Process for preparing Torasemide intermediate and its analogue
A compound and reaction technology, applied in the field of preparation of torasemide intermediates and analogs thereof, can solve the problems of low total yield, large distillation energy consumption, time-consuming and labor-intensive, etc., and achieves simple operation, high yield, high product good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
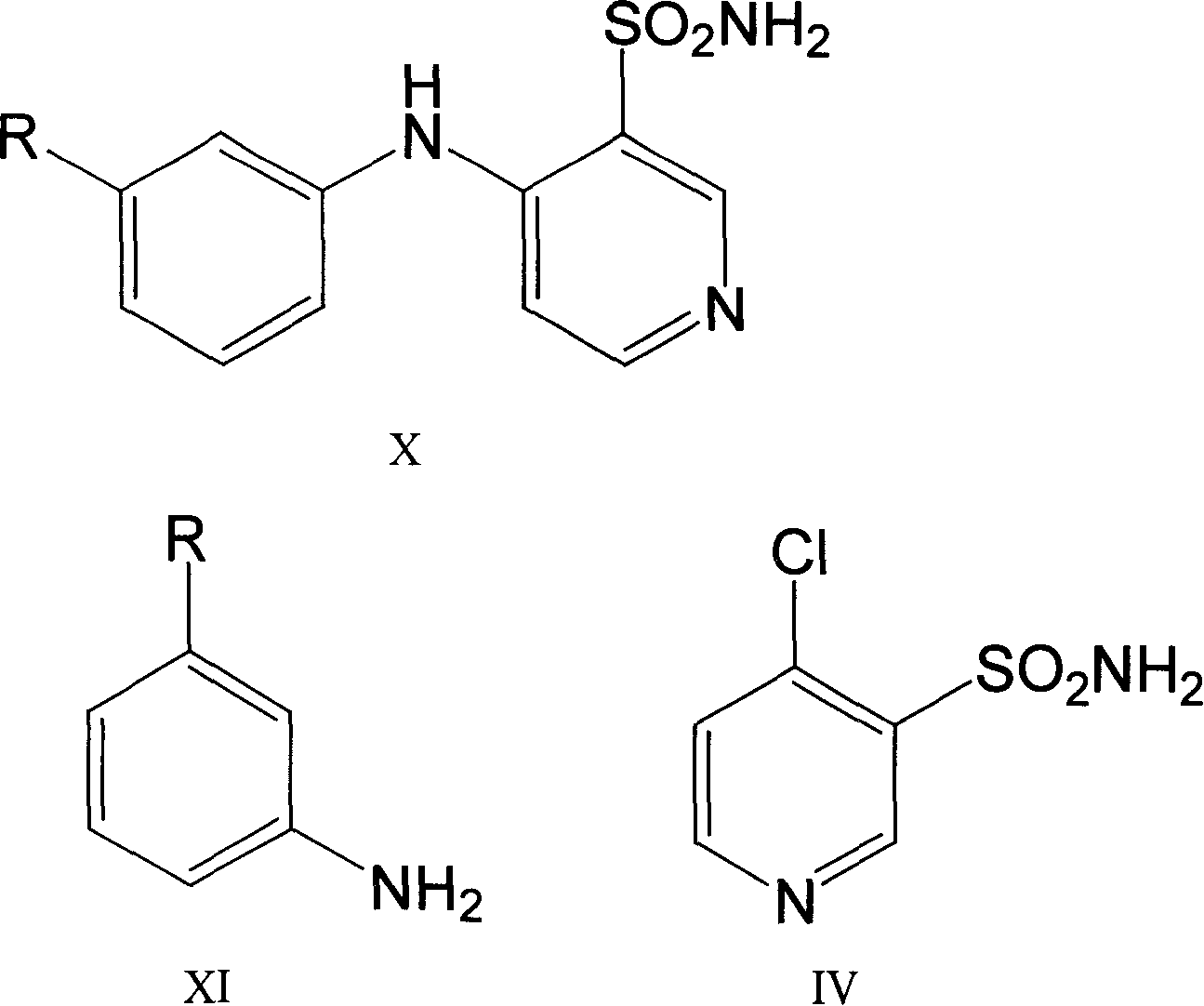
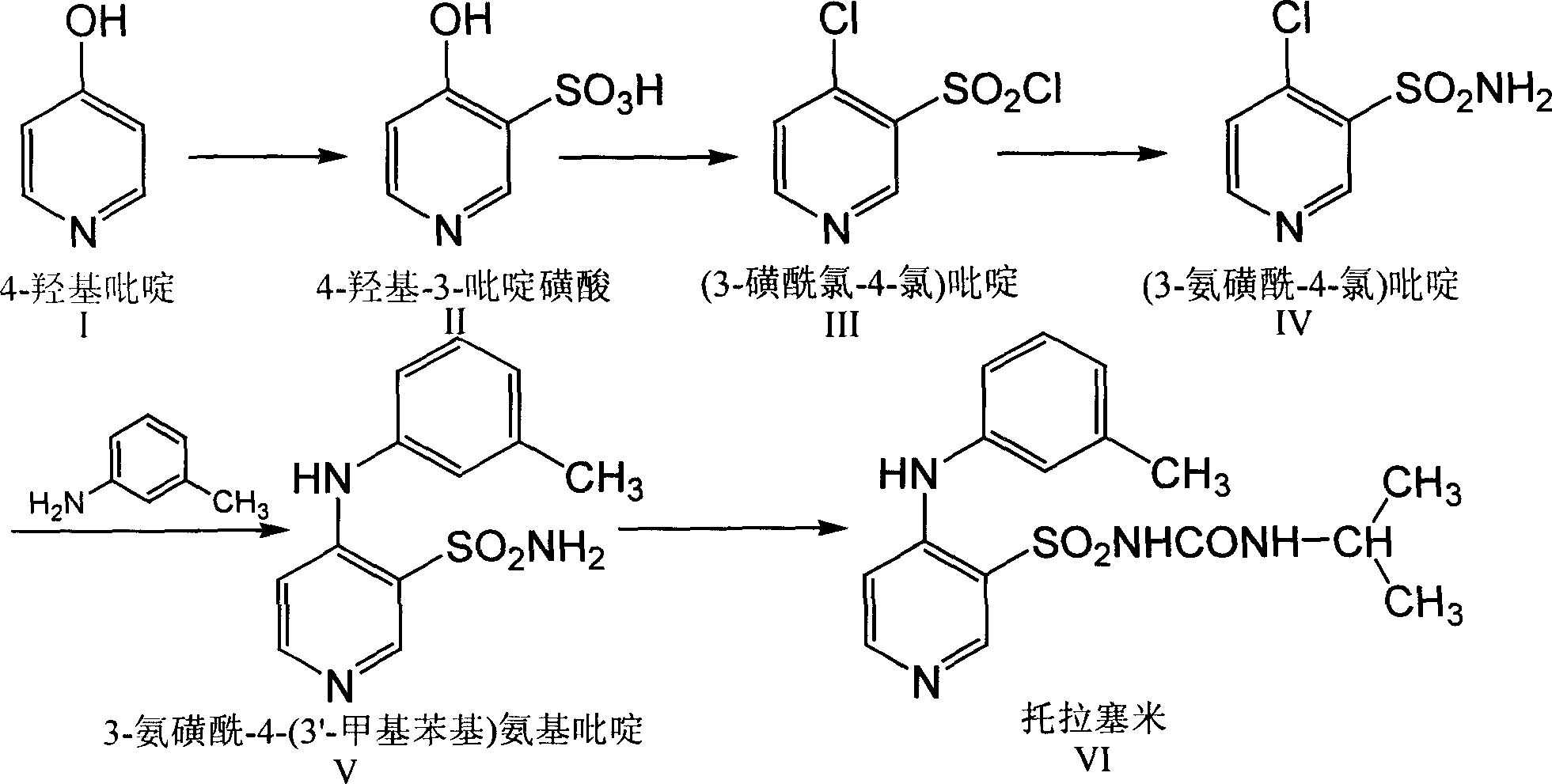
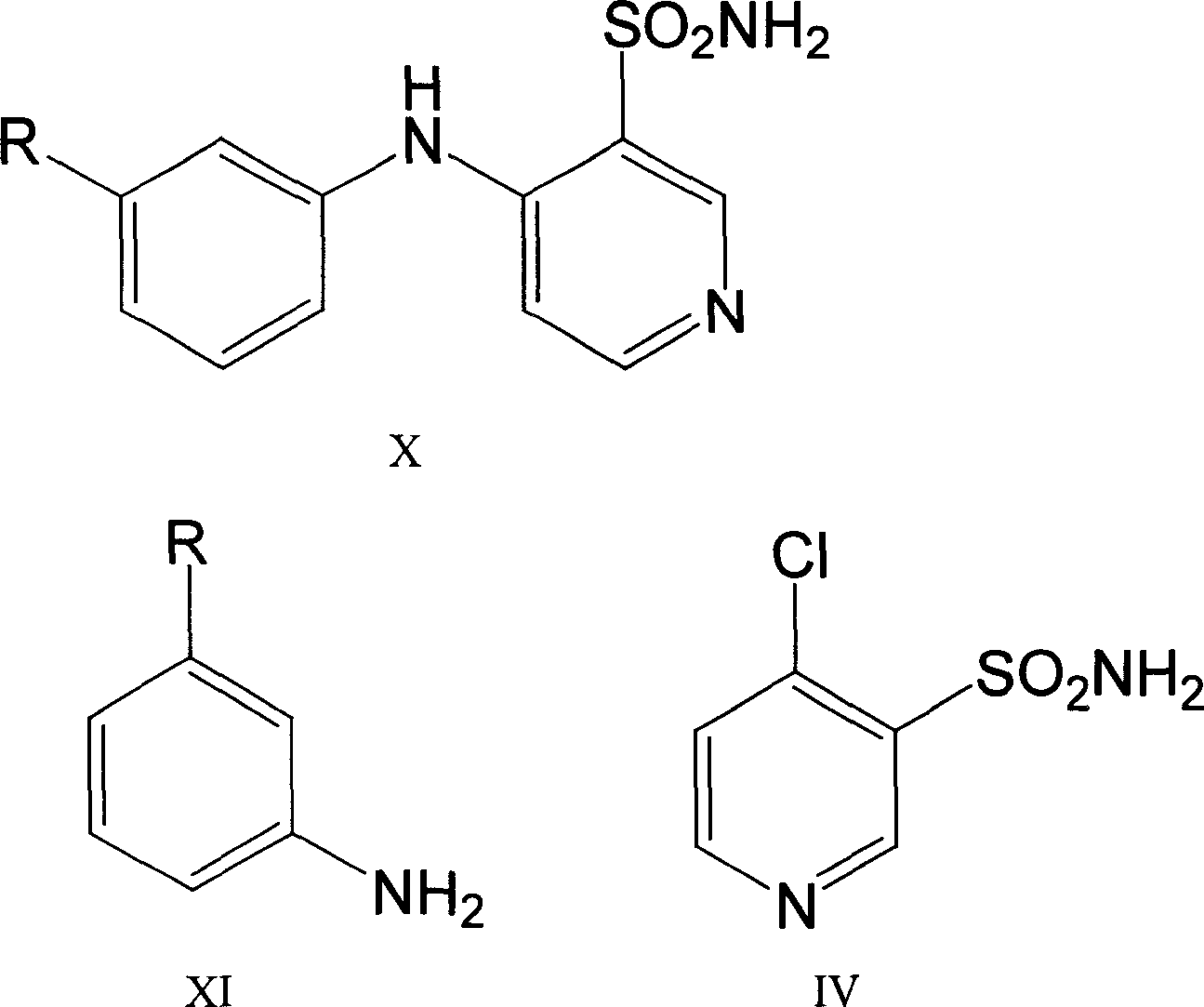
[0022] Synthesis of 3-sulfamoyl-4-(3'-methylphenyl)aminopyridine
[0023] In a 1000mL three-necked flask equipped with a mechanical stirrer, a condenser, and a thermometer, add (3-sulfamoyl-4-chloro)pyridine 20g (0.104mol), m-aminotoluene 27.8g (0.26mol) and n-butanol 400g, stir, heat and control the inner temperature to 95-105°C for 5h. The solvent was recovered under reduced pressure. While stirring, add water while hot. Cool down to 30°C and add 8% sodium hydroxide to dissolve it (pH12). Cool down to 20°C and extract the layers with 120g of isopropyl ether, and then extract the water layer with 60g x 2 times. The water layer was decolorized with activated carbon for 15 minutes and filtered, the filter cake was washed with a small amount of water, and the washed filtrates were combined, cooled to 5-10°C, adjusted to pH 7 with 10% hydrochloric acid solution, and stirred for 30 minutes. After filtering, the filter cake was washed three times with ice water, and dried at 80...
Embodiment 2
[0025] Synthesis of 3-sulfamoyl-4-(3'-methylphenyl)aminopyridine
[0026] In a 1000mL three-necked flask equipped with a mechanical stirrer, a condenser, and a thermometer, add (3-sulfamoyl-4-chloro)pyridine 20g (0.104mol), m-aminotoluene 27.8g (0.26mol) and N, 400 g of N-dimethylformamide was stirred, heated and controlled at an internal temperature of 95-105°C for 5 hours. The solvent was recovered under reduced pressure. While stirring, add water while hot. Cool down to 30°C and add 8% sodium hydroxide to dissolve it (pH12). Cool down to 20°C and extract the layers with 120g of isopropyl ether, and then extract the water layer with 60g x 2 times. The water layer was decolorized with activated carbon for 15 minutes and filtered, the filter cake was washed with a small amount of water, and the washed filtrates were combined, cooled to 5-10°C, adjusted to pH 7 with 10% hydrochloric acid solution, and stirred for 30 minutes. After filtering, the filter cake was washed three...
Embodiment 3
[0028] Synthesis of 3-sulfamoyl-4-(3'-trifluoromethylphenyl)aminopyridine
[0029] In a 1000mL three-neck flask equipped with a mechanical stirrer, a condenser, and a thermometer, add (3-sulfamoyl-4-chloro)pyridine 20g (0.104mol), m-aminotrifluorotoluene 41.9g (0.26mol) and DMSO 400g, stirred, heated and controlled internal temperature 95-105°C for 7h. The solvent was recovered under reduced pressure. While stirring, add water while hot. Cool down to 30°C and add 8% sodium hydroxide to dissolve it (pH12). Cool down to 20°C and extract the layers with 120g of isopropyl ether, and then extract the water layer with 60g x 2 times. The water layer was decolorized with activated carbon for 15 minutes and filtered, the filter cake was washed with a small amount of water, and the washed filtrates were combined, cooled to 5-10°C, adjusted to pH 7 with 10% hydrochloric acid solution, and stirred for 30 minutes. After filtering, the filter cake was washed three times with ice water, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com