Method for qualitatively and quantitatively detecting torasemide illegally added in food and application
A torasemide and detection method technology, applied in the detection field, can solve problems such as low sensitivity, false negative detection, inaccurate detection results, etc., and achieve the effects of simple and efficient operation, fast analysis speed, and improved sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
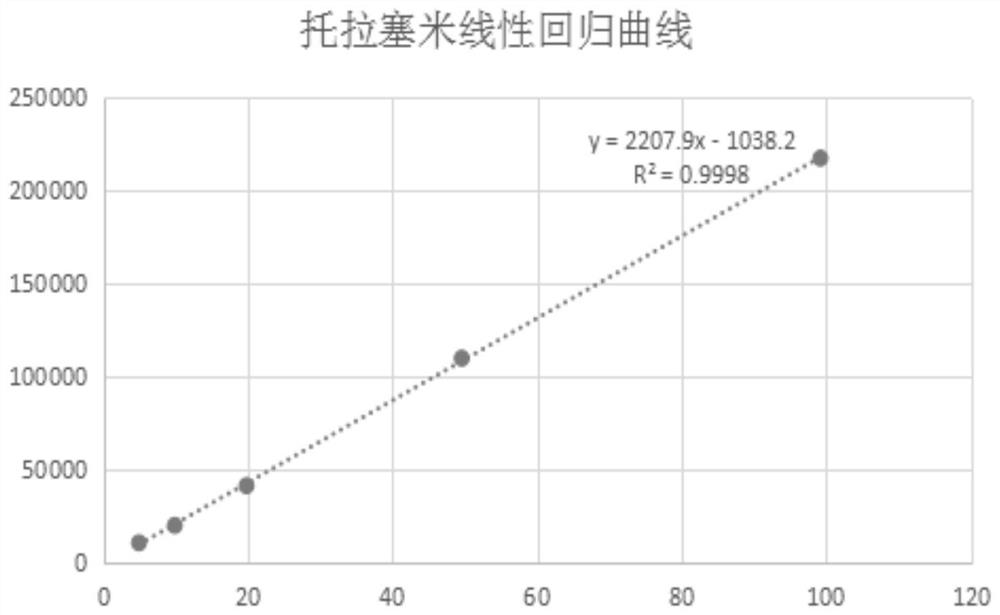
Image
Examples
Embodiment 1
[0050] Reagents used in this example: acetonitrile (CH 3 CN): chromatographically pure, ammonium acetate (CH 3 COONH 4 ): chromatographically pure, methanol (CH 3 OH): analytically pure, the water is the first-class water specified in GB / T 6682.
[0051] Preparation of 10mmol / L ammonium acetate aqueous solution: Weigh 1.54g of ammonium acetate, dilute with water to 2000mL, filter through a 0.45μm filter membrane and set aside.
[0052] The Chinese name, English name, CAS registration number, molecular formula, and relative molecular mass of the standard torasemide used are shown in Table 1. The purity of the standard is ≥95%.
[0053] Table 1 Torsemide standard substance information table
[0054]
[0055] The preparation of standard solution adopts the following method:
[0056] (1) Standard stock solution (100 μg / mL): Accurately weigh 10.0 mg of torasemide standard substance (accurate to 0.0001 g), place it in a 100 mL volumetric flask, dissolve it with methanol and ...
Embodiment 2
[0088] (1) Qualitative determination
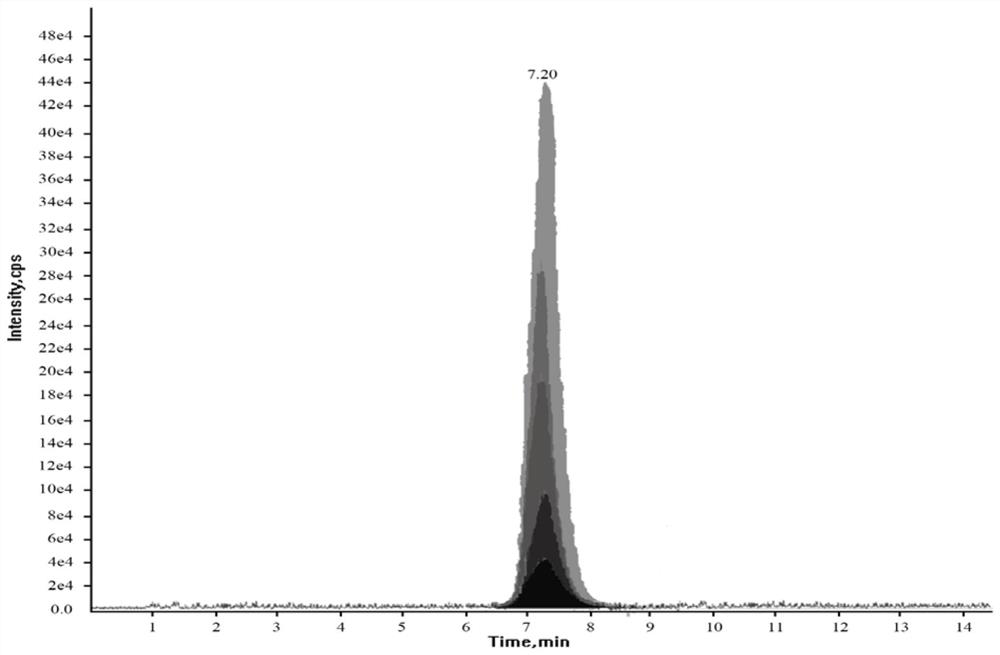
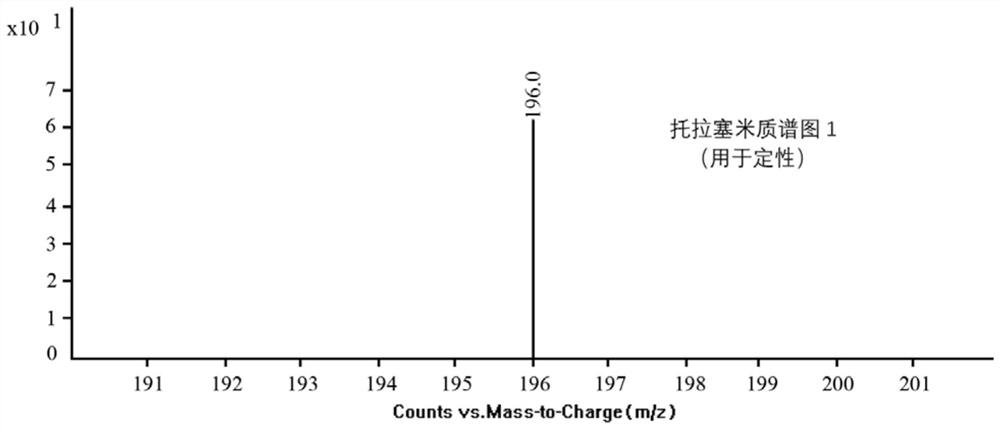
[0089] Determine the sample and standard series solutions according to the high-performance liquid chromatography-tandem mass spectrometry conditions, record the chromatographic retention time of each compound in the sample and standard series solutions, and use the percentage relative to the strongest ion abundance as the relative abundance of the qualitative ion pair , record the relative ion abundance of the corresponding components in the sample with the same concentration and the corresponding components in the standard series solution. When the chromatographic peak (variation range within ± 2.5%) consistent with the torasemide standard chromatographic peak retention time is detected in the sample, and the relative ion abundance tolerance does not exceed the scope specified in Table 3, the test can be determined. Torsemide was detected in the samples.
[0090] Table 3 The maximum allowable deviation of relative ion abundance in qual...
Embodiment 3
[0115] Embodiment 3 Sensitivity detection
[0116] Weigh 1g of blank solid sample (accurate to 0.0001g) or accurately measure 1mL of blank liquid sample, accurately add a certain concentration of reference substance solution into a 50mL measuring bottle, add 40mL of methanol, shake, ultrasonically extract for 30min, put Cool, add methanol to the scale, shake well, filter with a filter membrane (0.22 μm, organic phase type), take the subsequent filtrate, and analyze it by liquid chromatography-mass spectrometry. The signal-to-noise ratio is 3:1. The limit of detection was calculated based on the concentration of torasemide, and the limit of quantification was calculated based on the concentration of torsemide corresponding to the signal-to-noise ratio of 10:1. Detection was carried out according to the method in Example 2, and the quantification limit concentration obtained for direct detection of torasemide was 1.5 ng / mL, and the minimum detection limit concentration was 0.5 n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com