccharides, galacto-saccharides and inulin, and is in a form suitable for use in the composition of a mixture with combined feed, with a lignin content of from 30 to 95% by mass, in the form of powder of granules. The method for the prophylaxis and treatment of intoxication of diverse etiology, including mycotoxicoses, in animals, including birds, envisages that the animal will receive the above-mentioned veterinary pharmaceutical composition mixed with feed in a dosage of from 2 to 4.5 kg of the preparation per ton of combined feed, proportional to the content of mycotoxins in the combined feed. The method for the prophylaxis and treatment of diseases of the gastrointestinal tract, including dyspepsia, gastroenteritis, enteritis, colitis, hepatitis, and hepato-dystrophy, including in a toxic form, in animals, including cattle and pigs, envisages that the animal will receive the veterinary pharmaceutical composition according to claim 1 30±10 minutes prior to feeding, perorally individually or in a group method with water for watering or with the feed, 1 to 2 times per day in a dosage of from 0.2 to 0.3 g / kg of the mass of the animal. This ensures an increase in the effectiveness of the treatment and elimination of the above-mentioned defects in the use of hydrolyzed lignin, activated carbon and other generally known sorbents. At the same time, the joint use of lignin with a prebiotic as a means for the prophylaxis and treatment of animals makes it possible to produce a result in the form of a set of positive effects: minimization of side effects from the use of a sorbent; restoration of homeostasis in the gastrointestinal tract; and normalization of the metabolism indices, this finally resulting in a qualitative increase in the zootechnical and economic indices of animal husbandry.
Owner:ALEKSANDER VLADIMIROVICH DIKOVSKIY
Method for improving the aqueous solubility of poorly-soluble substances
ActiveCN102573912AImprove securityProductivity advantagePowder deliveryBiocideSolubilityCalcium biphosphate
Provided is a method for improving the solubility of poorly-soluble substances used in pharmaceuticals, veterinary pharmaceuticals, quasi-drugs, cosmetics, food products, agricultural chemicals, and the like, without using large amounts of additives. The provided method is characterized in that fine particles of a calcium compound such as calcium phosphate or calcium carbonate are used to cover the surfaces of particles of a poorly-soluble substance used in pharmaceuticals, veterinary pharmaceuticals, quasi-drugs, cosmetics, food products, agricultural chemicals, or the like.
Owner:SANGI CO LTD
Pharmaceutical compositions for direct systemic introduction
The invention relates to pharmaceutical compositions for direct systemic introduction, are also known as DSI pharmaceutical compositions, for used as human veterinary pharmaceutical compositions. In one embodiment, the invention relates to a pharmaceutical composition for direct system introduction comprising bovine gelatin, mannitol, an optional surfactant, an optional flavorant, and an active pharmaceutical ingredient. A DSI pharmaceutical composition of the invention has a disintegration time of 7 seconds or less in deionized water maintained at 37.0° C.±0.5° C. The invention also relates to a method of delivering an active pharmaceutical ingredient to an animal comprising the step of placing a DSI pharmaceutical composition of the invention into a mucosal cavity of an animal to be treated with the active pharmaceutical ingredient and to the corresponding methods of treatment.
Owner:NEW MARKET PHARMA
Compound coccidiostat pharmaceutical formulation
InactiveCN101129390ASolve solubilityFix stability issuesOrganic active ingredientsPharmaceutical delivery mechanismQuinoxalineAntioxidant
The invention discloses an animal medicine agent of compound coccidiostat, which comprises the following parts: 5-20%(W / V) promazine hydrochloride, 0. 25-1% (W / V) ethoxy amide benzenemethyl ester and 3-12%(W / V) sulfanilamide quinoxaline sodium, wherein the solvent, solutizer and necessary anti-oxidizing agent and pH value adjuster are added into the raw materials to produce the product with good anti-caecum coccidian effect. The invention is easy to dissolve in the water to solve the drug giving problem that the ill poultry is provided with water without fodder, which is convenient to spread with good spreading and using value.
Owner:青岛康地恩实业有限公司
Carbohydrate crosslinked glycoprotein crystals
InactiveUS20030170843A1Improve sealingCrystal fineHydrolasesWood working apparatusPersonal careImmunotherapeutic agent
The present invention relates to the field of carbohydrate crosslinked glycoprotein crystals. Advantageously, such crosslinked glycoprotein crystals display stability to harsh environmental conditions, while maintaining the structural and functional integrity of the glycoprotein backbone. According to one embodiment, this invention relates to methods for concentrating proteins that have been modified by carbohydrates and for releasing their activity at controlled rates. This invention also provides methods for producing carbohydrate crosslinked glycoprotein crystals and methods for using them in pharmaceutical formulations, vaccines, immunotherapeutics, personal care compositions, including cosmetics, veterinary pharmaceutical compositions and vaccines, foods, feeds, diagnostics, cleaning agents, including detergents and decontamination formulations. The physical and chemical characteristics of carbohydrate crosslinked glycoprotein crystals render them particularly useful as sorbents for separations, such as chiral chromatography, or affinity chromatography--which are based on specific interactions between the active binding site of the glycoprotein component of the crystals and the substance or molecule of interest. Such characteristics also render carbohydrate crosslinked glycoprotein crystals useful as catalytic and binding components for the production of biosensing devices.
Owner:ALTHEA TECH
Traditional Chinese medicine decoction liquid for treating swine erysipelas and preparation method thereof
InactiveCN105582066AGood control effectQuality improvementAntibacterial agentsPlant ingredientsSide effectSwine Erysipelas
The invention relates to traditional Chinese medicine decoction liquid for treating swine erysipelas and a preparation method thereof. The traditional Chinese medicine decoction liquid is prepared from the following ingredients in parts by weight: 10 to 15 parts of honeysuckle flowers, 10 to 15 parts of fructus forsythiae, 5 to 10 parts of dyer woad leaves, 10 to 15 parts of radix isatidis, 10 to 15 parts of radix scutellariae, 5 to 10 parts of dandelion, 5 to 10 parts of radix et rhizoma rhei, 5 to 10 parts of liquorice roots and 8 to 12 parts of radix bupleuri. The traditional Chinese medicine decoction liquid has the advantages that the efficacies of achieving antibacterial and anti-inflammation functions and clearing away heat and toxic materials are achieved; no medicine residue exists; no toxic and side effects exist; medicine resistance cannot be easily generated; the safety is high; the use is convenient; the absorption is easy; the requirements of safe animal remedy and animal-derived food safety guaranteeing are met; and the traditional Chinese medicine decoction liquid belongs to ideal traditional Chinese medicine decoction liquid for treating the swine erysipelas.
Owner:TIANJIN ZHONGAO FEED
Baicalin-aluminum dry suspension for treating pigling diarrhoea
ActiveCN106667915AAvoid Manufacturing ComplexityReduce manufacturing costAntibacterial agentsPowder deliverySide effectFiller Excipient
The invention provides a baicalin-aluminum dry suspension for treating pigling diarrhoea. Baicalin as an active component is extracted from radix scutellariae and is processed to form an aluminum salt, and the aluminum salt and reasonable auxiliary materials are mixed to form a veterinary pharmaceutical preparation. The baicalin-aluminum dry suspension comprises, by weight, 200-500 parts of an aluminum salt active component containing 50% of baicalin-aluminum, 2-10 parts of a suspending aid, 20-100 parts of a corrigent, and 390-750 parts of a filler. The baicalin-aluminum dry suspension is suitable for pigling before and after weaning, has remarkable effects, can replace the same kind of antibiotics, prevents resistance to drugs, prevents toxic or side effects caused by antibiotics and antibiotic residues and is beneficial to animal and human health.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Compositions and kits for compounding pharmaceuticals
The invention provides compositions and methods for the convenient compounding of pharmaceuticals. Single and multiple unit of use kits are provided which contain all the necessary components required for preparing a compounded pharmaceutical. The kits of the invention include a first container having a first active agent and a second container having at least one second inactive agent. The kits of the invention are also useful for compounding veterinary pharmaceuticals.
Owner:CUTISPHARMA
Veterinary use radix isatidis and dyers woad leaf granule and preparation method thereof
InactiveCN108186706ASimple materialImprove efficacyAntibacterial agentsOrganic active ingredientsSaccharumSucrose
The invention discloses a veterinary use radix isatidis and dyers woad leaf granule and a preparation method thereof, and belongs to the field of veterinary medicines. The veterinary use radix isatidis and dyers woad leaf granule is characterized by being prepared from the following raw materials including 500 to 800g of radix isatidis, 800 to 1000g of dyers woad leaves, 500 to 800g of radix bupleuri and 10 to 30g of aloe polysaccharide. The preparation method comprises the following steps of weighing the radix isatidis, the dyers woad leaves and the radix bupleuri; adding water for decorationand boiling for 2 to 4 times, wherein each time lasts 0.5 to 2h; merging decoration liquid; performing filtering; taking filter liquid to obtain a first solution; adding the aloe polysaccharide intothe first solution; performing concentration to obtain a second solution; performing precipitation treatment on the second solution; separating precipitates and supernate; concentrating the supernateto the thick paste state to obtain an extract; adding cane sugar and dextrin into the extract; after the uniform mixing, performing granulation and drying to obtain the veterinary use radix isatidis and dyers woad leaf granule. The veterinary use radix isatidis and dyers woad leaf granule has the advantages that the medication is simple and effective; the effects of clearing away heat and toxic materials and cooling the blood are achieved; the veterinary use radix isatidis and dyers woad leaf granule can be used for treating warm and heat diseases such as wind-heat type common cold, swollen sore throat and fever with eruption. Compared with the existing radix isatidis and dyers woad leaf granule, the veterinary use radix isatidis and dyers woad leaf granule provided by the invention has the advantages that the medicine effect is high; the effect taking speed is high.
Owner:山东信合生物制药有限公司
Chinese herbal medicine feed additive for preventing and curing colibacillosis
ActiveCN103202908AAntibacterial agentsAnimal feeding stuffChrysanthemum FlowerVeterinary pharmaceuticals
The invention belongs to the technical field of veterinary medicines, and in particular relates to a Chinese herbal medicine feed additive for preventing and curing colibacillosis. The feed additive mainly comprises the following components in parts by weight: 10-20 parts of coptis, 10-20 parts of phellodendron bark, 15-20 parts of wild chrysanthemum flower and 15-20 parts of cortex moutan radicis. The Chinese herbal medicine feed additive for preventing and curing colibacillosis disclosed by the invention has a prominent effect on preventing and curing colibacillosis, and is also effective to infection caused by gramnegativebacteria and diarrhoea caused by multiple reasons, and furthermore, the Chinese herbal medicine feed additive has antiviral efficacy, and can effectively avoid the problems of milk resistance and medicine residue and foodstuff safety.
Owner:广西南宁市桃源兽药厂
Unilateral mequindox injection and preparation method thereof
ActiveCN110893170AGuarantee product qualityAntibacterial agentsOrganic active ingredientsSodium salicylateVeterinary pharmaceuticals
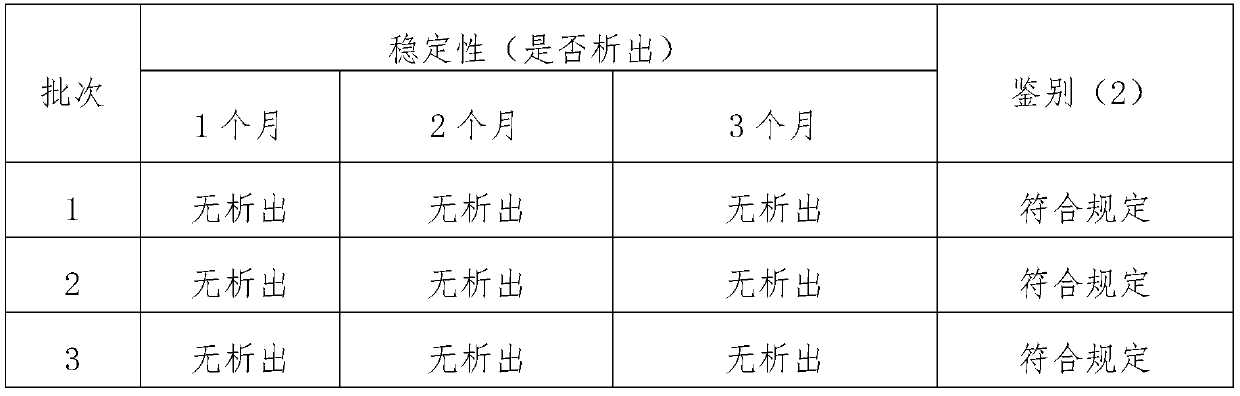
The present invention relates to the technical field of veterinary pharmaceuticals, and specifically relates to an unilateral mequindox injection and a preparation method thereof. The unilateral mequindox injection comprises the following feed compositions in mass concentrations: mequindox 18-22g / L; ethanol 180-220g / L; propylene glycol 90-110g / L; alpha-pyrrolidone 280-320g / L; and water for injection as a solvent. Compared to the prior art, sodium salicylate is removed from production process of the unilateral mequindox injection in the present invention, so as to obtain an injection product with stability comparable to an original injection, and maximum absorption can be detected at 242, 254 and 371 nm of wavelength, thereby ensuring quality of the product and meeting provisions in mequindox injection identification (2) section in Quality Standards for Veterinary Medicines, 2017 Edition, Chemicals Volume.
Owner:BEIJING LISHIDA PHARMA
Eupatorium clenbuterol preparation capable of preventing and treating respiratory diseases of livestock and poultry and production technique thereof
InactiveCN102172386AExtract completelyReduce lossesPowder deliveryClimate change adaptationDiseaseRespiratory disease
The invention relates to the field of a veterinary pharmaceutical and a feed additive, particularly relates to a production method of eupatorium (lindley eupatorium) clenbuterol powder and a granular formulation for preventing and treating respiratory diseases, namely a preparation method of eupatorium clenbuterol powder and the granular formulation. In the method, five Chinese medicinal crops, such as eupatorium and the like or decoction pieces and the like are taken as raw materials. The preparation method comprises the following steps: cutting the Chinese medicinal crops, such as eupatorium and the like into pieces; adding water; carrying out backflow extract; carrying out concentration, membrane separation or ordinary pressure concentration to obtain a concentrated solution; carrying out alcohol precipitation on the concentration solution or adding flocculant for clarification, membrane separation or resin absorption to remove ineffective components; concentrating, drying, smashing, adding a flavoring agent, an excipient or auxiliary materials composed by the flavoring agent and the excipient; and packaging. In the invention, the advantages of traditional Chinese medicine combination and a carrier or filler are combined; the process is optimized through experiments; the effective components of the traditional Chinese medicine are extracted completely, and the process is simple and feasible, and has easily controlled process. The formed product has no harmful residues, and is a safe and hormone-free green product. The preparation method provided by the invention is especially suitable for preventing and treating the respiratory diseases of livestock and poultry, and simultaneously also suitable for preventing and treating the respiratory diseases of animals, such as various pets and the like.
Owner:方希修 +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com