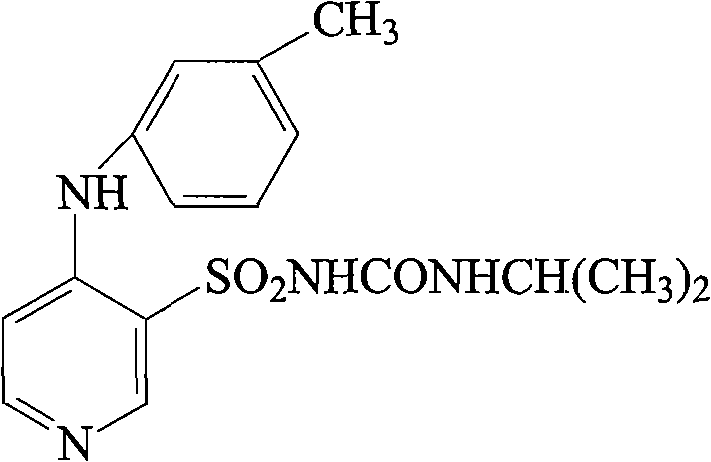
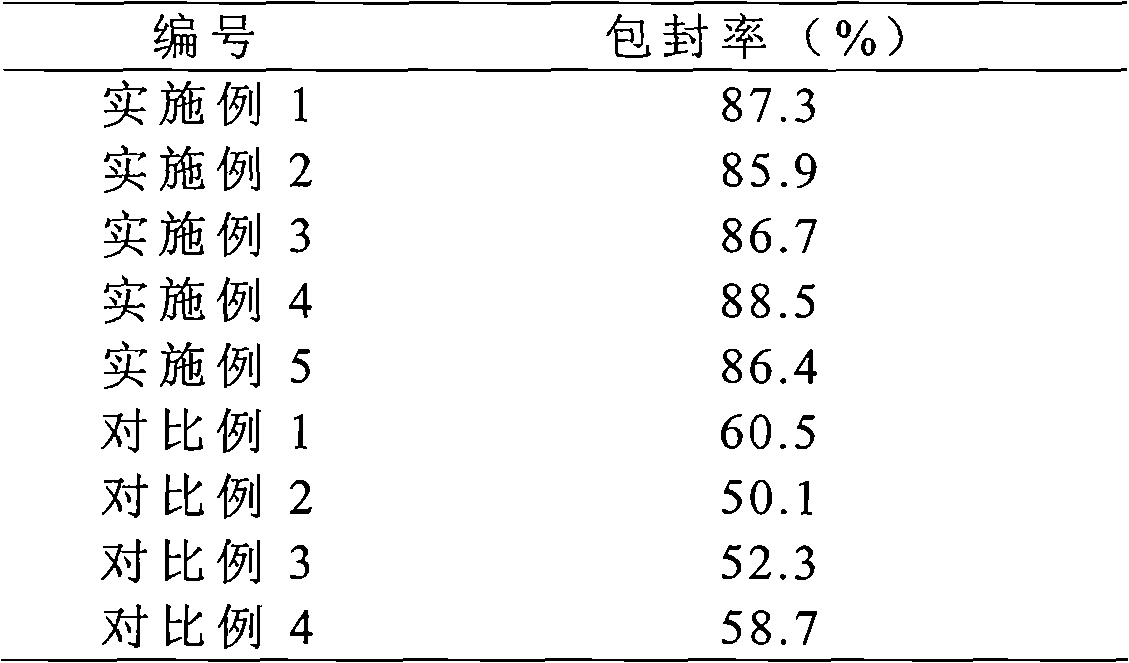
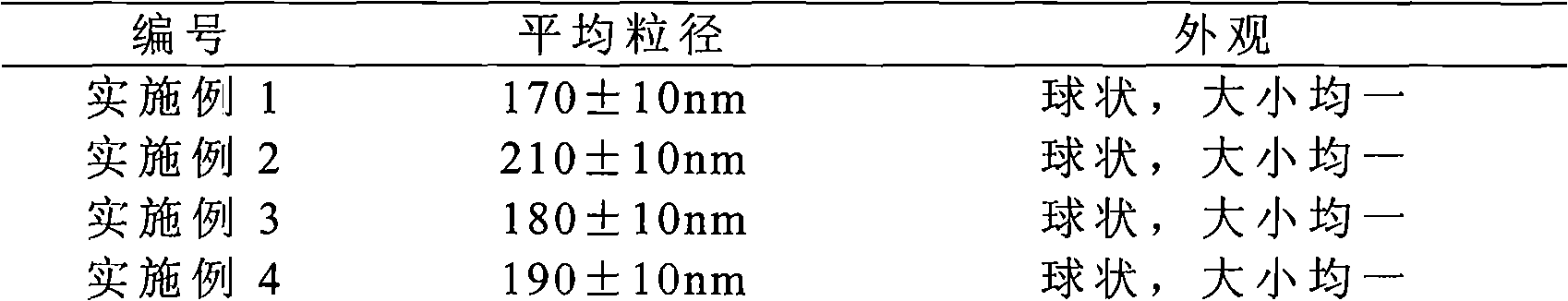
Solid preparation of Torasemide liposome
A technology of torasemide fat and solid preparations, which is applied in the field of medicine, can solve the problems of no improvement in the solubility and stability of torasemide, the increase of related substances, and the decrease of drug content, so as to improve bioavailability and reduce toxic and side effects Low, the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0070] On the other hand, the present invention provides the preparation method of above-mentioned torasemide liposome, and this method comprises the following steps:
[0071] (1) dissolving torasemide, soybean lecithin, cholesterol, sodium glycocholate and soybean sterol in an appropriate amount of ethanol to obtain a lipid solution;
[0072] (2) placing the above-mentioned lipid solution in a flask, in a constant temperature water bath at 35-40 °C, removing ethanol by rotary evaporation, and forming a uniform lipid film on the bottle wall;
[0073] (3) dissolve the sorbitol in water, slowly pour it into the flask and shake it gently, so that the lipid film is eluted and dispersed in a hydration medium to dissolve, to obtain a liposome suspension;
[0074] (4) The above suspension is filtered through a microporous filter membrane with a pore size of 0.45 μm, and spray-dried to obtain torasemide proliposome powder.
[0075] The torasemide liposome prepared by the method provi...
Embodiment 1
[0099] Example 1 Preparation of torasemide liposome tablet
[0100] The raw materials used are as follows:
[0101] Torsemide 5g
[0102] Soy Lecithin 40g
[0103] Cholesterol 5g
[0104] Sodium Glycocholate 7.5g
[0105] Sorbitol 40g
[0106] Soy sterol 4g
[0107] Lactose 50g
[0108] Starch 65g
[0109] Sodium Carboxymethyl Starch 10g
[0110] Povidone K30 10g
[0112] Preparation Process:
[0113](1) dissolve 5g torasemide, 40g soybean lecithin, 5g cholesterol, 7.5g sodium glycocholate and 4g soybean sterol in 800ml ethanol to obtain lipid solution;
[0114] (2) placing the above-mentioned lipid solution in a flask, in a constant temperature water bath at 40°C, and removing ethanol by rotary evaporation, a uniform lipid film is formed on the bottle wall;
[0115] (3) 40g of sorbitol was dissolved in 400ml of water, slowly poured into the flask and shaken gently, so that the lipid film was eluted and dispersed in a hydrated medium ...
Embodiment 2
[0120] Example 2 Preparation of torasemide liposome tablets
[0121] The raw materials used are as follows:
[0122] Torsemide 2.5g
[0123] Soy Lecithin 12g
[0124] Cholesterol 5g
[0125] Sodium Glycocholate 4g
[0126] Sorbitol 20g
[0127] Soy Sterol 1.5g
[0128] Lactose 26g
[0129] Starch 32g
[0130] Sodium Carboxymethyl Starch 5g
[0131] Povidone K30 5g
[0133] Preparation Process:
[0134] (1) Dissolve 2.5g torasemide, 12g soybean lecithin, 5g cholesterol, 4g sodium glycocholate and 1.5g soybean sterol in 400ml ethanol to obtain lipid solution;
[0135] (2) placing the above-mentioned lipid solution in a flask, in a constant temperature water bath at 40°C, and removing ethanol by rotary evaporation, and forming a uniform lipid film on the bottle wall;
[0136] (3) 20g of sorbitol was dissolved in 200ml of water, slowly poured into the flask and shaken gently, so that the lipid film was eluted and dispersed in a hydrated m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com