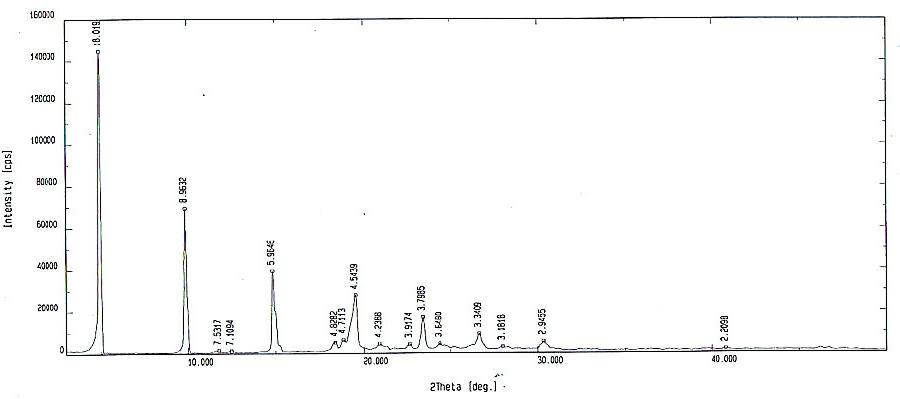
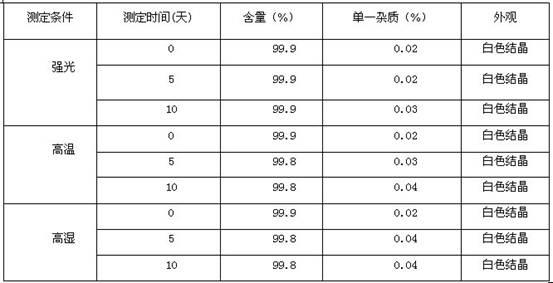
High-purity torasemide compound
A technology of torasemide and compounds, applied in the field of medicine, to achieve the effects of high yield, improved therapeutic effect, and low content of related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Take 5g of torasemide, add 115ml of isopropanol and 4ml of water, and heat to dissolve it. After all the solids are dissolved, continue heating, distill 36ml of liquid under normal pressure, cool to 0°C, stir, crystallize for 2 hours, filter after crystallization, and vacuum dry at 80°C for 4 hours to obtain 4.57g of pure crystals. The purity detected by HPLC was 99.9%.
Embodiment 2
[0060] Take torasemide 8, add 124 isopropanol and 6.4, and heat to dissolve it. After the solid is completely dissolved, add 54ml of isopropanol, cool to 0°C, stir, crystallize for 4 hours, filter after crystallization, and vacuum dry at 80°C for 4 hours to obtain pure crystalline product, 7.68g, which was detected by HPLC 99.9%.
Embodiment 3
[0062] Take 5g of torasemide, add 200ml of ethanol, heat to dissolve the solid, cool, stir at 10°C, crystallize for 2 hours, filter, and vacuum dry at 80°C for 4 hours to obtain 4.59g of pure crystalline product, which is detected by HPLC 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com